Abstract
Many Gram-positive bacteria can anchor their surface proteins to the cell wall peptidoglycan covalently by a common mechanism with Sortase A (SrtA), thus escaping from the host’s identification of immune cells. SrtA can complete this anchoring process by cleaving LPXTG motif conserved among these surface proteins and thus these proteins anchor on the cell wall. Moreover, those SrtA mutants lose this capability to anchor these relative proteins, with these bacteria no longer infectious. Therefore, SrtA inhibitors can be promising anti-infective agents to cure bacterial infections. Chinese herb medicines (CHMs) (chosen from Science Citation Index) have exhibited inhibition on SrtA of Gram-positive pathogens irreversibly or reversibly. In general, CHMs are likely to have important long-term impact as new antibacterial compounds and sought after by academia and the pharmaceutical industry. This review mainly focuses on SrtA inhibitors from CHMs and the potential inhibiting mechanism related to chemical structures of compounds in CHMs.
Introduction
Many Gram-positive bacteria are extremely prevalent and responsible for the mortality in the bacteria disease around the world. While for the bacteria themselves, surface proteins and/or pili proteins of Gram-positive pathogenic bacteria particularly play the key role during the infection process. These proteins could either adhere to host’s specific organ tissues or provide some way for bacteria to escape from host’s immune responseCitation1. Among these pathogens, Staphylococcus aureus (S. aureus) is particularly concerned. This Gram-positive pathogen is the leading cause of infections in the lower respiratory tract, bloodstream, skin and soft tissue nearly all over the world, causing diverse infections between human and animalsCitation2,Citation3. These extracellular proteins are covalently anchored to peptidoglycan by SrtA enzyme on bacterial cell wall after secreted by cells, making pathogens unrecognized during infectionCitation4,Citation5. In addition, the LPXTG motif is conserved among these special extracellular proteins with facilitating roles for bacteria to escape immune recognition, considering this we call them LPXTG-containing proteins. This SrtA-dependent anchoring of LPXTG-containing proteins is believed to be a necessary strategy for bacterial survival during infectionCitation6,Citation7.
Sortase is a kind of cell surface anchored transpeptidase, and cleaves specific target proteins at its specific sorting signal, for the covalent attachment to the peptidoglycan (). It is dispensable for microbial growth and survival, but necessary for bacterial infection processCitation8. Sortase homologs have been found in most available Gram-positive bacteria genomes; in most cases, more than one sortase genes have been identifiedCitation1,Citation9. Recently, four commonly described classes of sortases in gram-positive bacteria were dividedCitation10,Citation11. According to the sorting signals recognized and cleaved by the five subfamilies, subfamilies 1, 2 and 3 are, respectively, affiliated with the sortase A, B and C classes, respectively; and subfamilies 4 and 5 are affiliated to the sortase D class ().
Table 1. Cell wall sorting signals categorized by subfamily type.
One of the most important sortases among is the SrtA enzyme, which can recognize, cleave, and anchor specific LPXTG-containing proteins which contribute to bacterial infection process. As discussed above, SrtA inhibitors can interrupt the LPXTG-containing proteins anchoring process, and thus these proteins, which could combine to immunized host, failed in anchoring on the bacteria, making pathogens easily recognized by host during infection, which makes SrtA inhibitor a promising candidate for the development of treatments and/or prevents for major S. aureus’s infectionCitation11,Citation12. By contrast, there are significantly less SrtB identified than SrtACitation13. SrtB anchored its substrates deeper into the cell wall envelope due to the location of enzyme active site deep into cell wall, while proteins anchored by SrtA were displayed on the cell surfaceCitation14,Citation15. As membrane enzymes, SrtA would be more easily targeted by inhibitors and activity was wreaked, compared to intracellular bacterial targets. SrtC involves in flagella synthesisCitation9,Citation16, and similar to SrtA, SrtC could recognize and anchor the LPXTG-containing proteinsCitation17, and covalently tether the pilin subunits in Staphylococcus pneumonia, Enterococcus faecalis and Streptococcus agalactiaeCitation18–20, but it also requires help from SrtA to terminate assembly and to tether pili to the cellCitation10. The function of SrtD is not so clear up to now. Recent studies on the high GC % Gram-positive bacteria S. coelicolor suggested that SrtD enzymes may be related to mycelium formation in specific developmental conditionsCitation21,Citation22. In comparison, SrtA is well studied, and researches on the structure and catalytic mechanism of SrtA are extensive and detailed. In addition, considering the important role of SrtA paly in bacterial infection, we beleived SrtA inhibitors would be the key factor to cure bacterial infections.
S. aureus has the unique ability to generate suppurative lesions in virtually all organ systems by intruding into the deeper layers of host barriersCitation5. Significant reduction in virulence was observed in the S. aureus SrtA (SaSrtA) mutant (SrtA−); lacking of the SrtA activity, bacteria are unable to establish persistent infections, and bacterial cells are easily recognized by the immune system of host, while deletion or inactivation of other sortases does not obviously affect the infection of Gram-positive pathogensCitation23,Citation24, suggesting that the SrtA isoform plays a critical role in the pathogenesis of S. aureus by modulating the ability of the bacteria to adhere virulence-associated proteins to the host tissueCitation7,24–26. Moreover, there are no sortase homologs existing in eucaryon, and therefore selective toxicity is feasible. Above all, researches showed SrtA is not necessary for the growth and viability of S. aureus, and hence, SrtA inhibitors wouldn’t likely induce selection pressure for bacterial resistanceCitation27. Considering the important function of SrtA in S. aureus, SrtA inhibitors might consequently be promising novel candidates for the treatment and/or prevention of S. aureus infections without inducing selective pressures.
At prime tense, people tend to use antibiotics to treat bacterial infection disease. Afterwards, due to the abuse of antibiotics, bacterial strains resistant to antibiotics therapy appear, greatly threating the public health. Especially, the hospital strains of S. aureus are usually resistant to a variety of different antibioticsCitation28. Bacterial resistance to antibiotics is threatening the world, and has appeared in many fields of antibiotics applicationCitation29. Antibiotics treat bacterial infection diseases by preventing the assembly or synthesis of key materials which are essential for bacterial growth and reproduction of needs that are for growth. As a result, the target bacterium gradually formed resistant subpopulations under substantial stressCitation30. Most virulence factors produced by bacterium are not necessary for bacterial growth, and searching inhibitors for virulence factor is of great significance to cure bacterial infections by resistant bacteria, not inducing selective pressures at the same time. Surface adhesion proteins produced by S. aureus were the key virulence factors during infecting mammalian hosts, and SrtA is involved in the catalytic process of anchoring these surface adhesion proteinsCitation31. Hence, destruction the anchoring process of these proteins by inhibiting the activity of SrtA could reduce bacterial infection effectively. Moreover, SrtA inhibitors should be less likely to cause drug resistance or induce selective pressures. Currently, there have been some papers in the literature describing inhibitors of sortase running from synthetic products like small molecules to natural productsCitation32,Citation33. Mazmanian et al.Citation34 were the first researchers to publish a paper on SrtA inhibitors, where they showed that methanethiosulfonates or organomercurials displayed inhibitory effects on the SrtA reaction. However, people preferred to use CHMs for treatment now, especially in China, and therefore, novel antibacterial drug against SrtA are pressing needs. Plant-derived antimicrobial agents are always a source of novel therapeuticsCitation35, and CHMs have been accepted by doctors and patients as superior and a unique valuable property in China for a long timeCitation36,Citation37. The long history of CHMs exhibited it has enormous value in medicine fieldCitation38–40, and from this perspective, identifying antibacterial drugs from natural products like CHMs is urgently needed. Another reason for the renewed focus on medical plant antibacterial in the past 30 years has been own to increasing studies of CHMs treatment for bacterial infection diseases, and with the experiment conditions and technology greatly increased, many important components of CHMs has been clarified or are about to determine, which is of great use for us to determine the active inhibitors in the related CHM. Researches showed there were many plant extracts display strong inhibitory activity on SrtACitation34,Citation41, and the active substances of these CHMs could effectively inhibit SrtA activity at a concentration far lower than the minimal inhibitory concentration (MIC). That is, these CHMs could inhibit activity of SrtA without affecting the growth of bacteria or forming resistant subpopulations. Compared to traditional antibiotics, CHMs are easy to getCitation42,Citation43, and pharmacological activity of CHMs are based on their diversity of chemical components, and several components can simultaneously involve in the treatment of bacterial infections, while antibiotics nearly can’t be used simultaneously in case of adverse drug reactions or even death. Therefore, medicinal plants have received a significant attention as new sources of anti-microbial agents, and searches for effective SrtA inhibitors have shifted more attention to herbal medicine extracts.
Studies showed extracts from Curcuma longa, Fritillaria verticillata, Coptis chinensis, Radix isatidis, Radix Sophorae flavescentis, Radix Pulsatillae, both well-known traditional CHMs, have been widely used in medical practice in China for thousands of years. Moreover, the extracts of these CHMs exhibit inhibitory effect on SrtA with a relatively high MICCitation41.
Sortase A
Three-dimensional structure SrtA
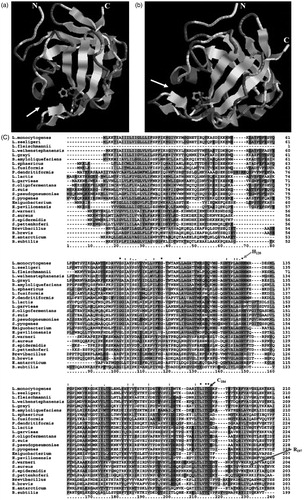
SrtA is a cell surface anchored thiol transpeptidase that cleaves LPXTG-containing proteins between threonine and glycine at the LPXTG motifCitation4. In Gram-positive pathogens, LPXTG-containing proteins promote adhesion to specific organ tissues, thus escaping from the identification of immune cellsCitation7,Citation44,Citation45. SaSrtA (SrtA of S. aureus, Genebank) is a 206-amino acid polypeptide with a hydrophobic N-terminal segment probably functioning as both a secretion signal peptide and a stop transfer signal for membrane anchoringCitation7. Quaternary structure of SaSrtA (modeled by SWISSMODELCitation46 http://swissmodel.expasy.org/) contains a unique β-barrel structure including eight β-pleated sheets, and three other shorter β-pleated sheets ().
Figure 1. The predicted quaternary structure of SaSrtA. The predicted His120, Cys184 and Arg197 in the active site are highlighted. The α-helix and β-sheet regions of the putative protein are indicated with cylinders and bands, respectively. Multiple sequence alignments revealed many conserved domains. The three amino acid residues thought to be involved in the formation of the catalytic site were conserved in those domains (highlights) of all 25 sequences. His120, Cys184 residues, participating in protein–protein interaction, are conserved between these bacteria above.
The key amino acids of SrtA
Three β-pleated sheets of the β-barrel structure were curved and folded, forming a notch, the active site of SrtA. The active site of hydrophobicity contains His120, Cys184, Arg197, Ala118, Val161 Val166, Ile182 (both of them are amino acids, and the number represents position on the peptide chain)Citation47–49, The catalytic site residue Cys184 is in close proximity with His120, forming a putative active site cysteine-histidine ion pairCitation50,Citation51. Histidine and Cysteine are both of great importance in maintaining the activity of SrtACitation50,Citation52, and so is Arg197Citation53, making the cleavage of Thr and Gly more easily by ionizing Cys184Citation54. The sulfhydryl group of Cys184 can also attack the T-G peptide bond of LPXTG-containing proteins, resulting in an enzyme-acyl intermediate formationCitation34,Citation55. While CHMs possess aryl (β-amino) ethyl ketones, vinyl sulfones as well as other compounds of a nucleophilic attack could inhibit SrtA activity by conjugating to the thiol of Cys184 of SrtACitation56, as well as those reacting with Histidine and Arginine, thus irreversibly inhibiting SrtA activityCitation57,Citation58. Besides, hydrophobic compounds could also penetrate into the active site though hydrophobic interaction, and threonine analogs can also inhibit the activity of SrtACitation59.
Phylogentic analysis of SrtA
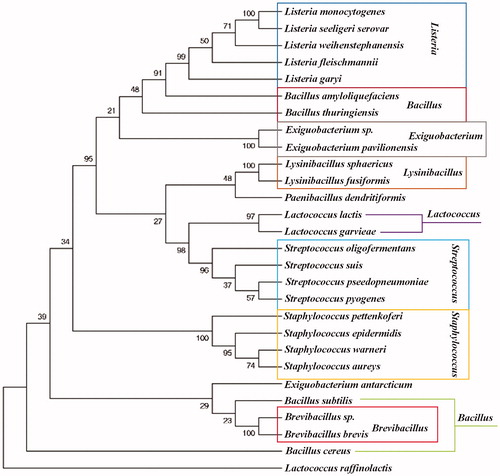
The available complete genome sequences enabled us to identify twenty-five SrtAs from nine Gram-positive bacteria species (Clustal X 2.0 program http://clustalx.software.informer.com/2.0/). Multiple sequence alignments revealed some conserved domains among these sequences (). As expected, the three amino acid residues (His120, Cys184, Arg197) thought to be involved in the formation of the catalytic site were conserved in those domains between these bacteria studied (highlights in ) of all twenty-five sequences. Nine groups of these twenty-five SrtA enzymes, were chosen to construct the phylogenetic tree () by using MEGA 6.0.6 softwareCitation60 (http://www.megasoftware.net/), Bacillus, Brevibacillus, Exiguobacterium, Lactococcus, Listeria, Lysinibacillus, Paenibacillus, Staphylococcus, Streptococcus. Universally, Brevibacillus, Listeria, Lysinibacillus, Staphylococcus, Streptococcus, and most of Exiguobacterium are clustered definitely into their own group, which implied a conserved evolutionary relationship among these species. Nevertheless, the Lactococcus exhibited an ambiguous relationship with a few Exiguobacterium (such as Exiguobacterium antarcticum), Paenibacillus and Bacillus (Bacillus subtilis and cereus). In conclusion, SrtAs exhibited a certain degree of homology among these nine groups, thus SrtA inhibitors of S. aureus can also reduce infection of pathogens among these nine groups other than S .aureus.
Figure 2. Phylogenetic relationship of the SrtA of various species. The SrtA used for phylogenetic tree construction are as the amino acid sequences used for homology alignment above. Numbers below the branches are the neighbor joining bootstrap values. The evolutionary distance is reflected by the numbers above the branches and the branch lengths proportional to the degree of amino acid substitutions. SrtAs exhibited a certain degree of homology among these nine groups.
The catalytic process of SrtA
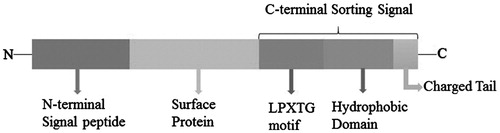
LPXTG-containing proteins, anchored by SrtA, mostly harbor an N-terminal signal peptide and a C-terminal cell wall sorting signal consisting of the LPXTG motif, hydrophobic domain and a positively charged tail in all pathogenic Gram-positive bacteria ()Citation61–63.
Figure 3. The structure of the LPXTG proteins precursor. (The length of rectangle does not stand for the real length of peptide). The LPXTG proteins, anchored by SrtA, mostly harbor an N-terminal signal peptide and a C-terminal cell wall sorting signal consisting of the LPXTG motif, hydrophobic domain and a positively charged tail have been found in all pathogenic Gram-positive bacteria, besides the anchored protein section.
After synthesized in the cytoplasm, LPXTG-containing proteins are released into extracellular space through the secretary pathway with N-terminal leader peptide moved. Then, SrtA catalyzes the breakage of amide bond between threonines and glycine in the LPXTG motif, and captures the C-terminal carboxyl of cleaved surface proteins by forming a thioester bond with its active site sulfhydryl on the cell surfaceCitation64. Subsequently, the nucleophilic attack of the amino group of the lipid II peptidoglycan precursor propels LPXTG-containing proteins to form an amide bond with the cell wall cross-bridge, regenerating the active site sulfhydryl of SrtACitation52,Citation64 (). During anchoring process, the scissile T-G peptide bond is positioned between the two active site residues, Cys184 and Arg197, and the active site is stabilized by His120Citation65, forming a hydrogen bond with Arg197Citation66. Altogether, SrtA completes the transpeptidation sorting reaction based on the surface protein secretion and cell wall synthesis pathways.
Figure 4. Cell wall sorting progress in Gram-positive bacteria. (1,2) Cell wall synthesis begins with the assembly of nucleotide linked wall peptides in the bacterial cytoplasm. Then the precursor, the lipid І, is transferred to bactoprenol pyrophosphate in the cytomembrane, next, N-Acetylglucosamine (G) connect to lipid І (MurNAc-(pentapeptide)-pyrophosphoryl-undecaprenol), forming the lipid II (GlcNAc-β-1,4-MurNAc-(pentapeptide)-pyrophosphoryl-undecaprenol). Finally lipid II molecules are transported across the cytoplasmic membrane for the polymerization reactions in bacteria. (3–6) Surface proteins are synthesized as precursors in the bacterial cytoplasm bearing an N-terminal signal peptide and a C-terminal sorting signal. The sorting signal contains an LPXTG sequence motif, a hydrophobic domain and a tail of positively charged residues. After surface protein synthesized, the N-terminal signal peptide will be removed before secreted out, and then the hydrophobic domain is embedded within the membrane and the charged tail remained in cell, retaining surface proteins in the secretory pathway. (5) The precursor cleaved by SrtA, a membrane-anchored transpeptidase, between threonines and glycine in the LPXTG motif, generating an acyl enzyme intermediate. (6) The thioester bond between SrtA and surface proteins, is resolved by the nucleophilic attack of the amino group of the cross-bridge (Gly5) within lipid II precursor. Finally, surface proteins linked to lipid II can be incorporated into the cell wall envelope.
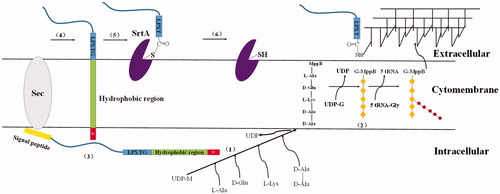
LPXTG-containing Proteins anchored by SrtA
SrtA anchors a number of proteins, such as protein A, fibronectin-binding proteins, collagen-binding proteins and pili proteins, covalently attached to the bacterial cell wall by a common mechanism that is ubiquitous amongst Gram-positive organisms.
Surface proteins
S. aureus is a human and animal pathogen, which causes diverse infectionsCitation67. Staphylococci lack pili and instead rely on surface protein-mediated adhesion to host cells or invasion of tissues as a strategy for escape from immune defenses by surface proteins. These surface proteinsCitation68 covalently anchored by SrtA include the fibronectin-binding proteinsCitation69,Citation70, collagen adhesion protein, and protein A. Fibronectin-binding proteins consist of FnbpA and FnbpBCitation71–73, which can interact with the fibronectin of mammalian cellsCitation74 (). In addition, Staphylococcal strains causing connective osteomyelitis or tissue infections regularly through the collagen adhesion proteinsCitation84,Citation85, and these collagen adhesion proteins can bind to host’s collagensCitation86. Besides, S. aureus protein A (Spa) binds to the Fc termini of mammalian immunoglobulins in a nonimmune fashion, resulting in the uniform coating of Staphylococci with antibodiesCitation87 ().
Table 2. Proteins anchored by SrtA.
Flagellin in Gram-positive bacteria
Expression of filamentous structures extending from bacterial surface is the common mechanism of bacteria to infect host tissues during colonization. The pili proteins have been demonstrated to improve the progression of infective diseases that affect periodontal, gastrointestinal, urinary, and respiratory tissue. Besides, flagella have been implicated in a number of biological processes, including biofilm formation, cell aggregation, host cell adhesion and invasion, and as potent inducers of the host’s inflammatory responseCitation88,Citation89. Particularly, the protein components of flagella are covalently polymerized by sortase enzymes in pathogenic bacteriaCitation90, similar to those surface proteins involved in the covalent attachments to the peptidoglycan cell wall of Gram-positive bacteriaCitation88. The adhesion moieties in flagella are distally located from the bacterial surface, which provide a significant advantage in the initial steps for establishing a successful colonization. Flagella are polymeric virulence factors necessary for bacterial colonization and pathogenesisCitation91, during the assembly process of flagella in Streptococci, SrtA is responsible for anchoring the polymerized pilus to the cell wallCitation92,Citation93, catalyzing proteins of LPXTG motif anchoringCitation94, and transferring the entire pilus structure onto lipid II, and ultimately, it becomes incorporated into peptidoglycan, forming the main site of infectionCitation90,Citation95. Deletion of SrtA releases a large portion of SpaA polymers into the culture mediumCitation96, indicating SrtA does indeed play some role in pilus assembly, specifically, in the anchoring phaseCitation20.
Chinese Herb medicines as inhibitors of SrtA
Fritillaria verticillata
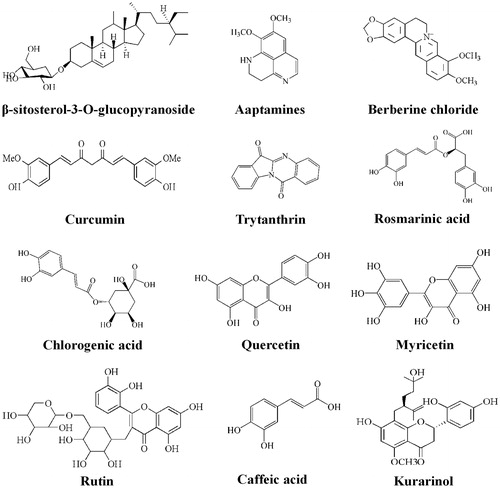
Fritillaria verticillata (Chinese name: Bei mu cao, Liliopsida) can be also used as one herbal medicine to inhibit SrtA activity, and it mainly produced in northern Xinjiang province, a member of Liliaceae familyCitation97. Bulbs of Fritillaria have been used as one of the most important antitussive expectorant and anti-hypertensive drugs in TCMs. Extracts from Fritillaria species bulbs are used to treat scrofula, coughs, asthma, tumors, etcCitation98. Extracts from the bulbs of Fritillaria verticillata had significant inhibitory effect on S. aureusCitation99. The β-sitosterol-3-O-glucopyranoside () isolated from the bulbs of Fritillaria verticillata by bioassay-guided chromatographic fractionation had an antibacterial activity against Bacillus subtilis, Staphylococcus aureus and Micrococcus leuteusCitation100 with MIC values of 50, 200 and 400 mg/mL, respectively. Once using extracts of Fritillaria verticillata bulbs to incubate S. aureus and analyzed the SrtA activity by fluorescence spectrophotometerCitation100, results showed that the isolated β-sitosterol-3-O-glucopyranoside from Fritillaria verticillata extracts exhibited inhibiting SrtA activity with an IC50 of 18.3 μg/ml (), almost two times more potent than the positive control, pHMB.
Table 3. Antibacterial activity of the herbal extracts on SrtA of S. aureus and effective material.
The β-sitosterol-3-O-glucopyranoside is one of sterolglycoside compoundes, of great hydrophobicity, and it can destroy S. aureus adhesion to fibronectin through fibronectin-binding proteinCitation101. The OH of the alkyl cyclopentane and alkyl cyclohexane in β-sitosterol-3-O-glucopyranoside can form H-bonds with the guanidine group of Arg197, and the aromatic rings can form strong lipophilic interactions with the hydrophobic active site of SrtACitation102, thus inhibiting the activity of SrtA. In addition, the hydroxyl group of the cyclohexane could also form another hydrogen bond with the backbone carbonyl of Gly192 and keeps the β7/β8 loop in a closed state. Moreover, the glucopyranoside side chain moiety was found to be the active component of the compound because elimination of this side chain moiety led to a reduction in inhibitory activityCitation103, probably also because OH of the cyclohexane moiety of the glucopyranoside side chain moiety can form hydrogen bonds with Arg197Citation104, both of which are important for Cysteine to complete the catalytic process. Hence, we can find out whether other CHMs containing sterol glycoside compounds can inhibit the activity of SrtA, which can provide one approach to find more herbs to inhibit SrtA, in addition figure out whether several kinds of CHMs taken together can strengthen inhibitory activities on SrtA.
Coptis chinensis Franch
Coptis chinensis Franch (Chinese name: Huang lian), a perennial herb, another valid inhibitor of SrtA, is a member of the Ranunculaceae family, grows in environment of low temperature and high air humidityCitation105. Coptis chinensis Franch as one TCM has a long history. As recorded in Compendium of Materia Medica, Coptis chinensis Franch has functions on many diseases. It has been used as antimicrobial agents, anti-inflammatory, hypolipidemic, hypoglycemicCitation106.
Generally, the extracts of Coptis chinensis Franch rhizome showed antibacterial effect especially on S. aureusCitation107. Berberine in Coptis chinensis Franch well inhibited the infection ability of S. aureusCitation108 and many other bacteriaCitation109, and S. aureus was most sensitive to berberine. Berberine chloride is one type of isoquinoline alkaloid (, ), from the rhizomes of Coptis chinensis Franch appeared a potent inhibitory effect on SrtA, with an IC50 value of 8.7 μg/ml and also had antibacterial activity against Gram-positive bacteria, with a MIC more than 100 μg/ml, made a comparison to available isoquinoline alkaloids berberine sulfate, palmatine chloride, and β-hydrastine, which are all antibacterial substancesCitation12.
As previously revealed, similar to curcumin, the aromatic rings of berberine chloride could combine to the hydrophobic side chains (the side chains of from the β6/β7 loop) through hydrophobic effect, embedded into the active site of SrtA, thus inhibiting SrtA activitiesCitation104. In addition, the nitrile group of Berberine chloride can form a hydrogen bond with SrtA catalytic residue Arg197 via hydrogen bondingCitation102. Coptis chinensis Franch as one CHM to treat bacterial infection contains many other compounds except isoquinoline alkaloid. Furthermore, isoquinoline alkaloids have been found mainly in some plant families, such as Papaveraceae, Magnoliaceae, Rutaceae and Ranunculaceae as well as Fumaria capnoides Linn, providing more herb medicines for us to study to find more inhibitors ().
Radix isatidis
Radix isatidis (Chinese name: Ban lan gen), a member of Cruciferae, Dicotyledoneae, is also known as Indigowoad Root, and this herb is cultivated in various regions of northern ChinaCitation110. The roots are harvested during the autumn and dried, and then get Radix isatidis, which are usually processed into granules called Banlangen granules. This product, commonly dissolved in hot water, is very popular throughout China, and used to remove toxic heat, soothe sore throat and to treat pharyngitis, laryngitis, erysipelas as well as carbuncle, in addition, it possesses an antibacterial, antiviral, improve immunity, antitumor effectCitation111,Citation112. Especially the prevention and cure of SARs, Radix isatidis again became the focus and hotspot of people’s research into medicinal value. In recent years, the study of Radix isatidis, especially the study of its chemical composition has been sharply increasingCitation110,Citation113,Citation114. The chemical composition isolated from this herb mainly contains the following categories: indole alkaloid and isoquinoline type alkaloid, flavonoid, lignin, organic acids, ketone, sterols and amino acidsCitation114. The extracts of Radix isatidis displayed inhibitory effect on Gram-positive bacteria as well as Gram-negative bacteria, S. aureus, S. epidermidis, B. subtilisCitation115. The method of systematic solvents was used to identify inhibitory effects of the different organic solvents extracts on S. aureusCitation116, and results showed that the total extract exhibited the higher antibacterial effect than others and that the antibacterial effects of Radix isatidis were not limited in one active portionCitation110.
As previously revealed, indole alkaloid and isoquinoline type alkaloid possess a prominent inhibitory effect on SrtACitation117, which were rich in nitrile groups and phenyl rings. The nitrile groups could form hydrogen bonds with the catalytic residue Arg197, and the aromatic rings formed strong lipophilic interactions with the amino acids in the hydrophobic binding pocket of the SrtA active site. Aaptamines from the tropical sponge, also in Radix isatidisCitation114, is one of indole alkaloid and can inhibit the activity of SrtACitation118. The extract of Radix isatidis contains lots of indole alkaloids, such as aaptamines, 5-hydroxyoxindole, indigo, indirubin, indole-3-acetonitrile-6-O-β-D-glucopyranoside, 2-[(3′-indole)-cyanomethylene]-3-indolinone, 2,3-dihydro-4-hydroxy-2-oxo-indole-3-acetonitril, 3-(3′,5′-Dimethoxy-4″-hydroxybenzylidene)-2-indolinone. These indole alkaloids possess acetonitrile groups mostly, which could combine to the sulfhydryl of Cys184 in SrtA, thus inhibiting the activity of SrtACitation59. Hence, the inhibitory effect of Radix isatidis can be due to the acetonitrile groups in these indole alkaloids. In addition, trytanthrin, another compound in the extract of Radix isatidis, is an isoquinoline type alkaloid. The aromatic rings in aaptamines, as hydrophobic groups, can combine to the hydrophobic side and occupy the sorting signal recognition region through hydrophobic interactions with the side chains from the β6/β7 loopCitation104 (), preventing LPXTG-containing proteins’ combination with SrtA, while the amide carbonyl, NH groups and the oxygen atom in aaptamines, can link to Cys184, leading to a failure of the amide bond between SrtA and LPXTG-containing proteins. According to the research about aaptamines, trytanthrin probably can inhibit the activity of SrtA. Moreover, there are still some flavonoids in Radix isatidis, such as quercetin and myricetin. Both myricetin and quercetin were potent SrtA inhibitors, and they inhibited SrtA activity with IC50 values of 44.03 μM (13.99 μg/ml) and 52.70 μM (15.91 μg/ml)Citation119, respectively. Both of the two flavonoids reduced bacterial clumping to fibrinogen in a dose-dependent manner trough fibronectin binding proteins (FnbpA and FnbpB), which anchoring process was catalysis by SrtA (). All these two flavonols tested in S.S. Kang’s studies showed they exhibited nearly no growth inhibition effect on S. aureus Newman strain with a MIC values greater than 300 μM.
Radix Sophorae flavescentis
Radix Sophorae flavescentis is another effective inhibitor of SrtA, a member of Leguminosae family, throughout Russia, Japan, India, Sorth Korea and China. The dried root of Radix Sophorae flavescentis, is well known to possess anti-inflammatory, anti-arrhythmic, anti-pyretic, anti-asthmatic, as well as anti-ulcerative effects and is used for the treatment of diarrhea, gastrointestinal hemorrhage and eczemaCitation120. Radix Sophorae flavescentis is rich in flavonoids, of a wide range of biological activities, including anti-cancer, anti-inflammatory and anti-bacterial propertiesCitation121. The flavonols kurarinol was the most effective inhibitors of SrtA compared to others isolated from Radix Sophorae flavescentis, with an IC50 of 48.63 μg/mL, and a MIC of 99.54 μg/mL almost with no effect on S. aureus growth activityCitation121 (), which is better than kurarinone, another compound from Radix Sophorae flavescentis, with one less hydroxyl group compared to kurarinone. The inhibitory activity of flavonols depend mainly on the hydroxyl substitution in flavonolsCitation119,Citation122. The mechanism of the inhibitory effect of kurarinol on SrtA may be due to the hydroxyl groups in kurarinol forming hydrogen bonds with both the side CHA amino of Arg197 and the backbone of Gly1925Citation4, resulting in SrtA activity weakened, and thus failing in intermediate product formation (SrtA-T-surface protein). Moreover, the aromatic rings could also interact with the side chains and combine to the hydrophobic active site of SrtA, occupying the sorting signal recognition region of SrtACitation104.
Kurarinol is one kind of flavonoids, hence, other Chinese herbs containing flavonols flavonoids maybe potentially possess SrtA inhibition. Flavones and flavonols are usually extracted from vegetables, fruit, tea, and red wine, and among natural flavonols, Flavonoids such as rutin, morinCitation123, myricetin, and quercetin are active against SrtACitation119. Rutin in Ruta graveolens L. could inhibit SrtA activity in vitro experiments, with an IC50 of 57 μg/mL and little bacteria inhibitionCitation124. Similar to the above mentioned CHMs, the aromatic rings of rutin can also make a difference in hindering the catalytic process of SrtA. Besides, the oxygen atom can form hydrogen bond with His120 and Arg197 residues of SrtA. In addition, there are two flavonoids, myricetin and quercetin in the bark of Rhus vernicifluaCitation119 also existing in Radix isatidis, exhibited strong SrtA inhibitory activity (see previous section in Radix isatidis). Moreover, although the two flavonoids structure is similar, they exhibited different inhibitory activity. The number of hydroxyl groups at the specific location of the particular aromatic rings gave them different inhibitory capacity on SrtACitation119. Another Chinese herb rich in flavonols is Rhizoma Scutellariae, and the major components of R. scutellariae include baicalein, baicalin, wogonin, and wogonoside. The root of Rhizoma Scutellariae is a traditional medicine, displaying a better anti-bacteria effect, and the extract of Rhizoma Scutellariae reveals an obvious inhibitory effect on S. aureus in vivo and vitroCitation125,Citation126. Although, we did not know which flavonoids exhibited inhibitory effect on S. aureus, according to the above conclusion that many flavonoids could inhibit SrtA activity due to their hydroxyl groups and aromatic rings, Rhizoma Scutellariae might be a potential candidate inhibitorCitation127 ().
Curcuma longa
Curcuma longa (Chinese name: Jiang Huang), an effective inhibitor of SrtA and also known as turmeric a perennial herb. It is a member of the Zingiberaceae (ginger) family, and can be cultivated extensively in India, China, and other countries with a tropical climateCitation116. The rhizome of Curcuma longa appears irregular ovoid, heavy yellow, rough and shrinking grain in the surface, and therefore the rhizome is widely used in foods for its flavor and color. Moreover, the rhizome of Curcuma longa has a long traditional use in the Chinese and Ayurvedic systems of medicine, particularly as an anti-inflammatory drug and also for the treatment of flatulence, jaundice, menstrual difficulties, hematuria, hemorrhage, and colicCitation128. Curcuma longa can also be applied topically in poultices to relieve pain and inflammationCitation129. The use of Curcuma longa extracts for the antimicrobial activity has long been known. Curcuma longa and its extracts have various beneficial effects on human healthCitation130. Curcumin derivatives is one kind of important ingredients from Curcuma longa extracts, and curcumin can exhibit antioxidativeCitation131, anticarcinogenicCitation132, anti-inflammatoryCitation133 and hypoglycemic potencyCitation134,Citation135.
Many pharmacological properties of Curcuma longa have been reported to date including its inhibitory effects on SrtACitation103, but its inhibitory mechanism on the Gram-positive bacteria SrtA has not been figured out. Analysis of the active compounds isolated from Curcuma longa, their inhibitory effects on SrtA and the minimum inhibitory concentrations (MICs) against S. aureus, showed that the curcumin could inhibited the S. aureus SrtA, resulting in failure of S. aureus cell adhesion to fibronectinCitation135. with an IC50 (50% inhibiting concentration) value of 0.7 μg/mL, more effective than pHMB (p-hydroxymercuribenzoic acid, IC50: 1.2 μg/mL)Citation135. In addition, curcumin showed no obvious growth inhibitory activity against S. aureus with MICs greater than 200 μg/mLCitation135, and therefore SrtA inhibitors should disrupt bacterial infections without inducing drug resistanceCitation7. Altogether, Curcuma longa is an excellent candidate in the development of a bacterial SrtA inhibitor.
The active site of SrtA was a large hydrophobic binding pocketCitation136, which was composed of lipophilic side chains of the amino acids, such as Val193, Trp194, Ala92, Ala104, Leu169, Val168, and Ile182Citation102 and the location of the hydrophobic binding pocket as shown in . These hydrophobic side chains have relatively strong lipophilic interactions with the phenyl rings of curcumin through the hydrophobic effect. In addition, two aromatic ring of curcumin are located in the trans orientation, similar to the structural formula of (Z)-3–(2,5-dimethoxyphenyl)-2–(4-methoxyphenyl) acrylonitrile (DMMA), one proved inhibitor of SrtACitation102,Citation137. Besides, the aromatic ring could form hydrophobic interactions with the side chains of residues from the β6/β7 loop (Val169, Leu168) and Arg197, occupying the sorting signal recognition region of SrtACitation104 (). In conclusion, curcumin of Curcuma longa, as an inhibitor of SrtA, maybe interdict the catalysis process of SrtA by embedding the two aromatic rings into its active site through hydrophobic interactionCitation111 and forming hydrogen bonds, and as a result, LPXTG-containing proteins can’t combine to cell wall of the bacteria by SrtA. Other curcumin derivatives from extracts of Curcuma longa also exhibited inhibitory effect on SrtACitation135, which would replenish Curcuma longa could be used for SrtA inhibitor as a potential medicine for bacterial infection. Besides, curcumin belongs to polyphenolic compounds, also known as a dicarbonyl compounds, shows an inhibitory effect on SrtA, thus we can find out whether other CHMs containing dicarbonyl compounds of aromatic rings can inhibit the activity of SrtA (, ).
Radix Pulsatillae
Radix Pulsatillae, Chinese Pulsatilla Root (shennong baicaojing) or the Korean pasque flower, one member of Ranunculaceae family, Dicotyledons class. Radix Pulsatillae is a perennial plant growing in China and Korea, and used as a traditional CHM for a long timeCitation138,Citation139. The rhizomes of Radix Pulsatillae have been used as a CHM for malaria, leucorrhea, epistaxis, scrofula, amebic dysentery and internal hemorrhoids, and the root is anti-inflammatory and anti-parasiticCitation140. It contains several medically active constituents including anemonin as well as saponinsCitation141.
The rosmarinic acid, phenyl propanoids caffeic acid, exhibited a potent inhibitory effect on SrtA of Streptococcus mutants isolated from human oral cavity; what’s more, these two compounds from the extract of Radix Pulsatillae demonstrate similar inhibition degreeCitation142. Rosmarinic acid, a kind of natural multifunctional phenylpropanoids compounds, has been used as anti-infective, anti-inflammatory, anti-virus and anti-tumor. Phenyl propanoids caffeic acid, one kind of phenylpropionic acids, possesses similar functions to rosmarinic acid.
The IC50 of rosmarinic acid and phenyl propanoids caffeic acid is 20.1 and 20.2 μM (IC50), respectively, with pMHB of 35.1 μMCitation142. One mechanism of the inhibitory effect of rosmarinic acid and phenyl propanoids caffeic acid on SrtA may be due to the location of hydroxyl group in these compounds. The presence of a hydroxyl group at the position 3 of caffeic acid exhibited greatly important compared with other phenylpropionic acids of Radix Pulsatillae, possessing an obviously higher degree of inhibition, so dose rosmarinic acid. The hydroxyl group at the position 9 could also affect inhibitory activity on SrtA. In addition, the aromatic rings of the compounds owns a similar function to those in the CHMs mentioned above. Besides, the carboxyl of these two compounds can be as strong nucleophilic groups connected the Cys184 of SrtA, resulting in the failure of intermediate product formation.
Natural phenyl propanoids caffeic acid mainly exists in feverfew, ranunculaceae, rutaceae plantsCitation143, and natural rosmarinic acid is widely distributed in plants, mainly contained in labiatae, boraginaceae, umbelliferae, cucurbitaceae and tiliaceae plantsCitation144. Natural chlorogenic acid, one phenylpropanoids compounds similar to rosmarinic acid, is rich in plants of Lonicera, Artemisia, such as Flos Lonicerae. Chlorogenic acid is one main antibacterial, antiviral active ingredient in Flos Lonicerae, and it could cause S. aureus infection failure through SrtA inhibition with an IC50 33.86 μg/ml, interfering in Fg/Fn binding and attenuate the virulence of S. aureusCitation104. Chlorogenic acid could localize to the “activity” region of SrtA, and the hydroxyl group of the cyclohexane in this phenylpropanoids compound formed hydrogen bonds with both amino acids Arg197 and the backbone of Gly192. Moreover, Chlorogenic acid could also form Van der Waals interactions with the side chains of Cys184 and Ile182Citation104. This new finding laid the foundation for us to search the phenylpropanoids-containing Chinese herbs as SrtA inhibitors to cure bacterial infectionCitation145 ().
Summary and conclusion
Sortase enzymes, anchoring proteins with different sorting signals, allow gram-positive bacteria to complete protein location and functional display within the cell wall envelope, and response to environment or specific host signals during infection. SrtA, one transpeptidase positioned on the outside of bacterial cells, catalyze the process of amide bond exchange, using LPXTG-containing proteins and peptidoglycan as substrates, play an very important role during Gram-positive bacterial infection process. Thus, finding SrtA inhibitors became the key point. However, in view of the abuse of antibiotics, the number of bacterial drug resistance is increasing, so we turn to CHMs, of medicinal value and with long history, to find out inhibitors of SrtA rather than antibiotics in case of more drug resistance.
Here, we list some traditional Chinese herbs as inhibitors of SrtA from S. aureus, Curcuma longa, Fritillaria verticillata, Coptis chinensis Franch, Radix isatidis, Radix Sophorae flavescentis and Radix Pulsatillae, and their effective constituents belong to which kind of chemical compounds. Hence, we can go on searching for other CHMs with similar above mentioned chemical compounds as new inhibitors of SrtA. In addition, these demonstrated inhibitors from other natural sources, like the sponge Spongosorites sp.Citation32 and ascidian Synoicum sp.Citation146 can also put a good foundation for studying herb medicines with similar compounds. Evidences from published data have shown that compounds isolated from these CHMs are effective against SrtA from S. aureus and other pathogenic microorganisms.
However, these research on CHMs are mostly conducted in vitro, thus, the inhibition of these herbs solely on the basis of IC50 values must be carefully considered. A determination of the mechanism about the inhibition on SrtA by these classes is required to completely and clearly understand, as well as their relative effectiveness and specificity; i.e., is the inhibition competitive in the presence of substrate? Do these inhibitors reversibly or irreversibly react with SrtA? What are the affinities and kinetics of inhibition? In light of the fact that many compounds can inhibit enzyme activity based on redox action, promiscuous aggregation, denaturation, pH changes, and so forth, not to mention possible quenching of fluorescence or spurious reaction with peptide substrate during the experiment process when considering the sortase activity assay, thus it seems prudent to carefully investigate the nature of the inhibitionCitation147,Citation148. Finally, we should also consider how to use these inhibitors and what form taken by us to get the best medicinal value in vivo. In addition, we should also consider how these inhibitors can aggregate in targeted points. In one word, we need further more experiments for minimum amount and the best curative effect of herb medicines in the future, unlike antibiotics resulting in drug abuse.
Current research and prospect forecast
Recently, there are more and more studies on the research about SrtA and biological function in Gram-positive pathogens, thus we figure out the catalytic mechanism of SrtA and its vital function in those pathogens. Considering that bacterial resistance to antibiotics has become a serious threat to public health, novel drugs against Gram-positive bacterial infection are urgently needed, and plant-derived antimicrobial agents are always a source of novel therapeutics, thus CHMs draw our attention on the treatment for bacterial diseaseCitation35,Citation149. Moreover, once we make it clear that how the active constituents of these herb medicine interact with SrtA, and make sure their chemical construction and spatial structure, as well as the key functional groups of these compounds interacting with the active site of SrtA, we can design some analog inhibitors similar to those natural being as new inhibitors of SrtA. Moreover, figuring out mechanisms between inhibitors and SrtA, reversible or irreversible, will undoubtedly be the focus of in-depth investigations in order to develop future environmental, biomedical applications.
Recent studies has also shown that SrtA can be used for protein modification, such as SrtA has been involved in site-specific modification, to find out their applications in the development of therapeutic antibodies, which allows the spatial and temporal control of proteins in vivo, as well as single molecule trackingCitation150. For example, Lactic acid bacteria (LAB) are a diverse group of Gram-positive bacteria found in a vast array of environments including dairy products and the human gastrointestinal tract. What’s more, genomic sequencing of LAB and annotation has allowed for the identification of sortase (especially SrtA) and sortase-dependent proteins (LPXTG-anchored proteins). Nowadays, we also use SrtA for protein modification by LPXTG motif; therefore, we can use SrtA for drug design. Specifically, the use of the LPXTG motif was investigated for in vitro vaccine delivery using food grade and probiotic lactobacilli as the presentation vector for the antigenCitation151. Intriguingly, SrtA was used for semienzymatic cyclization of disulfide-rich peptides for drug designCitation152.
Up to now, the sortase-mediated ligation (especially SrtA) has been successfully applied to many fields such as protein lipidationCitation152,Citation153, PEGylationCitation154, protein immobilization for drug-design and protein-modificationCitation155.
Declaration of interest
The authors state no conflict of interest. This work was supported by the National Natural Science Foundation of China (20776051) and the Scientific Returned Overseas Chinese Scholars, State Education Ministry (2005546).
References
- Pallen MJ, Lam AC, Antonio M, Dunbar K. An embarrassment of sortases – a richness of substrates? Trends Microbiol 2001;9:97–101
- Diekema D, Pfaller M, Schmitz F, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the Sentry Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis 2001;32:114–32
- Maresso AW, Wu R, Kern JW, et al. Activation of inhibitors by sortase triggers irreversible modification of the active site. J Biol Chem 2007;282:23129–39
- Mazmanian SK, Ton-That H, Su K, Schneewind O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci USA 2002;99:2293–8
- Osaki M, Takamatsu D, Shimoji Y, Sekizaki T. Characterization of Streptococcus suis genes encoding proteins homologous to sortase of Gram-positive bacteria. J Bacteriol 2002;184:971–82
- Kharat AS, Tomasz A. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect Immun 2003;71:2758–65
- Mazmanian SK, Liu G, Jensen ER, et al. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA 2000;97:5510–15
- Fitzgerald-Hughes D, Devocelle M, Humphreys H. Beyond conventional antibiotics for the future treatment of methicillin-resistant Staphylococcus aureus infections: two novel alternatives. FEMS Immunol Med Mic 2012;65:399–412
- Marraffini LA, Schneewind O. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Mol Microbiol 2006;62:1402–17
- Spirig T, Weiner EM, Clubb RT. Sortase enzymes in Gram-positive bacteria. Mol Microbiol 2011;82:1044–59
- Dramsi S, Trieu-Cuot P, Bierne H. Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res Microbiol 2005;156:289–97
- Kim S-H, Shin D-S, Oh M-N, et al. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by isoquinoline alkaloids. Biosci Biotech Biochem 2004;68:421–4
- Bradshaw WJ, Davies AH, Chambers CJ, et al. Molecular features of the sortase enzyme family. FEBS J 2015;282:2097–114
- Mazmanian SK, Skaar EP, Gaspar AH, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 2003;299:906–9
- Zong Y, Mazmanian SK, Schneewind O, Narayana SV. The structure of sortase B, a cysteine transpeptidase that tethers surface protein to the Staphylococcus aureus cell wall. Structure 2004;12:105–12
- Ton-That H, Schneewind O. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol 2003;50:1429–38
- Barnett TC, Scott JR. Differential recognition of surface proteins in Streptococcus pyogenes by two sortase gene homologs. J Bacteriol 2002;184:2181–91
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol 2002;45:1389–406
- Nallapareddy SR, Singh KV, Sillanpää J, et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006;116:2799–807
- Nobbs AH, Rosini R, Rinaudo CD, et al. Sortase A utilizes an ancillary protein anchor for efficient cell wall anchoring of pili in Streptococcus agalactiae. Infect Immun 2008;76:3550–60
- Claessen D, Rink R, de Jong W, et al. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev 2003;17:1714–26
- Elliot MA, Karoonuthaisiri N, Huang J, et al. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev 2003;17:1727–40
- Jacobitz AW, Wereszczynski J, Yi SW, et al. Structural and computational studies of the Staphylococcus aureus sortase B-substrate complex reveal a substrate-stabilized oxyanion hole. J Biol Chem 2014;289:8891–902
- Weiss WJ, Lenoy E, Murphy T, et al. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal models of infection. J Antimicrob Chemother 2004;53:480–6
- Jonsson M, Mazmanian SK, Schneewind O, et al. On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J Infect Dis 2002;185:1417–24
- Mazmanian SK, Ton-That H, Schneewind O. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol Microbiol 2001;40:1049–57
- Cascioferro S, Totsika M, Schillaci D. Sortase A: an ideal target for anti-virulence drug development. Microb Pathogenesis 2014;77:105–12
- Li M-Y, Huang R-J, Zhou X-D, Gregory RL. Role of sortase in Streptococcus mutans under the effect of nicotine. Int J Oral Sci 2013;5:206–11
- Chambers H. Ceftobiprole: in-vivo profile of a bactericidal cephalosporin. Clin Microbiol Infec 2006;12:17–22
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004;363:600–7
- Nandakumar R, Nandakumar M, Marten MR, Ross JM. Proteome analysis of membrane and cell wall associated proteins from Staphylococcus aureus. J Proteome Res 2005;4:250–7
- Oh K-B, Mar W, Kim S, et al. Bis(indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg. Med. Chem. Lett 2005;15:4927–31
- Digrak M, Alma MH, İlçim A, Sen S. Antibacterial and antifungal effects of various commercial plant extracts. Pharm Biol 1999;37:216–20
- Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999;285:760–3
- Clark AM. Natural products as a resource for new drugs. J Pharm Res 1996;13:1133–41
- Lao Y-M, Jiang J-G, Yan L. Application of metabonomic analytical techniques in the modernization and toxicology research of traditional Chinese medicine. Br J Pharmacol 2009;157:1128–41
- Harvey AL. Natural products in drug discovery. Drug Discov Today 2008;13:894–901
- Quave CL, Plano LR, Pantuso T, Bennett BC. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J Ethnopharmacol 2008;118:418–28
- Pesewu GA, Cutler RR, Humber DP. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. J Ethnopharmacol 2008;116:102–11
- De Lima MRF, de Souza Luna J, Dos Santos AF, et al. Anti-bacterial activity of some Brazilian medicinal plants. J Ethnopharmacol 2006;105:137–47
- Kim S-W, Chang I-M, Oh K-B. Inhibition of the bacterial surface protein anchoring transpeptidase sortase by medicinal plants. Biosci Biotechnol Biochem 2002;66:2751–4
- Talpir R, Rudi A, Ilan M, Kashman Y. Niphatoxin A and B; two new ichthyo and cytotoxic tripyridine alkaloids from a marine sponge. Tetrahedron Lett 1992;33:3033–4
- Gazaliev A, Zhurinov MZ, Tuleuov B. Isolation, analysis, biosynthesis, and modification of the alkaloid cytisine. Chem Nat Compd 1991;27:259–69
- Bierne H, Mazmanian SK, Trost M, et al. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol Microbiol 2002;43:869–81
- Garandeau C, Réglier-Poupet H, Dubail I, et al. The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence. Infect Immun 2002;70:1382–90
- Kiefer F, Arnold K, Künzli M, et al. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res 2009;37:387–92
- Ton-That H, Marraffini LA, Schneewind O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta 2004;1694:269–78
- Suree N, Yi SW, Thieu W, et al. Discovery and structure-activity relationship analysis of Staphylococcus aureus sortase A inhibitors. Bioorg Med Chem 2009;17:7174–85
- Kahlon AK, Negi AS, Kumari R, et al. Identification of 1-chloro-2-formyl indenes and tetralenes as novel antistaphylococcal agents exhibiting sortase A inhibition. Appl Microbiol Biotechnol 2014;98:2041–51
- Ton-That H, Mazmanian SK, Alksne L, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus Cysteine 184 and histidine 120 of sortase form a thiolate-imidazolium ion pair for catalysis. J Biol Chem 2002;277:7447–52
- Connolly KM, Smith BT, Pilpa R, et al. Sortase from Staphylococcus aureus does not contain a thiolate-imidazolium ion pair in its active site. J Biol Chem 2003;278:34061–5
- Ton-That H, Mazmanian SK, Faull KF, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates. J Biol Chem 2000;275:9876–81
- Ton-That H, Liu G, Mazmanian SK, et al. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA 1999;96:12424–9
- Marraffini LA, Ton-That H, Zong Y, et al. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. A conserved arginine residue is required for efficient catalysis of sortase A. J Biol Chem 2004;279:37763–70
- Huang X, Aulabaugh A, Ding W, et al. Kinetic mechanism of Staphylococcus aureus sortase SrtA. Biochemistry 2003;42:11307–15
- Frankel BA, Bentley M, Kruger RG, McCafferty DG. Vinyl sulfones: inhibitors of SrtA, a transpeptidase required for cell wall protein anchoring and virulence in Staphylococcus aureus. J Am Chem Soc 2004;126:3404–5
- Donahue EH, Dawson LF, Valiente E, et al. Clostridium difficile has a single sortase, SrtB, that can be inhibited by small-molecule inhibitors. BMC Microbiol 2014;14:219–32
- Quesne MG, Ward RA, de Visser SP. Cysteine protease inhibition by nitrile-based inhibitors: a computational study. Front Chem 2013;1:1–10
- Jung ME, Clemens JJ, Suree N, et al. Synthesis of (2R,3S) 3-amino-4-mercapto-2-butanol, a threonine analogue for covalent inhibition of sortases. Bioorg Med Chem Lett 2005;15:5076–9
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725–9
- Navarre WW, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol Microbiol 1994;14:115–21
- Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of Gram-positive bacteria. EMBO J 1993;12:4803–11
- Schneewind O, Model P, Fischetti VA. Sorting of protein A to the Staphylococcal cell wall. Cell 1992;70:267–81
- Ton-That H, Schneewind O. Anchor structure of staphylococcal surface proteins IV. Inhibitors of the cell wall sorting reaction. J Biol Chem 1999;274:24316–20
- Zong Y, Bice TW, Ton-That H, et al. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J Biol Chem 2004;279:31383–9
- Bentley ML, Lamb EC, McCafferty DG. Mutagenesis studies of substrate recognition and catalysis in the sortase A transpeptidase from Staphylococcus aureus. J Biol Chem 2008;283:14762–71
- Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 1998;6:484–8
- Pribyl T, Moche M, Dreisbach A, et al. Influence of impaired lipoprotein biogenesis on surface and exoproteome of Streptococcus pneumoniae. J Proteome Res 2014;13:650–67
- Beulin D, Yamaguchi M, Kawabata S, Ponnuraj K. Crystal structure of PfbA, a surface adhesin of Streptococcus pneumoniae, provides hints into its interaction with fibronectin. Int J Biol Macromol 2014;64:168–73
- Chen F, Liu B, Wang D, et al. Role of sortase A in the pathogenesis of Staphylococcus aureus-induced mastitis in mice. FEMS Microbiol Lett 2014;351:95–103
- Flock J, Fröman G, Jönsson K, et al. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J 1987;6:2351–7
- Jönsson K, Signäs C, Müller H-P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene Eur J Biochem 1991;202:1041–8
- Roche FM, Massey R, Peacock SJ, et al. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology (Reading, Engl.) 2003;149:643–54
- Wann ER, Gurusiddappa S, Höök M. The fibronectin-binding Mscramm FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem 2000;275:13863–71
- Grundmeier M, Hussain M, Becker P, et al. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect Immun 2004;72:7155–63
- Bingham RJ, Rudiño-Piñera E, Meenan NA, et al. Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci USA 2008;105:12254–8
- Maňásková SH, Nazmi K, van Belkum A, et al. Synthetic LPETG-containing peptide incorporation in the Staphylococcus aureus cell-wall in a sortase a-and growth phase-dependent manner. PLoS One 2014;9:e89260. doi: http://dx.doi.org/10.1371/journal.pone.0089260
- Matsumoto T, Takase R, Tanaka T, et al. Site-specific protein labeling with amine-containing molecules using Lactobacillus plantarum sortase. Biotechnol J 2012;7:642–8
- Hartford O, Francois P, Vaudaux P, Foster T. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol Microbiol 1997;25:1065–76
- McDevitt D, Francois P, Vaudaux P, Foster T. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol Microbiol 1995;16:895–907
- Yamaguchi M, Terao Y, Mori Y, et al. PfbA, a novel plasmin and fibronectin-binding protein of Streptococcus pneumoniae, contributes to fibronectin-dependent adhesion and antiphagocytosis. J Biol Chem 2008;283:36272–9
- Mandlik A, Swierczynski A, Das A, Ton-That H. Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol Microbiol 2007;64:111–24
- Chang C, Mandlik A, Das A, Ton-That H. Cell surface display of minor pilin adhesins in the form of a simple heterodimeric assembly in Corynebacterium diphtheriae. Mol Microbiol 2011;79:1236–47
- Patti JM, Jonsson H, Guss B, et al. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem 1992;267:4766–72
- Switalski LM, Patti JM, Butcher W, et al. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol 1993;7:99–107
- Rich RL, Deivanayagam CC, Owens RT, et al. Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors, α1β1 integrin and Staphylococcus aureus Cna MSCRAMM. J Biol Chem 1999;274:24906–13
- Jensen K. A normally occurring Staphylococcus antibody in human serum. Acta Pathologica Microbiologica Scandinavica 1958;44:421–8
- Soriani M, Telford JL. Relevance of pili in pathogenic Streptococci pathogenesis and vaccine development. Future Microbiol 2010;5:735–47
- Ton-That H, Schneewind O. Assembly of pili in Gram-positive bacteria. Trends Microbiol 2004;12:228–34
- Budzik JM, Oh S-Y, Schneewind O. Cell wall anchor structure of BcpA pili in Bacillus anthracis. J Biol Chem 2008;283:36676–86
- Danne C, Dubrac S, Trieu-Cuot P, Dramsi S. Single cell stochastic regulation of pilus phase variation by an attenuation-like mechanism. PLoS Pathog 2014;10:e1003860. doi: http://dx.doi.org/10.1371/journal.ppat.1003860
- Swaminathan A, Mandlik A, Swierczynski A, et al. Housekeeping sortase facilitates the cell wall anchoring of pilus polymers in Corynebacterium diphtheriae. Mol Microbiol 2007;66:961–74
- Clancy KW, Melvin JA, McCafferty DG. Sortase transpeptidases: insights into mechanism, substrate specificity, and inhibition. Biopolymers 2010;94:385–96
- Mandlik A, Swierczynski A, Das A, Ton-That H. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol 2008;16:33–40
- Oh S-Y, Budzik JM, Schneewind O. Sortases make pili from three ingredients. Proc Natl Acad Sci USA 2008;105:13703–4
- Ton-That H, Marraffini LA, Schneewind O. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol Microbiol 2004;53:251–61
- Tsuda Y, Sener B, Khalid A, Parvez M. New steroidal alkaloids from Fritillaria imperialis and their cholinesterase inhibiting activities. Chem Pharm Bull 2002;50:1013–16
- Kang D-G, Oh H, Cho D-K, et al. Effects of bulb of Fritillaria ussuriensis maxim on angiotensin converting enzyme and vascular release of NO/cGMP in rats. J Ethnopharmacol 2002;81:49–55
- Yang L, Xueru L, Ya Z, Ningping W. Study on antibacterial activity of total alkaloia from F. taitaiensis L. and shedan chuanbeiye in vitro. Ning Xia Med J 1996;3:147–8
- Kim S-H, Shin D-S, Oh M-N, et al. Microbiology & fermentation technology-inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by b-Sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci Biotech Biochem 2003;67:2477–9
- Oh K-B, Oh M-N, Kim J-G, et al. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl Microbiol Biotechnol 2006;70:102–6
- Oh K-B, Kim S-H, Lee J, et al. Discovery of diarylacrylonitriles as a novel series of small molecule sortase A inhibitors. J Med Chem 2004;47:2418–21
- Suree N, Jung M, Clubb R. Recent advances towards new anti-infective agents that inhibit cell surface protein anchoring in Staphylococcus aureus and other Gram-positive pathogens. Mini Rev Med Chem 2007;7:991–1000
- Wang L, Bi C, Cai H, et al. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front Microbiol 2015;6:1–12
- Liu X, Xia Y, Fang Y, et al. Interaction between bovine serum albumin and berberine chloride extracted from Chinese herbs of coptis chinensis franch. Chem J Chinese U 2003;25:2099–103
- Iwasa K, Lee D-U, Kang S-I, Wiegrebe W. Antimicrobial activity of 8-alkyl and 8-phenyl-substituted berberines and their 12-bromo derivatives. J Nat Prod 1998;61:1150–3
- Yu H-H, Kim K-J, Cha J-D, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food 2005;8:454–61
- Freile M, Giannini F, Sortino M, et al. Antifungal activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Acta Farmaceutica Bonaerense 2006;25:83–8
- Wei C-Z, Tian S-Q. In vitro antibacterial investigation of Zhili tablet. Chinese J Hosp Pharm 2002;22:167–72
- Kong W-J, Zhao Y-L, Shan L-M, et al. Investigation on the spectrum-effect relationships of EtOAc extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J Chromatogr B Analyt Technol Biomed Life Sci 2008;871:109–14
- Xiao S-s, Jin Y, Sun Y-q. Recent progress in the studies of chemical constituents, pharmacological effects and quality control methods on the roots of Isatis indigotica. J Shenyang Pharm Univ 2003;6:455–9
- Xu L, Huang F, Cheng T, Wu J. Antivirus constituents of radix of Isatis indigotica. Chin J Nat Med 2005;3:359–60
- Peng J, Fan G, Wu Y. Isolation and purification of clemastanin B and indigoticoside A from radix isatidis by high-speed counter-current chromatography. J Chromatogr A 2005;1091:89–93
- Liu Y-h, Wu X-y, Fang J-g, Tang J. Studies on chemical constituents from radix isatidis. Herald Med 2003;9:591–4
- Han G, Chen B-q, Xu Q-t. Studies on the antibiotic effects of banlangen buccal tablets. J Henan Univ (Med Sci) 2004;1:36–7
- Dobelis IN. Magic and medicine of plants. Pleasantville, NewYork: Ethnobotanical Leaflets; 1986. Vol. 5
- Lee Y-J, Han Y-R, Park W, et al. Synthetic analogs of indole-containing natural products as inhibitors of sortase A and isocitrate lyase. Bioorg Med Chem Lett 2010;20:6882–5
- Jang KH, Chung S-C, Shin J, et al. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg Med Chem Lett 2007;17:5366–9
- Kang SS, Kim J-G, Lee T-H, Oh K-B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol Pharm Bull 2006;29:1751–5
- Liu J, Zhu M, Shi R, Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am J Chinese Med 2003;31:337–54
- Oh I, Yang W-Y, Chung S-C, et al. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch Pharm Res 2011;34:217–22
- Escaich S. Antivirulence as a new antibacterial approach for chemotherapy. Curr Opin Chem Biol 2008;12:400–8
- Huang P, Hu P, Zhou SY, et al. Morin inhibits Sortase A and subsequent biofilm formation in Streptococcus mutans. Curr Microbiol 2014;68:47–52
- Ma R, Pang G-c. Role of rutin in modern civilization diseases. Food Sci 2013;7:307–11
- SUN D-m, KUANG Z-y, LI Y. Experiment on bacteriostasis function of baicalin against methicilin-resistant Staphlococcus aureus. Jilin Med J 2011;13:2587–8
- Meizhen Y, Xiaoshan G, Linxiang L, et al. Observation on inhibitory effect of coptis alone and its combination with scutellaria and liquorice on the growth of Staphylococcus aureus. China J Chinese Materia Medica 1998;6:1–4
- Chan BC-L, Bik-San Lau C, Jolivalt C, et al. Chinese medicinal herbs against antibiotic-resistant bacterial pathogens. Sci Against Microbial Pathogens 2011;1:773–81
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharmaceut 2007;4:807–18
- Long Y, Zhang W, Wang F, Chen Z. Simultaneous determination of three curcuminoids in Curcuma longa L. by high performance liquid chromatography coupled with electrochemical detection. J Pharm Anal 2013;4:3368–672
- Song J, Choi B, Jin E-J, et al. Curcumin suppresses Streptococcus mutans adherence to human tooth surfaces and extracellular matrix proteins. Eur J Clin Microbiol Infect Dis 2012;31:1347–52
- Sugiyama Y, Kawakishi S, Osawa T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 1996;52:519–25
- Huang M-T, Lou Y-R, Ma W, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res 1994;54:5841–7
- Hong J, Bose M, Ju J, et al. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004;25:1671–9
- Nishiyama T, Mae T, Kishida H, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem 2005;53:959–63
- Park B-S, Kim J-G, Kim M-R, et al. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. J Agric Food Chem 2005;53:9005–9
- Ilangovan U, Ton-That H, Iwahara J, et al. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc Natl Acad Sci USA 2001;98:6056–61
- Oh K-B, Nam K-W, Ahn H, et al. Therapeutic effect of (Z)-3-(2, 5-dimethoxyphenyl)-2-(4-methoxyphenyl) acrylonitrile (DMMA) against Staphylococcus aureus infection in a murine model. Biochem Biophys Res Commun 2010;396:440–4
- Ye W-C, Ji N-N, Zhao S-X, et al. Triterpenoids from Pulsatilla chinensis. Phytochemistry 1996;42:799–802
- Mimaki Y, Kuroda M, Asano T, Sashida Y. Triterpene saponins and lignans from the roots of Pulsatilla chinensis and their cytotoxic activity against HL-60 cells. J Nat Prod 1999;62:1279–83
- Ye W, He A, Zhao S, Che C-T. Pulsatilloside C from the roots of Pulsatilla chinensis. J Nat Prod 1998;61:658–9
- Sun Y, Liu J, Yu H, Gong C. Isolation and evaluation of immunological adjuvant activities of saponins from the roots of Pulsatilla chinensis with less adverse reactions. Int Immunopharmacol 2010;10:584–90
- Lee S, Song I-H, Lee J-H, et al. Sortase A inhibitory metabolites from the roots of Pulsatilla koreana. Bioorg Med Chem Lett 2014;24:44–8
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. The Plant Cell 1995;7:1085–97
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry 2003;62:121–5
- Deng F. Studies of Sortase A by total chemical synthesis. Chicago, Illinois: The University of Chicago; 2013
- Won TH, Jeon J-e, Lee S-H, et al. Beta-carboline alkaloids derived from the ascidian Synoicum sp. Bioorg Med Chem 2012;20:4082–7
- Seidler J, McGovern SL, Doman TN, Shoichet BK. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J Med Chem 2003;46:4477–86
- Kruger RG, Dostal P, McCafferty DG. Development of a high-performance liquid chromatography assay and revision of kinetic parameters for the Staphylococcus aureus sortase transpeptidase SrtA. Anal Biochem 2004;326:42–8
- Vogel H. Similarities between various systems of traditional medicine. Considerations for the future of ethnopharmacology. J Ethnopharmacol 1991;35:179–90
- van Vught R, Pieters R, Breukink E. Site-specific functionalization of proteins and their applications to therapeutic antibodies. Comput Struct Biotechnol J 2014;9:e201402001. doi: 10.5936/csbj.201402001
- Kajikawa A, Nordone SK, Zhang L, et al. Dissimilar properties of two recombinant Lactobacillus acidophilus strains displaying Salmonella FliC with different anchoring motifs. Appl Environ Microb 2011;77:6587–96
- Jia X, Kwon S, Wang C-IA, et al. Semienzymatic cyclization of disulfide-rich peptides using sortase A. J Biol Chem 2014;289:6627–38
- Swee LK, Guimaraes CP, Sehrawat S, et al. Sortase-mediated modification of αDEC205 affords optimization of antigen presentation and immunization against a set of viral epitopes. Proc Natl Acad Sci USA 2013;110:1428–33
- Popp MW, Dougan SK, Chuang T-Y, et al. Sortase-catalyzed transformations that improve the properties of cytokines. Proc Natl Acad Sci USA 2011;108:3169–74
- Hagemeyer CE, Alt K, Johnston AP, et al. Particle generation, functionalization and sortase A-mediated modification with targeting of single-chain antibodies for diagnostic and therapeutic use. Nat Protoc 2015;10:90–105