Abstract
Coumarin, a naturally occurring or synthesised phytochemical, displays a wide range of biological activities. However, chromen-2-ones fused with 1,3-benzoxazine rings is not well documented and there is a gap in the literature which required engaging. The substituted-2-thioxo-chromen-oxazine linear compounds 14a–i and angular compounds 16a–e were synthesised from the reaction of hydroxy-substituted-chromene-carboxylic 10–13 with freshly prepared Ph3P(SCN)2. 2-Morpholino-substituted-chromen-oxazine-4,8-dione and 8-morpholino-substituted-chromen-oxazine-2,10-dione 15a–f and 17 were synthesised from the reaction of the corresponding oxazines 14 and 16 with morpholine. PI3K activity was observed for the hydroxy-substituted-chromene-carboxylic acid of which compound 13b showed moderate PI3Kγ (IC50 = 5.56 μM) and PI3Kα (IC50 = 14.7 μM) activity. Additionally, 8-morpholino-chromen-oxazine-2,10-dione 17a showed isoform selective PI3Kδ activity with IC50 = 5.08 μM with non-DNA-PK ≥ 100 μM. Consequently compound 17a can be considered as a selective PI3Kδ inhibitor with non-DNA-PK at compound concentrations ≥100 μM.
Introduction
Coumarin, a naturally occurring phytochemical has a wide range of biological activities, such as anti-inflammatory, antitumorCitation1–3, anti-allergic and anti-HIV-1 propertiesCitation4,Citation5.
Coumarin and its bioisosteres – thioxocoumarin, thiocoumarin, dithiocoumarin and sulfocoumarin derivatives – were previously synthesised and utilised in the investigation of inhibitors of carbonic anhydrasesCitation6–8. The mechanism of action of this class of inhibitors is delineated in detail by resolving the X-ray crystal structure of CAII in complex with trans-2-hydroxy-cinnamic acid, the in situ hydrolysis product of simple coumarinCitation6–8.
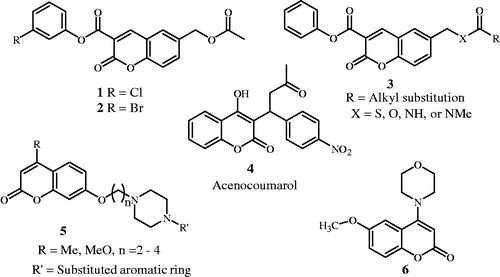
6-Acetoxymethyl-2-oxo-2H-1-benzopyran-3-carboxylate 1 and 3-bromophenyl-6-acetoxymethyl-2-oxo-2H-1-benzopyran-3-carboxylate 2 () expressed a marked potency in inhibiting cancer cell invasion in vitro and tumour growth in vivoCitation9.
Moreover coumarin derivative 3 proved to be effective on HT 1080 fibro-sarcoma cell invasionCitation1.
A major objective of our research is the development of potential potent and selective DNA-dependent protein kinase (DNA-PK) and phosphatidylinositol-3-kinase (PI3K) inhibitors, suitable for clinical evaluation as chemo- and radio-sensitisers in the treatment of cancer.
A novel class of coumarin (2H-1-benzopyran-2-one) derivatives 5 () exhibited high efficacies towards α1-adrenoceptors (α1-AR) in vitro pharmacological assaysCitation10,Citation11 while acenocoumarolCitation4 is marketed as an anticoagulantCitation12 and 6-methoxy-4-morpholino-2H-chromen-2-one 6 has displayed moderate DNA-PK with IC50 = 1.8 μMCitation13,Citation14.
Moreover the 2-morpholino substituted benzoxazines 7a-c were found to be effective inhibitors of ADP and/or collagen-induced platelet aggregationCitation15–18. The pharmacophore essential for platelet aggregation is the 1,3-benzoxazine skeleton, with a morpholino group at position 2 and substitution at position 8 and/or O-substitution at position 7, yet 2-(pyridin-3-yl(pyridin-3-ylmethyl)amino) analogue 8 shows less platelet aggregation activity compared with product 7 as result of replacing the 2-morpholine group ()Citation16.
Figure 2. Some benzoxazine structures of ADP and/or collagen-induced platelet aggregation, DNA-PK and PI3K inhibitors.
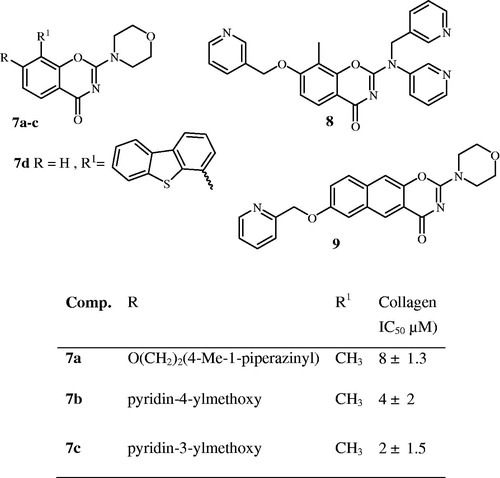
Furthermore, substituted-1,3-benzoxazines compounds 7 and 8 () were found to be moderately potent DNA-PK inhibitors with the most active being the 2-morpholino analogue 7c IC50 = 0.28 μM, while the most active 2-(pyridin-3-yl(pyridin-3-ylmethyl)amino) analogue 8 was found to be ∼10-fold less potent than 7c against DNA-PK with an IC50 of 2.5 μMCitation16–19.
As well as the most active 2-morpholino substituted-1,3-naphtha-oxazine was compound 9 () with an IC50 = 0.096 μMCitation17.
The PI3K inhibition studiesCitation16 revealed that compound 7c is potent and broad PI3K isoform inhibitor (IC50 for PI3Kα = 0.13 μM, β = 0.14 μM, γ = 0.72 μM and δ = 2.0 μM) while compound 7a with 7-[2-(4-methylpiperazin-1-yl)ethoxy] group shows selective inhibition of DNA-PK over PI3KCitation15.
Recently we synthesised 6-, and 8-aryl-substituted-1,3-benzoxazines compounds of which compound 7d (dibenzo[b,d]thiophen-4-yl) () showed extremely potent DNA-PK activity with IC50 = 0.034 μM, however, the PI3K activity was observed to be less potent (IC50 PI3Kβ = 5.8 and γ = 8.5 μM)Citation20.
The synthesis of chromen-2-ones fused with 1,3-benzoxazine rings is not well documented and there is a gap in the literature which required engaging.
In this study, the synthesis, DNA-PK, PI3K, anti-bacterial- and platelet-induced aggregation for some substituted-2-oxo-2H-chromene-6-, or 8-carboxylic acidsCitation10–13, their corresponding 1,3-oxazines (14 and 16) and 5, 6 or 10-substituted-2-morpholino-chromen[6,7-e][1,3]oxazine-4,8-dione (15 and 19) the linear chromen-oxazines and the 3, 4, 6-substituted-8-morpholino-chromen[8,7-e][1,3]oxazine-2,10-dioneCitation17 the angular chromen-oxazines are reported.
Experimental
Chemistry
Infrared spectra were obtained using a Perkin Elmer FT-IR 1720 spectrometer (Waltham, MA). 1H NMR and 13C NMR spectra were obtained using a Bruker AC 200 NMR spectrometer (Bremen, Germany) at 200 and 50 MHz, respectively. All 1H and 13C NMR spectral results are recorded as chemical shifts (δ). Chemical shifts recorded in CDCl3 are relative to the internal TMS (0 ppm) for 1H spectra and solvent peak (77.0 ppm) for 13C spectra. Chemical shifts recorded in d6-DMSO are relative to the solvent peak of 2.5 ppm for 1H spectra and 39.5 ppm for 13C spectra. 1H NMR multiplicities are expressed as singlet (s), broad singlet (bs) doublet (d), double doublet (dd), triplet (t), double triplet (dt), triple triplet (tt), quartet (q) and multiplet (m). High-resolution mass spectrometry (HRMS)analyses were carried out on an Agilent 6224 TOF LC/MS Mass Spectrometer coupled to an Agilent 1290 Infinity (Agilent, Palo Alto, CA). All data were acquired and reference mass corrected via a dual‐spray electrospray ionisation (ESI) source. Acquisition was performed using the Agilent Mass Hunter Data Acquisition software version B.05.00 Build 5.0.5042.2 and analysis was performed using Mass Hunter Qualitative Analysis version B.05.00 Build 5.0.519.13. Element analysis was performed by Chemical and Micro-analytical Services (CMAS), Australia. Melting point determinations were carried out using a Stuart Scientific (SMP3) melting point apparatus and all melting points are uncorrected.
Starting materials
The starting reagents, morpholine, 2,4-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid, were purchased from Sigma-Aldrich Chemical Company (NSW, Australia) and were used as received. 2,4-Dihydroxy-3-methylbenzoic was prepared according to the previously reported procedureCitation21.
Synthesis of 7-hydroxy-substituted-chromene-6-carboxylic acid and 7-hydroxy-4 substituted-chromene-8-carboxylic acid 10a–f and 12a–c
General procedure A (improved previously reported procedure)
2,4-Dihydroxybenzoic acid, 2,4-dihydroxy-3-methylbenzoic acid or 2,6-dihydroxybenzoic acid (10 mmol) and the appropriate β-ketoester (15 mmol) were combined in a 50 mL round bottom flask fitted with a magnetic stirrer. Conc. sulphuric acid (2.0 mL, 47 mmol) was added slowly drop-wise and the contents were heated at 75 °C for 30 min. Upon completion the reaction mixture was poured into a beaker containing ice-cold water (50 mL) and was then left to stir for 1 h. The crude solid was filtered by suction further washed with water and recrystallised from an appropriate solventCitation22.
General procedure B (modification of previously reported method)
10 mmol of 2,4-dihydroxybenzoic acid or 2,4-dihydroxy-3-methylbenzoic acid was added to 15 mmol of the appropriate β-keto ester in a 25 mL Erlenmeyer flask. Sulphuric acid 70% w/w (10 mL) was added drop-wise slowly with stirring and the mixture was left to stir overnight (∼18 h). Upon completion the reaction mixture was quenched with water (50 mL) and the product filtered and further washed with water to remove any remaining sulphuric acid and then recrystallised from an appropriate solventCitation23.
General procedure C (hydrolysis of methyl 7-hydroxy-substituted-chromene-6-carboxylate using the modified of the previously reported method)
10 mmol of methyl 7-hydroxy-3:4-cyclohexen-chromene-6-carboxylate was stirred overnight in a 25 mL round bottom containing 20 mL of 15% w/w aqueous NaOH. Upon completion the reaction was acidified with conc. HCL to pH 1–3. The solid which precipitated was filtered, further washed with water (10 mL) and then recrystallised from an appropriate solventCitation23.
7-hydroxy-4-methyl-2-oxo-2H-chromene-6-carboxylic acid 10a
2,4-dihydroxybenzoic acid was allowed to react with ethyl acetoacetate according to general procedure ACitation22. The crude solid was collected and recrystallised from abs. ethanol to give 10a (1.43 g, 65% yield), with similar physical and proton NMR data (see Supplementary Information for the experimental details of compound 10b bromination of substituted 7-hydroxy-chromene-6-carboxylic acid 10a, b and chromene-8-carboxylic acids 13a).
General procedure D (modification of the previously reported method)
1.0 g of 10a, 10b or 12a was combined with 20 mL of chloroform in a 100 mL round bottom flask fitted with a stirrer. Bromine was added (5 mmol for mono-bromination and 10 mmol for di-bromination) in 20 mL of chloroform drop-wise over a period of 5 min. Upon completion of the addition the dropping funnel was replaced with a condenser and the reaction was refluxed overnight. Upon completion the reaction mixture was filtered under reduced pressure, washed with chloroform (10 mL) and then recrystallised from an appropriate solventCitation24.
3-Bromo-7-hydroxy-4-methyl-2-oxo-2H-chromene-6-carboxylic acid 11a
7-Hydroxy-4-methyl-2-oxo-2H-chromene-6-carboxylic acid 10a (1.0 g) was allowed to react with bromine (0.73 g) according to general procedure D (for mono-bromination). The crude solid was collected and recrystallised from abs. ethanol to give 11a as a white powder (1.21 g, 81% yield), mp. 260–263 °C (Lit. 260 °CCitation24). vmax (KBr)/cm−1 3100–2900 br (OH), 1745s and 1659s (C=O), 1616s, 1567m (C=C). 1H NMR (200 MHz, d6-DMSO, 300 K) δ 8.15 (s, 1H, H-5), 6.91 (s, 1H, H-8), 4.15 (br, 2H, OH × 2 with the water peak), 2.56 (s, 3H, 4-CH3). 13C NMR (50 MHz, d6-DMSO, 300 K) δ 170.7 (6-COOH), 163.5 (C-2), 155.9 (C-7, C-8a), 151.5 (C-4), 128.9 (C-5), 112.4, 111.4, 109.2 (C-4a, C-3, C-6), 103.7 (C-8), 19.4 (4-CH3). This product was previously prepared however, non-IR, 1H and 13C NMR was reported (see Supplementary Information for the experimental details of compound 11b,c)Citation24.
7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carboxylic acid 12a
2,6-Dihydroxybenzoic acid (1.54 g) was allowed to react with ethyl acetoacetate according to general procedure A. The crude solid was collected and recrystallised from abs. ethanol to give 12a as a pale orange powder (1.59 g, 72% yield), mp. 266 °C (Lit. 266–268 °CCitation25). vmax (KBr)/cm−1 3000–2800 br (OH), 1698s and 1656s (C=O), 1597s, 1562 (C=C). 1H NMR (200 MHz, d6-DMSO, 300 K) δ 11.0 (bs, 1H, OH), 7.67 (d, 1H, JH5,H6 = 8.6 Hz, H-5), 6.92 (d, 1H, JH6,H5 = 8.8 Hz; H-6), 6.18 (s, 1H, H-3), 2.37 (s, 3H, 4-CH3). 13C NMR (50 MHz, d6-DMSO, 300 K) δ 166.2 (8-COOH), 159.5 (C-2), 158.5 (C-7), 153.6 (C-4), 151.3 (C-8a), 127.4 (C-5), 112.8 (C-6), 111.8 (C-8), 110.5 (C-3), 110.3 (C-8a), 18.3 (4-CH3). This product was previously prepared however, non-IR, 1H and 13C NMR was reportedCitation25 (see Supplementary Information for the experimental details of compound 12b,c).
3-Bromo-7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carboxylic acid 13a
7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carboxylic acid 12a was allowed to react with bromine according to general procedure D (for mono-bromination). The crude solid was collected and recrystallised from abs. ethanol to give 13a (1.28 g, 86% yield), mp. 253–255 °C decomp. vmax (KBr)/cm−1 3000–2800 br (OH), 1720s (C=O), 1663s and 1590s (C=C). 1H NMR (200 MHz, d6-DMSO, 340 K) δ 11.0 (br, 2H, OH × 2), 7.80 (d, 1H, JH5,H6 = 8.8 Hz, H-5), 6.96 (d, 1H, JH6,H5 = 8.6 Hz, H-6), 2.57 (s, 3H, 4-CH3). 13C NMR (50 MHz, d6-DMSO, 300 K) δ 165.7 (8-COOH), 158.7 (C-2), 156.0 (C-7), 152.1 (C-4), 149.6 (C-8a), 128.1 (C-5), 113.5 (C-6), 111.7, 110.3 (C-4a, C-8), 107.9 (C-3), 19.5 (4-CH3). Anal. Calcd. for C11H7BrO5 C, 44.18; H, 2.36. Found C, 44.26; H, 2.45 (see Supplementary Information for the experimental details of compound 13b).
Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compound 14a–i and angular compound 16a–e
General procedure E
A suspension of substituted-chromene-2-hydroxy-acid (4 mmol) in DCM (20 mL) was added to a mixture of Ph3P(SCN)2 which was freshly prepared according to the previously reported procedureCitation26,Citation27.
6-Methyl-2-thioxo-2,3-dihydro-4H,8H-chromen[6,7-e][1,3]oxazine-4,8-dione 14a
7-Hydroxy-4-methyl-2-oxo-2H-chromene-6-carboxylic acid 10a was allowed to react with freshly prepared triphenylphosphine thiocyanogen according to the general procedure E. The solid was collected and recrystallised from 1,4-dioxane to give 14a as fine yellow crystals (0.57 g, 55% yield), mp. >300 °C. vmax (KBr)/cm−1 3179w, 3071w (N–H), 1745s, 1692s (C=O), 1630s, 1615s, 1579m (C=C), 1139s (C = S). 1H NMR (200 MHz, d6-DMSO, 340 K) δ 13.60 (bs, 1H, NH), 8.22 (s, 1H, H-5), 7.53 (S, 1H, H-10), 6.47 (s, 1H, H-7), 2.50 (s, 3H, 4-CH3). 13C NMR (50 MHz, d6-DMSO, 340 K) δ 181.2 (C-2), 158.2, 157.5, 156.3, 156.3 (C-4, C-8, C-9a, C-10a) 152.0 (C-6), 124.3 (C-5), 117.9 (C-4a), 114.4 (C-7), 112.0 (C-5a), 103.8 (C-10), 17.8 (6-CH3). Anal. Calcd. for C12H7NO4S C, 55.17; H, 2.70; N, 5.36. Found C, 55.38; H, 2.81; N, 5.52 (see Supplementary Information for the experimental details of compound 14b-i).
8-Methyl-2-thioxo-2,3-dihydro-4H,6H-chromen[7,8-e][1,3]oxazine-4,6-dione 16a
7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carboxylic acid 12a was allowed to react with freshly prepared triphenylphosphine thiocyanogen according to the general procedure E. The solid was collected and recrystallised from 1,4-dioxane to give 16a as a yellow powder (0.67 g, 65% yield), mp. 292 °C. vmax (KBr)/cm−1 3081w (N–H), 1728s and 1704s (C=O), 1630m (C=C), 1170s (C = S). 1H NMR (200 MHz, d6-DMSO, 300 K) δ 13.56 (s, 1H, NH), 8.18 (d, 1H, JH9,H10 = 8.6 Hz, H-9), 7.47 (d, 1H, JH10,H9 = 8.2 Hz, H-10), 6.50 (s,1H, H-7), 2.48 (s, 3H, 8-CH3). 13C NMR (50 MHz, d6-DMSO, 350 K) δ 180.5 (C-2), 158.2, 157.1 (C-4, C-6), 153.8 (C-10a), 152.3 (C-8, C-4b), 132.2 (C-9), 116.9 (C-4a), 113.5 (C-7, 8a), 111.8 (C-10), 18.0 (8-CH3). Anal. Calcd. for C12H7NO4S C, 55.12; H, 2.70; N, 5.34. Found C, 55.32; H, 2.89; N, 5.34 (see Supplementary Information for the experimental details of compound 16b–e).
Synthesis of 2-morpholino-chromen [6,7-e][1,3]oxazine-4,8-dione 15 & 17
General procedure F
According to the earlier reported methodCitation15, the relevant 2-thioxo-2,3-dihydro-4H,8H-chromen[6,7-e][1,3]oxazine-dione 14a–f or 16 (1 mmol) was suspended in dry 1,4-dioxane (10 mL) in a 25 mL round bottom flask. Morpholine (5 mmol) was then added drop-wise, directly from the pipette, with stirring, the reaction mixture was heated to reflux (4–8 h). At the completion of the reaction, the mixture was cooled, evaporated to dryness under reduced pressure and triturated with minimal amount of diethyl ether. The resulting solid was collected by vacuum filtration and recrystallised from an appropriate solvent.
6-Methyl-2-morpholino-4H,8H-chromen[6,7-e][1,3]oxazine-4,8-dione 15a
6-Methyl-2-thioxo-2,3-dihydro-4H,8H-chromen[6,7-e][1,3]oxazine-4,8-dione 14a (0.26 g) was allowed to react with morpholine (0.43 g) for 4 h according to general procedure F. The crude material was collected and recrystallised from DMF to give 15a as white crystals (0.16 g, 52% yield), mp. > 300 °C. vmax (KBr)/cm−1 2969w, 2920w, 2859w (C-H), 1735s & 1717s (C=O), 1556s (C=N). 1H NMR (300 MHz, CDCl3, 300 K) δ 8.41 (s, 1H, H-5), 7.15 (s, 1H, H-10), 6.34 (s, 1H, H-7), 3.96–3.85 (bm, 8H, 4 × CH2 of morpholine), 2.52 (s, 3H, 6-CH3). 13C NMR (75 MHz, CDCl3, 300 K) δ 165.3, 159.1, 157.0, 156.5, 155.1 (C-2, C-4, C-8, C-9a, C-10a), 152.0 (C-6), 125.3 (C-5), 118.5 (C-4a), 115.0 (C-7), 114.0 (C-5a), 103.5 (C-10), 66.2 (C-3′), 44.9 (C-2′), 18.8 (6-CH3). HRMS ([C16H14N2O5] + H+) Calculated 315.0975 observed 315.0973 (see Supplementary Information for the experimental details of compound 15b–f).
4-Methyl-8-morpholino-2H,10H-chromen[8,7-e][1,3]oxazine-2,10-dione 17a
8-Methyl-2-thioxo-2,3-dihydro-4H,6H-chromen[7,8-e][1,3]oxazine-4,6-dione 16a (0.28 g) was allowed to react with morpholine (0.43 g) for 8 h according to general procedure F. The crude material was collected and recrystallised from DMF to give 17a as an off-white powder (0.13 g, 40% yield). 1H NMR (300 MHz, CDCl3, 305 K) δ 7.78 (d, 1H, JH9,H10 = 9.0 Hz, H-9), 7.12 (d, 1H, JH10,H9 = 9.0 Hz, H-10), 6.33 (s, 1H, H-7), 3.85–3.27 (bs, 8H, 4 × CH2 of morpholine), 2.43 (s, 3H, 8-CH3). Low CDCl3 solubility for 13C NMR (75 MHz, CDCl3, 310 K) δ 128.6 (C-9), 114.6 (C-7), 111.0 (C-10), 65.9 (C-3′), 44.2 (C-2′), 18.6 (8-CH3). HRMS ([C16H14N2O5] + H+) Calculated 315.0975 observed 315.0973 (see Supplementary Information for the experimental details of compound 17b).
Synthesis of bromo-substituted-2-morpholino-chromen[6,7-e][1,3]oxazine-4,8-dione 19
General procedure G
According to the previously reported methodCitation16, sodium hydrogen carbonate (1.0 g) was suspended in a 50 mL beaker containing 20 mL of 1:1 water and 2-propanol. The appropriate 2-thioxo-2,3-dihydro-4H,8H-chromen[6,7-e][1,3]oxazine-dione 14g–i (1.0 mmol) was added. The reaction mixture was warmed on a hotplate to ≈60 °C and then removed from heat and allowed to cool to room temperature with stirring. Iodomethane (0.5 mL, 8.0 mmol) was added drop-wise and allowed to stir at room temperature for at least 20 min or until a thick precipitation had formed. Morpholine (0.43 g, 5.0 mmol), 3-aminopyridine (0.28 g, 3.0 mmol) or benzylamine (0.53 g, 5.0 mmol) was added and the reaction mixture was stirred for an additional 3 h. The resulting solid was collected by vacuum filtration washed with water (∼30 mL) and recrystallised from an appropriate solvent.
7-Bromo-6-methyl-2-morpholino-4H,8H-chromen[6,7-e][1,3]oxazine-4,8-dione 19a
7-Bromo-6-methyl-2-thioxo-2,3-dihydro-4H,8H-chromen[6,7-e][1,3]oxazine-4,8-dione 14g was allowed to react with iodomethane and morpholine (0.43 g) according to general procedure G. The crude material was collected and recrystallised from ethanol/chloroform to give 19a as a white solid (0.32 g, 82% yield), mp. > 300 °C. νmax (ATR)/cm−1 3037w, 2962w, 2910w (C-H), 1737s, 1684m (C=O), 1618s (C=C), 1589m (C=N). 1H NMR (200 MHz, CDCl3, 300 K) δ 8.41 (s, 1H, H-5), 7.13 (s, 1H, H-10), 3.80 (bs, 8H, 4 × CH2 of morpholine), 2.65 (s, 3H, 6-CH3). 13C NMR (50 MHz, CDCl3, 300 K) δ 165.4 (C-4), 156.5, 155.9, 155.2, 155.0, 150.9 (C-2, C-6 C-8, C-9a, C-10a), 125.9 (C-5), 118.4, 114.6, 113.2 (C-4a, C-5a, C-7), 103.7 (C-10), 66.3 (C-3′), 45.2 (C-2′), 20.1 (6-CH3). Anal. Calcd. for C16H13BrN2O5 C, 48.88; H, 3.33; N, 7.12. Found C, 48.95; H, 3.42; N, 7.07 (see Supplementary Information for the experimental details of compound 19b,c).
Biological activity
PI3K inhibition assay
The PI3K assay was performed at Reaction Biology Corporation, Malvern, PA. All compounds were dissolved in DMSO and tested for their ability to inhibit PI3K. Compounds were tested in a 10-dose IC50 profile with 4-fold serial dilution starting at 100 μM. The control compound, PI-103 (3-[4-(4-morpholinyl)pyrido[3′,2′:4,5]furo[3,2-d]pyrimidin-2-yl]-phenol is a potent, cell-permeable, ATP-competitive inhibitor of PI3K family members) was tested in a 10-dose IC50 profile with 3-fold serial dilutions starting at 10 μM. Reactions were carried out at 10 μM ATP using the HTRF assay format.
DNA-PK inhibition assay
The DNA-PK assay was performed at Reaction Biology Corporation, Malvern, PA. All Compounds were dissolved in DMSO and tested for their ability to inhibit human DNA-PK. Compounds were tested in a 10-dose IC50 profile with 4-fold serial dilution starting at 100 μM. The control compound, LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one is a selective PI3K inhibitor) was tested in a 10-dose IC50 profile with 3-fold serial dilutions starting at 10 μM. Reactions were carried out using 20 μM Peptide substrate [EPPLSQEAFADLWKK], 10 μg/mL DNA and 10 μM ATP using the HTRF assay format.
Anti-platelet assay
Venous blood was collected from apparently healthy, drug-free volunteers into trisodium citrate 22.0 g/L. Ethics approval was obtained from La Trobe University Human Ethics Committee (FHEC Number 10/R73). The whole blood was centrifuged at 130 g for 12 min at room temperature to obtain platelet-rich plasma (PRP). The remaining blood was centrifuged for a further 10 min at 820 g in order to obtain platelet poor plasma (PPP). Platelet aggregation was determined by the optical method in a two-channel platelet aggregometer (Chrono-log Corporation Aggregometer Model 490-2D). Assays were carried out at 37 °C and had a total volume of 500 μL after the addition of the test compound and agonist. Stirring rate was 1000 rpm with the PRP and test compound being pre-incubated for 2 min before the addition of the appropriate agonist. The agonist used was 50 μL equine collagen (100 μg/mL). The assay was performed according to the previously reported assayCitation15 at an initial compound concentration of 80 μM.
Anti-bacteria assay
The disk diffusion method with Muller–Hinton agar was used to evaluate the antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylcoccus aureus and Enterococcus faecalis. All bacteria were grown from frozen cultures in nutrient broth by overnight incubation, diluted to give an OD600 of 0.95–1.05 then evenly applied to the appropriately labelled agar plates using a sterile swab. Blank antibacterial susceptibility disks (three per plate) were then placed on each agar plate. 2.5 μL of each compound as a 20.0 mmol solution in DMSO (50.0 nmol) was applied to the appropriate blank antibacterial susceptibility disks for each bacterial strain. Ciprofloxacin, of the same concentration, was used as positive control and a DMSO blank as a negative control. The diameter of the zone of inhibition was measured in millimetres (mm) after 24 h of incubation at 37 °C.
Results and discussion
Chemistry
Synthesis of substituted-7-hydroxy-2-oxo-2H-chromene-6-carboxylic acid 10a–f, 11a–c and chromene-8-carboxylic acids 12a–c & 13a,b
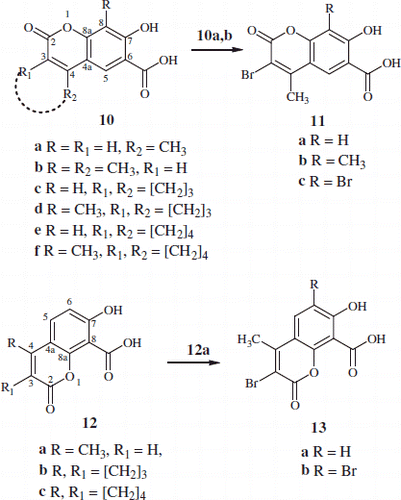
Substituted chromene-6-carboxylic acids 10a,b and chromene-8-carboxylic acids 12a were synthesised from the reaction of the appropriate substituted-2,4-dihydroxybenzoic acid and 2,6-dihydroxybenzoic acid with the correct β-keto ester using general procedure A. While the previously reported procedure B was utilised for the synthesis of compounds 10c–f, 12b and 12cCitation23. However, acids 10c and 10e were synthesised from the reaction of methyl 2,4-dihydroxy-benzoate with suitable β-keto ester according to general procedure B which gave the methyl ester analogue of the acid 10c and 10e which then was hydrolysed (procedure C) to give the corresponding carboxylic acidsCitation23,Citation28.
The mono-bromo-7-hydroxy-4-methyl-2-oxo-2H-chromene-6-or 8-carboxylic acid 11a,b and 13a were synthesised from the reaction of 1 mole of bromine in chloroform using the previously reported general procedure DCitation24 with the appropriate substituted-chromene-carboxylic acids 10a,b and 12a.
Similarly di-bromo-chromene-6-carboxylic acid 11c and chromene-8-carboxylic acid 13b were synthesised using 2 moles of bromineCitation24 with the relevant substituted-chromene-carboxylic acids 10a and 12a. The structures of the newly synthesised hydroxy-chromene-carboxylic acid 10b, d, f, 11b, 12b,c, 13a,b were verified using the analysed 1H, 13C NMR in addition to element analysis.
Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14a–i and angular compounds 16a–e
Substituted-2-thioxo-chromen-1,3-oxazine linear compound 14 and angular compound 16 were synthesised from the reaction of substituted-7-hydroxy-2-oxo-2H-chromene-6-carboxylic acid (compounds 10 and 11) and substituted-7-hydroxy-2-oxo-2H-chromene-8-carboxylic acid (compounds 12 and 13) with freshly prepared Ph3P(SCN)2 according to the previously reported procedureCitation26,Citation27 with no further modification (Scheme 2).
Synthesis of 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione 15a–f (linear) and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 17a–b (angular)
Compounds 15a–f and 17a,b (Scheme 2) were prepared from the reactions of the 2-thio-benzoxazines 14a–f and 16a and 16c with morpholine according to the previously reported procedures (Scheme 2)Citation18,Citation21.
Attempting the synthesis of the mono- and the di-bromo-2-morpholino-oxazines 19a–c by boiling the mono- and di-bromo-oxazines 14g–i with morpholine in dioxane unexpectedly gave the corresponding non-brominated products 15. The de-bromo-hydrogenated (hydrogenolysis) reaction was thought to be assisted by the presences of the excess morpholine used in the reaction, the dissolved H2S resulting from the formation of the corresponding non-brominated products or the free radical could be present in the solvent (dioxane).
To confirm this hypothesis the mono-bromo compound 19a and di-bromo compound 19b were boiled with morpholine in dioxane under similar condition as in the above procedure and afforded the de-brominated product 15a.
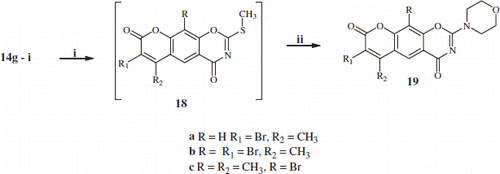
To overcome the above unexpected result, the mono- and di-bromo-oxazines 14 g–i were first converted to 2-S-methyl-1,3-benzoxazines 18a–c with good yield (90–95%), using previously reported procedureCitation16, from the reaction of substituted 2-thio-1,3-benzoxazine 14g–i with methyl iodide in the presence of NaHCO3 in 1:1 CH3OH/H2O at room temperature. Without isolation of intermediates 18a–c, morpholine was added drop-wise and allowed to stir at room temperature for 3 h to afford the mono-, and di-bromo-2-morpholino-substituted-benzoxazines 19a–c (Scheme 3).
The structures of the all new products were elucidated from the analysis of their IR, 1H NMR spectra and element analysis. The carbon-13 NMR could not be measured for compounds 19b,c as they were not soluble in most of deuterated organic solvent including d6-DMSO.
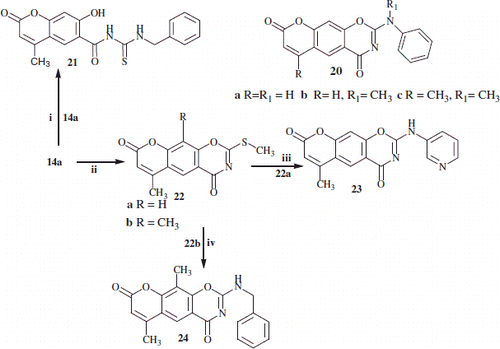
Reaction of substituted 2-thio-1,3-benzoxazine 14a with benzylamine and 14b with 3-aminopyridine (Scheme 4)
It is worth noting that only one reported method in the literatureCitation29 gave the synthesis of 2-(phenylamino)-4H,8H-chromeno[6,7-e][1,3]oxazine-4,8-dione and the 6-methyl-analogue 20a–c (Scheme 4).
In this study, the reaction of substituted 2-thio-1,3-benzoxazine 14a, with benzylamine/NaHCO3, in water/2-propanol stirred overnight at room temperature, gave N-(benzylcarbamothioyl)-7-hydroxy-4-methyl-2-oxo-2H-chromene-6-carboxamide 21. While the reaction of 3-aminopyridine or benzylamine with the 2-methylsulfanyl-chromeno-1,3-oxazines 23a and 23b, afford the corresponding 2-(pyrdin-3-ylamino) 23 and 2-benzylamino 24, respectively (Scheme 4). Experimental detail of products 22, 23 and 24 are reported in the Supplementary Information.
Biological activity
DNA-PK and PI3K activity
From the X-ray crystallography dataCitation14, the most crucial structural features of DNA-PK and PI3K inhibitors in 2-morpholino-8-phenyl-4H-chromen-4-one (LY294002) and 8-aryl analogues (NU7441 or KU-0060648) is the morpholine ring which forms a key hydrogen bond with Val882 (1.5 Å)Citation14. This moiety overlaps the volume occupied by the adenine in the ATP–enzyme complex. The chromones scaffold mimics the adenine ring of ATP: the 4-carbonyl group provides hydrogen bonding with Lys833 (3.2 Å), and the 8-aryl ring is located in a space corresponding to the ribose of ATP. It is stacked between Trp812 (π–π interaction, 3.6 Å) and Met804 (hydrophobic contact, 4.2 Å) on one side and Met953 (hydrophobic contact, 4.0 Å) on the other sideCitation14.
This kinase–ligand interaction plays a key role in the enzyme inhibition representing a cornerstone in drug development of novel DNA-PK and PI3K inhibitors. Unlike the morpholine ring, the other parts of the structure are not as crucial for affinity and their modification did not result in decrease in functional kinase inhibitionCitation14. 2-Morpholino substituted-1,3-naphtha-oxazine linear (such as compound 9, ) or angular naphtha-oxazine compounds showed very good selectivity of DNA-PK versus PI3KCitation19.
Furthermore, 6-methoxy-4-morpholino-2H-chromen-2-one 6 () gave good DNA-PK activity (IC50 = 1.7 μM)Citation13,Citation14. As a result, we consider DNA-PK testing of 7-hydroxy-4-oxo-1,2,3,4-tetrahydrocyclopenta[c]chromene-6-carboxylic acid 12b, 6-dibromo-7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carboxylic acid 13b, 2-morpholino-substituted-benzoxazines 15a, b, e, f, h (linear structure) and 17a, c (angular structure) which did not show appreciable activity below 100 μM. Although the products 15 and 17 included the 2-morpholino- and 4-C=O groups required for the DNA-PK activity. It is worth noting that DNA-PK activity did not tolerate the pyran-2-one ring fused to the benzoxazine moiety, however was able to tolerate a phenyl fused ring (1,3-naphtha-oxazines) which gave moderate DNA-PK activityCitation17.
Some PI3K activity () was observed for the hydroxy-carboxy-chromene 12b was low activity for PI3K isoforms β, γ and δ, IC50 = 49.10, 69.70 and 81.00 μM respectively, while 13b improved PI3Kγ (IC50 = 5.56 μM) and PI3Kα (IC50 = 14.7 μM). Hence compound 13b can be considered as a dual PI3Kγ and α inhibitor with non-DNA-PK activity tested at compound concentration ≥100 μM.
Table 1. PI3K inhibition IC50 of product 12b, 13b, 15a and 17b,c.
The 6-methyl-2-morpholino-chromen-oxazine-4,8-dione 15a (linear structure) showed similar PI3Kβ and δ activity with IC50 = 23.10 and 28.26 μM respectively. Furthermore, 4-methyl-8-morpholino-chromen-oxazine-2,10-dione 17a (angular structure) showed only PI3Kδ activity with an IC50 = 5.08 μM and no activity for the angular structure 17c > 100 μM (). Consequently product 17a can be considered as a selective PI3Kδ inhibitor with no observed DNA-PK activity when assayed at 100 μM concentrations.
Inhibition of collagen-induced platelet aggregation
Inhibition of collagen-induced platelet aggregation was performed according to the previously reported assay procedureCitation15 at an initial compound concentration of 80 μM.
Compound 7-hydroxy-2-oxo-2H-chromene-6-carboxylic acid 10a showed 24% inhibition while 10b, e, f and chromene-8-carboxylic acids 12a–c showed no inhibition at 80 μM.
Unexpectedly collagen-induced human platelet aggregation at compound concentration 80 μM for 2-amino-chromen-1,3-oxazine analogues (linear structure) 14c–i showed low or no inhibition. Compounds 14c, e, g, and h resulted in inhibition of 32, 13, 16 and 23% respectively versus the control.
However, the angular morpholino-chromene analogues 17a and b (Scheme 2) displayed improved inhibition activity over their linear analogue. Compound 17b was found to have an IC50 of 50 μM, while compound 17a was moderately active showing 35% inhibition when tested at 80 μM. It is worth noting that platelet aggregation activity did not tolerate pyran-2-one ring fused to the benzoxazine moiety similar to the results found for phenyl-fused rings (1,3-naphth-oxazines) which gave low platelet aggregation activityCitation17.
Antibacterial assay results
Compounds 10a–f, 12a–c, 11a–c, 13b, 14b–i, 15a, c, d, g and 19a, c (Schemes 1–3) were assayed for antibacterial activity using the disk diffusion method with Muller–Hinton agar against E. coli, P. aeruginosa, B. subtilis, S. aureus and E. faecalis. Each compound was prepared as a 20.0 mmol solution in DMSO then 2.5 μL of each compound solution (50 nmol) was applied to the appropriate blank antibacterial susceptibility disks. Ciprofloxacin was used as a positive control and DMSO blank as a negative control. The diameter of the zone of inhibition was measured in millimetres (mm) after 24 h of incubation at 37 °C. No zones of inhibition around the 6 mm blank susceptibility disks were observed for all the tested compounds as well for the DMSO blank as a negative control. While the ciprofloxacin positive control showed a zone of inhibition diameter as 35, 28, 39, 28 and 20 mm, respectively.
Scheme 1. Synthesis of substituted-7-hydroxy-2-oxo-2H-chromene-6-carboxylic acid 10a–f, 11a–c and chromene-8-carboxylic acids 12a–c & 13a,b.
Scheme 2. Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14, angular compounds 16, 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione 15 and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 17. Reaction conditions: (i) freshly prepared Ph3P(SCN)2; (ii) morpholine in dioxane/reflux.
![Scheme 2. Synthesis of substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14, angular compounds 16, 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione 15 and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 17. Reaction conditions: (i) freshly prepared Ph3P(SCN)2; (ii) morpholine in dioxane/reflux.](/cms/asset/eafdb003-f9f9-4040-9337-2d6e764369d3/ienz_a_1190710_sch0002.gif)
Scheme 3. Synthesis of mono- and di-bromo-2-morpholino-substituted-benzoxazines 19a–c. Reaction condition: (i) NaHCO3\in water/2-propanol/MeI; (ii) morpholine/RT.
Scheme 4. Outline the reactions of substituted-2-thioxo and 2-methyl thio-chromen-1,3-oxazine linear compounds 14a,b and 22a,b with benzylamine and 2-(pyrdin-3-yl-amino) respectively. Reaction conditions: (i) NaHCO3/benzylamine in water/2-propanol; (ii) NaHCO3 in water/2-propanol/MeI; (iii) 3-aminopyridine; (iv) benzylamine.
Conclusions
New substituted-7-hydroxy-2-oxo-2H-chromene-6-carboxylic acids 10a–f, chromene-8-carboxylic acids 12a–c, monobromo- and dibromo-acid analogues 11 and 13, the corresponding substituted-2-thioxo-chromen-1,3-oxazine linear compounds 14a–i and angular compounds 16a–e and 2-morpholino-substituted-chromen[6,7-e][1,3]oxazine-4,8-dione and 8-morpholino-substituted-chromen[8,7-e][1,3]oxazine-2,10-dione 15a–f and 17a–c were synthesised and characterised. DNA-PK, PI3K, anti-platelet and anti-bacteria activity of some of these products were assayed. No appreciable activity of DNA-PK at 100 μM, anti-platelet at 80 μM and anti-bacteria at 50.0 nmol were observed which indicated that the pyran-2-one ring fused to the benzoxazine moiety was not tolerated. Some of these products showed moderate to high isoforms selectivity of PI3K activity and compound 17a can be considered as a selective PI3Kδ inhibitor with non-DNA-PK ≥ 100 μM and compound 13b can be considered as a selective PI3Kγ, α inhibitor with non-DNA-PK ≥ 100 μM.
Ethics
Ethical approval for the use of human blood was obtained from La Trobe University Human Ethics Committee (FHEC10/R73).
Declaration of interest
The authors declare no conflict of interest.
Supplementary material available online
IENZ_1190710_Supplementary_Material.pdf
Download PDF (290.4 KB)References
- Kempen I, Hemmer M, Counerotte S, et al. 6-Substituted 2-oxo-2H-1-benzopyran-3-carboxylic acid derivatives in a new approach of the treatment of cancer cell invasion and metastasis. Eur J Med Chem 2008;43:2735–50
- Radanyi C, Le Bras G, Marsaud V, et al. Antiproliferative and apoptotic activities of tosylcyclonovobiocic acids as potent heat shock protein 90 inhibitors in human cancer cells. Cancer Lett 2009;274:88–94
- Nawrot-Modranka J, Nawrot E, Graczyk J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur J Med Chem 2006;41:1301–9
- Kucherenko A, Flavin MT, Boulanger WA, et al. Novel approach for synthesis of (±)-calanolide A and its anti-HIV activity. Tetrahedron Lett 1995;36:5475–8
- Chenera B, West ML, Finkelstein JA, Dreyer GB. Total synthesis of (±)-calanolide A, a non-nucleoside inhibitor of HIV-1 reverse transcriptase. J Org Chem 1993;58:5605–6
- Žalubovskis R. In a search for selective inhibitors of carbonic anhydrases: coumarin and its bioisosteres–synthesis and derivatization. Chem Heterocycl Comp 2015;7:607–12
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2009;53:335–44
- Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62
- Kempen I, Papapostolou D, Thierry N, et al. 3-Bromophenyl 6-acetoxymethyl-2-oxo-2H-1-benzopyran-3-carboxylate inhibits cancer cell invasion in vitro and tumour growth in vivo. Br J Cancer 2003;88:1111–18
- Zhou X, Chen Y-D, Wang T, et al. Rational design, synthesis, biological evaluation, homology and docking studies of coumarin derivatives as α1-adrenoceptor antagonists. Chem Biodiversity 2011;8:1052–64
- Tyagi YK, Tyagi S, Raj HG, Gupta RK. Synthesis of novel 4-methylcoumarins and comparative specificities of substituted derivatives for acetoxy drug: protein transacetylase. Sci Pharm 2008;76:395–414
- Amian A, Rodriguez JN, Muniz R, et al. Treatment with oral anticoagulants (acenocoumarol): influence of the initial doses in the incidence of hemorrhagic and thromboembolic episodes. Sangre (Barc) 1994;39:413–16
- Hollick JJ, Rigoreau LJM, Cano-Soumillac C, et al. Pyranone, thiopyranone, and pyridone inhibitors of phosphatidylinositol 3-kinase related kinases. Structure-activity relationships for DNA-dependent protein kinase inhibition, and identification of the first potent and selective inhibitor of the Ataxia Telangiectasia mutated kinase. J Med Chem 2007;50:1958–72
- Andrs M, Korabecny J, Jun D, et al. Phosphatidylinositol 3-kinase (PI3K) and phosphatidylinositol 3-kinase-related kinase (PIKK) inhibitors: importance of the morpholine ring. J Med Chem 2015;58:41–71
- Pritchard KM, Al-Rawi J, Bradley C. Synthesis, identification and antiplatelet evaluation of 2-morpholino substituted benzoxazines. Eur J Med Chem 2007;42:1200–10
- Ihmaid SK, Al-Rawi JMA, Bradley CJ, et al. Synthesis, DNA-PK inhibition, antiplatelet activity studies of 2-(N-substituted-3-aminopyridine)-substituted-1,3-benzoxazines and DNA-PK and PI3K inhibition, homology modeling studies of 2-morpholino-(7,8-di and 8-substituted)-1,3-benzoxazines. Eur J Med Chem 2012;57:85–101
- Ihmaid S, Al-Rawi J, Bradley C, et al. Synthesis, structural elucidation, DNA-PK inhibition, homology modelling and anti-platelet activity of morpholino-substituted-1,3-naphth-oxazines. Bioorg Med Chem 2011;19:3983–94
- Ihmaid S, Al-Rawi J, Bradley C. Synthesis, structural elucidation and DNA-dependent protein kinase and antiplatelet studies of 2-amino-[5, 6, 7, 8-mono and 7, 8-di-substituted]-1,3-benzoxazines. Eur J Med Chem 2010;45:4934–46
- Morrison R, Belz T, Ihmaid SK, et al. Dual and/or selective DNA-PK, PI3K inhibition and isoform selectivity of some new and known 2-amino-substituted-1,3-benzoxazines and substituted-1,3-naphthoxazines. Med Chem Res 2014;23:4680–91
- Morrison R, Al-Rawi JMA, Angove MJ, et al. Synthesis, structure elucidation, DNA-PK and PI3K and anti-cancer activity of 8- and 6-aryl-substituted-1-3-benzoxazines. Eur J Med Chem 2016;110:326–39
- Pritchard KM, Al-Rawi J. Reaction of Ph3P(SCN)2 with further ortho-hydroxy carboxylic acid systems, including substituted β-keto acids: synthesis of novel 2- thioxo-1,3-oxazines and their subsequent transformation with amines. Synth Commun 2008;38:4076–96
- Sethna SM, Shah RC. Pechmann condensation of methyl β-resorcylate with some β-ketonic esters. J Indian Chem Soc 1940;17:37–40
- Desai RD, Gaitonde MM, Hasan SM, Shah RC. Heterocyclic compounds. XVIII. Condensation of cyclic β-ketonic esters with methyl β-resorcylate and resacetophenone in the presence of anhydrous aluminum chloride. Proc – Indian Acad Sci Sect A 1947;25A:345–50
- Dalvi VJ, Sethna S. Bromination of coumarins. I. Bromination of 7-hydroxy-4-methylcoumarin, methyl 7-hydroxy-4-methyl-coumarin-6-carboxylate, 7-hydroxy-4-methylcoumarin-6-carboxylic acid, and their methyl ethers. J Indian Chem Soc 1949;26:359–65
- Rodriguez-Dominguez JC, Kirsch G. Zirconyl chloride: a useful catalyst in the Pechmann coumarin synthesis. Synthesis 2006;2006:1895–7
- Heppell J, Al-Rawi J. Functionalization of quinazolin-4-ones. Part 1: synthesis of novel 7-substituted-2-thioxo quinazolin-4-ones from 4-substituted-2-aminobenzoic acids and PPh3 (SCN)2. J Heterocycl Chem 2014;51:162–74
- Pritchard K, Al-Rawi J, Hughes A. Generalized method for the production of 1,3-benzoxazine, 1,3-benzothiazine, and quinazoline derivatives from 2-(hydroxy, thio, or amino) aromatic acids using triphenylphosphine thiocyanogen. Synth Commun 2005;35:1601–11
- Shah RC, Sethna SM, Banerjee BC, Chakravarti D. Pechmann's condensation of methyl β-resorcylate and β-resorcylic acid with ethyl acetoacetate. J Indian Chem Soc 1937;14:717–20
- Hedayatullah M, Huynh AH. Heterocyclic compounds from cyano esters. II. Cyanates and oxazinones of the coumarin series. Tetrahedron Lett 1976;31:1289–92