Abstract
The transmembrane serine protease, TMPRSS2 is an important target in the treatment of seasonal influenza infections and contributes to prostate carcinogenesis and metastasis. In this study, the effect of the synthetic TMPRSS2 inhibitor I-432 on jejunal IPEC-J2 cell monolayers cultured on membrane inserts was characterized. Using a fluorogenic substrate, it was found that the apical addition of I-432 could suppress trypsin-like activity in the supernatants of IPEC-J2 cells. The inhibition of TMPRSS2 did not affect physiologically produced hydrogen peroxide levels in the apical and in basolateral compartments. Loss of expression of the TMPRSS2 serine protease domain (28 kDa) was also observed when cells were pre-exposed to I-432. Partial decrease in immunofluorescent signal intensities derived from the altered distribution pattern of TMPRSS2 was detected after a 48 h long incubation of IPEC-J2 cells with the inhibitor indicating the efficacy of TMPRSS2 inhibition via I-432 administration in vitro.
Introduction
Cell surface-associated regulatory proteolytic processes appear to be responsible for degradation of extracellular matrix components, and contribute to tumor invasion, metastasis, blood coagulation, cell differentiation and apoptotic events. Type II transmembrane trypsin-like serine proteases (TTSPs) have been divided into four subgroups based on the phylogenetic analysis of their serine protease domains and the domain pattern of the extracellular stem region, including the human airway trypsin-like protease (HAT)/differentially expressed in squamous cell carcinoma (DESC), the hepsin/transmembrane protease serine (TMPRSS), the matriptase and the corin subfamiliesCitation1,Citation2. All TTSPs contain six conserved cysteine residues within their catalytic domain, which form three intradomain disulfide bonds as known for all serine proteases of the S1 fold. TTSPs have high affinity towards substrates containing an Arg residue in the P1 position compared to Lys containing sequences and they may be activated by other members of the family or via intermolecular autoactivation, as shown in vitro for HAT, matriptase, matriptase-2 and TMPRSS2Citation3–7. After activation, the C-terminal protease domain remains covalently linked to N-terminal located domains by a conserved disulfide bond and can develop their activity in cellular compartments or on the cell surfaceCitation8. Similarly to other TTSPs, TMPRSS2 contains an extracellularly located C-terminal serine protease domain as well as the stem region with a group A scavenger receptor cysteine-rich domain (SRCR) and a low-density lipoprotein receptor class A domain (LDLRA)Citation3.
TTSP-regulated proteolysis is a prerequisite for tissue homeostasis, however, uncontrolled expression and enhanced enzymatic activity of cell surface-attached TTSPs can lead to different disorders such as carcinogenesisCitation9 and contributes to influenza and other respiratory viral infectionsCitation10. Moreover, TMPRSS2 accumulated in the glandular lumen of normal and cancerous prostate tissues can be proteolytically cleaved and the rise in released protease fragment levels can confirm the presence of cancer cells. This can be used as diagnostic or prognostic marker for prostate cancerCitation7.
Expression of TMPRSS2 was detected in the luminal epithelial cells of mouse and human prostateCitation11, strong membranous protein presence was found in renal tubules, epididymis and ducts of pancreas, whereas moderate membranous staining was observed in gastrointestinal tractCitation7,12–14. However, the physiological role of this TTSP has not been completely revealed so far. TMPRSS2 knockout mice showed normal growth and reached normal adulthood without having abnormalities in organ histology and alteration in protein levels of prostatic secretions. This suggests that TMPRSS2 may maintain a specialized but nonvital function that is apparent only in association with systemic homeostasis perturbationCitation15. TMPRSS2 is expressed on MDCK-TMPRSS2 cell surface as full length zymogen (∼70 kDa) and in a processed form (∼30 kDa). The truncated form containing the catalytic domain can be shed in small amounts into supernatants, but interestingly, only a minor enzymatic activity could be detected for the soluble form of TMPRSS2Citation16.
TMPRSS2 present in the human airways is capable of cleaving influenza virus hemagglutinin (HA) thus facilitating fusion between viral and endosomal membranes. This suggests that potent TMPRSS2 inhibitors could be potential drugs for the treatment of influenza infections and virus spread. Suppression of HA cleavage and the inhibition of influenza virus spread in TMPRSS2-expressing MDCK cells by treatment with hydrophobic decanoylated peptide mimetic protease inhibitor, I-3 was observed after exposure of cells to A/Hamburg/09 (H1N1)Citation14,Citation16. I-432 was found to be one of the most potent 3-amidinophenylalanine-derived inhibitors of TMPRSS2 and matriptaseCitation17 and it was also shown that I-432 at 50 μM did not affect cell viability in IPEC-J2 cells after 48 h treatmentCitation18.
Enzymatic activity of TMPRSS2 was initially determined by the trypsin substrate, Cbz-Gly-Gly-Arg- (aminomethyl) coumarin (AMC). The amounts of matrix metalloproteases (MMP-2 and MMP-9) responsible for metastasis of tumor cells were reported to be elevated in prostate cancer upon activation of protease-activated receptor-2 (PAR-2), the substrate for TMPRSS2. By use of the PAR-2 antagonist Phe-Ser-Leu-Leu-Arg-Tyr-NH2 it was found that matriptase and TMPRSS2 can activate PAR2, which suggests an additional link between a membrane-anchored serine protease and prostate tumor metastasis. However, further studies should be conducted if excessive amount of TMPRSS2 in intracellular space may play a functional role in prostate tumorigenesis in addition to cell surface protease activityCitation9,Citation13,Citation19.
Another fluorogenic substrate, Boc-Leu-Gly-Arg-AMC was used as TMPRSS2 substrate to assess the possibility of proteolytic cleavage of the influenza virus surface glycoprotein HA by host cell proteasesCitation16. Improved substrate properties were found for the fluorogenic AMC derivative I-507 (Mes-D-Arg-Pro-Arg-AMC KM = 2.9 μM), which is more efficiently cleaved and was applied for a first inhibitor screening. In that work various substrate-analogue structures containing a 4-amidinobenzylamide as P1 residue and several arylsulfonylated amides of 3-amidinophenylalanine could be identified as potent TMPRSS2 inhibitorsCitation17.
In this study, non-tumorigenic jejunal epithelial IPEC-J2 cells grown on microporous membrane were used for mimicking in vivo conditions to study the effects of a potent combined inhibitor I-432 (i) on enzymatic activity of this TTSP in cell membrane and in soluble form using the fluorogenic substrate I-507 and (ii) on production of physiological reactive oxygen species in apical and basolateral compartments. In addition, the efficiency of TMPRSS2 inhibition was also investigated to see how the I-432 can affect this trypsin-like transmembrane serine protease at protein level.
Methods
Cell lines and culture conditions
The IPEC-J2 cell line was derived from jejunal epithelia of a neonatal piglet. The IPEC-J2 cell line was kindly provided by Dr. Jody Gookin and Dr. Stephen Stauffer, Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA and it was used between passage 50 and 75. Cells form a differentiated layer and are attached to each other via tight junctions. IPEC-J2 cells were seeded at a density of 1.5 × 105 per well on six-well plates with Transwell polyester membrane inserts (pore size 0.4 μm; surface area 4.67 cm2; Sigma-Aldrich, St. Louis, MO) coated with rat tail collagen (Sigma-Aldrich) in a 1.5 ml apical and 2.6 ml basolateral volume. Cells were maintained in complete medium containing 1:1 mixture of Dulbecco's Modified Eagle's Medium and Ham's F-12 Nutrient Mixture (DMEM/F12) supplemented with 5% FBS, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, 5 ng/ml epidermal growth factor and 1% penicillin-streptomycin (all from Fisher Scientific, Pittsburgh, PA). Cell cultures were tested by PCR and were found to be free of mycoplasma contamination. Cells were allowed to adhere for 24 h before being washed and re-fed every other day until confluence was reached. Transepithelial electrical resistance (TEER) measurement of monolayers was performed on alternate days after seeding, from day 5 to 21 of culture, using an EVOM Epithelial Tissue Volt/Ohmmeter (World Precision Instruments, Berlin, Germany). They were grown at 37 °C in a humidified atmosphere of 5% CO2.
Exposure of IPEC-J2 cells to TMPRSS2 inhibitor
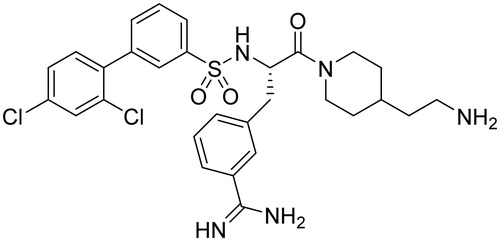
The stock solutions of dibasic TMPRSS2/matriptase inhibitor, I-432 (Ki = 0.9 nM for TMPRSS2 and Ki = 2 nM for matriptase, ) at 10 mM were prepared and it was kept at −20 °C. Before treatment, the confluent layers of IPEC-J2 cells were washed twice with supplement-free plain medium. The solutions of the TMPRSS2 inhibitor in phenol red free DMEM at 10, 25 and 50 μM were prepared freshly prior to enzyme activity assays and immunofluorescence experiments from a 10 mM stock solution. I-432 was added at the 50 μM in case of extracellular H2O2 measurements and Western blot analysis. After 48 h incubation, the cells were washed twice with plain medium before being subjected to the subsequent procedures.
Protein extraction
For protein extraction, the IPEC-J2 cells were washed with phosphate-buffered saline buffer (PBS, pH 7.4) and harvested with cell scraper after adding 250 μl extraction buffer (20 mM Tris pH 7.4, 2 mM EDTA, 150 mM NaCl, 1% Triton X-100 supplemented with 10 μl/ml phosphatase inhibitors (Sigma-Aldrich) and 5 μl/ml proteinase inhibitors (Sigma-Aldrich)). The pellet was collected in 1.5 ml Eppendorf tubes and lysed for 30 min on ice. Lysates were then centrifuged at 12 000 rpm for 15 min at 4 °C. The extracts were mixed with 5× Laemmli sample buffer containing 5% 2-mercaptoethanol (BioRad Laboratories, Philadelphia, PA) and heated to 95 °C for 5 min. Protein concentration in the supernatant was determined by the Bradford assay (BioRad Laboratories).
Western blot
Analysis of TMPRSS2 in cell extract was performed by Western blotting. Equal amounts of protein (20 μg) were loaded and run on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 180 V for 1 h. After running, the proteins were transferred onto Immobilion-P nitrocellulose membrane (Millipore, Darmstadt, Germany) at constant 75 mA at 4 °C overnight. After blotting, the membrane was stained with Ponceau S red (BioRad Laboratories) to visualize the transferred proteins. Nonspecific binding of the antibody was blocked by incubation with 5% nonfat milk (BioRad Laboratories) dissolved in 1 × 0.1 M Tris-buffered saline (TBS) containing 0.05% Tween 20 pH 7.4 (TBST) for 60 min at room temperature. The membrane was washed five times for 5 min with 1× TBST, and incubated overnight at 4 °C with rabbit polyclonal anti-TMPRSS2 (1:500, Sigma-Aldrich) antibody diluted in 1 × TBST containing 3% nonfat milk. Next day the membrane was washed 5 times for 5 min with TBST and incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody (1:1000, Cell Signaling Technology, Inc., Danvers, MA) diluted in 1× TBST containing 1% nonfat milk for 60 min at room temperature. For loading control rabbit, anti-β-actin (1:2000, Cell Signaling Technology, Inc.) antibody was used for 60 min. Detection was performed by Super Signal West Pico ECL reagent for 10 min (Pierce Biotechnology Inc., Rockford, IL). The molecular mass of specific bands was determined comparing to the Precision Plus Protein Standard (BioRad Laboratories) applied on the same gels. Densitometric analysis of the blots was done with Kodak Molecular Imaging Software 4.1 in a Kodak Image Station 4000 MM (Kodak, Rochester, NY).
Protease activity at the cell surface and in cell supernatants
To determine the trypsin-like protease activity, IPEC-J2 cells were cultured in 24-well plates. Prior to the protease activity measurements, the cells were treated with I-432 at 10, 25 and 50 μM for 48 h. Cells were washed with phenol red-free DMEM and the protease activity was assessed by incubation of cells with the fluorogenic substrate Mes-D-Arg-Pro-Arg-AMC × 2 TFA (I-507)Citation17. I-507 was used at the final concentration of 200 μM in 50 mM PBS (pH = 7.4) for 30 min at 37 °C. Hydrolysis of the peptide was monitored by the measurements of fluorescence intensity using a Victor X2 2030 fluorescence spectrometer at λex = 380 nm and λem = 460 nm.
To examine the enzymatic activity in the supernatants, IPEC-J2 cells were grown in 24-well plates and were incubated with I-432 at 10, 25 and 50 μM for 48 h. The supernatants were then removed, cleared by low-speed centrifugation (3000 rpm, 10 min at 4 °C), and treated with the same fluorescence substrate at the final concentration of 200 μM in the supernatant. After 30 min incubation time at 37 °C, fluorescence intensity was measured.
Extracellular H2O2 measurement by the Amplex red method
Fluorescent ROS measurement of cell supernatant was based on the detection of H2O2 using the Amplex Red Hydrogen Peroxide Assay Kit (Invitrogen/Life Technologies, Eugene, OR). In the presence of horseradish peroxidase (HRP), Amplex Red reacts with H2O2 in a 1:1 stoichiometry producing a highly fluorescent resorufinCitation20. IPEC-J2 cells were treated with I-432 at 50 μM for 48 h in phenol red free DMEM and the H2O2 concentrations in the medium were determined using a working solution of 100 μM Amplex Red and 0.2 U/ml HRP. After 30 min incubation with the dye at room temperature, the quantitative H2O2 contents of apical and basolateral compartments were measured using a Victor X2 2030 fluorometer (λex = 560 nm, λem = 590 nm).
Investigation of TMPRSS2 distribution via immunfluorescent staining
Inserts were fixed in methanol for 5 min followed by bovine serum albumin (BSA (5%), Sigma Aldrich) protein block for 20 min. Sections were incubated for 1 h in a humid chamber at room temperature with anti-TMPRSS2 rabbit polyclonal primary antibody (1:200, Sigma-Aldrich) and diluted in 5% BSA solution. For secondary antibody Alexa546 (orange-red) anti-rabbit Ig-s 1:200 diluted in PBS were used for 1 h. Sialic acid residues in cell membrane were stained with wheat germ agglutinin (WGA) (1:200 diluted in PBS, WGA Alexa Fluor 488, Invitrogen-Molecular Probes) for 10 min to visualize the localization of cell membrane and cell nuclei were stained in blue using 4′,6-diamidino-2-phenylindole (DAPI) (1:500 diluted in PBS, Invitrogen-Molecular Probes) for additional 10 min. Between incubations, the slides were washed in PBS for 3 × 2 min. Membranes were attached on glass slides using fluorescent mounting medium (DAKO, Glostrup, Denmark). The samples were analyzed using a Nikon Eclipse E600 epifluorescent microscope (Nikon, Amsterdam, Netherlands) with LUCIA™ Citogenetics 2.5 software.
Statistical analysis
For statistical evaluation, R 2.11.1 software package (2010; www.R-project.org) was applied. Differences between means were evaluated by one-way analysis of variance (one-way ANOVA) with post-hoc Tukey test, where data were of normal distribution and homogeneity of variances was confirmed.
Results
Expression of TMPRSS2 in IPEC-J2 cells
Different protein species for TMPRSS2 in whole cell lysates from IPEC-J2 cells untreated or exposed to I-432 analyzed by western blotting can be seen in . The serine protease domain of TMPRSS2 was absent (missing 28 kDa band) in cell lysates when IPEC-J2 cells were exposed to I-432 at 50 μM for 48 h (p = 0.02057). It was also observed that expression of truncated fragments of 62 kDa was significantly reduced after I-432 administration (p = 0.00357). However, full length form of TMPRSS2 with molecular mass of 72 kDa can be found mainly in I-432-treated samples and it can be detected only in trace quantities in control IPEC-J2 cells (p = 9.3535 × 10−5) ().
Figure 2. Expression of TMPRSS2 in IPEC-J2 cells. IPEC-J2 cells were untreated or exposed to I-432 for 48 h at 50 μM in phenol red free DMEM at pH = 7.4 at 37 °C. Cell lysates were prepared and analyzed by immunoblotting using polyclonal TMPRSS2 Ab. The full length of TMPRSS2 present in IPEC-J2 cells appeared as a 72 kD band, the truncated form as a 62 kDa band and serine protease domain as 28 kDa band on the blot, whereas β-actin (42 kDa) was used as reference housekeeping protein on the same blot. (A) Relative expression levels for full length form with apparent molecular mass of ∼72 kDa in I-432 treated samples versus control (p = 9.3535 × 10−5). (B) Relative expression levels for truncated form with apparent molecular mass of ∼62 kDa in I-432 treated samples versus control (p = 0.00357). (C) Relative expression levels for serine protease domain with apparent molecular mass of ∼28 kDa in I-432 treated samples versus control (p = 0.02057). Relative expression levels of detected protein products were compared in I-432 and in mock (Co) IPEC-J2 samples based on densitometric quantification of blot bands. Data are presented as average relative expression intensities ± SDs. Results shown are representative of three independent experiments.
Enzymatic activity of TMPRSS2
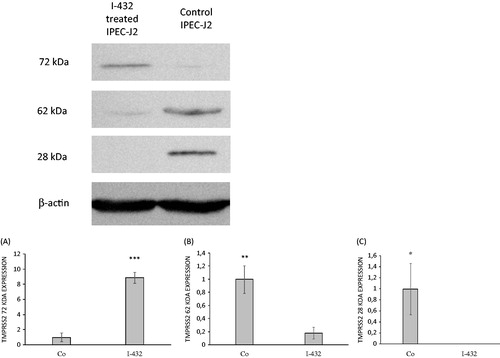
The cleavage rate of the substrate Mes-D-Arg-Pro-Arg-AMC was determined in the absence and in the presence of I-432 in cell-associated forms and in supernatants of non-tumorigenic IPEC-J2 cells. I-432 could significantly decrease the activity of shed TTSPs including that of TMPRSS2 of high potency towards I-507 thus lowering the extent of cleavage of applied fluorogenic substrate. Significant differences can be seen in fluorescence intensity values when IPEC-J2 cells were treated with I-432 at 10 μM (p = 0.02039), at 25 μM (p = 0.00113) and 50 μM (p < 0.001) compared to those of controls ().
Figure 3. Measurements of enzymatic TMPRSS2 activity using the fluorescence substrate I-507 at 200 μM in presence of inhibitor I-432 apically applied at 10, 25 and 50 μM for 48 h in IPEC-J2 cell system. (A) Enzymatic activity assay of shed TMPRSS2 in the supernatant of IPEC-J2. The data are indicated as average fluorescence intensities ± SDs (n = 3). Significant differences can be seen between IPEC-J2 cells exposed to I-432 at 10 μM (p = 0.02039), at 25 μM (p = 0.00113), at 50 μM (p < 0.001) for 48 h compared to those in untreated samples. (B) Activity measurements of cell-associated TMPRSS2 in control and in inhibitor-treated IPEC-J2, the data are indicated as average fluorescence intensities ± SDs. No significant differences were found in control and inhibitor-treated IPEC-J2 cells (n = 3, p > 0.05).
Tryptic activity of cell-associated TTSPs was also monitored in intact IPEC-J2 cells. In accordance with the measurement of soluble forms of proteins released from cells, we also detected proteolytic activity of cell-associated TTSP (p > 0.05, ).
Extracellular H2O2 produced by IPEC-J2 cells exposed to I-432
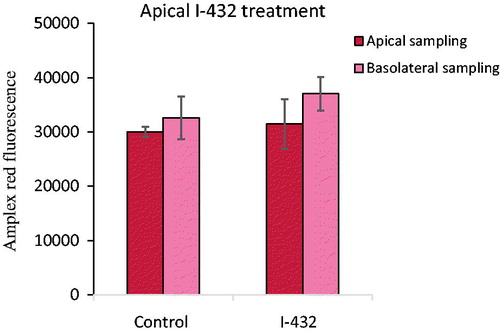
After a 48 h incubation time with apically administered inhibitor, I-432 at 50 μM fluorescence values of Amplex red were determined in IPEC-J2 cells compared to nontreated control cells (). The results showed that there were not any significant perturbations in redox balance in IPEC-J2 cells for the 50 μM concentration of the inhibitor compared to control values independently of the sampling type (p = 0.954 for apical sampling, p = 0.436 for basolateral sampling). It can be also seen that prior to and after I-432 treatment extracellular hydrogen peroxide levels did not differ in apical and in basolateral compartments (p = 0.795 and p = 0.265, respectively).
Figure 4. Detection of extracellular H2O2 production when IPEC-J2 cells were treated with 50 μM I-432 for 48 h apically using apical and basolateral sampling. Data are presented as average fluorescence intensities ± SDs. Apical treatment with I-432 did not result in significant alterations in peroxide level independently of the sampling type (p = 0.954 for apical sampling, p = 0.436 for basolateral sampling, n = 3).
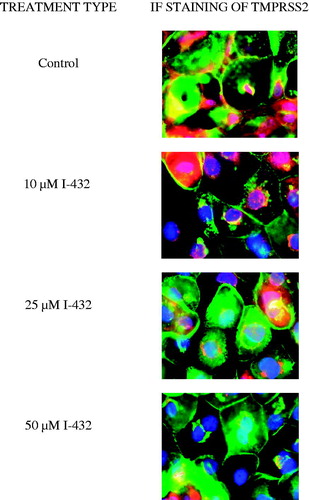
Subcellular localization of TMPRSS2 in IPEC-J2 cells
IPEC-J2 cells were exposed to inhibitor I-432 at 10, 25 and 50 μM for 48 h and cellular distribution of TMPRSS2 was analyzed by epifluorescent miscroscopy. In control cells, TMPRSS2 (show in red) can be found on the cell surface with a more intense staining pattern at cell-cell contacts and within the IPEC-J2 cells where the staining was rather diffuse. Immunfluorescence analysis revealed that in control samples wheat germ agglutinin (WGA, shown in green) was co-localized with TMPRSS2 confirming the cell membranous positivity in addition to perinuclear presence of TMPRSS2 under physiological conditions. With increasing concentration of I-432 only smaller amounts of TMPRSS2 could be detected (), presumably at the site of the synthesis with a visible decrease in the membrane presence of TMPRSS2.
Figure 5. Immunostaining of TMPRSS2 expression pattern in IPEC-J2 cells cultured on polyester membrane inserts treated with inhibitor I-432 at 10, 25 and 50 μM for 48 h. Cell nuclei are stained blue (DAPI), cell membranes are labeled with wheat germ agglutinin (Alexa 488, green). In control samples, TMPRSS2 (Alexa 564, red) is colocalized with wheat germ agglutinin. 48 h long treatment of I-432 induced significant fluorescent signal loss of TMPRSS2. TMPRSS2 occurence was mainly observed around synthesis sites in I-432-treated IPEC-J2 cells. 600× magnification. The scale bar (white) indicates 20 μm (n = 3).
Discussion
Prophylactic vaccination is widely accepted to stop seasonal flu epidemics, however, the vaccine has to be reformulated annually due to the alteration of the virus genome causing changes in virus surface proteins. Currently, there are only few approved therapeutic options with limited efficacies in the treatment of acute influenza virus infections, including administration of M2 ion channel blockers or neuraminidase inhibitorsCitation21. Very recently, the pyrazinecarboxamide RNA polymerase inhibitor, favipiravir was approved in Japan against influenzaCitation22.
Proteolysis of influenza virus HA by host cell proteases is a prerequisite for viral infectivity, but the precise protease responsible for HA cleavage has not been completely elucidated yet. It was previously shown that TMPRSS2 allows HA cleavage thus promoting viral spread. Knockout of TMPRSS2 could prevent the propagation of H1N1, H7N9 viruses into the lungs of mice, however, TMPRSS2 seemed to play only marginal role in H3N2-caused infectionCitation23,Citation24.
Development of synthetic inhibitors of TMPRSS2 can offer also possibilities in the therapy of human metapneumovirus (HMPV) infections by reduction in cleavage of the fusion protein FCitation25,Citation26 or in the treatment of severe acute respiratory syndrome coronavirus, where TMPRSS2 is involved in the spike protein S activationCitation27,Citation28. Calu-3 cells infected with H1N1 or H3N2 influenza viruses were treated with I-432 and related TMPRSS2 inhibitors at 50 μM for 48 h incubation time and it was found that the viral replication was significantly reduced. So far, the most potent inhibitor for suppression of H1N1 and H3N2 influenza viral spread in Calu-3 cells is I-432, which was also applied in our present studyCitation17.
IPEC-J2 behave similarly to human colon adenocarcinoma cells (Caco-2 and T84 cells) with the advantage of not being cancerous, and their glycolysation pattern, proliferation rate and colonization ability are closer to physiological functioning of enterocytesCitation29. Influenza A viruses in pigs can lead to both economic losses and can present permanent risks of cross-species transmission of new strains to humansCitation30. It was also reported that cleavage of HA in human colon adenocarcinoma cells appeared to take place intracellularlyCitation31 and reverse transcription-PCR analyzes revealed that Caco-2 cells express TMPRSS2Citation16. Further studies are still ongoing to ascertain the abundance and cellular localization of TMPRSS2 in different cell types and tissues. This is the first study in which expression of TMPRSS2 was determined and confirmed at protein level in a non-tumorigenic intestinal cell line, IPEC-J2 using Western blot technique.
In this work, we used the potent TMPRSS2 inhibitor I-432 to elucidate how this drug candidate can influence physiological parameters such as reactive oxygen species production as a key indicator of redox status. It was previously shown by us that I-432 at 50 μM applied for 48 h did not induce cell death compared to control cellsCitation18. It was found, however, that various inhibitors including I-432 could weaken intestinal barrier integrity by lowering transepithelial electrical resistance in vitroCitation32.
This is the first study in which the inhibitory effects of a potent 3-amidinophenylalanine-derived serine protease inhibitor was characterized on TMPRSS2 expression at protein level in IPEC-J2 cell line. Only the enzymatic activity of TTSP in cell supernatants could be suppressed in a concentration-dependent manner when cells were exposed to I-432 at 10, 25 and 50 μM. Under untreated conditions the baseline enzymatic activities were comparable in case of supernatants and membrane associated forms, but I-432 could not suppress cleavage of I-507 by cell-associated proteases suggesting that trypsin-like serine proteases other than TMPRSS2 could be present in the studied samples. Similarly to the previous results in MDCK cellsCitation16, enzymatic activity of TMPRSS2 at the cell surface and the soluble forms released from the plasma membrane were found to be very low. Evaluation of host cell contribution to influenza viral pathogenesis as a consequence of host-virus infection in swine, IPEC-J2 cell line seems to be a better in vitro model as an alternative to MDKC cells which differ significantly from either human or porcine cellsCitation33. The further advantage of the application for IPEC-J2 cell line originated from small intestine of unsuckled pigs is that it enables the replacement of animal infection models and supports the in-depth investigation of cross-species transmission and rare, but existing extrapulmonary effects of viral spread in digestive systemCitation34. Based on the fact that expression of TMPRSS2 in stomach and in colon was confirmed, it can be assumed that this serine protease might be responsible for putative extrapulmonary spread of influenza virusesCitation14,Citation35.
To evaluate the side effect of this potential drug candidate, the oxidative stress-inducing properties of I-432 was also monitored. The extracellular hydrogen peroxide level detected by Amplex red fluorescent probe was not altered when I-432 was used at 50 μM for 48 h incubation time. Protein species such as serine protease domain with molecular mass 28 kDa and the full length form of TMPRSS2 (72 kDa) were also identifiedCitation7,Citation16 in addition to 62 kDa truncated fragment. It was also confirmed that treatment with 50 μM I-432 induced complete loss of the band for serine protease domain on the blot. Another consequence of TMPRSS2 blockage via I-432 was the reduction in immunfluorescent signals indicating the lowered expression of this TTSP when the inhibitor was used at micromolar concentrations for 2 days. Taken together, it was ascertained that suppression of TMPRSS2 via I-432 resulted in dysregulated enzymatic activity and modified target protein expression profile compared to physiological serine protease functioning in non-tumorigenic jejunal cell monolayers.
Conclusion
Based on our data, it can be concluded that I-432 as potential drug candidate against influenza virus infection possesses favorable side effect profile considering the complete lack of oxidative stress induction after its administration thus allowing the maintenance of physiological redox status. The ability of this compound for TMPRSS2 inhibition was confirmed at protein level in vitro indicated by the lack of expression of serine protease domain and the decreasing membranous positivity of TMPRSS2 immunofluorescent signals.
Declaration of interest
The authors report no declaration of interest. The authors alone are responsible for the content and writing of this article. This research was supported by the 9877–3/2015/FEKUT grant of the Hungarian Ministry of Human Resources.
Acknowledgements
We are indebted to Dr. Jody Gookin and Dr. Stephen Stauffer, Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, USA for providing IPEC-J2 cells and for the valuable advice on handling them and to Dr. Nóra Meggyesházi, Semmelweis University, 1st Department of Pathology and Experimental Cancer Research for contributing to taking fluorescent images of the IPEC-J2 samples. This project was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the Hungarian Scientific Research Fund [grant number 115685].
References
- Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci 2011;99:1–50.
- Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol 2008;40:1297–316.
- Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem 2001;276:857–60.
- Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem 2009;284:23177–81.
- Takeuchi T, Harris JL, Huang W, et al. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 2000;275:26333–42.
- Velasco G, Cal S, Quesada V, et al. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem 2002;277:37637–46.
- Afar DE, Vivanco I, Hubert RS, et al. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res 2001;61:1686–92.
- Szabo R, Wu Q, Dickson RB, et al. Type II transmembrane serine proteases. Thromb Haemost 2003;90:185–93.
- Ko CJ, Huang CC, Lin HY, et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth and metastasis. Cancer Res 2015;75:2949–60.
- Esumi M, Ishibashi M, Yamaguchi H, et al. Transmembrane serine protease TMPRSS2 activates hepatitis C virus infection. Hepatology 2015;61:437–46.
- Vaarala MH, Porvari K, Kyllönen A, et al. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: detection of mutated TMPRSS2 form in a case of aggressive disease. Int J Cancer 2001;94:705–10.
- Lin B, Ferguson C, White JT, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 1999;59:4180–4.
- Lucas JM, True L, Hawley S, et al. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol 2008;215:118–25.
- Bertram S, Glowacka I, Blazejewska P, et al. TMPRSS2 and TMPRSS4 facilitate trypsin-independent spread of influenza virus in Caco-2 cells. J Virol 2010;84:10016–25.
- Kim TS, Heinlein C, Hackman RC, Nelson PS. Phenotypic analysis of mice lacking the TMPRSS2-encoded protease. Mol Cell Biol 2006;26:965–75.
- Böttcher-Friebertshäuser E, Freuer C, Sielaff F, et al. Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors. J Virol 2010;84:5605–14.
- Meyer D, Sielaff F, Hammami M, et al. Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation. Biochem J 2013;452:331–43.
- Pászti-Gere E, McManus S, Meggyesházi N, et al. Inhibition of matriptase activity results in decreased intestinal epithelial monolayer integrity in vitro. PLoS One 2015;10:e0141077.
- Wilson S, Greer B, Hooper J, et al. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J 2005;388:967–72.
- Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 1997;202:133–41.
- Steinmetzer T, Hardes K, Böttcher-Friebertshäuser E, Garten W. Strategies for the development of influenza drugs: basis for new efficient combination therapies. Top Med Chem 2015;15:143–82.
- Furuta Y, Gowen BB, Takahashi K, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013;100:446–54.
- Tarnow C, Engels G, Arendt A, et al. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. E J Virol 2014;88:4744–51.
- Garten W, Braden C, Arendt A, et al. Influenza virus activating host proteases: identification, localization and inhibitors as potential therapeutics. Eur J Cell Biol 2015;94:375–83.
- Schowalter RM, Smith SE, Dutch RE. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J Virol 2006;80:10931–41.
- Shirogane Y, Takeda M, Iwasaki M, et al. Efficient multiplication of human metapneumovirus in vero cells expressing the transmembrane serine protease TMPRSS2. J Virol 2008;82:8942–6.
- S, Nagata N, Shirato K, Kawase, et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 2010;84:12658–64.
- Glowacka I, Bertram S, Müller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 2011;85:4122–34.
- Cencic A, Langerholc T. Functional cell models of the gut and their applications in food microbiology – a review. Int J Food Microbiol 2010;141:S4–14.
- Peitsch C, Klenk HD, Garten W, Böttcher-Friebertshäuser E. Activation of influenza A viruses by host proteases from swine airway epithelium. J Virol 2010;84:5605–14.
- Zhirnov OP, Vorobjeva IV, Ovcharenko AV, Klenk HD. Intracellular cleavage of human influenza a virus hemagglutinin and its inhibition. Biochemistry Mosc 2003;68:1020–6.
- Pászti-Gere E, Barna RF, Ujhelyi G, Steinmetzer T. Interaction exists between matriptase inhibitors and intestinal epithelial cells. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. doi: 10.3109/14756366.2015.1060483.
- Sun Z, Huber VC, McCormick K, et al. Characterization of a porcine intestinal epithelial cell line for influenza virus production. J Gen Virol 2012;93:2008–16.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States 2005-2009. N Engl J Med 2009;360:2616–25.
- Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine 2008;26:59–66.