Abstract
In this work, 40 analogs with a natural maslinic acid core (from Olea europaea L.) and various aromatic azides were synthesized. A regiospecific, facile and practical synthesis of 1,5-triazolyl derivatives by Ru(II)-catalyzed azide-alkyne cycloaddition (RuAAC), and mono-, bis- and tri-1,4-triazolyl derivatives by Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) was described. All the reactions were assisted by microwave irradiation avoiding toxic reagents and solvents. The new products were obtained from the reaction mixture by simple purification in almost quantitative yields and the reaction times were in general shorter than those reported in the literature. Their chemical structures were elucidated on the basis of extensive spectroscopic methods including ESI-HRMS, 1D and 2D-NMR. Most of the compounds were evaluated for their anti-inflammatory activity using LPS-stimulated human peripheral blood mononuclear cells (PBMCs) and antiproliferative effects towards cultured murine EMT-6 (Breast) and human SW480 (colon) cancer cell lines.
Introduction
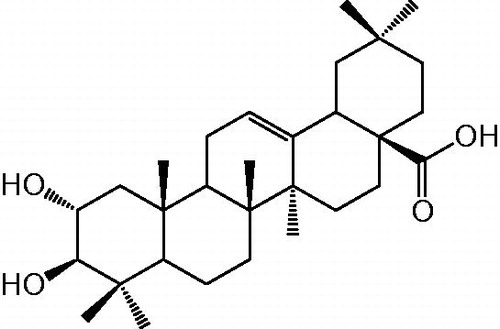
Recently, 1,2,3-triazoles have gained special attention in the field of drug discovery due to the growing use of “click” reaction such as ruthenium-catalyzed azide-alkyne cycloaddition (RuAAC)Citation1–3 and copper-catalyzed azide-alkyne cycloaddition (CuAAC)Citation4–6. Moreover, compounds containing a 1,2,3-triazole display a wide range of biological capacities such as anti-HIVCitation7, cytotoxicCitation8 and anti-inflammatoryCitation9 activities. On the other hand, natural products and their synthetic derivatives are very important in drug designCitation10,Citation11. Among these, natural pentacyclic triterpenoids and their derivatives are present in several classes of natural and synthetic biologically active compoundsCitation12,Citation13. In particular, maslinic acid (1, ) and its derivatives are of interest because of their wide spectrum of biological activities. Thus, for natural compound 1 an inhibitory activity for glycogen phosphorylasesCitation14, protein tyrosine phosphatase 1BCitation15, anti-inflammatoryCitation16, anti-tumorCitation17 as well as an anti-HIV-1 activitiesCitation18 was reported. However, the natural pentacyclic triterpenoid 1 may have utility in other pathological processes for its anti-inflammatory and anti-cancer properties. It has been found to attenuate intracellular oxidative stress via inhibition of NO and H2O2 production and reduction of pro-inflammatory cytokine generation in murineCitation19,Citation20. Furthermore, interesting reports from Reyes-Zurita et al.Citation21,Citation22 suggest that compound 1 is able to induce cell death by apoptosis in the colon cancer cell lines HT29 and in Caco-2. Among others, Allouche et al.Citation23 demonstrate that maslinic acid 1 was reported to be cytotoxic for the breast cancer cell line MCF7. Thus, maslinic acid 1 exhibited some selectivity between malignant and nonmalignant cell linesCitation24 and selective parasitostatic activities in miceCitation25. Recent research results correlate the low rate of cancer mortality in Mediterranean countries to a diet rich in olivesCitation26,Citation27.
In this context, maslinic acid 1 () which isolated from pomace olive (Olea europaea L.) cultivar: Chemlali, under ultra-sonication conditions with high quantities (17 g (8.5 mg/g DW)) was used herein as a starting material to synthesize new 1,5-disubstituted-1,2,3-triazolo-maslinic acid derivatives through well-established Ru(II)-catalyzed azide-alkyne cycloaddition (RuAAC) and to access to mono, bis- and tri-1,4-triazolyl derivatives via the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) conducted under microwave conditions. Most obtained compounds were evaluated for their anti-inflammatory and anti-proliferative activities in vitro.
Materials and methods
General experimental procedures
Solvents were distilled and dried using standard methods. Melting points were determined on a Büchi 510 apparatus (Mount Holly, NJ) using capillary tubes. Commercial TLC plates (Silica gel 60, F254, sds) were used to monitor the progress of the reaction. Column chromatography was performed with silica gel 60 (particle size 40–63 μm, sds). HRMS were acquired with a LCT Premier XE (Waters, ESI technique, positive mode) mass spectrometer. For ESI experiments, leucine-enkephaline peptide was employed as the LockSpray lockmass. 1H (300 MHz, 16–32 scans) and BB-decoupled 13C (75 MHz, 256–2048 scans) NMR spectra were recorded at room temperature (rt) on a Bruker AM-300 Fourier Transform spectrometer (Fitchburg, MA) equipped with a 10 mm probe in deuterated chloroform, acetone and pyridine with all chemical shifts (δ), reported in ppm, refereed to residual non-deuterated solvent. Coupling constants were measured in Hz. and signals are using the following abbreviations: s, singlet; d, doublet; t, triplet; m, multiplet, etc.
Chemistry
Synthesis of dipolarophiles 2–6
Method I: To a solution of compound 1 (3 g, 6.3 mmol) in dry DMF (5 mL), sodium hydride (12.6 mmol) and propargyl bromide (18.9 mmol) were added and the reaction mixture was stirred at room temperature for 2 h. Reaction was monitored by TLC. After the completion of the reaction, the residue was diluted with water (300 mL). The mixture was extracted with ethyl acetate (3 × 100 mL). The combined organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. The residue was chromatographed over silica gel and eluted with ethyl acetate:petroleum ether (2:8 then 3:7) to obtain the alkyl derivatives 2–6 in 44, 5, 15, 11 and 7% yield, respectively. Method II: With the aim to fully propargylate the maslinic acid 1 in C-2, C-3 and C-28 positions, we increased the number of equivalents of NaH (which goes from 2 to 4 equiv.) and propargyl bromide (which goes from 3 to 4 equiv.) and we extended the reaction time from 2 h to 8 h. The same protocol adopted in method I was applied and only the alkyl derivatives 2 and 4–6 was obtained in 25, 23, 20 and 31% yield, respectively.
Propargyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (2). White solid; m.p.: 297–299 °C; 1H NMR (300 MHz, CDCl3): δ 5.23 (1H, t, J = 3.6 Hz), 4.61 (1H, dd, J = 15.6; 2.4 Hz), 4.50 (1H, dd, J = 15.6; 2.7 Hz), 3.60 (1H, td, J = 9.3; 2.7 Hz), 2.93 (1H, d, J = 9.6 Hz), 2.80 (1H, dd, J = 13.8; 3.9 Hz), 2.34 (1H, t, J = 2.7 Hz), 1.99 (4H, m), 1.93–1.83 (4H, m), 1.62–1.46 (6H, m), 1.45–1.21 (6H, m), 1.06 (3H, s), 0.96 (3H, s), 0.91 (3H, s), 0.86 (3H, s), 0.83 (3H, s), 0.75 (3H, s), 0.67 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.3, 142.9, 121.9, 83.4, 77.6, 73.8, 68.4, 54.8, 51.1, 47.1, 46.2, 45.9, 45.3, 41.2, 40.7, 38.9, 38.6, 37.8, 36.5, 33.3, 32.5, 32.1, 31.7, 30.1, 28.1, 27.1, 26.7, 25.3, 23.0, 22.9, 22.5, 17.8, 16.6; HRMS (ESI+): calculated for (C33H51O4)+ [M + H]+ 511.3787, found 511.3778.
(2α)-2-(Propargyloxy)-(3β)-3-hydroxy-olean-12-en-28-oic acid (3). White solid; m.p.: 293–294 °C; 1H NMR (300 MHz, CDCl3): δ 5.29 (1H, s), 4.30 (1H, dd, J = 15.9; 2.4 Hz), 4.18 (1H, dd, J = 15.9; 2.4 Hz), 3.58 (1H, td, J = 9.3; 2.7 Hz), 2.93 (1H, d, J = 9.6 Hz), 2.84 (1H, dd, J = 13.8; 3.9 Hz), 2.47 (1H, t, J = 2.4 Hz), 2.05 (2H, m), 1.77–1.47 (8H, m), 1.37–1.23 (10H, m), 1.14 (3H, s), 1.06 (3H, s), 0.99 (3H, s), 0.94 (3H, s), 0.92 (3H, s), 0.85 (3H, s), 0.76 (3H, s); 13C NMR (75 MHz, CDCl3): δ 183.3, 143.2, 121.7, 80.4, 79.7, 76.5, 73.9, 55.8, 54.5, 47.1, 46.0, 45.3, 42.0, 41.1, 40.4, 38.8, 38.6, 37.7, 33.2, 32.5, 32.1, 31.9, 30.1, 29.2, 28.1, 27.1, 25.4, 23.0, 22.3, 17.6, 16.6, 16.4, 16.0; HRMS (ESI+): calculated for (C33H51O4)+ [M + H]+ 511.3787, found 511.3794.
Propargyl-(2αro2-(propargyloxy)-(3βro2-(propargolean-12-en-28-oate (4). White solid; m.p.: 296–298 °C; 1H NMR (300 MHz, CDCl3): δ 5.22 (1H, s), 4.67 (1H, dd, J = 15.6; 2.4 Hz), 4.56 (1H, dd, J = 15.3; 2.1 Hz), 4.26 (1H, dd, J = 15.9; 2.4 Hz), 4.15 (1H, dd, J = 15.9; 2.1 Hz), 3.54 (1H, td, J = 11.1; 4.2 Hz), 3.06 (1H, d, J = 9.6 Hz), 2.86 (1H, dd, J = 13.8; 3.9 Hz), 2.44 (3H, m), 2.04–1.94 (2H, m), 1.92–1.89 (2H, m), 1.70–1.53 (7H, m), 1.45–1.29 (4H, m), 1.24–1.16 (2H, m), 1.12 (3H, s), 1.04 (3H, s), 0.96 (3H, s), 0.92 (3H, s), 0.89 (3H, s), 0.83 (3H, s), 0.73 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.2, 143.0, 121.8, 80.8, 79.8, 77.6, 76.6, 73.9, 73.9, 55.8, 54.5, 51.1, 47.0, 46.2, 45.3, 42.1, 41.2, 40.7, 38.9, 38.6, 37.7, 33.3, 32.5, 32.1, 31.6, 30.1, 28.2, 27.1, 25.3, 23.1, 23.0, 22.4, 17.6, 16.6, 16.4, 16.0; HRMS (ESI+): calculated for (C36H53O4)+ [M + H]+ 549.3944, found 549.3938.
Propargyl-(3αropargyl-(3e8-oate-(3den-28-oate aolean-12-en-28-oate (5). White solid; m.p.: 296–298 °C; 1H NMR (300 MHz, CDCl3): δ 5.22 (1H, t, J = 3.3 Hz), 4.60 (1H, dd, J = 15.6; 2.4 Hz), 4.49 (1H, dd, J = 15.6; 2.4 Hz), 4.41 (1H, dd, J = 15.9; 2.4 Hz), 4.25 (1H, dd, J = 15.9; 2.4 Hz), 3.72 (1H, td, J = 11.1; 4.2 Hz), 2.79 (1H, dd, J = 13.8; 3.9 Hz), 2.74 (1H, d, J = 9.6 Hz), 2.42 (1H, t, J = 2.4 Hz), 2.33 (1H, t, J = 2.4 Hz), 2.07 (2H, m), 1.91–1.83 (4H, m), 1.59–1.47 (8H, m), 1.34 (2H, m), 1.30–1.22 (4H, m), 1.10 (3H, s), 0.94 (3H, s), 0.88 (3H, s), 0.85 (3H, s), 0.82 (3H, s), 0.73 (3H, s), 0.66 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.2, 142.8, 121.9, 93.0, 80.3, 77.6, 74.1, 73.9, 68.0, 61.0, 54.8, 51.1, 47.1, 46.2, 45.3, 41.2, 40.7, 39.7, 38.9, 37.5, 33.3, 32.5, 32.1, 31.7, 30.1, 28.4, 27.1, 26.4, 25.3, 23.1, 22.9, 22.4, 17.6, 16.6, 16.4, 16.0; HRMS (ESI+): calculated for (C36H53O4)+ [M + H]+ 549.3944, found 549.3940.
Propargyl-(2α,3β) − 2,3 − βισ(propargyloxy)-olean-12-en-28-oate (6). White solid; m.p.: 296–298 °C; 1H NMR (300 MHz, CDCl3): δ 5.23 (1H, s), 4.60 (1H, dd, J = 15.6; 2.4 Hz), 4.50 (1H, dd, J = 15.6; 2.4 Hz), 4.41 (1H, dd, J = 15.9; 2.4 Hz), 4.34 (1H, dd, J = 15.9; 2.4 Hz), 4.17 (2H, d, J = 2.1 Hz), 3.59 (1H, td, J = 11.1; 4.2 Hz), 2.82 (1H, d, J = 9.6 Hz), 2.79 (1H, dd, J = 13.8; 3.9 Hz), 2.33 (2H, m), 2.25 (1H, m), 1.96–1.83 (4H, m), 1.60–1.46 (8H, m), 1.34–1.20 (8H, m), 1.10 (3H, s), 0.94 (3H, s), 0.88 (3H, s), 0.85 (3H, s), 0.81 (3H, s), 0.73 (3H, s), 0.66 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.3, 142.9, 121.9, 89.2, 80.6, 77.7, 77.6, 73.8, 73.8, 73.2, 73.1, 60.2, 56.7, 54.8, 51.1, 47.1, 46.2, 45.3, 44.2, 41.2, 40.7, 39.5, 38.9, 37.4, 33.5, 33.3, 32.1, 31.7, 30.1, 29.1, 27.1, 26.4, 25.3, 23.1, 22.5, 17.8, 17.0, 16.5, 15.9; HRMS (ESI+): calculated for (C39H55O4)+ [M + H]+ 587.4100, found 587.4092.
General procedure for the RuAAC reaction
To a solution of alkyne 2 (0.1 g, 0.2 mmol) in DMF (1 mL), Cp*RuCl(PPh3)2 (5 mol %) was added at room temperature. To this mixture, aryl azide (0.4 mmol) was added and the reaction mixture was subjected to microwave irradiations at 250 W completed within 4–8 minutes. The crude mixture was diluted with water (75 mL) then extracted with EtOAc (3 × 25 mL) and the combined organic layer was dried over sodium sulfate, concentrated in vacuo and purified through a column chromatography to give pure the 1,5-triazolyl derivatives 8a–g in 83–98% yields.
(1-Phenyl-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8a). Yellowish gum; m.p.: 218–219 °C; 1H NMR (300 MHz, CDCl3): δ 7.77 (1H, s), 7.53–7.42 (5H, m), 5.19 (1H, t, J = 3.3 Hz), 5.09 (1H, d, J = 13.5 Hz), 5.01 (1H, d, J = 13.5 Hz), 3.63 (1H, td, J = 10.8; 4.2 Hz), 2.91 (1H, d, J = 9.6 Hz), 2.76 (1H, dd, J = 11.8; 3.8 Hz), 2.24 (2H, m), 1.93–1.75 (6H, m), 1.61–1.24 (12H, m), 1.23 (3H, s), 1.03 (3H, s), 0.89 (3H, s), 0.86 (3H, s), 0.81 (3H, s), 0.74 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.8, 143.4 136.7, 136.2, 131.4, 129.8, 129.6, 129.6, 124.7, 124.7, 122.6, 83.9, 68.9, 55.2, 53.7, 47.4, 46.9, 46.3, 45.7, 41.7, 41.2, 39.3, 39.1, 38.2, 33.7, 32.6, 31.9, 30.6, 29.3, 28.6, 27.5, 25.8, 23.5, 23.4, 18.2, 16.8, 16.7, 16.5, 14.0; HRMS (ESI+): calculated for (C39H56N3O4)+ [M + H]+ 630.4271, found 630.4274.
(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8b). Dark red solid; m.p.: 222–224 °C; 1H NMR (300 MHz, CDCl3): δ 7.83 (1H, s), 7.47 (2H, dd, J = 6.9; 2.1 Hz), 7.06 (2H, dd, J = 6.9; 2.1 Hz), 5.27 (1H, t, J = 3.3 Hz), 5.12 (1H, d, J = 13.5 Hz), 5.06 (1H, d, J = 13.8 Hz), 3.90 (3H, s), 3.71 (1H, td, J = 10.8; 3.9 Hz), 3.01 (1H, d, J = 9.6 Hz), 2.86 (1H, dd, J = 8.8; 3.4 Hz), 2.00–1.86 (4H, m), 1.69–1.56 (6H, m), 1.52–1.44 (6H, m), 1.29–1.22 (4H, m), 1.27 (3H, s), 1.13 (3H, s), 1.04 (3H, s), 1.01 (3H, s), 0.96 (6H, s), 0.83 (3H, s), 0.55 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.3, 160.3, 142.9, 134.7, 132.0, 128.6, 125.7, 125.7, 122.0, 114.2, 114.2, 83.4, 68.4, 55.1, 54.7, 53.3, 47.0, 46.3, 45.8, 45.2, 41.2, 40.7, 38.8, 38.6, 37.7, 33.8, 33.2, 32.4, 32.0, 31.8, 30.1, 29.1, 28.0, 27.0, 25.3, 24.4, 22.6, 17.7, 16.3, 15.2; HRMS (ESI+): calculated for (C40H58N3O5)+ [M + H]+ 660.4376, found 660.4381.
(1-(4-Chlorophenyl)-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8c). Dark orange solid; m.p.: 235–237 °C; 1H NMR (300 MHz, CDCl3): δ 7.84 (1H, s), 7.51–7.44 (4H, m), 5.25 (1H, t, J = 3.6 Hz), 5.14 (1H, d, J = 13.2 Hz), 5.10 (1H, d, J = 13.2 Hz), 3.67 (1H, td, J = 10.8; 4.2 Hz), 2.98 (1H, d, J = 9.6 Hz), 2.81 (1H, dd, J = 11.2; 4.1 Hz), 2.08–1.82 (6H, m), 1.68–1.54 (8H, m), 1.51–1.37 (6H, m), 1.25 (3H, s), 1.01 (3H, s), 0.98 (3H, s), 0.93 (3H, s), 0.90 (3H, s), 0.79 (3H, s), 0.50 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.3, 142.8, 135.5, 135.2, 133.9, 131.9, 129.4, 129.4, 125.4, 125.4, 122.1, 83.4, 68.3, 54.7, 53.1, 46.9, 46.4, 45.4, 45.2, 41.2, 40.7, 38.8, 38.6, 37.7, 33.2, 32.4, 32.0, 31.8, 30.1, 29.1, 28.1, 27.0, 27.1, 25.3, 22.9, 23.3, 18.1, 17.0, 16.7; HRMS (ESI+): calculated for (C39H55ClN3O4)+ [M + H]+ 664.3881, found 664.3885.
(1-(4-Bromophenyl)-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8d). White solid; m.p.: 276–277 °C; 1H NMR (300 MHz, CDCl3): δ 7.86 (1H, s), 7.53–7.46 (4H, m), 5.23 (1H, t, J = 3.3 Hz), 5.16 (1H, d, J = 13.5 Hz), 5.08 (1H, d, J = 13.5 Hz), 3.69 (1H, td, J = 11.1; 4.2 Hz), 3.02 (1H, d, J = 9.6 Hz), 2.86 (1H, dd, J = 11.2; 4.1 Hz), 2.05–1.83 (6H, m), 1.68–1.64 (6H, m), 1.58–1.50 (4H, m), 1.46–1.30 (4H, m), 1.25 (3H, s), 1.01 (3H, s), 0.98 (3H, s), 0.93 (3H, s), 0.90 (3H, s), 0.79 (3H, s), 0.51 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.5, 142.9, 135.5, 135.3, 134.0, 132.0, 129.4, 129.4, 125.4, 125.4, 122.1, 83.3, 68.4, 54.8, 53.7, 47.0, 46.4, 45.8, 45.2, 41.2, 40.8, 38.8, 38.6, 37.8, 33.2, 32.4, 32.0, 31.8, 30.1, 29.1, 28.1, 27.0, 27.1, 25.3, 22.8, 23.5, 18.3, 17.2, 16.5; HRMS (ESI+): calculated for (C39H55BrN3O4)+ [M + H]+ 708.3376, found 708.3379.
(1-(4-Nitrophenyl)-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8e). Yellow solid; m.p.: 308–310 °C; 1H NMR (300 MHz, CDCl3): δ 7.81 (1H, s), 7.51–7.40 (4H, m), 5.22 (1H, t, J = 3.0 Hz), 5.14 (1H, d, J = 13.5 Hz), 5.06 (1H, d, J = 13.5 Hz), 3.66 (1H, td, J = 10.5; 3.9 Hz), 3.00 (1H, d, J = 9.6 Hz), 2.87 (1H, dd, J = 11.2; 4.4 Hz), 2.09–1.87 (4H, m), 1.69–1.66 (4H, m), 1.63–1.58 (2H, m), 1.56–1.41 (6H, m), 1.39–1.28 (4H, m), 1.25 (3H, s), 1.01 (3H, s), 0.98 (3H, s), 0.93 (3H, s), 0.90 (3H, s), 0.79 (3H, s), 0.53 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.5, 144.5, 143.6, 142.9, 142.0, 131.8, 128.6, 128.6, 124.0, 124.0, 122.9, 83.6, 68.7, 54.8, 53.7, 47.0, 46.4, 45.8, 45.2, 41.2, 40.8, 38.8, 38.6, 37.8, 33.2, 32.4, 32.0, 31.8, 30.1, 29.1, 28.1, 27.0, 27.1, 25.3, 22.9, 23.6, 18.0, 17.5, 16.0; HRMS (ESI+): calculated for (C39H55N4O6)+ [M + H]+ 675.4122, found 675.4125.
(1-(3-Methylphenyl)-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8f). White solid; m.p.: 326–327 °C; 1H NMR (300 MHz, CDCl3): δ 7.84 (1H, s), 7.47–7.34 (4H, m), 5.28 (1H, t, J = 3.3 Hz), 5.16 (1H, d, J = 13.5 Hz), 5.10 (1H, d, J = 13.5 Hz), 3.66 (1H, td, J = 10.5; 3.9 Hz), 3.01 (1H, d, J = 9.6 Hz), 2.87 (1H, dd, J = 9.2; 3.8 Hz), 2.47 (3H, s), 2.04–7.86 (6H, m), 1.69–1.52 (8H, m), 1.50–1.33 (6H, m), 1.13 (3H, s), 1.04 (3H, s), 0.95 (3H, s), 0.90 (6H, s), 0.83 (3H, m), 0.55 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.8, 143.4, 139.9, 135.8, 135.4, 132.3, 130.5, 129.3, 125.3, 122.5, 121.7, 83.9, 68.9, 55.2, 53.7, 47.5, 46.8, 46.3, 45.7, 41.7, 41.2, 39.3, 39.1, 38.2, 33.7, 32.9, 32.5, 32.3, 31.9, 30.6, 29.6, 28.5, 27.5, 25.8, 23.5, 23.4, 23.0, 22.6, 21.3, 18.2; HRMS (ESI+): calculated for (C40H58N3O4)+ [M + H]+ 644.4427, found 644.4431.
(1-Naphthalen-1-yl-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (8 g). Eggplant solid; m.p.: 335–337 °C; 1H NMR (300 MHz, CDCl3): δ 8.09 (1H, d, J = 7.6 Hz), 7.98 (1H, d, J = 7.8 Hz), 7.94 (1H, s), 7.61–7.51 (4H, m), 7.24 (1H, d, J = 8.4 Hz), 5.18 (1H, t, J = 2.7 Hz), 4.96 (1H, d, J = 13.4 Hz), 4.91 (1H, d, J = 13.8 Hz), 3.69 (1H, td, J = 10.5; 3.9 Hz), 2.99 (1H, d, J = 9.6 Hz), 2.68 (1H, dd, J = 13.5; 4.2 Hz), 2.04 (1H, m), 1.93 (3H, m), 1.77–1.60 (4H, m), 1.43–1.18 (12H, m), 1.12 (3H, s), 1.05 (3H, s), 0.93 (3H, s), 0. 86 (3H, s), 0.84 (3H, s), 0.83 (3H, s), 0.49 (3H, s); 13C NMR (75 MHz, CDCl3): δ 176.1, 143.2, 142.7, 133.6, 133.4, 131.1, 128.2, 128.1, 128.1, 127.2, 126.2, 124.4, 124.4, 121.9, 121.6, 83.4, 68.5, 55.0, 53.4, 47.0, 46.3, 45.3, 41.2, 40.8, 38.3, 38.2, 37.9, 36.4, 33.3, 32.5, 31.9, 30.1, 27.6, 27.1, 26.6, 25.2, 23.1, 22.8, 22.5, 17.7, 16.5, 16.2, 16.0; HRMS (ESI+): calculated for (C43H58N3O4)+ [M + H]+ 680.4427, found 680.4429.
General procedure for the synthesis of mono-1,4-triazolyl derivatives 9a–g under CuAAC condition
Under solvent-free conditions, 0.1 g (0.2 mmol) of dipolarophile 2, Copper(I) iodide (0.5 equiv.) and triethylamine (1 equiv.) were added at room temperature. To this mixture, aryl- azide (0.4 mmol) was added and the reaction mixture was subjected to microwave irradiations at 200 W completed within 2–4 minutes. The crude mixture was extracted with EtOAc (3 × 25 mL) and the combined organic layer was dried over sodium sulfate, concentrated under reduced pressure and purified through a column chromatography or by recrystallization, to give pure 9a–g in 90–99% yields.
(1-Phenyl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9a). Yellow paste; m.p.: 202–204 °C; 1H NMR (300 MHz, CDCl3): δ 8.01 (1H, s), 7.70 (2H, dd, J = 7.5; 1.5 Hz), 7.50 (2H, dd, J = 7.2; 1.5 Hz), 7.42 (1H, m), 5.25 (3H, m), 3.15 (1H, dd, J = 11.2; 4.8 Hz), 2.87 (1H, dd, J = 7.7; 3.8 Hz), 1.94 (2H, m), 1.80 (2H, dd, J = 8.7; 3.5 Hz), 1.63–1.45 (12H, m), 1.34 (2H, m), 1.26 (4H, m), 1.08 (3H, s), 0.94 (3H, s), 0.89 (3H, s), 0.86 (3H, s), 0.74 (3H, s), 0.72 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 143.7, 143.5, 136.5, 129.7, 129.7, 129.7, 128.8, 122.5, 120.5, 120.5, 78.9, 57.3, 55.2, 47.5, 46.7, 45.8, 41.6, 41.4, 39.2, 38.7, 38.4, 36.9, 33.6, 33.0, 32.6, 32.4, 30.6, 29.6, 28.0, 27.6, 27.1, 25.7, 23.9, 23.8, 22.9, 18.2, 16.7, 15.5, 15.1; HRMS (ESI+): calculated for (C39H56N3O4)+ [M + H]+ 630.4271, found 630.4274.
(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9b). Dark purple solid; m.p.: 280–281 °C; 1H NMR (300 MHz, CDCl3): δ 7.96 (1H, s), 7.62 (2H, d, J = 7.8 Hz), 7.02 (2H, d, J = 8.1 Hz), 5.25 (3H, m), 3.87 (3H, s), 3.20 (1H, dd, J = 10.2; 4.2 Hz), 2.84 (1H, dd, J = 8.7; 3.8 Hz), 2.00 (2H, m), 1.80 (2H, dd, J = 9.0; 3.0 Hz), 1.63–1.45 (10H, m), 1.33–1.19 (8H, m), 1.10 (3H, s), 0.97 (3H, s), 0.91 (3H, s), 0.89 (3H, s), 0.78 (3H, s), 0.75 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 159.3, 143.1, 143.0, 129.8, 122.2, 122.9, 121.6, 121.6, 114.2, 114.2, 78.4, 56.8, 55.1, 46.9, 46.2, 45.3, 41.1, 40.8, 38.7, 38.2, 37.8, 36.4, 33.2, 32.6, 32.0, 31.8, 30.1, 29.2, 27.6, 27.1, 26.6, 25.2, 23.1, 22.8, 22.4, 17.7, 16.2, 15.0, 14.7; HRMS (ESI+): calculated for (C40H58N3O5)+ [M + H]+ 660.4376, found 660.4378.
(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9c). Dark red solid; m.p.: 286–287 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.69 (2H, dd, J = 6.9; 2.7 Hz), 7.51 (2H, dd, J = 6.6; 3.0 Hz), 5.28 (3H, m), 3.21 (1H, dd, J = 10.8; 4.2 Hz), 2.87 (1H, dd, J = 9.2; 3.8 Hz), 2.01 (1H, m), 1.80 (2H, dd, J = 7.8; 2.4 Hz), 1.68–1.45 (12H, m), 1.29 (3H, m), 1.18 (4H, m), 1.11 (3H, s), 0.97 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.78 (3H, s), 0.76 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.8, 143.5, 135.5, 134.6, 129.9, 129.9, 129.0, 122.5, 121.6, 121.6, 116.2, 78.9, 57.2, 55.1, 47.5, 46.7, 45.8, 41.6, 41.3, 39.2, 38.7, 38.4, 36.9, 33.8, 33.0, 32.6, 32.4, 31.8, 30.6, 28.0, 27.6, 27.1, 25.7, 23.6, 23.3, 22.9, 18.2, 16.7, 15.5; HRMS (ESI+): calculated for (C39H55ClN3O4)+ [M + H]+ 664.3881, found 664.3884.
(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9d). White solid; m.p.: 294–296 °C; 1H NMR (300 MHz, CDCl3): δ 8.01 (1H, s), 7.65 (2H, dd, J = 8.1; 3.4 Hz), 7.50 (2H, dd, J = 7.8; 3.2 Hz), 5.29 (3H, m), 3.21 (1H, dd, J = 10.8; 4.2 Hz), 2.87 (1H, dd, J = 9.2; 3.8 Hz), 1.99 (2H, m), 1.80 (2H, dd, J = 7.9; 2.8 Hz), 1.71–1.55 (10H, m), 1.33–1.27 (4H, m), 1.18 (4H, m), 1.11 (3H, s), 0.97 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.78 (3H, s), 0.76 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.9, 143.7, 136.1, 133.9, 129.2, 129.2, 129.0, 122.5, 120.8, 120.8, 115.9, 78.7, 56.8, 55.5, 47.6, 46.7, 45.8, 41.9, 41.3, 39.4, 38.7, 38.8, 36.7, 33.9, 33.3, 32.9, 32.4, 31.8, 30.6, 28.7, 27.9, 27.4, 25.7, 23.6, 23.3, 22.9, 18.2, 16.8, 15.2; HRMS (ESI+): calculated for (C39H55BrN3O4)+ [M + H]+ 708.3376, found 708.3379.
(1-(4-Nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9e). Yellow solid; m.p.: 298–300 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.60 (2H, d, J = 7.9 Hz), 7.00 (2H, d, J = 8.1 Hz), 5.28 (3H, m), 3.23 (1H, dd, J = 10.8; 3.8 Hz), 2.87 (1H, dd, J = 9.6; 4.0 Hz), 2.01 (1H, m), 1.83 (2H, dd, J = 7.8; 2.6 Hz), 1.68–1.44 (12H, m), 1.29 (3H, m), 1.22 (4H, m), 1.10 (3H, s), 0.98 (3H, s), 0.92 (3H, s), 0.90 (3H, s), 0.79 (3H, s), 0.76 (3H, s), 0.49 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.6, 143.1, 135.0, 134.4, 129.6, 129.6, 129.0, 122.4, 121.4, 121.4, 116.8, 78.9, 56.8, 55.0, 47.2, 46.6, 45.5, 41.3, 41.0, 39.2, 38.7, 38.2, 36.9, 33.8, 33.0, 32.7, 32.4, 31.8, 30.9, 28.2, 27.6, 27.0, 25.7, 23.9, 23.3, 22.9, 18.2, 16.8, 15.9; HRMS (ESI+): calculated for (C39H55N4O6)+ [M + H]+ 675.4122, found 675.4124.
(1-(3-Methylphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9f). Dark red solid; m.p.: 288–290 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.55 (1H, s), 7.50 (1H, d, J = 8.1 Hz), 7.39 (1H, t, J = 7.5 Hz), 7.25 (1H, d, J = 7.5 Hz), 5.28 (3H, m), 3.23 (1H, dd, J = 10.8; 4.2 Hz), 2.87 (1H, dd, J = 9.2; 3.8 Hz), 2.44 (3H, s), 1.95 (1H, td, J = 10.2; 3.8 Hz), 1.83 (2H, dd, J = 8.7; 3.3 Hz), 1.67–1.43 (10H, m), 1.38 (8H, m), 1.12 (3H, s), 1.04 (3H, s), 0.97 (3H, s), 0.94 (3H, s), 0.78 (3H, s), 0.75 (3H, s), 0.67 (1H, m), 0.45 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 143.6, 143.5, 139.9, 136.9, 129.5, 129.4, 122.6, 122.4, 121.2, 117.6, 78.9, 57.3, 55.1, 47.5, 46.7, 45.8, 41.6, 41.3, 39.2, 38.7, 38.4, 36.9, 33.8, 33.0, 32.6, 32.4, 31.8, 30.6, 29.6, 28.0, 27.6, 27.1, 25.7, 23.6, 23.3, 22.9, 18.2, 16.7, 15.5; HRMS (ESI+): calculated for (C40H58N3O4)+ [M + H]+ 644.4427, found 644.4432.
(1-Naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate (9 g). Eggplant solid; m.p.: 286–287 °C; 1H NMR (300 MHz, CDCl3): δ 8.03–7.94 (3H, m), 7.65–7.50 (5H, m), 5.38 (1H, d, J = 12.9 Hz), 5.30 (1H, d, J = 13.8 Hz), 5.26 (1H, s), 3.17 (1H, dd, J = 10.8; 5.1 Hz), 2.88 (1H, dd, J = 13.5; 4.2 Hz), 1.94 (1H, m), 1.80 (2H, m), 1.71–1.57 (3H, m), 1.60–1.47 (8H, m), 1.42–1.37 (2H, m), 1.28–1.19 (6H, m), 1.12 (3H, s), 0.96 (3H, s), 0.91 (3H, s), 0.88 (3H, s), 0.74 (3H, s), 0.73 (3H, s), 0.54 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 143.1, 142.7, 133.7, 133.0, 129.9, 127.8, 127.8, 127.4, 126.5, 126.2, 124.4, 122.9, 121.9, 121.8, 78.4, 59.8, 57.0, 54.6, 47.0, 46.3, 45.3, 41.2, 40.8, 38.3, 38.2, 37.9, 36.4, 33.3, 32.5, 31.9, 30.1, 27.6, 27.1, 26.6, 25.2, 23.1, 22.8, 22.5, 17.7, 16.5, 15.0, 14.6; HRMS (ESI+): calculated for (C43H58N3O4)+ [M + H]+ 680.4427, found 680.4429.
General procedure for the synthesis of bis-1,4-triazolyl derivatives 10a–g and 11a–g under CuAAC condition
Under solvent-free conditions, 0.1 g of bis-alkynes 4 or 5, Copper(I) iodide (0.8 equiv.) and triethylamine (2 equiv.) were added at room temperature. To this mixture, aryl azide (4 equiv.) was added and the reaction mixture was subjected to microwave irradiations at 250 W completed within 4–7 minutes. The crude mixture was extracted with EtOAc (3 × 25 mL) and the combined organic layer was dried over sodium sulfate, concentrated under reduced pressure and purified by recrystallization or silica gel column chromatography and eluted with petroleum ether:ethyl acetate (7:3) to obtain the desired new cycloadducts (10a–g and 11a–g).
(1-Phenyl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-3-hydroxy-olean-12-en-28-oate (10a). Yellowish solid; m.p.: 227–228 °C; 1H NMR (300 MHz, CDCl3): δ 8.06 (1H, s), 8.00 (1H, s), 7.74 (2H, m), 7.71 (2H, m), 7.56–7.50 (3H, m), 7.48–7.43 (3H, m), 5.32–5.22 (3H, m), 4.88 (1H, d, J = 12.3 Hz), 4.72 (1H, d, J = 12.3 Hz), 3.56 (1H, td, J = 11.2; 4.8 Hz), 3.13 (1H, d, J = 9.3 Hz), 2.87 (3H, m), 2.14–1.85 (4H, m), 1.68–1.48 (8H, m), 1.34 (3H, m), 1.26 (3H, m), 1.09 (3H, s), 1.04 (3H, s), 0.92 (3H, s), 0.89 (3H, s), 0.84 (3H, s), 0.80 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 143.3, 143.2, 143.2, 136.4, 136.3, 129.3, 129.3, 129.2, 129.2, 128.4, 128.4, 122.1, 121.7, 120.1, 120.1, 120.1, 120.0, 120.0, 81.0, 77.9, 61.8, 56.8, 54.5, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.4, 17.6, 16.4, 16.2, 15.9; HRMS (ESI+): calculated for (C48H63N6O4)+ [M + H]+ 787.4911, found 787.4908.
(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-hydroxy-olean-12-en-28-oate (10b). Dark solid; m.p.: 235–237 °C; 1H NMR (300 MHz, CDCl3): δ 7.94 (1H, s), 7.87 (1H, s), 7.60 (4H, d, J = 8.7 Hz), 7.03–7.00 (4H, m), 5.30–5.24 (3H, m), 4.86 (1H, d, J = 12.3 Hz), 4.70 (1H, d, J = 12.3 Hz), 3.89 (6H, s), 3.53 (1H, td, J = 11.2; 4.8 Hz), 3.16 (1H, d, J = 9.3 Hz), 2.88 (1H, dd, J = 13.5; 4.2 Hz), 2.84 (2H, m), 2.16–1.88 (4H, m), 1.70–1.49 (6H, m), 1.37 (4H, m), 1.29–1.18(4H, m), 1.10 (3H, s), 1.04 (3H, s), 0.93 (3H, s), 0.89 (3H, s), 0.85 (3H, s), 0.81 (3H, s), 0.47 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.6, 160.3, 158.9, 143.8, 143.8, 143.2, 136.4, 136.3, 134.8, 134.8, 125.8, 125.8, 125.7, 121.7, 121.6, 120.2, 114.3, 114.2, 114.0, 80.9, 77.8, 61.9, 55.1, 55.1, 55.0, 54.0, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 16.4, 16.3, 16.2, 16.0; HRMS (ESI+): calculated for (C50H67N6O6)+ [M + H]+ 847.5122, found 847.5120.
(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-hydroxy-olean-12-en-28-oate (10c). Dark solid; m.p.: 230–231 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.95 (1H, s), 7.68 (4H, d, J = 8.7 Hz), 7.50 (4H, dd, J = 5.1; 3.3 Hz), 5.30 (1H, m), 5.26 (2H, m), 4.86 (1H, d, J = 12.3 Hz), 4.70 (1H, d, J = 12.3 Hz), 3.55 (1H, td, J = 11.2; 4.8 Hz), 3.12 (1H, d, J = 9.6 Hz), 2.86 (1H, dd, J = 13.5; 4.2 Hz), 2.13–1.85 (6H, m), 1.67–1.44 (8H, m), 1.33 (2H, m), 1.26 (4H, m), 1.10 (3H, s), 1.04 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 144.8, 143.5, 143.2, 134.9, 134.9, 134.1, 134.1, 129.4, 129.4, 129.4, 129.4, 122.4, 122.2, 121.7, 121.7, 121.6, 121.6, 120.3, 81.1, 77.9, 61.9, 55.0, 54.0, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 16.4, 16.3, 16.2, 16.0; HRMS (ESI+): calculated for (C48H61Cl2N6O4)+ [M + H]+ 855.4131, found 855.4127.
(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-hydroxy-olean-12-en-28-oate (10d). White solid; m.p.: 227–229 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.95 (1H, s), 7.70 (4H, d, J = 8.7 Hz), 7.52 (4H, m), 5.31 (1H, t, J = 3.3 Hz), 5.28 (2H, m), 4.87 (1H, d, J = 12.3 Hz), 4.71 (1H, d, J = 12.3 Hz), 3.53 (1H, td, J = 11.2; 4.8 Hz), 3.15 (1H, d, J = 9.6 Hz), 2.83 (1H, dd, J = 13.5; 4.2 Hz), 2.11–1.81 (8H, m), 1.69–1.40 (4H, m), 1.33 (4H, m), 1.23 (4H, m), 1.11 (3H, s), 1.02 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.80 (3H, s), 0.75 (3H, s), 0.48 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 144.8, 143.5, 143.2, 134.9, 134.9, 134.1, 134.1, 129.4, 129.4, 129.4, 129.4, 122.4, 122.2, 121.7, 121.7, 121.6, 121.6, 120.3, 81.1, 77.9, 61.9, 55.0, 54.0, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 16.4, 16.3, 16.2, 16.0; HRMS (ESI+): calculated for (C48H61Br2N6O4)+ [M + H]+ 943.3121, found 943.3118.
(1-(4-Nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-hydroxy-olean-12-en-28-oate (10e). Yellowish solid; m.p.: 251–253 °C; 1H NMR (300 MHz, CDCl3): δ 8.00 (1H, s), 7.94 (1H, s), 7.77–7.68 (4H, m), 7.55–7.49 (4H, m), 5.31 (1H, t, J = 3.6 Hz), 5.28 (2H, m), 4.85 (1H, d, J = 12.3 Hz), 4.73 (1H, d, J = 12.3 Hz), 3.55 (1H, td, J = 11.2; 4.8 Hz), 3.17 (1H, d, J = 9.6 Hz), 2.81 (1H, dd, J = 13.5; 4.2 Hz), 2.09–1.85 (6H, m), 1.71–1.45 (6H, m), 1.33 (2H, m), 1.29–1.21 (6H, m), 1.11 (3H, s), 1.05 (3H, s), 0.90 (3H, s), 0.87 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 144.8, 143.5, 143.2, 134.9, 134.9, 134.1, 134.1, 129.4, 129.4, 129.4, 129.4, 122.4, 122.2, 121.7, 121.7, 121.6, 121.6, 120.3, 81.1, 77.9, 61.9, 55.0, 54.0, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 16.4, 16.3, 16.2, 16.0; HRMS (ESI+): calculated for (C48H61N8O8)+ [M + H]+ 877.4612, found 877.4610.
(1-(3-Methylphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-(3-methylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-hydroxy-olean-12-en-28-oate (10f). White solid; m.p.: 211–213 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.96 (1H, s), 7.55 (2H, m), 7.48 (2H, m), 7.41–7.36 (2H, m), 7.24 (2H, m), 5.32–5.21 (3H, m), 4.86 (1H, d, J = 12.3 Hz), 4.71 (1H, d, J = 12.3 Hz), 3.57 (1H, td, J = 11.2; 4.8 Hz), 3.17 (1H, d, J = 9.3 Hz), 2.87 (1H, dd, J = 13.5; 4.2 Hz), 2.45 (3H, s), 2.44 (3H, s), 2.13–1.86 (4H, m), 1.67–1.43 (6H, m), 1.33 (4H, m), 1.29–1.21 (6H, m), 1.11 (3H, s), 1.05 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.45 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.2, 145.2, 143.2, 139.4, 136.5, 136.4, 131.3, 129.0, 129.0, 128.5, 126.5, 122.2, 121.7, 120.7, 120.6, 119.9, 117.0, 113.6, 111.7, 81.0, 77.4, 61.9, 56.8, 54.5, 47.0, 46.2, 45.3, 42.4, 41.2, 40.8, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 20.8, 20.8, 16.4, 16.3, 16.3, 16.1; HRMS (ESI+): calculated for (C50H67N6O4)+ [M + H]+ 815.5224, found 815.5220.
(1-Naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-((1-naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methoxy)-3-hydroxy-olean-12-en-28-oate (10 g). dark purple solid; m.p.: 219–220 °C; 1H NMR (300 MHz, CDCl3): δ 8.05–7.96 (4H, m), 8.02 (1H, s), 7.91 (1H, s), 7.68–7.53 (10H, m), 5.40 (1H, d, J = 12.6 Hz), 5.34 (1H, d, J = 12.6 Hz), 5.31 (1H, t, J = 3.6 Hz), 4.95 (1H, d, J = 12.3 Hz), 4.81 (1H, d, J = 12.3 Hz), 3.61 (1H, td, J = 11.2; 4.8 Hz), 3.17 (1H, d, J = 9.3 Hz), 2.87 (1H, dd, J = 13.5; 4.2 Hz), 2.15–1.87 (4H, m), 1.75–1.43 (10H, m), 1.39–1.31 (3H, m), 1.25 (2H, m), 1.13 (3H, s), 1.06 (3H, s), 0.90 (3H, s), 0.87 (3H, s), 0.83 (3H, s), 0.80 (3H, s), 0.57 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 143.9, 142.7, 143.2, 133.7, 133.6, 133.2, 133.0, 133.0, 129.9, 129.9, 128.0, 127.8, 127.7, 127.7, 127.7, 127.7, 127.4, 126.6, 126.2, 124.4, 124.4, 123.0, 122.8, 121.8, 121.7, 81.0, 77.4, 62.0, 57.0, 54.5, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 17.6, 16.6, 16.6, 16.4, 16.0; HRMS (ESI+): calculated for (C56H67N6O4)+ [M + H]+ 887.5224, found 887.5221.
(1-Phenyl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11a). Yellowish solid; m.p.: 230–232 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.94 (1H, s), 7.73–7.69 (4H, m), 7.54–7.47 (4H, m), 7.45–7.39 (2H, m), 5.28–5.25 (3H, m), 4.98 (1H, d, J = 12.0 Hz), 4.95 (1H, d, J = 12.0 Hz), 3.88 (1H, td, J = 11.2; 4.8 Hz), 2.95 (1H, d, J = 9.3 Hz), 2.87 (1H, dd, J = 13.5; 4.2 Hz), 2.02 (2H, m), 2.00–1.85 (4H, m), 1.70–1.61 (4H, m), 1.64–1.43 (4H, m), 1.34 (4H, m), 1.26 (4H, m), 1.14 (3H, s), 1.10 (3H, s), 0.99 (3H, s), 0.89 (3H, s), 0.83 (3H, s), 0.79 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.2, 143.3, 143.0, 143.0, 136.4, 136.4, 129.2, 129.2, 129.2, 129.2, 129.2, 129.2, 128.3, 128.2, 122.1, 120.1, 120.1, 119.9, 119.9, 94.6, 68.2, 67.2, 56.8, 54.8, 47.0, 46.2, 46.1, 45.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.4, 17.6, 17.0, 16.2, 15.9; HRMS (ESI+): calculated for (C48H63N6O4)+ [M + H]+ 787.4911, found 787.4905.
(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11b). Dark solid; m.p.: 269–270 °C; 1H NMR (300 MHz, CDCl3): δ 7.94 (1H, s), 7.86 (1H, s), 7.60 (4H, dd, J = 6.9; 2.4 Hz), 7.00 (4H, dd, J = 6.9; 2.4 Hz), 5.30 (1H, m), 5.27 (1H, d, J = 12.9 Hz), 5.25 (1H, d, J = 12.9 Hz), 4.97 (1H, d, J = 12.3 Hz), 4.94 (1H, d, J = 12.3 Hz), 3.86 (1H, td, J = 11.2; 4.8 Hz), 3.85 (6H, s), 3.16 (1H, d, J = 9.3 Hz), 2.87 (1H, dd, J = 13.5; 4.2 Hz), 2.10–1.86 (4H, m), 1.66–1.48 (8H, m), 1.43 (4H, m), 1.35–1.19 (4H, m), 1.13 (3H, s), 1.03 (3H, s), 0.93 (3H, s), 0.89 (3H, s), 0.85 (3H, s), 0.81 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.2, 159.4, 159.3, 146.0, 143.1, 143.0, 129.9, 129.8, 122.1, 121.9, 121.7, 121.7, 121.5, 121.5, 119.8, 114.2, 114.2, 114.2, 114.2, 94.6, 68.2, 67.3, 56.9, 55.1, 55.1, 54.8, 47.0, 46.2, 46.1, 45.2, 41.1, 40.6, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 22.9, 16.4, 16.3, 16.2, 16.0; HRMS (ESI+): calculated for (C50H67N6O6)+ [M + H]+ 847.5122, found 847.5103.
(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11c). Yellowish solid; m.p.: 244–246 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.94 (1H, s), 7.73 (4H, dd, J = 6.9; 2.1 Hz), 7.50 (4H, dd, J = 6.9; 2.4 Hz), 5.29 (1H, m), 5.26 (2H, s), 4.98 (2H, s), 3.86 (1H, td, J = 11.2; 4.8 Hz), 3.12 (1H, d, J = 9.3 Hz), 2.86 (1H, dd, J = 13.5; 4.2 Hz), 2.13–1.85 (4H, m), 1.69–1.41 (10H, m), 1.36 (2H, m), 1.28 (4H, m), 1.10 (3H, s), 1.04 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.46 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 145.1, 144.0, 144.0, 139.9, 139.9, 134.6, 134.5, 129.9, 129.9, 129.9, 129.9, 122.4, 122.4, 121.7, 121.7, 121.6, 121.6, 120.0, 95.0, 68.8, 67.7, 57.2, 55.3, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 18.1, 17.6, 16.7, 16.4; HRMS (ESI+): calculated for (C48H61Cl2N6O4)+ [M + H]+ 855.4131, found 855.4123.
(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11d). White solid; m.p.: 256–258 °C; 1H NMR (300 MHz, CDCl3): δ 8.01 (1H, s), 7.91 (1H, s), 7.76 (4H, dd, J = 6.6; 2.1 Hz), 7.52 (4H, dd, J = 6.6; 2.1 Hz), 5.29 (3H, m), 4.99 (1H, d, J = 12.3 Hz), 4.96 (1H, d, J = 12.03 Hz), 3.89 (1H, td, J = 11.2; 4.8 Hz), 3.14 (1H, d, J = 9.3 Hz), 2.89 (1H, dd, J = 13.5; 4.2 Hz), 2.17–1.89 (4H, m), 1.69–1.46 (8H, m), 1.39 (2H, m), 1.35–1.26 (6H, m), 1.11 (3H, s), 1.05 (3H, s), 0.90 (3H, s), 0.86 (3H, s), 0.82 (3H, s), 0.78 (3H, s), 0.47 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.6, 146.0, 144.2, 144.4, 139.4, 139.4, 134.6, 134.5, 129.9, 129.9, 129.8, 129.8, 122.9, 122.9, 121.5, 121.5, 121.5, 121.5, 119.5, 95.0, 68.8, 67.7, 57.2, 55.3, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 18.1, 17.6, 16.7, 16.4; HRMS (ESI+): calculated for (C48H61Br2N6O4)+ [M + H]+ 943.3121, found 943.3115.
(1-(4-Nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11e). Yellowish solid; m.p.: 218–220 °C; 1H NMR (300 MHz, CDCl3): δ 8.03 (1H, s), 7.95 (1H, s), 7.75–1.71 (4H, m), 7.52–7.49 (4H, m), 5.28 (3H, m), 4.98 (2H, s), 3.89 (1H, td, J = 11.2; 4.8 Hz), 3.12 (1H, d, J = 9.3 Hz), 2.91 (1H, dd, J = 13.5; 4.2 Hz), 2.11–1.89 (6H, m), 1.71–1.55 (6H, m), 1.46 (4H, m), 1.31 (4H, m), 1.12 (3H, s), 1.04 (3H, s), 0.92 (3H, s), 0.86 (3H, s), 0.81 (3H, s), 0.75 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 145.1, 144.0, 144.0, 139.9, 139.9, 134.6, 134.5, 129.9, 129.9, 129.9, 129.9, 122.4, 122.4, 121.7, 121.7, 121.6, 121.6, 120.0, 95.0, 68.8, 67.7, 57.2, 55.3, 47.0, 46.2, 45.2, 42.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 23.0, 18.1, 17.6, 16.7, 16.4; HRMS (ESI+): calculated for (C48H61N8O8)+ [M + H]+ 877.4612, found 877.4604.
(1-(3-Methylphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1-(3-methylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11f). White solid; m.p.: 261–263 °C; 1H NMR (300 MHz, CDCl3): δ 8.02 (1H, s), 7.93 (1H, s), 7.55 (2H, s), 7.50 (2H, d, J = 8.1 Hz), 7.42–7.36 (2H, td, J = 7.8; 1.5 Hz), 7.25 (2H, d, J = 8.1 Hz), 5.31 (1H, m), 5.29 (1H, d, J = 12.6 Hz), 5.25 (1H, d, J = 12.6 Hz), 5.01 (1H, d, J = 12.3 Hz), 4.95 (1H, d, J = 12.3 Hz), 3.87 (1H, td, J = 11.2; 4.8 Hz), 3.17 (1H, d, J = 9.3 Hz), 2.88 (1H, dd, J = 13.5; 4.2 Hz), 2.45 (6H, s), 2.04 (2H, m), 1.89 (2H, m), 1.67–1.43 (10H, m), 1.36 (2H, m), 1.25–1.17 (4H, m), 1.11 (3H, s), 1.05 (3H, s), 0.97 (3H, s), 0.93 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.45 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.2, 143.7, 143.5, 143.5, 139.9, 139.9, 137.0, 136.9, 129.6, 129.6, 129.5, 129.5, 121.6, 122.4, 121.3, 121.2, 120.1, 117.6, 117.5, 95.1, 77.2, 68.7, 67.8, 57.3, 55.3, 47.1, 45.3, 42.4, 41.2, 40.8, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.6, 23.4, 22.9, 21.3, 21.3, 18.1, 17.6, 16.7, 16.5; HRMS (ESI+): calculated for (C50H67N6O4)+ [M + H]+ 815.5224, found 815.5218.
(1-Naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2-hydroxy-3-((1 naphthalen-1-yl -1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (11 g). Dark purple solid; m.p.: 244–245 °C; 1H NMR (300 MHz, CDCl3): δ 8.05–7.91 (4H, m), 8.01 (1H, s), 7.97 (1H, s), 7.67–7.52 (10H, m), 5.41–531 (3H, m), 5.09 (1H, d, J = 12.6 Hz), 5.03 (1H, d, J = 12.6 Hz), 3.92 (1H, td, J = 11.2; 4.8 Hz), 3.04 (1H, d, J = 9.6 Hz), 2.90 (1H, dd, J = 13.5; 4.2 Hz), 2.15–1.87 (6H, m), 1.75–1.43 (8H, m), 1.35(3H, m), 1.26 (2H, m), 1.13 (3H, s), 1.06 (3H, s), 0.90 (3H, s), 0.87 (3H, s), 0.83 (3H, s), 0.80 (3H, s), 0.57 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 143.6, 142.7, 143.2, 133.7, 133.6, 133.2, 133.0, 133.0, 129.9, 129.9, 128.0, 127.8, 127.7, 127.7, 127.7, 127.7, 127.4, 126.6, 126.2, 124.4, 124.4, 123.0, 122.4, 122.3, 122.2, 95.2, 77.2, 68.8, 67.8, 57.6, 55.4, 47.6, 46.8, 45.8, 41.7, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 17.6, 16.6, 16.6, 16.4, 16.0; HRMS (ESI+): calculated for (C56H67N6O4)+ [M + H]+ 887.5224, found 887.5220.
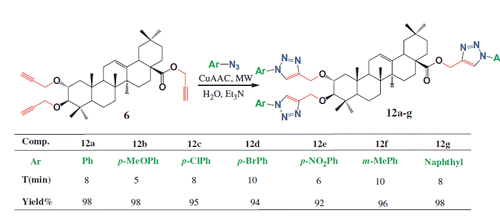
General procedure for the synthesis of tri-1,4-triazolyl derivatives 12a–g under CuAAC condition
Maslinic acid-tri-alkyne 6 (0.1 g, 0.17 mmol) and the appropriate azide (1 mmol) were dissolved in 3 mL H2O. While the mixture was being stirred, cuprous iodide (1 equiv) was added, followed by Et3N (0.5 mmol) at room temperature. The reaction mixture was then subjected to microwave irradiations at 300 W for 5–10 min, after which it was diluted with water and then extracted with ethyl acetate (3 × 50 mL). The organic layer was dried over Na2SO4. After removal of solvent in vacuo, the resulting residue was purified by recrystallization or silica gel column chromatography and eluted with petroleum ether:ethyl acetate (8:2) to obtain the desired new products (12a–g).
(1-Phenyl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12a). Purple solid; m.p.: 214–216 °C; 1H NMR (300 MHz, CDCl3): δ 8.27 (1H, s), 8.07 (1H, s), 8.05 (1H, s), 7.77–7.71 (6H, m), 7.57–7.36 (9H, m), 5.33–5.23 (5H, m), 4.90 (1H, d, J = 12.3 Hz), 4.81 (1H, d, J = 12.3 Hz), 3.73 (1H, td, J = 11.2; 4.8 Hz), 3.03 (1H, d, J = 9.6 Hz), 2.91 (1H, dd, J = 13.8; 4.2 Hz), 2.14–1.85 (6H, m), 1.78–1.48 (11H, m), 1.31 (2H, m), 1.20 (2H, m), 1.12 (3H, s), 1.07 (3H, s), 1.00 (3H, s), 0.89 (3H, s), 0.84 (3H, s), 0.80 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 143.6, 137.1, 137.1, 137.1, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.6, 129.6, 129.6, 128.8, 128.6, 128.4, 122.5, 122.3, 120.5, 120.4, 120.3, 120.3, 120.3, 120.3, 120.3, 120.3, 90.7, 77.9, 67.5, 63.3, 57.3, 47.5, 46.7, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.4, 17.6, 16.4, 16.2, 15.9; HRMS (ESI+): calculated for (C57H70N9O4)+ [M + H]+ 944.5551, found 944.5573.
(1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12b). Red solid; m.p.: 255–257 °C; 1H NMR (300 MHz, CDCl3): δ 8.16 (1H, s), 7.97 (1H, s), 7.95 (1H, s), 7.65–7.58 (6H, m), 7.05–6.95 (6H, m), 5.32–5.20 (5H, m), 4.90–4.77 (2H, m), 3.87 (6H, s), 3.84 (3H, s), 3.79 (1H, td, J = 11.2; 4.8 Hz), 3.02 (1H, d, J = 9.9 Hz), 2.92 (1H, dd, J = 13.8; 4.2 Hz), 2.14–1.85 (5H, m), 1.78–1.48 (10H, m), 1.31 (4H, m), 1.20 (2H, m), 1.12 (3H, s), 1.07 (3H, s), 1.00 (3H, s), 0.89 (3H, s), 0.84 (3H, s), 0.80 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 159.9; 159.7, 159.6, 146.6, 146.5, 143.6, 143.5, 130.6, 130.4, 122.2, 122.1, 122.1, 121.9, 121.9, 121.9, 121.0, 120.9, 114.8, 114.8, 114.8, 114.7, 114.7, 114.7, 114.7, 114.7, 114.7, 90.7, 77.9, 67.5, 63.3, 57.3, 55.5, 55.6, 55.5, 47.5, 46.7, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.6, 18.2, 17.5, 16.7, 16.4; HRMS (ESI+): calculated for (C60H76N9O4)+ [M + H]+ 1034.5868, found 1034.5917.
(1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12c). Yellowish solid; m.p.: 234–235 °C; 1H NMR (300 MHz, CDCl3): δ 8.20 (1H, s), 7.96 (1H, s), 7.93 (1H, s), 7.65–7.57 (6H, m), 7.44–7.35 (6H, m), 5.23–5.10 (5H, m), 4.90–4.77 (2H, m), 3.67 (1H, td, J = 11.2; 4.8 Hz), 2.95 (1H, d, J = 9.9 Hz), 2.87 (1H, dd, J = 13.8; 4.2 Hz), 2.14–1.85 (5H, m), 1.78–1.48 (10H, m), 1.31 (4H, m), 1.20 (2H, m), 1.12 (3H, s), 1.07 (3H, s), 0.97 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.74 (3H, s), 0.38 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 159.9; 159.7, 159.6, 146.6, 146.5, 143.6, 143.5, 130.6, 130.4, 122.2, 122.1, 122.1, 121.9, 121.9, 121.9, 121.0, 120.9, 114.8, 114.8, 114.8, 114.7, 114.7, 114.7, 114.7, 114.7, 114.7, 90.7, 77.9, 67.5, 63.3, 57.3, 47.5, 46.7, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.6, 18.2, 17.5, 16.7, 16.4; HRMS (ESI+): calculated for (C57H67Cl3N9O4)+ [M + H]+ 1046.4382, found 1046.4411.
(1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12d). White solid; m.p.: 221–223 °C; 1H NMR (300 MHz, CDCl3): δ 8.26 (1H, s), 8.01 (1H, s), 7.97 (1H, s), 7.75–7.55 (6H, m), 7.41–7.29 (6H, m), 5.25 (4H, m), 5.21–5.12 (4H, m), 4.93–4.79 (2H, m), 3.71 (1H, td, J = 11.2; 4.8 Hz), 2.99 (1H, d, J = 9.9 Hz), 2.87 (1H, dd, J = 13.8; 4.2 Hz), 2.14–1.85 (5H, m), 1.70–1.55 (6H, m), 1.48–1.38 (4H, m), 1.31 (2H, m), 1.25 (4H, m), 1.12 (3H, s), 1.07 (3H, s), 0.97 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.74 (3H, s), 0.49 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.9, 159.6; 159.3, 159.3, 145.1, 145.1, 151.1, 143.5, 130.6, 130.4, 122.2, 122.1, 122.1, 121.9, 121.9, 121.9, 121.0, 120.9, 115.9, 115.8, 115.8, 1116.0, 116.0, 116.0, 116.2, 116.2, 116.2, 90.7, 77.9, 67.5, 63.3, 57.3, 47.5, 46.7, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.6, 18.2, 17.8, 16.2, 16.0; HRMS (ESI+): calculated for (C57H67Br3N9O4)+ [M + H]+ 1178.2866, found 1178.2897.
(1-(4-Nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12e). Yellowish solid; m.p.: 211–212 °C; 1H NMR (300 MHz, CDCl3): δ 8.17 (1H, s), 7.91 (1H, s), 7.90 (1H, s), 7.68–7.55 (6H, m), 7.40–7.31 (6H, m), 5.24–5.16 (5H, m), 4.92–4.78 (2H, m), 3.69 (1H, td, J = 11.2; 4.8 Hz), 2.95 (1H, d, J = 9.9 Hz), 2.87 (1H, dd, J = 13.8; 4.2 Hz), 2.14–1.85 (5H, m), 1.78–1.48 (8H, m), 1.39 (4H, m), 1.22 (4H, m), 1.12 (3H, s), 1.07 (3H, s), 0.97 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.74 (3H, s), 0.44 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.7, 159.9; 159.7, 159.6, 146.6, 146.5, 143.6, 143.5, 130.6, 130.4, 122.2, 122.1, 122.1, 121.6, 121.6, 121.6, 121.0, 120.9, 115.2, 115.2, 115.1, 114.9, 114.8, 114.8, 114.7, 114.7, 114.7, 90.7, 77.9, 67.5, 63.3, 57.3, 47.5, 46.7, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.6, 18.2, 17.5, 16.7, 16.4; HRMS (ESI+): calculated for (C57H67N12O10)+ [M + H]+ 1046.5103, found 1072.5144.
(1-(3-Methylphenyl)-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-(3-methylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12f). White solid; m.p.: 211–213 °C; 1H NMR (300 MHz, CDCl3): δ 8.12 (1H, s), 8.02 (1H, s), 7.97 (1H, s), 7.57 (3H, m), 7.51–7.36 (6H, m), 7.40–7.35 (3H, m), 5.33 (1H, s), 5.30 (2H, m), 5.27 (2H, m), 4.99 (2H, m), 3.89 (1H, td, J = 11.2; 4.8 Hz), 3.17 (1H, d, J = 9.3 Hz), 2.88 (1H, dd, J = 13.5; 4.2 Hz), 2.46 (3H, s), 2.44 (6H, s), 2.06 (4H, m), 1.89 (4H, m), 1.67–1.43 (6H, m), 1.34 (2H, m), 1.25–1.19 (5H, m), 1.11 (3H, s), 1.05 (3H, s), 0.97 (3H, s), 0.93 (3H, s), 0.81 (3H, s), 0.78 (3H, s), 0.45 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.3, 143.7, 143.5, 143.5, 143.4, 139.9, 139.9, 139.8, 137.0, 136.9, 129.6, 129.4, 122.2, 122.1, 122.1, 121.0, 120.9, 115.2, 115.2, 115.1, 114.9, 114.8, 114.8, 114.8, 115.8, 115.7, 115.7, 90.0, 77.8, 67.5, 63.3, 57.3, 47.5, 46.7, 46.5, 46.5, 46.3, 45.8, 44.4, 44.3, 41.2, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 31.9, 30.2, 29.2, 28.2, 27.0, 25.3, 23.1, 23.0, 22.6, 18.2, 17.5, 16.7, 16.0; HRMS (ESI+): calculated for (C60H76N9O4)+ [M + H]+ 986.6020, found 986.6053.
(1-Naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methyl-(2α,3β)-2,3-bis((1-naphthalen-1-yl-1H-1,2,3-triazol-4-yl)methoxy)-olean-12-en-28-oate (12 g). Dark purple solid; m.p.: 204–205 °C; 1H NMR (300 MHz, CDCl3): δ 8.15–7.99 (12H, m), 7.77–7.64 (6H, m), 7.62–7.55 (6H, m), 5.41–531 (3H, m), 5.09 (2H, d, J = 12.6 Hz), 5.03 (2H, d, J = 12.6 Hz), 3.92 (1H, td, J = 11.2; 4.8 Hz), 3.04 (1H, d, J = 9.6 Hz), 2.90 (1H, dd, J = 13.5; 4.2 Hz), 2.15–1.87 (6H, m), 1.75–1.43 (8H, m), 1.35(3H, m), 1.26 (4H, m), 1.13 (3H, s), 1.06 (3H, s), 0.90 (3H, s), 0.87 (3H, s), 0.83 (3H, s), 0.80 (3H, s), 0.57 (3H, s); 13C NMR (75 MHz, CDCl3): δ 177.5, 143.6, 143.2, 143.2, 143.2, 142.7, 142.7, 133.7, 133.6, 133.2, 133.0, 133.0, 129.9, 129.9, 129.9, 129.9, 129.9, 129.9, 128.0, 127.8, 127.7, 127.7, 127.7, 127.7, 127.4, 126.6, 126.2, 124.4, 124.4, 123.0, 122.4, 122.4, 122.4, 122.4, 122.3, 122.3, 122.3, 122.2, 122.2, 122.2, 93.2, 77.2, 68.8, 67.8, 57.6, 55.4, 47.6, 46.8, 45.8, 41.7, 40.1, 38.8, 38.5, 37.6, 33.2, 32.5, 32.0, 31.6, 29.2, 28.2, 27.0, 25.3, 23.5, 23.2, 17.6, 16.6, 16.6, 16.6, 16.2; HRMS (ESI+): calculated for (C69H76N9O4)+ [M + H]+ 1094.6020, found 1094.6061.
Biological assay
Natural pentacyclic triterpenoid (Maslinic acid 1) and most of the synthesized triazoles were dissolved in dimethyl sulfoxide (DMSO) and added into the medium at final concentrations of 10, 30 and 100 μM. The final concentration of DMSO was 0.33% and was determined to have no cytotoxicity.
Determination of pro-inflammatory IL-1β cytokine production in PBMCs
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from healthy donors by density gradient centrifugation (Pancoll human, d.1.077 g/mL). Recovered PBMCs were washed thrice in Dulbecco’s phosphate buffered saline, seeded in a 96-well microplate at 6.67 × 105 cells/mL in RPMI-1640 containing 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), amphotericin B (250 ng/mL), and incubated at 5% CO2 and 37 °C. PBMCs were treated with compound 1 and the synthesized triazoles (8b–g; 9a–d,f,g; 10a,c,f,g; 11a–c,f; 12b,c,f,g) (10, 30 and 100 μM) then were stimulated for 24 h with LPS (E. coli, 0128:B12, Sigma) (10 ng/mL)Citation28,Citation29. Three anti-inflammatory standards were tested in the same conditions: ZVAD (5 μM), dexamethasone (DEXA, 1 μM) and prednisolone (PRED, 70 μM). After incubation, the supernatants were harvested and kept at −20 °C until use. Viability of PBMCs was assessed by XTT assay. Secretion IL-1β was measured only in the culture supernatants of PBMCs treated with compounds that showed low toxicity, using a sandwich enzyme-linked immunosorbent assay (ELISA) method (eBioscience, San Diego, CA), according to manufacturer instructions.
Determination of anti-proliferative activity in EMT-6 and SW480 cancer cell lines
Murine mammary carcinoma cell line EMT-6 and human colorectal adenocarcinoma cell line SW480, purchased from American Type Culture Collection (ATCC, Manassas, VA), were cultured in DMEM supplemented with 10% of fetal bovine serum and penicillin (100 U/mL), streptomycin (100 μg/mL), amphotericin B (250 ng/mL) and placed in CO2 incubator with 5% of CO2 at 37 °C. Cells were seeded in a 96-well microplate at 3.125 × 104 cells/mL and treated with compounds (10, 30 and 100 μM) for 48 h. Doxorubicin, etoposide, 5-fluorouracil and methotrexate (10 μM) were used as anti-cancer references. The anti-proliferative activity of compounds and drug references were determined using XTT assayCitation30,Citation31. This assay is based on the conversion of the water-soluble XTT (sodium 2,3,-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium) inner salt) reagent, in the presence of the electron-coupling reagent PMS (phenazine methylsulfate) to an orange formazan product by metabolically active cells. The amount of formazan produced was detected by measurement of the absorbance at 499 nm and a reference wavelength was used at 660 nm on a microplate reader Infinite M200 Pro TECAN (Männedorf, Switzerland). Cell viability is expressed as a percentage of control, which was taken as 100%.
Results and discussion
Chemistry
Synthesis of maslinic acid-alkyne derivatives 2–6 as dipolarophiles
Taking cue from the literature, we have undertaken a research program directed toward the structural modifications of maslinic acid 1 to fine tune its biological potential as anti-inflammatory and anti-proliferative agent. The synthesis of maslinic acid-alkyne derivatives 2–6 was done on maslinic acid (1) which isolated under green chemistry condition, from pomace olive (Olea europaea L.) cultivar: Chemlali with a large amount (17 g (8.5 mg/g DW) using an ultrasonic bath. The compound 1 was subjected to a propargylation of the hydroxyl groups at C-2, C-3 and carboxylic group at C-28. This reaction was tried under different conditions in dry N,N-dimethylformamide (DMF). Method I: 2 equiv. of NaH was used in the presence of propargyl bromide (3 equiv.) in dry DMF for 2 h at room temperature yielded a mixture of compounds in 82% yield (). Chromatography on a silica-gel column of this mixture resulted compounds 2 (44%), 3 (5%), 4 (15%), 5 (11%) and 6 (7%). Method II: When the amounts of NaH and propargyl bromide were increased to 4 equiv. of each in dry DMF at room temperature for 8 h, the yield of mixture has been improved (99%). Chromatography on a silica-gel column of this mixture resulted only the alkyl derivatives 2 and 4–6 in 25, 23, 20 and 21% yield, respectively ().
Table 1. Method I and II for the synthesis of dipolarophiles 2–6a.
The structures of the propargylated compounds 2–6 were unambiguously confirmed by ESI-HRMS and NMR. Thus, the molecular formula of compounds 2 and 3 (C33H50O4) as determined by ESI-HRMS (m/z 511.3778 and 511.3794, respectively [M + H]+, calculated for 511.3787), was in agreement with a monopropargylated structure of maslinc acid 1. While, the molecular formula of compounds 4 and 5 (C36H52O4) as established by ESI-HRMS (m/z 549.3938 and 549.3940, respectively [M + H]+, calculated for 549.3944), indicated that were they derived from the dipropargylation of maslinic acid 1. Moreover, the molecular formula of compound 6 (C39H54O4) as determined by ESI-HRMS (m/z 587.4092 [M + H]+, calculated for 587.4100), was found to be in concordance with the tripropargylation of maslinic acid 1. The position of the alkylation in the obtained derivatives was deduced by simple comparison of their NMR spectral data (1H and 13C) with those of the starting substrate 1.
RuAAC and CuAAC for the synthesis of 1,5- and 1,4-disubstituted triazoles
Aromatic azides were synthesized from the appropriate anilines using a two-step procedure by diazotization with sodium nitrite in acidic conditions followed by displacement with sodium azide. The desired aromatic azides 7a–g were obtained in yields ranging from 85 to 98%.
The thermal [3 + 2] cycloaddition method can suffer from low yields, side-reactions and poor regioselectivityCitation32,Citation33. While “click” reaction mediated preparations of 1,4- and 1,5-disubstituted triazoles have been reported to provide satisfactory yieldsCitation34,Citation35. The ruthenium-catalyzed version of the reaction (RuAAC), led mainly to 1,5-regioisomersCitation36. Although the Ru(II)-catalyzed Hüisgen 1,3-dipolar cyclization usually proceeds in high yields, this type of reaction has been reported to be highly dependent on the substrate and reaction conditions. At the outset of our studies, we investigated the cyclo-addition reaction between phenylazide 7a and maslinic acid-alkyne 2 with different concentrations of the catalyst Cp*RuCl(PPh3)2. The most relevant results of this study are shown in .
Table 2. Optimization of the 1,3-dipolar cyclization for the ruthenium-catalyzed click reactionTable Footnotea.
At the beginning, the reaction conditions were optimized in heating at 110 °C (reflux temperature of toluene) with different catalyst loading (, entries 1–3), and it was found that 5 mol % Ru(II) was the optimum amount of catalyst for these conditions (entry 2). When the temperature was decreased to room temperature, the yield of reaction, decreased significantly (30%) after even 48 h of reaction (entry 4). It should be noted that some catalyst deactivation has been encountered during long-heating times. Thus, to circumvent this limitation, we decided to work under microwave activation. Interestingly, from the different solvents, solvent/co-solvent mixtures and solvent-free tested under microwave conditions, DMF was found to be the most effective for this reaction (, compare entries 5–8). Thus, using 5 mol% Cp*RuCl(PPh3)2 under microwave heating in DMF, the reaction of dipolarophile 2 with phenylazide 7a, gave (1-phenyl-1H-1,2,3-triazol-5-yl)methyl-(2α,3β)-2,3-dihydroxyolean-12-en-28-oate 8a almost quantitatively in only 6 min of reaction time (, entry 5). It is interesting to note that anhydrous conditions are not necessary. Indeed, undistilled DMF, or DMF-containing 0.5 equiv. of water can be used to run this RuAAC (entries 5 and 7). While the use of water as solvent results in a significant reduction in yield (Traces) even after 15 min reaction (entry 6). It may be due to the poor solubility of the starting dipolarophile 2.
Under the optimized conditions, a region-specific approach using Huisgen 1,3-dipolar cyclo-addition reaction (RuAAC) of terminal alkyne 2 with various aromatic azides 7a–g in presence of Cp*RuCl(PPh3)2 as catalyst resulting into the formation of regiospecific 1,5-substituted-triazolyl derivatives 8a–g in excellent yields (Scheme 1). All the reactions were carried under microwave irradiation (250 W) completed within 4–8 minutes (). A similar reactivity was observed for most azides (, entries 1–4, 6 and 7). In contrast, 4-nitrophenylazide showed to be relatively less reactive under the same reaction conditions (, entry 5), probably due to the presence of a group with a negative mesomeric effect at the 4-position of the phenyl group, which could promote the dimerization of azides to give the corresponding secondary aminesCitation37,Citation38.
Scheme 1. Synthesis of the 1,5- and 1,4-triazolyl (8a–g, 9a–g) derivatives. Reagents and conditions: (a) [Cp*RuCl(PPh3)2], DMF, Microwave (250 W, 4–8 min), 83–98%. (b) Cuprous iodide (CuI), Et3N, solvent free, Microwave (200 W, 2–4 min), 90–99%.
![Scheme 1. Synthesis of the 1,5- and 1,4-triazolyl (8a–g, 9a–g) derivatives. Reagents and conditions: (a) [Cp*RuCl(PPh3)2], DMF, Microwave (250 W, 4–8 min), 83–98%. (b) Cuprous iodide (CuI), Et3N, solvent free, Microwave (200 W, 2–4 min), 90–99%.](/cms/asset/7caf71f1-f980-470b-a715-1baeda3eb7aa/ienz_a_1193733_sch0001.gif)
Table 3. Maslinic acid-1,5- and 1,4-disubstituted triazolyl derivatives varying at aromatic ring.
We have then investigated the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) under microwave conditions for the synthesis of 1,4-regiomers 9a–g. The copper (I)-catalyzed of the reaction (CuAAC) leading to the sole 1,4-regioisomers. Working under microwave conditions at 200 W, we first investigated the optimal conditions for the “click” reaction of dipolarophile 2 and phenylazide 7a in presence of triethylamine with different solvent and copper(I) iodide loading (). A 92% conversion was obtained only after 2 min of reaction with 0.5 equiv. catalyst. The total conversion of starting maslinic acid-alkyne 2 was obtained only after 2 min under solvent-free conditions and in 5 min in DMF for 0.5 equiv. catalyst loading. For lower CuI loading, conversion was not complete, even after 15 min reaction. Moreover, when we worked under H2O as solvent, the yield decreased (70%) even after 15 min reaction (entry 4). It may be due to the poor solubility of the maslinic acid-alkyne 2 or the possible complexation of the copper species in water.
Table 4. Optimization of CuAAC under microwave conditions for 9aa.
Starting with the maslinic acid-alkyne 2 the synthesis of various 1,4-disubstituted-1,2,3-triazolyl-maslinic acid was performed region-specifically using CuI as catalyst under microwave and solvent-free conditions (Scheme 1). The desired new 1,2,3-triazoles 9a–g were obtained in yields ranging from 90 to 99% (). This method has the advantage of being simple, low-cost and the products can be obtained from the reaction mixture by simple purification. The new products can be obtained from the reaction mixture in almost quantitative yields and the reaction times were in general shorter than those reported in the literatureCitation6.
The synthesized 1,5- and 1,4-disubstituted-1,2,3-triazolyl-maslinic acid (8a–g, 9a–g) were characterized by 1H/13C NMR, DEPT 135, NOESY and ESI-HRMS (Experimental section). The absence of 1H NMR signals of terminal alkyne 2 at δH 2.34 (1H, t, J = 2.7 Hz), and emerging of a singlet at δH 7.77–8.09 (H-4 of the triazole ring, for 1,5-isomers, 8a–g) and at δH 7.96–8.03 (H-5 of the triazole ring, for 1,4-isomers, 9a–g) provides a good support for the cycloaddition to form 1,5- and 1,4-disubstituted-triazoles. The same features are reflected in 13C NMR spectra, where the signal belongs to the terminal carbon of alkyne was disappeared and a new signal belongs to C–H ring carbon, was appeared at δC ∼114.2–136.7 after cyclo-addition.
The 1,5-regiochemistry thus adopted in compounds 8a–g was confirmed by NOESY spectra showing NOEs between the H-4triazole/H1′ and H1′/Harom, and the absence of any NOE between H-4triazole/Harom, under RuAAC conditions. While The NOESY spectra of regioisomers 9a–g showed NOEs between H-5triazole/H1′ and H-5triazole/Harom, and the absence of any NOE between H1′/Harom, under CuAAC conditions. Such observations are in perfect agreement with the proposed structures and with that cited in the literature.
CuAAC for the synthesis of bis-1,4-disubstituted triazolyl derivatives
Working under microwave and solvent-free conditions, we first investigated the optimal conditions for the cyclo-addition of bis-alkyne 4 and phenylazide 7a with different concentrations of catalyst (). A 85% conversion into the desired cyclo-adduct 10a was obtained only after 4 min of reaction with 0.7 equiv. catalyst. The total conversion of starting bis-alkyne 4 was obtained after 6 min with 0.7 equiv. catalyst loading and in 4 min for 0.8 equiv. catalyst loading. For lower catalyst loading, conversion was not complete, even after 12 min reaction (Scheme 2).
Scheme 2. Bis-1,4-disubstituted triazoles 10a–g and 11a–g prepared by the Cu(I)-catalyzed synthesis.
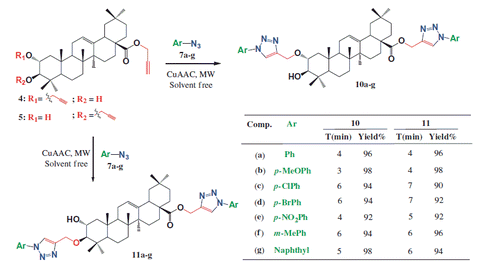
Table 5. Optimization of CuAAC under microwave and solvent free conditions for 10aa.
Thus, 0.8 equiv. catalyst, 2 equiv. Et3N and 4 equiv. of phenylazide 7a under microwave and solvent-free conditions for 4 min allowed the formation of the desired cyclo-adduct 10a. With this optimized condition, we probed the scope of the condensation of bis-alkyne 4 with various aromatic azides. The desired new bis-1,4-disubstituted-1,2,3-triazolyl-maslinic acid 10a–g were obtained in good yields (92–98%). The same methodology was successfully applied to bis-alkyne 5. The latter compound gave the desired new bis-1,4-disubstituted-1,2,3-triazolyl-maslinic acid 11a–g with various aromatic azides. All the reactions were carried under microwave irradiation (250 W) completed within 4–7 minutes (Scheme 2). A similar reactivity was observed for most azides. In contrast, with the electron-deficient azides, reactivity was decreased slightly under the same reaction conditions. Similarly the synthesized new bis-1,4-disubstituted triazoles 10a–g and 11 a–g were characterized by 1H/13C NMR, DEPT 135, NOESY and ESI-HRMS (Experimental section).
CuAAC for the synthesis of tri-1,4-disubstituted triazolyl derivatives
On the contrary of dipolarophiles 2–5, tri-alkyne 6 is fully soluble in water. Interestingly the use of water as solvent, we investigated the reactivity for the cyclo-addition of latter dipolarophile (6) and phenylazide 7a in the presence of triethylamine (3 equiv. referred to the starting tri-alkyne 6) with different catalyst (CuI) loading under microwave heating in water. The addition of CuI as catalyst (1 equiv. referred to the starting maslinic acid-tri-alkyne 6), was essential to obtain the maximum yield of the desired tri-1,4-disubstituted triazolyl 12a in minimum reaction time. For lower catalyst loading (0.8 and 0.9 equiv.), conversion was not complete. With the optimized condition in hand, the dipolarophile 6 was reacted with various aromatic azides, 3 equiv. of triethylamine and 1 equiv. of CuI catalyst. All the reactions were performed at 300 W under microwave conditions in water completed within 5–10 minutes. A similar reactivity was observed for all azides used. The desired new tri-triazoles 12a–g were obtained in yields ranging from 92 to 96% (Scheme 3).
All the synthesized new tri-1,4-disubstituted triazoles were characterized by 1H/13C NMR, DEPT 135, NOESY and ESI-HRMS (Experimental section).
Pharmacological screening
Anti-inflammatory activity
The anti-inflammatory activity of the tested compounds was studied using LPS-stimulated human peripheral blood mononuclear cells (PBMCs). Three anti-inflammatory references were tested in the same conditions: ZVAD (5 μM), dexamethasone (DEXA, 1 μM) and prednisolone (PRED, 70 μM). Secretion IL-1β was measured only in the culture supernatants of PBMCs treated with compounds that showed no or low toxicity, using a sandwich enzyme-linked immunosorbent assay (ELISA) method (eBioscience, San Diego, CA), according to manufacturer instructions.
As shown in , the % IL-1β production indicated that most compounds exhibited a significant anti-inflammatory activity. It has been found that all the synthesized triazoles exhibited more potent inhibitory activities than the starting natural compound 1. This finding showed that the introduction of the triazoles moieties in compound 1 considerably improve its anti-inflammatory effect. In the series of 1,5-regioisomers, compounds 8e (p-NO2) and 8f (m-C2H5) were found to be the most actives (% IL-1β production = 42 ± 1 and 46 ± 3 respectively; 100 μM). On the other hand, our findings showed that most of the tested 1,4-regioisomers 9a, 9b, 9d, 9f and 9 g displayed a significant anti-inflammatory activity, the derivative 9d with (p-Br) was found to be the most active (% IL-1β production = 21 ± 1; 100 μM). The comparison of % IL-1β production values of the tested compounds type 8 with those of the derivatives 9 substituted identically at the triazole ring revealed that these latter are more active (% IL-1β production = 40–91 and 21–82, respectively; 100 μM). However, we found that the regiochemistry in two analogs from the series 8 and 9 bearing the same substituent intervenes to reverse the activity, we cite the example of compounds 8d (p-Br) (% IL-1β production = 61 ± 5; 100 μM) and 9d (p-Br) (% IL-1β production = 21 ± 1; 100 μM). This finding showed the importance of both the regiochemistry of the triazole and the nature of the aryl attached to it.
Table 6. Effect of natural pentacyclic triterpenoid (Maslinic acid 1) and the synthesized triazoles on viability and inflammatory cytokine (IL-1β) production in LPS-stimulated PBMCs.
Regarding the bis-1,4-disubstituted triazoles type 10, only compounds 10a (H), 10c (p-Cl), showed a relatively-important activity (% IL-1β production = 71 ± 2 and 56 ± 2, respectively; 100 μM) compared to the remaining analogs and maslinic acid 1 (% IL-1β production = 109 ± 3; 30 μM). However, the % IL-1β production exhibited by the bis-1,4-disubstituted triazoles type 11 revealed that compounds with electron donating groups at an aromatic ring are generally more active than compounds without substitutions or substitutions with an electron withdrawing group. In fact, compounds 11b and 11f, with p-OCH3 and m-Me substitutions at the aromatic ring were the most promising (% IL-1β production = 34 ± 2 and 52 ± 1 respectively; 100 μM). The position of the triazole at C-2 (compounds 10) or C-3 (compounds 11) in maslinic acid 1 is sometimes decisive in this activity, we cite the case of 10f (% IL-1β production = 100 ± 4; 100 μM) and 11f (% IL-1β production = 52 ± 1; 100 μM) where the triazole bears the same aromatic system (m-MePh).
On the other hand, the tested compounds from the series of tri-1,4-disubstituted triazoles 12 were found to be the most potent among the whole synthesized compounds (% IL-1β production = 23–47; 30–100 μM). This finding showed the importance of the number and may be also the position of the triazole moieties to improve the anti-inflammatory activity of maslinic acid 1. Compounds 12f (m-Me) and 12 g with a naphthyl group on the triazole ring showed relatively-important activities (% IL-1β production = 23 ± 3 and 34 ± 3 respectively; 30 μM) compared to the remaining analogs and maslinic acid 1 (% IL-1β production = 109 ± 3; 30 μM).
In vitro anti-proliferative activity
The anti-proliferative activity of maslinic acid 1 and twenty-four of its triazole derivatives (8b–g; 9a–d,f,g; 10a,c,f,g; 11a–c,f; 12b,c,f,g) was studied using cultured murine EMT-6 (Breast) and human SW480 (colon) cancer cell lines. Doxorubicin, etoposide, 5-fluorouracil and methotrexate (10 μM) were used as anti-cancer references in this study. The obtained data revealed that maslinic acid 1 was found to be the most anti-proliferative against EMT-6 (Breast) and SW480 (colon) cancer cell lines (Viability (%/Control) = 5 and 9%, respectively; 100 μM) and most of its triazole derivatives showed moderate to good activities (). The percentage viability data indicated that most 1,5-regioisomers type 8 exhibited potent anti-proliferative activity. Compound 8d (p-Br) was displayed the most activity in this series against EMT-6 (Breast) (Viability (%/Control) = 13%) and SW480 (colon) (Viability (%/Control) = 34%; 100 μM) cancer cell lines. For the 1,4-regioisomers 9, compounds 9a, 9c and 9d exhibited higher anti-proliferative activity against the two used cancer cell lines at the concentration of 100 μM compared to the other analogs 9b, 9f and 9 g. Indeed, compound 9c (p-Cl) was found to be the most active against both EMT-6 (Breast) (Viability (%/Control) = 6%; 30 μM) and SW480 (colon) (Viability (%/Control) = 10%; 30 μM). In most cases, 1,4-regioisomers type 9 possess better anti-proliferative activity compared to that of 1,5-regioisomers type 8. This finding showed the signification of the region-chemistry of the triazole formed (1,4- or 1,5-) to this activity which may be explained by the approach in space of the aryl group attached to the triazole in relation to the triterpene moiety ().
Table 7. Effect of natural pentacyclic triterpenoid 1 and the synthesized triazoles on viability of EMT-6 and SW480 cell lines.
The anti-proliferative activity of the synthesized bis-triazoles 10 and 11 were investigated and they did not exhibit any marked activity. In the series of tri-1,4-disubstituted triazoles 12, only compounds 12f (m-Me) and 12 g (naphthyl) showed potent activity against EMT-6 (Breast) (Viability (%/Control) = 8%; 100 μM) and SW480 (colon) (Viability (%/Control) = 13%; 100 μM) cancer cell lines. The above data show the contribution of the free hydroxyl groups at C-2 and C-3 in maslinic acid 1 to this activity and also show the importance of nature and the number of triazoles fixed in these positions.
Conclusion
In conclusion, we have achieved the low-cost ultrasound irradiation-assisted isolation of maslinic acid 1 (17 g (8.5 mg/g DW)), under green chemistry condition, from pomace olive (Olea europaea L.) cultivar: Chemlali. On the other hand, we used it as starting material to develop an effective, facile and practical procedure for the regio-specific synthesis of 1,5-triazolyl derivatives by Ru(II)-catalyzed azide-alkyne cycloaddition (RuAAC), and mono, bis and tri-1,4-triazolyl derivatives by Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), using click-chemistry and microwave irradiation conditions, avoiding toxic reagents and solvents. This method afforded twenty-four maslinic acid triazole derivatives in quantitative yields. Most of the compounds were evaluated for their anti-inflammatory and anti-proliferative activities. Our findings showed the contribution of the nature of the introduced triazole ring and its regio-chemistry to improve in some cases the anti-inflammatory activity of maslinic acid 1 towards LPS-stimulated human peripheral blood mononuclear cells (PBMCs) and the importance of the two free hydroxyl groups in C-2 and C-3 positions to get anti-proliferative activity against EMT-6 (Breast) and human SW480 (colon) cancer cell lines.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article. This study was financially supported (LR11ES39) by the Ministry of Higher Education and Scientific Research of Tunisia.
Acknowledgements
The authors are grateful to Mrs Amna Benzarti and Miss Nadia Msaddek, NMR service at the Faculty of Monastir, University of Monastir for the 1D and 2D NMR analysis.
References
- Zhang L, Chen X, Xue P, et al Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J Am Chem Soc 2005;127:15998–9
- Boren BC, Narayan S, Rasmussen LK, et al Ruthenium-catalyzed azide-alkyne cycloaddition: scope and mechanism. J Am Chem Soc 2008;130:8923–30
- Rasmussen LK, Boren BC, Fokin VV. Ruthenium-catalyzed cycloaddition of aryl azides and alkynes. Org Lett 2007;9:5337–9
- Katritzky AR, Zhang Y, Singh SK. 1,2,3-Triazole formation under mild of acetylenes with azides. Heterocycles 2003;60:1225–39
- Wu P, Feldman AK, Nugent AK, et al Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(i)-catalyzed ligation of azides and alkynes. Angew Chem Int Ed Engl 2004;43:3928–1932
- Ifuku S, Matsumoto C, Wada M, et al Preparation of highly regioselective amphiprotic chitosan derivative via “click chemistry”. Int J Biol Macromol 2013;52:72–6
- Kumar D, Reddy VB, Varma RS. A facile and regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles using click chemistry. Tetrahedron Lett 2009;50:2065–8
- Kamal A, Shankaraiah N, Devaiah V, et al Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click’ chemistry: DNA-binding affinity and anticancer activity. Bioorg Med Chem Lett 2008;18:1468–73
- Kim TW, Yong Y, Shin SY, et al Synthesis and biological evaluation of phenyl-1H-1,2,3-triazole derivatives as anti-inflammatory agents. Bioorg Chem 2015;59:1–11
- Rocha GG, Simões M, Lúcio KA, et al Natural triterpenoids from Cecropia lyratiloba are cytotoxic to both sensitive and multidrug resistant leukemia cell lines. Bioorg Med Chem 2007;15:7355–60
- Odeku OA, Okunlola A, Lamprecht A. Microbead design for sustained drug release using four natural gums. Int J Biol Macromol 2013;58:113–20
- Sharma M, Sharma PD, Bansal MP, Singh J. Lantadene A-induced apoptosis in human leukemia HL-60 cells. Indian J Pharm 2007;39:140–4
- Kaur J, Sharma M, Sharma PD, Bansal MP. Antitumor activity of lantadenes in DMBA/TPA induced skin tumors in mice: expression of transcription factors. Am J Biomed Sci 2010;2:9–90
- Wen X, Zhang P, Liu J, et al Pentacyclic triterpenes. Part 2: Synthesis and biological evaluation of maslinic acid derivatives as glycogen phosphorylase inhibitors. Bioorg Med Chem Lett 2006;16:722–6
- Qiu WW, Shen Q, Yang F, et al Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem Lett 2009;19:6618–22
- Sheng H, Sun H. Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 2011;28:543–93
- Taniguchi S, Imayoshi Y, Kobayashi E, et al Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002;59:315–23
- Parra A, Rivas F, Lopez PE, et al Solution- and solid-phase synthesis and anti-HIV activity of maslinic acid derivatives containing amino acids and peptides. Bioorg Med Chem 2009;17:1139–45
- Montilla MP, Agil A, Navarro MC, et al Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Medica 2003;69:472–4
- Martin AM, Vazquez RP, Fernandez-Arche A, Ruiz-Gutierrez V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic Res 2006;40:295–302
- Reyes-Zurita FJ, Pachon-Pena G, Lizarraga D, et al The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011;11:154–62
- Reyes-Zurita FJ, Rufino-Palomares EE, Lupianez JA, Cascante M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett 2009;273:44–54
- Allouche Y, Warleta F, Campos M, et al Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on dna damage. J Agric Food Chem 2011;59:121–30
- Rodriguez RR, Perona JS, Herrera MD, Ruiz-Gutierrez V. Triterpenic compounds from “orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J Agric Food Chem 2006;54:2096–102
- Moneriz C, Marin-Garcia P, Garcia-Granados A, et al Parasitostatic effect of maslinic acid. I. Growth arrest of Plasmodium falciparum intraerythrocytic stages. Malaria J 2011;10:1–10
- Allouche Y, Beltran G, Gaforio JJ, et al Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem Toxicol 2010;48:2885–90
- Allouche Y, Jimenez A, Uceda M, et al Triterpenic content and chemometric analysis of virgin olive oils from forty olive cultivars. J Agric Food Chem 2009;57:3604–10
- Schindler R, Mancilla J, Endres S, et al Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990;1:40–7
- Shah VO, Ferguson JE, Hunsaker LA, et al Natural products inhibit LPS-induced activation of pro-inflammatory cytokines in peripheral blood mononuclear cells. Nat Prod Res 2010;24:1177–88
- Manase MJ, Mitaine-Offer A, Miyamoto T, et al Triterpenoid saponins from Polycarpaea corymbosa Lamk. var. eriantha Hochst. Phytochem 2014;100:150–5
- Jost LM, Kirkwood JM, Whitesided TL. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J Immunoll Methods 1992;147:153–65
- Norris P, Horton D, Levine BR. Cycloaddition of acetylenes with 5-azido-5-deoxy-D-aldopentose derivatives: synthesis of triazole reversed nucleoside analogs. Heterocycles 1996;43:2643–55
- Talekar RR, Wightman RH. Synthesis of some pyrrolo [2, 3-d] pyrimidine and 1, 2, 3-triazole isonucleosides. Tetrahedron 1997;53:3831–42
- Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today 2003;8:1128–37
- Tornoe CW, Meldal M. Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 2008;108:2952–3015
- Takasu K, Azuma T, Takemoto Y. Synthesis of trifunctional thioureas bearing 1,5-disubstituted triazole tether by Ru-catalyzed Huisgen cycloaddition. Tetrahedron Lett 2010;51:2737–40
- An IH, Seong HR, Ahn KH. Reductive dimerization of azides to secondary amines under hydrogenation conditions. Bull Korean Chem Soc 2004;25:420–2
- Lange M, Pettersen AL, Undheim K. Synthesis of secondary amines by reductive dimerization of azides. Tetrahedron 1998;54:5745–52