Abstract
Carbonic anhydrase inhibitors (CAIs) are of growing interest since various isoforms of the enzyme are identified as promising drug targets for treatment of disease. The principal drawback of the clinically used CAIs is the lack of isoform selectivity, which may lead to observable side effects. Studies aiming at the design of isoform-selective CAIs entail generation and biological testing of arrays of compounds, which is a resource- and time-consuming process. Employment of multicomponent reactions is an efficient synthetic strategy in terms of gaining convenient and speedy access to a range of scaffolds with a high degree of molecular diversity. However, this powerful tool appears to be underutilized for the discovery of novel CAIs. A number of studies employing multicomponent reactions in CAI synthesis have been reported in literature. Some of these reports provide inspiring examples of successful use of multicomponent chemistry to construct novel potent and often isoform-selective inhibitors. On critical reading of several publications, however, it becomes apparent that for some chemical series designed as CAIs, the desired inhibitory properties are only assumed and never tested for. In these cases, the biological profile is reported based on the results of phenotypical cellular assays, with no correlation with the intended on-target activity. Present review aims at critically assessing the current literature on the multicomponent chemistry in the CAI design.
Introduction
Carbonic anhydrase (CA EC 4.2.1.1) is a ubiquitous zinc enzyme, the crucial function of which is catalysis of reversible CO2 hydrationCitation1. Sixteen isoforms of CA differing in their activity and subcellular localization have been characterized in humans. Many CA isoforms play critical role in both normal physiological processes and pathogenic mechanisms where bicarbonate and/or protons are essential. These isozymes gain attention as promising targets for treatment of a range of diseasesCitation2. In particular, inhibition of cytosolic isoform CA II is used in glaucoma treatment for intraocular pressure controlCitation3. Transmembrane isoforms CA IX and XII were found to be overexpressed in many hypoxic tumorsCitation4,Citation5. Small molecules binding and inhibiting these isozymes can be used in tumor diagnostics as well as in therapy through suppression of extracellular acidification of cancer cellsCitation6. However, many of the clinically used carbonic anhydrase inhibitors (CAIs) do not possess significant selectivity with respect to a particular CA isoform and the associated side effects limit the use of these compounds. Therefore development of new, isoform-selective CAIs is highly desirableCitation7.
Any systematic quest for such new inhibitors is associated with creating and assaying of novel small molecule librariesCitation8. Besides identifying new compounds possessing the classical CA inhibitory pharmacophore, primary sulfonamide, such efforts have resulted in identification of new chemical classes endowed with the ability to inhibit CAsCitation9. These comprise recently reported inhibitors such as coumarinsCitation10, phenolsCitation11, polyaminesCitation12, etc. Moreover, crystallography studies of enzyme–inhibitor complexes help elucidate the mechanism of inhibitory action of various chemotypes. For instance, sulfonamides were found to coordinate with the catalytic zinc ion; coumarins bind at the entrance of the active site, and polyamines as well as phenols are able to interact with the zinc-coordinated water molecule.
Generation of novel small molecules libraries is a labor-intensive and time-consuming process. In this context, multicomponent approaches manifested themselves as powerful synthetic tools, providing convenient access to a great diversity of novel promising drug candidatesCitation13–15. Multicomponent chemistry is one of the effective strategies in modern lead discovery, gaining growing attention of the drug discovery community due to its resource economy and high productivity.
In this review our goal we aim at drawing attention to the fact that multicomponent chemistry in the context of synthesizing CAIs is fairly undeveloped. To this end, we summarize a fairly limited number of appropriate cases reported in the literature. In addition, we have encountered a challenge related to the data quality. Unfortunately, only a handful of reported compounds have acceptable grounds to be considered true CAIs having known inhibitory level supported by credible testing data. In particular, in many cases inhibitory activity of compounds was assumed based on the presence of a known CA inhibitory pharmacophore (mostly, primary sulfonamide) and the subsequently determined in vivo bioactivity or in vitro determined cytotoxity, and not on the accurate measurement of on-target activity, for instance, via the well-validated CO2-hydratase inhibition assay against an isolated recombinant protein. We chose to include such cases in the present review highlighting the synthetic aspects related to the use of multicomponent chemistry and underscoring the absence of on-target data for the resulting compounds.
Multicomponent synthesis of aminocyanopyrazole-based CAIs
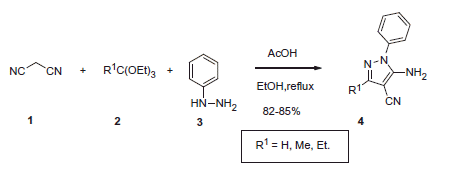
In 2013 Allouche et al.Citation16 suggested convenient access to substituted 2-amino-3-cyanopyrazoles through the reaction of malononitrile 1 with orthoesters 2 and phenylhydrazine 3 under acidic conditions. Several types of CAIs with hypothetically novel mechanism of action were successfully generated using this multicomponent approach. In particular, reaction involving phenylhydrazines 3 was realized in ethanol under reflux in the presence of acetic acid and provided N-phenyl substituted aminocyanopyrazoles 4 (Scheme 1). Inhibitory properties of these compounds toward human carbonic anhydrase (hCA) were then evaluated via classical stopped flow CO2 hydration assay.
Aminocyanopyrazoles 4 possessed micromolar and submicromolar inhibitory activity against cytosolic (hCA I and II) and transmembrane (hCA IX and XII). The authors hypothesized that compound 4 binds to the enzyme in a manner similar to the recently reported polyamine spermine inhibitors which anchor to the zinc-coordinated water/hydroxide ionCitation12. However, the mechanism of inhibitory action of 4 must be confirmed by X-ray crystallography of inhibitor-protein complexes.
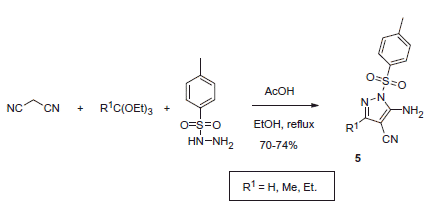
Furthermore, as suggested by Alp et al.Citation17, N-sulfonyl pyrazoles akin to 4 can act as CAIs by interacting with the coumarin-binding site at the entrance of the cavity. This mode of action was suggested for N-tosyl-substituted aminocyanopyrazoles 5, synthesized by a similar reaction involving tosyl hydrazine (Scheme 2).
These compounds also demonstrated inhibitory properties against hCA I, II, IX and XII with Ki’s in the range of 10−6–10−7 μM. Their hypothetical mechanism of CA inhibition also requires further investigation.
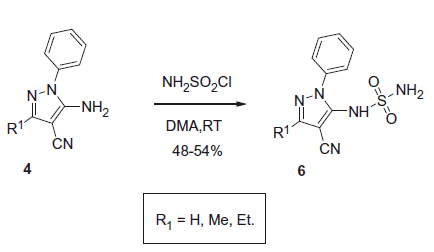
N-Sulfonamide derivatives 6 generated from 4 via the reaction with sulfamoyl chloride (Scheme 3) inhibited medicinally relevant isoforms of hCA most probably due to the canonical sulfonamide-Zn binding mechanismCitation18.
Ki values of sulfonamide derivatives 6 were found to be in the low nanomolar range. Best representatives of each CA inhibitor chemotype generated by Allouche et al.Citation16 are shown in .
Figure 1. Most potent CA inhibitors reported by Allouche et alCitation16.
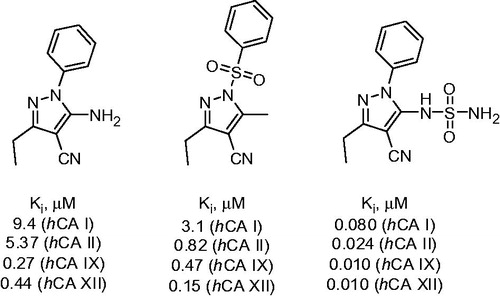
As it can be seen from these Ki values, the compounds synthesized via the multicomponent pyrazole synthesis demonstrated significant inhibitory activity, especially toward membrane-bound hCA isoforms IX and XII.
Dicarbonyl derivatives of methylaminobenzene-sulfonamide
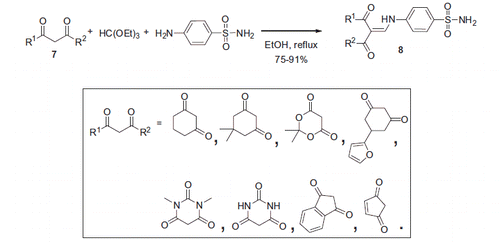
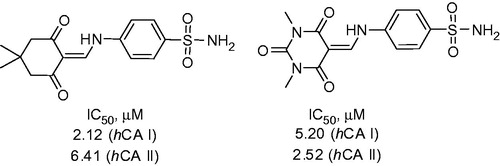
Dimirci and colleaguesCitation19 synthesized a series of 1,3-dicarbonyl 4-(N-methylamino)benzene sulfonamide (sulfanilamide) derivatives 8 using multicomponent chemistry. Eight novel sulfonamides were prepared from sulfanilamide, triethyl orthoformate and dicarbonyl compounds 7. Reaction in ethanol under heating furnished 8 in high yields (Scheme 4).
Sulfonamides 8 were assayed in CO2 hydration assay and their IC50 values toward cytosolic hCA I and II isoforms were determined to be in micromolar region. The most active compounds derived from dimedone and N,N-dimethylbarbituric acid are shown in .
Figure 2. Most potent inhibitors of hCA I and hCA II, respectively, reported by Dimirci et alCitation19.
Chromone-based sulfonamides
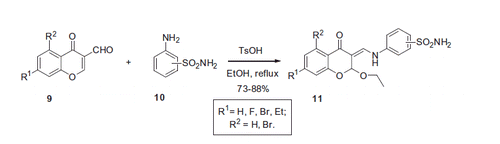
Sulfonamides incorporating a chromone moiety were recently reported to possess considerable activity as CAIs by al-Rashida et al.Citation20 The authors reported the formation of enamine adducts 11 by condensation of 3-formylchromones 9 with 3- and 4-amino benzenesulfonamides 10 in refluxing ethanol (with incorporation of the latter in the product’s molecule). Parallel to formation of the Shiff bases in reaction of amine with aldehyde group, nucleophilic attack at the electron-deficient C-2 atom took place resulting in 2-ethoxy-3-enamine-chromone derivatives in high yields (Scheme 5).
Inhibitory properties of 11 were assessed against bovine cytosolic CA. The assay was based on esterase activity of carbonic anhydrase (p-nitrophenyl acetate ester hydrolysis). Compound 11 exhibited high activity, being micromolar bCA inhibitors (i.e. comparable to the reference drug – acetazolamide displaying IC50 of 1.13 μM in the esterase assay). Although the CO2 hydrating and the ester hydrolyzing sites of CA show some similarity, the inhibition of the CA esterase activity demonstrates poor CO2 hydration activity. Therefore, leads showing activity in the ester hydrolysis assay should be cautiously considered as true CAIs. Taking this into account, we will only mention, that IC50’s of compound 11 were determined to be between 4.13 and 29.12 μM for bCA esterase activity, which is quite weak.
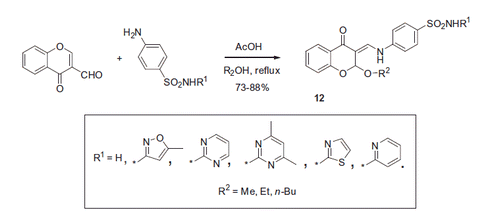
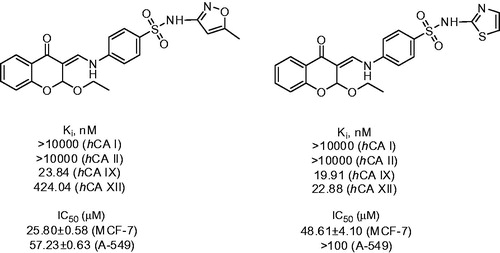
In 2015, Awadallah et al.Citation21 reported a similar reaction performed in methanol, ethanol and n-butanol, resulting in corresponding 2-alkoxy derivatives 12. Acetic acid was used as catalyst (Scheme 6).
Compounds 12 synthesized by this group were tested for inhibition of CA-catalyzed CO2 hydration in the classical stopped-flow kinetics assay and demonstrated selective inhibition of transmembrane tumor-associated isozymes IX and XII, with most promising Ki’s in the nanomolar range. Moreover, the most potent compounds from this series were tested in vitro against MCF-7 breast cancer and A-549 lung cancer cell lines and demonstrated fairly potent cytotoxic activity with IC50’s in micromolar range ().
Figure 3. Biological activity data for the lead compound 12 reported by Awadallah et alCitation21.
Some of compounds reported in this study also showed proapoptotic activity. Taking these results into account, the central role of CA inhibition in the observed cytotoxic effect was proposed. The high selectivity of the lead compounds toward hCA IX and XII (and no activity toward off-target cytosolic hCA I and II isoforms) makes them promising leads for further investigation.
Biginelli reaction of urea derivatives in the synthesis of CAIs
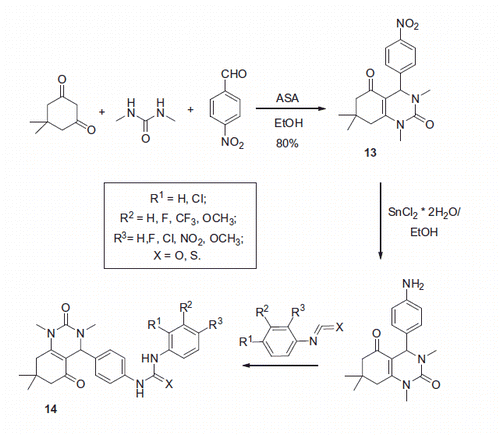
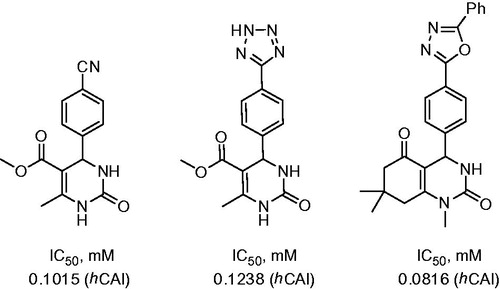
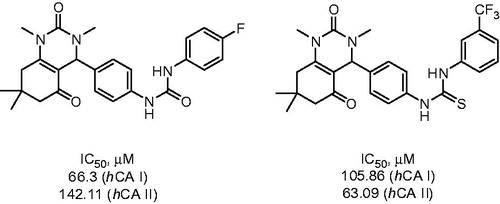
Celik et al.Citation22 employed a Biginelli reaction to prepare aryl-substituted dihydropyrimidines in order to study their CA inhibitory properties. Reaction of dimedone with dimethylurea and p-nitrobenzaldehyde was carried out in the ethanol in the presence of aluminia sulfuric acid (ASA) at 90 °C and afforded 13 in 80% yield. The nitro group of compound 13 was reduced with tin (II) chloride in ethanol and the resulting aniline was treated with sets of isocyanates or isothiocyanates in toluene at 60 °C resulting in 14 (Scheme 7).
The inhibitory properties of 14 toward hCA I and II were evaluated via CO2 hydration assay. IC50’s were found to be in the 66–198 μM range. Possible mechanism of inhibitory action of 14 proposed by the authors involved binding to the Zn-coordinated water molecule/hydroxide ion. However, this hypothesis remains to be verified by X-ray crystallographic structures of the respective inhibitor–protein complexes. The most potent inhibitors identified among nine compounds prepared are shown in .
Figure 4. Inhibitory properties of exemplary urea and thiourea derivatives reported by Celic et alCitation22.
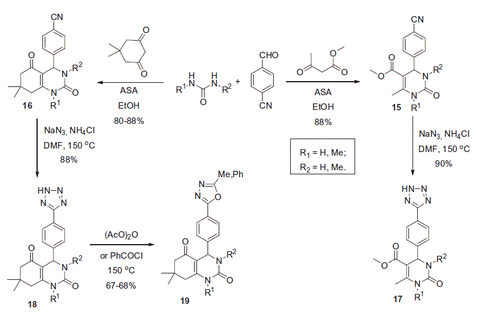
In the follow-on study, Celik and coworkers employed p-cyanobenzaldehyde in a similar reaction with ethyl acetoacetate or dimedone (Scheme 8).Citation23 Resulting compounds 15 and 16 were transformed into tetrazolyl derivatives 17 and 18 via reaction with sodium azide in DMF. Subsequent treatment of 18 with acetic anhydride or benzoyl chloride at 150 °C provided 1,3,4-oxadiazolyl substituted compound 19.
The IC50 values of compounds 15–19 toward hCA I were found to be in submillimolar region, i.e. the compounds displayed very low inhibitory activity (). Authors proposed that the mechanism of CA inhibition could be similar to that of coumarins (i.e. anchoring to the binding site at the entrance of the catalytic cavityCitation10). Again, such a hypothesis should be further substantiated by crystallography studies of the respective inhibitor-protein complexes. No inhibition data against other isoforms of hCA (besides hCA I) were reported for compounds 15–19.
Biginelli-type reaction involving phtalazine in the synthesis of CAIs
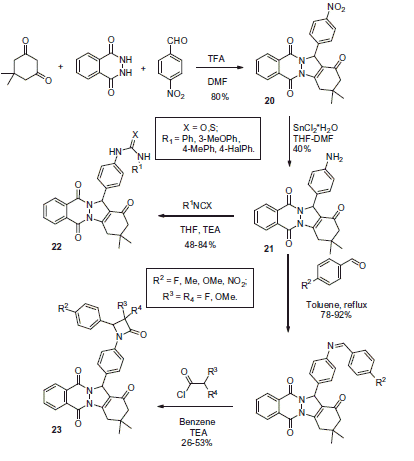
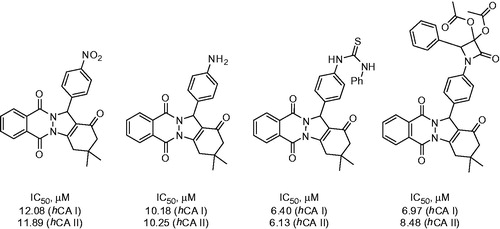
Beber and colleaguesCitation24,Citation25 from Sakaraya University reported the use of modified Biginelly-type reaction employing phtalazine as a 1,2-nucleophile in order to obtain indazolophtalazinetrione 20. This scaffold provided convenient access to two types of CA inhibitor, such as (thio)urea derivatives 22 and β-lactam phtalazines 23. The nitro group of 20 was reduced with tin (II) chloride. Resulting amino derivative 21 reacted with isocyanates or thioisocyanates (resulting in 22). Alternatively, it was converted to Schiff bases on treatment with aromatic aldehydes following azeotropic removal of water and the imines were reacted with ketenes generated from acetyl or acetoxyacetyl chloride, which led to moderate yields of 23 (Scheme 9).
Compound 21 as well as its (thio)urea (22) and β-lactam (23) derivatives reported by Beber were evaluated in hCA I and II hydratase inhibition assay. IC50 values were found in the range from 6.13 to 24.84 μM. The most potent compounds from this series are shown in .
Figure 6. Most active compounds of 28 phtalazine derivatives reported by Beber et alCitation24,Citation25.
Compounds 20–23 contain no traditional pharmacophores which are known to endow compounds with CA inhibitory activity. This suggested a novel mechanism of inhibition. It was hypothesized that the phtalazine scaffold is capable of binding at the entrance of CA catalytic cavity, similarly to coumarine derivatives. Urea and thiourea moieties had been reported to anchor Zn-coordinated waterCitation26 and β-lactams supposed to be capable of binding directly to the catalytic Zn ionCitation27. However, detailed mechanism of the CA inhibition by compounds 20–23 remains to be elucidated.
Multicomponent synthesis of thiazolidine-based sulfonamides
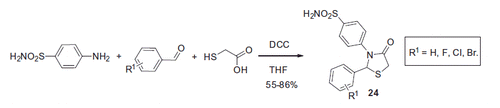
Suthar and coworkersCitation28 reported an efficient synthesis of arylthiazolidine substituted benzenesulfonamides 24 via the reaction of sulfanilamide with aromatic aldehydes and thioglycolic acid in the presence of dicyclohexylcarbodiimide (DCC) in THF (Scheme 10). hCA I, II and IX inhibitory properties of the compounds thus generated were studied via the stopped flow CO2 hydratase assay. Compound 24 demonstrated nanomolar inhibition of hCA IX and pronounced selectivity toward this transmembrane CA isoform over cytosolic isoforms hCA I and II. Ki values of studied compounds were in the range 112.3–1247.9 nM, 28.4–135.5 nM and 2.2–53.1 nM against hCA I, II and IX, respectively ().
Figure 7. Leading compound reported by Suthar et alCitation28.
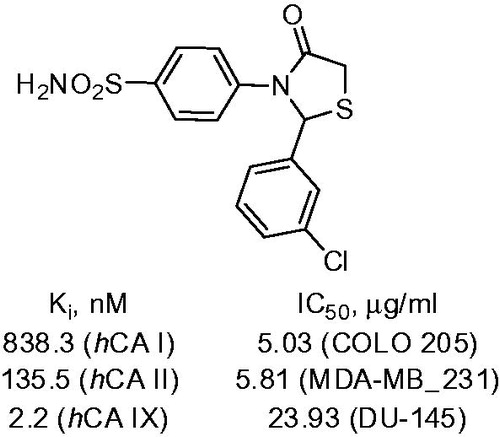
Upon evaluation of the compounds’ inhibitory properties toward CAs, their cytotoxic activity was determined in vitro against COLO-205, MDA-MB-231 and DU-145 human carcinoma cell lines. For the most promising compounds, the anticancer activity was further investigated in vivo. Compound 24 displayed cytotoxic and in vivo antitumor activity. The mechanism of antitumor activity was assumed to involve carbonic anhydrase inhibition. This assumption was supported with CA inhibitory activity for the most cytotoxic compounds as well as molecular docking studies.
Hantzsch-type reaction in synthesis of acridine-based sulfonamides
The Kaya group from Dumlupinar University investigated acridine-based sulfonamides 25 synthesized via a multicomponent Hantzsch reactionCitation29–32 (Scheme 11) as CAIs.
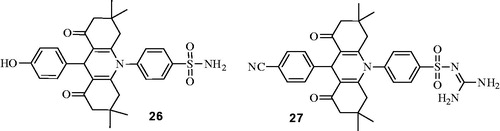
The exemplary compounds from this series (26 and 27) clearly are rather bulky compounds and they did not exert significant inhibition of the CA-catalyzed CO2-bicarbonate interconversionCitation29,Citation30 and only suppressed the esterase activity of hCA I and hCA II (in their earlier studies this group used p-nitrophenylacetate based esterase assay in order to test inhibitory properties of compounds) (). Based on these data, the authors drew a conclusion that these acridine-based sulfonamides have affinity to the enzyme.
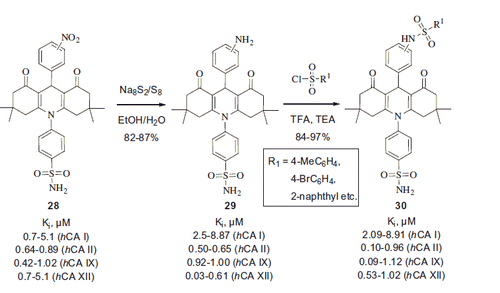
In subsequent studiesCitation31, closely related 3- and 4-nitrophenyl-substituted acridine derivatives 28 were reduced to give the respective anilines 29 and ensuing treatment with sulfonyl chlorides afforded corresponding bis-sulfonamides 30. Compounds 28–30 were tested via classical stopped flow CO2-hydratase assay and were found to be micromolar hCA I and low nanomolar hCA II, IX and XII inhibitors (Scheme 12). Interestingly, potent CA inhibitors 28–30 appear quite similar to compounds 25 which did not inhibit the CA activity in the CO2 hydration assay. Although the reasons for the striking difference is not clear in the case of nitrophenyl (28) and aminophenyl (29) derivatives, the presence of the pharmacophoric secondary sulfonamide moietyCitation18 in 30 was likely responsible for the observed high potency of the latter.
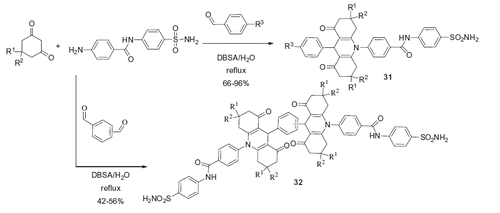
Compound 31 incorporating an amide linker between the benzenesulfonamide and the acridine moieties was prepared using a similar Hantzsch methodologyCitation32. When m- and p-phthalic dialdehyde was used (in combination with 2 equivalent of other components), bis-acridines 32 were obtained in good yields (Scheme 13).
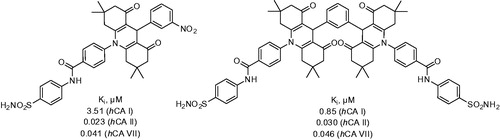
Both 31 and 32 manifested themselves as submicromolar to micromolar inhibitors of hCA I (Ki range 0.16–9.64 μM) and also inhibited hCA II and brain-associated hCA VII with the Ki range of 0.004–0.499 μM. The remarkable potency of representative compounds from this series () compared to that of 28–30 well illustrated the efficiency of tail approach in designing novel sulfonamide CA inhibitorsCitation33.
In summary, the Kaya group evaluated CA inhibitory properties of 55 acridine-based sulfonamides, and reported a range of novel inhibitors possessing micromolar to nanomolar-level potency.
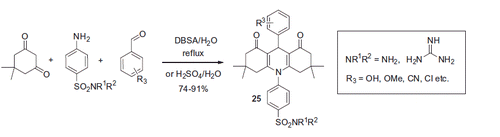
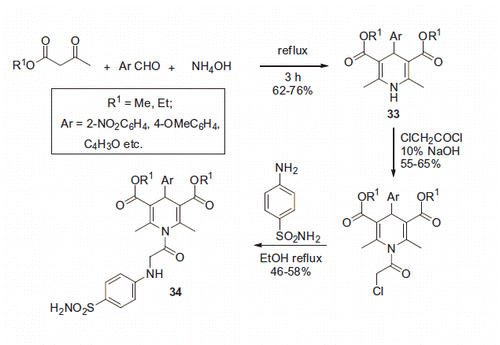
The use of a similar reaction of 1,3-dicarbonyl compounds with aromatic aldehydes and ammonia hydroxide was reported by Subudhi and coworkersCitation34. The process was conducted in refluxing ethanol furnishing 1,4-diydropyridines 33 in 67–76% yields. Several of the resulting 1,4-dihydropyridines 33 were decorated with a benzenesulfonamide moiety through the amide linkage, via sequential treatment with chloroacetyl chloride under basic conditions followed by sulfanilamide in refluxing ethanol (Scheme 14).
Unfortunately, the Subudhi team did not undertake evaluation of compound 34 for their CA inhibitory properties. The compounds were tested for anticonvulsant activity in mice. The observed anticonvulsant properties of 34 were assumed to be due to carbonic anhydrase inhibition, based on the presence of the primary sulfonamide pharmacophore. However, this conclusion should be taken with a due degree of caution since the compounds were not tested against carbonic anhydrase.
Nonsymmetrical Hantzsch-type reaction in synthesis of CAIs
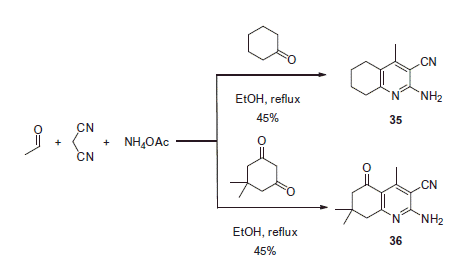
A nonsymmetrical Hantzsch reaction (i.e. a multicomponent reaction employing two different carbonyl components) was used in the synthesis of CAIs. In particular, a series of novel sulfonamides was synthesized from substituted 2-aminonicotinonitriles 35 and 36 by Ghorab and coworkers (Scheme 15)Citation35,Citation36.
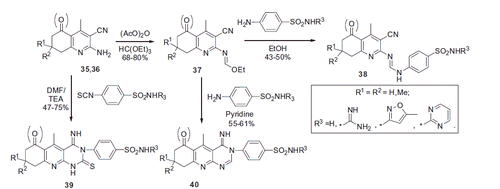
The multicomponent reaction of acetaldehyde with malononitrile, cyclohexanone (or dimedone) and ammonia acetate proceeded in refluxing ethanol affording 35 or 36 in modest yields. 2-Aminonicotinonitriles 35 and 36 were subjected to further modification in order to generate sulfonamide derivatives 38–40 (Scheme 16).
Sulfonamide 41 was obtained in a similar Hatzsch-type reaction using sulfanilamide in lieu of ammonia. It was used as a core building block for further modifications at the 2-amino-3-cyano-1,4-dihydropyridine moiety as illustrated by the examples in Scheme 17.
Scheme 17. Examples of derivatization of 2-amino-3-cyano-1,4-dihydropyridine moiety in sulfonamide 41.
Cytotoxic activity of sulfonamides thus synthesized was evaluated in vitro against human breast cancer cell line and the respective IC50 values were determined. Again, it was only hypothesized that the observed cytotoxic action was due to the compounds being CA inhibitors. However, 38–47 were not assayed against carbonic anhydrase, and their CA inhibitory properties remain to be substantiated by obtaining solid biochemical data. Cytotoxic (cytostatic or cytocydic) activity of small molecules can be exerted via a myriad of different targets and mechanismsCitation37 and compounds 38–47, too, can act via a mechanism completely different from CA inhibition (this possibility is perhaps underscored by the similar cytotoxic activity of primary and secondary sulfonamides, whereas the former are known to be a lot more potent CA inhibitors compared to the latter.
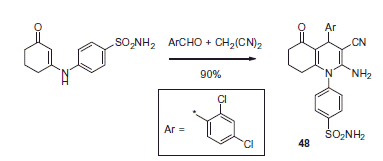
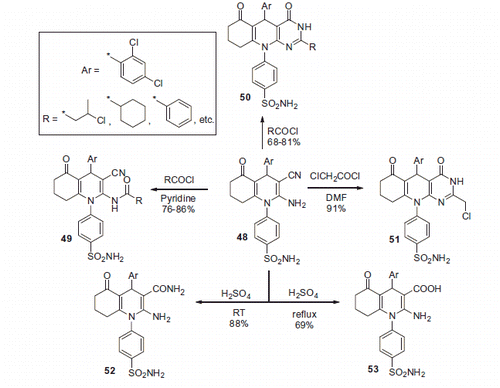
Attesting to the popularity of the 1,4-dihydropyridine scaffold in the CAI design, core 2-aminonicotinonitrile 48 was synthesized on multigram scale via a Hantzsch reaction by al-Said et al.Citation38 (Scheme 18).
Treatment of 48 with various reagents afforded a wide range of novel sulfonamide derivatives. In particular, reaction of 48 with acid chlorides in pyridine afforded 49, while in the absence of the basic solvent, a cyclization took place, furnishing 50. A similar cyclization occurred when 48 was treated with chloroacetyl chloride, affording 51. Treatment of 48 with concentrated sulfuric acid at room temperature provided primary carboxamide derivative 52, while raising the reaction temperature to reflux led to a complete hydrolysis and formation of carboxylic acid 53 (Scheme 19).
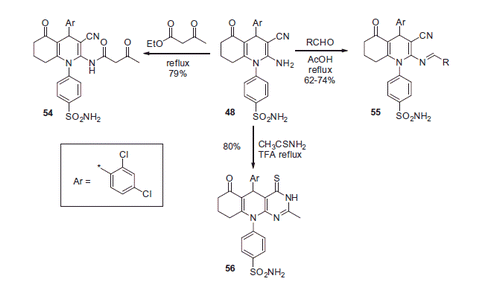
Several other benzenesulfonamide derivatives 54–56 were synthesized through treatment of 48 with various carbonyl compounds as well as thioacetamide or ethyl acetoacetate (Scheme 20).
In vitro cytotoxic activity of 22 synthesized compounds was evaluated and the principal mechanism of the observed cytotoxic action was assumed to involve CA inhibition. However, this assumption was only supported by computer modeling data, and the CA inhibitory activity of reported sulfonamides was not evaluated in a biochemical assay.
Mannich reaction in synthesis of CAIs
Mannich aminomethylation reaction has been amply used in synthesis of different types of CA inhibitors. Alkylamino groups can substantially influence inhibitor’s affinity to the enzyme active site and result in potent inhibitors. In addition to that, such “solubilizing side chains” can endow the resulting inhibitors with pronounced isoform selectivity.
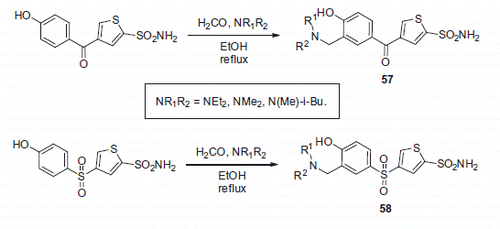
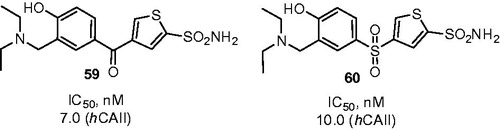
The aspect of increased potency is nicely illustrated by Mannich adducts 57 and 58 that were synthesized from corresponding 4-hydroxybenzoyl and 4-hydroxybenzenesulfonyl thiophene-2-sulfonamides by Hartman et al.Citation39 (Scheme 21). Compounds 57 and 58 (as well as their furan analogs) demonstrated inhibitory activity against hCA II and their IC50 values were found to be in the nanomolar range. Synthesized compounds also possessed remarkable results being tested ex vivo for their ability to penetrate the albino rabbit eye and to inhibit CA II in a homogenate of the iris-ciliary body. In addition, when evaluated in vivo in a rabbit model of ocular hypertension, most potent compounds (59 and 60) exhibited pronounced activity as intraocular pressure lowering agents, while, expectedly, demonstrating improved solubility compared to the starting phenolic compounds (). Compound 59 was found to be a lead candidate for further development as a potent topical agent for glaucoma treatment.
Figure 10. Most active Mannich adducts, reported by Hartman et alCitation39.
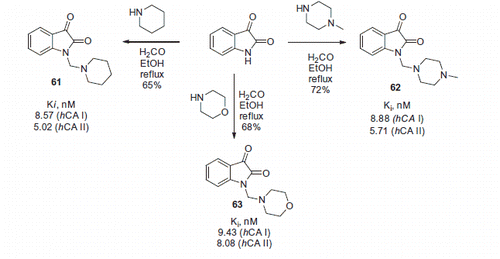
Ozgun and coworkersCitation40 recently reported isatin Mannich bases 61–63, bearing piperidine, morpholine or N-methylpiperazine appendages (Scheme 22). Compounds 61–63 were found to possess nanomolar inhibitory activity against cytosolic hCA I and hCA II isoforms.
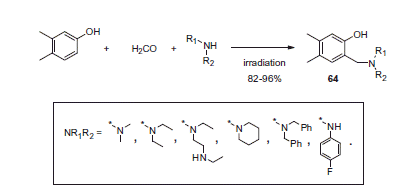
Büyükkidan et al.Citation41 synthesized 3,4-dimethylphenole Mannich derivatives 64 (Scheme 23) and investigated their inhibitory properties toward CA.
While none of the compounds (64) investigated inhibited CA-catalyzed CO2-to-bicarbonate interconversion, some of them were found to selectively inhibit the esterase activity of hCA II over hCA I. The observed CA esterase activity suppression was relatively weak, with Ki values ranging between 6.3 and 96.4 μM. Interestingly, the esterase activity was found to be potentiated, and not inhibited, by some of the analogs from series 64.
Among the CA inhibitors synthesized by Chow and colleaguesCitation42, two particularly potent Mannich bases 65 and 66 were reported. These compounds were found to strongly inhibit hCA II inhibition with IC50 values in nanomolar range ().
Figure 11. Mannich adducts reported by Chow et alCitation42.
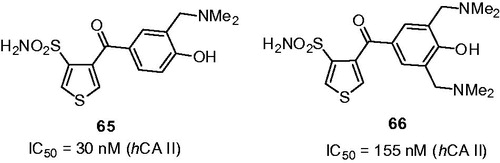
In contrast to compounds 59–60 where introduction of the aminomethyl side chains into the phenolic sulfonamide core resulted in an increased potency and solubility, Mannich derivatives 65 and 66, disappointingly, turned out to be less potent than the starting phenol-containing sulfonamides and, therefore, were not advanced into further ex vivo studies.
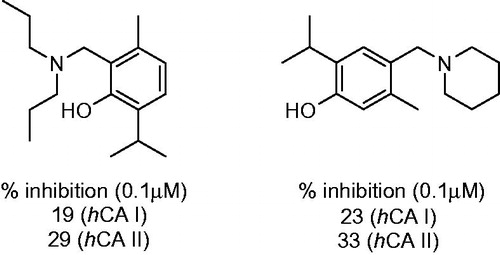
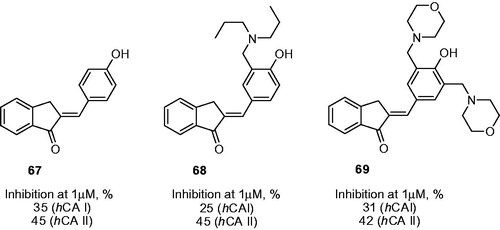
A case where introduction of the aminomethyl appendage did not seem to significantly influence inhibitory properties of the starting (phenolic) compound is illustrated by a series of phenolic Mannich bases including mono- and bis-dialkylamino dervatives such as 68 or 69, reported by Yamali et al.Citation43 These compounds possessed similar or slightly lower inhibitory properties compared to starting 67 as expressed in the single-concentration data reported ().
Figure 12. Percentage of inhibition data for progenitor compound 67 and its most potent Mannich derivatives 68–69.
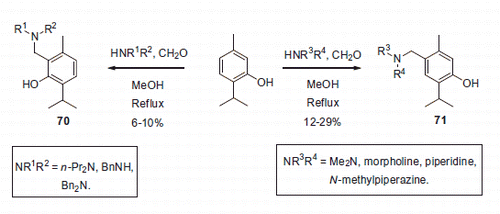
Similarly to compound 64 (vide supra), several thymol derivatives 70–71 were recently prepared via the Mannich reaction and evaluated for CA inhibition by Gul et al.Citation44. The position of the aminomethyl side chain was influenced by the nature of the secondary amine employed in the Mannich reaction and the resulting compounds were isolated in rather modest yields (Scheme 24).
The seven compounds synthesized within series 70–71 were found to possess substantial cytotoxic properties but demonstrated only a weak inhibition of hCA I and II (). Therefore, in their cytotoxic properties observed in the course of in vitro cell viability studies were assumed to be due to cellular mechanisms are than CA inhibition.
Conclusion
Multicomponent chemistry (primarily, the Biginelli, Hantzsch and Mannich reactions) is a powerful tool for generating arrays of novel compounds for biological evaluation. It has found significant utility in the construction of novel inhibitors of carbonic anhydrase. However, it is clear that a large body of literature reports scaffolds amenable via multicomponent approach, which contain the zinc-binding primary sulfonamide pharmacophore. For some of the compounds reported, CA inhibition was confirmed in a biochemical assay (through observation of the esterase or CO2 hydratase activity). However, with disappointing frequency, compounds are evaluated in phenotypical cellular assays (such cancer cell viability) and the CA inhibition is simply assumed to be the principal mechanism of the observed biological action. Such “CAIs” should be considered with a due degree of caution and should be reevaluated in an accurate assay using an isolated CA target. Likewise, it is clear that systematic studies of the structure-activity relationships around scaffolds amenable by the modern multicomponent reactions (such as the isocyanide-based Ugi reaction,Citation45 the Castagnoli–Cushman lactam synthesisCitation46 and related reactions) represent a significant knowledge void in the area of CA inhibitors. Directing research efforts toward this area of medicinal chemistry research will be a timely, prudent and much needed undertaking.
Declaration of interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by the Russian Scientific Fund [project grant 14-50-00069].
References
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229
- Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–73
- Vullo D, Innocenti A, Nishimori I, et al. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides – a new target for the design of antitumor and antiglaucoma drugs? Bioorg Med Chem Lett 2005;15:963–9
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Boehm HJ, Flohr A, Stahl M. Scaffold hopping. Drug Discov Today Technol 2004;1:217–24
- Supuran CT. Structure-based drug discovery of carbonic anhydrase Inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Maresca A, Scozzafava A, Supuran CT. 7,8-Di substituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 2010;20:7255–8
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: structural implications for substrate association. J Am Chem Soc 1994;116:3659–60
- Carta F, Temperini C, Innocenti A, et al. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J Med Chem 2010;53:5511–22
- Doemling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 2006;106:17–89
- Biggs-Houck JE, Younai A, Shaw JT. Recent advances in multicomponent reactions for diversity-oriented synthesis. Curr Opin Chem Biol 2010;14:371–82
- Gore RP, Rajput AP. A review on recent progress in multicomponent reactions of pyrimidine synthesis. Drug Invent Today 2013;5:148–52
- Allouche F, Chabchoub F, Carta F, Supuran CT. Synthesis of aminocyanopyrazoles via a multi-component reaction and anti-carbonic anhydrase inhibitory activity of their sulfamide derivatives against cytosolic and transmembrane isoforms. J Enzyme Inhib Med Chem 2013;28:343–9
- Alp C, Maresca A, Alp NA, et al. Secondary/tertiary benzenesulfonamides with inhibitory action against the cytosolic human carbonic anhydrase isoforms I and II. J Enzyme Inhib Med Chem 2012;28:294–8
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60
- Demirci T, Arslan M, Bilen Ç, et al. Synthesis and carbonic anhydrase inhibitory properties of 1,3-dicarbonyl derivatives of methylaminobenzene-sulfonamide. J Enzyme Inhib Med Chem 2014;29:132–6
- al-Rashida M, Ashraf M, Hussain M, et al. Discovery of new chromone containing sulfonamides as potent inhibitors of bovine cytosolic carbonic anhydrase. Eur J Med Chem 2015;19:3367–71
- Awadallah FM, El-Waei TA, Hanna MM, et al. Synthesis, carbonic anhydrase inhibition and cytotoxic activity of novel chromone based sulfonamide derivatives. Eur J Med Chem 2015;96:425–35
- Celik F, Arslan M, Yavuz E, et al. Synthesis and carbonic anhydrase inhibitory properties of novel 1,4-dihydropyrimidinone substituted diarylureas. J Enzyme Inhib Med Chem 2014;29:18–22
- Celik F, Arslan M, Kaya MO, et al. Synthesis and carbonic anhydrase inhibitory properties of tetrazole- and oxadiazole substituted 1,4-dihydropyrimidinone compounds. Artif Cells Nanomed Biotechnol 2014;42:58–62
- Berber N, Arslan M, Yavuz E, et al. Synthesis and evaluation of new phthalazine urea and thiourea derivatives as carbonic anhydrase inhibitors. J Chem 2013;2013:742178
- Berber N, Arslan M, Yavuz E, et al. Synthesis and evaluation of new phthalazine substituted β-lactam derivatives as carbonic anhydrase inhibitors. Russ J Bioorg Chem 2015;41:414–20
- Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902
- Koike T, Takamura M, Kimura E. Role of Zn(II) in β-lactamase II: a model study with a Zn(II)-cyclen complex. J Am Chem Soc 1994;116:8443–9
- Suthar SK, Bansal S, Lohan S, et al. Design and synthesis of novel 4-(4-oxo-2-arylthiazolidin-3-yl)benzenesulfonamides as selective inhibitors of carbonic anhydrase IX over I and II with potential anticancer activity. Eur J Med Chem 2013;66:372–9
- Kaya M, Basar E, Çakir E, et al. Synthesis and characterization of novel dioxoacridine sulfonamide derivatives as new carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:509–14
- Yeşildağ I, Ulus R, Başar E, et al. Facile, highly efficient, and clean one-pot synthesis of acridine sulfonamide derivatives at room temperature and their inhibition of human carbonic anhydrase isoenzymes. Monatsh Für Chemie 2014;145:1027–34
- Esirden I, Ulus R, Aday B, et al. Synthesis of novel acridine bis-sulfonamides with effective inhibitory activity against the carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem 2015;23:6573–80
- Ulus R, Yeşildağ I, Tanc M, et al. Synthesis of novel acridine and bis acridine sulfonamides with effective inhibitory activity against the cytosolic carbonic anhydrase isoforms II and VII. Bioorg Med Chem 2013;21:5799–05
- Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyaminopolycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–76
- Subudhi BB, Panda PK, Swain SP, Sarangi P. Synthesis, characterization and anticonvulsant activity evaluation of some 1,4-dihydropyridines and 3,5-(substituted)oxycarbonyl-1,4-dihydro-2,6-dimethyl-N-[2-(4-sulfamoylphenylamino)-acetyl]-4-(substituted)pyridines. Acta Pol Pharm Drug Res 2009;66:147–53
- Ghorab MM, Ragab FA, Hamed MM. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem 2009;4;4211–17
- Ghorab MM, Ragab FA, Heiba HI, et al. In vitro anticancer screening and radiosensitizing evaluation of some newquinolines and pyrimido[4,5-b]quinolines bearing a sulfonamide moiety. Eur J Med Chem 2010;45:3677–84
- Hoelder S, Clarke PA, Workman P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol Oncol 2012;6:155–76
- Al-Said MS, Ghorab MM, Al-Dosari MS, Hamed MM. Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem 2011;46:201–7
- Hartman GD, Halczenko W, Smith RL, et al. 4-Substituted thiophene- and furan-2-sulfonamides as topical carbonic anhydrase inhibitors. J Med Chem 1992;35:3822–31
- Ozgun DO, Yamali C, Gul HI, et al. Inhibitory effects of isatin Mannich bases on carbonic anhydrases, acetylcholinesterase, and butyrylcholinesterase. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2016.1149479
- Büyükkidan N, Büyükkıdan B, Bülbül M, et al. Synthesis and characterization of phenolic Mannich bases and effects of these compounds on human carbonic anhydrase isozymes I and II. J Enzyme Inhib Med Chem 2013;28:337–42
- Chow K, Lai R, Holmes JM, et al. 5-Substituted 3-thiophenesulfonamides as carbonic anhydrase inhibitors. Eur J Med Chem 1996;31:175–86
- Yamali C, Tugrak M, Gul HI, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2015.1126715
- Gul HI, Yamali C, Asiye TY, et al. Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. doi: 10.3109/14756366.2016.1140755
- Ivachtchenko AV, Ivanenkov YA, Kysil VM, et al. Multicomponent reactions of isocyanides in the synthesis of heterocycles. Russ Chem Rev 2010;79:787–817
- Krasavin M, Dar'in D. Current diversity of cyclic anhydrides for the Castagnoli-Cushman-type formal cycloaddition reactions: prospects and challenges. Tetrahedron Lett 2016;57:1635–40