Abstract
Carbonic anhydrase (CA) enzymes have been shown to play an important role in ion transport and in pH regulation in several organisms. Despite this information and the wealth of knowledge regarding the significance of CA enzymes, few studies have been reported about bee CA enzymes and the hazardous effects of chemicals. Using Apis mellifera as a model, this study aimed to determine the risk of pesticides on Apis mellifera Carbonic anhydrase enzyme (Am CA). CA was initially purified from Apis mellifera spermatheca for the first time in the literature. The enzyme was purified with an overall purification of ∼35-fold with a molecular weight of ∼32 kDa. The enzyme was then exposed to pesticides, including tebuconazole, propoxur, carbaryl, carbofuran, simazine and atrazine. The six pesticides dose-dependently inhibited in vitro AmCA activity at low micromolar concentrations. IC50 values for the pesticides were 0.0030, 0.0321, 0.0031, 0.0087, 0.0273 and 0.0165 μM, respectively. The AmCA inhibition mechanism of these compounds is unknown at this moment.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) is a zinc metalloenzymes, which regulates pH and CO2 levels in all living organismsCitation1,Citation2. A number of different CA isozymes have been described in higher vertebrates, whereas in other animals, e.g. insects, these enzymes are by far less investigated. Among the vertebrate CA isozymes, there are cytosolic (such as CA I, CA II, CA III, CA VII), membrane-bound (CA IV, CA IX, CA XII and CA XIV), mitochondrial (CA VA and CA VB), secretory forms (CA VI) and several acatalytic forms (CA VIII, CA X and CA XI)Citation3.
The physiological function of the CA isozymes is to facilitate the interconversion of CO2 and HCO3; therefore, they play key roles in diverse processes, such as physiological pH control and gas balance, calcification and photosynthesis. In addition, CA plays an important role in ion transport and pH regulation in eye, kidney, central nervous system (CNS) and inner earCitation4.
Pesticides are chemical substances used as biological agent, antimicrobial, disinfectant or device against any pest. Some of them are persistent organic pollutants and contribute to soil and contamination. They are also one of the considerable causes for plant pollutionCitation5. In many cases, pesticides and fungicides can interfere into rain water, irrigation water or river, plants and may be hazardous for specific enzymes. It is well known that enzymes catalyze almost all chemical reactions in the metabolism of the living systems, and many chemical substances including pesticides, fungicides, drugs and metal ions influence metabolism at low concentrations by decreasing or increasing enzyme activitiesCitation6.
Tebuconazole, propoxur, carbaryl, carbofuran, simazine and atrazine are well-known to interfere with a number processes since they have neurotoxic, hematotoxic, genotoxic, hepatic and renal effects on vertebrate. Nevertheless, little is known about their effects on specific enzymes in organisms especially honey bee. For this reason, we aimed in the current study to analyze the interactions between some pesticides and fungicides and carbonic anhydrase enzymes. To this end, we selected Apis mellifera as a model animal. We also aimed in this study to purify and characterize honey bee spermatheca CA enzyme for the first time. using affinity chromatography.
Materials and methods
Chemicals
Sepharose-4B, protein assay reagents, and chemicals for electrophoresis were purchased from Sigma–Aldrich (St. Louis, MO). All other chemicals were of analytical grade and obtained from Merck (Darmstadt, Germany).
Preparation of the homogenate
Apis mellifera samples were washed three times with 50 mM Tris-HCl +0.1 M Na2SO4 (pH 8.0). And each of bees were homogenized by liquid nitrogen, transferred to the same buffer and centrifuged at 4 °C, 15 000 g for 60 min. Supernatant was used in further studies.
Purification of carbonic anhydrase from honey bee by affinity chromatography
CNBr activated Sepharose-4B was washed with ddH2O. After that, aniline was attached to the activated gel as a spacer arm and finally diazotized sulfanilamide clamped to the para position of aniline molecule as ligand.
The homogenate was applied to the prepared Sepharose 4B-tyrosine-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The AmCA was eluted with 1M NaCl/25 mM Na2HPO4 (pH 6.3). All procedures were performed at 4 °CCitation7.
Hydratase activity determination
Enzyme activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation8. CO2-Hydratase activity as an enzyme unit (EU) was calculated by using the equation (t0–tc/tc), where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Protein determination
Quantitative protein determination was spectrophotometrically measured at 595 nm according to Bradford's methodCitation9, with bovine serum albumin as a standard.
SDS polyacrylamide gel electrophoresis (SDS-PAGE)
The control of enzyme purity was carried out using Laemmli’s procedureCitation10 in 3 and 8% acrylamide concentrations for running and stacking gel, respectively. 10% SDS was added to the gel solution. The gel was stabilized in a solution containing 50% propanol +10% TCA +40% distilled water for 30 min. Staining was performed for about 2 h in a solution of 0.1% Coommassie Brilliant Blue R-250 + 50% methanol +10% acetic acid. Finally, washing was carried out in a solution of 50% methanol +10% acetic acid +40% distilled water until the protein bands were cleared.
In vitro inhibition assays
The effects of increasing concentrations of tebuconazole, propoxur, carbaryl, carbofuran, simazine and atrazine on AmCA activities were determined colorimetrically using CO2-hydratase assay ().
The pesticides were also tested in the hydratase activity assay in triplicate at each concentration used. Different concentrations of pesticides were examined in preliminary assays. Enzyme activities were measured in the presence of different concentrations of tebuconazole, propoxur, carbaryl, carbofuran, simazine, and atrazine. Control enzyme activity in the absence of a pesticide was taken as 100%. For each pesticide, an activity % versus inhibitor concentration tube was drawn using conventional polynominal regression software (Microsoft Office 2000, Excel). Pesticide concentrations that produced 50% inhibition (IC50) were calculated from graphs.
Results and discussion
Here, we isolated the CA enzyme from Apis mellifera spermatheca for the first time. We achieved to purify the enzyme in a single step using affinity chromatography on Sepharose 4B tyrosine-sulfanilamide. The enzyme was purified 35-fold with a recovery ratio of 17.87% compared to the homogenate (). After the sample had completely passed through, the column was washed with 25 mM Tris-HCl/22 mM Na2SO4 buffer whose pH was 8.7. During washing, absorbencies of fractions were spectrophotometerically measured at 280 nm and 348 nm. These values of the absorbance showed that some proteins, bound to the affinity material, have been removed from the column by the washing solutions. Then, the enzyme was eluted with 1 M NaCl/25 mM Na2HPO4 pH 6.3. At the end of the last step, a highly purified enzyme was obtained exhibiting a single band on SDS-PAGE (). We used only one chromatographic technique, Sepharose 4B tyrosine-sulfanilamide affinity chromatography by modification of washing and elution conditions.
Figure 2. SDS-PAGE photograph. Lane 1: truncated β-galactosidase (83 kDa). Lane 2: standard proteins: truncated β-galactosidase (83 kDa), bovine albumin (66 kDa) and bovine carbonic anhydrase (29 kDa). Line 3: AmCA estimated molecular weight 37 kDa.
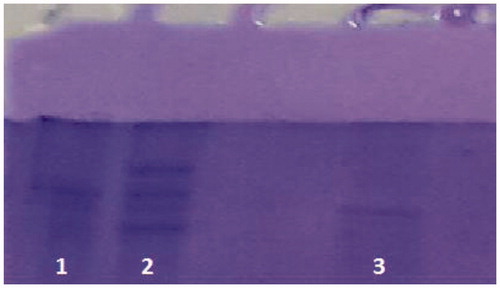
Table 1. Summary of purification procedure of CA from honey bee, AmCA.
The molecular weight was determined to be ∼37 kDa. Similar results have been observed for the enzyme from different sources. For example, human erythrocyte CA is 29 kDa, bovine erythrocyte CA is 29 kDa and European seabass liver CA is 30.2 kDaCitation3–6.
In addition to purification of the enzyme, tebuconazole, propoxur, carbaryl, carbofuran, simazine and atrazine were chosen to investigate their inhibitory effects honey bee spermatheca CA and I50 parameters of these pesticides were determined. Pesticides inhibited the enzyme activity at low concentrations.
Researches on influences of various chemicals on CA enzymes have gained a great attention recentlyCitation2,Citation11–17. For instance, in vitro effect of some heavy metals on enzymes, such as intestinal and branchial carbonic anhydrase and Na+–K+–ATPase, which play a key role in salt and osmoregulation and acid–base balance in the teleost fish, was studied. CA activities in gill and intestinal homogenates were significantly inhibited by heavy metalsCitation18. In another study, freshwater rainbow trout exposed to 10 μg l−1 Ag for 48 h had significantly lower activities of the branchial enzymes Na+/K+ ATPase (85% inhibition) and carbonic anhydrase (28% inhibition). The results suggested that a disturbance of branchial ionoregulation, as a result of inhibition of branchial enzymes involved in ion transport, is the principal mechanism of the physiological toxicity of silver nitrate to freshwater fish. Another study demonstrated that Cl− uptake in P. promelas acclimated to soft water occurs through both a Na+:K+:2Cl− co-transporter and a Cl−/HCO3− exchanger, but is not dependent on carbonic anhydraseCitation19.
CA which is a widespread metalloenzyme, which has previously been purified and characterized from many living organisms including animals. The isozymes of CA play important roles in different tissuesCitation2,Citation11–18. The similarities of CAs from various sources have been determined from their crystal structuresCitation2,Citation11–18. Hundreds of pollutants in the form of metals, acids, bases and other toxic compounds are being added to rivers, seas and the atmosphere a situation which has resulted in the destruction of the natural balanceCitation19. However, we have not found any data on AmCA enzyme in the literature and thus our study includes the investigation of the Apis mellifera CA enzyme for the first time. The inhibition mechanisms of these compounds against this α-CA are not known at this moment, also considering the fact that they do not incorporate functionalities usually present in most classes of reported inhibitorsCitation20–22. It should be also stressed that except Drosophila melanogaster, for which a β- and two α-CAs were described in detailCitation23,Citation24, the investigation of this enzyme superfamily in insects is still on its infancy, with very few literature reports available to date.
Conclusions
CA was purified from Apis mellifera spermatheca by affinity chromatography on Sepharose 4B-tyrosine-sulfanilamide (). SDS-PAGE gels revealed that CA migrated as a single band. The overall purification yield of CA was 17.87%, specific activity was 175 EU/mg protein and purification range was ∼35-fold (). shows the in vitro effects of tebuconazole. Tebuconazole has higher inhibition effects than propoxur, carbaryl, carbofuran, simazine, and atrazine. IC50 values were determined as 0.0030, 0.0321, 0.0031, 0.0087, 0.0273 and 0.0165 μM, for tebuconazole, propoxur, carbaryl, carbofuran, simazine and atrazine, respectively (). Our results showed that pesticides inhibit hbCA activity with rank order tebuconazole > carbaryl > carbofuran > atrazine > simazine > propoxur in in vitro conditions. Our findings indicate these pesticides are potent inhibitors for AmCA enzymes, and might cause undesirable results by disrupting acid–base regulation as well as salt transport, although their inhibition mechanism is unknown at this moment.
Table 2. AmCA inhibition data with studied pesticides (IC50 values), by an esterase assay with 4-nitrophenylacetate as substrate.
Disclosure statement
The authors report no declarations of interest.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:81–168.
- Ekinci D, Kurbanoglu NI, Salamci E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzym Inhib Med Chem 2012;27:845–8.
- Ozdemir ZO, Senturk M, Ekinci D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules. J Enzyme Inhib Med Chem 2013;28:316–19.
- Ekinci D, Cavdar H, Talaz O, et al. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–63.
- Ekinci D, Cavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73.
- Ceyhun SB, Senturk M, Yerlikaya E, et al. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Balaydin HT, Durdagi S, Ekinci D, et al. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012;27:467–75.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54.
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–3.
- Alp C, Ekinci D, Gultekin MS, et al. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis for carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–74.
- Ekinci D, Senturk M, Kufrevioglu OI. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat 2011;21:1831–41.
- Durdagi S, Şentürk M, Ekinci D, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–9.
- Balaydin HT, Soyut H, Ekinci D, et al. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzym Inhib Med Ch 2012;27:43–50.
- Ekinci D, Ceyhun SB, Senturk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8.
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2012;27:365–9.
- Ekinci D, Karagoz L, Ekinci D, et al. Carbonic anhydrase inhibitors: in vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J Enzyme Inhib Med Chem 2013;28:283–8.
- Lionett MG, Giordano ME, Vilella S, Schettino T. Inhibition of eel enzymatic activities by cadmium. Aquat Toxicol 2000;48:561–71.
- Bielmyer GK, Brix KV, Grosell A. Is Cl- protection against silver toxicity due to chemical speciation? Aquat Toxicol 2008;87:81–7.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016; 31:345–60.
- Syrjänen L, Tolvanen M, Hilvo M, et al. Characterization of the first beta-class carbonic anhydrase from an arthropod (Drosophila melanogaster) and phylogenetic analysis of beta-class carbonic anhydrases in invertebrates. BMC Biochem 2010;11:28.
- Syrjänen L, Tolvanen ME, Hilvo M, et al. Characterization, bioinformatic analysis and dithiocarbamate inhibition studies of two new α-carbonic anhydrases, CAH1 and CAH2, from the fruit fly Drosophila melanogaster. Bioorg Med Chem 2013;21:1516–21.