Abstract
Inhibitors of carbonic anhydrase (CA) have been carried out in many therapeutic applications, especially antiglaucoma activity. In this study, we investigated some uracil derivatives (4–12) to inhibit human CA I (hCA I) and II (hCA II) isoenzymes. The KI values of the compounds 4–12 are in the range of 0.085–428 µM for hCA I and of 0.1715–645 µM against hCA II, respectively. It is concluded from the kinetic investigations, all compounds used in the study act as competitive inhibitors with substrate, 4-NPA. Uracil derivatives are emerging agents for the inhibiton of carbonic anhydrase which could be used in biomedicine.
Introduction
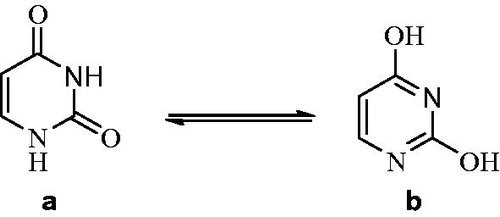
Uracil, one of the pyrimidine bases, is commonly present in ribonucleic acid (RNA)Citation1. It is chemically weak acid and exposed to two tautomeric forms at pH 7.0. This amide-imidic acid tautomeric shifts from lactam which is the amide tautomer to the imidic acid tautomer is referred to as the lactim structure ()Citation2. Uracils are used in the area of drug discovery due to their bioactivities, drug similarity in the perspectives of synthetic accessibility and ability. And it is reported that uracils show anti-viral and anti-tumour characteristics in addition to bactericidal, herbicidal and insecticidal featuresCitation3.
5-Fluorouracil (5-FU) (1) is an anti-tumour agent used for the treatment of cancerous tumours in colon or/and breastCitation4. Though 5-FU as a single agent has gained medical achievement, the molecule has been chemically modified with different processes to create new derivatives. Therefore, modified syntheses can improve their therapeutic indexes due to their well-known side effectsCitation2. 6-Amino-5-chlorouracil (2) and 6-amino-5-bromouracil (3) were the first thymidine phosphorylase inhibitors to be generated. However, their relatively less favourable IC50 values did not allow them to be developed into drug candidatesCitation2.
Carbonic anhydrases (CAs, EC 4.2.1.1) are metalloenzymes involving in numerous vital biochemical/physiological processesCitation5,Citation6 based on reversible hydration/dehydration process of CO2/HCO3-Citation7. The enzyme is found in all living beings of the three domains of lifeCitation8 with six evolutionary gene familiesCitation9. 15 CA isoenzymes encoded by α-CA gene family described in humansCitation10. Some of these human CA isoenzymes such as CA I, II, III, VII and XIII are cytosolic, some forms, CA IV, IX, XII and XIV, are membrane bound, two ones as CA VA and VB are mitochondrial and CA VI is found in saliva. The last three forms of human CAs (CA VIII, X and XI) are determined as noncatalyticCitation5.
The testing of CA inhibitors is carried out for the treatment of clinically important casesCitation11. Many chemical ligands have been used to inhibit CAs such as anionsCitation12, bischalconesCitation13, coumarinsCitation14, benzenesulfonamidesCitation15 and phenolesCitation16. And uracil derivatives have also been carried out to inhibit the catalytic activity of CAsCitation17.
Experimental
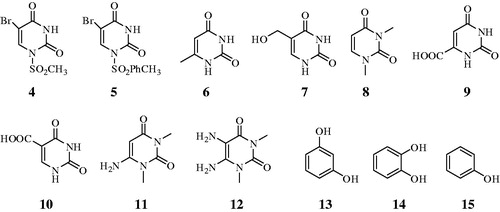
Orotic acid (9), isoorotic acid (10), 6-Amino 1,3-Dimethyluracil (11), 5,6-Diamino 1,3-Dimethyluracil (12), and other chemicals were obtained commercially from Sigma-Aldrich.
Purification of human carbonic anhydrase isozymes by affinity chromatography
Erythrocytes were purified from fresh human blood obtained from the Blood Centre of the Research Hospital at Atatürk University. The blood samples were centrifuged at 1500 rpm for 15 min and the plasma and buffy coat were removed. The red cells were isolated and washed twice with 0.9% NaCl, and hemolysed with 1.5 volumes of ice-cold water. The ghost and intact cells were removed by centrifugation at 20 000 rpm for 30 min at 4 °C. The pH of the hemolysate was adjusted to 8.7 with solid TrisCitation18. Firstly, benzoyl chloride and stirred for four hours at room temperature in CH2Cl2 cellulose. After the spacer arm cellulose added as a benzyl group and finally diazotized sulfanilamide clamped to the para position of benzyl group as ligand. The hemolysate was applied to the prepared cellulose-benzyl-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human carbonic anhydrase (hCA I and hCA II) isozymes were eluted with 1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), respectively. All procedures were performed at 4 °CCitation16.
CA inhibition
Enzyme activity was determined spectrophotometrically by following the change in absorbance at 348 nm of 4-nitrophenylacetate to 4-nitrophenylate over a period of 3 min at 25 °CCitation19–21. The enzymatic reaction contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution, in a total volume of 3.0 mLCitation22. Inhibitory effects of compounds 4–12 were compared with phenolic compounds 13–15. Different inhibitor concentrations were used and all compounds were tested in triplicate at each concentration used. Control cuvette activity was acknowledged as 100% in the absence of inhibitor. An Activity% – [Inhibitor] graph was drawn for each inhibitorCitation22–24. The curve-fitting algorithm allowed to obtain the IC50 values, working at the lowest concentration of substrate of 0.15 mM, from which KI values were calculatedCitation25,Citation26. The catalytic activity of these enzymes was calculated from Lineweaver–Burk plots, as reported earlier, and represent the mean from at least three different determinations. The CA I and II isoenzymes used here were purified from human blood as described earlierCitation19–24.
Results and discussion
Some research groups have studied with phenol and several phenolic compound types on 12 mammalian CA isoenzymes to show inhibitory properties of phenolsCitation27. We have extended previous investigations with some phenolic compounds which are globally used in food and medical industries. These kinds of compounds in different phenolic structures with antioxidant characteristics are essential in quenching reactive oxygen speciesCitation28–29. These type of compounds show several medical activities such as anticancer, anticarcinogenic, antimutagenic, antibacterial, antiviral or antiinflammatoryCitation5. Phenol, phenolic compounds and hydroxybenzoic acid derivatives are widely put into effect as prodrugs and/or drugs. Salicylic acid possesses the ability to reduce fevers and ease aches and pains. To date these medicinal features have been known and used as anti-inflammatory drugCitation27–34.
In the present study we have purified CA I and II (hCA I and hCA II) from human erythrocytes and examined the in vitro inhibition effects of above mentioned uracil derivatives 4–12 on these enzymes (), using the esterase activity of hCA I and II, with 4-NPA as substrate. Uracil derivatives 4 and 5 were synthesized from commercially available uracil. For this purpose uracil was converted to the brom uracil by using 1.1 Eq bromine in DMF at 120 °CCitation17. Condensation of brom uracil 2 with methanesulfonyl chloride, p-toluenesulfonyl chloride and acetic anhydryde to afford uracil derivatives 3 which has antitomour activity, 4, and 5 in comparable yieldCitation35,Citation36, respectively. The other tested compounds 6–15 were purchased from Sigma-Aldrich.
Table 1. hCA I and II inhibition data some compounds, by an esterase assay with 4-nitrophenylacetate as substrate.12b
(i) Against the slow cytosolic isozyme hCA I, compounds 4–8, and 15 behave as weak inhibitors, with Ki values in the range of 316.2–464 µM, similarly to the structurally related compounds 13 and 14 (KIs of 795 and 4003 µM). It is interesting that compounds 9–12 were much better hCA I inhibitors as compared to the corresponding compounds 7 and 9 from which it was derivated (). Kinetic investigations (Lineweaver–Burk plots, data not shown) indicate that similarly to sulfonamides and inorganic anionsCitation12–16, all the investigated natural compounds act as competitive inhibitors with 4-NPA as substrate.
(ii) A better inhibitory activity has been observed with compounds 9–12 investigated here for the inhibition of the rapid cytosolic isozyme hCA II (). Structure-activity relationship (SAR) is thus quite sharp for this small series of hydroxylic compounds: compounds 9–12 are effective leads, with two mono or di hidroxy moieties is already a submicromolar hCA II inhibitor. This effect is maintained when different groups are present in the meta position to the phenol OH moiety, such as in resorcinol. The best hCA II inhibitor in this series of derivatives were compound 11 with a KI of 0.1715 µM.
In a recent study it was reported that catechol and resorcinolCitation27 act as a CAI inhibitor, and could represent the starting point for a new class of inhibitors that may have advantages for patients with sulfonamide allergies. The sulfonamide zinc-binding group is thus superior to the thiol one (from the thioxolone hydrolysis product) for generating CA inhibitors with a varied and sometimes isozyme-selective inhibition profile against the mammalian enzymes. However, it is still important to explore further classes of potent CAIs in order to detect compounds with different inhibition profiles.
Compounds 3–12 used in this study affect the activity of CA isozymes due to the presence of the different functional groups (CH3, OH, Br, COOH, NH2, mesityl, and tosyl) present in their scaffold. Therefore, our findings indicate another class of possible CAIs of interest, in addition to the well-known inhibitors, the phenols/biphenyl diphenols bearing bulky ortho moieties in their molecules. Some hyroxylic compounds investigated here exhibited effective hCA I and II inhibitory activity, in the low-micromolar range, by the esterase method which usually gives KI-s an order of magnitude higher as compared to the CO2 hydrase assay. These findings point out that substituted hdroxylic compounds may be used as leads for generating potent CAIs eventually targeting other isoforms.
Disclosure statement
The authors report no declarations of interest
References
- Martinz Z, Botta O, Fogel ML, et al. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet Sci Lett 2008;270:130–6.
- Pałasz A, Cież D. In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur J Med Chem 2015;97:582–611.
- Arutyunyan AA, Mamyan SS, Stepanyan HM, Paronikyan RV. Synthesis and Antitumor and Antibacterial Properties of New N-Alkylated Pyrimidines. Pharm Chem J 2013;47:303–6.
- Smith NF, Figg WD, Sparreboom A. Recent advances in pharmacogenetic approaches to anticancer drug development. Drug Dev Res 2004;62:233–53.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Almajan GL, Barbuceanu S-F, Innocenti A, et al. Carbonic anhydrase inhibitors. Inhibiton of the cytosolic and tumor-associated carbonic anhydrase iszymes I, II and IX with some 1,3,4-oxadiazole- and 1,2,4-triazole-thiols. J Enzyme Inhib Med Chem 2008;23:101–7.
- Morgan PE, Supuran CT, Casey JR. Carbonic anhydrase inhibitors that directly inhibit anion transport by the human Cl-/HCO3- exchanger, AE1. Mol Membr Biol 2004;21:423–33.
- Uygun M, Karagözler AA, Denizli A. Molecularly imprinted cryogels for carbonic anhydrase purification from bovine erythrocyte. Artif Cells Nanomed Biotechnol 2014;42:128–37.
- Del Prete S, Vullo D, De Luca V, et al. Cloning, expression, purification and sulfoamide inhibition profile of the complyete domain of the η-carbonic anhydrasefrom plasmodium falciparum. Bioorg Med Chem Lett 2016;26:4184–4190.
- Le Darz A, Mingot A, Bouazza F, et al. Fluorinated pyrrolidines and piperidines incorporating tertiary benzenesulfonamide moieties are selective carbonic anhydrase II inhibitors. J Enzyme Inhib Med Chem 2015;30:737–45.
- Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Patents 2013;23:705–16.
- Orhan F, Senturk M, Supuran CT. Interaction of anions with a newly characterized alpha carbonic anhydrase from Halomonas sp. J Enzyme Inhib Med Chem 2015. [Epub ahead of print]. http://dx.doi.org/10.3109/14756366.2015.1100177.
- Arslan T, Celik G, Celik H, et al. Synthesis and biological evaluation of novel bischalcone derivatives as carbonic anhydrase inhibitors. Arch Pharm (Weinheim) 2016;349:741–8.
- Karatas MO, Alici B, Cakir U, et al. Syhthesis and carbonic anhydrase inhibitory properties of novel coumarin derivatives. J Enzyme Inhib Med Chem 2013;28:299–304.
- Yaseen R, Ekinci D, Senturk M, et al. Pyridazinone substituted benzenesulfonamides as potent carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2016;26:1337–41.
- Isık S, Vullo D, Durdagi S, et al. Interaction of carbonic anhydrase isozymes I, II, and IX with some pyridine and phenol hydrazinecarbothioamide derivatives. Bioorg Med Chem Lett 2015;25:5636–41.
- Guney M, Cavdar H, Senturk M, Ekinci D. Synthesis and carbonic anhydrase inhibitory properties of novel uracil derivatives. Bioorg Med Chem Lett 2015;25:3261–3.
- Ozdemir ZO, Senturk M, Ekinci D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules. J Enzyme Inhib Med Chem 2013;28:316–19.
- Balaydin HT, Senturk M, Menzek A. Synthesis and carbonic anhydrase inhibitory properties of novel cyclohexanonyl bromophenol derivatives. Bioorg Med Chem Lett 2012;22:1352–7.
- Cavdar H, Ekinci D, Talaz O, et al. α-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:148–54.
- Senturk E, Senturk M, Ekinci D. Comparison of inhibition effects of some anions on carbonic anhydrase isoenzymes from mammalian kidney tissues. Acta Physiol 2015;52:1101–6.
- Ekinci D, Senturk M, Senturk E. Purification and characterization of carbonic anhydrase enzyme from bovine heart tissue and investigation of inhibition effects of some sulfonamide derivative drugs. Acta Physiol 2015;215:99.
- Ekinci D, Cavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73.
- Ekinci D, Al-Rashida M, Abbas G, et al. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:744–7.
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–108.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I–XIV). Bioorg Med Chem Lett 2008;18:1583–7.
- Polat Kose L, Gulcin I, Ozdemir H, et al. The effects of some avermectins on bovine carbonic anhydrase enzyme. J Enzyme Inhib Med Chem 2016;31:773–8.
- Ozgeriş B, Goksu S, Kose Polat L, et al. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 2016;24:2318–29.
- Artunç T, Cetinkaya Y, Gocer H, et al. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2- dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Design 2016;87:594–607.
- Goksu H, Topal M, Keskin A, et al. 9,10-Dibromo-N-aryl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones: synthesis and ınvestigation of their effects on carbonic anhydrase ısozymes I, II, IX, and XII. Arch Der Pharm 2016;349:466–74.
- Urcar H, Senturk E, Senturk M, Gul M, Yildirim S. Investigation of the effects of some catecholamines on the activity of carbonic anhydrase enzyme purified from bovine kidney tissue. Acta Physiol 2016;218:57.
- Demirdag R, Comakli V, Senturk M, et al. Characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem 2013;21:1522–5.
- Balaydin HT, Senturk M, Goksu S, Menzek A. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols and their derivatives including natural products: Vidalol B. Eur J Med Chem 2012;54:423–8.
- Glavas-Obrovac L, Karner I, Pavlak M, et al. Synthesis and antitumor activity of 5-bromo-1-mesyluracil. Nucleosides Nucleotides Nucleic Acids 2005;24:557–69.
- Visnjevac A, Zinic M, Luic M, et al. Conformational chirality and chiral crystallization of N-sulfonylpyrimidine derivatives. Tetrahedron 2007;63:86–92.