Abstract
Melanogenesis is a process to synthesize melanin, which is a primary responsible for the pigmentation of human skin, eye and hair. Although numerous enzymatic catalyzed and chemical reactions are involved in melanogenesis process, the enzymes such as tyrosinase and tyrosinase-related protein-1 (TRP-1) and TRP-2 played a major role in melanin synthesis. Specifically, tyrosinase is a key enzyme, which catalyzes a rate-limiting step of the melanin synthesis, and the downregulation of tyrosinase is the most prominent approach for the development of melanogenesis inhibitors. Therefore, numerous inhibitors that target tyrosinase have been developed in recent years. The review focuses on the recent discovery of tyrosinase inhibitors that are directly involved in the inhibition of tyrosinase catalytic activity and functionality from all sources, including laboratory synthetic methods, natural products, virtual screening and structure-based molecular docking studies.
Introduction
It is estimated approximately 15% of the world population invest in skin whitening agents with Asia is being dominatedCitation1. Global industry analysts (GIA) have predicted that the universal market for skin lighteners will reach $23 billion by 2020, driven by new markets in Asia, particularly India, Japan and ChinaCitation2. According to the SIRONA biochem reportCitation1, approximately $13 billion spent on skin care products and cosmetics in Asia Pacific’s. In India alone, it is estimated that $432 million was spent in 2010 on skin lightening creams and skin care agents. A recent survey showed that 80% of Indian men use fairness creams and the number of consumer’s are growing 18% annuallyCitation1. The molecular mechanism of these skin lightening agents are to reduce the melanin, which is the main source of skin color.
Melanin is primarily responsible for the pigmentation of human skin, eyes and skin, which is produced from epidermis melanocytes in an approximate ratio of 1:36 with basal keratinocytesCitation3. In response to ultraviolet B (UVB)-irradiation, melanocyte synthesizes melanin through the process called melanogenesis. The synthesized melanin in melanosomes is transported to neighboring keratinocytes in epidermisCitation4. Under normal physiological conditions, pigmentation has a beneficial effect on the photo-protection of human skin against harmful UV injury and plays an important evolutionary role in camouflage and animal mimicryCitation5. However, an excessive production of melanin causes dermatological problems such as freckles, solar lentigo (age spots) and melasmaCitation6–9, as well as cancerCitation10 and postinflammatory melanodermaCitation11. In addition, continuous UV-irradiation can result in DNA damage, gene mutation, cancer development and impairment of the immune system or photoagingCitation12.
Regulation of melanogenesis
Melanogenesis is a complex pathway involving a combination of enzymatic and chemical catalyzed reactions. Melanocytes produce two types of melanin: eumelanin (brown-black) and pheomelanin (red-yellow) formed by the conjugation of cysteine or glutathione ()Citation13–15.
Figure 1. Melanogenesis pathway (production of eumelanin and pheomelanin)Citation13. (Tyr: tyrosinase; DQ: dopaquinone; L-Dopa: L-3:4-dihydroxyphenylalanine; DHICA: 5,6-dihydroxyindole-2 carboxylic acid; DHI: 5,6-dihydroxyindole; ICAQ: indole-2-carboxylic acid-5,6-quinone; IQ: indole-5,6-quinone; HBTA: 5-hydroxy-1,4-benzothiazinylalanine).
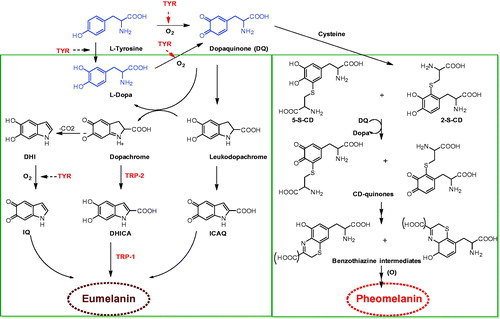
The melanogenesis process is initiated with the oxidation of L-tyrosine to dopaquinone (DQ) by the key enzyme, tyrosinase (TYR). The resulting quinone will serve as a substrate for the synthesis of eumelanin and pheomelaninCitation16,Citation17. The formation of DQ is a rate-limiting step in the melanin synthesis because remainder of the reaction sequence can proceed spontaneously at a physiological pH valueCitation17. After DQ formation, it undergoes intramolecular cyclization to produce indoline, leukodopachrome (cyclodopa). The redox exchange between leukodopachrome and DQ give rise to dopachrome and L-3,4-dihydroxyphenylalanine (L-DOPA), which is also a substrate for TYR and oxidized to DQ again by the enzyme. Dopachrome gradually decomposes to give dihydroxyindole (DHI) and dihydroxyindole-2-carboxylicacid (DHICA). The later process is catalyzed by TRP-2, now known as dopachrome tautomerase (DCT). Ultimately, these dihydroxyindoles (DHI and DHICA) are oxidized to eumelanin. TRP-1 is believed to catalyze the oxidation of DHICA to eumelanin. Alongside, DQ is converted to 5-S-cysteinyldopa or glutothionyldopa in the presence of cysteine or glutathione. Subsequent oxidation gives benzothiazine intermediates and finally to produce pheomelanin. Although three enzymes, TYR, TRP-1 and TRP-2 are involved in the melanogenesis pathway, tyrosinase is exclusively necessary for melanogenesis.
Tyrosinase and its key role in melanin synthesis
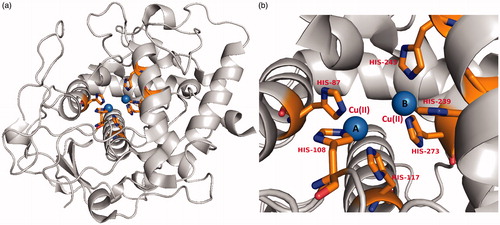
Tyrosinase (monophenol or o-diphenol, oxygen oxidoreductase, EC 1.14.18.1, syn.polyphenol oxidase), a multifunctional membrane bound type-3 copper-containing glycoprotein is located in the membrane of the melanosomeCitation18. Tyrosinase is produced only by melanocyte cells. Following its production and consequent processing in the endoplasmic reticulum and Golgi, it is trafficked to melanosomes, wherein the pigment melanin is synthesized. From the structural point of view, two copper ions are surrounded by three histidine residues that are responsible for the catalytic activity of tyrosinase ()Citation19. The active site has three states; oxy, met and deoxy forms in the formation of pigmentation. More specifically, at the active site, two copper ions interact with dioxygen to form a highly reactive chemical intermediate that directly participate in the hydroxylation of monophenols to diphenols (monophenolase activity) and in oxidation of o-diphenols to o-quinones (diphenolase activity)Citation20,Citation21. Tyrosinase is also catalyzing the process of neuromelanin production in which the oxidation of dopamine produces dopaquinones. However, excessive production of dopaquinones results in neuronal damage and cell death. This suggests that tyrosinase might play a significant role in neuromelanin formation in the human brain and responsible for the neurodegeneration associated with Parkinson’s disease and Huntington’s diseasesCitation22–26. Tyrosinase has also been linked in the browning of vegetables and fruits during postharvest and handing process, leading to quick degradationCitation27–29. The application of tyrosinase was further extended in the molting process of insectsCitation30. Thus, the regulation of melanin synthesis by inhibiting the tyrosinase enzyme is a major motivation for researchers in the context of preventing hyperpigmentation.
Figure 2. (a) A recent high resolution (1.8 Å) crystal structure of a plant tyrosinase (PDB ID: 5CE9, walnut leaves, Juglans regia)Citation19. (b) The active site of the enzyme contains two copper ions A and B which are coordinated by six histidine residues His87, His108, His117 for CuII (A) and His239, His243, His273 for CuII(B), respectively and represented in stick model.
Tyrosinase inhibitors
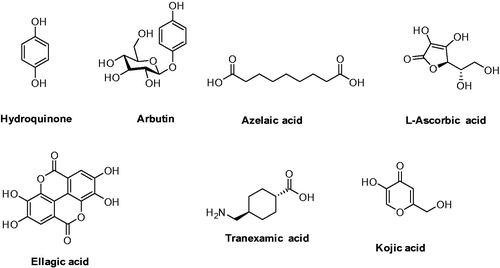
Since tyrosinase is a crucial enzyme in synthesizing melanin through melanogenesis, it becomes the most prominent and successful target for melanogenesis inhibitors that directly inhibit the tyrosinase catalytic activity. Most of cosmetics or skin lightening agents commercially available are tyrosinase inhibitors. The fact that the inhibitors target tyrosinase may specifically inhibit the melanogenesis in cells without side effects, as tyrosinase is exclusively produced only by melanocytes. Many tyrosinase inhibitors such as hydroquinone (HQ)Citation31–34, arbutin, kojic acidCitation35–37, azelaic acidCitation38,Citation39, L-ascorbic acidCitation40–42, ellagic acidCitation43–45, tranexamic acidCitation46–48 have been used as skin-whitening agents, with certain drawbacks ().
HQ is potentially mutagenic to mammalian cells and linked to a number of adverse reactions including contact dermatitis, irritation, transient erythema, burning, prickling sensation, leukoderma, chestnut spots on the nails, hypochromia and ochronosisCitation49–52. Arbutin, a prodrug of hydroquinone, is a natural product and reduces or inhibits melanin synthesis by inhibiting tyrosinase. However, natural forms of arbutin are chemically unstable and can release hydroquinone which is catabolized to benzene metabolites with the potential toxicity for bone marrowCitation53. Kojic acid use in cosmetics has been limited, due to its carcinogenicity and its instability during storageCitation54. L-Ascorbic acid is sensitive to heat and degrades easilyCitation55. Ellagic acid is insoluble and thus poorly bioavailableCitation56, and for the tranexamic acid the melanogenic pathway remains undeterminedCitation47. Thus, it is in great need of developing new tyrosinase inhibitors with drug-like properties.
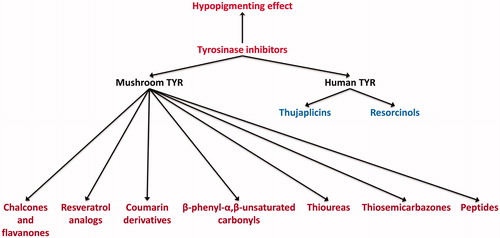
The review focuses on the recent discovery of tyrosinase inhibitors that are directly involved in the inhibition of tyrosinase catalytic activity and functionality from all sources, including laboratory synthetic methods, natural products, virtual screening and structure-based molecular docking studies. In the present review, the inhibitors were mainly categorized into two parts, mushroom and human tyrosinase inhibitors and further classified on the basis of their chemical functionalities (). We believe that this perspective will comprise a cumulative source of melanogenesis inhibitors for researchers and further understanding of anti-melanogenesis chemotherapy.
Mushroom tyrosinase inhibitors
Tyrosinase from the mushroom Agaricus bisporus is frequently used as an enzymatic in vitro model for developing the skin-whitening substances targeting human tyrosinase. Because of the commercial availability of mushroom tyrosinase (mTAR), most of the research has been studied with this enzyme. For screening of the compounds, the popular whitening agents, such as kojic acid, arbutin or hydroquinone, were used as a positive control. As a result, in recent years, numerous mushroom tyrosinase inhibitors from natural and synthetic sources have been identified. The inhibitory strength was expressed as IC50 value, which is the concentration of the inhibitor needed to inhibit half of the enzyme activity in the tested condition. The Ki value is the reflective of ligand-binding affinity to the enzyme. The lower Ki value means higher binding affinity, whereas higher Ki value means lower binding affinity. The Ki value for noncompetitive inhibitors is essentially the same numerical value as the IC50 of the inhibitors, whereas for competitive inhibitors, the Ki is about one-half that of the numerical values of IC50. For in vitro analysis, B16 cells were usually used because they are relatively easy to culture in vitro and shares the melanogenesis mechanisms of normal human melanocytes.
Chalcones and flavanone inhibitors
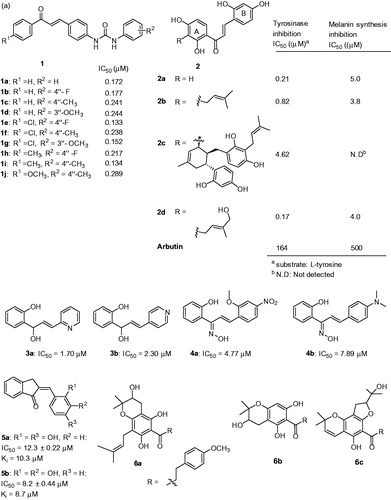
Chalcones belong to the class of natural products widely distributed in fruits, vegetables, spices, tea and soya-based foodstuff and exhibited inhibitory effect on tyrosinase with varying biological activities. A new series of 4-(phenylurenyl)chalcone (1) derivatives were synthesized and their inhibitory effects on the diphenolase activity of banana tyrosinase were evaluated (, 1a–1j)Citation57. The results showed that compounds 1a–1j inhibit the tyrosinase enzyme and in particular 1e and 1i were effectively inhibited with IC50 values of 0.133 and 0.134 μM, respectively. Furthermore, the compounds were identified as competitive inhibitors.
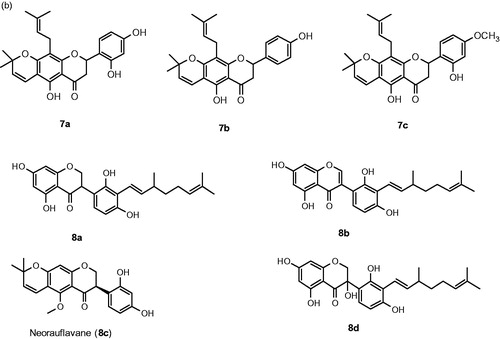
Figure 5. Chemical structure of chalcones, 1a–1j,Citation57 2a–2d,Citation58 3a–3b,Citation59 4a–4b,Citation60 5a–5bCitation61 and 6a–6cCitation62 (a) and flavanone inhibitors, 7a–7cCitation62 and 8a–8dCitation63 (b).
In another study, chalcones 2a–2d isolated from Morus australis were evaluated for their inhibitory activity on mushroom tyrosinase using L-tyrosine as the substrateCitation58. The results showed that all the four chalcone derivatives were extremely potent inhibitors for tyrosinase in comparison to the standard compound arbutin (, 2a–2d). Especially, compound 2a was 700-fold potent inhibition in comparison to arbutin. The structure–activity relationship (SAR) analysis showed that resorcinol construction at both ring A and ring B could be the reason for strong tyrosinase inhibition. In addition, the substitution at 3′ position of ring A plays an important role in enhancing the inhibitory potency. For example, the steric bulky substituent at ring A on 2c reduced the potency compared to the unsubstituted 2a, this is due to chelating ability towards the copper ions. Interestingly, compound 2d exhibited stronger inhibitory activity than 2a and 2c, indicate that 4-hydroxy-1-pentene group at 3′-position of 2d is responsible for the enhancement. Further, the effects of chalcones on melanin synthesis were tested in melanin-producing B16 murine melanoma cells and compounds 2a, 2b and 2d were strongly inhibited with little or no cytotoxicity.
Recently, Radhakrishnan et al., reported a library of azachalcones andCitation59 the inhibitory effects on mushroom tyrosinase using L-DOPA as substrate was investigated. Among the investigated compounds in the study, two compounds 3a and 3b, congeners (carbonyl reduction) of the pyridyl azachalcones were found to be potent enzyme inhibitors than kojic acid (IC50 = 27.30 μM) (, 3a–3b). Furthermore, kinetic analysis identified both 3a and 3b were competitive inhibitors with Ki values of 2.62 and 8.10 μM, respectively. The structure–function analysis showed that the nitrogen atom in the pyridine skeleton of the inhibitors could complex with the copper ions present in the tyrosinase active site. The same research group has reported another chalcone series with oxime functionality as inhibitors of tyrosinase and melanin formation in melanoma B16 cells.Citation60 Two of the compounds (4a: IC50 = 4.77 μM and 4b: IC50 = 7.89 μM) exhibited potent tyrosinase inhibitory activities (, 4a–4b) than the kojic acid (IC50 = 22.25 μM). Kinetic studies revealed as competitive inhibitors with Ki values of 5.25 and 8.33 μM. In α-melanocyte-stimulating hormone (α-MSH) induced B16 melanoma cells, these two compounds 4a and 4b inhibited the melanin formation and tyrosinase without cytotoxicity. In terms of SAR analysis, it was identified that the presence of ortho-methoxy with para-nitro substituents (ring B) were responsible for potent tyrosinase inhibition (4a). In addition, an electron donating para-dimethyl amino ring (ring B) exhibited the second most potent inhibition (4b).
In another study, a novel series of 2,3-dihydro-1H-inden-1-one chalcone-like derivatives were reported.Citation61 Two of them, 5a and 5b, were identified as potent inhibitors of diphenolase activity of tyrosinase with IC50 values of 12.3 and 8.2 μM (). Further exploration of the mechanism, both the inhibitors 5a and 5b were found to be reversible and competitive.
Wang et al. isolated dihydrochalcones (, 6a–6c) and flavanones (, 7a–7c) from Flemingia philippinensis and investigated for their inhibitory activities on tyrosinaseCitation62. The results showed that they inhibit the monophenolase (IC50 = 1.01 to 18.4 μM) and diphenolase (IC50 = 5.22 to 84.1 μM) actions of tyrosinase. In particular, dihydrochalcone (6c) effectively inhibited both monophenolase and diphenolase activities of tyrosinase with IC50 values of 1.28 and 5.22 μM, respectively. The SAR analysis is very interesting because the pharmacophore is not associated with tyrosinase inhibition and it lacks the α,β-unsaturated ketone motif which present in most of the inhibitors. In the case of flavanones, compound containing resorcinol group (7a) were competitive and significantly inhibited monophenolase (IC50 = 1.79 μM) and diphenolase (IC50 = 7.48 μM) of tyrosinase.
Figure 6. ResveratrolCitation64 and its analogs, 10a–10f,Citation66 11a–11e,Citation67 12a–12dCitation68 and 13a–13gCitation69. *The IC50 value of resveratrol is 26.63 ± 0.55 μMCitation65 and 57.05 μg/mLCitation66 according to the references 65 and 66.
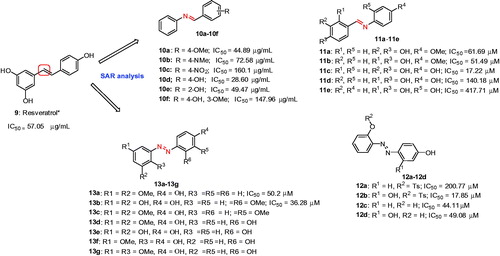
Figure 7. Chemical structure of coumarin derivatives, 14,Citation73 15a–15b,Citation74 16a–16dCitation75 and 17a–17dCitation76.
In search of new tyrosinase inhibitors, it was found that the extracts of Camylotropis hirtella show tyrosinase inhibitionCitation63. After successful purification and isolation of fourteen compounds, four compounds (, 8a–8d) showed potent inhibitory activities against tyrosinase. The most potent compound was found to be neorauflavane 8c exhibiting IC50 values of 30 and 500 nM against monophenolase and diphenolase activity of tyrosinase. Furthermore, comparing with kojic acid (13.2 μM), 8c was 400-fold more potent against monophenolase activity of tyrosinase. The second most potent compound was geranylated isoflavanone 8a inhibited monophenolase and diphenolase with IC50 values 2.9 and 128.2 μM, respectively, and identified as a competitive and reversible inhibitor. In addition, compounds 8a and 8c efficiently reduced the melanin content in α-MHS-induced B16 melanoma cells, without influencing cell viability. From the structural point of view, reduction of geranyl side chain improves the tyrosinase inhibitory activity.
Figure 8. Chemical structure of inhibitors with β-phenyl-α,β-unsaturated carbonyl functionality, 18a–18c,Citation77–80 19a–19c,Citation81 20a–20c,Citation8221a–21b,Citation83 22a–22cCitation85 (a); 23a–23bCitation85 and 23c–23gCitation88 (b).
Resveratrol analogs
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, 9) a widely distributed stilbenoid in nature such as in grapes, exhibited the inhibitory activity against mushroom tyrosinase through the mechanism of Kcat (suicide substrate) type inhibitionCitation64. In vitro analysis in α-MSH-stimulated B16 murine melanoma cells, resveratrol inhibited the cellular melanin production via suppression of melanogenesis-related proteins such as tyrosinase, TRP-1, TRP-2 and microphthalmia-associated transcription factor (MITF) expressionCitation65 without any cytotoxicity up to 200 μM.Citation64 The inhibitory effects of resveratrol have been confirmed in an in vivo model using UVB-irradiated brownish guinea pigs. In this study, treatment of resveratrol with UVB-irradiated dorsal skin of guinea pigs visually decreased the hyperpigmentation.
In an effort to improve the activity of resveratrol, Fenco et al., demonstrated a study with a series of resveratrol analogs (, 10a–10f), where one of -CH group was replaced with nitrogen atomCitation66. Among the tested analogs, compounds with 4-methoxy (10a), 4-hydroxy (10d) or 2-hydroxy (10e) substitutions show higher potent inhibition, suggest that changes to the basic resveratrol structure by a nitrogen atom resulted an increase in tyrosinase inhibitory effects. Moreover, the hydroxyl group at para position (10d) was found to be important for the potency. In another study, Bae et al designed, synthesized and evaluated a series of (E)-N-substituted benzylidene-hydroxy or methoxy-aniline derivatives (aza-resveratrol type) for their inhibitory effect on tyrosinase activity (, 11a–11e)Citation67. Derivatives with a 4-methoxy- or 4-hydroxy-anilino group (11a–11d) showed more potent inhibition against mushroom tyrosinase than 2-hydroxyanilino group 11e. (E)-4-((4-Hydroxyphenylimino)methyl)benzene-1,2-diol 11c exhibited the most potent and noncompetitive inhibition on mushroom tyrosinase showing an IC50 value of 17.22 μM and effective than kojic acid (51.11 μM). Compound 11a and 11c–11e were identified to be as noncompetitive inhibitors, whereas compound 11b as a competitive inhibitor. The study was further extended by synthesizing a series of (E)-2-((substitutedphenyl)diazenyl)phenyl-4-methylbenzenesulfonate (12a–b) and (E)-2-((substituted phenyl)diazenyl)phenol derivatives (12c–d) () and most of the compounds were strongly inhibit the tyrosinase in a dose-dependent manner. In particular, the novel compound, (E)-2-((2,4-dihydroxyphenyl)diazenyl)phenyl-4-methylbenzenesulfonate (12b) showed the best result with an IC50 of 17.85 μMCitation68. Lineweaver–Burk plot assay using L-tyrosine as substrate indicated 12b as a competitive inhibitor. Moreover, the compounds 12a–12d inhibited cellular tyrosinase activity and melanin synthesis in in murine B16F10 melanoma cells.
Recently, a series of azo-resveratrol (13a–13e and 13 g) and azo-oxyresveratrol (13f) were reported ()Citation69. Among these compounds, 13a and 13b exhibited high tyrosinase inhibitory activity of 56.25% and 72.75% at 50 μM, respectivelyCitation69. The 4-hydroxyphenyl moiety was found to be essential for higher inhibition and 3,5-dihydroxyphenyl or 3,5-dimethoxyphenyl derivatives showed better tyrosinase inhibition than 2,5-dimethoxyphenyl derivatives. Particularly, introduction of hydroxyl or methoxy group into the 4-hydroxyphenyl moiety diminished or significantly reduced mushroom tyrosinase inhibition. Among the synthesized azo compounds, azo-resveratrol (13b) was the most potent mushroom tyrosinase inhibition with an IC50 value of 36.28 μM. The results indicate that azo-resveratrol with high Log p value might be superior to resveratrol for the development of whitening agents and pharmaceutical drugs in the treatment of hyperpigmentation.
Coumarin derivatives
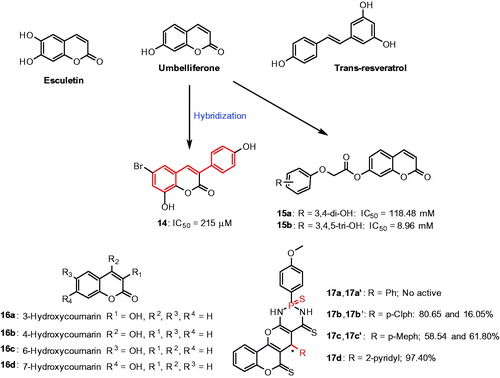
Coumarins are large family of benzopyrone compounds available from natural and synthetic origins with different pharmacological activitiesCitation70. In recent studies, few coumarins proved to inhibit the mushroom tyrosinase, which includes esculetin and umbelliferone with stronger tyrosinase inhibitory activityCitation71,Citation72. In a continuous effort, Matos et al., have demonstrated a series of coumarin-resveratrol hybrid compounds, 3-phenyl coumarins with hydroxyl or alkoxy and bromo substituent at various positions in the scaffoldCitation73 (). Among the series, compound with bromo atom and two hydroxyl groups in the 3-phenylcoumarin moiety (14), was identified as a best inhibitor with an IC50 value of 215 μM. This compound is a noncompetitive tyrosinase inhibitor with a Ki value of 0.189 mM.
In another study, a series of umbelliferone analogs were reported for their inhibitory effects on mushroom tyrosinaseCitation74. Specifically, compounds 15a and 15b possessing 3,4-dihydroxy and 3,4,5-dihydroxy phenyl scaffold showed more potent inhibitory activities against mushroom tyrosinase activity (). Asthana et al., demonstrated a series of hydroxycoumarins (16a–16d)Citation75 (). The SAR studies suggested that the position of hydroxyl substituent on the coumarin plays a role in enzymatic inhibition; the compounds with aromatic hydroxylated, 6-hydroxycoumarin (16c) and 7-hydroxycoumarin (16d), found to be weak substrates of the enzyme. Especially, 7-hydroxycoumarin strongly inhibited the dopachrome concentration in a range of 0.3 − 0.9 mM. At a maximum 7-hydroxycoumarin concentration, the inhibition reached 88%. The authors found the phenomenon was due to the specific inhibition of L-tyrosine conversion. On the other hand, the compounds with pyrone hydroxylated, 3-hydroxycoumarin (16a) and 4-hydroxycoumarin (16b) were not substrates of tyrosinase. 3-hydroxycoumarin (16a) was found to inhibit tyrosinase but not the compound with 4-hydroxycoumarin (16b), indicate the pyrone ring cannot be hydroxylated by tyrosinase.
Recently, a series of thiophosphonic acid diamides were screened for their tyrosinase inhibition activityCitation76. The results showed that the substituent attached to C-5 and stereochemistry of the two stereogenic centers (C-5 and phosphorus atom) were important for the tyrosinase inhibition (17a–17d). Diastereomers with unsubstituted phenyl did not show any inhibitory activity against tyrosinase (17a and 17a′). In contrast, compounds with a substituted phenyl showed various effects on tyrosinase activity, for example compound 17b with p-chlorophenyl (80.65% tyrosinase inhibition) moderately inhibited the tyrosinase but its diastereomer 17b′ 16.5% tyrosinase inhibition) was inactive. In another case, 17c (58.54% tyrosinase inhibition) and 17c′ contain p-methylphenyl (61.80% tyrosinase inhibition) exhibited good tyrosinase inhibition. Compound 17d consist of a 2-pyridinyl (97.40% tyrosinase inhibition) fragment was found to be the most potent tyrosinase inhibitor of the above study.
Inhibitors with β-phenyl-α,β-unsaturated carbonyl functionality
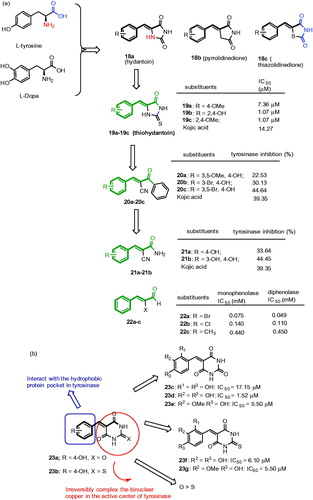
Recently, it was reported that benzylidenehydantoin 18a, benzylidenepyrrolidinedione 18b, and benzylidenethiazolidine-2,4-dione 18c () derivatives as potential tyrosinase inhibitors and using in vivo studies the compounds were proved to be an effective skin whitening agentsCitation77–80. They exhibited strong inhibitory activities than kojic acid and arbutin. In fact, the compound 18a was designed by mimicking the chemical structure of the L-tyrosine and L-DOPA, tyrosinase substrates. The SAR studies revealed that the amide NH at 1-position of hydantoin 18a has the ability to form hydrogen bonds with amino acids at the active site of tyrosinase. Furthermore, the imido group of 18a mimics the carboxylic acid group of the substrates. On the basis of this background, Kim et al. recently synthesized and evaluated a series of 5-(hydroxyl- or alkoxysubstituted benzylidene)thiohydantoin analogs possessing β-phenyl-α,β-unsaturated carbonyl scaffolds.Citation81 Among them, three compounds, 19a–19c, exhibited high inhibitory activities than kojic acid or resveratrol (). Especially, 2,4-dihydroxybenzylidene-2-thiohydantoin 19c (IC50 = 1.07 μM) was found to be the best inhibitor of this study. In addition, 19c inhibited the cellular tyrosinase activity in B16 cells without any significant cytotoxicity.
In a continuation, (E)-2-benzoyl-3-(substituted phenyl)acrylonitriles (BPA analogs) with a linear β-phenyl-α,β-unsaturated carbonyl scaffold were synthesized and evaluated as potential tyrosinase inhibitorsCitation82. Among them, three compounds 20a–20c effectively inhibited the mushroom tyrosinase activity (). Especially, compound 20c significantly suppressed the melanin biosynthesis and inhibited intracellular tyrosinase activity in B16 cells without influencing the cell viability. The SAR analysis revealed that all active compounds have 4-hydroxy group on the phenyl ring, and substitution of Br at 3-position or at 3 and 5-position were found to be associated with potent tyrosinase inhibitory activity.
Recently, the same research group continued to explore the SAR of 3-(substituted phenyl)acrylonitriles. Accordingly, a series of (E)-2-cyano-3-(substituted phenyl)acrylamide derivatives possessing a linear β-phenyl-α,β-unsaturated carbonyl scaffold showed inhibitory activity against mushroom tyrosinaseCitation83. Among the compounds, 21a and 21b exerted inhibitory activity against mushroom tyrosinase (). Especially, compound 21a showed excellent inhibitory activity. In B16 cells, 21a significantly suppressed tyrosinase activity in a dose-dependent manner without any influence of cytotoxic effect. From the structural point of view, a “linear” β-phenyl-α,β-unsaturated carbonyl scaffold plays an essential role in showing anti-melanogenic effect through the direct inhibition of tyrosinase enzyme.
It has been a long time known that cinnamaldehyde was able to inhibit L-DOPA oxidation by mushroom tyrosinaseCitation84. Recently, Cui et al., reported a series of α-substituted derivatives of cinnamaldehyde derivativesCitation85. The SAR studies showed that α-bromocinnamaldehyde 22a, α-chlorocinnamaldehyde 22b, and α-methylcinnamaldehyde 22c compounds reduced both monophenolase and diphenolase activity on tyrosinase (). The IC50 values of 22a–22c were 0.075, 0.140 and 0.440 mM on monophenolase and 0.049, 0.110 and 0.450 mM on diphenolase, respectively. Furthermore, it was suggested that the α-substituted cinnamaldehyde derivative were more potent compared to cinnamaldehyde.
Recently, thio/barbiturates have drawn attention in the field of tyrosinase inhibitorsCitation86, due to their attractive structural unit of β-phenyl-α,β-unsaturated carbonyl scaffold responsible for tyrosinase inhibitory function. In the literature, few 5-benzylidene (thio) barbiturates with hydroxyl substituent at 4-position of the phenyl ring had excellent inhibitory activities, for example, 23a and 23b inhibited with IC50 values of 13.98 and 14.49 μM, respectivelyCitation87 (). Inspired by this work, Chen et al., recently explored the SAR of thio/barbiturates emphasizing the position and the number of hydroxyl substituents for the influence of tyrosinase inhibitory activity. Accordingly, a series of hydroxy- or methoxy-substituted 5-benzylidene(thio)barbiturates were reported for their inhibitory effects on the diphenolase activity of mushroom tyrosinaseCitation88. The results show that compounds (23c–23 g) had potent tyrosinase inhibitory activities compared to kojic acid (IC50 = 18.25 μM). In particular, compound with 3,4-dihydroxy substituents 23e was identified as a best inhibitor with an IC50 value of 1.52 μM. The SAR studies revealed that barbiturates were potent than thiobarbiturates and 3,4-dihydroxyl groups on the phenyl ring improved the potency. Furthermore, these inhibitors were found to reversible type.
Thiourea derivatives
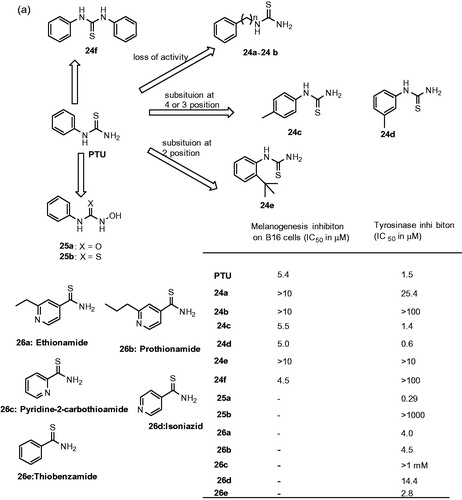
Phenylthiourea (PTU) is one of the most well-known tyrosinase inhibitors studied in the literatureCitation89–91. Sulfur atom of PTU binds to both copper ions at the active site of tyrosinase and blocks the enzyme activity. Professor Jung et al. recently explored the SAR of PTU derivatives on mushroom tyrosinaseCitation92,Citation93. Although the effects of PTU derivatives were primarily evaluated for their melanogenesis inhibition in B16 melanoma cells, later it was confirmed that it was due to inhibition of tyrosinase activity. The SAR studies has highlighted the important structural requirements for both melanogenesis and tyrosinase inhibition (): (i) direct connection of π-planar structure to thiourea (24a–24b), (ii) hydrophobic substituent at the para- or meta-position of the phenyl ring was accepted (24c–24d), substituent at the ortho-position was not tolerated (24e), suggested that C2-substituted phenyl may hinder the complex formation of thiourea with copper ions at the active site of tyrosinase. Moreover, (iii) free 3-amino hydrogens were important for tyrosinase inhibition but not for the melanogenesis inhibition on B16 cells, 1,3-disubsituted derivatives showed greater potency in melanogenesis inhibition (24f). These findings suggested that 1,3-disubsituted derivatives act through a pathway different from the tyrosinase catalytic activity to prevent melanogenesis. Molecular docking analysis of 1-phenylthiourea and 1,3,-diphenylthiourea revealed that direct connection of planar phenyl to thiourea unit and free 3-NH2 were prerequisite for the tyrosinase activity ().
Figure 9. Chemical structure of thiourea derivatives, 24a–24f,Citation92, Citation93 25a–25bCitation91 and 26a–26eCitation95 (a); 27a–27e,Citation97 28a–28d,Citation98 29a–29dCitation98 and 30a–30bCitation99 (b).
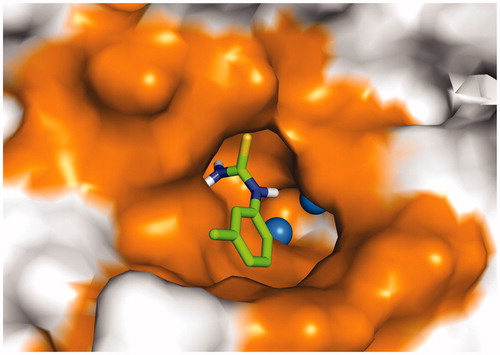
Figure 10. The docked pose of 24dCitation93 (stick model) is shown with the two copper ions (sphere representation) and the binding pocket (surface model) of tyrosinase from Bacillus megaterium (PDB ID: 3NQ1).
On the other hand, Crinton et al reported a series of N-hydroxy-N′-phenylurea derivatives, replacing sulfur with oxygen and 3-NH2 with N-hydroxylamine in PTUCitation91. The results showed the reported derivatives were more potent, in particular, N-hydroxy-N′-phenylurea (25a) showed 6-time more potent than PTU. In contrast N-hydroxy-N′-phenylthiourea (25b) derivatives showed no inhibition, suggested that the chelating ability of N-hydroxyurea is important for the tyrosinase inhibition ().
Apart from synthetic or natural sources, screening is another alterative strategy to find new inhibitors. Mainly screening of drugs that are clinically approved has become increasing for many biological targets. The data associated with an existing drug will reduce the time and cost associated with the intellectual rights for developing the novel pharmaceutics. This approach has several advantages; including availability, lower cost and safety/tolerability. Phenylthiourea has long been known as a tyrosinase inhibitorCitation89–91,Citation94. The chemical similarity analysis performing by ligand-based virtual or HTS screening identified ethionamide (26a) and its analogs (26c–26e), including prothionamide (26b), as tyrosinase inhibitorsCitation95 (). Ethionamide is an approved second-line antituberculosis drug used for the treatment of multidrug-resistant tuberculosis. In contrast, isoniazid, a structural analog and first-line antituberculosis drug was a poor inhibitor of tyrosinase. In B16 cells, inhibitors pyridine-2-carbothioamide and thiobenzamide substantially reduced the melanin content 44% and 37%, respectively. After an extensive structural analysis, the SAR data suggest that carbothioamide was a central moiety for the development of new and potent tyrosinase inhibitors.
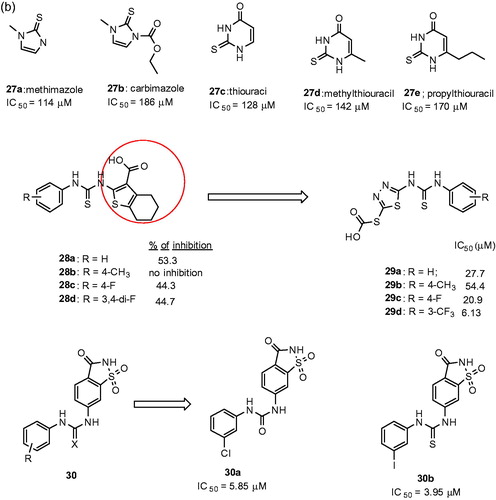
In a recent study, the researchers retrieved the thiourea-derived drugs in clinical use and investigated their effect on tyrosinase activities by using enzyme- and cell-based assaysCitation96. It was observed that the antithyroid agents methimazole 27a,Citation97 carbimazole 27b,Citation97 thiouracil 27c,Citation97 methylthiouracil 27dCitation97 and propylthiouracil 27eCitation97 inhibited mushroom tyrosinase (). In addition, kinetic studies assigned thiourea-containing drugs as noncompetitive inhibitors. The SAR studies explained that thiourea itself inhibits tyrosinase enzyme activity in a concentration-dependent manner. This shows the inhibitory activity of thiourea analogs must be originated from the sulfur and the nitrogen atoms.
In another study, a class of novel N-aryl-N′-substituted phenylthiourea derivatives was evaluated on the diphenolase activity of mushroom tyrosinaseCitation98. The results showed few 4,5,6,7-tetrahydro-2-[[(phenylamino)thioxomethyl]amino]-benzo[b]thiophene-3-carbo-xylic acid derivatives (28a–28d, ()) exhibited moderate inhibitory potency on diphenolase activity of tyrosinase. When the scaffold of 4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid was replaced with 2–(1,3,4-thiadiazol-2-yl)thio acetic acid, the inhibitory activity of compounds (e.g. 29a–29d, )Citation98 against tyrosinase was improved. Especially, 29d (IC50 = 6.13 μM) exhibited potent inhibitory activity than kojic acid (IC50 = 33.3 μM).
Since 1885, saccharin, 1,2-benzisothiazole-3-one-1,1-dioxide is a well-known heterocyclic compound and used as a sweetener in the form of its sodium salt. Besides, it is reported for many biological targets and thus can be viewed as a privileged scaffold. Recently, a novel series of 6-(phenylurenyl/thiourenyl) conjugated saccharin derivatives were evaluated for inhibitory effects on the diphenolase activity of banana tyrosinase enzymeCitation99. The results showed that all the compounds inhibited the tyrosinase activity. Among them, 6–(3-chlorophenylurenyl) saccharin 30a (Ki = 5.85 μM) and 6–(3-iodophenylthiourenyl) saccharin 30b (Ki = 3.95 μM) were found to be the most active compounds. The SAR studies showed 6-(phenylthiourenyl) saccharin derivatives exhibited higher inhibitory activity than 6-(phenylurenyl) saccharin derivatives. An electron withdrawing group at 3-position of phenylurenyl/thiourenyl-ring increased in activity, in particular, halogen series showed higher inhibitory activities.
Thiosemicarbazone-type inhibitors
Thiosemicarbazones occupy one of the major classes of tyrosinase inhibitors due to their classical structural unit and the ability to chelate copper ions at the active site of tyrosinase enzyme. Recently, a huge number of thiosemicarbazones by different research groups have been reportedCitation99–101 as potent tyrosinase inhibitors.
The SAR studies revealed that the thiosemicarbazide scaffold was a key unit for determining the tyrosinase inhibitory activity because it was able to effectively complex the two copper ions at the active site of tyrosinase. In an effort to improve the activity, a series of 4/3-aminoacetophenones and derived thiosemicarbazones were reported for their inhibitory activity of mushroom tyrosinaseCitation102. Results from the biological evaluation, the acylamino compounds (31a–31g, ) exhibited potent tyrosinase inhibitors than kojic acid (IC50 = 28.5 μM), and in contrast, the aminophenones (31 h and 31i) showed activation of tyrosinase activity. Compound 31d was found to be the most active compound with an IC50 value of 0.291 μM. The studies with SAR analysis also revealed that thiosemicarbazide group played a very vital role for tyrosinase inhibition, acylamino moieties crucial for enhancing the tyrosinase inhibitory activity and the acylamide substituent at 4-position on the phenyl ring increased the tyrosinase inhibitory potency. Moreover, the inhibition mechanism and kinetics study revealed that compound 31a was reversible and a noncompetitive inhibitor.
Figure 11. Chemical structure of thiosemicarbazone analogs, 31a–31iCitation102 and 32a–32iCitation103.
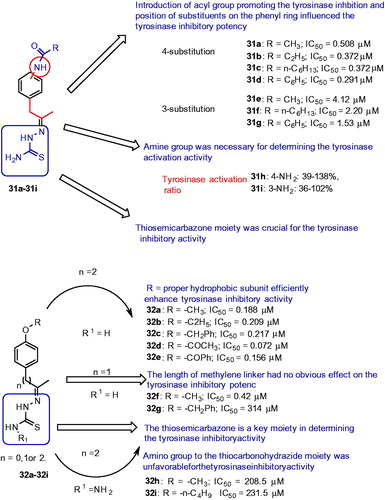
In continuing to search for potent compounds as highly efficient tyrosinase inhibitors, and understanding the SARs of thiosemicarbazone compounds, a series of novel 4-alkoxy- and 4-acyloxy-phenylethylenethiosemicarbazone analogs (32a–32i, ) were synthesized and evaluated for tyrosinase inhibitory activityCitation103. The results showed that most of the inhibitors displayed remarkable potency inhibiting tyrosinase with an IC50 value lower than 1.0 μM. In particular, compound 32d exhibited a remarkable tyrosinase inhibitory potency compared to other analogs from these series. The SAR studies revealed that the thiosemicarbazone moiety played a key role in determining the tyrosinase inhibitory activity. The length of methylene linker between the phenyl ring and the thiosemicarbazone moiety (32f–g) had no influence on the tyrosinase inhibitory potency. The introduction of thiocarbonohydrazide moiety (32 h–32i) was unfavorable for the tyrosinase inhibitory activity.
Peptide type inhibitors
In an effort to discover new tyrosinase inhibitors, the recent attention has been drawn to apply peptide sequences for tyrosinase inhibition. Several tyrosinase inhibitory peptides such as dipeptidesCitation104, cyclic peptidesCitation105, short-sequence oligopeptidesCitation106 and kojic acid tripeptide compoundsCitation107, have been investigated. Especially, oligopeptides have proven to be effective tyrosinase inhibitors. Two oligopeptides P3, an octapeptide (Arg-Ala-Asp-ser-Arg-Ala-Asp-Cys) and a decapeptide P4 (Tyr-Arg-ser-Arg-Lys-Tyr-Ser-Ser-Trp-Tyr) showed substantial inhibitory effects on mushroom and human tyrosinase with IC50 value of 123 and 40 μM, compared with hydroquinone. These oligopeptides did not show any effect on melanocytes cytotoxicityCitation108. After successful formulation into topical cream and favorable clinical results, a decapeptide P4 also known as decapeptide-12 has been commercialized as the main active ingredient in a skin-lightening productCitation109.
In a recent study, Hsiao et al., discovered dipeptide-like compound (A5) and tripeptides RCY and CRY effectively inhibit the tyrosinase activityCitation109. Especially, a novel tripeptide CRY showed the most striking inhibitory potency against mushroom tyrosinase (IC50 = 6.16 μM). This tripeptide is more potent than the known oligopeptides and comparable with kojic acid-tripeptides. CRY and RCY used the thiol group of the cysteine residue to coordinate with Cu ions at the active site of tyrosinase, thereby showing low tyrosinase activity. In another study, the cysteine-containing dipeptides were reported to inhibit the tyrosinase activity in an effective mannerCitation110. The authors suggested that these cysteine-containing dipeptides could directly block the active site of tyrosinase and thereby leading to potent inhibition. In particular, N-terminal cysteine-containing dipeptides markedly outperform the C-terminal and the cysteine-containing dipeptides, CE, CS, CY and CW show comparative bioactivities and tyrosine-containing dipeptides are substrate-like inhibitors. In addition, these dipeptides do not show significant cytotoxicity in melanocytes, and CA and PD attenuated 5.6 and 16.5% melanin content, respectively, at 100 μM ().
Table 1. Inhibition of melanin content in melanocytes by dipeptidesCitation111.
Li et al., reported a set of hydroxypyridinone-L-phenylalanine conjugates (33a and 33b) starting from kojic acid ()Citation111 for their inhibitory activities on tyrosinase. Evaluation against tyrosinase activity revealed that one of the compounds ((S)-(5-(benzyloxy)-1-octyl-4-oxo-1,4- dihydropyridin-2-yl)methyl 2-amino-3-phenylpropanoate, (33b) showed IC50 values 12.6 and 4.0 μM for monophenolase and diphenolase activities, respectively. Moreover, these conjugates were mixed-type inhibitors, suggesting they could bind to both the free enzyme and the enzyme − substrate complexes.
Figure 12. Chemical structure of peptide conjugates, 33a–33b,Citation112 34 and 35Citation113.
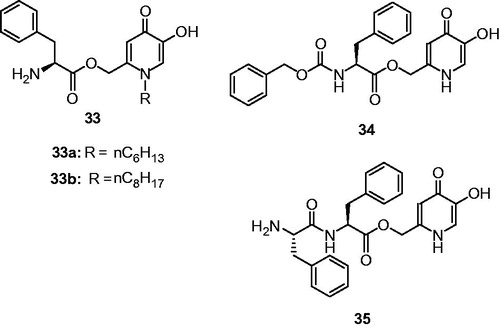
In another study, hydroxypyridinone-L-amino acid conjugates were designed and evaluated for their inhibitory activities on mushroom tyrosinaseCitation112. Among the investigated compounds, only two compounds exhibited both monophenolase (34, IC50 = 1.95 μM; 35, IC50 = 2.79 μM) and diphenolase inhibitory (34, IC50 = 8.97 μM; 35, IC50 = 26.20 μM) activity of tyrosinase (). Moreover, the compounds 34 and 35 were identified to be reversible and mixed-type inhibitors.
Miscellaneous mushroom tyrosinase inhibitors
Recently, the extracts and isolated compounds from natural sources have attracted much attention as tyrosinase inhibitors and have been accepted as popular skin whitening agentsCitation113–123. In an effort to find a safe and effective whitening substance, Chen et al., screened a number of natural products from herbal plants and isolated compounds 36 and 37 from the rhizome of Gastrodia elata, as mushroom tyrosinase inhibitorsCitation124. Subsequent SAR studies have identified analogs 38–40 (). Bis(4-hydroxybenzyl)sulfide 36 showed outstanding inhibitory potency against tyrosinase with an IC50 value of 0.5 μM and Ki value of 58 nM. The compound 37 connected through an ether linkage show 713-fold decrease in the inhibitory ability (IC50 = 378.11 μM) indicate the sulfur atom is very important in chelating with the copper ions and contributes in a greater inhibition tyrosinase activity. On the other hand, shortening the carbon linker, which connects the sulfur to benzene rings, resulted in the moderate tyrosinase inhibition of 38. The removal of hydroxyl group, 39 lead to poor tyrosinase inhibition, indicating that two hydroxyl groups are important. With the methoxy substitution, an analog of 36, reduces potency to IC50 value of 40.02 μM, suggesting that hydrogen bond interactions were favorable than the hydrophobic interactions provided by methoxide groups.
Figure 13. Chemical structures of miscellaneous tyrosinase inhibitors 36–40125 and 41–43Citation126.
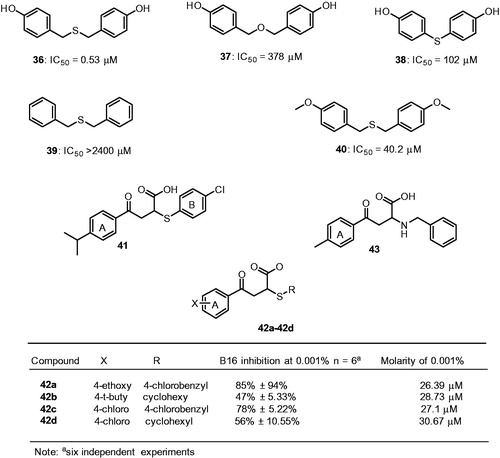
Compound 36 treated with 50 μM reduced 20% melanin content in the human melanocytes system without significant cytotoxicity. In addition, the zebrafish in vivo assay reveals that 36 effectively reduce melanogenesis with no adverse effect. Moreover, the acute oral toxicity study confirmed that the compound 36 was free of discernable cytotoxicity in mice. Thus compound 36 is a potential candidate in developing safe and effective pharmacological agent for skin-whitening. Recently, Ai et al., screened a chemical library using a virtual screening approach and identified a compound 41 as a potent mushroom tyrosinase inhibitorCitation125, with an IC50 value of 8 μM and yielded a 29%±17.64% blockage of melanin biosynthesis in B16 cells at a concentration of 0.002% that was equal to 27.5 μM.
The SAR studies examined around structure 41 showed the aromaticity of the ring B of compound 41 appears essential for activity, as replacement of ring B with cyclohexyl ring (compound 42b) losses the inhibitory activity of melanin synthesis in B16 cells. On the other hand, the substitution at 4-position of the ring A had a negligible effect on the inhibitory activity (42a–42d). These compounds were dose dependent and inhibit the melanin synthesis in B16 cells. However, further development of compound 41, lead to a potential formulation problem and eliminated as a candidate for cosmetic purposes. A further substructural analysis identified a compound 43 exhibited 79%±5.34% inhibition on melanin biosynthesis of B16 cells at a concentration of 0.001% (33.6 μM). These two compounds 41 and 43 possess good biochemical properties and satisfy Lipinski’s “rule of five” and exhibited a substantial inhibitory effect on melanin synthesis in B16 cells. This melanin synthesis inhibition was shown not to affect the cellular viability, which further underscores the potential commercial utility of these compounds.
It was reported that vanillin and vanillic acid isolated from Origanum vulgare may serve as agents for antimelanogenesisCitation126. Based on this background, a series of vanillin esters incorporating benzoic acid, cinnamic acid and piperazine have been reported for tyrosinase inhibitory activityCitation127. The results showed that compounds 44a–44d exhibited good to excellent inhibition of mushroom tyrosinase activity (). In particular, 44b exhibited the most potent inhibitory activity with an IC50 value of 16.13 μM. From the structural point of view, the substituted cinnamic acid esters and hydroxyl substitution played an important role in tyrosinase inhibition. The kinetic studies revealed compound 44b was a mixed-type tyrosinase inhibitor with Ki 13 μM and Ki′ 53 μM and formed reversible enzyme inhibitor complex.
Figure 14. Chemical structure of miscellaneous tyrosinase inhibitors, 44a–44c,Citation128 45 Citation129 46a–46c,Citation130 47a–47c,Citation131 48a–48b,Citation132 49 Citation133 50 Citation135 51a–51eCitation136 and 52.Citation139.
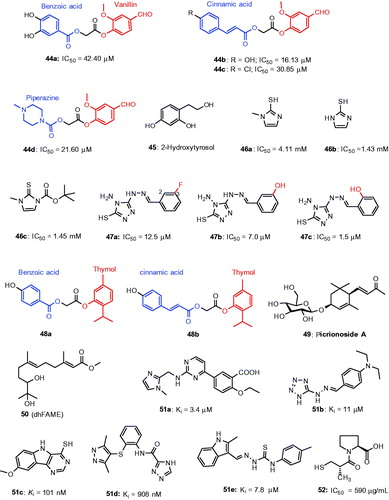
In another study, 2-hydroxytyrosol 45 (2-HT)Citation128 was found to inhibit mushroom tyrosinase with an IC50 value of 13.0 μmol/L which was equally potent as kojic acid (IC50 = 14.8 μmol/L). Furthermore, 2-HT dose-dependently inhibited tyrosinase activity (IC50 = 32.5 μmol/L) in the cell-free extract of B16 melanoma cells and α-MSH-stimulated melanin formation in intact B16 melanoma cells. Methimazole (2-mercapto-1-methylimidazole) derivatives were reported to inhibit mushroom tyrosinaseCitation129. 2-Mercaptoimidazole (46a), mercapto-1-methylimidazole (46b) and tert-butyl 3-methyl-2-sulfanylidene-2,3-dihydro-1H-imidazole-1-carboxylate (46c) significantly inhibited tyrosinase activity exhibiting an IC50 value of 4.11 mM, and 1.43 mM and 1.45 mM, respectively, (). Kinetic analysis indicated that compounds 46a and 46b as competitive tyrosinase inhibitors, while 46c as a noncompetitive tyrosinase inhibitor. Further, in vitro analysis on B16 cells, compounds of 46a–46c exerted potent inhibitory effect on intracellular melanin production without influencing the cytotoxicity.
A series of triazole Schiff’s base derivatives have been demonstrated for their inhibitory activity against mushroom tyrosinase activityCitation130. The results showed from the biological evaluation that only three compounds 47a–47c exhibit potent inhibitory effects with IC50 values of 12.5, 7.0 and 1.5 μM (). Kinetic analysis revealed that inhibitors are reversible and mixed type. Fluorescence quenching and copper interaction studies confirmed that interaction of inhibitors with tyrosinase and chelation ability with copper ions in the active site. From structural point of view, the substitution at 2-position of the benzene ring showed superior activity (47c) than 3-position (47b). A novel series of thymol analogs incorporating hydroxylated benzoic acids and cinnamic acids were reported as mushroom tyrosinase inhibitorsCitation131. In general, the cinnamic acid-derived thymol derivatives showed better tyrosinase inhibitory than the benzoic acid derivatives, exemplified with the comparison of 48a (IC50 15.20 μM) versus 48b (IC50 91.5 μM).
Recently, the effect of picrionoside A 49 isolated from the leaves of Korean ginseng (P. ginseng C.A. Mayer) was examinedCitation132 and it has inhibited mushroom tyrosinase activity with an IC50 value of 9.8 μM, about 6.8 to 10-fold higher than kojic acid and arbutin, respectively (). In melan-Ab cells, 49 reduced 17.1% melanin content in a dose-dependent manner without inducing the cell viability. In addition, Picrionoside A-treated zebrafish showed a remarkable inhibitory effect on body’s pigmentation. Taken together these results show that Picrionoside A may be an effective skin-whitening agentCitation133. In the course of screening, melanogenesis inhibitors in Streptomyces bikiniensis, it was found that S-(−)-10,11-dihydroxyfarnesoic acid methyl ester (dhFAME, 50), an insect juvenile hormone produced by Beauveria bassiana CS1029 Citation134 directly inhibited the tyrosinase activity. In addition, 50 significantly reduce the melanin content, inhibited the cellular tyrosinase activity as well as the intracellular accumulation of cAMP levels in melan-a cells without inducing the cell viability.
A new class of potent tyrosinase inhibitors was identified by structure-based virtual screening predictionCitation135. The structure of mushroom tyrosinase (PDB ID: 2Y9X) was used as a template for molecular dynamics (MD) simulation. Initially, an ensemble of 10 000 structures using molecular dynamics simulation was generated. Consequent screenings yielded top 61 molecules for evaluating against mushroom tyrosinase activity and the selective inhibitors (51a–51e) are indicated in . The results show that the moieties of tetrazole and triazole were able to interact with the di-copper catalytic center of the tyrosinase. In particular, a tetrazole compound 51b exhibiting the strongest activity. The authors found that many compounds displayed good reduction in melanin production in B16 melanoma cells with no cytotoxicity. Specifically, a thiosemicarbazone-containing compound 51e reduced melanin content by 55%. The results provide valuable insight into the modulation of the functions of type-3 copper enzymes.
Captopril ([2S]-N-[3-mercapto-2-methylpropionyl]-L-proline) is an angiotensin converting enzyme inhibitorCitation136,Citation137 which are widely used in the treatment of hypertension and heart failure. In order to identify novel and effective tyrosinase inhibitors, the inhibitory effect of captopril (52) was experimented for tyrosinase inhibitory activity. The result showed that 52 inhibited tyrosinase activity with an IC50 value of 590 μg/mL ()Citation138. Further in vitro studies in B16 cells, 52 was found to inhibit the tyrosinase activityCitation139,Citation140 in dose-dependent manner that leads to the inhibition of melanin formation without cytotoxicity.
Human tyrosinase inhibitors
Although a huge number of tyrosinase inhibitors were available and several of them with potent inhibitory activities were discussed earlier, almost all the inhibitors were evaluated against mushroom tyrosinase. In an effort to find novel inhibitors against human tyrosinase, Yoshimori et al demonstrated thujaplicins (52–54; α, β and γ isomers, ) for their inhibitory effects on both mushroom tyrosinase and human tyrosinase (hTYR), with comparisonCitation141. The results showed that β-and γ-thujaplicins (53 and 54) effectively inhibited the human tyrosinase activity in a dose-dependent manner with IC50 values of 8.98 and 1.15 μM, respectively. Especially, γ-thujaplicin 54 was extremely superior to kojic acid (IC50 = 17 μM). The SAR studies revealed the position of isopropyl on the tropolone scaffold was the determinant factor for the potency of thujaplicines. The potency of thujaplicins is in the following order: γ > β > α-thujaplicin.
Figure 15. Chemical structure of tyrosinase inhibitors; (a) thujaplicin analagoues (52–54),Citation142 (b) linderanolide B and subamolide ACitation143 and (c) resorcinol derivatives Citation144.
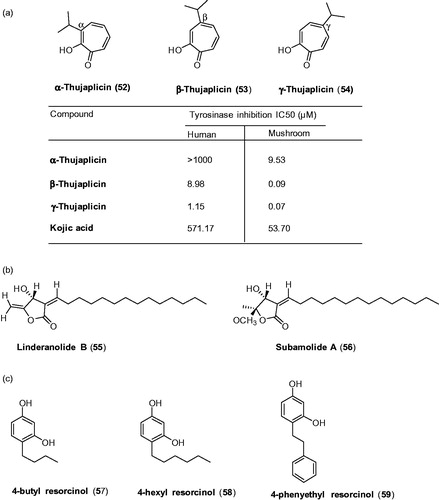
Researchers further evaluated the inhibitory effects of thujaplicins on mushroom tyrosinase inhibitory activities and compared with hTYR inhibitory activity. The results showed that huge differences in the inhibitory activities of thujaplicins against hTYR and mTYR were observed: kojic acid was found to be 10.64-fold weaker inhibition against hTYR than mTYR. In thujaplicins, α,β- and γ-thujaplicins were approximately >104.93-, 99.78-, and 16.43-fold, respectively, weaker inhibition against hTYR than mTYR. The activity differences of thujaplicins were explained by comparing the values obtained for hTYR and mTYR, and the amino acid compositions in the active sites of these enzymes.
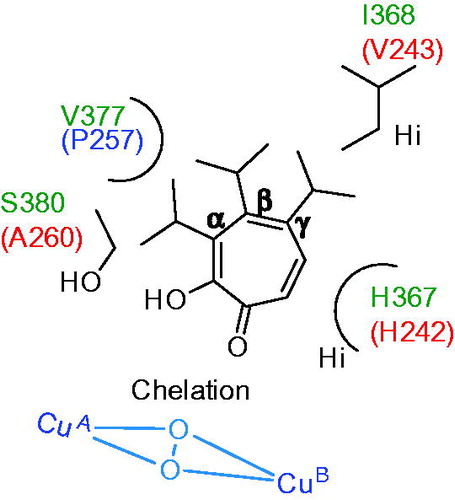
In order to understand the inhibitory mechanism, the binding mode of γ-thujaplicin was predicted using homology model of hTYR. It showed the carbonyl and hydroxyl group of the γ-thujaplicin chelate with two copper ions at the active site of hTYR. Tropolone scaffolds of thujaplicin forms stacking interaction with imidazole ring of His367 and hydrophobic interaction with isopropyl of Val377. The isopropyl group of thujaplicin forms hydrophobic interaction with Ile368. In addition, the results from comparative studies on the inhibitory effects of the other thujaplicins (α and β) indicated that van der Waals (VdW) clashes of the isopropyl group of α-thujaplicin with Val377 and S380 might reduce the inhibitory activity against hTYR. The main reason for higher inhibitory activity of γ–thujaplicin against hTYR among thujaplicins is considered to be the hydrophobic interaction of the isopropyl group with Ile368. In contrast, in mTYR, the Val377 and Ser380 are replaced with proline (P257) and alanine (A260), respectively, therefore it can be considered that there is little VdW clashes of the isopropyl group of α-thujaplicin with A260 and P257 (). This explains why the inhibitory effect of α-thujaplicin against mTYR (IC50 = 9.53 μM) is more than two-orders of magnitude stronger than hTYR (IC50> 1000 μM). Accordingly, it was suggested that the difference of Ala260 in mTYR and Ser380 in hTYR dramatically affect the inhibitory profile of α-thujaplicin.
Figure 16. Schematic representation of binding interaction of thujaplicins (α, β and γ) with hTYR (V377, I368, H367 and S380) and mTYR (P257, V243, H242 and A260)Citation142.
Wang and coworkers have recently found the natural products linderanolide B (55) and subamolide A (56) isolated from the stems of Cinnamomum were proved to have good in vitro inhibitory abilities of mushroom tyrosinase at low doses ()Citation142. Both compounds showed cell viability of human keratinocytes, melanocytes and fibroblasts treated with various concentrations of two compounds. Treatment at a low dose (0.01–1.0 μM) did not show significant cytotoxicity to human skin cells. The study revealed both compounds could reduce 50% of human tyrosinase activities at a dose of 1 μM after 48-h treatment and effectively inhibited (40% reduction) the melanin formation in HEMn-MP cells. Both 55 and 56 showed a remarkable inhibitory potential on zebrafish in vivo pigmentation even at low doses without observable toxicity. Therefore these two compounds are effective novel tyrosinase inhibitors to be considered as skin-whitening agent. In another study, Kolbe et al., examined the inhibitory effects of kojic acid, hydroquinone, arbutin with the other well-known class of compound 4-butyl resorcinol (57) on human tyrosinase as well as inhibition of production of MelanoDermTM skin model cultureCitation143. In 1995, 4-butyl resorcinol introduced a resorcinol derivative has inhibitory effect on both tyrosinaseCitation144 and TRP-1Citation145,Citation146. The results showed that 4-butyl resorcinol proved to be highly effective inhibitor of human tyrosinase with an IC50 value of 21 μmol/L and complete inhibition at concentrations above 100 μmol/L. 4-Butyl resorcinol exhibited 20 times more potent inhibitory activity than kojic acid, which showed an IC50 of 500 μmol/L and maximum inhibition (89%) was achieved at 5.6 mmol/L concentration. Arbutin and hydroquinone are poor inhibitors of human tyrosinase with IC50 values in the millimolar range, that is, 6500 μmol/L for arbutin and 4400 μmol/L for hydroquinone. However, none of both have completely inhibited the human tyrosinase.
In melanoDerm skin model, arbutin showed only marginal efficacy on melanin production with an IC50 value of 500 μmol/L, while kojic acid inhibited with an IC50 of 400 μmol/L. Interestingly, hydroquinone inhibited melanin production with an IC50 value below 40 μmol/L, which is probably due to different mechanisms of tyrosinase inhibition. 4-Butyl resorcinol was the most potent inhibitor with an IC50 of 13.5 μmol/L. The in vivo efficacy of 4-butyl resorcinol was confirmed in clinical studies. Patients with age spots on the forearm treated twice daily two age spots with a formula containing 4-n-butylresorcinol (57), 4-hexylresorcinol (58) and 4-phenylethylresorcinol (59). Within 8 weeks, 4-butylresorcinol (57) significantly reduced the appearance of age spots while 4-hexylresorcinol, 4-phenylethylresorcinol showed significant effects after 12 weeks. A second study showed that 4-butylresorcinol was more effective than 4-hexylresorcinol and 4-phenylethylresorcinol. The resulting clinical output on hyperpigmentation reveals that 4-butylresorcinol could be a valuable active compound for the management of pigmentation disorders. In fact, 4-butylresorcinol has been used for the treatment of melasma. In all published literatures, 4-butylresorcinol 0.1% cream showed rapid efficacy, safety and tolerability when it is used for the melasma treatmentCitation147,Citation148. The recent study reported that 4-butylresorcinol 0.3% cream is safe, effective and well tolerated in Indian patients with melismaCitation149.
Conclusions
Catalyzing the rate-limiting step of melanin synthesis, tyrosinase has become one of the most important targets for the development of hypopigmenting agents. In fact, tyrosinase is the most studied target for inhibiting the melanogenesis. Therefore, the inhibitors that target tyrosinase may specifically inhibit the melanogenesis in cells without other side effects. As a result, in recent years, numerous inhibitors have been developed and an overview of the inhibitors discussed in this review is shown in . Different classes of inhibitors include chalcones, resveratrols and flavanones were discussed in this review. Very interestingly, inhibitors with β-phenyl-α,β-unsaturated carbonyl scaffold were newly classified in this report and showed remarkable tyrosinase inhibitory activities. Especially, benzylidene-2-thiohydantoins and 5-benzylidene(thio)barbiturates showed greater inhibitory potency (). More medicinal chemistry efforts and structure activity relationships on these scaffolds would bring novel inhibitors in future. Another new scaffold bis(4-hydroxybenzyl)sulfide 36 showed outstanding inhibitory potency against tyrosinase with an IC50 value of 0.5 μM and Ki value of 58 nM. Compound 36 treated with 50 μM reduced 20% melanin content in the human melanocytes system without significant cytotoxicity. In addition, the zebrafish in vivo assay revealed that 36 effectively reduce melanin formation without adverse effects. Moreover, the acute oral toxicity study confirmed that the compound 36 was free of discernable cytotoxicity in mice. Thus, compound 36 is a potential candidate in developing safe and effective pharmacological agent for skin-whitening.
Repurposing of existing drugs has become one of the important approaches in the drug discovery program of developing potent melanogenesis inhibitors. The data associated with an existing drug will reduce the time and cost associated with the intellectual right for developing the novel pharmaceutics. This approach has several advantages; including availability, lower cost and safety/tolerability. Phenylthiourea has long been known as a tyrosinase inhibitor. The researchers retrieved the thiourea-derived drugs in clinical use and investigated their effect on tyrosinase activities. Ethionamide (26a) and its analogs (26c–26e), including prothionamide (26b), were identified as tyrosinase inhibitors (). Ethionamide is an approved second-line antituberculosis drug used for the treatment of multidrug-resistant tuberculosis. Many antithyroid drugs were identified as potent tyrosinase inhibitors; especially, methimazole 27a, carbimazole 27b, thiouracil 27c, methylthiouracil 27d, and propylthiouracil 27e inhibited mushroom tyrosinase ().
In general, mushroom tyrosinase is the most frequently used in vitro model for screening the hypopigmenting agents in the development of skin-whitening substance, while human and mouse melanocytic lysates were used to a lesser extent. This is because of the tyrosinase from the mushroom Agaricus bisporus is abundantly available and can be easily purified. However, in several aspects, the tyrosinase from mushroom is very different from the human tyrosinase. A secreted form of a mushroom tyrosinase is a tetramer enzyme present in the cytosol of the cells, while human tyrosinase is a monomeric and inactive glycosylated membrane bound form. Furthermore, it has been reported that human tyrosinase showed 6-fold higher affinity for L-DOPA oxidation activity than the mushroom tyrosinase, the Km value of human and mushroom tyrosinase for L-DOPA were 0.31 mM and 1.88 mM, respectively. In addition, the amino acid sequence identity between human and mushroom tyrosinase is 23%. These structural discrepancies were well correlated in the tyrosinase inhibitory activities assayed by AbTYR and hTYR. In fact, it was found that many melanogenesis inhibitors did not exhibit inhibitory effects on mushroom tyrosinase activity (see comparison of thujaplicins, section human tyrosinase inhibitors).
In conclusion, we hope that this review will be useful to medicinal chemists working on melanogenesis, especially on tyrosinase proteins, to identify novel inhibitors with drug-like properties.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Available from: http://www.sironabiochem.com/products/skinlightening/
- Available from: http://cosmetics.specialchem.com/news/industry-news/skin-lightening-products-market-to-reach-usd23-bn-by-2020-global-industry-analysts (Published on 2015-02-16)
- Francisco S, Stefania B, Mauro P, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res 2006;19:550–7.
- Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem 2002;50:125–33.
- Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 2007;21:976–94.
- Ahn SJ, Koketsu M, Ishihara H, et al. Regulation of melanin synthesis by selenium-containing carbohydrates. Chem Pharm Bull 2006;54:281–6.
- Iozumi K, Hoganson GE, Pennella R, et al. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J Invest Dermatol 1993;100:806–11.
- Li G, Ju HK, Chang HW, et al. Melanin biosynthesis inhibitors from the bark of Machilus thunbergii. Biol Pharm Bull 2003;26:1039–41.
- Unver N, Freyschmidt-Paul P, Horster S, et al. Alterations in the epidermal melanin axis and factor XIIIa melanophages in senile lentigo and ageing skin. Br J Dermatol 2006;155:119–28.
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol 2008;84:539–49.
- Urabe K, Nakayama J, Hori Y. In Norlund JJ, Boissy RE, et al. eds. The pigmentary system: physiology and pathophysiology. New York, NY: Oxford University Press; 1998:909–913.
- Yang JY, Koo JH, Song YG, et al. Stimulation of melanogenesis by scoparone in B16 melanoma cells. Acta Pharmacol Sin 2006;27:1467–73.
- Pillaiyar T, Manickam M, Jung SH. Inhibitors of melanogenesis: a patent review (2009–2014). Expert Opin Ther Pat 2015;7:775–88.
- Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol 2010;42:1094–104.
- Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004;84:1155–228.
- Hearing VJ. Mammalian tyrosinase-the critical regulatory control point in melanocyte pigmentation. Int J Biochem 1987;19:1141–7.
- Halaban R, Patton RS, Cheng E, et al. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem 2002;277:14821–8.
- Sánchez-Ferrer A, Rodríguez-López JN, García-Cánovas F, et al. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1995;1247:1–11.
- (a) Matoba Y, Kumagai T, Yamamoto A, et al. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 2008;281:8981–90. (b) Bijelic A, Pretzler M, Molitor C, et al. The Structure of a Plant Tyrosinase from Walnut Leaves Reveals the Importance of “Substrate-Guiding Residues” for Enzymatic Specificity. Angew Chem Int Ed Engl 2015;54:14677–80.
- Decker H, Tuczek F. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem Sci 2000;25:392–7.
- Jiménez-Atiénzar M, Escribano J, Cabanes J, et al. Oxidation of the flavonoid eriodictyol by tyrosinase. Plant Physiol Biochem 2005;43:866–73.
- Cavalieri EL, Li KM, Balu N, et al. Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis 2002;23:1071–7.
- Vontzalidou A, Zoidis G, Chaita E, et al. Design, synthesis and molecular studies of dihydrostilbene derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 2012;22:5523–6.
- Hasegawa T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int J Mol Sci 2010;11:1082–9.
- Tessari I, Bisaglia M, Valle F, et al. The reaction of alpha-synuclein with tyrosinase: possible implications for Parkinson disease. J Biol Chem 2008;283:16808–17.
- Greggio E, Bergantino E, Carter D. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson's disease. J Neurochem 2005;93:246–56.
- Yi W, Cao R, Peng W, et al. Synthesis and biological evaluation of novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors. Eur J Med Chem 2010;45:639–46.
- Friedman M. Food browning and its prevention: an overview. J Agric Food Chem 1996;44:631–53.
- Mayer AM. Polyphenol oxidases in plants-recent progress. Phytochemistry 1987;26:11–20.
- Liu SH, Pan IH, Chu IM. Inhibitory effect of p-hydroxybenzyl alcohol on tyrosinase activity and melanogenesis. Biol Pharm Bull 2007;30:1135–9.
- Arndt KA, Fitzpatrick TB. Topical use of hydroquinine as a depigmenting agent. J Am Med Assoc 1965;194:117–19.
- Fitzpatrick TB, Arndt KA, el-Mofty AM, et al. Hydroquinone and psoralens in the therapy of hypermelanosis and vitiligo. Arch Dermatol 1966;93:589–600.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol 1975;111:40–8.
- Heilgemeir GP, Balda BR. [Irreversible toxic depigmentation. Observations following use of hydroquinonemonobenzylether-containing skin bleaching preparations]. MMW Munch Med Wochenschr 1981;123:47–8.
- Kumar K, Vani MG, Wang SY, et al. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013;39:259–70.
- Gonçalez M, Corrêa M, Chorilli M. Skin delivery of kojic acid-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. BioMed Res Int 2013;2013:271–6.
- Ki DH, Jung HC, Noh YW, et al. Preformulation and formulation of newly synthesized QNT3-18 for development of a skin whitening agent. Drug Dev Ind Pharm 2013;39:526–33.
- Breathnach AC, Nazzaro-Porro M, Passi S, et al. Azelaic acid therapy in disorders of pigmentation. Clin Dermatol 1989;7:106–19.
- Verallo-Rowell VM, Verallo V, Graupe K, et al. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol Suppl (Stockh) 1989;143:58–61.
- Shivhare S, Malviya K, Malviya K, et al. A review: natural skin lighting and nourishing agents. Res J Top Cosmet Sci 2013; 4:21–5.
- Huang CH, Sung HC, Hsiao CY, et al. Transdermal delivery of three vitamin C derivatives by Er: YAG and carbon dioxide laser pretreatment. Lasers Med Sci 2013;28:807–14.
- Yao CL, Lin YM, Mohamed MS, et al. Inhibitory effect of ectoine on melanogenesis in B16-F0 and A2058 melanoma cell lines. Biochem Eng J 2013;78:163–9.
- Won YK, Loy CJ, Randhawa M, et al. Clinical efficacy and safety of 4-hexyl-1,3-phenylenediol for improving skin hyperpigmentation. Arch Dermatol Res 2014;306:455–65.
- Son K, Heo M. The evaluation of depigmenting efficacy in the skin for the development of new whitening agents in Korea. Int J Cosmet Sci 2013;35:9–18.
- Chen YS, Lee SM, Lin CC, et al. Kinetic study on the tyrosinase and melanin formation inhibitory activities of carthamus yellow isolated from Carthamus tinctorius L. J Biosci Bioeng 2013;115:242–5.
- Hsieh PW, Chen WY, Aljuffali A, et al. Co-drug strategy for promoting skin targeting and minimizing the transdermal diffusion of hydroquinone and tranexamic acid. Curr Med Chem 2013;20:4080–92.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol 2013;12:57–66.
- Eimpunth S, Wanitphadeedecha R, Manuskiatti W. A focused review on acne-induced and aesthetic procedure-related postinflammatory hyperpigmentation in Asians. J Eur Acad Dermatol 2013;27:7–18.
- Engasser PG. Ochronosis caused by bleaching creams. J Am Acad Dermatol 1984;10:1072–3.
- Fisher AA. Current contact news. Hydroquinone uses and abnormal reactions. Cutis 1983;250:240–4.
- Romaguera C, Grimalt F. Leukoderma from hydroquinone. Contact Dermatitis 1985;12:183.
- Curto EV, Kwong C, Hermersdörfer H, et al. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem Pharmacol 1999;57:663–72.
- Zhou H, Kepa JK, Siegel D, et al. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol Pharmacol 2009;76:579–87.
- Fujimoto N, Onodera H, Mitsumori K, et al. Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats. Carcinogenesis 1999;20:1567–71.
- Spínola V, Mendes B, Câmara JS, et al. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs. iodometric titration as analytical methods. LWT-Food Sci Technol 2013;50:489–95.
- Arulmozhi V, Pandian K, Mirunalini S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf B Biointerfaces 2013;110:313–20.
- Sonmez F, Sevmezler S, Atahan A, et al. Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg Med Chem Lett 2011;21:7479–82.
- Takahashi M, Takara K, Toyozato T, et al. A novel bioactive chalcone of Morus australis inhibits tyrosinase activity and melanin biosynthesis in B16 melanoma cells. J Oleo Sci 2012;61:585–92.
- Radhakrishnan SK, Shimmon RG, Conn C, et al. Azachalcones: a new class of potent polyphenol oxidase inhibitors. Bioorg Med Chem Lett 2015;25:1753–6.
- Radhakrishnan SK, Shimmon RG, Conn C, et al. Evaluation of novel chalcone oximes as inhibitors of tyrosinase and melanin formation in B16 cells. Arch Pharm (Weinheim) 2016;349:20–9.
- Radhakrishnan SK, Shimmon RG, Conn C, et al. Inhibitory kinetics of novel 2,3-dihydro-1H-inden-1-one chalcone-like derivatives on mushroom tyrosinase. Bioorg Med Chem Lett 2015;25:5495–9.
- Wang Y, Curtis-Long MJ, Lee BW, et al. Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg Med Chem 2014;22:1115–20.
- Tan X, Song YH, Park C, et al. Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking. Bioorg Med Chem 2016;24:153–9.
- Satooka H, Kubo I. Resveratrol as a kcat type inhibitor for tyrosinase: potentiated melanogenesis inhibitor. Bioorg Med Chem 2012;20:1090–9.
- Lee TH, Seo JO, Baek SH, et al. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol Ther (Seoul) 2014;22:35–40.
- Franco DC, de Carvalho GS, Rocha PR, et al. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules 2012;17:11816–25.
- Song YM, Ha YM, Kim JA, et al. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 2012;22:7451–5.
- Bae SJ, Ha YM, Kim JA, et al. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydroxyphenyl)diazenyl)phenyl 4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci Biotechnol Biochem 2013;77:65–72.
- Bae SJ, Ha YM, Park YJ, et al. Design, synthesis, and evaluation of (E)-N-substituted benzylidene-aniline derivatives as tyrosinase inhibitors. Eur J Med Chem 2012;57:383–90.
- Borges F, Roleira F, Milhazes N, et al. Simple coumarins: privileged scaffolds in medicinal chemistry. Front Med Chem 2009;4:23–85.
- Masamoto Y, Murata Y, Baba K, et al. Inhibitory effects of esculetin on melanin biosynthesis. Biol Pharm Bull 2004;27:422–5.
- Fais A, Corda M, Era B, et al. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009;14:2514–20.
- Matos MJ, Santana L, Uriarte E, et al. New halogenated phenylcoumarins as tyrosinase inhibitors. Bioorg Med Chem Lett 2011;21:3342–45.
- Ashraf Z, Rafiq M, Seo SY, et al. Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J Enzyme Inhib Med Chem 2015;30:874–83.
- Asthana S, Zucca P, Vargiu AV, et al. Structure-activity relationship study of hydroxycoumarins and mushroom tyrosinase. J Agric Food Chem 2015;63:7236–24.
- Gardelly M, Trimech B, Belkacem MA, et al. Synthesis of novel diazaphosphinanes coumarin derivatives with promoted cytotoxic and anti-tyrosinase activities. Bioorg Med Chem Lett 2016;26:2450–4.
- Ha YM, Kim JA, Park YJ, et al. Analogs of 5-(substituted benzylidene)hydantoin as inhibitors of tyrosinase and melanin formation. Biochim Biophys Acta Gen Subj 2011;1810:612–19.
- Ha YM, Kim JA, Park YJ, et al. Synthesis and biological activity of hydroxybenzylidenyl pyrrolidine-2,5-dione derivatives as new potent inhibitors of tyrosinase. Med Chem Comm 2011;2:542–9.
- Kim SH, Ha YM, Moon KMC, et al. Anti-melanogenic effect of (Z)-5-(2,4-dihydroxybenzylidene) thiazolidine-2,4-dione, a novel tyrosinase inhibitor. Arch Pharm Res 2013;36:1189–97.
- Chung KW, Park YJ, Choi YJ, et al. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4 dihydroxybenzylidene)pyrrolidine-2,5-dione (3-DBP). Biochim Biophys Acta (Gen Subj) 2012;1820:962–9.
- Kim HR, Lee HJ, Choi YJ, et al. Benzylidene-linked thiohydantoin derivatives as inhibitors of tyrosinase and melanogenesis: importance of the β-phenyl-α,β-unsaturated carbonyl functionality. Med Chem Comm 2014;5:1410–17.
- Yun HY, Kim do H, Son S, et al. Design, synthesis, and anti-melanogenic effects of (E)-2-benzoyl-3-(substituted phenyl)acrylonitriles. Drug Des Devel Ther 2015;9:4259–68.
- Son S, Kim H, Yun HY, et al. (E)-2-Cyano-3-(substituted phenyl)acrylamide analogs as potent inhibitors of tyrosinase: a linear β-phenyl-α,β-unsaturated carbonyl scaffold. Bioorg Med Chem 2015;23:7728–34.
- Isao K, Ikuyo KH. Tyrosinase inhibitory activity of the olive oil flavor compounds. J Agric Food Chem 1999;47:4574–8.
- Cui Y, Liang G, Hu YH, et al. Alpha-substituted derivatives of cinnamaldehyde as tyrosinase inhibitors: inhibitory mechanism and molecular analysis. J Agric Food Chem 2015;63:716–22.
- Yan Q, Cao R, Yi W, et al. Synthesis and evaluation of 5-benzylidene(thio)barbiturate-beta-D-glycosides as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:4055–8.
- Yan Q, Cao R, Yi W, et al. Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur J Med Chem 2009;44:4235–43.
- Chen Z, Cai D, Mou D, et al. Design, synthesis and biological evaluation of hydroxy- or methoxy-substituted 5-benzylidene(thio) barbiturates as novel tyrosinase inhibitors. Bioorg Med Chem 2014;22:3279–84.
- Ambati NB, Anand V, Hanumanthu P. A facile synthesis of 2-n(methyl amino) benzothiazoles. Synth. Commun 1997;27:1487–93.
- Pan B, Huang RZ, Han SQ, et al. Design, synthesis, and antibiofilm activity of 2-arylimino-3-aryl-thiazolidine-4-ones. Bioorg Med Chem Lett 2010;20:2461–4.
- Criton M, Le Mellay-Hamon V. Analogues of N-hydroxy-N'-phenylthiourea and N-hydroxy-N'-phenylurea as inhibitors of tyrosinase and melanin formation. Biorg Med Chem Lett 2008;18:3607–10.
- Thanigaimalai P, Hoang TA, Lee KC, et al. Structural requirement(s) of N-phenylthioureas and benzaldehyde thiosemicarbazones as inhibitors of melanogenesis in melanoma B 16 cells. Bioorg Med Chem Lett 2010;20:2991–3.
- Thanigaimalai P, Lee KC, Sharma VK, et al. Structural requirement of phenylthiourea analogs for their inhibitory activity of melanogenesis and tyrosinase. Bioorg Med Chem Lett 2011;21:6824–8.
- (a) Hall AM, Orlow SJ. Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation. Pigment Cell Res 2005;18:122–9. (b) Poma A, Bianchini S, Miranda M. Inhibition of L-tyrosine-induced micronuclei production by phenylthiourea in human melanoma cells. Mutat Res 1999;446:143–8. (c) Du BK, Erway WF. Studies on the mechanism of action of thiourea and related compounds; inhibition of oxidative enzymes and oxidations catalyzed by copper. J Biol Chem 1946;165:711–21.
- (a) Choi J, Park SJ, Jee JG. Analogues of ethionamide, a drug used for multidrug-resistant tuberculosis, exhibit potent inhibition of tyrosinase. Eur J Med Chem 2015;106:157–66. (b) Choi J, Jee JG. Repositioning of thiourea-containing drugs as tyrosinase inhibitors. Int J Mol Sci 2015;16:28534–48.
- Cooper DS. Antithyroid drugs. N Engl J Med 1984;311:1353–62.
- Liu P, Shu C, Liu L, et al. Design and synthesis of thiourea derivatives with sulfur-containing heterocyclic scaffolds as potential tyrosinase inhibitors. Bioorg Med Chem 2016;24:1866–71.
- Gençer N, Demir D, Sonmez F, et al. New saccharin derivatives as tyrosinase inhibitors. Bioorg Med Chem 2012;20:2811–21.
- (a) Zhu YJ, Song KK, Li ZC, et al. Antityrosinase and antimicrobial activities of transcinnamaldehyde thiosemicarbazone. J Agric Food Chem 2009;57:5518–23. (b) Li ZC, Chen LH, Yu XJ, et al. Inhibition kinetics of chlorobenzaldehyde thiosemicarbazones on mushroom tyrosinase. J Agric Food Chem 2010;58:12537–40. (c) Chen LH, Hu YH, Song W, et al. Synthesis and antityrosinase mechanism of benzaldehyde thiosemicarbazones: novel tyrosinase inhibitors. J Agric Food Chem 2012;60:1542–7. (d) Pan ZZ, Zhu YJ, Yu XJ, et al. Synthesis of 40-thiosemicarbazonegriseofulvin and its effects on the control of enzymatic browning and postharvest disease of fruits. J Agric Food Chem 2012;60:10784–8. (e) Yang MH, Chen CM, Hu YH, et al. Inhibitory kinetics of DABT and DABPT as novel tyrosinase inhibitors. J Biosci Bioeng 2013;115:514–27.
- *(a) Liu JB, Yi W, Wan YQ, et al. 1-(1-Arylethylidene)thiosemicarbazide derivatives: a new class of tyrosinase inhibitors. Bioorg Med Chem 2008;16:1096–102. (b) Liu JB, Cao RH, Yi W, et al. A class of potent tyrosinase inhibitors: alkylidenethiosemicarbazide compounds. Eur J Med Chem 2009;44:1773–8. (c) Yi W, Cao RH, Wen H, et al. Discovery of 4- functionalized phenyl-O-beta-D-glycosides as a new class of mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:6157–60. (d) Yi W, Cao RH, Chen ZY, et al. Design, synthesis and biological evaluation of hydroxy- or methoxy-substituted phenylmethylenethiosemicarbazones as tyrosinase inhibitors. Chem Pharm Bull 2009;57:1273–7. (e) Yi W, Cao RH, Chen ZY, et al. Rational design and synthesis of 4-o-substituted phenylmethylenethiosemicarbazones as novel tyrosinase inhibitors. Chem Pharm Bull 2010;58:752–4. (f) Yi W, Dubois C, Yahiaoui S, et al. Refinement of arylthiosemicarbazone pharmacophore in inhibition of mushroom tyrosinase. Eur J Med Chem 2011;46:4330–5. (g) Buitrago E, Vuillamy A, Boumendjel A, et al. Exploring the interaction of N/S compounds with a dicopper center: tyrosinase inhibition and model studies. Inorg Chem 2014;53:12848–58.
- (a) Thanigaimalai P, Lee KC, Sharma VK, et al. Ketonethiosemicarbazones: structure-activity relationships for their melanogenesis inhibition. Bioorg Med Chem Lett 2011;21:3527–30. (b) Lee KC, Thanigaimalai P, Sharma VK, et al. Structural characteristics of thiosemicarbazones as inhibitors of melanogenesis. Bioorg Med Chem Lett 2010;20:6794–6.
- You A, Zhou J, Song S, et al. Structure-based modification of 3-/4-aminoacetophenones giving a profound change of activity on tyrosinase: from potent activators to highly efficient inhibitors. Eur J Med Chem 2015;93:255–62.
- You A, Zhou J, Song S, et al. Rational design, synthesis and structure–activity relationships of 4-alkoxy- and 4-acyloxy-phenylethylenethiosemicarbazone analogues as novel tyrosinase inhibitors. Bioorg Med Chem 2015;23:924–31.
- Girelli AM, Mattei E, Messina A, et al. Inhibition of polyphenol oxidases activity by various dipeptides. J Agric Food Chem 2004; 52:2741–5.
- Morita H, Kayashita T, Kobata H, et al. Pseudostellarins D-F, new tyrosinase inhibitory cyclic peptides from Pseudostellaria heterophylla. Tetrahedron 1994;50:9975–82.
- Ubeid AA, Zhao L, Wang Y, et al. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J Invest Dermatol 2009;129:2242–9.
- Kim H, Choi J, Cho JK, et al. Solid-phase synthesis of kojic acid − tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorg Med Chem Lett 2004;14:2843–6.
- Reddy B, Jow T, Hantash BM. Bioactive oligopeptides in dermatology: part I. Exp Dermatol 2012;21:563–8.
- Hsiao NW, Tseng TS, Lee YC, et al. Serendipitous discovery of short peptides from natural products as tyrosinase inhibitors. J Chem Inf Model 2014;54:3099–111.
- Tseng TS, Tsai KC, Chen WC, et al. Discovery of potent cysteine-containing dipeptide inhibitors against tyrosinase: a comprehensive investigation of 20 × 20 dipeptides in inhibiting dopachrome formation. J Agric Food Chem 2015;63:6181–8.
- Li DF, Hu PP, Liu MS, et al. Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. J Agric Food Chem 2013;61:6597–603.
- Zhao DY, Zhang MX, Dong XW, et al. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg Med Chem Lett 2016;16:30486–93.
- Baek S, Kim J, Kim D, et al. Inhibitory effect of dalbergioidin isolated from the trunk of Lespedeza cyrtobotrya on melanin biosynthesis. J Microbiol Biotechnol 2008;18:874–9.
- Yanagihara M, Yoshimatsu M, Inoue A, et al. Inhibitory effect of gnetin C, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull 2012;35:993–6.
- Roh JS, Han JY, Kim JH, et al. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol Pharm Bull 2004;27:1976–8.
- Kim JH, Kim MR, Lee ES, et al. Inhibitory effects of calycosin isolated from the root of Astragalus membranaceus on melanin biosynthesis. Biol Pharm Bull 2009;32:264–8.
- Kong YH, Jo YO, Cho CW, et al. Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol Pharm Bull 2008;31:946–8.
- Cho Y, Kim KH, Shim JS, et al. Inhibitory effects of macelignan isolated from Myristica fragrans HOUTT. on melanin biosynthesis. Biol Pharm Bull 2008;31:986–9.
- Lee MY, Kim JH, Choi JN, et al. The melanin synthesis inhibition and radical scavenging activities of compounds isolated from the aerial part of Lespedeza cyrtobotrya. J Microbiol Biotechnol 2010;20:988–94.
- Kim JP, Kim BK, Yun BS, et al. Melanocins A, B and C, new melanin synthesis inhibitors produced by Eupenicillium shearii. I. Taxonomy, fermentation, isolation and biological properties. J Antibiot (Tokyo) 2003;56:993–9.
- Chen LG, Chang WL, Lee CJ, et al. Melanogenesis inhibition by gallotannins from Chinese galls in B16 mouse melanoma cells. Biol Pharm Bull 2009;32:1447–52.
- Kim SJ, Son KH, Chang HW, et al. Tyrosinase inhibitory prenylated flavonoids from Sophora flavescens. Biol Pharm Bull 2003;26:1348–50.
- Khan SB, Azhar-Ul-Haq, Afza N, et al. Tyrosinase-inhibitory long-chain esters from Amberboa ramosa. Chem Pharm Bull (Tokyo) 2005;53:86–9.
- Chen WC, Tseng TS, Hsiao NW, et al. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci Rep 2015;5:7995.
- Ai N, Welsh WJ, Santhanam U, et al. Novel virtual screening approach for the discovery of human tyrosinase inhibitors. PLoS One 2014;9:e112788.
- Chou TH, Ding HY, Hung WJ, et al. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp Dermatol 2010;19:742–50.
- Ashraf Z, Rafiq M, Seo SY, et al. Synthesis, kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg Med Chem 2015;23:5870–80.
- Uchida R, Ishikawa S, Tomoda H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm Sin B 2014;4:141–5.
- Chan CF, Lai ST, Guo YC, et al. Inhibitory effects of novel synthetic methimazole derivatives on mushroom tyrosinase and melanogenesis. Bioorg Med Chem 2014;22:2809–15.
- Yu F, Jia YL, Wang HF, et al. Synthesis of triazole schiff's base derivatives and their inhibitory kinetics on tyrosinase activity. PLoS One 2015;10:e0138578.
- Ashraf Z, Rafiq M, Seo SY, et al. Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur J Med Chem 2015;98:203–11.
- Lee DY, Jeong SC, Jeong YT, et al. Antimelanogenic effects of picrionoside A Isolated from the leaves of Korean Ginseng. Biol Pharm Bull 2015;38:1663–7.
- Millott N, Lynn WG. Ubiquity of melanin and the effect of phenylthiourea. Nature 1966;209:99–101.
- Baek SH, Ahn JW, Nam SH, et al. S-(-)-10,11-dihydroxyfarnesoic acid methyl ester inhibits melanin synthesis in murine melanocyte cells. Int J Mol Sci 2014;15:12750–63.
- Choi J, Choi KE, Park SJ, et al. Ensemble-based virtual screening led to the discovery of new classes of potent tyrosinase inhibitors. J Chem Inf Model 2016;56:354–67.
- Cleland JG, Dargie HJ, Hodsman GP, et al. Captopril in heart failure. A double blind controlled trial. Br Heart J 1984;52:530–5.
- Cleland JG. The clinical course of heart failure and its modification by ACE inhibitors: insights from recent clinical trials. Eur Heart J 1994;15:125–30.
- Kuo TC, Ho FM. Competitive inhibition of mushroom tyrosinase by captopril. Res J Biotechnol 2013;8:26–9.
- Espín JC, Wichers HJ. Effect of captopril on mushroom tyrosinase activity in vitro. Biochim Biophys Acta 2001;1544:289–300.
- Chu HL, Wang BS, Chang LC, et al. Effects of captopril on melanin formation in B16 cells. J Food Drug Anal 2012;20:668–73.
- Yoshimori A, Oyama T, Takahashi S, et al. Structure-activity relationships of the thujaplicins for inhibition of human tyrosinase. Bioorg Med Chem 2014;22:6193–200.
- Wang HM, Chen CY, Wen ZH. Identifying melanogenesis inhibitors from cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp Dermatol 2011;20:242–8.
- Kolbe L, Mann T, Gerwat W, et al. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J Eur Acad Dermatol Venereol 2013;27:19–23.
- Kim DS, Kim SY, Park SH, et al. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull 2005;28:2216–19.
- Katagiri T, Okubo T, Oyobikawa M, et al. Inhibitory action of 4-nbutylresorcinol on melanogenesis and its skin whitening effect. J Soc Cosmet Chem Jpn 2001;35:42–9.
- Okubo T, Oyohikawa M, Futaki K, et al. The inhibitory effects of 4-N-butyl-resorcinol on melanogenesis [abstract]. J Dermatol Sci 1995;10:88.
- Huh SY, Shin JW, Na JI, et al. The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: a randomized controlled split-face trial. Ann Dermatol 2010;22:21–5.
- Huh SY, Shin JW, Na JI, et al. Efficacy and safety of liposome-encapsulated 4-n-butylresorcinol 0.1%cream for the treatment of melasma: a randomized controlled split-face trial. J Dermatol 2010;37:311–15.
- Mohan NTM, Gowda A, Jaiswal AK, et al. Assessment of efficacy, safety, and tolerability of 4-n-butylresorcinol 0.3% cream: an Indian multicentric study on melasma. Clin Cosmet Investig Dermatol 2016;9:21–7.