Abstract
A novel family of cinnamic acid derivatives has been developed to be multifunctional cholinesterase inhibitors against AD by fusing N-benzyl pyridinium moiety and different substituted cinnamic acids. In vitro studies showed that most compounds were endowed with a noteworthy ability to inhibit cholinesterase, self-induced Aβ (1–42) aggregation, and to chelate metal ions. Especially, compound 5l showed potent cholinesterase inhibitory activity (IC50, 12.1 nM for eeAChE, 8.6 nM for hAChE, 2.6 μM for eqBuChE and 4.4 μM for hBuChE) and the highest selectivity toward AChE over BuChE. It also showed good inhibition of Aβ (1–42) aggregation (64.7% at 20 μM) and good neuroprotection on PC12 cells against amyloid-induced cell toxicity. Finally, compound 5l could penetrate the BBB, as forecasted by the PAMPA-BBB assay and proved in OF1 mice by ex vivo experiments. Overall, compound 5l seems to be a promising lead compound for the treatment of Alzheimer’s diseases.
Introduction
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder that is clinically associated with cognitive impairment, language skill loss and dementiaCitation1. To date almost 48 million elderly people are affected by AD, and this number is estimated to show unparalleled growth and increase to spread 131.5 million by 2050Citation2. Although the etiopathogenesis of AD is unclear, multiple factors such as amyloid-β (Aβ) deposits, low levels of acetylcholine (ACh), τ-protein aggregation, dyshomeostasis of biometals and oxidative stress, play a vital role in the pathogenesis of ADCitation3. The current therapeutic options against AD are composed by one N-methyl-d-aspartate (NMDA) receptor antagonist and three acetylcholinesterase (AChE) inhibitors, namely memantine (NMDA), donepezil, rivastigmine and galantamine (AChE)Citation4. Nevertheless, these marketed drugs modestly alleviate the symptoms but cannot cure brain damage or stop neuronal degeneration.
AChE and BuChE play a role in cholinergic signaling. According to the cholinergic hypothesis, the decrease in ACh levels results in memory loss and cognitive impairment, and the clinical restoration of cholinergic function is believed to alleviate AD symptomsCitation5,Citation6. Furthermore, studies have illustrated that AChE interacts with Aβ through the peripheral anionic site (PAS) promoting the formation of steady AChE-Aβ complexes, which are more toxic than single Aβ peptidesCitation7. Therefore, dual-site AChE inhibitors may be promising AD drug candidatesCitation8,Citation9.
Among multiple factors, neurotoxic Aβ plaques in the brain are a key contributing factor in the pathology of AD. Aβ (1–40) and Aβ (1–42) are the key isoforms of Aβ peptides. Aβ (1–42) may aggregate more rapidly and show stronger neuron cytotoxicity than Aβ (1–40) doesCitation10,Citation11. Preventing the formation and accumulation of Aβ is a probable therapeutic strategy for AD. The dyshomeostasis of metal ions such as Cu, Fe and Zn is commonly observed in many critical aspects of ADCitation12. The Cu2+ present in the brain at an abnormally high concentration interacts with Aβ, leading to the accelerated formation of neurofibrillary tangles (NFTs) and generate reactive oxygen species (ROS), which further induce oxidative impairment in the brainCitation13. Moreover, abnormally high levels of redox-active metal ions, such as Fe2+ and Cu2+, in the brain may lead to the formation of ROSCitation14. Notably, compared with the normal levels of metal ions in brain, the subtraction of physiologically essential metals may lead to risk. Consequently, reducing the abnormally high concentration of metals in the brain by chelating the metals is an additional logical approach for AD treatment. Oxidative stress also plays a crucial role in the development of neurodegenerative disorders such as AD. It has been hypothesised that the antioxidant defence system cannot neutralize oxidative species in elderly peopleCitation15. The oxidative stress theory of ageing also suggests that oxidative damage plays an important role in neuronal degenerationCitation16. Therefore, drugs that can scavenge oxygen radicals may be used to prevent AD.
Due to the pathological complication of AD, the multi-target-directed ligand (MTDL) design strategy has been proposed, and a range of compounds have been developed to act on various targetsCitation17–21. In AD, the effectiveness of multifunctional molecules with two or more distinct pharmacological properties being properly incorporated is higher than that of single-targeted drugsCitation22. Therefore, ChEIs with multiple effects, such as Aβ disaggregation, neuroprotection and oxidative load reduction may be a significant approach for AD management.
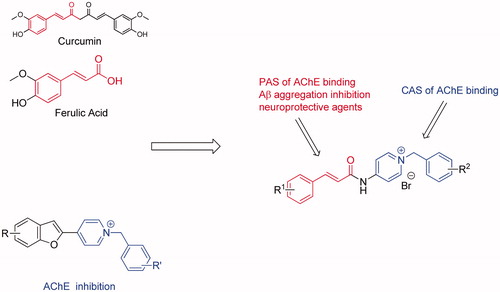
Cinnamic acid derivatives are naturally occurring compounds that possess various pharmacological properties for diverse neurological disordersCitation23. Ferulic acid (FA) and curcumin (Cur) are representative bioactive compounds, and they can prevent Aβ fibril aggregation, inhibit Aβ-mediated toxicity, scavenge ROS and reduce inflammatory effects both in vitro and in vivoCitation24–29. Consequently, cinnamic acid could serve as a beneficial fragment in the designed MTDL, such as tacrine-FA hybrids, FA-memoquin hybrids, FA-carbazole hybrids, donepezil-FA hybrids, cinnamic-N-benzylpiperidine hybridsCitation30–34.
In the search for new cinnamic acid-based derivatives as anti-AD, we have focused on the structure of benzylpyridinium salts (), which may represent a privileged scaffold that can be used to develop new AChE inhibitorsCitation35–37. Docking studies have shown that the N-benzyl pyridinium moiety interacts with the CAS of AChE, and the heterocyclic moiety interacts with the PAS of AChE forming stacking interactions. Proceeding with our researches on natural products with probable use as anti-ADCitation38–40, we reasonably combined the cinnamic acid with benzyl-pyridinium to achieve new hybrids that are anticipated to be dual-acting AChE. In this study, all designed compounds were synthesised and evaluated for their biological activities, including their anti-aggregation activity towards Aβ, ChE inhibition, antioxidant activity, metal chelating properties, neuroprotective effects against Aβ-induced PC12 cell injury and the ability to cross the blood–brain barrier (BBB). Moreover, kinetic and molecular modelling studies were performed to further explore their mechanism of interaction with AChE and Aβ.
Materials and methods
Materials
All chemicals (reagent grade) used were purchased from Sino Pharm Chemical Reagent Co., Ltd. (Shanghai, China). Reaction progress was monitored using analytical thin layer chromatography (TLC) on precoated silica gel GF254 (Qingdao Haiyang Chemical Plant, Qing-Dao, China) plates and the spots were detected under UV light (254 nm). Melting point was measured on an XT-4 micromelting point instrument and uncorrected. IR (KBr-disc) spectra were recorded by Bruker Tensor 27 spectrometer (Billerica, MA).
1H NMR and 13C NMR spectra were measured on a BRUKER AVANCE III spectrometer (Billerica, MA) at 25 °C and referenced to TMS. Chemical shifts are reported in ppm (δ) using the residual solvent line as internal standard. Splitting patterns are designed as s, singlet; d, doublet; t, triplet; m, multiplet. The purity of all compounds was confirmed to be higher than 95% through analytical HPLC performed with Agilent 1200 HPLC System (Santa Clara, CA). Mass spectra were obtained on a MS Agilent 1100 Series LC/MSD Trap mass spectrometer (ESI-MS) (Santa Clara, CA).
General procedure for the preparation of compounds 3a–b
A mixture of compound 1 (10 mmol) and DMAP (10 mmol) in 20 mL anhydrous CH2Cl2 was stirred at 0 °C for about 10 min and EDCI (20 mmol) was added to the mixture and stirred at room temperature for 1 h. The compound 2 (10 mmol) was added to the solution, and stirring was continued overnight. The reaction mixture was diluted with H2O and extracted with CH2Cl2. The organic extracts were combined, washed with brine, and dried with anhydrous Na2SO4, and the solvent was evaporated in vacuo to give the crude product, which was purified by silica gel chromatography with CH2Cl2:MeOH =15:1 as an eluent to afford corresponding target compound as a yellow solid.
N-(pyridin-4-yl)cinnamamide (3a)
Cinnamic acid was reacted with 4-aminopyridine following the general procedure to give the desired product 3a with a yield of 85%. ESI/MS m/z: 225.1 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 8.75 (s, 1H), 8.50 (dd, J = 4.9, 1.4 Hz, 2H), 7.79 (d, J = 15.5 Hz, 1H), 7.64 (dd, J = 4.8, 1.6 Hz, 2H), 7.49 (dd, J = 6.6, 3.0 Hz, 2H), 7.40–7.32 (m, 3H), 6.63 (d, J = 15.5 Hz, 1H).
(E)-3-(3,4-dimethoxyphenyl)-N-(pyridin-4-yl) acrylamide (3b)
(E)-3-(3,4-dimethoxy -phenyl) acrylic acid was reacted with 4-aminopyridine following the general procedure to give the desired product 3b with a yield of 88%. ESI/MS m/z: 285.2 [M + H]+; 1H NMR (400 MHz, CDCl3) δ 8.54 (d, J = 5.3 Hz, 2H), 7.96 (s, 1H), 7.75 (d, J = 15.4 Hz, 1H), 7.62 (d, J = 6.0 Hz, 2H), 7.15 (dd, J = 8.3, 1.8 Hz, 1H), 7.06 (d, J = 1.8 Hz, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.47 (d, J = 15.4 Hz, 1H), 3.95 (s, 3H), 3.89 (s, 3H).
General procedure for the preparation of compounds 5a–n
Compound 3 (10 mmol), appropriate benzyl chloride (12 mmol) and a catalytic amount of KI in dry acetonitrile (20 mL) was refluxed for 1–2 h. When the reaction was completed as indicated by TLC, the mixture was then concentrated under reduced pressure, and 20 mL of diethyl ether was added. On cooling, the precipitate was filtered and washed with diethyl ether to get the target compounds 5a–n with high yields.
1-Benzyl-4-cinnamamidopyridin-1-ium bromide (5a)
Yield 91%; yellow solid; IR (KBr) ν 3091, 3028, 2963, 1685, 1637, 1515, 1496, 1203, 1161, 966, 763, 752, 725 cm−1; m.p. >250 °C; ESI/MS m/z: 315.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.90 (s, 1H), 8.98 (d, J = 6.7 Hz, 2H), 8.25 (d, J = 6.5 Hz, 2H), 7.79 (d, J = 15.7 Hz, 1H), 7.75–7.65 (m, 2H), 7.55–7.37 (m, 8H), 7.05 (d, J = 15.8 Hz, 1H), 5.74 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.44, 152.24, 145.20, 145.20, 143.99, 134.68, 133.93, 130.81, 129.15, 129.15, 129.15, 129.15, 129.15, 129.15, 128.50, 128.50, 128.27, 128.27, 120.20, 115.21, 61.50.
4-Cinnamamido-1-(3-methylbenzyl) pyridin-1-ium bromide (5b)
Yield 90%; yellow solid; IR (KBr) ν 3074, 3023, 2969, 1701, 1633, 1596, 1527, 1456, 1166, 965, 849, 743 cm−1; m.p. >250 °C; ESI/MS m/z: 329.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.80 (s, 1H), 8.94 (d, J = 6.7 Hz, 2H), 8.22 (d, J = 6.4 Hz, 2H), 7.80 (d, J = 15.6 Hz, 1H), 7.70 (d, J = 4.9 Hz, 2H), 7.49 (d, J = 5.1 Hz, 3H), 7.33 (dd, J = 15.5, 8.0 Hz, 2H), 7.28 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 7.5 Hz, 1H), 6.99 (d, J = 15.7 Hz, 1H), 5.67 (s, 2H), 2.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.92, 152.72, 145.68, 145.68, 144.56, 139.03, 135.06, 134.42, 131.33, 131.03, 129.66, 129.66, 129.58, 129.51, 128.78, 128.78, 126.08, 120.66, 115.77, 115.77, 62.09, 21.39.
4-Cinnamamido-1-(4-methylbenzyl) pyridin-1-ium bromide (5c)
Yield 88%; yellow solid; IR (KBr) ν 3015, 1697, 1628, 1509, 1458, 1339, 1162, 975, 860, 761 cm−1; m.p. >250 °C; ESI/MS m/z: 329.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.93 (d, J = 6.9 Hz, 2H), 8.23 (d, J = 6.6 Hz, 2H), 7.79 (d, J = 15.7 Hz, 1H), 7.70 (d, J = 4.9 Hz, 2H), 7.49 (d, J = 5.1 Hz, 3H), 7.40 (d, J = 7.7 Hz, 2H), 7.26 (d, J = 7.6 Hz, 2H), 7.01 (d, J = 15.9 Hz, 1H), 5.67 (s, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.91, 152.68, 145.58, 145.58, 144.52, 139.18, 134.43, 132.16, 131.32, 130.18, 130.18, 129.65, 129.65, 129.08, 129.08, 128.77, 128.77, 120.68, 115.72, 115.72, 61.90, 21.22.
4-Cinnamamido-1-(3-fluorobenzyl) pyridin-1-ium bromide (5d)
Yield 89%; white solid; IR (KBr) ν 3075, 3018, 2966, 1701, 1632, 1589, 1526, 1448, 1146, 965, 848, 750, 676 cm−1; m.p. >250 °C; ESI/MS m/z: 333.2 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.96 (d, J = 6.7 Hz, 2H), 8.23 (d, J = 6.5 Hz, 2H), 7.80 (d, J = 15.8 Hz, 1H), 7.70 (d, J = 4.9 Hz, 2H), 7.54–7.45 (m, 4H), 7.42 (d, J = 9.7 Hz, 1H), 7.34 (d, J = 7.6 Hz, 1H), 7.28 (t, J = 8.6 Hz, 1H), 7.00 (d, J = 15.8 Hz, 1H), 5.74 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.93, 163.72 (d, 1JCF=246.44 Hz), 152.85, 145.78, 145.78, 144.60, 137.61 (d, 3JCF=7.58 Hz), 134.41, 131.80 (d, 3JCF=8.29 Hz), 131.34, 129.66, 129.66, 128.79, 128.79, 120.64, 116.51 (d, 2JCF=21.72 Hz), 116.07 (d, 2JCF=22.15 Hz), 115.80, 61.27.
4-Cinnamamido-1-(4-fluorobenzyl) pyridin-1-ium bromide (5e)
Yield 95%; yellow solid; IR (KBr) ν 3083, 3022, 2969, 1626, 1509, 1462, 1338, 1158, 964, 846, 767 cm−1; m.p. >250 °C; ESI/MS m/z: 333.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.95 (d, J = 6.7 Hz, 2H), 8.23 (d, J = 6.5 Hz, 2H), 7.79 (d, J = 15.8 Hz, 1H), 7.70 (d, J = 5.2 Hz, 2H), 7.63–7.57 (m, 2H), 7.49 (d, J = 5.1 Hz, 3H), 7.31 (t, J = 8.4 Hz, 2H), 7.00 (d, J = 15.8 Hz, 1H), 5.71 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.92, 163.15 (d, 1JCF=246.44 Hz), 152.76, 145.61, 144.56, 137.03, 134.42, 131.62 (d, 3JCF=7.98 Hz), 131.33, 129.65, 128.78, 120.66, 116.52 (d, 2JCF=21.37 Hz), 115.77, 61.19.
1-(3-Bromobenzyl)-4-cinnamamidopyridin-1-ium bromide (5f)
Yield 92%; yellow solid; IR (KBr) ν 3087, 3020, 2966, 1708, 1634, 1595, 1516, 1165, 1165, 1136, 966, 837 761, 703 cm−1; m.p. >250 °C; ESI/MS m/z: 393.0, 395.0 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.82 (s, 1H), 8.95 (d, J = 6.8 Hz, 2H), 8.23 (d, J = 6.5 Hz, 2H), 7.86–7.75 (m, 2H), 7.70 (d, J = 4.8 Hz, 2H), 7.64 (d, J = 8.3 Hz, 1H), 7.50 (dd, J = 12.0, 6.4 Hz, 4H), 7.43 (t, J = 7.8 Hz, 1H), 6.98 (d, J = 15.8 Hz, 1H), 5.72 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.93, 152.85, 145.76, 145.76, 144.62, 137.60, 134.41, 132.52, 131.91, 131.81, 131.35, 129.66, 129.66, 128.79, 128.79, 128.20, 122.70, 120.64, 115.83, 115.83, 61.14.
1-(4-Bromobenzyl)-4-cinnamamidopyridin-1-ium bromide (5g)
Yield 90%; yellow solid; IR (KBr) ν 3020, 1707, 1624, 1514, 1460, 1336, 1141, 972, 837, 762 cm−1; m.p. >250 °C; ESI/MS m/z: 393.0, 395.0 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 1H), 8.92 (d, J = 7.4 Hz, 2H), 8.22 (d, J = 7.2 Hz, 2H), 7.79 (d, J = 15.8 Hz, 1H), 7.72–7.69 (m, 2H), 7.68–7.65 (m, 2H), 7.50–7.45 (m, 5H), 6.97 (d, J = 15.8 Hz, 1H), 5.70 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.94, 152.81, 145.73, 145.73, 144.54, 134.47, 134.43, 132.56, 132.56, 131.35, 131.35, 129.65, 129.65, 128.77, 128.77, 123.06, 120.69, 115.73, 115.73, 61.19.
(E)-1-benzyl-4-(3-(3,4-dimethoxyphenyl) acrylamido) pyridin-1-ium bromide (5h)
Yield 91%; yellow solid; IR (KBr) ν 3087, 3022, 2962, 1706, 1641, 1596, 1512, 1463, 1264, 1132, 1025, 965, 746, 703 cm−1; m.p. >250 °C; ESI/MS m/z: 375.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 8.95 (d, J = 7.4 Hz, 2H), 8.22 (d, J = 7.3 Hz, 2H), 7.73 (d, J = 15.6 Hz, 1H), 7.51–7.41 (m, 5H), 7.28 (dd, J = 6.4, 1.9 Hz, 2H), 7.06 (d, J = 8.9 Hz, 1H), 6.89 (d, J = 15.7 Hz, 1H), 5.72 (s, 2H), 3.85 (s, 3H), 3.80 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.92, 157.60, 156.56, 154.20, 150.40, 150.40, 149.54, 139.97, 134.41, 134.41, 134.33, 133.72, 133.72, 131.95, 128.10, 122.89, 120.34, 117.04, 115.64, 115.64, 66.73, 60.90, 60.74.
(E)-4-(3-(3,4-dimethoxyphenyl) acrylamido)-1-(3-methylbenzyl) pyridin-1-ium bromide (5i)
Yield 93%; yellow solid; IR (KBr) ν 3016, 1697, 1626, 1509, 1458, 1257, 1134, 1015, 971, 840, 771, 709, 593 cm−1; m.p. >250 °C; ESI/MS m/z: 389.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.91 (d, J = 7.4 Hz, 2H), 8.20 (d, J = 7.1 Hz, 2H), 7.73 (d, J = 15.6 Hz, 1H), 7.36–7.22 (m, 6H), 7.06 (d, J = 8.9 Hz, 1H), 6.84 (d, J = 15.7 Hz, 1H), 5.66 (s, 2H), 3.85 (s, 3H), 3.80 (s, 3H), 2.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.17, 152.81, 151.81, 149.44, 145.59, 145.59, 144.83, 139.03, 135.07, 130.22, 129.57, 129.49, 127.18, 126.05, 123.39, 118.09, 115.59, 115.59, 112.27, 110.86, 62.04, 56.14, 55.99, 21.38.
(E)-4-(3-(3,4-dimethoxyphenyl) acrylamido)-1-(4-methylbenzyl) pyridin-1-ium bromide (5j)
Yield 94%; yellow solid; IR (KBr) ν 3068, 3014, 2948, 1683, 1622, 1508, 1462, 1293, 1132, 975, 794, 594 cm−1; m.p. >250 °C; ESI/MS m/z: 389.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.40 (s, 1H), 8.65 (d, J = 6.7 Hz, 2H), 8.52 (s, 2H), 7.74 (d, J = 15.5 Hz, 1H), 7.48 (d, J = 15.5 Hz, 1H), 7.31–7.27 (m, 3H), 7.22–7.17 (m, 4H), 6.83 (d, J = 8.3 Hz, 1H), 5.65 (s, 2H), 3.95 (s, 3H), 3.90 (s, 3H), 2.35 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.15, 152.77, 151.81, 149.45, 145.51, 145.51, 144.82, 139.17, 132.18, 130.18, 130.18, 129.07, 129.07, 127.02, 123.39, 118.10, 115.57, 115.57, 112.29, 110.85, 61.84, 56.16, 55.99, 21.22.
(E)-4-(3-(3,4-dimethoxyphenyl) acrylamido)-1-(3-fluorobenzyl) pyridin-1-ium bromide (5k)
Yield 88%; yellow solid; IR (KBr) ν 3014, 2964, 1711, 1688, 1592, 1518, 1445, 1258, 1137, 1014, 969, 850, 752, 571 cm−1; m.p. >250 °C; ESI/MS m/z: 393.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 8.95 (d, J = 7.4 Hz, 2H), 8.22 (d, J = 7.0 Hz, 2H), 7.74 (d, J = 15.6 Hz, 1H), 7.55–7.47 (m, 1H), 7.42 (d, J = 9.8 Hz, 1H), 7.34 (d, J = 7.7 Hz, 1H), 7.27 (dd, J = 14.2, 4.5 Hz, 3H), 7.06 (d, J = 8.9 Hz, 1H), 6.88 (d, J = 15.7 Hz, 1H), 5.74 (s, 2H), 3.83 (d, J = 4.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 173.89, 165.13 (d, 1JCF=228.26 Hz), 161.48, 152.96, 151.82, 149.45, 145.71, 145.71, 144.85, 137.67 (d, 3JCF=8.16 Hz), 131.79 (d, 3JCF=8.27 Hz), 127.19, 125.19, 123.38, 118.10, 116.50 (d, 2JCF=21.52 Hz), 116.06 (d, 2JCF=22.38 Hz), 115.85, 115.62, 115.62, 112.28, 110.88, 61.19, 56.14, 55.98.
(E)-4-(3-(3,4-dimethoxyphenyl) acrylamido)-1-(4-fluorobenzyl) pyridin-1-ium bromide (5l)
Yield 91%; yellow solid; IR (KBr) ν 3080, 3015, 1703, 1621, 1597, 1506, 1461, 1263, 1131, 1021, 964, 844, 802, 786, 596 cm−1; m.p. >250 °C; ESI/MS m/z: 393.1 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.91 (d, J = 7.3 Hz, 2H), 8.19 (d, J = 7.0 Hz, 2H), 7.72 (d, J = 15.6 Hz, 1H), 7.58 (dd, J = 8.7, 5.4 Hz, 2H), 7.31 (d, J = 8.9 Hz, 1H), 7.28 (dd, J = 4.2, 2.5 Hz, 2H), 7.05 (d, J = 8.9 Hz, 1H), 6.83 (d, J = 15.6 Hz, 1H), 5.69 (s, 2H), 3.82 (d, J = 3.7 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 166.17, 162.94 (d, 1JCF=251.49 Hz), 152.86, 151.82, 149.45, 145.55, 145.55, 144.82, 131.60 (d, 3JCF=8.08 Hz), 131.41, 127.19, 123.37, 118.11, 116.51 (d, 2JCF=21.47 Hz), 115.60, 112.28, 110.87, 61.13, 56.15, 55.98.
(E)-1-(3-bromobenzyl)-4-(3-(3, 4-dimethoxyphenyl) acrylamido) pyridin-1-ium bromide (5m)
Yield 92%; yellow solid; IR (KBr) ν 2928, 2831, 1592, 1511, 1460, 1313 1137, 990, 859, 762, 710 cm−1; m.p. >250 °C; ESI/MS m/z: 453.0, 455.0 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 8.93 (d, J = 6.9 Hz, 2H), 8.21 (d, J = 6.5 Hz, 2H), 7.80 (s, 1H), 7.74 (d, J = 15.6 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.42 (t, J = 7.9 Hz, 1H), 7.29 (s, 2H), 7.06 (d, J = 8.5 Hz, 1H), 6.85 (d, J = 15.6 Hz, 1H), 5.70 (s, 2H), 3.83 (d, J = 3.8 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 166.17, 152.96, 151.83, 149.45, 145.68, 144.90, 137.63, 132.51, 131.90, 131.81, 128.18, 127.17, 123.41, 122.70, 118.06, 115.68, 112.28, 110.87, 61.09, 56.15, 55.98.
(E)-1-(4-bromobenzyl)-4-(3-(3,4-dimethoxyphenyl) acrylamido) pyridin-1-ium bromide (5n)
Yield 88%; yellow solid; IR (KBr) ν 3012, 2948, 1681, 1618, 1508, 1460, 1268, 1130, 975, 841, 797 cm−1; m.p. >250 °C; ESI/MS m/z: 453.0, 455.0 [M]+; 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 8.91 (d, J = 7.3 Hz, 2H), 8.20 (d, J = 6.9 Hz, 2H), 7.73 (d, J = 15.6 Hz, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.32–7.25 (m, 2H), 7.06 (d, J = 8.9 Hz, 1H), 6.85 (d, J = 15.7 Hz, 1H), 5.69 (s, 2H), 3.82 (d, J = 3.7 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 166.17, 152.91, 151.83, 149.45, 145.66, 145.66, 144.87, 134.51, 132.57, 132.57, 131.32, 131.32, 127.18, 123.40, 123.05, 118.08, 115.62, 115.62, 112.28, 110.86, 61.18, 56.14, 55.98.
Biological activity
In vitro inhibition studies on AChE and BuChE
Acetylcholinesterase (E.C. 3.1.1.7) from electric eel and human erythrocytes, butyrylcholinesterase (BuChE, E.C. 3.1.1.8) from equine serum and human serum, 5, 5′-dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent, DTNB), S-butyrylthiocholine iodide (BTCI), acetylthiocholine iodide (ATCI) and donepezil hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). The capacity of the test compounds 5a–n to inhibit AChE and BuChE activities was assessed by Ellman’s methodCitation41. Test compound was dissolved in a minimum volume of DMSO (1%) and was diluted using the buffer solution (50 mM Tris–HCl, pH =8.0, 0.1 M NaCl, 0.02 M MgCl2·6H2O). In 96-well plates, 160 μL of 1.5 mM DTNB, 50 μL of AChE (0.22 U/mL prepared in 50 mM Tris–HCl, pH =8.0, 0.1% w/v bovine serum albumin, BSA) or 50 μL of BuChE (0.12 U/mL prepared in 50 mM Tris–HCl, pH =8.0, 0.1% w/v BSA) were incubated with 10 μL of various concentrations of test compounds (0.001–100 μM) at 37 °C for 6 min followed by the addition of the substrate (30 μL) ATCI (15 mM) or BTCI (15 mM) and the absorbance was measured at different time intervals (0, 60, 120 and 180 s) at a wavelength of 405 nm. The concentration of compound producing 50% of enzyme activity inhibition (IC50) was calculated by nonlinear regression analysis of the response-concentration (log) curve, using the Graph-Pad Prism program package (Graph Pad Software, San Diego, CA). Results are expressed as the mean ± SEM of at least three different experiments performed in triplicate.
Kinetic analysis of AChE inhibition
To obtain the mechanism of action 5l, reciprocal plots of 1/velocity versus 1/substrate were constructed at different concentrations of the substrate thiocholine iodide (0.05–0.5 mM) by using Ellman’s methodCitation35. Three concentrations of 5l were selected for the studies: 30.0, 15.0 and 7.5 nM for the kinetic analysis of AChE inhibition. The plots were assessed by a weighted least-squares analysis that assumed the variance of velocity (v) to be a constant percentage of v for the entire data set. Slopes of these reciprocal plots were then plotted against the concentration of 5l in a weighted analysis and Ki was determined as the intercept on the negative x-axis. Data analysis was performed with Graph Pad Prism 4.03 software (Graph Pad Software Inc., San Diego, CA).
Molecular modeling studies
Molecular modeling calculations and docking studies were performed using Molecular Operating Environment (MOE) software version 2008.10 (Chemical Computing Group, Montreal, Canada)Citation43. The X-ray crystallographic structures of AChE (PDB code 1EVE) and Aβ (1–42) (PDB code PDB 1IYT) were obtained from the Protein Data Bank. All water molecules in PDB files were removed and hydrogen atoms were subsequently added to the protein. The compound 5l was built using the builder interface of the MOE program and energy minimized using MMFF94x force field. Then the 5l was docked into the active site of the protein by the “Triangle Matcher” method, which generated poses by aligning the ligand triplet of atoms with the triplet of alpha spheres in cavities of tight atomic packing. The Dock scoring in MOE software was done using ASE scoring function and force field was selected as the refinement method. The best 10 poses of molecules were retained and scored. After docking, the geometry of resulting complex was studied using the MOE’s pose viewer utility.
ABTS radical cation scavenging activity assay49
2,2′-Azino-bis-2-ethybenz-thiazoline-6-sulfonic acid (ABTS) was dissolved in purified water to a 7 mM concentration. ABTS radical cation (ABTS.+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for at least 18 h before use. The stock solution of ABTS was serially diluted with sodium phosphate buffer (50 mM, pH 7.4) to 100 μM. Trolox and 5a–n at different concentrations (total volume of 50 μL) were added to 150 μL of 100 μM ABTS solution, respectively. After the addition of either trolox or another antioxidant to the ABTS solution, complete mixing of reactants was achieved by bubbling three to four times using plastic pipettes. The optical absorbance of ABTS at 415 nm was measured at 6 min after addition and equilibrated at 30 °C. Each individual treatment was repeated for three times and the results of the experiments were compared.
Inhibition of Aβ (1–42) self-induced aggregation
Inhibition of self-induced Aβ (1–42) aggregation was measured using a Thioflavin T (ThT)-binding assayCitation47. HFIP pretreated Aβ (1–42) samples (Anaspec Inc., Fremont, CA) were resolubilized with a 50 mM phosphate buffer (pH 7.4) to give a 25 μM solution. Each tested compound was firstly prepared in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and 1 μL of each was added to the well of black, opaque Corning 96-well plates such that the final solvent concentration was 10%. The final concentration of each compound was 20 μM and was prepared in independent triplicates. The solvent control was also included. Then, 9 μL of 25 mM Aβ (1–42) sample was added to each well and the samples mixed by gentle trapping. Plates were covered to minimize evaporation and incubated in dark at room temperature for 46–48 h with no agitation.
After the incubation period, 200 μL of 5 μM ThT in 50 mM glycine–NaOH buffer (pH 8.0) was added to each well. Fluorescence was measured on a SpectraMax M5 (Molecular Devices, Sunnyvale, CA) multi-mode plate reader with excitation and emission wavelengths at 446 nm and 490 nm, respectively. The fluorescence intensities were compared and the percent inhibition due to the presence of the inhibitor was calculated by the following formula: 100 − (IFi/IFo×100) where IFi and IFo are the fluorescence intensities obtained for Aβ (1–42) in the presence and in the absence of inhibitor, respectively.
Metal-chelating study
The study of metal chelation was performed in methanol at 298 K using UV–vis spectrophotometer (SHIMADZU UV-2450PC, Kyoto, Japan) with wavelength ranging from 200 to 500 nm. Due to the low solubility in PBS, the compounds were tested in methanol. The absorption spectra of compound 5l (50 μM, final concentration) alone or in the presence of CuSO4 and FeSO4 (100 μM, final concentration) for 30 min in methanol were recorded 1 cm-quartz cells. A fixed amount of compound 5l (50 μM) was mixed with growing amounts of Cu2+ (10–80 μM) and UV spectra were recorded. Variation of absorbance at 363 nm was used to monitor the formation of 5l/Cu2+ complexCitation44,Citation45.
Inhibition of Cu2+-induced Aβ (1–42) aggregation
For the inhibition of Cu2+-induced Aβ (1–42) aggregation experiment, the Aβ was diluted in 20 μM HEPES (pH 6.6) with 150 μM NaCl. The mixture of the peptide (10 μL, 25 μM, final concentration) with or without copper (10 μL, 25 μM, final concentration) and the test compound (10 μL, 50 μM, final concentration) were incubated at 37 °C for 24 h. The 20 μL of the sample was diluted to a final volume of 200 μL with 50 μM glycine–NaOH buffer (pH 8.0) containing ThT (5 μM). The detection method was the same as that of self-induced Aβ aggregation experimentCitation47.
Cell culture and measurement of cell viabilityCitation50
The toxicity effect of the tested compounds on the rat pheochromocytoma (PC12) cells was examined according to previously reported methods. PC 12 cells was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and routinely grown at 37 °C in a humidified incubator with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine calf serum, 100 units per mL penicillin, and 100 units per mL of streptomycin. Cells were sub-cultured in 96-well plates at a seeding density of 5000 cells per well and allowed to adhere and grow. When cells reached the required confluence, they were placed into serum-free medium and treated with compound 5l. Twenty-four hours later the survival of cells was determined by MTT assay. Briefly, after incubation with 20 μL of MTT at 37 °C for 4 h, living cells containing MTT formazan crystals were solubilized in 200 μL DMSO. The absorbance of each well was measured using a microculture plate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm. Results are expressed as the mean ± SD of three independent experiments.
PC 12 cells were grown in RPMI-1640 medium containing 10% (v/v) foetal bovine serum, 100 U penicillin/mL and 100 mg streptomycin/mL under 5% CO2 at 37 °C. The culture media were changed every other day. After pretreatment with different concentrations of test compound (0, 6, 12.5, 25, 50 μM) for 2 h, PC 12 cells were incubated with 25 μM of Aβ (1–42) for 24 h. The cell viability was evaluated using MTT assay. Briefly, the cells were treated with 10 μL MTT (5 mg/mL in PBS) for 4 h at 37 °C. Then, 200 μL of DMSO was added to dissolve the dark blue formazan crystals formed in intact cells, and the absorbance at 570 nm was detected by a microplate reader. PC12 cells were cultured without test compound or Aβ (1–42) as control group and the results were expressed by percentage of control.
In vitro BBB permeation assay
Brain penetration of compounds was evaluated using a parallel artificial membrane permeation assay (PAMPA) in a similar manner as described by Di et al.Citation51 Commercial drugs were purchased from Sigma (St. Louis, MO) and Alfa Aesar (Haverhill, MA). The porcine brain lipid (PBL) was obtained from Avanti Polar Lipids (Alabaster, AL). The donor microplate (PVDF membrane, pore size 0.45 mm) and the acceptor microplate were both from Millipore (Darmstadt, Germany). The 96-well UV plate (COSTAR@) was from Corning Incorporated (Harrodsburg, KY). The acceptor 96-well microplate was filled with 300 μL of PBS/EtOH (7:3), and the filter membrane was impregnated with 4 μL of PBL in dodecane (20 mg/mL). Compounds were dissolved in DMSO at 5 mg/mL and diluted 50-fold in PBS/EtOH (7:3) to achieve a concentration of 100 mg/mL, 200 μL of which was added to the donor wells. The acceptor filter plate was carefully placed on the donor plate to form a sandwich, which was left undisturbed for 16 h at 25 °C. After incubation, the donor plate was carefully removed and the concentration of compound in the acceptor wells was determined using an UV plate reader (Flexstation@ 3). Every sample was analyzed at five wavelengths, in four wells, in at least three independent runs, and the results are given as the mean ± SD. In each experiment, nine quality control standards of known BBB permeability were included to validate the analysis set.
Ex vivo brain penetrationCitation52
Fifteen male OF1 mice, weighing 25 g were used. Animals were housed under controlled light (with a 12-h light/12-h dark cycle, lights on at 7:00 a.m.) at 25 °C and proper humidity. Rats were given food and tap water ad libitum.
Donepezil hydrochloride and compound 5l were dissolved 10% DMSO. Animals were divided into three experimental handling groups: mice administered with (i) control (10% DMSO, n = 5); (ii) donepezil (10 μmol/kg, n = 5); (iii) compound 5l (10 μmol/kg, n = 5). Groups of 15 mice were treated i.p. with each compound. The animals were sacrificed 10 min later and brains were quickly removed and frozen on dry ice. Residual AChE activity was determined as previously described by the method of Ellman et al.Citation41 using brain homogenate preparations as a source of the enzyme: A homogenate of brain samples (10%, w/v) in 0.03 M sodium phosphate buffer (pH 7.4) was prepared. The brain homogenate in volume of 200 μL was mixed with 1% Triton X-100 and centrifuged at 3000 rpm at 4 °C for 10 min. Just before analysis of the enzymatic activity, an amount of 100 μL of homogenate was diluted 2.5 times in 0.1 M phosphate-buffered solution (pH 8.0). The reaction took place in a final volume of 300 μL of 0.1 M phosphate-buffered solution (pH 8.0) containing 100 μL of diluted homogenate and 333 μM DTNB solution. To avoid interferences between AChE and BChE activities, 100 μM ISOOMPA (specific BChE inhibitor) was present in the incubation medium. Prior to the addition of the substrate acetylthiocholine, a preincubation period of 5 min was used to eliminate the endogenous ACh present in the homogenates. The reaction was started by the addition of ATCI (450 μM) and the absorbance at 414 nm was evaluated 2 min after the substrate addition. Percent of inhibition was calculated by comparing AChE activity in brain of the drug-treated mice with activity from untreated controls.
Result and discussion
Chemistry
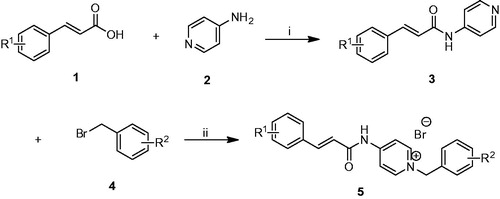
As shown in Scheme 1, the target compounds 5a–n were synthesized. First of all, the commercially available cinnamic acid derivatives 1 were activated with 4-dimethylaminopyridine (DMAP) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) at 0 °C in dichloromethane solutions, and subsequently condensation with compound 2 at room temperature overnight afforded the intermediates 3. Finally, the benzyl pyridinium bromide salts 5 were efficiently obtained by refluxing proper benzyl bromides 4 with the intermediates 3 in dry acetonitrile.
In vitro cholinesterase inhibitory activity and preliminary SAR studies
The activities of compounds 5a–n and the relative compound cinnamic acid against AChE (from electric eel) and BuChE (from equine serum) were examined using the spectrophotometric method of Ellman et al.Citation41 Donepezil was used as a standard compound for comparison. From , the novel cinnamic acid derivatives showed high activity towards AChE with IC50 values in the nanomolar range, and high selectivity for AChE over BuChE, indicating that these derivatives are selective AChE inhibitors. Among the cinnamic acid derivatives, compound 5l (IC50=12.1 nM) showed the most potent inhibitory activity against AChE, which was 3.3-fold higher than that of donepezil (IC50=40.2 nM). Moreover, compound 5l exhibited the highest selectivity level towards AChE over BuChE (SI =214.9). By contrast, compound 5n exhibited the highest inhibitory activity against BuChE (IC50=1.9 μM), resulting 2.3-times more potent than that of donepezil (IC50=4.5 μM). However, the AChE inhibitory activity of cinnamic acid was remarkably low (IC50>100 μM), suggesting that the N-benzyl pyridinium moiety is unavoidably required for higher activity. The AChE inhibition by benzyl pyridinium bromide was assayed to be in the micromolar rangeCitation40, which was lower than that of novel cinnamic acid derivatives. This finding suggests that the cinnamic acid skeleton was also essential for AChE activity.
Table 1. Inhibition of ChEs activity and selectivity index of compounds 5a–n.
To improve the inhibitory activity of the compounds against ChEs, we introduced substituents with different sizes and electronic properties on the benzene ring of cinnamic acid and on the benzyl group of the N-benzyl pyridinium moiety were varied. The IC50 value of compounds 5a–n indicated that the presence of methoxy groups at the positions 3 and 4 of the N-benzyl pyridinium moiety increased the ChE activity. For example, the ChE inhibitory activity of compounds 5i–n was higher than that of compounds 5b–g. Moreover, AChE inhibition was also affected by the substituents on the benzyl group. Compared with compound 5a, the introduction of methyl, fluorine and bromine on the meta-, para-position reduced AChE inhibitory activity (compounds 5b–g). For example, compound 5a (IC50=54.1 nM for AChE) was more potent than compound 5g (IC50=1450.5 nM for AChE) possessing 4-Br on the benzyl group. By contrast, compared with compound 5h, compounds 5i–n with different substituents on the benzyl group showed an increased AChE inhibitory activity. For example, compound 5l bearing 4-F on the benzyl group exhibited more potent AChE inhibition (11-fold) than did compound 5h. Similar to compound 5a–n for BuChE inhibition, compounds 5f (IC50=2.5 μM), 5g (IC50=2.1 μM), 5l (IC50=2.5 μM) and 5m (IC50=1.9 μM) that were characterized by a Br substituent showed more potent inhibition. Therefore, we can rationally deduce that the size of a substituent more crucially affects BuChE inhibition than its electronic properties.
Finally compounds 5i–n were selected for evaluation on human AChE. As listed in , all tested compounds presented IC50 values in the nanomolar range and were slightly more potent inhibitors for hAChE than for eeAChE. The SARs for hAChE were similar to those drawn for eeAChE inhibition (). Compound 5l (IC50=8.6 nM for hAChE) displayed the highest inhibition, which was fourfold higher than that of standard donepezil (IC50=33.5 nM).
Table 2. Inhibition of ChEs activity and selectivity index of compounds 5i–n.
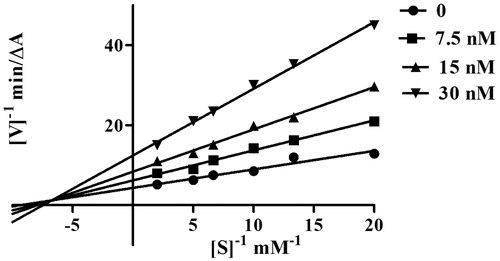
Kinetic study of AChE inhibition
To explore the AChE inhibitory mechanism of action of the cinnamic acid derivatives, the most potent inhibitor, compound 5l, was selected for a kinetic study using Lineweaver–Burk plotsCitation39. Graphical analysis () revealed both increasing slopes and increasing intercepts with increasing inhibitor concentration. According to this pattern, compound 5l is a mixed-type inhibitor for AChE and might be able to bind to the CAS and the PAS of AChE.
Figure 2. Kinetic study on the mechanism of EeAChE inhibition by compound 5l. Overlaid Lineweaver–Burk reciprocal plots of AChE initial velocity at increasing substrate concentration (0.05–0.50 mM) in the absence of inhibitor and in the presence of compound 5l are shown. Lines were derived from a weighted least-squares analysis of the data points.
Molecular modeling study of AChE inhibition
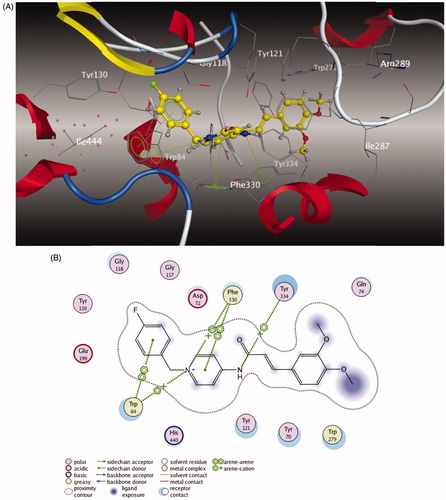
To further study the dual-site mode of compound 5l for AChE, a molecular docking study was performed using the software package MOE 2008.10Citation42,43. The X-ray crystal structure of the TcAChE complex with donepezil (hAChE, PDB code 1EVE) was applied to establish the starting model of AChE. As shown in , the N-benzyl pyridinium moiety of compound 5l was bound to the CAS of AChE, via aromatic π–π stacking interactions with the phenyl ring from Trp 84 with the ring-to-ring distance of 3.88 Å and the pyridine ring from Phe 330 with the ring-to-ring distance of 349 Å. Moreover, the charged nitrogen of the pyridine ring bound to the CAS was via a cation–π interaction with Trp 84 and Phe 330. The cinnamic acid moiety occupied the PAS formed by Trp 279 and Gln 74. All these results obviously indicated that compound 5l could simultaneously bind to the PAS and CAS of AChE.
Figure 3. (A) 3D docking model of compound 5l with TcAChE. Atom colors: yellow – carbon atoms of 5l, gray – carbon atoms of residues of TcAChE, dark blue – nitrogen atoms, red – oxygen atoms. The dashed lines represent the interactions between the protein and the ligand. (B) 2D schematic diagram of docking model of compound 5l with TcAChE. The figure was prepared using the ligand interactions application in MOE.
Metal-chelating properties of compound 5l
The chelating ability of compound 5l with biometals such as Cu2+ and Fe2+ was studied using UV–vis spectroscopy ()Citation44,Citation45. As shown in the UV–vis spectrum (), compound 5l without metal ions showed the absorption maximum at 216 nm and a shoulder at 353 nm. Upon the addition of CuSO4, a bathochromic shift in the maximum absorption from 216 nm to 224 nm and in the shoulder from 353 nm to 363 nm occurred, suggesting the formation of the 5l–Cu2+ complex. When FeSO4 was added, a red shift in the maximum absorption from 216 nm to 226 nm occurred, suggesting that compound 5l coordinates with Fe2+. The complexation ability of compound 5l might be ascribed to the dimethoxy group on the cinnamic acid moiety and to the amide moiety of the compoundCitation46,Citation33.
Figure 4. (A) UV absorbance spectrum of 5l (50 μM) alone (red) or in the presence of 100 μM CuSO4 (green) and 100 μM FeSO4 (yellow) in MeOH. (B) Determination of the stoichiometry of complex 5l−Cu2+ by molar ratio method.
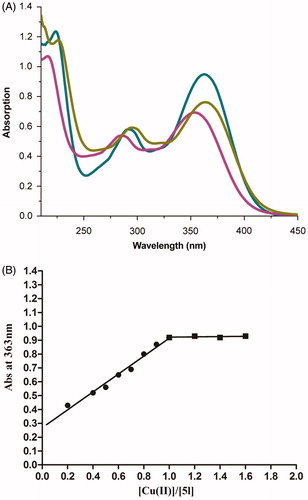
To determine the stoichiometry of the 5l–Cu2+ complex, the molar ratio method was used, for which solutions of compound 5l with accumulative amounts of CuSO4 were prepared. The UV spectra () showed that the absorbance at 363 nm, related to the formation of the Cu–5l complex, initially increased at increasing concentrations of CuCl2 and then plateaued, and the two straight lines intersected at a mole fraction of 1.02. Thus a 1:1 stoichiometry was hypothesised for the 5l–Cu2+ complex.
Inhibition of self-induced and Cu2+-induced Aβ (1–42)self-induced aggregation
All compounds tested for ChEs inhibition were also evaluated by a ThT-based fluorometric assay for their ability to inhibit self-induced Aβ (1–42) aggregationCitation47. Curcumin was used as a reference, because of its known inhibitory activity against Aβ (1–42) self-aggregation. The results are gathered in . Compounds 5a–n exhibited good potencies (46.3–65.6% at 20 μM) compared with Cur (54.6% at 20 μM). Notably, compounds 5k, 5l and 5n (65.6%, 64.7% and 66.3%, respectively, at 20 μM) showed the highest potency. Interestingly, compounds 5h–n with the dimethoxy group on the cinnamic acid moiety exhibited high inhibition against self-induced Aβ (1–42) aggregation with inhibition ranging from 53.9 to 66.3% at 20 μM. For example, compound 5l (64.7% at 20 μM) was more potent than that of compound 5e (52.8% at 20 μM). This finding led to the hypothesis that the dimethoxy group might favour Aβ aggregation inhibition. However, substituents on the benzyl group (see compounds 5i–n) did not seem to play a role in the inhibition of Aβ (1–42) self-aggregation.
Table 3. Inhibition of Aβ (1–42) self-induced aggregation and ABTS radical by target compounds.
As compound 5l showed good inhibitory activity against Aβ (1–42) self-aggregation and favourable chelating properties, its ability to inhibit Cu2+-induced Aβ (1–42) aggregation was investigated by a ThT-binding assayCitation48. Clioquinol was employed as a reference compound. As shown in , the fluorescence of Aβ treated with Cu2+ is 160.3% that of Aβ alone, which points out that Cu2+ hastens Aβ aggregation. In comparison, the fluorescence of Aβ treated with Cu2+ and the tested compound decreased dramatically (5l, 68.6% inhibition of Cu2+-induced Aβ aggregation; CQ, 60.2% inhibition). These results indicated that our compound could inhibit Cu2+-induced Aβ aggregation by effectively chelating Cu2+.
Docking study of compound 5l with Aβ (1–42) peptide
To further study the interaction mode with compound 5l for Aβ (1–42), a molecular docking study was performed using the software package MOE 2008.10Citation43. The X-ray crystal structure of the protein Aβ structure (PDB 1IYT) from the Protein Data Bank was used. As revealed in , the benzene ring of cinnamic acid interacted with the His 6 via a π–π stacking interaction. A hydrogen bond interaction was also observed between the acid amides group of compound 5l and Glu 3. These results indicated that the π–π stacking and the hydrogen bond interactions played crucial roles in the stability of the 5l/Aβ (1–42) complex.
Evaluation of compounds for antioxidant activity
The target compounds were evaluated for their antioxidant efficacy by using ABTS (ferric reducing antioxidant power) assaysCitation49. Compared with Cur, results showed that tested compounds exhibited no antioxidant ability (). This finding might be attributed to the absence of the hydroxy group of cinnamic acid moiety.
Cytotoxicity of compound 5l in PC12 cells and neuroprotection against Aβ (1–42)-induced toxicity
On the basis of the aforementioned screening results, the potential toxicity effect of compound 5l in PC12 cells was studiedCitation50. After incubating the cells with compound 5l for 24 h, the cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. As shown in , the result revealed that compound 5l at 3–50 μМ did not significantly affect cell viability, indicating that compound 5l was nontoxic to neuroblastoma PC12 cells.
Figure 7. (A) Effects of compound 5l on cell viability in PC12 cells. The cell viability was determined by the MTT assay after 24 h of incubation with various concentrations. The results were expressed as a percentage of control cells. Values are reported as the mean ± SD of three independent experiments. (B) Neuroprotection against Aβ (1–42) toxicity. Compound 5l was tested for neuroprotective activity against Aβ (1–42) toxicity in PC12 cells. Data represent the mean SD of three observations. *p < .05 and **p < .01 compared to the Aβ (1–42)-treated control group.
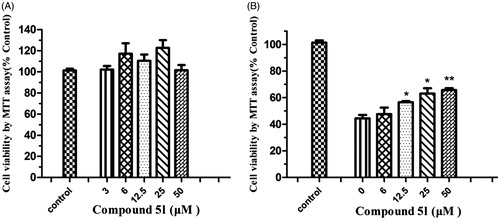
In the pathology of AD, Aβ-induced neuronal cell death is a serious event. To evaluate the neuroprotective effects of compound 5l against Aβ-induced neuronal death of PC12 cells, the data were recorded after the cells were exposed to increasing concentrations of compound 5l (6, 12.5, 25 and 50 μM) for 24 h. As can be seen in , treatment of cells with Aβ (1–42) (25 μM) to the growth medium markedly reduced cell viability to 44.2% compared with the untreated cells (control). Compound 5l exhibited neuroprotective effects at concentrations ranging from 6 to 50 μM (6 μM: 47.6 ± 4.2%; 12.5 μM: 57.5 ± 1.4%; 25 μM: 63.2 ± 3.5%; 50 μM: 66.6 ± 2.5%). These observations further showed that novel cinnamic acid derivatives bearing the N-benzyl pyridinium moiety can inhibit Aβ (1–42) self-aggregation for the treatment of AD.
In vitro BBB permeation assay
For successful central nervous system (CNS) drugs, the first requirement is crossing the BBB to reach brain. The potential ability of these compounds to penetrate into the brain was evaluated using a PAMPA as described by Di et al.Citation51 Assay validation was completed by comparing the experimental permeabilities of nine commercial drugs with previously reported values ()Citation51. A plot of the experimental data versus bibliographic values gave a good linear correlation, Pe (exp.) = 1.06 Pe (bibl.) − 1.69 (R2=.94). From this equation and taking into account the limit established by Di et al. for blood–brain barrier permeation, we classified the compounds as follows:
Table 4. Permeability (Pe×10−6 cm s−1) in the PAMPA-BBB assay for nine commercial drugs, used in the experiment validation.
“CNS +” (high BBB permeation predicted): Pe (10−6 cm s−1) > 2.55.
“CNS −” (low BBB permeation predicted): Pe (10−6 cm s−1) < 0.43.
“CNS +/−” (BBB permeation uncertain): Pe (10−6 cm s−1) from 2.55 to 0.43.
Compound 5l with good activities against Aβ (1–42) aggregation and AChE was selected. The Pe value of compound 5l was 2.89 ± 0.37 (CNS+). It indicated that compound 5l might be able to penetrate the BBB.
Ex vivo brain penetration study
To confirm the brain permeability of compound 5l predicted by the PAMPA, the AChE inhibitory activity was subjected to an ex vivo measurement after intraperitoneal (i.p.) injection of 10 μmol/kg of compound 5l and donepezil into miceCitation52. The mice were sacrificed 10 min after drug administration, and the inhibition (%) of brain AChE activity versus untreated controls was determined. Compared to control, mouse brain AChE activity was found to be inhibited by compound 5l (53.9 ± 2.9%) and donepezil (46.7 ± 3.2%). These results confirmed that compound 5l can cross the BBB in vivo.
Conclusions
In summary, novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety were designed, synthesised and evaluated as multifunctional cholinesterase inhibitors against AD. Among the synthesised compounds, most derivatives displayed potent AChE inhibitory activity and high selectivity for AChE over BuChE. Among them, compound 5l exhibited dual inhibitory potency on AChE and BuChE. The kinetic characterization suggested that compound 5l acted as a mixed-type inhibition, which was consistent with the result of the molecular modelling study. Furthermore, compound 5l showed metal-chelating ability, significant inhibition of Aβ aggregation and inhibition of Cu2+-induced Aβ aggregation, in addition to low neurotoxicity. Compound 5l also showed a neuroprotective effect against Aβ (1–42) toxicity in PC12 cells and was proven to penetrate into brain by the PAMPA-BBB assay in vitro and ex vivo experiments. Above all, compound 5l could be deemed as a multifunctional cholinesterase inhibitor and serve as a novel lead compound for treating AD.
Disclosure statement
The authors declare no conflicts of interest.
References
- Thies W, Bleiler L. Alzheimer's disease facts and figures. Alzheimer's Dement 2013;9:208–45.
- Tang H, Zhao HT, Zhong SM, et al. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg Med Chem Lett 2012;22:2257–61.
- Laferla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci 2007;8:499–509.
- Wilkinson D, Wirth Y, Goebel C. Memantine in patients with moderate to severe Alzheimer's disease: meta-analyses using realistic definitions of response. Dement Geriatr Cogn Disord 2014;37:71–85.
- Xie Q, Wang H, Xia Z, et al. Bis-(-)-nor-meptazinols as novel nanomolar cholinesterase inhibitors with high inhibitory potency on amyloid-beta aggregation. J Med Chem 2008;51:2027–36.
- Munoz-Torrero D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimers disease. Curr Med Chem 2008;15:2433–55.
- Reyes AE, Chacón MA, Dinamarca MC, et al. Acetylcholinesterase-Abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol 2014;164:2163–74.
- Fernández-Bachiller MI, Pérez C, Monjas L, et al. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer's disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J Med Chem 2012;55:1303–17.
- Özturan Özer E, Tan OU, Ozadali K, et al. Synthesis, molecular modeling and evaluation of novel N′-2-(4-benzylpiperidin-/piperazin-1-yl)acylhydrazone derivatives as dual inhibitors for cholinesterases and Aβ aggregation. Bioorg Med Chem Lett 2013;23:440–3.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–6.
- Paul S, Planque S, Nishiyama Y. Beneficial catalytic immunity to abeta peptide. Rejuvenat Res 2010;13:179–87.
- Bush AI. Drug development based on the metals hypothesis of Alzheimer's disease. J. Alzheimers Dis 2008;15:223–40.
- Fernández-Bachiller M, Pérez C, González-Muñoz GC, et al. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer's disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J Med Chem 2010;53:4927–37.
- Price KA, Crouch PJ, White AR. Therapeutic treatment of Alzheimer's disease using metal complexing agents. Recent Pat CNS Drug Discov 2007;2:180–7.
- Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 2010;69:155–67.
- Jiang CS, Fu Y, Zhang L, et al. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg Med Chem Lett 2015;25:216–20.
- Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 2005;48:6523–43.
- León R, Garcia AG, Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer's disease. Med Res Rev 2013;33:139–89.
- Agis-Torres A, Sölhuber M, Fernandez M, et al. Multi-target-directed ligands and other therapeutic strategies in the search of a real solution for Alzheimer's disease. Curr Neuropharmacol 2014;12:2–36.
- Bukhari SN, Jantan I. Synthetic curcumin analogs as inhibitors of β-amyloid peptide aggregation: potential therapeutic and diagnostic agents for Alzheimer's disease. Mini Rev Med Chem 2015;15:1110–21.
- Bajda M, Guzior N, Ignasik M, et al. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem 2011;18:4949–75.
- Chen X, Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr Med Chem 2013;20:1673–85.
- Sezgin Z, Dincer Y. Alzheimer's disease and epigenetic diet. Neurochem Int 2014;78:105–16.
- Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009;10:97–108.
- Kanski J, Aksenova M, Stoyanova A, et al. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure–activity studies. J Nutr Biochem 2002;13:273–81.
- Sgarbossa A, Giacomazza D, di Carlo M. Ferulic acid: a hope for Alzheimer's disease therapy from plants. Nutrients 2015;7:5764–82.
- Maruf AA, Lip H, Wong H, et al. Protective effects of ferulic acid and related polyphenols against glyoxal- or methylglyoxal-induced cytotoxicity and oxidative stress in isolated rat hepatocytes. Chem Biol Interact 2015;234:96–104.
- Hamaguchi T, Ono K, Yamada M. Review: curcumin and Alzheimer's disease. CNS Neurosci Ther 2015;16:285–97.
- Wang Y, Yin H, Wang L, et al. Curcumin as a potential treatment for Alzheimer's disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med 2013;41:59–70.
- Benchekroun M, Bartolini M, Egea J, et al. Novel tacrine-grafted Ugi adducts as multipotent anti-Alzheimer drugs: a synthetic renewal in tacrine-ferulic acid hybrids. ChemMedChem 2015;10:523–39.
- Pan W, Hu K, Bai P, et al. Design, synthesis and evaluation of novel ferulic acid-memoquin hybrids as potential multifunctional agents for the treatment of Alzheimer's disease. Bioorg Med Chem Lett 2016;26:2539–43.
- Fang L, Chen M, Liu Z, et al. Ferulic acid-carbazole hybrid compounds: combination of cholinesterase inhibition, antioxidant and neuroprotection as multifunctional anti-Alzheimer agents. Bioorg Med Chem 2016;24:886–93.
- Xu W, Wang XB, Wang ZM, et al. Synthesis and evaluation of donepezil–ferulic acid hybrids as multi-target-directed ligands against Alzheimer's disease. Med Chem Commun 2016;7:990–8.
- Estrada M, Herrera-Arozamena C, Pérez C, et al. New cinnamic – N-benzylpiperidine and cinnamic – N,N-dibenzyl(N-methyl)amine hybrids as Alzheimer-directed multitarget drugs with antioxidant, cholinergic, neuroprotective and neurogenic properties. Eur J Med Chem 2016;121:376–86.
- Baharloo F, Moslemin MH, Nadri H, et al. Benzofuran-derived benzylpyridinium bromides as potent acetylcholinesterase inhibitors. Eur J Med Chem 2015;93:196–201.
- Mostofi M, Mohammadi Ziarani G, Mahdavi M, et al. Synthesis and structure–activity relationship study of benzofuran-based chalconoids bearing benzylpyridinium moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem 2015;103:361–9.
- Wang C, Wu Z, Cai H, et al. Design, synthesis, biological evaluation and docking study of 4-isochromanone hybrids bearing N-benzyl pyridinium moiety as dual binding site acetylcholinesterase inhibitors. Bioorg Med Chem Lett 2015;25:5212–16.
- Lan JS, Xie SS, Li SY, et al. Design, synthesis and evaluation of novel tacrine-(β-carboline) hybrids as multifunctional agents for the treatment of Alzheimer's disease. Bioorg Med Chem 2014;22:6089–104.
- Xie SS, Lan JS, Wang XB, et al. Multifunctional tacrine-trolox hybrids for the treatment of Alzheimer's disease with cholinergic, antioxidant, neuroprotective and hepatoprotective properties. Eur J Med Chem 2015;93:42–50.
- Xie SS, Lan JS, Wang X, et al. Design, synthesis and biological evaluation of novel donepezil-coumarin hybrids as multi-target agents for the treatment of Alzheimer's disease. Bioorg Med Chem 2016;24:1528–39.
- Ellman GL, Courtney KD, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Benek O, Musilek K, Horova A, et al. Preparation, in vitro screening and molecular modelling of monoquaternary compounds related to the selective acetylcholinesterase inhibitor BW284c51. Med Chem 2014;11:21–9.
- Muzammil S, Armstrong AA, Kang LW, et al. Unique thermodynamic response of tipranavir to human immunodeficiency virus type 1 protease drug resistance mutations. J Virol 2007;81:5144–54.
- Li S-Y, Jiang N, Xie S-S, et al. Design, synthesis and evaluation of novel tacrine-rhein hybrids as multifunctional agents for the treatment of Alzheimer's disease. Org Biomol Chem 2014;12:801–14.
- Sugimoto H, Tsuchiya Y, Sugumi H, et al. Novel piperidine derivatives. Synthesis and anti-acetylcholinesterase activity of 1-benzyl-4-[2-(N-benzoylamino)ethyl]piperidine derivatives. J Med Chem 1990;33:1880–7.
- Xie SS, Wang XB, Li JY, et al. Design, synthesis and evaluation of novel tacrine-coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer's disease. Eur J Med Chem 2013;64:540–53.
- Bartolini M, Bertucci C, Bolognesi ML, et al. Insight into the kinetic of amyloid beta (1–42) peptide self-aggregation: elucidation of inhibitors' mechanism of action. Chembiochem 2007;8:2152–61.
- Lu C, Guo Y, Yan J, et al. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer's disease. J Med Chem 2013;56:5843–59.
- Miller NJ, Rice-Evans CA. Factors influencing the antioxidant activity determined by the ABTS.+ radical cation assay. Free Radic Res 1997;26:195–9.
- Magliaro BC, Saldanha CJ. Clozapine protects PC-12 cells from death due to oxidative stress induced by hydrogen peroxide via a cell-type specific mechanism involving inhibition of extracellular signal-regulated kinase phosphorylation. Brain Res 2009;1283:14–24.
- Di L, Kerns EH, Fan K, et al High throughput artificial membrane permeability assay for blood?brain barrier. Eur J Med Chem 2003;38:223–32.
- Galdeano C, Viayna E, Sola, et al. Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases. J Med Chem 2012;55:661–9.

![Figure 5. Inhibition of Cu2+-induced Aβ (1–42) aggregation by compound 5l comparing with that of clioquinol (CQ) ([Aβ = 25 μM, [5l] = 50 μM, [CQ] = 50 μM, [Cu2+] = 25 μM, 37 °C, 24 h). Values are reported as the mean ± SD of three independent experiments. #p < .05, **p < .01.](/cms/asset/0c808715-5902-4a59-ac71-38a45ca9cf50/ienz_a_1256883_f0005_b.jpg)