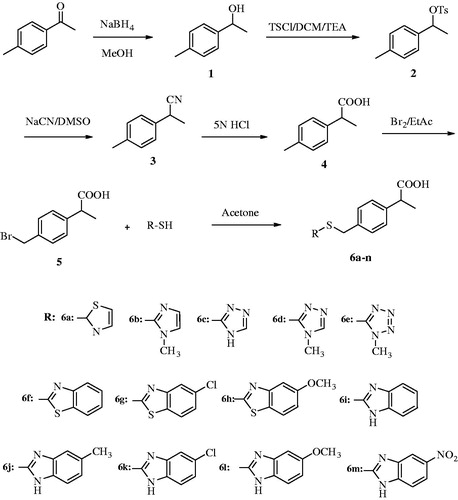
Abstract
A series of 2-(4-substitutedmethylphenyl)propionic acid derivatives (6a–6m) were synthesized, characterized and evaluated for cyclooxygenase (COX) enzyme inhibitory and antimicrobial activity. Test compounds that exhibited good COX inhibition and antibacterial activity were further screened for their cytotoxicity and genotoxicity. Compounds 6h and 6l showed better COX-1 and COX-2 inhibition when compared to ibuprofen. Inhibition potency of these compounds against COX-2 was very close to that of nimesulide. The compounds 6d, 6h, 6l and 6m displayed promising antibacterial property when compared to chloramphenicol. However, the compound 6l was emerged as the best dual COX inhibitory-antibacterial agent in this study. The ADME prediction of the compounds revealed that they may have a good pharmacokinetic profile. Docking results of the compounds 6h and 6l with COX-1 (PDB ID: 1EQG) also exhibited a strong binding profile.

1. Introduction
Prostaglandin H synthase (PGHS) also known as cyclooxygenase (COX) is a dimeric membrane enzyme that is in charge of production of prostaglandins, prostacyclins and thromboxanesCitation1. Prostaglandins are lipid autacoids associated with physiologic and pathologic processes, including inflammationCitation2. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most prescribed medicines for the therapy of diverse inflammatory diseases. The mechanism of action of NSAIDs is based on the repression of prostaglandin biosynthesis from arachidonic acid via inhibiting the enzyme COXsCitation3.
COX has both COX and peroxidase activities. The COX activity of COX enzymes forms PGG2 by incorporation of two oxygen molecules to arachidonic acid via catalytic residue Tyr385. As a consequence of peroxidase activity of COXs, PGG2 is reduced to PGH2 that is converted to prostaglandins, prostacyclins and thromboxanesCitation4. Two isoformsCitation5 of COX exist, COX-1 and COX-2. The constitutive COX-1 isoform is produced in most tissues and responsible for the synthesis of cytoprotective PGs in the gastrointestinal system, vascular homeostasis and platelet aggregation, whereas the inducible COX-2 is expressed in some tissues in order to produce prostaglandins thus initializes the inflammationCitation6.
The structures and sequence of COX-1 and COX-2 enzymes are quite similar. Each enzyme consists of three structural domains: N-terminal epidermal growth factor (EGF) domain, membrane-binding motif and C-terminal catalytic domain that includes both the COX and peroxidase active sitesCitation7. Ibuprofen, flurbiprofen and naproxen are prominent members of NSAIDs containing 2-arylpropionic acid scaffold. Depending on numerous studies, it is regarded that the free carboxylic acid group situated in these molecules composes critical interactions with Arg120, Glu524 and Tyr355 in the COX active siteCitation8–10. The carboxylic acid structure, hence, is considered as an essential pharmacophoric core for COX activityCitation11. According to studies, esterification or amidation of the free carboxylic acid group cause reduced COX inhibition activityCitation12.
Azole compounds are electron-rich nitrogen heterocycles, playing an extremely essential role in medicinal area. Hence, they have been gained a special attentionCitation13. Due to their heteroatomic ring system and electron-rich property, azole-based compounds can easily interact with the enzymes and receptors in organisms as a result of coordination bonds, hydrogen bonds, ion-dipole, cation–π, π–π stacking and hydrophobic effect as well as van der Waals force, etc., thereby exhibiting various bioactivitiesCitation14. The design, synthesis and antimicrobial activity of azole derivatives have been widely examined and have become one of the highly important highlights in recent years, and the progress is quite rapid. Particularly, a large number of azole-based antibacterial and antifungal compounds have been penetratingly studied as candidates and even some of them have been used at the clinic, which have indicated the excessive potential and development value of azole compoundsCitation15–22. Furthermore, azole-based compounds were reported to exhibit biologically important activities as anti-inflammatory and analgesic agentsCitation23.
Markedly, inflammation and infection are not identical, even in the case where infection is the primary reason of the inflammation. Moreover, the inflammatory response elicited by an invading organism can result in host damage, raise the availability of nutrients and facilitate access to host tissues. Additionally, inflammation may cause accumulation of fluid in the injured area, which may stimulate bacterial growthCitation24. Other reports revealed that NSAIDs may increase the progression of bacterial infectionCitation25,Citation26. Furthermore, in the management of infectious and inflammatory diseases, the use of multidrug therapy is an increasing concern for patients with damaged liver or kidney functions, patients with diseases of the gastrointestinal system or patients suffering from diverse side effects of other drugs. Monotherapy would be preferred with regards to both the pharmacoeconomics and the patient complianceCitation27. Therefore, a dual COX inhibitory-antibacterial agent with an improved safety profile is necessary for enhanced therapeutic benefits and better patient compliance. Prompted from this requirement lot of studies have been reportedCitation28–43.
As a result, above-mentioned information directed us to synthesize some novel 2-(4-substitutedmethylphenyl)propionic acid derivatives and investigate their inhibitory activity against COX-1, COX-2 enzymes and various microbial strains.
2. Experimental
2.1. Materials and methods
Entire chemicals used in the syntheses were purchased from Sigma-Aldrich Chemicals (Sigma-Aldrich Corp., St. Louis, MO) or Merck Chemicals (Merck KGaA, Darmstadt, Germany). Melting points of the synthesized compounds were determined by MP90 digital melting point apparatus (Mettler Toledo, Columbus, OH) and were uncorrected. 1H NMR and 13C NMR spectra were recorded by a Bruker 300 and 75 MHz digital FT-NMR spectrometer (Bruker Bioscience, Billerica, MA) in DMSO-d6, respectively. In the NMR spectra, splitting patterns were designated as follows: s: singlet; d: doublet; t: triplet; and m: multiplet. Coupling constants (J) were reported as Hertz. The IR spectra were obtained on a Shimadzu, IR Affinity-1 S (Shimadzu, Tokyo, Japan). HRMS studies were performed on Shimadzu LCMS-IT-TOF system (Shimadzu, Tokyo, Japan). The purities of compounds were checked by TLC on silica gel 60 F254 (Merck KGaA, Darmstadt, Germany).
2.1.1. Synthesis of 1-(4-methylphenyl) ethanol (1)
4-Methyl acetophenone (0.05 mol, 6.70 g) was dissolved in methanol (100 mL) and NaBH4 (0.05 mol, 1.89 g) was added in portions. Once the reaction was completed, methanol was evaporated and precipitated product was washed with water. The precipitate was extracted with dichloromethane in portions (3 × 100 mL), extracts were combined and dried with anhydrous sodium sulfate. The solvent was evaporated, and the residue was recrystallized from ethanol to give the 1-(4-methylphenyl) ethanol (1)Citation44. Yield; 74%.
2.1.2. Synthesis of 1-(4-methylphenyl)ethyl 4-methylbenzenesulfonate (2)
1-(4-Methylphenyl)ethanol (1) (0.04 mol, 5.45 g) and TEA (0.04 mol, 5.58 mL) in dichloromethane (100 mL) was taken in a saturated CaCl2 ice bath into ice bath. p-Tosyl chloride (0.012 mol, 2.29 g) in dichloromethane was added dropwise and the mixture was stirred for 40 h. The precipitated product was filtered, washed with 10% tartaric acid and then 5 N potassium chloride solution to give 1-(4-methylphenyl)ethyl 4-methylbenzenesulfonate (2). Yield; 72%Citation44.
2.1.3. Synthesis of 2-(4-methylphenyl) propionitrile (3)
1-(4-Methylphenyl)ethyl 4-methylbenzenesulfonate (2) (0.02 mol, 5.81 g) was dissolved in dimethyl sulfoxide (20 mL) and NaCN (0.02 mol, 0.98 g) was added. The solution was refluxed at 90 °C for 18 h. After completion of reaction, the mixture was poured into iced-water and extracted with diethyl ether in portions (3 × 100 mL). The extracts were combined and dried with anhydrous sodium sulfate. The solvent was evaporated to obtain 2-(4-methylphenyl) propionitrile (3). Yield; 68%Citation44.
2.1.4. Synthesis of 2-(4-methylphenyl) propionic acid (4)
2-(4-Methylphenyl)propionitrile (0.015 mol, 2.18 g) was dissolved in 5 N HCl (40 mL). The mixture was refluxed for 1 h. The precipitated product was extracted with ethyl acetate in portions (3 × 100 mL). The extracts were combined and dried with anhydrous sodium sulfate. The solvent was evaporated, and then raw product was recrystallized from ethanol to give 2-(4-methylphenyl) propionic acid (4)Citation44. Yield; 78%.
2.1.5. Synthesis of 2-(4-(bromomethyl)phenyl) propionic acid (5)
2-(4-Methylphenyl)propionic acid (0.01 mol, 1.64 g) was dissolved in ethyl acetate (50 mL) and catalytic amount of HBr was added. This solution was taken into ice bath and bromine (0.012 mol, 0.61 mL) in ethyl acetate (20 mL) was added dropwise. After completion of dropping the reaction mixture was stirred at room temperature for 2 h. The solvent was evaporated and precipitated product was washed with water, dried and then recrystallized from ethanol to afford 2-(4-(bromomethyl)phenyl) propionic acid (5). Yield; 79%Citation45.
2.1.6. General procedure for the synthesis of 2-(4-substitutedmethylphenyl)propionic acid (6a–6n)
2-(4-Bromo-methylphenyl) propionic acid (0.001 mol, 0.243 g) and appropriate (benz)azolylthiol derivative (0.001 mol) were dissolved in acetone. The solution was refluxed at 40 °C for 12 h. Acetone was evaporated, residue was washed with water, filtered, dried and recrystallized from ethanol to obtain final products (6a–6n)Citation46.
2.1.6.1. 2-(4-(((4,5-Dihydrothiazol-2-yl)thio)methyl)phenyl)propanoic acid (6a)
Yield: 77%, M.P.=154.2–156.3 °C, FTIR (ATR, cm−1): 3410 (O–H), 1701 (C=O), 1047, 845, 777. 1H-NMR (300 MHz, DMSO-d6): δ = 7.36 (2 H, d, J = 8.2 Hz, 1,4-disubs. benzene–CH–), 7.25 (2 H, d, J = 8.2 Hz, 1,4-disubs. benzene–CH–), 4.46 (2 H, s, –CH2–), 4.21 (1 H, t, J = 8.2 Hz, –CH2), 3.64 (1 H, q, J = 7.1 Hz, –CH–), 3.59 (1 H, t, J = 8.2 Hz, –CH2–), 1.33 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (75 MHz, DMSO-d6): δ = 175.7, 156.1, 141.3, 134.9, 129.6, 128.2, 60.4, 44.8, 37.2, 35.1 and 18.9. HRMS (m/z): [M + H]+ calcd for C13H15NO2S2: 282.0617; found 282.0603.
2.1.6.2. 2-(4-(((1-Methyl-1H-imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6b)
Yield: 76%, M.P.=liquid, FTIR (ATR, cm−1): 3391 (O–H), 1717 (C=O), 1038, 860, 698. 1H-NMR (300 MHz, DMSO-d6): δ = 7.82 (1 H, d, J = 2.0 Hz, imidazole –CH–), 7.78 (1 H, d, J = 2.0 Hz, imidazole –CH–), 7.19 (2 H, d, J = 8.3 Hz, 1,4-disubs. benzene –CH–), 7.14 (2 H, d, J = 8.3 Hz, 1,4-disubs. benzene –CH–), 4.41 (2 H, s, –CH2–), 3.64 (1 H, q, J = 7.1 Hz, –CH–), 3.54 (3 H, s, –CH3), 1.34 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (75 MHz, DMSO-d6): δ = 175.6, 141.6, 139.2, 135.1, 129.3, 128.2, 125.9, 121.6, 44.8, 35.3 and 18.9. HRMS (m/z): [M + H]+ calcd for C14H16N2O2S: 277.1005; found 277.1000.
2.1.6.3. 2-(4-(((1H-1,2,4-Triazol-3-yl)thio)methyl)phenyl)propanoic acid (6c)
Yield: 85%, M.P.=liquid, FTIR (ATR, cm−1): 3393 (O–H), 1717 (C=O), 1022, 858. 1H-NMR (300 MHz, DMSO-d6): δ = 9.02 (1 H, d, J = 8.2 Hz, triazole –CH–), 7.18 (2 H, d, J = 8.2 Hz, 1,4-disubs. benzene –CH–), 7.15 (2 H, d, J = 8.3 Hz, 1,4-disubs. benzene –CH–), 4.41 (2 H, s, –CH2), 3.64 (1 H, q, J = 7.1 Hz, –CH–), 1.29 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (75 MHz, DMSO-d6): δ = 175.7, 153.6, 141.2, 135.4, 129.5, 127.7, 44.8, 35.5 and 19.0. HRMS (m/z): [M + H]+ calcd for C12H13N3O2S: 264.0801; found 264.0789.
2.1.6.4. 2-(4-(((4-Methyl-4 H-1,2,4-triazol-3-yl)thio)methyl)phenyl)propanoic acid (6d)
Yield: 81%, M.P.=liquid, FTIR (ATR, cm−1): 3420 (O–H), 1721 (C=O), 1024, 822, 760. 1H-NMR (300 MHz, DMSO-d6): δ = 8.28 (1 H, s, triazole –CH–), 7.19 (2 H, d, J = 8.3 Hz, 1,4-disubs. benzene –CH–), 7.14 (2 H, d, J = 8.3 Hz, 1,4-disubs. benzene –CH–), 4.41 (2 H, s, –CH2–), 3.64 (1 H, q, J = 7.1 Hz, –CH–), 3.55 (3 H, s, –CH3), 1.29 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (75 MHz, DMSO-d6): δ = 175.6, 156.8, 143.2, 140.7, 136.8, 130.0, 127.6, 44.7, 35.3, 27.03 and 18.9. HRMS (m/z): [M + H]+ calcd for C13H15N3O2S: 278.0958; found 278.0952.
2.1.6.5. 2-(4-(((1-Methyl-1 H-tetrazol-5-yl)thio)methyl)phenyl)propanoic acid (6e)
Yield: 82%, M.P.=liquid, FTIR (ATR, cm−1): 3374 (O–H), 1717 (C=O), 1038, 860, 698. 1H-NMR (300 MHz, DMSO-d6): δ = 7.31 (2H, d, J = 8.2 Hz, 1,4-disubs. benzene –CH–), 7.19 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 4.45 (2 H, s, –CH2–), 3.77 (3 H, s, –CH3), 3.61 (1 H, q, J = 7.1 Hz, –CH–), 1.28 (3 H, d, J = 7.1 Hz, –CH3. 13C-NMR (75 MHz, DMSO-d6): δ = 175.7, 153.6, 141.2, 135.4, 129.5, 128.0, 44.8, 34.0 and 18.8. HRMS (m/z): [M + H]+ calcd for C12H14N4O2S: 279.0910; found 279.0912.
2.1.6.6. 2-(4-((Benzo[d]thiazol-2-ylthio)methyl)phenyl)propanoic acid (6f)
Yield: 80%, M.P.=128.2–130.8 °C. FTIR (ATR, cm−1): 3120 (O–H), 1734 (C=O), 1067, 854, 742. 1H-NMR (300 MHz, DMSO-d6): δ = 13.87 (1 H, s, –COOH), 7.62–7.67 (2 H, m, BT –CH–), 7.45 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 7.34–7.32 (2 H, m, BT –CH–), 7.25 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 4.67 (2 H, s, –CH2–), 3.67 (1 H, q, J = 7.4 Hz, –CH–),1.34 (3 H, d, J = 7.4 Hz, –CH3). 13C-NMR (75 MHz, DMSO-d6): δ = 175.7, 164.3, 151.7, 141.2, 135.5, 131.2, 129.9, 129.6, 128.1, 125.1, 118.8, 110.7, 44.8, 35.7 and 18.9. HRMS (m/z): [M + H]+ calcd for C17H15NO3S: 330.0617; found 330.0617.
2.1.6.7. 2-(4-(((5-Chlorobenzo[d]thiazol-2-yl)thio)methyl)phenyl)propanoic acid (6g)
Yield: 85%, M.P.=162.3–164.7 °C, FTIR (ATR, cm−1): 3030 (O–H), 1715 (C=O), 1063, 860, 799. 1H-NMR (500 MHz, DMSO-d6): δ = 8.06 (1 H, d, J = 8.6 Hz, benzothiazole –CH–), 7.98 (1 H, d, J = 2.0 Hz, benzothiazole –CH–), 7.47 (2 H, d, J = 8.2 Hz, 1,4-disubs. benzene –CH–), 7.43 (1 H, dd, J = 8.6–2.0 Hz, benzothiazole –CH–), 7.27 (2 H, d, J = 8.2 Hz, 1,4-disubs. benzene –CH–), 4.64 (2 H, s, –CH2–), 3.67 (1 H, q, J = 7.2 Hz, –CH–), 1.35 (3 H, d, J = 7.2 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6): δ = 175.7, 169.5, 154.0, 141.2, 135.3, 133.9, 131.7, 129.7, 128.2, 125.0, 123.7, 121.1, 44.8, 36.8 and 18.9. HRMS (m/z): [M + H]+ calcd for C17H14NO2S2Cl: 364.0227; found 364.0218.
2.1.6.8. 2-(4-(((5-Methoxybenzo[d]thiazol-2-yl)thio)methyl)phenyl)propanoic acid (6 h)
Yield: 77%, M.P.=166.5–168.2 °C, FTIR (ATR, cm−1): 3071 (O–H), 1724 (C=O), 1082, 835, 694. 1H-NMR (500 MHz, DMSO-d6): δ = 7.87 (1 H, d, J = 8.8 Hz, benzothiazole –CH–), 7.46–7.45 (3 H, m, 1,4-disubs. benzene –CH–, benzothiazole –CH–), 7.27 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 7.01 (1 H, dd, J = 8.0–2.5 Hz, benzothiazole –CH–), 4.62 (2 H, s, –CH2–), 3.84 (3 H, s, –OCH3), 3.67 (1 H, q, J = 7.1 Hz, –CH–), 1.35 (3 H, d, J = 7.1 Hz, –CH3).13C-NMR (125 MHz, DMSO-d6): δ = 175.6, 158.0, 148.5, 141.6, 134.6, 134.5, 129.5, 128.4, 128.0, 115.1, 114.7, 96.6, 56.4, 44.8, 37.0 and 18.9. HRMS (m/z): [M + H]+ calcd for C18H17NO3S2: 360.0723; found 360.0724.
2.1.6.9. 2-(4-(((1H-benzo[d]imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6i)
Yield: 78%, M.P.=208.7–210.9 °C, FTIR (ATR, cm−1): 3142 (O–H), 1699 (C=O), 1072, 851, 735. 1H-NMR (500 MHz, DMSO-d6): δ = 7.69–7.66 (2 H, m, benzimidazole –CH–), 7.44–7.41 (4 H, m, 1,4-disubs. benzene –CH–, benzimidazole –CH–), 7.27 (2 H, d, J = 8.0 Hz, 1,4-disubs. benzene –CH–), 4.72 (2 H, s, –CH2–), 3.66 (1 H, q, J = 7.1 Hz, –CH–). 13C-NMR (125 MHz, DMSO-d6): δ = 175.6, 150.2, 141.6, 134.7, 134.6, 129.5, 128.4, 125.0, 113.9, 44.8, 35.2 and 18.9. HRMS (m/z): [M + H]+ calcd for C17H16N2O2S: 313.1005; found 313.1009.
2.1.6.10. 2-(4-(((5-Methyl-1H-benzo[d]imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6j)
Yield: 80%, M.P.=209.1–211.6 °C, FTIR (ATR, cm−1): 3051 (O–H), 1701 (C=O), 1070, 856, 799. 1H-NMR (500 MHz, DMSO-d6): δ = 7.58 (1 H, d, J = 8.4 Hz, benzimidazole –CH–), 7.49 (1 H, s, benzimidazole –CH–), 7.40 (2 H, d, J = 7.9 Hz, 1,4-disubs. benzene –CH–), 7.30–7.26 (3 H, m, 1,4-disubs. benzene –CH–, benzimidazole –CH–), 4.73 (2 H, s, –CH2–), 3.66 (1 H, q, J = 7.1 Hz, –CH–), 2.47 (3 H, s, –CH3), 1.33 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6): δ = 175.6, 149.3, 141.6, 135.4, 134.5, 133.9, 129.5, 128.4, 126.8, 113.5, 113.3, 44.8, 36.8, 21.6 and 18.9. HRMS (m/z): [M + H]+ calcd for C18H18N2O2S: 327.1162; found 327.1149.
2.1.6.11. 2-(4-(((5-Chloro-1H-benzo[d]imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6k)
Yield: 81%, M.P.=208.8–211.5 °C, FTIR (ATR, cm−1): 3096 (O–H), 1703 (C=O), 1063, 851, 806. 1H-NMR (500 MHz, DMSO-d6): δ = 7.69 (1 H, s, benzimidazole –CH–), 7.61 (1 H, d, J = 8.6 Hz, benzimidazole –CH–), 7.41 (2 H, d, J = 7.9 Hz, 1,4-disubs. benzene –CH–), 7.35 (1 H, d, J = 8.6 Hz, benzimidazole –CH–), 7.26 (2 H, d, J = 7.9 Hz, 1,4-disubs. benzene –CH–), 4.68 (2 H, s, –CH2–), 3.66 (1 H, q, J = 7.1 Hz, –CH–), 1.33 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6): δ = 175.6, 152.0, 141.4, 137.4, 135.1, 129.5, 128.4, 128.2, 127.5, 124.1, 115.2, 113.9, 44.8, 36.1, 21.6 and 18.9. HRMS (m/z): [M + H]+ calcd for C17H15N2O2SCl: 347.0616; found 347.0608.
2.1.6.12. 2-(4-(((5-Methoxy-1H-benzo[d]imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6 l)
Yield: 82%, M.P.=175.6–178.4 °C, FTIR (ATR, cm−1): 3034 (O–H), 1730 (C=O), 1068, 845, 760. 1H-NMR (500 MHz, DMSO-d6): δ = 7.60 (1 H, d, J = 9.0 Hz, benzimidazole –CH–), 7.39 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 7.26 (2 H, d, J = 8.1 Hz, 1,4-disubs. benzene –CH–), 7.15 (1 H, d, J = 2.3 Hz, benzimidazole –CH–), 7.08 (1 H, dd, J = 9.0–2.3 Hz, benzimidazole –CH–), 4.72 (2 H, s, –CH2–), 3.85 (3 H, s, –OCH3), 3.66 (1 H, q, J = 7.2 Hz, –CH–), 1.33 (3 H, d, J = 7.2 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6): δ = 175.6, 158.0, 148.5, 141.6, 134.6, 134.5, 129.5, 128.4, 128.0, 115.1, 114.7, 96.6, 56.4, 44.8, 37.0 and 18.9. HRMS (m/z): [M + H]+ calcd for C18H18N2O3S: 343.1111; found 343.1099.
2.1.6.13. 2-(4-(((5-Nitro-1H-benzo[d]imidazol-2-yl)thio)methyl)phenyl)propanoic acid (6 m)
Yield: 79%, M.P.=176.5–178.7 °C, FTIR (ATR, cm−1): 3098 (O–H), 1699 (C=O), 1063, 823, 748. 1 H-NMR (500 MHz, DMSO-d6): δ = 8.35 (1 H, d, J = 1.0 Hz, benzimidazole –CH–), 8.08 (1 H, dd, J = 7.9–1.0 Hz, benzimidazole –CH–), 7.64 (1 H, d, J = 7.9 Hz, benzimidazole –CH–), 7.44 (2 H, d, J = 7.9 Hz, 1,4-disubs. benzene –CH–),7.25 (2 H, d, J = 7.8 Hz, 1,4-disubs. benzene –CH–), 4.63 (2 H, s, –CH2–), 3.65 (1 H, q, J = 7.1 Hz, –CH–), 1.34 (3 H, d, J = 7.1 Hz, –CH3). 13C-NMR (125 MHz, DMSO-d6): δ = 175.7, 156.4, 142.8, 141.0, 135.9, 129.5, 128.3, 128.1, 118.1, 113.9, 110.8, 44.8, 35.2 and 18.9. HRMS (m/z): [M + H]+ calcd for C17H15N3O4S: 358.0856; found 358.0858.
2.2. COX-1 and COX-2 inhibition assay
Inhibitory potency of the compounds against COX-1 and COX-2 enzymes was determined using fluorometric COX-1 and COX-2 inhibitor screening kits (Biovision, Zurich, Switzerland). Experimental protocol was followed as described in the guides of the supplierCitation47,Citation48. All of the pipettings in the assay were performed by Biotek Precision robotic system (BioTek Instruments, Inc., Winooski, VT). Fluorescence (Ex/Em =535/587 nm) of the samples were kinetically measured by BioTek-Synergy H1 multimode microplate reader (BioTek Instruments, Inc., Winooski, VT) at 25 °C for 5–10 min. Appropriate two points (T1 and T2) in the linear range of the plot were chosen, and the corresponding fluorescence values (RFU1 and RFU2) were obtained. The slope for all samples, including enzyme control (EC), by dividing the net ΔRFU (RFU2–RFU1) values by the time ΔT (T2–T1) were calculated by using following equation:
This initial in vitro assay was done with two concentrations (10−3 and 10−4 M) for all compounds. The compounds, showing inhibition above 50%, were further assayed by the same protocol at varying concentrations (10−5 and 10−9 M) to determine their IC50 against COX-1 and COX-2 enzymes. The IC50 value was calculated from the plots of enzyme activity against concentrations by applying regression analyses on GraphPad Prism Version 5 (GraphPad Software, La Jolla, CA).
2.3. Enzyme kinetics
Enzyme kinetics study was performed to assess the nature of inhibition by the most active derivatives (6h and 6l) on the COX-1 enzyme. The enzyme kinetics were determined, wherein the arachidonic acid substrate either in the absence or presence of selected derivatives at different concentrations (IC50/4, IC50/2, IC50, 2 × IC50 and 4 × IC50). The mode of inhibition was determined by following the Lineweaver–Burk double reciprocal plot analysis of the data and calculated as per the Michaelis–Menten kinetics. To understand the possible mode of action, Km and Vmax were also calculated. The slopes of the Lineweaver–Burk plots were plotted versus the inhibitor concentration, and the Ki values were determined from the x-axis intercept as inhibition constant − Ki.
2.4. Antimicrobial activity
Microbiological studies were performed according to following guides: CLSI reference M07-A9 broth microdilution methodCitation49 for bacterial strains and EUCAST definitive (EDef 7.1) methodCitation50 for Candida species. Synthesized compounds were tested for their in vitro growth inhibitory activity against Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Listeria monocytogenes (ATCC 1911), Klebsiella pneumoniae (NCTC 9633), Escherichia coli (ATCC 35218), E. coli (ATCC 25922) Candida albicans (ATCC 24433) Candida krusei (ATCC 6258) and Candida parapsilosis (ATCC 22019). Chloramphenicol and ketoconazole were used as control drugs.
The cultures were obtained from the Mueller–Hinton broth (Difco) for the bacterial strains after overnight incubation at 37 °C. The yeasts were maintained in Roswell Park Memorial Institute (RPMI) after overnight incubation at 37 °C. The inocula of test microorganisms adjusted to match the turbidity of a Mac Farland 0.5 standard tube as determined with a spectrophotometer and the final inoculum size was 0.5–2.5 × 105 cfu/mL for antibacterial and antifungal assays. Testing was carried out in Mueller–Hinton broth and RPMI at pH =7, and the two-fold serial dilutions technique was applied. The last well on the microplates containing only inoculated broth was kept as controls and the last well with no growth of microorganism was recorded to represent the minimum inhibitory concentration (MIC) expressed in µg/mL. For both the antibacterial and antifungal assays, the compounds were dissolved in DMSO. Further dilutions of the compounds and standard drugs in test medium were prepared at the required quantities of 1000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9 and 1.95 µg/mL concentrations with Mueller–Hinton broth and RPMI mediums. The completed plates were incubated for 24 h. At the end of the incubation, resazurin (20 µg/mL) was added into each well and plates were incubated for 2 h. MIC values were determined using a microplate reader at 590 nm excitation, 560 nm emission.
2.5. Cytotoxicity test
Cytotoxicity was tested using the NIH/3T3 mouse embryonic fibroblast cell line (ATCC® CRL-1658™, London, UK). NIH/3T3 cells were incubated according to the supplier’s recommendations. NIH/3T3 cells were seeded at 1 × 104 cells into each well of 96-well plates. MTT assay was performed as previously describedCitation51,Citation52. The compounds were tested between 1000 and 0.316 µM concentrations. Inhibition percentage was calculated for each concentration according to the formula below, and IC50 values were determined by plotting a dose-response curve of inhibition percentage versus compound concentrations testedCitation53.
2.6. Genotoxicity test
The genotoxicity of the most effective compounds was determined by Ames assay using Ames MPF 98/100 mutagenicity assay sample kit (Xenometrix AG, Allschwil, Switzerland) as previously describedCitation52,Citation54. Salmonella typhimurium strains, TA98 (frameshift mutations) and TA100 (base-pair substitutions), are used in this assay. Concentration range of the compounds was between 16 and 5000 µg/mL according to the previous guidelinesCitation55. Compounds were prepared in six different concentrations (5, 2.5, 1.25, 0.625, 0.3125 and 0.156 mg/mL) in DMSO. Mutagenic potential was determined in absence and presence of Aroclor™-1254 induced male Sprague–Dawley rat liver microsomal enzyme (S9) mix (Xenometrix AG, Switzerland). Positive controls without S9 mix were 2-Nitrofluorene (2 µg/mL) and 4-nitroquinoline N-oxide (0.1 µg/mL) whereas 1 and 2.5 µg/mL of 2-aminoanthracene solutions were used as positive controls with S9 against TA 98 and TA100, respectively. Solvent control was prepared with 4% DMSO. At the end of the experiment, revertant bacteria dropped the pH of solution and indicator medium color was changed to yellow. Yellow wells were counted as positive and compared with the negative control. Fold induction over the negative control and fold induction over the baseline were calculated. Fold induction over the negative control is the ratio of the mean number of positive wells for the dose concentration divided by the mean number of positive wells for the zero dose (negative) control. Fold induction over the baseline is the ratio of the mean number of positive wells for the dose concentration divided by zero dose baseline. The zero dose baseline is obtained by adding one standard deviation to the mean number of positive wells of the zero dose control. Mutagenicity was determined according to the criteria from previous studiesCitation52,Citation56. For a value ≤3 and significant increases between two and three-fold the baselines were classified as a weak mutagen, and increases ≥ three-fold the baselines were classified as a mutagen. For a value >3 and significant increases between 1.5 and 2.5-fold the baselines were classified as a weak mutagen, and increases ≥2.5-fold the baselines were classified as a mutagen. As a rule, at least two adjacent doses with significant increases or a significant increase at the highest dose level should be observed for a mutagenic compound. All doses were compared according to Student’s t-test at p < .05 for statistical significance. Compounds, which did not have any of the properties mentioned above were classified as a non-mutagenic compound.
2.7. Theoretical calculation of ADME parameters
Some physicochemical parameters, which were used to evaluate ADME properties of the compounds (6a–6m) were analyzed by online Molinspiration property calculation programCitation57.
2.8. Molecular docking
A structure based in silico procedure was applied to discover the binding modes of the compounds 6h and 6l to COX-1 enzyme active site. The crystal structures of COX-1 enzyme (PDB ID: 1EQG), crystallized with the reversible inhibitor ibuprofen, was retrieved from the Protein Data Bank server (www.pdb.org).
The docking study was performed by using Maestro 10.6 software (Koingo Software, Inc., Kelowna, Canada)Citation58. The X-ray crystal structure was submitted to the Protein Preparation Wizard protocol of the Schrödinger Suite 2016 Update 3Citation59 to follow similar procedures described previouslyCitation60. Ligand preparation was applied by the LigPrep 3.8Citation61 to assign the protonation states and atom types of a molecule, correctly. The grid generation was formed using Glide 7.1Citation62 program and docking runs were performed with single precision docking mode (SP).
3. Results and discussion
3.1. Chemistry
Target compounds were synthesized in six steps following literature methods (Scheme 1). In the first step, 4-methylacetophenone (1) was reduced to 1-(4-methylphenyl)ethanol (2) in MeOH using NaBH4. Second, in a saturated CaCl2 ice bath, the compound 2 was treated with p-tosyl chloride to obtain 1-(4-methylphenyl)ethyl 4-methylbenzenesulfonate (3), which was reacted with NaCN to give 2-(4-methylphenyl)propionitrile (4) in the third step. Hydrolysis of compound 3 with 5 N HCl afforded the 2-(4-methylphenyl)propionic acid (4) in the next step. Bromination of the compound 4 in EtOAc gave the 2-(4-bromomethylphenyl)propionic acid (5), which was subjected to substitution reaction with various (benz)azolylthiols to obtain final compounds 6a–6m. As a result of synthesis path, the intermediate compounds were obtained in varying yields of 68–79%, whereas final compounds were isolated in 76–85% yields. Structural elucidation of the synthesized compounds was performed by spectral analyses. In the IR spectra, O–H and C=O stretching absorption belonging to carboxylic acid group were observed over 3000 cm−1 as broad bands and around 1700 cm−1 as sharp bands, respectively. In the NMR spectra, –CH3 protons recorded as doublet at 1.28–1.35 ppm and –CH3 carbon was recorded at 18.8–19.0 ppm. A quartet peak at 3.61–3.67 ppm was observed for –CH– proton and carbon of –CH– was assigned at 34.0–37.0 ppm. Protons of –SCH2– were observed as singlet at 4.41–4.73 ppm and carbon of this group was recorded at 44.7–44.8 ppm. The O–H proton of carboxylic acid group was recorded as a singlet at 13.87 ppm in only compound 6f, whereas the other compounds did not gave the same peak due to exchangeable carboxylic acid proton. Carbonyl carbon gave a peak at 175.6–175.7 ppm. All the other protons and carbons were recorded at expected values. All measured mass and isotope ratios were compatible with theoretical values in HRMS spectra.
3.2. COX enzymes inhibitory activity of the compounds
The in vitro COX-1 and COX-2 inhibitory activity of the compounds 6a–6m was evaluated with a fluorescence-based COX assay (“COX-1 Fluorescent Inhibitor Screening Kit, Catalog No: K547-100” and “COX-2 Fluorescent Inhibitor Screening Kit, Catalog No: K548–100”, Biovision, Milpitas, CA) that utilizes the COX-mediated reduction of PGG2 to PGH2 to oxidize 10-acetyl-3,7-dihydroxyphenoxazine to resorufin. This highly fluorescent compound can easily be analyzed with an excitation wavelength of 530–540 nm and emission wavelength of 585–595 nm. The results of the COX inhibitory activity of the 2-(4-Substituted-methylphenyl)propionic acid derivatives (6a–6m) are summarized in the . Ibuprofen and nimesulide were used as nonselective COX inhibitor and selective COX-2 inhibitor, respectively. Selectivity indexes (SI) were expressed as IC50 (COX-1)/IC50 (COX-2). Selectivity toward COX-2 decreases as the corresponding SI decreases while selectivity toward COX-1 isoform increases as the corresponding SI decreases. It was noted that the compounds indicated SI of 0.52–0.63. This result suggested that the compounds had selectivity toward COX-1 isoenzyme. The compounds 6a–6e indicated lower inhibition potency than the compounds 6f–6m against both isoenzymes. It has been determined that compounds 6f, 6g, 6h and 6l have important inhibitory activity against both COX-1 and COX-2 enzymes. IC50 values of these compounds were comparable with that of nimesulide against the COX-2 enzyme. Furthermore, they were more effective than ibuprofen and nimesulide against COX-1 enzyme. The most active compounds 6h and 6l displayed IC50 values of 1.76 and 1.40 µM against COX-1 and IC50 values of 2.96 and 2.34 µM against COX-2.
Table 1. IC50 (μM) values of the compounds 4, 6a–6m and reference drugs against COX-1 and COX-2 enzymes.
In order to observe contribution of variable groups to activity, COX inhibition potency of the intermediate product 2-(4-methylphenyl)propionic acid (4) was also evaluated. As seen in , the compound 4 has a lower potency than those of final compounds (6a–6n).
3.3. Enzyme kinetics
Substrate dependent kinetic parameters were determined to characterize the mechanism of inhibition of COX isoforms by compounds 6h and 6l. The kinetic parameters of this study were determined based on Michaelis–Menten equation followed by a Lineweaver–Burk double reciprocal analysis of data set regarding 1/Vmax versus 1/[S] plot. The Lineweaver–Burk plot analysis of the compounds 6h and 6l revealed them as competitive inhibitors. As shown in , the 1/Vmax (y-intercept) values for five different concentrations (IC50/4, IC50/2, IC50, 2 × IC50 and 4 × IC50) of compounds 6h and 6l are as same as that of no inhibitor, confirming their competitive inhibitory nature for COX-1 on the substrate arachidonic acid. The Ki (intercept on the x-axis) value of the compounds 6h and 6l was determined from the secondary plot of the slope versus varying concentrations (). The compounds 6h and 6l displayed Ki values of 2.07 and 1.70 µM for COX-1 enzyme, respectively.
Figure 1. (a) Lineweaver–Burk plots for the inhibition of COX-1 enzyme by compound 6h. [S], substrate concentration (mM); V, reaction velocity (nmol/min/mg protein). Inhibitor concentrations are shown at the left. Km values from 4 × IC50 to Control; 0.070, 0.048, 0.036, 0.029, 0.021 and 0.014 (mM). Vmax value of the competitive inhibition; 0.830 ± 0.011 (nmol/min/mg protein). (b) Secondary plot for calculation of steady-state inhibition constant (Ki) of compound 6h. Ki was calculated as 2.07 μM.
![Figure 1. (a) Lineweaver–Burk plots for the inhibition of COX-1 enzyme by compound 6h. [S], substrate concentration (mM); V, reaction velocity (nmol/min/mg protein). Inhibitor concentrations are shown at the left. Km values from 4 × IC50 to Control; 0.070, 0.048, 0.036, 0.029, 0.021 and 0.014 (mM). Vmax value of the competitive inhibition; 0.830 ± 0.011 (nmol/min/mg protein). (b) Secondary plot for calculation of steady-state inhibition constant (Ki) of compound 6h. Ki was calculated as 2.07 μM.](/cms/asset/0d70a70a-dcc3-42f3-8b85-cda6a92cac84/ienz_a_1310726_f0001_b.jpg)
Figure 2. (a) Lineweaver–Burk plots for the inhibition of COX-1 enzyme by compound 6l. [S], substrate concentration (mM); V, reaction velocity (nmol/min/mg protein). Inhibitor concentrations are shown at the left. Km values from 4 × IC50 to Control; 0.064, 0.043, 0.030, 0.025, 0.017 and 0.013 (mM). Vmax value of the competitive inhibition; 0.669 ± 0.003 (nmol/min/mg protein). (b) Secondary plot for calculation of steady-state inhibition constant (Ki) of compound 6l. Ki was calculated as 1.70 μM.
![Figure 2. (a) Lineweaver–Burk plots for the inhibition of COX-1 enzyme by compound 6l. [S], substrate concentration (mM); V, reaction velocity (nmol/min/mg protein). Inhibitor concentrations are shown at the left. Km values from 4 × IC50 to Control; 0.064, 0.043, 0.030, 0.025, 0.017 and 0.013 (mM). Vmax value of the competitive inhibition; 0.669 ± 0.003 (nmol/min/mg protein). (b) Secondary plot for calculation of steady-state inhibition constant (Ki) of compound 6l. Ki was calculated as 1.70 μM.](/cms/asset/26f642e6-56dd-4e9a-9ece-3bcebf875b01/ienz_a_1310726_f0002_b.jpg)
3.4. Antimicrobial activity
Synthesized compounds (6a–6m) were evaluated for antimicrobial activity against various microorganisms such as Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Listeria monocytogenes (ATCC 1911), Klebsiella pneumoniae (NCTC 9633), Escherichia coli (ATCC 35218), E. coli (ATCC 25922) Candida albicans (ATCC 24433) Candida krusei (ATCC 6258) and Candida parapsilosis (ATCC 22019). MIC values () were revealed by fluorometric measurements using resazurin solutionCitation63,Citation64. Chloramphenicol and ketoconazole were used as standard drugs in the activity test. As seen in , the synthesized compounds (6a–6m) have more potency against bacteria than fungi and display similar antibacterial spectrum to the chloramphenicol. The MIC value of 6.25 µg/mL against E. coli (ATCC 35218) was observed for all compounds as well as chloramphenicol. Besides, the compounds 6d, 6h, 6l and 6m indicated stronger antibacterial activity than the other compounds in the series. These compounds found to be more effective against Enterococcus faecalis (ATCC 29212), Listeria monocytogenes (ATCC 1911) than chloramphenicol. The compound 6m, carrying 5-nitrobenzimidazole substructure, was the most active in the series with a better antibacterial spectrum than chloramphenicol. This finding may be explained by the well-known antibacterial effects of 5-nitrobenzimidazolesCitation65.
Table 2. Antimicrobial activity (MIC μg/mL) of compounds 4, 6a–6m and reference drugs against pathogenic microorganisms.
Antimicrobial activity of the intermediate product 2-(4-methylphenyl)propionic acid (4) was also investigated to compare its activity to those of final compounds (6a–6m). As seen in the , the compound 4 is as not active against any bacterial strains.
3.5. Cytotoxicity
There are a number of requirements to be fulfilled for successful new drug development. The drug candidate should not only possess intrinsic activity, but should also be able to reach its target and not exhibit toxic effects. Thus, cytoxicity of compounds 6 h and 6 l, which demonstrated significant COX inhibition and promising antibacterial activity, was investigated by MTT assay. This assay is based upon the reduction of yellow MTT dye by metabolically active eukaryotic and prokaryotic cells to form the purple formazan product. The assay is generally used to examine cell viability and to estimate cell culture growthCitation66,Citation67. MTT assay was carried out using healthy NIH/3T3 mouse embryonic fibroblast cell lines (ATCC CRL1658), which is recommended for cytotoxicity screening by ISO (10993-5, 2009)Citation68. Ibuprofen and nimesulide were also subjected to MTT assay in order to compare cytotoxicity of the compounds 6h and 6l with those of reference agents. presents the results, in which the synthesized compounds and reference agents displayed IC50 of ≥1000 µM. These findings show that the antibacterial activity of the compounds 6h and 6l is not due to general toxicity, but can be ascribed to its selective action against bacteria. Furthermore, it may be concluded that the compounds 6 h and 6 l are not cytotoxic, because their IC50 values against COX enzymes are about 500 fold lower than IC50 values against NIH/3T3 cells.
Table 3. IC50 (μM) values of the ibuprofen. nimesulide and the compounds 6h and 6l against NIH/3T3 cell line.
3.6 Genotoxicity
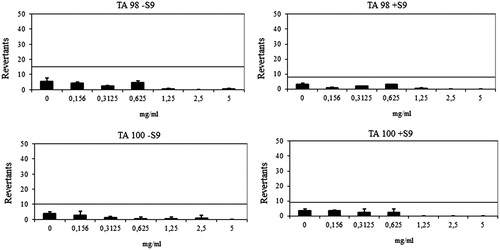
Ames assay was performed to investigate the genotoxicity of compounds 6h and 6l. In AmesMPF assay, more than 25 positive wells were observed with positive controls and negative control wells also showed less than eight positive wells in the presence and absence of S9 with TA98 and TA100, which complied with the requirements for the validation of the AmesMPF and also as described in previous studiesCitation56. Results are presented in .
Table 4. The AMESMPF results of the compounds.
The compound 6h showed a baseline of 7.71 with TA98 in the absence of S9 and 1.91 in the presence of S9. Any of the concentrations did not reach the mentioned values above the base-line and also did not show any significance. Therefore, the compound 6h was classified as non-mutagenic against TA98 in the presence/absence of metabolic activation (S9) (). The compound 6h had a baseline of 1.91 with TA 100 in the absence of S9 and a baseline of 6.65 in the presence of S9. Fold inductions over baseline did not reach values more than 2 or 1.5 and statistically different results did not reveal a dose-response tendency. According to these findings, the compound 6h did not show any mutagenicity against TA 100 ().
Figure 3. Dose-response curve of compound 6h against TA98 and TA100 in the presence and absence of S9 according to AMESMPF test.
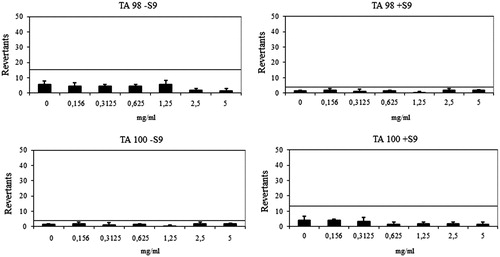
The compound 6l showed a baseline of 7.64 and 4.00 against TA 98 with/without S9, respectively. Mentioned-fold increases over the baseline according to the criteria were not determined with the compound 6l, and significant results did not reach these values and did not show any dose-response tendency. The compound 6l was also found to be non-mutagenic against TA100 in the presence or absence of metabolic activation (). The compound 6l had a baseline of 5 with TA 100 in the absence of S9 and a baseline of 4.49 in the presence of S9. Fold inductions over baseline were less than 1.5 in each concentration of the compounds and there were not any significant differences. The compound 6l was accepted as non-mutagenic against TA98 and TA100 with and without metabolic activation (). According to the AmesMPF results, the compounds 6h and 6l were classified as non-mutagens, which increases the pharmacological importance of the compounds.
3.7. Prediction of ADME properties
In addition to essential biological activity, drug candidates should also have an ideal pharmacokinetic profile. Lipinski’s rule evaluates the absorption, distribution, metabolism and elimination (ADME) properties of drug like compounds and is important for the optimization of a biologically active compound. The rule requires that an orally active drug should not have more than one violationCitation69. In order to determine pharmacokinetic properties of the synthesized compounds 6a–6m, the theoretical calculations of the physicochemical parameters (molecular weight (MW), log octanol/water partition coefficient (log P), topological polar surface area (tPSA), number of hydrogen donors (nON), number of hydrogen acceptors (nOHNH), number of rotatable bonds (nRotb) and molecular volume (MV)) are presented in along with violations of Lipinski’s rule. According to this data, all of the compounds (6a–6m) follow Lipinski’s rule by causing no more than one violation. For compounds 6h and 6l, all calculated physicochemical parameters are compatible with Lipinski’s rule. Thus, it may be suggested that synthesized compounds may have a good pharmacokinetic profile, which is crucial for a drug candidate.
Table 5. In silico physicochemical parameters of the compounds 6a–6m.
3.8. Molecular docking
Docking studies were performed in order to gain more insight into the binding mode of the compounds 6h and 6l, and to evaluate the effects of structural modifications on the inhibitory activity against COX-1 enzyme. Studies were carried out by using the X-ray crystal structure of COX-1 enzyme (PDB ID: 1EQG)Citation10 obtained from Protein Data Bank server (www.pdb.org). The docking poses of the compounds 6h and 6l are presented in .
Figure 5. The binding site of COX-1 containing compound 6h (a) and 6l (b). The interacting side chain amino acid residues are shown in sticks style.
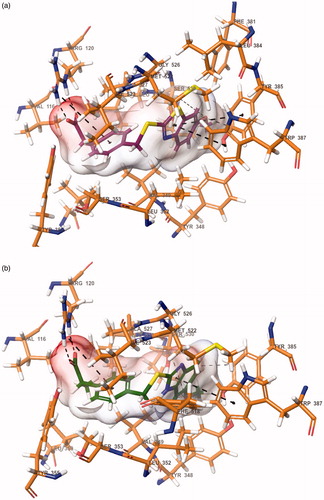
When the docking studies are analyzed, it is seen that the inhibitor ibuprofen binds in the COX active site, which is consisted of a long narrow hydrophobic channel lining from the membrane binding surface to the center of the protein. The propionic acid group of ibuprofen is very essential in terms of binding to the active site. This group takes part in a network of polar interactions, which include two hydrogen bonds between the propionic acid (carbonyl and hydroxyl groups) and Arg120Citation8,Citation10.
The compounds 6h and 6l are settled in the hydrophobic channel very concordantly, likewise ibuprofen. Phenyl propionic acid is the common group of the ibuprofen and the compounds 6h and 6l. Propionic acid moiety forms two hydrogen bonds with Arg120. Furthermore, the phenyl ring constitutes a salt bridge with Arg120. Benzothiazole and benzimidazole provide aromaticity for compounds 6h and 6l, respectively. These structures interact with the phenyl of Tyr385 and indole of Trp387 by doing π–π interactions in both compounds 6h and 6l.
In terms of chemical structures of the synthesized compounds (6a–6m), only the compounds 6h and 6l have methoxy substituents in fifth position of benzothiazole and benzimidazole. The methoxy group ensures significant polar interaction with the amino group of Leu534 by doing a hydrogen bond. By virtue of this interaction, compounds 6h and 6l could bind to the active site, efficiently and may have a higher COX inhibition potency than other derivatives in the series.
3.9. Structure activity relationships (SARs)
The substitution pattern was explored using various (benz)azolylthio moieties in 2-[4-methylphenyl]propionic acid main substructure. Thus, determination of contribution of the various bioisosteric (benz)azolylthio moieties to COX inhibitory and/or antimicrobial activity and evaluation of SARs were planned. The noteworthy results of enzyme inhibition, antimicrobial, physicochemical parameters calculation and docking studies also required to discuss structure activity relationships (SARs). However, SARs cannot be discussed for antifungal activity due to high MIC values of the compounds (6a–6m). Moreover, observation of very similar antibacterial activity, displayed by the compounds (6a–6m) indicates that there is no important difference between contributions of azolylthio moieties to antibacterial activity and makes consideration of the SARs very difficult. Only presence of the 5-nitro substitution benzimidazolylthio moiety in compound 6l results with enhanced antibacterial activity. Hence, it can be assumed that promising antibacterial activity of the compounds (6a–6m) is related to their general structural characteristics. Lower antibacterial activity results, observed in the compound 4, also support this approach and highlight the importance of (benz)azolylthio moiety on antibacterial activity.
Against COX enzymes, all target compounds (6a–6m) exhibited better COX inhibition than intermediate compound 4. This finding displays that incorporation of (benz)azolylthio and 2-(4-methylphenyl)propionic acid structures has a positive contribution to COX inhibitory activity. However, the compounds 6a–6e have lower inhibition potency than the compounds 6f–6m. The first suggestion of this observation can be the logP values of the compounds. Increasing logP in compounds 6f–6m may enhance the enzyme inhibition potency (). Second, it can be suggested that in the compounds 6f–6m presence of a benzazolylthio moiety, which is absent in 6a–6e, promotes the enzyme inhibition as a result of π–π interaction in the active site of enzyme. Among the compounds 6f–6m, the most active compounds are 6 h and 6 l. The common feature of the 6h and 6l separating from other compounds is a methoxy substituent in the fifth position of benzothiazolylthio and benzimidazolylthio substructures. Thus it may be suggested that methoxy group creates more inhibition potency than the other substituents. This proposal may be explained by hydrogen accepting ability of alkyloxy groups and has been supported by the docking study (). It is well known that 2-phenylpropionic acid is the main substructure, being responsible to COX inhibition, in lots of well-known marketing drugs. Thus, this substructure has been fixed in all compounds. Importance of 2-phenylpropionic acid in COX inhibition has also been observed in the docking studies ().
4. Conclusions
In summary, preliminary evaluation of new 2-(4-substitutedmethylphenyl)propionic acid derivatives as dual COX inhibitory-antibacterial agents resulted with promising findings. The compounds 6h and 6l displayed a good antibacterial profile along with significant COX-1 and COX-2 inhibition. Furthermore, these compounds did not show cytotoxicity and mutagenicity. Docking study indicated the significant interactions between both compounds and COX-1 enzyme. Consequently, findings of this study will not only direct our research group to further studies, but also may have an impact on medicinal chemists, stimulating them to synthesize more effective and safer compounds bearing chemical structures similar to those of the compounds 6h and 6l as dual COX inhibitory-antibacterial agents.
Acknowledgements
This study was financially supported by Anadolu University Scientific Projects Fund, Project No: 1409S385.
Disclosure statement
The authors declare no conflicts of interest.
References
- Gupta K, Selinsky BS, Kaub CJ, et al. The 2.0 Angstrom resolution crystal structure of prostaglandin H2 synthase-1: structural insights into an unusual peroxidase. J Mol Biol 2004;335:503–18.
- Botting RM. Cyclooxygenase: past, present and future. Atribute to John R. Vane (1927–2004). J Therm Biol 2006;31:208–19.
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 2004;56:387–437.
- Smith WL, Marnett LJ. Prostaglandin endoperoxide synthase: structure and catalysis. Biochim Biophys Acta 1991;1083:1–17.
- Bakhle YS. Structure of COX-1 and COX-2 enzymes and their interaction with inhibitors. Drugs Today (Barc) 1999;35:237–50.
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000;69:145–82.
- Garavito RM, Malkowski MG, DeWitt DL. The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat 2002;68–69:129–52.
- Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature 1994;367:243–9.
- Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996;384:644–8.
- Selinsky BS, Gupta K, Sharkey CT, Loll PJ. Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry 2001;40:5172–80.
- Narayana B, Raj KKV, Ashalatha BV, Kumari NS. Synthesis of some new 2-(6-methoxy-2-naphthyl)- 5-aryl-1,3,4-oxadiazoles as possible non-steroidal anti-inflammatory and analgesic agents. Arch Pharm (Weinheim) 2005;338:373–7.
- Borne R, Levi M, Wilson N. Nonsteroidal anti-inflammatory drugs (Chap. 31). In: Lemke TL, Williams DA, Roche VF, Zito SW, eds. Foye’s principles of medicinal chemistry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2013:987–1044.
- Downer-Riley NK, Jackson YA. Recent advances in the synthesis of 1,3-azoles. Curr Top Med Chem 2016;16:3617–26.
- Peng XM, Cai GX, Zhou CH. Recent developments in azole compounds as antibacterial and antifungal agents. Curr Top Med Chem 2013;13:1963–2010.
- Zhao S, Zhao L, Zhang X, et al. Design, synthesis, and structure-activity relationship studies of benzothiazole derivatives as antifungal agents. Eur J Med Chem 2016;123:514–22.
- Vijesh AM, Isloor AM, Shetty P, et al. New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 2013;62:410–5.
- Moraca F, De Vita D, Pandolfi F, et al. Synthesis, biological evaluation and structure-activity correlation study of a series of imidazol-based compounds as Candida albicans inhibitors. Eur J Med Chem 2014;83:665–73.
- Ansari KF, Lal C. Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur J Med Chem 2009;44:2294–9.
- Zhang HZ, Damu GL, Cai GX, Zhou CH. Design, synthesis and antimicrobial evaluation of novel benzimidazole type of fluconazole analogues and their synergistic effects with chloromycin, norfloxacin and fluconazole. Eur J Med Chem 2013;64:329–44.
- Kant R, Kumar D, Agarwal D, et al. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur J Med Chem 2016;113:34–49.
- Morjan RY, Al-Attar NH, Abu-Teim OS, et al. Synthesis, antibacterial and QSAR evaluation of 5-oxo and 5-thio derivatives of 1,4-disubstituted tetrazoles. Bioorg Med Chem Lett 2015;25:4024–8.
- Rostom SA, Ashour HM, El Razik HA, et al. Azole antimicrobial pharmacophore-based tetrazoles: synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg Med Chem 2009;17:2410–22.
- Secci D, Bolasco A, D'Ascenzio M, et al. Conventional and microwave‐assisted synthesis of benzimidazole derivatives and their in vitro inhibition of human cyclooxygenase. J Heterocyclic Chem 2012;49:1187–95.
- Umaru T, Nwamba OC, Kolo I, Nwodo UU. Antimicrobial activity of non-steroidal anti-inflammatory drugs with respect to immunological response: diclofenac sodium as a case study. Afr J Biotechnol 2009;8:7332–9.
- Weng TC, Chen CC, Toh HS, Tang HJ. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect 2011;44:418–23.
- Leroy S, Marc E, Bavoux F, et al. Hospitalization for severe bacterial infections in children after exposure to NSAIDs. A prospective adverse drug reaction reporting study. Clin Drug Invest 2010;30:179–85.
- Bekhit AA, Abdel-Azeim T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem 2004;12:1935–45.
- Sharshira EM, Hamada NM. Synthesis and antimicrobial evaluation of some pyrazole derivatives. Molecules 2012;17:4962–71.
- Handler N, Jaeger W, Kuen-Krismer B, Erker T. Cyclooxygenase-1 and cyclooxygenase-2 inhibition of novel 1,2-disubstituted imidazoles. Arch Pharm (Weinheim) 2005;338:602–4.
- Parab RH, Dixit BC. Synthesis, characterization and antimicrobial activity of imidazole derivatives based on 2-chloro-7-methyl-3-formylquinoline. J Chem 2012;9:1188–95.
- Ahmadi F, Ghayahbashi MR, Sharifzadeh M, et al. Synthesis and evaluation of anti-inflammatory and analgesic activities of new 1,2,4-triazole derivatives. Med Chem 2015;11:69–76.
- Eswaran S, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur J Med Chem 2009;44:4637–47.
- Deb PK, Kaur R, Chandrasekaran B, et al. Synthesis, anti-inflammatory evaluation, and docking studies of some new thiazole derivatives. Med Chem Res 2014;23:2780–92.
- Bondock S, Khalifa W, Fadda AA. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem 2007;42:948–54.
- Alegaon SG, Hirpara MB, Alagawadi KR, et al. Synthesis of novel pyrazole–thiadiazole hybrid as potential potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett 2014;24:5324–9.
- Bekhit AA, El-Sayed OA, Aboulmagd E, Park JY. Tetrazolo[1,5-a]quinoline as a potential promising new scaffold for the synthesis of novel anti-inflammatory and antibacterial agents. Eur J Med Chem 2004;39:249–55.
- Parveen H, Mukhtar S, El Sayed NH, Hayat F. Synthesis, characterization and antimicrobial activity of long chain fatty alkenoates of metronidazole and their novel tetrazole derivatives. Asian J Chem 2014;26:8134–8.
- Lu X, Zhang H, Li X, et al. Design, synthesis and biological evaluation of pyridine acyl sulfonamide derivatives as novel COX-2 inhibitors. Bioorg Med Chem 2011;19:6827–32.
- Jyothi MV, Rajendra Prasad Y, Venkatesh P, Sureshreddy M. Synthesis and antimicrobial activity of some novel chalcones of 3-acetyl pyridine and their pyrimidine derivatives. Chem Sci Trans 2012;1:716–22.
- Moneer AA, Mohammed KO, El-Nassan HB. Synthesis of novel substituted thiourea and benzimidazole derivatives containing a pyrazolone ring as anti-inflammatory agents. Chem Biol Drug Des 2016;87:784–93.
- Altıntop MD, Abu Mohsen U, Özkay Y, et al. Synthesis and antimicrobial activity of benzimidazole-based acetamide derivatives. Turk J Pharm Sci 2015;12:29–38.
- Paramashivappa R, Phani Kumar P, Subba Rao PV, Srinivasa Rao A. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett 2003;13:657–60.
- Ören İ, Temiz Ö, Yalçın İ, et al. Synthesis and antimicrobial activity of some novel 2,5- and/or 6-substituted benzoxazole and benzimidazole derivatives. Eur J Pharm Sci 1999;7:153–60.
- Vázquez MT, Rosell G, Pujol MD. Synthesis and anti-inflammatory activity of rat-2-(2,3-dihydro-1,4-benzodioxin)propionic acid and its R and S enantiomers. Eur J Med Chem 1997;32:529–34.
- Xingxian Z, Kebin H, Jiankun N, et al. Process for preparation of loxoprofen sodium, Patent CN 101412670, 2009.
- Özkay Y, Işıkdağ İ, İncesu Z, et al. Synthesis of 2-substituted-N-[4-(1-methyl-4,5-diphenyl-1H-imidazole-2-yl)phenyl]acetamide derivatives and evaluation of their anticancer activity. Eur J Med Chem 2010;45:3320–8.
- Biovision COX-1 Fluorescent Inhibitor Screening Kit (Catalog No: K548-100) manual. Available from: http://www.biovision.com/manuals/K548.pdf [last accessed Aug 2016].
- Biovision COX-2 fluorescent inhibitor screening kit (Catalog No: K547-100) manual. Available from: http://www.biovision.com/manuals/K547.pdf [last accessed Aug 2016].
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard-Ninth Edition. Pennsylvania, USA: CLSI document M07-A9.
- EUCAST. Definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Copenhagen, Denmark: EUCAST.
- Sağlık BN, Ilgın S, Özkay Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur J Med Chem 2016;124:1026–40.
- Demir Özkay Ü, Can ÖD, Sağlık BN, et al. Design, synthesis, and AChE inhibitory activity of new benzothiazole-piperazines. Bioorg Med Chem Lett 2016;26:5387–94.
- Patel S, Gheewala N, Suthar A, Shah A. In-vitro cytotoxicity activity of Solanum nigrum extract against hela cell line and vero cell line. Int J Pharm Pharm Sci 2009;1:38–46.
- Altıntop MD, Özdemir A, Turan-Zitouni G, et al. Synthesis and biological evaluation of some hydrazone derivatives as new anticandidal and anticancer agents. Eur J Med Chem 2012;58:299–307.
- Chandrasekaran CV, Sundarajan K, Gupta A, et al. Evaluation of the genotoxic potential of standardized extract of Glycyrrhiza glabra (GutGard™). Regul Toxicol Pharmacol 2011;61:373–80.
- Flückiger-Isler S, Kamber M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames preincubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat Res 2012;747:36–45.
- M. Cheminformatics, Bratislava, Slovak Republic. Available from: http://www.molinspiration.com/services/properties.html [last accessed Aug 2016].
- Maestro, version 10.6. New York, NY: Schrödinger, LLC; 2016.
- Schrödinger, LLC, New York, NY, 2016.
- Kaserer T, Temml V, Kutil Z, et al. Prospective performance evaluation of selected common virtual screening tools. Case study: cyclooxygenase (COX) 1 and 2. Eur J Med Chem 2015;96:445–57.
- LigPrep, version 3.8. New York, NY: Schrödinger, LLC; 2016.
- Glide, version 7.1. New York, NY: Schrödinger, LLC; 2016.
- Borra RC, Lotufo MA, Gagioti SM, et al. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz Oral Res 2009;23:255–62.
- Palomino JC, Martin A, Camacho M, et al. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2002;46:2720–2.
- Larina L, Lopyrev V. Nitroazoles: synthesis, structure and applications. New York: Springer Science, 2009.
- Pozzolini M, Scarfı′ S, Benatti U, Giovine M. Interference in MTT cell viability assay in activated macrophage cell line. Anal Biochem 2003;313:338–41.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- International Organization for Standardization. Biological evaluation of medical devices-part 5: tests for in vitro cytotoxicity ISO-10993-5. 3rd ed. Geneva, Switzerland: International Organization for Standardization; 2009.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 2001;46:3–26.