Abstract
Cancer stem cells (CSCs) have been objects of intensive study since their identification in 1994. Adopting a structural rigidification approach, a novel series of 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d was designed and synthesised, in an attempt to develop potent anticancer agent that can target the bulk of tumour cells and CSCs. The anti-proliferative activity of the synthesised compounds was evaluated against two cell lines, namely; colon cancer HT-29 and triple negative breast cancer MDA-MB-468 cell lines. Also, their inhibitory activity against the cell surface expression of CD133 was examined. In particular, compound 4b emerged as a promising hit molecule as it manifested good antineoplastic potency against both tested cell lines (IC50 = 9 and 12 μM, respectively), beside its ability to inhibit the cell surface expression of CD133 by 50% suggesting a promising potential of effectively controlling the tumour by eradicating the tumour bulk and inhibiting the proliferation of the CSCs. Moreover, compounds 4a and 4c showed moderate activity against HT-29 (IC50 = 21 and 29 μM, respectively) and MDA-MB-468 (IC50 = 23 and 24 μM, respectively) cell lines, while they inhibited the CD133 expression by 14% and 48%, respectively. Finally, a single crystal X-ray diffraction was recorded for compound 4d.
Introduction
Cancer stem cells (CSCs) paradigm spurted over the past few decades as an answering solution for the ambiguity of haematological malignancies as well as solid tumours regarding intra-tumoural heterogeneity and tumour dormancyCitation1,Citation2. Moreover, the observation that tumour has the proclivity to resist chemotherapy and even radiotherapyCitation3, besides metastatic relapse that can occur more than a decade post initial treatment and clinical cureCitation4,Citation5, suggests a more circuitous aetiology for the malignancies. This surveillance devoted the scientists to abandon the postulation that tumour is a mass of homogeneous cancer cell population but rather to adopt the concept that malignancy is conceived as a “disorganised tissue”, having the CSCs at the top of the hierarchy of heterogeneous tumour tissuesCitation6–8.
Avalanche of scientific research postulated two key hypothetical explanations for the existence of CSCsCitation8,Citation9. CSCs may arise from normal stem cells via gene mutation rendering the stem cells neoplastic, or alternatively their origin might be genetic alterations of the differentiated tumour cells that ultimately acquire CSC-like featuresCitation9.
In this view, CSCs are beheld as a distinct subpopulation of tumour cells that exhibit exclusive characteristics. Indeed, three main key properties of CSCs render them highly distinguishableCitation10, (1) differentiation, as they are capable to give rise to a hierarchy of progenitor and aberrantly differentiated cells, (2) self-renewal capacity, which conserves an intact stem cell pool, (3) homeostatic control that guarantees a balance between differentiation and self-renewal in response to environmental stimuliCitation10. Moreover, cunningly, the CSCs mimic their normal counterparts as they possess slow rate of proliferation rendering them resistant to conventional treatmentCitation10,Citation11. Accordingly, CSCs are regarded as the main culprit that fuels tumour development, progression, metastasis, and relapse. In this insight, targeting CSCs that are the “beating heart” of the tumour is a judicious goal for establishing a platform of effective cancer therapyCitation4,Citation5,Citation11.
Pertaining to their close similarity to normal cells, it is problematic to segregate CSCs from non-CSCs within a tumour. But for the presence of surface cell antigens, the identification and separation of tumour initiating cells from more differentiated tumour cells would not have been possibleCitation12. Five surface antigens whose expression is thought to indicate stem cell like properties namely, CD133, CD44, CD24, CDCP1, and CXCR4 proved to be useful for the identification and characterisation of CSCs within a tumourCitation12. CD133 (Prominin-1 or AC133) is a transmembrane pentaspan protein antigenCitation13 found on stem-like cells of various tissues and cancers, like brainCitation14, colonCitation15, breastCitation16, liverCitation17, pancreasCitation18, kidneyCitation19, lungCitation20, endometriumCitation21, ovaryCitation22, and boneCitation23. The supporting evidence that CD133 (+) cells had the ability to maintain survival, recurrence, metastasis, and chemotherapy resistance of neoplasms further proved that CD133 is a useful CSC markerCitation24. Accordingly, it is thought to be a predictive indicator for neoplasm identification. Targeting CD133 might be a successful strategy for combating cancer.
In our previous work, we synthesised a series of 2-((benzimidazol-2-yl) thio)-1-arylethan-1-ones (Series 1, ) that proved to possess good anti-proliferative activity toward HT-29 colon cancer cell line besides its capability to inhibit cell surface expression of CD133 in HT-29 cancer cellsCitation2. Inspired by these findings and as a part of our ongoing efforts towards developing potent anticancer agentsCitation25, we designed a new series of 3-phenylthiazolo[3,2-a]benzimidazoles (Series 2, ) based on a benzimidazole scaffold that proved to be affirmative for the anti-proliferative activity beside the CD133 inhibitory potential.
Figure 1. Judicious design of target 3-phenylthiazolo[3,2-a]benzimidazoles (Series 2) based on cyclisation of disclosed compounds 2-((benimidazol-2-yl)thio)-1-arylethan-1-ones (Series 1).
![Figure 1. Judicious design of target 3-phenylthiazolo[3,2-a]benzimidazoles (Series 2) based on cyclisation of disclosed compounds 2-((benimidazol-2-yl)thio)-1-arylethan-1-ones (Series 1).](/cms/asset/5fc4cbb1-e1d9-4fba-a424-9ef30569d25a/ienz_a_1347166_f0001_c.jpg)
Our judicious design aimed at improving the potency of the disclosed compounds by increasing the selectivity of the synthesised compounds. This was achieved through limiting the free rotation around the single bonds in the thioethanone linker by incorporating the linker in a cyclised thiazole ring. This intervention afforded compounds that are frozen into a rigid structure thus exhibiting less isomers which augments their selectivity at the target proteins. Moreover, our design was based on previous SAR findings that highlighted the substitution of the pendent phenyl ring by an electron-donating group to be profitable for the inhibition of both the bulk tumour cells and the CSCs.
Materials and methods
Chemistry
Melting points were determined using a Gallenkamp melting point apparatus and are uncorrected. Infrared (IR) Spectra were recorded as KBr disks using the Perkin Elmer FT-IR (Fourier transform infrared) Spectrum BX apparatus. Mass spectra were measured on an Agilent Triple Quadrupole 6410 QQQ LC/MS (Liquid chromatography/Mass spectroscopy) equipped with an ESI (electrospray ionisation). NMR spectra were recorded on a Bruker NMR spectrometer. 1H spectrum was run at 500 MHz and 13C spectrum was run at 125 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Chemical shifts are expressed in δ values (ppm) using the solvent peak as internal standard. All coupling constant (J) values are given in hertz. The abbreviations used are as follows: s, singlet; d, doublet; m, multiplet. Elemental analyses were carried out at the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. Analytical thin layer chromatography (TLC) on silica gel plates containing UV indicator was employed routinely to follow the course of reactions and to check the purity of products. All reagents and solvents were purified and dried by standard techniques.
General procedure for synthesis of sulphate salts 3a–d
To a solution of the appropriate acetophenone 1a–d (5 mmol) in glacial acetic acid (10 ml), 2-mercaptobenzimidazole 2 (0.75 g, 5 mmol) and conc. sulphuric acid (50 mmol) were added. The reaction mixture was heated under reflux for 2 h. The solid product obtained upon cooling was filtered off, washed with cold water then with petroleum ether, and recrystallised from ethanol to afford the corresponding sulphate salts 3a–d, respectively.
General procedure for preparation of 3-(3-anyl)benzo[4,5]imidazo[2,1-b]thiazoles 4a–d
To a suspension of the appropriate sulphate salts 3a–d (2 mmol) in water (10 ml), an aqueous solution of sodium bicarbonate was added. The reaction mixture was stirred at room temperature for 2 h. The solid formed was collected by filtration, washed with water, dried, and crystallised from ethanol to afford compounds 4a–d, respectively.
3-(3-Methoxyphenyl)benzo[4,5]imidazo[2,1-b]thiazole (4a)
White crystals (yield 80%), m.p. 173–175 °C; 1H NMR (DMSO-d6) δ ppm: 3.84 (s, 3H, OCH3), 7.13 (d, 1H, Ar–H, J = 7.5 Hz), 7.20–7.32 (m, 6H, Ar–H), 7.54 (t, 1H, Ar–H, J = 8.0 Hz), 7.71 (d, 1H, Ar–H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ ppm: 55.86 (OCH3), 109.23, 112.01, 114.68, 116.44, 119.23, 120.86, 121.47, 123.61, 130.61, 130.69, 133.66, 148.64, 157.03, 159.92; Anal. Calcd. for C16H12N2OS: C, 68.55; H, 4.31; N, 9.99; Found C, 68.73; H, 4.28; N, 10.12.
4-(Benzo[4,5]imidazo[2,1-b]thiazol-3-yl)-2-methoxyphenol (4b)
White crystals (yield 78%), m.p. 190–193 °C; 1H NMR (DMSO-d6) δ ppm: 3.82 (s, 3H, OCH3), 6.99 (d, 1H, Ar–H, J = 8.0 Hz), 7.09 (s, 1H, Ar–H), 7.12 (t, 2H, Ar–H, J = 7.5 Hz), 7.27–7.31 (m, 3H, Ar–H), 7.69 (d, 1H, Ar–H, J = 8.0 Hz), 9.67 (s, 1H, OH, D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 56.23 (OCH3), 107.63, 112.07, 113.40, 116.14, 119.11, 120.06, 120.72, 122.38, 123.48, 130.22, 134.22, 148.17, 148.67, 148.77, 156.94; Anal. Calcd. for C16H12N2O2S: C, 64.85; H, 4.08; N, 9.45; Found C, 65.14; H, 4.02; N, 9.34.
3-(3,4,5-Trimethoxyphenyl)benzo[4,5]imidazo[2,1-b]thiazole (4c)
White crystals (yield 83%), m.p. 177–179 °C; 1H NMR (DMSO-d6) δ ppm: 3.79 (s, 6H, 2 OCH3), 3.83 (s, 3H, OCH3), 7.07 (s, 2H, Ar–H), 7.22–7.38 (m, 4H, Ar–H), 7.71 (d, 1H, Ar–H, J = 8.0 Hz); 13C NMR (DMSO-d6) δ ppm: 56.62 (OCH3), 60.71 (OCH3), 106.95, 108.79, 112.23, 119.19, 120.89, 123.58, 124.64, 130.21, 133.83, 139.20, 148.68, 153.59, 156.93; Anal. Calcd. for C18H16N2O3S: C, 63.51; H, 4.74; N, 8.23; Found C, 63.69; H, 4.69; N, 8.11.
4-(Benzo[4,5]imidazo[2,1-b]thiazol-3-yl)aniline (4d)
White crystals (yield 75%), m.p. 185–186 °C; 1H NMR (DMSO-d6) δ ppm: 5.28 (s, 2H, NH2), 6.73 (d, 2H, Ar–H, J = 8.0 Hz), 7.22–7.38 (m, 4H, Ar–H), 7.59 (d, 2H, Ar–H, J = 8.0 Hz), 7.68 (d, 1H, Ar–H, J = 8.0 Hz); Anal. Calcd. for C15H11N3S: C, 67.90; H, 4.18; N, 15.84; Found C, 68.15; H, 4.14; N, 15.73.
X-ray crystallographic analysis
The measurements of the crystal of compound 4d were performed on a Bruker SMART APEX II D8 Venture diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 100 K. The structure was solved by direct method and refined with SHELXTL. E-maps provided the positions of all the non-H-atoms. The full-matrix least-squares refinement was carried out on F2’s using anisotropic temperature factors for all non-H-atoms. Crystallographic data for the structure reported in this paper have been deposited at the Cambridge Crystallographic Data Centre and allocated with the deposition number: CCDC 1429525.
Biological evaluations
In vitro anti-proliferative activity
Anti-proliferative activity of the synthesised 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d was evaluated at Stem Cell Therapy and Tissue Reengineering Program, King Faisal Specialized Hospital and Research Center, Riyadh, Saudi Arabia. In vitro anti-proliferative activity was measured by the cell growth inhibition assay. This assay was conducted by use WST-1(water soluble tetrazolium-1) reagentCitation26 for determination of IC50 for each compound. HT-29 colon cancer cell line and MDA-MB-468 triple negative breast cancer cell line were purchased from the American Type Culture Collection. Cells were maintained in RPMI 1640 (Sigma-Aldrich, St. Louis, MO), supplemented with 10% FBS (Fetal Bovine Serum) (Lonza Group, Basel, Switzerland), 100 IU/mL penicillin, 100 mg/mL streptomycin, and 2 mmol/L L-glutamine (Sigma). Cells were seeded into 96-well plates at 0.4 * 104/well and incubated overnight. The medium was replaced with fresh one containing the desired concentrations of the test compounds. After 48 h, 10 μl of the WST-1 reagent were added to each well and the plates were re-incubated for 4 h at 37 °C. The amount of formazan was quantified using ELISA (Enzyme Linked Immunosorbent Assay) reader at 450 nm.
CD133 expression measure by flow cytometry
HT-29 and MDA-MB-468 cells harvested, washed, and then the cells were stained with conjugated monoclonal antibodies CD133-APC (Miltenyi Biotec, Bergisch Gladbach, Germany). The analyses were performed on a BD LSR II™ (BD Biosciences, San Jose, CA). Debris and cell clusters were excluded during side-scatter and forward-scatter analyses.
Results and discussion
Chemistry
In the present work, target compounds 4a–d were prepared according to Scheme 1. In a one-pot two-components heterocyclisation process, sulphate salts 3a–d were obtained via the reaction of acetophenone 1a–d with 2-mercaptobenzimidazole 2 in refluxing acetic acid in the presence of five equivalents of sulphuric acid. Next, neutralisation of such sulphate salts 3a–d was carried out through stirring with aqueous solution of sodium bicarbonate to furnish the 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d, with 75–83% yield (Scheme 1). All spectral and elemental analyses were consistent with the proposed structures of the prepared compounds.
X-ray crystallographic study for 4d
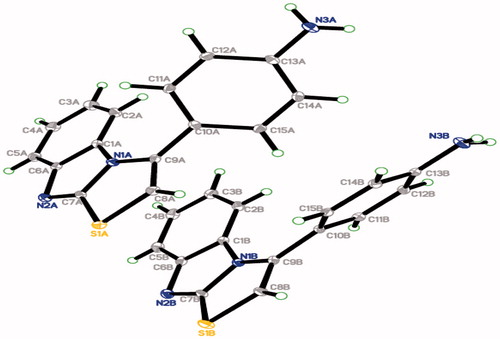
Characterisation of compound 4d was conducted by a single crystal X-ray structural analysis. The structure was solved with direct method and refined by SHELXTLCitation27. Crystallographic data of compound 4d is deposited with the Cambridge Crystallographic Data Centre with deposition number CCDC 1429525. The crystallographic structure of 4d is represented in . The single crystal X-ray study for such compound unambiguously defines its exact structure. Crystal packing of 4d showed the intermolecular hydrogen bonds N3A—H1NA···N2A and N3B—H1NB···N2B (Supplementary data). The crystallographic data and hydrogen-bond geometry of 4d are presented in and , respectively, while selected geometric parameters are illustrated in the Supplementary data.
Table 1. Crystallographic data and refinements for compound 4d.
Table 2. Hydrogen-bond geometry (Å, °) of 4d.
Biological evaluation
Anti-proliferative activity of the prepared 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d was evaluated against human colon cancer cell line HT-29 and triple negative breast cancer cell line MDA-MB-468 using the WST-1 assay as described by Ngamwongsatit et alCitation26. 5-Fluorouracil was used as a positive control for its well-known clinical utility for managing malignant carcinomas. The anti-proliferative activities were expressed as growth inhibitory concentration (IC50) values. Among the tested compounds, compound 4b bearing a terminal phenyl ring substituted with two electron-donating groups proved to be the most potent against both cell lines; HT-29 and MDA-MB-468 with IC50 values of 9 and 12 μM, respectively. Compared to the positive control 5-FU (IC50 values of 15 and 41 μM, respectively), these results proclaimed compound 4b to be superior to the reference drug. Moreover, compounds 4a and 4c showed moderate activity against HT-29 cell line with IC50 values of 21 and 29 μM, respectively, which are comparable to that of 5-FU (IC50 = 15 μM). Luckily, both compounds evinced good antineoplastic potency against MDA-MB-468 cell line relative to 5-FU (IC50 values of 23, 24, and 41 μM, respectively). Unfortunately, compound 4d bearing a p-amino phenyl group did not manifest any significant anti-proliferative activity against the cancer colon cell line HT-29 while it demonstrated moderate activity against triple negative breast cancer cell line MDA-MB-468 (53 μM) ().
Table 3. In vitro anti-proliferative activity of compounds 4a–d against colon HT-29 and breast MDA-MB-468 cancer cell lines.
Moreover, the inhibitory effect of the prepared 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d on cell surface expression of CD133 was evaluated at 10 μM via flow cytometry. The analysis was performed on a BD LSR II™ (BD Biosciences). The results expressed as a side population inhibition (%). Scrutinizing the obtained results disclosed that compound 4a bearing a m-methoy phenyl group only limitedly inhibited the CD133 by 13%, whereas compound 4b that possesses an extra electron donating group; an additional p-hydroxy group significantly inhibited the CD133 expression by 50%. Coherent to these findings, compound 4c that has three methoxy groups grafted on its terminal phenyl ring produced a comparable inhibition of CD133 (48%). These findings are in accordance with the previous conclusion that outlined the importance of incorporation of a lipophilic electron-donating group represented by a terminal phenyl ring substituted with a methoxy or a hydroxyl group. Regrettably, compound 4d containing a p-amino phenyl ring did not display any marked activity against the cell surface expression of CD133 ().
Table 4. Inhibition (%) of cell surface expression of CD133 on HT-29 cancer cells at 10 μM.
It is worth noting that the cytotoxic activities were decreased in the order of 4b > 4a > 4c > 4d, while the order was 4b > 4c > 4a > 4d for the inhibitory activity towards CD133 surface expression, which suggesting absence of a correlation between the two activities that may be attributable to the different phenotypic characteristics and different proliferative potentials.
Conclusions
In conclusion, we designed and synthesised a novel series of 3-arylthiazolo[3,2-a]benzimidazoles 4a–d (Series 2) based on structural rigidification of a series of 2-((benzimidazol-2-yl) thio)-1-arylethan-1-ones (Series 1) that proved to have antineoplastic activity and inhibitory activity of cell surface expression of CD133. The anti-proliferative activity of the synthesised compounds was evaluated against two cell lines; colon cancer cell line HT-29 and triple negative breast cancer cell line MDA-MB-468. Moreover, their inhibitory activity against the cell surface expression of CD133 was determined in an attempt to explore their potential to eradicate CSCs as well as the tumour bulk cells. Compound 4b emerged as a promising hit molecule as it manifested excellent antineoplastic potency against both tested cell lines (IC50 values of 9 and 12 μM, respectively) beside its ability to inhibit the cell surface expression of CD133 by 50% suggesting a promising potential of effectively controlling the tumour by eradicating the tumour bulk and inhibiting the proliferation of the CSCs. Moreover, compounds 4a and 4d exhibited good anti-proliferative activity against both cell lines and also significant inhibition potential of cell surface expression of CD133. On the other hand, structure of compound 4d was further substantiated via X-ray single crystal analysis.
IENZ_1347166_Supplementary_Material.pdf
Download PDF (566.7 KB)Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RG-1436–038.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012;12:133–43.
- Abdel-Aziz HA, Ghabbour HA, Eldehna WM, et al. 2-((Benzimidazol-2-yl)thio)-1-arylethan-1-ones: synthesis, crystal study and cancer stem cells CD133 targeting potential. Eur J Med Chem 2015;104:1–10.
- Denisenko TV, Sorokina IV, Gogvadze V, Zhivotovsky B. Mitotic catastrophe and cancer drug resistance: a link that must to be broken. Drug Resist Updat 2016;24:1–2.
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 2009;138:822–9.
- Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol 2009;27:44–7.
- Wahl GM, Spike BT. Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. NPJ Breast Cancer 2017;3:14.
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011;17:313–9.
- Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma 2005; 52:435.
- Nguyen LV, Vanner R, Dirks P, Eaves C. Cancer stem cells: an evolving concept. J Nat Rev Cancer 2012;12:133–43.
- Suresh R, Ali S, Ahmad A, et al. The role of cancer stem cells in recurrent and drug-resistant lung cancer. In: Ahmad A, Gadgeel SM, ed. Lung cancer and personalized medicine: novel therapies and clinical management. Switzerland: Springer International Publishing; 2016:57–74.
- Anthony H, Norbert F. Cancer stem cells: a promising concept and therapeutic challenge. Int J Cancer 2011;129:2309.
- Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393–5.
- Grosse‐Gehling P, Fargeas CA, Dittfeld C, et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol 2013;229:355–78.
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821–8.
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445:111–5.
- Han L, Gao X, Gu X, et al. Prognostic significance of cancer stem cell marker CD133 expression in breast cancer. Int J Clin Exp Med 2017;10:4829–37.
- Ma S. Biology and clinical implications of CD133+ liver cancer stem cells. Exp Cell Res 2013;319:126.
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–23.
- Yanagisawa S, Kadouchi I, Yokomori K, et al. Identification and metastatic potential of tumor-initiating cells in malignant rhabdoid tumor of the kidney. Clin Cancer Res 2009;15:3014–22.
- Salnikov AV, Gladkich J, Moldenhauer G, et al. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients . Int J Cancer 2010;126:950–8.
- Rutella S, Bonanno G, Procoli A, et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res 2009;15:4299–311.
- Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem‐like cells via NF‐κB‐mediated MMP‐9 upregulation. Stem Cells 2012;30:2309–19.
- Tirino V, Desiderio V, d'Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PloS One 2008;3:e3469.
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106–10.
- (a) Eldehna WM, Almahli H, Al-Ansary GH, et al. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J Enzyme Inhib Med Chem 2017;32:600–13. (b) Abdel-Aziz HA, Eldehna WM, Ghabbour HA, et al. Synthesis, crystal study, and anti-proliferative activity of some 2-benzimidazolylthioacetophenones towards triple-negative breast cancer MDA-MB-468 cells as apoptosis-inducing agents. Int J Mol Sci 2016;17:1221. (c) Eldehna WM, Abou-Seri SM, El Kerdawy AM, et al. Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl) phthalazine derivatives. Eur J Med Chem 2016;113:50–62. (d) Eldehna WM, Fares M, Ibrahim HS, et al. Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016;21:762. (e) Eldehna WM, Fares M, Ceruso M, et al. Amido/ureidosubstituted benzenesulfonamides-isatin conjugates as low nanomolar/subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform XII. Eur J Med Chem 2016;110:259–66. (f) Alafeefy AM, Ahmad R, Abdulla M, et al. Development of certain new 2-substituted-quinazolin-4-yl-aminobenzenesulfonamide as potential antitumor agents. Eur J Med Chem 2016;109:247–53. (g) Abou-Seri SM, Eldehna WM, Ali MM, El Ella DAA. 1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: synthesis and in vitro biological evaluation. Eur J Med Chem 2016;107:165–79. (h) Eldehna WM, Fares M, Ibrahim HS, et al. Indoline ureas as potential anti-hepatocellular carcinoma agents targeting VEGFR-2: synthesis, in vitro biological evaluation and molecular docking. Eur J Med Chem 2015;100:89–97. (i) Eldehna WM, Altoukhy A, Mahrous H, Abdel-Aziz HA. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur J Med Chem 2015;90:684–94. (j) Abdel-Aziz HA, Ghabbour HA, Eldehna WM, et al. Synthesis, crystal structure, and biological activity of cis/trans amide rotomers of (Z)-N'-(2-oxoindolin-3-ylidene)formohydrazide. J Chem 2014;2014:760434.
- Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J Microbiol Methods 2008;73:211–5.
- Sheldrick GM. A short history of SHELX. Acta Crystallogr A 2008;64:112–22.

![Scheme 1. Synthesis of target 3-phenylthiazolo[3,2-a]benzimidazoles 4a–d.](/cms/asset/c8e9f737-342c-43e0-8dc4-e797641cd8d5/ienz_a_1347166_sch0001.jpg)