Abstract
Identification of a new class of antitumor agent capable to induce apoptosis without triggering necrotic cell death event is challenging. The present communication describes the multicomponent synthesis of seven new (1S,4S)-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates and their in vitro antiproliferative activity on cervical cancer cell line (CaSki), breast cancer cell line (MDA-MB231), lung cancer cell line (SK-Lu-1) and human lymphocytes. Among the synthesized dithiocarbamates, compound 9e displayed significant antiproliferative activity without inducing any necrotic cell death (both on tumour cells and lymphocytes) and induced apoptosis in tumor cells by the caspase dependent apoptotic pathway. The compound 9e also exhibited greater tumor selectivity than human lymphocytes. In silico ADME predictions revealed that compound 9e has the potential to be developed as a drug candidate. Rapid chemical modifications of this lead are thus highly necessary for further investigation as a drug like safer antitumor candidate and also to achieve compounds with better activity profile.
Introduction
During the design of safer antiproliferative agents, it would be desirable to take into account the actual side effects related with different cell death processesCitation1. Apoptosis and necrosis represent two fundamental types of cell death processesCitation2. Apoptotic cell death is a regulated cellular mechanism, however the plasma membrane retains the integrity during the processCitation3. In contrast, necrotic cells undergo plasma membrane rupture, nuclear and cellular swellingCitation4. Necrosis is usually followed by an inflammatory response to the released cellular contents, often resulting in further tissue damageCitation5. Majorly of the cytotoxic drugs not only target neoplastic cells but are also toxic to normal cells and organs. As a result, chemotherapy is always associated with adverse effects, including substantial impacts on the immune system. Such kind of undesired effects very often are detrimental to the health of the patients.
Literature revealed that many dithiocarbamate derivatives displayed potent anticancer activity () both in in vitro and in vivo model and might act by induction of apoptosisCitation6. Molecular hybridization technique had been widely adopted in designing new dithiocarbamate cytotoxic agents which includes tetrahydrocarbazoleCitation7, 1,2,3-triazolesCitation8, quinazolinesCitation9, emetineCitation10, chromonesCitation11, benzodioxoleCitation12 dithiocarbamate derivatives, etcCitation13,Citation14. However, the molecular hybridization of dithiocarbamate with bridged bicyclic compounds to achieve new class of antiproliferative agents have not been reported so far. Among the bridged N-heterocycles conformationally restricted rigid piperazine homolog 2,5-diazabicyclo[2.2.1]heptane has been extensively used in medicinal chemistry for synthesizing potent drug candidatesCitation15. Surprisingly, only two literatures (from Merck Research Laboratories and Wyeth Research) are currently available applying 2,5-diazabicyclo[2.2.1]heptane to achieve antitumor agents (, VII, VIII)Citation16,Citation17. In the context of current drug discovery strategies, empirical approaches without particular target largely depends on the quality of the newly synthesized molecules in respect to molecular complexity and diversity which could be built easily through multi-component reactionsCitation18. Until now, there are no reports available for the functionalization of 2,5-diazabicyclo[2.2.1]heptanes through multicomponent reaction pathway to achieve rapid diversity in the framework.
Figure 1. Structures of some dithiocarbamates and (1S,4S)-2,5-diazabicyclo[2.2.1]heptanes displaying potent anticancer activity.
![Figure 1. Structures of some dithiocarbamates and (1S,4S)-2,5-diazabicyclo[2.2.1]heptanes displaying potent anticancer activity.](/cms/asset/9dab9ccf-723e-4255-aa75-b8de18357955/ienz_a_1363197_f0001_b.jpg)
In view of the above, here in we have reported the first multicomponent synthesis of seven new (1S,4S)-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates and in vitro biological evaluation of their antitumor activity, apoptosis-inducing effect, necrotic effect, selectivity and ADME profiling.
Experimental
Materials and methods
Melting points were determined in open capillaries in a MEL-TEMP® melting point apparatus. 1H and 13C NMR spectra were obtained at 300/400 MHz and 75/100 MHz respectively using Mercury-Plus400 spectrometer and CDCl3/DMSO-d6 as solvent; chemical shifts were recorded in parts per million (ppm) with TMS as the internal reference. Mass spectra (FAB-MS) were measured on a MS Station, MARCA JEOL, JMS-700 equipment and signals were given in m/z. Optical rotations were determined in a Perkin-Elmer 341 polarimeter using a 1-dm cell path length (sodium D-line 589 nm), at 20 °C sample compartment temperature.
Chemistry
Synthesis of (1S,4S)-2-Boc-2,5-diazabicyclo[2.2.1]heptane (7, Scheme 2)
Free base 5 of the (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane dihydrobromide salt 4Citation19 was obtained by the treatment of sodium methoxide (in methanol) solution and was used in the next stage without storage. In a 250 ml round bottom flask, 28 g (148.72 mmol) of (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane in 200 ml of dichloromethane was added and flask was placed in an ice-bath. About 41.24 g (188.52 mmol) of di-tert-butyl dicarbonate was added in portions into the reaction flask followed by the addition of 26.25 ml (188.52 mmol) triethylamine. The reaction flask was slowly warmed to room temperature and the stirring was continued. After completion of the reaction (as monitored by TLC), the reaction mixture was washed with distilled water (3 × 200 ml), dried over sodium sulphate and concentrated under vacuum to get white solid product 6 with 90% yield (53 g). After that, 53 g (183 mmol) of 6 was dissolved in 300 ml dry methanol and placed in a 500 ml hydrogenation flask (60 psi) in presence of 10% by weight of Pearlman’s catalyst. After completion of the hydrazinolysis, the catalyst was filtered off and the filtrate containing the product was concentrated to obtain a white solid as N-Boc-DBH 7 with 90% yield (40 g).
Multicomponent synthesis of (1S,4S)-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates (9a–9g, Scheme 3)
An oven-dried screw cap reaction tube was charged with a magnetic stir bar, N-Boc-DBH (1 mmol), catalyst (MgO; 0.5 mmol) and 3 ml methanol. Carbon disulphide (1.5 mmol) was added drop wise to the stirred mixture at 0 °C temperature. After 30 min of stirring, reactant 8 (8a–8g, 1 mmol) was added slowly to the stirred reaction mixture, and the stirring was continued for overnight at ambient temperature. The progress of the reaction was monitored by thin-layer chromatography (TLC). On completion of the reaction, methanol was evaporated on a rotary evaporator and the product was extracted with dichloromethane followed by column chromatographic purification over silica gel (heptane/ethyl acetate) to provide the pure product. Although this procedure was described on the mmol scale, gram-scale reactions also provided uniform results.
Biological activity
Cell proliferation assays
CaSki (cervical cancer cell line), MDA-MB-231 (breast cancer cell line) and SK-Lu-1 (lung cancer cell line) were purchased from the American Type Culture Collection (ATCC Rockville, MD) and assays were performed by seeding 7500 cells/well in 96-well tissue culture plates at a volume of 100 µL of RPMI-1640 medium supplemented with 5% NCS per well. Cells were allowed to grow for 24 h in the culture medium prior to exposure with the compounds. Also, 1% of vehicle (ETOH or ETOH:DMSO 1:1) was added to the control cells. Antiproliferative activity was determined after 24 h by crystal violet stainingCitation21. Cell counts were determined by measuring absorbance at 590 nm in an enzyme-linked immunosorbent assay (ELISA) plate reader.
Determination of necrotic effect
Lactate dehydrogenase (LDH) release from cells was determined using LDH assay kit to confirm cell necrosisCitation22. The experiments were carried out following the manufacturer’s protocol (CytoTox 96® Non-Radioactive; Cytotoxicity Assay; Promega, Littleton, CO).
CFSE-labelling assay
Lymphocytes were obtained from peripheral blood of healthy human volunteers, and isolated by density gradient centrifugation and cultured in 96-well plates. Lymphocyte proliferation was induced with phytohemaglutinin and were treated with the compounds. The proliferation was evaluated after 72 h by the incorporated CFSE-labelling assayCitation23.
Immunolocalisation of active caspase-3 by fluorescence microscopy
Cells were cultured in glass coverslips and treated with the compounds during 24 h. The cells were fixed in 2% paraformaldehyde. The cells were permeabilised with 0.05% Triton X-100 and incubated with anti-active caspase-3 antibody (Novous Biologicals, Littleton, CO). Next, the samples were washed and incubated with a secondary goat anti-rabbit antibody with fluorescein isothiocyanate. Finally, they were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Immunoassays were evaluated under a Nikon Eclipse E600 Microscope and images were recorded with a Nikon Digital DXM1 200F Camera.
Results and discussion
The synthetic routes for the preparation of (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates 9a–9g have been outlined in Schemes 1–3. The starting compound 4 was synthesized according to the procedure described by Regla (our group member) and Juaristi et al.Citation19 (Scheme 1). Compound 5 was treated with di-tert-butyl dicarbonate followed by hydrogenolysis to remove the benzyl group and afford compound 7 in good yield (Scheme 2). Multicomponent reaction strategy was then applied for straightforward synthesis of the title dithiocarbamate derivatives (, 9a–9g) in good yields following the reaction between N-Boc-DBH 7, carbon disulphide and various electrophiles (8a–8g) in the presence of magnesium oxide as heterogeneous catalyst and methanol as solvent (Scheme 3). The reaction profile was very clean and energy efficient.
Scheme 1. Synthesis of (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane dihydrobromide. Reagents and conditions. (a) TsCl, Na2CO3, H2O, 94%; (b) NaBH4, BF3–Et2O, THF, 85%; (c) TsCl, C5H5N, toluene, 20 h, 83%; (d) PhCH2NH2, toluene, reflux, 96%; (e) HBr 40%, 96%.
![Scheme 1. Synthesis of (1S,4S)-2-benzyl-2,5-diazabicyclo[2.2.1]heptane dihydrobromide. Reagents and conditions. (a) TsCl, Na2CO3, H2O, 94%; (b) NaBH4, BF3–Et2O, THF, 85%; (c) TsCl, C5H5N, toluene, 20 h, 83%; (d) PhCH2NH2, toluene, reflux, 96%; (e) HBr 40%, 96%.](/cms/asset/db809420-54f7-44bd-a3d2-f3fa97ba8ef5/ienz_a_1363197_sch0001.gif)
Figure 2. Structures of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.
![Figure 2. Structures of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.](/cms/asset/9de34a29-2a64-4c64-97da-346bd1a8b65e/ienz_a_1363197_f0002_b.jpg)
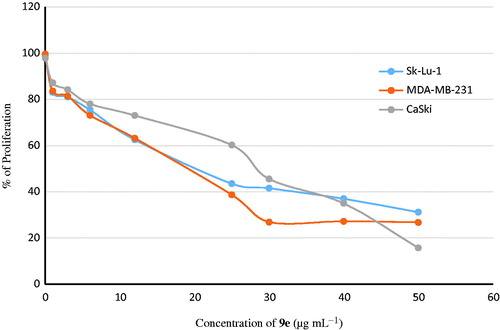
Having synthesized a variety of (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates, we set out to evaluate the compounds for their possible antitumor activities. All the compounds were subjected to measure antiproliferative activity against cervical cancer cell line (CaSki), breast cancer cell line (MDA-MB-231) and lung cancer cell line (SK-Lu-1), respectively, employing ETOH and ETOH:DMSO 1:1 as a vehicle and the corresponding IC50 values have been shown in . Among the seven test compounds, compound 9e showed moderate antiproliferative activity with IC50 values 28, 18 and 20 µg/mL against CaSki, MDA-MB231 and SK-Lu-1 cell line, respectively (). To identify the preliminary cell death processes induced by this compound, the necrotic effect of the compound was evaluated on CaSki, MDA-MB231 and SK-Lu-1 cell lines as well as on human lymphocytes using lactate dehydrogenase (LDH) assay (). It is our delight to mention that compound 9e did not induce any necrotic cell death on the three tumour cells and human lymphocytes, unlike cisplatin which induced necrotic cell death (). In the preliminary apoptosis experiment CaSki, MDA-MB-231 and SK-Lu-1 cultures were stimulated at the level of their determined IC50 values and the morphological changes, chromatin condensation including the formation of apoptotic bodies were determined through staining with fluorochrome 4′,6-diamidino-2-phenylindole (DAPI). Compact nuclei and apoptotic bodies were clearly observed in the cultures (). The condensed chromatin in treated cells suggested that compound 9e induced cell death by apoptosis in the concerned cancer cell lines. In the present study, we had detected active caspase-3 by immunodetection. shows that compound 9e induced the expression of active caspase-3 in CaSki, MDA-MB-231 and SK-Lu-1 cultures, implying that apoptosis could be triggered through a caspase dependent process. It is well known that during chemotherapy the immune system is usually affected. Thus to evaluate the selectivity, the proliferation of enriched lymphocyte population (ELP) was evaluated with compound 9e using CFSE-labelling assay (). The results indicated that with compound 9e, proliferative potential of lymphoid cells was not negatively affected after 72 h, implying a greater degree of antiproliferative selectivity towards malignant cell lines than with lymphocytes. In silico ADME profiling study revealed that compound 9e has the potential to be developed as oral drug candidate ().
Figure 4. Necrotic effect of 9e (at the IC50 values) on both the tumour and lymphocytes cell lines by LDH leakage assay.
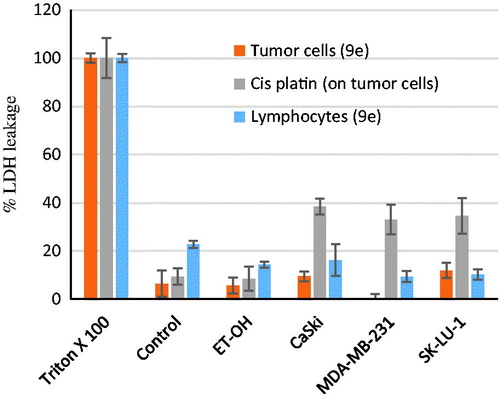
Figure 5. Compound 9e induced apoptotic death. Immunodetection of active caspase-3 by compound 9e on CaSki (A), MDA-MB-231 (B) and SK-Lu-1cultures (C).
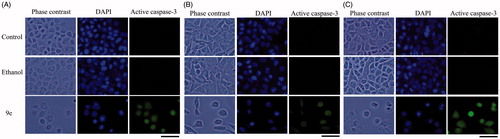
Figure 6. Effect of the compound 9e on lymphocyte proliferation by CFSE-labelling assay [at the concentrations of 18 (left) and 20 (right) µg mL−1].
![Figure 6. Effect of the compound 9e on lymphocyte proliferation by CFSE-labelling assay [at the concentrations of 18 (left) and 20 (right) µg mL−1].](/cms/asset/ca301556-bfee-4304-b80e-914b459f2380/ienz_a_1363197_f0006_c.jpg)
Table 1. Experimental data of the compounds 9a–9g.
Table 2. Spectral data of the compounds 9a–9g.
Table 3. Antiproliferative activities of the synthesized (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates (9a–9g).
Table 4. In silico prediction of physicochemical pharmacokinetic propertiesCitation24.
Conclusions
The objective of the present study was to synthesize new (1S,4S)-2,5-diazabicyclo[2.2.1]heptanes bearing dithiocarbamate moiety through MCR pathway and to study the effect on antitumor activity, apoptosis induction, necrosis as well as selectivity. One compound displayed significant antiproliferative activity against CaSki, MDA-MB-231 and SK-Lu-1 tumour cell lines (with IC50 values 28, 18 and 20 µg/mL, respectively) and induced apoptotic cell death through caspase-3 activation without triggering any necrosis. It also showed greater degree of tumour selectivity compared with peripheral blood lymphocytes. Thus, chemical modifications of this compound are highly necessary to afford drug like potency. Therefore, such compound could serve as promising safer antitumor agent and certainly augur well for deeper assays on mechanistic effects in the next stage of research.
Acknowledgements
The authors acknowledge instrumental and infrastructural facility from FES Zaragoza, UNAM. Sujay Laskar acknowledges a postdoctoral fellowship from DGAPA/UNAM.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci 2011;119:3–19.
- Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 2015;16:329–44.
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008;9:231–41.
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 2004;16:663–9.
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 2008;3:99–126.
- Li Y, Qi H, Li X, et al. A novel dithiocarbamate derivative induces cell apoptosis through p53-dependent intrinsic pathway and suppresses the expression of the E6 oncogene of human papillomavirus 18 in HeLa cells. Apoptosis 2015;20:787–95.
- El-Nassan HB. Synthesis and antitumor activity of tetrahydrocarbazole hybridized with dithioate derivatives. J Enzyme Inhib Med Chem 2015;30:308–15.
- Duan YC, Ma YC, Zhang E, et al. Design and synthesis of novel 1,2,3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur J Med Chem 2013;62:11–19.
- Ding PP, Gao M, Mao BB, et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur J Med Chem 2016;108:364–73.
- Akinboye ES, Bamji ZD, Kwabi-Addo B, et al. Design, synthesis and cytotoxicity studies of dithiocarbamate ester derivatives of emetine in prostate cancer cell lines. Bioorg Med Chem 2015;23:5839–45.
- Huang W, Ding Y, Miao Y, et al. Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur J Med Chem 2009;44:3687–96.
- Altıntop MD, Sever B, Akalın Çiftçi G, et al. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and hCA-II inhibitors. Eur J Med Chem 2017;125:190–6.
- Ren JL, Zhang XY, Yu B, et al. Discovery of novel AHLs as potent antiproliferative agents. Eur J Med Chem 2015;93:321–9.
- Zheng YC, Duan YC, Ma JL, et al. Triazole-dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J Med Chem 2013;56:8543–60.
- Murineddu G, Asproni B, Pinna G, et al. Synthesis of biologically active bridged diazabicycloheptanes. Curr Med Chem 2012;19:5342–63.
- Hamblett CL, Methot JL, Mampreian DM, et al. The discovery of 6-amino nicotinamides as potent and selective histone deacetylase inhibitors. Bioorg Med Chem Lett 2007;17:5300–9.
- Wang X, Berger DM, Salaski EJ, et al. Discovery of highly potent and selective type I B-Raf kinase inhibitors. Bioorg Med Chem Lett 2009;19:6571–4.
- Ruijter E, Scheffelaar R, Orru RV. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew Chem Int Ed Engl 2011;50:6234–46.
- Melgar-Fernández R, González-Olvera R, Olivares-Romero JL, et al. Synthesis of novel derivatives of (1S,4S)-2,5-diazabicyclo[2.2.1]heptane and their evaluation as potential ligands in asymmetric catalysis. Eur J Org Chem 2008;2008:655–72.
- Beinat C, Banister SD, McErlean CSP, et al. Practical synthesis of (1S,4S)-2,5-diazabicyclo[2.2.1]heptane. Tetrahedron Lett 2013;54:5345–7.
- Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem 1989;182:16–19.
- Legrand C, Bour JM, Jacob C, et al. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium. J Biotechnol 1992;25:231–43.
- Lyons AB, Hasbold J, Hodgkin PD. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol 2001;63:375–98.
- Molinspiration Cheminformatics, Bratislava, Slovak Republic. Available from: http://www.molinspiration.com/cgi-bin/properties [last accessed 26 Jul 2016].

![Scheme 2. Synthesis of (1S,4S)-tert-butyl 2,5-diazabicyclo[2.2.1]heptane-2-carboxylate.](/cms/asset/f5e8bdfc-dae1-4132-8f79-c44f3ea3b1af/ienz_a_1363197_sch0002.gif)
![Scheme 3. One-pot synthesis of (1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane-dithiocarbamates.](/cms/asset/71cdd8a0-1834-4540-b528-2d569a1768fc/ienz_a_1363197_sch0003.gif)