Abstract
A library of benzenesulphonamides incorporating 1,2,3-triazole rings functionalised with ester, carboxylic acid, carboxamide, carboxyhydrazide, and hydroxymethyl moieties were synthesised. The carbonic anhydrase (CAs, EC 4.2.1.1) inhibitory activity of the new compounds was assessed against four human (h) isoforms, hCA I, hCA II, hCA IV, and hCA IX. Among them, hCA II and IV are anti-glaucoma drug targets, being involved in aqueous humour secretion within the eye. hCA I was inhibited with Ki’s ranging between 8.3 nM and 0.8737 µM. hCA II, the physiologically dominant cytosolic isoform, was excellently inhibited by these compounds, with Ki’s in the range of 1.6–9.4 nM, whereas hCA IV was effectively inhibited by most of them, with Ki’s in the range of 1.4–55.3 nM. Thirteen of the twenty sulphonamides were found to be excellent inhibitors of tumour associated hCA IX with Ki’s ≤ 9.5 nM. Many of the new compounds reported here showed low nM inhibitory action against hCA II, IV, and IX, isoforms involved in glaucoma and some tumours, making them interesting candidates for further medicinal chemistry/pharmacologic studies.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) belong to family of zinc metalloenzymes found in variety of organisms, including higher vertebrates and humansCitation1–3. As all the seven families of CAs known to date (α, β, γ, δ, ζ, η, and ɵ-class)Citation4–6 are involved in the reversible hydration–dehydration of carbon dioxide and bicarbonate ions, therefore, play vital roles in various important physiological processes, such as respiration, electrolyte secretion in variety of tissues/organs, biosynthetic reactions (i.e. lipogenesis, glucogenesis, and ureagenesis), bone resorption, calcification, etc.Citation1,Citation2,Citation7,Citation8. However, several studies demonstrated that abnormal levels or activities of these enzymes have been associated with various human diseases. Out of the sixteen isoforms of human associated α-class of CAs, some isoforms are involved in pertinent pathologies, such as glaucomaCitation9, epilepsyCitation10, obesityCitation11, altitude sickness,1 retinitis pigmentos1 cancerCitation12–15, etc. Therefore, carbonic anhydrase inhibitors (CAIs) have applications as therapeutic agents, such as antiglaucoma, antiobesity, antidiuretic, antiepileptic, anti-altitude sickness, antipain, and anti-infective agentsCitation1,Citation16–20. However, designing and synthesising isoform-selective inhibitors are a challenging task for obtaining a drug with minimum side effect.
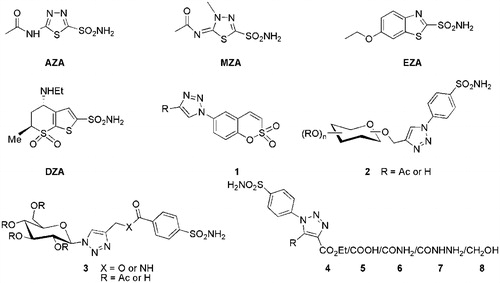
Primary sulphonamide bearing heterocyclic compounds form a part of potent CAIs in which binding group binds to the Zn(II) ion as anion in a tetrahedral geometry. A large number of drugs belonging to this class, like acetazolamide (AZA), methazolamide (MZA), ethoxzolamide (EZA), dorzolamide (DZA), etc., are in clinical use from past many years targeting different therapeutic areasCitation21. Recently our research group has reported some fused 1,2,4-triazoles and 4-functionalised pyrazoles bearing benzenesulphonamide as selective inhibitors of CA IX and XIICitation22–24. Further 1,2,3-triazole ring containing compounds are gaining interest in diverse therapeutic areas like antiproliferative,Citation25 antitubercular, antimicrobialCitation26–28, anticancerCitation29,Citation30, anti-HIVCitation31, antifungal, antibacterialCitation32, anti-inflammatoryCitation33, antiobesityCitation34, antiviralCitation35, etc., as well as in several DNA-alkylating, crosslinking agentsCitation36,Citation37, and β-lactamase inhibitorsCitation38. Also some 1,2,3-triazole ring containing selective CAIs have been reported (1–3)Citation39,Citation40. Motivated by these findings and continuing our interest in the design of various classes of heterocyclic based compounds of potential medicinal interestCitation22–24,Citation41–46, we turned our attention towards the synthesis of a small library of novel 4-functionalised 1-aryl-5-alkyl/aryl-1,2,3-triazoles (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) bearing a primary sulphonamide group on the phenyl ring at N-1 position of 1,2,3-triazole scaffold with different functionalities at C-4, such as ester, carboxylic acid, carboxamide, hydrazinocarbonyl, and hydroxymethyl () in order to investigate their carbonic anhydrases inhibition against isoforms hCA I, II, IV, and IX.
Materials and methods
General
All the commercially available chemicals were used without further purification. All the solvents were dried and/or purified according to standard procedures prior to use. All the reactions were monitored by thin layer chromatography (TLC) on TLC silica gel on F254 aluminium plates using a mixture of chloroform and methanol as eluent while UV lamp was used to visualise the spots. Melting points were determined in open capillaries in an electrical melting point apparatus and are uncorrected. IR spectra were recorded on ABB MB 3000 DTGS IR instrument using the KBr pellet technique. 1H NMR spectra were recorded on 400 MHz, while 13C NMR spectra were registered at 100 MHz, using deuterated dimethyl sulphoxide (DMSO-d6) as solvent, and tetramethylsilane (TMS) as internal standard at room temperature. Chemical shifts are reported as δ values in parts per million (ppm) downfield from TMS. High resolution mass spectra were obtained from a MicroMass ESI-TOF MS spectrometer. Multiplicities are described as singlet (s), doublet (d), doublet of doublet (dd), doublet of a doublet of a doublet (ddd), doublet of triplet (dt), triplet (t), quartet (q), multiplet (m), exchangeable proton (ex) for NMR assignments and strong (s), medium (m), broad (br) for IR assignments. The coupling constants are expressed in hertz (Hz).
Synthesis of ethyl 1-[4-(aminosulfonyl)phenyl]-5-(alkyl/aryl)-1H-1,2,3-triazole-4-carboxylate (4a–4d)
General procedure: A mixture of appropriate β-diketoester 11a–11d (2.00 mmol) and piperidine (5 mol%) dissolved in 3 ml DMSO were heated at 70 °C in silicon oil bath for 5 min followed by addition of 4-azidobenzenesulphonamide (2.02 mmol). After addition, reaction mixture was allowed to stir at 70 °C for 4–6 h. Reaction was monitored through thin-layer chromatography. After completion, reaction mixture was poured into water after cooling to afford required product 4a–4d. The crude product thus obtained was recrystalised with ethanol.
Ethyl 1-[4-(aminosulfonyl)phenyl]-5-methyl-1H-1,2,3-triazole-4-carboxylate (4a)
Yield 91%; white solid; mp: 212 °C; IR(KBr) (ν, cm−1): 3302, 3070 (m, N–H stretch), 2924 (m, –CH3 stretch), 1728 (s, C=O stretch),1335, 1165 (s, SO2stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.08 (dd, J = 8.8 Hz, J = 2.0 Hz, 2H, Ar), 7.88 (dd, J = 8.8 Hz, J = 2.0 Hz, 2H, Ar), 7.61 (s, 2H, SO2NH2), 4.37 (q, J = 7.2 Hz, 2H, CH2), 2.58 (s, 3H, –CH3), 1.35 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 160.92, 145.31, 139.48, 137.47, 136.08, 127.14, 125.98, 60.50, 14.12, 9.73; HRMS (ESI-MS) m/z 333.0640 (M + Na)+, C12H14N4O4SNa+, calcd 333.0634.
Ethyl 1-[4-(aminosulfonyl)phenyl]-5-phenyl-1H-1,2,3-triazole-4-carboxylate (4b)
Yield 75%; dirty white solid; mp: 184 °C; IR(KBr) (ν, cm−1): 3371, 3263, 3061 (m, N–H stretch), 1704 (s, C=O stretch), 1342, 1159 (s, SO2 stretch); 1H NMR (400 MHz, CDCl3) δ (ppm):): 7.96 (dd, J = 7.2 Hz, J = 1.2 Hz, 2H, Ar), 7.56–7.48 (m, 5H, Ar), 7.32 (dd, J = 8.4 Hz, J = 1.2 Hz, 2H, Ar), 7.06 (s, 2H, SO2NH2), 4.33 (q, J = 7.2 Hz, 2H, CH2), 1.28 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 165.33, 149.56, 145.64, 142.84, 141.97, 135.02, 134.95, 133.38, 132.17, 130.08, 130.05, 65.94, 18.81; HRMS (ESI-MS) m/z 395.0785 (M + Na)+, C17H16N4O4SNa+, calcd 395.0789.
Ethyl 1-[4-(aminosulfonyl)phenyl]-5-(4-methoxyphenyl)-1H-1,2,3-triazole-4-carboxylate (4c)
Yield 74%; pale yellow solid; mp: 176 °C; IR(KBr) (ν, cm−1): 3364, 3271 (m, N–H stretch), 2970 (m, –CH3 stretch), 1713 (s, C=O stretch), 1342, 1157 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.93 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.59 (d, J = 8.8 Hz, 2H, Ar), 7.54 (s, 2H, SO2NH2), 7.32 (d, J = 8.8 Hz, 2H, Ar), 6.97 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 4.23 (q, J = 7.2 Hz, 2H, CH2), 3.77 (s, 3H, –CH3), 1.18 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 160.31, 160.26, 144.94, 141.09, 137.89, 136.28, 131.88, 126.82, 126.37, 116.99, 113.72, 60.46, 55.21, 13.89; HRMS (ESI-MS) m/z 403.1072 (M + H)+, C18H18N4O5SH+, calcd 403.1076.
Ethyl 1-[4-(aminosulfonyl)phenyl]-5-(2-naphthyl)-1H-1,2,3-triazole-4-carboxylate (4d)
Yield 92%; dirty white solid; mp: 180 °C; IR(KBr) (ν, cm−1): 3325, 3240 (m, N–H stretch), 1728 (s, C=O stretch), 1335, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.07 (d, J = 8.4 Hz, 1H, Ar), 8.00 (d, J = 8.0 Hz, 1H, Ar), 7.78 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.65 (dd, J = 7.2 Hz, J = 1.2 Hz, 1H, Ar),7.58 (d, J = 8.8 Hz, 3H, Ar), 7.54 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, Ar),7.48 (dt, J = 8.4 Hz, J = 1.6 Hz, 1H, Ar), 7.45–7.43 (m, 3H, Ar, SO2NH2), 4.04–4.00 (m, 2H, CH2), 0.81 (t, J = 7.6 Hz, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 159.80, 145.04, 139.66, 138.14, 137.71, 132.67, 131.10, 130.40, 129.42, 128.41, 127.33, 126.73, 126.42, 125.66, 125.06, 124.32, 123.12, 60.29, 13.25; HRMS (ESI-MS) m/z 423.1124 (M + H)+, C21H18N4O4SH+, calcd 423.1127.
Synthesis of 1-[4-(aminosulfonyl)phenyl]-5-alkyl/aryl-1H-1,2,3-triazole-4-carboxylic acid (5a–5d)
General procedure: An appropriate 1,2,3-triazolic ester 4a–4d was dissolved in 20% aq NaOH solution (5 ml) and refluxed for 4 h. Then cooled the solution to room temperature, added ice to it and neutralised with concd HCl which resulted into the precipitation of a white solid. The solid was filtered off, washed with water, dried and recrystallised from appropriate solvent which afforded the pure products 5a–5d.
1-[4-(Aminosulfonyl)phenyl]-5-methyl-1H-1,2,3-triazole-4-carboxylic acid (5a)
Yield 94%; white solid; mp: 198 °C; IR(KBr) (ν, cm−1): 3348, 3225 (m, N–H stretch), 3078 (br, O–H stretch), 2905 (m, –CH3 stretch), 1697 (s, C=O stretch),1334, 1150 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.24 (s, br, 1H, –COOH), 8.07 (d, J = 8.4 Hz, 2H, Ar), 7.87 (d, J = 8.4 Hz, 2H, Ar), 7.62 (s, 2H, SO2NH2), 2.56 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 162.42, 145.24, 139.22, 137.63, 136.83, 127.16, 125.94, 9.74; HRMS (ESI-MS) m/z 305.0325 (M + Na)+, C10H10N4O4SNa+, calcd 305.0321.
1-[4-(Aminosulfonyl)phenyl]-5-phenyl-1H-1,2,3-triazole-4-carboxylic acid (5b)
Yield 85%; white solid; mp: 156 °C; IR(KBr) (ν, cm−1): 3379, 3263 (m, N–H stretch), 3063 (br, O–H stretch),1713 (s, C=O stretch), 1342, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.66 (s, br, 1H, –COOH), 7.90 (dd, J = 6.8 Hz, J = 1.6 Hz, 2H, Ar), 7.59–7.57 (m, 4H, Ar, SO2NH2), 7.45–7.40 (m, 5H, Ar); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 162.12, 145.41, 141.41, 138.33, 137.72, 130.84, 130.29, 128.71, 127.27, 126.92, 126.16; HRMS (ESI-MS) m/z 367.07473 (M + Na)+, C15H12N4O4SNa+, calcd 367.0477.
1-[4-(Aminosulfonyl)phenyl]-5-(4-methoxyphenyl)-1H-1,2,3-triazole-4-carboxylic acid (5c)
Yield 98%; dirty white solid; mp: 148–150 °C; IR(KBr) (ν, cm−1): 3333, 3242 (m, N–H stretch), 3055 (br, O–H stretch), 2905 (m, –CH3 stretch), 1705 (s, C=O stretch), 1335, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.02 (s, br, 1H, –COOH), 7.90 (d, J = 8.8 Hz, 2H, Ar), 7.56 (d, J = 8.8 Hz, 2H, Ar), 7.55 (s, 2H, SO2NH2), 7.30 (d, J = 8.8 Hz, 2H, Ar), 6.95 (d, J = 8.8 Hz, 2H, Ar), 3.77 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 161.69, 160.16, 144.85, 140.75, 137.98, 137.01, 131.85, 126.78, 126.32, 117.31, 113.72, 55.18; HRMS (ESI-MS) m/z 397.0577 (M + Na)+, C16H14N4O5SNa+, calcd 397.0582.
1-[4-(Aminosulfonyl)phenyl]-5-(2-naphthyl)-1H-1,2,3-triazole-4-carboxylic acid (5d)
Yield 93%; pale yellow solid; mp: 198–200 °C; IR(KBr) (ν, cm−1): 3356, 3256 (m, N–H stretch), 3078 (br, O–H stretch), 1690 (s, C=O stretch), 1335, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 12.98 (s, br, 1H, COOH), 8.04 (d, J = 8.4 Hz, 1H, Ar), 7.97 (d, J = 8.0 Hz, 1H, Ar), 7.76 (dd, J = 8.8 Hz, J = 2.0 Hz, 2H, Ar), 7.66 (dd, J = 7.6 Hz, J = 1.2 Hz, 1H, Ar), 7.58–7.39 (m, 8H, Ar, SO2NH2); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 161.28, 144.94, 139.32, 138.88, 137.83, 132.67, 131.06, 130.29, 129.48, 128.42, 127.30, 126.66, 126.40, 125.61, 125.11, 124.32, 123.39; HRMS (ESI-MS) m/z 395.0800 (M + H)+, C19H14N4O4SH+, calcd 395.0814.
Synthesis of 1-[4-(aminosulfonyl)phenyl]-5-alkyl/aryl-1H-1,2,3-triazole-4-carboxamide (6a–6d)
General procedure: A solution of appropriate 1,2,3-triazolic ester 4a–4d (1.29 mmol) in cold concentrated aq. ammonia solution (3 ml) was stirred for 22 h in a stoppered flask. The white precipitates of carboxamide derivatives 6a–6d thus obtained were filtered, washed with excess of cold water, dried at 120 °C and recrystallised from ethanol.
1-[4-(Aminosulfonyl)phenyl]-5-methyl-1H-1,2,3-triazole-4-carboxamide (6a)
Yield 74%; white solid; mp: 314–316 °C; IR(KBr) (ν, cm−1): 3456, 3356, 3271 (m, N–H stretch), 1674 (s, C=O stretch), 1333, 1173 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.06 (dd, J = 8.8 Hz, J = 2.0 Hz, 2H, Ar), 7.94 (s, ex, 1H, OH/NH), 7.87 (dd, J = 8.8 Hz, J = 2.0 Hz, 2H, Ar), 7.52–7.49 (m, ex, 3H, OH/NH, SO2NH2), 2.57 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 162.59, 145.10, 138.47, 137.72, 137.01, 127.10, 125.80, 9.31; HRMS (ESI-MS) m/z 282.0656 (M + H)+, C10H11N5O3SH+, calcd 282.0661.
1-[4-(Aminosulfonyl)phenyl]-5-phenyl-1H-1,2,3-triazole-4-carboxamide (6b)
Yield 76%; pale yellow solid; mp: 210 °C; IR(KBr) (ν, cm−1): 3479, 3379, 3178, 3101 (m, N–H stretch), 1674 (s, C=O stretch), 1335, 1157 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.03 (s, ex, 1H, OH/NH), 7.88 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.58–7.54 (m, 5H, Ar, SO2NH2, OH/NH), 7.42–7.37 (m, 5H, Ar); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 162.00, 145.35, 139.71, 139.33, 138.48, 131.00, 130.02, 128.57, 127.26, 126.91, 126.24; HRMS (ESI-MS) m/z 366.0629 (M + Na)+, C15H13N5O3SNa+, calcd 366.0635.
1-[4-(Aminosulfonyl)phenyl]-5-(4-methoxyphenyl)-1H-1,2,3-triazole-4-carboxamide (6c)
Yield 76%; white solid; mp: 280–282 °C; IR(KBr) (ν, cm−1): 3441, 3325, 3240, 3101 (m, N-H stretch), 2916 (m, –CH3 stretch), 1690 (s, C=O stretch), 1342, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.95 (s, 1H, OH/NH), 7.90 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.58–7.53 (m, 5H, OH/NH, Ar, SO2NH2), 7.29 (d, J = 8.8 Hz, 2H, Ar), 6.94 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 3.77 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 161.64, 160.03, 144.77, 138.93, 138.69, 138.15, 131.98, 126.79, 126.33, 117.40, 113.60, 55.17; HRMS (ESI-MS) m/z 396.0754 (M + Na)+, C16H15N5O4SNa+, calcd 396.0743.
1-[4-(Aminosulfonyl)phenyl]-5-(2-naphthyl)-1H-1,2,3-triazole-4-carboxamide (6d)
Yield 75%; white solid; mp: 278–280 °C; IR(KBr) (ν, cm−1): 3472, 3364, 3217, 3063 (m, N–H stretch), 1682 (s, C=O stretch), 1350, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.03 (d, J = 8.8 Hz, 2H, OH/NH, Ar), 7.97 (d, J = 8.0 Hz, 1H, Ar), 7.76 (d, J = 8.8 Hz, 2H, Ar), 7.62–7.36 (m, 10H, Ar, SO2NH2, OH/NH); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 161.10, 144.86, 140.91, 137.94, 137.25, 132.71, 131.17, 130.07, 129.54, 128.36, 127.09, 126.64, 126.30, 125.55, 125.09, 124.45, 123.58; HRMS (ESI-MS) m/z 416.0805 (M + Na)+, C19H15N5O3SNa+, calcd 416.0794.
Synthesis of 4-[4-(hydrazinocarbonyl)-5-alkyl/aryl-1H-1,2,3-triazol-1-yl]benzenesulphonamide (7a)
General procedure: A solution of appropriate 1,2,3-triazolic ester 4a–4d (1.93 mmol) and hydrazine hydrate (5.81 mmol) in ethanol (15 ml) was refluxed for 10–12 h. The reaction was monitored through thin-layer chromatography. After completion, reaction mixture was concentrated and allowed to cool to room temperature. Solid thus obtained was filtered and crystallised from EtOH:THF (1:1) to afford the desired compounds 7a–7d in good yields.
4-[4-(Hydrazinocarbonyl)-5-methyl-1H-1,2,3-triazol-1-yl]benzenesulphonamide (7a)
Yield 92%; white solid; mp: 216–218 °C; IR(KBr) (ν, cm−1): 3325, 3178, 3078, 3016 (m, N–H stretch), 1670 (s, C=O stretch), 1342, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.79 (s, ex, 1H, NH), 8.06 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.88 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.57 (s, 2H, SO2NH2), 4.50 (s, ex, 2H, NH2), 2.58 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 160.00, 145.06, 137.79, 137.69, 136.40, 127.11, 125.72, 9.21; HRMS (ESI-MS) m/z 319.0576 (M + Na)+, C10H12N6O3SNa+, calcd 319.0590.
4-[4-(Hydrazinocarbonyl)-5-phenyl-1H-1,2,3-triazol-1-yl]benzenesulphonamide (7b)
Yield 70%; white solid; mp: 206 °C; IR(KBr) (ν, cm−1): 3402, 3317, 3186, 3094 (m, N–H stretch), 1668 (s, C=O stretch), 1335, 1157 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.88 (s, ex, 1H, NH), 7.89 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.58 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.54 (s, 2H, SO2NH2), 7.42–7.38 (m, 5H, Ar), 4.49 (s, ex, 2H, NH2); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 159.67, 145.35, 139.22, 138.69, 138.45, 130.93, 130.10, 128.64, 127.31, 126.82, 125.99; HRMS (ESI-MS) m/z 381.0640 (M + Na)+, C15H14N6O3SNa+, calcd 381.0745.
4-[4-(Hydrazinocarbonyl)-5-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl]benzenesulphonamide (7c)
Yield 70%; white solid; mp: 170–172 °C; IR(KBr) (ν, cm−1): 3333, 3232, 3103 (m, N-H stretch), 1659 (s, C=O stretch), 1335, 1165 (s, SO2 stretch);1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.80 (s, ex, 1H, NH), 7.91 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.57 (d, J = 8.8 Hz, 2H, Ar), 7.53 (s, 2H, SO2NH2), 7.29 (d, J = 8.8 Hz, 2H, Ar), 6.94 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 4.48 (s, ex, 2H, NH2), 3.76 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 160.10, 159.34, 144.78, 138.46, 138.13, 138.08, 131.90, 126.85, 126.24, 117.22, 113.69, 55.20; HRMS (ESI-MS) m/z 389.1026 (M + H)+, C16H16N6O4SH+, calcd 389.1032.
4-[4-(Hydrazinocarbonyl)-5-(2-naphthyl)-1H-1,2,3-triazol-1-yl]benzenesulphonamide (7d)
Yield 72%; white solid; mp: 156 °C; IR(KBr) (ν, cm−1): 3279, 3063 (m, N–H stretch), 1659 (s, C=O stretch), 1327, 1165 (s, SO2 stretch); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.53 (s, ex, 1H, NH), 8.04 (d, J = 8.0 Hz, 1H, Ar), 7.98 (d, J = 8.0 Hz, 1H, Ar), 7.76 (d, J = 8.8 Hz, 2H, Ar), 7.63 (d, J = 7.2 Hz, 1H, Ar), 7.58–7.44 (m, 7H,Ar, SO2NH2), 7.35 (d, J = 8.4 Hz, 1H, Ar), 4.45 (s, ex, 2H, NH2); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 159.26, 145.30, 140.74, 138.42, 137.31, 133.22, 131.73, 130.67, 130.19, 128.90, 127.66, 127.20, 126.84, 126.01, 125.61, 124.94, 123.88; HRMS (ESI-MS) m/z 431.0894 (M + Na)+, C19H16N6O3SNa+, calcd 431.0903.
Synthesis of 4-(4-(hydroxymethyl)-5-methyl-1H-1,2,3-triazol-1-yl)benzenesulphonamide (8a–8d)
General procedure: Appropriate 1,2,3-triazolic ester 4a–4d (3.22 mmol) was dissolved in dry tetrahydrofuran (20 ml) and a suspension of LiAlH4 (6.44 mmol) in dry tetrahydrofuran (5 ml) was slowly added under anhydrous conditions. After 20 min of reaction at 20 °C, reaction mixture was refluxed for 2 h. After completion of the reaction, an aqueous solution of HCl 1 N was added dropwise until a neutral pH. The reaction mixture was concentrated under reduced pressure; the residue was taken into ethyl acetate and washed with water and brine. The organic layer was dried over MgSO4 and concentrated under reduced pressure. The residue was recrystalised in ethanol.
4-(4-(Hydroxymethyl)-5-methyl-1H-1,2,3-triazol-1-yl)benzenesulphonamide (8a)
Yield 55%; pale yellow solid; mp: 184 °C; IR(KBr) (ν, cm−1): 3495 (br, O–H stretch), 3294, 3186, 3086 (m, N–H stretch), 1336, 1157 (s, SO2 stretch); 1H NMR (400 MHz,DMSO-d6) δ (ppm): 8.05 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.85 (dd, J = 6.8 Hz, J = 2.0 Hz, 2H, Ar), 7.58 (s, 2H, SO2NH2), 5.20 (t, ex, J = 5.6 Hz, 1H, OH), 4.59 (d, J = 5.6 Hz, 2H, CH2), 2.39 (s, 3H, CH3); 13C NMR (100 MHz,DMSO-d6) δ (ppm): 145.71, 144.96, 139.01, 132.34, 127.65, 125.56, 54.68, 8.95.
4-(4-(Hydroxymethyl)-5-phenyl-1H-1,2,3-triazol-1-yl)benzenesulphonamide (8b)
Yield 68%; pale yellow solid; mp: 174–176 °C; IR(KBr) (ν, cm−1): 3325 (br, O–H stretch), 3225, 3171, 3070 (m, N–H stretch), 1342, 1157 (s, SO2 stretch); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.97 (d, J = 8.8 Hz, 2H, Ar), 7.48–7.44 (m, 5H, Ar, SO2NH2), 7.37–7.35 (m, 2H, Ar), 7.28 (d, J = 7.2 Hz, 2H, Ar), 5.25 (s, ex, 1H, OH), 4.65 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ (ppm): 150.68, 149.26, 143.74, 140.40, 134.54, 134.33, 133.81, 132.11, 131.06, 129.72, 59.36.
4-(4-(Hydroxymethyl)-5-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)benzenesulphonamide (8c)
Yield 69%; yellow solid; mp: 180–182 °C; IR(KBr) (ν, cm−1): 3481 (br, O–H stretch), 3295 (m, N–H stretch), 2916 (m, –CH3 stretch), 1342, 1142 (s, SO2 stretch); 1H NMR (400 MHz, CDCl3) δ (ppm): 7.99–7.95 (m, 2H, Ar), 7.48–7.44 (m, 2H, Ar), 7.30–7.25 (m, 4H, Ar, SO2NH2), 6.96–6.92 (m, 2H, Ar), 5.22 (t, J = 5.6 Hz, 1H, OH), 4.63 (d, J = 5.6 Hz, 2H, CH2), 3.85 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ (ppm): 165.14, 150.34, 149.16, 143.87, 140.36, 135.92, 132.09, 129.70, 122.94, 119.31, 60.14, 59.41.
4-(4-(Hydroxymethyl)-5-(2-naphthyl)-1H-1,2,3-triazol-1-yl)benzenesulphonamide (8d)
Yield 71%; dirty white solid; mp: 212–214 °C; IR(KBr) (ν, cm−1): 3431 (br, O–H stretch), 3295 (m, N–H stretch), 1335, 1157 (s, SO2 stretch); 1H NMR (400 MHz, CDCl3) δ (ppm): 8.02 (dd, J = 6.8 Hz, J = 2.4 Hz, 1H, Ar), 7.94 (d, J = 6.0 Hz, 1H, Ar), 7.76 (d, J = 8.4 Hz, 2H, Ar), 7.58–7.57 (m, 2H, Ar), 7.53–7.49 (m, 1H, Ar), 7.42–7.38 (m, 4H, Ar), 7.27 (s, 2H, SO2NH2), 5.11 (t, J = 5.2 Hz, 1H, OH), 4.48 (ddd, J = 58.4 Hz, J = 12.4 Hz, J = 5.2 Hz, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ (ppm):152.24, 149.19, 143.80, 138.52, 138.23, 136.38, 135.34, 134.64, 133.53, 132.28, 131.96, 131.53, 130.37, 129.40, 128.73, 128.67, 59.26.
Results and discussion
Chemistry
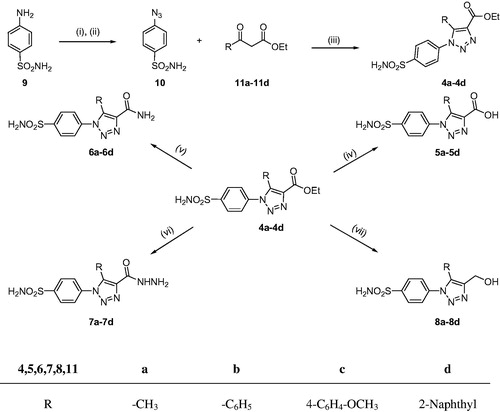
The synthetic route adopted for the synthesis of 4-functionalised 1,2,3-triazole compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) is outlined in Scheme 1. 1,2,3-Triazole-4-carboxylates 4a–4d, the supreme compounds to carry out the complete conversion, were synthesised by reactions of 4-azidobenzenesulphonamide (10) with differently substituted β-ketoesters (11a–11d) in the presence of organic base. 4-Azidobenzenesulphonamide (10) in turn was prepared from sulphanilamide (9) via diazotisation followed by treatment with sodium azideCitation47. After the synthesis, 1,2,3-triazole-4-carboxylates 4a–4d were converted to corresponding carboxylic acids 5a–5d by hydrolysis with a strong base and corresponding carboxamide derivatives 6a–6d by treatment with ammonia solution. 1,2,3-Triazole-4-hydrazinocarbonyl derivatives 7a–7d were obtained by treating their corresponding esters 4a–4d with hydrazine hydratein ethanol while 1,2,3-triazole-4-hydroxymethyl derivatives 8a–8d were prepared by treating esters 4a–4d with lithium aluminium hydrideCitation48 (Scheme 1).
Scheme 1. Synthetic pathway to the sulphonamides 4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d. Reagents and conditions: (i) HCl, NaNO2, H2O, 0 °C, 15 min; (ii) NaN3, 0 °C, 30 min; (iii) Piperidine, DMSO, 70 °C, 4 h; (iv) NaOH, reflux, 3 h then H3O+; (v) NH3 solution, stir, 22 h; (vi) NH2NH2.H2O, EtOH, reflux, 10–12 h; (vii) LiAlH4, dry THF, reflux, 2 h then H3O+.
The structures of all the newly synthesised compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) were characterised by a rigorous analysis of their IR, 1H NMR and 13C NMR spectral data. Structures were further confirmed by their HRMS data. In FT-IR, a strong characteristic absorption band for C=O stretch was observed in the range 1704–1728 cm−1 for 1,2,3-triazole-4-carboxylates 4a–4d, 1690–1713 cm−1 for 1,2,3-trazole-4-carboxylic acids 5a–5d, 1674–1690 cm−1 for 1,2,3-triazole-4-carboxamides 6a–6d and 1659–1670 cm−1 for 1,2,3-triazole-4-hydrazinocabonyl derivatives 7a–7d while no such absorption band was observed in 1,2,3-triazole-4-hydroxymethyl compounds 8a–8d showing the complete reduction of ester group to hydroxymethyl group. The NMR spectra of ethyl esters of 1,2,3-triazole-4-caboxylic acids 4a–4d displayed a quartet in the range δ 4.37–4.00 ppm of two protons and a triplet in the range δ 0.81–1.35 ppm of three protons for ethyl group. Conversion of ester compounds 4a–4d to the corresponding carboxylic acids 5a–5d was confirmed by a downfield exchangeable singlet around δ 13.00 ppm due to COOH with disappearance of signals due to ethyl group protons. Similarly, 1,2,3-triazole-4-carboxamides 6a–6d were characterised by two exchangeable singlets in the range of δ 7.94–8.04 ppm and 7.52–7.54 ppm corresponding to NH and OH protons, target 1,2,3-triazole-4-hydrazinocarbonyls 7a–7d showed two exchangeable singlets in the range δ 9.53–9.88 ppm due to NH proton and δ 4.45–4.50 ppm due to NH2 protons whereas 1,2,3-triazole-4-hydroxymethyl compounds 8a–8d displayed a triplet around δ 5.11–5.25 ppm of OH and a doublet around δ 4.48–4.65 ppm of CH2 protons. The presence of sulphonamide group in all the target 4-functionalised 1,2,3-triazole compounds (4–8) was evident from a broad singlet, exchangeable in D2O, appearing in the range δ 7.44–7.56 ppm.
CA inhibition
All the newly synthesised 4-functionalised 1,2,3-triazole compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) were evaluated against cytosolic isoenzymes hCA I & hCA II and membrane bound isoenzymes hCA IV & hCA IX for their CA inhibition potential by using stopped-flow CO2hydrase assay methodCitation49 and acetazolamide (AZA) was chosen as reference drug for the assay. In general, all the assayed compounds have shown significant inhibitory action against the reported isoforms, with low nanomolar inhibition constant (Ki). Inhibition data of the compounds as given in let the following insights regarding CAs inhibitory properties.
Table 1. Inhibitory potency data for compounds 4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d against isozymes hCAI, hCA II, hCA IV, and hCA IX.
The cytosolic isoform hCA I was in general significantly inhibited by all the newly synthesised compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) with Ki in the range 8.3 nM–0.8737 µM (). It is pertinent to mention that 5-CH3 substituted derivatives of newly synthesised compounds except 8a were most effective inhibitors of hCA I with Ki ≤ 15.1nM as compared to corresponding 5-aryl derivatives. At the same time some compounds namely 5b, 5c, 6c, 6d, 8a, and 8c showed weaker inhibition potential as compared to reference drug AZA (Ki =250 nM) against hCA I that is off-target while inhibiting hCA II and IV in glaucoma and hCA IX in tumours.
Nearly all the newly synthesised compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) showed better inhibitory potential in low nanomolar range with Ki ≤ 9.4 nM except three compounds namely 5b, 7b, and 8a against the most abundant isoform hCA II as compared to standard drug AZA (Ki =12.1 nM). Some of the tested compounds mainly 5-CH3 derivatives (4a, 5a, and 6a) and two other compounds (7d and 8c) exhibited inhibitory potency (Ki ≤ 4 nM) several times better than AZA ().
All the tested compounds except some 5-CH3 derivatives namely 5a, 6a, 7a, and 8a showed excellent inhibition potential with Ki in the range of 1.4–55.3 nM against membrane bound isoform, hCA IV as compared to standard drug AZA. Most of the compounds (4a–4d, 5c–5d, 6b–6d, 7c–7d, and 8b–8d) have their inhibitory potency (Ki ≤ 8.4 nM) several folds superior than AZA (Ki ≤ 74 nM) against hCA IV which is one of the drug target for designing antiglaucoma drugs ().
In general, all the tested compounds except few (4c, 5b, 5c, 6b, 7b, 8a, and 8b) have shown better activity profile (Ki ≤ 9.5 nM) against tumour associated membrane bound isozyme hCA IX as compared to reference drug AZA (Ki =25.8 nM). It is significant to mention here that, in the broader sense, derivatives with 5-CH3and 5-(naphtha-2-yl) substitution have shown better activity as compared to other derivatives ().
Interestingly compounds possessing rather bulky scaffolds were milder inhibitors of cytosolic isoform hCA I, over other isoforms (hCA II, IV, and IX) and is mainly due to the fact that the active site cavity of hCA I is smaller than other isozymes hCA II, IV and IX, because of the presence of two His residues (i.e. His 200 and His 67)Citation50. Overall comparison of activity, in terms of SAR, reveals that all the compounds except derivatives with 5-CH3 group were better selective for hCA II and IV over hCA I in the broader sense. Therefore, these compounds can be important candidates for designing antiglaucoma drugs. However, their good activity against both hCA II and hCA IX suggests that structure of compounds needs further modification for getting better selectivity for tumour associated hCA IX over hCA II.
Conclusions
In this paper, we report a series of twenty novel compounds of 4-functionalised 1-aryl-5-alkyl/aryl-1,2,3-triazole compounds (4a–4d, 5a–5d, 6a–6d, 7a–7d, and 8a–8d) bearing a primary sulphonamide group on the phenyl ring at N-1 position of 1,2,3-triazole scaffold with different functionalities at C-4, such as ester, carboxylic acid, carboxamide, hydrazinocarbonyl, and hydroxymethyl which were evaluated against four isozymes, hCA I, II, IV, and IX. Most of the compounds performed better against aforementioned isoforms showing low nanomolar potency as compared to reference drug acetazolamide. Out of twenty newly synthesised compounds, seventeen compounds (except 5 b, 7 b, and 8a) showed low nanomolar affinity (Ki ≤ 9.4 nM) for hCA II, sixteen compounds except the derivatives with 5-CH3 substitution have displayed excellent activity (Ki ≤ 55.3 nM) for hCA IV and thirteen compounds (except 4c, 5 b, 5c, 6 b, 7 b, 8a, and 8 b) have shown better activity (Ki ≤ 9.5) for hCA IX while most of compounds with bulkier substitution at C-5 were medium to weaker inhibitors of hCA I with Ki values in the range of 56.2–873.7 nM. In short, reported compounds have shown remarkable activity against hCA I, II, IV, and IX isoforms from which it can be concluded that 1,2,3-triazoles scaffold deserve to be investigated further as a novel scaffold for CAIs.
Acknowledgements
One of the authors (Rajiv Kumar) is grateful to University Grants Commission, New Delhi, India, for the award of Senior Research Fellowship and the other (Vikas Sharma) to the Council of Scientific and Industrial Research, New Delhi, India, for the award of Junior Research Fellowship.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68.
- Prete SD, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorg Med Chem Lett 2014; 24:4389–96.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012; 27:759–72.
- Kikutani S, Nakajima K, Nagasato C, et al. Thylakoid luminal θ-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci 2016; 113:9828–33.
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30.
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs–antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014; 29:379–87.
- Bozdag M, Carta F, Vullo D, et al. Synthesis of a new series of dithiocarbamates with effective human carbonic anhydrase inhibitory activity and antiglaucoma action. Bioorg Med Chem 2015;23:2368–76.
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91.
- Arechederra RL, Waheed A, Sly WS, et al. Effect of sulfonamides as carbonic anhydrase VA and VB inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem 2013;21:1544–8.
- Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor‐associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45.
- Gawad NMA, Amin NH, Elsaadi MT, et al. Synthesis of 4-(thiazol-2-ylamino)-benzenesulfonamides with carbonic anhydrase I, II and IX inhibitory activity and cytotoxic effects against breast cancer cell lines. Bioorg Med Chem 2016;24:3043–51.
- Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000;60:7075–83.
- Guler OO, De Simone G, Supuran CT. Drug design studies of the novel antitumor targets carbonic anhydrase IX and XII. Curr Med Chem 2010;17:1516–26.
- Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Patents 2013;23:705–16.
- Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Patents 2013;23:725–35.
- Yaseen R, Ekinci D, Senturk M, et al. Pyridazinone substituted benzenesulfonamides as potent carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2016;26:1337–41.
- Maresca A, Vullo D, Scozzafava A, et al. Inhibition of the β-class carbonic anhydrases from Mycobacterium tuberculosis with carboxylic acids. J Enzyme Inhib Med Chem 2013;28:392–406.
- Monti SM, Supuran CT, Simone GD. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Patents 2013;23:737–49.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Ram S, Celik G, Khloya P, et al. Benzenesulfonamide bearing 1, 2, 4-triazole scaffolds as potent inhibitors of tumor associated carbonic anhydrase isoforms hCA IX and hCA XII. Bioorg Med Chem 2014;22:1873–82.
- Ram S, Ceruso M, Khloya P, et al. 4-Functionalized 1, 3-diarylpyrazoles bearing 6-aminosulfonylbenzothiazole moiety as potent inhibitors of carbonic anhydrase isoforms hCA I, II, IX and XII. Bioorg Med Chem 2014;22:6945–52.
- Kumar R, Bua S, Ram S, et al. Benzenesulfonamide bearing imidazothiadiazole and thiazolotriazole scaffolds as potent tumor associated human carbonic anhydrase IX and XII inhibitors. Bioorg Med Chem 2017; 25:1286–93.
- Zhang SY, Fu DJ, Yue XX, et al. Design, Synthesis and structure-activity relationships of novel chalcone-1, 2, 3-triazole-azole derivates as antiproliferative agents. Molecules 2016;21:653.
- Gill C, Jadhav G, Shaikh M, Kale R. Clubbed [1, 2, 3] triazoles by fluorine benzimidazole: a novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg Med Chem Lett 2008;18:6244–7.
- Holla BS, Mahalinga M, Karthikeyen MS, et al. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur J Med Chem 2005;40:1173–8.
- Ebner DC, Culhane JC, Winkelman TN, et al. Synthesis of novel oxazolidinone antimicrobial agents. Bioorg Med Chem 2008;16:2651–6.
- Pagliai F, Pirali T, Grosso ED, et al. Rapid synthesis of triazole-modified resveratrol analogues via click chemistry. J Med Chem 2006;49:467–70.
- Khazir J, Hyder I, Gayatri JL, et al. Design and synthesis of novel 1,2,3-triazole derivatives of coronopilin as anti-cancer compounds. Eur J Med Chem 2014;82:255–62.
- Olomola TO, Klein R, Lobb KA, et al. Towards the synthesis of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Tetrahedron Lett 2010;51:6325–8.
- Sangshetti JN, Nagawade RR, Shinde DB. Synthesis of novel 3-(1-(1-substituted piperidin-4-yl)-1H-1, 2, 3-triazol-4-yl)-1, 2, 4-oxadiazol-5 (4H)-one as antifungal agents. Bioorg Med Chem Lett 2009;19:3564–7.
- Wuest F, Tang X, Kniess T, et al. Synthesis and cyclooxygenase inhibition of various (aryl-1, 2, 3-triazole-1-yl)-methanesulfonylphenyl derivatives. Bioorg Med Chem 2009;17:1146–51.
- Poulsen SA, Wilkinson BL, Innocenti A. Inhibition of human mitochondrial carbonic anhydrases VA and VB with para-(4-phenyltriazole-1-yl)-benzenesulfonamide derivatives. Bioorg Med Chem Lett 2008;18:4624–7.
- Jordao AK, Afonso PP, Ferreira VF, et al. Antiviral evaluation of N-amino-1,2,3-triazoles against Cantagalo virus replication in cell culture. Eur J Med Chem 2009;44:3777–83.
- Lin H, Walsh CT. A chemoenzymatic approach to glycopeptide antibiotics. J Am Chem Soc 2004;126:13998–4003.
- Casas-Solvas JM, Vargas-Berenguel A, CapitànVallvey LF, Santovo-Gonzalez F. Convenient methods for the synthesis of ferrocene − carbohydrate conjugates. Org Lett 2004;6:3687–90.
- Bennett I, Broom NJP, Bruton G, et al. 6-(Substituted methylene) penems, potent broad spectrum inhibitors of bacterial β-lactamase. J Antibiot 1991;44:331–7.
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- Nocentini A, Carta F, Ceruso M, et al. Click-tailed coumarins with potent and selective inhibitory action against the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem 2015;23:6955–66.
- Kumar P, Chandak N, Kaushik P, et al. Synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory–antibacterial agents. Med Chem Res 2012;21:3396–405.
- Kumar S, Namkung W, Verkman AS, Sharma PK. Novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids as calcium activated chloride channel inhibitors. Bioorg Med Chem 2012;20:4237–44.
- Khloya P, Celik G, Ram S, et al. 4-Functionalized 1, 3-diarylpyrazoles bearing benzenesulfonamide moiety as selective potent inhibitors of the tumor associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 2014;76:284–90.
- Khloya P, Ceruso M, Ram S, et al. Sulfonamide bearing pyrazolylpyrazolines as potent inhibitors of carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem Lett 2015;25:3208–12.
- Kumar S, Ceruso M, Tuccinardi T, et al. Pyrazolylbenzo[d]imidazoles as new potent and selective inhibitors of carbonic anhydrase isoforms hCA IX and XII. Bioorg Med Chem 2016;24:2907–13.
- Chandak N, Ceruso M, Supuran CT, Sharma PK. Novel sulfonamide bearing coumarin scaffolds as selective inhibitors of tumor associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem 2016;24:2882–6.
- Morimoto Y, Matsuda F, Shirahama H. Synthetic studies on virantmycin. 1. Total synthesis of (±)-virantmycin and determination of its relative stereochemistry. Tetrahedron 1996;52:10609–30.
- Rogez-Florent T, Meignan S, Foulon C, et al. New selective carbonic anhydrase IX inhibitors: synthesis and pharmacological evaluation of diarylpyrazole-benzenesulfonamides. Bioorg Med Chem 2013;21:1451–64.
- Khalifah RJ. The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Bio Chem 1971;246:2561–73.
- Ferraroni M, Tilli S, Briganti F, et al. Crystal structure of a zinc-activated variant of human carbonic anhydrase I, CA I Michigan 1: evidence for a second zinc binding site involving arginine coordination. Biochemistry 2002;41:6237–44.