Abstract
Compounds containing nitrogen and sulfur atoms can be widely used in various fields such as industry, medicine, biotechnology and chemical technology. Therefore, the reactions of aminomethylation and alkoxymethylation of mercaptobenzothiazole, mercaptobenzoxazole and 2-aminothiazole were developed. Additionally, the alkoxymethyl derivatives of mercaptobenzoxazole and 2-aminothiazole were synthesized by a reaction with hemiformals, which are prepared by the reaction of alcohols and formaldehyde. In this study, the inhibitory effects of these molecules were investigated against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) enzymes and carbonic anhydrase I, and II isoenzymes (hCA I and II). Both hCA isoenzymes were significantly inhibited by the recently synthesized molecules, with Ki values in the range of 58–157 nM for hCA I, and 81–215 nM for hCA II. Additionally, the Ki parameters of these molecules for BChE and AChE were calculated in the ranges 23–88 and 18–78 nM, respectively.
Introduction
Chemists are interested in derivatives of mercaptobenzothiazole and mercaptobenzoxazole because a number of biologically and physiologically active compounds with bactericidal, fungicidal, tuberculostatic, anti-inflammatory, parasympatholytics and anesthetic properties have been synthesized based on them.Citation1
The carbonic anhydrases (CAs, E.C.4.2.1.1) are a superfamily of metalloenzymes that catalyze a crucial and simple biochemical reaction, the reversible hydration of carbon dioxide (CO2) and water (H2O) to bicarbonate () and protons (H+).Citation2–5 This reaction, in the absence of CA cannot proceed with a perceptible rate under physiological positions.Citation6–8
CAs are widely distributed in all kingdoms of life and are categorized in seven distinct classes: α-, β-, γ-, δ-, ζ-, η- and θ-CAs. Each CA family demonstrates proper specific characteristics in the primary amino acid sequence.Citation9,Citation10 α-CAs are found in mammals. α-CAs, which have sixteen isoenzymes are expressed predominantly in vertebrates and are the only class observed in humans. They are catalytically active and differ in their subcellular localization, distribution in organs and tissues, kinetic properties, expression levels, and inhibitor binding affinities.Citation11–13 Additionally, CAs play important roles in a multitude of physiological activities in eukaryotes, such as CO2 transport, respiration, photosynthesis and electrolyte secretion.Citation14–16
The production of novel CA inhibitors (CAIs) is a growing priority for pharmaceutical research and discovery. In addition to the defined role of CAIs as antiglaucoma drugs and diuretics, their potential as anti-obesity, anti-convulsant, anti-infective and anticancer has been recently described.Citation17,Citation18 hCA II inhibitors has been widely studied from structural and design points-of-view and in dynamics simulations.Citation19–21 In addition, it is the most widespread physiologically relevant CA isoenzyme.
Alzheimer’s disease (AD) is the most prevalent cause of dementia in elderly people.Citation22–24 Recoveries in cognitive capabilities in AD patients were obtained by disrupting or blocking the acetylcholinesterase (AChE) activity with inhibitor compounds.Citation25–27 Alkaloid compounds are some of the strongest acetylcholinesterase inhibitors (AChEIs); therefore searches for novel alkaloids with inhibitory compounds have been conducted.Citation28–30 The AChE enzyme by prompting hydrolyzes of the neurotransmitter acetylcholine (ACh), concluding an impulse transmissions at the cholinergic synapses in neurons.Citation31,Citation32 As can be seen in , the active site of AChE’s consists of two parts: (i) the anionic part that accommodates the positively charged section of acetylcholine and (ii) the catalytic part where the ester bond is hydrolysed.Citation33,Citation34 AChE is the target of many drugs and neurotoxins that bind particularly to its active site.Citation35,Citation36 Inhibition of AChE is used for the treatment of senile dementia, AD, myasthenia gravis, ataxia and Parkinson’s disease.Citation37–39 AChE can also serve as a probe for biosensors that are capable of binding to and potentially discovering new AChE inhibitor compounds; these compounds have applications as possible neurotoxins, such as nerve factors, pesticides and therapeutic drugs.Citation40,Citation41 X-ray structures have indicated that the although the butyrylcholinesterase (BChE) and AChE structures are similar, multiple structural discrepancies in the active-site gorges and the active sites have been observed.Citation42,Citation43 BChE has of toxicological and pharmacological importance because it scavenges ChEIs, including potent organophosphorus nerve factors, before they bind synapses and hydrolyzes ester-containing drugsCitation44. BChE is also important for drug metabolism such as cocaine.Citation45 Both BChE and AChE, which have molecular roles beyond normal neurons and differentiated kinetics recorded in the brain, accumulate within tangles and amyloid plaques.Citation46
Figure 1. The hydrolyze reaction of acetylcholine in the presence of acetylcholinesterase enzyme (AChE).
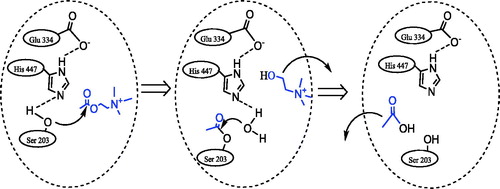
The goal of this paper is to design and synthesize some novel aminomethyl and alkoxymethyl derivatives (1–17) and to generate more potent BChE and AChE enzymes, CA II and I isoforms.
Experimental
Chemistry
Synthesis of aminomethyl derivatives of benzothiazole and benzoxazolthiones (1–10)
Aminomethylation was carried out at the temperature of 10°C by adding the corresponding aminal to a solution of mercaptobenzothiazole (or mercaptobenzoxazole) in ethanol. The resulting product was recrystallized from methanol. The aminomethyl derivatives of benzothiazole and benzoxazolthiones 2–8 were reported in the literature.Citation47–53 However, there is no information about the synthesis of compounds 9 and 10 in the literature.
Initial aminals were obtained by condensing of secondary amines with formaldehyde. The physico-chemical characteristics of the obtained products are shown in .
Table 1. Physico-chemical characteristics of aminomethyl derivatives of benzothiazol- and benzoxazolthiones
Formaldehyde was used as a form of paraformaldehyde. The reaction was carried out in an absolute ethanol solution. Hemiformals reacted immediately after its preparation without isolation. The resulting reaction water was separated by azeotropic distillation with benzene. The crystals were obtained after distilling the solvents, including ethanol and benzene, and recrystallizing. The melting points and yields are given in .
Table 2. Physico-chemical characteristics of the alkoxymethyl derivatives of benzoxazolthione and 2-aminothiazoles.
Synthesis of the alkoxymethyl derivatives of benzoxazolthione and 2-aminothiazole (11–17)
To do this, hemiformal was obtained from 0.05 mol of a formaldehyde (used as paraformaldehyde) and 40 ml of the corresponding alkanol (taken in excess as a solvent). Hemiformal reacted immediately after its preparation without isolation. Then, 0.05 mol of mercaptobenzoxazole (or 2-aminothiazole) dissolved in ethanol was added to hemiformal at the temperature of 10 °C. The resulting reaction water was separated by azeotropic distillation with benzene. The crystalline substances were obtained after distilling off the solvent (ethanol, benzene) and recrystallization.
Biological studies
Purification of carbonic anhydrase I and II isoforms and inhibition studies
To observe of inhibition effects of novel aminomethyl and alkoxymethyl derivatives (1–17) on CA I, and II isoforms, which purified from fresh human erythrocyte using an affinity chromatography procedure.Citation54,Citation55 CA activity was determined using the previously described spectrophotometric procedure of Verpoorte et al.Citation56 as explained previously.Citation21,Citation57,Citation58 In this procedure, changes in activity were obtained during 3 min at 22 °C. p-Nitrophenylacetate (PNA) compound was used as a substrate, and it was converted by both isoforms to p-nitrophenolate ions.Citation59,Citation60 The quantity of protein was measured according to the previously described by Bradford method.Citation61–64 and bovine serum albumin was used as the standard.Citation65,Citation66 After the purification method of the CA isoforms, samples were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE).Citation67–69 The change in activity was spectrophotometrically obtained at 348 nm.Citation70,Citation71 The IC50 values were calculated from activity (%) against compounds inhibition.Citation72–74 Three various concentrations were used to calculate Ki values.Citation75–77
AChE/BChE activity determination and inhibition studies
The inhibitory effects of novel aminomethyl and alkoxymethyl derivatives (1–17) on AChE and BChE activities were measured according to Ellman et al.Citation78 Acetylthiocholine iodide (AChI) and butyrylthiocholine iodide (BChI) were used as substrates for the reaction. 5,5′-Dithio-bis(2-nitro-benzoic)acid (DTNB) was used for the measurement of the AChE/BChE activities. Briefly, 1.0 ml of Tris/HCl buffer (1.0 M, pH 8.0), and 10 µL of sample solution were dissolved in deionized water at different concentrations and 50 µL AChE/BChE solution were mixed and incubated for 10 min at 25 °C. Next 50 µL of DTNB (0.5 mM) was added. The reaction was then initiated by the addition of 50 µL of AChI or BChI. The hydrolysis of these substrates was monitored spectrophotometrically by the formation of the yellow 5-thio-2-nitrobenzoate anion, as a result of the reaction of DTNB with thiocholine, which released by enzymatic hydrolysis of AChI or BChI, with absorption maximum at 412 nm.
Results and discussion
Synthesis
Many physiologically active natural compounds contain > N-CH2-O- > N-CH2-N < structural fragments. This study sought to build a structure that combines physiologically active benzothiazole or benzoxazole groups with alkoxymethyl or aminomethyl fragments. Therefore, the aminomethylation and alkoxymethylation reactions of mercaptobenzothiazole, mercaptobenzoxazole and 2-aminothiazole are developed.
The structure of the products was established by NMR spectroscopy and the composition was confirmed by elemental analysis. Spectra were measured on a Bruker device in acetone. A singlet at 4.6 ppm corresponding to N-CH2-N was observed in the 1H NMR spectra of all aminomethyl derivatives. A singlet at 5.2–5.8 ppm characterized the presence of the fragment N-CH2-O in the 1H NMR spectra of alkoxymethyl derivatives. Methylene-bis-amines, which have good alkylation (amino-methylation) properties, were used as amino-methylation reagents.
The alkoxymethyl derivatives of mercaptobenzoxazole and 2-aminothiazole were synthesized by reacting them with hemiformals, which were prepared by the reaction of alcohols with formaldehyde.

Biological results
Sulfamate and sulfonamide CAIs demonstrated fundamental anti-glaucoma and anti-tumour activities in vivo and in vitro; therefore new therapeutic approaches targeting either hCA IX/XII (for antitumor activity) or hCA II (for antiglaucoma action) have been developed.Citation79,Citation80 hCA II enhances sodium bicarbonate secretion in the anterior uvea of the eye causing glaucoma and visual dysfunction.Citation81 Heterocyclic molecules with primitive sulfonamide compounds are the most extensively evaluated class of CAIs, which has led to the advancement of diverse classes of clinical drugs like methazolamide (MZA), acetazolamide (AZA) and others.Citation82 In this work, both the Ki and IC50 of the aminomethyl and alkoxymethyl derivatives (1–17) were calculated and they are given in .
Table 3. AChE, human carbonic anhydrase I, and II isoforms (hCA I, and II) AChE and BChE enzymes inhibition effects of aminomethyl and alkoxymethyl derivatives (1–17) and proportion of AChE to BChE enzymes.
Cytosolic hCA I, and II isoenzymes are widely distributed throughout the human body and interference with these enzymes may cause side effects. For the cytosolic hCA I enzyme, aminomethyl and alkoxymethyl derivatives (1–17) had Ki values in the range of 58 ± 15 to 157 ± 38 nM (). Especially, compound 8 (Ki: 58 ± 15 nM); N-morfolinomethylbenzoxazoline-2-thion and compound 5 (Ki: 70 ± 15 nM); N-diethylaminomethylbenzothiazoline-2-thione) inhibited the hCA I isoform more potently than the standard compound AZA (Ki: 333 ± 28 nM), which is used to treat glaucoma, cystinuria, periodic paralysis, epileptic seizure, dural estasia and central sleep apnea. hCA I is involved in retinal edema and cerebral and the inhibition of hCA I can be a significant factor for eliminating of these conditions.Citation83
The role of hCA II in diseases such as glaucoma has been well characterized. Indeed,
production serves as a mechanism to transport sodium ions (Na+) into the eye along with the influx of water, which leads to an increase in intraocular pressure.Citation84 Inhibition of CA II decreases
production and subsequently aqueous humor secretion, which leads to decreased pressure in the eye.Citation85 For the ubiquitous cytosolic isoform hCA II, novel aminomethyl and alkoxymethyl derivatives (1–17) had Ki values ranging from 81 ± 19–215 ± 40 nM. In addition, AZA compound applied as a standard CA inhibitor, which obtained Ki value of 353 ± 60 nM. As can be observed in hCA II, the most considerable inhibition result was recorded by N-oxazinomethylbenzothiazoline-2-thione (1) (81 ± 19) ().
BChE and AChE were very significantly inhibited by novel aminomethyl and alkoxymethyl derivatives (1–17). It was calculated that Ki values were in the range of 23 ± 3–88 ± 11 nM for BChE and 18 ± 2–78 ± 36 nM for AChE, respectively (). Additionally, tacrine (TAC) was used as clinically BChE and AChE inhibitor, which had Ki values of 128 ± 16 and 109 ± 5 nM, respectively. The results are shown that entire of test molecules have perfect inhibition activity against BChE and AChE compared to TAC.
In this work, we calculated AChE/BChE selectivity. The most promising compound 14 obtained 2.22-fold of inhibitory activity against AChE/BChE than that of TAC. It can be as a potential factor for the therapy of AD. Also, as is shown in , the compound 14 (N-(methoxyethoxy)methyl-benzoxazoline-2-thione) showed the highest selectivity for AChE over BChE (ratio: 0.388) and weakest compound was 6 (N-diethylaminomethylbenzoxazoline-2-thione) (ratio 1:500).
Discussion
The synthesized molecules are shown to inhibit hCA II and I isoenzymes by the interplay of aminomethyl and alkoxymethyl derivatives (1–17) with cofactor Zn2+ ions in the structure of the isoforms. For hCA I isoform (generally defined an important isoform when CAIs for anticancer activity or antiglaucoma are encountered) was good inhibited by entire of the evaluated molecules, the best inhibitors of them were N-diethylaminomethylbenzothiazoline-2-thione (5), N-morfolinomethylbenzoxazoline-2-thion (8) and N-oxazolidinomethylbenzoxazoline-2-thione (9) (). The 2-isopropoxymethylaminothiazole (16) and 2-(methoxy)methylaminothiazole (17) compounds are weaker inhibitors compared to other compounds for this isoenzyme. The molecule 8 was shown to had the excellent inhibitory efficacy on hCA I isoenzyme activity while the molecule 1 was shown to had the excellent inhibitory efficacy on hCA II isoenzyme activity. For hCA II isoform, the best inhibitors of them were N-oxazinomethylbenzothiazoline-2-thione (1) and N-isopropoxymethylbenzoxazoline-2-thione (13). The 2-(methoxyethoxy) methylaminothiazole (15) and 2-methoxymethylaminothiazole (17) molecules are weaker inhibitors compare with other molecules for this isoform. As seen in and , IC50 values are in the range of 89–187 nM towards hCA II, while for hCA I is in the range of 79–156 nM. The IC50 values for standard molecule AZA towards hCA II and I are 520 and 373 nM, respectively. All molecules have lower IC50 value compare with AZA toward hCA II and hCA I isoenzymes.
Figure 2. (a) IC50 values of aminomethyl and alkoxymethyl derivatives for hCA I, and II isoenzymes. (b) IC50 values of aminomethyl and alkoxymethyl derivatives for AChE and BChE enzymes. (c) Ki values of aminomethyl and alkoxymethyl derivatives for hCA I, and II isoenzymes. (d) Ki values of aminomethyl and alkoxymethyl derivatives for AChE and BChE enzymes.
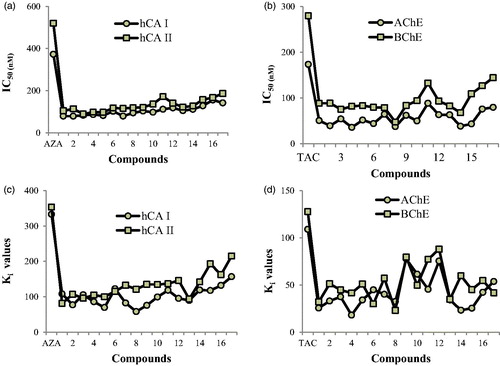
As seen in and , IC50 amounts were in the range of 36–89 nM towards AChE, while they were in the range of 48–145 nM towards BChE (). The IC50 amounts of the standard compound TAC towards AChE and BChE were 174 and 280 nM, respectively. Entire compounds have lower IC50 amount than TAC toward AChE and BChE. ChEIs have shown excellent efficacy than placebo in clinical tests and are extensively prescribed as symptomatic therapy to ameliorate behavior and recognition in AD patients with moderate dementiaCitation27,Citation28. TAC (9-Amino-1,2,3,4-tetrahydroacridine) compound is a reversible inhibitor of BChE and AChE and the first drug to be agreed by the Drugs and Foods Administration of America for the placative therapy of AD.Citation35 For AChE and BChE enzymes were good inhibited by entire of the evaluated compounds, the best inhibitors of AChE were N-Piperidinomethylbenzothiazoline-2-thione (4), N-(methoxyethoxy)methyl-benzoxazoline-2-thione (14) and also for BChE were N-diethylaminomethylbenzoxazoline-2-thione (6) and N-morfolinomethylbenzoxazoline-2-thion (8), respectively.
Conclusions
In this paper, nanomolar levels of Ki amounts were obtained for entire novel aminomethyl and alkoxymethyl derivatives (1–17) and these molecules can be considerable inhibitor of AChE, BChE enzymes and both hCA isoforms. The molecules 5 and 8 towards hCA I and molecules 1 and 13 towards hCA II and molecules 4 and 14 towards AChE and molecules 6 and 8 towards BChE enzymes recorded which can to be the leader molecules of the parts for subsequent evaluations.
Acknowledgements
S. Alwasel would like to thank the Distinguished Scientist Fellowship Program, King Saud University for their support.
Disclosure statement
The authors declare no conflict of interest.
References
- Cressier D, Procullac C, Hernande P, et al. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg Med Chem 2009;17:5275–84.
- Kocyigit UM, Budak Y, Gürdere MB, et al. Synthesis, characterization, anticancer, antimicrobial and carbonic anhydrase inhibition profiles of novel (3aR,4S,7R,7aS)-2-(4-((E)-3-(3-aryl)acryloyl) phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives. Bioorg Chem 2017;70:118–25.
- Gokcen T, Al M, Topal M, et al. Synthesis of some natural sulphonamide derivatives as carbonic anhydrase inhibitors. Org Commun 2017;10:15–23.
- Karalı N, Akdemir A, Göktas F, et al. Novel sulfonamide-containing 2-indolinones that selectively inhibit tumor-associated alpha carbonic anhydrases. Bioorg Med Chem 2017;25:3714–8.
- Aksu K, Ozgeris B, Taslimi P, Naderi A, et al. Antioxidant activity, acetylcholinesterase, and carbonic anhydrase inhibitory properties of novel ureas derived from phenethylamines. Arch. Pharm. (Weinheim) 2016; 349:944–54.
- Topal F, Gulcin İ, Dastan A, Guney M. Novel eugenol derivatives: potent acetylcholinesterase and carbonic anhydrase inhibitors. Int J Biol Macromol 2017;94:845–51.
- Ceylan M, Kocyigit UM, Usta NC, et al. Synthesis, carbonic anhydrase I and II isoenzymes inhibition properties and antibacterial activities of novel tetralone based 1,4-benzothiazepine derivatives. J Biochem Mol Toxicol 2017;31:e21872.
- Del Prete S, Vullo D, Osman SM, et al. Sulfonamide inhibition profiles of the b-carbonic anhydrase from the pathogenic bacterium Francisella tularensis responsible of the febrile illness tularemia. Bioorg Med Chem 2017;25:3555–61.
- Oktay K, Polat Kose L, Sendil K, et al. Synthesis of 3-chloro-1-substituted aryl pyrrolidine-2,5-dione derivatives: discovery of potent human carbonic anhydrase inhibitors. Med Chem Res 2017;26:1619–27.
- Bayrak Ç, Taslimi P, Gülçin İ, Menzek A. The first synthesis of 4-phenylbutenone derivative bromophenols including natural products and their inhibition profiles for carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Bioorg Chem 2017;72:359–66.
- Supuran CT, Capasso C. The η-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin Ther Targets 2015;19:551–63.
- Akocak S, Lolak N, Nocentini A, et al. Synthesis and biological evaluation of novel aromatic and heterocyclic bis-sulfonamide Schiff bases as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg Med Chem 2017;25:3093–7.
- Koçyiğit UM, Aslan OM, Gu¨lçin İ, et al. inhibition of novel 2-(4-(aryl)thiazole-2-yl)-3a,4,7,7a-tetrahydro-1h-4,7-methanoisoindole-1,3(2h)-dione derivatives. Arch Pharm 2016;349:955–63.
- Akbaba Y, Bastem E, Topal F, et al. Synthesis and carbonic anhydrase inhibitory effects of novel sulfamides derived from 1-aminoindanes and anilines. Arch. Pharm. (Weinheim) 2014;347:950–7.
- Innocenti A, Gulcin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenol natural products effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3.
- Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drugs Des 2011;77:494–9.
- Çoban TA, Beydemir S, Gülçin İ, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–61.
- Coban TA, Beydemir S, Gülçin İ, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–70.
- Topal F, Topal M, Gocer H, et al. Antioxidant activity of taxifolin: an activity-structure relationship. J Enzyme Inhib Med Chem 2016;31:674–83.
- Innocenti A, Öztürk Sarıkaya SB, Gülçin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64.
- Şentürk M, Gülçin İ, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Göçer H, Akıncıoğlu A, Öztaşkın N, et al. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of dopamine-related compounds. Arch. Pharm. (Weinheim) 2013;346:783–92.
- Akıncıoğlu A, Topal M, Gülçin İ, Göksu S. Novel sulfamides and sulfonamides incorporating tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch Pharm 2014;347:68–76.
- Göçer H, Akıncıoğlu A, Göksu S, et al. Carbonic anhydrase and acetylcholine esterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2015;30:316–20.
- Aksu K, Topal F, Gülçin I, et al. Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch. Pharm. (Weinheim) 2015;348:446–55.
- Akıncıoğlu A, Akıncıoğlu H, Gülçin I, et al. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602.
- Oztaşkın N, Çetinkaya Y, Taslimi P, Göksu S, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem 2015;60:49–57.
- Polat Köse L, Gu¨lçin İ, Gören AC, et al. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crops Prod 2015;74:712–21.
- Scozzafava A, Kalın P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6.
- Topal M, Gocer H, Topal F, et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem 2016;31:266–75.
- Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2016;31:441–7.
- Özgeriş B, Göksu S, Köse Polat L, et al. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 2016;24:2318–29.
- Gülçin İ, Scozzafava A, Supuran CT, et al. The effect of caffeic acid phenethyl ester (CAPE) metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione s-transferase, lactoperoxidase and carbonic anhydrase ısoenzymes I, II, IX and XII. J Enzyme Inhib Med Chem 2016;31:1095–101.
- Yılmaz S, Akbaba Y, Özgeriş B, et al. Synthesis and inhibitory properties of some carbamates on carbonic anhydrase and acetylcholine esterase. J Enzyme Inhib Med Chem 2016;31:1484–91.
- Sujayev A, Garibov E, Taslimi P, et al. Synthesis of some tetrahydropyrimidine-5-carboxylates, determination of their metal chelating effects and inhibition profiles against acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:1531–9.
- Gül HI, Tuğrak M, Sakagami H, et al. Synthesis and bioactivity studies on new 4-(3-(4-substitutedphenyl)-3a,4-dihydro-3h-indeno[1,2-c]pyrazol-2-yl) benzenesulfonamides. J Enzyme Inhib Med Chem 2016;31:1619–24.
- Gülçin İ, Scozzafava A, Supuran CT, et al. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:1698–702.
- Genç H, Kalin R, Köksal Z, et al. Discovery of potent carbonic anhydrase and acetylcholinesterase inhibitors: 2-aminoindan β-lactam derivatives. Int J Mol Sci 2016;17:1736.
- Koçak R, Turan A, E, Kalin P, et al. Synthesis of some novel norbornene-fused pyridazines as potent inhibitors of carbonic anhydrase and acetylcholinesterase. J Heterocyc Chem 2016;53:2049–56.
- Turan B, Sendil K, Sengul E, et al. The synthesis of some β-lactams and investigation of their metal chelating activity, carbonic anhydrase and achetylcholinesterase inhibition profiles. J Enzyme Inhib Med Chem 2016;31(Suppl 1):79–88.
- Özbey F, Taslimi P, Gulcin İ, et al. Synthesis, acetylcholinesterase, butyrilcholinesterase, carbonic anhydrase inhibitory and metal chelating properties of some novel diaryl ether. J Enzyme Inhib Med Chem 2016;31(Suppl 2):79–85.
- Işık M, Beydemir S, Yılmaz A, et al. Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed Pharmacother 2017;87:561–7.
- Gul HI, Demirtas A, Ucar G, et al. Synthesis of Mannich bases by two different methods and evaluation of their acetylcholine esterase and carbonic anhydrase inhibitory activities. Lett Drug Des Discov 2017;14:573–80.
- Woreka F, Schilha M, Neumaier K, et al. On-site analysis of acetylcholinesterase and butyrylcholinesterase activity with the ChE check mobile test kit: determination of reference values and their relevance for diagnosis of exposure to organophosphorus compounds. Toxicol Lett 2016;249:22–8.
- Chen X, Zheng X, Zhou Z, et al. Effects of a cocaine hydrolase engineered from human butyrylcholinesterase on metabolic profile of cocaine in rats. Chem Biol Interact 2016;259:104–9.
- Taslimi P, Sujayev A, Mamedova S, et al. Synthesis and bioactivity of several new hetaryl sulfonamides. J Enzyme Inhib Med Chem 2017;32:137–45.
- Sorokin VG. Izvestiya Vysshikh Uchebnykh Zavedenii. Khimiya I Khimicheskaya Tekhnologiya 1975;18:74–6.
- Franklin S, Tamilvendan D, Venkatesa Prabhu G, Balasubramanian T. Structural and spectral analysis of a Mannich Base: 3-(Morpholin-4-ylmethyl)-1,3-benzothiazole-2-thione. J Chem Crystallog 2012;42:29–33.
- Hatayama, Kazuya Jpn. Kokai Tokkyo Koho. 1995; JP 07041604 A 19950210.
- Valiuliene S, Kuodis Z, Rutavicius A, Chemija. 1994;(2):81-5n.
- Dhal PN, Nayak A. Ind J Pharm 1975;37:92–4.
- Yamaguchi J, Washisu S, Jpn. Kokai Tokkyo Koho. 1989; JP 01017048 A 19890120.
- Erdogan B, Seyhan E, Atay O, Isikdag I. Gazi Universitesi Eczacilik Fakultesi Dergisi 1989;6:163–72.
- Öztürk Sarıkaya SB, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
- Nar M, Çetinkaya Y, Gülçin İ, Menzek A. (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors. J Enzyme Inhib Med Chem 2013;28:402–6.
- Verpoorte JA, Metha S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9.
- Coban TA, Beydemir S, Gücin İ, et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg Med Chem 2009;17:5791–5.
- Ozturk Sarıkaya SB, Gulcin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20.
- Artunc¸ T, Çetinkaya Y, Göçer H, et al. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Des 2016;87:594–607.
- Akıncıoğlu A, Akbaba Y, Göçer H, et al. Novel sulfamides as potential carbonic anhydrase isoenzymes inhibitors. Bioorg Med Chem 2013;21:1379–85.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51.
- Atasaver A, Özdemir H, Gülçin İ, Küfrevioğlu Öİ. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem 2013;136:864–70.
- Şişecioğlu M, Gülçin İ, Çankaya M, Özdemir H. The inhibitory effects of L-Adrenaline on lactoperoxidase enzyme (LPO) purified from buffalo milk. Int J Food Propert 2012;15:1182–9.
- Köksal E, Ağgül AG, Bursal E, Gülçin İ. Purification and characterization of peroxidase from sweet gourd (Cucurbita Moschata Lam. Poiret). Int J Food Propert 2012;15:1110–9.
- Şıktar E, Ekinci D, Şıktar E, et al. Protective role of L-carnitine supplementation against exhaustive exercise-induced oxidative stress in rats. Eur J Pharmacol 2011;668:407–13.
- Aksu K, Nar M, Tanç M, et al. The synthesis of sulfamide analogues of dopamine related compounds and their carbonic anhydrase inhibitory properties. Bioorg Med Chem 2013;21:2925–31.
- Akbaba Y, Akıncıoğlu A, Göçer H, et al. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J Enzyme Inhib Med Chem 2014;29:35–42.
- Çetinkaya Y, Göçer H, Gülçin İ, Menzek A. Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm (Weinheim) 2014;347:354–9.
- Topal M, Gülçin İ. Rosmarinic acid: a potent carbonic anhydrase isoenzymes inhibitor. Turk J Chem 2014;38:894–902.
- Güney M, Coşkun A, Topal F, et al. Oxidation of cyanobenzocycloheptatrienes: synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg Med Chem 2014;22:3537–43.
- Göksu S, Naderi A, Akbaba Y, et al. Carbonic anhydrase inhibitory properties of novel benzylsulfamides using molecular modeling and experimental studies. Bioorg Chem 2014;56:75–82.
- Arabaci B, Gülçin İ, Alwasel S. Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014;19:10103–14.
- Ozturk Sarikaya SB, Sisecioglu M, Cankaya M, et al. Inhibition profile of a series of phenolic acids on bovine lactoperoxidase enzyme. J Enzyme Inhib Med Chem 2015;30:479–83.
- Boztaş M, Çetinkaya Y, Topal M, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxy-bromophenol derivatives incorporating cyclopropane moieties. J Med Chem 2015;58:640–50.
- Yıldırım A, Atmaca U, Keskin A, et al. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem 2015;23:2598–605.
- Scozzafava A, Passaponti M, Supuran CT, et al. Carbonic anhydrase inhibitors: Guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:586–91.
- Aydin B, Gülcin I, Alwasel SH. Purification and characterization of polyphenol oxidase from Hemşin apple (Malus communis L.). Int J Food Propert 2015;18:2735–45.
- Ellman GL, Courtney KD, Andres V, Featherston RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Gocer H, Aslan A, Gülçin İ, Supuran CT. Spirobisnaphthalenes effectively inhibit carbonic anhydrase. J Enzyme Inhib Med Chem 2016;31:503–7.
- Küçük M, Gulcin İ. Purification and characterization of carbonic anhydrase enzyme from black sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on the enzyme activity. Environ Toxicol Pharmacol 2016;44:134–9.
- Go¨ksu H, Topal M, Keskin A, et al. 9,10-Dibromo-N-aryl-9,10-dihydro-9,10-[3,4]epipyrroloanthracene-12,14-diones: synthesis and investigation of their effects on carbonic anhydrase isozymes I, II, IX, and XII. Arch Pharm 2016;349:466–74.
- Polat Kose L, Gülçin İ, Özdemir H, et al. The effects of some avermectins on bovine carbonic anhydrase enzyme. J Enzyme Inhib Med Chem 2016;31:773–8.
- Sujayev A, Polat Kose L, Garibov E, et al. Synthesis of N-alkyl (aril)-tetra pyrimidine thiones and investigation of their human carbonic anhydrase I and II inhibitory effects. J Enzyme Inhib Med Chem 2016;31:1192–7.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Rogez-Florent T, Foulona C, Drucbert AS, et al. Chiral separation of new sulfonamide derivatives and evaluation of their enantioselective affinity for human carbonic anhydrase II by microscale thermophoresis and surface plasmon resonance. J Pharm Biomed Anal 2017;137:113–22.
