Abstract
A series of 13 compounds having a monoindolizine mono-salt skeleton was designed and synthesised in order to evaluate their antimycobacterial activity. The synthesis is efficient, involving only three steps: two alkylations and one 3 + 2 dipolar cycloaddition. The antimicrobial activity against Mycobacterium tuberculosis H37Rv grown under aerobic conditions was evaluated, eight compounds showing a very good antimycobacterial activity. SAR correlation reveals a certain influence of the R substituent from the para position of benzoyl moiety at position 3 of indolizine. The most active five compounds passed the second stage of anti-TB testing, the assay demonstrating that they are potent against both replicating and non-replicating Mtb, have a bactericidal mechanism of action, are active against drug-resistant Mtb strains, present a moderate to good activity against nontuberculous mycobacteria, a good intracellular activity, and a moderate to high cytotoxicity. For one compound showing a promising anti-TB profile, a complete ADMET study has been performed.
Introduction
Tuberculosis is one of the major causes of disability and death worldwideCitation1, remaining one of the top 10 causes of deaths in 2015Citation2. More than 95% of TB deaths occur in low- and middle-income countries, according to the World Health OrganisationCitation2. In 2015, 10.4 million people became ill with TB, and 1.4 million people died from the disease. An additional 0.4 million deaths resulted from TB disease among people living with HIVCitation2. Globally in 2015, an estimated 480,000 people developed multidrug-resistant TB (MDR-TB) and around 100,000 people developed rifampicin-resistant TBCitation2. The emergence of MDR-TB and, more recently, extensively drug-resistant (XDR) TB has intensified the need for new TB drugs. Major efforts are done for the discovery and development of new TB drug targets and candidate drugs, and evaluation of novel TB drugs and optimal drug combinations in preclinical and clinical studiesCitation2,Citation3. There are currently two important strategies used for discovery of new anti-TB drugsCitation4,Citation5. One involves the synthesis of analogous of the existing drugs, and the other refers to the search for novel structures. Between the various classes of organic compounds, fused N-heterocyles, especially pyridine fused systems, showed promising anti-TB activity against replicating Mycobacterium tuberculosis (Mtb) H37Rv, similar to isoniazidCitation6,Citation7.
In our previous work, we showed that several new classes of compounds with fused heterocyclic structure possess antimicrobial activityCitation8–14, antimycobacterial includingCitation8–12. Recently, we reported compound 1 with monoindolizine mono-pyridinium salt structure showing a promising antimycobacterial activity against both replicating and non-replicating MtbCitation8.
These results prompted us to extend our study to a series of compounds having the same monoindolizine mono-pyridinium skeleton, but different substituents on adjacent phenyl rings, in order to have a better understanding of the acting mode of these compounds and to be able to see any influence the substituents have on the antimycobacterial activity. The results presented herein refer to the synthesis and antimycobacterial evaluation of a new series of thirteen compounds.
Methods
General
Melting points were recorded on a A. Krüss Optronic Melting Point Meter KSPI and are uncorrected. Proton and carbon nuclear magnetic resonance (δH, δC) spectra were recorded on a DRX-500 Bruker (Bruker, Bremen, Germany) (500 MHz). All chemical shifts are quoted on the δ-scale in ppm. Coupling constants are given in Hz. IR spectra were recorded on a FTIR Shimadzu spectrometer. Thin layer chromatography (TLC) was carried out on Merck silica gel 60F254 plates. Visualisation of the plates was achieved using a UV lamp (λmax = 254 or 365 nm).
General procedure for synthesis of quaternary salts 6a–m
The monoindolizine 5 (1 mmol, 1 equiv., 0.37 g 5a, 0.40 g 5 b, 0.45 g 5c, 0.38 g 5d, 0.40 g 5e) and bromacetophenone derivative (p or/and m substituted, 2 mmol, 2 equiv.) was suspended in anhydrous acetone (20 ml) and magnetically stirred over night at reflux. The resulting precipitate was collected by filtration and then washed with acetone. All products were purified by crystallisation (CHCl3:MeOH 1:1, v:v).
4-(3-Benzoyl-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-oxo-2-(p-tolyl)ethyl)pyridin-1-ium bromide (6a). Orange powder (0.52 g, 89% yield), mp = 279–282 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.36 (t, J = 7.2 Hz, 3H, H12), 2.47 (s, 3H, CH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.53 (s, 2H, H22), 7.50 (d, J = 8.0 Hz, 2H, H26, H28), 7.63 (t, J = 7.2 Hz, 2H, 2 × H16), 7.70 (s, 1H, H2), 7.71 (t, J = 7.2 Hz, 1H, H17), 7.84 (d, J = 7.2 Hz, 2H, 2 x H15), 7.96 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, H6), 8.01 (d, J = 8.0 Hz, 2H, H25, H29), 8.79 (d, J = 6.8 Hz, 2H, 2 × H19), 8.93 (as, 1H, H8), 9.14 (d, J = 6.8 Hz, 2H, 2 × H20), 9.92 (d, J = 7.6 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 21.3 CH3, 60.1 C11, 65.5 C22, 108.1 C1, 113.7 C6, 118.8 C8, 123.0 C3, 124.6 2 × C19, 127.9 C2, 128.3 C25, C29, 128.6 2 × C15, 128.7 2 × C16, 129.1 C5, 129.6 C26, C28, 131.0 C24, 132.1 C17, C7, 137.8 C9, 138.7 C14, 145.4 C27, 146.6 2 × C20, 152.3 C18, 162.6 C10, 184.9 C13, 190.1 C23. IR (KBr, ν(cm−1): 3399, 3032, 3974, 1707, 1643, 1622, 1642, 1205. Anal. Calcd. for C32H27BrN2O4: C, 65.87; H, 4.54; N, 4.66; Found: C, 65.93; H, 4.50; N, 4.75.
4-(3-Benzoyl-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(3-methoxyphenyl)-2-oxoethyl)pyridin-1-ium (6b). Yellow powder (0.53 g, 89% yield), mp 255–256 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.37 (t, J = 7.2 Hz, 3H, H12), 3.89 (s, 3H, OCH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.58 (s, 2H, H22), 7.40 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H, H27), 7.59–7.64 (m, 4H, H26, H29, 2 × H16), 7.68–7.73 (m, 3H, H2, H17, H25), 7.84 (d, J = 7.2 Hz, 2H, 2 × H15), 7.96 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, H6), 8.80 (d, J = 6.8 Hz, 2H, 2 × H19), 8.92 (as, 1H, H8), 9.15 (d, J = 6.8 Hz, 2H, 2 × H20), 9.90 (d, J = 7.6 Hz, 1H, H5). 13C-NMR (125 MHz, DMSO-d6): δ 14.2 C12, 55.6 OCH3, 60.1 C11, 65.7 C22, 108.1 C1, 112.9 C29, 113.6 C6, 118.8 C8, 120.5 C27, 120.6 C25, 123.0 C3, 124.6 2 × C19, 127.8 C2, 128.6 2 × C15, 128.7 2 × C16, 129.1 C5, 130.4 C26, 134.8 C24, 132.0 C17, 132.1 C7, 137.7 C9, 138.6 C14, 146.5 2 × C20, 152.2 C18, 159.5 C28, 162.6 C10, 184.9 C13, 190.6 C23. IR (KBr, ν(cm−1): 3032, 2976, 1697, 1624, 1342, 1198. Anal. Calcd. for C32H27BrN2O5: C, 64.11; H, 4.54; N, 4.67; Found: C, 64.23; H, 4.45; N, 4.70.
4-[3-(4-Chlorophenyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)pyridine-1-ium bromide (6c). Yellow powder (0.59 g, 93% yield), mp 288 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.36 (t, J = 7.2 Hz, 3H, H12), 3.91 (s, 3H, OCH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.49 (s, 2H, H22), 7.20 (d, J = 8.8 Hz, 2H, H26, H28), 7.68 (d, J = 8.4 Hz, 2H, 2 × H16), 7.73 (s, 1H, H2), 7.86 (d, J = 8.4 Hz, 2H, 2 × H15), 7.96 (dd, J = 7.2 Hz, J = 2.0 Hz, 1H, H6), 8.08 (d, J = 8.8 Hz, 2H, H25, H29), 8.78 (d, J = 7.2 Hz, 2H, 2 × H19), 8.92 (d, J = 1.2 Hz, 1H, H8), 9.12 (d, J = 6.8 Hz, 2H, 2 × H20), 9.88 (d, J = 7.2 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 55.9 OCH3, 60.3 C11, 65.4 C22, 108.3 C1, 113.9 C6, 114.5 C26, C28, 118.9 C8, 122.9 C3, 124.7 2 × C19, 126.3 C24, 128.0 C2, 128.8 2 × C16, 129.3 C5, 130.8 2 × C15, C25, C29, 132.3 C7, 137.0 C17, 137.4 C14, 138.0 C9, 146.7 2 × C20, 152.3 C18, 162.7 C10, 164.3 C27, 183.7 C13, 189.0 C23. IR (KBr, ν(cm−1): 3395, 3022, 2936, 1707, 1680, 1642, 1242, 1206, 1173. Anal. Calcd. for C32H26BrClN2O5: C, 60.63; H, 4.13; N, 4.42; Found: C, 60.70; H, 4.10; N, 4.45.
4-(3-(4-Chlorobenzoyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(3-methoxyphenyl)-2-oxoethyl)pyridin-1-ium (6d). Yellow powder (0.46 g, 73% yield), mp 252–254 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.36 (t, J = 7.2 Hz, 3H, H12), 3.88 (s, 3H, OCH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.52 (s, 2H, H22), 7.40 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H, H27), 7.58 (as, 1H, H29), 7.62 (t, J = 8.0 Hz, 1H, H26), 7.68–7.71 (m, 3H, 2 × H16, H25), 7.75 (s, 1H, H2), 7.87 (d, J = 8.4 Hz, 2H, 2 × H15), 7.97 (dd, J = 7.6 Hz, J = 2.0 Hz, 1H, H6), 8.80 (d, J = 6.8 Hz, 2H, 2 × H19), 8.92 (d, J = 0.8 Hz, 1H, H8), 9.11 (d, J = 6.8 Hz, 2H, 2 × H20), 9.91 (d, J = 7.2 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 55.6 OCH3, 60.2 C11, 65.8 C22, 108.3 C1, 113.0 C29, 113.8 C6, 118.9 C8, 120.5 C25, 120.7 C27, 123.0 C3, 124.7 2 × C19, 128.0 C2, 128.8 2 × C16, 129.3 C5, 130.4 C26, 130.7 2 × C15, 132.3 C7, 134.9 C24, 137.0 C17, 137.0 C14, 138.0 C9, 146.6 2 × C20, 152.4 C18, 159.6 C28, 162.7 C10, 183.7 C13, 190.6 C23. IR (KBr, ν(cm−1): 3030, 2920, 1701, 1643, 1248, 1198. Anal. Calcd. for C32H26BrClN2O5: C, 60.63; H, 4.13; N, 4.42; Found: C, 60.65; H, 4.10; N, 4.48.
4-(3-(4-Chlorobenzoyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(4-fluorophenyl)-2-oxoethyl)pyridin-1-ium bromide (6e). Orange powder (0.55 g, 89% yield), mp 307–310 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.37 (t, J = 7.2 Hz, 3H, H12), 4.38 (q, J = 7.2 Hz, 2H, H11), 6.51 (s, 2H, H22), 7.55 (t, J = 8.8 Hz, 2H, H26, H28), 7.70 (d, J = 8.4 Hz, 2H, 2 × H16), 7.76 (s, 1H, H2), 7.88 (d, J = 8.4 Hz, 2H, 2 × H15), 7.98 (ad, J = 7.2 Hz, 1H, H6), 8.20 (dd, J = 8.4 Hz, J = 5.6 Hz, 2H, H25, H29), 8.81 (d, J = 6.4 Hz, 2H, 2 × H19), 8.97 (as, 1H, H8), 9.11 (d, J = 6.4 Hz, 2H, 2 × H20), 9.92 (d, J = 7.2 Hz, 1H, H5). 13C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 60.2 C11, 65.5 C22, 108.2 C1, 113.8 C6, 116.3 (d, C26, C28, J= 22 Hz), 118.8 C8, 122.9 C3, 124.7 2 × C19, 127.9 C2, 128.7 2 × C16, 129.2 C5, 130.3 (d, C24, J= 3.0 Hz), 130.7 2 × C15, 131.4 (d, C25, C29, J= 10.0 Hz), 132.2 C7, 137.0 C17, 137.3 C14, 137.9 C9, 146.6 2 × C20, 152.3 C18, 162.6 C10, 165.7 (d, C27, J= 253 Hz), 183.6 C13, 189.4 C23. IR (KBr, ν(cm−1): 3024, 3926, 1713, 1624, 1348, 1204. Anal. Calcd. for C31H23BrClFN2O4: C, 59.87; H, 3.73; N, 4.50; Found: C, 59.93; H, 3.70; N, 4.54.
4-(3-(4-Bromobenzoyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-oxo-2-(p-tolyl)ethyl)pyridin-1-ium bromide (6f). Orange powder (0.61 g, 92% yield), mp 283–284 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.37 (t, J = 7.2 Hz, 3H, H12), 2.47 (s, 3H, CH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.51 (s, 2H, H22), 7.50 (d, J = 8.0 Hz, 2H, H26, H28), 7.74 (s, 1H, H2), 7.79 (d, J = 8.4 Hz, 2H, 2 × H16), 7.83 (d, J = 8.4 Hz, 2H, 2 × H15), 7.96 (ad, J = 7.6 Hz, 1H, H6), 8.01 (d, J = 8.0 Hz, 2H, H25, H29), 8.78 (d, J = 7.2 Hz, 2H, 2 × H19), 8.94 (as, 1H, H8), 9.12 (d, J = 6.4 Hz, 2H, 2 × H20), 9.90 (d, J = 7.2 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.4 C12, 21.4 CH3, 60.3 C11, 65.6 C22, 108.3 C1, 113.9 C6, 118.9 C8, 122.9 C3, 124.7 2 × C19, 126.1 C17, 128.1 C2, 128.4 C25, C29, 129.3 C5, 129.7 C26, C28, 130.9 2 × C16, 131.1 C24, 131.8 2 × C15, 132.4 C7, 137.8 C14, 138.0 C9, 145.6 C27, 146.7 2 × C20, 152.4 C18, 162.7 C10, 183.9 C13, 190.2 C23. IR (KBr, ν(cm−1): 3419, 3021, 2930, 1705, 1682, 1622, 1344, 1206. Anal. Calcd. for C32H26Br2N2O4: C, 58.03; H, 3.96; N, 4.23; Found: C, 58.07; H, 3.93; N, 4.25.
4-[3-(4-Bromophenyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(4-methoxyphenyl)-2-oxoethyl)pyridine-1-ium bromide (6g). Orange powder (0.56 g, 82% yield), mp 277–278 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.36 (t, J = 7.2 Hz, 3H, H12), 3.92 (s, 3H, OCH3), 4.37 (q, J = 7.2 Hz, 2H, H11), 6.45 (s, 2H, H22), 7.20 (d, J = 8.8 Hz, 2H, H26, H28), 7.76 (s, 1H, H2), 7.79 (d, J = 8.4 Hz, 2H, 2 × H16), 7.84 (d, J = 8.4 Hz, 2H, 2 × H15), 7.97 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, H6), 8.08 (d, J = 8.8 Hz, 2H, H25, H29), 8.78 (d, J = 7.2 Hz, 2H, 2 × H19), 8.96 (as, 1H, H8), 9.10 (d, J = 6.8 Hz, 2H, 2 × H20), 9.92 (d, J = 7.2 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 55.8 OCH3, 60.2 C11, 65.3 C22, 108.2 C1, 113.8 C6, 114.4 C26, C28, 118.8 C8, 122.8 C3, 124.9 2 × C19, 126.0 C17, 126.2 C24, 127.9 C2, 129.2 C5, 130.7 C25, C29, 130.8 2 × C16, 131.6 2 × C15, 132.2 C7, 137.6 C14, 137.9 C9, 146.6 2 × C20, 152.2 C18, 162.6 C10, 164.2 C27, 183.7 C13, 188.9 C23. IR (KBr, ν(cm−1): 3406, 3018, 2932, 1713, 1680, 1622, 1346, 1205. Anal. Calcd. for C32H26Br2N2O5: C, 56.66; H, 3.86; N, 4.13; Found: C, 56.69; H, 3.85; N, 4.16.
4-(3-(4-Bromobenzoyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(3-methoxyphenyl)-2-oxoethyl)pyridin-1-ium (6h). Yellow powder (0.67 g, 99% yield), mp 256–259 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.37 (t, J = 7.2 Hz, 3H, H12), 3.89 (s, 3H, OCH3), 4.38 (q, J = 7.2 Hz, 2H, H11), 6.55 (s, 2H, H22), 7.40 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H, H27), 7.59 (as, 1H, H29), 7.62 (t, J = 8.0 Hz, 1H, H26), 7.70–7.74 (m, 2H, H2, H25), 7.79 (d, J = 8.4 Hz, 2H, 2 × H16), 7.83 (d, J = 8.4 Hz, 2H, 2 × H15), 7.97 (dd, J = 7.6 Hz, J = 2.0 Hz, 1H, H6), 8.80 (d, J = 6.8 Hz, 2H, 2 × H19), 8.94 (d, J = 1.2 Hz, 1H, H8), 9.13 (d, J = 6.8 Hz, 2H, 2 × H20), 9.89 (d, J = 7.6 Hz, 1H, H5). 13 C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 55.6 OCH3, 60.2 C11, 65.8 C22, 108.3 C1, 113.0 C29, 113.8 C6, 118.8 C8, 120.5 C27, 120.7 C25, 122.9 C3, 124.7 2 × C19, 126.0 C17, 128.0 C2, 129.2 C5, 130.4 C26, 130.8 2 × C16, 131.7 2 × C15, 132.3 C7, 134.8 C24, 137.7 C14, 137.9 C9, 146.6 2 × C20, 152.3 C18, 159.6 C28, 162.7 C10, 183.8 C13, 190.6 C23. IR (KBr, ν(cm−1): 3025, 2930, 1701, 1642, 1248, 1200, 1171. Anal. Calcd. for C32H26Br2N2O5: C, 56.66; H, 3.86; N, 4.13; Found: C, 56.68; H, 3.83; N, 4.16.
4-(3-(4-Chlorobenzoyl)-1-(ethoxycarbonyl)indolizine-7-yl)-1-(2-(2,4-hydroxyphenyl)-2-oxoethyl)pyridin-1-ium (6m). Yellow powder (0.35 g, 56% yield), mp 265–267 °C. 1H-NMR (400 MHz, DMSO-d6): δ 1.37 (t, J = 7.2 Hz, 3H, H12), 4.37 (q, J = 7.2 H, 2H, H11z), 6.43 (s, 2H, H22), 7.02 (d, J = 8.4 Hz, 1H, H28), 7.49 (s, 1H, H25), 7.52 (d, J = 8.4 Hz, 1H, H29), 7.69 (d, J = 8.0 Hz, 2H, 2 × H16), 7.73 (s, 1H, H2), 7.87 (d, J = 8.0 Hz, 2H, 2 × H15), 7.96 (d, J = 7.2 Hz, 1H, H6), 8.76 (d, J = 6.0 Hz, 2H, 2 × H19), 8.93 (s, 1H, H8), 9.12 (d, J = 6.0 Hz, 2H, 2 × H20), 9.89 (d, J = 7.2 Hz, 1H, H5), 9.70 (s, 1H, OH), 10.41 (s, 1H, OH). 13C-NMR (125 MHz, DMSO-d6): δ 14.3 C12, 60.2 C11, 65.2 C22, 108.2 C1, 115.0 C25, 115.5 C28, 113.8 C6, 118.8 C8, 121.8 C29, 122.9 C3, 124.6 2 × C19, 125.1 C24, 128.0 C2, 128.8 2 × C16, 129.2 C5, 130.7 2 × C15, 132.3 C7, 137.0 C17, 137.4 C14, 137.9 C9, 146.6 2 × C20, 145.7 C26, 152.1 C18, 152.3 C27, 162.7 C10, 183.7 C13, 189.6 C23. IR (KBr, ν(cm−1): 3420, 3030, 2974, 1693, 1609, 1526, 1204, 1084. Anal. Calcd. for C31H24Cl2N2O6: C, 62.95; H, 4.09; N, 4.74; Found: C, 62.98; H, 4.03; N, 4.76.
Microbiology
Compounds were evaluated for antimycobacterial activity against M. Tuberculosis, as a part of the TAACFTB screening program under direction of the US National Institute of Health, the NIAID division. Antimycobacterial activities of the compounds were performed by Center of Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) at Southern Research Institute. All protocols concerning the antimycobacterial evaluation of tested compounds can be found in the Supplementary Appendix.
Results and discussion
Design and synthesis
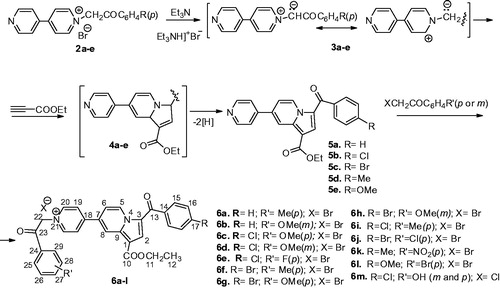
Our strategy included the synthesis of compounds with the same 4-(indolizine-7-yl)-pyridin-1-ium scaffold as in compound 1, but with various substituents on both phenyl rings. Having in mind the observation that a (p)substituted-benzoyl moiety is usefully pharmacophoric unit for the antimycobacterial activityCitation10,Citation12,Citation16, but as well the structure of model compound 1, we considered the synthesis of new derivatives having as para substituents at benzoyl rest from position 3 of indolizine: H, Cl, Br, Me and OMe. On the second phenyl ring, we chose as substituents in para: Me, OMe, F, Cl, Br and NO2 groups. In order to allow structure–activity relationship (SAR) comparisons with (p)substituted-benzoyl salts, we synthesized as well compounds having the OMe group as substituent in meta, and one compound having two OH groups in meta and para positions.
The synthesis of the new 4,4′-bipyridine derivatives was in line with the strategy reported previously by usCitation8,Citation15 and is presented in Scheme 1. Thus, 4,4′-bipyridine mono salts 2a–e (obtained by 4,4′-bipyridine alkylationCitation17) were used for the in situ generation of ylides 3a–e which reacted with ethyl propiolate in [3 + 2] cycloaddition, leading to the intermediate compound 4a–e, and finally to the completely aromatized monoindolizines 5a–e. Another alkylation of indolizines 5a–e using ω-bromoacetophenones led to the compounds 6a–m (compounds 6a–h and 6m are new entities, while compounds 6i–l were previously synthetized in our groupCitation15 (Scheme 1).
Alkylation towards 2a–e is high yielding while cycloadditions led to indolizine 5a–e in ∼50% yield. All compounds were fully characterised using elemental and spectral (NMR and IR) analysis.
Activity against mycobacterium tuberculosis
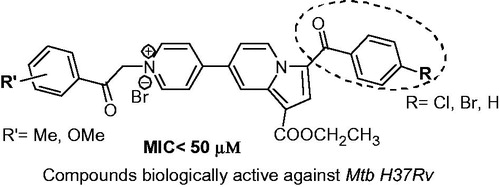
The antimicrobial activity of compounds 6 against Mycobacterium tuberculosis H37Rv grown under aerobic conditions was evaluated as part of the TAACF TB screening program under direction of the US National Institute of Health, the NIAID division. The standard primary in vitro screen was assessed by determining the minimum inhibitory concentration at which growth was completely inhibited (MIC), and the concentrations that resulted in 50% and 90% inhibition of growth (IC50 and IC90 respectively)Citation18–21. As can be seen in , eight compounds showed activity against Mtb H37Rv, five of them with a MIC <15 µM. Compound 6i showed the best value of MIC, IC50 and IC90 from all tested compounds (), being superior to the model compound 1 ().
Figure 1. The structure of the reported compound 1 having anti-TB activityCitation8.
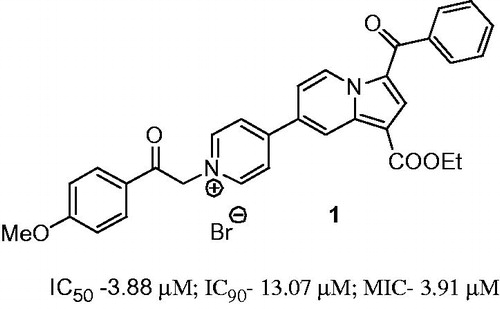
Table 1. Results of antimycobacterial activity of compounds 6 against M. tuberculosis H37Rv grown under aerobic conditions.
Interestingly, the active compounds are only the ones having para substituent R= –Cl, –Br and –H and R’ = –Me(para) or –OMe(para or meta). Replacing para R group with –Me or –OMe and/or R’ group with –F(p), –Cl(p), –Br(p), –NO2(p) or –OH (m and p) led to a dropping of antimycobacterial activity (MIC >100 µM) (). We thus hypothesised that the activity of these compounds bearing a p-halogen-benzoyl or a benzoyl moiety at position 3 of indolizine and a p-methylbenzoyl or methoxy(p or m)benzoyl moiety is somehow connected with a specifically interactions with putative binding sites.
Compounds 6a, 6c, 6d, 6h and 6i that showed promising anti-TB activity in the primary assay, were subjected to the advanced antimycobacterial susceptibility profiling including MIC, IC50 and IC90 (repeated at lower starting concentrations), MIC under low oxygen, minimal bactericidal concentration (MBC), testing on drug-resistant Mtb, intracellular activity and cytotoxicity.
MIC, IC50 and IC90 determinations were repeated using similar assays for compounds 6a, 6c, 6d, 6h and 6i and the obtained values () were comparable with the previous ones (). The bactericidal activity of compounds was assessed against Mtb H37Rv grown in aerobic conditions. Viable cell counts are measured over 3 weeks of exposure to determine the rate of kill. MBC was defined as the minimum concentration required to achieve a 2-log kill in 21 d. For compounds with >1-log kill, an assessment of time- and/or concentration dependence was determined from the kill kinetics (DMSO was used as a positive control for growth). For compounds 6a, 6c, 6d and 6i, the effect of concentration predominates over that of time; therefore, these compounds display concentration-dependent effects that are significantly associated with an optimal free drug maximum concentration to MIC ratioCitation22. For compound 6h the effect of time is greater, displaying a time-dependent effect, and bacterial outcome is associated with free drug concentrations remaining above the MIC for a defined portion of the dosing intervalCitation22. The MIC value used in this experiment was taken from the first MIC assay presented herein. Encouraging, all five tested compounds are bactericidal against replicating cultures with MBCs equal or smaller then MICs.
Table 2. Revaluation of antimycobacterial activity of compounds 6a, 6c, 6d, 6h and 6i against M. tuberculosis H37Rv grown under aerobic conditions.
Traditional screening of drugs against Mtb only addressed or targets the organisms in an active replicating state. It is now widely accepted that Mtb can reside in a state of non-replicating persistence which has not been adequately assessed in the development of new antimicrobials. Therefore, we determined the antimycobacterial activity (MIC, IC50 and IC90) of the compounds 6 against Mtb H37Rv grown under hypoxic conditions using the low oxygen recovery assay (LORA)Citation23–25. Bacteria are first adapted to low oxygen conditions and then exposed to compounds under hypoxia for 10 d followed by incubation under aerobic conditions (outgrowth) for 28 h. Parallel, oxygen-deprived bacteria were also inoculated into compound assay plates and incubated under aerobic conditions for 5 d. The growth in both assays was measured using luminescence (). Rifampicin was included in each plate and metronidazole was included in each run as positive controls for aerobic and anaerobic killing of Mtb, respectivelyCitation23–25.
Table 3. The bactericidal activity (MBC) of compounds 6a, 6c, 6d, 6h and 6i.
As can be seen in , all tested compounds showed a better antimycobacterial activity in anaerobic conditions than the control Metronidazole. Interestingly, for compounds 6a, 6c, 6d and 6i, MIC values in anaerobic conditions were smaller than the values obtained in aerobic conditions. Usually, the antimycobacterial agents targeting the cell wall are inactive in anaerobic conditionsCitation26; therefore, we presume that tested compounds 6 hit other cellular targets of Mtb.
Table 4. Results of antimycobacterial activity of compounds 6a, 6c, 6d, 6h and 6i against M. tuberculosis H37Rv under low oxygen.
A good antimycobacterial activity of compounds 6a, 6c, 6d and 6i is maintained against five resistant isolates of Mtb strains under aerobic conditionsCitation18–21, especially for compounds 6c and 6i (see MIC, IC50 and IC90 values in ). Strains tested were two isoniazid resistant strains (INH-R1 and INH-R2), two rifampicin resistant strains (RIF-R1 and RIF-R2) and a fluoroquinolone resistant strain (FQ-R1).
Table 5. MIC, IC50 and IC90 of compounds 6 against M. tuberculosis resistant at different treatments and non-tuberculous mycobacteria.
The antimycobacterial activity against nontuberculous mycobacteria (NTM) Mycobacterium avium and Mycobacterium abscessus was as well evaluated under aerobic conditionsCitation18,Citation21,Citation27. As can be seen in , only compounds 6c and 6i showed activity against M. abscessus, while compounds 6a, 6c and 6i showed similar moderate activity (MIC = 50 µM) against M. avium.
The cytotoxixity of compounds towards eukaryotic cells was determined using the THP-1 human monocytic cell line, by calculating the concentration of compound causing 50% loss in viability (IC50)Citation27 (). The cytotoxicity of tested compounds proved to be moderate to high, all compounds having a selectivity index (SI) < 1 (SI = IC50/MIC). These findings were somewhat disappointing, since structural elements of these compounds are part of different used drugs.
Table 6. Results of cytotoxicity evaluation.
Since the overall efficacy of any anti TB drug will be improved by its ability to traffic into the macrophage phagosome containing replicating bacteria, we evaluated the intracellular activity of compoundsCitation24. This was measured by using THP-1 cell line infected with Mtb. Infected cells were exposed to compounds for 72 h and viable bacterial counts were measured using luminescence as a measure of intracellular growth. The IC50 and IC90 were defined as the compounds concentrations that produced 50% and 90% inhibition of bacterial growth, respectively.
All tested compounds exhibited a good intracellular activity (IC50 = 7–13 µM), even if the results are inferior to the control Isoniazid ().
Taking into considerations the promising anti-TB activity of compound 6i, a complete absorption, distribution, metabolism, excretion and toxicity (ADMET) study has been performed for it.
First, plasma protein binding (PPB) for compound 6i was determined by equilibrium dialysis using a semi-permeable membrane which separates two compartments containing protein (human plasma) and bufferCitation28,Citation29. The experiments used propranolol as internal binding standard and warfarin as a high-binding control. Molecules can penetrate freely, but proteins cannot pass through the membrane. Compound 6i was strongly bound to the plasma proteins ().
Table 7. Results of plasma protein binding assay for compound 6i.
Usually high PPB is associated with a lower clearance rate resulting in a greater half-time in vivo compared with low protein binding compounds. Despite the fact that drugs with low protein binding are believed to be more efficacious because of higher free drug concentration, there are studies concluding that the binding of a drug to plasma proteins has little effect on the in vivo efficacy of that drugCitation29,Citation30.
The Caco-2 cell layer permeability assay is widely used as a more predictive in vitro model of absorption through the intestinal epitheliumCitation31. Therefore, the permeability (measured in both directions) of compound 6i was assessed using a Caco-2-cell monolayer. For A–B permeability, compound 6i was added to the apical side of the Caco-2 monolayer and the transport to the basal side monitored. For B–A permeability, test compound was added to the basal side of the Caco-2 monolayer and the transport of the compound to the apical side monitored. The amount of compound present in each compartment was quantified by LC-MS/MS. Each experiment included the control compounds atenolol (low permeability, paracellular transport), propranolol (high permeability, passive transcellular transport) and talinolol (P-gp efflux control)Citation32–36 ().
Table 8. Permeability evaluation of compound 6i.
Compound 6i can be considered poorly permeable with a A→B Papp < 2, and shows a low active efflux (Re = 1.7). This led us to suppose that the mechanism of absorption of compound 6i is almost a paracellular one with basically no involvement of transporter proteins. However, recent studies proved no significant correlation between antimycobacterial activity and Caco-2 permeability, indicating that permeability is not a predictor of activity inside of mycobacteriumCitation31.
Drug metabolism via the cytochrome P450 system has emerged as an important determinant in the occurrence of several drug-drug interactions that can result in drug toxicities, reduced pharmacological effect, and adverse drug reactionsCitation37. Therefore, compound 6i was tested for inhibition of six cytochrome P450 enzyme isoforms: CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. For each assay, human liver microsomes are incubated with a probe substrate for each CYP isoform in the presence of compound. The formation of metabolites for each isoform was quantified by LC-MS/MS as a measure of enzyme activityCitation38–40. For compound 6i, enzyme activity was calculated and IC50 generated ().
Table 9. Cytochrome P450 inhibition results.
Compound 6i showed a high inhibition of CYP3A4-catalyzed testosterone and a moderate inhibition of midazolam 1’-hydroxylation, this profile suggesting a high potential for drug-drug interactions. No other CYPs were directly inhibited by 6i, IC50s of 6i on these CYPs being >5 µM.
Compound 6i was tested for microsomal stability using pooled human liver S9 microsomes. Microsomes are incubated with the test compound at 37 °C in the presence of the co-factor NADPH; the reaction was terminated, the supernatant recovered and test compound quantified by LC-MS/MS. The stability of compound is expressed as a function of timeCitation41,Citation42 ().
Table 10. In vitro microsomal stability assay.
Compound 6i proved to be a very highly cleared compound. Compounds with this profile are generally considered that they are likely to be rapidly cleared in vivo resulting in a short duration of action and, it should be cleared strongly in vivo by CYP metabolismCitation43.
The cytotoxicity of compound 6i was also tested towards eukaryotic cell using the human liver cells (HepG2), and Staurosporine as control (IC50= 0.0086 µM). The IC50 was determined as the concentration of compound causing a 50% loss of viabilityCitation44–47. Compound 6i showed an IC50 value of 7.0 µM, similar with its MIC value (8.0 µM), which maintains its cytotoxicity profile.
Conclusion
In summary, we have employed the 4-(indolizine-7-yl)-pyridin-1-ium scaffold as core for the synthesis of 13 compounds in order to test their antimycobacterial activity. The reaction pathway is efficient and straight applicable, involving two N-alkylations of the 4,4,-bipyridine and, a Huisgen [3 + 2] dipolar cycloaddition of resulting ylides to ethyl propiolate. The primary antimycobacterial screening reveals that eight of the 13 tested compounds had a good activity against Mycobacterium tuberculosis H37Rv under aerobic conditions. SAR correlation reveals a certain influence of the R substituent from the para position of benzoyl moiety at position 3 of indolizine, the most active being compounds with R= –H, –Cl, –Br. The most active five compounds (namely 6a, 6c, 6d, 6h, 6i) passed the second stage of anti TB testing, these including MIC, IC50 and IC90 (repeated at lower starting concentrations), MIC under low oxygen, MBC, testing on drug-resistant Mtb strains and nontuberculous mycobacteria, intracellular activity and cytotoxicity. These assay proved that our compounds are potent against both replicating and non-replicating Mtb, have a bactericidal mechanism of action, are active against drug-resistant Mtb strains, present a moderate to good activity against nontuberculous mycobacteria, a good intracellular activity, and a moderate to high cytotoxicity. The ADMET studies of compound 6i show poor results, but motivating in the same time for further studies within the area of monoindolizine mono-salt.
IENZ_1375483_Supplementary_Material.pdf
Download PDF (462.4 KB)Acknowledgements
Part of this work (biological tests) was supported by National Institutes of Health and the National Institute of Allergy and Infectious Diseases, Contract no. HHSN27220110009I. Special thanks for Jim P. Boyce, senior officer to Division of Microbiology and Infectious Diseases, NIAID. The authors also thank the POSCCE-O 2.2.1, SMIS-CSNR 13984-901, Project no. 257/28.09.2010, CERNESIM, for the NMR experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science 2010;328:856–61.
- WHO. Global Tuberculosis Report. Geneva, Switzerland: WHO; 2016. Available from: http://www.who.int/tb/publications/global_report/en/ [last accessed 10 Oct 2017].
- Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012;397:1902–13.
- Ma Z, Lienhardt C, Mclleron H, et al. Global tuberculosis drug development pipeline: the need and the reality. Lancet 2010;5:1–10.
- Crabb C. Global alliance at full steam for new TB drugs. Bull World Health Organ 2002;80:517.
- Dulla B, Wan B, Franzblau SG, et al. Construction and functionalization of fused pyridine ring leading to novel compounds as potential antitubercular agents. Bioorg Med Chem Lett 2012;22:4629–35.
- Moraski GC, Markley LD, Chang M, et al. Generation and exploration of new classes of antitubercular agents: the optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. Bioorg Med Chem 2012;20:2214–20.
- Danac R, Mangalagiu II. Antimycobacterial activity of nitrogen heterocycles derivatives: bipyridine derivatives. Part III. Eur J Med Chem 2014;74:664–70.
- Mantu D, Luca C, Moldoveanu C, et al. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur J Med Chem 2010;45:5164–8.
- Al Matarneh CM, Ciobanu CI, Mangalagiu II, et al. Design, synthesis and antimycobacterial evaluation of some new azaheterocycles with 4,7-phenanthroline skeleton. Part VI. J Serb Chem Soc 2016;81:133–40.
- Danac R, Al Matarneh CM, Shova S, et al. New indolizinees with phenanthroline skeleton: synthesis, structure, antimycobacterial and anticancer evaluation. Bioorg Med Chem 2015;23:2318–27.
- Danac R, Daniloaia T, Antoci V, et al. Design, synthesis and antimycobacterial activity of some new azaheterocycles: phenanthroline with p-halo-benzoyl Skeleton. Part V. Lett Drug Des Discov 2015;12:14–17.
- Butnariu RM, Mangalagiu II. New pyridazine derivatives: synthesis, chemistry and biological activity. Bioorg Med Chem 2009;17:2823–9.
- Caprosu M, Butnariu R, Mangalagiu II. Synthesis and antimicrobial activity of some new pyridazine derivatives. Heterocycles 2005;65:1871–9.
- Rotaru A, Druta I, Avram E, et al. Synthesis and properties of fluorescent 1,3-substituted mono and biindolizines. Arkivoc 2009;13:287–99.
- Dholariya HR, Patel KS, Patel JC, et al. Dicoumarol complexes of Cu (II) based on 1, 10-phenanthroline: synthesis, X-ray diffraction studies, thermal behaviour and biological evaluation. Spectrochim Acta A 2013;108:319–28.
- Rotaru A, Danac R, Druta I, et al. The synthesis and the biological activity of diquaternary salts derivatives of 4,4`-bipyridyl. Rev Chim (Bucharest) 2005;56:179–83.
- Ollinger J, Bailey MA, Moraski GC, et al. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS One 2013;8:e60531.
- Zelmer A, Carroll P, Andreu N, et al. A new in vivo model to test anti-tuberculosis drugs using fluorescence imaging. J Antimicrob Chemother 2012;67:1948–60.
- Carroll P, Schreuder LJ, Muwanguzi-Karugaba J, et al. Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters. PLoS One 2010;5:e9823.
- Lambert RJ, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J Appl Microbiol 2000;88:788–90.
- Kuti JL. Optimizing Antimicrobial Pharmacodynamics: a guide for stewardship program. Rev Med Clin Condes 2016;27:615–24.
- Cho SH, Warit S, Wan B, et al. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 2007;51:1380–5.
- Andreu N, Zelmer A, Fletcher T, et al. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS One 2010;5:e10777.
- Wayne LG. In vitro model of hypoxically induced nonreplicating persistence of Mycobacterium tuberculosis. In: Parish T, Stoker NG, eds. Mycobacterium tuberculosis protocols. Totowa, NJ: Humana Press; 2001:247–270.
- Favrot L, Ronning DR. Targeting the mycobacterial envelope for tuberculosis drug development. Expert Rev Anti Infect Ther 2012;10:1023–36.
- Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue Assay. J Clin Microbiol 1998;36:362–6.
- Banker MJ, Clark TH, Williams JA. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J Pharm Sci 2003;92:967–74.
- Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov 2010;9:929–39.
- Ramesh R, Shingare RD, Kumar V, et al. Repurposing of a drug scaffold: Identification of novel sila analogues of rimonabant as potent antitubercular agents. Eur J Med Chem 2016;122:723–30.
- Lakshminarayana SB, Huat TB, Ho PC, et al. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. J Antimicrob Chemother 2015;70:857–67.
- Stewart BH, Chan OH, Lu RH, et al. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm Res 1995;12:693–9.
- Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 2001;46:27–43.
- Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res 1997;14:763–6.
- Endres CJ, Hsiao P, Chung FS, et al. The role of transporters in drug interactions. Eur J Pharm Sci 2006;27:501–17.
- Balimane PV, Han YH, Chong S. Current industrial practices of assessing permeability and P-glycoprotein interaction. APS J 2006;8:E1–E13.
- Ogu CC, Maxa JL. Drug interactions due to cytochrome P450. *Proc (Bayl Univ Med Cent) 2000;13:421–3.
- Kim MJ, Kim H, Cha IJ, et al. High-throughput screening of inhibitory potential of nine cytochrome P450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2005;19:2651–8.
- Walsky RL, Obach RS. Validated assays for human cytochrome P450 activities. Drug Metab Dispos 2004;32:647–60.
- Fowler S, Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug–drug interactions. AAPS J 2008;10:410–24.
- Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 1994;47:1469–79.
- Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and non-specific binding to microsomes. Drug Metab Dispos 1999;27:1350–9.
- Di L, Kerns EH, Ma XJ, et al. Applications of high throughput microsomal stability assay in drug discovery. Comb Chem High Throughput Screen 2008;11:469–76.
- Crouch SP, Kozlowski R, Slater KJ, et al. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods 1993;160:81–8.
- Lundin A, Hasenson M, Persson J, et al. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Meth Enzymol 1986;133:27–42.
- Maehara Y, Anai H, Tamada R, et al. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol 1987;23:273–6.
- Slater K. Cytotoxicity tests for high-throughput drug discovery. Curr Opin Biotechnol 2001;12:70–4.