Abstract
Carbonic anhydrase (CA, EC 4.2.1.1) plays crucial physiological roles in many different organisms, such as in pH regulation, ion transport, and metabolic processes. CA was isolated from the European bee Apis mellifera (AmCA) spermatheca and inhibitory effects of pesticides belonging to various classes, such as carbamates, thiophosphates, and pyrethroids, were investigated herein. The inhibitory effects of methomyl, oxamyl, deltamethrin, cypermethrin, dichlorodiphenyltrichloroethane (DDT) and diazinon on AmCA were analysed. These pesticides showed effective in vitro inhibition of the enzyme, at sub-micromolar levels. The IC50 values for these pesticides ranged between of 0.0023 and 0.0385 μM. The CA inhibition mechanism with these compounds is unknown at the moment, but most of them contain ester functionalities which may be hydrolysed by the enzyme with the formation of intermediates that can either react with amino acid residues or bid to the zinc ion from the active site.
1. Introduction
Pesticides are chemical compounds that are used against various pests as a biological control agent. Some pesticides are persistent organic contaminants in the soil and environment. They also constitute one of the most significant causes of pollution worldwideCitation1. The rapid growth of agriculture and animal processing has caused bees to be exposed to such contaminants with which they had never previously come into contact. Growing food demand has forced farmers to use more mineral fertilisers and pesticides for producing higher yieldsCitation2. In recent decades the increasing concern about the effect of pesticides on pollinators has been expressed in the scientific literatureCitation3. Fort his reason, some new data were collected from laboratory and semi-field studies on the toxic effects of pesticides on bees, especially bumble beesCitation3,Citation4. A variety of articles have highlighted the significance of bees as natural pollinators not only for our crops but also for wildflowers and woodland plants, in temperate and tropical habitatsCitation5–7. For example, it has been reported that about 60 crop plant species may not bear growing fruit without bees as impollinatorsCitation8; with devastating economic implications.
Carbonic anhydrases (Cas, EC 4.2.1.1) are found in almost all living organisms and control pH and CO2/bicarbonate levelsCitation9. Many different CA isoenzymes have been identified in higher vertebrates, although these enzymes are less investigated in other species, such as the arthropods, including insectsCitation10–12. The physiological role of CA isozymes is to promote CO2 to HCO3− interconversion, thus, playing vital functions in various biochemical/physiological processes, including physiological pH regulation, gas balancing, calcification, photosynthesis, metabolism, etcCitation9–12. Additionally, in vertebrates, CAs play a significant role in the eye, kidneys, central nervous system (CNS), inner ear, and many other organs, in terms of ion transfer, pH regulation, and metabolismCitation13–15.
Pesticides and fungicides interfering with rainwater, irrigation water, or groundwater, plants, can inhibit particular enzymesCitation13–15. In our group’s previous research, we investigated the effects of several widely used pesticides, such as tebuconazole, propoxur, carbaryl, carbofuran, simazine, and atrazine on the recently discovered bee CA, termed AmCACitation15. However, little is known about the effects of other chemical agents on this enzyme. Therefore, in this study, we purified honey bee spermatheca AmCA enzyme and analysed its interactions with pesticides and fungicides belonging to other classes.
2. Materials and methods
2.1. Chemicals
All chemicals for the affinity system were provided by Sigma-Aldrich (St. Louis, MO). Other reagents were obtained from Merck (Darmstadt, Germany).
2.2. Homogenate
Bee spermatheca samples were washed three times with 50 mM Tris/Sulphate (pH 7.8). Spermatecha samples taken from 200 bees were combined and homogenised with liquid nitrogen, then placed in the same buffer and centrifuged at 4 °C, 15,000 g for 30 min. The precipitate portion was discarded and the supernatant portion was separated and used in subsequent studies.
2.3. Purification of the enzyme
The enzyme was purified using Sepharose-4B-sulfanilamide affinity gel prepared by our groupCitation15. Aniline was used as a spacer arm in the chromatography column. The column was then equilibrated with 25 mM Tris-HCl/0.1M Na2SO4 (pH 8.7) and the gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). Finally, elution was performed with 1 M NaCl/25 mM Na2HPO4 (pH 6.3). The temperature was kept at 4 °C during all experiments.
2.4. In vitro inhibition experiments for AmCA enzyme
The effects of methomyl, oxamyl, deltamethrin, dichlorodiphenyltrichloroethane (DTT), cypermethrin and diazinon on AmCA activities were assayed colorimetrically using the CO2 hydrase assayCitation16, in triplicate at each concentration of inhibitor (ranging from 1 nM to 10 mM). Control enzyme activity was taken as 100%. An activity % versus inhibitor graph was drawn for all pesticides (Microsoft Office 2000, Excel). Pesticide concentrations that caused 50% inhibition (IC50) of the enzyme activity were thus obtained graphically using a regression software. The enzyme concentration in the assay system was 8.9 nM.
3. Results and discussion
The collapse of honeybee (Apis mellifera) colonies poses an important problem in many developed countriesCitation17–20. In fact, bee colonies are economically important for honey and wax products. The collapse of hive clones is generally related to two phenomena: (i) parasites, such as virusCitation21, nosema infectionsCitation22, mitesCitation23, and hive insectsCitation24, which attack the colony; and (ii) pesticides use, which negatively impacts the insects due to their toxicityCitation25. Furthermore, such low pesticide levels can also make bees an easier target to biological infectionsCitation20–28.
The majority of the problems in beehives faced by beekeepers are due to biological factorsCitation27. However, these factors are improbable to be the most important reason for the recent decrease of bumblebees in North America and Europe or the disappearing of several wild beespeciesCitation22,Citation28. Agrochemicals, including pesticides, are probably the most significant factors that provoke honeybee and wild bee colonies extinction. Much research has been conducted ultimately in order to understand this problem in North AmericaCitation29, FranceCitation30, SpainCitation31 and IndiaCitation32. Such research focussed on determining the amount and prevalence of pesticide residues in honey, pollen, wax, and other different beehive matrices (e.g. combs)Citation29–31. A dataset was thus established for explaining the effects of pesticide residue both on honeybees and, potentially wild beesCitation33,Citation34.
Recently, we isolated and characterised the α-CA enzyme from Apis mellifera spermatheca, AmCACitation15. In that study, we were able to purify the enzyme in a single step using Sepharose 4B tyrosine-sulfanilamide affinity chromatographyCitation15. Furthermore, pesticides such as tebuconazole, carbaryl, carbonfuran, atrazine, simazin, and propoxur were tested on the amCA enzyme and IC50 values were determined as 0.0030, 0.0031, 0.0087, 0.0165, 0.0273, and 0.0321 μM, respectively ()Citation15. The efficiency order of pesticides AmCA inhibition was: tebuconazole > carbaryl > carbofuran > atrazine > simazine > propoxur.
Table 1. AmCA inhibition data from our previous studyCitation15.
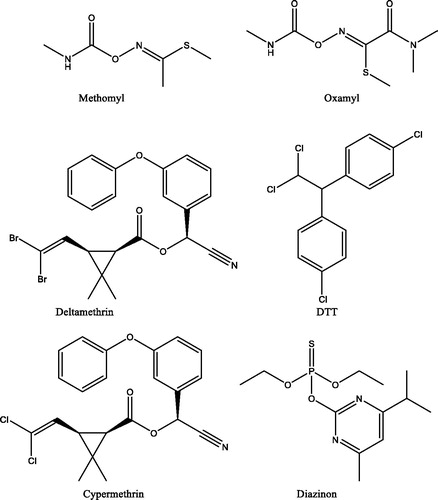
Here we report the inhibitory effects of 6 pesticides belonging to various classes (Fıgure Citation1) on AmCA. Indeed, carbamates, thiophosphates, and pyrethroids were considered as potential CA inhibitors in the present study ().
Besides the enzyme purification already reported in the previous workCitation15, the inhibitory effects of six different pesticides on honeybee spermatheca CA and their IC50 parameters were investigated here. The activity of AmCA was inhibited by pesticides shown in at low micromolar concentrations. Indeed, the IC50 values were determined to be: 0.0023 ± 0.0001, 0.0025 ± 0.0001, 0.0028 ± 0.0001, 0.0034 ± 0.0001, 0.0078 ± 0.0003, and 0.0385 ± 0.0012 μM for methomyl, oxamyl, deltamethrin, dichlorodiphenyltrichloroethane (DTT), cypermethrin, and diazinon, respectively ().
Table 2. AmCA inhibition data for new pesticides shown in .
It should be mentioned that a detailed study on pesticide residues in bees was conducted by Sanchez-Bayo and GokaCitation20. In this study, topical LD50 and oral LD50 values of pesticides against honey bees and bumblebees were reportedCitation20. The topical lethal dose (LD50) rates found in this studyCitation20 were as follows: deltamethrin 0.02 μg/bee, cypermethrin 0.03 μg/bee, methomyl 0.49 μg/bee, and diazinon 0.38 μg/bee. In the same study, the oral dose rates were as follows: cypermethrin 0.06 μg/bee, methomyl 3.38 μg/bee, diazinon 0.21 μg/bee, and DDT 5.08 μg/beeCitation20. Compared to this literature resultsCitation20, the results obtained in the present study show a low level of IC50 for the inhibition of AmCA with pesticides presented in and widely used in agriculture (). Thus, our results may prove that pesticides as those presented in may induce a strong inhibition on honey bee spermatheca CA enzyme. It is not known at the moment whether this inhibition may lead to physiological consequences but studies are ongoing in the field in our laboratories.
There has been a great interest in recent studies focussed on the effects of several classes of chemicals on CA enzymesCitation35–37. CA is a classical metalloenzyme, whose isozymes have significant roles in many tissues, that has been characterized and purified from several organisms, including animalsCitation35–37. A huge number of pollutants, such as metals, acids, bases, and other toxic compoundsCitation38 are being mixed in water sources and also in the atmosphere, which increasingly damages our environment. The potent AmCA inhibitory effects of compounds shown in may represent a potential explanation of why bees (domestic and wild ones) are under pressure worldwide with an increasing level of extinction of many diverse such insect species.
This also brings us to the possible mechanism of action of these pesticides investigated here. The carbamates, thiophosphates, and pyrethroids investigated here () possess ester bonds which can be hydrolysed by the esterase activity of the α-CAs. In fact, it has been thoroughly documented that these enzymes are esterases/thioesterases/selenoestearses with carboxylic, phosphoric, thiocarboxylic, and even selenol estersCitation39–42. Only DDT does not have this functionality, but this compound was reported by Bitman et al. to act as a CAI in the 70sCitation43.
The two carbamates from , methomyl, and oxamyl can be substrates of CAs which may hydrolyse their ester/thioester bonds with the formation of small molecules which can bind to the metal centre (acetate, methyl-thiol, or Me2N–COCOOH in the case of the second carbamate). The pyrethroids may also be hydrolysed at their ester functionality, with the generation of carboxylic acids and alcohols which were shown to act as CAIsCitation12,Citation13,Citation36. Diazinon has thiophosphate functionalities which were shown to be hydrolysed by the esterase activity of CAs in previous work from this laboratory, leading to suicide inhibitors of the enzymeCitation39. However, these hypotheses should be verified by X-ray crystallography, one of the most powerful techniques useful to assess CA inhibition mechanisms, especially the innovative ones. This technique also has its weak points, especially when used in an inattentive manner. The best example is the report by Liljas’ group that cyanide and cyanate do not coordinate the metal ion from CA active siteCitation44. Subsequent work from other laboratories showed those data to be false, as both cyanate and cyanide were observed coordinated to the metal ion, as most other anion inhibitors investigated to dateCitation45,Citation46. Furthermore, cyanate was also shown to be a suicide substrate that can be hydrolysed by the CA activity with the formation of carbamateCitation45. However, bitter and dubious comments from the above-mentioned crystallography group continued even 30 years laterCitation47.
4. Conclusions
AmCA was purified from Apis mellifera spermatheca by affinity chromatography. Pesticides such as methomyl, oxamyl, deltamethrin, and DDT showed inhibition effects, comparable to those of cypermethrin and diazinon. The IC50 values were determined as 0.0023 ± 0.0001, 0.0025 ± 0.0001, 0.0028 ± 0.0001, 0.0034 ± 0.0001, 0.0078 ± 0.0003 and 0.0385 ± 0.0012 μM, respectively (). Our results showed that pesticides inhibit AmCA activity with the following order: methomyl > oxamyl > deltamethrin > DDT > cypermethrin > diazinon. Our findings indicate these pesticides to act as potent inhibitors of AmCA, which might cause undesirable biological effects in bees, by disrupting their acid–base regulation as well as salt transport. Further studies are warranted in order to understand whether these inhibition data are relevant for the significant diminution of domestic and wild bee species worldwide. Several studies on bees as well as other organisms showed that sperm life depends on different parameters, among which the pH. It was observed that sperm loses its vitality if the pH level increases. This supports the relevance of the present findings. However, the data presented here need a careful in vivo (or in the hive) validation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ekinci D, Senturk M, Interactions of fungicides and pesticides with specific enzymes. In: Carisse O. ed. Fungicides. London, UK: In-Tech Open Publisher, 2010: 38–404.
- Sattarı SZ, Bouwman AF, Martınez Rodríguez R, et al. Negative global phosphorus budgets challenge sustainable intensification of grasslands. Nat Commun 2016;7:10696.
- Osborne JL. Ecology: bumblebees and pesticides. Nature 2012;491:43–5.
- Marletto F, Patetta A, Manino A. Laboratory assessment of pesticide toxicity to bumble bees. Bull Insectology 2003;56:155–8.
- Klein A-M, Vaissière BE, Cane JH, et al. Importance of pollinators in changing landscapes for world crops. P Roy Soc B-Biol Sci 2007;274:303–13.
- Allen-Wardell G, Bernhardt P, Bitner R, et al. The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol 1998;12:8–17.
- Potts SG, Biesmeijer JC, Kremen C, et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 2010;25:345–53.
- Heard TA. The role of stingless bees in crop pollination. Annu Rev Entomol 1999;44:183–206.
- (a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168. (b) Supuran CT. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin Drug Discov 2020;15:671–86.
- (a) Demirdag R, Comakli V, Senturk M, et al. Characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem 2013;21:1522–5. (b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. (c) Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68.
- (a) Ekinci D, Kurbanoglu NI, Salamci E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzyme Inhib Med Chem 2012;27:845–8. (b) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- (a) Özdemir ZÖ, Şentürk M, Ekinci D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules. J Enzyme Inhib Med Chem 2013;28:316–9. (b) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (c) Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97. (d) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- a)Ceyhun SB, Sentürk M, Yerlikaya E, et al. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74. (b) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25. (c) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12. (d) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21. (e) Supuran CT, Altamimi ASA, Carta F. Carbonic anhydrase inhibition and the management of glaucoma: a literature and patent review 2013-2019. Expert Opin Ther Pat 2019;29:781–92.
- Ceyhun SB, Şentürk M, Ekinci D, et al. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Phys C 2010;152:215–23.
- Soydan E, Guler A, Biyik S, et al. Carbonic anhydrase from Apis mellifera: purification and inhibition by pesticides. J Enzyme Inhib Med Chem 2017;32:47–50.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54.
- Wang D, Hu J, Bobulescu IA, et al. A sperm-specific Na+/H + exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci USA 2007;104:9325–30.
- Pettis JS, Rice N, Joselow K, et al. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS One 2016;11:e0155833.
- Paynter E, Harvey M, Welch M, et al. Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Sci Rep 2017;7:40236.
- Sanchez-Bayo F, Goka K. Pesticide residues and bees – a risk assessment. PLoS One 2014;9:e94482.
- Cox-Foster DL, Conlan S, Holmes EC, et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007;318:283–7.
- Cameron SA, Lozier JD, Strange JP, et al. Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 2011;108:662–7.
- Thompson H, Ball R, Brown M, et al. Varroa destructor resistance to pyrethroid treatments in the United Kingdom. Bull Insect 2003;56:175–81.
- Underwood RM, vanEngelsdorp D. Colony Collapse Disorder: have we seen this before? Bee Culture 2007;135:13–15.
- Maini S, Medrzycki P, Porrini C. The puzzle of honey bee losses: a brief review. Bull Insect 2010;63:153–60.
- Vidau C, Diogon M, Aufauvre J, et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS One 2011;6:e21550.
- Williams GR, Tarpy DR, vanEngelsdorp D, et al. Colony collapse disorder in context. BioEssays 2010;32:845–6.
- Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annual Rev Entomol 2008;53:191–208.
- Stoner KA, Eitzer BD. Using a hazard quotient to evaluate pesticide residues detected in pollen trapped from honey bees (Apis mellifera) in Connecticut. PLoS One 2013;8:e77550.
- Chauzat MP, Carpentier P, Martel AC, et al. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ Entomol 2009;38:514–23.
- Bernal J, Garrido-Bailon E, Nozal MJd, et al. Overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain. J Econ Entomol 2010;103:1964–71.
- Choudhary A, Sharma DC. Dynamics of pesticide residues in nectar and pollen of mustard (Brassica juncea (L.) Czern.) grown in Himachal Pradesh (India). Environ Monit Assess 2008;144:143–50.
- Thompson HM. Assessing the exposure and toxicity of pesticides to bumblebees (Bombus sp.). Apidologie 2001;32:305–21.
- Greig-Smith PW, Thompson HM, Hardy AR, et al. Incidents of poisoning of honeybees (Apis mellifera) by agricultural pesticides in Great Britain 1981–1991. Crop Prot 1994;13:567–81.
- (a) Ekinci D, Sentürk M, Küfrevioğlu Öİ. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat 2011;21:1831–41. (b)Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I – XIV). Bioorg Med Chem Lett 2008;18:1583–7. (c)Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I – XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–8.
- (a) Ekinci D, Ceyhun SB, Şentürk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8. (b) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29. (c) Supuran CT. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95.
- (a) Dizdaroglu Y, Albay C, Arslan T, et al. Design, synthesis and molecular modelling studies of some pyrazole derivatives as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2020;35:289–97. (b) Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010-2018). Expert Opin Ther Pat 2018;28:493–504. (c) Supuran CT. Carbonic anhydrase activators. Future Med Chem 2018;10:561–73.
- Bielmyer GK, Brix KV, Grosell A. Is Cl- protection against silver toxicity due to chemical speciation? Aquat Toxicol 2008;87:81–7.
- (a) Innocenti A, Scozzafava A, Parkkila S, et al. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–71. (b) Innocenti A, Supuran CT. Paraoxon, 4-nitrophenyl phosphate and acetate are substrates of α- but not of β-, γ- and ζ-carbonic anhydrases. Bioorg Med Chem Lett 2010;20:6208–12.
- (a) Lopez M, Vu H, Wang CK, et al. Promiscuity of carbonic anhydrase II. Unexpected ester hydrolysis of carbohydrate-based sulfamate inhibitors. J Am Chem Soc 2011;133:18452–62. (b) Truppo E, Supuran CT, Sandomenico A, et al. Carbonic anhydrase VII is S-glutathionylated without loss of catalytic activity and affinity for sulfonamide inhibitors. Bioorg Med Chem Lett 2012;22:1560–4.
- Tanc M, Carta F, Scozzafava A, Supuran CT. α-Carbonic anhydrases possess thioesterase activity. ACS Med Chem Lett 2015;6:292–5.
- Angeli A, Carta F, Donnini S, et al. Selenolesterase enzyme activity of carbonic anhydrases. Chem Commun 2020;56:4444–7.
- Bitman J, Cecil HC, Fries GF. DDT-induced inhibition of avian shell gland carbonic anhydrase: a mechanism for thin eggshells. Science 1970;168:594–6.
- Lindahl M, Svensson LA, Liljas A. Metal poison inhibition of carbonic anhydrase. Proteins 1993;15:177–82.
- Supuran CT, Conroy CW, Maren TH. Is cyanate a carbonic anhydrase substrate? Proteins 1997;27:272–8.
- West D, Pinard MA, Tu C, et al. Human carbonic anhydrase II-cyanate inhibitor complex: putting the debate to rest. Acta Crystallogr F Struct Biol Commun 2014;70:1324–7.
- Jonsson BH, Liljas A. Perspectives on the classical enzyme carbonic anhydrase and the search for inhibitors. Biophys J 2020;119:1275–80.