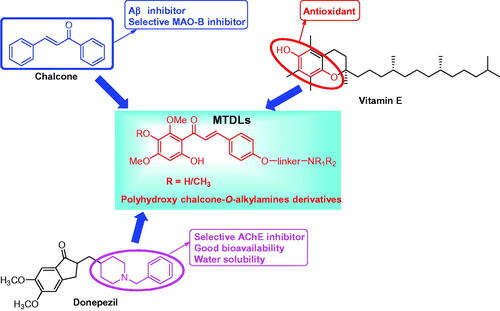
Abstract
A novel series of chalcone-Vitamin E-donepezil hybrids was designed and developed based on multitarget-directed ligands (MTDLs) strategy for treating Alzheimer’s disease (AD). The biological results revealed that compound 17f showed good AChE inhibitory potency (ratAChE IC50 = 0.41 µM; eeAChE IC50 = 1.88 µM). Both the kinetic analysis and docking study revealed that 17f was a mixed type AChE inhibitor. 17f was also a good antioxidant (ORAC = 3.3 eq), selective metal chelator and huMAO-B inhibitor (IC50 = 8.8 µM). Moreover, it showed remarkable inhibition of self- and Cu2+-induced Aβ1–42 aggregation with a 78.0 and 93.5% percentage rate at 25 µM, respectively, and disassembled self-induced and Cu2+-induced aggregation of the accumulated Aβ1–42 fibrils with 72.3 and 84.5% disaggregation rate, respectively. More importantly, 17f exhibited a good neuroprotective effect on H2O2-induced PC12 cell injury and presented good blood-brain barrier permeability in vitro. Thus, 17f was a promising multi-target-directed ligand for treating AD.
1. Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterised by deterioration of memory, language, and other cognitive impairments in elder peopleCitation1. Newly available data exhibits that over 50 million dementia patients and the number will be projected to 152 million in 2050Citation2. The current therapeutic agents approved by FDA, such as the acetylcholinesterase (AChE) inhibitors (rivastigmine, donepezil, and galantamine) and the N-methyl-D-aspartate receptor antagonist (menantine), only present modest symptomatic effects and cannot stop, prevent, or reverse the progression of ADCitation3,Citation4. Thus, the development of disease-modifying drugs is a great unmet medical need for AD patients.
Up to now, the exact pathogenesis of AD remains unclear. The amyloid cascade hypothesis states that Aβ aggregates are the triggering event in the pathogenesis of ADCitation5. The accumulation of soluble Aβ oligomers leads to the damage and death of neurons and further accelerates the spread of tau-related neurofibrillary tangles, neuroinflammation, and neuronal degeneration and deathCitation6,Citation7. So, Aβ serves as a major therapeutic target for treating AD. Recently, the failures of current and previous trials of immunotherapy reveal that targeting of Aβ alone might not be enough to prevent or slow AD progression, as multiple mechanisms are involved in AD pathogenesis and their relative contributions might vary at different stages of the diseaseCitation8. The use of appropriate and specific therapeutic targets at different stages of the disease might be a promising way to cure or prevent AD in the future.
Due to the complexity of AD, the success of a therapeutic approach is likely to depend on the simultaneous modulation of more than one AD-relevant target, which leads to a new paradigm in drug discovery for AD, namely the multi-target-directed ligands (MTDLs). MTDLs can hit two or more AD-relevant complementary targets and produce synergistic effects on the disease network by improving clinical outcomesCitation9–12. In particular, the MTDLs involving AChE inhibitors have drawn great attention because selective AChE inhibitors could improve cognitive impairment and many promising AChE inhibitor-based multifunctional agents are in progressCitation13–15.
In addition, the oxidative stress hypothesis states that the generation of excess reactive oxygen species (ROS) is also a major contributor to the progression of AD. The accumulation of ROS leads to the generation of oxidative damage and further damages protein, lipid, and DNACitation16. Moreover, the metal ion hypothesis states that high levels and dysregulation of Cu2+, Fe2+, and Zn2+ exist in the brain of AD, which accelerates the aggregation of Aβ and neurotoxic oxidative processesCitation17,Citation18. Therefore, antioxidants and biometal chelators offer a promising therapy for the treatment of AD.
Increasing evidences also reveal that high levels of monoamine oxidase-B (MAO-B) are observed in the brain of AD. The excess MAO-B produces hydroxyl radicals, accelerating the former of Aβ plaquesCitation19. Rasagiline, a selective MAO-B inhibitory drug, has been performed a phase 2 trial in people with mild-to-moderate AD, and it reveals trends to better performanceCitation20.
Chalcones are prominent secondary-metabolite precursors of flavonoids and isoflavonoids in plants. Chalcones possess widely biological activities, particularly, radical-scavenging, anti-inflammatory, MAO-B inhibition, and neuroprotective property contribute to the treatment of ADCitation21,Citation22. Many chalcone derivatives have been designed and applied for the development of anti-ADCitation22–25. Vitamin E (α-tocopherol) is a fat-soluble antioxidant that can regulate the production of ROS and reactive nitrogen species (RNS) while displaying weak water solubility, and the hydroxy of Vitamin E plays a key role in the antioxidant activityCitation26. Donepezil is a commercial AChE inhibitor for the treatment of mild-to-moderate AD, and it has been widely attracted by its AChE inhibitory potency, high selectivity, low toxicity, and good bioavailability. The 1-benzylpiperidine fragment of donepezil is the key pharmacophore of AChE inhibition and indicates good water solubility, and many donepezil hybrids have been developed as MTDLsCitation13,Citation14,Citation27. This work plans to create multi-target active small molecules by fusing chalcone, Vitamin E, and donepezil, and then evaluate whether the novel derivatives possess various multifunctional potency and good drug-like properties.
In this work, a series of novel chalcone-Vitamin E-donepezil hybrids were synthesised and developed as multitarget-directed ligands (). The evaluation of biological activities includes AChE inhibitory activity, metals chelation property, antioxidant activity, Aβ aggregation inhibition/disaggregation, and MAO-B inhibitory potency.
2. Result and discussion
2.1. Chemistry
In Schemes 1, 2, the synthesis of chalcone-Vitamin E-donepezil hybrids was described. Firstly, the starting material 1 was reacted with amounts of 1,3-dibromopropane, 1,4-dibromobutane, or 1,6-dibromohexane in anhydrous CH3CN with K2CO3 at 65 °C to get compounds 2a–c. And then, compounds 2a–c were reacted with secondary amines 3a–bCitation23–25 and diethylamine 3c to afford compounds 4a–b, 5a–c, and 6a–b. Finally, the products 8, 9a–f, and 10 were obtained by the condensation of intermediates 7 with the corresponding benzaldehydes 1, 4a–b, 5a–c, and 6a–b in 50% KOH solution.
Scheme 1. Synthesis of target chalcone-Vitamin E-donepezil hybrids 8, 9a–f, and 10. Reaction conditions: (i) Br(CH2)nBr, K2CO3, CH3CN, 65 °C, 6–10 h; (ii) NHR1R2 (3a–c), K2CO3, CH3CN, refluxed, 6–8 h; (iii) 1, 4a–b, 5a–c, and 6a–b, 50% KOH, r.t., 3–4 days.
Scheme 2. Synthesis of target chalcone-Vitamin E-donepezil hybrids 16 and 17a–f. Reagents and conditions: (i) chloromethyl methyl ether, (i-Pr)2EtN, acetone, 50 °C, 6–8 h; (ii) 4a–b, 5a–b, and 6a–b, 50% KOH, r.t., 3–4 days; (iii) 1, 50% KOH, r.t., 3–4 days; (iv) 10% HCl, room temperature, overnight.
For the target compounds 17a–f. Primarily, material 11 was reacted with chloromethyl methyl ether (MOMCl) to give the compound 12, then the key intermediates 13a–b, 14a–b, and 15a–b were synthesised through the condensation of 12 with the corresponding benzaldehydes 1, 4a–b, 5a–b, and 6a–b in alcoholic 50% KOH solution, which was treated with 10% HCl to get the desired products 16 and 17a–f.
2.2. Pharmacology
2.2.1. Ache and BuChE inhibition assay
Ellman’s method was employed to assess the AChE and BuChE inhibitory activities of target chalcone-Vitamin E-donepezil hybridsCitation27,Citation28. AChE was from cortex homogenate of rat (RatAChE) and electric eel (eeAChE), ratBuChE from rat serum. In the above experiments, donepezil, compounds 8 and 16 were applied as positive compounds. As displayed in , all the target chalcone-Vitamin E-donepezil hybrids displayed good and high selective AChE inhibitory activity. Compounds 9d and 17f demonstrated the best AChE inhibitory potency in the two skeleton series, respectively, with their IC50 values of 0.32 and 0.41 µM, respectively.
Table 1. AChE and BuChE inhibitory potency and oxygen radical absorbance capacity (ORAC, Trolox equivalent) by chalcone-Vitamin E-donepezil hybrids, Vitamin E, and donepezil.
In the preliminary experiments, as expected, compounds 8 (IC50 > 500 µM) and 16 (IC50 > 500 µM) exhibited no significant inhibition of AChE. Based on our previous work, the 2-methoxybenzyl groups were in favour of improving AChE inhibitory activity, thus the 2-methoxybenzyl substituent groups were introduced into the parent compounds 8 and 16 to obtain compounds 9a–f and 17a–f, respectively, dramatically showing significant AChE inhibitory activities (IC50 was 3.45–0.32 µM). In particular, the compounds 9d and 17f exhibited good inhibitory potency with their IC50 values of 0.32 and 0.41 µM, respectively. Moreover, the compounds (9b, 9d, 9f, 17b, 17d, and 17f) possessing the N-ethyl group on the terminal nitrogen showed better inhibition potency than the corresponding compounds (9a, 9c, 9e, 17a, 17c, and 17e) with N-methyl group, which was supported by our previous workCitation24. In addition, to study the importance of benzylamines groups, we replaced the N-ethyl-2-methoxy-benzenemethanamine group of compound 9d with diethylene group to furnish compound 10 (IC50 = 4.94 µM), giving a dropped obviously AChE inhibitory potency. These results indicated that the electron density of the aromatic ring, especially the N-ethyl-2-methoxy-benzenemethanamine group, contributed to the AChE inhibitory activity. Furthermore, compared with our previous work of chalcone derivatives, when introducing dual O-alkylamines into the chalcone skeleton, the AChE inhibitory activity was obviously enhancedCitation24. When the O-alkylamines fragment was replaced at 3 positions, the chalcone derivatives showed significant selective BuChE inhibitory activityCitation23.
For BuChE inhibition assay, all chalcone-Vitamin E-donepezil hybrids proved to be inactive or weak active on ratBuChE. Based on the above results, compounds 9d and 17f were selected for metal chelation abilities.
2.2.2. Antioxidant activity
The Oxygen Radicals Absorbance Capacity by Fluorescence (ORAC-FL) method was employed to evaluate antioxidant activities of the synthesised target compounds with Trolox as a reference compoundCitation29,Citation30. As shown in , the positive compound Vitamin E showed good antioxidant activity with an ORAC-FL value of 2.6 eq, all the target chalcone-Vitamin E-donepezil hybrids demonstrated good antioxidant potency with ORAC-FL values ranging from 0.90 to 3.4 eq, but displayed lower potency than the parent compounds 8 (5.4 eq) and 16 (7.8 eq). Among these compounds, compounds 17a–f (ORAC-FL values of 2.6–3.4 eq) with two hydroxyl groups exhibited more potent antioxidant activity than other analogues 9a–f and 10 (ORAC-FL values of 0.93–1.04 eq) with one hydroxyl group. The data revealed that the number of free hydroxyls contributed to the antioxidant potency. Compounds 9d and 17f showed significant antioxidant potency with ORAC-FL values of 0.96 and 3.3 eq, respectively. According to the above data, compound 17f was chosen for kinetic characterisation study and molecular modelling study.
2.2.3. Kinetic characterisation of AChE inhibition
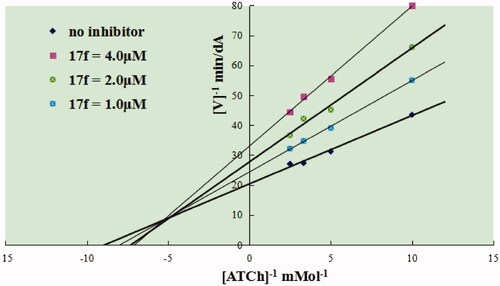
To explore the possible mechanism for chalcone-Vitamin E-donepezil hybrids on AChE. The significant compound 17f, with good AChE inhibitory activity and antioxidant activity, was selected to investigate the inhibition mechanism of AChECitation27,Citation28. The reciprocal Lineweaver-Burk plots analysis () suggested that both increasing slopes (decreased Vmax) and intercepts (higher Km) at increasing concentration of 17f. The intersection point fell in the second quadrant, revealing a mixed-type inhibition.
2.2.4. Molecular docking of 17f with AChE
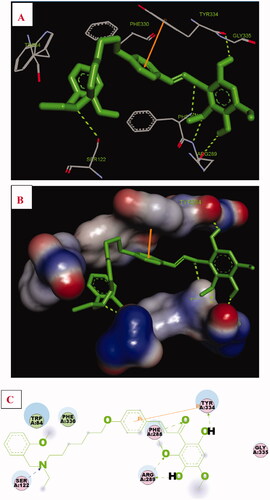
The possible interacting mechanism of 17f with AChE (PDB code: 1EVE) was performed using AUTODOCK 4.2 packageCitation25. As displayed in , the O atom of the methoxyl group interacted with important residues Phe288 and Arg289, respectively. The H atom of one of the hydroxyl groups presented one intermolecular hydrogen bonding with Arg289. The H atom of another hydroxyl group presented one intermolecular hydrogen bonding with residue Tyr334. In addition, the carbonyl group displayed one intermolecular hydrogen bonding with residue Phe288. Moreover, the N atom of N-(2-methoxybenzyl)ethanamine fragment interacted with important residue Ser122 via one intermolecular hydrogen bonding. Furthermore, the benzene ring of compound 17f formed one π–π interaction with residue Phe330. Besides, some hydrophobic interactions were presented between compound 17f and residues (such as Trp84, Ser122, Arg289, Phe288, Tyr334, Gly335, and Phe330). The result also revealed that 17f occupied the catalytic site, the mid-gorge site, and the peripheral site, offering a possible mechanism for the high AChE inhibitory activity.
2.2.5. Molecular dynamics (MD) simulations
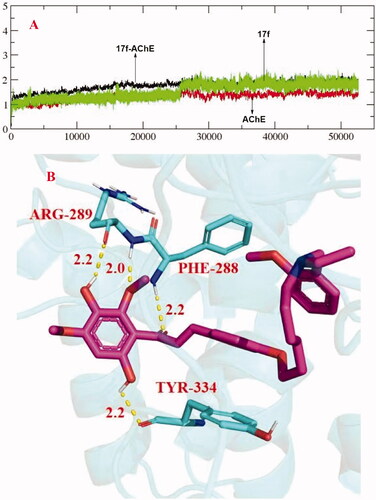
The stability of docked binding pose of the compound 17f-AChE complex was analysed by molecular dynamics simulation analysis using Amber 16Citation31. displayed that the root means square deviations (RMSD) analysis of compound 17f with the amino acid residues of AChE. The results indicated that the RMSDs of all the replicas for the six simulated systems show relatively stable fluctuations after 50 ns of the MSMD simulations, suggesting that the six simulated systems basically reach equilibrium. showed the key residues and interactions modes of 17f with AChE, and four intermolecular hydrogen bonding were observed. The two hydroxy groups of 17f formed one intermolecular hydrogen bonding with the Arg289 (2.2 Å) and Tyr334 (2.2 Å) residues, respectively. The oxygen atom of the methoxy group formed a key intermolecular hydrogen bonding with the Arg289 (2.0 Å) and the oxygen atom of the carbonyl group interacted with key residue Phe288 via one intermolecular hydrogen bonding.
2.2.6. Propidium iodide displacement assay
Given the results from the kinetic study, molecular docking and dynamic simulations, compound 17f has significant interactions with PAS residues of AChE. Therefore, the affinity of compound 17f at 10 and 50 µM concentrations for the PAS-binding was tested by propidium iodide displacement assayCitation32–34. As listed in , the binding of compound 17f to PAS displaced propidium iodide and resulted in decreased fluorescence intensity. Compared with donepezil (10 µM = 20.4%, 50 µM = 32.7%), compound 17f displayed slightly higher displacement of propidium iodide (10 µM = 23.9%, 50 µM = 34.2%), which was in agreement with computational studies.
Table 2. The results of propidium iodide displacement assay and inhibition of huAChE-induced Aβ aggregation towards compound 17f and donepezil.
2.2.7. Effects on self-mediated Aβ1–42 aggregation
To determine the effects of the chalcone-Vitamin E-donepezil hybrids on self-induced Aβ1–42 aggregation, the inhibition assay and disaggregation assay were performed using the thioflavin T (ThT) fluorescence assayCitation29. Curcumin compounds 8 and 16 were also tested. The data were collected in . For the inhibition assay, the precursor compounds 8 (23.1%) and 16 (27.7%) indicated lower inhibitory activity than curcumin (47.3%), and donepezil exhibited no significant inhibition potency (under 5% inhibition ratio at 25 µM). The target compounds (9a–f, 10, and 17a–f) showed exhibited more inhibitory potency than curcumin. Generally, compounds 17a–f indicated better inhibitory activity than other compounds. Seen from the screen data, replacing the N-ethyl-2-methoxy-benzenemethanamine group of 9b (63.4%) with the diethylene group to obtain the compound 10, getting a waned dramatically inhibition ratio of 37.7%. Compound 17f showed higher inhibition potency with an inhibition ratio of 78.0%. These results demonstrated that the hydroxyl group at the 2- and 4-position in the acetophenone moiety (A ring) could play an important role in inhibiting self-mediated Aβ1–42 aggregation, and the 2-methoxybenzyl substitutions contributed to the inhibition potency, however, the diethylamine group might be the disadvantageous effect on inhibitory potency.
Table 3. Inhibition and disaggregation potency of Aβ1–42 aggregation by donepezil, curcumin, and chalcone-Vitamin E-donepezil hybrids.
For disaggregation assay. The data in displayed that target compounds exhibited different disaggregation abilities, inhibition ratio ranging from 22.2 to 75.9%. Compound 17f exhibited remarkably disaggregation potency (72.3%).
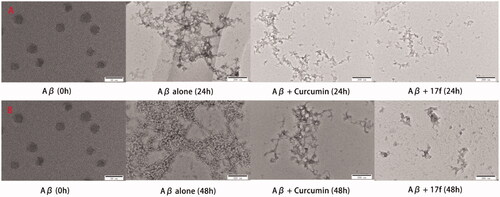
Further, transmission electron microscopy (TEM) was employed to observe the degree of Aβ1–42 aggregation. As presented in , the fresh Aβ1–42 aggregated into amyloid fibrils after 24 h incubation, when treating with curcumin and compound 17f, respectively, only small Aβ aggregates were observed, which supported the ThT binding assay results. A similar phenomenon was also observed in disaggregation self-induced Aβ1–42 aggregation experiments (). Therefore, both the ThT assay and TEM images suggested 17f produced significant inhibition and disaggregation effect on self-mediated Aβ1–42 aggregation.
2.2.8. Effects on huAChE-induced Aβ1–40 aggregation by 17f
Accumulated evidence showed that the PAS of AChE could bind to the Aβ and accelerated the formation of amyloid fibrils. Compound 17f was chosen to perform the inhibition experiment of hAChE-induced Aβ1–40Citation30. As indicated in , 17f significantly inhibited huAChE-induced Aβ1–40 aggregation with a 53.9% inhibition rate, which was better than that of donepezil (24.3%).
2.2.9. Metal chelation properties
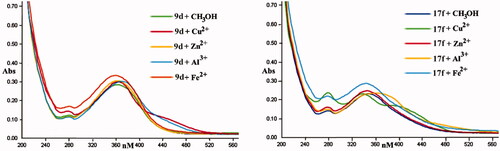
The representative AChE inhibitors 9d and 17f were selected to assess the chelation properties using Cu2+, Zn2+, Al3+, and Fe2+ by UV-vis spectrometryCitation29,Citation30. As displayed in , after CuCl2 and AlCl3 were added to a solution of 9d, the characteristic peak produced a red shift from 362 to 444 and 376 nm, respectively, revealing the formation of 9d-Cu2+ and 9d-Al3+ complex. Correspondingly, the characteristic peak presented no obvious shift when FeSO4 and ZnCl2 were added. For compound 17f, the characteristic peak generated a red shift from 350 to 422 and 370 nm after adding CuCl2 and AlCl3, respectively, however, there was no significant shift when FeSO4 and ZnCl2 were added. This phenomenon suggested that the compounds 9d and 17f were selective chelating agents.
Figure 6. The UV spectrum of compounds 9d and 17f alone or in the presence of CuCl2, AlCl3, ZnCl2, and FeSO4. The final concentration was 37.5 μM.
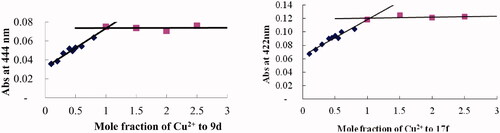
In addition, the molar ratio method was employed to evaluate the stoichiometry of the 17f-Cu2+ complex through preparing the solution of 17f with increasing CuCl2 at 422 nm. As displayed in , the absorbance linearly increased at first and then tended to be stable. The two straight lines intersected at a mole fraction of 1.03, revealing a 1:1 stoichiometry for complex 17f-Cu2+. A similar method was carried out, for compound 9d with CuCl2 revealed a break at 1.1 and 1.06, revealing a 1:1 stoichiometry for the 9d-Cu2+ complex.
2.2.10. Effects on Cu2+-mediated Aβ1–42 aggregation and disaggregation
Similarly, the inhibition assay and disaggregation assay were employed for the Cu2+-mediated Aβ1–42 aggregation to assess the ability of chalcone-Vitamin E-donepezil hybrids using ThT binding assayCitation29,Citation30. For inhibition potency. As shown in , the precursor compounds 8 (33.2%) and 16 (38.1%) exhibited low inhibition potency, target compounds exhibited moderate-to-good inhibition potencies compared with curcumin (76.5%). Compound 17f demonstrated remarkable inhibition potency (93.5%). In general, the compounds 17a–f with two hydroxyl groups displayed better inhibitory activity than the other target compounds. In addition, replacing the N-ethyl-2-methoxy-benzenemethanamine group of 9b (71.6%) with the diethylene group to get compound 10 (49.4%) with the lowest inhibitory activity. These results implied that the hydroxyl groups acted as a key role in the inhibition of Cu2+-mediated Aβ1–42 aggregation, the 2-methoxybenzyl groups substitutions contributed to the inhibition potency, the aliphatic amine (such as diethylamine) provided an adverse influence on inhibition potency.
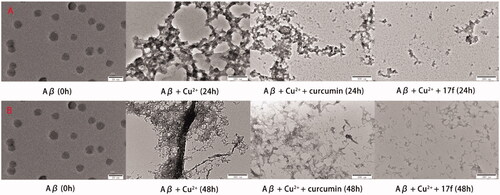
For disaggregation potency, most target compounds exhibited significant disaggregation potency. Among them, compound 10 (26.2%) showed the lowest disaggregation ability and it might be that the diethylene group produced disadvantageous effects on disaggregating potency. Compound 17f indicated significant disaggregation potency with a disaggregation ratio of 84.5% and it showed that 17f disaggregated Cu2+-mediated Aβ1–42 fibrils. In short, compound 17f can inhibit and disaggregate Cu2+-mediated Aβ1–42 fibrils, which were also supported by the TEM images in .
2.2.11. Molecular docking of 17f with Aβ
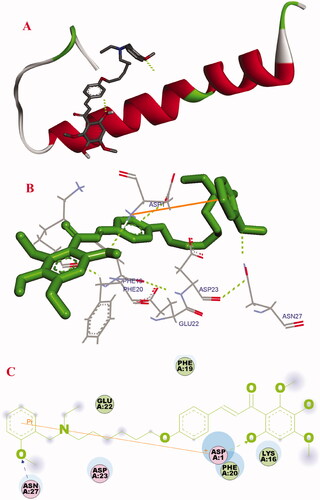
Molecular docking was employed to investigate the binding mechanism of 17f with Aβ1–42 (PDB: 1BA4). As displayed in , compound 17f was located at the C-terminus hydrophobic area of Aβ. The oxygen atom of the hydroxyl group presented one intermolecular hydrogen bonding with ASP1, the methoxy group of N-(2-methoxybenzyl)ethanamine fragment formed one intermolecular hydrogen bonding with Asn27, and the benzene ring of N-(2-methoxybenzyl)ethanamine fragment interacted with Asp 1 via one δ–π interaction. Besides, compound 17f presented some hydrophobic interactions with the amino acid residues LYS16, ASP1, Phe19, PHE20, Glu22, Asp23, and ASN27. These observed interactions might provide a rational mechanism for the binding of Aβ1–42 with 17f.
2.2.12. Inhibitory potency of huMAO-a and huMAO-B
The fluorescence method was employed to evaluate the inhibitory activity of huMAO-A and huMAO-B (recombinant human enzyme) by target chalcone-Vitamin E-donepezil hybridsCitation26. The data from showed that most of the target derivatives were significantly selective huMAO-B inhibitors, except compounds 17a, 17b, and 17c. Among these compounds, compound 9e (IC50 = 2.5 µM) displayed the best MAO-B inhibitory potency, and the selectivity index value was 15.1. In general, compounds 17a–f with two hydroxyl groups exhibited slightly weaker inhibitory activity than compounds 9a–f with one hydroxyl group. Compound 17f presented selective MAO-B inhibitory activity (IC50 = 8.8 µM; SI = 4.3).
Table 4. Inhibition potency of huMAO-A and huMAO-B) and selectivity index (SI) values of clorgyline, rasagiline, iproniazid, and chalcone-Vitamin E-donepezil hybrids.
2.2.13. Molecular modelling of 17f with MAO-B
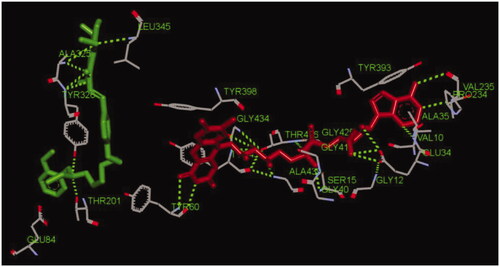
Compound 17f was selected to perform the docking with human MAO-B (PDB code: 2V60)Citation23. As indicated in , the O atom of methoxyl group in compound 17f interacted with key residues Leu345 and Ala325Thr 201 via one intermolecular hydrogen bonding, respectively. The O atom of the hydroxyl group in 17f interacted with residue Ala325 via one intermolecular hydrogen bonding. Moreover, the carbonyl group interacted with residues Ala325 and Tyr326 via one intermolecular hydrogen bonding, respectively. Furthermore, the N atom of N-(2-methoxybenzyl)ethanamine moiety interacted with Tyr326 and Thr201 via one intermolecular hydrogen bonding, respectively. In addition, there were some hydrophobic interactions could be found between compound 17f and residues (such as Leu345, Tyr60, Thr201, Glu84, Tyr326, and Ala325). Therefore, the observed interactions offered a rational mechanism for the high MAO-B inhibitory potency towards 17f.
2.2.14. Neuroprotective effects
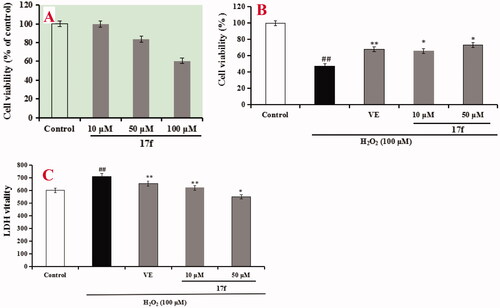
Firstly, the cytotoxicity of compound 17f was tested using an MTT assay. As shown in , compound 17f did not show obvious cytotoxicity until the concentration increased up to 50 µM, showing a wide safety range. Subsequently, the neuroprotective effects of 17f against H2O2–induced PC12 cell injury were investigated through MTT assay and lactate dehydrogenase (LDH) assay, and Vitamin E (VE) acted as the positive drugCitation29,Citation30. As shown in , PC12 cells were cultured and exposed to 100 µM H2O2 for 1 h to establish the oxidative damage model and the cell viability fleetly declined to 47.1% (p < 0.01) compared with the normal group. When the PC12 cells were treated with 100 µM Vitamin E (VE), the cell viability increased to 68.2% (p < 0.01). Subsequently, the PC12 cells were treated with 10 and 50 µM compound 17f, respectively. The cell viability added up to 65.7% (p < 0.05) and 73.4% (p < 0.05), respectively, indicating a better neuroprotective effect than VE. Further, the experiment was also investigated by LDH assay. As shown in , the LDH vitality suddenly enhanced to 712.7 (p < 0.01) compared with the vehicle group (602.3) after PC12 cells were exposed to 100 µM H2O2. When PC12 cells were treated with 100 µM VE, the LDH vitality was 654.7 (p < 0.01). Subsequently, when PC12 cells were treated with 10 and 50 µM compound 17f, respectively, the LDH vitality declined to 621.3 (p < 0.01) and 549.7 (p < 0.05), respectively. In short, the above results exhibited that compound 17f exhibited a good neuroprotective effect against H2O2-induced PC12 cell injury by MTT and LDH assay.
Figure 11. (A) Cytotoxicity of 17f in PC12 cells. (B) Attenuation of H2O2-induced PC12 cell injury by compound 17f was tested using MTT assay. (C) The LDH activity of compound 17f on H2O2-induced PC12 cell injury was evaluated using LDH assay. Three independent experiments were carried out. Data were expressed as mean ± SD and percentage of control value. ##p < 0.01 vs. control; **p < 0.01, *p < 0.05 vs. H2O2 group. VE: Vitamin E.
2.2.15. Blood–brain barrier assay in vitro
The parallel artificial membrane permeation assay of the blood–brain barrier (PAMPA-BBB) was employed to investigate BBB permeability of 17fCitation35,Citation36. Eleven commercial drugs were chosen and the permeability was compared with reported data to validate this method and produced a good linear correlation, Pe(exp) = 0.9163Pe(bibl.) − 0.2247 (R2 = 0.9558). Based on this equation and the limit established, we concluded that derivatives with permeability Pe (×10−6 cm/s) > 3.44 × 10−6 cm/s possess high BBB permeation; 3.44 > Pe > 1.61 displayed uncertain BBB permeation; Pe < 1.61 showed low BBB permeation. The measured data in revealed that the positive compounds verapamil and diazepam could cross the BBB, while enoxacin could not cross the BBB. The data also displayed 17f could cross the BBB.
Table 5. The predictive penetration of 17f by PAMPA-BBB assay.
3. Conclusions
In conclusion, a novel series of chalcone-Vitamin E-donepezil hybrids were designed and developed as multi-target-directed ligands against AD. Most of the derivatives displayed good to better AChE inhibitory potency and high selectivity towards BuChE. Derivatives 9d and 17f displayed the best inhibitory potency with IC50 values of 0.32 and 0.41 µM, respectively. Moreover, compound 17f displayed good antioxidant activity ORAC-FL values of 3.3 trolox equivalents and acted as an MAO-B inhibitor (IC50 = 8.8 µM). Both molecular docking and kinetic analysis revealed that 17f demonstrated a mixed-type AChE inhibition, binding to both CAS and PAS of AChE. UV-vis spectrometry confirmed compounds 9d and 17f were good biometal chelators. Meanwhile, both the ThT assay and TEM images revealed that the compound 17f had remarkable inhibition potency of self-induced, huAChE-induced, and Cu2+-induced Aβ aggregation, and could decompose self-induced and Cu2+-induced Aβ1–42 aggregation. Furthermore, compound 17f exhibited a good neuroprotective effect and displayed high BBB permeability in vitro. These results declared that compound 17f was a potential multitarget lead compound against AD. Further investigations of AD therapeutic candidates based on these results are in progress.
4. Experiment section
4.1. Chemistry
All the reaction was detected by TLC, and the purity was performed by high-performance liquid chromatography (HPLC). HPLC analysis was performed on a Shimadzu LC-10Avp plus system using a Kromasil C18 column (4.6 × 250 mm, 5 um). 1H NMR and 13 C NMR spectra were recorded and collected using a Variant INOVA spectrometer at 400 NMR and 100 NMR, respectively. Mass spectra were analysed and collected on Agilent-6210 TOF LC-MS Spectrometer.
4.1.1. General procedure for the synthesis of derivatives 2a–c
Compounds 2a–c were synthesised, and their characterisation data were consistent with the reported previously.
4.1.2. General procedure for the synthesis of derivatives 3a–b
Compounds 3a–b were synthesised referenced our previous workCitation27.
4.1.3. General procedure for the synthesis of derivatives 4a–b, 5a–c, and 6a–b
To a mixture of the appropriate derivatives 2a–c (5 mmol), anhydrous K2CO3 (6 mmol) in CH3CN (10 ml), secondary amines 3a–b, and diethylamine (5.5 mmol) were added. The reaction mixture was heated to 65 °C for 6–8 h. After a complete reaction, the solvent was concentrated in a vacuum. Then water (30 ml) and dichloromethane (30 ml) were added, respectively, and extracted. And then saturated aqueous NaCl (30 ml) was added to wash the combined organic phases and dried with Na2SO4. The organic phases were evaporated and the residue was purified on a silica gel chromatography using mixtures of petroleum/acetone as eluent to obtain the oil products 4a–b, 5a–c, and 6a–b.
4.1.3.1. 4-[[3-[Methyl(2-methoxyphenyl)methyl]amino]propyl]oxy]benzaldehyde (4a). Intermediate 2a was treated with N-methyl-2-methoxy-benzenemethanamine (3a) to obtain 4a as colourless oil, yield 89.5%. 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.82 (d, J = 8.8 Hz, 2H), 7.32 (d, J = 7.2 Hz, 1H), 7.24 (t, J = 7.2 Hz, 1H), 6.98 (d, J = 8.8 Hz, 2H), 6.90 (t, J = 7.2 Hz, 1H), 6.86 (d, J = 8.0 Hz, 1H), 4.14 (t, J = 6.4 Hz, 2H), 3.80 (s, 3H), 3.61 (s, 2H), 2.65 (t, J = 6.4 Hz, 2H), 2.30 (s, 3H), 2.11–2.07 (m, 2H).
4.1.3.2. 4-[[3-[Ethyl(2-methoxyphenyl)methyl]amino]propyl]oxy]benzaldehyde (4b). Intermediate 2a was treated with N-ethyl-2-methoxy-benzenemethanamine (3b) to obtain 4b as colourless oil, yield 87.5%. 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.81 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 7.2 Hz, 1H), 7.22 (dt, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 6.90 (t, J = 7.2 Hz, 1H), 6.85 (d, J = 8.0 Hz, 1H), 4.10 (t, J = 6.4 Hz, 2H), 3.81 (s, 3H), 3.69 (s, 2H), 2.71 (t, J = 6.8 Hz, 2H), 2.62 (q, J1 = 13.2 Hz, J2 = 6.8 Hz, 2H), 2.05–2.00 (m, 2H), 1.11 (t, J = 6.8 Hz, 3H).
4.1.3.3. 4-[[4-[Methyl(2-methoxyphenyl)methyl]amino]butyl]oxy]benzaldehyde (5a). Intermediate 2b was treated with N-methyl-2-methoxy-benzenemethanamine (3a) to obtain 5a as colourless oil, yield 85.5%. 1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 7.2 Hz, 1H), 7.28 (t, J = 7.6 Hz, 1H), 6.96 (d, J = 8.4 Hz, 2H), 6.94 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 8.4 Hz, 1H), 4.05 (t, J = 4.8 Hz, 2H), 3.83 (s, 3H), 3.74 (s, 2H), 2.67–2.62 (m, 2H), 2.37 (s, 3H), 1.90–1.84 (m, 4H).
4.1.3.4. 4-[[4-[Ethyl(2-methoxyphenyl)methyl]amino]butyl]oxy]benzaldehyde (5b). Intermediate 2b was treated with N-methyl-2-ethoxy-benzenemethanamine (3b) to obtain 5b as colourless oil, yield 81.2%. 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.81 (d, J = 8.4 Hz), 7.43 (d, J = 7.2 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 6.96 (d, J = 8.8 Hz, 2H), 6.92 (t, J = 7.2 Hz, 1H), 6.85 (d, J = 8.0 Hz, 1H), 4.02 (t, J = 6.4 Hz, 2H), 3.81 (s, 3H), 3.65 (s, 2H), 2.59–2.55 (m, 4H), 1.86–1.80 (m, 2H), 1.72–1.68 (m, 2H), 1.09 (t, J = 7.2 Hz, 3H).
4.1.3.5. 4-[3-(Diethylamino)propoxy]benzaldehyde (5c). Intermediate 2b was treated with diethylamine to obtain 5c as colourless oil, yield 90.5%. 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.83 (d, J = 8.8 Hz, 2H), 6.99 (d, J = 8.4 Hz, 1H), 4.08 (t, J = 6.0 Hz, 2H), 2.64–2.55 (m, 6H), 1.86–1.81 (m, 2H), 1.73–1.68 (m, 2H), 1.08 (t, J = 6.8 Hz, 6H).
4.1.3.6. 4-[[6-[Methyl(2-methoxyphenyl)methyl]amino]hexyl]oxy]benzaldehyde (6a). Intermediate 2c was treated with N-methyl-2-methoxy-benzenemethanamine (3a) to obtain 6a as colourless oil, yield 86.1%. 1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.34 (d, J = 7.2 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 6.98 (d, J = 8.8 Hz, 2H), 6.93 (t, J = 7.2 Hz, 1H), 6.87 (d, J = 8.0 Hz, 1H), 4.03 (t, J = 6.4 Hz, 2H), 3.82 (s, 3H), 3.57 (s, 2H), 2.45 (t, J = 6.8 Hz, 2H), 2.25 (t, J = 6.8 Hz, 2H), 1.84–1.79 (m, 2H), 1.63–1.57 (m, 2H), 1.51–1.46 (m, 2H), 1.43–1.40 (m, 2H).
4.1.3.7. 4-[[6-[Ethyl(2-methoxyphenyl)methyl]amino]hexyl]oxy]benzaldehyde (6b). Intermediate 2c was treated with N-ethyl-2-methoxy-benzenemethanamine (3b) to obtain 6b as colourless oil, yield 87.6%. 1H NMR (400 MHz, CDCl3) δ 9.87 (s, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 6.4 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 6.97 (d, J = 8.8 Hz, 2H), 6.94 (t, J = 7.2 Hz, 1H), 6.86 (d, J = 8.0 Hz, 1H), 4.01 (t, J = 6.4 Hz, 2H), 3.82 (s, 3H), 3.72 (s, 2H), 2.65–2.63 (m, 2H), 2.58–2.54 (m, 2H), 1.83–1.76 (m, 2H), 1.63–1.60 (m, 2H), 1.50–1.43 (m, 2H), 1.40–1.33 (m, 2H), 1.12 (t, J = 7.2 Hz, 3H).
4.1.4. 1-[6-Hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl]ethanone (12)
A mixture of material 11 (5 mmol) and (i-Pr)2EtN (6 mmol) in acetone 20 ml and chloromethyl methyl ether (5.5 mmol) was added slowly at −5 °C for 0.5 h. Then the mixture was heated at 50 °C for 6–8 h. Finally, the solvents were evaporated, then water (30 ml) and dichloromethane (30 ml) were added, respectively, and extracted. And then saturated aqueous NaCl (30 ml) was added to wash the combined organic phases and dried with Na2SO4. The organic phases were evaporated and the residue was purified by column chromatography on silica gel (petroleum/acetone as eluent) to obtain the colourless oil product 12 as colourless oil, yield 67.3%. 1H NMR (400 MHz, CDCl3) δ 13.43 (s, 1H), 5.02 (s, 2H), 3.96 (s, 3H), 3.88 (s, 3H), 3.61 (s, 3H), 2.66 (s, 3H).
4.1.5. General procedure for the synthesis of 8, 9a–f, 10, 13a–b, 14a–b, and 15a–b
The acetophenone derivatives (1 mmol) were reacted with the appropriate benzaldehyde derivatives (1 mmol) in EtOH (3 ml) by slowly adding 50% KOH (4 mmol). After for 72 h to 100 h reaction, 10% HCl was added to adjust pH = 2, and then added NaHCO3 powder to the mixture, and then extracted with CH2Cl2 (10 ml × 3). Then the organic phase was washed using aqueous NaHCO3 (30 ml × 2) and aqueous NaCl (30 ml) and dried with Na2SO4. Finally, the organic phase was evaporated and the residue was purified on a silica gel chromatography by mixtures of CH2Cl2/acetone as eluent to get target derivatives 8, 9a–f, 10, 13a–b, 14a–b, and 15a–b.
4.1.5.1. (E)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one (8). Material 7 was treated with p-hydroxybenzaldehyde to obtain 8 as yellow solid, 61.5% yield, mp: 142.2–143.2 °C. 1H NMR (400 MHz, CDCl3) δ 13.78 (s, 1H), 7.85 (d, J = 16.0 Hz, 1H), 7.80 (d, J = 16.0 Hz, 1H), 7.56 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.4 Hz, 2H), 6.30 (s, 1H), 5.37 (brs, 1H), 3.93 (s, 3H), 3.90 (s, 3H), 3.84 (s, 3H).
4.1.5.2. (E)-1-(6-Hydroxy-2,3,4-trimethoxyphenyl)-3-(4-(3-((2-methoxybenzyl)(methyl)amino)propoxy)phenyl)prop-2-en-1-one (9a). Material 7 was treated with intermediate 4a to obtain 9a as yellow oil, 50.5% yield, 98.4% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.78 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.42–7.38 (m, 1H), 7.29–7.26 (m, 1H), 6.99–6.88 (m, 4H), 6.30 (s, 1H), 4.11 (t, J = 6.0 Hz, 2H), 3.93 (s, 3H), 3.91 (s, 3H), 3.84 (s, 3H), 3.83 (s, 3H), 3.72 (brs, 2H), 2.78–2.75 (m, 2H), 2.39 (s, 3H), 2.19–2.16 (m, 2H). MS (ESI) m/z: 522.2 [M + H]+.
4.1.5.3. (E)-3-(4-(3-(ethyl(2-methoxybenzyl)amino)propoxy)phenyl)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)prop-2-en-1-one (9b). Material 7 was treated with intermediate 4b to obtain 9b as yellow oil, 60.5% yield, 97.9% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.80 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.58 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 6.8 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 6.92–6.89 (m, 3H), 6.85 (d, J = 8.0 Hz, 1H), 6.30 (s, 1H), 4.07 (t, J = 6.4 Hz, 2H), 3.93 (s, 3H), 3.90 (s, 3H), 3.84 (s, 3H), 3.81 (s, 3H), 3.64 (s, 2H), 2.69–2.65 (m, 2H), 2.58–2.55 (m, 2H), 2.01–1.95 (m, 2H), 1.07 (t, J = 6.4 Hz, 3H). 13 C NMR (100 MHz, CDCl3) δ 192.72, 162.54, 161.09, 159.80, 157.56, 154.88, 143.50, 135.18, 131.85, 130.13, 130.02, 127.72, 123.65, 120.20, 114.84, 114.66, 110.11, 108.67, 96.49, 66.29, 61.83, 61.20, 55.80, 55.19, 51.36, 49.59, 47.72, 26.79, 11.72. MS (ESI) m/z: 536.3 [M + H]+.
4.1.5.4. (E)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)-3-(4-(4-((2-methoxybenzyl)(methyl)amino)butoxy)phenyl)prop-2-en-1-one (9c). Material 7 was treated with intermediate 5a to obtain 9c as yellow oil, 60.5% yield, 98.5% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.87 (d, J = 15.6 Hz, 1H), 7.82 (d, J = 15.6 Hz, 1H), 7.59 (d, J = 9.2 Hz, 2H), 7.44–7.40 (m, 1H), 7.29 (t, J = 7.2 Hz, 1H), 6.96 (t, J = 7.6 Hz, 1H), 6.92–6.89 (m, 3H), 6.30 (s, 1H), 4.03 (t, J = 6.0 Hz, 2H), 3.93 (s, 3H), 3.91 (s, 3H), 3.85 (s, 3H), 3.84 (s, 3H), 3.82 (s, 2H), 2.75–2.72 (m, 2H), 2.45–2.41 (m, 2H), 1.90–1.85 (m, 4H). 13 C NMR (100 MHz, CDCl3) δ 192.79, 162.58, 160.94, 159.88, 157.87, 154.94, 143.44, 135.24, 131.37, 130.21, 129.03, 127.91, 123.85, 120.47, 114.87, 110.51, 108.72, 96.53, 67.68, 61.90, 61.27, 56.57, 56.05, 55.40, 54.76, 41.58, 26.85, 23.08. MS (ESI) m/z: 536.3 [M + H]+.
4.1.5.5. (E)-3-(4-(4-(ethyl(2-methoxybenzyl)amino)butoxy)phenyl)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)prop-2-en-1-one (9d). Material 7 was treated with intermediate 5b to obtain 9d as yellow oil, 60.5% yield, 98.1% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.78 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.58 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 6.8 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 6.95 (t, J = 8.0 Hz, 1H), 6.91–6.86 (m, 3H), 6.30 (s, 1H), 4.00 (t, J = 5.6 Hz, 2H), 3.93 (s, 3H), 3.90 (s, 3H), 3.84 (s, 3H), 3.83 (s, 3H), 3.79 (s, 2H), 2.70–2.65 (m, 4H), 1.84–1.79 (m, 4H), 1.18 (t, J = 6.4 Hz). MS (ESI) m/z: 550.3 [M + H]+.
4.1.5.6. (E)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)-3-(4-(6-((2-methoxybenzyl)(methyl)amino)hexyloxy)phenyl)prop-2-en-1-one (9e). Material 7 was treated with intermediate 6a to obtain 9e as yellow oil, 45.5% yield, 98.7% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.59 (d, J = 8.8 Hz, 2H), 7.37 (d, J = 4.8 Hz, 1H), 7.26 (t, J = 6.8 Hz, 1H), 6.96–6.87 (m, 4H), 6.30 (s, 1H), 4.00 (t, J = 6.8 Hz, 2H), 3.93 (s, 3H), 3.90 (s, 3H), 3.84 (s, 3H), 3.83 (s, 3H), 3.65 (s, 2H), 2.54–2.50 (m, 2H), 2.30 (s, 3H),1.83–1.78 (m, 2H), 1.68–1.63 (m, 2H), 1.50–1.46 (m, 2H), 1.44–1.40 (m, 2H). 13 C NMR (100 MHz, CDCl3) δ 192.67, 162.48, 160.96, 159.78, 157.70, 154.82, 143.36, 135.13, 131.41, 130.11, 129.20, 127.71, 123.68, 120.60, 114.77, 114.58, 110.35, 108.60, 96.42, 67.78, 61.78, 61.16, 55.95, 55.29, 52.33, 50.51, 47.17, 28.78, 26.85, 25.63, 25.23, 10.40. MS (ESI) m/z: 564.3 [M + H]+.
4.1.5.7. (E)-3-(4-(6-(ethyl(2-methoxybenzyl)amino)hexyloxy)phenyl)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)prop-2-en-1-one (9f). Material 7 was treated with intermediate 6b to obtain 9f as yellow oil, 63.5% yield, 97.6% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.60–7.55 (m, 3H), 7.30 (t, J = 6.8 Hz, 1H), 6.99 (t, J = 6.8 Hz, 1H), 6.93–6.88 (m, 3H), 6.30 (s, 1H), 3.99 (t, J = 6.4 Hz, 2H), 3.93 (s, 3H), 3.90 (s, 3H), 3.84 (s, 3H), 3.83 (s, 3H), 3.87 (s, 2H), 2.78–2.71 (m, 4H), 1.83 (m, 4H), 1.51–1.45 (m, 2H), 1.41–1.36 (m, 2H), 1.26–1.21 (m, 3H). MS (ESI) m/z: 578.3 [M + H]+.
4.1.5.8. (E)-3-(4-(4-(diethylamino)butoxy)phenyl)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)prop-2-en-1-one (10). Material 7 was treated with intermediate 5c to obtain 10 as yellow oil, 60.3% yield, 98.2% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.77 (s, 1H), 7.87 (d, J = 15.6 Hz, 1H), 7.82 (d, J = 15.6 Hz, 1H), 7.60 (d, J = 8.8 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H), 4.06 (t, J = 5.6 Hz, 2H), 3.95 (s, 3H), 3.93 (s, 3H), 3.84 (s, 3H), 2.94–2.83 (m, 8H), 1.93–1.84 (m, 4H), 1.28 (t, J = 6.8 Hz, 6H). 13 C NMR (100 MHz, CDCl3) δ 192.55, 162.38, 160.51, 159.74, 154.71, 143.09, 135.03, 130.03, 127.86, 123.78, 114.66, 108.46, 96.31, 67.11, 61.69, 61.07, 55.88, 51.31, 46.36, 26.52, 21.42, 9.54. MS (ESI) m/z: 458.3 [M + H]+.
4.1.5.9. (E)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)-3-(4-(3-((2-methoxybenzyl)(methyl)amino)propoxy)phenyl)prop-2-en-1-one (13a). Compound 12 was treated with intermediate 4a to obtain 13a as yellow oil, 51.2% yield. 1H NMR (400 MHz, CDCl3) δ 13.80 (s, 1H), 7.87 (d, J = 15.6 Hz, 1H), 7.82 (d, J = 15.6 Hz, 1H), 7.58 (d, J = 8.8 Hz, 2H), 7.32 (d, J = 7.6 Hz, 1H), 7.23 (t, J = 8.0 Hz, 1H), 6.93–6.89 (m, 3H), 6.86 (d, J = 8.0 Hz, 1H), 6.31 (s, 1H), 5.08 (s, 2H), 4.10 (t, J = 6.4 Hz, 2H), 3.89 (s, 3H), 3.89 (s, 3H), 3.81 (s, 3H), 3.64 (s, 3H), 3.59 (s, 2H), 2.64 (t, J = 6.8 Hz, 2H), 2.29 (s, 3H), 2.08–2.05 (m, 2H).
4.1.5.10. (E)-3-(4-(3-(ethyl(2-methoxybenzyl)amino)propoxy)phenyl)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)prop-2-en-1-one (13b). Compound 12 was treated with intermediate 4b to obtain 13b as yellow oil, 51.2% yield. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.87 (d, J = 15.6 Hz, 1H), 7.83 (d, J = 15.6 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 6.8 Hz, 1H), 7.22 (t, J = 8.0 Hz, 1H), 6.93–6.89 (m, 3H), 6.85 (d, J = 8.0 Hz, 1H), 6.31 (s, 1H), 5.08 (s, 2H), 4.07 (t, J = 6.4 Hz, 2H), 3.89 (s, 3H), 3.89 (s, 3H), 3.81 (s, 3H), 3.67 (s, 2H), 3.64 (s, 3H), 2.74–2.65 (m, 2H), 2.63–2.58 (m, 2H), 2.04–2.00 (m, 2H), 1.09 (t, J = 6.8 Hz, 3H).
4.1.5.11. (E)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)-3-(4-(4-((2-methoxybenzyl)(methyl)amino)butoxy)phenyl)prop-2-en-1-one (14a). Compound 12 was treated with intermediate 5a to obtain 14a as yellow oil, 58.3% yield. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.87 (d, J = 16.0 Hz, 1H), 7.83 (d, J = 16.0 Hz, 1H), 7.58 (d, J = 8.4 Hz, 2H), 7.44–7.36 (m, 1H), 7.29–7.26 (m, 1H), 6.96–6.88 (m, 4H), 6.31 (s, 1H), 5.08 (s, 2H), 4.03 (t, J = 5.2 Hz, 2H), 3.89 (s, 3H), 3.89 (s, 3H), 3.83 (s, 3H), 3.64 (s, 2H), 3.61 (s, 3H), 2.62–2.58 (m, 2H), 2.36–2.31 (m, 3H), 1.87–1.83 (m, 4H).
4.1.5.12. (E)-3-(4-(4-(ethyl(2-methoxybenzyl)amino)butoxy)phenyl)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)prop-2-en-1-one (14b). Compound 12 was treated with intermediate 5b to obtain 14b as yellow oil, 51.2% yield. 1H NMR (400 MHz, CDCl3) δ 13.78 (s, 1H), 7.87 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.62–7.54 (m, 3H), 7.34 (t, J = 8.0 Hz, 1H), 6.98 (t, J = 7.2 Hz, 1H), 6.92–6.88 (m, 3H), 6.31 (s, 1H), 5.07 (s, 2H), 4.06 (s, 2H), 4.01 (t, J = 6.0 Hz, 2H), 3.89 (s, 3H), 3.88 (s, 3H), 3.86 (s, 3H), 3.64 (s, 3H), 2.92–2.85 (m, 4H), 2.01–1.96 (m, 2H), 1.87–1.82 (m, 2H), 1.33 (t, J = 6.8 Hz, 3H).
4.1.5.13. (E)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)-3-(4-(6-((2-methoxybenzyl)(methyl)amino)hexyloxy)phenyl)prop-2-en-1-one (15a). Compound 12 was treated with intermediate 6a to obtain 15a as yellow oil, 48.3% yield. 1H NMR (400 MHz, CDCl3) δ 13.79 (s, 1H), 7.87 (d, J = 16.0 Hz, 1H), 7.83 (d, J = 16.0 Hz, 1H), 7.59 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 6.8 Hz, 1H), 7.33 (t, J = 7.2 Hz, 1H), 6.99 (t, J = 7.2 Hz, 1H), 6.93–6.90 (m, 3H), 6.31 (s, 1H), 5.08 (s, 2H), 4.00 (t, J = 6.0 Hz, 2H), 3.89 (s, 3H), 3.89 (s, 3H), 3.87 (s, 2H), 3.85 (s, 3H), 3.64 (s, 3H), 2.72–2.76 (m, 2H), 2.45 (s, 3H), 1.85–1.78 (m, 4H), 1.55–1.48 (m, 2H), 1.43–1.39 (m, 2H).
4.1.5.14. (E)-3-(4-(6-(ethyl(2-methoxybenzyl)amino)hexyloxy)phenyl)-1-(6-hydroxy-2,4-dimethoxy-3-(methoxymethoxy)phenyl)prop-2-en-1-one (15b). Compound 12 was treated with intermediate 6b to obtain 15b as yellow oil, 54.1% yield. 1H NMR (400 MHz, CDCl3) δ 13.80 (s, 1H), 7.87 (d, J = 16.0 Hz, 1H), 7.83 (d, J = 16.0 Hz, 1H), 7.62 (d, J = 7.6 Hz, 1H), 7.59 (d, J = 8.8 Hz, 2H), 7.36 (t, J = 7.2 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.93–6.90 (m, 3H), 6.31 (s, 1H), 5.08 (s, 2H), 4.09 (s, 2H), 3.99 (t, J = 6.4 Hz, 2H), 3.89 (s, 3H), 3.89 (s, 3H), 3.86 (s, 3H), 3.64 (s, 3H), 2.95–2.91 (m, 2H), 2.86–2.81 (m, 2H), 1.83–1.77 (m, 4H), 1.52–1.47 (m, 2H), 1.42–1.38 (m, 2H), 1.33–1.31 (m, 3H).
4.1.5.15. 3′,4,6′-Trihydroxy-2′,4′-dimethoxy-chalcone (16). Compound 11 was treated with p-hydroxybenzaldehyde to obtain 16 as yellow oil, 24.3% yield. 1H NMR (400 MHz, CDCl3) δ 13.38 (s, 1H), 7.86 (d, J = 15.2 Hz, 1H), 7.82 (d, J = 15.2 Hz, 1H), 7.56 (d, J = 8.4 Hz, 1H), 6.88 (d, J = 8.8 Hz, 2H), 6.33 (s, 1H), 5.66 (brs, 1H), 3.95 (s, 3H), 3.87 (s, 3H).
4.1.6. General procedure for the synthesis of 17a–f
A mixture of intermediates 13a–b, 14a–b, and 15a–b (1.5 mmol) in EtOH (1 ml) was added 10% HCl (1 ml) for 24 h at room temperature. And then the mixture was adjusted pH = 8 using NaHCO3 powder and extracted with CH2Cl2 (10 ml × 3). The organic phases were washed with 30 ml saturated aqueous NaCl and dried with Na2SO4. Finally, the solvent was evaporated and the residue was purified using mixtures of CH2Cl2/acetone as eluent to obtain products 17a–f.
4.1.6.1. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-(3-((2-methoxybenzyl)(methyl)amino)propoxy)phenyl)prop-2-en-1-one (17a). Compound 13a was treated with 10% HCl to obtain 17a as orange oil, 87.2% yield, 98.3% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.41 (s, 1H), 7.87 (d, J = 15.2 Hz, 1H), 7.83 (d, J = 15.2 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.32 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 8.0 Hz, 1H), 6.92 (d, J = 8.8 Hz, 1H), 6.88–6.84 (m, 2H), 6.32 (s, 1H), 5.32 (brs, 1H), 4.10 (t, J = 6.4 Hz, 2H), 3.94 (s, 3H), 3.87 (s, 3H), 3.80 (s, 3H), 3.57 (s, 2H), 2.62 (t, J = 6.8 Hz, 2H), 2.28 (s, 3H), 2.09–2.02 (m, 2H). MS (ESI) m/z: 508.2 [M + H]+.
4.1.6.2. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-(3-(ethyl(2-methoxybenzyl)amino)propoxy)phenyl)prop-2-en-1-one (17b). Compound 13b was treated with 10% HCl to obtain 17b as orange oil, 81.6% yield, 97.8% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.41 (s, 1H), 7.87 (d, J = 15.2 Hz, 1H), 7.83 (d, J = 15.2 Hz, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.44–7.41 (m, 1H), 7.23 (t, J = 8.0 Hz, 1H), 6.94–6.84 (m, 4H), 6.33 (s, 1H), 5.22 (brs, 1H), 4.07 (t, J = 6.0 Hz, 2H), 3.94 (s, 3H), 3.87 (s, 3H), 3.78 (s, 3H), 3.59 (s, 2H), 2.69–2.61 (m, 4H), 2.02–2.00 (m, 2H), 1.02–1.00 (m, 3H). 13 C NMR (100 MHz, CDCl3) δ 192.46, 160.99, 159.47, 157.61, 154.26, 146.95, 143.54, 131.76, 130.40, 130.13, 128.11, 127.67, 123.40, 120.26, 114.82, 110.17, 108.46, 96.05, 66.18, 61.67, 56.11, 55.19, 51.15, 49.49, 47.60, 26.41, 11.35. MS (ESI) m/z: 522.2 [M + H]+.
4.1.6.3. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-(4-((2-methoxybenzyl)(methyl)amino)butoxy)phenyl)prop-2-en-1-one (17c). Compound 14a was treated with 10% HCl to obtain 17c as orange oil, 85.8% yield, 98.0% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.41 (s, 1H), 7.87 (d, J = 15.2 Hz, 1H), 7.83 (d, J = 15.2 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.39 (d, J = 6.8 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 6.96–6.87 (m, 4H), 6.33 (s, 1H), 5.25 (brs, 1H), 4.02 (t, J = 5.6 Hz, 2H), 3.94 (s, 3H), 3.87 (s, 3H), 3.83 (s, 3H), 3.70 (s, 2H), 2.62–2.60 (m, 2H), 2.34 (s, 3H), 1.86–1.84 (m, 4H). 13 C NMR (100 MHz, CDCl3) δ 192.53, 161.01, 159.55, 157.87, 154.20, 146.93, 143.57, 131.77, 131.32, 130.23, 128.90, 127.83, 123.52, 120.42, 114.89, 110.48, 108.54, 96.13, 67.72, 61.80, 56.62, 56.22, 55.37, 54.79, 41.65, 26.88, 23.15. MS (ESI) m/z: 522.2 [M + H]+.
4.1.6.4. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-(4-(ethyl(2-methoxybenzyl)amino)butoxy)phenyl)prop-2-en-1-one (17d). Compound 14b was treated with 10% HCl to obtain 17d as orange oil, 82.9% yield, 97.8% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.40 (s, 1H), 7.87 (d, J = 15.2 Hz, 1H), 7.83 (d, J = 15.2 Hz, 1H), 7.59–7.56 (m, 3H), 7.31 (t, J = 8.0 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 6.91–6.87 (m, 3H), 6.33 (s, 1H), 5.23 (brs, 1H), 4.01 (t, J = 6.0 Hz, 2H), 3.98 (s, 2H), 3.94 (s, 3H), 3.91 (s, 3H), 3.87 (s, 3H), 2.79–2.77 (m, 4H), 1.84–1.82 (m, 4H), 1.26–1.24 (m, 3H). MS (ESI) m/z: 536.3 [M + H]+.
4.1.6.5. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-((6-((2-methoxybenzyl)(methyl)amino)hexyl)oxy)phenyl)prop-2-en-1-one (17e). Compound 15a was treated with 10% HCl to obtain 17e as orange oil, 87.2% yield, 97.7% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.40 (s, 1H), 7.87 (d, J = 15.2 Hz, 1H), 7.83 (d, J = 15.2 Hz, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 6.0 Hz, 1H), 7.37 (t, J = 7.2 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 6.94–6.90 (m, 3H), 6.33 (s, 1H), 5.23 (brs, 1H), 4.05 (s, 2H), 4.00 (t, J = 5.6 Hz, 2H), 3.94 (s, 3H), 3.87 (s, 6H), 2.83–2.80 (m, 2H), 2.55 (s, 3H), 1.84–1.82 (m, 4H), 1.53–1.50 (m, 2H), 1.44–1.41 (m, 2H). MS (ESI) m/z: 550.3 [M + H]+.
4.1.6.6. (E)-1-(3,6-dihydroxy-2,4-dimethoxyphenyl)-3-(4-((6-(ethyl(2-methoxybenzyl)amino)hexyl)oxy)phenyl)prop-2-en-1-one (17f). Compound 15b was treated with 10% HCl to obtain 17f as orange oil, 80.6% yield, 98.1% HPLC purity. 1H NMR (400 MHz, CDCl3) δ 13.42 (s, 1H), 7.86 (d, J = 16.0 Hz, 1H), 7.82 (d, J = 16.0 Hz, 1H), 7.57 (d, J = 8.4 Hz, 2H), 7.52 (d, J = 6.4 Hz, 1H), 7.29–7.25 (m, 1H), 6.96 (t, J = 7.6 Hz, 1H), 6.91–6.86 (m, 3H), 6.31 (s, 1H), 5.26 (brs, 1H), 3.98 (t, J = 6.4 Hz, 2H), 3.94 (s, 3H), 3.85 (s, 3H), 3.83 (s, 3H), 3.82 (s, 2H), 2.73–2.71 (m, 2H), 2.65–2.62 (m, 2H), 1.80–1.76 (m, 2H), 1.68–1.66 (m, 2H), 1.49–1.43 (m, 2H), 1.39–1.34 (m, 2H), 1.19 (t, J = 6.4 Hz, 3H). 13 C NMR (100 MHz, CDCl3) δ 192.42, 160.98, 159.43, 157.69, 154.25, 146.96, 143.43, 131.77, 131.32, 130.80, 130.10, 129.04, 127.64, 127.53, 123.40, 120.51, 114.77, 110.31, 108.40, 95.98, 67.79, 61.60, 56.07, 55.25, 52.36, 50.52, 47.13, 28.87, 26.87, 25.63, 25.30, 10.46. MS (ESI) m/z: 564.3 [M + H]+.
4.2. Biological assay
4.2.1. Ache and BuChE inhibition assay
The ratAChE was from 5% rat cortex homogenate, ratBuChE was from rat serum, and eeAChE was from electric eel (Sigma–Aldrich Co.). The tested method applied Ellman assay and the detailed procedure could reference our previous workCitation27,Citation28.
4.2.2. Molecular docking
The crystal structure of the AChE complexed with donepezil (code ID: 1EVE) was obtained from the Protein Data Bank after eliminating the original inhibitors and water molecules. The 3 D Structure of 17f was built and performed geometry optimisation by molecular mechanics. After the addition of Gasteiger charges, removal of hydrogen atoms, the addition of their atomic charges to skeleton atoms, and the assignment of proper atomic types, the further preparation of the inhibitor was accomplished. Autotors were then used to define the rotatable bonds in the ligands. Docking studies were performed using the AUTODOCK 4.2 program. By using Autodock Tools (ADT; version 1.5.6), polar hydrogen atoms were added to amino acid residues, and Gasteiger charges were assigned to all atoms of the enzyme. The resulting enzyme structure was used as an input for the AUTOGRID program. AUTOGRID performed pre-calculated atomic affinity grid maps for each atom type in the ligand, plus an electrostatics map and a separate desolvation map presented in the substrate molecule. All maps were calculated with 0.375 Å spacing between grid points. The centre of the grid box was placed at the centre of donepezil with coordinates x = 2.023, y = 63.295, z = 67.062. The dimensions of the active site box were set at 50 × 50 × 50 Å. Flexible ligand docking was performed for the compounds. Each docked system was performed by 100 runs of the AUTODOCK search by the Lamarckian genetic algorithm (LGA). Other than the referred parameters above, the other parameters were accepted as default. A cluster analysis was performed on the docking results using a root mean square (RMS) tolerance of 1.0 and the lowest energy conformation of the highest populated cluster was selected for analysis. Graphic manipulations and visualisations were done by Autodock Tools or Discovery Studio 2.1 softwareCitation27,Citation28.
4.2.3. Propidium iodide displacement assay
Propidium iodide displacement assay was applied to determine the binding of compound 17f to the peripheral site of AChE by competitively displacing the propidium iodideCitation32–34. The assay mixture of eeAChE (5 U) was incubated with or without test compound 17f (final concentration 10 and 50 µM, 150 µl) for 6 h at 25 °C. After incubation, propidium iodide (final concentration 1 µM, 50 µl) was added to make the final assay volume of 200 µl. After 10 min, fluorescence intensity was measured at excitation and emission wavelength of λex = 535 nm and λem = 595 nm, respectively using Varioskan Flash Multimode Reader (PerkinElmer). The percentage inhibition was calculated by the following expression: 100 − (IFi/IF0 × 100), where IFi and IF0 are the fluorescence intensities with and without inhibitor, respectively. Each assay was performed in triplicates, as three separate experiments.
4.2.4. Molecular dynamics simulations
AMBER16 was used for solvation, molecular dynamics simulation, and trajectory analysis. During the simulation, the SHAKE method was used to constrain the expansion and contraction of the chemical bond connected to the hydrogen atom, the simulation integration step was set to 2 fs, and the PME method was used to calculate the long-range electrostatic interaction, and the periodic boundary conditions (PBC) were used to eliminate the edge effect of the solvent box. The following protocols were implemented for each system: (1) The limiting potential of the proteins, ligands, and counter ions were all restricted by a force constant of 200 kcal/mol Å2, and the energy of the solvent water molecules was minimised to make the water molecules reach a relaxed state; (2) the restriction potential for the proteins and ligands were both constrained by a force constant of 300 kcal/mol Å2, which further minimised the energy of the system; (3) the restriction potential of the protein backbone was restricted by a force constant of 20 kcal/mol Å2, and only the side chain was allowed to relax and minimise the energy; (4) Restrictions were not imposed on the entire system and the energy was minimised. The NPT ensemble (Isotherm and Isobaric) to heat from 10 to 300 K with constant volume during 100 ps to ensure that the system reached equilibrium. Finally, with constant temperature and pressure, an unconstrained molecular dynamics simulation of 50 ns was performed. The MMGBSA and residue-free energy decomposition calculations were made based on the generated trajectory. The whole simulation process used CUDA8.0 software to support the GPU acceleration workCitation31,Citation32.
4.2.5. Metal binding studies
The metal chelation property was studied by Shimadzu UV-2450 spectrophotometer using CuCl2, ZnCl2, FeSO4, and AlCl3 at 200–600 nm. Moreover, stoichiometry was investigated by titrating the solution of derivatives with increasing CuCl2. The procedure referenced our previous workCitation29,Citation30.
4.2.6. Antioxidant activity assay
The ORAC-FL assay was employed to determine the antioxidant potency and referenced our previous workCitation29,Citation30.
4.2.7. Effect of test compounds on self-induced Aβ1–42 aggregation assay
The inhibition and disaggregation experiments towards self- and Cu2+-mediated Aβ1–42 aggregation were performed using ThT fluorescence assay. The detailed procedure is described in the previous workCitation25,Citation29,Citation30.
4.2.8. Inhibition of monoamine oxidase
The recombinant huMAO-A and huMAO-B were obtained from Sigma–Aldrich. All enzymatic reactions were quantified on a Varioskan Flash Multimode Reader (PerkinElmer) and the detailed experiments referenced our previous workCitation23,Citation24.
Author contributions
Zhipei Sang, Yong Deng, and Li Zhang originated the concept and design. Zhipei Sang was responsible for synthesis, evaluation of in vitro, data analysis, and wrote the manuscript. Qing Song and Zhongcheng Cao were responsible for the synthesis of target compounds and evaluation in vitro.
Supplemental Material
Download PDF (657.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barnett R. Alzheimer's disease. Lancet 2019;393:1589.
- Patterson C. World Alzheimer Report 2018—the state of the art of dementia research: new frontiers. London: Alzheimer’s Disease International (ADI); 2018: 1–48.
- Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov 2010;9:387–98.
- Viña J, Sanz-Ros J. Alzheimer's disease: only prevention makes sense. Eur J Clin Invest 2018;48:e13005.
- Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci 2018;19:687–700.
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297: 353–6.
- Nalivaeva NN, Turner AJ. Targeting amyloid clearance in Alzheimer's disease as a therapeutic strategy. Br J Pharmacol 2019;176:3447–63.
- Wang YJ. Alzheimer disease: lessons from immunotherapy for Alzheimer disease. Nat Rev Neurol 2014;10:188–9.
- Cavalli A, Bolognesi ML, Minarini A, et al. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem 2008;51:347–72.
- Zhang P, Xu S, Zhu Z, Xu J. Multi-target design strategies for the improved treatment of Alzheimer's disease. Eur J Med Chem 2019;176:228–47.
- de Freitas Silva M, Dias KST, Gontijo VS Jr., et al. Multi-target directed drugs as a modern approach for drug design towards Alzheimer's disease: an update. Curr Med Chem 2018;25:3491–525.
- Lee S, Zheng X, Krishnamoorthy J, Savelieff MG, et al. Rational design of a structural framework with potential use to develop chemical reagents that target and modulate multiple facets of Alzheimer's disease. J Am Chem Soc 2014;136:299–310.
- Li Q, He S, Chen Y, et al. Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer's disease. Eur J Med Chem 2018;158:463–77.
- Unzeta M, Esteban G, Bolea I, et al. Multi-target directed donepezil-like ligands for Alzheimer's disease. Front Neurosci 2016;10:205.
- Anand A, Patience AA, Sharma N, Khurana N. The present and future of pharmacotherapy of Alzheimer's disease: a comprehensive review. Eur J Pharmacol 2017;815:364–75.
- Cheignon C, Tomas M, Bonnefont-Rousselot D, et al. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol 2018;14:450–64.
- Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol 2004;3:431–4.
- Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol 2014;10:9–17.
- Schedin-Weiss S, Inoue M, Hromadkova L, et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res Ther 2017;9:57.
- Youdim MBH. Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases. J Neural Transm 2018;125:1719–33.
- Zhuang C, Zhang W, Sheng C, et al. Chalcone: a privileged structure in medicinal chemistry. Chem Rev 2017;117:7762–810.
- Zhang X, Rakesh KP, Bukhari SNA, et al. Multi-targetable chalcone analogs to treat deadly Alzheimer's disease: current view and upcoming advice. Bioorg Chem 2018;80:86–93.
- Sang Z, Wang K, Zhang P, et al. Design, synthesis, in-silico and biological evaluation of novel chalcone derivatives as multi-function agents for the treatment of Alzheimer's disease. Eur J Med Chem 2019;180:238–52.
- Bai P, Wang K, Zhang P, et al. Development of chalcone-O-alkylamine derivatives as multifunctional agents against Alzheimer's disease. Eur J Med Chem 2019;183:111737.
- Sang Z, Song Q, Cao Z, et al. Design, synthesis and evaluation of novel dimethylamino chalcone-O-alkylamines derivatives as potential multifunctional agents against Alzheimer's disease. Eur J Med Chem 2021;216:113310.
- Lee GY, Han SN. The role of vitamin E in immunity. Nutrients 2018;10:1614.
- Qiang X, Sang Z, Yuan W, et al. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem 2014;76:314–31.
- Sang Z, Qiang X, Li Y, et al. Design, synthesis and evaluation of scutellarein-O-alkylamines as multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem 2015;94:348–66.
- Sang Z, Wang K, Shi J, et al. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer's disease. Eur J Med Chem 2020;192:112180.
- Sang Z, Wang K, Shi J, et al. Apigenin-rivastigmine hybrids as multi-target-directed ligands for the treatment of Alzheimer's disease. Eur J Med Chem 2020;187:111958.
- Wang LL, Du Y, Li SM, et al. Design, synthesis and evaluation of tetrahydrocarbazole derivatives as potential hypoglycemic agents. Bioorg Chem 2021;115:105172.
- Sharma P, Tripathi A, Tripathi PN, et al. Design and development of multitarget-directed N-benzylpiperidine analogs as potential candidates for the treatment of Alzheimer's disease. Eur J Med Chem 2019;167:510–24.
- Tripathi A, Choubey PK, Sharma P, et al. Design and development of molecular hybrids of 2-pyridylpiperazine and 5-phenyl-1,3,4-oxadiazoles as potential multifunctional agents to treat Alzheimer's disease. Eur J Med Chem 2019;183:111707.
- Tripathi PN, Srivastava P, Sharma P, et al. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg Chem 2019;85:82–96.
- Di L, Kerns EH, Fan K, et al. High throughput artificial membrane permeability assay for blood-brain barrier. Eur J Med Chem 2003;38:223–32.
- Sang Z, Wang K, Bai P, et al. Design, synthesis and biological evaluation of novel O-carbamoyl ferulamide derivatives as multi-target-directed ligands for the treatment of Alzheimer's disease. Eur J Med Chem 2020;194:112265.