Abstract
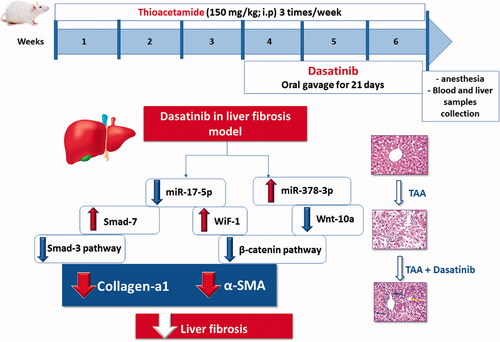
Hepatic stellate cells activation (HSCs) plays a crucial role in the pathogenesis of liver fibrosis. Specific microRNAs have been suggested to affect the activation of HSCs via various signalling pathways including TGF-β/smads and Wnt/β-catenin pathways. Dasatinib is a multitarget inhibitor of many tyrosine kinases has recently studied for its anti-fibrotic effects in a variety of fibrous diseases. This study investigated the role of modulation of miRNA-378 and miRNA-17 in the pathogenesis of liver fibrosis through altering Wnt/β-catenin and TGF-β/smads pathways and evaluated the beneficial effect of the tyrosine kinase inhibitor, dasatinib, in thioacetamide-induced liver fibrosis model in mice. Treatment with dasatinib down-regulated miRNA-17 expression, leading to the restoration of WiF-1 and smad-7 which cause the inhibition of both Wnt/β-catenin and TGF-β/smads signalling. In addition, it upregulated miRNA-378 leading to the decrease of Wnt-10 which contributes to the suppression of activated HSCs.
Graphical Abstract
Keywords:
1. Introduction
Chronic liver diseases (CLDs) are a major public health issue that affects people all over the world. CLDs affect 844 million people worldwide, resulting in two million deaths each year, according to estimatesCitation1. Liver fibrosis is characterised by an excessive deposition of extracellular matrix (ECM) proteins in liver, mainly synthesised by activated hepatic stellate cells (HSCs)Citation2. Hundreds of thousands of people worldwide suffer from liver fibrosis, which is caused in part by the obesity epidemic, as well as the high prevalence of alcohol addiction and viral hepatitisCitation3. Hepatitis C virus (HCV) is the leading cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (HCC). Around 55–85% of HCV-infected patients become chronic active cases and go through the stages of fibrosis, cirrhosis, and possibly HCCCitation4.
TGF-β/smad, p38 MAPK, and other pathways have all been linked to the development of liver fibrosis. However, the molecular mechanism is unknown, and there is no effective treatment availableCitation5. Exploration of new signal pathways and the development of novel therapeutic strategies are thus urgently required. Previous studies have shown that an abnormal Wnt/β-catenin signalling pathway plays a key role in the development of organ fibrosis, accelerates HSC activation including cell proliferation and ECM accumulation, and may be a novel therapeutic target in fibrotic disordersCitation6. Single Wnt ligands can activate multiple signalling pathways, increased gene expression of Wnt-1, Wnt-10, and β-catenin were observed in lung fibrosisCitation7. Overexpression of Wnt-10 results in progressive loss of subcutaneous adipose tissue accompanied by dermal fibrosis and increased expression of fibrotic genesCitation8. Several secreted protein families antagonise Wnt/β-catenin signalling. The function of Wnt inhibitors depends on their expression levels and the cellular context. The secreted Frizzled-related proteins (sFRPs) and Wnt inhibitory factor (WIF), which exhibit a high degree of homology with the Wnt ligand-binding domains of Fzd, both bind to Wnt ligands, and thereby function as Wnt antagonists for both β-catenin and noncanonical signallingCitation9.
MicroRNAs are short RNA sequences that regulate gene expression by destabilising mRNA and inhibiting mRNA translationCitation10. Specific microRNAs have been recognised to play a role in the activation of HSCs through a variety of signalling pathwaysCitation11,Citation12. HSC activation involves many signalling pathways such as TGFβ/smads signalling and Wnt/β-catenin pathwayCitation13. MiR-17 is reported to synergistically trigger fibrosis development via its target genes smad-7Citation14 and WIFICitation15. MiR-17 is increased in carbon tetrachloride (CCl4)-treated rat liver fibrotic tissues due to the negative role of smad-7 in TGF-β/smad signalling. Inhibition of miR-17 suppressed proliferation of HSCs, ECM production and α-smooth muscle actin (α-SMA) expression induced by TGF-β1Citation16. In addition, overexpression of miR-17–5 and suppression of WIFI enhanced the Wnt/β-catenin pathway in liver fibrotic tissues; earlier research has shown that WIFI is a direct downstream target of miR-17Citation15,Citation17. Recently, miR-378a has been reported to be down-regulated in fibrotic liver tissues and inhibits HSC activation via targeting of Wnt-10Citation18. Overexpression of miR-378a resulted in the suppression of HSC activation including HSC proliferation, α-SMA and type-I collagenCitation19.
Dasatinib is a second-generation oral multitarget inhibitor of many tyrosine kinasesCitation10,Citation20. Dasatinib was designed to treat some types of cancers including chronic myeloid leukaemia (CML)Citation21. Dasatinib has recently been studied for its anti-fibrotic effects in a variety of fibrous diseases, including systemic sclerosis, lung fibrosis, and chronic pancreatitis. Through the TKs/GSK3/β-catenin pathway, dasatinib inhibits the proliferation and activation of pancreatic stellate cells (PSCs)Citation21,Citation22. The current study aims to investigate the potential efficacy and the molecular mechanisms of dasatinib in the treatment of thioacetamide-induced liver fibrosis by modulating miR-378 and miR-17 via the Wnt/β catenin and TGF-/smad pathways.
2. Materials and methods
2.1. Animals
Male albino mice weighing between 15 and 20 g were used in this study. The Egyptian Company for the Production of Vaccines, Sera, and Drugs provided these mice (EGYVAC; Cairo, Egypt). Mice were housed in plastic cages at October University for Modern Sciences and Arts’ animal house under constant conditions (temperature 25 ± 3 °C and humidity 50%). Free water and standard pellet chow (El-Nasr Co., Egypt) were available. The study was approved from the ethics committee of October University for Modern Sciences and Arts.
2.2. Drugs and chemicals
Dasatinib and thioacetamide were purchased from Sigma-aldrich (Saint Louis, MO, USA). The other chemicals used were all of analytical grade.
2.3. Induction of liver fibrosis
For the induction of liver fibrosis, thioacetamide (150 mg/kg; i.p) dissolved in saline was injected three times a week for 6 weeks. The method of induction of liver fibrosis was chosen based on previous researchCitation23,Citation24.
2.4. Experimental design
Mice were categorised into three groups (n = 6) at random. The first set of mice served as the normal control group. thioacetamide (150 mg/kg; i.p.) was given to the liver fibrosis control group. The third group was treated with dasatinib (20 mg/kg/day; p.o.) for 21 days starting from the 4th week of the experiment. Based on previous research, the dose and route of administration of dasatinib were determinedCitation22.
At the end of the 6th week, blood samples were collected via the retro-orbital plexus for serum separation and liver enzymes investigation. Liver enzymes were analysed using commercial kits (Biodiagnostic; cairo, Egypt).
The mice were then sacrificed via cervical dislocation under ether anaesthesia, and the livers were quickly dissected out and washed in ice-cold saline. RNA extraction from the isolated livers were used for analysis of the expression of miR-378-3p, miR-17-5p, Wnt-10a, WiF-1, β-catenin, smad-7, smad-3 and collagen-a1 through qRT-PCR. Sections of the isolated livers were fixed in formalin and used for the histopathological examination as well as the investigation of the immunohistochemical reactivity of TNF-α and α-SMA.
2.5. Quantitative real-time polymerase chain reaction (RT-PCR)
The isolated livers were used for total RNA isolation using Trizol (Invitrogen; Auckland, New Zealand), according to the manufacturer’s instructions and reverse-transcribed into cDNA with the Reverse Transcriptase M-MLV (Promega, Madison, WI, USA).
Primer sequences to be used in the experiment were as follows:
For miRNA quantitative reverse transcriptase PCR, small RNA species-enriched RNA was isolated according to the manufacturer’s instructions (mirVana miRNA isolation kit; Ambion, Austin, TX, USA). miRNA was reverse-transcribed by using Ncode miRNA first-strand complementary DNA synthesis kits (Invitrogen). Quantitative reverse transcriptase PCR was performed by using a Power SYBR Green PCR Master Mix on the CFX96 Instrument (Bio-Rad, USA). Data analysis was determined by using the relative standard curve method.
2.6. Histopathologic assessment of hepatic tissue damage
The livers from the different groups were fixed in 10% formalin solution. Sections of the livers were collected on glass slides, deparaffinised and stained by haematoxylin and eosin stain for routine histopathological examination using electric light microscope. This is according to the method previously described by JD Bancroft and M GambleCitation25.
2.7. Immunohistochemical reaction of TNF-α and α-SMA
Sections from liver tissue of around 3 µm thickness embedded in paraffin were used for detection of TNF-α and α-SMA through the immunostaining with primary antibody polyclonal immunoglobulin-G of mice TNF-α and α-SMA according the method previously described byCitation26. Finally, grading of immunohistochemical reactivity was measured from four randomly chosen fields in each section and averaged using image analysis software (Image J, Fiji version; MD, USA).
2.8. Statistical analysis
Data are presented in the form of mean ± SEM. The comparisons among means of different groups were done via one-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparisons post-test Citation27. Kruskal–Wallis test was used for analysing the histopathological scores and followed by Dunn’s multiple comparisons test. The level of significance was taken as p ˂ .05. All the statistical tests carried out using GraphPad Prism software package, version 5 (GraphPad Software, Inc., USA).
3. Results
3.1. Effect of dasatinib on serum levels of liver enzymes in liver fibrosis
Thioacetamide resulted in significant increase in the serum levels of liver enzymes. Alanine transaminase (ALT) was increased by 1.67-fold and aspartate transaminase was increased by 2.44-fold in the inducted liver fibrosis group compared to the control group. On the other hand, treatment with dasatinib significantly lowered the serum levels of ALT by 10.67% and AST by 46.32% compared to the mice with liver fibrosis ().
Figure 1. (A, B) Effect of dasatinib treatment on serum levels of the liver enzymes; alanine transaminase (ALT) and aspartate transaminase (AST) in mice with thioacetamide-induced liver fibrosis. The data are presented as mean ± SEM (n = 6). aSignificant difference from the control group; bsignificant difference from liver fibrosis-inducted group (at p ˂ .05)

3.2. Effect of dasatinib on miR-378 and Wnt-10a/β-catenin signalling in liver fibrosis
The mice with liver fibrosis exhibited a significantly suppressed expression of miR-378-3p in the liver (with a 67% decrease compared to the control group). This effect was accompanied by a significantly elevated expression of Wnt-10a and β-cantenin (475 and 4.64-fold elevation compared to the control group). These effects were abolished by treatment with dasatinib which significantly increased the liver expression level of miR-378-3p with 2.24-fold and decreased the expression level of Wnt-10a and β-cantenin in the liver by 40.0% and 32.5%, respectively compared to the mice with liver fibrosis ().
Figure 2. Effect of dasatinib treatment on relative expression of miRNA-378-3p, Wnt-10a and β-catenin in the liver tissue of mice with thioacetamide-induced liver fibrosis. The data are presented as mean ± SEM (n = 6). aSignificant difference from the control group; bsignificant difference from liver fibrosis-inducted group (at p ˂ .05).

3.3. Effect of dasatinib on miR-17 and smad-7/smad-3 signalling in liver fibrosis
A significant 9.9-fold elevation was observed in the hepatic level of miR-17-5p in the liver fibrosis group compared to the control group. On the other hand, treatment with dasatinib significantly lowered the hepatic level of miR-17-5p by 50.5% compared with the liver fibrosis mice ().
Figure 3. (A–E) Effect of dasatinib treatment on relative expression of miRNA-17-5p, smad-7, smad-3, collagen a1 and Wnt inhibitory factor-1 (WIF-1) in liver tissue of mice with thioacetamide-induced liver fibrosis. The data are presented as mean ± SEM (n = 6). aSignificant difference from the control group; bsignificant difference from liver fibrosis inducted-group (at p ˂ .05).

In addition, the results of the current study indicated that the hepatic level of smad-7 was significantly reduced by 58.0%, whereas hepatic levels of smad-3 and collagen a1 were raised by 4.5- and 6.3-fold, respectively in the liver fibrosis group compared to the control group. Conversely, treatment with dasatinib resulted in a significant 3.1-fold elevation in the hepatic level of smad-7 and a significant decline in smad-3 and collagen a1 by 49.2% and 51.9%, respectively compared to the liver fibrosis group ().
Furthermore, the impact of the modulation of miR-17 was reflected on the hepatic expression level of WiF-1 that is significantly reduced by 62% in the liver fibrosis group compared to the control group. Treatment with dasatinib significantly elevated the hepatic expression level of Wif-1 by 3.09-fold compared to the liver fibrosis group ().
3.4. Effect of dasatinib on immunohistochemical reactivity of TNF-α and α-SMA in liver fibrosis
Liver sections from normal control mice () showed relatively negative expression of α-SMA. Thioacetamide resulted in significantly increased expression of α-SMA in the hepatic parenchyma (). The group treated with dasatinib showed only weak expression of α-SMA in the hepatic tissue () with no significant difference from the control group. The immuno-staining for TNF-α revealed weak expression in the hepatic tissue of the normal control mice (). The expression of TNF-α increased markedly in the hepatocytes surrounding the central vein and the hepatocytes surrounding the portal area upon induction of liver fibrosis (). Hepatic tissue from the dasatinib-treated mice showed noticeable suppression in TNF-α expression (). Comparative quantification of the immunohistochemical expression for α-SMA and TNF-α in hepatic tissue of mice from all groups is presented in expressed as area % of the brown colour according to image J software.
Figure 4. Immunostaining of α-smooth muscle actin (α-SMA) and tumour necrosis factor-α (TNF-α) in the liver tissue of mice with thioacetamide-induced liver fibrosis (H&E × 40). (A) α-SMA/control group, (B) α-SMA/liver fibrosis group, (C) α-SMA/dasatinib-treated group, (E) TNF-α/control group, (F) TNF-α/liver fibrosis group, (G) TNF-α/dasatinib-treated group, (D, H) represent a comparative quantification of the immunohistochemical expression for α-SMA and TNF-α in hepatic tissue of mice from all groups: The severity of the immunoactivity is depending on the intensity and distribution of the brown colour. aRepresents a significant difference from the normal control group, ba significant difference from liver fibrosis inducted-group (at p ˂ .05).

3.5. Effect of dasatinib treatment on histopathological alterations of liver
Histological examination of liver sections from control mice revealed normal histological structures of the central vein and the surrounding hepatocytes on the parenchyma with no histopathological alteration (). Liver sections from mice received thioacetamide alone showed degeneration and necrobiotic changes observed in the hepatocytes and associated with focal inflammatory cells infiltration in a diffuse manner in between (). Treatment with dasatinib resulted in the presence of minor degeneration in some hepatocytes with marked improvement from the liver fibrosis-inducted group in addition to the presence of some inflammatory cells infiltration (). Scoring of the histological observations in the hepatic tissue is presented in .
Figure 5. Effect of dasatinib treatment on the histopathological alterations in the liver tissue in mice with thioacetamide-induced liver fibrosis (H&E × 16): (A) control group, (B) liver fibrosis group, (C) dasatinib-treated group, (D) scoring of the histological observations in the hepatic tissue from all groups. Data are presented as mean ± SEM of 6 random non-overlapping fields/section. aSignificant difference from the control group, bsignificant difference from liver fibrosis inducted-group (at p ˂ .05).

Discussion
Aside from the TGF/smads signalling pathwayCitation28, the Wnt/β-catenin pathway has been shown to play a role in stellate cell/fibroblast activation and fibrosis in a number of organs, including the liver, kidney, lung, and pancreas, making it a possible therapeutic target for liver fibrosis. Some microRNAs are supposed to be essential regulators of these signalling pathways and these hepatic stellate cells activation (HSCs)Citation28–30. The present study hypothesised that dasatinib would attenuate liver fibrosis and ameliorate the inflammatory responses. For this purpose, we investigated the potential efficacy and mechanisms of dasatinib in the treatment of thioacetamide-induced liver fibrosis in mice through the modulation of miR-378 and miR-17 that can target Wnt-10 and WIF1, respectively, and inhibit the Wnt/β-catenin pathway.
Many biological processes are controlled by the interaction of receptor tyrosine kinase signalling with Wnt/β-catenin signalling, but the mechanisms of this interaction are still unknown. The role of various receptor tyrosine kinase systems in activating canonical Wnt signalling is suggested by the potent activation of Wnt/β-catenin by FGFR2, FGFR3, EGFR, and TRKA kinases.Citation31 Dasatinib is a second-generation oral multitarget inhibitor of multiple tyrosine kinases that was originally designed to treat CML. Dasatinib was recently investigated for having an anti-fibrotic effect in some of the fibrous diseases, including systemic sclerosis, lung, and pancreatic fibrosisCitation22,Citation32. Dasatinib inhibited TGF-induced myofibroblast differentiation and ECM fibronectin expression in both HFLFs and NIH3T3 cells, according to Abdalla et alCitation33.
Generally, HSC activation is characterised by the accumulation of collagens, the enhancement of α-SMA expression and the increase of cell proliferationCitation34. Wnt-10 overexpression results in progressive loss of subcutaneous adipose tissue, dermal fibrosis, and up-regulation of fibrotic gene expression, which increased collagen aggregation and α-SMA expressionCitation22. MicroRNAs, have been implicated in the pathogenesis of many diseasesCitation35. In the current study, we observed that miR-378a expression was markedly decreased in fibrotic liver tissues, while Wnt-10 expression increased significantly. Furthermore, the dasatinib-treated group revealed a significant increase in the miR-378 expression accompanied by marked suppression of Wnt-10 expression in liver tissue. Thus, we suggest that dasatinib can suppress the HSCs activation which is a critical event in the development of liver fibrosis through the markedly increased expression of miR-378a accompanied by the suppression of Wnt-10 expression. Our finding is matched with another study reported that miR-378a was down-regulated accompanied by increased activation of HSCs in rats with CCl4-induced liver fibrosisCitation18. In the current study, the increased expression of miR-378a-3p upon treatment with dasatinib can have a direct role in suppression of the activated HSCs. This effect was reflected through the significantly decreased levels α-SMA and type-I collagen as markers for HSCs activation.
Aberrant Wnt/β-catenin pathway contributes to the development of liver fibrosisCitation36. Wnt/β-catenin pathway activation contributes also to HSC activation and ECM accumulationCitation37. In this study, we demonstrated that miR-17 expression was increased in fibrotic liver tissues, along with a marked reduction in WIF1 and smad-7 expression levels. Dasatinib inhibits miR-17, resulting in increased expression of both WIF1 and smad-7, which are miR-17’s targets. Our findings are consistent with previous research that found WIF1, a Wnt antagonist, can reduce hepatic fibrosis by inhibiting the Wnt/β-catenin pathwayCitation38. WIF1 was predicted to be a putative target of miR-17, which induced HSC activation, according to Peng et alCitation39. Our findings are supported by Yu et al. study, which found that miR-17 promotes HSC activation by reducing smad-7, implying that it may be useful as a new therapeutic target for liver fibrosisCitation14. Smad-7 overexpression inhibits smad-3 phosphorylation, which decreases TGF-mediated fibrogenesis and protects against liver damageCitation40, consequently, smad-7 acts as a negative regulator of HSC activation and hepatic fibrosis. Loss of smad-7 has been reported in fibrotic liver and during HSC activation induced by TGF-β1Citation41.
Our results not only provide a new insight into the role of miRNA-activated TGF-β1/smad and Wnt/β-catenin signalling in liver fibrosis but also show a new anti-fibrotic mechanism of dasatinib in liver fibrosis. The current study demonstrates that dasatinib can down-regulate miR-17 expression, leading to the restoration of WIF1 and smad-7 which further cause the inhibition of both Wnt/β-catenin and TGF-β/smads signalling. In addition, dasatinib can upregulate miR-378a leading to decrease in Wnt-10 expression which contributes to the suppression of activated HSCs. To sum up, we suggest that dasatinib can be a potential therapeutic drug for liver fibrosis due to its crucial role in suppressing various fibrotic signalling pathways and its ability to suppress HCS activation and EMC deposition.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large‐scale screening. Liver Int 2018;38:2–6.
- Huang YH, Chen MH, Guo QL, et al. Interleukin-10 induces senescence of activated hepatic stellate cells via STAT3-p53 pathway to attenuate liver fibrosis. Cell Signal 2020;66:109445.
- Duval F, Moreno-Cuevas JE, González-Garza MT, et al. Protective mechanisms of medicinal plants targeting hepatic stellate cell activation and extracellular matrix deposition in liver fibrosis. Chinese Med 2014;9:27.
- Gomaa A, Allam N, Elsharkawy A, et al. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 2017;9:17–25.
- Tang PM-K, Zhang Y-Y, Lan H-Y. LncRNAs in TGF-β-driven tissue fibrosis. Non-Coding RNA 2018;4:26.
- Nishikawa K, Osawa Y, Kimura K. Wnt/β-catenin signaling as a potential target for the treatment of liver cirrhosis using antifibrotic drugs. Int J Mol Sci 2018;19:3103.
- Baarsma H, Königshoff M. ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax 2017;72:746–59.
- Kruglikov IL. Interfacial adipose tissue in systemic sclerosis. Curr Rheumatol Rep 2017;19:4.
- Zeng X, Zhang Y, Xu H, et al. Secreted frizzled related protein 2 modulates epithelial-mesenchymal transition and stemness via Wnt/β-catenin signaling in choriocarcinoma. Cell Physiol Biochem 2018;50:1815–31.
- Zaafan MA, Abdelhamid AM. The cardioprotective effect of microRNA-103 inhibitor against isoprenaline-induced myocardial infarction in mice through targeting FADD/RIPK pathway. Eur Rev Med Pharmacol Sci 2021;25:837–44.
- Shaker OG, Ayeldeen G, Abdelhamid AM. Circulating microRNA-944 and its target gene EPHA7 as a potential biomarker for colorectal cancer. Arch Physiol Biochem 2020:1–7.
- Lambrecht J, Mannaerts I, van Grunsven LA. The role of miRNAs in stress-responsive hepatic stellate cells during liver fibrosis. Front Physiol 2015;6:209.
- Dituri F, Cossu C, Mancarella S, Giannelli G. The interactivity between tgfβ and bmp signaling in organogenesis, fibrosis, and cancer. Cells 2019;8:1130.
- Yu F, Guo Y, Chen B, et al. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab Invest 2015;95:781–9.
- Yu F, Lu Z, Huang K, et al. MicroRNA-17-5p-activated Wnt/β-catenin pathway contributes to the progression of liver fibrosis. Oncotarget 2016;7:81–93.
- Ganguly N, Chakrabarti S. Role of long non‑coding RNAs and related epigenetic mechanisms in liver fibrosis. Int J Mol Med 2021;47:1.
- Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 2017;8:90579–604.
- Yu F, Fan X, Chen B, et al. Activation of hepatic stellate cells is inhibited by microRNA-378a-3p via Wnt10a. Cell Physiol Biochem 2016;39:2409–20.
- Machado IF, Teodoro JS, Palmeira CM, Rolo AP. miR-378a: a new emerging microRNA in metabolism. Cell Mol Life Sci 2020;77:1947–58.
- Araujo JC, Mathew P, Armstrong AJ, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1-2 study. Cancer 2012;118:63–71.
- McCafferty EH, Dhillon S, Deeks ED. Dasatinib: a review in pediatric chronic myeloid leukemia. Paediatr Drugs 2018;20:593–600.
- Zeng X-P, Wang L-J, Guo H-L, et al. Dasatinib ameliorates chronic pancreatitis induced by caerulein via anti-fibrotic and anti-inflammatory mechanism. Pharmacol Res 2019;147:104357.
- Choi S, Jung HJ, Kim MW, et al. A novel STAT3 inhibitor, STX-0119, attenuates liver fibrosis by inactivating hepatic stellate cells in mice. Biochem Biophys Res Commun 2019;513:49–55.
- Yang HY, Kim KS, Lee YH, et al. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int J Biol Sci 2019;15:800–11.
- Bancroft JD, Gamble M. Theory and practice of histological techniques. Amsterdam, The Netherlands: Elsevier Health Sciences; 2008.
- Zaafan MA, Haridy AR, Abdelhamid AM. Amitriptyline attenuates bleomycin-induced pulmonary fibrosis: modulation of the expression of NF-κβ, iNOS, and Nrf2. Naunyn-Schmiedeberg’s Arch Pharmacol 2019;392:279–86.
- Stoline M. The status of multiple comparisons: simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. J Am Stat Assoc. 1981;35:134–41.
- Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 2013;25:264–68.
- Cao H, Wang C, Chen X, et al. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Scientific Rep 2018;8:13644.
- Xiao W, Jiang W, Shen J, et al. Retinoic acid ameliorates pancreatic fibrosis and inhibits the activation of pancreatic stellate cells in mice with experimental chronic pancreatitis via suppressing the Wnt/β-catenin signaling pathway. PLoS One 2015;10:e0141462.
- Krejci P, Aklian A, Kaucka M, et al. Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS One 2012;7:e35826.
- Martyanov V, Kim G-HJ, Hayes W, et al. Novel lung imaging biomarkers and skin gene expression subsetting in dasatinib treatment of systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12:e0187580.
- Abdalla M, Thompson L, Gurley E, et al. Dasatinib inhibits TGFβ-induced myofibroblast differentiation through Src-SRF Pathway. Eur J Pharmacol 2015;769:134–42.
- He Y, Huang C, Zhang S-P, et al. The potential of microRNAs in liver fibrosis. Cell Signal 2012;24:2268–72.
- Shaker O, Ayeldeen G, Abdelhamid A. The impact of single nucleotide polymorphism in the long non-coding MEG3 gene on microRNA-182 and microRNA-29 expression levels in the development of breast cancer in Egyptian women. Front Genet 2021;12:683809.
- Kim KK, Wei Y, Szekeres C, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 2009;119:213–24.
- Kordes C, Sawitza I, Häussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun 2008;367:116–23.
- Cheng JH, She H, Han YP, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2008;294:G39–49.
- Peng H, Wan LY, Liang JJ, et al. The roles of lncRNA in hepatic fibrosis. Cell and Biosci 2018;8:1–8.
- Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chemico-Biol Inter 2018;292:76–83.
- Meng X-m, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nature Rev Nephrol 2016;12:325–38.
