?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Isocoumarins, isomeric to comarins which act as effective carbonic anhydrase (CA, EC 4.2.1.1) inhibitors, were investigated for the first time as inhibitors of this enzyme. A series of 3-substituted and 3,4-disubstituted isocoumarins incorporating phenylhydrazone, 1-phenyl-pyrazole and pyrazolo-substituted pyrimidine trione/thioxo-pyrimidine dione moieties were investigated for their interaction with four human (h) CA isoforms, hCA I, II, IX and XII, known to be important drug targets. hCA I and II were not inhibited by these compounds, whereas hCA IX and XII were inhibited in the low micromolar range by the less bulky derivatives. The inhibition constants ranged between 2.7–78.9 µM against hCA IX and of 1.2–66.5 µM against hCA XII. As for the coumarins, we hypothesise that the isocoumarins are hydrolysed by the esterase activity of the enzyme with formation of 2-carboxy-phenylacetic aldehydes which act as CA inhibitors. Isocoumarins represent a new class of CA inhibitors.
1. Introduction
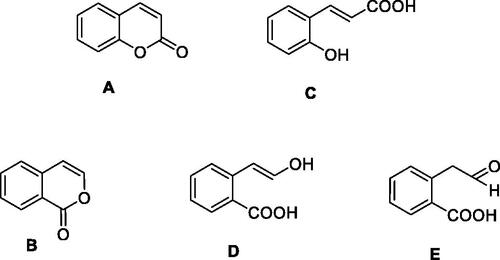
Isocoumarins, both naturally occurringCitation1 and synthetic such derivativesCitation2, similar to the isomeric comarinsCitation3, possess a multitude of applications in the drug design of pharmacologically relevant derivativesCitation3,Citation4. These two privileged scaffolds A and B () probably find many such applications due to the fact that the bicyclic ring system found in them combines a rather stable, planar aromatic scaffold with a good reactivity due to the lactone ring present in both derivatives, combined with the relative facility of derivatization at diverse pharmacophoric points with the possibility to generate new chemical spaceCitation1–4. A salient feature of coumarins and isocoumarins is the relatively facile hydrolysis of their lactone ring with formation of 2-hydroxycinammic acid C (from coumarin) and 2-carboxy-phenylacetic aldehyde E from isocoumarins, as the enol D is unstable and spontaneously converts to ECitation1–4 ().
Coumarins were by far the most investigated class of such compounds, also because some of them are clinically used as anticoagulants for decadesCitation5 and were more recently investigated in detail as carbonic anhydrase (CA, EC 4.2.1.1) inhibitorsCitation6.
2. Materials and methods
2.1. General
All chemicals and anhydrous solvents were purchased from Sigma-Aldrich, Merck, Across Organics and TCI and used without further purification. Melting points (mp) were determined with SMP30 melting point apparatus in open capillaries and are uncorrected. FT-IR spectra were recorded by using Perkin Elmer Spectrum 100 FT-IR spectrometer. Nuclear Magnetic Resonance (1H-NMR and 13C-NMR) spectra of compounds were recorded using a an Agilent-NMR-vnmrs400 MHz and Bruker 300 MHz spectrometer in DMSO-d6 and TMS as an internal standard operating at 300 MHz for 1H-NMR and 75 MHz for 13C-NMR. Thin layer chromatography (TLC) was carried out on Merck silica gel 60 F254 plates.
2.2. General procedure for the synthesis of 3–(1-(2-phenylhydrazine) ethyl)-isochrom-1-one derivatives X(1–5)
The methyl ketone (10 mmol) and phenylhydrazine derivative compounds were added to a reaction flask by adding 20 ml EtOH with a catalytic amount of acetic acid and refluxed for 2 h. After the reaction complete, the obtained compounds were filtered off and crystallised from ethanol. The final products X(1–5) were dried under vacuum and fully characterised by FT-IR, 1H-NMR, 13C-NMR, and melting points.
3–(1-(2-phenylhydrazono)ethyl)-1H-isochromen-1-one (X1) Yield: 90%; mp: 202–204 °C; FT-IR (cm−1): 3294 (NH), 1695 (C = O); 1H-NMR (DMSO, δ, ppm): 2.21 (s, 3H), 6.94–7.69 (m, 10H), 8.28 (s, 1H).
3–(1-(2–(4-chlorophenyl) hydrazono)ethyl)-1H-isochromen-1-one (X2) Yield: 80%; mp: 238°–240 °C; FT-IR (cm−1): 3287 (NH), 1692 (C = O); 1H-NMR (DMSO, δ, ppm): 2.47 (s, 3H), 7.20–8.11 (m, 9H), 9.79 (s, 1H).
4–(2-(1–(1-oxo-1H-isochromen-3-yl)ethylidene)hydrazinyl) benzonitrile (X3) Yield: 82%; mp: 250°–252 °C; FT-IR (cm−1): 3271 (NH), 2222 (C≡N), 1692 (C = O), 1597 (C = N);1H-NMR (DMSO, δ, ppm): 2.45 (s, 3H), 7.29–8.12 (m, 9H), 10.18(s, 1H). 13C-NMR (DMSO, δ, ppm): 12.2, 82.9, 99.3, 101.1, 103.8, 113.8, 120.3, 120.4, 127.2, 129.2, 133.9, 135.7, 137.5, 137.7, 148.9, 152, 161.5, 175.4.
4-methyl-3–(1-(2-phenylhydrazono)ethyl)-1H-isochromen-1-one (X4) Yield: 82%; mp: 210°–212 °C; FT-IR (cm−1): 3271 (NH), 1733, 1709 (C = O); 1H-NMR (DMSO, δ, ppm): 2.17 (s, 3H), 2.47 (s, 3H), 7.18–8.17 (m, 9H), 9.57 (s, 1H). 13C-NMR (DMSO, δ, ppm): 13.4, 39.4, 109.3, 112.8, 113.4, 120.2, 124.6, 128.5, 129.3, 129.4, 135.6, 135.9, 138.9, 145.6, 149.6, 161.4.
3–(1-(2–(4-chlorophenyl) hydrazono)ethyl)-4-methyl-1H-isochromen-1-one (X5) Yield: 83%; mp: 240°–242 °C; FT-IR (cm−1): 3320 (NH), 1715 (C = O); 1H-NMR (DMSO, δ, ppm): 2.17 (s, 3H), 2.41 (s, 3H), 7.17–8.18 (m, 8H), 9.70 (s, 1H). 13C-NMR (DMSO, δ, ppm): 13.4, 40.6, 109.5, 114.8, 120.2, 123.5, 124.6, 128.6, 129.2, 129.3, 135.7, 136.8, 138.8, 144.6, 149.4, 161.3
2.3. General procedure for the synthesis of 3–(1-oxo-1H-isochromen-3-yl)-1-phenyl-pyrazole-4-carbaldehyde X(6–10) derivatives
The phenylhydrazone derivatives X (1–5) (10 mmol) and DMF (0.88 g, 12 mmol) were placed in a reaction flask and the POCl3 (1.84 g, 12 mmol) was added dropwise over the reaction mixture by keeping the temperature between 0° and 5 °C. After the completion of adding, the mixture was allowed to stir overnight at room temperature. Next day, the mixture was poured into the ice-water and it was triturated with 10% NaOH solution. The precipitate was filtered off and dried under vacuum at room temperature. The final products X(6–10) were fully characterised by FT-IR, 1H-NMR, 13C-NMR, and melting points.
3–(1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (X6) Yield: 82%; mp: 228°–230 °C; FT-IR (cm−1): 3124, 2921 (C-H), 1728 (C = O isocoumarin), 1676 (C = O aldehyde); 1H-NMR (DMSO, δ, ppm): 7.22–8.32 (m, 10H), 8.53 (s, 1H), 10.54 (s, 1H).
1–(4-chlorophenyl)-3–(1-oxo-1H-isochromen-3-yl)-1H-pyrazole-4-carbaldehyde (X7) Yield: 80%; mp: 258°–260 °C; FT-IR (cm−1): 3287, 3025 (C-H), 1716 (C = O isocoumarin), 1682 (C = O aldehyde); 1H-NMR (DMSO, δ, ppm): 7.58–8.55 (m, 9H), 9.03 (s, 1H), 10.58 (s, 1H).
4–(4-formyl-3–(1-oxo-1H-isochromen-3-yl)-1H-pyrazol-1-yl) benzonitrile (X8) Yield: 78%; mp: >300oC; FT-IR (cm−1): 3120–3078 (CH), 2228 (C≡N), 1712 (C = O isocoumarin), 1673 (C = O aldehyde); 1H-NMR (DMSO, δ, ppm): 7.69–8.24 (m, 9H), 9.50 (s, 1H), 10.31 (s, 1H).
3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (X9) Yield: 80%; mp: 201°–203 °C; FT-IR (cm−1): 3117, 2921 (C-H), 1718 (C = O isocoumarin), 1679 (C = O aldehyde); 1H-NMR (DMSO, ppm): 2.60 (s, 3H), 7.28–8.43 (m, 8H), 8.59 (s, 1H), 10.31 (s, 1H). 13C-NMR (DMSO, δ, ppm): 12.9, 113.5, 119.7, 121.1, 123.9, 124.7, 128.3, 128.8, 129.6, 129.8, 129.9, 134.9, 138.2, 138.8, 142.9, 146.8, 161.4, 185.5.
1–(4-chlorophenyl)-3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1H-pyrazole-4-carbaldehyde (X10) Yield:78%; mp: 288°–290 °C; FT-IR (cm−1): 3124, 2911 (C-H), 1715 (C = O isocoumarin), 1679 (C = O aldehyde); 1H-NMR (DMSO, δ, ppm): 2.41 (s, 3H), 7.65–8.30 (m, 8H), 9.40 (s, 1H), 10.09 (s, 1H) .
2.4. General procedure for the synthesis of X(11–20) derivatives
The aldehyde derivatives X(6–10) (10 mmol) was dissolved in acetic acid and the barbituric acid/2-thiobarbituric acid (10 mmol) was added over the mixture and stirred overnight at room temperature. Then, the mixture was filtered off and crystallised from ethanol to yield compounds X(11–20). The obtained final products were dried under vacuum and fully characterised by FT-IR, 1H-NMR, 13C-NMR, and melting points.
5-((3–(1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazol-4-yl) methylene) pyrimidine-2,4,6(1H,3H,5H)-trione (X11) Yield: 82%; mp: >300 °C; FT-IR (cm−1): 3235, 3081 (NH), 1738, 1722, 1680 (C = O), 1574 (C = N); 1H-NMR (DMSO, δ, ppm): 7.44–8.20 (m, 10H), 8.68 (s, 1H), 9.74 (s, 1H), 12.41 (s, 1H, NH), 12.45 (s, 1H, NH).
5-((1–(4-chlorophenyl)-3–(1-oxo-1H-isochromen-3-yl)-1H-pyrazol-4-yl) methylene) pyrimidine-2,4,6(1H,3H,5H)-trione (X12) Yield: 81%; mp: >300oC; FT-IR (cm−1): 3238, 3058 (NH), 1735, 1699, 1666 (C = O), 1571 (C = N); 1H-NMR (DMSO, δ, ppm): 7.20–8.20 (m, 9H), 8.62 (s, 1H), 9.69 (s, 1H), 11.29 (s, 1H, NH), 11.36 (s, 1H, NH).
4–(3-(1-oxo-1H-isochromen-3-yl)-4-((2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene) methyl)-1H-pyrazol-1-yl) benzonitrile (X13) Yield: 80%; mp: >300oC; FT-IR (cm−1): 3192, 3068 (NH), 2231 (-C≡N), 1735, 1712, 1676 (C = O), 1565 (-C = N); 1H-NMR (DMSO, δ, ppm): 7.37–8.17 (m, 9H), 8.56 (s, 1H), 9.71 (s, 1H), 11.28 (s, 1H, NH), 11.36 (s, 1H, NH).
5-((3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazol-4-yl) methylene) pyrimidine-2,4,6(1H,3H,5H)-trione (X14) Yield: 81%; mp: >300oC; FT-IR (cm−1): 3248, 3084 (NH), 1731, 1712, 1686 (C = O), 1568 (C = N); 1H-NMR (DMSO, δ d, ppm): 2.46 (s, 3H), 7.59–7.91 (m, 9H), 8.11 (s, 1H), 9.76 (s, 1H), 11.32 (s, 1H, NH), 11.33 (s, 1H, NH). 13C-NMR (DMSO, δ, ppm): 13.3, 114.8, 116, 117.2, 120.3, 120.8, 125, 128.8, 129.6, 130, 130.4, 134.8, 136.1, 137.6, 138.8, 142, 142.1, 150.2, 150.5, 161.1, 162.9, 163.6…
5-((1–(4-chlorophenyl)-3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1H-pyrazol-4-yl) methylene) pyrimidine-2,4,6(1H,3H,5H)-trione (X15) Yield: 82%; mp: >300oC; FT-IR (cm−1): 3163, 3042 (NH), 1738, 1709, 1666 (C = O), 1575 (C = N); 1H-NMR (DMSO, δ, ppm): 2.46 (s, 3H), 7.63–8.25 (m, 8H), 8.27 (s, 1H), 9.75 (s, 1H), 11.32 (s, 1H, NH), 11.33 (s, 1H, NH).
5-((3–(1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazol-4-yl) methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (X16) Yield: 82%; mp: >300oC; FT-IR (cm−1): 3143, 3055 (NH), 1761, 1715 (C = O), 1565 (C = N); 1H-NMR (DMSO, δ, ppm): 7.42–8.20 (m, 10H), 8.64 (s, 1H), 9.69 (s, 1H), 11.28 (s, 1H, 1NH), 11. 35 (s, 1H, 1NH). 13C-NMR (DMSO, δ, ppm): 107.3, 116.3, 116.5, 120.2, 120.6, 127.4, 128.9, 129.4, 129.9, 130.3, 135.5, 135.9, 136.8, 138.6, 143.9, 147.3, 149, 160.7, 161, 162, 162.7, 178.8.
5-((1–(4-chlorophenyl)-3–(1-oxo-1H-isochromen-3-yl)-1H-pyrazol-4-yl) methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (X17) Yield: 82%; mp: >300oC; FT-IR (cm−1): 3137, 3075 (NH), 1754, 1712, (C = O), 1558 (C = N); 1H-NMR (DMSO, δ, ppm): 7.42–8.18 (m, 9H), 8.65 (s, 1H), 9.72 (s, 1H), 12.39 (s, 1H, 1NH), 12. 45 (s, 1H, 1NH).
4–(4-((4,6-dioxo-2-thioxotetrahydropyrimidin-5(2H)-ylidene) methyl)-3–(1-oxo-1H-isochromen-3-yl)-1H-pyrazol-1-yl) benzonitrile (X18) Yield: 80%; mp: >300oC; FT-IR (cm−1): 3215, 3137 (NH), 2235 (C≡N), 1751, 1715 (C = O), 1574 (C = N); 1H-NMR (DMSO, δ, ppm): 2.46 (s, 3H), 7.47–8.26 (m, 9H), 8.28 (s, 1H), 9.79 (s, 1H), 12.43 (s, 2H, 2NH).
5-((3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1-phenyl-1H-pyrazol-4-yl) methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (X19) Yield: 81%; mp: >300oC; FT-IR (cm−1): 3147, 2902 (NH), 1705, 1666 (C = O), 1568 (C = N); 1H-NMR (DMSO, δ, ppm): 2.46 (s, 3H), 7.61–8.26 (m, 9H), 8.28 (s, 1H), 9.78 (s, 1H), 12.43 (s, 2H, 2NH).13C-NMR (DMSO, δ, ppm): 13.4, 115, 116.1, 117.4, 120.3, 120.8, 125, 128.9, 129.6, 130, 130.4, 135, 136.1, 137.6, 138.7, 141.9, 143, 150.4, 160.7, 161, 161.9, 172.9, 178.8.
5-((1–(4-chlorophenyl)-3–(4-methyl-1-oxo-1H-isochromen-3-yl)-1H-pyrazol-4-yl) methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (X20) Yield: 82%; mp: >300oC; FT-IR (cm−1): 3130, 2915 (NH), 1715, 1669 (C = O), 1568 (C = N); 1H-NMR (DMSO, δ, ppm): 7.47–8.26 (m, 8H), 8.42 (s, 1H), 9.76 (s, 1H), 11.35 (s, 2H, 2NH).
2.5. Ca inhibition assay
An SX.18 MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the inhibition of various CA isozymesCitation7. Phenol Red (at a concentration of 0.2 mM) has been used as an indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.4) as a buffer, 0.1 M Na2SO4 or NaClO4 (for maintaining constant the ionic strength; these anions are not inhibitory in the used concentration), following the CA-catalyzed CO2 hydration reaction for a period of 5–10 s. Saturated CO2 solutions in water at 25 °C were used as substrate. Stock solutions of inhibitors were prepared at a concentration of 10 mM (in DMSO-water 1:1, v/v) and dilutions up to 0.01 nM done with the assay buffer mentioned above. At least seven different inhibitor concentrations have been used for measuring the inhibition constant. Inhibitor and enzyme solutions were pre-incubated together for 15 min–6 h at 4 °C prior to assay, in order to allow for the formation of the E-I complex. Triplicate experiments were done for each inhibitor concentration, and the values reported throughout the paper is the mean of such results. The inhibition constants were obtained by nonlinear least-squares methods using the Cheng-Prusoff equation, as reported earlier, and represent the mean from at least three different determinationsCitation1,Citation8–14. All CA isozymes used here were recombinant proteins obtained as reported earlier by our group.
3. Results and discussion
3.1. Chemistry
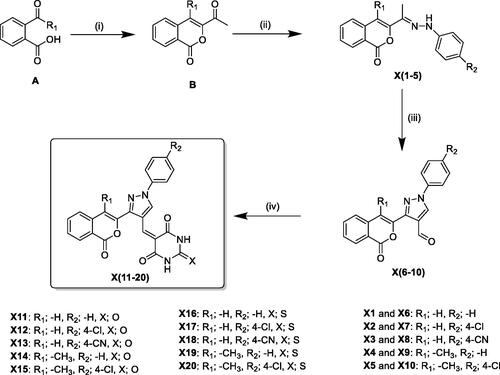
The structurally diverse isocoumarin derivatives X(1–20) were synthesised according to the general synthetic route shown in Scheme 1. 3-Acetylisocoumarin-substituted compounds B were synthesised as previously described by some of usCitation15. The hydrazone derivatives X(1–5) were obtained by reacting B with substituted hydrazinesCitation16. The aldehydes X(6–10) were synthesised in high yields by using the Vilsmeier-Haack procedureCitation17. These aldehydes were condensed with barbituric acid/2-thiobarbituric acid under acid condition at reflux to produce the final derivatives X(10–20). The chemical structures of the novel isocoumarin-substituted derivatives reported here were confirmed by analytical and spectral data (see Materials and methods for details).
Scheme 1. General synthetic route for the synthesis of the isocoumarin-substituted compounds X(1-20). Reagent and conditions: (i) chloroacetone, TEA, 170 °C, (ii) substituted phenylhydrazine hydrochloride, EtOH, sodium acetate, 2 h reflux, (iii) DMF/POCl3, 0–5 °C, then 3 h reflux, (iv) barbituric acid/2-thiobarbituric acid, acetic acid.
3.2. Carbonic anhydrase inhibition
Coumarins act as prodrug inhibitors, being hydrolysed by the esterase activity of CAs to the corresponding hydroxy-cinnamic acids which per se act as inhibitors, binding at the entrance of the enzyme active site and occluding itCitation6. Thus, unlike other inhibitors, such as the anions, the sulphonamides and their isosteres, etc., Citation6, the enzyme and the inhibitor are incubated for at least 6 h in order to allow for the hydrolysis to occur. This was also the protocol that we used for assaying the CA inhibition with isocoumarins, since incubation times of 15 min–3 h led to low but increasing levels of inhibition (data not shown). However, after 6 h incubation, the inhibition levels remained constant and are shown in .
Table 1. Inhibition data of human CA I, II, IX and XII with compounds X1-20 and the standard sulphonamide inhibitor acetazolamide (AAZ) by a stopped-flow CO2 hydrase assayCitation7. 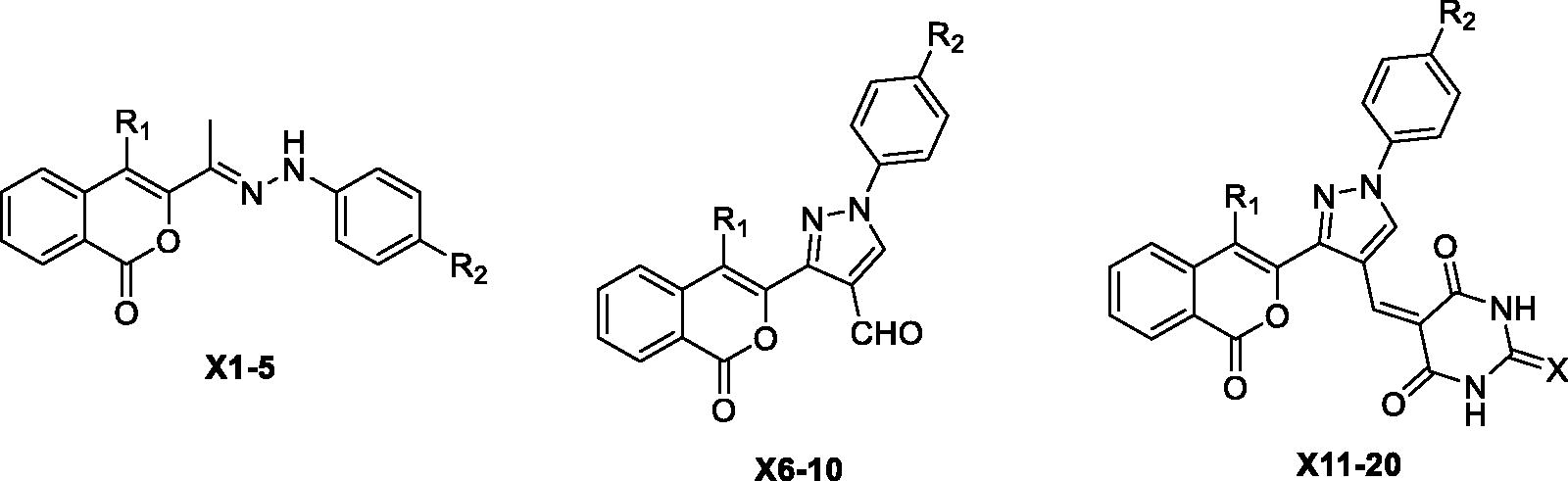
Four human (h) CA isoforms, known to be relevant drug targets (hCA I, II, IX and XII)Citation18 were included in the work for assessing their inhibition by the isocoumarins reported here (). It may be observed that as for many coumarinsCitation6, hCA I and II were not inhibited by isocoumarins up until 100 µM concentrations of inhibitor in the assay system. On the contrary, many isocoumarins (except X12, X17 and X20) showed low micromolar inhibitory power against these isoforms, with KIs in the range of 2.7 − 78.9 µM against hCA IX and of 1.2 − 66.5 µM against hCA XII, respectively (). It can be observed that the less bulky isocoumarins X1-5 and X6-10 were the most effective CAIs in the investigated series, with KI-s against hCA IX and XII < 15 µM, whereas the compounds incorporating bulkier moieties, such as X11–20 showed a reduced inhibitory power. This is to be expected, since the active site cavity of these enzymes may not easily accommodate two bulky moieties (phenylpyrazole and pyrimidine-trione/thioxo-pyrimidine-dione) present in some of these compounds.
4. Conclusions
We investigated here whether isocoumarins, which are isomeric compounds to comarins known to act as effective CAIs, also act as inhibitors of this enzyme. A series of 3-substituted and 3,4-disubstituted isocoumarins incorporating phenyl-hydrazone, 1-phenyl-pyrazole and pyrazolo-substituted pyrimidine trione/thioxo-pyrimidine dione moieties prepared by an original approach were investigated for their interaction with hCA I, II, IX and XII, known to be important drug targets. hCA I and II were not inhibited by these compounds, whereas hCA IX and XII were inhibited in the low micromolar range by the less bulky derivatives. The inhibition constants ranged between 2.7 − 78.9 µM against hCA IX and of 1.2 − 66.5 µM against hCA XII. As for the coumarins, we hypothesise that the isocoumarins are hydrolysed by the esterase activity of the enzyme with formation of 2-carboxy-phenylacetic aldehydes which act as CA inhibitors. Isocoumarins represent a new class of CAIs.
Acknowledgements
The authors thank Harran University Scientific Research Projects Coordination Department for financial support (HÜBAP, Project number: 21089). This research was also financed by the Italian Ministry for Education and Science (MIUR), grant PRIN: rot. 2017XYBP2R and by Ente Cassa di Risparmio di Firenze (ECRF), grant CRF2020.1395.
Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- (a) Reveglia P, Masi M, Evidente A. Melleins-intriguing natural compounds. Biomolecules 2020;10:772. (b) Shabir G, Saeed A, El-Seedi HR. Natural isocoumarins: structural styles and biological activities, the revelations carry on. Phytochemistry 2021;181:112568. (c) Pellissier L, Koval A, Marcourt L, et al. Isolation and identification of isocoumarin derivatives with specific inhibitory activity against Wnt pathway and metabolome characterization of Lasiodiplodia venezuelensis. Front Chem 2021;9:664489. (d) Coronado L, Zhang XQ, Dorta D, et al. Semisynthesis, antiplasmodial activity, and mechanism of action studies of isocoumarin derivatives. J Nat Prod 2021;84:1434–41.
- (a) Weber A, Breugst M, Pietruszka J. Experimental and computational investigations of the reactions between α,β-unsaturated lactones and 1,3-dienes by cooperative Lewis acid/Brønsted acid catalysis. Angew Chem Int Ed Engl 2020;59:18709–16. (b) Yata T, Nishimoto Y, Chiba K, Yasuda M. Indium-catalyzed C-F Bond transformation through oxymetalation/β-fluorine elimination to access fluorinated isocoumarins. Chemistry 2021;27:8232. (c) Saeed A, Larik FA. Metal-free synthesis of isocoumarins. Chem Heterocycl Compd 2016; 52:450–2.
- (a) Riveiro ME, De Kimpe N, Moglioni A, et al. Coumarins: old compounds with novel promising therapeutic perspectives. Curr Med Chem 2010; 17:1325–38. (b) Stefanachi A, Leonetti F, Pisani L, et al. Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018; 23:250. (c) Carradori S, Secci D, Petzer JP. MAO inhibitors and their wider applications: a patent review. Expert Opin Ther Pat 2018; 28:211–26. (d) Menezes JC, Diederich M. Translational role of natural coumarins and their derivatives as anticancer agents. Future Med Chem 2019; 11:1057–82.
- (a) Giovannuzzi S, Hewitt CS, Nocentini A, et al. Coumarins effectively inhibit bacterial α-carbonic anhydrases. J Enzyme Inhib Med Chem 2022;37:333–8. (b) Petreni A, Osman SM, Alasmary FA, et al. Binding site comparison for coumarin inhibitors and amine/amino acid activators of human carbonic anhydrases. Eur J Med Chem 2021;226:113875. (c) Supuran CT. Multitargeting approaches involving carbonic anhydrase inhibitors: hybrid drugs against a variety of disorders. J Enzyme Inhib Med Chem 2021;36:1702–14. (d) Supuran CT. Coumarin carbonic anhydrase inhibitors from natural sources. J Enzyme Inhib Med Chem 2020;35:1462–70.
- Lippi G, Gosselin R, Favaloro EJ. Current and emerging direct oral anticoagulants: state-of-the-Art. Semin Thromb Hemost 2019;45:490–501.
- (a) Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009; 131:3057–62. (b) Vu H, Pham NB, Quinn RJ. Direct screening of natural product extracts using mass spectrometry. J Biomol Screen 2008; 13:265–75. (c) Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44. (d) Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- a) Draghici B, Vullo D, Akocak S, et al. Ethylene bis-imidazoles are highly potent and selective activators for isozymes VA and VII of carbonic anhydrase, with a potential nootropic effect. Chem Commun (Camb) 2014;50:5980–3. (b) Akocak S, Lolak N, Vullo D, et al. Synthesis and biological evaluation of histamine Schiff bases as carbonic anhydrase I, II, IV, VII, and IX activators. J Enzyme Inhib Med Chem 2017;32:1305–12. (c) Akocak S, Lolak N, Bua S, et al. α-Carbonic anhydrases are strongly activated by spinaceamine derivatives. Bioorg Med Chem 2019;27:800–4. (d) Akocak S, Lolak N, Bua S, et al. Activation of human α-carbonic anhydrase isoforms I, II, IV and VII with bis-histamine schiff bases and bis-spinaceamine substituted derivatives. J Enzyme Inhib Med Chem 2019;34:1193–8.
- (a) Carradori S, Secci D, De Monte C, et al. A novel library of saccharin and acesulfame derivatives as potent and selective inhibitors of carbonic anhydrase IX and XII isoforms. Bioorg Med Chem 2016;24:1095–105. (b) Nocentini A, Bua S, Lomelino CL, et al. Discovery of new sulfonamide carbonic anhydrase IX inhibitors incorporating nitrogenous bases. ACS Med Chem Lett 2017;8:1314–9. (c) Winum JY, Temperini C, El Cheikh K, et al. Carbonic anhydrase inhibitors: clash with Ala65 as a means for designing inhibitors with low affinity for the ubiquitous isozyme II, exemplified by the crystal structure of the topiramate sulfamide analogue. J Med Chem 2006;49:7024–31. (d) Casey JR, Morgan PE, Vullo D, et al. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004; 47:2337–47.
- (a) Pastorekova S, Casini A, Scozzafava A, et al. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett 2004;14:869–73. (b) Akocak S, Lolak N, Bua S, et al. Synthesis and biological evaluation of novel N,N'-diaryl cyanoguanidines acting as potent and selective carbonic anhydrase II inhibitors. Bioorg Chem 2018;77:245–51. (c) Akocak S, Alam MR, Shabana AM, et al. PEGylated bis-sulfonamide carbonic anhydrase inhibitors can efficiently control the growth of several carbonic anhydrase IX-expressing carcinomas. J Med Chem 2016;59:5077–88.
- (a) Oztürk Sarikaya SB, Topal F, Sentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62. (b) Lolak N, Akocak S, Bua S, Supuran CT. Design, synthesis and biological evaluation of novel ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as potent carbonic anhydrase IX inhibitors. Bioorg Chem 2019; 82:117–22. (c) Lolak N, Akocak S, Bua S, et al. Discovery of new ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as carbonic anhydrase I, II, IX and XII inhibitors. Bioorg Med Chem 2019; 27:1588–94.
- (a) Krall N, Pretto F, Decurtins W, et al. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors . Angew Chem Int Ed Engl 2014; 53:4231–5. (b) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: structure-activity correlations for a series of isozyme II activators. J Pharm Sci 1994; 83:768–73. (c) Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011; 99:424–31.
- (a) Gulçin İ, Abbasova M, Taslimi P, et al. Synthesis and biological evaluation of aminomethyl and alkoxymethyl derivatives as carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2017;32:1174–82. (b) Guzel-Akdemir O, Akdemir A, Karali N, et al. Discovery of novel isatin-based sulfonamides with potent and selective inhibition of the tumor-associated carbonic anhydrase isoforms IX and XII. Org Biomol Chem 2015;13:6493–9. (c) Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902.
- (a) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005; 15:3102–8. (b)Sentürk M, Gülçin I, Beydemir S, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011; 77:494–9. (c) Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem 2012;55:5591–600.
- (a) Koca M, Ertürk AS, Umaz A. Microwave-assisted intermolecular aldol condensation: Efficient one-step synthesis of 3-acetyl isocoumarin and optimization of different reaction conditions. Arab J Chem 2018;11:538–45. (b) Kurt A, Kilinc I, Koca M. Preparation of copolymer systems of 2-Isocoumarin-3-yl)-2-oxoethyl metacrylate with methyl methacrylate and thermal decomposition kinetics. Iran J Sci Technol Trans A Sci 2020;44:1039–50. (c) Reddy GM, Garcia JR, Yuvaraja G, et al. Design, synthesis of tri-substituted pyrazole derivatives as promising antimicrobial agents and investigation of structure activity relationships. J Heterocyclic Chem 2020;57:2288–96. (d) Karuk Elmas SN, Dincer ZE, Erturk AS, et al. A novel fluorescent probe based on isocoumarin for Hg2+ and Fe3+ ions and its application in live-cell imaging. Spectrochim Acta A Mol Biomol Spectrosc 2020;224:117402. (e) Shablykina OV, Shablykin OV, Ishchenko VV, et al. Synthesis of 3-hetaryl-1H-isochromen-1-ones based on 3-(2-bromoacetyl)-1H-isochromen-1-one. Chem Heterocycl Compd 2013;48:1621–7. (f) Kurt A, Avcı HI, Koca M. Synthesis and characterization of a novel isocoumarin-derived polymer and the investigation of its thermal decomposıtıon kinetics. Maced J Chem Chem Eng 2018;37:173–84.
- Kenchappa R, Bodke YD, Chandrashekar A, et al. Synthesis of coumarin derivatives containing pyrazole and indenone rings as potent antioxidant and antihyperglycemic agents. Arab J Chem 2017;10:3895–906.
- Chodankar NK, Sequeira S, Seshadri S. Short communication synthesis of 3-hetarylcoumarins. Dye Pigment 1986;7:231–6.
- (a) Supuran CT. Emerging role of carbonic anhydrase inhibitors. Clin Sci (Lond) 2021; 135:1233–49. (b) Supuran CT. Novel carbonic anhydrase inhibitors. Future Med Chem 2021; 13:1935–7. (c) Supuran CT. Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs. 2022:30(12):1197–1208.