Abstract
Rab geranylgeranyltransferase (GGTase-II, RGGT) catalyses the post-translational modification of eukaryotic Rab GTPases, proteins implicated in several pathologies, including cancer, diabetes, neurodegenerative, and infectious diseases. Thus, RGGT inhibitors are believed to be a potential platform for the development of drugs and tools for studying processes related to the abnormal activity of Rab GTPases. Here, a series of new α-phosphonocarboxylates have been prepared in the first attempt of rational design of covalent inhibitors of RGGT derived from non-covalent inhibitors. These compounds were equipped with electrophilic groups capable of binding cysteines, which are present in the catalytic cavity of RGGT. A few of these analogues have shown micromolar activity against RGGT, which correlated with their ability to inhibit the proliferation of the HeLa cancer cell line. The proposed mechanism of this inhibitory activity was rationalised by molecular docking and mass spectrometric measurements, supported by stability and reactivity studies.
GRAPHICAL ABSTRACT

1. Introduction
Over the past two decades, targeted covalent inhibitors have gained recognition as an effective approach in drug discovery.Citation1,Citation2 Such trend results from the increased awareness of the benefits that this approach provides (e.g. improved efficacy and beneficial pharmacokinetics, resulting in a longer duration of action), as well as technological development, especially in the field of metabolomics and proteomics.Citation3 The latter has enabled credible determination of mechanisms of action of new compounds, their effectiveness, and identification of potential side effects. Recent years have brought to the market new therapeutics with the covalent mechanism of action, e.g. carfilzomib (multiple myeloma), abiraterone (prostate cancer), and afatinib (lung cancer).Citation1,Citation3 The very last reports show the efficacy of covalent cysteine binding inhibitor paxlovid as the potential drug for COVID-19.Citation4 This strategy is also applicable in the design of molecular probes for studying the function of biomolecules and biochemical processes.Citation5–7
Encouraged by the possible benefits of this approach, we designed first covalent inhibitors of Rab geranylgeranyltransferase, derived from α-phosphonocarboxylates (PCs). RGGT is involved in the post-translational modification of eukaryotic Rab GTPases, the primary regulators of the formation, transport, docking, and fusion of vesicles during membrane transport.Citation8 The dysfunction of Rab GTPases leads to various diseases ranging from infections to cancer.Citation9 RGGT catalyses double prenylation of most Rab GTPases, with the formation of a thioether bond between two C-terminal cysteines and isoprenoid chains derived from geranylgeranyl pyrophosphate. This modification is necessary for the proper functioning of Rab GTPases, making RGGT a potential drug target. RGGT is a heterodimer composed of an α subunit encoded by the RABGGTA gene and a β subunit encoded by the RABGGTB gene.Citation10
Five classes of RGGT inhibitors have been reported to date.Citation11–15 Among them, tetrahydrobenzodiazepine derivatives show the highest inhibition efficiency,Citation13 while the natural product, psoromic acid, is the only covalent inhibitor of RGGT.Citation14 α-Phosphonocarboxylates inhibit the introduction of the second geranylgeranyl group to Rabs, making them more selective, by affecting only proteins that require double prenylation.Citation15 Inhibition of Rab prenylation by PCs is also correlated with their cytotoxic properties.Citation16,Citation17 We demonstrated that the most active derivatives of currently known phosphonocarboxylates contain imidazo[1,2-a]pyridineCitation17 or the imidazole ringCitation18 (). As no structural data describing phosphonocarboxylate – RGGT complexes exist, we proposed the model for their interaction based on the molecular docking results for imidazo[1,2-a]pyridine analogues.Citation17 Importantly, a few cysteines are present near the proposed site of interaction that could be targeted by the electrophilic moiety of the covalent inhibitor. Developing a covalent inhibitor could increase the effectiveness of inhibition and confirm the place of interaction with the studied enzyme.
Figure 1. Structures of the studied compounds: (A) potential inhibitors with the covalent mode of action and (B) the non-covalent control analogues, derived from analogues with the non-covalent mechanism of action (C).
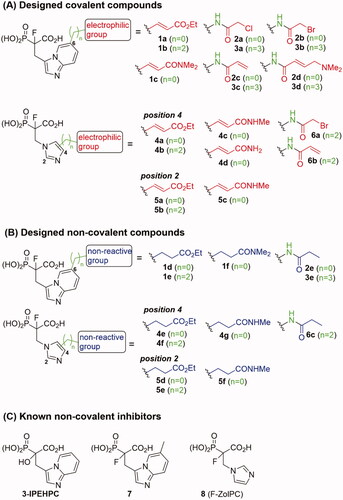
Herein we describe the first reported attempt to develop covalent inhibitors of RGGT. The novel compounds were derived from two PC inhibitors of RGGT: compound 7, the 6-substituted analogue of 3–(3-pyridyl)-2-hydroxy-2-phosphonopropanoic acid (3-IPEHPC) and compound 8, 2-fluoro-3-(1H-imidazol-1-yl)-2-phosphonopropanoic acid (F-ZolPC) ().Citation17,Citation19 The modifications vary in linker length, size, geometry, and reactivity of substituents, aiming at thiol residue of cysteine located in the catalytic cavity of the studied enzyme (). As electrophilic traps, we chose moieties present in the known drugs with a covalent mechanism of action, targeting cysteines.Citation6,Citation20 Most synthesised compounds were Michael acceptors but we have also studied chloro- and bromoacetamide fragments (). We also synthesised a group of negative controls, in which the electrophilic group was replaced by structurally similar but non-reactive moiety ().
Compounds 1–6 were examined for their potency against RGGT, using a procedure previously developed in our group.Citation17 We also tested their selectivity by assessing the prenylation of Rap1A/Rap1B, a substrate of a structurally similar prenyltransferase, GGTase-I. The results were supported by covalent docking studies and the LC-MS-based peptide mapping.
2. Materials and methods
2.1. Chemistry experimental procedures
All reagents were purchased from commercial sources and used as obtained unless specified otherwise. Compounds 9–29 were purified using Gilson PLC 2250 purification system coupled with ‘The Advion expression compact mass spectrometer’ (mode of ionisation: APCI). Appropriate products’ fractions were identified based on the value of peak [M + H+]. Preparative HPLC for purification of compounds 1–6 was performed using Gilson Prep equipped with a UV − vis-156 detector and semipreparative column Kromasil 100 − 5-C18 (5 µm, 10 mm × 250 mm). NMR spectra were measured at 250.13 or 700 MHz for 1H NMR, 62.90 or 170 MHz for 13 C NMR, 283 or 101.30 MHz for 31 P NMR on Bruker Avance DPX 250 and Bruker Avance II Plus 700 spectrometers, respectively. Chemical shifts (δ) are reported in parts per million (ppm) relative to internal residual CHCl3 in CDClCitation3 (δ 7.26 1H NMR) or CDCl3 signal in 13 C NMR (δ 77.16); internal residual HDO in D2O (δ 4.79 1H NMR) or external 85% H3PO4 (δ 0 ppm 31 P NMR). 31 P NMR and 13 C NMR spectra were proton-decoupled. Coupling constants (J) are quoted in Hz. The assignment of the signals in 1H NMR and 13 C NMR was supported by two-dimensional experiments (COSY, HMQC, HMBC). Several signals of quaternary 13 C were only observed in the HMBC spectrum (signals from PCF or FCCO2H); therefore their chemical shifts (δ) are reported with an accuracy of 1 ppm. All compounds showing activity against RGGT are >95% pure by elemental analysis. A monomode microwave reactor (CEM Discover SP) equipped with an IntelliVent pressure control system was used. The standard method was applied, and maximum pressure was set to 250 psi. Temperatures of the reaction mixtures were measured with an external infra-red sensor. All synthetic procedures are included in Supplemental Material.
2.2. Stability studies
Compound 1–3 (about 1–1.5 mg, final concentration 5 mM) was dissolved in 10 mM PBS (PBS prepared by dissolving Gibco® PBS tablets in D2O according to the manufacturer's instructions) in the NMR tube (diameter 5 mm). Next, the sample’s pH was adjusted to 7 using 3–6 µL of 1 M NaOH/D2O. The sample was measured by 1H NMR immediately after its preparation and after 24, 48, and 72 h of incubation at 37° C. All compounds (except for analogues 2b and 3b) proved to be stable under the applied conditions. After 72 h, only about 35% of 2b was left untouched, while 3b completely decomposed. Numerous unidentified products of decomposition were observed in such reaction mixture. The molar ratio of starting compounds 2b, 3b compared with new ones, was estimated based on the integration of signals in the 1H NMR spectrum derived from -CH2Br protons and the summed up integrations of aromatic ring protons from compound 2b/3b and its products of decomposition.
2.2.1. Reactivity studies
Compounds 1–3 (about 1–1.5 mg), 100 mM PBS (500 µL, prepared by dissolving Gibco® PBS tablets in D2O) and DTPA (diethylenetriaminepentaacetic acid, 10 µL of 10 mM solution in D2O) were placed in the NMR tube. As an internal standard dimethylformamide (DMF, 3 µL) was used and added to the sample [δ 8.2 ppm (s, HCONMe2, 1H)]. The prepared sample was mixed thoroughly by shaking and degassed by placing in an ultrasonic bath. To avoid the oxidation of glutathione, the tube was purged with argon. Next, the sample was measured by 1H NMR allowing observation of the integration ratio of signals of compound 1–3 and internal standard at t = 0 min. Then, GSH (100 µL) was added and mixed thoroughly, degassed and purged with argon. The final concentration of the test compound was 5 mM and glutathione was 40 mM (8 x concentration of the test compound). Next, the 1H NMR spectrum was recorded after 10–20 min at 37 °C and then every 1 h for 11–16 h. For the duration of the reaction, the sample was incubated at 37 °C. The assessment of the progress of the reaction was made based on the decreasing integration of signals from the electrophilic group of the test compounds or based on the increasing integration of signals from the reaction product with glutathione. The effect of the oxidation of GSH on its effective concentration during the reaction was negligible (approx. 5% of oxidised GSH after 24 h of incubation at 37 °C) and does not affect the speed of the reaction with the tested compounds.
2.3. Biological materials and methods
2.3.1. General
PrestoBlue® Cell Viability Reagent, Mem-PER™ Plus Membrane Protein Extraction Kit, and all reagents for cell culture were purchased from Life Technologies (Carlsbad, CA, USA). Bradford Protein Assay and Clarity™ Western ECL Substrate were obtained from Bio-Rad (Hercules, CA, USA). Protease inhibitor cocktail and lovastatin were purchased from Sigma (Saint Louis, MO, USA). Primary antibodies against Rab11A and Rap1A/Rap1B were obtained from Abcam (Cambridge, UK) and primary antibodies against β-actin along with secondary HRP-linked antibodies were purchased from Cell Signalling Technology (Beverly, MA, USA).
Iodoacetamide and WaLP were purchased from Sigma (Saint Louis, MO, USA), DTT, CuSO4, TCEP from Fluorochem (Derbyshire, United Kingdom) and THPTA, biotin-PEG3-azide from Click Chemistry Tools (Scottsdale, AZ, USA). Sequencing grade chymotrypsin was purchased from Promega (Madison, WI, USA).
2.3.2. HeLa cell culture
The cervical epithelial carcinoma HeLa cell line was purchased from the American Type Cell Collection (ATCC). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS) containing 100 IU/mL penicillin, 0.25 µg/mL amphotericin B and 50 µg/mL neomycin. Cells were incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2. For biological studies, compounds were dissolved just before use in PBS at a stock concentration of 10 mM and then pH adjusted to about 7.
2.3.3. Determination of cytotoxicity
HeLa cells were seeded into 96-well cell culture plates at a density of 4 × 103 cells/well in 100 µL of complete, serum-containing culture medium. On the following day, cells were washed with phosphate-buffered saline (PBS) and 100 µL of serum-free (fasting) medium was added as described previously.Citation17 Subsequently, HeLa cells were treated with PCs at eight concentrations and 72 h later PrestoBlue® Cell Viability Reagent was applied. Following 50 min incubation time at 37 °C and 5% CO2, cell viability was determined by measuring the fluorescent signal (Ex/Em = 530/590 nm) on a Synergy 2 Microplate Reader (BioTek, Vermont, USA). The obtained fluorescence magnitudes were used to calculate cell viability expressed as a percent of the viability of the untreated control cells. The data expressed as the mean of at least 3 independent experiments were used to calculate the IC50 parameter.
2.3.4. Assessment of inhibition of Rab11A and Rap1A/Rap1B prenylation
HeLa cells were seeded into 6-well cell culture plates at a density of 4 × 105 cells/well in 3 ml of complete medium. On the following day, 1.5 ml of fresh serum-free medium was supplemented with PCs or lovastatin. The latter was used as a control acting as a hydroxymethylglutaryl (HMG)-coenzyme A (CoA) reductase inhibitor and preventing the downstream biosynthesis of cholesterol and prenylation with the geranylgeranyl or farnesyl moieties.Citation21 After 48 h of incubation, cell monolayers were rinsed with PBS and detached using trypsin-EDTA solution. The cytosolic and membrane-rich fractions, containing protease inhibitor cocktail, were isolated from cell pellets using Mem-PER™ Plus Membrane Protein Extraction Kit according to the manufacturers’ instructions. The protein concentration in both fractions was determined using Bradford Protein Assay. Equal amounts of protein (30 µg) from cytosolic fractions were resolved by 12% SDS-PAGE gels and transferred to 0.2 µm nitrocellulose membrane. Membranes were probed with β-actin, Rab11A, or Rap1A/Rap1B antibodies and detected using the appropriate HRP-conjugated secondary antibody, followed by an ECL assay. Visualisation of the chemiluminescent protein bands was performed using ChemiDoc™ MP Imaging System (Bio-Rad). Densitometry analysis was performed with ImageLab™ Software (Bio-Rad) and relative unprenylated protein band intensity was normalised to β-actin and quantified with respect to controls (untreated cells).
2.4. Statistical analysis
Unless stated otherwise, all the biological results are presented as means of 3–6 repeated experiments ± SEM. Statistical differences between mean values of the inhibitor treated and untreated samples were analysed using one-way ANOVA followed by Dunnett’s multiple comparisons test using GraphPad Prism (version 6.01 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com). Confidence p-levels are indicated by asterisks, with * denoting p ≤ 0.05, ** denoting p ≤ 0.01, *** denoting p ≤ 0.001, and **** denoting p ≤ 0.0001.
2.5. Molecular docking
Compounds were drawn with Maestro 2019.2 (Schrödinger release 2019–2: Maestro, Schrödinger, LLC, New York, NY, 2019). Both stereoisomers were drawn for all compounds selected for molecular docking (covalent compounds 2a, 2b, 2c, and 2d, and reference compounds 1d, 1e, and 2e; ). Protein structure for docking (PDB 4GTS) was downloaded from the Protein Data Bank and prepared with Protein Preparation Wizard. In docking, the protein was held rigid, except hydroxyl and thiol groups were allowed to rotate. Binding modes/transition state poses were generated with Glide (Schrödinger release 2019–2: Glide, Schrödinger, LLC, New York, NY, 2019) with standard precision (SP) mode. Covalent docking was done with CovDock in Glide (Maestro 2019–2;Citation22) with customised reaction chemistry. Pose for 7 was acquired with Induced fit docking (IFD; more detailsCitation17).
Table 2. Effects of Imidazo[1,2-a]pyridine Analogues of α-phosphonocarboxylates on HeLa Cell Viabilitya and Rab11A prenylationb
2.6. Determination of 2b binding site to RGGT by mass spectrometry
IA-light and IA-heavy reactants were prepared according to the previously published procedure.Citation23 RGGT, Rab7, and REP-1 were expressed in Escherichia coli and purified according to the published procedure (expression vectors were a kind gift from prof. Kirill Aleksandrov, University of Queensland, Australia).Citation24–27 Protein complex formation was performed in 50 mM HEPES pH 7.2, 50 mM NaCl, 1 mM MgCl2 and 1 mM DTT in two quadruplicates (4 controls and 4 samples for inhibitor treatment). The reaction mixture containing 2 µM REP-1, 100 nM RGGT, 4 µM Rab7, and 100 µM inhibitor was incubated for 1 h at 37 °C with agitation. Simultaneously, inhibitor-free control samples were prepared. Solutions were treated with 0.2 mM probes (IA-light for inhibitor-treated or IA-heavy for control) at 25 °C for 1 h with constant mixing. Subsequently, the solutions were treated with 10 mM DTT at 65 °C for 15 min, and then 20 mM iodoacetamide at 37 °C for 30 min. One sample from the inhibitor-treated set was combined with one control sample and 250 µg of BSA after denaturation and alkylation steps. BSA was added to obtain a visible pellet and limit the loss of proteins of interest during the precipitation step. Methanol, chloroform, and ultrapure water were added sequentially (1:4:1:3 probe to methanol to chloroform to water ratio), the mixtures were vortexed and centrifuged (14 000 x g, 5 min). The top layers were removed, leaving the protein layers at the phase interface intact. Methanol (2 volumes) was added, the samples were gently mixed, and the protein pelleted as before. All supernatants were discarded, and the precipitated proteins were air-dried in an inverted tube for 5 min. Samples were resuspended in 2% SDS in PBS (100 µL). Once dissolved, the samples were diluted to a final concentration of 0.2% SDS by adding PBS. For each 1 ml of proteins solutions, click reagent mixture was prepared as follows: 20 µL CuSO4 (50 mM in H2O), 10 µL tris(2-carboxyethyl)phosphine hydrochloride (TCEP, 100 mM in H2O), 20 µL tris(3-hydroxypropyltriazolymethyl)amine (THPTA, 10 mM in DMSO) and 20 µL biotin-PEG3-azide (5 mM in DMSO) were added sequentially to a microcentrifuge tube and mixed. The click reagent mixtures were added to the protein solutions, giving the following final concentration of components: 1 mM CuSO4, 1 mM TCEP, 200 µM THPTA, 100 µM azide-PEG3-biotin, and the solutions were incubated on a shaker at 25 °C for 1 h. The click reactions were terminated by the addition of 10 mM EDTA. Protein samples were precipitated as described. Samples were resuspended in 100 µL of 6 M urea in 100 mM Tris-HCl pH 8.0 and 10 mM CaCl2, if they were digested with chymotrypsin, or 100 mM Tris-HCl pH 8.5, if they were digested with α-lytic protease, and then diluted to a final concentration of 0.6 M urea by addition of the buffer appropriate for selected proteases. Subsequently, after measurement of the total protein concentration in samples, chymotrypsin or α-lytic protease was added to a final protease:protein ratio of 1:30 (w/w) and incubated for 18 h at 25 °C for chymotrypsin or 37 °C for α-lytic protease. The reactions were terminated by the addition of 1:100 Protease Inhibitor Cocktail.
Peptide mixtures were then desalted with the use of AttractSPE™ Discs Bio – C18 (Affinisep, catalogue no. SPE-Discs-Bio-C18-100.T1.47.20) using a published stage-tip protocol,Citation28 and dried in a centrifugal vacuum concentrator at 45 °C. Prior to LC-MS measurement, the samples were resuspended in 0.1% TFA, 2% acetonitrile in water. Chromatographic separation of peptides was performed on an Easy-Spray Acclaim PepMap column 50 cm long ×75 µm inner diameter (Thermo Fisher Scientific) at 45 °C by applying 60–90 min acetonitrile gradients in 0.1% aqueous formic acid at a flow rate of 300 nl/min. An UltiMate 3000 nano-LC system was coupled to a Q Exactive HF-X mass spectrometer via an easy-spray source (all Thermo Fisher Scientific). The Q Exactive HF-X was operated in data-dependent mode with survey scans acquired at a resolution of 60,000–120,000 at m/z 200 and MS/MS scans acquired at a resolution of 15,000–45,000 at m/z 200. Isotope patterns with charges 2–6 from the survey scan were selected (up to 12 per duty cycle) with an isolation window of 1.3 m/z and fragmented by higher-energy collision dissociation (HCD) with normalised collision energies of 27. Raw files generated from these measurements were processed in PEAKS Studio 10.5 (Bioinformatics Solutions Inc., Waterloo, Canada) and the peptides were identified from the MS/MS spectra searched against a short database containing the proteins subjected to incubation with 2b as well as common proteinous contaminants. Methionine oxidation was set as variable modifications. For WaLP digested samples cleavages of Tyr/Ala/Ser/Val followed by any amino acid were allowed, for chymotrypsin digested samples cleavages of Phe/Leu/Tyr/Trp followed by any amino acid except Pro were allowed. Up to four missed cleavages were allowed. Parent mass error tolerance was set to 5 ppm and fragment mass error tolerance to 0.01 Da. The peptide-spectrum match quality threshold was set to p = 0.01. SILAC-based quantification method was applied with the light label set as Cys + 629.2996 Da and the heavy label was set as Cys + 635.3197 Da, and the maximal number of labelled amino acids per peptide set to 2. Other parameters were used as pre-set in the software. Peptide intensities were exported as csv files and formatted to the final version () using Microsoft Office Excel 2016.
Table 3. Peptides identified in MS and intensity ratio of untreated vs treated with 2b inhibitor samples.
3. Results and discussion
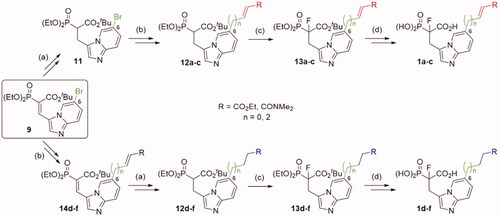
Synthesis of novel imidazo[1,2-a]pyridine analogues of α-phosphonocarboxylates required four different routes (Schemes 1–2, for experimental details see: Schemes S2–S9). The first step involves the synthesis of 6-substituted-imidazo[1,2-a]pyridin-3-yl-2-(diethoxyphosphoryl)acrylates 9–10 according to the published procedure.Citation17 In the synthesis of the compounds 1a,c (Scheme 1), the double bond Cα=Cβ in 9 was reduced and thus obtained analogue 11 was subject to the Mizoroki-Heck reaction with appropriate olefin, giving compounds 12a,c.Citation29 In the synthesis of control analogues 1d,f, the order of reactions was reversed, first the Mizoroki-Heck reactionCitation29 and then the reduction of carbon-carbon double bonds was applied. In the case of analogues 1 b,e with elongated carbon linker (n = 2), the Mizoroki-Heck reaction was carried out between allyl alcohol and 9, giving appropriate aldehyde (Schemes S3 and S5),Citation29 which was later used in Horner-Wadsworth-Emmons reaction with triethyl phosphonoacetate. Then, the products 12a-f were fluorinated using N-fluorobenzenesulfonimide (NFSI) in the presence of sodium hydride.Citation19 The ester groups of obtained compounds 13 were deprotected, applying bromotrimethylsilane for phosphonate, and TFA for tert-butyl carboxyester cleavage. After purification by HPLC, we obtained pure compounds 1a-f.
Scheme 1. Strategies used for the synthesis of α-phosphonocarboxylates 1. Reagents and conditions: (a) NaBH4, NiCl2·6H2O, MeOH; (b) for 12a,c and 14d,f (n = 0): N,N-dimethylacrylamide or ethyl acrylate, Pd(AcO)2, tri-o-tolylphosphine, DIPEA, propionitrile, microwave irradiation; for 12b and 14e (n = 2): (I) allyl alcohol, Pd(AcO)2, tri-o-tolylphosphine, DIPEA, propionitrile, microwave irradiation; (II) triethylphophonoacetate, NaH, THF; (c) NaH, NFSI, THF; (d) (I) bromotrimethylsilane, TEA, acetonitrile, (II) EtOH, (III) TFA.
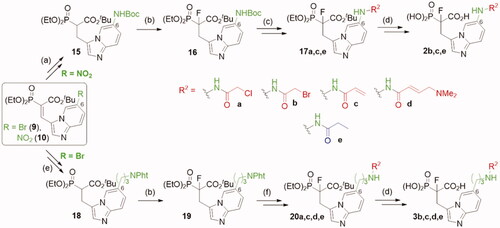
Analogues 10 and 9 were used to synthesise compounds 2 and 3 (Scheme 2), which bear the amine group as an anchoring point for electrophilic modification, either directly connected with imidazo[1,2-a]pyridine ring or via a 3-carbon linker, respectively. For the synthesis of analogues 2, compound 10 was first subjected to reduction of the nitro group, its protection by the Boc group, and the reduction of the double C=C bond, which led to compound 15. In the case of analogues 3, compound 9 was subjected to Mizoroki-Heck reaction with 2-allylisoindoline-1,3-dioneCitation29 and after reduction of two double C=C bonds, we obtained compound 18. Next, analogues 15, 18 were subjected to the fluorination with NFSI and NaH and the deprotection of the amine group, followed by its coupling with an acyl halide or a carboxylic acid. Thus obtained 17, 20 were subjected to deprotection of the ester phosphonoacetate group, using bromotrimethylsilane and TFA. That approach worked for compounds 2 b,c,e, and 3 b-e, but failed for compounds 2a,d and 3a. Therefore, compounds 2a,d were synthesised from 16 applying the reverse order of reactions, first deprotection of phosphonoacetate ester, followed by the introduction of the electrophilic moiety (Scheme S7). In the case of analogue 3a, during the reaction of 20a with bromotrimethylsilane, an exchange of chlorine atom for bromine was observed in chloroacetamide residue (Scheme S9). Therefore, 3b was transformed into compound 3a in the reaction with an excess of NaCl.
Scheme 2. Strategies used for the synthesis of α-phosphonocarboxylates 2–3. Reagents and conditions: (a) (I) 8, H2, Pd/C, (II) Boc2O, DCM, (III) NaBH4, NiCl2·6H2O, MeOH; (b) NaH, NFSI, THF; (c) for 2 b,c: (I) 3 M HCl/EtOH, (II) acyl halide, DCM; (d) (I) bromotrimethylsilane, TEA, acetonitrile, (II) EtOH, (III) TFA; (e) (I) 7, 2-allylisoindoline-1,3-dione, Pd(AcO)2, tri-o-tolylphosphine, DIPEA, propionitrile, microwave irradiation, (II) H2, Pd/C, (III) NaBH4, NiCl2·6H2O, MeOH; (f) (I) hydrazine hydrate, MeOH; (II) acyl halide or carboxylic acid with coupling agent in DCM or DMF; For details of synthesis of 2a,d, 3a see Schemes S7, S9.
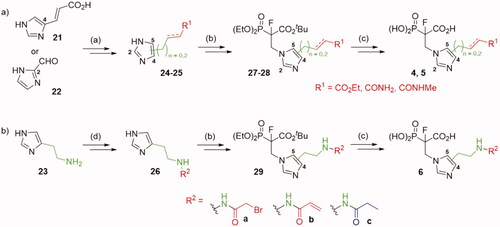
We also synthesised analogues derived from 8, modified at either the 2- or 4-position of the imidazole ring (Scheme 3). The first stages of the synthesis required the preparation of synthons 24–26, which took from one to seven steps (Schemes S10–S14). The compounds 24–26 reacted with Michael acceptor, tert-butyl 2-(diethoxyphosphoryl)acrylate.Citation19 The products were subjected to fluorination, using NaH and NFSI.Citation19 Thus obtained 27–29 were subjected to deprotection of phosphonoacetate ester using the procedure optimised for analogues 1–3.
Scheme 3. Strategies used for the synthesis of α-phosphonocarboxylates 4–6. Reagents and conditions: (a) Scheme S10–14; (b) tert-butyl 2-(diethoxyphosphoryl)acrylate, NaH, THF, then NFSI, THF; (c) (I) bromotrimethylsilane, TEA, acetonitrile, (II) EtOH, (III) TFA; (d) (I) 23, phthalic anhydride, AcOH, (II) Ph3Cl, TEA, (III) hydrazine hydrate, MeOH, (IV) acyl halide, DCM, (V) TFA, DCM.
We conducted stability studies of compounds 1–3 in phosphate buffer saline at physiological pH and temperature, monitored by 1H NMR. Compounds 4–6 were excluded from these studies as they did not show biological activity. All analogues were stable after 72 h, except for analogues of bromoacetamide 2b, 3b. After 72 h, only 35% of 2b was left untouched, while 3b completely decomposed.
We assessed the reactivity of 1–3 in the reaction with a model nucleophile containing cysteine, glutathione (GSH)Citation30 at 37 °C in PBS/D2O by 1H NMR with dimethylformamide as a quantitative standard (Scheme S1). GSH has been used previously to screen and evaluate the reactivity of diverse electrophiles and as a model compound to establish a suitable ‘reactivity window’Citation31 for covalent inhibitors. The progress of the reaction was based on the integration of disappearing signals corresponding with electrophilic groups, except for analogue 2a, where the increase of integration of signals from the reaction product with GSH was measured. The highest reactivity was shown by bromoacetamide analogues 2b and 3b, whose complete conversion was observed within 15 min ().
Table 1. Effect of exchanging electrophilic residue on half-Life Values t1/2 for reaction with GSH.
The biological activity of synthesised analogues was tested using human cervical carcinoma HeLa cells. Firstly, IC50 inhibitory concentrations of viability were estimated (Figures S1 and S2) since inhibition of prenyltransferases decreases cell growth.Citation15,Citation17
Among the first set of α-phosphonocarboxylates, only compounds 1a and 1c, bearing Cα = Cβ double bond adjacent to a heterocycle, showed antiproliferative activity, while 1b with an elongated carbon linker (n = 2) was not active. Within their non-covalent counterparts (1d-f), only 1d and its version with an elongated carbon linker, 1e, reduced HeLa cells viability, however, 1f was not active ().
Analogues 1d, 1e, 2b, 2e demonstrated inhibitory activity against RGGT at 100 µM () and therefore were selected to evaluate the lowest effective dose (LED) of inhibition of Rab11A and Rap1A/Rap1B prenylation (). Rap1A/Rap1B served as an indicator of PCs selectivity against RGGT because Rap1A/Rap1B is geranylgeranylated by GGTase-I.
Figure 2. Effect of imidazo[1,2-a]pyridine (1–3; A) and imidazole (4–6; B) PC analogues on Rab11A prenylation in HeLa cells. Cells were treated for 48 h with PCs (100 µM). Rab11A and β-actin were detected in cytosolic fraction using Western blot.
![Figure 2. Effect of imidazo[1,2-a]pyridine (1–3; A) and imidazole (4–6; B) PC analogues on Rab11A prenylation in HeLa cells. Cells were treated for 48 h with PCs (100 µM). Rab11A and β-actin were detected in cytosolic fraction using Western blot.](/cms/asset/b6aa540e-d5d0-4c2f-8008-098f5e5ef030/ienz_a_2053525_f0002_b.jpg)
Potential covalent inhibitors 1a, 1b, and 1c did not inhibit Rab11A prenylation. Therefore, their potency to reduce HeLa cell viability is probably associated with a different mechanism of action. Among the non-covalent counterparts 1d-f, 1d, and 1e inhibited RGGT at 50 µM or 25 µM, respectively, and simultaneously, they did not affect the activity of GGTase-I (). Non-covalent analogues of 7 (6-substituted analogues of 3-IPEHPC), 1d and 1e, are selective micromolar RGGT inhibitors with similar or slightly weaker activity than the reference compound 7.Citation17
Figure 3. Effect of 6-substituted 3-IPEHPC analogue with the 3-ethoxy-3-oxopropyl group (1d), its version with an elongated carbon linker (n = 2; 1e), bromoacetamide analogue (2b) and its non-covalent counterpart (2e) on Rab11A and Rap1A/Rap1B prenylation in HeLa cells. Cells were treated for 48 h with the indicated concentrations of PCs (µM) or 10 µM lovastatin (Lov), acting as a positive control. Cytosolic fractions containing unprenylated proteins were Western blotted for Rab11A, Rap1A/Rap1B, and β-actin (A), and the bands were quantified by densitometry, normalised to β-actin, and presented as a percentage of controls (B). Data represented mean ± SEM from at least three independent experiments, *p ≤ 0.05, ****p ≤ 0.0001.
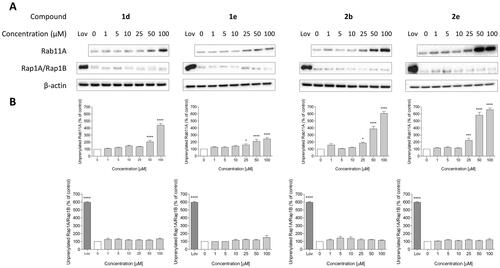
The second group constituted compounds bearing amine group connected with imidazo[1,2-a]pyridine ring directly (2) or via a 3-carbon linker (3). 3e and its covalent counterparts (3a-d) could not deplete Rab11A from HeLa cell membranes at 100 µM in contrary to the shorter non-covalent analogue, 2e. Among analogues of 2e, only 2b was active against RGGT. It might be correlated with the highest reactivity of bromoacetamide modification observed in studies with GSH. Compound 2b and its non-covalent version 2e inhibited RGGT activity at 25 µM, but they were not active against other prenyltransferases or enzymes of the mevalonate pathway ().
Analogues bearing bromoacetamide (2b, 3b) and chloroacetamide (2a, 3a) moieties were cytotoxic, while only 2b showed inhibitory activity towards RGGT. The cytotoxicity of 2a and 3a was probably an effect of different mechanisms of action, due to the presence of highly reactive electrophilic moiety.Citation30 Compounds 2c-3c and 2d-3d were the least reactive, which corresponded with their non-cytotoxic character.
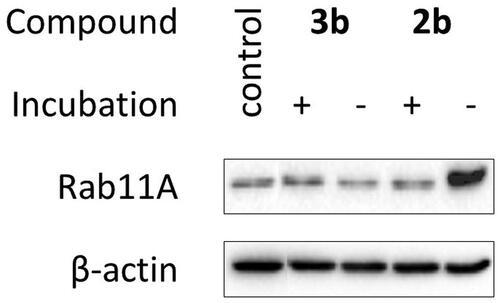
Because 2b and 3b were unstable, we supplemented HeLa cells with decomposition products. They turned out cytotoxic (), but none of them disrupted Rab11A prenylation (). It indicates that inhibition of Rab11A protein prenylation by 2b may arise from the action of the parent compound. Also, we cannot exclude that the biological effect of 2b is comparable with its non-covalent analogue 2e, only due to the instability of the former. The cytotoxic character of 3b might result from the activity of degradation products and/or reactivity of the bromoacetamide group.
Figure 4. Effect of decomposition products of 2b and 3b on Rab11A prenylation in HeLa cells. Cells were treated for 48 h with 100 µM of freshly prepared 2b and 3b (-) and their solutions after incubation in PBS/D2O at 37 °C for 72 h (+). Cytosolic fractions containing unprenylated proteins were Western blotted for Rab11A and β-actin.
Imidazole analogues of α-phosphonocarboxylates 4–6, did not exert any cytotoxic effect on HeLa cell proliferation (Figure S2) and they did not suppress prenylation of Rab11A at 100 µM (). It confirms data from our previous studies, where compounds with imidazole ring bearing two substituents, at nitrogen and C2 or C4, were inactive against RGGT.Citation19
The proposed mechanism of the inhibitory activity of these compounds was rationalised with molecular docking. Several cysteines are present in the active site of the RGGT, which could be targeted by the electrophilic groups. We suggested that interactions of the acidic residues, phosphonic and carboxylic groups in α-phosphonocarboxylates, guide the location of the inhibitors in the RGGT binding area (; compound 7). The interaction between REP-1-Rab complex and RGGT could change the conformation of the binding cavity,Citation10 therefore in our previous studies, we applied induced-fit docking in which side chains of specific amino acids are allowed to move freely, and thus subtleties of the interactions between the ligand and the protein can be considered more accurately than with traditional molecular docking.Citation17 Novel non-covalent compounds seem to behave similarly (Figure S3). The scaffold of the active covalent compound 2b follows the suggested conformation of the previous compounds like 7 (). Additionally, the R144B could stabilise 2b to the proximity of C196B in the transition state (). In addition, the hydrophobic part of the electrophilic moiety in position 6 faces the W244B indole side chain, yielding favourable packing. Finally, the covalent bond could be formed between 2b and C196B ().
Figure 5. Possible covalent bond formation by compound 2b suggested by docking simulations using 4GTS structure. 2b adopts a similar binding mode as the non-covalent compound 717 (A). In not covalently bound state R144B stabilises 2b into the proximity of C196B (B) before the formation of the covalent interaction (C). The black dashed lines indicate interactions between the inhibitor and the protein. Black arrows indicate the formation of covalent bond. Used atom colours: C in protein amino acids, 7, 2b in unbound state, and 2b in bound state are grey, orange, light green, and light blue, respectively. O = red, N = blue, P = pink, S = yellow, F = dark green, Zn = golden, Br = magenta.
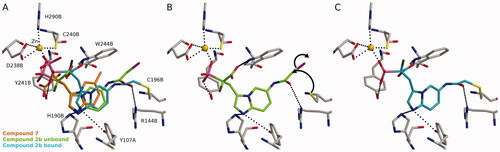
Also, other compounds (2a, 2c and 2d) seem to behave similarly in the docking experiment as 2b. However, bond formation is sensitive to the correct angle and distance between the cysteine thiol group in RGGT and the electrophilic residue of an inhibitor. Therefore, small structural differences, such as the length of the linker or steric hindrance, should influence the efficiency of nucleophilic attack. Both compounds, 2c and 2d are longer than 2b or 2a (by one carbon) in terms of the carbon position, which is attacked. Additionally, 2d bears steric hindrance making it less reactive. Interestingly, although 2a and 2b are structurally highly similar (the presence of either chlorine or bromine, in the electrophilic moiety), they significantly differ in activity, which might be the result of a weaker C-Br bond, making Br a better leaving group.Citation30,Citation32
Next, we performed mass spectrometry (MS) measurements to acquire further evidence for the covalent binding between 2b and C196B. For the RABGGTB binding site determination, we adapted the active cysteine profiling method.Citation23 Briefly, the proteins (RGGT, Rab7, REP-1) were incubated in the presence or absence of compound 2b. Subsequently, the reaction mixtures were treated in parallel with a pan-cysteine reactive reagent, N-benzyl-2-iodo-N-(prop-2-yn-1-yl)acetamide, in two isotopic forms (IA-light: six 12 C in benzene ring; IA-heavy: six 13 C in benzene ring), readily distinguishable by MS (Figure S4). After denaturation and alkylation, the mixtures were combined, and proteins were digested with one of the two peptidases (chymotrypsin or wild-type α-lytic protease WaLP) to generate orthogonal peptidic fragments, with complete coverage of cysteines in RABGGTB. Ions corresponding to cysteine-containing peptides modified by IA-light and IA-heavy were identified and quantified based on the relative intensities measured for these isotopically distinct ions (). Any cysteine reacted with compound 2b would lose its ability to subsequentially react with IA-light, and the concentration of the peptide containing that cysteine modified by IA-light would therefore decrease in the protein sample treated with 2b in comparison with the sample not subjected to the treatment. The only RABGGTB cysteine-containing peptides for which intensities were severely decreased upon incubation with 2b were peptides that correspond to sequences CCTGFLAITSQL (digestion with chymotrypsin) and GQIYCCTGFLA (digestion with WaLP), which contain C196B site, in addition to the inseparable vicinal C197B site. Guided by the molecular docking experiments, we anticipate the C196B as the major site of covalent binding to the inhibitor.
In conclusion, we present the first studies on the rational design of covalent Rab geranylgeranyltransferase inhibitor. For that purpose, we equipped the known non-covalent inhibitors with diverse electrophilic moieties. Using biological assaysCitation17 combined with mass spectrometric measurementsCitation23 and supported by molecular docking, we identified compound 2b as an RGGT inhibitor. We propose that 2b forms covalent bonding with the thiol group of the cysteine in the active site of RGGT.
The identification of the site of interaction has been achieved by using isotopically labelled probes and mass spectrometric analysis. It required optimisation in terms of finding the right digesting enzyme, as the standard trypsin could lead to very poor coverage of RABGGTB sequence, as predicted. Instead, we recommend the use of chymotrypsin and WaLP as they give complete coverage of cysteines. The experiment showed a significant decrease in the labelling of cysteines, C196 or/and C197, among 12 cysteines present in RABGGTB. While this experiment cannot distinguish between modifications of these neighbouring cysteines, the results of molecular docking indicate that the most optimal arrangement of bromoacetamide residue of the inhibitor and the thiol group of cysteine is possible for C196B. Additionally, the MS measurement did not reveal a substantial degree of modification by 2b of the cysteine residues from the C-terminus of Rab7, the RGGT substrate, even though these are present in the active site during prenylation. This observation makes us think that the inhibitory character of 2b results only from modification of RGGT.
Summarising, our data indicated that 2b is the first selective RGGT inhibitor equipped with highly reactive bromoacetamide moiety, which presumably enables the formation of the covalent bond between analogue and the enzyme. The comparable activity against RGGT of 2b and its non-covalent counterpart, propionamide analogue 2e, might result from the low stability of bromoacetamide moiety.Citation33 Efforts to increase the stability of inhibitor by changing bromine into chlorine in acetamide residue resulted in analogue 2a with no activity against RGGT. Further work is needed in order to tune the reactivity of electrophilic moiety.
Supplemental Material
Download PDF (20.5 MB)Acknowledgements
The authors thank Dr. Marek Domin (Boston College) for HRMS of final compounds. The authors thank Dr. Barbara Pacholczyk-Sienicka and Grzegorz Ciepielowski for carrying out NMR experiments. The authors thank Dr. Marzena Jędrzejczak-Krzepkowska for valuable comments on the expression of recombinant proteins and preparing the necessary documentation. We acknowledge CSC − IT Center for Science, Finland, for generous computational resources (O.T.P.: jyy2516 and jyy2585).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- De Cesco S, Kurian J, Dufresne C, et al. Covalent inhibitors design and discovery. Eur J Med Chem 2017;138:96–114.
- Mukherjee H, Grimster NP. Beyond cysteine: recent developments in the area of targeted covalent inhibition. Curr Opin Chem Biol 2018;44:30–8.
- Zhang T, Hatcher JM, Teng M, et al. Recent advances in selective and irreversible covalent ligand development and validation. Cell Chem Biol 2019;26:1486–500.
- Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study | Pfizer [Internet]. 2021 [cited 2021 Nov 6]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate.
- Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem 2008;77:383–414.
- Shannon DA, Weerapana E. Covalent protein modification: the current landscape of residue-specific electrophiles. Curr Opin Chem Biol 2015;24:18–26.
- Deng H, Lei Q, Wu Y, et al. Activity-based protein profiling: recent advances in medicinal chemistry. Eur J Med Chem 2020;191:112151.
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011;91:119–49.
- Gendaszewska-Darmach E, Garstka MA, Błażewska KM. Targeting small GTPases and their prenylation in diabetes mellitus. J Med Chem 2021;64:9677–710.
- Pylypenko O, Rak A, Reents R, et al. Structure of Rab escort protein-1 in complex with Rab geranylgeranyltransferase. Mol Cell 2003;11:483–94.
- Watanabe M, Fiji HDG, Guo L, et al. Inhibitors of protein geranylgeranyltransferase I and Rab geranylgeranyltransferase identified from a library of allenoate-derived compounds. J Biol Chem 2008;283:9571–9.
- Tan KT, Guiu-Rozas E, Bon RS, et al. Design, synthesis, and characterization of peptide-based rab geranylgeranyl transferase inhibitors. J Med Chem 2009;52:8025–37.
- Stigter EA, Guo Z, Bon RS, et al. Development of selective, potent RabGGTase inhibitors. J Med Chem 2012;55:8330–40.
- Deraeve C, Guo Z, Bon RS, et al. Psoromic acid is a selective and covalent rab-prenylation inhibitor targeting autoinhibited rabggtase. J Am Chem Soc 2012;134:7384–91.
- Coxon FP, Helfrich MH, Larijani B, et al. Identification of a novel Phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J Biol Chem 2001;276:48213–22.
- McKenna CE, Kashemirov BA, Błazewska KM, et al. Synthesis, chiral high performance liquid chromatographic resolution and enantiospecific activity of a potent new geranylgeranyl transferase inhibitor, 2-hydroxy-3-imidazo[1,2-a]pyridin-3-yl-2-phosphonopropionic Acid. J Med Chem 2010;53:3454–64.
- Kaźmierczak A, Kusy D, Niinivehmas SP, et al. Identification of the privileged position in the imidazo[1,2-a]pyridine ring of phosphonocarboxylates for development of Rab Geranylgeranyl Transferase (RGGT) inhibitors. J Med Chem 2017;60:8781–800.
- Coxon F, Joachimiak Ł, Najumudeen AK, et al. Synthesis and characterization of novel phosphonocarboxylate inhibitors of RGGT. Eur J Med Chem 2014;84:77–89.
- Joachimiak Ł, Marchwicka A, Gendaszewska-Darmach E, et al. Synthesis and biological evaluation of imidazole-bearing α-phosphonocarboxylates as inhibitors of Rab Geranylgeranyl Transferase (RGGT). ChemMedChem 2018;13:842–51.
- Lonsdale R, Burgess J, Colclough N, et al. Expanding the armory: predicting and tuning covalent warhead reactivity. J Chem Inf Model 2017;57:3124–37.
- Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006;6:358–70.
- Zhu K, Borrelli KW, Greenwood JR, et al. Docking covalent inhibitors: a parameter free approach to pose prediction and scoring. J Chem Inf Model 2014;54:1932–40.
- Abo M, Li C, Weerapana E. Isotopically-labeled iodoacetamide-alkyne probes for quantitative cysteine-reactivity profiling. Mol Pharm 2018;15:743–9.
- Kalinin A, Thomä NH, Iakovenko A, et al. Expression of mammalian geranylgeranyltransferase type-II in escherichia coli and its application for in vitro prenylation of Rab proteins. Protein Expr Purif 2001;22:84–91.
- Sidorovitch V, Niculae A, Kan N, et al. Expression of mammalian Rab Escort Protein-1 and -2 in yeast Saccharomyces cerevisiae. Protein Expr Purif 2002;26:50–8.
- Alexandrov K, Simon I, Yurchenko V, et al. Characterization of the ternary complex between Rab7, REP-1 and Rab geranylgeranyl transferase. Eur J Biochem 1999;265:160–70.
- Rak A, Niculae A, Kalinin A, et al. In vitro assembly, purification, and crystallization of the rab geranylgeranyl transferase: substrate complex. Protein Expr Purif 2002;25:23–30.
- Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2007;2:1896–906.
- Kusy D, Wojciechowska A, Małolepsza J, et al. Functionalization of the imidazo[1,2-a]pyridine ring in α-phosphonoacrylates and α-phosphonopropionates via microwave-assisted Mizoroki-Heck reaction. Beilstein J Org Chem 2020;16:15–21.
- Flanagan ME, Abramite JA, Anderson DP, et al. Chemical and computational methods for the characterization of covalent reactive groups for the prospective design of irreversible inhibitors. J Med Chem 2014;57:10072–9.
- Petri L, Ábrányi-Balogh P, Varga PR, et al. Comparative reactivity analysis of small-molecule thiol surrogates. Bioorg Med Chem 2020;28:115357.
- Lundblad RL, Chapter 2: alkylating agents. In: Chemical reagents for protein modification. 4th ed. CRC Press, Taylor & Francis Group; 2014:48–54.
- Ábrányi-Balogh P, Petri L, Imre T, et al. A road map for prioritizing warheads for cysteine targeting covalent inhibitors. Eur J Med Chem 2018;160:94–107.
