Abstract
Helicobacter pylori can cause chronic gastritis, peptic ulcer, and gastric carcinoma. This study compares chemical composition and anti-H. pylori activity of mandarin leaves and marjoram herb essential oils, and their combined oil. GC/MS analysis of mandarin oil revealed six compounds (100% identified), mainly methyl-N-methyl anthranilate (89.93%), and 13 compounds (93.52% identified) of marjoram oil, mainly trans-sabinene hydrate (36.11%), terpinen-4-ol (17.97%), linalyl acetate (9.18%), and caryophyllene oxide (8.25%)). Marjoram oil (MIC = 11.40 µg/mL) demonstrated higher activity than mandarin oil (MIC = 31.25 µg/mL). The combined oil showed a synergistic effect at MIC of 1.95 µg/mL (same as clarithromycin). In-silico molecular docking on H. pylori urease, CagA, pharmacokinetic and toxicity studies were performed on major compounds from both oils. The best scores were for caryophyllene oxide then linalyl acetate and methyl-N-methyl anthranilate. Compounds revealed high safety and desirable properties. The combined oil can be an excellent candidate to manage H. pylori.
Graphical Abstract
1. Introduction
Despite enormous progress in medicinal strategies for the treatment of many human health problems, infectious diseases continue to pose a significant threat to public healthCitation1. Helicobacter pylori is an extracellular gram-negative spiral bacterium, that is now recognised as a major cause of gastroduodenal diseases such as chronic gastritis, which affects nearly everyone and leads to peptic ulcers or gastric adenocarcinoma, the second most common cause of cancer death worldwideCitation2,Citation3. Standard medications can cure the infection in more than 80% of H. pylori-infected patients. Patient compliance, antibiotic resistance, and recurring infections, on the other hand, are all the major concerns that limit the use of antibiotics in the treatment of H. pylori infection that needs to be addressedCitation4,Citation5.
Natural products are reported to demonstrate various biological activitiesCitation6–10 and as promising antimicrobialsCitation11,Citation12. The importance of plant-based products for disease treatment is growing exponentially due to the increased incidence of adverse drug reactionsCitation13 and the development of microbial resistance to the available antimicrobial drugsCitation14.
Essential oils derived from aromatic and medicinal plants have recently gained popularity and great scientific interest as they are a part of traditional medicine predominating all over the globe for the alleviation of various health problems. Essential oils have been shown to possess potential antibacterial, antifungal, antiviral, anticancer, and antioxidant properties such as cinnamon, orange, lemon, pepper, thyme, and SchinusCitation15–20. Besides, they act as an important milestone in alternative medicine as well as natural therapiesCitation1. Therefore, it is reasonable to expect that a variety of plant compounds in these oils have antimicrobial effects.
Among several essential oils that may be useful as antimicrobial agents, marjoram oil (Origanum majorana L., Lamiaceae) is an aromatic medicinal plant with the greatest potential for industrial applications because it shows different biological activities, including antibacterial, antifungal, antihypertensive, anti-inflammatory, and antioxidant propertiesCitation21–23. Origanum majorana leaves and essential oil have been claimed to be useful for the treatment of respiratory and gastrointestinal problemsCitation24. It is one of the most popular spices used in cooking, arousing interest not only in the use of its leaves but also in its essential oil for therapeutic purposesCitation25.
On the other hand, the genus Citrus (Rutaceae) has been one of the most popular and commercially important crops for thousands of years. Citrus fruits are known for their nutritional values as an excellent source of vitamin C, their unique flavour, and their medicinal propertiesCitation26. Interestingly, essential oil (EO) is the most vital by-product of citrus processing. Petitgrain mandarin essential oil is extracted from Citrus reticulata leaves. It could relieve stress and digestive problems while helping with flatulence, diarrhoea, and constipation. It is mostly used to increase circulation to the skin, reducing fluid retention and helping prevent stretch marks. Mandarin oil is used to calm the nervous system and has a tonic effectCitation27,Citation28. Moreover, it showed broad-spectrum antibacterial and antifungal agents. It inhibited the growth of several bacterial and fungal strainsCitation29–31. Furthermore, petitgrain mandarin essential oil showed potential antioxidant, anticancer (HL-60 and NB4), and radical scavenging activitiesCitation32.
The increasing emergence of H. pylori infections worldwide as well as the emerging tolerance against most currently available antibiotics has necessitated the urgent need to discover novel and highly effective antimicrobial regimens due to the lack of therapies available to control H. pylori infections. Meanwhile, it has been noticed that a few plants have been investigated recently for their H. pylori bactericidal activity. Antibacterial drug interactions can change the efficacy and either synergistic or antagonistic action, interaction between different compounds can lead to the reduction of the inhibitory activityCitation33.
This has driven our interest to assess the constituents of essential oils of marjoram (Origanum majorana L.) and mandarin leaves by using GC-MS, as well as evaluate the synergistic anti-H. pylori activity in-vitro of these oils, as compared to clarithromycin. An in-silico study was performed, where molecular docking was carried out on the major compounds identified from both oils on H. pylori virulent factors domains such as urease and CagA. Further in-silico pharmacokinetic and toxicity studies were performed on these major components to determine their safety margins and properties.
2. Materials and methods
2.1. Essential oils
The whole herb of Origanum majorana was subjected to steam distillation for 5 h. The oil produced has a pale yellow colour and herbaceous sweet odour. Citrus reticulata oil was prepared by water distillation of the leaves using the Clevenger apparatus for 5 h. Its colour is pale yellow and of intensely sweet and fresh scent. Both oils were purchased from Somitt Aromatic Company that were kept in dark bottles.
2.2. Analysis of essential oils by gas chromatography
2.2.1. GC/FID analysis
The GC/FID analyses were carried out on a Varian 3400 apparatus (Varian GmbH, Darmstadt, Germany) equipped with an FID detector and an Rtx-5MS fused-bonded silica column (30 m x 0.25 mm i.d., film thickness 0.25 µm; Ohio Valley, Ohio, USA); the operating conditions were: The initial column temperature was kept at 45 °C for 2 min (isothermal), and then programmed rising at a rate of 5 °C/min to 300 °C and held for 5 min. Detector and injector temperatures were 300 °C and 250 °C, respectively. The sample volume was 0.03 µl, Helium carrier gas flow rate was 2 ml/min. Peak Simple 2000 chromatography data system (SRI Instruments, Torrance, USA) was used for recording and integrating the chromatograms.
2.2.2. GC/MS analysis
The analyses were carried out on a Hewlett Packard gas chromatograph (GC HP 5890 II; Hewlett Packard GmbH, Bad Homburg, Germany) equipped with the same column and conditions as for the GC/FID. The capillary column was directly coupled to a quadrupole mass spectrometer (SSQ 7000; Thermo-Finnigan, Bremen, Germany). The injector temperature was 250 °C. Helium carrier gas flow rate was 2 ml/min. All the mass spectra were recorded with the following analytical conditions: filament emission current, 60 mA; electron energy, 70 eV; ion source temperature, 200 °C; and scan range was from 40 to 400 Amu. The diluted samples (0.5% v/v n-hexane used as solvent) were injected with split mode (split ratio, 1:15). Compounds were identified by comparison of their mass spectral data and retention indices with Wiley Registry of Mass Spectral Data 8th edition and NIST Mass Spectral Library (December 2005). The identification was further confirmed by the calculation of the retention indices (RI) relative to a homologous series of n-alkanes (C6 - C22), under identical experimental conditions, as well as matching with the literatureCitation34–37.
2.3. Assessment of the anti-Helicobacter pylori activity
2.3.1. Determination of the minimal inhibitory concentration (MIC)
The micro-well dilution method was used to evaluate the antibacterial activity of the Marjoram and Mandarin oils against Helicobacter pylori (ATCC 43504, the reference strain being obtained from the American Type Culture Collection) adopting the NCCLS guidelines (1998) and as previously described by Cerda et al.Citation38. 100 µg of the tested samples were combined with 100 µL of 20% (v/v) bacterial suspensions (OD at 600 nm = 1.0) in a flat bottomed 96-well microplate. Serial two-fold dilutions of the oils and the standard were prepared directly in a sterile 96-well microtiter plate. Deionised water was used as a negative control meanwhile clarithromycin was used as a positive control. The reaction mixture was incubated using Mueller-Hinton broth. After incubation at 37 °C for 3 days under microaerophilic conditions (10% CO2 and 80% humidity). 25 µL of 10 mM 3–(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) freshly prepared in water were added into the mixture (final volume was 225 µL) to each well and incubate for 30 min. The developed purple colour was measured at 550 nm using a microplate readerCitation1. All tests were performed in triplicate. Inhibition (%) was calculated as follows: [(Initial control absorbance-final absorbance)/(Initial control absorbance)] × 100.
The Agar dilution checkerboard method is used to evaluate the synergic action of both essential oils. The MIC90, the concentration of samples with 90% inhibition was calculated from dose-response curves. The MIC values were assessed in triplicate, using an automatic ELISA microplate reader.
2.4. In-silico studies
2.4.1. Molecular docking
The X-ray 3D structures of human H. pylori Urease and Cag A oncogenic proteins were downloaded from the protein data bank using the following IDs: 1e9y and 4dvy, respectively. All the docking studies were conducted using MOE 2019Citation39 which was also used to generate the 2D and 3D interaction diagrams between the docked ligands and their potential targets. In the beginning, the two enzymes and the seven isolated major compounds were prepared using the default parameters. The active site of each target was determined and the seven isolated compounds were saved into a single file with MDB extension. Finally, the docking was finalised by docking the mdb file containing the seven compounds into the active site of both the enzymes.
2.4.2. In-silico toxicity and ADME prediction
Both the Toxicity and pharmacokinetic properties of the seven compounds were predicted by the online servers ProTox II (https://tox-new.charite.de/protox_II/index.php?site=compound_input) and Swiss adme (http://www.swissadme.ch/index.php), respectively.
3. Results
3.1. Determination of the volatile oil compositions using GC/MS
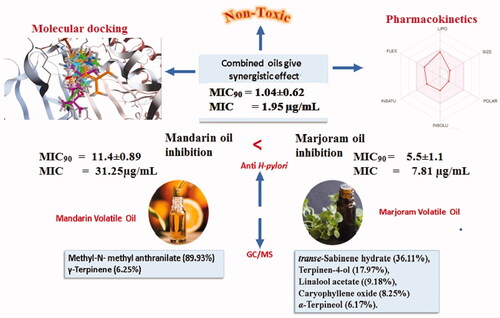
Six compounds were identified from the oil of mandarin leaves (, ). The predominant constituents of the oil were methyl-N-methyl anthranilate (89.93%) and γ-terpinene (6.25%), respectively. While 93.52% of marjoram oil was identified (, ), the major peaks were for trans-sabinene hydrate (36.11%), terpinen-4-ol (17.97%), linalyl acetate (9.18%), caryophyllene oxide (8.25%), and α-terpineol (6.17%), respectively.
Table 1. The essential oil composition of mandarin leaves oil.
Table 2. The essential oil composition of marjoram oil.
3.2. Determination of anti-Helicobacter pylori activity
This study’s focal objective is to compare the antimicrobial activities of essential oils of mandarin leaves and marjoram against Helicobacter pylori. The MIC results for the two tested oils separately and combined with clarithromycin as a positive control are shown in ), respectively.
Figure 3. The MIC90 & MIC graphs of Anti-Helicobacter pylori activity of mandarin oil leaves (A), marjoram oil (B), equal amounts of both oils(C), and Clarithromycin (D) as standard. All determinations were carried out in a triplicate manner and values are expressed as the mean ± SD.

The results of this study revealed that marjoram oil showed higher antibacterial activity against H. pylori at a MIC of 11.4 µg/mL, () relative to mandarin essential oil, which exhibited a MIC value of 31.25 µg/mL (). This may be attributed to the presence of high content of oxygenated compounds in marjoram oil that were identified as shown in (), including trans-sabinene hydrate (36.11%), terpinen-4-ol (17.97%), linalyl acetate (9.18%), caryophyllene oxide (8.25%), and α-terpineol (6.17%).
While methyl-N-methyl anthranilate (89.93%) has been identified as the major constituent of mandarin leaf oil, followed by γ-terpinene (6.25%) as shown in (). A combined mixture of both oils exhibited a potentially synergistic inhibitory effect against H. pylori at a MIC of 1.95 µg/ml, yielding higher inhibition () relative to marjoram and mandarin oils separately. Furthermore, clarithromycin demonstrated the same MIC value (1.95 µg/mL).
3.3. Docking study
A molecular docking study was carried out on the major compounds identified from both oils on H. pylori virulent factors domains such as urease and CagA. Results showed that caryophyllene oxide showed the best fitting scores followed by linalyl acetate and methyl-N-methyl anthranilate, as demonstrated in . The binding affinities of caryophyllene oxide, linalyl acetate and methyl-N-methyl anthranilate to urease and CagA are further demonstrated in , respectively. Caryophyllene oxide showed the best binding affinities to the urease enzyme by 4 hydrogen bonds (H-bonds) and solvent interaction, while it revealed an interaction with only 2 H-bonds with cagA. Linalyl acetate exhibited 5 H-bonds and 2 metal (Nickel) co-ordinates with urease and 3 H-bonds with cagA. Regarding, methyl-N-methyl anthranilate, it showed 3 H-bonds and solvent interaction with urease, while it demonstrated 2 H-bonds and 2 hydrophobic interactions with the active sites of CagA. revealed concomitant interactions of seven major compounds identified in both oils with the active sites of urease and cagA, respectively, to reveal more of the synergistic effect of these components as anti-H. pylori.
Figure 4. 2D and 3D-binding affinities of caryophyllene oxide (A,D), linalyl acetate (B,E), methyl-N-methyl anthranilate (C,F) and concomitant interactions of seven major compounds identified in marjoram and mandarin oils with the active sites of H. pylori urease domain.

Figure 5. 2D and 3D-binding affinities of caryophyllene oxide (A,D), linalyl acetate (B,E), methyl-N-methyl anthranilate (C,F) and concomitant interactions of seven major compounds identified in marjoram and mandarin oils with the active sites of H. pylori CagA domain.

Table 3. Docking scores of marjoram and mandarin major oil compounds on H. pylori virulent factors domains (urease and CagA).
3.4. In-silico toxicity study
As demonstrated in all the compounds have high margins of safety and they were predicted to have no potential toxicity.
Table 4. The predicted toxicity of marjoram and mandarin major oil compounds.
3.5. Pharmacokinetics study
It is important for therapeutic candidates to have both acceptable pharmacokinetics and pharmacodynamics profiles. Accordingly, the pharmacokinetic profiles of the seven major compounds were computed using the online server of swiss adme. As depicted in . All the compounds were predicted to have high GIT absorption making them excellent oral candidates against H. pylori.
This high bioavailability of the seven compounds is attributed to their desired physicochemical properties including FLEX (Flexibility), LIPO (Lipophilicity), INSATU (Saturation), INSOLU (Solubility), SIZE and POLAR (Polarity) as demonstrated in and . In addition, all compounds showed no or minimal interaction with microsomal cytochromes and then could be taken concurrently with other medications. Most importantly, no compound was found to be a substrate for the p-glycoprotein known to be one of the resistance mechanisms of H. pylori for existing antibioticsCitation40. A worthy note, the seven compounds were aligned with all of Lipinski's rules, besides none of them had any reported Pan Assay Interference (PAINS).
Figure 6. The predicted physicochemical properties for selected compounds, such as linalyl acetate (A) and trans-sabinene hydrate (B).

Table 5. The predicted pharmacokinetics of marjoram and mandarin major oil compounds.
4. Discussion
The continuous evolution of many drawbacks with the current therapies for H. pylori, such as the prevalence of antibiotic-resistant, drug interventions, side effects, and poor satisfaction, all highlight the search for safe and effective non-antibiotic alternative medicinesCitation5. This has driven our interest in evaluating the bactericidal activity of marjoram and mandarin oils against H. pylori. At present, interest in essential oils has increased because of their bactericidal activity against several bacteria without the marked toxic effects of synthetic drugs.
Bactericidal activity is a well-known property of volatile oils, particularly those of marjoram and mandarin. Numerous studies have confirmed the antimicrobial activity of marjoram essential oilsCitation21,Citation22,Citation25,Citation41,Citation42 and mandarin leaf essential oilsCitation26,Citation43–46. This study investigates the bactericidal activity of the hydro-distilled essential oils of the leaves of mandarin and marjoram. The results of this study revealed that marjoram oil showed a higher effect against H. pylori than mandarin essential oil. This may be attributed to the presence of high content of oxygenated compounds identified in marjoram oil, including trans-sabinene hydrate (36.11%), terpinen-4-ol (17.97%), linalyl acetate (9.18%), caryophyllene oxide (8.25%), and α-terpineol (6.17%). While methyl-N-methyl anthranilate (89.93%) was identified as the major constituent of mandarin leaf oil, followed by γ-terpinene (6.25%).
Many studies of in vitro antimicrobial activity of marjoram and mandarin oils in the literature may be probably due to the action of the major compounds which have been previously tested for their bactericidal activity, such as terpinen-4-ol, α-terpineol, and γ-terpinene were found as the predominant components of the essential oils obtained from the aerial parts of Origanum scabrum and Origanum microphyllum, both endemic species in Greece, exhibited a very interesting antimicrobial profile after they were tested against six Gram-negative and Gram-positive bacteria and three pathogenic fungiCitation47. Furthermore, the acetone crude extract of the stem bark of Sclerocarya birrea is a promising source for anti-H pylori compounds, with terpinen-4-ol, an essential oxygenated monoterpene oil, being the most abundant agent (35.83%), and it was reported as a major mediator of the anti-H pylori activityCitation48. The inhibitory activity of terpinen-4-ol in this study was similar to that of amoxicillin, one of the most effective drugs used in the eradication of H. pylori infections worldwideCitation49. Additionally, trans-sabinene hydrate, terpinen-4-ol, α-terpineol, and γ-terpinene have been reported as major components of marjoram oil, which exhibited antibacterial activity against food-related bacteria like E. coli, Salmonella cholraesius, and S. aureus in fresh sausage. Because of their antimicrobial activity against food-borne bacteria EOs could be added to food products to extend their shelf life, but changes in the taste, as well as formulation problems, could represent a problem there inCitation21.
Linalool (8.5%), α-terpineol (4.4%), and linalyl acetate (4.2%) are considered the most important components of Myrtle oil that showed significant antimicrobial activities against Salmonella typhimorium, Lactobacillus spp., Yersinia enterocolitica, Helicobacter pyloriCitation50, and significant antifungal activity when combined with amphotericin BCitation51. Moreover, the antimicrobial activity of the essential oil of Thymus capitatus was tested using the broth dilution method. γ-Terpinene in Thymus capitatus essential oil (10%) induced strong bactericidal activity against H. pylori strainsCitation52.
β-Caryophyllene, is a natural bicyclic sesquiterpene, which is extracted from clove and tested for the eradication of H. pylori in a mouse model, and its effects on the inflammation of the gastric mucosa. Interestingly, β-caryophyllene showed potent antimicrobial activity against H. pylori by direct killing action. In addition, it improved the inflammation of the gastric mucosa by decreasing H. pylori numberCitation53. The use of compounds with natural origin has gained popularity in scientific research focussed on drug innovation against H. pylori because of their broad flexibility and low toxicity. For example, monoterpenes limonene and β-pinene resulted in MICs against H. pylori of 75 µg/mL and 500 µg/mL respectivelyCitation54.
Regarding the biological properties, we know that essential oils are complex mixtures of numerous constituents. As a result, their biological effects can be the result of the synergism of all their constituents, thus components are working together. In this case, the effect of the mixture would be greater than the pure sum of its single partsCitation55. The essential oils could be used on their own, as well as in combination with other oils or synthetic active agents since synergy was observed by combining these substances. Various studies showed that the extent of antimicrobial activity and the mode of action is dependent on the additive, synergistic, or even antagonistic effects of the individual constituents.
Results of this study clearly showed the synergistic effect of both marjoram and petitgrain mandarin oils on their anti-H. pylori activity. The combined oil sample showed the highest inhibitory effect against H. pylori at MIC 1.95 µg/mL. Clarithromycin, the used reference drug, demonstrated the same MIC value as the combined oil, at the same concentration used. Thus, it should be noted that the combined oils' effects are comparable to clarithromycin.
An in-silico study was carried out to further verify the observed results. Docking studies are performed to reveal the binding affinity of the major components to the target enzymesCitation56,Citation57, where caryophyllene oxide showed the best fitting scores followed by linalyl acetate and methyl-N-methyl anthranilate. Furthermore, all the tested compounds showed high margins of safety in-silico, they were predicted to have no potential toxicity and were aligned with all Lipinski's rules. Additionally, all the tested compounds showed no or minimal interaction with microsomal cytochromes, thus, they could be taken concomitantly with other medications. Moreover, neither of the tested compounds was found to be a substrate for the p-glycoprotein (one of the resistance mechanisms of H. pylori for existing antibiotics)Citation40 or had any reported Pan Assay Interference (PAINS). From this perspective, the two oil extracts are considered promising inhibitors for both sensitive and resistant strains of H. pylori with a notable safety margin and good desirable pharmacokinetic properties.
5. Conclusion
Marjoram and mandarin oils are the most widely available and highly consumed by humans due to their nutritional and medicinal values and very low toxic effects. The current study revealed the promising synergistic effects of the volatile constituents from marjoram and mandarin leaves against Helicobacter pylori, offering a natural remedy for pharmaceutical industries to combat this bacterial infection. Furthermore, the major compounds of both oils were evaluated in-silico, and demonstrated superior biological activities, excellent safety margins and desired pharmacokinetic properties making them excellent candidates to manage H. pylori.
Disclosure statement
The authors declare that they do not have any conflict of financial interests or personal relationships that could influence the reported work.
Additional information
Funding
References
- Miethke M, Pieroni M, Weber T, et al. Towards the sustainable discovery and development of new antibiotics. Nature Rev Chem 2021;5:1610–49.
- Peek RJ, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002;2:28–37.
- Piscione M, Mazzone M, Di Marcantonio MC, et al. Eradication of Helicobacter pylori and gastric cancer: a controversial relationship. Front Microbiol 2021;12:630852.
- Esmaeili D, Mohabati AM, Tohidpour A. Anti-helicobacter pylori activities of Shoya powder and essential oils of Thymus vulgaris and Eucalyptus globulus. Open Microbiol J 2012;6:65–9.
- Grande R, Carradori S, Puca V, et al. Selective inhibition of Helicobacter pylori carbonic anhydrases by carvacrol and thymol could impair biofilm production and the release of outer membrane vesicles. Int J Mol Sci 2021;22:11583.
- Abdallah SH, Mostafa NM, Mohamed MA, et al. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat Prod Res 2021;1–7.
- Mostafa NM, Edmond MP, El-Shazly M, et al. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat Prod Res 2021;1–6.
- Moussa AY, Mostafa NM, Singab ANB. Pulchranin A: first report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat Prod Res 2020;34:2715–22.
- El-Nashar HA, Mostafa NM, Eldahshan OA, et al. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat Prod Res 2022;36:1182–90.
- El-Nashar HAS, Mostafa NM, El-Shazly M, et al. The role of plant-derived compounds in managing diabetes mellitus: a review of literature from 2014 To 2019. Curr Med Chem 2021;28:4694–730.
- Al-Madhagy SA, Mostafa NM, Youssef FS, et al. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct 2019;10:6267–75.
- El-Nashar HA, Mostafa NM, El-Badry MA, et al. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat Prod Res 2020;9:1–4.
- El-Zahar H, Menze ET, Handoussa H, et al. UPLC-PDA-MS/MS profiling and healing activity of polyphenol-rich fraction of Alhagi maurorum against oral ulcer in rats. Plants 2022;11:455.
- Abdul W, Hajrah N, Sabir J, et al. Therapeutic role of Ricinus communis L. and its bioactive compounds in disease prevention and treatment. Asian Pac J Trop Med 2018;11:177.
- Singab AS, Mostafa N, Eldahshan OA, et al. Profile of volatile components of hydrodistilled and extracted leaves of Jacaranda acutifolia and their antimicrobial activity against foodborne pathogens. Nat Prod Commun 2014;9:1007–10.
- Omayma AE, Halim AF. Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of egyptian navel orange (Citrus sinensis (L.) Osbeck var. malesy). Chem Biodivers 2016;13:681–5.
- Todirascu-Ciornea E, El-Nashar HAS, Mostafa NM, et al. Schinus terebinthifolius essential oil attenuates scopolamine-induced memory deficits via cholinergic modulation and antioxidant properties in a zebrafish model. Evid Based Complement Alternat Med 2019;2019:5256781.
- Ashmawy A, Mostafa N, Eldahshan O. GC/MS analysis and molecular profiling of lemon volatile oil against breast cancer. J Essent Oil Bear Plants 2019;22:903–16.
- Ayoub N, Singab AN, Mostafa N, et al. Volatile constituents of leaves of Ficus carica Linn. grown in Egypt. J Essent Oil Bear Plants 2010;13:316–21.
- Mostafa NM, Mostafa AM, Ashour ML, et al. Neuroprotective effects of black pepper cold-pressed oil on scopolamine-induced oxidative stress and memory impairment in rats. Antioxidants 2021;10:1993.
- Busatta C, Vidal RS, Popiolski AS, et al. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiol 2008;25:207–11.
- Erenler R, Sen O, Aksit H, et al. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities . J Sci Food Agric 2016;96:822–36.
- Tahraoui A, El-Hilaly J, Israili ZH, et al. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in South-Eastern Morocco (Errachidia province). J Ethnopharmacol 2007;110:105–17.
- Charles DJ, Marjoram sweet. In: Antioxidant properties of spices, herbs and other sources. New York: Springer; 2013. 393–399 p.
- Juliana DLM, Lisiane MV, Graciele DF, et al. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind Crops Prod 2015;77:444–50.
- Dalia IH, Rehab HA, Maged EM, et al. Chemical composition and biological activity of essential oils of Cleopatra mandarin (Citrus reshni) cultivated in Egypt. J Pharmacogn Phytother 2013;5:83–90.
- Fleisher Z, Fleisher A. Aromatic plants of the holy land and the Sinai, Part III. Mandarin leaf oil (Citrus recticulata Blanco). J Essent Oil Res 1990;2:331–4.
- Davies FS, Albrigo LG, Citrus. Wallingford UK: CAB International, 1994. 1 p.
- Yi F, Jin R, Sun J, et al. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT Food Sci Technol 2018;95:346–53.
- Tao N, Jia L, Zhou H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem 2014;153:265–71.
- Omayma AE. Comparison of chemical and antimicrobial studies of egyptian mandarin leaves and green branches volatile oil. Eur J Med Plants 2015;5:248–53.
- Sayed AF. Antioxidant and anticancer activities of Citrus reticulate (Petit grain Mandarin) and Pelargonium graveolens (Geranium) essential oils. Res J Agric Biol Sci 2009;5:740–7.
- Mayar AI, Omar WS, Maryam RA, et al. Potentiation of anti-helicobacter pylori activity of clarithromycin by Pelargonium graveolens oil. Arab J Gastroentrol 2021;22:224–8.
- Esraa A, Shahat RO, Omayma AE, et al. Chemical composition and biological activities of the essential oil from leaves and flowers of Pulicaria incisa sub. candolleana growing in Egypt. Chemistry and Biodiversity 2017;14.
- Azab SS, Abdel Jaleel GA, Eldahshan OA. Anti-inflammatory and gastroprotective potential of leaf essential oil of Cinnamomum glanduliferum in ethanol-induced rat experimental gastritis. Pharm Biol 2017;55:1654–61.
- Adams RP, Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. 4th Ed., Illinois, USA: Allured Publishing Corporation. 2007. 456 p.
- Younis MM, Ayoub IM, Mostafa NM, et al. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase, and anti-hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants 2022;11:918.
- Cerda O, Rivas A, Toledo H. Helicobacter pylori strain ATCC700392 encodes a methyl-accepting chemotaxis receptor protein (MCP) for arginine and sodium bicarbonate. FEMS Microbiol Letters 2003;224:175–81.
- Vilar S, Cozza G, Moro S. Medicinal chemistry and the Molecular Operating Environment (MOE): application of QSAR and molecular docking to drug discovery. Curr Top Med Chem 2008;8:1555–72.
- Damanhuri NS, Kumolosasi E, Omar MS, et al. The influence of P-glycoprotein expression in the standard treatment of Helicobacter pylori infection in Sprague Dawley rats. Daru J Faculty Pharm Tehran University Med Sci 2021;29:13–22.
- Deans SG, Katerina PS. The antimicrobial properties of marjoram (Origanum majorana L,) volatile oil. Flavor Fragr J 1990;5:187–90.
- Shimaa TO, Sherein IA, Amany MM. Antibacterial effect of Origanum majorana L. (Marjoram) and Rosmarinus officinalis L. (Rosemary) in Egypt. Global Veterinaria 2014;13:1056–64.
- Tripoli E, Guardia ML, Giammanco S, et al. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chemistry 2007;104:466–79.
- Manners GD. Citrus limonoids: analysis, bioactivity, and biomedical prospects. J Agric Food Chem 2007;55:8285–94.
- Tao NG, Liu YJ, Tang YF, et al. Essential oil composition and antimicrobial activity of citrus reticulata. Chem Natural Comp 2009;45:437–8.
- Jayaprakasha GK, Negi PS, Sikder S, et al. Antibacterial activity of citrus reticulata peel extracts. Z Naturforsch C J Biosci 2000;55:1030–4.
- Aligiannis N, Kalpoutzakis E, Mitaku S, et al. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 2001;49:4168–70.
- Njume C, Afolayan AJ, Green E, et al. Volatile compounds in the stem bark of Sclerocarya birrea (Anacardiaceae) possess antimicrobial activity against drug-resistant strains of Helicobacter pylori. Int J Antimicrob Agents 2011;38:319–24.
- Tanih NF, Okeleye BI, Naido N, et al. Marked susceptibility of South African Helicobacter pylori strains to ciprofloxacin and amoxicillin: clinical implication. S Afr Med J 2010;100:49–52.
- Deriu A, Branca G, Molicotti P, et al. In vitro activity of essential oil of Myrtus communis L. against H. pylori. Int J Antimicrobial Agent 2007;30:262–5.
- Mahboubi M, Bidgoli FG. In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine 2010;17:771–4.
- Meryem G, Duygu H, Azmi H, et al. Antimicrobial activity of the essential oil of Thymus capitatus against Helicobacter pylori. Acta Poloniae Pharmaceutica ñ Drug Res 2020;77:1.
- Bonifácio BV, dos Santos Ramos MA, da Silva PB, et al. Antimicrobial activity of natural products against. Helicobacter pylori: a review. Ann Clin Microbiol Antimicrob 2014;13:54.
- Rath CC, Devi S, Dash SK, et al. Antibacterial potential assessment of jasmine essential oil against E. coli. Indian J Pharm Sci 2008;70:238–24.
- Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995;47:331–85.
- Mostafa NM, Ashour ML, Eldahshan OA, et al. Cytotoxic activity and molecular docking of a novel biflavonoid isolated from Jacaranda acutifolia (Bignoniaceae). Nat Prod Res 2016;30:2093–100.
- Mostafa NM, Ismail MI, AM E-A, et al. Investigation of SARS-CoV-2 main protease potential inhibitory activities of some natural antiviral compounds via molecular docking and dynamics approaches. Phyton Int J Exp Botany 2022;91:1089–104.


