Abstract
Glycogen synthase kinase 3β (GSK-3β) catalyses the hyperphosphorylation of tau protein in the Alzheimer’s disease (AD) pathology. A series of novel thieno[3,2-c]pyrazol-3-amine derivatives were designed and synthesised and evaluated as potential GSK-3β inhibitors by structure-guided drug rational design approach. The thieno[3,2-c]pyrazol-3-amine derivative 16b was identified as a potent GSK-3β inhibitor with an IC50 of 3.1 nM in vitro and showed accepted kinase selectivity. In cell levels, 16b showed no toxicity on the viability of SH-SY5Y cells at the concentration up to 50 μM and targeted GSK-3β with the increased phosphorylated GSK-3β at Ser9. Western blot analysis indicated that 16b decreased the phosphorylated tau at Ser396 in a dose-dependent way. Moreover, 16b effectively increased expressions of β-catenin as well as the GAP43, N-myc, and MAP-2, and promoted the differentiated neuronal neurite outgrowth. Therefore, the thieno[3,2-c]pyrazol-3-amine derivative 16b could serve as a promising GSK-3β inhibitor for the treatment of AD.
1. Introduction
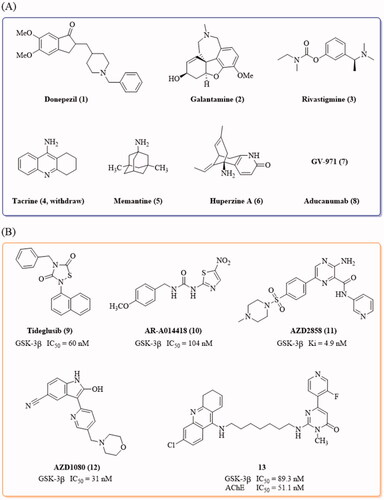
Alzheimer’s disease (AD), characterised by memory loss and cognitive impairments, is a chronic neurodegenerative disorder that disturbs more than 50 million people’s healthy life worldwideCitation1,Citation2. At present, only a few drugs, donepezil, galantamine, rivastigmine, tacrine, memantine, huperzine A (NMPA), GV-971 (NMPA) and aducanumab (), are available for the treatment of this diseaseCitation3–6; however, there are no drugs that can effectively block or reverse the progression of AD, possibly due to the complicated aetiology of this disease. A number of hypotheses have been proposed for AD pathogenesisCitation7–18, among which, the β-amyloid (Aβ) deposit and tau protein hyperphosphorylation are the key concernsCitation19,Citation20. The coexistence of Aβ plaques and tau intracellular neurofibrillary tangles (NFTs) in the neocortex is associated with the collapse of neural circuits and cognitive decline, and the interactions between Aβ and tau exaggerate the pathology of ADCitation21,Citation22.
Figure 1. (A) Drugs approved by FDA and NMPA for the treatment of AD by June 2021; (B) Representative GSK-3β inhibitors with anti-AD activity in vivo.
Glycogen synthase kinase-3 (GSK-3), a proline-directed serine/threonine kinase, is closely associated with Aβ deposits and tau hyperphosphorylation. GSK-3 has two subtypes, GSK-3α (51 kDa) and GSK-3β (47 kDa) in mammals. Most notably, in the brain, GSK-3β is the primary isoform and acts as the dominator for tau hyperphosphorylationCitation23,Citation24. The overactivation of GSK-3β was identified and co-localized with neurofibrillary tangles (NFTs) in postmortem AD brainCitation25,Citation26. Hyperphosphorylated tau lost the physiological ability to bind to tubulin, and therefore, detached from tubulin, resulting in the formation of paired helical filaments (PHFs) and subsequently aggregated to NFTsCitation27,Citation28. The abnormal deposition of NFTs led to extensive damage to the normal transport and signalling pathways, cell cytoskeleton, mitochondria, and neuronal cell deathCitation29. In addition to the tau pathway, GSK-3β could promote the Aβ fibril generation and induce Aβ aggregationCitation30. In transgenic AD mice, the inhibition of GSK-3β could reduce the Aβ-induced toxicity and improve cognition performancesCitation31. Moreover, the overactivation of GSK-3β could cause neuroinflammation, neuronal death, and apoptosisCitation32,Citation33. In the light of the multifunctional roles of GSK-3β in AD pathology, GSK-3β becomes a potential target for the development of anti-AD drugsCitation34,Citation35.
Tideglusib () is the small thiadiazolidinone GSK-3β inhibitor that entered the clinical trial for the treatment of ADCitation36. Besides, the GSK-3β inhibitors, AR-A014418, AZD2858, AZD1080, as well as the GSK-3β and acetylcholinesterase (AchE) dual inhibitor 13, demonstrated anti-AD effects in AD animalsCitation26,Citation37–39 (). Most of the reported GSK-3β inhibitors feature with the “double-sites occupation” pharmacophore model: a key skeleton interacted with the hinge region by forming two hydrogen bonds with Asp133 and/or Val135, a moiety connected to the key skeleton as hydrogen bond acceptor to interact with Lys85 side chainCitation40. Following this model, we used the thieno[3,2-c]pyrazol-3-amine as the key framework and designed a series of thieno[3,2-c]pyrazol-3-amine derivatives as the potential GSK-3β inhibitors. The thieno[3,2-c]pyrazol-3-amine framework has the possibility to form triple hydrogen bonds with the hinge region so as to enhance its binding with the enzyme (). The N atom of pyridine moiety connected with the thieno[3,2-c]pyrazol-3-amine may act as hydrogen bond acceptor to interact with Lys85 side chain. A variety of the substituents (RCitation1, R2 and R3) were introduced to investigate their effects on the GSK-3β inhibitory activities.
2. Results and discussion
2.1. Chemistry
The newly designed thieno[3,2-c]pyrazol-3-amine derivatives were synthesised as outlined in Scheme 1. The 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine 14 was prepared according to the reported method from 3-bromothiophene in seven stepsCitation41. The reaction of 14 with acyl chloride or sulphonyl chloride generated the thieno[3,2-c]pyrazol-3-amine intermediates 15a–15e. The Suzuki coupling of 15a–15e with pyridylboronic acids or pyridylboronic acid esters provided the thieno[3,2-c]pyrazol-3-amine derivatives 16a–16e, 17a–17d and 18a–18n in 11.5–55.9% yields. A total of 23 thieno[3,2-c]pyrazol-3-amine derivatives were prepared.
Scheme 1. Synthesis of thieno[3,2-c]pyrazol-3-amine derivatives 16a–16e, 17a–17d and 18a–18n. Reagents and conditions: (a) various acyl chlorides or propane-1-sulphonyl chloride, pyridine, 110 °C; (b) substituted arylboronic acids or arylboronic acid esters, Pd(dppf)Cl2, CH3CO2K, DMF/EtOH/H2O or DMF/1,4-dioxane/H2O, 100 °C.
![Scheme 1. Synthesis of thieno[3,2-c]pyrazol-3-amine derivatives 16a–16e, 17a–17d and 18a–18n. Reagents and conditions: (a) various acyl chlorides or propane-1-sulphonyl chloride, pyridine, 110 °C; (b) substituted arylboronic acids or arylboronic acid esters, Pd(dppf)Cl2, CH3CO2K, DMF/EtOH/H2O or DMF/1,4-dioxane/H2O, 100 °C.](/cms/asset/127c3b76-dd81-4870-b2ed-ea7cb6fdbfb1/ienz_a_2086867_sch0001_b.jpg)
2.2. GSK-3β inhibitory activity and kinase selectivity
All the targeted compounds were evaluated for their GSK-3β inhibitory activities in the calliper mobility shift assay in vitro. AR-A014418 (10, ), a prototypical GSK-3β-specific inhibitor, was used as the positive controlCitation37.
At first, the effects of the acyl or sulphonyl groups at the thieno[3,2-c]pyrazol-3-amine on GSK-3β inhibitory activities were investigated. As shown in Scheme 1 and , the cyclopropanecarbonyl and the isobytyryl group showed similar effects on the GSK-3β inhibitory potency. Compounds 16a and 16b were very potent GSK-3β inhibitors with the IC50 values of 4.4 nM and 3.1 nM, respectively. When the thieno[3,2-c]pyrazol-3-amine was substituted by the n-butyryl (16c) or benzoyl (16e), the resulting compound 16c or benzoyl 16e maintained high potency, but was about 10-fold less active than 16b. However, the sulphonamide 16d showed very weak GSK-3β inhibitory activity. The introduction of a phenyl group at the meta-position of the pyridine ring in 16a–16d showed subtle influences on the activity of their parent compounds. Compounds 17a and 17b were about 4-fold less active than 16a and 16b, but compound 17c was active as that of 16c. The sulphonamide 17d was not active. Therefore, the substitution of the thieno[3,2-c]pyrazol-3-amine with a sulphonyl group was not preferred.
Table 1. Inhibitory effects of compounds against GSK-3β.
As compound 16b showed very potent GSK-3β inhibitory activity, further structural modifications based on 16b were made at the para-position of the pyridine ring. In general, inducing a phenyl or substituted phenyl, a biphenyl, a naphthalenyl group at this position decreased the potency of 16b. The phenyl substituted analogue 18a showed modest GSK-3β inhibitory activity with the IC50 of 84 nM. For the methyl or methoxy substituted phenyl derivatives, the activity was in the order of ortho- > para- > meta-. The ortho-methyl and the ortho-methoxy derivatives 18b and 18e were slightly more active than 18a, with IC50 values of 64 and 64 nM, respectively. For the fluorine substituted phenyl analogues, the 4-F-phenyl derivative 18j was highly active, with an IC50 of 15 nM, possibly due to the special features of fluorine atom with the smallest size and the largest electron-withdrawing property. The trifluoromethyl group (18k and 18l) also decreased the potency of 18a slightly. The biphenyl derivative 18m showed much weak activity with an IC50 of 387 nM. The naphthalenyl derivative 18n was about 2-fold less active than 18a.
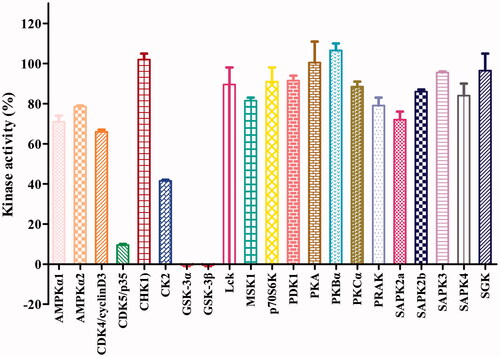
The potent GSK-3β inhibitor 16b was next subjected to kinase selectivity assay. A panel of kinases which were structurally related to GSK-3β was used for the GSK-3β selectivity studiesCitation26,Citation37,Citation38. Among a panel of 21 diverse kinases, at the concentration of 1.0 μM (>320-fold IC50 value on GSK-3β), 16b showed in general good selectivity over most of the kinases except for the low selectivity over the GSK-3α and CDK5 and moderate selectivity over CK2 (). GSK-3α and GSK-3β are known to share a 98% sequence homology at the catalytic siteCitation42. CDK5 and GSK-3 belong to the CMGC protein kinase family, which shared highly homology with each otherCitation43,Citation44. CDK5 is known abnormally activated and also responsible for the tau hyperphosphorylation in ADCitation45. It was known that Aβ could increase CK2 activity, which in turn accelerated the tau phosphorylation in ADCitation46. Therefore, the inhibition of CDK5 and CK2 by 16b may be beneficial for its anti-AD activities.
Figure 3. Effects of compound 16b on the activities of 21 protein kinases in vitro. Protein kinases were of human origin and assayed in the presence of 1.0 μM compound 16b or vehicle (DMSO). The enzymatic activity was measured in the presence of Km ATP. Kinase activities were given as the mean of twice determinations. AMPKα1, AMP-activated protein kinase 1; AMPKα2, AMP-activated protein kinase 2; CDK4/cyclinD3, cyclin-dependent protein kinase-4/cyclinD3; CDK5/p35, cyclin-dependent protein kinase-5/p35; CHK1, checkpoint kinase-1; CK2, casein kinase-2; Lck, lymphocyte kinase; MSK1, mitogen- and stress-activated protein kinase-1; p70S6K, p70 ribosomal protein S6 kinase; PDK1, 3-phosphoinositide-dependent protein kinase-1; PKA, cAMP-dependent protein kinase; PKBα, protein kinase Bα; PKCα, protein kinase Cα; PRAK, p38-regulated/activated kinase; SAPK2a, stress-activated protein kinase-2a; SAPK2b, stress-activated protein kinase-2b; SAPK3, stress-activated protein kinase-3; SAPK4, stress-activated protein kinase-4; SGK, serum- and glucocorticoid-induced protein kinase.
2.3. Molecular docking study
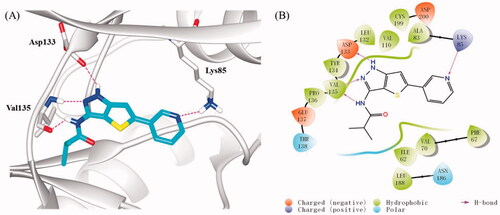
To investigate the possible binding mode of compound 16b with GSK-3β, molecular docking was performed on GSK-3β (PDB: 4ACG)Citation38 using Sybyl-X 2.0 softsuite. All docked conformations were ranked based on docking scores. As depicted in , compound 16b fitted well into the ATP binding pocket of GSK-3β. The thieno[3,2-c]pyrazol-3-amine skeleton occupied the adenine pocket and formed triple hydrogen bonds with backbone atoms of Asp133 and Val135 in the hinge region, which is necessary for the ligand recognition. In addition to these hydrogen bonds, the thieno[3,2-c]pyrazol-3-amine portion also made hydrophobic interactions with the hydrophobic pocket formed by the residues of Ala83, Val110, Leu132, Asp133, Tyr134, Val135, and Leu188. The pyridine ring also participated in hydrophobic interactions with Phe67, Val70, and Cys199. Meanwhile, the N atom of pyridine served as a hydrogen bond acceptor to interact with Lys85 which located at the β-strand of N-terminal domain. Besides, the terminal isobytyryl group located at a position adjacent to the hinge region and produced hydrophobic interactions with Ile62 and Pro136. As expected, 16b followed the “double-sites occupation” pharmacophore model well that may contribute to its high inhibitory activity with GSK-3β.
Figure 4. (A) Docking model of compound 16b in the ATP binding pocket of GSK-3β (PDB: 4ACG). Compound 16b was shown in green colour stick model, and hydrogen-bonding interactions were shown as purple dotted lines; (B) 2D interactions diagram of 16b. For clarity, only the polar hydrogen atoms were shown.
2.4. Cytotoxicity of compound 16b on SH-SY5Y cells
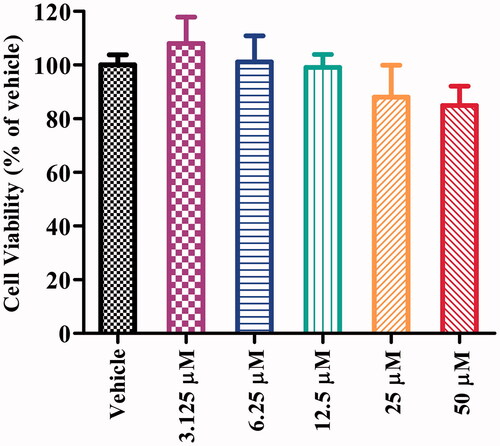
To evaluate the neuronal cell cytotoxicity of compound 16b, we examined its cytotoxic profile on human neuroblastoma SH-SY5Y cells after incubation for 24 h by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Differential SH-SY5Y cells possess more neuron-like morphology and biochemical processes to human mature neurons, such as extensively branched neurites and neuro-special markers and were widely used in many neuronal activity studies in vitroCitation47. As depicted in , compound 16b exhibited no significant cytotoxicity at concentration up to 50 μM, which corresponded to >16000-fold the IC50 value of GSK-3β inhibitory activity.
Figure 5. Cell viability of SH-SY5Y cells exposed to compound 16b at different concentrations (range from 3.125 − 50 μM) for 24 h. Vehicle treater cells were used as control. The results were expressed as the percentage of viable cells observed after treatment with compound 16b respect to vehicle-treated cells (100%) and shown as the mean ± SD from at least three separate experiments.
2.5. The effects of 16b on GSK-3β and β-catenin expressions in cells
GSK-3β is activated by phosphorylation at Tyr216 and is inhibited by phosphorylation at Ser9 Citation48. As one of the substrates of Akt (protein kinase B), GSK-3 can inhibit AktCitation49. The inhibition of GSK-3β increases the p-Akt expression and activates the PI3K/Akt pathway, and that, in turn, phosphorylates the GSK-3β at the Ser9 siteCitation50,Citation51. Since 16b showed excellent inhibitory potency against GSK-3β in vitro, we further investigated its effect on the phosphorylation of GSK-3β at Ser9 on SH-SY5Y cells by Western blot assay, using LiCl as a positive control. As shown in , LiCl greatly promoted GSK-3β phosphorylation at Ser9 (p-GSK-3β/GAPDH: 0.60 vs 0.33). The treatment with 16b at 10 μM and 20 μM dose-dependently increased the p-GSK-3β level at Ser9 compared with the control group, with the p-GSK-3β/GAPDH ratio was 0.41 and 0.50, respectively. Therefore, in cellular level, compound 16b was confirmed to have a direct effect on GSK-3β.
Figure 6. (A) The effect of 16b on phosphorylation of GSK-3β at Ser9; (B) the effect of 16b on β-catenin abundance. Protein expressions were detected by immunoblot analysis with a specific antibody. Values are reported as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs control.
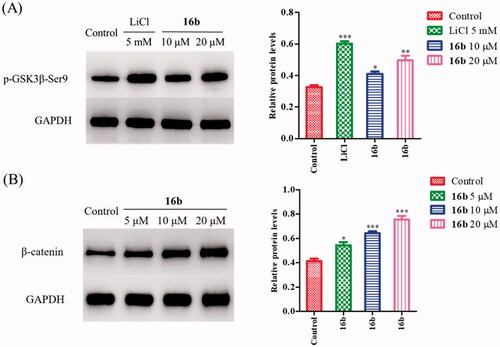
GSK-3β is implicated in the Wnt/β-catenin signalling pathway, which plays an important role in neuronal developmentCitation29. GSK-3β, together with adenomatous polyposis coli (APC), Axin, and casein kinase 1 (CK1), form a ploy-protein complex that regulates the hyperphosphorylation of β-catenin. Phospho-β-catenin is recognised by ubiquitin and degraded by proteasomesCitation52–54. Pharmacological inhibition of GSK-3β leads to the activation and stabilisation of β-catenin, subsequently resulting in the accumulation of β-catenin in cytoplasmCitation55–57. The activation of Wnt/β-catenin signalling pathway can promote synaptic growth, alleviate spatial memory impairment and neurodegeneration in Alzheimer’s models Citation58–60. Moreover, β-catenin also plays a pivotal role in cell adhesion complexes. The combination of β-catenin and N-cadherin elevates cell-to-cell interactions which is prerequisite for neuronal differentiationCitation61,Citation62. Therefore, we further evaluated the effect of 16b on β-catenin. In agreement with its GSK-3β inhibitory activity on SH-SY5Y cells, 16b increased β-catenin abundance in a dose-dependent manner. As shown in , after treatment with 16b at the concentration of 5 μM, 10 μM and 20 μM, the β-catenin/GADPH ratio increased from 0.41 of the control to 0.54, 0.64, 0.76, respectively.
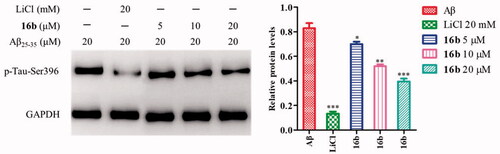
2.6. Inhibition of Aβ-induced tau protein hyperphosphorylation
GSK-3β phosphorylates tau at sites (Ser199, Ser396, and Ser443), and the hyperphosphorylated tau aggregate into NFTs in ADCitation63. Inhibition of GSK-3β could reduce tau hyperphosphorylation. Therefore, the cell-based assay examining Aβ-induced tau phosphorylation at Ser396 represents a direct functional assay to measure the cellular activity of GSK-3β inhibitorsCitation64. To investigate the effect of 16b on tau phosphorylation, Western blot analysis was carried out to check Aβ-induced tau protein hyperphosphorylation at Ser396 in SH-SY5Y cells, using LiCl as the control. As shown in , the treatment with 20 μM Aβ25-35 caused the tau protein hyperphosphorylation at Ser396 with a p-tau/GAPDH ratio of 0.83. At the concentration of 5 μM, 10 μM and 20 μM, 16b decreased the phosphorylation tau level to 0.70, 0.52, 0.40, respectively.
2.7. Effects of 16b on neuronal neurite outgrowth and GAP43, N-myc, and MAP-2 expressions in SH-SY5Y cells
Among kinds of pathological symptoms, neurogenesis impairment and neuronal loss play important roles in neurodegeneration in AD. Therefore, regulating neurogenesis is considered to be a promising therapeutic option for ADCitation65.
Compelling evidence indicated that GSK-3β plays a large part in synaptic plasticity and neurogenesisCitation66,Citation67. Differentiated SH-SY5Y cells express neurogenesis-related markers, including growth-associated protein 43 (GAP43), the N-myc gene as well as neuronal polarity marker microtubule-associated protein 2 (MAP-2). GAP43 is an intrinsic determinant of neuronal development and plasticity which regulates axon growth and regenerationCitation68,Citation69. N-myc is indispensable to normal neurogenesis in the expansion of progenitor cell populationsCitation70. MAP-2 regulates neuronal development, structural stability and synaptic plasticity through the formation of axonal and dendritic processesCitation71,Citation72. Inhibition of GSK-3β could induce neurogenesis and promote the expressions of the neurogenesis-related markersCitation73. Firstly, we used SH-SY5Y cells to study the effect of 16b on neurite outgrowth, using retinoic acid (RA) as a positive controlCitation74. After incubating with 16b (10 μM) or RA (10 μM) for 72 h, the morphology of differentiated neuronal neurite outgrowth were obtained. As depicted in , 16b exhibited a substantial ability in inducing SH-SY5Y cells neurite outgrowth compared with RA.
Figure 8. (A) Effect of 16b (10 μM) on neurite outgrowth (72 h). Red arrows indicated cells bearing neurites. Pictures were taken at 200 × magnification; (B) Effect of 16b (10 μM) on neurogenesis markers expressions (24 h). ***p < 0.001 vs control.
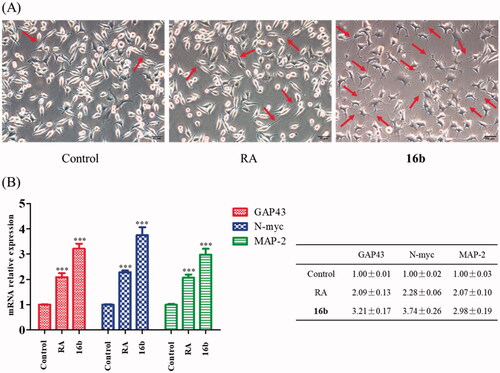
We next quantified the neurogenesis-related markers to evaluate the effect of 16b on neurogenesis. Co-incubated 16b (10 μM) or RA (10 μM) with SH-SY5Y cells for 24 h, the mRNA expressions of GAP43, N-myc and MAP-2 were measured by the quantitative real-time reverse transcription-PCR (RT-PCR) analysis. As shown in , RA was able to up-regulate the mRNA expressions of GAP43, N-myc and MAP-2. Meanwhile, compound 16b exhibited more potent effects on inducing the expressions of GAP43, N-myc and MAP-2 compared with RA.
3. Conclusion
In summary, a series of novel thieno[3,2-c]pyrazol-3-amine derivatives were designed, synthesised, and evaluated as potential GSK-3β inhibitors. Compound 16b exhibited potent GSK-3β inhibitory activity with IC50 at single-digit nanomolar level. In a panel of 21 kinases, 16b showed in overall good selectivity over most of them except for the CDK5 and CK2 kinases. In cellular level, 16b showed no cytotoxicity against SH-SY5Y cells at the concentration up to 50 μM and inhibited GSK-3β through the up-regulation of the phosphorylation at Ser9. Meanwhile, 16b inhibited the Aβ-induced tau protein hyperphosphorylation at Ser396. In addition, β-catenin plays a crucial role in neurogenesis. Compound 16b inhibited GSK-3β, interfered the physiological degradation of β-catenin, resulting in the abundance of β-catenin. Moreover, 16b could increase the mRNA expressions of the recognised neurogenesis-related markers and promote the differentiated neuronal neurite outgrowth, which is very useful in face of progressive neurogenesis impairment and neuronal loss in AD. Compelling evidence have verified GSK-3β to process function link between Aβ and tau. In view of the involvement in multiple pathways in AD progress, GSK-3β is being an interesting drug discovery target for the treatment of AD. As a potent GSK-3β inhibitor, 16b could serve as a promising lead for further investigation in facing the complicated pathogenesis of AD.
4. Experimental section
4.1. Chemistry
All solvents used were commercially available and were used without further purification unless otherwise noted. Starting materials used were either available from commercial sources or prepared according to literature procedures. For examined compounds, 1H and 13 C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker-400 or Bruker-600 NMR spectrometer, respectively. The following reference signals were used: TMS δ 0.00, or the residual solvent signal of DMSO-d6 δ 2.50 (1H), δ 39.52 (13 C). MS spectra data were obtained using an API 4000 instrument. High-resolution mass spectrometry (HRMS) data were acquired by an AB-Triple TOF5600 or Agilent Q-TOF6540 instrument. Melting points (Mp) were measured on an X-6 micromelting point apparatus (Beijing Tech. Co., Ltd., Beijing, China). The reactions were followed by thin-layer chromatography (TLC) and visualised in an iodine chamber or with a UV lamp. Compounds were purified by column chromatography using silica gel (200 − 300 mesh). The purity (> 95%) of samples were determined using a Shimadzu LC-20AT series system (column, Shim-pack GWS C18, 4.6 × 250 mm, 5 μm; mobile phase, methanol/H2O = 80/20; flow rate, 1.0 ml/min; UV wavelength, 254 nm).
4.1.1. N-(5-Bromo-1H-thieno[3,2-c]pyrazol-3-yl)cyclopropanecarboxamide (15a)
To a stirred solution of 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine (14, 218 mg, 1.00 mmol) in pyridine (5 ml) was added dropwise cyclopropanecarbonyl chloride (115 mg, 1.10 mmol). The reaction solution was heated to reflux at 110 °C for 12 h. The reaction was quenched up by the addition of methanol (5 ml), and concentrated under reduced pressure. The residues were purified by silica gel chromatography (hexane/EtOAc = 2:1) to give 15a (231 mg, 80.8%) as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) 12.53 (s, 1H), 11.14 (s, 1H), 7.24 (s, 1H), 1.92 − 1.81 (m, 1H), 0.89 − 0.75 (m, 4H); 13 C NMR (100 MHz, DMSO-d6) δ 171.69, 145.36, 138.95, 120.18, 113.00, 110.42, 13.49, 7.38; ESI (MS): calcd. for C9H981BrN3OS [M + H]+: 287.96, found: 288.08.
4.1.2. N-(5-Bromo-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (15b)
According to the same procedures for preparing 15a, compound 15b was obtained from 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine (14) and isobutyryl chloride in 72.2% yield as a yellow solid. Mp: >240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.53 (s, 1H), 10.80 (s, 1H), 7.24 (s, 1H), 2.71 − 2.60 (m, 1H), 1.08 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 175.20, 145.35, 138.92, 120.18, 113.03, 110.48, 33.77, 19.37; ESI (MS): calcd. for C9H11BrN3OS [M + H]+: 287.98, 289.98, found: 288.07, 290.07.
4.1.3. N-(5-Bromo-1H-thieno[3,2-c]pyrazol-3-yl)butyramide (15c)
According to the same procedures for preparing 15a, compound 15c was obtained from 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine (14) and butyryl chloride in 76.9% yield as a white solid. Mp: 228 − 230 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.51 (s, 1H), 10.79 (s, 1H), 7.24 (s, 1H), 2.29 (t, J = 7.4 Hz, 2H), 1.64 − 1.52 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H); 13 C NMR (150 MHz, DMSO-d6) δ 171.12, 145.34, 138.85, 120.17, 113.00, 110.51, 36.96, 18.38, 13.60; HRMS (ESI): calcd. for C9H1181BrN3S [M + H]+: 289.9780, found: 289.9789.
4.1.4. N-(5-Bromo-1H-thieno[3,2-c]pyrazol-3-yl)propane-1-sulphonamide (15d)
According to the same procedures for preparing 15a, compound 15d was obtained from 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine (14) and 1-propanesulfonyl chloride as a yellow solid without further purification.
4.1.5. N-(5-Bromo-1H-thieno[3,2-c]pyrazol-3-yl)benzamide (15e)
According to the same procedures for preparing 15a, compound 15e was obtained from 5-bromo-1H-thieno[3,2-c]pyrazol-3-amine (14) and benzoyl chloride as a yellow solid without further purification.
4.1.6. N-(5-(pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)cyclopropanecarboxamide (16a)
To a solution of 15a (85.8 mg, 0.3 mmol) and 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine (123 mg, 0.6 mmol) in DMF (2 ml), EtOH (1 ml) and distilled water (1 ml) was added Pd(dppf)Cl2 (11 mg, 0.015 mmol) and CH3CO2K (88 mg, 0.9 mmol). The mixture was stirred at 100 °C for 12 h. Distilled water (5 ml) was added, and the mixture was extracted with EtOAc (20 ml × 3). The organic phase was washed with brine and dried over Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (CH2Cl2/MeOH = 40:1) to give 16a (53.1 mg, 18.7%) as a white solid. Mp: 224 − 226 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.56 (s, 1H), 11.11 (s, 1H), 8.94 (s, 1H), 8.53 (d, J = 4.8 Hz, 1H), 8.10 − 8.06 (m, 1H), 7.54 (s, 1H), 7.46 (dd, J = 8.0, 4.8 Hz, 1H), 1.97 − 1.84 (m, 1H), 0.94 − 0.74 (m, 4H); 13 C NMR (150 MHz, DMSO-d6) δ 171.93, 149.43, 148.44, 146.61, 146.46, 140.03, 133.11, 131.16, 124.52, 110.97, 107.23, 13.94, 7.75; HRMS (ESI): calcd. for C14H13N4OS [M + H]+: 285.0805, found: 285.0811. Purity: 95.2%.
4.1.7. N-(5-(pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (16b)
According to the same procedures for preparing 16a, compound 16b was obtained from 15b and 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 18.0% yield as a white solid. Mp: > 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.56 (s, 1H), 10.76 (s, 1H), 8.95 (d, J = 1.8 Hz, 1H), 8.54 (d, J = 4.8 Hz, 1H), 8.11 − 8.07 (m, 1H), 7.54 (s, 1H), 7.46 (dd, J = 8.0, 4.8 Hz, 1H), 2.73 − 2.65 (m, 1H), 1.11 (d, J = 6.9 Hz, 6H); 13 C NMR (150 MHz, DMSO-d6) δ 175.45, 149.44, 148.42, 146.62, 146.45, 140.03, 133.11, 131.17, 124.52, 111.06, 107.23, 34.21, 19.88; HRMS (ESI): calcd. for C14H15N4OS [M + H]+: 287.0961, found: 287.0971. Purity: 99.3%.
4.1.8. N-(5-(pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)butyramide (16c)
According to the same procedures for preparing 16a, compound 16c was obtained from 15c and 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 55.9% yield as a white solid. Mp: 238 − 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.55 (s, 1H), 10.77 (s, 1H), 8.95 − 8.93 (m, 1H), 8.52 (dd, J = 4.8, 1.2 Hz, 1H), 8.10 − 8.05 (m, 1H), 7.53 (s, 1H), 7.45 (dd, J = 8.0, 4.8 Hz, 1H), 2.32 (t, J = 7.4 Hz, 2H), 1.61 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13 C NMR (150 MHz, DMSO-d6) δ 171.35, 149.45, 148.42, 146.63, 146.46, 139.92, 133.14, 131.16, 124.53, 111.06, 107.24, 37.44, 18.83, 14.09; HRMS (ESI): calcd. for C14H15N4S [M + H]+: 287.0961, found: 287.0975. Purity: 99.9%.
4.1.9. N-(5-(pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)propane-1-sulphonamide (16d)
According to the same procedures for preparing 16a, compound 16d was obtained from 15d and 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 11.5% yield as a white solid. Mp: 208 − 210 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.83 (s, 1H), 10.43 (s, 1H), 8.97 (d, J = 1.8 Hz, 1H), 8.56 (dd, J = 4.8, 1.2 Hz, 1H), 8.12 − 8.08 (m, 1H), 7.62 (s, 1H), 7.48 (dd, J = 8.0, 4.8 Hz, 1H), 3.20 − 3.12 (m, 2H), 1.81 − 1.70 (m, 2H), 0.96 (t, J = 7.5 Hz, 3H); 13 C NMR (150 MHz, DMSO-d6) δ 149.69, 149.59, 146.78, 146.44, 138.20, 133.37, 130.82, 124.55, 111.39, 107.77, 54.05, 17.29, 13.07; HRMS (ESI): calcd. for C13H15N4O2S2 [M + H]+: 323.0631, found: 323.0634. Purity: 95.8%.
4.1.10. N-(5-(pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)benzamide (16e)
According to the same procedures for preparing 16a, compound 16e was obtained from 15e and 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 16.3% yield as a white solid. Mp: > 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.77 (s, 1H), 11.33 (s, 1H), 9.00 (s, 1H), 8.56 (s, 1H), 8.13 (d, J = 7.5 Hz, 1H), 8.09 (d, J = 7.4 Hz, 2H), 7.61 (s, 2H), 7.54 (t, J = 7.3 Hz, 2H), 7.49 (d, J = 5.2 Hz, 1H); 13 C NMR (150 MHz, DMSO-d6) δ 165.29, 149.50, 148.47, 146.66, 146.52, 140.12, 133.66, 133.21, 132.44, 131.17, 128.89, 128.42, 124.59, 111.74, 107.32; HRMS (ESI): calcd. for C17H13N4OS [M + H]+: 321.0805, found: 321.0794. Purity: 98.7%.
4.1.11. N-(5-(5-Phenylpyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)cyclopropanecarboxamide (17a)
According to the same procedures for preparing 16a, compound 17a was obtained from 15a and 3-phenyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 13.2% yield as a white solid. Mp: > 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.61 (s, 1H), 11.13 (s, 1H), 8.91 (d, J = 2.3 Hz, 1H), 8.85 (d, J = 2.2 Hz, 1H), 8.30 (t, J = 2.2 Hz, 1H), 7.84 (d, J = 7.3 Hz, 2H), 7.70 (s, 1H), 7.54 (t, J = 7.6 Hz, 2H), 7.47 (t, J = 7.6 Hz, 1H), 1.96 − 1.88 (m, 1H), 0.90 − 0.79 (m, 4H); 13 C NMR (150 MHz, DMSO-d6) δ 171.96, 148.46, 147.51, 146.22, 145.42, 140.06, 137.01, 136.28, 131.30, 130.88, 129.62, 128.91, 127.63, 111.10, 107.84, 13.95, 7.76; HRMS (ESI): calcd. for C20H17N4OS [M + H]+: 361.1118, found: 361.1123. Purity: 99.9%.
4.1.12. N-(5-(5-Phenylpyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (17b)
According to the same procedures for preparing 16a, compound 17b was obtained from 15b and 3-phenyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 15.7% yield as a white solid. Mp: > 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.61 (s, 1H), 10.79 (s, 1H), 8.92 (d, J = 2.1 Hz, 1H), 8.85 (d, J = 2.1 Hz, 1H), 8.32 (t, J = 2.1 Hz, 1H), 7.88 − 7.83 (m, 2H), 7.70 (s, 1H), 7.54 (t, J = 7.6 Hz, 2H), 7.47 (t, J = 7.6 Hz, 1H), 2.73 − 2.68 (m, 1H), 1.12 (d, J = 6.9 Hz, 6H); 13 C NMR (150 MHz, DMSO-d6) δ 175.48, 148.44, 147.51, 146.21, 145.43, 140.05, 137.01, 136.29, 131.31, 130.87, 129.62, 128.91, 127.63, 111.20, 107.83, 34.22, 19.88; HRMS (ESI): calcd. for C20H19N4OS [M + H]+: 363.1274, found: 363.1265. Purity: 99.8%.
4.1.13. N-(5-(5-Phenylpyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)butyramide (17c)
According to the same procedures for preparing 16a, compound 17c was obtained from 15c and 3-phenyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 38.6% yield as a white solid. Mp: > 240 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.60 (s, 1H), 10.79 (s, 1H), 8.91 (d, J = 1.9 Hz, 1H), 8.85 (d, J = 1.9 Hz, 1H), 8.32 (t, J = 1.9 Hz, 1H), 7.87 − 7.83 (m, 2H), 7.70 (s, 1H), 7.56 − 7.52 (m, 2H), 7.47 (t, J = 7.4 Hz, 1H), 2.34 (t, J = 7.3 Hz, 2H), 1.67 − 1.58 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H); 13 C NMR (150 MHz, DMSO-d6) δ 170.28, 147.38, 146.47, 145.15, 144.39, 138.89, 135.96, 135.24, 130.26, 129.84, 128.56, 127.85, 126.58, 110.13, 106.81, 36.37, 17.76, 13.03; HRMS (ESI): calcd. for C20H18N4OS [M + H]+: 363.1274, found: 363.1288. Purity: 99.6%.
4.1.14. N-(5-(5-Phenylpyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)propane-1-sulphonamide (17d)
According to the same procedures for preparing 16a, compound 17d was obtained from 15d and 3-phenyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine in 12.7% yield as a white solid. Mp: 112 − 114 °C. 1H NMR (600 MHz, DMSO-d6) δ 12.88 (s, 1H), 10.45 (s, 1H), 8.91 (s, 1H), 8.86 (s, 1H), 8.33 (s, 1H), 7.84 (d, J = 7.5 Hz, 2H), 7.77 (s, 1H), 7.54 (t, J = 7.5 Hz, 2H), 7.46 (t, J = 7.3 Hz, 1H), 3.21 − 3.03 (m, 2H), 1.83 − 1.63 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H); 13 C NMR (150 MHz, DMSO-d6) δ 149.14, 147.32, 145.72, 145.11, 137.76, 136.49, 135.88, 130.68, 130.52, 129.15, 128.48, 127.21, 111.08, 107.94, 53.60, 16.84, 12.61; HRMS (ESI): calcd. for C19H19N4O2S2 [M + H]+: 399.0944, found: 399.0929. Purity: 96.5%.
4.1.15. N-(5-(4-Phenylpyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18a)
To a solution of 15b (83 mg, 0.289 mmol) and (4-phenylpyridin-3-yl)boronic acid (115 mg, 0.578 mmol) in DMF (2 ml), 1,4-dioxane (1 ml) and distilled water (0.5 ml) was added Pd(dppf)Cl2 (9.94 mg, 0.0136 mmol) and CH3CO2K (53.3 mg, 0.544 mmol). The mixture was stirred at 100 °C for 15 h. Distilled water (5 ml) was added, and the mixture was extracted with EtOAc (20 ml × 3). The organic phase was washed with brine and dried over Na2SO4. After filtration and evaporation, the residue was purified by silica gel chromatography (hexane/EtOAc = 1:1) to give 18a (21 mg, 20.0%) as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.32 (s, 1H), 10.69 (s, 1H), 8.71 (s, 1H), 8.63 (d, J = 4.8 Hz, 1H), 7.43 (d, J = 4.8 Hz, 1H), 7.41 − 7.32 (m, 5H), 6.68 (s, 1H), 2.71 − 2.59 (m, 1H), 1.06 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.83, 150.41, 149.42, 147.46, 147.35, 145.28, 139.25, 138.03, 129.17, 128.75, 128.55, 128.35, 124.82, 111.55, 110.09, 33.72, 19.36; HRMS (ESI): calcd. for C20H19N4OS [M + H]+: 363.1274, found: 363.1270. Purity: 99.3%.
4.1.16. N-(5-(4-(2-Methylphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18b)
According to the same procedures for preparing 18a, compound 18b was obtained from 15b and (4-(2-methylphenyl)pyridin-3-yl)boronic acid in 20.6% yield as a white solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H), 10.67 (s, 1H), 8.79 (s, 1H), 8.61 (d, J = 4.4 Hz, 1H), 7.37 − 7.17 (m, 5H), 6.46 (s, 1H), 2.69 − 2.59 (m, 1H), 1.94 (s, 3H), 1.06 (d, J = 6.4 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 175.29, 149.88, 149.36, 147.81, 147.59, 145.47, 139.71, 138.49, 135.24, 130.55, 130.24, 129.58, 128.93, 126.54, 125.66, 111.70, 109.76, 34.19, 19.94, 19.83; HRMS (ESI): calcd. for C21H21N4OS [M + H]+: 377.1431, found: 377.1426. Purity: 97.6%.
4.1.17. N-(5-(4-(3-Methylphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18c)
According to the same procedures for preparing 18a, compound 18c was obtained from 15b and (4-(3-methylphenyl)pyridin-3-yl)boronic acid in 19.0% yield as a yellow solid. Mp: 236 − 238 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H), 10.71 (s, 1H), 8.69 (s, 1H), 8.61 (d, J = 5.0 Hz, 1H), 7.41 (d, J = 5.0 Hz, 1H), 7.27 − 7.16 (m, 3H), 7.07 (d, J = 7.4 Hz, 1H), 6.67 (s, 1H), 2.69 − 2.60 (m, 1H), 2.30 (s, 3H), 1.06 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.84, 150.36, 149.33, 147.55, 147.36, 145.29, 139.25, 138.04, 137.82, 129.22, 129.13, 128.99, 128.29, 125.91, 124.85, 111.45, 110.04, 33.72, 21.03, 19.37; HRMS (ESI): calcd. for C21H21N4OS [M + H]+: 377.1431, found: 377.1441. Purity: 96.6%.
4.1.18. N-(5-(4-(4-Methylphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18d)
According to the same procedures for preparing 18a, compound 18d was obtained from 15b and (4-(4-methylphenyl)pyridin-3-yl)boronic acid in 24.1% yield as a yellow solid. Mp: 239 − 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H), 10.70 (s, 1H), 8.67 (s, 1H), 8.61 (d, J = 5.0 Hz, 1H), 7.40 (d, J = 5.0 Hz, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 6.70 (s, 1H), 2.70 − 2.58 (m, 1H), 2.30 (s, 3H), 1.06 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.83, 150.49, 149.41, 147.46, 147.37, 145.46, 139.25, 138.66, 137.75, 135.08, 129.17, 128.65, 124.78, 111.51, 110.02, 33.71, 20.77, 19.35; HRMS (ESI): calcd. for C21H21N4OS [M + H]+: 377.1431, found: 377.1435. Purity: 95.7%.
4.1.19. N-(5-(4-(2-Methoxyphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18e)
According to the same procedures for preparing 18a, compound 18e was obtained from 15b and (4-(2-methoxyphenyl)pyridin-3-yl)boronic acid in 12.2% yield as a white solid. Mp: 213 − 215 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 10.65 (s, 1H), 8.71 (s, 1H), 8.58 (s, 1H), 7.42 − 7.28 (m, 2H), 7.18 (d, J = 6.4 Hz, 1H), 7.09 − 6.95 (m, 2H), 6.51 (s, 1H), 3.53 (s, 3H), 2.69 − 2.60 (m, 1H), 1.06 (d, J = 9.0 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.76, 155.97, 149.33, 148.71, 147.33, 145.72, 144.74, 139.20, 130.42, 130.18, 127.06, 125.67, 120.72, 111.62, 111.07, 108.48, 99.52, 55.32, 33.73, 19.38; HRMS (ESI): calcd. for C21H21N4O2S [M + H]+: 393.1380, found: 393.1381. Purity: 98.1%.
4.1.20. N-(5-(4-(3-Methoxyphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18f)
According to the same procedures for preparing 18a, compound 18f was obtained from 15b and (4-(3-methoxyphenyl)pyridin-3-yl)boronic acid in 20.0% yield as a white solid. Mp: 212 − 214 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H), 10.70 (s, 1H), 8.70 (s, 1H), 8.63 (d, J = 5.0 Hz, 1H), 7.45 (d, J = 5.0 Hz, 1H), 7.28 (t, J = 8.2 Hz, 1H), 6.97 − 6.85 (m, 3H), 6.72 (s, 1H), 3.70 (s, 3H), 2.70 − 2.61 (m, 1H), 1.06 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.84, 159.11, 150.39, 149.39, 147.38, 147.32, 145.25, 139.36, 139.25, 129.64, 129.15, 124.74, 121.01, 114.44, 113.80, 111.51, 110.10, 55.05, 33.72, 19.36; HRMS (ESI): calcd. for C21H21N4O2S [M + H]+: 393.1380, found: 393.1381. Purity: 99.7%.
4.1.21. N-(5-(4-(4-Methoxyphenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18g)
According to the same procedures for preparing 18a, compound 18g was obtained from 15b and (4-(4-methoxyphenyl)pyridin-3-yl)boronic acid in 14.7% yield as a yellow solid. Mp: >240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.35 (s, 1H), 10.71 (s, 1H), 8.65 (s, 1H), 8.59 (d, J = 4.8 Hz, 1H), 7.41 (d, J = 4.8 Hz, 1H), 7.28 (d, J = 8.2 Hz, 2H), 6.94 (d, J = 8.2 Hz, 2H), 6.73 (s, 1H), 3.76 (s, 3H), 2.69 − 2.60 (m, 1H), 1.06 (d, J = 6.4 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.83, 159.32, 150.53, 149.39, 147.40, 147.12, 145.67, 139.28, 139.26, 130.13, 130.02, 129.06, 124.71, 114.05, 109.91, 55.13, 33.72, 19.36; HRMS (ESI): calcd. for C21H21N4O2S [M + H]+: 393.1380, found: 393.1379. Purity: 99.8%.
4.1.22. N-(5-(4-(2-Fluorophenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18h)
According to the same procedures for preparing 18a, compound 18h was obtained from 15b and (4-(2-fluorophenyl)pyridin-3-yl)boronic acid in 25.7% yield as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.53 (s, 1H), 10.77 (s, 1H), 8.70 (d, J = 4.8 Hz, 2H), 7.88 (t, J = 7.2 Hz, 1H), 7.65 (d, J = 4.8 Hz, 2H), 7.58 (t, J = 7.2 Hz, 1H), 7.46 − 7.40 (m, 2H), 2.72 − 2.65 (m, 1H), 1.11 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.95, 155.42 (d, JC-F = 253.1 Hz), 149.89, 147.69, 142.54, 142.24, 142.21, 139.39, 130.19, 130.16, 129.51 (d, JC-F = 3.2 Hz), 126.86 (d, JC-F = 14.0 Hz), 125.50 (d, JC-F = 3.9 Hz), 123.76 (d, JC-F = 1.8 Hz), 123.22 (d, JC-F = 13.4 Hz), 111.13, 109.01, 33.74, 19.38; HRMS (ESI): calcd. for C20H18FN4OS [M + H]+: 381.1180, found: 381.1194. Purity: 99.7%.
4.1.23. N-(5-(4-(3-Fluorophenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18i)
According to the same procedures for preparing 18a, compound 18i was obtained from 15b and (4-(3-fluorophenyl)pyridin-3-yl)boronic acid in 20.5% yield as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.54 (s, 1H), 10.78 (s, 1H), 8.68 (d, J = 4.7 Hz, 2H), 7.95 (t, J = 8.2 Hz, 1H), 7.92 − 7.74 (m, 4H), 7.50 (s, 1H), 2.75 − 2.66 (m, 1H), 1.12 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.97, 158.90 (d, JC-F = 249.3 Hz), 150.35, 147.72, 144.77, 144.75, 142.00, 141.96, 139.41, 138.36, 138.28, 129.10 (d, JC-F = 3.8 Hz), 123.30 (d, JC-F = 2.7 Hz), 122.94 (d, JC-F = 12.7 Hz), 120.99, 114.73 (d, JC-F = 24.1 Hz), 108.76, 33.75, 19.38; HRMS (ESI): calcd. for C20H18FN4OS [M + H]+: 381.1180, found: 381.1192. Purity: 99.0%.
4.1.24. N-(5-(4-(4-Fluorophenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18j)
According to the same procedures for preparing 18a, compound 18i was obtained from 15b and (4-(4-fluorophenyl)pyridin-3-yl)boronic acid in 32.6% yield as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.57 (s, 1H), 10.78 (s, 1H), 8.66 (d, J = 5.6 Hz, 2H), 8.15 (dd, J = 7.2, 2.2 Hz, 1H), 7.85 − 7.78 (m, 3H), 7.58 (s, 1H), 7.55 − 7.47 (m, 1H), 2.74 − 2.65 (m, 1H), 1.11 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.98, 159.05 (d, JC-F = 251.9 Hz), 150.24, 147.63, 145.57, 142.05, 139.42, 134.33, 128.26 (d, JC-F = 9.1 Hz), 127.01, 123.11, 122.98, 121.39, 117.42 (d, JC-F = 23.0 Hz), 109.00, 33.75, 19.40; HRMS (ESI): calcd. for C20H18FN4OS [M + H]+: 381.1180, found: 381.1190. Purity: 98.1%.
4.1.25. N-(5-(4-(3-(trifluoromethyl)phenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18k)
According to the same procedures for preparing 18a, compound 18k was obtained from 15b and (4-(3-(trifluoromethyl)phenyl)pyridin-3-yl)boronic acid in 19.4% yield as a yellow solid. Mp: 202 − 204 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H), 10.70 (s, 1H), 8.75 (s, 1H), 8.68 (d, J = 3.0 Hz, 1H), 7.73 (s, 2H), 7.62 (s, 2H), 7.54 (d, J = 3.0 Hz, 1H), 6.69 (s, 1H), 2.68 − 2.58 (m, 1H), 1.06 (d, J = 5.6 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.81, 150.45, 149.56, 147.28, 145.78, 144.47, 139.20, 138.93, 132.95, 129.53, 129.26 (q, JC-F = 32.1 Hz), 129.11, 125.39 (q, JC-F = 3.7 Hz), 125.01 (q, JC-F = 3.7 Hz), 124.72, 123.90 (q, JC-F = 271 Hz), 111.55, 110.48, 33.68, 19.28; HRMS (ESI): calcd. for C21H18F3N4OS [M + H]+: 431.1148, found: 431.1154. Purity: 98.6%.
4.1.26. N-(5-(4-(4-(trifluoromethyl)phenyl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18l)
According to the same procedures for preparing 18a, compound 18l was obtained from 15b and (4-(4-(trifluoromethyl)phenyl)pyridin-3-yl)boronic acid in 21.1% yield as a yellow solid. Mp: >240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H), 10.69 (s, 1H), 8.76 (s, 1H), 8.69 (d, J = 4.8 Hz, 1H), 7.76 (d, J = 7.9 Hz, 2H), 7.58 (d, J = 7.9 Hz, 2H), 7.49 (d, J = 4.8 Hz, 1H), 6.72 (s, 1H), 2.68 − 2.59 (m, 1H), 1.06 (d, J = 6.7 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.84, 150.47, 149.51, 147.32, 145.95, 144.54, 142.19, 139.24, 129.70, 129.06, 128.65 (q, JC-F = 31.8 Hz), 125.37 (q, JC-F = 3.7 Hz), 124.62, 124.03 (q, JC-F = 271 Hz), 111.70, 110.40, 33.67, 19.27; HRMS (ESI): calcd. for C21H18F3N4OS [M + H]+: 431.1148, found: 431.1156. Purity: 98.9%.
4.1.27. N-(5-(4-([1,1'-Biphenyl]-4-yl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18m)
According to the same procedures for preparing 18a, compound 18m was obtained from 15b and (4-([1,1′-biphenyl]-4-yl)pyridin-3-yl)boronic acid in 13.5% yield as a yellow solid. Mp: 159 − 161 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.36 (s, 1H), 10.70 (s, 1H), 8.72 (s, 1H), 8.65 (d, J = 5.0 Hz, 1H), 7.74 − 7.68 (m, 4H), 7.50 − 7.47 (m, 2H), 7.46 − 7.43 (m, 3H), 7.38 (d, J = 7.4 Hz, 1H), 6.76 (s, 1H), 2.69 − 2.59 (m, 1H), 1.05 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.83, 150.52, 149.45, 147.39, 146.99, 145.25, 139.81, 139.27, 139.11, 137.03, 129.40, 129.10, 128.96, 127.74, 126.67, 126.59, 124.74, 111.58, 110.15, 33.68, 19.32; HRMS (ESI): calcd. for C26H23N4OS [M + H]+: 439.1587, found: 439.1588. Purity: 95.0%.
4.1.28. N-(5-(4-(naphthalen-2-yl)pyridin-3-yl)-1H-thieno[3,2-c]pyrazol-3-yl)isobutyramide (18n)
According to the same procedures for preparing 18a, compound 18n was obtained from 15b and (4-(naphthalen-2-yl)pyridin-3-yl)boronic acid in 24.2% yield as a yellow solid. Mp: > 240 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.30 (s, 1H), 10.68 (s, 1H), 8.76 (s, 1H), 8.68 (d, J = 5.0 Hz, 1H), 8.03 (s, 1H), 7.97 − 7.90 (m, 2H), 7.86 (d, J = 8.5 Hz, 1H), 7.59 − 7.54 (m, 3H), 7.37 (dd, J = 8.5, 1.4 Hz, 1H), 6.68 (s, 1H), 2.66 − 2.59 (m, 1H), 1.04 (d, J = 6.8 Hz, 6H); 13 C NMR (100 MHz, DMSO-d6) δ 174.80, 150.39, 149.41, 147.37, 147.32, 145.11, 139.19, 135.75, 132.85, 132.38, 129.29, 128.21, 127.89, 127.73, 127.57, 126.76, 126.52, 126.49, 125.13, 111.45, 110.29, 33.66, 19.30; HRMS (ESI): calcd. for C24H21N4OS [M + H]+: 413.1431, found: 413.1431. Purity: 98.3%.
4.2. GSK-3β kinase assay
The GSK-3β inhibition assay was performed by calliper mobility shift assay using the method described previouslyCitation64,Citation75 and AR-A014418 was used as a positive control. In brief, compounds or AR-A014418 were tested from 1 μM or 5 μM, 3-fold dilution for IC50 determination. GSK-3β protein and the tested compound were loaded in 384-well plate (Corning). After incubation for 10 min, the FAM-labeled peptide 15 (GL Biochem, Shanghai, China) and ATP prepared in the reaction buffer were added and ran for 1 h at 28 °C. Stop buffer (25 μL) was added and conversion data were collected on a LabChip EZ Reader (PerkinElmer, Shanghai, China) at each concentration through the direct detection of both substrate and product via Laser-Induced Fluorescence (LIF) at 492 nm. The IC50 values were calculated from dose-response curves using XLfit (curve fitting software for Excel).
4.3. Kinase selectivity screen
Compound 16b was evaluated for kinase selectivity at Eurofins Cerep SA (Celle-L’Evescault, France) in enzymatic radioactive assays in a panel of 21 different kinases (including GSK-3β) from diverse families. Protein kinase were assayed in the presence of 1.0 μM compound 16b or vehicle (DMSO). The enzymatic activity was measured in the presence of Km ATP.
4.4. Molecular docking
Molecular docking was performed on the Sybyl-X 2.0 software (Tripos, St. Louis, MO) and the X-ray crystal structure of GSK-3β (PDB: 4ACG) was obtained from the RCSB Protein Data Bank. The protein was added with hydrogen atoms and charges. Waters were removed from the PDB file. Native ligand (6LQ) was extracted from the protein and used as a standard to generate the protomol. The binding pocket was defined as all residues within 5 Å of the original ligand. Finally, docking was performed by using the Surflex-Dock mode, and the conformations were used to analyse the interactions between ligand and GSK-3β. UCSF Chimaera 1.16 was used to visualise the result of docking Citation76. Maestro 11.9 was used to show the 2D interactions diagram Citation77.
4.5. Cytotoxicity on SH-SY5Y cell line
SH-SY5Y cells were cultured in DMEM/F12 (Dulbecco’s modified Eagle medium and Ham’s F-12, 1:1) with 10% FBS (fetal bovine serum), 1% penicillin and 1% streptomycin under 5% CO2 atmosphere at 37 °C. SH-SY5Y cells (1 × 105) were seeded in 96-well plates and incubated for 24 h. Different concentrations of compound 16b were added into each well and incubated for another 24 h. The survival of cells was determined by MTT assay and the absorbance of each well were measured using a SpectraMax M5 multimode plate reader at 570 nm. Results were expressed as percentage of control and statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA).
4.6. Western blot analysis on p-GSK-3β and β-catenin
SH-SY5Y cells (1 × 106) were seeded in 12-well plates (Corning, Los Altos, MA, USA) and incubated with compound 16b or LiCl at the indicated concentrations for 2.5 h at 37 °C in 5% CO2. At the end of incubation, cells were lysed by addition of ice-cold RIPA buffer containing a protease inhibitor cocktail. The protein quantification was determined using a BCA protein assay kit (Jiangsu KeyGEN BioTECH Co., Ltd., Nanjing, China). Cellular lysates were mixed with an equal volume of SDS (sodium dodecyl sulfate) loading buffer (Jiangsu KeyGEN BioTECH Co., Ltd., Nanjing, China) and separated by electrophoresis (Bio-rad Power Supplies Basic, Shanghai, China) in polyacrylamide gel. Proteins were transferred from acrylamide gels to nitrocellulose membranes and blocked in a blocking buffer (PBS, 5% non-fat milk) for 1.5 to 2 h at 20 °C. After overnight incubation at 4 °C with primary p-GSK3β-Ser9 (Cell Signalling Technology, Danvers, MA, USA), or β-catenin (Cell Signalling Technology, Danvers, MA, USA), and GAPDH (Santa Cruz Biotechnology, Shanghai, China), the blots were washed in Tween 20-TBS (TBST, Jiangsu KeyGEN BioTECH Co., Ltd., Nanjing, China) for 20 min and then incubated with secondary antibody (IgG-HRP; Jiangsu KeyGEN BioTECH Co., Ltd., Nanjing, China) for 1 h at room temperature. The blots were washed by TBST for 20 min and detected with ECL chemiluminescent reagent (Jiangsu KeyGEN BioTECH Co., Ltd., Nanjing, China) for 3 min. Pixel intensity was quantitated using gel imaging system (SYNGENE G:BOX/iChemi XR5, ISS, San Diego, CA, US) and Gel-Pro32 software (Media Cybernetics, Bethesda, MD, USA). GAPDH was used as an internal control.
4.7. Inhibition of Aβ-induced tau hyperphosphorylation
SH-SY5Y cells were seeded in 12-well plates until 80% confluence, serum-deprived for 12 h. Cells were pre-incubated with compound 16b or LiCl for 1 h, thereafter stimulated with Aβ25–35 (Sigma) for another 6 h. According to the previously reported methodCitation64,Citation75, the phosphorylated tau was determined.
4.8. Neuronal neurite outgrowth assay and quantitative RT-PCR
SH-SY5Y cells (5 × 103) were planted in 96-well plates and cultivated at 37 °C for 24 h. Compound (RA or 16b, 10 μM) was then added and cultivated for 72 h. The morphology of neurite outgrowth was examined under an inverted microscope (2 × 100; Olympus, Tokyo, Japan). After the SH-SY5Y cells were cultivated for 24 h, total RNA was extracted, and quantitative RT-PCR was performed according to the previously reported methodCitation75.
Supplemental Material
Download PDF (4.4 MB)Disclosure statement
The authors declare no competing financial interests.
Additional information
Funding
References
- Patterson C, World Alzheimer report 2018. London: Alzheimer’s Disease International; 2018.
- Srivastava S, Ahmad R, Khare SK. Alzheimer's disease and its treatment by different approaches: A review. Eur J Med Chem 2021;216:1724.
- Liu W, Liu X, Liu W, et al. Discovery of novel β-carboline derivatives as selective AChE inhibitors with GSK-3β inhibitory property for the treatment of Alzheimer's disease. Eur J Med Chem 2022; 229:114095.
- Verma A, Kumar Waiker D, Bhardwaj B, et al. The molecular mechanism, targets, and novel molecules in the treatment of Alzheimer's disease. Bioorg Chem 2022;119:105562.
- Syed YY. Sodium Oligomannate: First approval. Drugs 2020;80:441–4.
- Dhillon S. Aducanumab: First approval. Drugs 2021;81:1437–43.
- Wang K, Na L, Duan M. The pathogenesis mechanism, structure properties, potential drugs and therapeutic nanoparticles against the small oligomers of Amyloid-β. Curr Top Med Chem 2021;21:151–67.
- Chen G-F, Xu T-H, Yan Y, et al. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 2017;38:1205–35.
- Anu Kunnath R, Subham D, Alex J, et al. Neurodegenerative pathways in Alzheimer's Disease: A Review. Curr Neuropharmacol 2021;19:679–92.
- Tan CC, Zhang XY, Tan L, et al. Tauopathies: Mechanisms and therapeutic strategies. J Alzheimers Dis 2018;61:487–508.
- Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 2018;14:399–415.
- Li Y, Jiao Q, Xu H, et al. Biometal dyshomeostasis and toxic metal accumulations in the development of Alzheimer’s disease. Front Mol Neurosci 2017;10:339.
- Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer's disease. Biomed Rep 2016;4:519–22.
- Wang T, Xu SF, Fan YG, et al. Iron pathophysiology in Alzheimer's Diseases. Adv Exp Med Biol 2019;1173:67–104.
- Kinney JW, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575–90.
- Tong BC, Wu AJ, Li M, et al. Calcium signaling in Alzheimer's disease & therapies. Biochim Biophys Acta Mol Cell Res 2018;1865:1745–60.
- Gerakis Y, Hetz C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer's disease. Febs J 2018;285:995–1011.
- Hill E, Wall MJ, Moffat KG, et al. Understanding the pathophysiological actions of tau oligomers: A critical review of current electrophysiological approaches. Front Mol Neurosci 2020;13:155.
- Tripathi T, Kalita P. Synergistic effect of Amyloid-β and tau disrupts neural circuits. ACS Chem Neurosci 2019;10:1129–30.
- Yin X, Qiu Y, Zhao C, et al. The role of amyloid-beta and tau in the early pathogenesis of Alzheimer's Disease. Med Sci Monit 2021;27:e933084.
- He Z, Guo JL, McBride JD, et al. Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med 2018;24:29–38.
- Bennett RE, DeVos SL, Dujardin S, et al. Enhanced tau aggregation in the presence of amyloid β. Am J Pathol 2017;187:1601–12.
- Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs 2007;185:73–84.
- La Pietra V, La Regina G, Coluccia A, et al. Design, synthesis, and biological evaluation of 1-phenylpyrazolo[3,4-e]pyrrolo[3,4-g]indolizine-4,6(1H,5H)-diones as new glycogen synthase kinase-3β inhibitors. J Med Chem 2013;56:10066–78.
- Pei JJ, Braak E, Braak H, et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 1999;58:1010–9.
- Georgievska B, Sandin J, Doherty J, et al. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral target engagement in humans. J Neurochem 2013;125:446–56.
- Iqbal K, Grundke-Iqbal I. Discoveries of tau, abnormally hyperphosphorylated tau and others of neurofibrillary degeneration: A personal historical perspective. J Alzheimers Dis 2006;9:219–42.
- Wischik CM, Harrington CR, Storey JM. Tau-aggregation inhibitor therapy for Alzheimer's disease. Biochem Pharmacol 2014;88:529–39.
- De Simone A, Tumiatti V, Andrisano V, et al. Glycogen synthase kinase 3β: A new gold rush in Anti-Alzheimer's Disease Multitarget Drug Discovery? J Med Chem 2021;64:26–41.
- Llorens-Martin M, Jurado J, Avila J, et al. GSK-3β, a pivotal kinase in Alzheimer disease. Front Mol Neurosci 2014;7:46.
- Forlenza OV, De-Paula VJR, Diniz BSO. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer's disease and related neurodegenerative disorders. ACS Chem Neurosci 2014;5:443–50.
- Wang H, Brown J, Martin M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011;53:130–40.
- Gómez-Sintes R, Hernández F, Lucas JJ, Avila J. er al. GSK-3 mouse models to study neuronal apoptosis and neurodegeneration. Front Mol Neurosci 2011;4:45.
- Sayas CL, Ávila J. GSK-3 and tau: A key duet in Alzheimer’s disease. Cells 2021;10:721.
- Ruiz A, Eldar-Finkelman SM. H. Glycogen synthase kinase-3 inhibitors: Preclinical and clinical focus on CNS-a decade onward. Front Mol Neurosci 2021;14:792364.
- del T. S. Phase IIa clinical trial on Alzheimer’s disease with NP12, a GSK3 inhibitor. Alzheimers Dement 2010;6:S147.
- Bhat R, Xue Y, Berg S, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 2003;278:45937–45.
- Berg S, Bergh M, Hellberg S, et al. Discovery of novel potent and highly selective glycogen synthase kinase-3β (GSK3β) inhibitors for Alzheimer's disease: design, synthesis, and characterization of pyrazines. J Med Chem 2012;55:9107–19.
- Yao H, Uras G, Zhang P, et al. Discovery of novel tacrine-pyrimidone hybrids as potent dual AChE/GSK-3 inhibitors for the treatment of Alzheimer's Disease. J Med Chem 2021;64:7483–506.
- Sivaprakasam P, Han X, Civiello RL, et al. Discovery of new acylaminopyridines as GSK-3 inhibitors by a structure guided in-depth exploration of chemical space around a pyrrolopyridinone core. Bioorg Med Chem Lett 2015;25:1856–63.
- Choi S, Park K, Seo HJ, et al. Preparation of pyrazole derivatives as TNIK, IKKε and TBK1 inhibitor and pharmaceutical composition comprising same. US20160289196. 2016.
- Ramurthy S, Pfister KB, Boyce RS, et al. Discovery and optimization of novel pyridines as highly potent and selective glycogen synthase kinase 3 inhibitors. Bioorg Med Chem Lett 2020;30:126930.
- Liu SL, Wang C, Jiang T, et al. The role of CDK5 in Alzheimer's Disease. Mol Neurobiol 2016;53:4328–42.
- Hanger DP, Anderton BH, Noble W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol Med 2009;15:112–9.
- Kimura T, Ishiguro K, Hisanaga S. Physiological and pathological phosphorylation of tau by CDK5. Front Mol Neurosci 2014;7:65.
- Perez DI, Gil C, Martinez A. Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases. Med Res Rev 2011;31:924–54.
- Agholme L, Lindström T, Kågedal K, et al. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis 2010;20:1069–82.
- Augello G, Emma MR, Cusimano A, et al. The role of GSK-3 in cancer immunotherapy: GSK-3 inhibitors as a new frontier in cancer treatment. Cells 2020;9:1427.
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol Ther 2015;148:114–31.
- Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A 1999;96:8745–50.
- Buttrick GJ, Wakefield JG. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle 2008;7:2621–5.
- Ikeda S, Kishida S, Yamamoto H, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin . Embo J 1998;17:1371–84.
- Wu D, Pan W. GSK3: A multifaceted kinase in Wnt signaling. Trends Biochem Sci 2010;35:161–8.
- Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol 2013;5:a007898.
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases . Dev Cell 2009;17:9–26.
- Salcedo-Tello P, Ortiz-Matamoros A, Arias C. GSK3 function in the brain during development, neuronal plasticity, and neurodegeneration. Int J Alzheimers Dis 2011;2011:189728.
- Skardelly M, Gaber K, Schwarz J, et al. Neuroprotective effects of the beta-catenin stabilization in an oxygen- and glucose-deprived human neural progenitor cell culture system. Int J Dev Neurosci 2011;29:543–7.
- Jin N, Zhu H, Liang X, et al. Sodium selenate activated Wnt/β-catenin signaling and repressed amyloid-β formation in a triple transgenic mouse model of Alzheimer's disease. Exp Neurol 2017;297:36–49.
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry 2010;15:272–85,
- Lu W, Yamamoto V, Ortega B, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 2004;119:97–108.
- Lin CC, Chou CH, Howng SL, et al. GSKIP, an inhibitor of GSK3beta, mediates the N-cadherin/beta-catenin pool in the differentiation of SH-SY5Y cells. J Cell Biochem 2009;108:1325–36.
- Yap AS, Brieher WM, Pruschy M, et al. Lateral clustering of the adhesive ectodomain: A fundamental determinant of cadherin function. Curr Biol 1997;7:308–15.
- González JF, Alcántara AR, Doadrio AL, et al. Developments with multi-target drugs for Alzheimer's disease: an overview of the current discovery approaches. Expert Opin Drug Discov 2019;14:879–91.
- Shi X-L, Wu J-D, Liu P, et al. Synthesis and evaluation of novel GSK-3β inhibitors as multifunctional agents against Alzheimer's disease. Eur J Med Chem 2019;167:211–25.
- Moradi HR, Hajali V, Khaksar Z, et al. The next step of neurogenesis in the context of Alzheimer's disease. Mol Biol Rep 2021;48:5647–60.
- Jaworski T, Banach-Kasper E, Gralec K. GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019;2019:4209475.
- Giese KP. GSK-3: A key player in neurodegeneration and memory. IUBMB Life 2009;61:516–21.
- Benowitz LI, Routtenberg A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci 1997;20:84–91.
- Kawasaki A, Okada M, Tamada A, et al. Growth cone phosphoproteomics reveals that GAP-43 phosphorylated by JNK is a marker of axon growth and regeneration. iScience 2018;4:190–203.
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev 2002;16:2699–712.
- Nothias F, Vernier P, von Boxberg Y, et al. Modulation of NCAM polysialylation is associated with morphofunctional modifications in the hypothalamo-neurohypophysial system during lactation. Eur J Neurosci 1997;9:1553–65.
- Sánchez Martin C, Ledesma D, Dotti CG, et al. Microtubule-associated protein-2 located in growth regions of rat hippocampal neurons is highly phosphorylated at its proline-rich region. Neuroscience 2000;101:885–93.
- Rodriguez-Jimenez FJ, Vilches A, Perez-Arago MA, et al. Activation of neurogenesis in multipotent stem cells cultured in vitro and in the spinal cord tissue after severe injury by inhibition of glycogen synthase kinase-3. Neurotherapeutics 2021;18:515–33.
- De Simone A, La Pietra V, Betari N, et al. Discovery of the first-in-class GSK-3β/HDAC dual inhibitor as disease-modifying agent to combat Alzheimer’s disease. ACS Med Chem Lett 2019;10:469–74.
- Shi XL, Yan N, Cui YJ, et al. A unique GSK-3β inhibitor B10 has a direct effect on Aβ, targets tau and metal dyshomeostasis, and promotes neuronal neurite outgrowth. Cells 2020;9:649.
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12.
- Maestro, version 11.9.011; Schrödinger, LLC, New York, NY, 2019.

![Figure 2. Design of thieno[3,2-c]pyrazol-3-amine derivatives as GSK-3β inhibitors.](/cms/asset/6c0a9c8f-cce3-4369-821a-a283223df1b9/ienz_a_2086867_f0002_c.jpg)