Abstract
Glucosamine-6-phosphate synthase (GlcN-6-P synthase) is known as a promising target for antimicrobial agents and antidiabetics. Several compounds of natural or synthetic origin have been identified as inhibitors of this enzyme. This set comprises highly selective l-glutamine, amino sugar phosphate or transition state intermediate cis-enolamine analogues. Relatively low antimicrobial activity of these inhibitors, poorly penetrating microbial cell membranes, has been improved using the pro-drug approach. On the other hand, a number of heterocyclic and polycyclic compounds demonstrating antimicrobial activity have been presented as putative inhibitors of the enzyme, based on the results of molecular docking to GlcN-6-P synthase matrix. The most active compounds of this group could be considered promising leads for development of novel antimicrobial drugs or antidiabetics, provided their selective toxicity is confirmed.
1. Introduction – the target
l-Glutamine:d-fructose-6-phosphate amidotransferase, also known under the name of glucosamine-6-phosphate synthase (GlcN-6-P synthase), is a ubiquitous enzyme of primary anabolism, present in almost all known living organisms and tissues and known under the abbreviated name of GFA or GFAT. The reaction catalysed by GlcN-6-P synthase constitutes the first committed step of a branch of glycolysis, leading to the ultimate formation of 5′-diphospho-N-acetyl-D-glucosamine (UDP-GlcNAc), known as the hexosamine biosynthesis pathway (HBP). In mammals, the HBP has been identified as one of the biochemical pathways that could contribute to insulin resistance, which is a molecular basis of type-2 diabetesCitation1. The elevated activity of GlcN-6-P synthase was found to be correlated with insulin resistance, postprandial hyperglycaemia and diabetic complications. In consequence, the human enzyme is considered a potential diabetes targetCitation2–4.
The human GlcN-6-P synthase, i.e. hGFAT, has been also assigned a prominent role in the close relationship between HBP and cancer. In this respect, it is worth mentioning that in humans, two GFAT paralogs exist, namely hGFAT1 encoded by the gfpt1 gene and gfpt2-encoded hGFAT2, that primarily differ in their tissue-specific expression patternsCitation5. Expression of gfpt1 was found to be upregulated in breastCitation6, prostateCitation7 and hepaticCitation8 cancers, while gfpt2 is considerably overexpressed in pancreatic adenocarcinomaCitation9, colorectal cancerCitation10 and non-small-cell lung cancerCitation11. Inhibition of GFPT2 selectively reduced KRAS/LKB1 co-mutant tumour cell growth in culture, xenografts and genetically modified miceCitation12. The GlcN-6-P synthase inhibitor: nanoparticle conjugates were found to exhibit remarkable cytotoxicity against human cervical cancer (HeLa) and hypopharyngeal carcinoma cell linesCitation13. Moreover, an inhibitor of GlcN-6-P synthase in combination with the established anticancer agent, cisplatin, demonstrated a synergistic effectCitation14. Therefore, GlcN-6-P synthase is also considered a possible target for anticancer agents, at least in some cancer types.
On the other hand, in prokaryotic and eukaryotic microorganisms, cell walls of which are composed of amino sugar-containing macromolecules, like peptidoglycan and lipopolysaccharides in bacteria or chitin and N-glycosylated mannoproteins in fungi, GlcN-6-P synthase is an enzyme of crucial importance for cell survival and growth. Deletion of the GlcN-6-P synthase encoding gene in fungi and bacteria is lethalCitation15,Citation16 and even a short-term inhibition of GlcN-6-P synthase activity in cell wall containing microorganisms results in fungicidal or bactericidal effect. On the other hand, short-term inhibition of GlcN-6-P synthase activity in mammals is not lethalCitation17 and in the case of infectious diseases in diabetic patients could be even beneficial, so the potential of this enzyme as a target for antibacterials and antifungals is unquestionableCitation18.
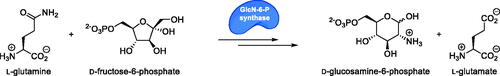
GlcN-6-P synthase catalyses an irreversible reaction between l-glutamine and d-fructose-6-phosphate, resulting in the formation of D-glucosamine-6-phosphate and L-glutamate (Scheme 1). The enzyme is a member of the so-called amidotransferase subfamily of enzymes, transferring amide nitrogen from L-glutamine to an acceptor substrate but is highly specific for its substrates, especially L-Gln. This specificity makes GlcN-6-P synthase unique among other enzymes of the subfamily, which are able to use ammonia as an alternative amino donorCitation19,Citation20.
GlcN-6-P synthase is a relatively large, dimeric or tetrameric protein. Its monomeric subunit containing 589–716 amino acid residuesCitation18, is composed of two domains, the N-terminal domain (GAH) involved in L-Gln binding and hydrolysis and the d-Fru-6-P -binding C-terminal isomerase domain (ISOM). The prokaryotic GlcN-6-P synthase is a dimer of two identical subunits, as shown in , while the eukaryotic enzyme is homotetramericCitation18. The prokaryotic (bacteria) and eukaryotic (fungi, mammals) enzyme versions differ also in terms of physiological modes of regulation of catalytic activity. In bacteria, expression of the GlcN-6-P synthase encoding gene is regulated posttranscriptionally by siRNACitation21, whereas the eukaryotic enzyme is a subject of allosteric feedback inhibition by UDP-GlcNAcCitation22,Citation23 and protein kinase A-mediated phosphorylationCitation24.
Figure 1. Molecular structure of GlcN-6-P synthase. Top – Structure of E. coli GFA dimer, with GAH and ISOM active centres indicated as red and green spheres, respectively. Based on the pdbid: 1jxa matrix. Bottom – a single subunit of GlcN-6-P synthase, with a detailed presentation of active centres’ crucial residues and 5-oxo-L-norleucine covalently bound to the Cys1 residue at GAH and Glc-6-P in an open ring form at ISOM. Side chains of residues present within the radius of 4.5 Å of both ligands are drawn as thin sticks and ligands as thicker sticks. Crucial residues of superimposed C. albicans (pdbid: 2poc) and H. sapiens (pdbid: 6r4f) GlcN-6-P synthases are shown, to visualise the cross-species conservation of both the structure and conformation of the crucial amino acid residues. The observed significant variations of Cys1, Trp74 and Glu488 confirmations are due to their conformational mobility during the catalytic act.
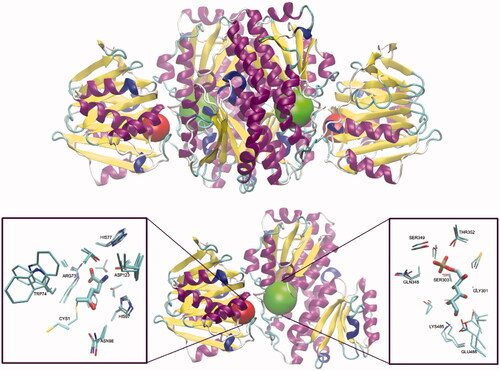
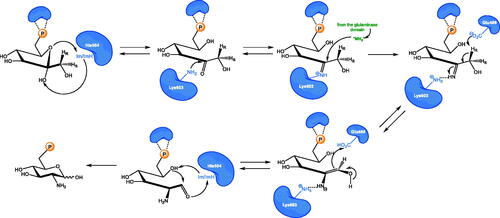
In GlcN-6-P synthase, there is not any single defined active centre but two active centres located at GAH and ISOM domains, respectively, are connected through the intramolecular, solvent inaccessible channelCitation25. The only catalytic residue at GAH, namely N-terminal Cys1, catalyses the hydrolysis of l-Gln amide and three residues, namely Glu488, His504 and Lys603 (E. coli GlcN-6-P synthase numbering), participate in ketose-aldose isomerisation of fructosamine-6-P intermediate at ISOMCitation26. All the catalytic residues and another five involved in substrate binding are highly conserved among GlcN-6-P synthases of different sourcesCitation18. The molecular mechanism of GlcN-6-P synthase catalytic action is complex and involves three main steps: hydrolysis of glutamine at GAH, transfer of ammonia from GAH to ISOM and isomerisation of the resulting fructosamine-6-P at ISOM. At first, the Fru-6-P molecule binds to ISOM and the opening of its hexose ring triggers the conformational changes of two domains, namely closing access to the ISOM active site and promoting rearrangement of Cys1 at GAH into an active conformation. The subsequent binding of l-Gln at GAH induces another conformational change of the enzyme molecule, which ensures hydrolysis of glutamine amide and ammonia transfer through the intramolecular channel to ISOM. In the third step, the fructosamine-6-P is isomerised through the cis-enamine intermediate and finally, the reaction products, i.e. GlcN-6-P and l-Glu are released (Scheme 2)Citation27.
2. Search for the GlcN-6-P synthase inhibitors
Due to the target potential of GlcN-6-P synthase, an extensive search for its inhibitors as potential antimicrobials or antidiabetics has been continued for several years. A number of such compounds have been found in Nature or synthesised as rationally designed molecules. Some of them or their derivatives exhibited expected biological activity. In another approach, several heterocyclic compounds exhibiting antimicrobial activity have been found potential GlcN-6-P synthase inhibitors by molecular docking studies. GlcN-6-P synthase inhibitors known so far fall into four groups: substrate analogues, transition state or intermediate analogues, product analogues and compounds binding outside the active centre. Some of them are alkylating agents, mechanism-based suicide inhibitors or transition metal complexes. Herein, we would like to present a comprehensive and concise review of the most significant examples of the GlcN-6-P synthase inhibitors, particularly focussing on their syntheses and antimicrobial or antidiabetic properties.
3. Glutamine analogues targeting GlcN-6-P synthase
Tetaine, also known under the name of bacilysin (Scheme 3A), is a natural compound produced by Bacillus subtilisCitation28 that exhibits both antibacterial and antifungal activityCitation29. The C-terminal amino acid of this dipeptide, anticapsin (Scheme 3B), produced independently by Streptomyces griseoplanusCitation30, was identified as an irreversible inhibitor of GlcN-6-P synthase (Ki = 9.5 µM)Citation31. This antimetabolite acts as a structural analog of l-glutamine and binds to the enzyme active site via alkylation of the catalytic Cys1 residue by its epoxide moietyCitation31. In a tetaine analogue, known under the name of chororotetaine (Scheme 3A), the anticapsin residue is replaced by a structurally related, another GlcN-6-P synthase targeting glutamine analogue, containing a chlorocyclohexenone ringCitation32.
Scheme 3. (A) Antibiotics containing glutamine analogues targeting GlcN-6-P synthase. (B) Synthesis of anticapsin reported by Baldwin et al. Citation36
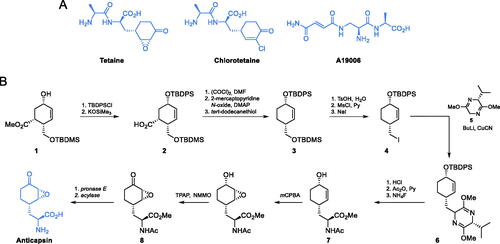
In the structure of anticapsin, an E configuration was mistakenly assigned to the epoxide ringCitation33 and corrected for the proper Z configuration two decades laterCitation34. Due to that error, there are reports before the year 1993 which claim to be synthetic methods of anticapsin preparation, but in reality concerned synthesis of its enantiomerCitation35. The first enantioselective synthesis of anticapsin was presented by Baldwin and co-workersCitation36 (Scheme 3B). All other known methods of anticapsin synthesis can be found in the review paper on natural epoxycyclohexanesCitation37.
Baldwin’s synthesis of anticapsin started with a chiral alcohol 1, the preparation of which was designed by Kobayashi et al.Citation38. The secondary hydroxyl group of this alcohol was transformed into the tert-butyldiphenylsilyl ether and then saponified using potassium trimethylsilanolate, to yield compound 2. A three-step decarboxylation of 2 via thiohydroxamic ester afforded bis-silyl ether 3, which was then gradually transformed into primary iodide 4. Alkylation of this halide with 5 led to the formation of compound 3 which was subsequently hydrolysed to enantiomerically pure amino acid derivative 7. Next, two selective oxidation reactions were performed, to obtain derivative 8 which was treated with specific enzymes to yield a free anticapsin Citation36.
During the screening program of Streptomyces, Molloy et al. isolated compound A190106 that exhibited growth inhibitory activity on Salmonella gallinarum (MIC = 8 μg/ml)Citation39. Structural analysis of this metabolite revealed that it was a dipeptide containing a fumaramic acid moiety (Scheme 3A). Van der Baan et al.Citation40 synthesised the N-terminal amino acid of this dipeptide, FCDP, using the route shown in Scheme 4A, path A. The authors used fumaric acid, which can be easily obtained from maleic anhydride, as a starting material, transformed into amide 9 via ammonolysis and coupled it with a methyl ester of N-α-tert-butoxycarbonyl-l-2,3-diaminopropanoic acid, using EEDQ as a coupling agent. The formed product was then hydrolysed with aqueous KOH and then with TFA, what led to the eventual formation of FCDPCitation40.
Scheme 4. (A) Synthesis of GlcN-6-P synthase inhibitors containing l-2,3-diaminopropanoic moiety. (B) Molecular mechanism of GlcN-6-P synthase inactivation at GAH by FMDPCitation43.
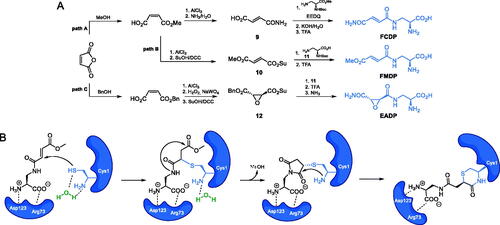
Chmara et al. suggested that the biological activity of FCDP-Ala can be attributed to the inactivation of GlcN-6-P synthase by FCDP (Ki = 85 µM)Citation41. After discovering of inhibitory activity of FCDP on Salomonella typhimurium GlcN-6-P synthase, Andruszkiewicz et al. synthesised FCDP analogues. In this series of compounds, one of them, namely FMDP, demonstrated significantly higher inhibitory potential against bacterial and yeast GlcN-6-P synthase (IC50 = 15–21 µM) than FCDP (82–100 µM) and inactivated the Candida albicans enzyme with Ki = 0.1 µMCitation42. To obtain this compound, the authors converted maleic anhydride into an active ester of monomethyl fumarate 10, coupled it with the terminal amino group of N-α-tert-butoxycarbonyl-L-2,3-diaminopropanoic acid 11 and then hydrolysed with trifluoroacetic acid, to obtain FMDP (Scheme 4A, path B)Citation42. Kucharczyk et al. found that FMDP formed a covalent bond with the Cys1 residue of bacterial GlcN-6-P synthase, upon Michael-type nucleophilic addition of -SH functionality to the conjugated double bond system of the inhibitor molecule, which is followed by the formation of the 1,4-thiazin-3-one derivative, containing the substantial part of the Cys1 backboneCitation43, as shown in Scheme 4B.
In the following works, Andruszkiewicz and co-workers tested the inhibitory activity of epoxysuccinic derivatives of 2,3-diaminopropanoic acid and found EADP (Scheme 4A, path C), along with some of its enantiomers and diastereoisomers, to be competitive inhibitors of GlcN-6-P synthase from Saccharomyces cerevisae (for EADP, Ki = 40 µM)Citation44. EADP was synthesised starting from maleic anhydride, which was transformed into methyl fumarate, as shown previously and then its C = C bond was oxidised to epoxide 12 which was subsequently coupled with 11 and then subjected to a two-step deprotection process (Scheme 4A, path C)Citation44.
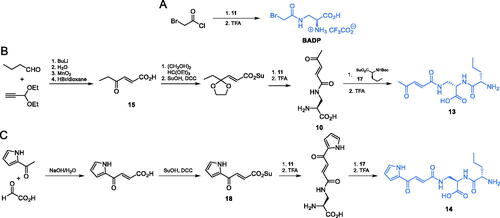
To explore the topology of the glutamine-binding site of GlcN-6-P synthase, Auvin et al. obtained N-ω-haloacetyl derivatives of α,ω-diaminoalkanoic acids, out of which Nω–bromoacetyl-L-2,3-diaminopropionic acid (BADP) (Scheme 5A) showed parameters of GlcN-6-P synthase inactivation comparable to those of FMDP (Ki = 0.1 µM)Citation45. To obtain that inhibitor, the authors used the Boc-protected l-2,3-diaminopropionic acid 11 which was acetylated with bromoacetyl chloride. Deprotection of the intermediate led to the final product in the form of a TFA salt (Scheme 5A)Citation45.
Another series of l-2,3-diaminopropanoic acid derivatives that exhibit GlcN-6-P synthase inhibiting properties, including compounds 13 and 14 (Scheme 5B and C) were obtained by Walkowiak et al.Citation46 The antifungal activity of these compounds was poor, with MIC values in the 62.5–125 µg/mL range. To obtain compound 13, the authors utilised a condensation reaction between butanal and propionaldehyde diethyl acetate, followed by oxidation and hydrolysis, to obtain ketone 15 (Scheme 5B). The carbonyl group of this ketone intermediate was protected via acetal formation and then treated with TFA to obtain compound 16, which was subsequently coupled with appropriately protected L-norvaline derivative 17 and once again treated with TFA, to finally yield dipeptide 13. On the other hand, compound 14 was prepared via an aldol condensation of 2-acetylpyrrole and 2-oxoacetic acid, which yielded pseudofumarate 18 (Scheme 5C). Carboxylic acid activation using DCC/HOBt and consecutive conjugation of the protected amino acid was performed similarly as in the B route and led to the ultimate formation of 14Citation46.
It is noteworthy, that all L-2,3-diaminopropanoic acid-based inhibitors of GlcN-6-P synthase exhibit high selectivity towards this amidotransferaseCitation47. Such a feature is unique because other glutamine analogs, like 6-diazo-5-oxo-l-norleucine (DON) or azaserine are inhibitors of many glutamine-utilising enzymesCitation48. Interestingly, anticapsin, not based on the l-2,3-diaminopropanoic acid scaffold, is also a selective inhibitor of GlcN-6-P synthase.
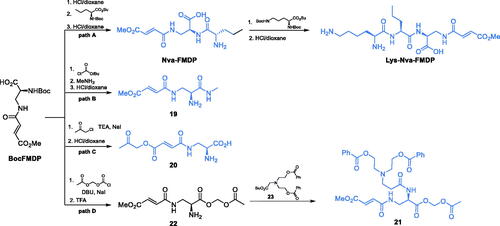
Due to the high affinity of FMDP to GlcN-6-P synthase, this compound was selected as a leader in the search for antimicrobial agents targeting that enzyme. Unfortunately, its intrinsic antimicrobial activity was low (MICs in the 100–200 µg/mL range). A substantial improvement was achieved upon the incorporation of FMDP into oligopeptide structures, with two compounds, namely Nva-FMDP and Nva-Lys-FMDP, designed and obtained by Andruszkiewicz et al. (Scheme 6, path A)Citation49. Those compounds exhibited the highest in vitro and in vivo activity against C. albicans and several other human pathogenic fungiCitation50 but were also active against bacteriaCitation51. The FMDP-oligopeptides are transported into microbial cells by oligopeptide permeases and then cleaved by intracellular peptidasesCitation50,Citation52. Recently, Nva-FMDP was found to exhibit promising growth inhibitory activity against Fluconazole-resistant cells of the emerging fungal pathogen Candida aurisCitation53.
Conversion of FMDP into its more lipophilic derivatives was another way of potentiation of its antimicrobial activity. Zgódka et al. synthesised the acetoxymethyl ester of FMDP, which demonstrated about 20 times lower MIC value against C. albicans than the parent amino acidCitation54. Pawlak et al. designed another series of compounds, trying to take advantage of the antimicrobial potential of FMDP. The authors focussed their attention on the alpha-carboxyl group of FMDP as a suitable site of the structural modification and transformed it into amides, one of which (19) is shown on Scheme 6, path BCitation55. To obtain that compound, the authors used N-Boc-FMDP as a precursor and transformed it with isobutyl chloroformate into a mixed anhydride, which was subsequently aminolysis with methylamine and treated with dry HCl, to yield the final compoundCitation55. That FMDP amide showed some in vitro antifungal activity, however, it was limited exclusively to C. albicans. The same team also obtained esters and α-hydroxy ketones of FMDP (Scheme 6, path C). In that case, N-Boc-FMDP was also used as starting point in the synthesis. The 2-oxoalkoxyl function was introduced by the reaction of BocFMDP with chloroacetone. Subsequent deprotection led to the formation of an ester 20. That compound showed some moderate antifungal in vitro activity against C. albicans and Candida glabrataCitation56. Koszel et al.Citation57 synthesised derivatives of bicine conjugated with FMDP. Most of the synthesised compounds showed moderate antifungal activity, while retaining a good water solubility. The most potent compound 21 (Scheme 6, path D) was obtained from BocFMDP. The starting compound was treated with ((chlorocarbonyl)oxy)methyl acetate in the presence of DBU and sodium iodide. After deprotection of intermediate 22 with trifluoroacetic acid, the amino function was coupled with bicine derivative 23, which led to the formation of 21Citation57.
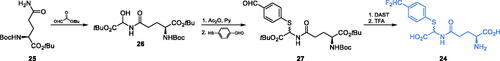
Prompted by the need to find more specific inhibitors that target the glutamine-binding site of GlcN-6-P synthase, Massiere et al. designed compound 24 (Scheme 7)Citation58. The authors proposed a mechanism of action of this inhibitor, in which after binding at the GAH active site it is decomposed to products that spontaneously generate powerful electrophilic species reacting with the nucleophilic residues at the GAH enzyme and in consequence, inactivating the enzyme. However, in practice, this potential mechanism-based inhibitor exhibited only moderate enzyme-inhibiting properties (Ki = 36 mM). That inhibitor was synthesised using the suitably protected glutamine which reacted with tert-butyl glyoxalate to give a glycine derivative 25. The formed compound 26 was then acetylated and treated with 4-mercaptobenzaldehyde, which led to the formation of 27. The aromatic group of this compound was then converted into difluoromethyl group. One-step de-protection of amino and carboxyl function led to the final compound 24 (Scheme 7)Citation58.
Scheme 7. Synthesis of the mechanism-based GlcN-6-P synthase inhibitor, according to Massiere et al.Citation58
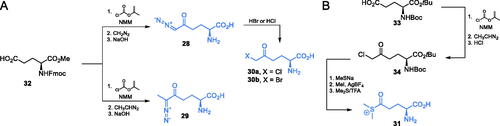
A series of other electrophilic glutamine analogues targeting GlcN-6-P synthase were reported in the literature. Walker et al.Citation59 described irreversible inhibition of GlcN-6-P synthase by diazoalkyl derivatives: 28 (DON) and 29, halometyl derivatives 30a-b (Scheme 8A) and dimethyl sulfonium ketone (DSOK) 31 (Scheme 8B). To obtain diazoalkyl glutamine analogues 28 and 29, the authors used the appropriately protected glutamic acid derivative 32 and reacted it with in situ generated N-nitroso-β-isobutylalkyl ketone, which led to the formation of appropriate diazoalkyl ketone. Compound 28 was also used as a precursor for compounds 30a-b. By treating diazoketone 28 with HBr or HCl, compounds 30a or 30 b were obtained respectively. To obtain DSOK 31, a protected glutamate 33 was transformed into respective chloromethyl ketone 34. This intermediate compound was transformed into methyl sulfide with sodium methanethiolate and then methylated with methyl iodide to form a dimethylsulfonium ion. Deprotection with trifluoroacetic acid led to compound 31. DSOK (31) appeared one of the strongest inactivators of GlcN-6-P synthase (Ki = 0.37 µM)Citation59.
Several other glutamine analogues, like 6-diazo-5-oxo-L-norleucine, azaserine or γ-glutamate semialdehydeCitation60 are effective inhibitors of GlcN-6-P synthaseCitation18 but for the lack of enzyme inhibitory selectivity, they cannot be considered promising drug candidates.
4. Fructose-6-phosphate and glucosamine-6-phosphate analogues
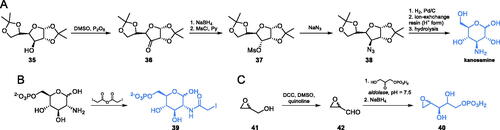
Kanosamine, i.e. 3-amino-3-deoxy-d-glucose, is an antibiotic substance produced by Bacillus aminoglucosidicus. Mechanism of its antifungal action comprises uptake by the glucose transport system, intracellular conversion into kanosamine-6-phosphate and inhibition of GlcN-6-P synthase by this derivative, competitive in regard to Fru-6-P, with Ki = 5.9 mMCitation61. The synthesis of kanosamine was reported by Meyer zu Reckendorf et al. (Scheme 9A). In the presented approach, the authors used the sugar derivative 35 which was oxidised to ketone 36. The formed ketone was then stereoselectively reduced with sodium borohydride and transformed into a corresponding mesylate 37, which was subsequently reacted with sodium azide via SN2 reaction, to obtain azide 38. Catalytic hydrogenation of 38, followed by ion-exchange resin-mediated hydrolysis, led to N-acetylkanosamine which can be hydrolysed to the free amino sugarCitation62.
The first irreversible inhibitor of GlcN-6-P synthase targeting the ISOM domain was described by Bearne et al.Citation63 In their studies, they obtained N-iodoacetylglucosamine-6-phosphate 39, designed as a structure that mimics the final product formed at the active centre of this domain. Compound 39 was obtained through the reaction of commercially available glucosamine-6-phosphate and iodoacetic anhydride (Scheme 9B). The authors strictly controlled the pH of the reaction, which led to the formation of the final product with a high yield. Compound 39 inactivated GlcN-6-P synthase with Ki = 0.22 mMCitation63.
Leriche et al.Citation64 studied the Fru-6-P binding site at the ISOM domain of E. coli GlcN-6-P synthase, using anhydro-1,2-hexitol-6-phosphate 40 (Scheme 9C), a structural analogue of an open ring form of Fru-6-P, which was previously identified as an irreversible inhibitor of phosphoglucose isomerase. To obtain 40, the authors used racemic glycidol 41 as a starting material, which was oxidised to a corresponding aldehyde 42 via the Moffat oxidation. The formed aldehyde was then chemoenzymatically condensed with glycerone phosphate, using aldolase. The resulting intermediate was then reduced using sodium borohydride which led to the racemic 40. This compound inactivated GlcN-6-P synthase, with Ki = 1.4 mMCitation64.
5. Analogues of transition state intermediates at the ISOM active site
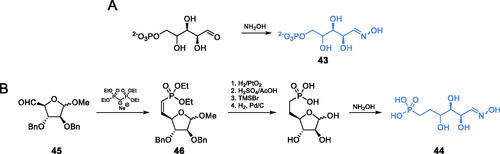
Le Camus and co-workers found arabinose-5-phosphate oxime 43 (APO) to be a potent inhibitor of GlcN-6-P synthaseCitation65. The authors obtained the aforementioned compound by converting the commercially available arabinose-5-phosphate to its oxime with hydroxylamine (Scheme 10A). Due to the low hydrolytic stability of phosphate moiety in 43, the authors decided to obtain its homolog 44. This compound was obtained using d-arabinose-derived aldehyde 45, which was converted into vinylphosphonate 46 using the Horner-Emmons reaction. A Series of selective deprotection reactions, followed by a reaction with hydroxylamine, led to the final formation of oxime 44 (Scheme 10B). Both compounds, 43 and 44, can be considered as structural analogues of open ring fructosamine-6-P, formed at the ISOM active site from Fru-6-P after its amination with glutamine-derived ammonia. The enzyme inhibitory potential of 43 was quite high (Ki = 14.3 µM), while that of 44 was much lower (Ki = 0.36 mM)Citation65.
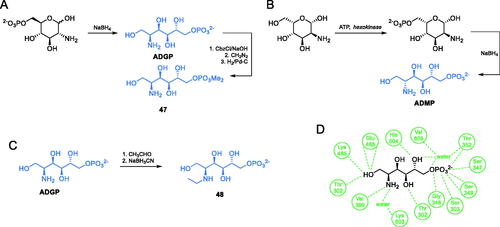
Investigating the Fru-6-P binding site of E. coli GlcN-6-P synthase, Badet-Denisot and co-workers synthesised 2-amino-2-deoxyglucitol-6-phosphate (ADGP, Scheme 11A), which turned out to be a strong inhibitor of the enzyme, with Ki = 25 µMCitation66. This compound is considered a structural mimic of cis-enamine intermediate. The authors obtained ADGP using a standard reduction of GlcN-6-P with sodium borohydride. The antimicrobial activity of ADGP is low (MIC = 5 mg/mL), but its high enzyme inhibitory potential inspired Janiak et al. to obtain and determine the enzyme inhibitor activity of ADGP derivatives. While most of them were found to be poorer inhibitors of GlcN-6-P synthase than the parent compound, some of them presented better antifungal in vitro activity. The most active compound was the dimethyl ester of ADGP − 47, with MIC values in the 0.3–0.6 mg/mL rangeCitation67. This compound was obtained by the transformation of ADGP to its N-benzyloxycarbonyl derivative, followed by phosphate methylation using diazomethane. Subsequent deprotection of amino function by standard hydrogenation on Pd/C, led to the formation of 47Citation67. In continuation of this study, Milewski et al. reported that 2-amino-2-deoxy-d-mannitol-6-phosphate (ADMP, Scheme 10B) was found to be another potent inhibitor of GlcN-6-P synthase, actually stronger than ADGPCitation68. That inhibitor was prepared using a commercially available 2-amino-2-deoxy-d-mannose via chemoenzymatic phosphorylation and subsequent reduction with sodium borohydride (Scheme 10B)Citation68. Melcer et al. studied N-alkyl and N,N-dialkyl derivatives of ADGP, which exhibited higher antifungal activity than the parent compound, due to the better uptake by fungal cells. The most potent inhibitor 48 (Scheme 11B) was prepared by subsequent exhaustive reductive amination with acetaldehydeCitation69.
6. Heterocyclic inhibitors targeting the active sites of GlcN-6-P synthase
6.1. Inhibitors based on five-membered ring scaffolds
Vijesh et al.Citation70 reported the synthesis of the imidazole derivatives containing imidazole-based scaffold, supposed to be potent antimicrobials. Derivative of 2-thiooxoimidazolidin-4-one imidazole 49 was obtained in excellent yield by refluxing 3-aryl-1H-pyrazole-4-carbaldehyde 50 with thiosemicarbazone in the presence of anhydrous sodium acetate and then refluxed with dimethylacetylenedicarboxylate (DMAD) (Scheme 12A)Citation71. The in vitro antimicrobial activity studies showed that some of the trisubstituted imidazole derivatives exhibited growth inhibitory effect against tested microorganisms, with compound 49 emerging as the most active. Moreover, that compound exhibited higher activity against Pseudomonas aeruginosa than streptomycin. Molecular docking of 49 to the GlcN-6-P synthase matrix (PDB 2VF5) revealed that this ligand may bind to the active site of ISOM due to the interactions with Thr352 and Lys603, with estimated Ki of 8.56 µMCitation71.
Scheme 12. (A) Syntheses of putative GlcN-6-P synthase inhibitors, according to Vijesh et al.Citation70 (B) Syntheses of presumable GlcN-6-P synthase inhibitors, according to Tomi et al.Citation72
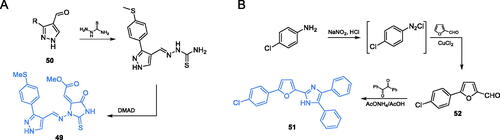
Some promising antimicrobial activity of triaryl-substituted imidazoles was reported by Tomi and co-workersCitation72. In those studies, three imidazole derivatives were obtained and their antibacterial and antifungal activity was evaluated. Synthesis of compound 51 started with the conversion of p-chloroaniline to diazonium salt, followed by conjugation with furfural in the presence of CuCl2, thus giving the 5-substituted furfural 52. Formation of the imidazole ring was accomplished by reaction of the obtained aldehyde with benzil and ammonium acetate under acidic conditions. As a result of condensation, the final derivative 51 was obtained (Scheme 12B)Citation72. The studies on the antimicrobial potential of 51 showed that this compound exhibited the highest activity against Gram-negative bacteria (E. coli, P. aeruginosa and Klebsiella pneumoniae), actually higher than that of ampicillin. Moreover, results of molecular docking studies revealed that 51 may bind to the ISOM domain active site of GlcN-6-P synthase (PDB 1MOQ) by interaction with Gly301, with the estimated inhibition constant Ki = 2.59 µM and binding energy of −7.62 kcal/molCitation72.
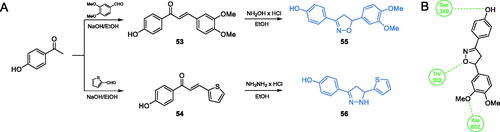
Ismail and co-workersCitation73 characterised a series of 1,2-diazole- and 1,2-oxazole-based compounds as potential antimicrobial agents. The synthesis of inhibitors was a two-step procedure, in which p-hydroxy acetophenone was condensed in an aldol manner with 3,3-dimethoxybenzaldehyde or thiophene-2-carbaldehyde, resulting in chalcones 53 and 54. Subsequently, chalcones served as substrates for cyclisation reactions with hydroxylamine or hydrazine hydrochloride that led to final oxazole- and diazole-based compounds 55 and 56, respectively (Scheme 13)Citation73. Derivatives 55 and 56 exhibited the highest antimicrobial potential in disc diffusion tests, actually higher than that of amoxicillin. The molecular docking studies of 55 and 56 to GlcN-6-P synthase (pdbid: 1moq) showed that obtained inhibitors may bind to the ISOM active site via interactions with Ala602, Ser349 and Thr302 residues. Estimated values of Ki for 55 and 56 were 0.769 and 4.21 µM and their binding energies were −8.34 and −7.33 kcal/mol, respectivelyCitation73.
Scheme 13. (A) Synthesis of disubstituted 1,2-oxazole and 1,2-diazole based possible inhibitors of GlcN-6-P synthase, according to Ismail et al.Citation73 (B) Predicted binding mode of compound 55 at the ISOM active site; H-bonds are shown by dashed lines.
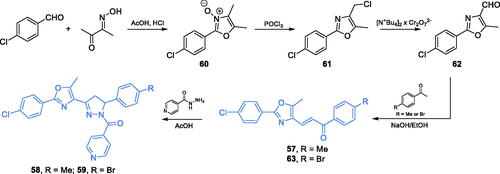
Antimicrobial agents based on 1,3-oxazole and 1,2-diazole scaffolds were synthesised by Katariya and co-workersCitation74. The synthetic approach for 57–59 depended on the generation of the 1,3-oxazole ring of compound 62. To accomplish the cyclisation, p-chlorobenzaldehyde and diacetyl monooxime were condensed, resulting in N-oxide 60, which was converted to chloride 61 by treatment with POCl3. Subsequent oxidation with bis-tetrabutylammonium dichromate (bis-TBAC) resulted in aldehyde 62 that reacted in an aldol manner with appropriately substituted acetophenone, giving chalcone 63 and compound 57. Finally, cyclisation of chalcones 57 and 63 with isoniazid in glacial acetic acid led to compounds 58 and 59 (Scheme 14)Citation74. Compounds 57–59 exhibited the highest activity against bacterial and fungal cells, with MIC values as low as 6.25 µg/mL. In silico molecular docking studies accomplished on GlcN-6-P synthase (PDB 2VF5) revealed that 58 and 59 may interact with the ISOM domain active site via H-bonding with Thr302, Thr352, and Ser347 residues, while derivative 57 formed H-bonds with Thr352 and Asp354. The docking scores obtained for 57–59 were −52.263, −62.113, and −57.586, respectivelyCitation74.
Scheme 14. Synthesis of 1,3-oxazole- and 1,2-diazole-based putative inhibitors of GlcN-6-P synthase, according to Katariya et al.Citation74
Bahare and co-workers described 2,4-thiazolidinedione-based compounds exhibiting significant antimicrobial activitiesCitation75. The synthesis of 64 started with condensation of thiourea and chloroacetic acid, which led to 2,4-thiazolidinedione 65, which underwent the aldol reaction with 2,5-dimethylbenzaldehyde, resulting in compound 66. The final alkylation of the nitrogen atom with alkyl chloride 67, gave 64 as a final compound (Scheme 15)Citation75. In vitro evaluation accomplished on two fungal strains showed that compound 64 was a strong antifungal agent, with MIC values of 3.12 and 6.25 µg/mL against C. albicans and Aspergillus niger, respectively. It is noteworthy, that those values were lower than that for fluconazole (12.5 µg/mL). Further molecular docking studies were done on GlcN-6-P synthase (PDB 2VF5) revealed presumable three important H-bond interactions of 64 with Thr302, Val399 and Ala602 residues of the ISOM domain active siteCitation75.
Scheme 15. (A) Synthesis of a potential GlcN-6-P synthase inhibitor, according to Bahare et al.Citation75 (B) Synthesis of GlcN-6-P synthase inhibitor, according to Omar et al.Citation76 (C) Predicted binding mode of 67c at the ISOM active site; H-bonds are shown by dashed lines; hydrophobic interactions are shown by wavy lines.
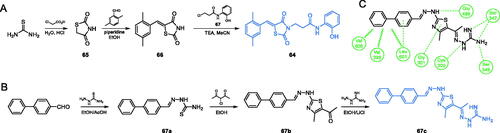
Omar and co-workersCitation76 reported the synthesis of a series of four compounds based on a thiazole scaffold. Obtained compounds possessed hydrophilic guanidine moiety as well as a lipophilic hydrocarbon-based substituent of aromatic nature, both responsible for interaction with particular residues at the ISOM. The synthesis of the most potent inhibitor 67c (Scheme 15B) was accomplished in a three-step procedure starting from the condensation of a biphenyl aldehyde and a thiosemicarbazide in acidic conditions of acetic acid. The resulting carbothioamide 67a was subsequently cyclized to thiazole derivative 67b by reaction with 3-choloro-2,4-dioxopentane and finally guanidine residue was introduced by imine bond formation with aminoguanidine hydrochloride in the presence of LiCl. Antimicrobial evaluation of obtained compounds, accomplished on Gram-positive bacteria (S. aureus and B. subtilis) and fungi (C. albicans and A. oryzae) revealed that derivative 67c was an excellent antibacterial agent, with MIC values comparable to those of ciprofloxacin (1 µg/mL for S. aureus and 0.5 µg/mL for B.subtilis). The antifungal activity of 67c was even better and in the case of C. albicans MIC value was twice as better (4 µg/mL) when compared to standard fluconazole (8 µg/mL). Moreover, compound 67c exhibited significant activity against the MRSA strain (MIC value 32 µg/mL) when compared to standard linezolid and vancomycin (1 µg/mL for both drugs). Under in vitro conditions, 67c inhibited GlcN-6-P synthase activity with IC50 = 3.47 µM. Molecular docking studies performed on GlcN-6-P synthase matrix (pdbid: 2vf5) showed that 67c interacts with the ISOM domain active site in the mode similar to that of GlcN-6-P and is stabilised by interactions with Val399, Ser347, Gln348, Glu488 (catalytic residue), Val605 and Leu601Citation76, as shown in Scheme 15C.
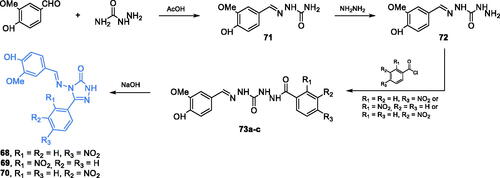
Triazole derivatives 68–70 were synthesised by Rajasekaran and co-workers as potential GlcN-6-P synthase inhibitors. These compounds were obtained from 4-hydroxy-3-methoxybenzaldehyde and hydrazinecarboxamide, which were condensed under acidic conditions to semicarbazone 71. A subsequent substitution of NH2 group with hydrazine resulted in derivative 72 that was acylated with appropriately substituted benzoyl chloride, resulting in 73a–c. The final cyclisation, accomplished in basic conditions, gave the final compounds 68–70 (Scheme 16)Citation77. The antimicrobial evaluation revealed that obtained compounds exhibited significant activity against Gram-negative and Gram-positive bacteria, with MIC values of 3.125–6.25 µg/mL. Good antifungal activity, especially against C. albicans, was observed for compounds 69 and 70, with MIC values of 3.125–12.5 µg/mL. Results of the docking study of 68–70 performed on GlcN-6-P synthase matrix (pdbid: 1jxa) showed the putative high affinity of those derivatives to the active site of the ISOM domain and three common H-bond interactions with non-crucial Ser347, Thr352 and Val399 residues were identified. The docking scores obtained for 68–70 were −9.17, −9.98, and −8.98, respectivelyCitation77.
Scheme 16. Synthesis of triazole-based putative inhibitors of GlcN-6-P synthase, according to Rajasekaran et al.Citation77
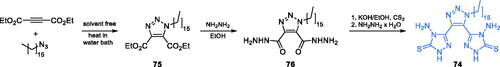
Trisubstituted 1,2,3-triazole 74 synthesised by Aouad and co-workers has been recognised as a good inhibitor of microbial growthCitation78. Click chemistry and solvent-free approach were applied for the synthesis of triazole intermediate 75 which was obtained by 1,3-dipolarcycloaddition of diethyl acetylenedicarboxylate and 1-azidopentadecane. The resulting heterocycle was subsequently aminolysis with hydrazine, thus giving 76, which eventually was converted into the desired inhibitor in a two-step manner depending on treatment with carbon disulphide under basic conditions, followed by the addition of hydrazine (Scheme 17)Citation78. The MIC values obtained against Gram-positive and Gram-negative bacteria ranged between 1 and 16 µg/mL, compared to 1–8 µg/mL obtained for ciprofloxacin. It is noteworthy, that in the case of Bacillus cereus and P. aeruginosa, the obtained MIC values were two times lower than that for the standard drug. Derivative 74 exhibited also good activity against fungal strains, especially C. albicans. The MIC value for this compound was the same as that for a standard antifungal, fluconazole (1 µg/mL). In silico studies on 74 docking to GlcN-6-P synthase (pdbid: 2vf5) revealed possible strong binding to the ISOM domain active site, with estimated Ki = 0.17 µM and binding energy of −9.23 kcal/molCitation78.
Scheme 17. Synthesis of trisubstituted 1,2,3-triazole as a potential inhibitor of GlcN-6-P synthase, according to Aouad et al.Citation78
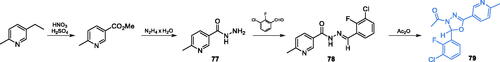
Similarly to compounds based on imidazole or pyrazole rings, the 1,3,4-oxadiazole-based systems also exhibited significant biological activities, presumed to be due to the GlcN-6-P synthase inhibitionCitation79. Shyma et al. reported synthesis, characterisation and biological studies of 1,3,4-oxadiazoles containing 6-methyl pyridine moiety, as a continuation of their search for biologically potent moleculesCitation80,Citation81. Starting from the 2-methyl 5-ethyl pyridine, the methyl 6-methyl nicotinate was prepared via oxidation using a nitrating mixture. This compound was treated with hydrazine hydrate to obtain carbohydrazide 77, which subsequently reacted with 3-chloro-2-fluorobenzaldehyde, giving the corresponding hydrazone 78. The final step, leading to the target inhibitor 79 involved 1,3,4-oxadiazole ring formation, which was accomplished by cyclisation of 78 in the presence of acetic anhydride (Scheme 18)Citation79. The obtained product was screened for antibacterial and antifungal properties. Compound 79 emerged as most active against all tested bacterial strains, with growth inhibitory potential similar to that of the standard drug, streptomycin. Moreover, some antifungal activity of compound 79 was also found, although lower than that of the standard drug fluconazole. As revealed in the molecular docking studies, compound 79 should bind to the active pocket of GlcN-6-P synthase (pdbid: 2vf5) at the ISOM domain with Ki = 2.24 µMCitation79.
Scheme 18. Synthesis of 1,3,4-oxadiazoles derivative as a potential inhibitor of GlcN-6-P synthase, according to Shyma et al. Citation79
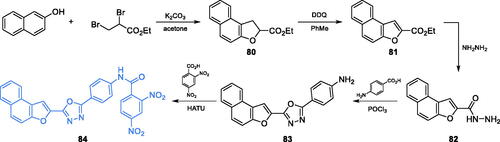
A series of 2,5-disubstituted-1,3,4-oxadiazole agents exhibiting significant antimicrobial activity were obtained by Sindhe and co-workersCitation82. The synthesis started from naphthofuran-2-hydrazide 82. This compound could be obtained with good yields from 2-naphtol and ethyl 2,3-dibromopropionate, which underwent condensation and subsequent cyclisation to derivative 80. Aromatisation of 80 with DDQ resulted in aromatic ethyl ester 81Citation83 that could be aminolysed with hydrazine to naphthofuran-2-hydrazide 82. The cyclisation of 82 with p-aminobenzoic acid gave the new aromatic ring of oxadziazole 83, which was eventually condensed with 2,4-dinitrobenzoic acid, using HATU as a coupling reagent, giving the final compound 84 (Scheme 19)Citation82. Oxadiazole 84 exhibited moderate antimicrobial activity, with MICs ranging between 0.2 and 0.4 mg/mL, as compared to that of standard drugs, ciprofloxacin (0.2 mg/mL) against bacterial strains and fluconazole (0.2 mg/mL) against fungal strains. In silico molecular docking experiments performed on GlcN-6-P synthase (pdbid: 2vf5) revealed that 84 presumably interacts via five hydrogen bonds with non-crucial Val399, Ser303, Ser349, Thr302 and Ser401 residues at the ISOM domain active site, with the calculated binding energy of −9.3 kcal/molCitation82.
Scheme 19. Synthesis of 2,5-disubstituted oxadiazole derivative as a potential inhibitor of GlcN-6-P synthase, according to Sindhe et al.Citation82
6.2. Inhibitors based on six-membered ring scaffolds
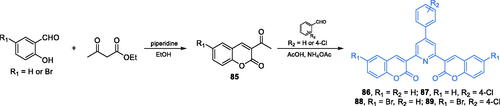
Some 2,6-bis(1-coumarin-2yl)-4–(4-substitutedphenyl)pyridine derivatives exhibiting moderate to good antimicrobial activity were reported by Kenchappa and co-workersCitation84. The synthesis of compounds 86–89 started from the preparation of appropriately substituted coumarin 85, which was obtained according to Vijesh et al.Citation85, who condensed 2-hydroxybenzaldehyde or 5-bromo-2-hydroxybenzaldehyde with ethyl acetoacetate. Subsequently, the reaction of 85 with appropriate benzaldehyde derivative and ammonium acetate was accomplished leading to the formation of the pyridine ring of final products 86–89 (Scheme 20)Citation84. The obtained compounds exhibited remarkable antibacterial activity, with MIC values comparable to those found for amoxicillin and gentamicin. Moreover, docking studies on GlcN-6-P synthase (pdbid: 2vf5) revealed the ability of those agents to bind to the ISOM domain active site via H-bonding with Gln348 and catalytic Lys603 residues. Estimated inhibition constants for 86–89 were 193.05, 226.14, 281.02, and 367.79 µM, respectively. Their binding energies ranged between −5.07 for 86 and −4.69 kJ/mol for 89Citation84.
Scheme 20. Synthesis of 2,4,6-trisubstituted pyridine-based putative inhibitors of GlcN-6-P synthase, according to Kenchappa et al.Citation84
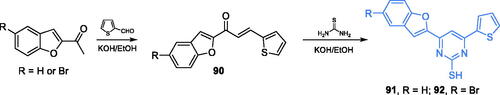
Pyrimidine-based trisubstituted derivatives with good antibacterial and antifungal activity were synthesised and biologically evaluated by Venkatesh and co-workersCitation86. The inhibitors were prepared in two-step synthesis depending on the formation of chalcone 90 by aldol condensation of appropriately substituted 2-acetylbenzofuran with thiophene carbaldehyde, followed by cyclisation reaction with thiourea (Scheme 21)Citation86. Compounds 91 and 92 exhibited MICs in the 12–18 µg/mL range, in comparison to 17–19 µg/mL found for streptomycin. Evaluation of antifungal potency revealed MIC values in the 10–15 µg/mL range, compared to 17–18.5 µg/mL for fluconazole. Derivatives 91 and 92 showed the lowest binding energy (−5.77 and −5.29 kcal/mol) to the GAH domain active site (pdbid: 1xff) and ability to H-bonding with catalytic Cys1 and Trp74 residues, thus suggesting their potential as relatively good inhibitors of this enzymeCitation86.
Scheme 21. Synthesis of trisubstituted pyrimidine-based putative inhibitors of GlcN-6-P synthase, according to Venkatesh et al.Citation86
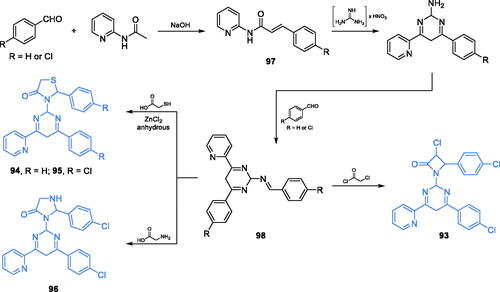
2,4,6-trisubstituted-1,3-diazines 93–96 (Scheme 22) were reported by Bakr and co-workers as promising antimicrobial agentsCitation87. Synthesis of these compounds started from condensation of an appropriately p-substituted benzaldehyde and 2-(acetylamino)pyridine that gave the α,β-unsaturated product 97. The cyclisation accomplished with 97 and guanidine nitrate gave diazine scaffold, which was subsequently subjected to reaction with benzaldehyde derivative, thus leading to imine 98. The imine was the starting material for three different cyclisation reactions. Condensation of 98 with 1-chloroacetic acid chloride resulted in the formation of an azetidine-2-on ring of inhibitor 93, while reactions with 1-mercaptoacetic acid and glycine gave five-membered rings of inhibitors 94–96, respectively (Scheme 22)Citation87. Derivative 93, possessing azetidine-2-one substituent, occurred to be the most potent antibacterial agent. On the other hand, the highest antifungal activity, comparable to that of amphotericin B, was observed for compounds 94–96 bearing thiazolidine-4-one and imidazoline-4-one substituents. Molecular docking studies based on the GAH domain of GlcN-6-P synthase (pdbid: 1xff) revealed the possible formation of H-bonds with Gly99 and arene-arene interaction with Trp74 located at the active centre. The binding energies for 93, 95, and 96 were −13.06, −16.89, and −15.76 kcal/mol, respectivelyCitation87.
Scheme 22. Synthesis of 2,4,6-trisubstituted 1,3-diazine-based potential inhibitors of GlcN-6-P synthase, according to Bakr et al.Citation87
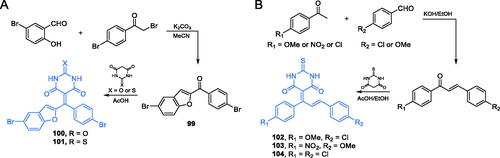
A series of barbiturate- and thiobarbiturate-based derivatives containing benzofuran moieties were obtained and characterised by Kenchappa and co-workersCitation88. The synthesis of two compounds (100 and 101) was accomplished by Knoevenagel condensation of appropriate methanone derivative 99 with barbituric or thiobarbituric acid (Scheme 23A)Citation88. It is noteworthy, that the structural analogues of 100 and 101, incorporating Meldrum’s acid, instead of barbitone moiety, were also synthesised and biologically evaluated by the research group of KenchappaCitation89. Compounds 100 and 101 displayed good antibacterial and antifungal activity, with MIC values ranging from 12.5 to 32 µmol/L. Further molecular docking studies on GlcN-6-P synthase (pdbid: 2vf5) revealed the protein-ligand interaction, depending on H-bond formation, with amino acid residues at the ISOM domain active site. In that simulations, compound 101 was found to interact with Gln348, Ser349, and Thr352 residues, with Ki = 280.61 µM and binding energy of −4.85 kJ/mol, while 100, exhibiting estimated Ki = 229.07 µM and binding energy of −5.27 kJ/mol), formed H-bonds with Gln348 and Thr352 residuesCitation89.
Scheme 23. Synthesis of barbiturate- and thiobarbiturate-based possible GlcN-6-P synthase inhibitors, according to Kenchappa et al.Citation88,Citation90
Kenchappa et al. obtained also some chalcone derivatives of thiobarbituratesCitation90. These compounds were obtained analogously to the previous ones, using the Knovenagel condensation (Scheme 23B)Citation90. The biological evaluation of compounds 102–104 demonstrated some antimicrobial activity. Their MIC values for antibacterial action ranged from 28 to 37 µg/mL, compared to 25–27 µg/mL of the standard streptomycin. In the case of antifungal evaluation, MIC values of 23–42 µg/mL against three fungal strains have been obtained, in comparison to 20–24 µg/mL found for griseofulvin as a standard antifungal drug. In silico experiments accomplished on GlcN-6-P synthase (pdbid: 2vf5) exhibited that molecules 102 and 104 were supposed to form hydrogen bonds with Gln348 and Ser349 residues at the active site of the ISOM domain. The inhibition constants for 102 and 104 were estimated as 589.5 and 487.8 µM, respectively, and their binding energies were −4.41 and −4.52 kJ/molCitation90.
6.3. Inhibitors based on indene scaffolds
Aswathanarayanappa et al. proposed the synthesis of substituted 5-phenyl-1-benzofuran-2-yl derivativesCitation91. The starting material was a benzofuran derivative 105, which was prepared from 2-bromo-1–(4-bromophenyl)ethanone and 5-bromo-2-hydroxybenzaldehyde. Under basic conditions, a nucleophilic attack occurred on the aldehyde moiety, followed by cyclisation to form a benzofuran ring. The resulting compound 105 was subsequently treated with a boron compound in the presence of a tetrakis-(triphenylphosphine) palladium catalyst. Substitution of bromide atoms by introduced aryl or alkyl residues in aromatic rings was obtained via Suzuki coupling reaction, resulting in inhibitor 106 (Scheme 24A). Compound 106 appeared to be the most promising antimicrobial agent, thus it was active against Staphylococcus aureus (MIC of 1 µg/mL), B. subtilis (MIC of 10 µg/mL) and C. albicans (MIC of 10 µg/mL). Molecular docking studies (pdbid: 2vf5) showed possible binding of 106 to the active site of GlcN-6-P synthase ISOM domain, with an inhibition constant of 8.09 µMCitation91.
Scheme 24. (A) Synthesis of benzofuran-2-yl derivative-based potential inhibitor of GlcN-6-P synthase, according to Aswathanarayanappa et al.Citation91 (B) Synthesis of β-amino carbonyl derivatives of benzofuran as potential GlcN-6-P synthase inhibitors, according to Kenchappa et al.Citation92
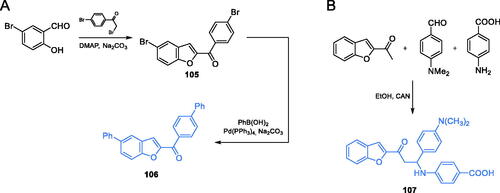
Other benzofuran-derived compounds were proposed by Kenchappa and co-workersCitation92. The synthesis was based on the known method developed by Vijesh et al.Citation85 Compound 107 was obtained by the one-pot, three-component reaction between p-substituted benzaldehyde, p-substituted aromatic amine and 1–(1-benzofuran-2-yl) ethenone, in the presence of cerric ammonium nitrate (CAN) as a catalyst. CAN showed the best catalytic properties among all Lewis acids (ZnCl2, CuCl2, AlCl3, FeCl3, LaCl3, InCl3) proposed in this study. All products were obtained with good yields, on average 81% (Scheme 24B)Citation92. The docking studies presented the benzofuran derivative 107 as a good inhibitor of GlcN-6-P synthase. That compound was docked to GlcN-6-P synthase (pdbid: 2vf5) matrix and showed to form hydrogen bonds with non-crucial Ser347 and Cys300 at the active site of the ISOM domain, with an inhibition constant of 22.19 µMCitation92. The MIC values of 107 in the evaluation of its antimicrobial potential ranged between 40 and 50 µg/mL for all tested microorganisms (three bacterial and three fungal species), compared to those of the standard drugs, fluconazole (40 µg/mL) and streptomycin (30 µg/mL).
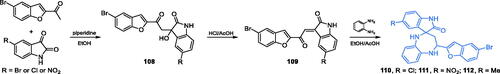
In another work, Kenchappa and co-workers characterised twelve spiro-derivatives of diazepine heterocycle, incorporating benzofuran- and indole-based moietiesCitation93. The proposed four-step synthesis started with an aldol reaction between 2-acetyl-5-bromobenzofuran and appropriately substituted isatin. Condensation product 108 was subsequently dehydrated under acidic conditions of HCl giving α,β-unsaturated derivative 109. Eventual intramolecular cyclisation with four reaction centres took place between 109 and 2-aminoaniline and gave the final products 110–112 (Scheme 25)Citation93. Compounds 110–112 displayed moderate antimicrobial activity when tested against four bacterial and four fungal strains. The results showed that heterocycles 110–112 were as good antibacterial agents as the standard drug ciprofloxacin and displayed MIC values ranging from 0.3 to 0.2 mg/mL. Antifungal activity of tested derivatives was similar to that of the standard fluconazole. The molecular docking of 110–112 to GlcN-6-P synthase (pdbid: 2vf5) demonstrated that those molecules interacted with residues Gly301, Cys300, Val399, and Ala602 of the ISOM domain active site. The estimated binding energies for 110 and 111 were −6.9 and −8.1 kcal/mol, respectivelyCitation93.
Scheme 25. Synthesis of benzodiazepine-based potential inhibitors of GlcN-6-P synthase, according to Kenchappa et al.Citation93
Novel imidazo[4,5-c]pyridine derivatives were synthesised and described by Jose et al.Citation94 Synthesis of imidazo[4,5-C]pyridine derivative 113 started from the halogenation of 4-amino-2-chloro pyridine with ICl in the presence of glacial acetic acid and potassium acetate solution. From the resulting mixture of iodopyridines, 4-amino-2-chloro-5-iodo pyridine was separated. The subsequent nitration reaction resulted in pyridine derivative 114, which was then treated with piperidine carboxamine, where the chlorine atom was substituted with the piperidine derivative. In the next step, the iodine group was replaced via Suzuki reaction in the presence of potassium carbonate as a required base and [PdCl2(dppf)]CH2Cl2 complex as a catalyst. The nitro group of compound 115 was reduced to diamine 116 and then cyclized to final product 113. The final step was carried out in the presence of DBU and T3P under microwave irradiation with the use of furan-2-carboxylic acid, which caused the formation of an imidazole ring and introduced a furan-2-yl substituent (Scheme 26)Citation94. Five out of twelve obtained imidazole derivatives showed moderate to good antimicrobial activity. Derivative 113 inhibited growth of Gram-negative bacteria E. coli (IC50 = 74.5 µg/mL) and that of S. cerevisiae yeast (IC50 = 99 µg/mL). Molecular docking to the GlcN-6-P synthase matrix (pdbid: 1jxa) showed the interaction between 113 as a ligand and His77 at the active site of the GAH domain, with Ki = 4.96 nMCitation94, surprisingly low, assuming the only interaction with a single His residue.
Scheme 26. Synthesis of imidazo[4,5-C]pyridine-based potential GlcN-6-P synthase inhibitor, according to Jose et al.Citation94 and its predicted binding mode to the GAH active centre of GlcN-6-P synthase; H-bonds are shown by dashed lines.
![Scheme 26. Synthesis of imidazo[4,5-C]pyridine-based potential GlcN-6-P synthase inhibitor, according to Jose et al.Citation94 and its predicted binding mode to the GAH active centre of GlcN-6-P synthase; H-bonds are shown by dashed lines.](/cms/asset/5f4fda1a-d3d1-4747-93eb-1446acb8c1bf/ienz_a_2096018_sch0026_c.jpg)
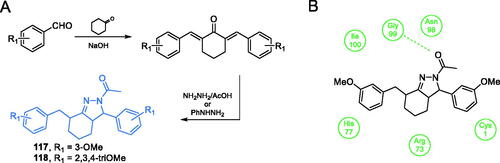
Kumar et al. proposed the synthesis of curcumin derivatives, antimicrobial properties of which were explained by their inhibitory action towards GlcN-6-P synthase. All compounds were obtained through Claisen-Schmidt condensation of substituted benzaldehydes with cyclohexanone in the presence of a base, followed by reflux with appropriate hydrazine derivatives (Scheme 27)Citation95. Kumar’s work was based on previous results reported by Minu et al.Citation96 about the synthesis of 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives. Those authors observed enhanced biological activity of compounds that contained electron withdrawing groups, such as halogen groups, at the third position of hexahydroindazole. Kumar et al. analogously proposed a series of novel hexahydro indazole derivatives of curcumin with various substituents on the aryl ring. Antimicrobial activities of compounds 117 and 118 (Scheme 27), determined against Gram-positive and Gram-negative human pathogenic bacteria (E. coli, P. aeruginosa, S. typhimurium and S. aureus) and fungal microorganisms of the Candida spp. was promising. In molecular docking studies, both compounds are bound to the active site of the GAH domain. Compound 117 is supposed to interact with Gly99, with Ki = 0.045 µM and 118 interacted with Arg73 and His77, with Ki = 8.57 µMCitation95.
Scheme 27. (A) Synthesis of curcumin derivatives as potential inhibitors of GlcN-6-P synthase, according to Kumar et al.Citation95 (B) Predicted binding mode of compound 117 to the GAH domain of GlcN-6-P synthase; H-bonds are shown by dashed lines.
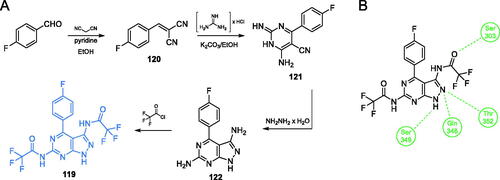
Khan et al.Citation97 proposed compound 119 (Scheme 28), which exhibited good activity against S. aureus, Streptococcus pyogenes, S. typhimurium, and E. coli. In the synthetic approach for bicyclic aromatic system, the pyrimidine ring was obtained in the first step. To achieve that, p-fluorobenzaldehyde and malononitrile were condensed, resulting in alkene 120, in which the nitrile moieties served as electrophiles in cyclisation reaction with guanidine hydrochloride, giving the heterocyclic ring of 121. Subsequently, another cyclisation was performed, using hydrazine as a double nucleophile. The eventual acylation of primary amine groups of 122 with trifluoroacetic acid chloride resulted in the final inhibitor 119 (Scheme 28)Citation97. The MIC values of this compound against bacteria ranged from 16 to 32 µg/mL when compared to that of the standard drug, chloramphenicol (32 µg/mL). Docking studies performed on bacterial GlcN-6-P synthase (pdbid: 2vf4) revealed the ability of 119 to bind to the ISOM domain active site by interaction with Ser303, Ser349, Gln348 and Thr352 residues, with the binding energy of −7.3 kcal/molCitation97.
Scheme 28. (A) Synthesis of fluorine-substituted pyrazolopyrimidine-based putative inhibitor of GlcN-6-P synthase, according to Khan et al.Citation97 (B) Presumable binding mode of 119 to GlcN-6-P synthase at the ISOM active site; H-bonds are shown by dashed lines.
Satyendra et al. described the synthesis, molecular docking studies and biological properties of 5,7-dichloro-1,3-benzoxazole-2-thiol derivativesCitation98,Citation99. The synthesis started with the formation of ethyl [(5,7-dichloro-1,3-benzoxazole-2-yl)sulfanyl]acetate 124 due to the reaction of commercially available 5,7-dichloro-1,3-benzoxazole-2-thiol 123 with ethyl chloroacetate (Scheme 29, path A). The resulting compound is treated with hydrazine to obtain 5,7-dichloro-2-hydrazino-1,3-benzoxazole 125, which subsequently reacted with carbon disulphide in the presence of a strong base, resulting in compound 126. Compound 126 has two tautomeric forms 126a and 126b and due to this property, two routes of further transformations were taken: acylation of a thiol group or substitution of the nitrogen atom in the newly obtained ring (Scheme 29, path A)Citation98,Citation99. All final compounds and some intermediates were screened for antimicrobial activity and potential inhibition of GlcN-6-P synthase. Compound 127a emerged as the most active against all tested microorganisms and exhibited good antimicrobial activity, with MIC values of 3.125 µg/mL, compared to MIC of 3.125 µg/mL obtained for ciprofloxacin. Molecular docking to GlcN-6-P synthase (pdb: 1gdo) revealed that 127a possibly binds at the active site of the GAH domain by interaction with Cys1 (Ki = 1.04 µM)Citation99.
Scheme 29. Synthesis of 5,7-dichloro-1,3-benzoxazole-2-thiol derivatives as potential inhibitors of GlcN-6-P synthase, according to Satyendra et al. (path A)Citation98,Citation99. Synthesis of 5,7-dichloro-1,3-benzoxazole-2-yl derivatives as potential inhibitors of GlcN-6-P synthase according to Jayanna et al. (path B)Citation100.
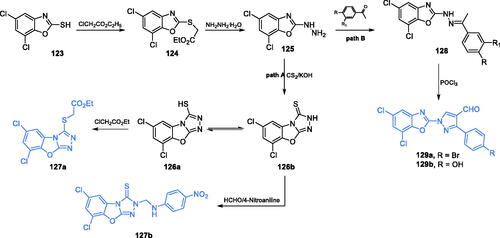
Benzoxazole ring was also an integral part of compounds, whose synthesis was proposed and described by Jayanna et al.Citation100 Novel derivatives of benzoxazole were linked to pyrazole moiety containing an aldehyde group. The first step of synthesis was imine formation between 5,7-dichloro-2-hydrazino-1,3-benzoxazole 125 and appropriate 3,4-disubstituted acetophenone. The resulting compound 128 was subjected to a Vilsmeier-Haack reaction with POCl3 in DMF, to obtain five final products, including 129 (Scheme 29, path B)Citation100. Compounds 129a and 129 b emerged as the most promising inhibitors of GlcN-6-P synthase, due to good antimicrobial activities (MIC values of 20–30 µg/mL for all strains for both compounds) and results of molecular docking to the target enzyme (pdbid: 2vf5). Compound 129a formed hydrogen bonds with Thr352, with Ki = 280.61 µM and the binding energy of −4.85 kJ/mol, while 129 b (binding energy of −8.19 kJ/mol) interacted with Thr352 and Glu488, at the active site of ISOM domainCitation100.
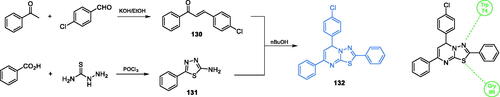
Efficient synthesis and promising antimicrobial activities of 5,7-disubstituted-2-phenyl-5H-[1,3,4]-thiadiazolo[3,2-a]pyrimidine derivatives were reported by Venkatesh and co-workersCitation101. The synthesis of the final compounds called for the preparation of chalcone 130 and thiadiazole 131Citation102. The chalcone was obtained by an aldol reaction between acetophenone and p-chlorobenzaldehyde. The single-step reaction was also applied for the production of thiadiazole 131, which was synthesised by condensation of benzoic acid and hydrazinecarbothioamide. Eventual conjugation of 130 and 131 connected with cyclisation reaction gave the final inhibitor 132 (Scheme 30)Citation101,Citation102. The most potent antimicrobial derivative 132 exhibited good activity against both bacterial and fungal cells. The MIC value in antibacterial tests ranged between 18 and 20 µg/mL, which was comparable to that of the standard drug, ciprofloxacin (20–23 µg/mL). Evaluation of the antifungal activity of 132 showed MIC values in the 25–28 µg/mL range, which was comparable to that of the standard fluconazole (30–32 µg/mL). Molecular docking simulations accomplished on the GAH domain of GlcN-6-P synthase (pdbid: 1xff) showed that derivative 132 could interact via H-bonding with Trp74 and Gly99 residues of the GAH domain active site, with the binding energy estimated as −10.1 kcal/molCitation101.
Scheme 30. Synthesis of a potential GlcN-6-P synthase inhibitor, according to Venkatesh et al.Citation101,Citation102 and its predicted binding mode to the GAH domain; H-bonds are shown by dashed lines.
6.4. Inhibitors based on naphthalene-based scaffolds
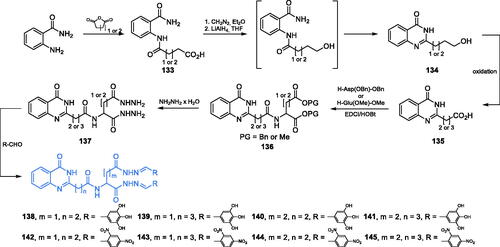
The preparation of a series of eight potent antimicrobial agents based on a quinazolinone structure was reported by Kumara and co-workersCitation103. The quinazolinone scaffold of the inhibitors was obtained from 2-aminobenzamide and succinic or glutaric anhydride. Condensation of mentioned resulted in diamide 133, which after esterification with diazomethane, followed by reduction with LiAlH4 and aqueous workup, gave the desired quinazolinone scaffold 134Citation104. Oxidation to carboxylic acid 135 and amide bond formation with appropriately protected aspartic or glutamic acid (EDCI/HOBt technique) led to diester 136, which was subsequently aminolysis to 137 with hydrazine. Eventually, conjugation with appropriate aromatic aldehyde resulted in final compounds 138–145 (Scheme 31)Citation103. The obtained derivatives exhibited promising antibacterial and antifungal activities in disc diffusion tests. Molecular docking studies on the GlcN-6-P synthase matrix (pdbid: 2vf5) showed that the proposed compounds interacted with the ISOM domain active site, including Glu488, Ala602, Ser401, Gln348, Ser303, Gly301 and Thr352 residues. The docking scores obtained for 138–145 ranged between −8.969 and −12.238100.
Scheme 31. Synthesis of quinazolinone-based putative inhibitors of GlcN-6-P synthase, according to Kumara et al.Citation103
Coumarin is a natural compound known for its broad spectrum of biological and pharmacological activities, such as antifungal, antioxidant, antibacterialCitation105,Citation106 or anticancerCitation106 properties. Over the years, many pharmacologically potent derivatives based on the coumarin structure were proposed. Kenchappa et al.Citation92 described the synthesis of some novel coumarin derivatives via three component Mannich reaction in the presence of CAN as a catalyst. The reaction occurred between 3-acetyl coumarin 146 (Scheme 32A, path A), 4-chlorobenzaldehyde and 4-aminobenzoic acid. Evaluation of antifungal properties of thus obtained compound 148 revealed its good inhibitory effect on Aspergillus flavus and Chrisosporium keratinophilum growth, with MIC of 40 µg/mL. Molecular docking to GlcN-6-P synthase (pdbid: 2vf5) confirmed, that 148 had an ability of binding to the active centre of ISOM domain of the target enzyme via hydrogen bonds with Ser347 and one hydrogen bond with Cys300 (estimated Ki = 22.19 µM)Citation92.
Scheme 32. (A) Syntheses of coumarin based potential inhibitors of GlcN-6-P synthase, according to Kenchappa (path A)Citation92, Kumar (path B)Citation107 and Helmy et al. (path C)Citation108. (B) Synthesis of 4-chromone-based inhibitor of GlcN-6-P synthase, according to Devi et al.Citation109
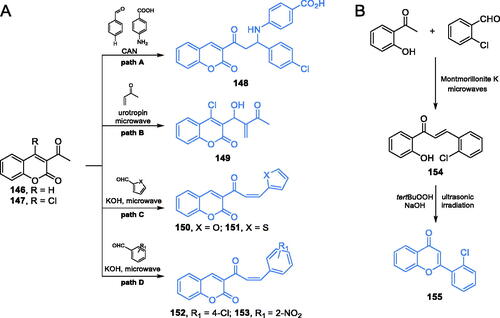
Another potential GlcN-6-P synthase inhibitor based on the coumarin derivatives was proposed by Kumar et al.Citation107 The authors described the synthesis of Baylis-Hillman adducts of coumarin, according to which a coumarin derivative 147 (Scheme 32A, path B) reacted with an appropriate alkene in the presence of 5 mol% of urotropine as a catalyst. Moreover, all compounds were prepared by the use of ionic liquids [BMIM]BF4 and [BIPIM]BF4 and under microwave irradiation. Ionic liquids, aside from being green chemical solvents, also played a catalytic role. The synthesised compound 149 was supposed to be a good GlcN-6-P synthase inhibitor, based on the results of molecular docking studies and antibacterial activity evaluation. This compound exhibited some growth inhibitory effect against Gram-positive bacteria S. aureus and B. subtilis. Molecular docking simulations of 149 interactions with GlcN-6-P synthase (pdbid: 2vf5) exhibited interactions with Cys300, Thr352, Gly301, Val605, Ser604, Lys603, and Ala602 residues at the active centre of the ISOM domain, with a binding energy of −4.747 kcal/molCitation107.
The microwave method in the synthesis of coumarin derivatives was also applied by Helmy et al.Citation108 as a greener approach in organic synthesis, compared to the conventional method. The one-step synthesis involved a reaction between 3-acetyl coumarin 147 and an appropriate carbonyl compound. Due to the presence of the strong base, an aldol condensation occurred, resulting in products 150–151 and 152–153Citation108. In vitro growth, inhibitory activity against four selected strains (Gram-positive bacteria S. aureus and B. subtilis and Gram-negative bacteria E. coli and Proteus vulgaris) revealed that compound 153 (Scheme 32A, path D) emerged as the most active, especially against E. coli (MIC = 98 µg/mL, compared to amikacin, MIC = 121 µg/mL). Coumarin derivative 151 (Scheme 32A, path C) also showed good activity against all the tested microorganisms and was found to be the most active against B. subtilis (MIC of 123 µg/mL, compared to amikacin, MIC = 98 µg/mL). Both compounds showed the lowest docking scores (−13.9 for 151 and −17.72 for 153) and interaction with Ala602 at the active site of the ISOM domain (pdbid: 2vf5)Citation108.
Devi and co-workersCitation109 reported a 4-chromone-based compound 155 (Scheme 32B) as an antimicrobial agent. According to the authors, the synthesis of 155 involving microwave and ultrasonic irradiation should be considered a “green” one. The chalcone 154, obtained from 2-hydroxyacetophenone and 2-chlorobenzaldehyde in the presence of Montmorillonite K under microwave irradiation, was cyclised to 4-chromone 155 in an alkaline environment of NaOH and the presence of tert-butyl hydroperoxide under ultrasonic irradiation (Scheme 32B)Citation109. In a disc diffusion test, derivative 155 exhibited antifungal activity, comparable to that of the standard drug, fluconazole. Molecular docking studies on GlcN-6-P synthase (podbid: 2vf5) showed that 155 interacted with the ISOM domain active site (docking score −89.68) via hydrogen bond formation with Glu384, Ser349 and Lys603 residuesCitation109.
Promising antimicrobial activity was observed for bicyclic 156 and tricyclic 157 derivatives (Scheme 33) incorporating pyridine- and indole-based scaffolds. According to Elkanzi and co-workersCitation110, the synthesis of 156 and 157 started with condensation of 3,4-dihydro-2H-pyran, diethyl malonate and acetamide, which resulted in the production of a nitrogen-containing ring of derivative 158. Subsequent basic hydrolysis gave carboxylic acid 159, which was decarboxylated to 160 by heating in diphenyl ether. Chlorination of 160 with POCl3 followed by a substitution reaction with phenylhydrazine gave inhibitor 156 that underwent the coupling reaction with ethyl propiolate followed by cyclisation to inhibitor 157 (Scheme 33)Citation110. Compound 156 which was an intermediate in the synthesis of 157 exhibited comparable antifungal activity as that showed by the standard drug ketoconazole towards Candida sp. In the disc diffusion test, derivative 157 occurred to be an effective antibacterial agent, with the activity comparable with that of the standard ampicillin. Molecular docking experiments accomplished on the GAH domain of GlcN-6-P synthase matrix (pdbid: 1xff) revealed that both derivatives 156 and 157 could bind at the GAH active site by hydrogen bonding with Gly99 for 156 (binding energy −20.52 kcal/mol) and Thr76 and His97 in the case of derivative 157 (binding energy −19.4 kcal/mol)Citation110.
Scheme 33. Synthesis of potential GlcN-6-P synthase inhibitors, according to Elkanzi et al.Citation110
6.5. Complexes of d and f block elements
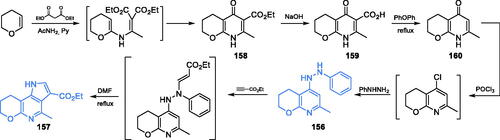
Transition metal complexes of some organic compounds were reported as antifungal, antibacterial, analgesic or anti-inflammatory agentsCitation111,Citation112. Ebrahimipour et al. characterised an antimicrobial activity of uranyl(VI) Schiff base complexes and suggested this activity as a consequence of GlcN-6-P synthase inhibitionCitation113. The synthesis of complex 162 started with the formation of ligand 161 which was obtained by condensation of 1,2-diaminobenzene with 5-bromo-2-hydroxybenzaldehyde. Ligand 161 was subjected to complexation with a methanolic solution of UO2(OAc)2×2H2O, giving target uranyl complex 162 (Scheme 34A)Citation113. Complex 162 (Scheme 34A) exhibited some activity against Gram-negative bacteria, with MIC values of 0.937–3.75 mg/mL, and against C. albicans (MIC = 7.5 mg/mL). In silico study done on GlcN-6-P synthase matrix (pdbid: 2vfc)revealed that the uranyl(VI) complex 162 may be considered a good inhibitor of GlcN-6-P synthase, assuming its binding at the active site of ISOM domain, with a binding energy of −8.9 kcal/molCitation113.
Scheme 34. Syntheses of: (A) uranyl(VI) complex; (B) vanadium(V) complex; (C) cobalt(II) complex with antimicrobial activity, according to Ebrahimipour et al.Citation113–115
The same research group also synthesised and evaluated the antimicrobial activity of three tridentate oxido-vanadium(V) complexes, including 164 (Scheme 34B). The synthesis of the ligand for complex 164 was a one-step reaction depending on the condensation of benzohydrazide and 2-hydroxynaphthaldehyde in ethanol, resulting in hydrazone 163. The final addition of VO(acac)2 in methanol to 163 gave the final complex 164 (Scheme 34B)Citation114. In vitro experiments showed that all complexes exhibited significant activity against both Gram-positive and Gram-negative bacteria, as well as against methicillin-resistant S. aureus (MIC = 19.5 µg/mL). Docking studies proceeded on GlcN-6-P synthase matrix (pdbid: 2vf5) suggesting that complex 164 should be a good inhibitor of this enzyme, binding at the ISOM domain. The inhibition constant and binding energy were estimated as 123.33 µM and −5.33 kcal/mol, respectivelyCitation114.
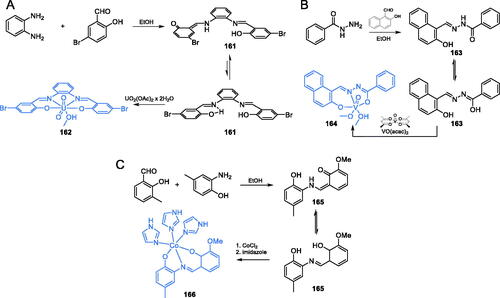
In another work, Ebrahimipour et al.Citation115 reported novel cobalt(III) complexes 166 incorporating imidazole and 2-((3-methoxy-2-oxidobenzylidene)amino)-4-methylphenolate ligand (Scheme 34C). Synthesis of 165 was accomplished by condensation of 2-hydroxy-3-methoxybenzaldehyde and 2-amino-4-methylphenol. Subsequent sequential complexation with CoCl2 and imidazole resulted in target complex 166 (Scheme 34C)Citation115. The obtained complex 166 was tested against some Gram-negative and Gram-positive bacteria as well as against human pathogenic yeasts C. albicans. Complex 166 exhibited significant activity against all tested strains, with MIC values ranging between 0.78 and 3.125 mg/mL. Molecular docking studies to GlcN-6-P synthase (pdbid: 2vf5) revealed binding of 166 to the ISOM domain active site via interactions with Cys300, Gly301, Leu484, Leu601, Glu488, and Lys487 residues, with a binding energy of −6.69 kcal/molCitation115.
Palladium(II) and platinum(II) complexes (168 and 169) of N-butyl-N-phenyldithiocarbamate (Scheme 35A) were reported by Onwudiwe et al.Citation116 as potential GlcN-6-P synthase inhibitors. The synthesis of target complexes required for N-butyl-N-phenyldithiocarbamate ligand 167, which was obtained as ammonium salt from N-butylaniline and carbon disulphide in ammonia condition. Subsequent treatment of ligand 167 with Na2PdCl4 or K2PtCl4 in aqueous media led to desired palladium and platinum complexes 168 and 169 (Scheme 35A)Citation116,Citation117. The antimicrobial activity of the mentioned complexes was tested against two bacterial (E. coli and S. aureus) and two fungal (A. flavus and Fasiparium exosporium) strains. The results showed that both palladium and platinum complexes exhibited some antifungal activity (MIC = 50–100 µg/mL), comparable to that of ketoconazole (MIC = 65–80 µg/mL). In silico docking experiments on GlcN-6-P synthase (pdbid: 2vf5) revealed potential strong binding interactions with the ISOM domain active site by interaction with Thr302, Gln348, Ser303, Ala400, and Val605 residues. The binding energies for 168 and 169 were estimated as −6.113 and −6.54 kcal/mol, respectivelyCitation116.
Scheme 35. Synthesis of metal complexes, potential GlcN-6-P synthase inhibitors, according to (A) Onwudiwe et al.Citation116 and (B) Wang et al.Citation118
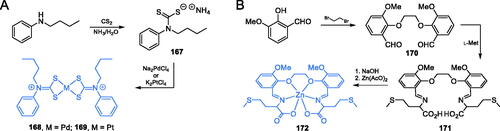
The research group of Wang proposed three zinc(II) complexes with a sexidentate bis-Schiff base ligandCitation118. The ligand 171 was prepared from 2-hydroxy-3-methoxybenzaldehyde, 1,2-dibromoethane and l-methionine in two synthetic steps. Firstly, the benzaldehyde derivative was condensed with 1,2-dibrompethane resulting in dialdehyde 170 and then imine bond formation was accomplished with l-methionine leading to ligand 171. The target complex 172 was obtained by the addition of zinc acetate to the alkaline solution of ligand 171 (Scheme 35B)Citation118. Obtained complexes were tested against E. coli and S. aureus by the agar-well diffusion method. Complex 172 (Scheme 35B) exhibited good antimicrobial activity against both bacterial strains with the average diameter of the inhibition zone comparable to that of ampicillin. The docking simulation accomplished on the GlcN-6-P synthase matrix (pdbid: 2vf5) showed that complex 172 was well embedded into the active site of the ISOM domain and interacted via H-bonding with Thr302 and Val605 residues, with the binding energy estimated as −9.97 kcal/molCitation118.
7. Inhibitors binding outside the active centres of GlcN-6-P synthase
One of the two products of the reaction catalysed by GlcN-6-P synthase, namely d-glucosamine-6-P, is a natural inhibitor of the bacterial version of this enzyme. On the other hand, eukaryotic GlcN-6-P synthase is a subject of feedback inhibition by UDP-GlcNAcCitation18. The UDP-GlcNAc binding site is localised at ISOM domain but outside the active centreCitation119. Other binding sites for potential inhibitors are the intramolecular channel and the contact areas between two dimers constituting a tetrameric structure of the eukaryotic GlcN-6-P synthase.
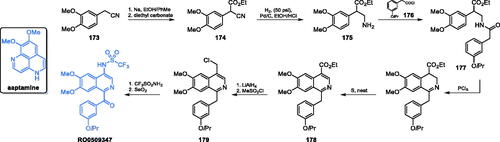
Aaptamine (Scheme 36), a heterocyclic compound isolated from the sea sponge Aaptos aaptos, was found to be an inhibitor of GlcN-6-P synthase, with ICS0 = 120 µMCitation120. Several aaptamine derivatives exhibited antifungal activityCitation121. A much stronger inhibitor of human GlcN-6-P synthase, compound RO0509347 (IC50 = 1 µM) resulted from the high throughput screening and subsequent hit identification and optimisation at Hoffman La-Roche Inc. That compound demonstrated significant efficacy in reducing glucose excursion in oral glucose tolerance tests in diet-induced obesity miceCitation122. Synthesis of that compound started with commercially available (3,4-dimethoxy)acetonitrile 173, which underwent base-catalysed condensation with diethyl carbonate, resulting in α-cyanoester 174. Subsequent reduction of nitrile moiety with gaseous hydrogen on Pd/C catalyst under elevated pressure gave amine 175, which after acylation with acyl chloride 176, resulted in amide 177. The formation of the isoquinoline ring of 178 was accomplished in the Bischler-Napieralski manner by treatment of 177 with PCl5, followed by aromatisation reaction utilising neat sulphur. Then, the obtained isoquinoline ester 178 was converted to alkyl halide 179 in a two-step manner, depending on the reduction of ester moiety with LiAlH4, followed by the treatment with methanesulfonyl chloride. Subsequent substitution of chloride atom with trifluoromethanesulfonyl amide and oxidation with SeO2 gave the final compound RO0509347 (Scheme 36)Citation122.
Scheme 36. Structure of aaptamine and synthesis of RO0509347 developed at Hoffman La-Roche Inc.Citation122
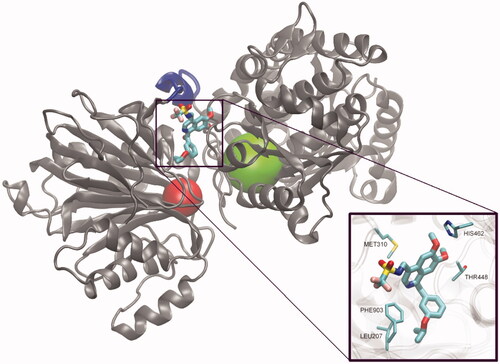
The binding site for RO0509347 at GlcN-6-P synthase has not been unequivocally identified. However, a molecular docking of this compound to the human GlcN-6-P synthase (hGFAT2) matrix, results of which are shown in , revealed the cavity located in the vicinity of the intramolecular channel connecting GAH and ISOM domains as the most probable binding site. A putative binding site is located in a narrow cleft between the two domains near the interdomain linker. Since the precise interdomain communication is crucial for the catalysis, despite the fact that the bound inhibitor does not directly interfere with any of the enzyme active sites, binding the ligand at this site should hinder the interdomain communication and thus disturb catalytic reaction. In the bound ligand conformation, the polar sulphonamide group sticks out of the cleft to the aqueous surrounding, the isoquinoline moiety participates in the favourable MET-π interactions, while the aliphatic part of isopropoxy moiety is trapped in a small hydrophobic pocket formed by Phe903 and Leu207. This pose and interactions of the bound ligand correlate well with the SAR data for this group of compoundsCitation122.
Figure 2. The hypothetical complex of RO0509347 with human GlcN-6-P synthase. Drawing based on the results of docking calculations to the hGFAT2 matrix (pdbid: 6r4f)Citation123, performed with the use of Autodock 4.2, according to the procedure described previouslyCitation124. A single subunit of the tetrameric enzyme is shown, with GAH and ISOM active centres indicated as red and green spheres respectively. A flexible linker joining both domains of the enzyme is coloured blue.
7.1. Inhibitors based on five-membered ring scaffolds
Khan and co-workersCitation125 described trisubstituted pyrazole-based potential inhibitors of GlcN-6-P synthase. The microwave-assisted synthesis was applied to the production of final inhibitors, as well as intermediate chalcone 180, which was obtained by aldol reaction between 3-acetyl-2,5-dimethylthiphene and 9-ethyl-9H-carbazole-3-carbaldehyde. The eventual formation of a 1,2-diazole-based ring by cyclisation reaction with appropriate hydrazine derivative resulted in final inhibitors 181 and 182 (Scheme 37A)Citation125. Derivatives 181 and 182 showed antibacterial (against S. aureus, S. pyogenes, S. typhimurium and E. coli) activity, with MIC values identical or twice the time lower that of the standard drug chloramphenicol (32 µg/mL). Molecular docking experiments accomplished on E. coli GlcN-6-P synthase (pdbid: 2vf4) revealed that the obtained compounds may bind to the enzyme ISOM domain outside the active site, via interaction with Asp474, Ser310, Trp312, Ala404 and Glu569 residues. Calculated binding energies for 181 and 182 were −8.5 and −9.2 kcal/mol, respectivelyCitation125.
Scheme 37. (A) Synthesis of 1,2-diazole-based inhibitors of GlcN-6-P synthase according to Khan et al.Citation125 (B) Synthesis of trisubstituted 1,2-diazole-based inhibitors of GlcN-6-P synthase according to Ebenezer et al.Citation126
A set of 1,2-diazol-based compounds were synthesised and biologically evaluated by Ebenezer and co-workersCitation126. The synthesis of inhibitors began with an initial condensation of phenylhydrazine and appropriately substituted acetophenone giving phenyl hydrazone 183, which subsequently underwent the Vilsmeier-Haack formylation followed by cyclisation, using a mixture of POCl3 and dimethylformamide. The obtained 1,2-diazole 184 was allowed to undergo a one-pot three-component reaction with 2-aminopyridine and phenylacetylene, catalysed by CuSO4/sodium ascorbate to produce the final putativeinhibitors 185–189 (Scheme 37B)Citation126. Those five derivatives exhibited good bactericidal activity against both Gram-positive and Gram-negative bacteria. Moreover, derivative 186 showed significant activity against MRSA, reaching the minimum bactericidal concentration (MBC) value of 2.5 µg/mL, compared to 1.84 µg/mL of the standard drug ciprofloxacin. The in silico investigations proceeded on GlcN-6-P synthase (pdbid: 1jxa) suggesting that antibacterial activity of 185–189 could be due to their binding outside the active sites of GlcN-6-P synthase, mainly by interaction with Arg21, Arg22, Glu24, Tyr251, and Ile397 residues. The binding energies for proposed compounds ranged between −9.5 and −10.5 kcal/molCitation126.
Sarojini et al. described the synthesis of new series of 2-substituted-4–(2,5-dichloro thienyl)-1,3-thiazolesCitation127 (Scheme 38) based on thiazole derivatives proposed by Narayana et al.Citation128, some of which showed excellent antifungal and antibacterial activity. The 2,5-dichloro substituted thienyl derivative was synthesised by the reaction of 2-bromo-1–(2,5-dichlorothien-3-yl) ethenone with 8-quinolyl substituted thioamide. Nucleophilic attack of the amino group of thioamide on ethenone derivative was followed by a second ring formation. An intermediate product was obtained through bromination of 1–(2,5-dichlorothien-3-yl) ethanone in acetic acid (Scheme 38)Citation127,Citation128. While most of the newly synthesised thienyl derivatives did not exhibit satisfactory antimicrobial activity, one of them −190 emerged as highly active against all tested microorganisms, with MIC values ranging between 6.25 and 12.5 µg/ml (6.25 µg/ml for ampicillin). Molecular docking to GlcN-6-P synthase matrix (pdbid: 1jxa) revealed that 190 may be a good inhibitor of this enzyme, as it is expected to bind to Gln451 residue outside the active site (estimated Ki = 0.957 µM)Citation127.
Scheme 38. (A) Syntheses of a possible GlcN-6-P inhibitor, according to Sarojini et al.Citation127,Citation128 and its predicted binding mode to GlcN-6-P synthase; H-bonds are shown by dashed lines.
Another triazole derivative was designed, synthesised and described by Krishna et al.Citation129 The proposed compound was synthesised in four steps manner, beginning with the conversion of 3-amino-2-bromo-5-chloropyridine to imine 191 by treatment with an aqueous solution of hydrochloric acid and sodium nitrite, followed by addition of ethyl 2-chloro acetoacetate and sodium acetate. Subsequent treatment of 191 with gaseous ammonia resulted in azaenol product 192, which was cyclized to final inhibitor 193 by condensation with 2,5-difluorobenzaldehyde (Scheme 39)Citation129. Compound 193 was subjected to in vitro, in vivo and in silico biological activity screening, including antibacterial, antiproliferative and anti-inflammatory activity determination. Derivative 193 emerged as an agent effective against all tested bacterial strains in disc diffusion tests, compared to the standard drug nitrofurazone. Molecular docking to GlcN-6-P synthase (pdbid: 1jxa) revealed that 193 could bind to the enzyme outside the active centres, with minimum docking energy of −175.9 kJ/molCitation129.
Scheme 39. Synthesis of trisubstituted 1,2,4-triazole as a potential inhibitor of GlcN-6-P synthase, according to Krishna et al.Citation129
Sarojini results were mentioned by Siwek et al.Citation130 in their work on 1,3,4-thiadiazole and s-triazole derivatives as potent GlcN-6-P synthase inhibitors. The synthesis of proposed structures was published in a previous work of the same group on s-triazoles as antibacterial agentsCitation131. The thiadiazole-based derivative was prepared using as a starting material 4-methyl-1,2,3-thiadiazole-5-carboxylic acid hydrazide 194 (Scheme 40). The reaction of the 194 with ethyl isothiocyanate gave thiosemicarbazide 195, which subsequently was treated with sulphuric acid to obtain compound 196. The proposed compound, exhibited some antimicrobial inhibitory effect, exclusively against Candida spp. Comparing the results of the biological activity with molecular docking studies, 196 may be considered a potential inhibitor of GlcN-6-P synthase. This derivative showed minimal binding energy of −1.7 kcal/mol in molecular docking at the GAH domain (pdbid: 1xff). Despite the fact that most of the prepared derivatives did not show antimicrobial effect, the presented results might be useful, for example as a reference set of inactive structures in the construction of QSAR modelsCitation130.
Scheme 40. Synthesis of the 1,3,4-thiadiazole derivative as a putative inhibitor of GlcN-6-P synthase, according to Siwek et al.Citation130
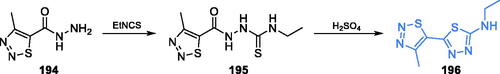
Recently Askri and co-workersCitation132 reported antimicrobial activity of some spiro-derivatives based on pyrrolidine scaffold. A three-component cascade reaction of (E)-3-arylidene-1-methylpyrrolidine-2–5-diones, l-valine and isatine derivatives, involving 1,3-dipolar cycloaddition was applied for the synthesis of 197 and 198 (Scheme 41)Citation132. Obtained compounds exhibited good activity (MIC = 3.9 µg/mL) against S. aureus, compared to that of the standard drug, tetracycline (24 µg/mL) and moderate activity against C. albicans (MIC = 78 µg/mL). Molecular docking experiments accomplished on GlcN-6-P synthase (pdbid: 1jxa) revealed that derivatives 197 and 198 interacted with several amino acid residues via H-bonding, including Asp192 and Glu351 in the case of 197 (docking score −15.33) and Pro377 in the case of 198 (docking score −13.4)Citation132.
Scheme 41. Synthesis of spiro pyrrolidine-based putative inhibitor of GlcN-6-P synthase, according to Askri et al.Citation132
7.2. Inhibitors based on six-membered ring scaffolds
Sowmya et al. proposed a synthesis of novel fluorinated pyridazinone derivativesCitation133. The synthesis involved microwave irradiation, which made possible application of the solvent free conditions. Synthesis of six 3-(2H)-pyridazinone derivatives started with grounding up 4–(3,5-difluorophenyl)-butanoic acid and an appropriate hydrazine hydrochloride derivative in the presence of a catalytic amount of acetic acid. The resulting solid was subsequently exposed onto a microwave initiator. The use of microwaves resulted in high yields, over 85% for all prepared compounds and the duration of each synthesis did not exceed 10 min. Moreover, solvent-free conditions made that synthesis more environmentally friendlyCitation133. Compounds 199 and 200 (Scheme 42A) were the most active against three bacterial strains (E. coli, B. cereus, S. aureus) compared to streptomycin, with diameters of the zones of inhibition obtained for 199 and 200 ranging between 65–76% and 54–65%, respectively, of that of the standard drug. The molecular docking studies confirmed that both compounds are potential inhibitors of GlcN-6-P synthase (PDB 1XFF). Compound 199 showed three interactions, with Pro198, Thr200 and Arg202, while 200 demonstrated interactions with Arg201 and Thr200 at GAH, outside the active centre of this domainCitation133.
Scheme 42. (A) Synthesis of fluorinated pyridazinone derivatives as a potential inhibitor of GlcN-6-P synthase, according to Sowmya et al.Citation133 (B) Synthesis of pyridazinone derivatives as a putative inhibitor of GlcN-6-P synthase, according to Nagle et al.Citation134
Another synthesis of compounds based on the pyridazinone ring was described by Nagle et al.Citation134 The proposed diazine derivatives contained a thymol group in their structure, which may contribute to the overall biological activity of the final compounds since thymol is known for a wide spectrum of biological propertiesCitation135,Citation136. The first step of the synthesis involved a nucleophilic attack of the thymol hydroxyl group on n-butyl bromide in the presence of a strong base and phase transition catalyst, TBABCitation137. The resulting ether 201 was subsequently treated with succinic anhydride and aluminium chloride with EDCI. The acylation of the aromatic ring was followed by esterification, which occurred for the newly introduced carboxyl moiety of 202, resulting in ester 203. Subsequently, pyridazinone ring was formed by the use of hydrazine hydrate and then the alkyl group was introduced on nitrogen atom in the presence of sodium hydride, affording the final compound 204 (Scheme 42B)Citation134. The obtained derivative exhibited relatively good antimicrobial activity. Molecular docking to GlcN-6-P (pdbid: 1jxa) revealed that 204 may bind at the ISOM domain, interacting with Glu534 and Glu79, outside the active centreCitation134.
7.3. Inhibitors based on naphthalene-based scaffolds
Preveena and co-workersCitation138 reported a series of naphthalene-based compounds with highly promising pharmacological properties. The proposed synthesis started from appropriate para-substituted acetanilide 204a-c, which underwent a cyclisation reaction in DMF/POCl3 conditions. The obtained quinoline derivatives 205a-c were subjected to Darzens condensation with 2,4-disubstituted-α-bromoketone 206a-b under mild basic conditions, resulting in final epoxides 207–209. In the case of 208 and 209, predominantly trans isomers were obtained, with small quantities of the corresponding cis isomers. Derivative 207 was obtained as an almost equimolar mixture of trans and cis isomers (Scheme 43, path B)Citation138. Obtained compounds exhibited good antibacterial activity against B. subtilis (207), E. coli (208), and S. aureus (209), comparable with that of the standard antibiotics (ofloxacin and ampicillin). Moreover, derivatives 208 and 209 showed the best antifungal activity, similar to that of the standard drug, fluconazole. Both cis and trans isomers of 207–209 exhibited similar antimicrobial activity. The docking experiments indicated good interaction of mentioned agents with the GlcN-6-P synthase GAH domain (pdbid: 1xff) outside the active site. The trans isomers created more hydrogen bonds with the GAH domain in comparison to the cis ones. In the case of trans isomers, H-bonding was observed with Arg201, Asp11, Gly66, Thr200 and Arg22 residues, while the cis ones created H-bonds with Glu14, Arg217, Gly66 and Asp11 residues. The binding energies for 207–209 ranged between −7.96 and −8.35 kcal/molCitation138.
Scheme 43. Synthesis of 2-chloroquinolin-3-yl ester derivatives as potential GlcN-6-P inhibitors, according to Tabassum et al. (path A)Citation139. Synthesis of quinoline-based epoxides, according to Preveena et al. (path B)Citation138.
Another series of quinoline derivatives were synthesised and described by Tabassum et al.Citation139 The novel 2-chloroquinolin-3-yl ester derivative 210 was obtained in a manner similar to that of 205a–c, using Veismeier-Haack cyclisation of appropriate acetanilide derivative to quinolone derivatives. Subsequently, the resulting aldehyde 205a was reduced with sodium borohydride and the formed alcohol was eventually esterified by chlorinated phenylacetic acid chloride under alkaline conditions (Scheme 43, path A)Citation139. Among all prepared quinoline derivatives, the best antimicrobial activity in disc diffusion tests was exhibited by compound 210 (Scheme 43, path A). Docking to GlcN-6-P synthase matrix (pdbid: 1xff) revealed binding of 210 due to its interaction with Met184, Arg10, Arg216 and Arg217 residues outside the active site of the GAH domain (estimated Ki = 0.0764 µM)Citation139.
Borse et al.Citation140 described the two-step synthesis of 12 isoquinoline derivatives. The first step of the synthesis involved a reaction between an appropriate carboxylic acid and ethyl 3,4-dimethoxyphenyl acetate, in the presence of phosphorous oxide. As a part of the second step, resulting intermediates were treated with ammonium acetate under solvent-free conditions, to be finally irradiated with microwaves (Scheme 44)Citation140. All obtained products were found to exhibit moderate to good antimicrobial activity and compared to the results of the molecular docking study, suggesting that all derivatives may be considered good GlcN-6-P synthase inhibitors. However, compounds 211 and 212 emerged as the most promising antimicrobials among all tested isoquinoline derivatives. Compound 211 showed promising activity against S. aureus and C. albicans and 212 against S. aureus only. Molecular docking to the GlcN-6-P synthase matrix (pdbid: 1jxa) confirmed, that both compounds may be considered as potential GlcN-6-P synthase inhibitors, possibly binding to the ISOM domain (E = −109,41 kJ/mol for 211 and E = −135.48 kJ/mol for 212)Citation140.
Scheme 44. Synthesis of isoquinoline derivatives as potential inhibitors of GlcN-6-P synthase, according to Borse et al.Citation140
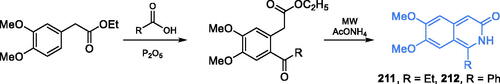
8. Conclusions and perspectives
GlcN-6-P synthase is one of the enzymes most extensively studied as a molecular target for potential novel antimicrobial or antidiabetic drugs. Inhibitors targeting GAH or ISOM active sites, rationally designed or of natural origins, such as FMDP, DSOK, APO or ADGP are highly selective for GlcN-6-P synthase. However, most of them are hydrophilic compounds, poorly penetrating biological membranes. In consequence, their antimicrobial activity is low. Hopefully, their antimicrobial potential could be improved upon conversion into derivatives of the pro-drug type, especially by employing molecular nanocarriersCitation141–143 that could ensure efficient delivery of nanocarrier: GlcN-6-P synthase inhibitor to the microbial cell interior. Nevertheless, cleavable conjugates, able to release the active inhibitor in the cytosol may have potential as antimicrobial drug candidates of a broad spectrum, covering human pathogenic bacteria and fungi.
A huge number of heterocyclic compounds exhibiting antimicrobial activity have been reported as possible GlcN-6-P inhibitors, based on the results of their molecular docking into bacterial GlcN-6-P synthase matrix. In some cases, especially for compounds 55, 67c, 74 and 113, the calculated values of docking score, binding energy or inhibitory constants have suggested their strong enzyme inhibitory potential but only for 67c, this potential has been confirmed by experimental data. Moreover, little if not at all is known about the selectivity of these compounds as GlcN-6-P synthase inhibitors and selective toxicity in the pathogenic microorganism: the human host system. Compounds, for which selective toxicity due to the GlcN-6-P synthase inhibition will be confirmed, are surely worth further investigating.
Very few confirmed GlcN-6-P synthase inhibitors bind outside the GAH and ISOM active sites. Among them, the aaptamine derivatives, such as RO0509347, presumably interfering with interdomain communication between GAH and ISOM, seem especially interesting. Although originally developed as antidiabetics, they may also have potential as antimicrobials. This possibility should be thoroughly further examined.
Disclosure statement
The authors declare no conflicts of interest.
References
- Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab 2006;290:E1–E8.
- Chou KC. Molecular therapeutic target for type-2 diabetes. J Proteome Res 2004;3:1284–8.
- Zhang H, Jia Y, Cooper JJ, et al. Common variants in glutamine: fructose-6-phosphate amidotransferase 2 (GFPT2) gene are associated with type 2 diabetes, diabetic nephropathy, and increased GFPT2 mRNA levels. J Clin Endocrinol Metab 2004;89:748–55.
- Srinivasan V, Sandhya N, Sampathkumar R, et al. Glutamine fructose-6-phosphate amidotransferase (GFAT) gene expression and activity in patients with type 2 diabetes: inter-relationships with hyperglycaemia and oxidative stress. Clin Biochem 2007;40:952–7.
- Oki T, Yamazaki K, Kuromitsu J, et al. cDNA cloning and mapping of a novel subtype of glutamine: fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics 1999;57:227–34.
- Dong T, Kang X, Liu Z, et al. Altered glycometabolism affects both clinical features and prognosis of triple-negative and neoadjuvant chemotherapy treated breast cancer. Tumour Biol 2016;37:8159–68.
- Ren S, Shao Y, Zhao X, et al. Integration of metabolomics and transcriptomics reveals major metabolic pathways and potential biomarker involved in prostate cancer. Mol Cell Proteomics 2016;15:154–63.
- Li LL, Shao MM, Peng PK, et al. High expression of GFAT1 predicts unfavorable prognosis in patients with hepatocellular carcinoma. Oncotarget 2017;8:19205–17.
- Guillaumond F, Leca J, Olivares O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci USA 2013;110:3919–24.
- Vasconcelos-dos-Santos A, Loponte H, Mantuano NR, et al. Hyperglycemia exacerbates colon cancer malignancy through hexosamine biosynthetic pathway. Oncogenesis 2017;6:e306–e306.
- Szymura SJ, Zaemes JP, Allison DF, et al. NF-κB upregulates glutamine-fructose-6-phosphate transaminase 2 to promote migration in non-small cell lung cancer. Cell Commun Signal 2019;17:24.
- Kim J, Lee HM, Cai F, et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1-mutant lung cancer. Nat Metab 2020;2:1401–12.
- Kertmen A, Przysiecka Ł, Coy E, et al. Emerging anticancer activity of candidal glucoseamine-6-phosphate synthase inhibitors upon nanoparticle-mediated delivery. Langmuir 2019;35:5281–93.
- Chen W, Do KC, Saxton B, et al. Inhibition of the hexosamine biosynthesis pathway potentiates cisplatin cytotoxicity by decreasing BiP expression in non–small‐cell lung cancer cells. Mol Carcin 2019;58:1046–55.
- Whelan WL, Ballou CE. Sporulation in d-glucosamine auxotrophs of Saccharomyces cerevisiae: meiosis with defective ascospore wall formation. J Bacteriol 1975;124:1545–57.
- Sarvas M. Mutant of Escherichia coli K-12 defective in D-glucosamine biosynthesis. J Bacteriol 1971;105:467–71.
- Bates CJ, Adams WR, Handschumacher RE. Control of the formation of uridine diphospho-N-acetyl-hexosamine and glycoprotein synthesis in rat liver. J Biol Chem 1966;241:1705–12.
- Milewski S. Glucosamine-6-phosphate synthase – the multi-facets enzyme. Biochim Biophys Acta 2002;1597:173–92.
- Zalkin H, Smith JL. Enzymes utilizing glutamine as an amide donor. Adv Enzymol Relat Areas Mol Biol 1998;72:87–144.
- Massière F, Badet-Denisot MA. The mechanism of glutamine-dependent amidotransferases. Cell Mol Life Sci 1998;54:205–22.
- Göpel Y, Khan MA, Görke B. Ménage à trois: post-transcriptional control of the key enzyme for cell envelope synthesis by a base-pairing small RNA, an RNase adaptor protein and a small RNA mimic. RNA Biol 2014;11:433–42.
- Kornfeld R. Studies on L-glutamine:D-fructose-6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem 1967;242:3135–41.
- Milewski S, Kuszczak D, Jedrzejczak R, et al. Oligomeric structure and regulation of Candida albicans glucosamine-6-phosphate synthase. J Biol Chem 1999;274:4000–8.
- Ruegenber S, Mayr FAMC, Atanassov I, et al. Protein kinase A controls the hexosamine pathway by tuning the feedback inhibition of GFA-1. Nat Commun 2021;12:2176.
- Teplyakov A, Obmolova G, Badet B, Badet-Denisot MA. Channeling of ammonia in glucosamine-6-phosphate synthase. J Mol Biol 2001;313:1093–102.
- Durand P, Golinelli-Pimpaneau B, Mouilleron S, et al. Highlights of glucosamine-6P synthase catalysis. Arch Biochem Biophys 2008;474:302–17.
- Mouilleron S, Badet-Denisot MA, Badet B, Golinelli-Pimpaneau B. Dynamics of glucosamine-6-phosphate synthase catalysis. Arch Biochem Biophys 2011;505:1–12.
- Walker JE, Abraham EP. The structure of bacilysin and other products of Bacillus subtillis. Biochem J 1970;118:563–70.
- Kenig M, Abraham EP. Antimicrobial activities and antagonists of bacilysin and anticapsin. J Gen Microbiol 1976;94:37–45.
- Shah R, Neuss N, Gorman M, Boeck LD. Isolation, purification and characterization of anticapsin. J Antibiot 1970;23:613–7.
- Borowski E, Milewski S, Chmara H. Anticapsin: an active site directed inhibitor of glucosamine-6-phosphate synthetase from Candida albicans. Drugs Exptl Clin Res 1986;12:577–83.
- Rapp C, Jung G, Katzer W, Loeffler C. Chlorotetain from Bacillus subtilis, an antifungal dipeptide with an unusual chlorine-containing amino acid. Angew Chem 1988;27:1733–4.
- Neuss N, Molloy BB, Shah R, DeLaHiguera N. The structure of anticapsin, a new biologically active metabolite of Streptomyces griseoplanus. Biochem J 1970;118:571–5.
- Crossley MJ, Stamford AW. Concise, stereocontrolled synthesis of the C4 epimers of anticapsin and bacilysin: revision of the configurations of the natural products. Aust J Chem 1993;46:1443–6.
- Souchet M, Baillargé M, Le Goffic F. A new and stereoselective synthesis of the antibiotic anticapsin. Tetrahedron Lett 1988;29:191–4.
- Baldwin JE, Adlington RM, Mitchell MB. Stereocontrolled enantiospecific synthesis of anticapsin. Tetrahedron 1995;51:5193–206.
- Marco-Contelles J, Molina MT, Anjum S. Naturally occurring cyclohexane epoxides: sources, biological activities, and synthesis. Chem Rev 2004;104:2857–900.
- Kobayashi S, Shibata J, Shimada M, Ohno M. An enantioselective synthesis of the A-ring synthon for vitamin D3 metabolites by chemicoenzymatic approach. Tetrahedron Lett 1990;31:1577–80.
- Molloy BB, Lively DH, Gale RM, et al. A new dipeptide antibiotic from Streptomyces collinus. J Antibiot 1972;25:137–40.
- Van der Baan JL, Barnick JWFK, Bickelhaut F. Antibiotic A 19009. Structural investigation and synthesis. J Antibiot 1983;36:784–92.
- Chmara H, Andruszkiewicz R, Borowski E. Inactivation of glucosamine-6-phosphate synthetase from Salmonella typhimurium LT 2 SL 1027 by Nβ-fumarylcarboxyamido-l-2,3-diaminopropionic acid. Biochem Biophys Res Commun 1984;120:865–72.
- Andruszkiewicz R, Chmara H, Milewski S, Borowski E. Synthesis of N3-fumaramoyl-L-2,3-diaminopropanoic acid analogues, the irreversible inhibitors of glucosamine synthetase. Int J Pept Protein Res 1986;27:449–53.
- Kucharczyk N, Denisot MA, Le Goffic F, Badet B. Glucosamine-6-phosphate synthase from Escherichia coli: determination of the mechanism of inactivation by N3-fumaroyl-L-2,3-diaminopropionic derivatives. Biochemistry 1990;29:3668–76.
- Andruszkiewicz R, Milewski S, Borowski E. Amide and ester derivatives of N3-transepoxysuccinoyl-L-2,3-diaminopropanoic acid: Inhibitors of glucosamine-6-phosphate synthase. J Enzyme Inhib 1995;9:123–33.
- Auvin S, Cochet O, Kucharczyk N, et al. Synthesis and evaluation of inhibitors for Escherichia coli glucosamine-6-phosphate synthase. Bioorg Chem 1991;19:143–51.
- Walkowiak A, Wakieć M, Bontemps-Gracz M, Andruszkiewicz A. Glutamine analogues containing a keto function – novel inhibitors of fungal glucosamine-6- phosphate synthase. J Enzyme Inhib Med Chem 2005;20:439–47.
- Wojciechowski M, Milewski S, Mazerski J, Borowski E. Glucosamine-6-phosphate synthase, a novel target for antifungal agents. Molecular modelling studies in drug design. Acta Biochim. Polon 2005;52:647–53.
- Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene 2016;35:3619–25.
- Andruszkiewicz R, Milewski S, Zieniawa T, Borowski E. Anticandidal properties of N3-(4-methoxyfumaroyl)-L-2,3-diaminopropanoic acid oligopeptides. J Med Chem 1990;33:132–5.
- Milewski S, Chmara H, Andruszkiewicz R, et al. Antifungal peptides with novel specific inhibitors of glucosamine 6-phosphate synthase. Drugs Exp Clin Res 1988;14:461–5.
- Chmara H, Milewski S, Andruszkiewicz R, et al. Antibacterial action of dipeptides containing an inhibitor of glucosamine-6-phosphate isomerase. Microbiology 1998;144:1349–58.
- Milewski S, Andruszkiewicz R, Kasprzak L, et al. Mechanism of action of anticandidal dipeptides containing inhibitors of glucosamine-6-phosphate synthase. Antimicrob Agents Chemother 1991;35:36–43.
- Shahi G, Kumar M, Skwarecki AS, et al. Fluconazole resistant Candida auris clinical isolates have increased levels of cell wall chitin and increased susceptibility to a glucosamine-6-phosphate synthase inhibitor. Cell Surface 2022;8:100076.
- Zgódka D, Milewski S, Borowski E. A diffusible analogue of N3-(4-methoxyfumaroyl)-L-2,3-diaminopropanoic acid with antifungal activity. Microbiology 2001;147:1955–9.
- Pawlak D, Stolarska M, Wojciechowski M, Andruszkiewicz R. Synthesis, anticandidal activity of N3-(4-methoxyfumaroyl)-(S)-2,3-diaminopropanoic amide derivatives – novel inhibitors of glucosamine-6-phosphate synthase. Eur J Med Chem 2015;90:577–82.
- Pawlak D, Schielmann M, Wojciechowski M, Andruszkiewicz R. Synthesis and biological activity of novel ester derivatives of N3-(4-metoxyfumaroyl)-(S)-2,3-diaminopropanoic acid containing amide and keto function as inhibitors of glucosamine-6-phosphate synthase. Bioorg Med Chem. Lett 2016;26:3586–9.
- Koszel D, Lącka I, Kozłowska-Tylingo K, Andruszkiewicz R. The synthesis and biological activity of lipophilic derivatives of bicine conjugated with N3-(4-methoxyfumaroyl)-L-2,3-diaminopropanoic acid (FMDP)—an inhibitor of glucosamine-6-phosphate synthase. J Enzyme Inhib Med Chem 2012;27:167–73.
- Massière F, Badet-Denisot M-A, René L, Badet B. Design, Synthesis and evaluation of the first mechanism-based inhibitor of glucosamine 6-phosphate synthase. J Am Chem Soc 1997;119:5748–9.
- Walker B, Brown MF, Lynas JF, et al. Inhibition of Escherichia coli glucosamine synthetase by novel electrophilic analogues of glutamine—comparison with 6-diazo-5-oxo-norleucine. Bioorg Med Chem Lett 2000;10:2795–8.
- Griffiths M, Keast D, Crawford M, et al. The role of glutamine and glucose analogues in metabolic inhibition of human myeloid leukaemia in vitro. Int J Biochem 1993;25:1749–55.
- Janiak AM, Milewski S. Mechanism of antifungal action of kanosamine. Med Mycol 2001;39:401–8.
- Meyer zu Reckendorf W. A simple synthesis of 3-amino-3-deoxy- D-glucose (kanosamine). Angew Chem Int Ed Engl 1966;5:967.
- Bearne SL. Active site-directed inactivation of Escherichia coli glucosamine-6-phosphate synthase. J Biol Chem 1996;271:3052–7.
- Leriche C, Badet-Denisot MA, Badet B. Affinity labeling of Escherichia coli glucosamine-6-phosphate synthase with a fructose 6-phosphate analog: evidence for proximity between the N-terminal cysteine and the fructose-6-phosphate-binding site. Eur J Biochem 1997;245:418–22.
- Le Camus C, Badet-Denisot MA, Badet B. Arabinose-5-phosphate oxime vs its methylenephosphonate mimetic as high energy intermediate of the glucosamine-6P synthase catalysed reaction. Tetrahedron Lett 1998;39:2571–2.
- Badet-Denisot M-A, Leriche C, Massière F, Badet B. Nitrogen transfer in E. coli glucosamine-6P synthase. Investigations using substrate and bisubstrate analogs. Bioorg Med Chem Lett 1995;5:815–20.
- Janiak AM, Hoffmann M, Milewska MJ, Milewski S. Hydrophobic derivatives of 2-amino-2-deoxy-D-glucitol-6-phosphate: a new type of D-glucosamine-6-phosphate synthase inhibitors with antifungal action. Bioorg Med Chem 2003;11:1653–62.
- Milewski S, Janiak A, Wojciechowski M. Structural analogues of reactive intermediates as inhibitors of glucosamine-6-phosphate synthase and phosphoglucose isomerase. Arch Biochem Biophys 2006;450:39–49.
- Melcer A, Łacka I, Gabriel I, et al. Rational design of N-alkyl derivatives of 2-amino-2-deoxy-d-glucitol-6P as antifungal agents. Bioorg Med Chem Lett 2007;17:6602–6.
- Vijesh AM, Isloor AM, Telkar S, et al. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arabian J Chem 2013;6:197–204.
- Vijesh AM, Isloor AM, Telkar S, et al. Synthesis, characterization and antimicrobial studies of some new pyrazole incorporated imidazole derivatives. Eur J Med Chem 2011;46:3531–6.
- Tomi IHR, Al-Daraji AHR, Abdula AM, Al-Marjani MF. Synthesis, antimicrobial and docking study of three novel 2,4,5-triarylimidazole derivatives. J Saudi Chem Soc 2016;20:509–16.
- Ismail AH, Abdula AM, Tomi IHR, et al. Synthesis, antimicrobial evaluation and docking study of novel 3,5-disubstituted-2-isoxazoline and 1,3,5-trisubstituted-2-pyrazoline derivatives. Med Chem 2019;17:462–73.
- Katariya KD, Vennapu DR, Shah SR. Synthesis and molecular docking study of new 1,3-oxazole clubbed pyridyl-pyrazolines as anticancer and antimicrobial agents. J Mol Struct 2021;1232:130036.
- Bahare RS, Ganguly S, Choowongkomon K, Seetaha S. Synthesis, HIV-1 RT inhibitory, antibacterial, antifungal and binding mode studies of some novel N-substituted 5-benzylidine-2,4-thiazolidinediones. Daru 2015;23:6.
- Omar AM, Ihmaid S, Habib E-SE, et al. The rational design, synthesis, and antimicrobial investigation of 2-amino-4-methylthiazole analogues inhibitors of GlcN-6-P synthase. Bioorg Chem 2020;99:103781.
- Rajasekaran A, Sivakumar KK, Sureshkumar K, Manjushree M. Design, synthesis, characterisation and in-vitro antimicrobial activity of some hybridized triazole scaffolds. Futur J Pharm Sci 2017;3:1–10.
- Aouad MR, Mayaba MM, Naqvi A, et al. Design, synthesis, in silico and in vitro antimicrobial screenings of novel 1,2,4-triazoles carrying 1,2,3-triazole scaffold with lipophilic side chain tether. Chem Cent J 2017;11:117.
- Shyma PC, Balakrishna K, Peethambar SK, et al. Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methyl pyridine moiety for antimicrobial property. Eur J Med Chem 2013;68:394–404.
- Sujith KV, Jyothi NR, Shetty P, Kalluraya B. Regioselective reaction: synthesis and pharmacological study of Mannich bases containing ibuprofen moiety. Eur J Med Chem 2009;44:3697–702.
- Girisha KS, Kalluraya B, Narayana V, Padmashree . Synthesis and pharmacological study of 1-acetyl/propyl-3-aryl-5-(5-chloro- 3-methyl-1-phenyl-1H-pyrazol-4-yl)-2-pyrazoline. Eur J Med Chem 2010;45:4640–4.
- Sindhe MA, Bodke YD, Kenchappa R, et al. Synthesis of a series of novel 2,5-disubstituted-1,3,4-oxadiazole derivatives as potential antioxidant and antibacterial agents. J Chem Biol 2016;9:79–90.
- Arrault A, Touzeau F, Guillaumet G, Merour JY. A straightforward synthesis of 1,2-dihydronaphtho[2,1-b]furans from 2- naphthols. Synthesis 1999;1999:1241–5.
- Kenchappa R, Yadav D, Bodke D, Chandrashekar A, et al. Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-substituted phenyl) pyridine derivatives as potent biological agents. Arab J Chem 2017;10:1336–44.
- Vijesh AM, Isloor AM, Prabhu V, et al. Synthesis, characterization and anti-microbial studies of some novel 2,4-disubstituted thiazoles. Eur J Med Chem 2010;45:5460–4.
- Venkatesh T, Bodke YD, Joy MN, et al. Synthesis of some benzofuran derivatives containing pyrimidine moiety as potent antimicrobial agents. Iran J Pharm Res 2018;17:75–86.
- Bakr RB, Elkanzi NAA. Preparation of some novel thiazolidinones, imidazolinones, and azetidinone bearing pyridine and pyrimidine moieties with antimicrobial activity. J Heterocycl Chem 2020;57:2977–89.
- Kenchappa R, Bodke YD, Asha B, et al. Synthesis, antimicrobial, and antioxidant activity of benzofuran barbitone and benzofuran thiobarbitone derivatives. Med Chem Res 2014;23:3065–81.
- Kenchappa R, Bodke YD, Telkar S, et al. Synthesis, characterization, and antimicrobial activity of new benzofuran derivatives. Russ J Gen Chem 2016;86:2827–36.
- Venkatesh T, Bodke YD, Telkar S. Synthesis, antimicrobial and antioxidant activity of chalcone derivatives containing thiobarbitone nucleus. Med Chem 2016;6:440–8.
- Aswathanarayanappa C, Bheemappa E, Bodke YD, et al. 5-phenyl-1-benzofuran-2-yl derivatives: synthesis, antimicrobial and antioxidant activity. Med Chem Res 2013;22:78–87.
- Kenchappa R, Bodke Y, Peethambar SK, et al. Synthesis of β-amino carbonyl derivatives of coumarin and benzofuran and evaluation of their biological activity. Med Chem Res 2013;22:4787–97.
- Kenchappa R, Bodke YD, Telkar S, Nagaraja O. Synthesis and antimicrobial activity of fused isatin and diazepine derivatives derived from 2-acetyl benzofuran. Russ J Gen Chem 2017;87:2027–38.
- Jose G, Kumara TH, Nagendrappa G, et al. New polyfunctional imidazo[4,5-C]pyridine motifs: synthesis, crystal studies, docking studies and antimicrobial evaluation. Eur J Med Chem 2014;77:288–97.
- Kumar D, Harish BG, Gangwar M, et al. Synthesis, molecular docking and in vitro antimicrobial studies of new hexahydroindazole derivatives of curcumin. Lett Drug Des Discovery 2012;10:119–28.
- Minu M, Thangadurai A, Wakode SR, et al. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg Med Chem 2009;19:2960–4.
- Khan SA, Asiri AM, Rahman RM, et al. Multistep synthesis of fluorine-substituted pyrazolopyrimidine derivatives with higher antibacterial efficacy based on in vitro molecular docking and density functional theory. J Heterocycl Chem 2017;54:3099–107.
- Satyendra RV, Vishnumurthy KA, Vagdevi HM, et al. Synthesis, in vitro antioxidant, anthelmintic and molecular docking studies of novel dichlorosubstituted benzoxazole-triazolo-thione derivatives. Eur J Med Chem 2011;46:3078–84.
- Satyendra RV, Vishnumurthy KA, Vagdevi HM, et al. In vitro antimicrobial and molecular docking of dichloro substituted benzoxazole derivatives. Med Chem Res 2011;19:617–716.
- Jayanna ND, Vagdevi HM, Dharshan JC, et al. Synthesis, antimicrobial, analgesic activity, and molecular docking studies of novel 1-(5,7-dichloro-1,3-benzoxazol-2-yl)-3-phenyl-1H- pyrazole-4-carbaldehyde derivatives. Med Chem Res 2013;22:5814–9.
- Venkatesh T, Bodke Y, Joy MN, et al. Synthesis of some novel 5,7-disubstituted-2-phenyl-5H-[1,3,4]thiadiazolo [3,2-a]pyrimidine derivatives and evaluation of their biological activity. Lett Org Chem 2016;13:661–71.
- Keerthi Kumar CT, Keshavayya J, Rajesh TN, et al. Synthesis, characterization and biological activity of 5-Phenyl-1,3,4-thiadiazole-2-amine incorporated azo dye derivatives. Org Chem Int 2013;2013:1–3.
- Kumara HK, Suhas R, Suyoga Vardhan DM, et al. A correlation study of biological activity and molecular docking of Asp and Glu linked bis-hydrazones of quinazolinones. RSC Adv 2018;8:10644–53.
- Mhaske SB, Argade NP. Concise and efficient synthesis of bioactive natural products pegamine, deoxyvasicinone, and (-)-vasicinone. J Org Chem 2001;66:9038–40.
- Melagraki G, Afantitis A, Markopoulou OI, et al. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem 2009;44:3020–6.
- Sandhya B, Giles D, Vinod M, et al. Synthesis, pharmacological evaluation and docking studies of coumarin derivatives. Eur J Med Chem 2011;46:4649–701.
- Kumar AS, Kanakaraju S, Prasanna B, Chandramouli GVP. Synthesis, molecular docking studies and antibacterial evaluation of Baylis-Hillman adducts of coumarin and pyran derivatives using ionic liquid under microwave irradiation. Chem Sci Tran 2013;2:561–9.
- Helmy MM, Abdellattif MH, Eldeab HA. New methodology for synthesis of coumarin derivatives as potent antimicrobial agents. Int J Adv Pharm Biol Chem 2014;3:983–90.
- Devi AP, Dhingra N, Bhardwaj U, et al. 2-(phenyl)-4H-chromen-4-ones: green synthesis, characterization, in vitro antifungal evaluation and molecular docking approach toward Aspergillus fumigatus. Curr Res Green Sustain Chem 2022;5:100234.
- Elkanzi NAA, Bakr RB, Ghoneim AA. Design, synthesis, molecular modeling study and antimicrobial activity of some novel pyrano[2,3-b]pyridine and pyrrolo[2,3-b]pyrano[2.3-d]pyridine derivatives. J Heterocycl Chem 2019;56:406–16.
- Chandra S, Jain D, Sharma AK, Sharma P. Coordination modes of a Schiff base pentadentate derivative of 4-aminoantipyrine with cobalt(II), nickel(II), and copper(II) metal ions: synthesis, spectroscopic and antimicrobial studies. Molecules 2009;14:174–90.
- Raman N, Sobha S, Thamaraichelvan A. A novel bioactive tyramine derived Schiff base and its transition metal complexes as selective DNA binding agents. Spectrochim Acta Part A 2011;78:888–98.
- Ebrahimipour SY, Sheikhshoaie I, Castro J, et al. Synthesis, spectral characterization, structural studies, molecular docking and antimicrobial evaluation of new dioxidouranium(VI) complexes incorporating tetradentate N2O2 Schiff base ligands. RSC Adv 2015;5:95104–17.
- Yousef Ebrahimipour S, Sheikhshoaie I, Simpson J, et al. Antimicrobial activity of aroylhydrazone-based oxido vanadium(v) complexes: in vitro and in silico studies. New J Chem 2016;40:2401–12.
- Yousef Ebrahimipour S, Machura B, Mohamadi M, Khaleghi M. A novel cationic cobalt(III) schiff base complex: preparation, crystal structure, hirshfeld surface analysis, antimicrobial activities and molecular docking. Microb Pathog 2017;113:160–7.
- Onwudiwe DC, Ekennia AC, Mogwase BMS, et al. Palladium(II) and platinum(II) complexes of N-butyl-N-phenyldithiocarbamate: synthesis, characterization, biological activities and molecular docking studies. Inorgan Chim Acta 2016;450:69–80.
- Onwudiwe DC, Ajibade PA. Synthesis and characterization of metal complexes of N-alkyl-N-phenyl dithiocarbamates. Polyhedron 2010;29:1431–6.
- Wang H, Zhang X, Zhao Y, et al. Three Zn(II) complexes with a sexidentate N2O4-donor bis-Schiff base ligand: synthesis, characterization, DFT studies, in vitro antimicrobial evaluation and molecular docking studies. Inorg Chim Acta 2017;466:8–15.
- Raczynska J, Olchowy J, Konariev PV, et al. The crystal and solution studies of glucosamine-6-phosphate synthase from Candida albicans. J Mol Biol 2007;372:672–88.
- Bobzin SC, Yang S, Kasten TP. Application of liquid chromatography-nuclear magnetic resonance spectroscopy to the identification of natural products. J. Chromatography B 2000;748:259–67.
- Yu H-B, Yang F, Sun F, et al. Aaptamine derivatives with antifungal and anti-HIV-1 activities from the South China Sea sponge Aaptos aaptos. Marine Drugs 2014;12:6003–13.
- Qian Y, Ahmad M, Chen S, et al. Discovery of 1-arylcarbonyl-6,7-dimethoxyisoquinoline derivatives as glutamine fructose-6-phosphate amidotransferase (GFAT) inhibitors. Bioorg Med Chem Lett 2011;21:6264–9.
- Oliveira IA, Allonso D, Fernandes TVA, et al. Enzymatic and structural properties of human glutamine: fructose-6-phosphate amidotransferase 2 (hGFAT2). J Biol Chem 2021;296:100180.
- Skarbek K, Gabriel I, Szweda P, et al. Synthesis and antimicrobial activity of 6-sulfo-6-deoxy-D-glucosamine and its derivatives. Carbohydr Res 2017;448:79–87.
- Khan SA, Asiri AM, Al-Ghamdi NSM, et al. Microwave assisted synthesis of chalcone and its polycyclic heterocyclic analogues as promising antibacterial agents: in vitro, in silico and DFT studies. J Mol Struct 2019;1190:77–85.
- Ebenezer O, Awolade P, Koorbanally N, Singh P. New library of pyrazole–imidazo[1,2-α]pyridine molecular conjugates: synthesis, antibacterial activity and molecular docking studies. Chem Biol Drug Des 2020;95:162–73.
- Sarojini BK, Krishna BG, Darshanraj CG, et al. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole, derivatives for antimicrobial properties. Eur J Med Chem 2010;45:3490–6.
- Narayana B, Ashalatha BV, Vijaya Raj KK, Suchetha Kumari N. Synthesis of some new 4-{2-[(Aryl)amino]-1,3-thiazol4-yl}benzene-1,2-diols as possible antibacterial and antifungal agents. Phosphorus Sulfur Silicon Relat Elem 2006;181:1381–9.
- Krishna BG, Srojini BK, Darshanraj CG. Synthesis, characterization, molecular docking and evaluation of antibacterial, antiproliferative, and anti-inflammatory properties of new pyridinyl substituted triazole derivatives. Der Pharma Medica 2014;6:345–61.
- Siwek A, Plech T, Stefańska J, et al. Molecular properties prediction, docking studies and antimicrobial screening of 1,3,4-thiadiazole and S-triazole derivatives. Curr Comput-Aided Drug Des 2014;10:3–14.
- Siwek A, Wujec M, Dobosz M, Wawrzycka-Gorczyca I. Study of direction of cyclization of 1-azolil-4-aryl/alkyl-thiosemicarbazides. Heteroat Chem 2010;21:521–32.
- Askri S, Dbeibia A, Mchiri C, Boudriga S, et al. Antimicrobial activity and in silico molecular docking studies of pentacyclic spiro[oxindole-2,3’-pyrrolidines] tethered with succinimide scaffolds. Appl Sci 2021;12:360.
- Sowmya HBV, Kumara THS, Nagendrappa G, et al. Solvent free synthesis, crystal studies, docking studies and antibacterial properties of some novel fluorinated pyridazinone derivatives. J Mol Struct 2013;1054-1055:179–87.
- Nagle P, Pawar Y, Sonawane A, et al. Docking simulation, synthesis and biological evaluation of novel pyridazinone containing thymol as potential antimicrobial agents. Med Chem Res 2014;23:918–26.
- Desai JM, Shah VH. Synthesis and biological activity of cyanopyridine, isoxazole, and pyrazoline derivatives having thymol moiety. Indian J Chem Sect B: Org Chem Incl Med Chem 1996;42B:382–5.
- Mastelic J, Jerkovic I, Blazevic I, et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J Agric Food Chem 2008;56:3989–96.
- Samar AA. An efficient synthesis and reactions of novel indolylpyridazinone derivatives with expected biological activity. Molecules 2007;12:25–42.
- Preveena N, Nagendrappa G, Kumara THS, et al. Synthesis of (3-(2-chloroquinolin-3-yl)oxiran-2-yl)(phenyl)methanone derivatives and in vitro and in silico study of their various biological activities. Int J Pharm Sci Invent 2015;4:53–76.
- Tabassum S, Kumara THS, Jasinski JP, et al. Synthesis, crystal structure, ABTS radical-scavenging activity, antimicrobial and docking studies of some novel quinoline derivatives. J Mol Struct 2014;1070:10–20.
- Borse AU, Patil NL, Patil MN, et al. Microwave assisted synthesis of 1-substituted 6,7-dimethoxy-3-oxo-2,3-dihydroisoquinolines under solvent free conditions as potential antimicrobial agents and their docking study. J Pharm Res 2012;5:3223–6.
- Skwarecki AS, Milewski S, Schielmann M, Milewska MJ. Antimicrobial molecular nanocarrier-drug conjugates. Nanomedicine 2016;12:2215–40.
- Pham TN, Loupias P, Dassonville-Klimpt A, Sonnet P. Drug delivery systems designed to overcome antimicrobial resistance. Med Res Rev 2019; 39:2343–96.
- Cheng AV, Wuest WM. Signed, sealed, delivered: conjugate and prodrug strategies as targeted delivery vectors for antibiotics. ACS Infect Dis 2019;5:816–28.