Abstract
Carbonic anhydrases, catalysing the reversible CO2 hydration reaction, contribute in all living organisms to the maintenance of stable metabolic functions depending on intracellular concentrations of carbon dioxide, bicarbonate, and protons. Recent studies have examined how CAs affect bacterial lifecycle, considering these enzymes druggable targets due to interference with their activities by using inhibitors or activators. Here, we propose Escherichia coli cells as a model for testing the effect of acetazolamide (AZA), a potent CA inhibitor, on bacterial survival by evaluating E. coli growth through its glucose consumption. AZA, at concentrations higher than 31.2 µg/mL, was able to impair E. coli growth and glucose uptake. AZA is a good inhibitor of the two recombinant E. coli CAs, the β-CA CynT2, and the γ-CA EcoCAγ, with KIs of 227 and 248 nM, respectively. This study provides a proof-of-concept, low-cost method for identifying effective CA inhibitors capable of impairing bacterial metabolism.
1. Introduction
Over the past few years, the knowledge of bacterial carbonic anhydrases (CAs, EC 4.2.1.1) has significantly increased. Many reports showed that these metalloenzymes catalyse the physiologically crucial reversible CO2 hydration reaction (CO2 + H2O ⇋ HCO3– + H+) with high catalytic rates (kcat) ranging from 104 to 106 s−1.Citation1 It is thus readily apparent that CAs help all living organisms in balancing the endogenous levels of carbon dioxide (CO2), bicarbonate (HCO3–), and protons (H+), which are essential for sustaining a range of metabolic activitiesCitation2–6. The non-catalytically occurring CO2 hydration reaction cannot provide bicarbonate to support/bicarbonate to support the central metabolism rates, since the kcat hydration of 0.15 s−1 and kcat dehydration of 50.0 s−1 are too low at physiological pH to satisfy the organism's metabolic demandsCitation7. Because of their central role in catalysing critical reactions in metabolic pathways, these enzymes have ultimately been considered as attractive druggable targets whose inhibition might impair the microorganism lifecycleCitation6–10. In all life kingdoms, eight CA classes have been identified so far, and they are indicated with the Greek letters α, β, γ, δ, ζ, η, θ, and ιCitation7,Citation9–12. Up to date, four CA-classes (α, β, γ, and ι) have been identified in bacteriaCitation2–6. In our previous papers and at the time when only three CA-classes were known to be present in bacteria (α, β, and γ), we allocated them to Gram-negative bacterial compartments (periplasmic space and cytoplasm), considering their amino acid sequence featuresCitation7,Citation9,Citation11,Citation12.
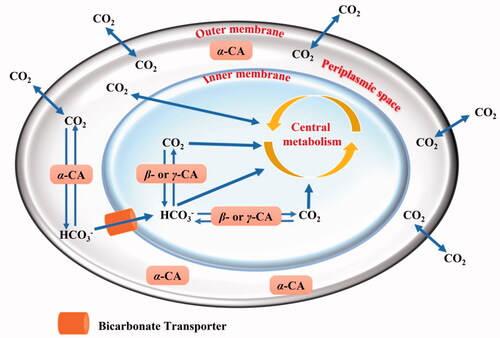
As shown in , we proposed that α-CAs, characterised by a signal peptide at the N-terminus of the polypeptide chain, reside in the periplasmic region and have the function of avoiding the loss of CO2 through diffusion from the bacteria cells, converting it into bicarbonate, which is transported inside the cytoplasm by bicarbonate transportersCitation7. In contrast, the β- or γ-classes which are localised in the cytoplasm, perform intracellular functions, such as CO2/HCO3– transport/homeostasis and pH regulationCitation7. Recently, in some Gram-negative bacteria lacking α-CAs, it has been discovered the presence of β-, γ-, and ι-CAs with a potential signal peptide at their N-terminus, which probably may have a function similar to that performed by α-CAs in the species where this class is presentCitation10,Citation11,Citation13–16. The function of CAs in bacteria and how these enzymes influence the bacterial lifecycle have been recently examined in the literature. For example, the β-CA encoded in the genome of Ralstonia eutropha is essential for its growth at ambient CO2 concentrations.Citation17 In Escherichia coli, the β-CA (CynT) produces the HCO3-, preventing bacteria from running out of bicarbonate due to the breakdown of cyanate as well as other metabolic processes. A second E. coli β-CA isoform (CynT2) was shown to be critical for bacterial proliferation at atmospheric CO2 concentrationsCitation18,Citation19. Intriguingly, multiple examples of correlations between CA activity and the ability of bacteria, such as Vibrio choleraeCitation20, Mycobacterium tuberculosisCitation21–25, Salmonella entericaCitation26–28, Pseudomonas aeruginosaCitation29, Helicobacter pyloriCitation30–32, etc., to persist, become pathogenic and cause disease in humans can be found in the literature. Thus, it is readily evident that the inhibition of bacterial CA may decrease the pathogen survival/fitness. Fortunately, many specific chemical classes of CA inhibitors (CAIs) were reported in the scientific literatureCitation33–36. Recently, several FDA-approved CAIs were modified to target vancomycin-resistant enterococci (VRE)Citation37. Ethoxzolamide (EZA), an authorised diuretic and CAI, was shown to kill Helicobacter pylori in vitro, suggesting it could be turned into an anti-H. pylori medicationCitation38. Finally, acetazolamide inhibited the growth of the Gram-negative bacterium Neisseria gonorrhoeae both in vitro as well as in in vivo mouse models of gonococcal genital tract infectionCitation39.
Figure 1. Schematic representation of a gram-negative bacterium with the inner and outer membranes delimiting the periplasmic (in white) and cellular cytoplasm (in light blue). The passive CO2 diffusion through the bacterial cell and the bicarbonate transporter (orange colour), which actively vehicles bicarbonate from the periplasmic space to the cytoplasm are shown together with various CAs and their putative role(s).
In the present work, we propose the use of Escherichia coli cells, which can be easily manipulated in the lab, as a model for testing the effect of CAIs on the bacterial lifecycle. We used as example of CAI acetazolamide (AZA), a potent CA inhibitor, and evaluated E. coli growth as well as its glucose consumption. Glucose was added as the only carbon source to a liquid minimal culture media for bacterial growth. It has been demonstrated earlier by our groups that AZA was able to inhibit in vitro the two recombinant E. coli CAs, β-CA (CynT2) and γ-CA (EcoCAγ), with KIs of 227 and 248 nM, respectivelyCitation40,Citation41.
The present work proposes a proof-of-concept, inexpensive method for selecting CAIs able to impair bacterial metabolism, which could be employed in future applications in the fight against pathogenic bacteria, which are difficult to be managed in normal laboratories without the appropriate biosafety precautions.
2. Materials and methods
2.1. Cloning, expression, and purification of the recombinant E.coli β- and γ-CAs
The synthetic genes encoding for the E.coli β- and γ-CA were synthesised by the Invitrogen GeneArt (ThermoFisher Scientific), a company specialised in gene synthesis. The genes were cloned into the expression vector pET100D-Topo and heterologously expressed as described previouslyCitation40–43. The recovered E. coli β- and γ-CAs were >90% pure. The protein quantification was carried out by the Bradford method (BioRAD)Citation44.
2.2. Protonography
Following the electrophoresis, the SDS-PAGE gel was subject to protonography to detect the yellows bands due to the CO2 hydratase activity. The protonogram was developed as previously described by our groupsCitation15,Citation45,Citation46.
2.3. Stopped flow CA assay
The CO2 hydratase activity of the soluble recombinant enzymes, the corresponding kinetic constants, and AZA inhibition constants were determined using the stopped-flow technique as previously describedCitation41.
2.4. E. Coli cells preparation
E. coli DH5α cells were streaked under sterile conditions on an LB Agar plate with no selection from a strain stock stored at −80 °C mixed with 60% (v/v) glycerol (final concentration 30% v/v). Plates were incubated overnight at 37 °C. The day after, a few E. coli single colonies were picked up with a sterile applicator stick and inoculated in a 250 ml flask containing 50 ml of LB medium under non-selective conditions and grown overnight at 37 °C with aeration (≥150 rpm). The cells from the overnight growth were harvested by centrifugation at 4 °C, centrifuged at 1500×g for 30 min, and suspended in fresh LB media to a final optical density (OD) of 0.15 at 600 nm and growth up to 0.6 OD and subsequently counted.
2.5. E. Coli growth in presence of AZA
Eight acetazolamide (AZA) concentrations were used to test the effect of this pharmacological inhibitor on bacterial lifecycle (4000, 2000, 1000, 500, 250, 125, 62.5, and 31.2 µg/mL). AZA was dissolved in 100% dimethyl sulfoxide (DMSO) at 20 mg/mL stock concentration. The highest final concentration of DMSO in the assay was 5% (this concentration has no effects on the bacterial growth). Cells were seeded in a 24 multi-well plate at a final concentration of 100,000 cells/mL, containing Mueller Hinton (MH) broth as a medium. Cells were grown in an incubator for 6 h at 37 °C. As a control, we used E. coli cells with no AZA added. Afterward, 1 ml of bacterial growth from each well was collected and OD was read at 600 nm using a Varian Cary 50 Scan UV Visible Spectrophotometer.
2.6. Effect of AZA on E. coli glucose uptake
AZA was tested at 4000, 2000, 1000, 500, 250, 125, 62.5, and 31.2 µg/mL for its effect on glucose uptake. AZA stock solution was prepared as described in paragraph 2.5. The assay highest DMSO concentration was 5%, which has no effects on bacterial glucose uptake, as demonstrated by incubating the cells in the presence of glucose and 5% DMSO. One hundred thousand cells/mL were seeded in a 24 multi-well plate containing minimal medium (M9) supplemented with glucose at a final concentration of 0.4% and incubated at 37 °C. Cells with no AZA were used as control. An aliquot of the sample (5 µL) was used to analyse the sugar concentration. It was diluted up to 200 µL with H2O, 1 mM EDTA and subjected to the Phenol Sulphuric Acid assay as described in the literatureCitation47. Briefly, to determine the glucose uptake, 200 µL of Phenol 5% and 1 ml of concentrated sulphuric acid were added to the sample aliquots to reach a final reaction volume of 1.4 ml. The reactions were let to stand for 15 min. Then, the sample was transferred to a cuvette at 485 nm using a Varian Cary 50 Scan UV Visible Spectrophotometer. The assay standard curve was performed with the following concentrations of glucose: 0, 2, 4, 8, 10, 12, and 14 µg/mL, prepared from a glucose 100 mg/L stock solution diluted in distilled water and 1 mM EDTA.
3. Results and discussion
3.1. Cynt2 (β-CA) and the EcoCAγ (γ-CA) production and characterisation
The E. coli genome encodes for β-, γ-, and ι-CAs. Our groups heterologously overexpressed the CynT2 (β-CA) and the EcoCAγ (γ-CA) as fusion proteins with six tandem histidines (His6-Tag) at the polypeptide chain N-terminus. A high degree of purity of both proteins was accomplished by isolating them from the soluble cytoplasmic protein fraction of the bacterial host cells with a nickel-charged resin that had a high affinity for the polyhistidine motif. Protonography of the two purified CAs is shown in .
Figure 2. Protonogram of the two heterologously expressed E. coli CAs. The yellow band on the protonogram corresponds to the enzyme activity, which is responsible for the pH decrease from 8.2 to 6.8 due the dye transition point at acidic pH values. Legend: Lane STD, molecular markers; Lane 1, purified CynT2; Lane 2, purified EcoCAγ.
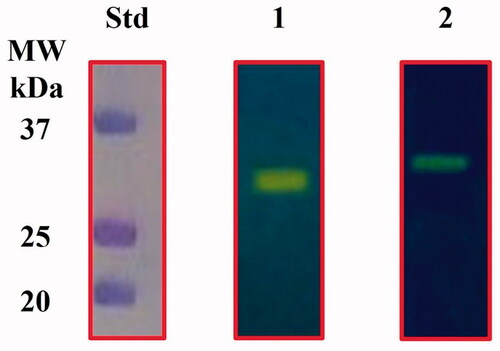
The catalytic activity of the recombinant enzymes is revealed by the yellow band due to the CO2 hydratase activity that correlated with the molecular weight of the CynT2 (29.0 kDa) and EcoCAγ (33.0 kDa) monomers. β- and γ-CAs are enzymes active as dimers and trimers, respectively. However, the yellow band appeared in the position of the monomeric forms of these enzymes, because SDS removal during the protonogram development leads to the rearrangement of the β- and γ-CAs monomers in the gel, which probably restore the active dimeric or tetrameric forms of the β-CA as well as the trimeric form of the γ-CA.
The stopped-flow technique was utilised to ascertain the CO2 hydratase activity as well as the respective kinetic constants of the purified enzymes. Therefore, we report these measurements in comparison with those obtained for the two human isoenzymes (CAI and CAII), previously purified in our labsCitation41.
CynT2 and EcoCAγ resulted to be excellent catalysts for the CO2 hydration reaction (kcat of 5.3 × 105 s−1 and 5.7 × 105 s−1, respectively) and were inhibited by acetazolamide (AAZ), a well-known pharmacological CA inhibitor, with a KI of 227 nM and 248, respectively ().
Table 1. Kinetic characteristics of the CO2 hydration reaction catalysed by α-, β-, and γ-CA enzymes. Human (h) hCA I and II (α-CAs), at 20° C and pH 7.5 in 10 mM HEPES buffer; CynT2 and EcoCAγ determined at 20° C, pH 8.3 in 20 mM TRIS buffer and 20 mM NaClO4. We also present inhibition data using the clinically relevant sulphonamide AZA (5-acetamido-1,3,4-thiadiazole-2-sulphonamide).
3.2. CAIs as antibacterials
The drug-approach method involves three critical points: i) finding out which metabolic pathways are necessary for the pathogen to live; ii) finding out the essential enzymes, which govern a specific metabolic pathway. The optimal approach would be to find target enzymes that are only found in the pathogen and not in the host. Even if, often this situation is uncommon since most important metabolic pathways are the same in all living things; iii) finding/designing small molecules and/or peptides that can selectively inhibit the target enzyme, and thus the pathogen growth. It is readily apparent that the two E. coli CAs meet these three points since CAs have a central role in catalysing critical reactions in bacterial metabolic pathways, such as the CO2/HCO3- bacterial homeostasis. Besides, they can be effectively inhibited by many inhibitors, such as simple aromatic/heterocyclic sulphonamides, which are frequently used as building blocks in the development of novel effective, and selective families of such pharmaceutical drugs. Of course, the criterium ii) is respected only in part because CAs are ubiquitous enzymes. In this scenario, the limitation can be circumvented by developing and isolating compounds that selectively inhibit bacterial enzymes while leaving host protein homologs unaffected or only marginally inhibited. Trimethoprim, for example, was discovered to specifically block the bacterial enzyme dihydrofolate reductase (DHFR), but not the human DHFR.
The possibility of inhibiting bacterial CAs is remarkable, especially when one considers the emergence of drug resistance to the existing antimicrobial medications, which is one of the most severe problems the human population needs to face. Thus, understanding the inhibitory patterns of CAs from various bacterial species and identifying potent and selective inhibitors that can impair bacterial growth is essential.
3.2.1. Effect of AZA on the bacterial growth
In this context, we have tested the effect of acetazolamide (AZA), an FDA-approved effective sulphonamide CAI, on bacterial survival by evaluating E. coli growth. The growth of E. coli was monitored after 6 h incubation with the CAI, by measuring the OD at the wavelength of 600 nm, which is related to the cell number, and incubating 1.0 × 105 E. coli cells in an MH medium containing different concentrations of AZA. As a control, we used E. coli cells with no AZA. The result is shown in .
Figure 3. Effect of AZA on E. coli cells grown in MH for 6 h in presence of the eight different concentrations of AZA reported on the x-axis. (A) Bacterial growth has been monitored by measuring the optical density at 600 nm. (B) reports the wells showing the E. coli growth and the control represented by the bacterial cells with no AZA. Each data point is the mean value of at least three independent experiments.
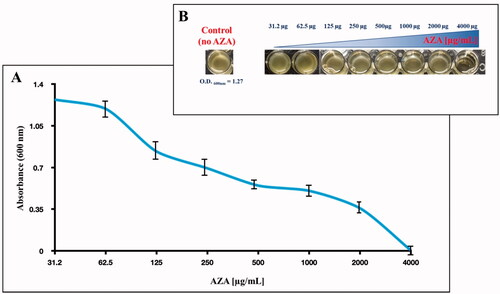
It is readily apparent that the increased AZA concentrations in the MH medium impair bacterial growth (). As shown in , the inhibiting effect of AZA on E. coli growth was maximal at 4000 µg/mL (absorbance 0.0025, and no bacterial cells were visible in the well, Panel B)). Lowering the AZA concentration (from 4000 to 31.2 µg/mL), the absorbance reached the value that corresponds to the control (OD600nm= 1.2) at the inhibitor concentrations in the range of 62.5 to 31.2 µg/mL (), meaning that at these concentrations, the inhibitor did not affect cell growth.
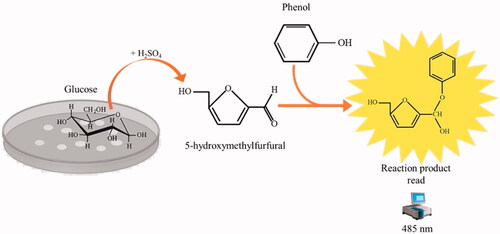
Figure 4. The reaction of phenol–sulphuric acid assay with glucose. Phenol in the presence of sulphuric acid is used for the quantitative colorimetric determination of glucose. Glucose by the action of concentrated sulphuric acid is dehydrated to hydroxymethylfurfural. These compounds then react with phenol to produce the reaction product (phenol- hydroxymethylfurfural), having a yellow-gold colour. The phenol-hydroxymethylfurfural is directly proportional to the amount of glucose present in the medium.
3.2.2. Effect of AZA on bacterial glucose uptake
Microbial metabolism relies on carbon sources, which are essential for biosynthetic activities and in many cases, energy metabolism. Besides, the rate at which carbon sources are used is closely related to metabolic activity. Glucose is arguably the most used and monitored carbohydrate, and it is quickly monitored by utilising the assays of the phenol–sulphuric acid ()Citation47.
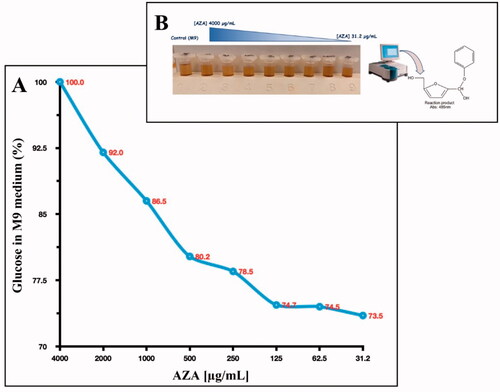
Thus, we explored the glucose consumption by E. coli cells. The experiment was performed by adding glucose at a final concentration of 0.4% to a liquid minimal culture media (M9 medium) as the only carbon source. The M9 contained 1.0 × 105 E. coli cells and different concentrations of AZA ranging from 4000 to 31.2 µg/mL (the same concentration used to monitor the bacterial growth). The glucose consumption was detected after 6 h by prevailing an aliquoted of the M9 containing glucose and measuring the phenol-hydroxymethylfurfural optical density at 485 nm (see ). As a control, we used the M9 with 0.4% glucose. As shown in , after 6 h, the E. coli maximum glucose consumption resulted in the lowest concentration of AZA (31.2 µg/mL). It is readily apparent that the increased AZA concentration avoids sugar consumption because of its inhibitory effect on bacterial CAs, which are directly involved in providing CO2/HCO3− necessary for the bacterial metabolic demand.
Figure 5. Glucose consumption by E. coli cells. Panel A shows the glucose consumption curve at different AZA concentrations (from 4000 to 31.2 g/mL) and using 1.0 × 105 E. coli cells. The phenol–sulphuric acid assay was performed after the E. coli cells were incubated for 6 h in the M9 medium supplemented with a final concentration of 0.4% glucose. Panel B shows the tubes containing the different concentrations of AZA and the glucose at 04%. The yellow-gold colour was developed by the reaction of the phenol with the 5- hydroxymethylfurfural, which was produced by the interaction of sulphuric acid with glucose. Each data point is the mean value of at least three independent experiments.
4. Conclusions
In this article we focalise our attention on bacterial metabolic pathways in which bacterial CAs have a central role. These enzymes are thus attractive drug targets. To demonstrate their draggability, we used E. coli cells as a model system, since they can be easily manipulated in the lab, and used facile general methods for determining bacterial survival, bacterial growth and carbohydrate consumption. In pharmacological application, these assays are mostly used to evaluate the viability and compare the impact of various products on microbial cultures. As a result, we demonstrated that AZA, a sulphonamide FDA-approved CA inhibitor, impairs bacterial growth as well as the glucose uptake, which E. coli (and other bacteria) use as a carbon source for growth and metabolism. Intriguingly, the inhibitory effect of AZA on the E. coli culture became apparent at concentrations a slightly higher than 31.2 µg/mL. Thus, we demonstrate here that the AZA that inhibits in vitro the two bacterial CAs, in the nanomolar range, also inhibits in vivo the growth of the bacterium. The strategy used in the present work affords a proof-of-concept study and brings new insights into the choice of novel and efficient CAIs capable of impairing bacterial metabolism, especially in relation to the fact that such eventually newly found compounds might be potentially used to combat drug resistance of many pathogens to the existing antimicrobial drugs.
Acknowledgements
The authors are grateful to Chiara Nobile, Valentina Brasiello and Marco Petruzziello for technical assistance.
Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. C Capasso and CT Supuran thank the Italian Ministry for University and Research (MIUR), for the project FISR2019_04819 BacCAD with which this research was funded.
Additional information
Funding
References
- (a) Flaherty DP, Seleem MN, Supuran CT. Bacterial carbonic anhydrases: underexploited antibacterial therapeutic targets. Future Med Chem 2021;13:1619–22. (b) Campestre C, De Luca V, Carradori S, et al. Carbonic anhydrases: new perspectives on protein functional role and inhibition in helicobacter pylori. Front Microbiol. 2021;12:629163.
- (a) An W, Holly KJ, Nocentini A, et al. Structure-activity relationship studies for inhibitors for vancomycin-resistant Enterococcus and human carbonic anhydrases. J Enzyme Inhib Med Chem 2022;37:1838–44. (b) Annunziato G, Angeli A, D'Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. Chem Med Chem 2016;11:1904–14.
- Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94.
- Del Prete S, Vullo D, De Luca V, et al. Sulfonamide inhibition studies of the beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:1115–20.
- Del Prete S, De Luca V, De Simone G, et al. Cloning, expression and purification of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. J Enzyme Inhib Med Chem 2016;31:54–9.
- Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: streptococcus mutans and porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56–74.
- Supuran CT, Capasso C. Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin Ther Pat 2020;30:963–82.
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- Supuran CT, Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J Enzyme Inhib Med Chem 2016;31:1254–60.
- Nocentini A, Supuran CT, Capasso C. An overview on the recently discovered iota-carbonic anhydrases. J Enzyme Inhib Med Chem 2021;36:1988–95.
- Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- De Luca V, Petreni A, Carginale V, et al. Effect of amino acids and amines on the activity of the recombinant iota-carbonic anhydrase from the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2021;36:1000–6.
- De Luca V, Petreni A, Nocentini A, et al. Effect of sulfonamides and their structurally related derivatives on the activity of iota-carbonic anhydrase from burkholderia territorii. Int J Mol Sci 2021;22:571.
- Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial iota-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8.
- Petreni A, De Luca V, Scaloni A, et al. Anion inhibition studies of the Zn(II)-bound iota-carbonic anhydrase from the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2021;36:372–6.
- Kusian B, Sultemeyer D, Bowien B. Carbonic anhydrase is essential for growth of Ralstonia eutropha at ambient CO2 concentrations. J Bacteriol 2002;184:5018–26.
- Cronk JD, Endrizzi JA, Cronk MR, et al. Crystal structure of E. coli beta-carbonic anhydrase, an enzyme with an unusual pH-dependent activity. Protein Sci 2001;10:911–22.
- Merlin C, Masters M, McAteer S, Coulson A. Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 2003;185:6415–24.
- Abuaita BH, Withey JH. Bicarbonate Induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 2009;77:4111–20.
- Nishimori I, Minakuchi T, Maresca A, et al. The beta-carbonic anhydrases from Mycobacterium tuberculosis as drug targets. Curr Pharm Des 2010;16:3300–9.
- Kohler S, Ouahrani-Bettache S, Winum JY. Brucella suis carbonic anhydrases and their inhibitors: Towards alternative antibiotics? J Enzyme Inhib Med Chem 2017;32:683–7.
- Singh S, Supuran CT. 3D-QSAR CoMFA studies on sulfonamide inhibitors of the Rv3588c beta-carbonic anhydrase from Mycobacterium tuberculosis and design of not yet synthesized new molecules. J Enzyme Inhib Med Chem 2014;29:449–55.
- Ceruso M, Vullo D, Scozzafava A, Supuran CT. Sulfonamides incorporating fluorine and 1,3,5-triazine moieties are effective inhibitors of three beta-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2014;29:686–9.
- Carta F, Maresca A, Covarrubias AS, et al. Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active beta-carbonic anhydrase from Mycobacterium tuberculosis, Rv3588c. Bioorg Med Chem Lett 2009;19:6649–54.
- Rollenhagen C, Bumann D. Salmonella enterica highly expressed genes are disease specific. Infect Immun 2006;74:1649–60.
- Nishimori I, Minakuchi T, Vullo D, et al. Inhibition studies of the beta-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium with sulfonamides and sulfamates. Bioorg Med Chem 2011;19:5023–30.
- Vullo D, Nishimori I, Minakuchi T, et al. Inhibition studies with anions and small molecules of two novel beta-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium. Bioorg Med Chem Lett 2011;21:3591–5.
- Lotlikar SR, Kayastha BB, Vullo D, Khanam SS, et al. Pseudomonas aeruginosa β-carbonic anhydrase, psCA1, is required for calcium deposition and contributes to virulence. Cell Calcium 2019;84:102080.
- Modak JK, Tikhomirova A, Gorrell RJ, et al. Anti-Helicobacter pylori activity of ethoxzolamide. Journal of Enzyme Inhibition and Medicinal Chemistry 2019;34:1660–7.
- Ronci M, Del Prete S, Puca V, et al. Identification and characterization of the alpha-CA in the outer membrane vesicles produced by Helicobacter pylori. J Enzyme Inhib Med Chem 2019;34:189–95.
- Buzás GM, Supuran CT. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puşcaş (1932-2015). J Enzyme Inhib Med Chem 2016;31:527–33.
- (a) Supuran CT. Emerging role of carbonic anhydrase inhibitors. Clin Sci (Lond) 2021;135:1233–49. (b) Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80.
- Supuran CT. Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2021;30:1197–208.
- Giovannuzzi S, Hewitt CS, Nocentini A, et al. Coumarins effectively inhibit bacterial alpha-carbonic anhydrases. J Enzyme Inhib Med Chem 2022;37:333–8.
- Giovannuzzi S, Hewitt CS, Nocentini A, et al. Inhibition studies of bacterial alpha-carbonic anhydrases with phenols. J Enzym Inhib Med Ch 2022;37:666–71.
- Kaur J, Cao X, Abutaleb NS, et al. Optimization of acetazolamide-based scaffold as potent inhibitors of vancomycin-resistant enterococcus. J Med Chem 2020;63:9540–62.
- Buzas GM. Helicobacter pylori - 2021. Orv Hetil 2021;162:1275–82.
- (a) Abutaleb NS, Elhassanny AEM, Seleem MN. In vivo efficacy of acetazolamide in a mouse model of Neisseria gonorrhoeae infection. Microb Pathog 2022;164:105454. (b) Giovannuzzi S, Abutaleb NS, Hewitt CS, et al. Dithiocarbamates effectively inhibit the α-carbonic anhydrase from Neisseria gonorrhoeae. J Enzyme Inhib Med Chem 2022;37:1–8. (c) Abutaleb NS, Elhassanny AEM, Nocentini A, Hewitt CS, et al. Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. J Enzyme Inhib Med Chem 2022;37:51–61.
- Del Prete S, Bua S, Supuran CT, Capasso C. Escherichia coli gamma-carbonic anhydrase: characterisation and effects of simple aromatic/heterocyclic sulphonamide inhibitors. J Enzyme Inhib Med Chem 2020;35:1545–54.
- Del Prete S, De Luca V, Bua S, et al. The effect of substituted benzene-sulfonamides and clinically licensed drugs on the catalytic activity of CynT2, a carbonic anhydrase crucial for escherichia coli life cycle. Int J Mol Sci 2020;21:4175–88.
- Del Prete S, De Luca V, Nocentini A, et al. Anion inhibition studies of the beta-carbonic anhydrase from Escherichia coli. Molecules 2020; 25:2564.
- Nocentini A, Del Prete S, Mastrolorenzo MD, et al. Activation studies of the beta-carbonic anhydrases from Escherichia coli with amino acids and amines. J Enzyme Inhib Med Chem 2020;35:1379–86.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Del Prete S, De Luca V, Iandolo E, et al. Protonography, a powerful tool for analyzing the activity and the oligomeric state of the gamma-carbonic anhydrase identified in the genome of Porphyromonas gingivalis. Bioorg Med Chem 2015;23:3747–50.
- Del Prete S, De Luca V, Supuran CT, Capasso C. Protonography, a technique applicable for the analysis of eta-carbonic anhydrase activity. J Enzyme Inhib Med Chem 2015;30:920–4.
- Dubois M, Gilles K, Hamilton JK, et al. A colorimetric method for the determination of sugars. Nature 1951;168:167.
