Abstract
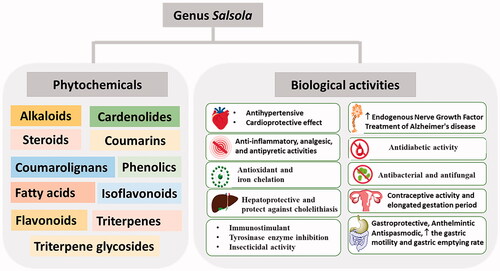
Salsola is an important genus in the plant kingdom with diverse traditional, industrial, and environmental applications. Salsola species are widely distributed in temperate regions and represent about 45% of desert plants. They are a rich source of diverse phytochemical classes, such as alkaloids, cardenolides, triterpenoids, coumarins, flavonoids, isoflavonoids, and phenolic acids. Salsola spp. were traditionally used as antihypertensive, anti-inflammatory, and immunostimulants. They attracted great interest from researchers as several pharmacological activities were reported, including analgesic, antipyretic, antioxidant, cytotoxic, hepatoprotective, contraceptive, antidiabetic, neuroprotective, and antimicrobial activities. Genus Salsola is one of the most notorious plant genera from the taxonomical point of view. Our study represents a comprehensive review of the previous phytochemical and biological research on the old world Salsola secies. It is designed to be a guide for future research on different plant species that still belong to this genus or have been transferred to other genera.
Graphical Abstract
1. Introduction
Plants are considered as a latent treasure and a vital source for the discovery of medicines. They include a plethora of secondary metabolites that act as modulators for the enzymes involved in human diseasesCitation1,Citation2. Plant extracts and their derived natural products or analogues are extensively reported to exert promising effects on human devastating diseases including different types of cancerCitation3–6. They are also reported to protect humans against different types of microbesCitation7 and recently evolved infectious diseases as COVID-19Citation8,Citation9.
The genus Salsola (commonly known as saltwort) belongs to the family Amaranthaceae, previously Chenopodiaceae. The genus name is from the Latin words “salsus” or “sallere” meaning salty because they are halophytes capable of living in saline environments or due to their content of alkaline salts, such as potassium and sodium carbonatesCitation10–12. The old genus Salsola comprised about 150 sp. growing in extreme climatic conditions as arid, semi-arid, and temperate regions worldwideCitation11,Citation13. They represented about 45% of the desert plantsCitation11 and some of them are invasive speciesCitation14. Various plants of the genus Salsola are edible and some of them have been used in traditional medicineCitation15. Some of them are also reported to be rich in fibre contentCitation16. They have important value as animal feed and they are beneficial in the reclamation and phytoremediation of soil contaminated with heavy metalsCitation11,Citation14. Plants belonging to this genus also represent a rich source for endophytic microbes that could be used for potential biological applicationsCitation17,Citation18. Furthermore, different plants of the genus Salsola were reported to have industrial value as the use of S. soda and S. kali as a source of sodium carbonate, in linin, and cotton bleaching, and in glass and soap makingCitation14,Citation19,Citation20.
Despite the importance of plants belonging to the genus Salsola, they do not receive great research attention. Most of the research is done on the respiratory diseases and the hypersensitivity caused by the pollen grains of some Salsola spp. and developing vaccines for itCitation21–23. Very limited reviews are made on the genus Salsola such as the one made by Altay and OzturkCitation11 that discuss its fodder value. Hanif et al.Citation14 discussed the environmental, industrial, and traditional uses of Salsola spp. and they mentioned a small fraction of the biological studies made on them. This article addresses almost all the research articles concerning the phytochemistry and the biological activity of the plants belonging to the old genus Salsola until 2021.
2. Morphological characters
Members of the genus Salsola are shrubs, sub-shrubs, annual or perennial herbs. They are characterised by small, sessile, often succulent leaves that may be opposite or alternate. Most have bisexual axillary flowers that can be solitary or clustered to form loose or dense spikes (). Each flower is subtended by two prominent bracteoles, with a frequently hard 5-segmented perianth (often winged in fruit), and a superior ovary. Seeds are horizontal, subglobose, with a spiral embryoCitation11,Citation24,Citation25.
Figure 1. Photographs of selected Salsola spp.; a. S. kali (adapted from kali https://gobotany.nativeplanttrust.org/sp./salsola/kali/), b. S. collina, c. S. tragus, d. S. imbricata (adapted from https://www.floraofqatar.com/amaranthaceae.htm), e. S. komarovii, f. S. oppositifolia Desf. (adapted from adapted from https://powo.science.kew.org/), g. S. soda (adapted from https://eunis.eea.europa.eu/sp./168053), h. S. laricifolia (adapted from https://panama.inaturalist.org/taxa/985676-Salsola-laricifolia).

3. Taxonomic classification
Genus Salsola belongs to the flowering plant family Amaranthaceae descending from the order CaryophyllalesCitation26. Salsola has a long history of being considered as one of the largest genera within the family Chenopodiaceae containing 100 to 190 sp.Citation27. While it is classified now as one of the Amaranthaceae genera after merging family Chenopodiaceae with the family Amaranthaceae according to the angiosperm phylogeny group (AGP-IV)Citation26,Citation28–30. Plants belonging to the genus Salsola have the following taxonomic classificationCitation27,Citation30–32.
Kingdom: Plantae - Plants
Subkingdom: Tracheobionta - Vascular plants
Superdivision: Spermatophyta - Seed plants
Division: Magnoliophyta - Flowering plants
Class: Magnoliopsida - Dicotyledons
Subclass: Caryophyllidae
Order: Caryophyllales
Family: Amaranthaceae (previously, Chenopodiaceae)
Subfamily: Salsoloideae
Tribe: Salsoleae
Genus: Salsola
The taxonomy of Salsola spp. is debateable and confusing due to their diversity and distribution in the Asian and the middle east deserts that lead to difficulties in their collection and investigationCitation31. The close relationship between Salsola spp. and the dependence on minor morphological differences in their old classification together with the recent use of molecular techniques in plant systematics led to major changes in the classification of the genus SalsolaCitation27. The classification of the genus Salsola has been revised by Akhani et al. (2007) and it was spitted into 10 different genera. The transfer of different sp. from the old world Salsola to other genera, such as Caroxylon genus resulted in decreasing the number of its sp. to 25Citation27.
The type of the genus Salsola was Salsola sodaCitation27,Citation31, which has been recently changed by the International Code of Nomenclature into Salsola Kali as suggested by Mosyakin et al.Citation33. This resulted in changing the name of many traditionally known Salsola spp. into SodaCitation28.
These taxonomical and nomenclatural changes together with the presence of different synonyms for several Salsola spp. would obscure the determination of the phytochemical constituents and the biological activities of the old world Salsola species.
Therefore, in this article, we will review the phytochemical content and the biological activities of the old world Salsola spp. and indicate their current taxonomic status as illustrated in .
Table 1. Current taxonomic status and synonyms of Salsola plants mentioned in this review article.
4. Chemistry
4.1. Volatile constituents
Hexahydro-farnesyl acetone and benzoic acid esters were reported as the major constituents of S. cyclophylla volatile oilCitation15,Citation41. However, GC analysis of the volatile fractions of different parts of S. vermiculate L. plant revealed that carvone and β-caryophylline were the major components in leaves (52.2% and 5.8%, respectively), while carvone and cuminaldehyde were the major components in roots (49.9% and 4.4%, respectively). Additionally, carvone, limonene, and linalool were detected as the major constituents of the stems of S. vermiculate L. (53%, 17.4%, and 11.3%, respectively)Citation42.
4.1.1. Non-volatile constituents
Previous phytochemical investigations of plants belonging to the genus Salsola indicates the presence of diverse groups of secondary metabolites, such as alkaloidsCitation43–49, cardenolides and steroidsCitation50,Citation51, coumarins and coumarolignansCitation52, fatty acidsCitation50,Citation51,Citation53, flavonoids and isoflavonoidsCitation54–59, phenolicsCitation60, and triterpene glycosidesCitation61–64.
4.1.2. Alkaloids and nitrogenous compounds
Different classes of alkaloids and other nitrogenous compounds have been reported from plants of the genus Salsola, . A unique group of optically active l-methyl-tetrahydro-isoquinoline alkaloids have been early detected by Proskurnina and OrekhovCitation45 from Salsola richteri Karel and the isolated alkaloids were identified as carnegine 1.2, salsoline 1.16, and N-norcarnegine (salsolidine) 1.19. The southern Turkmenistan salsola, S. richteri Karel yielded 0.16% of salsolineCitation44. A fourth related derivative, N-methylisosalsoline 1.12, was detected by GC/MS in the aerial parts of S. oppositofolia, S. soda and S. tragusCitation65. In addition, 3,4-dihydro-6,7-dihydroxy-1(2H)-isoquinolinone; namely pericampylinone-A (iseluxine) 1.14, was also isolated from S. collina Pall.Citation66. The presence of optically active (-) pyrrolo[2,1-a]isoquinoline type alkaloids has been reported from S. collina Pall.Citation43,Citation45,Citation47–49. Particularly, Zhao and DingCitation47 isolated and identified the first alkaloid of this group namely, salsoline A (trolline) 1.17; (S)-8,9-dihydroxy-1,2,5,6-tetrahydropyrrolo[2,1-a]isoquinolin-3(10bH)-one followed by Xiang et al.Citation43 who were able to isolate and identify another related positional isomer namely; salsoline B 1.18 from the same plant. Another group of nitrogenous derivatives, moupinamides has been reported from different Salsola spp. in both free and combined (glucoside) forms. They possess a skeleton of N-trans-feruloyltyramine or N-trans-feruloyldopamine structures. The structures of N-trans-feruloyl-3-O-methyldopamine 1.9 and N-trans-feruloyl-3′″-methoxydopamine 4′-O-β-D-glucopyranoside 1.6, were reported in S. collinaCitation43 whereas, N-trans-feruloyltyramine 1.13 and 7′-hydroxy N-trans- feruloyltyramine 1.10, were found in S. collina and S. tetrandraCitation43,Citation53,Citation66. Also, trans-N-feruloyl tyramine-4′″-O-β-D-glucopyranoside 1.7, was reported from S. inermis ForsskCitation51. The only reported moupinamide derivative with a "cis" double bond configuration of the cinnamoyl moiety was cis-N-feruloyltyramine 1.5 which was isolated from the aerial parts of S. baryosomaCitation67. It is worth noting that several tentatively (incompletely) defined structures were reported by UPLC/qTOF-MS analysis of the aerial parts and roots of S. vermiculata and S. tetrandraCitation68. They included N-caffeoyl tyramine, N-(3′,4′-dimethoxy-cinnamoyl)-norepinephrine, N-(4′-methoxy-cinnamoyl)-norepinephrine, N-feruloyl-3′″-methoxytyramine However, further spectral analysis, such as 1 D and 2 D NMR are required to confirm their structures.
Figure 2. Structures of alkaloids and nitrogenous compounds (1.1–1.23) reported in the genus Salsola.

Another miscellaneous group of nitrogenous compounds was reported from different Salsola spp., including simple nitrogenous compounds, such as methyl carbamate 1.11 from S. tetrandra, S. kali, S. longifolia and S. rigidaCitation69. The amino acid derivative, N-acetyltryptophan 1.1 was isolated from S. collina Pall. and S. grandis Freitag, Vural & AdiguzelCitation66,Citation70. Pericampylinone-A 1.14, terrestric acid 1.20, uracil 1.22, and uridine 1.23 were reported by Jin et al.Citation66 from S. collina Pall. While salisomide 1.15 was reported by Saleem et al.Citation57 from S. imbricata Forssk. The alkylamine, tridecanamine 1.21, was also reported from the aerial parts of S. terrandra ForsskCitation71.
4.1.3. Cardenolides and steroids
Steroids are a group of natural products biosynthesized from the isoprenoid pathway via the 2,3-oxidosqualene (C30) route. Cardenolides are cardioactive steroidal lactones with a 5-membered (furanones) or 6-membered (pyranone) ring at C-17. They are naturally present free or glycosylated with mono- or multi-sugar moieties. Several families are known for their high cardenolides content, such as Asclepidaceae, Apocynaceae, and othersCitation72. However, only one report on cardenolides from the Amaranthaceae family has been described. It addressed the isolation of five cardenolides, salsotetragonin 2.1, calactin 2.2, 12-dehydroxyghalakinoside 2.3, desglucouzarin 2.4, and uzarigenin 2.5 from the Algerian plant, Salsola tetragona Delile, Citation50. Other reported steroids comprised several phytosterols with diversity in the alkyl side chains at C-17, including campesterol 2.6, cholesterol 2.7, and desmosterol 2.8 from S. collinaCitation73, β-sitosterol 2.9, stigmastanol 2.10, and stigmasterol 2.11, in addition to a combined phytosterol, stigmasterol-3-O-β-D-glucopyranoside 2.12 from the aerial parts of S. inermisCitation51.
The existence of fatty acid esters or acylated sterols was reported by Mayakova et al.Citation73 from the genus Salsola. They investigated the contents of the saponified acylsterols fraction of the pentane extract of S. collina. The neutral fraction indicated the presence of four sterols, including β-sitosterol, stigmasterol, cholesterol, and campesterol, whereas the acyl fraction of the hydrolysed esters composed of stearic, palmitic, and oleic acidsCitation73.
4.1.4. Coumarins and coumarinolignans
Coumarins are bioactive secondary metabolites biosynthesized in plants from the phenylpropanoid (C6C3) pathway by cyclisation of cinnamic acid. They contribute to diverse biological activities, such as anticoagulant, antimicrobial, antiviral, and anticancer activitiesCitation74. Several studies reported the presence of simple coumarins in members of the genus Salsola. These reported coumarins are either free or glycosylated with mostly methoxylated C-6 and oxygenated C-7 positions. Two simple coumarins, namely umbelliferone 3.1 and scopoletin 3.2 were reported from the aerial parts of S. inermisCitation51. Whereas S. kali showed the presence of fraxidin 3.3Citation75 . However, the highest record of coumarins from this genus was noted to S. laricifolia that included several simple coumarins (3.3–3.10) and two unusual coumarinolignans; cleomiscosin B 3.11, cleomiscosin D 3.12, formed by the association with another cinnamic acid moiety (C6C3)Citation52. Calycantoside 3.10, a compound possessing the structure of 6,8-dimethoxy-coumarin-7-O-β-glucopyranoside was reported with the miss-spelled name, calicantoside from the epigeal (aerial) parts of S. laricifoliaCitation76 .
4.1.5. Fatty acids and their derivatives
Few saturated fatty acids compared to unsaturated ones were reported from Salsola plants, and . Ghorab et al.Citation50 reported the isolation of the fatty acid ester, 2,3-dihydroxypropylpalmitate 4.1 from the aerial parts of S. tetragona. Whereas free palmitic acid 4.10, in addition to three unsaturated fatty acids, including linoleic, linolenic, and oleic acids (4.5, 4.6, and 4.9, respectively) were detected by UPLC/qTOF-MS analysis of S. vermiculata and S. tetrandraCitation68. Also, oleic acid 4.9 was isolated from the aerial parts of S. tetragonaCitation50. A characteristic group of trihydroxylated mono-, di-, and tri-unsaturated fatty acids was reported from several plants of the genus Salsola, including 9,12,13-trihydroxyoctadeca-10(E),15(Z)-dienoic acid 4.13 and 9,12,13-trihydroxy-10(E)-octadecenoic acid 4.14 from the aerial parts of S. tetrandraCitation53 and 9,12,13-trihydroxydocosan-10,15,19-trienoic acid 4.15 from the aerial parts of S. inermisCitation51. Additionally, several fatty acids, including hydroxyoctadecenoic acid, dihydroxyoctadecenoic acid, hydroxyoctadecatrienoic acid, hydroxyoctadecadienoic acid, and trihydroxyoctadecadienoic acid were also tentatively identified from the aerial parts and roots of S. vermiculata and S. tetrandra by UPLC/qTOF-MS analysis methodCitation68.
Table 2. Non-volatile constituents from the genus Salsola.
4.1.6. Flavonoids and isoflavonoids
Flavonoids and isoflavonoids are predominant plant polyphenols having a C6-C3-C6 skeleton and are considered as one of the frequently studied plant phytochemicalsCitation94. Flavonoids are yellow-colored compounds possessing a highly distinctive biosynthetic pathway as they are synthesised from the mixed phenylpropanoid (4-coumaroyl-CoA) and polyketide (3 malonyl-CoA) pathwayCitation95. The isoflavonoids subclass is characterised by the presence of a 2-phenyl instead of 3-phenyl substitution at the benzo-γ-pyrone moietyCitation94. Concerning the biological activities, flavonoids are the main dietary antioxidants due to their action as scavengers of harmful free radicals. In addition, they act as signalling molecules by their modulatory effect on several protein kinases, such as MAP kinase (mitogen-activated protein kinase). The latter mechanism can explain their neuroprotection, cardioprotection, and anticancer activitiesCitation96. Isoflavonoids are much limited in their distribution in plant families (e.g. Leguminosae) compared to flavonoids and are characterised by their phytoestrogenic activity as in the case of genisteinCitation97. In the genus Slasola, the reported flavonoids () can be classified into flavones (such as apigenin 5.1, chrysin 5.2, luteolin-7-O-β-D-glucoside 5.17, and tricin 5.28, from S. imbricata Forssk, S. kali L., and S. collina Pall., respectivelyCitation60,Citation66,Citation75,Citation84, flavonols (such as isorhamnetin 5.4, quercetin 5.18, and kaempferol derivatives 5.13–5.16), flavanols (such as catechin 5.33), and flavanones (such as hesperidin 5.34, hesperitin 5.35, and naringenin 5.36). The free flavonol aglycone, kaempferol was incompletely identified by UPLC/qTOF-MS analysis of the aerial parts and roots of S. vermiculata and S. Tetrandra plantsCitation68. The presence of OCH3 groups (i.e. methoxylated flavonoids) was mainly observed at C-3`and C-4′ in the B-ring of flavones (in tricin and its derivatives 5.28–5.32), and at C-3′ of flavonols (in the isorhamnetin derivatives 5.4–5.13). However, diversity in methoxylation positions was recorded for the isoflavonoids group (5.37–5.52), as both the A-ring (positions C-5, 6, 7, and 8) and the B-ring (positions C-2′, 3′, and 5′) acquired OCH3 groups. For detailed references and the plant source, see . Finally, a unique 8,2′-dimethoxylated isoflavan derivative, salisoflavan 5.53, was reported from the arial parts S. imbricata ForsskCitation57.
4.1.7. Lignans
Lignans are natural secondary metabolites biosynthesized from the oxidative coupling of two p-hydroxyphenylpropane moieties (C6-C3) linked by a bond connecting the middle (β-β`) carbons of their side chainsCitation98. Regarding the genus Salsola, six derivatives from two major subclasses, lignans and cylolignans, were identified. For the lignans subclass, three tetrahydrofuran derivatives, alangilignoside C 6.2, conicaoside 6.3, and lariciresinol-9-O-β-D-glucopyranoside 6.5 were isolated from the aerial parts of S. komaroviiCitation89. Regarding the cylolignans subclass, two tetrahydronaphthalene derivatives, namely (8S,8`R,7`R)-9′-[(β-glucopyranosyl)oxy]lyoniresinol 6.4 and (+)-lyoniresinol 9′-O-β-D-glucopyranoside 6.6, were isolated from the same plantCitation89, and . In addition, another bicyclolignan derivative having a 3,7-dioxabicyclo[3.3.0]octane ring system, namely acanthoside D 6.1 was isolated from S. collina plantCitation60.
4.1.8. Triterpenoids and their derivatives
Triterpenoids are structurally diverse widely distributed natural phytochemicals possessing a C30-skeleton and are biosynthesized from the isoprenoid precursor, squaleneCitation99. Pentacyclic triterpenoids of the C–C–C–C(–C) 6–6-6–6-6 rings were reported in some Salsola spp. categorised as triterpenoids and nortriterpenoids ( and ). The triterpenoids group included mainly ursane, and oleanane skeletons, both free and combined. However, oleanane derivatives are the predominant group. Free hydroxylated oleanolic acid/derivatives are represented by guavenoic acid 7.2, 1α,2α,3β,19α,23-pentahydroxyursa-12,20(30)-dien-28-oic acid 7.8, salsolin A 7.10, and salsolic acid 7.12 were isolated from S. baryosmaCitation90 and oleanolic acid 7.6 from S. inermis and S. sodaCitation10,Citation51. Whereas, only olean-12-en-3,28-diol 7.5 found in S. inermis showed the presence of a primary alcoholic group (28-CH2OH) instead of a COOH at C-17 51. One ursane derivative, namely salsolin B 7.11 was identified from S. baryosmaCitation90. Concerning the reported combined triterpenoids, two positions of the triterpenoid's skeleton were noticed to possess sugar moieties; the first position is C-3 that showed the presence of a sugar chain of variable length ranging from 1–3 sugars (e.g. glucose, xylose, and glucuronic acid). The second one is C-28 which showed the presence of glucosyl esters. Of these saponins, three characteristic salsolosides were reported, including salsoloside C 7.13 from S. micranthera Botsch, S. grandis Freitag, Vural, and S. sodaCitation10,Citation62,Citation70,Citation77, salsolosides D 7.14, and E 7.15 from S. micranthera BotschCitation63. Two 3-β-hydroxy 30-noroleana-12,20(29)-dien-28-oic acid (syn. akebonic acid) derivatives were isolated from the roots of S. imbricata Forssk and identified as 3-O-β-D-glucuronopyranosyl-30-norolean-12,20(dien-28-O-[β-D-glucopyranosyl] ester 7.17 and 3-O-β-D-xylopyranosyl-(1 → 2)-O-β-D-glucuronopyranosyl-akebonic acid 28-O-β-D-glucopyranoside 7.18Citation61.
4.1.9. Phenolic acids and simple phenols
Simple phenols are a minor class of natural products defined as aromatic compounds with at least one hydroxyl group attached to a benzene ring, such as catechol, resorcinol, and phloroglucinol. However, phenolic acids/derivatives represent a major class of plant-derived natural products, categorised into benzoic acids, such as protocatechuic and gallic acids (C6-C1) and cinnamic acids, such as caffeic and coumaric acids (C6-C3)Citation100. HPLC analysis of the aerial parts and root of S. kali revealed the presence of two simple phenols viz, catechol 8.6 and resorcinol 8.21Citation12,Citation75. The presence of simple aromatic aldehydes was reported from S. tuberculatiformis Botsch. (4-hydroxybenzaldehyde 8.15) and S. collina Pall. (protocatechuic aldehyde 8.19 and vanillin 8.29)Citation66. However, diverse benzoic acids were found in several plants of the genus Salsola, the most characteristic of which are gentisic acid 8.12, α-resorcylic acid 8.22, and β-resorcylic acid 8.23 from the herb and root of S. kaliCitation12, and the dihydrostilbene, tetranin A 8.27 from the roots of S. tetrandra FolskCitation59. In addition, various free cinnamic acids and their esters were reported from the plants of this genus. Regarding free cinnamic acids, previous phytochemical studies on S. kali, S. imbricata Forssk, S. vermiculata, S. tetrandra, S. cyclophylla, and S. collina Pall. showed the presence of caffeic 8.4, cinnamic 8.8, p-coumaric 8.9, and ferulic acids 8.10Citation12,Citation15,Citation66,Citation68,Citation80,Citation84. Whereas cinnamic acid esters were described in two Salsola spp. viz., S. cyclophylla and S. imbricata Forssk., including β-phenylethyl caffeate 8.5, chlorogenic acid 8.7, and rosmarinic acid 8.24Citation15,Citation84, and .
4.1.10. Miscellaneous glycosides
Several miscellaneous glycosides with both phenolic and isoprenoid aglycones were reported from several plants of the genus Salsola. The glycone part in most cases is either glucose or β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranose. The phenolic glycosides, benzyl 6-O-β-D-apiofuranosyl-β-D-glucopyranoside 9.1, biophenol 2 9.2, cuneataside C 9.9, and 2–(3,4-dihydroxy)-phenyl-ethyl-β-D-glucopyranoside 9.10 were isolated from the aerial parts of S. komaroviiCitation89. The cyanogenic glycosides, taxiphyllin 9.17 and 3,4,5-trimethoxyphenyl-β-D-glucopyranoside 9.18 were reported in the aerial parts of S. tetrandraCitation53. Whereas the isoprenoid glycosides comprised the acyclic monoterpene, 9-hydroxylinaloyl glucoside 9.11 from S. tetrandraCitation53, in addition to several ionone derivatives with different unsaturation and oxidation status, such as roseoside A 9.4 and blumenyl B β-D-glucopyranoside 9.5 from S. komaroviiCitation89 and the epoxy derivatives icariside B2 9.12 and lyohebecarpin A 9.14 from S. komarovii and S. tetrandra, respectivelyCitation53,Citation89 were reported, and .
4.1.11. Biphenylpropanoids
Biphenylpropanoids ( and ) were isolated from the aerial parts of S. villosa Delile. ex Schul. and the roots of S. imbricata. They are formed of dimeric C6C3 residues (linked head to head) with a characteristic oxirane ring formed by epoxidation of either one of the side chains' double bond as in biphenylsalsinol 10.1Citation91 and biphenylsalsonoid A 10.2Citation92 or both as in case of biphenylsalsonoid B 10.3Citation92.
4.1.12. Polyhydric alcohols and carbohydrates
Syrchina et al.Citation93 described the presence of a few monosaccharide derivatives, including two simple ethyl glucosides namely, ethyl β-D-fructopyranoside 11.1 and ethyl β-D-glucopyranoside 11.2 from S. collina Pall. In addition, they reported the presence of two polyhydric alcohols (D-mannitol 11.5 and myoinositol 11.6) from the same plantCitation93, and .
4.1.13. Miscellaneous group
Only two compounds are included in this group; the first one is a dimeric methylcyclopentenyl alcohol namely, salsolanol 12.1 isolated from the aerial parts of S. villosa Delile. ex Schul. Citation91. While, the second compound is an isohexyl 2- pentyl ester of sulphurous acid 12.2 detected by GC-MS analysis of the aerial parts of S. tetrandraCitation71, and .
5. Pharmacological activities
Plants of the genus Salsola are widely used in the folk medicine of different countries for the treatment of several diseases, such as hypertension, broken bones as well as for boosting the immunity ().
Table 3. Traditional medical uses of Salsola species.
Research studies showed that extracts of different Salsola spp. and compounds isolated from them exert a wide range of variable pharmacological activities. These activities will be discussed in detail in this section. They are also summarised in and .
Table 4. Reported pharmacological activities of Salsola species.
5.1. Effect on the cardiac system and blood pressure
One of the early reported pharmacological activities of Salsola spp. is their antihypertensive action. Different Salsola spp. are used as ingredients in different Chinese patents obtained from Faming Zhuanli Shenqing for treating hypertension. Of these, S. collina was the most extensively used sp. as indicated by the number of patents addressed this particular plant. Also, S. ruthenica and S. arbuscula were used in some Chinese patents. Likewise, S. ruthenic, a synonym for S. tragus, was reported as a potential treatment for essential hypertensionCitation105. Ammon et al.Citation81 attributed the antihypertensive activity of S. kali and S. longifolia Forsk to salsoline 1.16 and salsolidine 1.19 alkaloids due to their ability to stimulate respiration and to decrease blood pressureCitation81.
Loizzo et al.Citation106 investigated the inhibitory activity of different extracts of the aerial parts of S. oppositifolia Desf., S. soda L., and S. tragus against the angiotensin-converting enzyme (ACE). The ethyl acetate extracts of S. oppositifolia and S. soda showed interesting activities with IC50 values of 181.04 and 284.27 µg/mL, respectively which further support the traditional antihypertensive use of these species.
The aqueous extract of the whole shrub of S. kali was reported to display a cardioprotective effect against adriamycin-induced cardiotoxicity in male Swiss albino miceCitation107. This effect was attributed to lowering the oxidative stress in the heart and inhibiting lipid peroxidationCitation107.
5.2. Anti-inflammatory, analgesic, and antipyretic activities
Janbaz et al.Citation108 tested the aqueous-ethanol extract of the aerial parts of S. imbricata to assess its traditional use in inflammatory conditions. They confirmed the anti-inflammatory activity of S. imbricata as it significantly inhibited carrageenan-induced paw edoema in rats. The same research group also tested the analgesic activity of S. imbricata extract using NaCl-induced writhing and formalin-induced paw licking models in rats. Their obtained results indicated that S. imbricata exhibited a dose-dependant analgesic activity by reducing the number of abdominal writhing mediated by 4% NaCl intraperitoneal injection at all tested doses (100, 300, and 500 mg/kg)Citation108. Nevertheless, it decreased the time of paw licking by rats only at the dose of 500 mg/kg. Also, S. imbricata showed significant antipyretic activity in the brewer’s yeast-induced pyrexia model in ratsCitation108. The aqueous methanolic extract of S. imbricata leaves and the phenolic compounds isolated from it decreased the NO production levels in in-vitro LPS-induced inflammation in RAW 264.7 macrophage cells and were found to be non-toxic at the concentration of 100 µg/mLCitation80. Regarding the tested phenolic compounds, isorhamnetin-3-O-glucopyranoside 5.8 displayed higher activity than its corresponding galactopyranoside glycoside 5.7 and aglycone 5.4Citation80.
The anti-inflammatory and antinociceptive activities of S. grandis were tested using the carrageenan-induced paw edoema model in rats and p-benzoquinone-induced nociception tests in mice, respectivelyCitation77. The ethanolic extract obtained from the aerial parts of S. grandis was fractionated and the most bioactive fraction (n-BuOH) was further subjected to a bioassay-guided fractionation to isolate the compounds responsible for S. grandis’s activity. The flavonoidal compounds, tiliroside 5.14 and quercetin-3-O-β-D-galactoside 5.19 displayed the highest activities in the used modelsCitation77.
The anti-inflammatory activity of different extracts of the aerial parts of S. cyclophylla was evaluated by Mohammed et al.Citation15 using the carrageenan-induced paw edoema method. The aqueous-ethanolic extract showed the highest anti-inflammatory activity among the tested extracts and its activity was close to the well-known anti-inflammatory drug, diclofenac. Mohammed et al. attributed this anti-inflammatory activity to the antioxidant potential of the phenolic and flavonoid components present in the aqueous-ethanolic extract. The same research group also investigated the analgesic activity of S. cyclophylla using the hot-plate and acetic-acid writhing models in mice. The aqueous ethanolic extract showed the highest activity with 87.50– 99.66% pain reduction rates after different time intervals, which was comparable to the diclofenac activityCitation15.
Seo et al.Citation37 reported that the ethanol extract of S. komarovii showed effective anti-inflammatory activity as hydrocortisone by reducing the production of LPS-induced IL-6. It also exerted glucocorticoid receptor binding activity and interfered with NF-κB nuclear translocationCitation37.
The synthetic analogue of the active principle of S. tuberculata, 2–(4-acetoxyphenyl)2-chloro-N-methylethylammonium-chloride, was reported to inhibit UVB induced intracellular interleukin-1 alpha (icIL-1α) in the UVB in-vitro model for inflammationCitation122. Contrarily, the methanol extract of S. tuberculata exerted a pro-inflammatory activity by boosting the UVB induced-icIL-1α production and enhanced cytotoxicity. While the dichloromethane extract showed no significant effect on skin cells inflammationCitation122. The investigated synthetic analouge was also suggested to exert its anti-inflammatory and contraceptive activities by competitive inhibition of glucocorticoid binding to corticosteroid-binding globulin (CBG) leading to increased levels of the in-vivo free corticosteroneCitation123,Citation124.
5.3. Antioxidant and iron chelation activities
The antioxidant potential is one of the most extensively studied activities of Salsola species. It could be concluded from the reported results that the used plant parts and the extraction solvent could greatly affect the antioxidant activity. Flavonoids and their glucosidal derivatives are mostly the responsible compounds for antioxidant activities. While other compounds, such as essential oil components, alkaloids, and biphenylpropanoids showed only moderate activities.
The antioxidant activity of S. cyclophylla extracts was tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) colorimetric assay methodCitation15. The best DPPH-free radicals scavenging potential was observed for the aqueous-ethanolic extract that showed comparable activity to the used standard, quercetin. While, the ethyl acetate extract showed the highest ferrous ions (Fe2+) chelating activity using ferrozine-based assayCitation15. The same group reported the antioxidant activity of the essential oil obtained by water distillation of S. cyclophylla that showed only one-half of the quercetin activity. They attributed this activity to the benzoic acid esters and the hexahydrofarnesyl acetone components that occur in the essential oil in high concentrationsCitation41.
Antioxidant and iron chelation activities of the methanolic extract of different plant parts of S. kali were also investigated by Boulaaba et al.Citation75 using the same methods used for S. cyclophylla extracts. Leaf and stem extracts showed the highest antioxidant activity while leaf and root extracts showed the highest iron chelation activityCitation75.
The alkaloidal extracts of S. oppositofolia, S. soda, and S. tragus were prepared by extraction of their aerial parts with methanol, alkalinization with NH4OH then extraction with ethyl acetate. The three alkaloidal extracts showed significant antioxidant activity when tested using the DPPH method. Remarkably, S. oppositifolia showed the highest activity with an IC50 value of 16.30 µg/mLCitation48.
Oueslati et al.Citation92 investigated the antioxidant activity of biphenylsalsonoids A (10.2) and B (10.3) isolated from the ethyl acetate fraction of the roots of S. imbricata using DPPH and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS+) assay methods. The two compounds showed moderate antioxidant activityCitation92. Trans-N-feruloyltyramine derivatives isolated from S. foetida (1.3, 1.4, and 1.8) exhibited moderate antioxidant activity with IC50 ranging from 378 to 427 µM using DPPH radical scavenging assayCitation78.
The ethyl acetate extract of S. komarovii aerial parts was subjected to HPLC separation and the obtained elutes were tested for antioxidant activity using ABTS+ radical scavenging method. The components responsible for the antioxidant activity were identified by HPLC-MS as the flavonoids, isorhamnetin 5.4, astragalin 5.15, isoquercitrin 5.20, and rutin 5.25Citation79.
The ethyl acetate fraction of S. baryosma showed 77% DPPH radicals scavenging activity while other tested fractions showed lower activities below 57%Citation109. This result is contradictory with that obtained by Khacheba et al.Citation125 who reported weak antioxidant activity of S. baryosma ethyl acetate extract using DPPH assay.
The antioxidant activity of 80% (v/v) aqueous-methanol extracts of S. vermiculata and S. baryosma in addition to other Algerian herbs was tested using the hydroxyl (OH•), nitroxide (NO•) and (ABTS+) radicals scavenging assays, and Fe3+–TPTZ complex reductive power assay. The results showed that S. baryosma exhibited the highest antioxidant activity in OH• radical assay with an EC50 of 0.26 ppm despite its low phenolic contentCitation22.
Beyaoui et al.Citation59 investigated the antioxidant activity of two compounds, tetranins A and B, isolated from the ethyl acetate extract of S. tetrandra roots using DPPH and ABTS assays. The dihydrostilbene, tetranin A 8.27 exerted higher antioxidant activity than the isoflavonoid, tetranin B 5.37. However, both compounds showed lower activity than the standard antioxidant, TroloxCitation59.
The ethanol extract of S. collina Pall demonstrated anti-oxidative activity through DPPH radical scavenging capacity (Oh et al., 2014).
5.4. Cytotoxic activity
Only a few studies were made for investigating the cytotoxic activity of a small number of Salsola spp., including S. cyclophylla, S. oppositifolia, S. collina Pall, and S. baryosma. The cytotoxic activity of 95% aqueous-ethanolic extract of the aerial parts of S. cyclophylla was investigated using MTT assay against M14 melanoma derived epithelial breast cancer (MDA cells), human pancreatic cancer (PANC-1), Michigan Cancer Foundation-7 (MCF-7) breast cancer cells, and the normal human fibroblast cells. The aqueous-ethanolic extract of S. cyclophylla showed low to moderate cytotoxic activity only at high concentrations (50–400 µg/mL) against the tested cell lines and no significant cytotoxic effect was observed at low concentration (< 50 µg/mL)Citation15.
Different fractions obtained from the extract of the aerial parts of S. oppositifolia were screened for cytotoxic activity against a panel of cancer cell linesCitation85. The n-hexane fraction showed the highest cytotoxic activity on lung carcinoma (COR-L23) and amelanotic melanoma (C32) cell lines with IC50 values of 19.1 μg/mL and 24.4 μg/mL, respectively. The dichloromethane fraction also demonstrated cytotoxic activity against these two cell lines with IC50 values of 30.4 μg/mL and 33.2 μg/mL for COR-L23 and C32 cell lines, respectively. The ethyl acetate fraction exhibited a selective moderate cytotoxic activity against breast cancer, MCF-7 cells (IC50 67.9 μg/mL). The major constituents isolated from the ethyl acetate fraction, isorhamnetin-3-O-glucopyranoside 5.8 and isorhamnetin-3-O-rutinoside 5.5 also demonstrated a potential activity against MCF-7 with IC50 values of 18.2 and 25.2 μg/mL, respectively. Additionally, isorhamnetin-3-O-rutinoside 5.5 showed high activity against the hormone-dependent prostate carcinoma cell line (LNCaP) with an IC50 value of 20.5 μg/mLCitation85.
The ethanol extract of S. collina Pall showed cytotoxic activity against human colon carcinoma cells (HT29). It resulted in a reduction in the number and size of the cells through cell cycle regulation and caused cell arrest in the G2/M phaseCitation110.
The ethanol extract of S. baryosma whole plant showed no significant cytotoxic activity when tested with other plant extracts using the brine shrimp methodCitation126. The same result was reported by Ahmed et al.Citation109, while 80% ethanol extract of S. baryosma did not exhibit cytotoxic activity against brine shrimp larvae and only the ethyl acetate fraction showed 50% cytotoxic activity. However, all tested fractions of S. baryosma showed phytotoxicity against Lemna minor plant growthCitation109.
5.5. Effect on the immune system
Interestingly, S. laricifolia Turcz is reported to be one of the immune system-boosting drugs, and a pharmaceutical product derived from it “Salimon” represents one of the best-selling immunostimulant drugs in the Mongolian drug marketCitation76.
5.6. Effect on the liver and the gallbladder
Lochein, a liquid extract of the Russian thistle S. collina Pall., was reported to show a significant hepatoprotective effect on patients with chronic hepatitisCitation127. It also has been approved as an active food supplement by the Ministry of Health of the Russian FederationCitation111. Ethanol extract (25%) of the aerial parts of S. collina Pall. was reported to decrease the signs of paracetamol-induced liver damage in rats and to exert a better hepatoprotective activity than the reference drug, silymarinCitation111. It was also reported to decrease the levels of the liver enzymes and lipid peroxidation products and to enhance the detoxification of bilirubin, and ammoniaCitation111. Moreover, S. collina aqueous extract was reported to protect against cholelithiasis in rabbits through enhancing cholesterol and water absorption and decreasing inflammation and formation of biliary sloughCitation112.
Oral administration of S. imbricata methanol extract was reported to prevent liver toxicity in CCl4-induced hepatotoxicity in mice. This hepatoprotective activity was attributed to the ability of the phenolic content of S. imbricata to enhance the antioxidant capacity of the liverCitation84.
Ethanol extracts (70%) of S. tetrandra and S. baryosma showed a prophylactic and therapeutic hepatoprotective activity against paracetamol-induced hepatorenal toxicity in ratsCitation113. The results showed that S. tetrandra was more active and showed a higher ability to decrease the levels of inflammatory markers, such as interleukin-1β (IL-1β) and tumour necrosis factor alpha (TNF-α)Citation113.
The alcoholic extracts of S. volkensii and S. villosa showed hepatoprotective effects with a broad safety margin against CCl4-induced hepatotoxicity in Sprague Dewaly rats indicating their potential use for the treatment of liver damageCitation114,.
5.7. Effects on the gastrointestinal system
Different plants of the Salsola genus were reported to exert several effects on the gastrointestinal tract, including gastroprotective activity against ulcer, anthelmintic, and antispasmodic activities.
Alcoholic extract (50%) of S. komarovi in 500 mg/kg concentration was found to significantly protect against gastric ulcer and to be more potent than Ranitidine (300 mg/kg) in 60% HCl-ethanol induced gastritis modelCitation115. While 70% alcoholic extract of S. tetrandra showed a similar gastroprotective effect to that of Ranitidine against aspirin-induced gastric ulceration in ratsCitation71.
Chloroform extract of S. imbricata bark demonstrated anthelmintic activity against Haemonchus contortus wormsCitation116. Ethanol extract (80%) of S. baryosma (synonym for S. imbricata) demonstrated antispasmodic activity as it inhibited the rabbit jejunum contraction at a concentration of 0.3–3 mg/mLCitation109. It was suggested to act as a calcium channel blocker because it resulted in 70% inhibition of K+-induced contractions in rabbit jejunum at the concentration of 1–5 mg/mLCitation109. The ethyl acetate fraction of the aerial parts extract of the same sp. showed the highest spasmolytic and bronchorelaxant activities on isolated rabbit jejunum and tracheal preparations which were suggested to be due to its agonist action on β-adrenergic receptors and Ca+2 antagonising activityCitation117.
On the other hand, the ethyl acetate extract of S. collina was reported to increase the gastric motility and gastric emptying rate through activating M-cholinergic receptor, increasing ghrelin and gastrin plasma levels and increasing the expression of the vasoactive intestinal peptide receptors in ratsCitation118,Citation128.
5.8. Antidiabetic activity
Decreasing post-prandial hyperglycaemia by inhibiting digestive enzymes involved in carbohydrate hydrolysis, such as α-amylase and α-glucosidase enzymes is a commonly used therapeutic approach for the management of diabetes. Therefore extensive studies were made on the α-amylase and α-glucosidase inhibitory activity of different Salsola spp.Citation22,Citation65.
The α-amylase inhibitory activity of different fractions of the aerial parts of S. kali, S. soda, and S. oppositifolia was investigated by Tundis et al.Citation65. The ethyl acetate fraction of S. kali showed the highest α-amylase inhibitory activity with an IC50 value of 0.022 mg/mL. The bioassay-guided chromatographic separation of this most active fraction resulted in the isolation of two flavonol glycosides, of which isorhamnetin-3-O-rutinoside 5.5 displayed significant α-amylase inhibitory activity with an IC50 value of 0.129 mMCitation65.
Djeridane et al.Citation22 investigated the antidiabetic potential of the aqueous-methanol extracts of S. vermiculata and S. baryosma by testing their ability to inhibit α-amylase and α-glucosidase enzymes activities. The results indicated that S. baryosma exhibited the highest competitive inhibitory activity with inhibition constant (Ki) values of 7 and 16 µM against α-amylase and α-glucosidase, respectively suggesting its potential for type 2 diabetes managementCitation22. Similarly, N-acetyltryptophan 1.1 isolated from S. collina Pall by Jin et al.Citation66 showed 44% inhibition of α-amylase enzyme activity.
Iannuzzi et al.Citation10 studied the chemical profile of the cultivated buds of S. soda and compared it to that of the wild plant. They also screened the inhibitory activity of the compounds isolated from their n-BuOH fraction against three enzymes of the aldo/keto reductase superfamily, namely aldose reductase (hAKR1B1), aldose-reductase-like protein (hAKR1B10), and carbonyl reductase 1 (hCBR1). They found that quercetin-3-O-glucuronopyranoside 5.22, the only flavonoid identified in both plant types was the most effective inhibitor for the tested enzymes and suggested its use as a functional nutraceutical to counteract diabetic complicationsCitation10.
5.9. Effect on neurodegenerative diseases
The effect of the isolated compounds from the methanol extract of S. komarovii aerial parts on the production of the endogenous Nerve Growth Factor (NGF) in C6 glioma cells was investigated by Cho et al.Citation89. The lignan derivative, conicaoside 6.3 showed the highest NGF-production stimulating activity and the lowest toxicity among the tested compounds indicating its potential for the regulation of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseasesCitation89. Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases that is combined with acetylcholine deficiency. Therefore, it can be improved by inhibiting the enzymes affecting the cleavage of acetylcholine, such as acetylcholinesterase (AChE) and butyrylcholinesterase (BChE).
The ethanolic extract of the aerial parts of S. grandis and the different compounds isolated from its n-BuOH sub-extract were investigated for AChE inhibitory activity by Orhan et al.Citation70. Only N-acetyltryptophan 1.1 showed AChE inhibitory activity suggesting its neuroprotective potential against Alzheimer’s diseaseCitation70.
The methanolic extract of S. vermiculata root demonstrated strong anti-acetylcholinesterase inhibitory activity which was higher than that of S. vermiculata aerial parts and S. tetrandra roots and aerial parts. It showed an IC50 of 0.45 ± 0.17 mg/mL. While the standard drug, eserine showed IC50 of 0.27 ± 0.1 mg/mLCitation68. This activity could be attributed to the rich catecholamines content in S. vermiculata rootCitation68.
The alkaloidal extracts of S. tragus, S. soda, and S. oppositifolia Desf. were screened for AChE and BChE inhibitory activitiesCitation48. S. tragus showed the highest inhibitory activity with IC50 of 30.2 and 26.5 µg/mL against AChE and BChE, respectively. While S. soda and S. oppositifolia Desf. showed selective inhibition of BChE with IC50 values of 34.3 and 32.7 µg/mL, respectivelyCitation48. Salsolic acid 7.12 and other two triterpenes 7.2 & 7.8 isolated by Ahmad et al.Citation90 from the chloroform extract of S. baryosma were reported to inhibit the BChE enzymeCitation90.
5.10. Effect on fertility
The contraceptive activity of Salsola plants was firstly described by Ploss in 1960. He reported the use of the aqueous extract of an undefined Salsola sp. as an oral contraceptive in AlgeriaCitation40. The aqueous extract of S. tuberculatiformis (previously known as S. tuberculate and commonly known as Gannabos) was reported to be used by Bushmen women as an oral contraceptive and to cause prolonged gestation and foetal post-maturity in Karakul sheep in Namibia region, South AfricaCitation40,Citation119,Citation129. Swart et al.Citation40 investigated the phytochemicals responsible for this activity in S. tuberculatiformis. The compound responsible for this activity was reported to be a labile synephrine analogue with a reactive aziridine group. Therefore, they synthesised the compound, 2–(4-acetoxyphenyl)2-chloro-N-methylethylammonium-chloride, as a stable analogue for the active principle of S. tuberculatiformis. This compound was found to disturb the mammalian steroid hormones homeostasis and to inhibit adrenal steroidogenesisCitation40.
The ethanolic extract of S. imbricata was reported to cause a slight decrease in the testis weight and to cause a significant decline in the sperm count when administered orally to male albino rats suggesting its potential use as a reversible male contraceptive, with a high safety marginCitation104. They attributed this contraceptive activity to the phenolic content of the plant, especially quercitrinCitation104.
5.11. Effect on melanin biosynthesis
Trans-N-feruloyltyramine derivatives (1.3, 1.4, and 1.8) isolated from S. foetida were reported to exhibit significant tyrosinase enzyme inhibitory activity with IC50 ranging from 0.40–2.61 µM which was lower than that of the standard tyrosinase inhibitors, kojic acid and L-mimosine, with IC50 of 16.67 and 3.68 µM, respectively. Therefore, these derivatives could have promising activities on melanocytes and skin pigmentation abnormalitiesCitation78.
5.12. Antimicrobial activity
The chloroform extract of the aerial parts of S. villosa and the compounds isolated from it were tested against different bacterial strains using the paper disc diffusion methodCitation91. The isolated compound biphenylsalsinol 10.1 showed the highest antimicrobial activity against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa bacterial strains with an inhibitory zone diameter (IZD) ranging from 12.33 to 28.66 mm. While salsolanol 12.1 showed slight activity against S. aureus, E. coli, S. epidermidis with IZD ranging from 9.33 to 12.66 mmCitation91. Oueslati et al.Citation92 also investigated the antibacterial activity of the roots of S. imbricata and the bioactive compounds, biphenylsalsonoid A 10.2 and B 10.3, isolated from its ethyl acetate fraction. The two isolated compounds showed similar antibacterial activity against S. aureus, S. epidermidis and E. coli with MIC values ranging from 16–32 µg/mLCitation92. While biphenylsalsonoid A 10.2 was two times more active than biphenylsalsonoid B 10.3 against Micrococcus luteus. It is worth noting that both compounds showed lower activity than the standard drug, Kanamycin which showed MIC values ranging from 2–8 µg/mLCitation92
The antimicrobial activities of the methanol extract of S. kali leaves and stems were investigated by Boulaaba et al.Citation75. The stem extract showed higher activity than the leaf extract. It showed antibacterial activity against P. aeruginosa and M. luteus with an inhibition zone diameter (IZD) of 10 mm. It showed weak or slight activity against other bacterial pathogens and Candida sp.Citation75.
Mohammed et al.Citation41 investigated the antimicrobial activity of S. cyclophylla essential oil against different microorganisms using the agar well-diffusion method. It showed good antibacterial activity against the Gram + ve, S. aureus and Streptococcus pyogenes, and the Gram -ve, P. aeruginosa, and E. coli. However, it had no activity against S. epidermidis. It also demonstrated powerful antifungal activity against C. albicansCitation41.
Gannoun et al.Citation42 investigated the antimicrobial activities of S. vermiculate leaf, root, and stem extracts and their volatile fractions towards different pathogens. They reported that the ethanolic roots extract showed the highest activity against S. aureus with a MIC value of 0.28 mg/mLCitation42. The used extracts showed low antifungal activity against the tested fungal sp. with IZD ranging from 6–9.5 mmCitation42. On the other hand, S. vermiculata aqueous extract was reported to be an effective antifungal agent that can be used as a preservative during grain storage. This activity was examined by the decrease of fungal growth on wheat samples that were coated with S. vermiculata aqueous extract, dried, and stored for one yearCitation120.
Terrestric acid 1.20 isolated from S. collina Pall by Jin et al.Citation66 showed antifungal activity against Candida albicans with a minimum 80% inhibitory concentration (MIC80) of 8 µg/mLCitation66. The alkaloid salsoline A (trolline) 1.17, present in S. collina Pall. and the flowers of Trollius chinensis, was reported to exhibit significant antibacterial activity against S. aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae. It also exhibited moderate antiviral activity against influenza viruses A and BCitation46.
5.13. Insecticidal activity
The ethanol extract of S. baryosma was reported to cause moderate insecticidal activity (22.08% mortality) against Trogoderma granarium insects (Everts) which was lower than the standard insecticidal compound, cypermethrin (37.64% mortality)Citation121.
6. Conclusion
The impressive diversity of the pool of phytochemicals of Salsola spp. is comprehensively studied in this review. Furthermore, up-to-date taxonomic classification and description of the important morphological characteristics of the plants of this genus were discussed herein. The phytochemical profile of Salsola spp. is composed of alkaloids, nitrogenous compounds, flavonoids and isoflavonoids, triterpenoids, cardenolides and steroids, coumarins, coumarolignans, lignans and diphenylpropanoids, and simple phenolic acids. These secondary metabolites represent a great interest for the chemotaxonomy of the genus. Furthermore, they would support the diverse traditional medicinal uses and pharmacological activities of Salsola species demonstrated by many reports as antihypertensive, immunostimulant, anti-inflammatory, hepatoprotective, anthelmintic, antispasmodic, and antidiabetic. The current study represents a guiding light for researchers studying such widely distributed wild medicinal plants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- D'Ambola M, Fiengo L, Chini MG, et al. Fusicoccane diterpenes from hypoestes forsskaolii as heat shock protein 90 (Hsp90) modulators. J Natural Products 2019;82:539–49.
- Bader A, Tuccinardi T, Granchi C, et al. Phenylpropanoids and flavonoids from Phlomis kurdica as inhibitors of human lactate dehydrogenase. Phytochemistry 2015;116:262–8.
- El-Naggar MH, Mira A, Bar FMA, et al. Synthesis, docking, cytotoxicity, and LTA4H inhibitory activity of new gingerol derivatives as potential colorectal cancer therapy. Bioorgan Medic Chem 2017;25:1277–85.
- Abo-Elghiet F, Ibrahim MH, El Hassab MA, et al. LC/MS analysis of Viscum cruciatum Sieber ex Boiss. Extract with anti-proliferative activity against MCF-7 cell line via G0/G1 cell cycle arrest: An in-silico and in-vitro study. J Ethnopharmacol 2022;295:115439.
- AlQathama A, Bader A, Al-Rehaily A, et al. In vitro cytotoxic activities of selected Saudi medicinal plants against human malignant melanoma cells (A375) and the isolation of their active principles. Euro J Integr Medic 2022;49:102083.
- El-Naggar MH, Abdel Bar FM, Harsha C, et al. Synthesis of new selective cytotoxic ricinine analogues against oral squamous cell carcinoma. Natural Product Res 2021;35:2145–56.
- El-Naggar MH, Elgaml A, Abdel Bar FM, Badria FA. Antimicrobial and antiquorum-sensing activity of Ricinus communis extracts and ricinine derivatives. Natural Product Res 2019;33:1556–62.
- Abdelgawad SM, El Hassab MA, Abourehab MA, et al. Olive Leaves as a Potential Phytotherapy in the Treatment of COVID-19 Disease, A Mini-Review. Front Pharmacol 2022;13:879118.
- Khalid S, Almalki FA, Hadda TB, et al. Medicinal applications of cannabinoids extracted from Cannabis sativa (L.): a new route in the fight against COVID-19? Curr Pharmaceutical Design 2021;27:1564–78.
- Iannuzzi AM, Moschini R, De Leo M, et al. Chemical profile and nutraceutical features of Salsola soda (agretti): anti-inflammatory and antidiabetic potential of its flavonoids. Food Biosci 2020;37:100713.
- Altay V, Ozturk M. The genera salsola and suaeda (Amaranthaceae) and their value as fodder. In: Grigore MN. (eds) Handbook of Halophytes. Springer, Cham. https://doi.org/10.1007/978-3-030-17854-3_97-1
- Sokołowska-Krzaczek A, Skalicka-WoźniakK, Czubkowska K. Variation of phenolic acids from herb and roots of Salsola kali L. Acta Societatis Botanicorum Poloniae 2009;78:197–201.
- Botschantzev V. A synopsis of Salsola (Chenopodiaceae) from south and south-west Africa. Kew Bulletin 1974;29:597–614. )
- Hanif Z, Ali HH, Rasool G, et al. Genus Salsola: its benefits, uses, environmental perspectives and future aspects-a review. J Rangeland Sci 2018;8:315–28.
- Mohammed HA, Al-Omar MS, Mohammed SA, et al. Phytochemical analysis, pharmacological and safety evaluations of halophytic plant, Salsola cyclophylla. Molecules 2021;26:2384.
- Al-Malki MA, OSMAN HE, El-Morsy MH. Ecological and nutritional values of halophytes in the Al-Qunfudhah, Saudi Arabia. J Umm Al-Qura University Appl Sci 2021;7:27–33.
- Bibi F, Strobel GA, Naseer MI, et al. Microbial Flora associated with the halophyte–Salsola imbricate and its biotechnical potential. Frontiers in Microbiology 2018;9:65.
- Mukhtar S, Mehnaz S, Mirza MS, Malik KA. Isolation and characterization of bacteria associated with the rhizosphere of halophytes (Salsola stocksii and Atriplex amnicola) for production of hydrolytic enzymes. Brazil J Microbiol 2019;50:85–97.
- Wisniak J, (2003) Sodium carbonate—From natural resources to Leblanc and back.
- Tite MS, Shortland A, Maniatis Y, et al. The composition of the soda-rich and mixed alkali plant ashes used in the production of glass. J Archaeol Sci 2006;33:1284–92.
- Colás C, Monzón S, Venturini M, Lezaun A. Double-blind, placebo-controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol 2006;117:810–6.
- Djeridane A, Hamdi A, Bensania W, et al. The in vitro evaluation of antioxidative activity, α-glucosidase and α-amylase enzyme inhibitory of natural phenolic extracts. Diabetes Metab Syndrome Clin Res Rev 2015;9:324–31.
- Ferrer A, Larramendi C, Huertas A, et al. Allergenic differences among pollens of three Salsola species. Inter Arch Allergy Immunol 2010;151:199–206.
- Borger C, Yan G, Scott J, et al. Salsola tragus or S. australis (Chenopodiaceae) in Australia - Untangling taxonomic confusion through molecular and cytological analyses. Aust J Bot 2008;56:600–8.
- Boulos L. Flora of Egypt. Vol. 1, Cairo: Al Hadara; 1999.
- Nazish M, Zafar M, Ahmad M, et al. Palyno‐morphological investigations of halophytic taxa of Amaranthaceae through SEM from Salt range of Northern Punjab, Pakistan. Microsc Res Techniq 2019;82:304–16.
- Akhani H, Greuter W, Roalson EH. Notes on the typification and nomenclature of Salsola and Kali (Chenopodiaceae). Taxon 2014;63:647–50.
- Rudov A, Mashkour M, Djamali M, Akhani H. A review of C4 plants in southwest Asia: an ecological, geographical and taxonomical analysis of a region with high diversity of C4 eudicots. Front. Plant Sci 2020;11:546518.
- Chase MW, Christenhusz M, Fay M, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botan J Linnean Soc 2016;181:1–20.
- Kanwal D, Abid R. Taxonomic assessment of the family Amaranthaceae with special emphasis on seed morphology. Pak J Bot 2017;49:43–68.
- Akhani H, Edwards G, Roalson E. Diversification of the old World Salsoleae s.l. (Chenopodiaceae): molecular phylogenetic analysis of nuclear and chloroplast data sets and a revised classification. Int J Plant Sci 2007;168:931–56.
- Hernández-Ledesma P, Berendsohn WG, Borsch T, et al. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015;45:281–383.
- Mosyakin SL, Rilke S, Freitag H. 2323) Proposal to conserve the name Salsola (Chenopodiaceae s. str.; Amaranthaceae sensu APG) with a conserved type. Taxon 2014;63:1134–5.
- POWO. Plants of the world online. Facilitated by the Royal Botanic Gardens, Kew; 2019.
- IPNI. Salsola, The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. 2021.
- Boulos L. The identity, typification and distribution of Salsola imbricata Forsskål: Studies in the Chenopodiaceae of Arabia 1. Kew Bull 1991;46:137–40.
- Seo JH, Jin MH, Chang YH. Anti-inflammatory effect of Salsola komarovii extract with dissociated glucocorticoid activity. BMC Complementary Med Therap 2020;20:1–9.
- Feodorova T, M.V. Lomonosov Moscow State University. New nomenclatural combinations in Nitrosalsola (Chenopodiaceae). Ukr Bot J 2015;72:442–5.
- Mucina L. Caroxylon (Chenopodiaceae s. str.) in continental southern Africa and Madagascar: a preliminary nomenclatural synopsis and biogeographical considerations. Phytotaxa 2017;312:151.
- Swart P, Swart AC, Louw A, van der Merwe KJ. Biological activities of the shrub Salsola tuberculatiformis Botsch.: contraceptive or stress alleviator? Bioessays 2003;25:612–9.
- Mohammed HA, Al-Omar MS, Aly MS, Hegazy MM. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J Essential Oil Bearing Plants 2019;22:82–93.
- Gannoun S, Mahfoudhi A, Flamini G, et al. Chemical composition and antimicrobial activities of Tunisian Salsola vermiculate L. J Chem Pharm Res 2016;8;1087–92.
- Xiang Y, Li Y-B, Zhang J, et al. A new alkaloid from Salsola collina. Yao Xue Xue Bao = Acta Pharmaceutica Sinica 2007;42:618–20.
- Orekhov AP, Proskurnina N. Alkaloids from Salsola richteri Karel, Khim.-Farm. Prom-st., 8–10. 1934.
- Proskurnina N, Orekhov A. The alkaloids of Salsola richteri. III. The optically active salsoline and the isolation of two new alkaloids. Bull Soc Chim Fr Mem 1937;4:1265–71.
- Wang RF, Yang XW, Ma CM, et al. A bioactive alkaloid from the flowers of Trollius chinensis. Heterocycles Sendai Institute Heterocyc Chem 2004;63:1443–8.
- Zhao Y, Ding X. Studies on the alkaloids from Salsola collina Pall. Yaoxue Xuebao 2004;39:598–600.
- Tundis R, Menichini F, Conforti F, et al. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer's disease. J Enzyme Inhib Medic Chem 2009;24:818–24.
- Pässler U, Knölker H-J. The pyrrolo [2, 1-a] isoquinoline alkaloids. Alkaloids Chem Biol 2011;70:79–151.
- Ghorab H, Khettaf A, Lehbili M, et al. A new cardenolide and other compounds from Salsola tetragona. Natural Product Commun 2017;12:3–5.
- Elsharabasy FS, Hosney AM. Chemical constituents from the aerial parts of Salsola inermis. Egypt Pharmac J 2013;12:90.
- Proksa B, Uhrin D, Narantuyaa S, Batsuren D. Cleomiscosins B and D, new coumarino-lignoids from Salsola laricifolia. Pharmazie 1990;45:804–6.
- Oueslati MH, Ben Jannet H, Mighri Z, et al. Phytochemical constituents from Salsola tetrandra. J Nat Products 2006;69:1366–9.
- Tomas F, Morenilla A, Barberan F. Two flavonol glycosides from Salsola kali, Fitoterapia. 1985.
- Abegaz BM, Woldu Y. Isoflavonoids from the roots of Salsola somalensis. Phytochemistry 1991;30:1281–4.
- Syrchina A, Gorshkov A, Shcherbakov V, et al. Flavonolignans of Salsola collina. Chem Nat Compounds 1992;28:155–8.
- Saleem M, Akhter N, Shaiq Ali M, et al. Structure determination of salisomide and salisoflavan, two new secondary metabolites from Salsola imbricata, by 1D and 2D NMR spectroscopy. Mag Res Chem 2009;47:263–5.
- Xiang Y, Yao Y, Zhou Q, et al. A new flavone glycoside from Salsola collina. Zhongcaoyao 2009;40:1858–60.
- Beyaoui A, Chaari A, Ghouila H, et al. New antioxidant bibenzyl derivative and isoflavonoid from the Tunisian Salsola tetrandra Folsk. Nat Product Res 2012;26:235–42.
- Xiang Y, Li Y, Zhang J, et al. Studies on chemical constituents of Salsola collina. Zhongguo Zhongyao Zazhi 2007;32:409–13.
- Hamed AI, Masullo M, Sheded MG, et al. Triterpene saponins from Salsola imbricata. Phytochem Lett 2011;4:353–6.
- Annaev C, Isamukhamedova M, Abubakirov N. Triterpene glycosides of Salsola micranthera. I. Structures of salsolosides C and D. Chem Nat Compounds 1983;19:691–5.
- Annaev C, Isamukhamedova M, Abubakirov N. Triterpene glycosides of Salsola micranthera. II. Structure of salsoloside E. Khimiya Prirodnykh Soedinenii 1984;1:65–9.
- Ahmad Z, Mehmood S, Fatima I, et al. Structural determination of salsolins A and B, new antioxidant polyoxygenated triterpenes from Salsola baryosma, by 1D and 2D NMR spectroscopy. Magn Res Chem 2008;46:94–8.
- Tundis R, Loizzo M, Statti G, Menichini F. Inhibitory effects on the digestive enzyme α-amylase of three Salsola species (Chenopodiaceae) in vitro. Die Pharmazie An Inter J Pharmac Sci 2007;62:473–5.
- Jin Y-S, Du J-L, Yang Y, et al. Chemical and biologically active constituents of Salsola collina. Chem Nat Comp 2011;47:257–60.
- Hussein NS, El-Bassuony AA. Hydroxycinnamoylamides from Salsola baryosoma. Rev Latinoam Quim 2004;32:15–20.
- Rasheed DM, El Zalabani SM, Koheil MA, et al. Metabolite profiling driven analysis of Salsola species and their anti-acetylcholinesterase potential. Nat Product Res 2013;27:2320–7.
- Karawya MS, Wassel GM, Baghdadi HH, Ahmed ZF. Isolation of methyl carbamate from four egyptian Salsola species. Phytochemistry 1972;11:441–2.
- Orhan IE, Kucukboyaci N, Calis I, et al. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis Freitag, Vural & Adiguzel and molecular modeling studies on N-acetyltryptophan. Phytochem Lett 2017;20:373–8.
- Elsharabasy FS, Al-Mushhin AAM, Araffa S, Farrag A. R J J o P, and Phytochemistry Phytochemical screening and gastroprotective effect of the aerial parts of Salasola terrandra Forssk. Against aspirin induced gastric ulceration in rats. J Pharmacogn Phytochem 2015;3:221–32.
- Townsend CA, Ebizuka Y. Natural product structural diversity-I, secondary metablites: organization and biosynthesis, In: Lew Mander H-WL, ed. Comprehensive natural product II; chemistry and biology. Oxford: Elsevier Science ltd.; 2010.
- Mayakova TI, Leont'eva VG, Zharkaya TI, et al. Sterols of Salsola Collina. Khim Prir Soedin 1984;4:531–2.
- Stringlis IA, de Jonge R, Pieterse CMJ. The age of coumarins in plant-microbe interactions. Plant Cell Physiol 2019;60:1405–19.
- Boulaaba M, Medini F, Hajlaoui H, et al. Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. South Afric J Bot 2019;123:193–9.
- Cooper R, Deakin JJ. Natural products of silk road plants. CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; 2020.
- Kucukboyaci N, Süntar I, Calis I. In vivo anti-inflammatory and antinociceptive activities of the extracts and chemical constituents of an endemic turkish plant, Salsola grandis. Rec Nat Prod 2016;10:369–79.
- Khan KM, Maharvi GM, Abbaskhan A, et al. Three tyrosinase inhibitors and antioxidant compounds from Salsola foetida. Helv Chim Acta 2003;86:457–64.
- Lee HJ, Pan C-H, Kim E-S, Kim CY. Online high performance liquid chromatography (HPLC)-ABTS + based assay and HPLC-electrospray ionization mass spectrometry analysis of antioxidant phenolic compounds in Salsola komarovii. J Korean Soc Appl Biol Chem 2012;55:317–21.
- Osman SM, El KWA, Wink M, El RMA. New isorhamnetin derivatives from Salsola imbricata Forssk. Leaves with distinct anti-inflammatory activity. Pharmacogn Mag 2016;12:S47–S51.
- Ammon HL, Prasad SM, Barnhart DM, et al. Structures of salsoline hydrochloride hydrate and salsolidine hydrochloride dihydrate. Acta Crystallogr C 1987;43:567–70.
- WHO. Medicinal plants in Mongolia. Manila: WHO Regional Office for the Western Pacific; 2013. p. 181–2.
- Narantuyaa S, Batsurén D, Batirov ÉK, Malikov VM. A chemical study of plants of the Mongolian flora lariside—A new scopoletin glycoside from Salsola laricifolia. Chem Nat Compounds 1986;22:267–9.
- Shehab NG, Abu-Gharbieh E, Bayoumi FA. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complementary Alternative Med 2015;15:12.
- Tundis R, Loizzo MR, Bonesi M, et al. In vitro cytotoxic activity of Salsola oppositifolia Desf.(Amaranthaceae) in a panel of tumour cell lines. Zeitschrift Für Naturforschung C 2008;63:347–54.
- Kinghorn AD, Falk H, Kobayashi J. Fortschritte Der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products, Springer: Wien New York. 2010;93.
- Wang X, Zhao Y, Jia X, Ding X. Chemical constituents of Salsola collina. Zhongyaocai 2011;34:230–1.
- Woldu Y, Abegaz B. Isoflavonoids from Salsola somalensis. Phytochemistry 1990;29:2013–5.
- Cho HK, Suh WS, Kim KH, et al. Phytochemical constituents of Salsola komarovii and their effects on NGF induction. Nat Prod Sci 2014;20:95–101.
- Ahmad Z, Mehmood S, Ifzal R, et al. Butyrylcholinesterase inhibitory triterpenes from Salsola baryosma. Pol J Chem 2007;81:1427–32.
- Oueslati MH, Al-Ghamdi FA, Noubigh A. Two new bioactive salsolanol and biphenylsalsinol from the aerial parts of Salsola villosa Delile. ex Schul. (Chenopodiaceae) growing in Saudi Arabia. Asian Pac J Trop Biomed 2015;5:624–8.
- Oueslati MH, Bouajila J, Jannet H. Two new bioactive biphenylpropanoids from the roots of Salsola imbricata (Chenopodiaceae) Growing in Saudi Arabia. OJC 2017;33:1871–8.
- Syrchina AI, Chernykh EA, Rafeichikova IV, et al. Carbohydrates, carbohydrate ethers, and alcohols of Salsola collina. Khim Prir Soedin 1991;3:420–1.
- Wang J-F, Liu S-S, Song Z-Q, et al. Naturally occurring flavonoids and isoflavonoids and their microbial transformation: a review. Molecules 2020;25:5112.
- Falcone Ferreyra ML, Rius S, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 2012;3:222.
- Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants or signalling molecules?, Free Radic. Biol Med 2004;36:838–49.
- Dixon RA, Ferreira D. Genistein. Phytochemistry 2002;60:205–11.
- Agrawal PK, Thakur RS. 13C NMR Spectroscopy of lignan and neolignan derivatives. Magn Reson Chem 1985;23:389–418.
- Xu R, Fazio GC, Matsuda SP. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004;65:261–91.
- Kougan G, Tabopda T, Kuete V, Verpoorte R. Simple phenols, phenolic acids, and related esters from the medicinal plants of Africa. Medicinal Plant Research in Africa 2013; 225–49.
- Hammiche V, Maiza K. Traditional medicine in Central Sahara: pharmacopoeia of Tassili N'ajjer. J Ethnopharmacol 2006;105:358–67.
- Al-Saleh F, Ali H, Mirza M. Chemical constituents of some medicinal plants growing in Bahrain. Fitoterapia 1993;64:251.
- Vijendra N, Kumar K. Traditional knowledge on ethno-medicinal uses prevailing in tribal pockets of Chhindwara and Betul Districts, Madhya Pradesh, India. Afr J Pharmacy Pharmacol 2010;4:662–70.
- Shehab NG, Abu-Gharbieh E. Phenolic profiling and evaluation of contraceptive effect of the ethanolic extract of Salsola imbricata Forssk. in male albino rats. Evid Based Complement Alternative Med 2014;2014:1–8.
- Fu S. Salsola ruthenic in treatment of essential hypertension. Zhonghua Nei ke za Zhi 1959;7:977–81.
- Loizzo MR, Tundis R, Statti GA, et al. In vitro angiotensin converting enzyme inhibiting activity of Salsola oppositifolia Desf., Salsola soda L. and Salsola tragus L. Nat Product Res 2007;21:846–51.
- Aniss HA, Said AEM, El Sayed IH, Adly C. Amelioration of adriamycin-induced cardiotoxicity by Salsola kali aqueous extract is mediated by lowering oxidative stress. Redox Report 2014;19:170–8.
- Janbaz K, Aslam N, Imran I, Jabeen Q. Evaluation of anti-inflammatory, analgesic and antipyretic activities of Salsola imbricata forssk in rats. J Animal Plant Sci 2021;31:2021.
- Ahmed S, Ashraf M, Jabbar A, et al. Pharmacological screening of Salsola baryosma. J Chem Soc Pak 2006;28:82–3.
- Oh Y, Jin S, Park H-j, et al. Anti-oxidative and anti-cancer activities by cell cycle regulation of Salsola collina extract. Korean J Microbiol Biotechnol 2014;42:73–81.
- Vengerovskii A, Melent’eva A, Burkova V. Hepatoprotective and antioxidant actions of an extract of the russian thistle in paracetamol hepatitis in rats. Pharm Chem J 2010;44:138–40.
- Nikiforov S, Semenov A, Syrchina A. Effect of an Aqueous Extract of Salsola collina on the Course of Experimental Cholelithiasis in Rabbits. Pharm Chem J 2002;36:496–9.
- Mahmoud AH, Soliman MS, and, et al. Tremendous effect of Salsola tetrandra and Salsola baryosma on a liver toxicity using paracetamol overdose. Der Pharma Chemica 2016;8:117–26.
- Nofal S, Nada S, Hassan N, et al. Preventive effect of Salsola villosa and Salsola volkensii aqueous alcoholic extract on acute and chronic liver injury in albino rats: some pharmacological, histological and histochemical studies. Egypt Med J 2002;17:115–39.
- Hong S, Lee H-A, Lee Y-S, et al. Protective effect of halophyte Salsola komarovi Iljin against gastric ulcer Induced by alcohol treatment in rats. J Biomed Res 2014;15:170–5.
- Ajaib M, Farooq S, Khan K, et al. Phytochemical analysis and anthelmintic activity of Salsola imbricata. J Chem Soc Pak 2019;41:198–202.
- Aslam N, Janbaz KH. Antispasmodic and bronchorelaxant activities of Salsola imbricata are mediated through dual Ca+ 2 antagonistic and β-adrenergic agonistic effects. Pharmac Biol 2017;55:1131–7.
- Zhao X, Wang H, Zhang Z, et al. Effects of ethyl acetate extract of Salsola collina on brain-gut peptides and interstitial cells of gastric Cajal in rats with diabetic gastroparesis. Iran J Basic Med Sci 2020;23:1218.
- Basson P, Morgenthal J, Bilbrough R, et al. (1969) “Grootlamsiekte”, a specific syndrome of prolonged gestation in sheep caused by a shrub Salsola tuberculata (Fenzl ex Moq) Schinz var. tomentosa CA Smith ex Aellen.
- Moghtet S, Menad N, Meddah B, Moussaoui A. Effect of Salsola vermiculata on fungi of french soft wheat and test of grain storage by the coating method. Int J Fundam Appl Sci 2018;10:226.
- Hasan M-U-H, Siddique M, Sagheer M, Aleem M. Comparative efficacy of ethanol leaf extracts of Amaranthus viridis L. and Salsola baryosma (Schultes) and cypermethrin against Trogoderma granarium (Everts). Pak J Agri Sci 2005;42:1–4.
- Magcwebeba T, Zyl AV, Swart A, Swart P. 121: The effect of Salsola tuberculata extracts and Compound A against intracellular interleukin-1alpha (icIL-1α) in the UVB in vitro model for inflammation and chemoprevention in skin. Cytokine 2014;70:56–7.
- Louw A, Swart P. Salsola tuberculatiformis Botschantzev and an aziridine precursor analog mediate the in vivo increase in free corticosterone and decrease in corticosteroid-binding globulin in female Wistar rats. Endocrinology 1999;140:2044–53.
- De Bosscher K, Vanden Berghe W, Beck IM, et al. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA 2005;102:15827–32.
- Khacheba I, Djeridane A, Kameli A, Yousfi M. The inhibitory effect of some algerian plants phenolics extracts on the α - glucosidase and α - amylase Activities and their Antioxidant Activities. Curr Enzym Inhib 2013;10:59–67.
- Taha A, Alsayed H. Brine shrimp bioassay of ethanol extracts of Sesuvium verrucosum, Salsola baryosma and Zygophyllum quatarense medicinal plants from Bahrain. Phytother Res PTR 2000;14:48–50.
- Beloborodova EI, Saratikov AS, Vengerovskiĭ AI, Shalovaĭ AA. [Lochein - a novel hepatoprotective drug]. Klinicheskaia Meditsina 2000;78:56–9.
- Wang S, Yan M, Guo Y, et al. In vivo and in vitro effects of Salsola collina on gastrointestinal motility in rats. Iran J Basic Med Sci 2020;23:383–9.
- De Lange M. Prolonged gestation in karakul ewes in South West Africa. 1960.












