Abstract
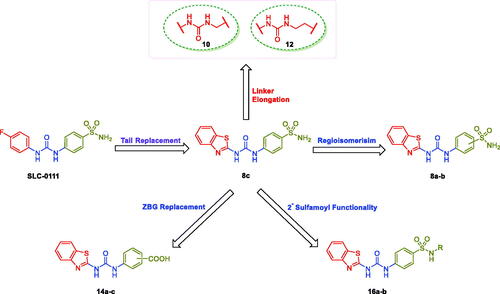
In this work, different series of benzothiazole-based sulphonamides 8a-c, 10, 12, 16a-b and carboxylic acids 14a-c were developed as novel SLC-0111 analogues with the goal of generating potent carbonic anhydrase (CA) inhibitors. The adopted strategy involved replacing the 4-fluorophenyl tail in SLC-0111 with a benzothiazole motif that attached to the ureido linker to produce compounds 8c and its regioisomers 8a-b. In addition, the ureido spacer was elongated by methylene or ethylene groups to afford the counterparts 10 and 12. In turn, the primary sulfamoyl zinc binding group (ZBG) was either substituted or replaced by carboxylic acid functionality in order to provide the secondary sulphonamide-based SLC-0111 analogues 16a-b, and the carboxylic acid derivatives 14a-c, respectively. All compounds (8a-c, 10, 12, 14a-c and 16a-b) were tested for their ability to inhibit CA isoforms CA I, II, IX and XII. Additionally, the in vitro anticancer properties of the developed CAIs were evaluated.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are family of ubiquitous zinc-metalloenzymes present in the whole organismsCitation1. These enzymes catalyse the essential conversion of carbon dioxide and water to bicarbonate and proton in a crucial process accountable for diverse cellular activities such as electrolyte secretion, bone resorption, maintenance of acid-base balance, gluconeogenesis, CO2 and pH homeostasis, calcification and tumorgenicityCitation2–4. The human CAs (hCAs) are relevant to α-CAs isozymes and sub-categorized into fifteen isoforms displaying distinct cellular distribution, levels of expression, kinetics and molecular featuresCitation5,Citation6. Of special interest, the catalytic activity of CAs I-IV, VA, VB, VI, VII, IX, XII-XIV isoforms is due to the presence of three histidine residues in the active site in coordination with zincCitation7. Furthermore, hCAs are classified upon cellular distribution into cytosolic (hCAs I, II, III, VII and XIII), trans membrane (hCAs IV, IX, XII, and XIV), mitochondrial (hCAs VA and VB), and hCA VI is secreted in milk and salivaCitation8,Citation9. The abnormal levels of these enzymes have been associated with many diseases; thus inhibitors of the CAs have potential applications in the treatment of glaucoma, edoema, obesity and mental problemsCitation1,Citation7,Citation9,Citation10.
Interestingly, the trans-membranal hCA IX and XII have shown diverse peculiarities over the other isoforms. hCA IX/XII isozymes have shown elevated expression in tumour cells and are associated to hypoxic solid tumours inducing tumour growth and metastasisCitation11,Citation12. Accordingly, the extremely desired selective inhibition of cancer-associated hCA IX/XII has been described as cutting-edge approach for the discovery of new small molecules for cancer treatmentCitation13–19. As a consequence, several approaches have been employed to develop selective inhibitors for hCA IX/XII. Strikingly, tail approach stood out as the most successful and effective tool to improve the potency and selectivity of sulphonamide-based carbonic anhydrase inhibitors (CAIs)Citation20. In this context, tail approach has been devoted to generate selective sulphonamide CAIs which involves grafting of different molecular motifs (tails) to the aromatic/heterocyclic ring bearing a zinc binding group (ZBG) through a flexible spacerCitation21,Citation22.
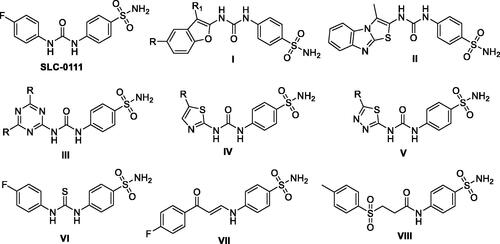
The ureido benzenesulfonamide SLC-0111 () has been developed utilising the tail approach as the first-in-class hCA IX inhibitor which, for the management of metastatic hypoxic solid tumours, is currently being investigated within phase I/II clinical trialsCitation3,Citation23,Citation24. To date, numerous SLC-0111 analogues have been described with the prime aim to enhance potency and selectivity towards hCA IX exploiting bioisosteric replacement approach via replacement of the SLC-0111aryl tail by various sets of chemical scaffolds () like benzofuran ICitation23, thiazolo[3,2-a]benzimidazole IICitation25, triazine IIICitation26, thiazole IV, and thiadiazole VCitation27. In addition, the replacement of the ureido linker in SLC-0111 with another spacers () for instance, thioureido VICitation14, enaminone VIICitation28 and sulfonylpropanamide VIIICitation3 was adopted for developing novel SLC-0111 analogous. Remarkably, these strategies resulted in an enhancement of tumour-associated hCA IX inhibitory action.
The aforesaid results motivated our research interest to adopt the tail approach for the development of new SLC-0111 analogues featured with potent and selective hCA IX/XII inhibitory influence. In the herein study, the benzothiazole motif has been appended to the ureido linker rather than the phenyl tail in SLC-0111 in order to provide the target inhibitor 8c (). Thereafter, the regioisomers 8a-b were designed through shifting of sulfamoyl functionality in 8c to ortho and para positions, respectively (. In addition to this, the ureido spacer in the SLC-0111 analogue 8c was lengthened by either methylene or ethylene groups, which resulted in the counterparts 10 and 12, respectively. Moreover, the functionality of the primary ZBG was either replaced by carboxylic acid or it was substituted in order to produce the carboxylic acid derivatives 14a-c and the secondary sulphonamide-based SLC-0111 analogues 16a-b, respectively.
The target benzothiazole-based SLC-0111 analogues 8a-c, 10, 12, 14a-c and 16a-b were developed and screened for their inhibitory impact towards CA I, II, IX and XII isoforms. Furthermore, the designed CAIs were assessed for their potential in vitro anticancer effects.
Experimental
Chemistry
General
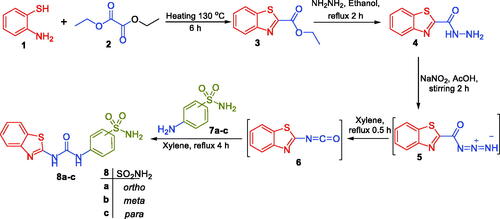
Uncorrected melting points were measured using a Stuart melting point device. In addition, the Schimadzu FT-IR 8400S spectrophotometer was used to record the IR spectra, whereas the Bruker spectrophotometer (400 MHz) was used to record the NMR spectra. 13C NMR spectra were run at 100 MHz in deuterated dimethylsulphoxide (DMSO-d6). All coupling constant (J) values are reported in hertz. Both ethyl 1,3-benzothiazole-2-carboxylate 3 and benzo[d]thiazole-2-carbohydrazide 4 were prepared as previously reportedCitation29.
Ethyl benzo[d]thiazole-2-carboxylate 3
White crystals, m.p. = 69–72 °C (reported m.p. = 68–69 °C)Citation29, yield = 77%.
Benzo[d]thiazole-2-carbohydrazide 4
White crystals, m.p. = 174–175 °C (reported m.p. = 173–174 °C)Citation29, yield = 84%.
General procedures for the preparation of target benzothiazole-derived sulphonamides 8a-c, 10, 12, 16a-b and the carboxylic acids 14a-c
A mixture of benzo[d]thiazole-2-carbohydrazide 4 (0.58 g, 3 mmol) and sodium nitrite (0.41 g, 6 mmol) in glacial acetic acid was stirred in an ice bath for 2 h. Azide 4 was produced by air-drying the generated solid, washing it with water (3 × 4 mL), and collecting it using filtration. Azide 4 was then heated for 30 min at reflux in dry xylene. To the prepared xylene solution, the appropriate amine derivative (aminobenzenesulfonamides 7a-c, 4-(aminomethyl)benzenesulfonamide 9, 4–(2-aminoethyl)benzenesulfonamide 11, aminobenzoic acids 13a-c, and secondary sulphonamides 15a-b) was added. After being refluxed for four hours, the reaction mixture was allowed to settle down to room temperature. The desired benzothiazole sulphonamides and carboxylic acids were obtained by filtering, washing the formed precipitate with methylene chloride (3 × 3 mL), drying it, and recrystallizing it from isopropyl alcohol.
Both spectral (NMR and IR) and elemental analysis for the newly prepared sulphonamides (8a-c, 10, 12, and 16a-b) and carboxylic acids (14a-c) were described in the Supplementary Materials.
Biological evaluation
The procedures that were used for the conducted biological tests were carried out in the same manner that was stated earlier; stopped-flow CACitation30–34, NCI-single doseCitation35–37 and MTT cytotoxicityCitation38–41 assays, and they were detailed in the Supplementary Materials.
Results and discussion
Chemistry
Schemes 1, 2, and 3 illustrate the synthetic routes that were used in order to prepare the target benzothiazole-derived sulphonamides 8a-c, 10, 12, and 16a-b, as well as the carboxylic acids 14a-c.
Synthesis was started by cyclisation of 2-aminothiophenol 1 with diethyl oxalate 2 to afford ethyl benzo[d]thiazole-2-carboxylate 3. Hydrolysis of ester 2, via refluxing with hydrazine hydrate in ethanol, afforded benzo[d]thiazole-2-carbohydrazide 4 in 84% yield. Thereafter, stirring of intermediate 4 with sodium nitrite in glacial acetic acid at 0 °C produced the corresponding azide derivative 5. In the last step, preparation of target benzothiazole-derived sulphonamides 8a-c was performed via the addition of the appropriate aminobenzenesulfonamide derivative 7a-c to a pre‐heated azide 4 solution in xylene (Scheme 1).
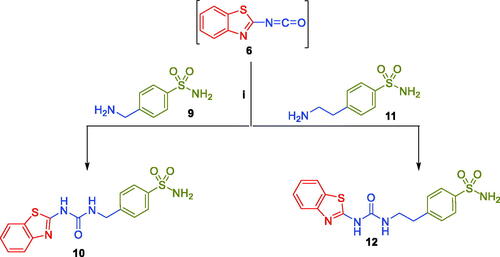
Furthermore, 2-isocyanatobenzothiazole intermediate 6 was reacted with 4-(aminomethyl)benzenesulfonamide 9 and 4–(2-aminoethyl)benzenesulfonamide 11 in refluxing xylene to yield target benzothiazole-derived sulphonamides 10 and 12, respectively (Scheme 2).
In the last Scheme, benzothiazole-based carboxylic acids 14a-c and sulphonamides 16a-b were obtained through a nucleophilic addition reaction of 2-isocyanatobenzothiazole intermediate 6 with aminobenzoic acids 13a-c and secondary sulphonamides 15a-b, respectively (Scheme 3).
Biological evaluation
Carbonic anhydrases inhibition
The herein synthesised benzothiazole-derived sulphonamides 8a-c, 10, 12, 16a-b and the carboxylic acids 14a-c were assessed for their inhibitory action against the widespread cytosolic hCA I and II, and cancer-related IX and XII isoforms employing a stopped flow CO2 hydrase assay and the CAI acetazolamide (AAZ) was adopted as a controlCitation30. The provided inhibition constants (KI) manifested in can be exploited to delineate the structure activity relationships (SARs).
Table 1. Inhibition constants for benzothiazole-based derivatives (8a-c, 10, 12, 14a-c and 16a-b) and the standard sulphonamide inhibitor acetazolamide (AAZ) towards hCA I, II, IX and XII, determined with a stopped-flow CO2 hydrase assay.
The herein tested benzothiazole-based sulphonamides 8a-c, 10, 12, 16a-b and the carboxylic acids 14a-c displayed diverse off-target hCA I inhibition profile spanning from nanomolar to high micromolar inhibitory constants (KIs = 61.5 nM to > 100 µM), . In the regard of benzothiazole-bearing sulphonamides with ureido linker 8a-c, they exerted low to moderate inhibitory activities towards the dominant hCA I with KIs ranged from 361.7 nM to 12.59 µM. Noteworthy, the switching of sulphonamide anchoring moiety from ortho- and meta positions (8a-b; KIs = 12.59 and 4.04 µM, respectively) to para position potentially elevated the inhibition constant to the nanomolar level (8c; KI = 361.7 nM). Notably, the elongation of the ureido spacer in the p-benzenesulfonamide counterpart 8c (KI = 361.7 nM) by one carbon sharply reduced hCA I inhibitory potency (10; KI = 945.9 nM), whereas its elongation by two carbons led to sensible enhancement in hCA I inhibitory impact providing the most potent hCA I inhibitor within the current study (12; KI = 61.5 nM). In contrast, the bioisosteric replacement of the sulphonamide zinc binding functionality in 8a-c by carboxylic acid moiety 14a-c dramatically diminished hCA I inhibition constants to high micromolar values (14a-c; KIs = 68.09, 82.75 and 11.82 µM, respectively). Furthermore, it was noted that the inclusion of secondary sulfamoyl functionality totally abolished hCA I inhibitory effect (16a-b; KIs > 100 µM) compared to the primary sulfamoyl-appended sulphonamides 8a-c.
Interestingly, the in vitro kinetic data towards the physiologically relevant hCA II isoform presented inhibition pattern similar to hCA I, . In a similar fashion, the benzothiazole-derived analogues 8a-c exhibited moderate to high hCA II inhibitory potential with KIs spanning between 54.1 and 785.2 nM. While the incorporation of ortho or meta sulphonamide demonstrated moderate hCA II inhibition (8a-b; KI = 785.2 and 652.7 nM, respectively), the shifting of this sulfamoyl to para position interestingly improved hCA II inhibition constant to two-digits value (8c; KI = 54.1 nM). To explore the impact of linker length, the obtained results revealed that elongation of the ureido linker in 8c (KI = 54.1 nm) by one carbon significantly decreased the inhibitory action against hCA II by 4-fold (10; KI = 204.3 nM), whereas the elongation of this linker by two carbons was more advantageous furnishing the most powerful hCA II inhibitor within this series (KI = 28.5 nM) in a similar way to the hCA I inhibition profile. Similarly, the appending of carboxyl group as a zinc anchoring moiety 14a-c in place of the sulfamoyl functionality 8a-c (KIs range 54.1– 785.2 nM) drastically declined hCA II inhibition to micromolar level (14a-c; KIs equal 75.94, 94.13 and 9.47 µM, respectively). In addition, the applying of secondary sulfamoyl group 16a-b completely abolished hCA II inhibitory power similar to hCA I inhibition data.
Concerning the inhibitory influence of the here evaluated benzothiazole-based SLC-0111 analogues towards cancer-associated hCA IX isozyme (), the primary sulfamoyl-bearing derivatives 8a-c, 10 and 12 exerted the most efficient potencies within this study against such enzyme displaying two-digits nanomolar KIs spanning a range between 16.4 and 65.3 nM. It is worth to mention that switching of the sulphonamide from ortho- and meta positions 8a-b (KIs equal 65.3 and 48.9 nM, respectively) to para position 8c enhanced the hCA IX inhibitory power (KI equals 31.5 nM), while the elongation of ureido linker in 8c by one carbon decreased the inhibition potency by the half (10; KI = 58.8 nM). On the other hand, extending such a linker by two carbons resulted in the production of the most efficient hCA IX inhibitor within the scope of the present study (12; KI = 16.4 nM). Additionally, the replacement of the sulfamoyl zinc binding group in 8a-c (KI range 31.5 to 65.3 nM) by carboxylic acid functionality sharply lowered the hCA IX inhibition constants (14a-c; KI = 44.62, 16.28 and 2.41 µM, respectively). Like the hCA I and II inhibition outcomes, the incorporation of 3,5-dimethyl-1,2-oxazole-bearing secondary sulphonamide entirely revoked the hCA IX inhibitory efficiency (16b; KI > 100 µM), whereas the inclusion of thiazole-appended secondary sulphonamide 16a resulted in very weak hCA IX inhibition (KI of 56.09 µM).
In the context of inhibitory activities towards the second tumour-related hCA XII isoform, the herein assessed benzothiazole-derived SLC-0111 analogues demonstrated diverse potencies in a similar behaviour as hCA I, II and IX isoforms as depicted in . The primary sulfamoyl-bearing analogues 8a-c, 10 and 12 showed the most favourable inhibition profile against hCA XII isoform demonstrating inhibition constants KIs ranged from 29.3 to 57.5 nM. For the ureido linker-grafted sulphonamides 8a-c, the para regioisomer 8c was the most potent hCA XII inhibitor with KI = 29.3 nM, similarly the ortho and meta regioisomers exhibited potential hCA XII inhibition (8a-b; KIs equal 41.2 and 57.5 nM, respectively). Moreover, the elongation of the ureido linker in 8c (KI = 29.3 nM) by one or two carbons slightly reduced the inhibition constants (10 and 12; KIs of 51.2 and 34.7 nM, respectively). As obtained from hCA I, II and IX inhibitory investigations, the bioisosteric replacement of the sulphonamide functionality in 8a-c (KIs range 29.3 − 57.5 nM) with carboxylic acid group dramatically decreased the hCA XII inhibitory effect (14a; KI = 39.86 µM, 14b; KI = 9.14 µM and 14c; KI = 8.54 µM). Furthermore, it was noted that the inclusion of secondary sulfamoyl functionality 16a-b markedly declined hCA XII inhibition data, while the grafting of thiazole-bearing sulfamoyl 16a resulted in KI = 32.5 µM, the introduction of 3,5-dimethyl-1,2-oxazole-bearing secondary sulphonamide 16b completely abolished the hCA XII inhibition (KI > 100 µM).
Collectively, the elicited SAR hinted out that the replacement of 4-fluorophenyl tail in SLC-0111 with benzothiazole motif while maintaining the para primary sulfamoyl functionality in conjunction with the elongation of its ureido linker by two carbons furnished the most potent inhibitors towards the tumour-related hCA IX with inhibition constants better than the lead SLC-0111 (8c, KI = 31.5 nM; 12, KI = 16.4 nM vs SLC-0111, KI = 45 nM). Undesirably such improvement in hCA IX inhibition for the promising candidates (8c and 12) was concomitant with the enhancement in inhibition of the ubiquitous hCA I (IX/I S.I. = 11.5, 3.75, respectively) and II isoforms (IX/II S.I. = 1.7 for both) compared to SLC-0111 (IX/I S.I. = 112.9; IX/II S.I. = 21.3). Consequently, the resulting potent benzothiazole-tethered SLC-0111 analogues 8c and 12 can be employed as leads for further optimisation to develop promising candidates with superior potency and selectivity towards the cancer-associated isozymes over the physiologically dominant hCA I and II isoforms.
Antitumor activity towards NCI-60 cancer cell lines
All herein developed benzothiazole-derived sulphonamides 8a-c, 10, 12, 16a-b and the carboxylic acids 14a-c were explored for their potential antitumor activities at the National Cancer Institute (NCI-USA) within the Developmental Therapeutic Program, utilising the US-NCI protocol and the sulforhodamine B (SRB) colorimetric assay for cell growth and viability evaluationCitation32,Citation37,Citation42,Citation43.
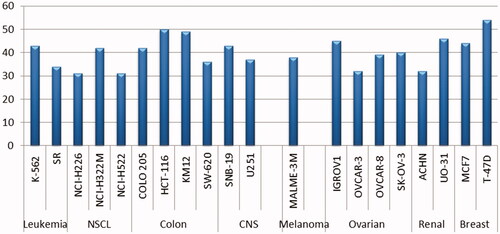
The obtained results revealed that the examined molecules have weak or non-significant antitumor activities towards most NCI cancer cell lines, except 8b. Sulphonamide 8b displayed selective anti-proliferative activity (GI > 30%) towards twenty cancer cell lines belonging to all tumour subpanels, except the prostate cancer subpanel, with GI% range of 31–54% (). The best growth inhibitory activity of 8b (GI = 54%) was observed for the breast T-47D cancer cell line. provides a summary of the cancer cell lines that are most vulnerable to the effects of benzothiazole-derived sulphonamide 8b.
Sulphonamide 8c exerted cell growth inhibition (GI) equals about 20% towards non-small cell lung cancer (EKVX) and breast cancer (MCF7 and T-47D) cell lines, as well as GI = 24% towards renal cancer (UO-31) cell line. Sulphonamide 10 displayed GI equals about 20% against non-small cell lung cancer (NCI-H226 and EKVX), and breast cancer (MCF7 and T-47D) cell lines, in addition, it exerted about 25% GI towards ovarian cancer (IGROV1) and CNS cancer (SNB-75) cell lines. Superiorly, the renal cancer (UO-31) cell line was the most sensitive one to the effect of sulphonamide 10 with a GI value of 33%. Moreover, non-small cell lung cancer (EKVX), ovarian cancer (IGROV1) and CNS cancer (SNB-75), and renal cancer (UO-31) and breast cancer (MCF7 and T-47D) cell lines were the most susceptible cells to the impact of sulphonamides 16a and 16b with GI % = 27, 28, 29, 37, 24, 22 for 16a, and GI % = 19, 24, 37, 29, 24, 16 for 16b.
Regarding the anticancer activities of series 14, carboxylic acid derivative 14a was found to possess a moderate growth inhibitory effect against non-small cell lung cancer (EKVX), ovarian cancer (IGROV1), CNS cancer (SNB-75), and renal cancer (CAKI-1 and UO-31) cell lines with inhibition % 22, 27, 20, 20, and 32, respectively. Also, carboxylic acid derivative 14b displayed GI more than 20% against ovarian cancer (IGROV1), CNS cancer (SNB-75), and renal cancer (UO-31) cell lines, whereas compound 14c exerted GI more than 20% against ovarian cancer (IGROV1), non-small cell lung cancer (EKVX), renal cancer (UO-31) and breast cancer (MCF7 and T-47D) cell lines.
Anti-proliferative activity towards breast cancer cell lines
The inhibition constants presented in highlighted that sulphonamide 8b elicited an excellent selectivity towards hCA IX and XII over the off-target hCA I, with selective indexes (SIs) equal 82.6 and 70.2, respectively. In addition, only sulphonamide 8b demonstrated excellent selectivity towards hCA IX and XII in comparison to hCA II, with SI values of 13.3 and 11.3, respectively. As a consequence of this, sulphonamide 8b maintained both its activity and its selectivity with regard to the hCA IX and XII isoforms that are associated with cancer. In addition, according to the findings of the US-NCI assay that was discussed before, sulphonamide 8b was determined to be the most effective anticancer molecules herein reported. As a consequence, sulphonamide 8b was evaluated for its anticancer effect towards breast cancer (T-47D and MCF-7) cell lines within the hypoxic conditions, using the SRB assay. The assay results that presented in showed that sulphonamide 8b had good IC50 values (6.73 ± 0.28 and 9.16 ± 0.70) against T-47D and MCF-7 cells, respectively.
Table 2. Anticancer activity of target benzothiazole-based sulphonamide 8b towards breast T-47D and MCF-7 cancer cell lines under hypoxic conditions.
Conclusions
This study developed different series of benzothiazole-based sulphonamides 8a-c, 10, 12, 16a-b and carboxylic acids as novel SLC-0111 analogues, and assessed their CA inhibitory effects towards CA I, II, IX and XII isoforms. Different drug design approaches were utilised. The benzothiazole motif was appended to the ureido linker instead of the SLC-0111 fluorophenyl tail to produce compounds 8c and its regioisomers 8a-b. In addition, the ureido spacer was elongated by methylene or ethylene groups to afford the counterparts 10 and 12. In turn, the functionality of the primary ZBG was either replaced by carboxylic acid or it was substituted in order to produce the carboxylic acid derivatives 14a-c and the secondary sulphonamide-based SLC-0111 analogues 16a-b, respectively. The elicited SAR, from this study, hinted out that the introduction of para primary sulfamoyl functionality along with elongation of the ureido linker by two carbons were more beneficial for the cancer-related hCA IX and XII inhibition. The primary sulfamoyl-bearing analogues 8a-c, 10 and 12 disclosed the most favourable inhibition profile against tumour-related hCA IX and XII isoforms demonstrating inhibition constants ranged from 16.4 to 65.3 nM and from 29.3 to 57.5 nM, respectively. Moreover, all herein developed benzothiazole-derived sulphonamides 8a-c, 10, 12, 16a-b and the carboxylic acids 14a-c were explored for their potential antitumor activities at the NCI-National Cancer Institute. The examined molecules have weak or non-significant antitumor activities towards most NCI cancer cell lines, except sulphonamide 8b which displayed selective anti-proliferative activity (GI > 30%) towards twenty cancer cell lines belonging to all tumour subpanels, except the prostate cancer subpanel. It is interesting to mention that 8b elicited an excellent selectivity towards hCA IX and XII over the off-target hCA I, with selective indexes equal to 82.6 and 70.2, respectively. Additionally, it demonstrated good selectivity towards hCA IX and XII over hCA II with selective indexes of 13.3 and 11.3, respectively. Furthermore, the cytotoxicity SRB assay showed that sulphonamide 8b had good IC50 values (6.73 ± 0.28 and 9.16 ± 0.70) against breast cancer T-47D and MCF-7 cells, respectively.
Supplemental Material
Download PDF (146 KB)Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem Rev. 2012;112(8):4421–4468.
- Kumar S, Rulhania S, Jaswal S, Monga V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur J Med Chem. 2021;209:112923.
- Elbadawi MM, Eldehna WM, Nocentini A, Abo-Ashour MF, Elkaeed EB, Abdelgawad MA, Alharbi KS, Abdel-Aziz HA, Supuran CT, Gratteri P, et al. Identification of N-phenyl-2- (phenylsulfonyl) acetamides/propanamides as new SLC-0111 analogues: synthesis and evaluation of the carbonic anhydrase inhibitory activities. Eur J Med Chem. 2021;218:113360.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J. 2016;473(14):2023–2032.
- Buabeng ER, Henary M. Developments of small molecules as inhibitors for carbonic anhydrase isoforms. Bioorg Med Chem. 2021;39:116140.
- Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008–2018). Expert Opin Ther Pat. 2018;28(10):729–740.
- Angeli A, Carta F, Nocentini A, Winum J-Y, Zalubovskis R, Akdemir A, Onnis V, Eldehna WM, Capasso C, Simone GD, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites. 2020;10(10):412.
- Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: Where are we today? Med Res Rev. 2020;40(6):2485–2565.
- Eldehna WM, Taghour MS, Al-Warhi T, Nocentini A, Elbadawi MM, Mahdy HA, Abdelrahman MA, Alotaibi OJ, Aljaeed N, Elimam DM, et al. Discovery of 2,4-thiazolidinedione-tethered coumarins as novel selective inhibitors for carbonic anhydrase IX and XII isoforms. J Enzyme Inhib Med Chem. 2022;37(1):531–541.
- Elimam DM, Eldehna WM, Salem R, Bonardi A, Nocentini A, Al-Rashood ST, Elaasser MM, Gratteri P, Supuran CT, Allam HA. Natural inspired ligustrazine-based SLC-0111 analogues as novel carbonic anhydrase inhibitors. Eur J Med Chem. 2022;228:114008.
- Narella SG, Shaik MG, Mohammed A, Alvala M, Angeli A, Supuran CT. Synthesis and biological evaluation of coumarin-1,3,4-oxadiazole hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem. 2019;87:765–772.
- Zengin Kurt B, Sonmez F, Ozturk D, Akdemir A, Angeli A, Supuran CT. Synthesis of coumarin-sulfonamide derivatives and determination of their cytotoxicity, carbonic anhydrase inhibitory and molecular docking studies. Eur J Med Chem. 2019;183:111702.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31(3):345–360.
- Angeli A, Tanini D, Peat TS, Di Cesare Mannelli L, Bartolucci G, Capperucci A, Ghelardini C, Supuran CT, Carta F. Discovery of new Selenoureido Analogues of 4-(4-Fluorophenylureido)benzenesulfonamide as Carbonic Anhydrase Inhibitors. ACS Med Chem Lett. 2017;8(9):963–968.
- Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64.
- Nerella SG, Singh P, Arifuddin M, Supuran CT. Anticancer carbonic anhydrase inhibitors: a patent and literature update 2018–2022. Expert Opin Ther Pat. 2022;32(8):833–847.
- McDonald PC, Chafe SC, Supuran CT, Dedhar S. Cancer therapeutic targeting of hypoxia induced carbonic anhydrase IX: from bench to bedside. Cancers. 2022;14(14):3297.
- Peerzada MN, Hamel E, Bai R, Supuran CT, Azam A. Deciphering the key heterocyclic scaffolds in targeting microtubules, kinases and carbonic anhydrases for cancer drug development. Pharmacol Ther. 2021;225:107860.
- Mishra CB, Mongre RK, Prakash A, Jeon R, Supuran CT, Lee M-S. Anti-breast cancer action of carbonic anhydrase IX inhibitor 4-[4-(4-Benzo [1, 3] dioxol-5-ylmethyl-piperazin-1-yl)-benzylidene-hydrazinocarbonyl]-benzenesulfonamide (BSM-0004): in vitro and in vivo studies. J Enzyme Inhib Med Chem. 2021;36(1):954–963.
- Tanpure RP, Ren B, Peat TS, Bornaghi LF, Vullo D, Supuran CT, Poulsen S-A. Carbonic anhydrase inhibitors with dual-tail moieties to match the hydrophobic and hydrophilic halves of the carbonic anhydrase active site. J Med Chem. 2015;58(3):1494–1501.
- Elbadawi MM, Eldehna WM, Nocentini A, Somaa WR, Al-Rashood ST, Elkaeed EB, El Hassab MA, Abdel-Aziz HA, Supuran CT, Fares M. Development of 4-((3-oxo-3-phenylpropyl) amino) benzenesulfonamide derivatives utilizing tail/dual-tail approaches as novel carbonic anhydrase inhibitors. Eur J Med Chem. 2022;238:114412.
- Fares M, Eldehna WM, Bua S, Lanzi C, Lucarini L, Masini E, Peat TS, Abdel-Aziz HA, Nocentini A, Keller PA, et al. Discovery of potent dual-tailed benzenesulfonamide inhibitors of human carbonic anhydrases implicated in glaucoma and in vivo profiling of their intraocular pressure-lowering action. J Med Chem. 2020;63(6):3317–3326.
- Shaldam M, Eldehna WM, Nocentini A, Elsayed ZM, Ibrahim TM, Salem R, El-Domany RA, Capasso C, Abdel-Aziz HA, Supuran CT. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur J Med Chem. 2021;216:113283.
- Sarnella A, Ferrara Y, Auletta L, Albanese S, Cerchia L, Alterio V, De Simone G, Supuran CT, Zannetti A. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J Exp Clin Cancer Res. 2022;41(1):1–16.
- Alkhaldi AAM, Al-Sanea MM, Nocentini A, Eldehna WM, Elsayed ZM, Bonardi A, Abo-Ashour MF, El-Damasy AK, Abdel-Maksoud MS, Al-Warhi T, et al. 3-Methylthiazolo[3,2-a]benzimidazole-benzenesulfonamide conjugates as novel carbonic anhydrase inhibitors endowed with anticancer activity: design, synthesis, biological and molecular modeling studies. Eur J Med Chem. 2020;207:112745.
- Lolak N, Akocak S, Bua S, Sanku RKK, Supuran CT. Discovery of new ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as carbonic anhydrase I, II, IX and XII inhibitors. Bioorg Med Chem. 2019;27(8):1588–1594.
- Abo-Ashour MF, Eldehna WM, Nocentini A, Ibrahim HS, Bua S, Abdel-Aziz HA, Abou-Seri SM, Supuran CT. Novel synthesized SLC-0111 thiazole and thiadiazole analogues: determination of their carbonic anhydrase inhibitory activity and molecular modeling studies. Bioorg Chem. 2019;87:794–802.
- Eldehna WM, Abo-Ashour MF, Berrino E, Vullo D, Ghabbour HA, Al-Rashood ST, Hassan GS, Alkahtani HM, Almehizia AA, Alharbi A, et al. SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg Chem. 2019;83:549–558.
- (a) Liu K, Ding Y, Kang C. Synthesis and antiproliferative activity of new n-acylhydrazone derivatives containing Benzothiazole and indole based moiety. Pharm Chem J. 2020;54(4):345–352. (b) Al-Rashood KA, Abdel-Aziz HA. Thiazolo [3, 2-a] benzimidazoles: synthetic strategies, chemical transformations and biological activities. Molecules. 2010;15(6):3775–3815.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246(8):2561–2573.
- (a) Alafeefy AM, Abdel-Aziz HA, Vullo D, Al-Tamimi A-MS, Awaad AS, Mohamed MA, Capasso C, Supuran CT. Inhibition of human carbonic anhydrase isozymes I, II, IX and XII with a new series of sulfonamides incorporating aroylhydrazone-,[1,2,4]triazolo[3, 4-b][1, 3,4] thiadiazinyl-or 2-(cyanophenylmethylene)-1,3,4-thiadiazol-3(2H)-yl moieties. J Enzyme Inhib Med Chem. 2015;30(1):52–56. (b) Ibrahim HS, Abdelrahman MA, Nocentini A, Bua S, Abdel-Aziz HA, Supuran CT, Abou-Seri SM, Eldehna WM. Insights into the effect of elaborating coumarin-based aryl enaminones with sulfonamide or carboxylic acid functionality on carbonic anhydrase inhibitory potency and selectivity. Bioorg Chem. 2022;126:105888.
- Tawfik HO, Shaldam MA, Nocentini A, Salem R, Almahli H, Al-Rashood ST, Supuran CT, Eldehna WM. Novel 3-(6-methylpyridin-2-yl) coumarin-based chalcones as selective inhibitors of cancer-related carbonic anhydrases IX and XII endowed with anti-proliferative activity. J Enzyme Inhib Med Chem. 2022;37(1):1043–1052.
- Elimam DM, Elgazar AA, Bonardi A, Abdelfadil M, Nocentini A, El-Domany RA, Abdel-Aziz HA, Badria FA, Supuran CT, Eldehna WM. Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: design, synthesis and biological evaluation. Eur J Med Chem. 2021;225:113800.
- Eldehna WM, Nocentini A, Elsayed ZM, Al-Warhi T, Aljaeed N, Alotaibi OJ, Al-Sanea MM, Abdel-Aziz HA, Supuran CT. Benzofuran-based carboxylic acids as carbonic anhydrase inhibitors and antiproliferative agents against breast cancer. ACS Med Chem Lett. 2020;11(5):1022–1027.
- Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res. 1995;34(2):91–109.
- Elbadawi MM, Eldehna WM, Wang W, Agama KK, Pommier Y, Abe M. Discovery of 4-alkoxy-2-aryl-6, 7-dimethoxyquinolines as a new class of topoisomerase I inhibitors endowed with potent in vitro anticancer activity. Eur J Med Chem. 2021;215:113261.
- Eldehna WM, Salem R, Elsayed ZM, Al-Warhi T, Knany HR, Ayyad RR, Traiki TB, Abdulla M-H, Ahmad R, Abdel-Aziz HA, et al. Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with apoptosis inducing mechanism in Colon cancer. J Enzyme Inhib Med Chem. 2021;36(1):1423–1434.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Taghour MS, Elkady H, Eldehna WM, El-Deeb NM, Kenawy AM, Elkaeed EB, Alsfouk AA, Alesawy MS, Metwaly AM, Eissa IH. Design and synthesis of thiazolidine-2, 4-diones hybrids with 1, 2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: in-vitro anticancer evaluation and in-silico studies. J Enzyme Inhib Med Chem. 2022;37(1):1903–1917.
- Yousef RG, Ibrahim A, Khalifa MM, Eldehna WM, Gobaara IMM, Mehany AB, Elkaeed EB, Alsfouk AA, Metwaly AM, Eissa IH. Discovery of new nicotinamides as apoptotic VEGFR-2 inhibitors: virtual screening, synthesis, anti-proliferative, immunomodulatory, ADMET, toxicity, and molecular dynamic simulation studies. J Enzyme Inhib Med Chem. 2022;37(1):1389–1403.
- Eldehna WM, El Hassab MA, Elsayed ZM, Al-Warhi T, Elkady H, Abo-Ashour MF, Abourehab MA, Eissa IH, Abdel-Aziz HA. Design, synthesis, in vitro biological assessment and molecular modeling insights for novel 3-(naphthalen-1-yl)-4, 5-dihydropyrazoles as anticancer agents with potential EGFR inhibitory activity. Sci Rep. 2022;12(1):1–13.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112.
- Dweedar HE, Mahrous H, Ibrahim HS, Abdel-Aziz HA. Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono) indolin-2-ones as anticancer agents. Eur J Med Chem. 2014;78:275–280.