Abstract
The present study aimed to develop potent carbonic anhydrase inhibitors (CAIs). The design of the target compounds was based on modifying the structure of the ureido-based carbonic anhydrase inhibitor SLC-0111. Six series of a substituted benzoylthioureido core were prepared featuring different zinc-binding groups; the conventional sulphamoyl group 4a–d and 12a–c, its bioisosteric carboxylic acid group 5a–d and 13a–c or the ethyl carboxylate group 6a–d and 14a–c as potential prodrugs. All compounds were assessed for their carbonic anhydrase (CA) inhibitory activity against a panel of four physiologically relevant human CA isoforms hCA I and hCA II, and hCA IX, and hCA XII. Compounds 4a, 4b, 4c, 4d, 5d, 12a, and 12c revealed significant inhibitory activity against hCA I that would highlight these compounds as promising drug candidates for the treatment of glaucoma.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) enzyme is a well-known protein that exists in humans as fifteen distinct isoforms of the α-genetic familyCitation1. CAs enzymes are metalloproteins containing zinc that efficiently catalyses the reversible conversion of carbon dioxide to bicarbonate and release protonCitation2. CA enzyme has eight distinct classes (α, β, γ, δ, ζ, η, θ, and ι) that have no significant sequence identity and were discovered independently. As a result, the carbonic anhydrase classes are excellent examples of catalytic function of biological evolutionCitation3. CAs are involved in important physiological processes such as acid–base regulation, gluconeogenesis, biosynthetic reactionsCitation4, electrolyte secretion, bone resorption/calcification, and tumorigenicityCitation5. Consequently, inhibition of CAs can be a target for the treatment of glaucoma, obesity, neuropathic pain, arthritis, Alzheimer’s disease and cancerCitation6,Citation7. Some CA isoforms notably isoform IX are found in high concentrations in many solid cancers and in lower concentrations in a variety of normal tissues with strong correlation between its expression and hypoxiaCitation8–10 and where high CA levels are associated with poor prognosisCitation11. Therefore, the design of CAIs have been a beneficial strategy for the treatment of many diseases since the late 1950s where the primary sulphonamides and their isosteres emerged as promising drugs for decadesCitation12–14. Most of the potent CAIs have been correlated with the presence of suitable zinc binding group (ZBG) to establish the required interaction within the hCAs active sitesCitation15, nevertheless many non-zinc binding CAIs have been recently developedCitation16.
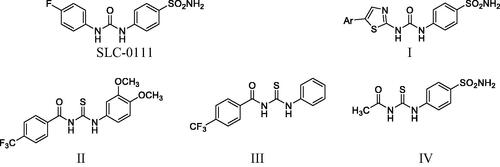
Literature survey unveiled that many ureido and thioureido phenyl derivatives exhibit remarkable CA inhibition activity (). Interestingly, the ureido-substituted benzenesulfonamide CA inhibitor SLC-0111 was developed with a highly effective hCA IX/XII inhibitory activity and it was progressed to phase I/II clinical trials for the treatment of advanced metastatic solid cancersCitation17,Citation18. Many studies focussed on the development of various SLC-0111 analogues through replacement of the 4-fluorophenyl tail, with either substituted thiazole compound ICitation19, 4-trifluoromethylbenzoyl compounds II and III (IC50 = 1.90 and 2.48 µM) respectively against hCAIICitation20 or acetyl moiety compound IV (IC50 = 2.14 µM) against hCAIICitation21 where they showed significant carbonic anhydrase inhibitory activity.
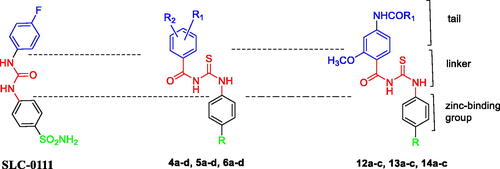
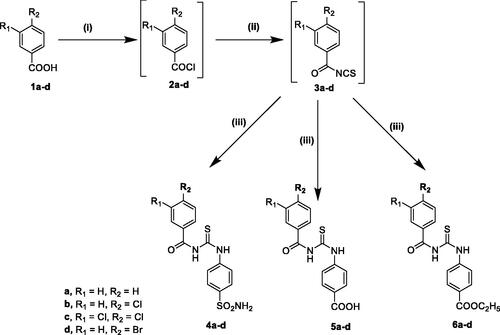
Accordingly, built on the reported carbonic anhydrase inhibitory activity of SLC-0111 and substituted benzoylthioureido compounds II–IV, we planned to synthesise new benzoylthioureido derivatives with potential carbonic anhydrase inhibitory activity. This is attained via replacement of 4-fluorophenyl ureido moiety of SLC-0111 with un/substituted benzoylthioureido ones, where the substitution at the benzoyl moiety involves either 3-chloro, 3,4-dichloro or 3-bromo substituent while retaining sulphamoyl phenyl of SLC-0111 to produce compounds 4a–d, or bioisosteric replacement of the sulphamoyl phenyl with benzoic acid to obtain 5a–d, or with ethyl benzoate moiety as potential prodrugs to give 6a–d.
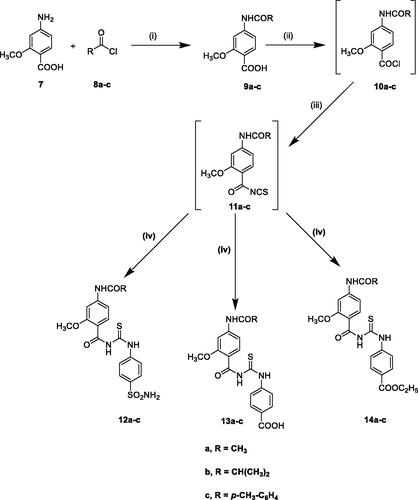
Further modification includes substitution of 4-fluorophenyl ureido of SLC-0111 with 2-methoxy-4-substituted benzamido moiety while keeping the same substitution pattern of the zinc binding groups (sulphamoyl phenyl or benzoic acid) to afford 12a–c and 13a–c, respectively, or via the prodrug moiety (ethyl benzoate) to obtain 14a–c ().
Table 1. Inhibition data (KI, nM) of human CA isoforms hCA I, II, IX and XII with compounds 4a–d, 5a–d, 6a–d, 12a–c, 13a–c, and 14a–c against SLC-0111 and AAZ; by a stopped flow CO2 hydrase assay.
This amendment aimed to explore the effect of such modification on the potency and/or selectivity of the designed compounds. Meanwhile, a highly flexible carbonyl thioureido linker was retained in all compounds ().
Results and discussion
Chemistry
The synthetic routes adopted for the synthesis of the target compounds 4a–d, 5a–d, 6a–d, 12a–c, 13a–c, and 14a–c are depicted in Schemes 1 and 2, respectively.
The synthesis of compounds 4a–d, 5a–d, and 6a–d was carried out by conversion of the appropriate benzoic acid derivative 1a–d to the corresponding acid chloride 2a–d, followed by reaction with ammonium thiocyanate to give the key intermediate benzoyl isothiocyanates 3a–d that were treated similarly with sulphanilamide, 4-aminobenzoic acid or ethyl 4-aminobenzoate to furnish the target compounds (Scheme 1). IR spectra of compounds 4a–d showed the appearance of characteristic bands at 3360–3255 cm−1corresponding to NH2 and NH, in addition to two stretching vibration bands at 1330–1160 cm−1 attributed to the characteristic SO2 group. 1H NMR spectra revealed the appearance of D2O exchangeable signal in the aromatic region around 7.42 ppm corresponding to two NH2 protons of the SO2NH2 group as a singlet.
Scheme 1. Reagents and reaction conditions: (i) SOCl2, methylene chloride, reflux, 4–5 h, (ii) NH4SCN, acetone, reflux, 1–3 h, (iii) sulphanilamide or 4-aminobenzoic acid or ethyl 4-aminobenzoate, acetone, reflux, 2–3 h.
IR spectrum of compound 5c showed the appearance of characteristic carboxylic OH stretching vibration band at 3402 cm−1 and carbonyl band at 1654 cm−1. IR spectra of compounds 6a–d displayed characteristic bands at 3380–3350 cm−1 corresponding to the NHs groups in addition to carbonyl bands of the ester at range of 1745–1712 cm−1. 1H NMR spectra of 6a–d revealed the appearance of triplet signal at 1.30–1.42 ppm for protons of methyl group of esters (CH3CH2-) and quartette signal at 4.33 ppm corresponding to (CH3CH2-) protons. Further, 13C NMR spectra of 6b, 6c, and 6d displayed ethyl carbons CH3CH2 at 14.6 ppm, CH3CH2 carbon at 61.2 ppm along with carbonyl carbons at 165.7 ppm and CS carbon at 176.4–179.3 ppm.
Additionally, the synthesis of the target acetamido-benzoylureido derivatives 12a–c, 13a–c, and 14a–c was performed by acylation of 4-amino-2-methoxybenzoic acid 7 with different acyl chlorides 8a–c in dry acetonitrile in the presence of potassium hydroxide to afford compounds 9a–c. The key intermediates 10a–c and 11a–c were obtained in situ through the formation of the corresponding acid chlorides via the reaction of the acid derivatives with thionyl chloride followed by ammonium thiocyanate. The intermediates 11a–c were not isolated but rather used in the synthesis of the target compounds by reaction with sulphanilamide, 4-aminobenzoic acid or ethyl 4-aminobenzoate in dry acetone to afford the target compounds 12a–c, 13a–c, and 14a–c, respectively in high yields (Scheme 2). The IR spectra of these compounds declared the presence of the expected new functional groups. The characteristics of the 1H NMR spectra of 10a–c revealed the appearance of exchangeable NH protons at 7.27–7.41 ppm due to the sulphamoyl group, whereas compounds 11a–c showed –COOH proton at 12.80–13.05 ppm. Also compounds 12a–c displayed triplet-quartette signals for the ethyl protons at 1.32–1.43 and 4.32–4.46, respectively. This is in addition to the signals characteristic of the methyl, isopropyl, tolyl protons of the amide group of compounds 12a–c, 13a–c, and 14a–c. On the other hand, 13C NMR spectra of 12a, 13a and 14a showed signal at 24.7 ppm corresponding to CH3 of acetamido group. Also, 13C NMR spectra of 12b and 13b revealed the isopropyl carbons (CHCH3)2 at 19.7 ppm and (CHCH3)2 carbon at 35.6 ppm. In addition, 13C NMR spectra of 13c displayed a signal at 21.5 ppm attributed for CH3 carbons of the tolyl moiety.
Biological evaluation
Carbonic anhydrase inhibitory activity
The CA inhibitory activities of all synthesised compounds 4a–d, 5a–d, 6a–d, 12a–c, 13a–c, and 14a–c, as well as acetazolamide (AAZ) as a standard inhibitor were screened against four CA isoforms: hCA I, hCA II, hCA IX, and hCA XII. The selection of these four isoforms was based on the fact that hCA II is an antiglaucoma medication target, hCA IX and XII are well established targets for the therapy and prognosis of hypoxic malignancies, whereas, hCA I is one of the most common off-target isoforms for antiglaucoma and anticancer CAIs therapeutic applicationCitation22.
The results showed that the inhibitory activity of the tested compounds against the four CA isoforms were highly dependent on the nature of the ZBG. In this context, the sulphamoyl derivatives (4a–d and 12a–c) appeared as the most potent inhibitors among other derivatives, which revealed that the –COOH group failed to be a good ZBG. Interestingly, the ubiquitous cytosolic isoform hCA I, which is highly abundant in the gastrointestinal tract and red blood cells, was the most inhibited isoform among the others. The tested compound elicited mild activity against hCA II and hCA IX and a poor activity against hCA XII. A closer look on the results pointed out that seven compounds 4a, 4b, 4c, 4d, 6d, 12a and 12c exhibited the best inhibitory activity against hCA I with KI values ranging from 40.40–91.00 nM and better than the reference drug AAZ (250 nM). All of these compounds, except 6d, featured a free sulphamoyl group which strongly supported the binding rational. Trying to figure out the effect of the tail substitution on the activity, it could be noticed that a small acetamido group as in compound 12a was correlated to the highest activity against all hCA isoforms. Unsubstituted phenyl ring (compound 4a) was associated with good to moderate activity, notably this compound was the most potent against hCA XII with KI value = 57.40 nM. The presence of 3-chloro substitution (compound 4b) gave the best activity agasinst hCA I, hCA II and h CA IX among the halogenated derivatives. The 3,4-dichloro and the 3-bromo derivatives, 4c and 4d, respectively, demonstarted good to moderate activity against hCA I, hCA II and h CA XII, where compound 4c elicited the most potent inhibitory activity against hCA I (KI = 40.40 nM). It is noteworthy that the 3-bromo ethylbenzoate derivative 6d showed good to moderate activity against hCA I and hCA II.
Since the tested compounds were designed as modified derivatives of the lead compound SLC-0111, it deemed of interest to explore the impact of such modifications on the activity and selectivity of the targeted compounds. The results revealed a great increase in potency of the new compounds against hCA I and hCA II, especially compounds 4a–d, 12a and 12c compared to SLC-0111. On the other hand, there was much reduction in potency against hCA IX and hCA XII in all compounds. Therefore, it could be claimed that the modifications performed on the structure of SLC-0111 in the present investigation led to a switch in selectivity of the compounds to the non-cancer related isoforms hCA I and hCA II.
Conclusions
Twenty-one target compounds bearing a sulphamoyl, carboxylic or ethyl carboxylate substitutions and a diversely substituted phenyl tail moiety were designed as analogues of SLC-0111 and were synthesised through simple chemical procedures. All target compounds were assessed for their CA inhibitory activity against four relevant isoforms, namely hCA I, hCA II, hCA IX and hCA XII. The study revealed that the sulphamoyl group was the most efficient ZBG. Modifications of the substitution on the tail moiety had only a minor effect on activity and/or selectivity. Seven compounds 4a, 4b, 4c, 4d, 6d, 12a and 12c were selective against hCA I (Kis = 40.40–91.00 nM) compared to AAZ (Ki = 250.00 nM) which might present them as potential antiglaucoma drug candidates. Compounds bearing 4-acetamido-2-methoxy benzamido 12a or 2-methoxy- (4-methylbenzamido) 12c displayed superior activity (Kis= 67.60 and 91.00 nM) against hCAI more than that expressed by AAZ. Unlike SLC-0111, the targeted compounds were more selective to hCA I and hCA II rather than to hCA IX and hCA XII which would highlight these compounds as promising drug candidates for the treatment of glaucoma.
Materials and methods
Chemistry
General
All chemicals and solvents, which purchased and used without further purification. The melting points were measured using the SMP30 melting point apparatus. Thermo Scientific Nicolet iS10 spectrometer was used to record FT-IR spectra. 1HNMR spectra were run on a Bruker 400 MHz spectrophotometer. 13CNMR spectra were recorded in δ scale given in ppm on a Bruker 101 MHz spectrophotometer. Both types of spectra were performed in DMSO-d6. Elemental analyses were performed on a Thermo Scientific Flash 2000 elemental analyser at the Regional Center for Mycology and Biotechnology, Al-Azhar University, Egypt. All reactions were monitored by silica gel 60 F254 TLC and visualised under UV light (254 nm). Compounds 2a–dCitation24, 3a–dCitation25, 4a,bCitation26, 5a,b,d, 6aCitation27–29, 9a–cCitation24, 10a–cCitation25, 11a–cCitation26 were prepared according to the reported procedures.
General procedure for the preparation of target compounds 4a–d, 5a–d, 6a–d, 12a–c, 13a–c and 14a–c
The freshly prepared benzoyl isothiocyanate derivative 3a–d or 11a–c (1 mmol) was treated with sulphanilamide, 4-aminobenzoic acid or ethyl 4-aminobenzoate (1 mmol) in refluxing anhydrous acetone (10 ml) for 2–3 h. The reaction mixture was cooled to room temperature and the formed precipitate was collected by filtration and recrystallized from ethanol to give the final target compounds in high yieldsCitation28.
N-[(4-Sulphamoylphenyl)carbamothioyl]benzamide (4a)
Yellow crystals, (yield: 85%), m.p. 237–242 °CCitation30.
4-Chloro-N-[(4-sulfamoylphenyl)carbamothioyl]benzamide (4b)
Yellow crystals, (yield: 76%), m.p. 226–234 °CCitation30.
3,4-Dichloro-N-[(4-sulphamoylphenyl)carbamothioyl]benzamide (4c)
Yellow crystals, (yield: 75%), m.p. 206–208 °C; IR (KBr, νmax/cm−1): 3360, 3255 (NHs), 1670 (C=O), 1531 (C=S), 1327, 1157 (SO2); 1H NMR (DMSO-d6) δ ppm: 7.41 (s, 2H, NH2, D2O exchangeable), 7.82 (d, 1H, J = 8.4 Hz, Ar-H), 7.85–7.94 (m, 5H, Ar-H), 8.25 (d, 1H, J = 2.0 Hz, Ar-H), 11.89 (s, 1H, NH, D2O exchangeable), 12.53 (s, 1H, NH, D2O exchangeable); MS (m/z): 404.20 [M]+, 406.47 [M + 2]+; Anal. Calcd. for C14H11Cl2N3O3S2 (404.28): C, 41.59; H, 2.74; N, 10.39; Found C, 41.78; H, 2.95; N, 10.57.
4-Bromo-N-[(4-sulfamoylphenyl)carbamothioyl]benzamide (4d)
Yellow crystals, (yield: 75%), m.p. 215–217 °C; IR (KBr, νmax/cm−1): 3350–3245 (NHs), 1665 (C = O), 1520 (C = S), 1330, 1160 (SO2); 1H NMR δ ppm: 7.43 (s, 2H, NH2, D2O exchangeable), 7.63 (d, 2H, J = 8.6 Hz, Ar-H), 7.83–7.87 (m, 4H, Ar-H), 8.02 (d, 2H, J = 8.6 Hz, Ar-H), 11.83 (s, 1H, NH, D2O exchangeable), 12.63 (s, 1H, NH, D2O exchangeable); Anal. Calcd. for C14H12BrN3O3S2 (414.29): C, 40.59; H, 2.92; N, 10.14; Found C, 40.47; H, 2.67; N, 10.03.
4-(3-Benzoylthioureido)benzoic acid (5a)
Yellow crystals, (yield: 75%), m.p. 212–224 °CCitation31.
4-[3-(4-Chlorobenzoyl)thioureido]benzoic acid (5b)
Yellow crystals, (yield: 72%), m.p. 232–240 °CCitation31.
4-[3-(3,4-Dichlorobenzoyl)thioureido]benzoic acid (5c)
Yellow crystals, (yield: 67%), m.p. 216–218 °C; IR (KBr, νmax/cm−1): 3402 (OH), 3290 (br, NHs), 1678, 1654 (C = O), 1523 (C = S); 1H NMR δ ppm: 7.84–7.89 (m, 2H, Ar-H), 7.90–7.98 (m, 4H, Ar-H), 8.26 (s, 1H, Ar-H), 11.91 (s, 1H, NH, D2O exchangeable), 12.56 (s, 1H, NH, D2O exchangeable), 12.97 (s, 1H, OH, D2O exchangeable); Anal. Calcd. for C15H10Cl2N2O3S (369.22): C, 48.80; H, 2.73; N, 7.59; Found C, 49.07; H, 2.89; N, 7.80.
4-[3-(4-Bromobenzoyl)thioureido]benzoic acid (5d)
Yellow crystals, (yield: 60%), m.p. 206–215 °CCitation31.
Ethyl-4-(3-benzoylthioureido)benzoate (6a)
Yellow crystals, (yield: 67%), m.p. 223–235 °CCitation32.
Ethyl 4-[3-(4-chlorobenzoyl)thioureido]benzoate (6b)
Yellow crystals, (yield: 75%), m.p. 219–221 °C; IR (KBr, νmax/cm−1): 3350–3360 (NHs), 1740, (C = O) ester, 1665, (C = O) amide, 1535 (C = S); 1H NMR δ ppm: 1.42 (t, 3H, J = 1.6 Hz, CH3), 4.33 (q, 2H, J = 6.0 Hz, CH2), 7.29–7.36 (m, 4H, Ar-H), 7.94–8.00 (m, 4H, Ar-H), 8.97 (s, 2H, 2NH, D2O exchangeable); 13C NMR δ ppm: 14.6, 61.3, 110.3, 129.4, 129.7, 130.02, 130.08, 131.3, 142.9, 145.9, 165.7, 176.4; Anal. Calcd. for C17H15ClN2O3S (362.83): C, 56.28; H, 4.17; N, 7.72; Found C, 56.43; H, 4.28; N, 7.94.
Ethyl 4-[3-(3,4-dichlorobenzoyl)thioureido]benzoate (6c)
Yellow crystals, (yield: 67%), m.p. 216–218 °C; IR (KBr, νmax/cm−1): 3186 (NHs), 1712, 1693 (C = O), 1535 (C = S); 1H NMR δ ppm: 1.33 (t, 3H, J = 7.1 Hz, CH3), 4.33 (q, 2H, J = 10.0 Hz, CH2), 7.34 (d, 2H, J = 8.4 Hz, Ar-H), 7.48 (d, 2H, J = 8.6 Hz, Ar-H), 7.90–8.02 (m, 4H, Ar-H), 8.95 (s, 1H, Ar-H), 11.87 (s, 1H, NH, D2O exchangeable), 12.58 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: 14.6, 61.2, 110.3, 124.0, 129.4, 129.7, 130.2, 131.7, 133.0, 136.3, 142.6, 145.9, 165.7, 176.4, 179.2; MS (m/z): 397.27 [M]+, 396.68 [M]+, 399.34 [M + 2]+; Anal. Calcd. for C17H14Cl2N2O3S (397.27): C, 51.40; H, 3.55; N, 7.05; Found C, 51.67; H, 3.62; N, 7.19.
Ethyl-4-[3-(4-bromobenzoyl)thioureido]benzoate (6d)
Yellow crystals, (yield: 75%), m.p. 218–220 °C; IR (KBr, νmax/cm−1): 3360–3380 (NHs), 1745, (C = O) ester, 1660, (C = O) amide, 1535 (C = S); 1H NMR δ ppm: 1.33 (t, 3H, J = 7.0 Hz, CH3), 4.32 (q, 2H, J = 7.0 Hz, CH2), 7.76 (d, 2H, J = 8.5 Hz, Ar-H), 7.91 (d, 4H, J = 8.6 Hz, Ar-H), 8.00 (d, 2H, J = 8.6 Hz, Ar-H), 11.77 (s, 1H, NH, D2O exchangeable), 12.69 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: 14.6, 61.2, 123.9, 127.5, 127.6, 130.2, 131.2, 131.7, 131.9, 142.6, 165.5, 167.7, 179.3; Anal. Calcd. for C17H15BrN2O3S (407.28): C, 50.13; H, 3.71; N, 6.88; Found C, 50.39; H, 3.85; N, 7.12.
4-Acetamido-2-methoxy-N-[(4-sulfamoylphenyl)carbamothioyl]benzamide (12a)
Yellow crystals, (yield: 75%), m.p. 225–227 °C; IR (KBr, νmax/cm−1): 3340, 3302 (NHs), 1697, 1666 (C = O), 1519 (C = S), 1338, 1157 (SO2); 1H NMR δ ppm: 2.11 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 7.35 (d, J = 8, 1H, Ar-H), 7.41 (s, 2H, NH2,D2O exchangeable), 7.65–7.87 (m, 3H, Ar-H), 7.95–7.98 (m, 3H, Ar-H), 10.41 (s, 1H, NH, D2O exchangeable), 11.12 (s, 1H, NH, D2O exchangeable), 12.78 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: 24.7, 57.0, 102.5, 112.1, 113.2, 124.6, 126.7, 133.0, 141.1, 141.8, 146.3, 159.0, 164.8, 169.8; MS (m/z): 422.66 [M]+; Anal. Calcd. for C17H18N4O5S2 (422.47): C, 48.33; H, 4.29; N, 13.26; Found C, 48.61; H, 4.45; N, 13.50.
4-Isobutyramido-2-methoxy-N-[(4-sulfamoylphenyl)carbamothioyl] benzamide (12b)
White crystals, (yield: 79%), m.p. 248–250 °C; IR (KBr, νmax/cm−1): 3300–3310 (NHs), 1710, 1690, (C = O) amide, 1520 (C = S), 1336, 1135 (SO2); 1H NMR δ ppm: 1.13 (d, 6H, J = 6.8 Hz,-CH(CH3)2), 2.61–2.68 (m, 1H, -CH(CH3)2), 4.01 (s, 3H, OCH3), 7.36 (d, 1H, J = 7.1 Hz, Ar-H), 7.40 (s, 2H, NH2, D2O exchangeable), 7.73 (s, 1H, Ar-H), 7.86 (d, 2H, J = 8.6 Hz, Ar-H), 7.92–7.99 (m, 3H, Ar-H), 10.32 (s, 1H, NH, D2O exchangeable), 11.11 (s, 1H, NH, D2O exchangeable), 12.77 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: 19.7, 35.6, 57.0, 102.6, 112.2, 113.1, 124.6, 126.7, 133.0, 141.1, 141.9, 146.5, 159.0, 164.7, 176.6, 178.7; Anal. Calcd. for C19H22N4O5S2 (450.53): C, 50.65; H, 4.92; N, 12.44; Found C, 50.89; H, 4.81; N, 12.67.
2-Methoxy-4-(4-methylbenzamido)-N-[(4-sulfamoylphenyl)carbamothioyl] benzamide (12c)
Yellow crystals, (yield: 82%), m.p. 250–252 °C; IR (KBr, νmax/cm−1): 3350–3205 (NHs), 1680, 1650, (C = O) amide, 1530 (C = S), 1320, 1154 (SO2); 1H NMR δ ppm: 2.40 (s, 3H, CH3), 3.82 (s, 3H, OCH3), 7.27 (s, 2H, NH2, D2O exchangeable), 7.36 (d, 2H, J = 7.9, Ar-H), 7.40 (d, 1H, J = 8.4, Ar-H), 7.46–7.50 (m, 1H, Ar-H), 7.69–7.72 (m, 1H, Ar-H), 7.80 (d, 2H, J = 8.8, Ar-H), 7.90 (d, 2H, J = 8.0, Ar-H), 7.96 (d, 2H, J = 8.6, Ar-H), 10.38 (s, 1H, NH, D2O exchangeable), 10.48 (s, 2H, 2NH, D2O exchangeable); Anal. Calcd. for C23H22N4O5S2 (498.57): C, 55.41; H, 4.45; N, 11.24; Found C, 55.63; H, 4.61; N, 11.48.
4-[3-(4-Acetamido-2-methoxybenzoyl)thioureido]benzoic acid (13a)
Brown crystals, (yield: 82%), m.p. 245–247 °C; 1H NMR δ ppm: 2.10 (s, 3H, CH3), 4.00 (s, 3H, OCH3), 7.34 (d, 1H, J = 7.1, Ar-H), 7.64 (s, 1H, Ar-H), 7.90–8.00 (m, 5H, Ar-H), 10.42 (s, 1H, NH, D2O exchangeable), 11.11 (s, 1H, NH, D2O exchangeable), 12.85 (s, 1H, NH, D2O exchangeable), 13.05 (s, 1H, OH, D2O exchangeable). 13C NMR δ ppm: 24.7, 57.0, 102.5, 112.0, 113.1, 123.7, 128.6, 130.4, 131.5, 133.0, 142.1, 146.3, 159.0, 164.8, 167.1, 169.8, 178.2; Anal. Calcd. for C18H17N3O5S (387.41): C, 55.81; H, 4.42; N, 10.85; Found C, 56.08; H, 4.56; N, 11.07.
4-[3-(4-Isobutyramido-2-methoxybenzoyl)thioureid])benzoic acid (13b)
Brown crystals, (yield: 76%), m.p. 256–258 °C; IR (KBr, νmax/cm−1): 3313 (br NHs, OH), 1689, 1670 (C = O), 1516 (C = S); 1H NMR δ ppm: 1.12 (d, 6H, J = 6.8 Hz, CH(CH3)2), 2.60–2.67 (m, 1H, CH(CH3)2), 4.00 (s, 3H, OCH3), 7.35 (d, 1H, J = 7.5 Hz, Ar-H), 7.72 (s, 1H, Ar-H), 7.92 (t, 3H, J = 8.2 Hz, Ar-H), 7.97 (t, 2H, J = 6.7 Hz, Ar-H), 10.30 (s, 1H, NH, D2O exchangeable), 11.09 (s, 1H, NH, D2O exchangeable), 12.86 (s, 1H, NH, D2O exchangeable), 12.97 (s, 1H, OH, D2O exchangeable). 13C NMR δ ppm: 19.7, 35.6, 56.9, 102.5, 112.1, 112.7, 123.4, 128.5, 130.4, 133.0, 142.0, 146.6, 158.9, 164.6, 167.1, 176.6, 178.1; MS (m/z): 416.32 [M + 1]+; Anal. Calcd. For C20H21N3O5S (415.46): C, 57.82; H, 5.10; N, 10.11; Found C, 57.95; H, 5.23; N, 10.40.
4-(3-(2-Methoxy-4-(4-methylbenzamido)benzoyl)thioureido]benzoic acid (13c)
Yellow crystals, (yield: 78%), m.p. 281–283 °C; IR (KBr, νmax/cm−1): 3353 (NHs), 3286 (OH) acide, 1687 (C = O) acide, 1670, 1650 (C = O) amide, 1553 (C = S); 1H NMR δ ppm: 2.09 (s, 3H, CH3), 4.05 (s, 3H, OCH3), 7.36 (d, 2H, J = 10.0 Hz, Ar-H), 7.67–7.95 (m, 7H, Ar-H), 8.00 (d, 2H, J = 8.6 Hz, Ar-H), 10.46 (s, 1H, NH, D2O exchangeable), 10.85 (s, 1H, NH, D2O exchangeable), 11.15 (s, 1H, NH, D2O exchangeable), 12.80 (s, 1H, OH, D2O exchangeable). 13C NMR δ ppm: 21.5, 57.1, 113.6, 119.9, 123.7, 125.8, 128.6, 129.4, 129.5, 130.4, 130.6, 132.1, 142.4, 143.8, 146.4, 158.8, 164.8, 166.2, 167.4, 178.3; Anal. Calcd. for C24H21N3O5S (463.51): C, 62.19; H, 4.57; N, 9.07; Found C, 61.97; H, 4.73; N, 9.34.
Ethyl-4-[3-(4-acetamido-2-methoxybenzoyl)thioureido]benzoate (14a)
Brown crystals, (yield: 68%), m.p. 237–239 °C; IR (KBr, νmax/cm−1): 3487, 3205 (NHs), 1700 (C = O) ester, 1675, 1635 (C = O) amide, 1531 (C = S); 1H NMR δ ppm: 1.32 (t, 3H, J = 7.1 Hz, CH2CH3), 2.09 (s, 3H, CH3), 4.01 (s, 3H, OCH3), 4.33 (q, 2H, J = 7.1 Hz, CH2CH3), 7.34 (d, 1H, J = 8.6, Ar-H), 7.65 (s, 1H, Ar-H), 7.96 (d, J = 8.5 Hz, 3H, Ar-H), 7.98–8.02 (m, 2H, Ar-H), 10.42 (s, 1H, NH, D2O exchangeable), 11.12 (s, 1H, NH, D2O exchangeable), 12.88 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: δ 14.6, 24.7, 57.0, 61.2, 102.5, 112.0, 113.1, 123.8, 127.6, 130.2, 133.0, 142.4, 146.3, 159.0, 164.8, 165.5, 169.8, 178.3; Anal. Calcd. for C20H21N3O5S (415.46): C, 57.82; H, 5.10; N, 10.11; Found C, 58.04; H, 5.19; N, 10.37.
Ethyl-4-[3-(4-isobutyramido-2-methoxybenzoyl)thioureido]benzoate (14b)
White crystals, (yield: 75%), m.p. 245–247 °C; IR (KBr, νmax/cm−1): 3302, 3186 (NHs), 1712, 1693,1666 (C = O), 1512 (C = S); 1H NMR δ ppm: 1.13 (d, J = 6.8 Hz, 6H, CH(CH3)2), 1.33 (t, 3H, J = 7.1 Hz, -CH2CH3), 2.58–2.67 (m, 1H, CH(CH3)2), 4.01 (s, 3H, OCH3), 4.32 (q, 2H, J = 4.1 Hz, -CH2CH3), 7.33–7.37 (m, 2H, Ar-H), 7.73 (s, 1H, Ar-H), 7.93–8.01 (m, 4H, Ar-H), 10.31 (s, 1H, NH, D2O exchangeable), 11.10 (s, 1H, NH, D2O exchangeable), 12.88 (s, 1H, NH, D2O exchangeable); 13C NMR δ ppm: 14.6, 19.7, 31.0, 57.0, 61.3, 102.6, 110.7, 112.2, 123.6, 127.6, 129.4, 133.0, 142.4, 146.5, 159.0, 164.7, 165.5, 165.7, 178.2. MS (m/z): 444.25 [M + 1]+; Anal. Calcd. for C22H25N3O5S (443.52): C, 59.58; H, 5.68; N, 9.47; Found C, 59.79; H, 5.80; N, 9.71.
Ethyl-4-[3-(2-methoxy-4-(4-methylbenzamido)benzoyl)thioureido]benzoate (14c)
White crystals, (yield: 80%), m.p. 257–259 °C; IR (KBr, νmax/cm−1): 3205 (br, NHs), 1712 1693,1633 (C = O), 1531 (C = S); 1H NMR δ ppm: 1.43 (t, 3H, J = 6.3 Hz, CH2CH3), 2.44 (s, 3H, CH3), 4.02 (s, 3H, OCH3), 4.46 (q, 2H, J = 7.0 Hz, -CH2CH3), 7.38 (s, 1H, Ar-H), 7.55 (d, J = 7.9 Hz, 2H, Ar-H), 7.71 (d, J = 7.4 Hz, 1H, Ar-H), 8.01–8.08 (m, 7H, Ar-H), 10.57 (s, 1H, s, 1H, NH. D2O exchangeable), 11.82 (s, 1H, NH, D2O exchangeable), 12.90 (s, 1H, NH, D2O exchangeable); Anal. Calcd. for C26H25N3O5S (491.56): C, 63.53; H, 5.13; N, 8.55; Found C, 63.39; H, 5.31; N, 8.79.
Biological evaluation
Carbonic anhydrase inhibitory activity
A stopped flow CO2 hydrase assay was adopted using an SX.18 MV-R Applied Photophysics (Oxford, UK) stopped-flow instrument to assess the inhibition against the various CA isozymesCitation33. Phenol red(at a concentration of 0.2 mM has been used as an indicator, working at the absorbance maximum of 557 nm, with 20 Mm Hepes (pH 7.5) as buffer, and 20 mM Na2SO4. The initial rates of the CA-catalysed CO2 hydration reaction was run for a period of 10–100 s then completing as the reported protocol. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, and represent the mean for at least three different determinationsCitation34–36. All CA isofoms were recombinant ones obtained in-house as previously reportedCitation37–42.
Supplemental Material
Download PDF (515.3 KB)Disclosure statement
C. T. Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Additional information
Funding
References
- Borges A, Abreu AC, Dias C, Saavedra MJ, Borges F, Simões M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules. 2016;21(7):877.
- Hassan MI, Shajee B, Waheed A, Ahmad F, Sly WS. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem. 2013;21(6):1570–1582.
- Hirakawa Y, Senda M, Fukuda K, Yu HY, Ishida M, Taira M, Kinbara K, Senda T. Characterization of a novel type of carbonic anhydrase that acts without metal cofactors. BMC Biol. 2021;19(1):15.
- Ghorai S, Pulya S, Ghosh K, Panda P, Ghosh B, Gayen S. Structure-activity relationship of human carbonic anhydrase-II inhibitors: detailed insight for future development as anti-glaucoma agents. Bioorg Chem. 2020;95:103557.
- Dar’in D, Kantin G, Kalinin S, Sharonova T, Bunev A, Ostapenko GI, Nocentini A, Sharoyko V, Supuran CT, Krasavin M. Investigation of 3-sulfamoyl coumarins against cancer-related IX and XII isoforms of human carbonic anhydrase as well as cancer cells leads to the discovery of 2-oxo-2H-benzo [h] chromene-3-sulfonamide – a new caspase-activating proapoptotic agent. Eur J Med Chem. 2021;222:113589.
- Akocak S, Ilies MA. Next-generation primary sulfonamide carbonic anhydrase inhibitors. Target Carbon Anhydrases. 2014;1:35.
- Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1, 3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem. 2018;33(1):1575–1580.
- Elimam DM, Elgazar AA, Bonardi A, Abdelfadil M, Nocentini A, El-Domany RA, Abdel-Aziz HA, Badria FA, Supuran CT, Eldehna WM. Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: design, synthesis and biological evaluation. Eur J Med Chem. 2021;225:113800.
- Angeli A, Carta F, Nocentini A, Winum J-Y, Zalubovskis R, Akdemir A, Onnis V, Eldehna WM, Capasso C, Simone GD, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites. 2020;10(10):412.
- Gieling RG, Williams KJ. Carbonic anhydrase IX as a target for metastatic disease. Bioorg Med Chem. 2013;21(6):1470–1476.
- Supuran CT, Alterio V, Di Fiore A, D' Ambrosio K, Carta F, Monti SM, De Simone G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev. 2018;38(6):1799–1836.
- Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf Dem Keller U, Leung S, Huntsman D, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–3376.
- Winum J, Rami M, Scozzafava A, Montero J, Supuran C. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev. 2008;28(3):445–463.
- Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–368.
- Ibrahim HS, Allam HA, Mahmoud WR, Bonardi A, Nocentini A, Gratteri P, Ibrahim ES, Abdel-Aziz HA, Supuran CT. Dual-tail arylsulfone-based benzenesulfonamides differently match the hydrophobic and hydrophilic halves of human carbonic anhydrases active sites: selective inhibitors for the tumor-associated hCA IX isoform. Eur J Med Chem. 2018;152:1–9.
- Liguori F, Carradori S, Ronca R, Rezzola S, Filiberti S, Carta F, Turati M, Supuran CT. Benzenesulfonamides with different rigidity-conferring linkers as carbonic anhydrase inhibitors: an insight into the antiproliferative effect on glioblastoma, pancreatic, and breast cancer cells. J Enzyme Inhib Med Chem. 2022;37(1):1857–1869.
- Pacchiano F, Carta F, McDonald PC, Lou Y, Vullo D, Scozzafava A, Dedhar S, Supuran CT. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54(6):1896–1902.
- Pacchiano F, Aggarwal M, Avvaru BS, Robbins AH, Scozzafava A, McKenna R, Supuran CT. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb). 2010;46(44):8371–8373.
- Abo-Ashour MF, Eldehna WM, Nocentini A, Ibrahim HS, Bua S, Abdel-Aziz HA, Abou-Seri SM, Supuran CT. Novel synthesized SLC-0111 thiazole and thiadiazole analogues: determination of their carbonic anhydrase inhibitory activity and molecular modeling studies. Bioorg Chem. 2019;87:794–802.
- Qaiser S, Mubarak MS, Ashraf S, Saleem M, Ul-Haq Z, Safdar M, Rauf A, Abu-Izneid T, Qadri MI, Maalik A. Benzilydene and thiourea derivatives as new classes of carbonic anhydrase inhibitors: an in vitro and molecular docking study. Med Chem Res. 2021;30(3):552–563.
- Saeed A, Khan SU, Saeed M, Shabir G, Hasan A, Khera RA, El-Seedi H, Halim SA, Khan A, Al-Harrasi A, et al. Synthesis of novel hybrid pharmacophore of N-((4 sulfamoylphenyl(carbamothioyl)alkanamides as potent carbonic anhydrase-II and 15-lipoxygenase inhibitors. Drug Dev. Res. 2022;83:745–754.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246(8):2561–2573.
- Elbadawi MM, Eldehna WM, Nocentini A, Abo-Ashour MF, Elkaeed EB, Abdelgawad MA, Alharbi KS, Abdel-Aziz HA, Supuran CT, Gratteri P, et al. Identification of N-phenyl-2-(phenylsulfonyl)acetamides/propanamides as new SLC-0111 analogues: Synthesis and evaluation of the carbonic anhydrase inhibitory activities. Eur J Med Chem. 2021;218:113360.
- Plutín AM, Suárez M, Ochoa E, Machado T, Mocelo R, Concellón JM, Rodríguez-Solla H. Synthesis of new acyl, furoyl, and benzoylthiocarbamates as polydentate systems. Structural study of isopropyl N-(2-furoyl) thiocarbamate. Tetrahedron. 2005;61:5812–5817.
- Larsen JS, Zahran MA, Pedersen EB, Nielsen C. Synthesis of triazenopyrazole derivativesas potential inhibitors of HIV-1. Monatshefte Fuer Chem. 1999;130(9):1167–1173.
- Nitulescu GM, Draghici C, Olaru OT. New potential antitumor pyrazole derivatives: synthesis and cytotoxic evaluation. Int J Mol Sci. 2013;14(11):21805–21818.
- Saeed A, Mumtaz A, Ishida H. Synthesis, characterization of some new 1-aroyl-3-(4-aminosulfonylphenyl) thioureas and crystal structure of 1-(3, 4, 5-trimethoxybenzoyl)-3-(4-aminosulfonylphenyl) thiourea. J Sulfur Chem. 2011;32(1):45–54.
- Oudah KH, Najm MAA, Samir N, Serya RAT, Abouzid KAM. Design, synthesis and molecular docking of novel pyrazolo[1,5-a][1,3,5]triazine derivatives as CDK2 inhibitors. Bioorg Chem. 2019;92:103239.
- Carradori S, Guglielmi P. Mechanisms of action of carbonic anhydrase inhibitors: compounds that bind “out of the binding site” and compounds with an unknown mechanism of action. New York (NY): Elsevier Inc.; 2019. https://doi.org/10.1016/B978-0-12-816476-1.00012-5
- Mahdavi M, Shirazi MS, Taherkhani R, Saeedi M, Alipour E, Moghadam FH, Moradi A, Nadri H, Emami S, Firoozpour L, et al. Synthesis, biological evaluation and docking study of 3-aroyl-1-(4-sulfamoylphenyl)thiourea derivatives as 15-lipoxygenase inhibitors. Eur J Med Chem. 2014;82:308–313.
- El-Sayed NS, El-Bendary ER, El-Ashry SM, El-Kerdawy MM. Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo [3, 2-a] pyrimidines. Eur J Med Chem. 2011;46(9):3714–3720.
- Saeed S, Bhatti MH, Yunus U, Jones PG. Ethyl 4-(3-benzoylthioureido) benzoate. Acta Crystallogr Sect E Struct Rep Online. 2008;64(Pt 8):o1485.
- Vullo D, Carta F. Mechanisms of action of carbonic anhydrase inhibitors: zinc binders, in: Carbon. Anhydrases. New York (NY): Elsevier; 2019. p. 187–222.
- Heravi YE, Bua S, Nocentini A, Del Prete S, Saboury AA, Sereshti H, Capasso C, Gratteri P, Supuran CT. Inhibition of Malassezia globosa carbonic anhydrase with phenols. Bioorg Med Chem. 2017;25(9):2577–2582.
- Zhang Z, Lau J, Zhang C, Colpo N, Nocentini A, Supuran CT, Bénard F, Lin K-S. Design, synthesis and evaluation of 18F-labeled cationic carbonic anhydrase IX inhibitors for PET imaging. J Enzyme Inhib Med Chem. 2017;32(1):722–730.
- Nocentini A, Supuran CT. Chapter 1 - Carbonic anhydrases: an overview. In: Supuran CT, Nocentini A, editors. Carbon Anhydrases. Academic Press; 2019. p. 3–16.
- Ebrahimi H, Hadi JS, Al-Ansari HS. A new series of Schiff bases derived from sulfa drugs and indole-3-carboxaldehyde: synthesis, characterization, spectral and DFT computational studies. J Mol Struct. 2013;1039:37–45.
- Nocentini A, Bua S, Lomelino CL, McKenna R, Menicatti M, Bartolucci G, Tenci B, Di Cesare Mannelli L, Ghelardini C, Gratteri P, et al. Discovery of new sulfonamide carbonic anhydrase IX inhibitors incorporating nitrogenous bases. ACS Med Chem Lett. 2017;8(12):1314–1319.
- Nocentini A, Vullo D, Del Prete S, Osman SM, Alasmary FAS, AlOthman Z, Capasso C, Carta F, Gratteri P, Supuran CT. Inhibition of the β-carbonic anhydrase from the dandruff-producing fungus Malassezia globosa with monothiocarbamates. J Enzyme Inhib Med Chem. 2017;32(1):1064–1070.
- Naqvi A, Shahnawaaz M, Rao AV, Seth DS, Sharma NK. Synthesis of Schiff bases via environmentally benign and energy-efficient greener methodologies. E-Journal Chem. 2009;6(s1):S75–S78.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777.
- Vullo D, Lehneck R, Donald WA, Pöggeler S, Supuran CT. Sulfonamide inhibition studies of the β-class carbonic anhydrase CAS3 from the filamentous Ascomycete Sordaria macrospora. Molecules. 2020;25(5):1036.