Abstract
The present investigation reports the design and synthesis of three series of benzoylthioureido derivatives bearing either benzenesulfonamide 7a–f, benzoic acid 8a–f or ethylbenzoate 9a–f moieties. The synthesised compounds were screened for their carbonic anhydrase inhibitory activity (CAI) against four isoforms hCA I, II, IX, and XII. Compounds 7a, 7b, 7c, and 7f exhibited a potent inhibitory activity towards hCAI (Kis = 58.20, 56.30, 33.00, and 43.00 nM), respectively compared to acetazolamide (AAZ) and SLC-0111 (Kis = 250.00 and 5080.00 nM). Compounds 7a, 7b, 7c, 7e, and 7f elicited selectivity over h CA II (Kis = 2.50, 2.10, 56.60,39.60 and 39.00 nM) respectively, relative to AAZ and SLC-0111(Kis = 12.10 and 960.00 nM). Also, compounds 7c, 7f, and 9e displayed selectivity against the tumour-associated isoform hCA IX (Kis = 31.20, 30.00 and 29.00 nM) respectively, compared to AAZ and SLC-0111 (Kis = 25.70 and 45.00 nM). Additionally, compounds 8a and 8f revealed a moderate to superior selectivity towards hCAXII (Kis = 17.00 and 11.00 nM) relative to AAZ and SLC-0111(Kis = 5.70 and 45.00 nM). Molecular docking and ADME prediction studies were performed on the most active compounds to shed light on their interaction with the hot spots of the active site of CA isoforms, in addition to prediction of their pharmacokinetic and physicochemical properties.
Introduction
In the 1930s, the enzyme carbonic anhydrase (CAs, EC 4.2.1.1) was identified which are zinc metalloenzymes that catalyse the reversible hydration of CO2 and H2O to HCO3− and H+ ions in all organisms (CO2 + H2O ⇌ HCO3− +H+).Citation1 Only the α-form is present in humans, however there are 15 isoforms of CA that have been identified, each with its own subcellular location. hCA I, III, VII, and XIII are cytosolic, hCA IV, IX, XII, and XIV are membrane bound; hCA VA and VB are mitochondrial; isoform VI is secreted; while CA VIII, X, and XI are catalytic. Cells can readily adjust the extracellular and intracellular pH and CO2/HCO3 pools by using any of these CA isoforms.Citation2 Several metabolic reactions (such as lipogenesis, ureagenesis, and gluconeogenesis), pH and CO2 homeostasis, electrolyte secretion, respiration, bone resorption, and tumorigenicity, are all dependent on this CA-catalysed reactionCitation3,Citation4. Increased expression of CA isoforms in humans has been linked to a variety of illness conditions. Oedema of the retina and brain, glaucoma, epilepsy, and altitude sickness have all been associated to the cytosolic hCA I and II isoformsCitation5. Some human isoenzymes, especially hCA IX and hCA XII, have been linked to cancer development. However, hCA IX and hCA XII are involved in the regulation of extracellular and intracellular pH, as well as the metabolism of tumour cells, so they are promising novel targets in anticancer drug research and development for the management of hypoxic tumours due to their overexpression in a variety of human malignanciesCitation6. Due to recent epidemic outbreaks, scientists have been looking for potent compounds that can affect a wide range of diseases and designing them to be used in a variety of targets. Sulphonamide is one of these classes, with applications ranging from antibiotics to current anti-cancer therapyCitation7,Citation8. The sulfamoyl group, which binds to the Zn+2 ion at the active site, certainly, many heterocyclic based sulphonamides are the most versatile scaffolds used to make selective and potent CA inhibitors, and they play a key role in CA inhibitionCitation9–12.
Because of its ability to form many stable hydrogen bonds with targeted protein, the ureido moiety is one of the most prevalent functional groups within medications of synthetic or natural originCitation13,Citation14. For N,N-alkylated compounds of the class reported by ZhangCitation15 and Liguori et al.,Citation16 the significant function of the ureido moiety in promoting the selectivity of carbonic anhydrase inhibitors (CAIs) was clearly demonstrated. On the other hand, Akgul et al.Citation17,Citation18. demonstrated that thioureido moiety, a bioisoster for ureido, is another potent scaffold for CAIs ().
Figure 1. General structure of taurine sulphonamides terminated with thioureido tailsCitation15.

In recent years, many approaches have been used to synthesise effective and selective carbonic anhydrase inhibitors. “Tail” approach is the most popular one, in which a flexible linker is used to attach tails of various chemical nature to an aromatic/heterocyclic ring with a zinc binding group (ZBG), such as primary sulfamoyl and carboxylic acid functionalitiesCitation19. Another approach is the addition of multiple ''tails’' to the aromatic sulphonamide moiety to improve interaction with hydrophobic or hydrophilic portions of the active site consequently it can interact with non-traditional amino acid residues on the middle and outer regions of the active site cavity, resulting in better ligand binding and isozyme selectivity, this is called the ‘two-tail’ approachCitation20.
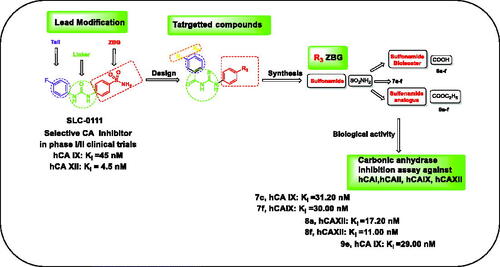
SLC-0111, is the first ureidobenzene sulphonamide CAI, currently in phase I/II clinical studies for the treatment of advanced hypoxic solid tumours with CA IX and XII overexpression. It was discovered by using ‘tail’ approach. SLC-0111 has high selectivity towards IX, XII isoformsCitation6. Additionally, the benzoylthioureido benzene sulphonamide derivative compound ACitation18 () is reported as a highly potent and selective inhibitor of CA IX (IC50 = 0.12 nM). Based on the aforementioned facts, this work suggests the synthesis of some novel benzoylthioureido benzenesulfonamides and their analogues as promising carbonic anhydrase inhibitors.
Figure 2. The design for the targeted compounds benzoylthioureido benzenesulfonamide derivatives (7a–f) and their analogues (8a–f) and (9a–f).

The present study includes several approaches to design the targeted compounds. This is achieved by replacement of the substituted phenyl moiety of SLC-0111 and compound A with substituted benzamide ones either by keeping the acetamido moiety of AAZ or incorporating other substituted amide, maintaining the carbonyl thioureido linker of compound A or spacer elongation of the ureido linker in SLC-0111 along with retaining the benzenesulfonamide moiety to afford compounds 7a–f.
Based on the bioisosteric replacement strategy promoted by numerous research organizationsCitation18,Citation21–24, the zinc binding group (sulfamoyl) of 7a–f is replaced by its carboxylic acid bioisostere to give 8a–f.
Furthermore, structural modification involves replacement of the sulfamoyl moiety with ethylcarboxylate as prodrug to the respective carboxylic acid group to obtain 9a–f.
All the newly synthesised compounds 7a–f, 8a–f, and 9a–f were characterised and biologically evaluated towards a panel of hCA I, II, IX, and XII isoforms.
Materials and methods
Chemistry
Starting materials, solvents, and reagents were purchased from Sigma-Aldrich and used without further purification. Melting points were recorded on a Stuart SMP10 digital melting point apparatus and were uncorrected. The purities of compounds were monitored by analytical TLC, performed on silica gel 60 F254 packed on Aluminium sheets, purchased from Merck, with visualisation under UV light (254 nm). Infra-red (IR) Spectra were recorded as KBr discs using a Shimadzu FT-IR 8400S infra-red spectrophotometer. 1H NMR and 13C NMR spectra were determined in DMSO-d6 and recorded on 400 MHz spectrophotometer for 1H NMR and 100 MHz spectrophotometer for 13C NMR (Bruker AG, Switzerland) at Faculty of Pharmacy, Cairo University; chemical shift (δ) values are expressed in parts per million (ppm) and coupling constants (J) in Hertz (Hz). The abbreviations used are as follows: s, singlet; d, doublet; m, multiplet. Elemental microanalyses and mass spectra were performed at the Regional Centre for Mycology and Biotechnology, Al-Azhar University, Egypt.
Compounds 3a–f, 4a–f, and 5a–f have been synthesised as reportedCitation25–38.
General procedure for the preparation of compounds 7a–f
A solution of freshly prepared benzoyl chloride derivatives 4a–f (1 mmol) and ammonium thiocyanate (0.08 g/1 mmol) in acetone (10 ml) was heated under reflux for 1–3 h. After completion of reaction (monitored by TLC), the reaction mixture was cooled to room temperature and the formed precipitate (NH4Cl) was filtered off. To the freshly prepared solution of appropriate benzoyl isothiocyanate derivative 5a–f, sulphanilamide 6a (0.17 g/1 mmol) was added and the mixture was stirred under reflux for 2–3 h. Then the reaction was cooled to room temperature, the formed precipitate was collected by filtration and recrystallized from ethanol to give the pure product 7a–f.
3-Acetamido-N-((4-sulfamoylphenyl)carbamothioyl)benzamide 7a
Brown crystals, (yield 80%), m.p. 256–258 °C; IR (KBr, νmax/cm−1): 3430–3342 (NHs), 1660, 1610 (2C = O), 1350 & 1190 (SO2); 1H NMR δ 2.09 (s, 3H, CH3), 7.40 (s, 2H, D2O exchangeable, -SO2NH2), 7.45 (t, J = 7.9 Hz, 1H, Ar-H), 7.64 (d, J = 7.6 Hz, 1H, Ar-H), 7.84 − 7.87 (m, 3H, Ar-H), 7.91 (d, J = 8.7 Hz, 2H, Ar-H), 8.18 (s, 1H, Ar-H), 10.20 (s, 1H, D2O exchangeable, -NH),11.66 (s, 1H, D2O exchangeable, -NH),12.66 (s, 1H, D2O exchangeable, -NH); Anal. Calcd. for C16H16N4O4S2 (392.45): C, 48.97; H, 4.11; N, 14.28; Found C, 49.15; H, 4.29; N, 14.35.
3-Isobutyramido-N-((4-sulfamoylphenyl)carbamothioyl)benzamide 7b
Light brown crystals, (yield 76%), m.p. 240–242 °C; IR (KBr, νmax/cm−1): 3435–3329 (NHs), 1659, 1615 (2C = O), 1340 & 1170 (SO2); 1H NMR δ 1.12 (d, J = 6.8 Hz, 6H, CH(CH3)2), 2.60–2.66 (m, 1H, CH(CH3)2), 7.40 (s, 2H, D2O exchangeable, -SO2NH2), 7.45 (t, J = 7.9 Hz, 1H, Ar-H), 7.63 (d, J = 7.9 Hz, 1H, Ar-H), 7.85–7.93 (m, 5H, Ar-H), 8.21 (s, 1H, Ar-H), 10.09 (s, 1H, D2O exchangeable, -NH),11.67 (s, 1H, D2O exchangeable, -NH), 12.66 (s, 1H, D2O exchangeable, -NH). 13C NMR δ: 19.92 (CH(CH3)2), 35.46 (CH(CH3)2), 119.74, 123.53, 124.00, 124.89, 126.73, 129.30, 133.16, 140.01, 141.38, 141.81, 168.65 (C = O), 176.02 (C = O), 179.85 (C = S); MS (m/z): 420.83 [M]+; Anal. Calcd. for C18H20N4O4S2 (420.50): C, 51.41; H, 4.79; N, 13.32; Found C, 51.70; H, 4.88; N, 13.57
3-(4-Methylbenzamido)-N-((4-sulfamoylphenyl)carbamothioyl)benzamide 7c
White crystals, (yield 80%), m.p. 210–212 °C; IR (KBr, νmax/cm−1): 3425–3336 (NHs), 1681, 1651 (2C = O), 1330 & 1157 (SO2); 1H NMR δ 2.41 (s, 3H, CH3), 7.36 (d, J = 7.9 Hz, 2H, Ar-H), 7.41 (s, 2H, D2O exchangeable, -SO2NH2,), 7.51 (t, J = 8.0 Hz, 1H, Ar-H), 7.71 (d, J = 7.8 Hz, 1H, Ar-H), 7.85 (d, J = 8.8 Hz, 2H, Ar-H), 7.91–7.94 (m, 4H, Ar-H), 8.06 (d, J = 7.9 Hz, 1H, Ar-H), 8.42 (s, 1H, Ar-H), 10.42 (s, 1H, s, 1H, D2O exchangeable, -NH), 11.68 (s, 1H, s, 1H, D2O exchangeable, -NH), 12.68 (s, 1H, D2O exchangeable, -NH). 13C NMR δ 21.25 (CH3), 120.71, 123.87, 124.66, 124.97, 126.49, 127.96, 129.01, 129.22, 131.90, 132.84, 139.63, 141.15, 141.56, 142.13, 165.81 (C = O), 168.36 (C = O), 179.60 (C = S); MS (m/z): 468.24 [M]+; Anal. Calcd. for C22H20N4O4S2 (468.55): C, 56.40; H, 4.30; N, 11.96; Found C, 56.63; H, 4.28; N, 12.19.
4-Acetamido-N-((4-sulfamoylphenyl)carbamothioyl)benzamide 7d
Light yellow crystals, (yield 75%), m.p. 218–220 °C; IR (KBr, νmax/cm−1): 3380–3275 (NHs), 1675, 1614 (C = O), 1540 (C = S), 1345 &1185 (SO2); 1H NMR δ 2.09 (s, 3H, CH3), 7.40 (s, 2H, D2O exchangeable, -SO2NH2), 7.44 (d, J = 8.0 Hz, 1H, Ar-H), 7.64 (d, J = 7.6 Hz, 1H, Ar-H), 7.83 − 7.93 (m, 6H, Ar-H), 10.19 (s, 1H, D2O exchangeable, -NH),11.66 (s, 1H, D2O exchangeable, -NH), 12.65 (s, 1H, D2O exchangeable, -NH); Anal. Calcd. for C16H16N4O4S2 (392.45): C, 48.97; H, 4.11; N, 14.28; Found C, 49.13; H, 4.07; N, 14.15.
4-Isobutyramido-N-((4-sulfamoylphenyl)carbamothioyl)benzamide 7e
White crystals, (yield 79%), m.p. 248–250 °C; IR (KBr, νmax/cm−1): 3375–3271 (NHs), 1670, 1650 (C = O) 1338 & 1161 (SO2); 1H NMR δ 1.12 (d, J = 6.7 Hz, 6H,-CH(CH3)2), 2.63–2.66 (m, 1H, -CH(CH3)2), 7.40 (s, 2H, D2O exchangeable, -SO2NH2), 7.76 (d, J = 8.5 Hz, 2H, Ar-H), 7.84 (d, J = 8.4 Hz, 2H, Ar-H), 7.91 (d, J = 8.4 Hz, 2H, Ar-H), 7.99 (d, J = 8.5 Hz, 2H, Ar-H), 10.22 (s, 1H, D2O exchangeable, -NH), 11.52 (s, 1H, D2O exchangeable, -NH), 12.81 (s, 1H, D2O exchangeable, -NH); 13C NMR δ 19.85 (-CH(CH3)2), 35.57 (-CH(CH3)2), 118.67, 124.79, 126.08, 126.74, 130.49, 141.40, 141.74, 144.49, 167.87 (C = O), 176.40 (C = O), 179.96 (C = S); MS (m/z): 420.03 [M]+; Anal. Calcd. for C18H20N4O4S2 (420.50): C, 51.41; H, 4.79; N, 13.32; Found C, 51.69; H, 4.85; N, 13.54.
4-Methyl-N-(4(((4sulfamoylphenyl)carbamothioyl)carbamoyl)phenyl) benzamide 7f
Yellow crystals, (yield 82%), m.p. 228–230 °C; IR (KBr, νmax/cm−1): 3350–3268 (NHs), 1680, 1650 (2C = O), 1320 & 1116 (SO2); 1H NMR δ 2.41 (s, 3H,CH3), 7.37 (d, J = 7.9 Hz, 3H, Ar-H), 7.42 (s, 2H, D2O exchangeable, -SO2NH2), 7.78–7.86 (m, 3H,Ar-H), 7.88–7.95 (m, 6H, Ar-H), 10.49 (s, 1H, D2O exchangeable, -NH), 11.63 (s, 1H, D2O exchangeable, -NH), 12.78 (s, 1H, D2O exchangeable, -NH).; 13C NMR δ; 21.34 (CH3), 1224.58, 126.51, 126.74, 129.08, 129.23, 129.30, 129.35, 131.25, 141.16, 141.54, 144.01, 165.96 (C = O), 168.24 (C = O), 179.71 (C = S); Anal. Calcd. for C22H20N4O4S2 (468.55): C, 56.40; H, 4.30; N, 11.96; Found C, 56.57; H, 4.24; N, 12.23.
General procedure for the preparation of compounds 8a–f
A solution of freshly prepared benzoyl chloride derivatives 4a–f (1 mmol) and ammonium thiocyanate (0.08 g/1 mmol) in acetone (10 ml) was heated under reflux for 1–3 h. After completion of reaction (monitored by TLC), the reaction mixture was cooled to room temperature and the formed precipitate (NH4Cl) was filtered off. To the freshly prepared solution of benzoyl isothiocyanate derivatives 5a–f, 4-aminobenzoic acid 6b (0.014 g/1 mmol) was added and the mixture was stirred under reflux for 2–3 h. The reaction mixture was cooled and the resulting precipitate was collected by filtration and recrystallized from ethanol to give the pure products 8a–f.
4-(3-(3-Acetamidobenzoyl)thioureido)benzoic acid 8a
Brown crystals, (yield 76%), m.p. 247–249 °C; IR (KBr, νmax/cm−1): 3286 (br, NHs), 2672–2558 (OH carboxylic), 1720, 1680, 1630 (3C = O); 1H NMR δ 2.09 (s, 3H, CH3), 7.44 (t, J = 7.9 Hz, 1H, Ar-H), 7.64 (d, J = 7.92 Hz, 1H, Ar-H), 7.84 (d, J = 8.1 Hz, 1H, Ar-H), 7.89 (d, J = 8.6 Hz, 2H, Ar-H), 7.98 (d, J = 8.6 Hz, 2H, Ar-H), 8.17 (s, 1H, Ar-H), 10.20 (s, 1H, D2O exchangeable, -NH), 11.63 (s, 1H, D2O exchangeable, -NH), 12.72 (s, 1H, D2O exchangeable, -NH), 12.93 (s, 1H, D2O exchangeable, -OH). 13C NMR δ 24.47 (CH3), 119.57, 123.54, 123.84, 124.01, 128.55, 129.32, 130.39, 133.18, 139.91, 142.41, 167.17 (C = O), 168.62 (C = O), 169.13 (C = O), 179.43 (C = S); MS (m/z): 357.25 [M]+; Anal. Calcd. for C17H15N3O4S (357.38): C, 57.13; H, 4.23; N, 11.76; Found C, 56.82; H, 4.18; N, 11.69.
4-(3-(3-Isobutyramidobenzoyl)thioureido)benzoic acid 8b
Light brown crystals, (yield 76%), m.p. 240–242 °C; IR (KBr, νmax/cm−1): 3286 (br, NHs), 2661–2548 (OH carboxylic), 1710, 1681, 1589 (3C = O); 1H NMR δ 1.12 (d, J = 6.4 Hz, 6H, CH(CH3)2), 2.59–2.64 (m, 1H, CH(CH3)2), 7.30 (d, J = 8.5 Hz, 1H, Ar-H), 7.44 (t, J = 8.0 Hz, 1H, Ar-H), 7.63 (d, J = 8.2 Hz, 1H, Ar-H), 7.89 − 8.00 (m, 4H, Ar-H), 8.20 (s, 1H, Ar-H) 10.08 (s, 1H, D2O exchangeable, -NH),11.63 (s, 1H, D2O exchangeable, -NH), 12.72 (s, 1H, D2O exchangeable, -NH), 12.97 (s, 1H, D2O exchangeable,-OH). 13C NMR δ 19.89 (CH(CH3)2), 31.03 (CH(CH3)2), 119.76, 124.04, 129.98, 130.41, 131.13, 131.44, 140.00, 142.41, 145.68, 167.19 (C = O), 176.05 (C = O), 176.50 (C = O), 179.46 (C = S); MS (m/z): 385.44 [M]+, 385.89 [M]+; Anal. Calcd. for C19H19N3O4S (385.44): C, 59.21; H, 4.97; N, 10.90; Found C, 59.44; H, 5.13; N, 11.08.
4-(3-(3-(4-Methylbenzamido)benzoyl)thioureido)benzoic acid 8c
White crystals, (yield 78%), m.p. 254–256 °C; IR (KBr, νmax/cm−1): 3286 (br, NHs), 2665–2550 (OH carboxylic), 1710, 1655, 1615 (3C = O); 1H NMR δ 2.41 (s, 3H, CH3), 7.36 (d, J = 8.8 Hz, 2H, Ar-H), 7.51 (t, J = 7.9 Hz, 1H, Ar-H), 7.71 (d, J = 8.4 Hz, 1H, Ar-H), 7.88–8.07 (m, 7H, Ar-H), 8.41 (s, 1H, Ar-H), 10.42 (s, 1H, D2O exchangeable, -NH), 11.65 (s, 1H, D2O exchangeable, -NH), 12.74 (s, 1H, D2O exchangeable, -NH), 12.94 (s, 1H, D2O exchangeable,-OH); Anal. Calcd. for C23H19N3O4S (433.48): C, 63.73; H, 4.42; N, 9.69; Found C, 63.50; H, 4.63; N, 9.93.
4-(3-(4-Acetamidobenzoyl)thioureido)benzoic acid 8d
Brown crystals, (yield 82%), m.p. 245–247 °C; IR (KBr, νmax/cm−1): 3185 (br, NHs), 2680–2530 (OH carboxylic), 1675, 1600 (3C = O); 1H NMR δ 2.10 (s, 3H, CH3), 7.73 (d, J = 8.5 Hz, 2H, Ar-H), 7.90 (d, J = 8.4 Hz, 1H, Ar-H), 7.92 − 7.94 (m, 4H, Ar-H), 7.95 (d, J = 8.5 Hz, 1H, Ar-H), 10.26 (s, 1H, D2O exchangeable, -NH),10.41 (s, 1H, D2O exchangeable, -NH),12.74 (s, 1H, D2O exchangeable, -NH),13.03 (s, 1H, D2O exchangeable,-OH). 13C NMR δ 21.82 (CH3), 111.58, 118.12, 131.20, 131.57, 131.75, 131.84, 132.34, 132.63, 162.65 (C = O), 164.32 (C = O), 168.53 (C = O), 177.67. (C = S); MS (m/z): 357.08 [M]+; Anal. Calcd. for C17H15N3O4S (357.38): C, 57.13; H, 4.23; N, 11.76; Found C, 57.18; H, 4.20; N, 11.73.
4-(3-(4-Isobutyramidobenzoyl)thioureido)benzoic acid 8e
Yellow Crystals, (yield 76%), m.p. 240–242 °C; IR (KBr, νmax/cm−1):, 3190 (br NHs), 2665–2548 (OH carboxylic), 1685, 1604 (C = O); 1H NMR δ 1.14 (d, J = 6.8 Hz, 6H,CH(CH3)2), 2.63–2.69 (m, 1H, CH(CH3)2), 7.77 (d, J = 9.6, 2H, Ar-H), 7.90 − 7.98 (m, 6H, Ar-H), 10.22 (s, 1H, D2O exchangeable, -NH),11.50 (s, 1H, D2O exchangeable, -NH),12.88 (s, 1H, D2O exchangeable, -NH), 12.96 (s, 1H, D2O exchangeable,-OH). 13C NMR δ 19.84 (CH3), 35.57 (CH), 118.66, 123.93, 126.08, 128.49, 130.40, 130.47, 142.43, 144.47, 167.17 (C = O), 167.90 (C = O), 176.40 (C = O), 179.56 (C = S); MS (m/z): 385.90 [M]+; Anal. Calcd. for C19H19N3O4S (385.44): C, 59.21; H, 4.97; N, 10.90; Found C, 59.43; H, 5.08; N, 11.18.
4-(3-(4–(4-Methylbenzamido)benzoyl)thioureido)benzoic acid 8f
White crystals, (yield 78%), m.p. 254–256 °C; IR (KBr, νmax/cm−1):, 3414, 3309 (NHs), 2669–2553 (OH carboxylic), 1683, 1681 (C = O); 1H NMR δ 2.41 (s, 3H, CH3), 7.36 (d, J = 7.9 Hz, 2H, Ar-H), 7.90 (d, J = 8.1 Hz, 4H, Ar-H), 7.96–8.00 (m, 4H, Ar-H), 8.04 (d, J = 8.8 Hz, 2H, Ar-H), 10.51 (s, 1H, D2O exchangeable, -NH), 11.54 (s, 1H, D2O exchangeable, -NH), 12.88 (s, 1H, D2O exchangeable, -NH), 12.96 (s, 1H, D2O exchangeable,-OH). 13C NMR δ 21.52 (CH3), 119.73, 123.94, 126.65, 128.34, 128.50, 129.48, 130.34, 130.40, 132.08, 142.44, 142.56, 144.37, 166.31 (C = O), 167.17 (C = O), 167.94 (C = O), 179.56 (C = S); MS (m/z): 433.31[M] +; Anal. Calcd. for C23H19N3O4S (433.48): C, 63.73; H, 4.42; N, 9.69; Found C, 63.50; H, 4.63; N, 9.93.
General procedure for the preparation of compounds 9a–f
A solution of freshly prepared benzoyl chloride derivatives 4a–f (1 mmol) and ammonium thiocyanate (0.08 g/1 mmol) in acetone (10 ml) was heated under reflux for 1–3 h. After completion of reaction (monitored by TLC), the reaction mixture was cooled to room temperature and the formed precipitate (NH4Cl) was filtered off. To the freshly prepared solution of benzoyl isothiocyanate derivative 5a–f, ethyl 4-aminobenzoate 6c (0.017 g/1 mmol) was added and the mixture was stirred under reflux for 2–3 h. The reaction mixture was cooled and the resulting precipitate was filtered and recrystallized from ethanol to give the pure product 9a–f.
Ethyl 4–(3-(3-acetamidobenzoyl)thioureido)benzoate 9a
Brown crystals, (yield 65%), m.p. 242–244 °C; IR (KBr, νmax/cm−1): 3350–3322 (NHs), 1710 (C = O) ester, 1668, 1620 (C = O) amide; 1H NMR δ 1.32 (t, J = 7.1 Hz, 3H, CH2CH3), 2.09 (s, 3H, CH3), 4.31 (q, J = 7.1 Hz, 2H, CH2CH3), 7.45 (t, J = 7.9 Hz, 1H, Ar-H), 7.64 (d, J = 7.9 Hz, 1H, Ar-H), 7.84 (d, J = 8.1 Hz, 1H, Ar-H), 7.93 (d, J = 8.6 Hz, 2H, Ar-H), 8.00 (d, J = 8.7 Hz, 2H, Ar-H), 8.17 (s, 1H, Ar-H), 10.19 (s, 1H, D2O exchangeable, -NH),11.64 (s, 1H, D2O exchangeable, -NH), 12.73 (s, 1H, D2O exchangeable, -NH). 13C NMR δ 14.65 (CH3 CH2), 24.48 (CH3), 61.21 (CH3 CH2), 119.57, 123.54, 123.84, 124.07, 127.58, 129.31, 130.21, 133.17, 139.92, 142.73, 165.59 (C = O), 168.61 (C = O), 169.10 (C = O), 179.45 (C = S); Anal. Calcd. for C19H19N3O4S (385.44): C, 59.21; H, 4.97; N, 10.90; Found C, 59.47; H, 5.09; N, 11.13.
Ethyl 4–(3-(3-isobutyramidobenzoyl)thioureido)benzoate 9b
Brown crystals, (yield 73%), m.p. 245–247 C; IR (KBr, νmax/cm−1): 3290 (br, NHs), 1697 (C = O) ester, 1666, 1589 (C = O) amide; 1H NMR δ 1.12 (d, J = 6.8 Hz, 6H, -CH(CH3)2), 1.32 (t, J = 7.1 Hz, 3H, -CH2CH3), 2.59–2.66 (m, 1H, CH(CH3)2), 4.30 (q, J = 7.1 Hz, 2H, -CH2CH3), 7.44 (t, J = 8.0 Hz, 1H, Ar-H), 7.63 (d, J = 7.9 Hz, 1H, Ar-H), 7.88– 7.95 (m, 3H, Ar-H), 8.00 (d, J = 8.7 Hz, 2H, Ar-H), 8.21 (s, 1H, Ar-H), 10.08 (s, 1H, D2O exchangeable, -NH), 11.65 (s, 1H, D2O exchangeable, -NH), 12.74 (s, 1H, D2O exchangeable, -NH). 13C NMR δ 14.48 (CH3 CH2), 19.75 (CH(CH3)2), 35.28 (CH(CH3)2), 61.06 (CH3 CH2), 119.53, 123.38, 123.93,128.63, 129.13, 130.06, 132.98, 135.09, 139.83, 142.56, 165.42 (C = O), 168.50 (C = O), 173.30 (C = O), 175.84 (C = S); MS (m/z): 413.13 [M]+; Anal. Calcd. for C21H23N3O4S (413.49): C, 61.00; H, 5.61; N, 10.16; Found C, 61.17; H, 5.85; N, 10.38.
Ethyl 4–(3-(3–(4-methylbenzamido)benzoyl)thioureido)benzoate 9c
White crystals, (yield 80%), m.p. 236–238 °C; IR (KBr, νmax/cm−1): 3278 (br, NHs), 1716 (C = O) ester, 1662, 1593 (C = O) amide; 1H NMR δ 1.35 (t, J = 7.1 Hz, 3H, -CH2CH3), 2.41 (s, 3H, CH3), 4.34 (q, J = 2.2 Hz, 2H, -CH2CH3), 7.35–7.37 (m, 3H, Ar-H), 7.51 (d, J = 7.8 Hz, 1H, Ar-H), 7.71 (d, J = 7.4 Hz, 1H, Ar-H), 7.91–8.08 (m, 6H, Ar-H), 8.42 (s, 1H, Ar-H), 10.43 (s, 1H, s, 1H, D2O exchangeable, -NH), 11.68 (s, 1H, s, 1H, D2O exchangeable, -NH), 12.76 (s, 1H, s, 1H, D2O exchangeable, -NH). 13C NMR δ 14.42 (CH3CH2), 21.27 (CH3), 60.98 (CH3CH2), 120.73, 123.85, 124.97, 127.36, 127.98, 129.08, 129.22, 129.28, 129.99, 131.93, 132.84, 139.67, 142.11, 142.50, 165.36 (C = O), 165.79 (C = O), 168.38 (C = O), 179.23 (C = S); MS (m/z): 461.26 [M + 1] +; Anal. Calcd. for C25H23N3O4S (461.54): C, 65.06; H, 5.02; N, 9.10; Found C, 64.97; H, 5.18; N, 9.37.
Ethyl 4–(3-(4-acetamidobenzoyl)thioureido)benzoate 9d
Brown crystals, (yield 68%), m.p. 237–239 °C; IR (KBr, νmax/cm−1): 3345–3320 (NHs), 1700 (C = O) ester, 1675, 1635 (C = O) amide; 1H NMR δ 1.33 (t, J = 7.1 Hz, 3H, CH2CH3), 2.12 (s, 3H,CH3), 4.38 (q, J = 7.1 Hz, 2H,CH2CH3), 7.71–7.76 (m, 2H, Ar-H), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.97–8.03 (m, 4H, Ar-H), 10.34 (s, 1H, D2O exchangeable, -NH),11.52 (s, 1H, D2O exchangeable, -NH),12.91 (s, 1H, D2O exchangeable, -NH); Anal. Calcd. for C19H19N3O4S (385.44): C, 59.21; H, 4.97; N, 10.90; Found C, 59.43; H, 5.14; N, 11.17.
Ethyl 4–(3-(4-isobutyramidobenzoyl)thioureido)benzoate 9e
White crystals, (yield 75%), m.p. 245–247 C; IR (KBr, νmax/cm−1): 3275 (br, NHs), 1712 (C = O) ester, 1662, 1608 (C = O) amide; 1H NMR δ 1.12 (d, J = 6.8 Hz, 6H,-CH(CH3)2), 1.31 (t, J = 7.1 Hz, 3H, -CH2CH3), 2.61–2.68 (m, 1H, CH(CH3)2), 4.30 (q, J = 7.1 Hz, 2H, -CH2CH3), 7.76 (d, J = 8.8 Hz, 2H, Ar-H), 7.92–7.95 (m, 2H, Ar-H), 8.00 (d, J = 7.6 Hz, 4H, Ar-H), 10.21 (s, 1H, D2O exchangeable, -NH), 11.50 (s, 1H, D2O exchangeable, -NH), 12.90 (s, 1H, D2O exchangeable, -NH). 13C NMR δ 14.64 (CH3 CH2), 19.84 (CH(CH3)2), 35.56 (CH(CH3)2), 61.20 (CH3 CH2), 118.61, 123.98, 126.03, 127.46, 130.22, 130.50, 142.73, 144.47, 163.84 (C = O), 165.58 (C = O), 171.64 (C = O),176.39 (C = S); MS (m/z): 413.45 [M]+; Anal. Calcd. for C21H23N3O4S (413.49): C, 61.00; H, 5.61; N, 10.16; Found C, 60.78; H, 5.84; N, 10.40.
Ethyl 4–(3-(4–(4-methylbenzamido)benzoyl)thioureido)benzoate 9f
Light yellow crystals, (yield 80%), m.p. 237–239 °C; IR (KBr, νmax/cm−1): 3340 (br, NHs), 1705 (C = O) ester, 1658, 1597 (C = O) amide; 1H NMR δ 1.45 (t, J = 6.3 Hz, 3H, -CH2CH3), 2.45 (s, 3H, CH3), 4.47 (m, 2H, -CH2CH3), 7.38 (d, J = 7.9 Hz, 2H, Ar-H), 7.55 (d, J = 7.9 Hz, 1H, Ar-H), 7.71 (d, J = 7.9 Hz, 1H, Ar-H), 8.01–8.08 (m, 8H, Ar-H), 10.58 (s, 1H, s, 1H, D2O exchangeable, -NH), 11.84 (s, 1H, s, 1H, D2O exchangeable, -NH), 12.92 (s, 1H, s, 1H, D2O exchangeable, -NH); MS (m/z): 462.21 [M + 1]+; Anal. Calcd. for C25H23N3O4S (461.54): C, 65.06; H, 5.02; N, 9.10; Found C, 64.90; H, 5.23; N, 9.34.
Carbonic anhydrase inhibition assay
An applied photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity.Citation28 Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 Mm Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, as reported earlier,Citation29–32 and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation33–35.
Molecular modeling study
Molecular docking
Molecular docking of the promising candidates 7a, 7b, 7c, 7f, 8a, 8f, and 9e into the three-dimensional X-ray structure of human carbonic anhydrase (hCA) was conducted using MOE software package 2019.0102, to shed light on the possible interactions of the designed compounds. The crystal structures of human carbonic anhydrase isoforms I, II, IX, and XII in complex with their corresponding inhibitor were downloaded from the protein data bank (PDB: 3WXH, 3HS4, 3IAI, and 1JDO, respectively)Citation39. All bound water molecules with exception of the ones that appear to participate in the ligand–receptor interaction were eliminated. The non-necessary molecules and cofactors were eliminated from the protein and the polar hydrogen was added. A light energy minimisation step with tethering the heavy atoms was performed on the protein using the MMFF94 forcefield. Docking of the lowest energetic conformer for each compound into the active site of human carbonic anhydrase isoforms I, II, IX, and XII using the default settings in MOE and the docking scores (S) were calculated.
Physicochemical, ADME, and pharmacokinetic properties prediction
The Swiss Institute of Bioinformatics’ (SIB) free Swiss ADME online tool was employed to evaluate physicochemical properties and determine ADME descriptors, pharmacokinetic features, medicinal chemistry compatibility, and drug-like characteristics of the most powerful newly synthesised 7a, 7b, 7c, 7f, 8a, 8f, and 9e compounds. ChemDraw Professional 19.0 was used to draw the ligands, which were exported in SMILE format and then uploaded to an online server for evaluation.
Results and discussion
Chemistry
Different substituted benzoylthioureido benzenesulfonamide derivatives and their analogues 7a–f, 8a–f, and 9a–f were synthesised using the described synthetic methods as illustrated in Scheme 1. Acylation of the amino functionality of 3-amino or 4-amino benzoic acid 1a or 1b was performed using either acetyl chloride 2a, isobutyryl chloride 2b or 4-methylbenzoyl chloride 2c in dry acetonitrile in the presence of potassium hydroxide to afford compounds 3a–f, respectively. Then, in situ preparation of acid chloride derivatives 4a–f was carried out by addition of thionyl chloride to the appropriate 3a–f in methylene chloride under reflux. After removing thionyl chloride under vacuum, ammonium thiocyanate was added to the freshly prepared acid chlorides 4a–f in dry acetone to give the key intermediate 5a–f. Next, heating 5a–f under reflux with sulphanilamide 6a in dry acetone furnished 7a–f in good yields (75–82%). IR spectra of compounds 7a–f showed the appearance of characteristic bands at 3435–3268 cm−1 corresponding to NH2 and NH, in addition to two stretching vibration bands at 1320–1116 cm−1 attributed to the characteristic SO2 group.
Scheme 1. Synthetic pathways for compounds 7a–f, 8a–f, 9a–f. Reagents and conditions: (i) KOH, acetonitrile, R.T, 1–2 h, (ii) SOCl2, methylene chloride, reflux, 4–5 h, (iii) NH4SCN, acetone, reflux, 1–3 h, (iv) acetone, reflux, 2–3 h.

1H NMR spectra revealed the appearance of D2O exchangeable signal in the aromatic region around 7.40 ppm corresponding to two protons of the SO2NH2 group as a singlet, along with D2O exchangeable three NH signals at about 10.08–12.81 ppm assigned to thioureido and benzamide protons. 1H NMR spectra of compounds 7a–f elicited an increase in the integration of aromatic protons. Also, the aliphatic region of 7a and 7d showed the appearance of singlet signal at 2.09 ppm corresponding to (CH3) protons of acetamido moiety. The isopropyl derivatives 7b and 7e revealed the appearance of isopropyl protons as doublet at 1.12 ppm representing the two methyl protons and a multiplet at 2.60–2.66 ppm of the CH protons. Compounds 7c and 7f; showed a singlet signal at 2.41 ppm integrated for three protons of the tolyl moiety.
On the other hand, 13C NMR spectra of 7c and 7f displayed a signal at 21.25 and 21.34 ppm attributed to CH3 carbons of the tolyl moiety. The two carbonyl carbons and C = S carbon of 7a–f appeared at 165–180 ppm.
Reacting the key intermediates 5a–f with ethyl 4-aminobenzoic acid 6b afforded 8a–f in good yields 76–78%. IR spectra of compounds 8a–f revealed the appearance of characteristic carboxylic OH stretching vibration bands in a wide range at nearly 2680–2530 cm−1. On the other hand, 1H NMR spectra showed the presence of an OH acidic exchangeable signal at 12.60–12.97 ppm, as well as three NH protons at 10.08–12.88 ppm. Also compounds 8a and 8d displayed the appearance of singlet signal at 2.09 and 2.10 ppm, respectively, corresponding to the (CH3) protons of the acetamido moiety. Compounds 8b and 8e were in accordance with their expected structures in 1HNMR by the appearance of isopropyl protons as doublet at 1.12 and 1.14 ppm, respectively, and multiplet signals at 2.59–2.69 ppm, whereas compounds 8c and 8f; elicited a singlet signal at 2.41 ppm integrated for the three protons of the tolyl (CH3) group. 13C NMR spectra of 8a and 8d showed signal at 24.47 and 21.82 ppm, respectively corresponding to (CH3) of acetamido group. Also, 13C NMR spectra of 8a-f revealed the presence of additional carbonyl carbon of acid.
On the other hand, reacting 5a–f with ethyl 4-aminobenzoate 6c gave compounds 9a–f in good yields 65–80%. IR spectra showed characteristic bands at 3350–3275 cm−1 corresponding to the NH groups along with carbonyl bands of the ester at range of 1697–1716 cm−1. 1H NMR spectra of 9a–f elicited three exchangeable NH signals at 10.08–12.92 ppm, in addition to an increase in the aromatic region integration. Compounds 9a and 9d; revealed a singlet signal at 2.09, 2.12 ppm integrated for the three protons of the acetamido group. On the other hand, the isopropyl derivatives 9b and 9e showed the appearance of isopropyl protons as doublet at 1.12 ppm and multiplet signals at range 2.59–2.68 ppm. Moreover, compounds 9c and 9f; showed a singlet signal at 2.41, 2.45 ppm, respectively, integrated for the three protons of the tolyl (CH3) group. This is in addition to the usual triplet-quartet pattern of the ethyl group.
Further, 13C NMR spectra of 9a displayed ethyl carbons (CH3CH2) at 14.65 ppm, (CH3CH2) carbon at 61.21 and CH3 acetamido carbon at 24.48 ppm along with two carbonyl carbons at 165.59 and 168.61 ppm and CS carbon at 179.45 ppm. 13C NMR spectra of 9b and 9e revealed the isopropyl carbons (CHCH3)2 at 19.75 and 19.84 ppm, respectively and (CHCH3)2 carbon at 35.28 and 35.56 ppm respectively, whereas, the tolyl (CH3) carbon of 9c appeared at 21.27 ppm.
Carbonic anhydrase inhibitory activity
All synthesised compounds 7a–f, 8a–f, and 9a–f were tested using the standard inhibitor acetazolamide (AAZ) in a stopped flow CO2 hydrase assay for their capacity to suppress the physiologically relevant hCA isoforms, hCA I, II, IX, and XIICitation40. The selection of these four isoforms was based on the fact that hCA II is antiglaucoma medication targetCitation41 while hCA IX and XII have been validated as targets for the treatment and prognosis of hypoxic malignanciesCitation42,Citation43. Otherwise, hCA I is one of the most important off-target isoforms for antiglaucoma and anticancer CAI therapeutic applicationsCitation5,Citation10. The inhibitory data presented in can be used to create the structural activity relationship (SAR) shown below.
Table 1. Carbonic anhydrase inhibitory activity of compounds 7a–f, 8a–f, and 9a–f and the standard sulphonamide inhibitor acetazolamide (AAZ) using a stopped flow CO2 hydrase assay.

The following structure–activity relationship (SAR) was attained from the inhibition data listed in :
The highly prevalent isoform hCA I was the highly inhibited isoform in this study with inhibition constants ranging from 33.00 nM to more than 100 000 nM. Compounds 7a, 7b, 7c, and 7f (Kis = 58.20, 56.30, 33.00 and 43.00 nM) showed significant activity ranging from 4 to 8 times more than AAZ (Ki = 250.00 nM). Moreover, compounds 7d and 7e have moderate inhibition activity towards hCAI isoform (Kis = 5451.10 and 333.40 nM), respectively.
Obviously, replacement of the sulfamoyl moiety with its isosteres ethyl ester and carboxylic group reduced activity as demonstrated in compounds 8a–f and 9a–f. Compound 8a and 8c displayed mild inhibition activity with (Kis = 8177.40 and 9171.50 nM), compound 8f has reasonable inhibitory activity with (Ki = 422.20 nM). While compounds 9a, 9b, and 9e showed moderate inhibiting activity (Kis = 9036.70, 8661.20, and 8495.20 nM), others have inhibition range up to 100 µM. Noteworthy, the two most active compounds 7c and 7f against hCAI isoform were sulphonamide derivatives as ZBG with tolyl substitution at position 3 and 4, respectively, whereas position 3 was the most active one 7c (Ki=33.00 nM) while 7f has Ki = 43.00 nM. Furthermore, other active compounds 7a and 7b (Kis = 58.20 and 56.30 nM), were sulphonamide derivatives with aliphatic amide substituent at position 3 of phenyl group as a tail, and replacement of substituent to position 4 as in compounds 7d and 7e moderately affected the inhibitory activity with Kis = 5451.10 and 333.40 nM.
hCA II was affected efficiently by benzenesulfonamide derivatives as demonstrated by compounds 7a, 7b, that show selective and potent activity inhibition constants (Kis = 2.50, 2.10 nM), respectively, which is fivefold more active than AAZ. Whereas compounds 7c, 7e, and 7f elicited significant activity (Kis = 56.60, 39.60, and 39.00 nM), respectively, relative to AAZ (Ki = 12.10 nM)
Also, replacing the (-SO2NH2) moiety with its isostere (COOH) decreased the inhibitory action (8a, 8c, 8d, and 8e) (Kis = 5401.30, 295.10, 855.10, and 429.60 nM), respectively. Grafting with various substituents at the position 3 of the benzamide has a significant influence on the inhibitory efficacy with the following order -NHCOCH(CH3)2< -NHCOCH3< -NHCO(4-CH3C6H4). Whereas changing the substituent to position 4 diminished the inhibitory effect of compounds 7d and 7e (Kis = 701.20, 39.60 nM) except compound 7f where the activity (Ki = 39.00 nM).
Interestingly, compounds 9b and 9e (-COOC2H5) showed significant activity (Kis = 57.10 and 73.20 nM), respectively. Replacement of sulphonamide moiety to ester analogue (-COOC2H5) 9a, 9c, 9d, 9f reduced the inhibitory effect to less than 100 nM while, compounds 9b and 9e showed moderate activity with Kis = 57.10 and 73.20 nM, respectively. Unfortunately, replacement of SO2NH2 with its isostere (COOH) did not show any significant activity.
Concerning hCA IX, the tumour-associated isoform, it was as well considerably inhibited by the compounds 7c and 7f bearing (-SO2NH2) as ZBG (Kis = 31.20 and 30.00 nM) relative to AAZ (Ki = 25.70 nM).
Surprisingly, replacing the (-SO2NH2) moiety with (COOC2H5) produced a slight increase in the inhibitory action as in compound 9e (Ki = 29.00 nM). Although, replacing the (-SO2NH2) moiety with its (COOH) isostere in compounds 8a–f did not reveal any significant inhibitory activity range (Kis = 176.00–971.10 nM).
Noticeably, the inhibitory profiles of carbonic anhydrase in suggested that only thioureido benzenesulfonamide bearing (3-(4-methyl benzamide)) (7c) or 4(4-methyl benzamide (7f), ester-based compound containing (3-(4-isobutyramidobenzoyl)) (9e) show a significant activity for the tumour-related hCA IX isoform.
Regarding hCA XII, Compounds bearing benzoic acid moiety along with 3- acetamidobenzoyl 8a (Ki = 17.00 nM) or 4-(4-methylbenzamide) 8f (Ki = 11.00 nM) were the most effective among all the tested compounds .
Furthermore, compounds containing sulphonamide as ZBG 7a–f showed considerable inhibitory values as compounds 7d, 7e, and 7f (Kis = 97.00, 94.00, and 106.00 nM, respectively). The inhibitory impact was decreased to 100 µM by replacing the sulphonamide moiety with (-COOC2H5) 9a–f, with the exception of 9e and 9f, which display moderate activity (Kis = 201.50 and 155.40 nM).
Finally, using a bioisosteric replacement strategy to replace the SLC-0111 ureido linker with a flexible thioureido one, as well as elongation strategies, the inhibitory activity against the hCA IX isoform was successfully increased. such as compounds 7c, 7f, and 9e (Ki = 31.20, 30.00, and 29.00 nM), respectively, vs (Ki = 45.0 for SLC-0111)Citation44 with selectivity index for inhibition of hCA IX over hCA I (SI = 293 for 8e) vs (SI = 113 for SLC-0111) . Unfortunately, the increased activity towards hCA II was coupled by decreased activity against the hCA XII isoform, resulting in a worse hCA II/XII selectivity index for target sulphonamide compounds. On the other hand, acid isostere compounds especially 8a and 8f exhibited potent activity against tumour associated isoform hCA XII with Kis =17.00 and 11.00 nM () and with selectivity index for inhibition of hCA XII over hCA II (SI = 318 and >9091, respectively) vs SLC-0111 (SI = 213; ). To improve hCA II/XII selectivity for other compounds, more structural changes are required.
Table 2. Calculated selectivity indexes (S.I.s) for inhibition of hCA IX over hCA I and hCA II isoforms for compounds 7c, 7f, 9e, SLC-0111, and AAZ.
Table 3. Calculated selectivity indexes (S.I.s) for inhibition of hCA XII over hCA I and hCA II isoforms for compounds 8a, 8f, SLC-0111, and AAZ.
Molecular modeling study
Molecular docking
Molecular docking is conducted for candidates 7c and 7f against hCAI, 7a and 7b against hCAII, 7f and 9e against hCAIX and 8a and 8f against hCAXII, to investigate their binding affinity in a correlation with their significant CA inhibitory activities. All of the targeted compounds coordinated with the Zn ion through the (O = S and NH) of sulphonamide (7a, 7b, 7c, and 7f), (COO) of carboxylic acid (8a and 8f) or (COOEt) (9e). As elaborated from the docking simulation, most of the benzenesulfonamide derivatives are engaged also in H-bonding with Thr199 through SO2 of sulphonamoyl except 7f in hCAI, in addition to the hydrophobic interaction, and other residues that participate in binding such as His200, leu198, Ala131, Ser135 and leu141. Docking scores for the targeted compounds fall in the range of (−11.25 to −17.24 Kcal/mol) and were highly consistent with their inhibitory activity ranking against hCA isoforms ().
2D diagram of other compounds are provided in the supplementary data.
Physicochemical, ADME, and pharmacokinetic properties prediction
The most active compounds 7a, 7b, 7c, 7f, 8a, 8f, and 9e based on hCAs inhibitory activity results, are evaluated for their physicochemical characteristics as well as prognosis ADME parameters, pharmacokinetic properties, and drug-like nature using the Swiss ADME online web application, which is provided by the Swiss Institute of Bioinformatics (SIB). This is done to make sure that they are favourable congeners from both a pharmacokinetic perception as well as biological effect.
The presented compounds are expected to have promising physicochemical and pharmacokinetic characteristics. They exhibit a portended logPo/w in a range of 1.26–4.24, good water solubility for 7a, 7b, and 8a compounds while 7c, 7f, 8f, and 9e compounds have moderate solubility, with high GIT absorption for 8a and 9e, while other compounds have low GIT absorption. No BBB permeability for all selected compounds, therefore no expected CNS side effects. demonstrates the BOILED-Egg plot of the WLOGP vs. TPSA (Topological Polar Surface Area)Citation45,Citation46 of the tested compounds.
Figure 9. Expected boiled-egg plot from Swiss ADME online web tool for compounds 7a, 7b, 7c, 7f, 8a, 8f, and 9e.

Compounds 8a and 9e demonstrate a high human intestine absorption (HIA) area out of the seven compounds; they are expected to be absorbed in the gastrointestinal system due to their ideal physicochemical properties, which fall within the range of acceptable physicochemical parameters for oral bioavailability (GIT). While none of the selected compounds show blood-brain barrier permeability (BBB) which indicates their safety against CNS. The plot also demonstrates that none of the seven tested compounds are P-glycoprotein substrates (PGP-), and as a result, none of them are subject to the transporter’s efflux process, which many cancer cell lines use as a mechanism for drug resistanceCitation47,Citation48. Furthermore, Swiss ADME revealed that all of the tested compounds, fulfil Lipinski’s (Pfizer)Citation49 and Ghose’s (Amgen)Citation50 the observed results predicted that the tested compounds have promising drug-likeness criteria. Nevertheless, they are not categorised as lead-like because their molecular weights exceed 350 and number of rotatable bonds is more than seven. Moreover, Swiss ADME data classified the seven most active compounds as non-PAINS (pan-assay interference compounds), notably the remarkable selectivity of our target compounds.
The computational analysis of the physicochemical and pharmacokinetic properties of the newly synthesised compounds revealed that the majority of them exhibit potential biological effectiveness and pharmacokinetic characteristics.
Conclusion
The present study describes the design, synthesis, and carbonic anhydrase inhibitory activity of some benzoylthioureido benzenesulfonamide derivatives 7a–f and their analogues 8a–f and 9a–f using replacement and elongation tail strategy of SLC-0111 aiming to increase potency and selectivity compared with acetazolamide AAZ as standard CAI. The synthesised compounds were assessed as CAIs against hCA I, II, IX, and XII. The benzenesulfonamides 7a, 7b, 7c, 7e, and 7f bearing amide moiety at position 3 or 4 showed moderate to superior inhibitory activity against hCA I, II and IX with high selectivity towards hCA II isoforms, particularly the 3- acetamido derivative 7a and 3-isobutyramido 7b (Kis = 2.50 and 2.10 nM), respectively, relative to that expressed by AAZ (Kis = 12.10 nM) and reported SLC-0111 (Ki =960.00 nM).
On the other hand, compounds 7a, 7b, 7c, and 7f exhibited a superior selectivity over hCAI (Kis =33.00–58.20 nM) compared with AAz (Ki = 250.00 nM).
Despite of designing compounds 8a–f as bioisosters to 7a–f, only two compounds of the benzoic acid series 8a and 8f elicited a moderate inhibitory activity against hCAXII (Kis = 17.00 and 11.00 nM), respectively, in comparison to AAZ and reported SLC-0111 values (Kis = 5.70 and 4.50 nM), respectively. Compounds 7c, 7f, and 9e were selective towards hCAIX (Kis =31.20, 30.00 and 29.00 nM) relative to AAZ and reported SLC-0111 (Kis =25.70 and 45.00, respectively). On the other hand, the CA inhibitory activity was reduced when SO2NH2 moiety was replaced by their analogues COOH or COOC2H5 with the exception of the ethylbenzoate derivative 9e which displays a significant activity against hCAIX (Ki =29.00 nM). The best inhibitory activity was observed in compounds bearing the amide moieties at position 3.
In addition, Simulation of docking for the most active compounds 7a, 7b, 7c, 7f, 8a, 8f, and 9e based on their docking binding patterns and scores, predicted their binding mode and binding affinity to the hCA I, II, IX, and XII active sites and explained their selectivity. Moreover, compounds 8a and 9e have favourable pharmacokinetic characteristics in addition to exhibiting significant CA inhibitory activity towards CA IX and XII respectively.
Supplemental Material
Download PDF (783 KB)Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181.
- Supuran CT. Carbonic anhydrases as drug targets - an overview. Curr Top Med Chem. 2007;7(9):825–833.
- Abdalkareem Jasim S, Kzar HH, Haider Hamad M, Ahmad I, Al-Gazally ME, Ziyadullaev S, Sivaraman R, Abed Jawad M, Thaeer Hammid A, Oudaha KH, et al. The emerging role of 27-hydroxycholesterol in cancer development and progression: An update. Int Immunopharmacol. 2022;110:109074.
- Nocentini A, Donald WA, Supuran CT. Human carbonic anhydrases: tissue distribution, physiological role, and druggability. In: Supran CT, Nocentini A, editors. Carbonic anhydrases. Cambridge: Academic press; 2019. p. 151–185.
- Aspatwar A, Parvathaneni NK, Barker H, Anduran E, Supuran CT, Dubois L, Lambin P, Parkkila S, Winum J-Y. Design, synthesis, in vitro inhibition and toxicological evaluation of human carbonic anhydrases I, II and IX inhibitors in 5-nitroimidazole series. J Enzyme Inhib Med Chem. 2020;35(1):109–117.
- Supuran CT. Experimental carbonic anhydrase inhibitors for the treatment of hypoxic tumors. J Exp Pharmacol. 2020;12:603–617.
- Oudah KH, Najm MAA, Roomi AB, Al-Sa’idy HA, Awadallah FM. The recent progress of sulfonamide in medicinal chemistry. Syst Rev Pharm. 2020;11(12):1473–1477.
- Raya I, Chupradit S, Mustafa YF, et al. Carboxymethyl chitosan nano-fibers for controlled releasing 5-fluorouracil anticancer drug. J Nanostruct. 2022;12(1):136–143.
- Cakmak EB, Zengin Kurt B, Ozturk Civelek D, Angeli A, Akdemir A, Sonmez F, Supuran CT, Kucukislamoglu M. Quinoline-sulfamoyl carbamates/sulfamide derivatives: synthesis, cytotoxicity, carbonic anhydrase activity, and molecular modelling studies. Bioorg Chem. 2021;110:104778.
- Lolak N, Akocak S, Bua S, Sanku RKK, Supuran CT. Discovery of new ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as carbonic anhydrase I, II, IX and XII inhibitors. Bioorg Med Chem. 2019;27(8):1588–1594.
- Shaldam M, Nocentini A, Elsayed ZM, Ibrahim TM, Salem R, El-Domany RA, Capasso C, Supuran CT, Eldehna WM. Development of novel quinoline-based sulfonamides as selective cancer-associated carbonic anhydrase isoform ix inhibitors. IJMS. 2021;22(20):11119.
- Awadallah FM, El-Waei TA, Hanna MM, Abbas SE, Ceruso M, Oz BE, Guler OO, Supuran CT. Synthesis, carbonic anhydrase inhibition and cytotoxic activity of novel chromone-based sulfonamide derivatives. Eur J Med Chem. 2015;96:425–435.
- Awadallah FM, Bua S, Mahmoud WR, Nada HH, Nocentini A, Supuran CT. Inhibition studies on a panel of human carbonic anhydrases with N1-substituted secondary sulfonamides incorporating thiazolinone or imidazolone-indole tails. J Enzyme Inhib Med Chem. 2018;33(1):629–638.
- Al-Bahadily DCH, Chaloob R, Oudah KH, Al-Salman HNK, Shari FH, Hussein HH. Fasten, simple, and specific stability of the avant-garde RP-HPLC method for estimation and validation of nystatin in pharmaceutical formulations. Int J Res Pharm Sci. 2019;10(4):3717–3727.
- Zhang Z. Aryl sulfonamide compounds as carbonic anhydrase inhibitors and their therapeutic use. WO 2017004543. Published online 2017.
- Liguori F, Carradori S, Ronca R, Rezzola S, Filiberti S, Carta F, Turati M, Supuran CT. Benzenesulfonamides with different rigidity-conferring linkers as carbonic anhydrase inhibitors: an insight into the antiproliferative effect on glioblastoma, pancreatic, and breast cancer cells. J Enzyme Inhib Med Chem. 2022;37(1):1857–1869.
- Akgul O, Singh S, Andring JT, McKenna R, Selleri S, Carta F, Angeli A, Supuran CT. Handling drug-target selectivity: a study on ureido containing carbonic anhydrase inhibitors. Eur J Med Chem. 2021;212:113035.
- Liu L, Wang W, Huang J, Zhao Z, Li H, Xu Y. Novel benzoyl thioureido benzene sulfonamides as highly potent and selective inhibitors of carbonic anhydrase IX: optimization and bioactive studies. Medchemcomm. 2018;9(12):2100–2105.
- Elbadawi MM, Eldehna WM, Nocentini A, Somaa WR, Al-Rashood ST, Elkaeed EB, El Hassab MA, Abdel-Aziz HA, Supuran CT, Fares M, et al. Development of 4-((3-oxo-3-phenylpropyl)amino)benzenesulfonamide derivatives utilizing tail/dual-tail approaches as novel carbonic anhydrase inhibitors. Eur J Med Chem. 2022;238:114412.
- Bonardi A, Nocentini A, Bua S, Combs J, Lomelino C, Andring J, Lucarini L, Sgambellone S, Masini E, McKenna R, et al. Sulfonamide inhibitors of human carbonic anhydrases designed through a three-tails approach: improving ligand/isoform matching and selectivity of action. J Med Chem. 2020;63(13):7422–7444.
- Said MF, George RF, Petreni A, Supuran CT, Mohamed NM. Synthesis, molecular modelling and QSAR study of new N-phenylacetamide-2-oxoindole benzensulfonamide conjugates as carbonic anhydrase inhibitors with antiproliferative activity. J Enzyme Inhib Med Chem. 2022;37(1):701–717.
- Nada H, Elkamhawy A, Abdellattif MH, Angeli A, Lee CH, Supuran CT, Lee K. 4-Anilinoquinazoline-based benzenesulfonamides as nanomolar inhibitors of carbonic anhydrase isoforms I, II, IX, and XII: design, synthesis, in-vitro, and in-silico biological studies. J Enzyme Inhib Med Chem. 2022;37(1):994–1004.
- Sarnella A, Ferrara Y, Auletta L, Albanese S, Cerchia L, Alterio V, De Simone G, Supuran CT, Zannetti A. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J Exp Clin Cancer Res. 2022;41(1):1–16.
- Bonardi A, Bua S, Combs J, Lomelino C, Andring J, Osman SM, Toti A, Di Cesare Mannelli L, Gratteri P, Ghelardini C, et al. The three-tails approach as a new strategy to improve selectivity of action of sulphonamide inhibitors against tumour-associated carbonic anhydrase IX and XII. J Enzyme Inhib Med Chem. 2022;37(1):930–939.
- Zeng F, Li S, Yang G, Luo Y, Qi T, Liang Y, Yang T, Zhang L, Wang R, Zhu L, et al. Design, synthesis, molecular modeling, and biological evaluation of acrylamide derivatives as potent inhibitors of human dihydroorotate dehydrogenase for the treatment of rheumatoid arthritis. Acta Pharm Sin B. 2021;11(3):795–809.
- Srivastav M, Shantakumar SM. Synthesis and anti-inflammatory activity of some novel 3-(6-Substituted-1, 3-Benzothiazole-2-Yl)-2-[{(4-Substituted Phenyl) Amino} Methyl] quinazolines-4 (3H)-ones. 2009;6(4):1055–1062.
- AydIn F, Ünver H, Aykaç D, Iskeleli NO. Spectroscopic studies and structure of 4-(3-benzoylthioureido)benzoic acid. J Chem Crystallogr. 2010;40(12):1082–1086.
- Saeed A, Mumtaz A, Florke U. Synthesis, characterization and crystal structure of 1-(4-methylbenzoyl)-3-(4 aminosulfonylphenyl)thiourea. Eur J Chem. 2010;1(2):73–75.
- Oudah KH, Najm MAA, Samir N, Serya RAT, Abouzid KA. Design, synthesis and molecular docking of novel pyrazolo[1,5-a][1,3,5]triazine derivatives as CDK2 inhibitors. Bioorg Chem. 2019;92:103239.
- Yazdani E, Kazemi Miraki M, Salamatmanesh A, Azarnia J, Azizi K, Ghandi L, Heydari A. A magnetically recoverable copper–salen complex as a nano-catalytic system for amine protection via acetylation using thioacetic acid. Res Chem Intermed. 2019;45(4):1775–1793.
- Das VK, Devi RR, Thakur AJ. Recyclable, highly efficient and low cost nano-MgO for amide synthesis under SFRC: A convenient and greener “NOSE” approach. Appl Catal A Gen. 2013;456:118–125.
- Song B, Xiao T, Qi X, Li L-N, Qin K, Nian S, Hu G-X, Yu Y, Liang G, Ye F, et al. Design and synthesis of 8-substituted benzamido-phenylxanthine derivatives as MAO-B inhibitors. Bioorg Med Chem Lett. 2012;22(4):1739–1742.
- Gawel JM, Shouksmith AE, Raouf YS, Nawar N, Toutah K, Bukhari S, Manaswiyoungkul P, Olaoye OO, Israelian J, Radu TB, et al. PTG-0861: A novel HDAC6-selective inhibitor as a therapeutic strategy in acute myeloid leukaemia. Eur J Med Chem. 2020;201:112411.
- Basanagouda M, Kulkarni MV, Kalkhambkar RG, Kulkarni GM. ChemInform abstract: new, efficient, selective, and one-pot method for acylation of amines. ChemInform. 2008;38(17):2929–2940.
- Das VK, Devi RR, Raul PK, Thakur AJ. Nano rod-shaped and reusable basic Al2O3 catalyst for N-formylation of amines under solvent-free conditions: A novel, practical and convenient NOSE’ approach. Green Chem. 2012;14(3):847–854.
- Saeed S, Bhatti MH, Yunus U, Jones PG. Ethyl 4-(3-benzoylthioureido)benzoate. Acta Crystallogr Sect E Struct Reports Online. 2008;64(8):o1485.
- Mahdavi M, Shirazi MS, Taherkhani R, Saeedi M, Alipour E, Moghadam FH, Moradi A, Nadri H, Emami S, Firoozpour L, et al. Synthesis, biological evaluation and docking study of 3-aroyl-1-(4- sulfamoylphenyl)thiourea derivatives as 15-lipoxygenase inhibitors. Eur J Med Chem. 2014;82:308–313.
- Noori SD, Kadhi MS, Najm MAA, Oudah KH, Qasim QA, Al-Salman HNK. In-vitro evaluation of anticancer activity of natural flavonoids, apigenin and hesperidin. Mater Today Proc. 2022;60:1840–1843.
- Burley SK, Bhikadiya C, Bi C, Bittrich S, Chen L, Crichlow GV, Christie CH, Dalenberg K, Di Costanzo L, Duarte JM, et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49(D1):D437–D451.
- Khalifah RG. The Carbon Dioxide Hydration Activity of Carbonic Anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246(8):2561–2573.
- Singh P, Purnachander Yadav P, Swain B, Thacker PS, Angeli A, Supuran CT, Arifuddin M. Discovery of a novel series of indolylchalcone-benzenesulfonamide hybrids acting as selective carbonic anhydrase II inhibitors. Bioorg Chem. 2021;108(January):104647.
- Dar’in D, Kantin G, Kalinin S, Sharonova T, Bunev A, Ostapenko GI, Nocentini A, Sharoyko V, Supuran CT, Krasavin M, et al. Investigation of 3-sulfamoyl coumarins against cancer-related IX and XII isoforms of human carbonic anhydrase as well as cancer cells leads to the discovery of 2-oxo-2H-benzo[h]chromene-3-sulfonamide – A new caspase-activating proapoptotic agent. Eur J Med Chem. 2021;222:113589.
- Yamali C, Inci Gul H, Ozli G, Angeli A, Ballar Kirmizibayrak P, Erbaykent Tepedelen B, Sakagami H, Bua S, Supuran CT. Exploring of tumor-associated carbonic anhydrase isoenzyme IX and XII inhibitory effects and cytotoxicities of the novel N-aryl-1-(4-sulfamoylphenyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxamides. Bioorg Chem. 2021;115(July):105194.
- Elbadawi MM, Eldehna WM, Nocentini A, Abo-Ashour MF, Elkaeed EB, Abdelgawad MA, Alharbi KS, Abdel-Aziz HA, Supuran CT, Gratteri P, et al. Identification of N-phenyl-2-(phenylsulfonyl)acetamides/propanamides as new SLC-0111 analogues: Synthesis and evaluation of the carbonic anhydrase inhibitory activities. Eur J Med Chem. 2021;218:113360.
- Daina A, Zoete V. A Boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11(11):1117–1121.
- Ursu O, Oprea TI. Model-free drug-likeness from fragments. J Chem Inf Model. 2010;50(8):1387–1394.
- Subik K, Lee J-F, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung M-C, Bonfiglio T, Hicks DG, et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, KI-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer. 2010;4(1):35–41.
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):1–13.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3–26.
- Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb Chem. 1999;1(1):55–68.