Abstract
A series of 1,2,3-benzoxathiazine-2,2-dioxides possessing various substituents in the 5, 7, or 8 position was obtained from corresponding 2-hydroxybenzaldehydes in their reaction with sulfamoyl chloride. 5-, 7-, and 8-aryl substituted 1,2,3-benzoxathiazine-2,2-dioxides were prepared from aryl substituted 2-hydroxybenzaldehydes obtained from 3-, 4-, or 6-bromo-2-hydroxybenzaldehydes via two-step protocol. 1,2,3-Benzoxathiazine-2,2-dioxides were investigated for the inhibition of four human carbonic anhydrase (hCA, EC 4.2.1.1) isoforms, cytosolic hCA I and II and tumour-associated transmembrane hCA IX and XII. Twenty four derivatives of 1,2,3-benzoxathiazine 2,2-dioxide were obtained. Most of them act as nanomolar inhibitors of hCA IX and XII. Almost all compounds except 2d and 5a-e also express nanomolar inhibitory activity for hCA II. hCA I is poorly inhibited or not inhibited by 1,2,3-benzoxathiazine 2,2-dioxides. Some of the new derivatives exhibit promising selectivity towards CA IX/XII over hCA I, although none of the compounds are selective towards CA IX/XII over both hCA I and II.
Introduction
Human carbonic anhydrases (hCAs, EC 4.2.1.1) are a superfamily of widespread metalloenzymes in most living organisms that catalyse a reversible hydration of carbon dioxide (CO2) into bicarbonate ion (HCO3–) and proton (H+)Citation1–5.
CAs are involved in such important physiological processes as respiration, metabolism, pH regulation, ion transport, bone resorption, secretion of gastric, cerebrospinal fluid, and pancreatic juices, biosynthetic reactions such as gluconeogenesis and ureagenesis, etcCitation1–3.
Sixteen different CA isoforms have been identified and characterised in mammals so farCitation1,Citation3.
Human CAs are considered as promising therapeutic targets for a wide range of diseases, such as epilepsy, cerebral and retinal oedema, glaucoma (hCA I and hCA II), haemolytic anaemia, obesity, sterility, and cancerCitation1,Citation6.
Most efforts in the last few decades have been focussed on the tumour-associated isoforms (hCA IX and XII) that were shown to possess an important role in hypoxic tumour physiopathology and thus validated as biomarkers and therapeutic targets for various cancer types. Their inhibition has been related to the reduction of primary tumour growth, inhibition of invasion and metastasis, and a reduction in the cancer stem cell populationCitation7–9.
hCA IX expression is associated with a bad prognosis in cancer, whereas hCA XII is expressed in normal tissues and overexpressed in a number of malignant tumorsCitation4,Citation10.
hCA IX has been considered as a valuable marker for cancer, and the development of hCA IX inhibitors with selectivity over widely distributed isoforms hCA I/II is a potential strategy for designing anticancer agentsCitation3.
During the last decade, a wide range of novel CA inhibitors has been discovered, such as saccharin derivativesCitation11–13, thiophene moiety containing sulfonamidesCitation14, furagin derivativesCitation15, and many more.
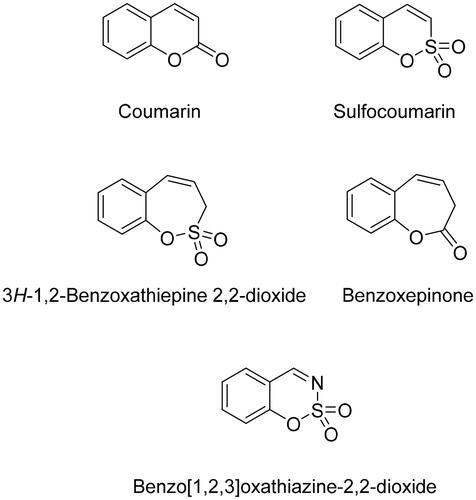
Sulphonamide moiety is the most widespread zinc-binding group (ZBG) of CA inhibitors. Non-classical CA inhibitors such as coumarins, carboxylic acids, phenols, polyamines inhibit the catalytic activity of CA by different mechanisms rather than coordinating to the zincCitation10.
Coumarin ring showed exceptional anticancer properties acting through various mechanisms of actionCitation10,Citation16,Citation17. Coumarin derivatives were introduced by Supuran’s group as a non-classical type of CAIsCitation18.
The mechanism of action of coumarin as CA inhibitor is based on hydrolysis forming cis-2-hydroxy-cinnamic acid, instead of binding the CA active site with its intact coumarin moietyCitation10.
The ability of coumarin to inhibit CA triggered the investigation of coumarin derivatives and its bioisosteres as CA inhibitors.Citation19
In 2013, coumarin bioisosteres 1,2-benzoxathiine 2,2-dioxides, also referred as sulfocoumarins, were reported as a new class of prodrug-type CA inhibitors, some of them showing low nanomolar inhibitory activities.Citation20 It was demonstrated that sulfocoumarins (1,2-benzoxathiine 2,2-dioxides) possess a similar mechanism of action as coumarins, acting as effective CA inhibitors. The sulfocoumarins were hydrolysed by the esterase CA activity to 2-hydroxyphenyl-vinylsulfonic acids, which then bind to the enzyme in a region rarely occupied by other classes of inhibitors.Citation20 Later a wide range of sulfocoumarin derivatives has been investigated and tested, showing significant isoform-selective CA inhibition propertiesCitation21–26.
In 2017, homologs of sulfocoumarin-3H-1,2-benzoxathiepine 2,2-dioxides, i.e. homo-sulfocoumarins, were introduced as CA inhibitors for the first time. As the results of the biological screening show, homo-sulfocoumarins possess very high level of CA isoform selectivity as wellCitation27–30.
In 2020, one more novel isoform-selective class of compounds – the bioisosteres of homo-sulfocoumarins, namely benzoxepinones (), were reported as CA inhibitors for the first time.Citation31
Taking into account that coumarin bioisosteres and their homologs described above show excellent CA inhibitory properties along with significant selectivity for the inhibition of hCA IX and hCA XII over hCA I and hCA II, we report here a series of nitrogen-containing analogues of 1,2-benzoxathiine 2,2-dioxides, namely derivatives of 1,2,3-benzoxathiazine 2,2-dioxide.
Materials and methods
Chemistry
Reagents, starting materials, and solvents were obtained from commercial sources and used as received. Thin-layer chromatography was performed on silica gel, spots were visualised with UV light (254 and 365 nm). Melting points were determined on an OptiMelt automated melting point system. IR spectra were recorded on Shimadzu FTIR IR Prestige-21 spectrometer. NMR spectra were recorded on Bruker Avance Neo (400 MHz) spectrometer with chemical shifts values (δ) in ppm relative to TMS using the residual DMSO-d6 signal (1H 2.50; 13C 39.52) or CDCl3 signal (1H 7.26; 13C 77.16) as an internal standard. High-resolution mass spectra (HRMS) were recorded on a mass spectrometer with a Q-TOF micro mass analyser using the ESI technique. Elemental analyses were performed on Carlo Erba CHNS-O EA-1108 apparatus. GC-MS analyses were performed on Agilent Technologies 7890 A gas chromatograph, column – HP-5HS (df = 0.25 μm, ID = 0.25 mm, length − 30 m) with Agilent Technologies 5975C masselective detector.
Synthesis
Sulfamoyl chloride
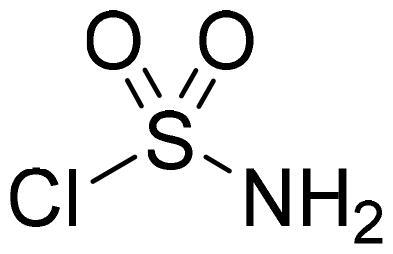
Chlorosulfonylisocyanate (3.80 ml, 43.3 mmol) was cooled to +5 °C. Formic acid (1.67 ml, 43.3 mmol) was added dropwise in temperature range between +5 - +13 °C. Reaction mixture was stirred at 0 °C for additional 45 min, and then allowed to warm to room temperature. Toluene (30 ml) was added to the reaction mixture, precipitate was filtered, filtrate was evaporated. Obtained as yellow oily solid (6.58 g, 98%). Mp 36–37 °C.
General procedure for the preparation of 1,2,3-benzoxathiazine 2,2-dioxides 2a–k
The derivative of 2-hydroxybenzaldehyde (1 equiv) was dissolved in dry DMA (6 ml). Reaction mixture was cooled to 0 °C. Sulfamoyl chloride (2.5 equiv) was slowly added to the reaction mixture. The stirring was continued at room temperature under argon atmosphere for 24–72 h. Reaction mixture was then poured into ice-water (25 ml), extracted with DCM (3 × 25 ml), washed with satd. NaHCO3 (3 × 25 ml) and brine (3 × 25 ml). Organic phase was dried over Na2SO4, filtered, evaporated. The product was purified by column chromatography on silica gel with PE/EtOAc (2:1) followed by recrystallisation from EtOH.
6-Methyl-1,2,3-benzoxathiazine 2,2-dioxide (2a)

Compound 2a was obtained from 2-hydroxy-4-methylbenzaldehyde (0.20 g, 1.47 mmol) and sulfamoyl chloride (0.42 g, 3.68 mmol). Reaction mixture was stirred for 48 h. Obtained as white solid (0.19 g, 64%). Mp 94–95 °C.
1H NMR (400 MHz, CDCl3): δ 8.61 (1H, s), 7.55 (1H, dd, J= 0.7 Hz, J= 2.2 Hz), 7.46 (1H, dd, J= 0.4 Hz, J= 1.6 Hz), 7.19 (1H, d, J= 8.5 Hz), 2.44 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 167.9, 152.4, 138.6, 136.5, 130.7, 118.5, 115.3, 20.8 ppm.
Anal. Calcd. for C8H7NO3S: C, 48.72; H, 3.58; N 7.10. Found: C, 48.24; H, 3.57; N, 7.00.
GC-MS (m/z, %): 51 (21), 77 (27), 78 (53), 104 (40), 106 (20), 132 (87), 197 (100).
IR (KBr, cm−1), νmax = 1382 (S = O), 1185 (S = O).
6-Methoxy-1,2,3-benzoxathiazine 2,2-dioxide (2b)
Compound 2b was obtained from 2-hydroxy-5-methoxybenzaldehyde (0.16 ml, 1.32 mmol) and sulfamoyl chloride (0.38 g, 3.30 mmol). Reaction mixture was stirred for 72 h. Purified by column chromatography with PE/EtOAc (1:1). Obtained as light yellow solid (0.10 g, 36%). Mp 121–122 °C.
1H NMR (400 MHz, CDCl3): δ 8.67 (1H, s), 7.33 (1H, dd, J= 2.9 Hz, J= 9.1 Hz), 7.29 (1H, d, J= 8.5 Hz), 7.14 (1H, d, J= 2.9 Hz), 3.92 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 167.7, 157.3, 148.2, 124.6, 119.9, 115.8, 113.2, 56.3 ppm.
Anal. Calcd. for C8H7NO4S: C, 45.07; H, 3.31; N 6.57. Found: C, 45.04; H, 3.32; N, 6.50.
GC-MS (m/z, %): 79 (39), 107 (26), 134 (100), 149 (40), 213 (51).
IR (KBr, cm−1), νmax = 1375 (S = O), 1362 (S = O), 1183 (S = O), 1168 (S = O).
6-Fluoro-1,2,3-benzoxathiazine 2,2-dioxide (2c)
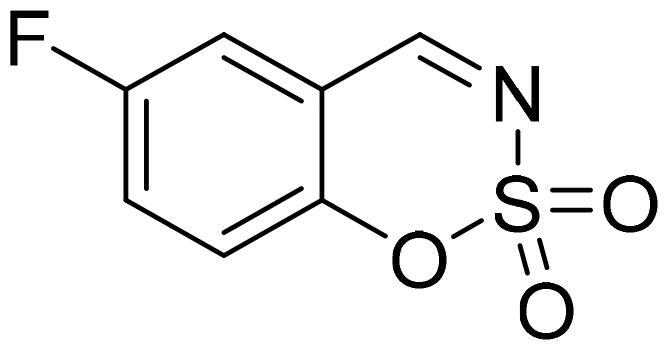
Compound 2c was obtained from 5-fluoro-2-hydroxybenzaldehyde (0.20 g, 1.43 mmol) and sulfamoyl chloride (0.41 g, 3.58 mmol). Reaction mixture was stirred for 72 h. Obtained as white solid (0.14 g, 48%). Mp 142–143 °C.
1H NMR (400 MHz, CDCl3): δ 8.64 (1H, s), 7.48 (1H, ddd, J= 3.0 Hz, J= 7.6 Hz, J= 9.1 Hz), 7.39 (1H, dd, J= 3.3 Hz, J= 6.9 Hz), 7.32 (1H, dd, J= 4.1 Hz, J= 9.1 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 166.7 (d, J= 2.3 Hz), 159.3 (d, J= 249 Hz), 150.4 (d, J= 2.5 Hz), 125.0 (d, J= 24.0 Hz), 120.8 (d, J= 7.9 Hz), 116.5 (d, J= 24.5 Hz), 116.0 (d, J= 8.1 Hz) ppm.
Anal. Calcd. for C7H4FNO3S: C, 41.79; H, 2.00; N 6.96. Found: C, 41.51; H, 2.18; N, 6.70.
GC-MS (m/z, %): 81 (31), 109 (92), 137 (54), 201 (100).
IR (KBr, cm−1), νmax = 1388 (S = O), 1353 (S = O), 1189 (S = O), 1154 (S = O).
6-Bromo-1,2,3-benzoxathiazine 2,2-dioxide (2d)
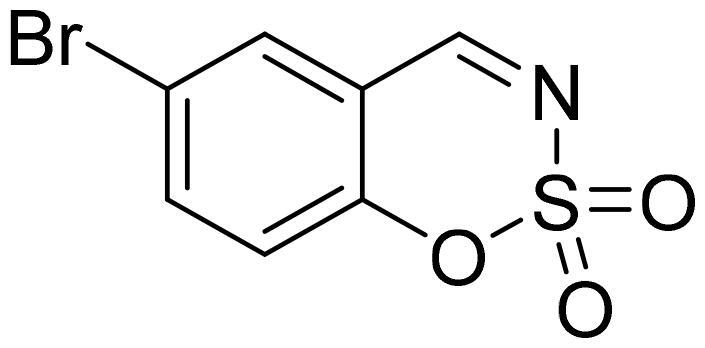
Compund 2d was obtained from 2-hydroxy-6-bromobenzaldehyde (0.20 g, 1.00 mmol) and sulfamoyl chloride (0.29 g, 2.50 mmol). Reaction mixture was stirred for 48 h. Obtained as white solid (0.12 g, 47%). Mp 154–155 °C.
1H NMR (400 MHz, CDCl3): δ 8.62 (1H, s), 7.85 (1H, dd, J= 2.2 Hz, J= 8.7 Hz), 7.82 (1H, d, J= 2.2 Hz), 7.21 (1H, d, J= 8.7 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 166.4, 153.3, 140.4, 133.1, 120.6, 118.9, 116.6 ppm.
Anal. Calcd. for C7H4BrNO3S: C, 32.08; H, 1.54; N 5.34. Found: C, 32.08; H, 1.40; N, 5.20.
GC-MS (m/z, %): 62 (30), 63 (100), 90 (27), 171 (56), 197 (53), 263 (83).
IR (KBr, cm−1), νmax = 1381 (S = O), 1345 (S = O), 1179 (S = O).
7-Methyl-1,2,3-benzoxathiazine 2,2-dioxide (2e)

Compound 2e was obtained from 2-hydroxy-4-methylbenzaldehyde (0.20 g, 1.47 mmol) and sulfamoyl chloride (0.42 g, 3.68 mmol). Reaction mixture was stirred for 72 h. Purified by column chromatography with PE/EtOAc (1:1). Obtained as light yellow solid (0.20 g, 70%). Mp 79–80 °C.
1H NMR (400 MHz, CDCl3): δ 8.60 (1H, s), 7.55 (1H, d, J= 7.9 Hz), 7.20–7.24 (1H, m), 7.10 (1H, s), 2.50 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 167.6, 154.5, 150.5, 130.7, 127.3, 118.9, 113.3, 22.5 ppm.
Anal. Calcd. for C8H7NO3S: C, 48.72; H, 3.58; N 7.10. Found: C, 48.70; H, 3.67; N, 7.05.
GC-MS (m/z, %): 77 (22), 78 (49), 104 (50), 132 (55), 197 (100).
IR (KBr, cm−1), νmax = 1381 (S = O), 1195 (S = O).
7-Methoxy-1,2,3-benzoxathiazine 2,2-dioxide (2f)
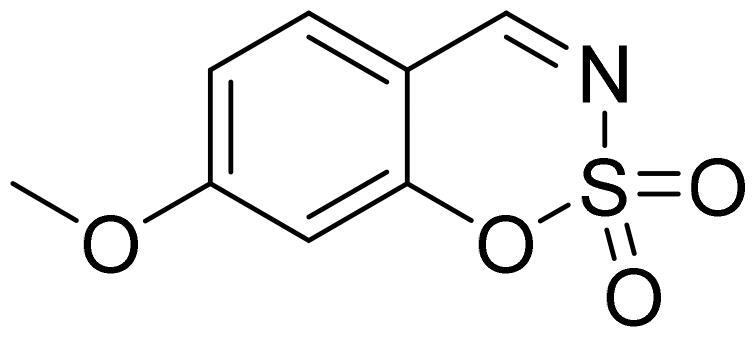
Compound 2f was obtained from 2-hydroxy-4-methoxybenzaldehyde (0.20 g, 1.34 mmol) and sulfamoyl chloride (0.39 g, 3.35 mmol). Reaction mixture was stirred for 48 h. Obtained as light brown solid (0.19 g, 67%). Mp 125–126 °C.
1H NMR (400 MHz, CDCl3): δ 8.51 (1H, s), 7.56 (1H, d, J= 8.7 Hz), 6.90 (1H, dd, J= 2.4 Hz, J= 8.7 Hz), 6.73 (1H, d, J= 2.4 Hz), 3.94 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 167.4, 166.9, 157.0, 132.7, 113.7, 109.3, 103.0, 56.6 ppm.
Anal. Calcd. for C8H7NO4S: C, 45.07; H, 3.31; N 6.57. Found: C, 45.15; H, 3.27; N, 6.51.
GC-MS (m/z, %): 106 (44), 134 (49), 149 (18), 213 (100).
IR (KBr, cm−1), νmax = 1383 (S = O), 1357 (S = O), 1184 (S = O), 1156 (S = O).
7-Fluoro-1,2,3-benzoxathiazine 2,2-dioxide (2g)
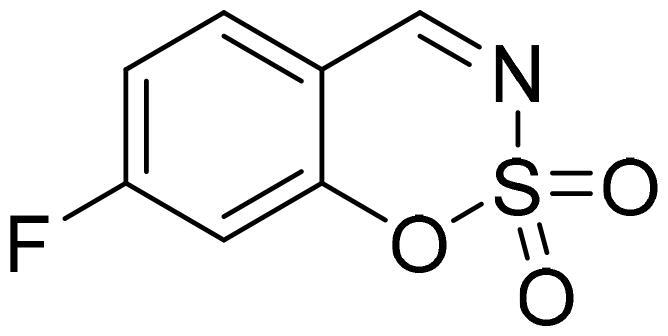
Compund 2g was obtained from 2-hydroxy-4-fluorobenzaldehyde (0.20 g, 1.43 mmol) and sulfamoyl chloride (0.41 g, 3.58 mmol). Reaction mixture was stirred for 72 h. Purified by column chromatography with PE/EtOAc (1:1). Obtained as light brown solid (0.15 g, 51%). Mp 67–68 °C.
1H NMR (400 MHz, CDCl3): δ 8.62 (1H, s), 7.72 (1H, dd, J= 5.8 Hz, J= 8.5 Hz), 7.14 (1H, ddd, J= 2.4 Hz, J= 7.9 Hz, J= 8.5 Hz), 7.03 (1H, dd, J= 2.4 Hz, J= 8.5 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.7 (d, J= 263.8 Hz), 166.8, 156.3 (d, J= 13.7 Hz), 133.4 (d, J= 11.4 Hz), 114.5 (d, J= 22.9 Hz), 112.4 (d, J= 3.1 Hz), 107.0 (d, J= 26.1 Hz) ppm.
Anal. Calcd. for C7H4FNO3S: C, 41.79; H, 2.00; N 6.96. Found: C, 41.75; H, 2.00; N, 6.90.
GC-MS (m/z, %): 81 (21), 82 (49), 109 (74), 110 (26), 137 (37), 201 (100).
IR (KBr, cm−1), νmax = 1395 (S = O), 1385 (S = O), 1142 (S = O).
7-Bromo-1,2,3-benzoxathiazine 2,2-dioxide (2h)
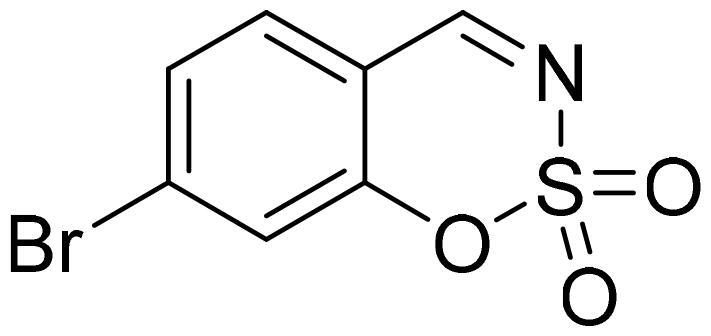
Compound 2h was obtained from 4-bromo-2-hydroxybenzaldehyde (0.20 g 1.00 mmol) and sulfamoyl chloride (0.29 g, 2.50 mmol). Reaction mixture was stirred for 72 h. Obtained as white solid (0.14 g, 55%). Mp 120–121 °C.
1H NMR (400 MHz, CDCl3): δ 8.63 (1H, s), 7.58 (1H, dd, J= 1.6 Hz, J= 8.2 Hz), 7.54 (1H, d, J= 8.2 Hz), 7.50 (1H, d, J= 1.6 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.0, 154.5, 132.6, 131.6, 129.9, 122.3, 114.2 ppm.
Anal. Calcd. for C7H4BrNO3S: C, 32.08; H, 1.54; N 5.34. Found: C, 32.28; H, 1.57; N, 5.17.
GC-MS (m/z, %): 62 (27), 63 (83), 90 (42), 169 (60), 197 (34), 263 (100).
IR (KBr, cm−1), νmax = 1384 (S = O).
8-Methoxy-1,2,3-benzoxathiazine 2,2-dioxide (2i)

Compound 2i was obtained from 2-hydroxy-3-methoxybenzaldehyde (0.20 g, 1.34 mmol) and sulfamoyl chloride (0.39 g, 3.35 mmol). Reaction mixture was stirred for 24 h. Obtained as white solid (0.09 g, 30%). Mp 140–141 °C.
1H NMR (400 MHz, CDCl3): δ 8.57 (1H, s), 7.22–7.31 (2H, m), 7.15–7.20 (1H, m), 3.90 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 168.1, 148.5, 143.7, 126.2, 121.6, 120.0, 116.1, 56.8 ppm.
Anal. Calcd. for C8H7NO4S: C, 45.07; H, 3.31; N 6.57. Found: C, 44.97; H, 3.26; N, 6.42.
GC-MS (m/z, %): 51 (32), 106 (37), 107 (57), 119 (22), 134 (38), 149 (35), 213 (100).
IR (KBr, cm−1), νmax = 1383 (S = O), 1359 (S = O), 1185 (S = O), 1171 (S = O).
8-Fluoro-1,2,3-benzoxathiazine 2,2-dioxide (2j)
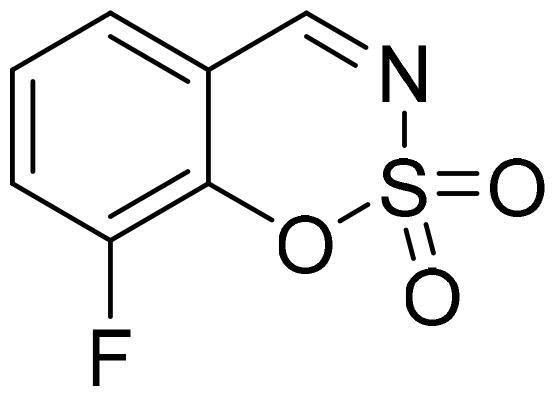
Compound 2j was obtained from 2-hydroxy-3-fluorobenzaldehyde (0.20 g, 1.43 mmol) and sulfamoyl chloride (0.41 g, 3.58 mmol). Reaction mixture was stirred for 72 h. Purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.12 g, 42%). Mp 83–84 °C.
1H NMR (400 MHz, CDCl3): δ 8.69 (1H, d, J= 1.2 Hz), 7.53–7.59 (1H, m), 7.47–7.51 (1H, m), 7.39 (1H, dq, J= 4.3 Hz, J= 7.9 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3 (d, J= 3.0 Hz), 150.3 (d, J= 256.5 Hz), 142.5 (d, J= 13.7 Hz), 126.4 (d, J= 6.6 Hz), 125.9 (d, J= 3.8 Hz), 124.6 (d, J= 17.6 Hz), 117.0 ppm.
Anal. Calcd. for C7H4FNO3S: C, 41.79; H, 2.00; N 6.96. Found: C, 41.80; H, 1.98; N, 6.73.
GC-MS (m/z, %): 81 (30), 82 (59), 109 (74), 137 (63), 201 (100).
IR (KBr, cm−1), νmax = 1399 (S = O), 1363 (S = O), 1191 (S = O).
8-Bromo-1,2,3-benzoxathiazine 2,2-dioxide (2k)
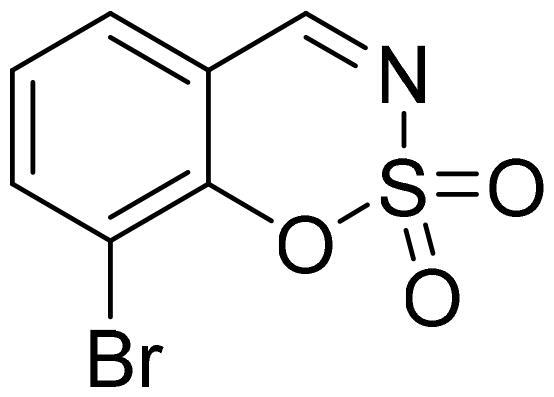
Compound 2k was obtained from 3-bromo-2-hydroxybenzaldehyde (0.20 g, 1.00 mmol) and sulfamoyl chloride (0.29 g, 2.50 mmol). Reaction mixture was stirred for 72 h. Purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.10 g, 39%). Mp 130–131 °C.
1H NMR (400 MHz, CDCl3): δ 8.63 (1H, s), 7.96 (1H, dd, J= 1.5 Hz, J= 8.1 Hz), 7.65 (1H, dd, J= 1.5 Hz, J= 7.7 Hz), 7.33 (1H, t, J= 7.8 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3, 151.5, 141.0, 129.9, 126.9, 116.7, 112.6 ppm.
Anal. Calcd. for C7H4BrNO3S: C, 32.08; H, 1.54; N 5.34. Found: C, 32.22; H, 1.58; N, 5.30.
GC-MS (m/z, %): 62 (33), 63 (100), 64 (27), 90 (54), 118 (57), 169 (36), 263 (99).
IR (KBr, cm−1), νmax = 1378 (S = O), 1355 (S = O), 1180 (S = O).
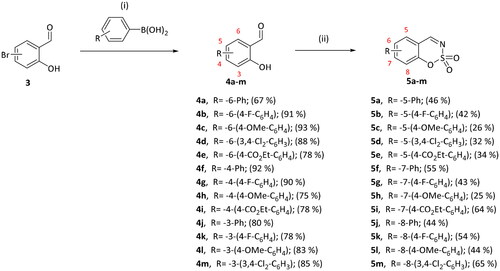
General procedure for the preparation of 2-hydroxybenzaldehydes 4a-m
The derivative of 2-hydroxybenzaldehyde (1 equiv), the derivative of benzeneboronic acid (1.3 equiv), K2CO3 (2.5 equiv), and Pd(PPh3)4 (0.05 equiv) were suspended in toluene/water (5:1, 20 ml) mixture in pressure tube. Reaction mixture was heated to 90 °C and stirred for 24 h, then cooled to room temperature, filtered through celite. EtOAc (30 ml) was added, reaction mixture was washed with satd. NaHCO3 (3× 25 ml) and brine (2× 25 ml). Organic phase was dried over Na2SO4, filtered, evaporated. The product was purified by column chromatography with PE/EtOAc (10:1) followed by recrystallisation from EtOH.
3-Hydroxy-1,1'-biphenyl-2-carbaldehyde (4a)
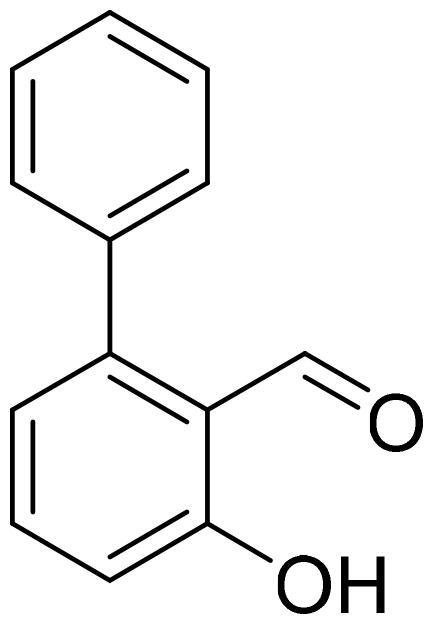
Compound 4a was obtained from 6-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), benzeneboronic acid (0.39 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol), and Pd(PPh3)4 (0.14 g, 0.12 mmol). Reaction mixture was stirred for 48 h, the product was purified by column chromatography with PE/EtOAc (2:1) followed by EtOAc (100%). Obtained as yellow solid (0.33 g, 67%). Mp 32–33 °C.
1H NMR (400 MHz, CDCl3): δ 11.92 (1H, s), 9.84 (1H, d, J= 0.6 Hz), 7.50–7.60 (1H, m), 7.43–7.49 (3H, m), 7.35–7.40 (2H, m), 6.98–7.02 (1H, m), 6.89 (1H, dd, J= 1.1 Hz, J= 7.5 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 197.3, 162.9, 147.6, 137.6, 136.7, 130.2, 128.5, 128.4, 121.6, 118.1, 117.1 ppm.
HRMS (ESI, m/z): calcd for C13H9O2 [M-H]- 197.0603, found 197.0606.
GC-MS (m/z, %): 115 (25), 141 (22), 152 (20), 197 (100).
IR (KBr, cm−1), νmax = 3058 (OH), 1656 (C = O).
4'-Fluoro-3-hydroxy-1,1'-biphenyl-2-carbaldehyde (4b)

Compound 4b was obtained from 6-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-fluorobenzeneboronic acid (0.45 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol), and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as white solid (0.49 g, 91%). Mp 72–73 °C.
1H NMR (400 MHz, CDCl3): δ 11.89 (1H, s), 9.82 (1H, d, J= 0.6 Hz), 7.53 (1H, dd, J= 7.7 Hz, J= 8.5 Hz), 7.31–7.38 (2H, m), 7.16 (2H, t, J= 8.6 Hz), 6.99–7.02 (1H, m), 6.86 (1H, dd, J= 1.1 Hz, J= 7.5 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 197.0, 163.1, 163.0 (d, J= 248.9 Hz), 146.5, 136.9, 133.7 (d, J= 3.5 Hz), 131.9 (d, J= 8.3 Hz), 121.8, 118.3, 117.4, 115.8 (d, J= 21.5 Hz) ppm.
HRMS (ESI, m/z): calcd for C13H8O2F [M-H]- 215.0508, found 215.0510.
GC-MS (m/z, %): 120 (22), 133 (24), 159 (20), 170 (25), 215 (78), 216 (100).
IR (KBr, cm−1), νmax = 3044 (OH), 1653 (C = O).
4'-Methoxy-3-hydroxy-1,1'-biphenyl-2-carbaldehyde (4c)

Compound 4c was obtained from 6-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-methoxybenzeneboronic acid (0.49 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as yellow solid (0.53 g, 93%). Mp 99–100 °C.
1H NMR (400 MHz, CDCl3): δ 11.91 (1H, s), 9.85 (1H, d, J= 0.5 Hz), 7.51 (1H, dd, J= 7.7 Hz, J= 8.3 Hz), 7.29 (2H, d, J= 8.8 Hz), 6.99 (2H, d, J= 8.8 Hz), 6.94–6.98 (1H, m), 6.87 (1H, dd, J= 1.1 Hz, J= 7.5 Hz), 3.87 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 197.4, 163.0, 159.9, 147.4, 136.7, 131.4, 129.8, 121.7, 118.3, 116.6, 114.1, 55.5 ppm.
HRMS (ESI, m/z): calcd for C14H13O3 [M+ H]+ 229.0865, found 229.0863.
GC-MS (m/z, %): 128 (22), 157 (26), 185 (21), 213 (23), 227 (37), 228 (100).
IR (KBr, cm−1), νmax = 3007 (OH), 1652 (C = O).
3',4'-Dichloro-3-hydroxy-1,1'-biphenyl-2-carbaldehyde (4d)

Compound 4d was obtained from 6-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 3,4-dichlorobenzeneboronic acid (0.62 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as white solid (0.58 g, 88%). Mp 144–145 °C.
1H NMR (400 MHz, CDCl3): δ 11.87 (1H, s), 9.82 (1H, d, J= 0.5 Hz), 7.52–7.58 (2H, m), 7.49 (1H, d, J= 2.1 Hz), 7.21 (1H, dd, J= 2.1 Hz, J= 8.3 Hz), 7.01–7.05 (1H, m), 6.85 (1H, dd, J= 1.1 Hz, J= 7.5 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 196.3, 163.1, 144.7, 137.5, 137.0, 133.1, 133.0, 131.8, 130.5, 129.4, 121.5, 118.1, 117.9 ppm.
HRMS (ESI, m/z): calcd for C13H7O2Cl2 [M-H]- 264.9823, found 254.9820.
GC-MS (m/z, %): 92 (24), 120 (57), 139 (57), 168 (24), 202 (24), 230 (21), 231 (88), 233 (28), 266 (100), 267 (59), 268 (71).
IR (KBr, cm−1), νmax = 3072 (OH), 1652 (C = O).
Ethyl 2'-formyl-3'-hydroxy-1,1'-biphenyl-4-carboxylate (4e)
Compound 4e was obtained from 6-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-(ethoxycarbonyl)benzeneboronic acid (0.63 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as yellow solid (0.52 g, 78%). Mp 92–93 °C.
1H NMR (400 MHz, CDCl3): δ 11.89 (1H, s), 9.80 (1H, d, J= 0.5 Hz), 8.14 (2H, d, J= 8.5 Hz), 7.55 (1H, dd, J= 7.6 Hz, J= 8.3 Hz), 7.45 (2H, d, J= 8.5 Hz), 7.02–7.06 (1H, m), 6.89 (1H, dd, J= 1.1 Hz, J= 7.5 Hz), 4.43 (2H, q, J= 7.1 Hz), 1.42 (3H, t, J= 7.1 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 196.6, 166.2, 163.1, 146.4, 142.1, 136.8, 130.6, 130.2, 129.8, 121.5, 118.0, 117.8, 61.4, 14.5 ppm.
HRMS (ESI, m/z): calcd for C16H13O4 [M-H]- 269.0814, found 269.0816.
GC-MS (m/z, %): 115 (22), 139 (32), 197 (100), 225 (46), 241 (46), 270 (91).
IR (KBr, cm−1), νmax = 3056 (OH), 1716 (C = O), 1651 (C = O).
3-Hydroxy-1,1'-biphenyl-4-carbaldehyde (4f)
Compound 4f was obtained from 4-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), benzeneboronic acid (0.39 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as white solid (0.45 g, 92%). Mp 81–82 °C.
1H NMR (400 MHz, CDCl3): δ 11.05 (1H, s), 9.86 (1H, d, J= 0.6 Hz), 7.53–7.58 (3H, m), 7.33–7.44 (3H, m), 7.19 (1H, dd, J= 1.6 Hz, J= 8.0 Hz), 7.14–7.16 (1H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 196.2, 162.1, 150.0, 139.5, 134.2, 129.1, 129.0, 127.5, 119.7, 119.1, 116.0 ppm.
HRMS (ESI, m/z): calcd for C13H9O2 [M-H]- 197.0603, found 197.0603.
GC-MS (m/z, %): 115 (22), 197 (100).
IR (KBr, cm−1), νmax = 3057 (OH), 1683 (C = O).
4'-Fluoro-3-hydroxy-1,1'-biphenyl-4-carbaldehyde (4g)

Compound 4g was obtained from 4-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-fluorobenzeneboronic acid (0.45 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as light yellow solid (0.48 g, 90%). Mp 108–109 °C.
1H NMR (400 MHz, CDCl3): δ 11.12 (1H, s), 9.92 (1H, d, J= 0.6 Hz), 7.57–7.63 (3H, m), 7.13–7.23 (4H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 196.1, 163.5 (d, J= 248.6 Hz), 162.1, 148.9, 135.6 (d, J= 3.2 Hz), 134.3, 129.2 (d, J= 8.3 Hz), 119.7, 118.8, 116.1 (d, J= 21.5 Hz), 115.8 ppm.
HRMS (ESI, m/z): calcd for C13H8O2F [M-H]- 215.0508, found 215.0513.
GC-MS (m/z, %): 133 (19), 215 (100).
IR (KBr, cm−1), νmax = 3057 (OH), 1652 (C = O).
3-Hydroxy-4'-methoxy-1,1'-biphenyl-4-carbaldehyde (4h)

Compound 4h was obtained from 4-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-methoxybenzeneboronic acid (0.49 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as white solid (0.43 g, 75%). Mp 129–130 °C.
1H NMR (400 MHz, CDCl3): δ 11.12 (1H, s), 9.89 (1H, d, J= 0.4 Hz), 7.56–7.61 (3H, m), 7.22 (1H, dd, J= 1.7 Hz, J= 8.1 Hz), 7.18 (1H, d, J= 1.7 Hz), 7.00 (2H, d, J= 8.9 Hz), 3.87 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 195.8, 162.1, 160.6, 149.6, 134.2, 131.7, 128.7, 119.3, 118.5, 115.1, 114.6, 55.5 ppm.
HRMS (ESI, m/z): calcd for C14H13O3 [M+ H]+ 229.0865, found 229.0868.
IR (KBr, cm−1), νmax = 3453 (OH), 1671 (C = O).
GC-MS (m/z, %): 227 (53), 228 (100).
Ethyl 4'-formyl-3'-hydroxy-1,1'-biphenyl-4-carboxylate (4i)
Compound 4i was obtained from 4-bromo-2-hydroxybenzaldehyde (0.50 g, v2.49 mmol), 4-(ethoxycarbonyl)benzeneboronic acid (0.63 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as white solid (0.52 g, 78%). Mp 110–111 °C.
1H NMR (400 MHz, CDCl3): δ 11.11 (1H, s), 9.95 (1H, d, J= 0.6 Hz), 8.14 (2H, d, J= 8.6 Hz), 7.68 (2H, d, J= 8.6 Hz), 7.65 (1H, d, J= 8.0 Hz), 7.28 (1H, d, J= 1.7 Hz, J= 8.0 Hz), 7.24 (1H, d, J= 1.7 Hz), 4.41 (2H, q, J= 7.2 Hz), 1.42 (3H, t, J= 7.2 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 196.2, 166.3, 162.0, 148.7, 143.7, 134.3, 130.8, 130.3, 127.5, 120.1, 119.1, 116.4, 61.3, 14.5 ppm.
HRMS (ESI, m/z): calcd for C16H15O4 [M+ H]+ 271.0970, found 271.0975.
GC-MS (m/z, %): 225 (100), 242 (25), 270 (64).
IR (KBr, cm−1), νmax = 3421 (OH), 1711 (C = O), 1665 (C = O).
2-Hydroxy-1,1'-biphenyl-3-carbaldehyde (4j)
Compound 4j was obtained from 3-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), benzeneboronic acid (0.39 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Purified by column chromatography with PE/EtOAc (10:1) → (5:1). Obtained as light yellow solid (0.39 g, 80%). Mp 46–47 °C.
1H NMR (400 MHz, CDCl3): δ 11.54 (1H, d, J= 0.5 Hz), 9.96 (1H, s), 7.55–7.65 (4H, m), 7.43–7.52 (2H, m), 7.34–7.41 (1H, m), 7.11 (1H, t, J= 7.6 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 197.0, 159.0, 138.0, 136.4, 133.3, 130.6, 129.4, 128.4, 127.8, 121.0, 120.0 ppm.
HRMS (ESI, m/z): calcd for C13H11O2 [M+ H]+ 199.0759, found 199.0759.
GC-MS (m/z, %): 115 (28), 141 (22), 152 (42), 169 (24), 197 (62), 198 (100).
IR (KBr, cm−1), νmax = 3051 (OH), 1656 (C = O).
4'-Fluoro-2-hydroxy-1,1'-biphenyl-3-carbaldehyde (4k)

Compound 4k was obtained from 3-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-fluorobenzeneboronic acid (0.45 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol), and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as light yellow solid (0.42 g, 78%). Mp 95–96 °C.
1H NMR (400 MHz, CDCl3): δ 11.55 (1H, d, J= 0.5 Hz), 9.96 (1H, s), 7.54–7.61 (4H, m), 7.08–7.18 (3H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 196.9, 162.5 (d, J= 247.3 Hz), 158.9, 137.8, 133.4, 132.3 (d, J= 3.8 Hz), 131.1 (d, J= 7.9 Hz), 129.6, 121.0, 120.1, 115.4 (d, J= 21.3 Hz) ppm.
HRMS (ESI, m/z): calcd for C13H8O2F [M-H]- 215.0508, found 215.0509.
GC-MS (m/z, %): 138 (25), 159 (24), 170 (33), 215 (60), 216 (100).
IR (KBr, cm−1), νmax = 3039 (OH), 1658 (C = O).
2-Hydroxy-4'-methoxy-1,1'-biphenyl-3-carbaldehyde (4l)
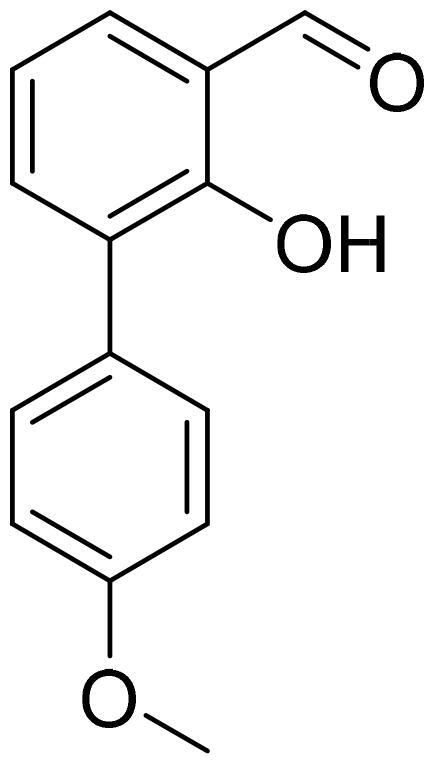
Compound 4l was obtained from 3-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 4-methoxybenzeneboronic acid (0.49 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol) and Pd(PPh3)4 (0.14 g, 0.12 mmol). Obtained as light yellow solid (0.47 g, 83%). Mp 81–82 °C.
1H NMR (400 MHz, CDCl3): δ 11.53 (1H, d, J= 0.6 Hz), 9.95 (1H, s), 7.60 (1H, ddd, J= 0.6 Hz, J= 1.7 Hz, J= 7.6 Hz), 7.54 (2H, d, J= 8.8 Hz), 7.53 (1H, dd, J= 1.7 Hz, J= 7.6 Hz), 7.09 (1H, t, J= 7.6 Hz), 6.99 (2H, d, J= 8.8 Hz), 3.86 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 197.0, 159.3, 159.0, 137.6, 132.9, 130.5, 130.3, 128.8, 121.0, 120.0, 113.9, 55.5 ppm.
HRMS (ESI, m/z): calcd for C14H13O3 [M+ H]+ 229.0865, found 229.0870.
GC-MS (m/z, %): 128 (22), 228 (100).
IR (KBr, cm−1), νmax = 3037 (OH), 1658 (C = O).
3',4'-Dichloro-2-hydroxy-1,1'-biphenyl-3-carbaldehyde (4m)

Compound 4m was obtained from 3-bromo-2-hydroxybenzaldehyde (0.50 g, 2.49 mmol), 3,4-dichlorobenzeneboronic acid (0.62 g, 3.23 mmol), K2CO3 (0.86 g, 6.22 mmol), and Pd(PPh3)4 (0.14 g, 0.12 mmol). Purified by column chromatography with PE/EtOAc (10:1) → (5:1). Obtained as white solid (0.57 g, 85%). Mp 143–144 °C.
1H NMR (400 MHz, CDCl3): δ 11.59 (1H, d, J= 0.5 Hz), 9.96 (1H, s), 7.71 (1H, d, J= 2.1 Hz), 7.57–7.63 (2H, m), 7.51 (1H, d, J= 8.3 Hz), 7.45 (1H, dd, J= 2.1 Hz, J= 8.3 Hz), 7.12 (1H, t, J= 7.7 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 196.9, 158.9, 137.5, 136.3, 134.1, 132.5, 131.9, 131.3, 130.3, 128.7, 128.1, 121.1, 120.2 ppm.
HRMS (ESI, m/z): calcd for C13H7O2Cl2 [M-H]- 264.9823, found 264.9823.
GC-MS (m/z, %): 139 (31), 168 (23), 202 (23), 220 (18), 265 (43), 266 (100), 268 (63).
IR (KBr, cm−1), νmax = 3045 (OH), 1657 (C = O).
General procedure for the preparation of 1,2,3-benzoxathiazine 2,2-dioxides 5a-m
The derivative of 2-hydroxybenzaldehyde 4a–m (1 equiv) was dissolved in dry DMA (6 ml). Reaction mixture was cooled to 0 °C. Sulfamoyl chloride (2.5 equiv) was slowly added to the reaction mixture. The stirring was continued at room temperature under argon atmosphere for 24 h. Reaction mixture was then poured into ice-water (25 ml), extracted with DCM (3× 25 ml), washed with satd. NaHCO3 (3× 25 ml) and brine (3× 25 ml). Organic phase was dried over Na2SO4, filtered and evaporated. The product was purified by column chromatography with PE/EtOAc (2:1) followed by recrystallisation from EtOH.
5-Phenyl-1,2,3-benzoxathiazine 2,2-dioxide (5a)
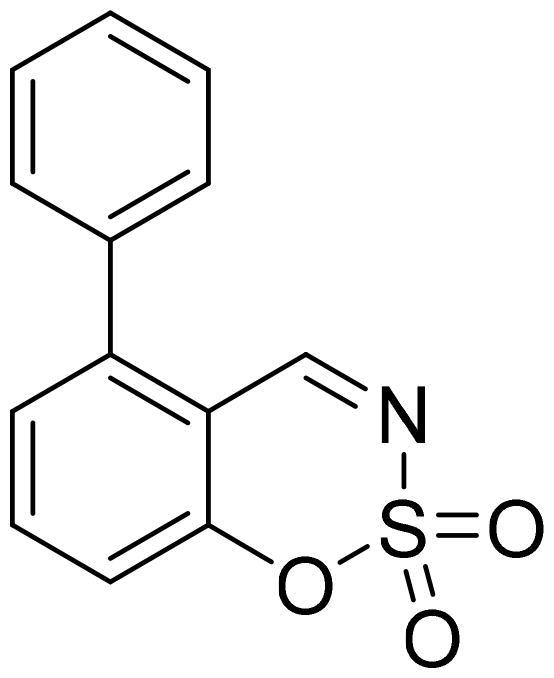
Compound 5a was obtained from 3-hydroxy-1,1′-biphenyl-2-carbaldehyde (4a) (0.20 g, 1.01 mmol) and sulfamoyl chloride (0.29 g, 2.53 mmol). Obtained as white solid (0.12 g, 46%). Mp 156–157 °C.
1H NMR (400 MHz, CDCl3): δ 8.59 (1H, d, J= 0.4 Hz), 7.77 (1H, dd, J= 7.8 Hz, J= 8.3 Hz), 7.51–7.57 (3H, m), 7.37–7.43 (3H, m), 7.28–7.32 (1H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 167.7, 155.0, 145.7, 137.0, 135.7, 130.2, 129.6, 129.3, 127.4, 117.5, 114.1 ppm.
HRMS (ESI, m/z): calcd for C13H10NO3S [M+ H]+ 260.0381, found 260.0381.
GC-MS (m/z, %): 139 (42), 166 (39), 167 (80), 195 (30), 259 (100).
IR (KBr, cm−1), νmax = 1389 (S = O), 1193 (S = O), 1183 (S = O).
5-(4-Fluorophenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5b)
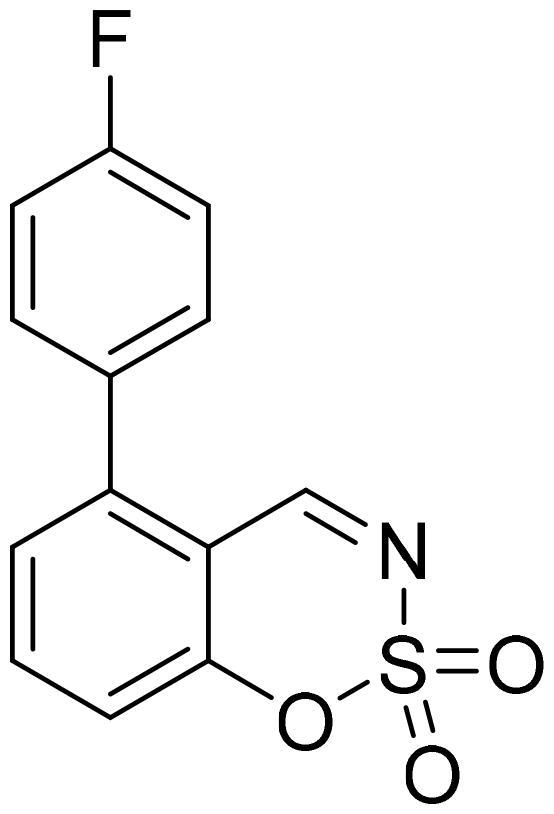
Compound 5b was obtained from 4′-fluoro-3-hydroxy-1,1′-biphenyl-2-carbaldehyde (4b) (0.28 g, 1.28 mmol) and sulfamoyl chloride (0.37 g, 3.20 mmol). Reaction mixture was stirred for 48 h. Obtained as white solid (0.14 g, 42%). Mp 186–187 °C.
1H NMR (400 MHz, CDCl3): δ 8.56 (1H, d, J= 0.4 Hz), 7.77 (1H, dd, J= 7.8 Hz, J= 8.3 Hz), 7.35–7.42 (3H, m), 7.29–7.33 (1H, m), 7.21–7.28 (2H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3, 163.7 (d, J= 250.8 Hz), 155.1, 144.5, 137.1, 132.0 (d, J= 8.4 Hz), 131.8 (d, J= 3.1 H), 127.4, 117.7, 116.5 (d, J= 22.1 Hz), 114.1 ppm.
HRMS (ESI, m/z): calcd for C13H9NO3SF [M+ H]+ 278.0287, found 278.0283.
GC-MS (m/z, %): 157 (41), 184 (37), 185 (72), 213 (31), 277 (100).
IR (KBr, cm−1), νmax = 1388 (S = O), 1191 (S = O).
5–(4-Methoxyphenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5c)
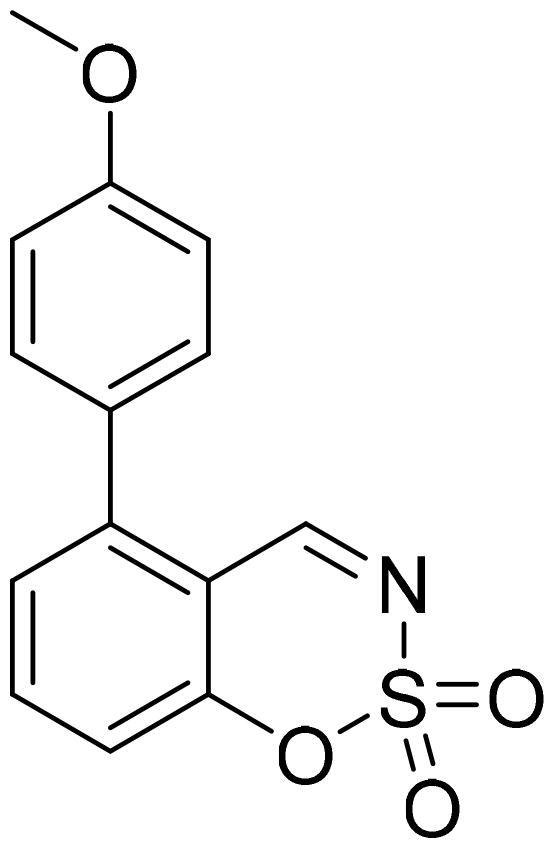
Compound 5c was obtained from 4′-methoxy-3-hydroxy-1,1′-biphenyl-2-carbaldehyde (4c) (0.30 g, 1.31 mmol) and sulfamoyl chloride (0.38 g, 3.28 mmol). Obtained as yellow solid (0.10 g, 26%). Mp 177–178 °C.
1H NMR (400 MHz, CDCl3): δ 8.59 (1H, d, J= 0.5 Hz), 7.74 (1H, dd, J= 7.8 Hz, J= 8.3 Hz), 7.38 (1H, dd, J= 1.1 Hz, J= 7.8 Hz), 7.31 (2H, d, J= 8.8 Hz), 7.23–7.27 (1H, m), 7.06 (2H, d, J= 8.8 Hz), 3.89 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 168.0, 160.9, 155.1, 145.6, 137.0, 131.6, 128.0, 127.2, 116.9, 114.8, 114.1, 55.7 ppm.
HRMS (ESI, m/z): calcd for C14H12NO4S [M+ H]+ 290.0487, found 290.0494.
GC-MS (m/z, %): 127 (22), 154 (28), 182 (23), 289 (100).
IR (KBr, cm−1), νmax = 1378 (S = O), 1376 (S = O), 1184 (S = O).
5-(3,4-Dichlorophenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5d)
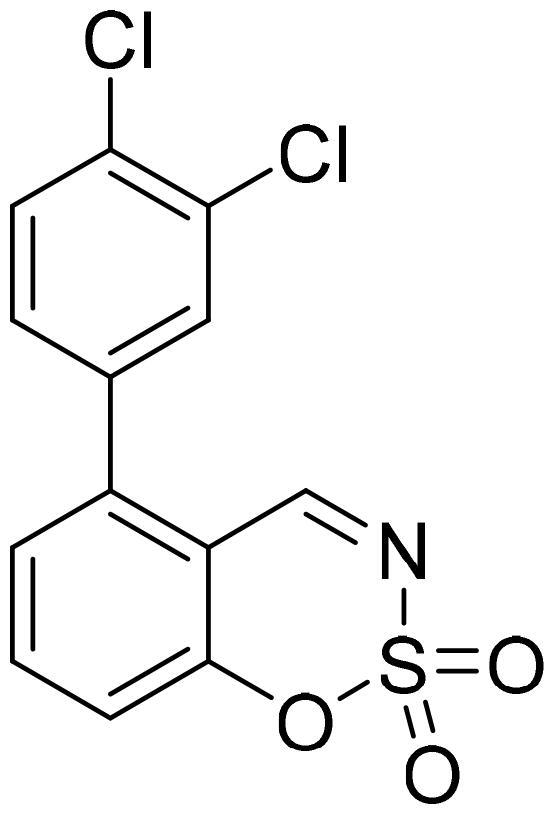
Compound 5d was obtained from 3′,4′-dichloro-3-hydroxy-1,1′-biphenyl-2-carbaldehyde (4d) (0.30 g, 1.12 mmol) and sulfamoyl chloride (0.32 g, 2.8 mmol). Obtained as white solid (0.12 g, 32%). Mp 230–231 °C.
1H NMR (400 MHz, CDCl3): δ 8.56 (1H, d, J= 0.5 Hz), 7.79 (1H, dd, J= 7.8 Hz, J= 8.3 Hz), 7.63 (1H, d, J= 8.3 Hz), 7.52 (1H, d, J= 2.2 Hz), 7.33–7.39 (2H, m), 7.22 (1H, dd, J= 2.2 Hz, J= 8.2 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 166.7, 155.1, 142.7, 137.3, 135.5, 134.6, 133.9, 131.7, 131.3, 129.4, 127.3, 118.5, 113.9 ppm.
HRMS (ESI, m/z): calcd for C13H8NO3SCl2 [M+ H]+ 327.9602, found 327.9605.
IR (KBr, cm−1), νmax = 1384 (S = O), 1190 (S = O), 1166 (S = O).
Ethyl 4-(2,2-dioxido-1,2,3-benzoxathiazin-5-yl)benzoate (5e)
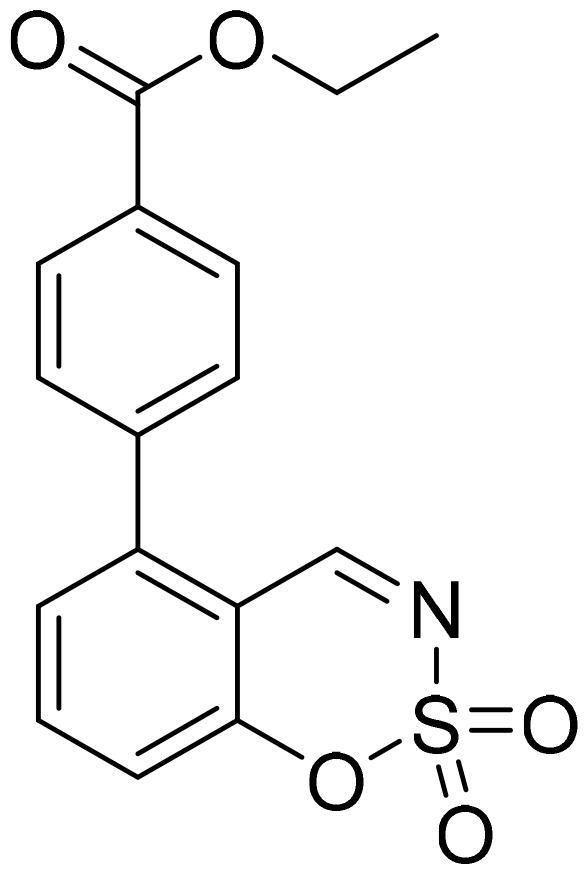
Compound 5e was obtained from ethyl 2′-formyl-3′-hydroxy-1,1′-biphenyl-4-carboxylate (4e) (0.20 g, 0.74 mmol) and sulfamoyl chloride (0.21 g, 1.85 mmol). Reaction mixture was stirred for 48 h. Obtained as white solid (0.08 g, 34%). Mp 135–136 °C.
1H NMR (400 MHz, CDCl3): δ 8.55 (1H, d, J= 0.5 Hz), 8.21 (2H, d, J= 8.4 Hz), 7.80 (1H, dd, J= 7.8 Hz, J= 8.4 Hz), 7.48 (2H, d, J= 8.4 Hz), 7.41 (1H, dd, J= 1.1 Hz, J= 7.7 Hz), 7.33–7.36 (1H, m), 4.42 (2H, q, J= 7.1 Hz), 1.44 (3H, t, J= 7.1 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.0, 165.9, 155.1, 144.4, 139.9, 137.2, 131.7, 130.4, 130.2, 127.3, 118.2, 114.0, 61.6, 14.5 ppm.
HRMS (ESI, m/z): calcd for C16H14NO5S [M+ H]+ 332.0593, found 332.0590.
GC-MS (m/z, %): 216 (20), 253 (100), 254 (23), 331 (40).
IR (KBr, cm−1), νmax = 1715 (C = O), 1383 (S= O), 1197 (S= O).
7-Phenyl-1,2,3-benzoxathiazine 2,2-dioxide (5f)

Compound 5f was obtained from ethyl 3-hydroxy-1,1′-biphenyl-4-carbaldehyde (4f) (0.20 g, 1.01 mmol) and sulfamoyl chloride (0.29 g, 2.52 mmol). Obtained as white solid (0.14 g, 55%). Mp 171–172 °C.
1H NMR (400 MHz, CDCl3): δ 8.68 (1H, d, J= 0.4 Hz), 7.73 (1H, d, J= 8.1 Hz), 7.61–7.66 (3H, m), 7.48–7.55 (4H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 167.4, 154.9, 151.2, 137.9, 131.3, 130.0, 129.5, 127.5, 124.8, 116.8, 114.1 ppm.
HRMS (ESI, m/z): calcd for C13H10NO3S [M+ H]+ 260.0381, found 260.0382.
GC-MS (m/z, %): 139 (50), 167 (24), 195 (57), 259 (100).
IR (KBr, cm−1), νmax = 1379 (S= O), 1193 (S= O).
7-(4-Fluorophenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5g)
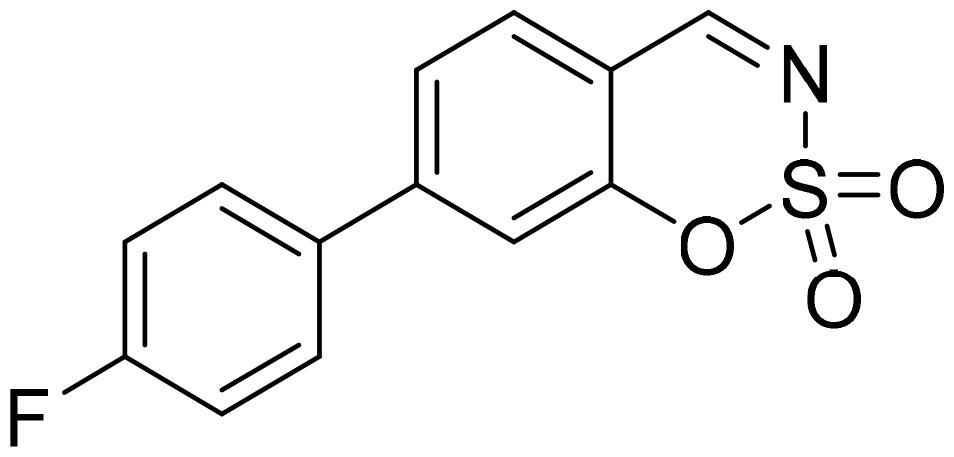
Compound 5g was obtained from 4′-fluoro-3-hydroxy-1,1′-biphenyl-4-carbaldehyde (4g) (0.20 g, 0.93 mmol) and sulfamoyl chloride (0.27 g, 2.31 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.11 g, 43%). Mp 164–165 °C.
1H NMR (400 MHz, CDCl3): δ 8.68 (1H, d, J= 0.4 Hz), 7.72 (1H, d, J= 8.1 Hz), 7.57–7.65 (3H, m), 7.45 (1H, d, J= 1.6 Hz), 7.22 (2H, t, J= 8.6 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3, 164.0 (d, J= 251.1 Hz), 154.9, 150.0, 134.1 (d, J= 3.4 Hz), 131.3, 129.4 (d, J= 8.6 Hz), 124.7, 116.6, 116.7 (d, J= 21.4 Hz), 114.1 ppm.
HRMS (ESI, m/z): calcd for C13H9NO3SF [M+ H]+ 278.0287, found 278.0288.
GC-MS (m/z, %): 157 (57), 158 (21), 185 (28), 213 (36), 277 (100).
IR (KBr, cm−1), νmax = 1381 (S = O), 1195 (S = O), 1162 (S = O).
7-(4-Methoxyphenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5h)
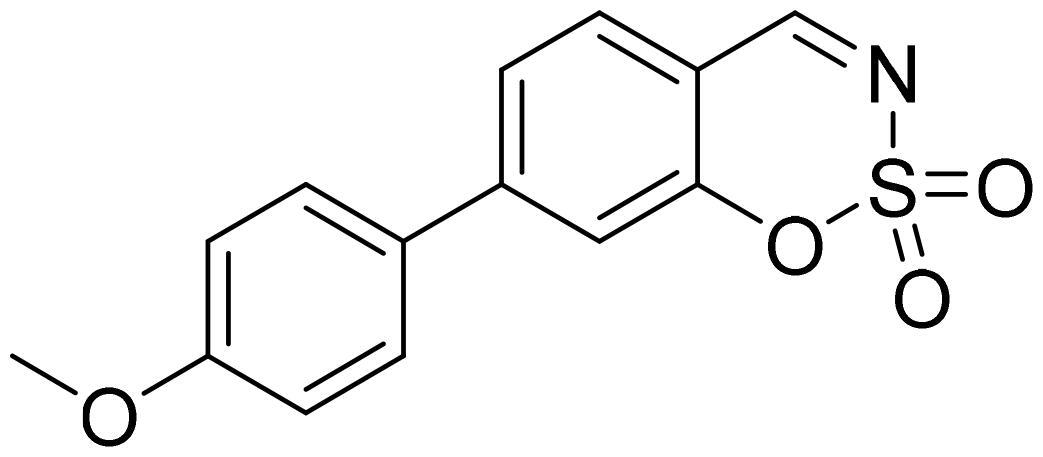
Compound 5h was obtained from 3-hydroxy-4′-methoxy-1,1′-biphenyl-4-carbaldehyde (4h) (0.20 g, 0.88 mmol) and sulfamoyl chloride (0.25 g, 2.19 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as light yellow solid (0.07 g, 25%). Mp 181–182 °C.
1H NMR (400 MHz, CDCl3): δ 8.65 (1H, d, J= 0.5 Hz), 7.68 (1H, d, J= 8.0 Hz), 7.57–7.62 (3H, m), 7.45 (1H, d, J= 1.7 Hz), 7.03 (2H, d, J= 8.8 Hz), 3.88 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3, 161.4, 155.0, 150.8, 131.2, 130.1, 128.9, 124.1, 115.9, 115.0, 113.6, 55.6 ppm.
HRMS (ESI, m/z): calcd for C14H12NO4S [M+ H]+ 290.0487, found 290.0498.
IR (KBr, cm−1), νmax = 1377 (S= O), 1363 (S= O), 1188 (S= O).
Ethyl 4-(2,2-dioxido-1,2,3-benzoxathiazin-7-yl)benzoate (5i)

Compound 5i was obtained from ethyl 4′-formyl-3′-hydroxy-1,1′-biphenyl-4-carboxylate (4i) (0.20 g, 0.74 mmol) and sulfamoyl chloride (0.21 g, 1.85 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.16 g, 64%). Mp 204–205 °C.
1H NMR (400 MHz, CDCl3): δ 8.71 (1H, d, J= 0.4 Hz), 8.18 (2H, d, J= 8.6 Hz), 7.76 (1H, d, J= 8.0 Hz), 7.69 (2H, d, J= 8.6 Hz), 7.66 (1H, dd, J= 1.6 Hz, J= 8.0 Hz), 7.53 (1H, d, J= 1.6 Hz), 4.43 (2H, q, J= 7.1 Hz), 1.43 (3H, t, J= 7.1 Hz) ppm.
13C NMR (100 MHz, CDCl3): δ 167.3, 166.0, 154.9, 149.8, 142.0, 131.7, 131.3, 130.6, 127.5, 125.0, 117.2, 114.7, 61.5, 14.5 ppm.
HRMS (ESI, m/z): calcd for C16H14NO5S [M+ H]+ 332.0593, found 332.0611.
IR (KBr, cm−1), νmax = 1701 (C = O), 1377 (S= O), 1194 (S= O).
8-Phenyl-1,2,3-benzoxathiazine 2,2-dioxide (5j)
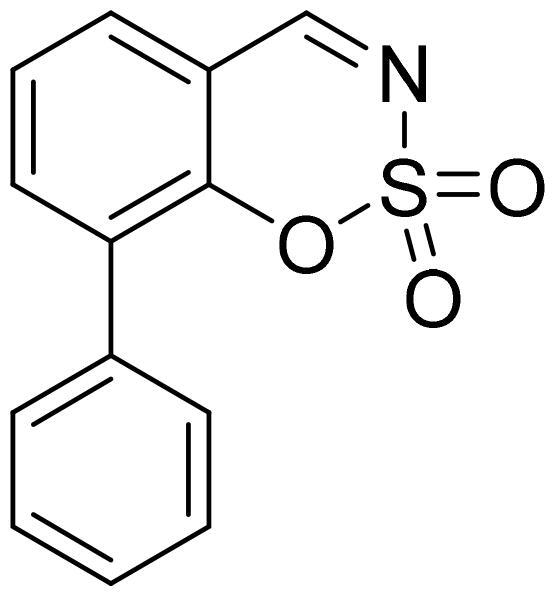
Compound 5j was obtained from 2-hydroxy-1,1′-biphenyl-3-carbaldehyde (4j) (0.20 g, 1.01 mmol) and sulfamoyl chloride (0.29 g, 2.52 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.12 g, 44%). Mp 164–165 °C.
1H NMR (400 MHz, CDCl3): δ 8.71 (1H, s), 7.80 (1H, dd, J= 1.6 Hz, J= 7.7 Hz), 7.66 (1H, dd, J= 1.6 Hz, J= 7.7 Hz), 7.42–7.56 (6H, m) ppm.
13C NMR (100 MHz, CDCl3): δ 168.1, 151.1, 138.8, 133.4, 132.8, 130.0, 129.5, 129.0, 128.9, 126.3, 116.1 ppm.
HRMS (ESI, m/z): calcd for C13H10NO3S [M+ H]+ 260.0381, found 260.0379.
GC-MS (m/z, %): 139 (64), 140 (21), 168 (42), 194 (74), 195 (53), 259 (100).
IR (KBr, cm−1), νmax = 1387 (S= O), 1174 (S= O).
8-(4-Fluorophenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5k)
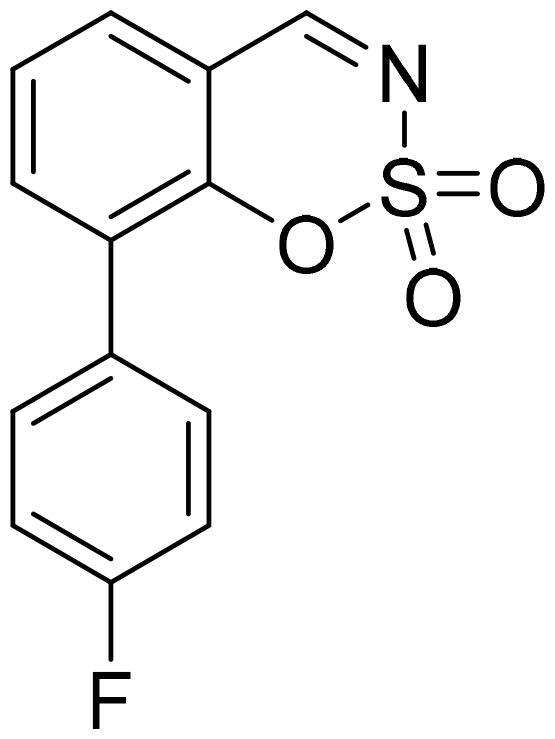
Compound 5k was obtained from 4′-fluoro-2-hydroxy-1,1′-biphenyl-3-carbaldehyde (4k) (0.20 g, 0.93 mmol) and sulfamoyl chloride (0.27 g, 2.31 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as white solid (0.14 g, 54%). Mp 123–124 °C.
1H NMR (400 MHz, DMSO-d6): δ 9.29 (1H, s), 8.04 (1H, dd, J= 1.6 Hz, J= 7.7 Hz), 7.98 (1H, dd, J= 1.6 Hz, J= 7.7 Hz), 7.65 (1H, t, J= 7.7 Hz), 7.57–7.63 (2H, m), 7.41 (2H, t, J= 8.9 Hz) ppm.
13C NMR (100 MHz, DMSO-d6): δ 171.3, 162.4 (d, J= 246.2 Hz), 149.7, 138.9, 131.8, 131.4 (d, J= 8.3 Hz), 129.8, 129.6 (d, J= 3.1 Hz), 126.7 ppm.
HRMS (ESI, m/z): calcd for C13H9NO3SF [M+ H]+ 278.0287, found 278.0294.
GC-MS (m/z, %): 157 (66), 158 (21), 184 (20), 186 (31), 213 (64), 277 (100).
IR (KBr, cm−1), νmax = 1383 (S= O), 1171 (S= O).
8-(4-Methoxyphenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5l)
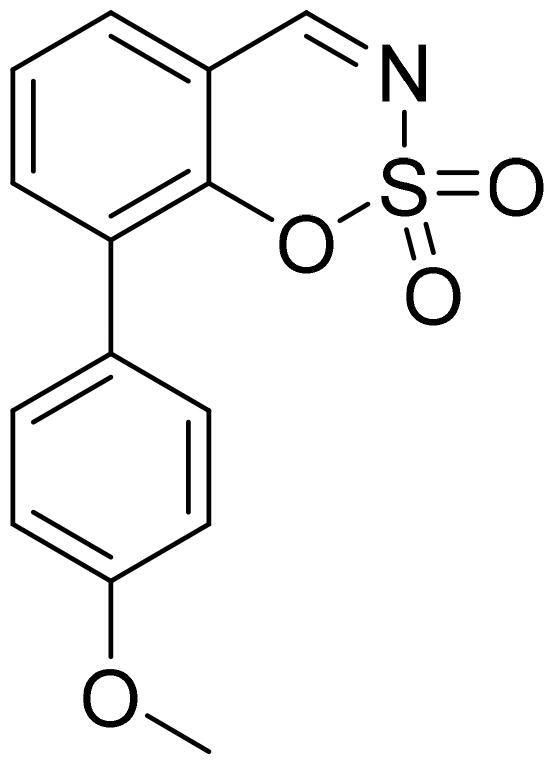
Compound 5l was obtained from 2-hydroxy-4′-methoxy-1,1′-biphenyl-3-carbaldehyde (4l) (0.20 g, 0.88 mmol) and sulfamoyl chloride (0.25 g, 2.19 mmol). The product was purified by column chromatography with PE/EtOAc (1:1). Obtained as yellow solid (0.11 g, 44%). Mp 133–134 °C.
1H NMR (400 MHz, CDCl3): δ 8.70 (1H, s), 7.77 (1H, dd, J= 1.7 Hz, J= 7.7 Hz), 7.61 (1H, dd, J= 1.7 Hz, J= 7.7 Hz), 7.44–7.51 (3H, m), 7.02 (2H, d, J= 9.0 Hz), 3.87 (3H, s) ppm.
13C NMR (100 MHz, CDCl3): δ 168.2, 160.3, 151.0, 138.5, 132.4, 130.7, 129.4, 126.2, 125.6, 116.2, 114.5, 55.5 ppm.
HRMS (ESI, m/z): calcd for C14H12NO4S [M+ H]+ 290.0487, found 290.0488.
GC-MS (m/z, %): 177 (49), 155 (21), 183 (23), 198 (23), 210 (39), 225 (31), 289 (100).
IR (KBr, cm−1), νmax = 1373 (S = O), 1370 (S = O), 1180 (S = O).
8-(3,4-Dichlorophenyl)-1,2,3-benzoxathiazine 2,2-dioxide (5m)
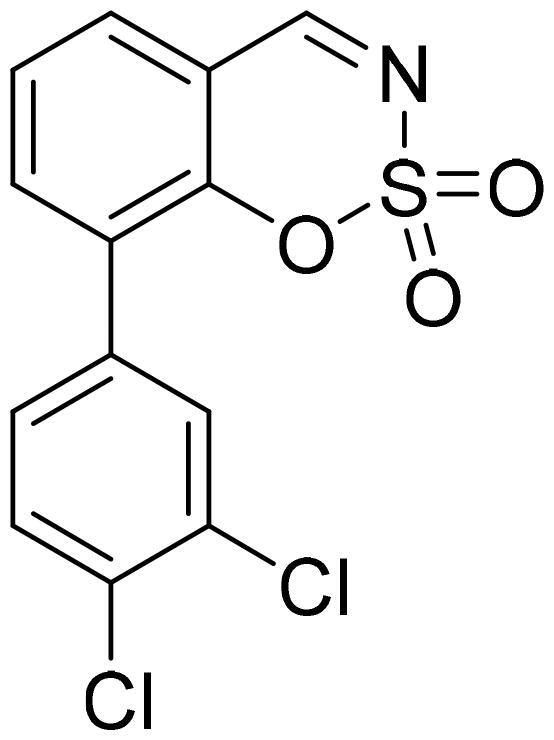
Compound 5m was obtained from 3′,4′-dichloro-2-hydroxy-1,1′-biphenyl-3-carbaldehyde (4m) (0.20 g, 0.75 mmol) and sulfamoyl chloride (0.22 g, 1.88 mmol). The product was purified by column chromatography with PE/EtOAc (1:2). Obtained as white solid (0.16 g, 65%). Mp 165–166 °C.
1H NMR (400 MHz, DMSO-d6): δ 9.31 (1H, s), 8.08 (1H, d, J= 1.6 Hz, J= 7.7 Hz), 8.04 (1H, d, J= 1.6 Hz, J= 7.7 Hz), 7.82–7.87 (2H, m), 7.67 (1H, t, J= 7.8 Hz), 7.55 (1H, dd, J= 2.1 Hz, J= 8.3 Hz) ppm.
13C NMR (100 MHz, DMSO-d6): δ 171.3, 149.7, 138.9, 133.7, 132.5, 131.8, 131.6, 131.0, 129.5, 128.2, 126.8, 115.8 ppm.
HRMS (ESI, m/z): calcd for C13H8NO3SCl2 [M+ H]+ 327.9602, found 327.9609.
IR (KBr, cm−1), νmax = 1393 (S = O), 1180 (S = O).
Ca inhibitory assay
An applied photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity.Citation32
Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5), and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10 − 100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5 − 10% of the reaction have been used for determining the initial rate. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled – deionised water, and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay in order to allow for the formation of the E – I complex. Data from were obtained after 6 h incubation of enzyme and inhibitor. The inhibition constants were obtained by nonlinear least-squares methods using PRISM 3 and the Cheng – Prusoff equation, as reported earlierCitation15,Citation33–37, and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation12,Citation18,Citation38–45.
Table 1. Inhibition data of human CA isoforms hCA I, II, IX, XII with compounds JI reported here and the standard inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.
Results and discussion
Chemistry
Benzo[1,2,3]oxathiazine-2,2-dioxides 2a–-k were obtained from corresponding 2-hydroxybenzaldehydes 1a–k in their reaction with sulfamoyl chloride that was prepared from chlorosulfonyl isocyanate (Scheme 1).Citation46
Scheme 1. Reagents and conditions: (i) HCOOH, 0 °C, 15 min, then RT, 45 min; (ii) ClSO2NH2, DMA, RT, 24–72 h.
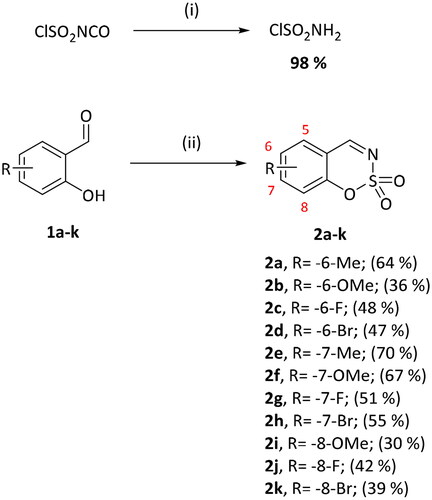
Eleven target compounds were obtained with moderate yields ().
5-, 7-, and 8-aryl substituted benzo[1,2,3]oxathiazine-2,2-dioxides 5a–m were obtained from aryl substituted 2-hydroxybenzaldehydes 4a–m (Scheme 2) prepared from 3-, 4-, or 6-bromo-2-hydroxybenzaldehydes 3. The first step was Suzuki-Miyaura coupling of aldehydes 3 with various boronic acids followed by cyclisation using sulfamoyl chloride. The yields of intermediates 4a–m were from moderate to high, but the yields of the products 5a–m were lower due to the loss during purification process. All inhibitors obtained exceeded 95% purity according HPLC analysis.
Carbonic anhydrase inhibition
Twenty four derivatives of 1,2,3-benzoxathiazine 2,2-dioxide were screened for the inhibition of four human CA isoforms – the cytosolic off-targets hCA I and II as well as transmembrane, tumour-associated hCA IX and XII that are anticancer drug targetsCitation18,Citation47–52.
Inhibition data of 1,2,3-benzoxathiazine 2,2-dioxides 2a–k and 5a–m (as well as acetazolamide AAZ as standard) against hCA I, II, IX, and XII after 15 min of incubation period of the enzyme and inhibitor solutions are presented in Citation32.
Data obtained show that some derivatives of 1,2,3-benzoxathiazine 2,2-dioxide (2 b, 2d, 5a–e) did not inhibit cytosolic isoform hCA I at all while other ones showed micromolar inhibitory activity in a quite wide range (0.36 − 46 µM).
hCA II, which is off-target in this case, was not inhibited only by two compounds, 5d and 5e. In case of 1,2,3-benzoxathiazine 2,2-dioxide derivatives 2a–k with small substituents like Me, MeO, Br, F, inhibition constants in nanomolar range (39 − 169 nM) were obtained, except compound 2d with Br in position 6 which has Ki = 8.3 µM. 5-Aryl substituted 1,2,3-benzoxathiazine 2,2-dioxide derivatives 5a–e poorly inhibited hCA II or did not inhibit it at all, whereas 7- and 8-aryl derivatives showed nanomolar inhibitory activity towards hCA II.
The transmembrane isoform hCA IX was effectively inhibited by 1,2,3-benzoxathiazine 2,2-dioxides 2a–k and 5f–m with Ki in the range of 22 − 74 nM, although 5-aryl substituted derivatives showed micromolar inhibitory activity (5a–c, Ki = 4.1 − 10.9 µM), but 5d-e did not show any inhibitory activity towards hCA IX.
Another transmembrane tumour-associated isoform hCA XII was also effectively inhibited by most of investigated compounds with Ki in the range of 5.7 − 186.4 nM. 5-Aryl derivatives 5a–d showed lower inhibitory activity while compound 5e did not exhibit any inhibitory properties towards hCA XII.
It may be seen that some of the new derivatives exhibit promising selectivity towards hCA IX/XII over hCA I, although none of the compounds are selective towards hCA IX/XII over both hCA I and II. The most promising tendence to selectivity towards hCA IX/XII over both hCA I and II is observed in case of compound 2d which has Br in position 6.
Conclusions
We report here a series of novel 1,2,3-benzoxathiazine-2,2-dioxide derivatives 2a–k with small substituents (Me, MeO, F, Br) in 6, 7, or 8 position prepared by straightforward synthesis from corresponding 2-hydroxybenzaldehydes in their reaction with sulfamoyl chloride. A series of 5-, 7-, or 8-aryl substituted 1,2,3-benzoxathiazine-2,2-dioxides 5a–m were obtained by two-step protocol from aryl substituted 2-hydroxybenzaldehydes in Suzuki-Miyaura reaction with selected boronic acids followed by cyclisation using sulfamoyl chloride.
The new derivatives were assayed as inhibitors of four hCA isoforms, the cytosolic hCA I and II, and the transmembrane, tumour-associated hCA IX and XII.
1,2,3-Benzoxathiazine-2,2-dioxides generally do not inhibit or show low inhibitory activity towards cytosolic widespread hCA I.
Ubiquitous hCA II was inhibited by the new derivatives in nanomolar and micromolar range whereas two 5-aryl derivatives did not show any hCA II inhibitory activity.
Transmembrane isoforms hCA IX and XII were inhibited in nanomolar range by most of 1,2,3-benzoxathiazine-2,2-dioxides although 5-aryl derivatives showed lower inhibitory activity or did not inhibit these two isoenzymes.
Some 1,2,3-benzoxathiazine-2,2-dioxides exhibit promising selectivity towards hCA IX/XII over hCA I, although none of the compounds are selective towards hCA IX/XII over both hCA I and II.
Supplemental Material
Download PDF (1.2 MB)Disclosure statement
No potential conflict of interest was reported by all author(s) except CTS. CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Elbadawi MM, Eldehna WM, Nocentini A, Somaa WR, Al-Rashood ST, Elkaeed EB, El Hassab MA, Abdel-Aziz HA, Supuran CT, Fares M, et al. Development of 4-((3-oxo-3-phenylpropyl)amino)benzenesulfonamide derivatives utilizing tail/dual-tail approaches as novel carbonic anhydrase inhibitors. Eur J Med Chem. 2022; 238:114412.
- Singh P, Sridhar Goud N, Swain B, Kumar Sahoo S, Choli A, Angeli A, Singh Kushwah B, Madhavi Yaddanapudi V, Supuran CT, Arifuddin M, et al. Synthesis of a new series of quinoline/pyridine indole-3-sulfonamide hybrids as selective carbonic anhydrase IX inhibitors. Bioorg Med Chem Lett. 2022; 70:128809.
- Siwach K, Kumar A, Panchal H, Kumar R, Bhardwaj JK, Angeli A, Supuran CT, Sharma PK. Selective inhibition of carbonic anhydrase IX by sulphonylated 1,2,3-triazole incorporated benzenesulphonamides capable of inducing apoptosis. J Enzyme Inhib Med Chem. 2022;37(1):1454–1463.
- Angeli A, Carta F, Nocentini A, Winum J-Y, Zalubovskis R, Akdemir A, Onnis V, Eldehna WM, Capasso C, Simone GD, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites. 2020;10(10):412.
- Nocentini A, Angeli A, Carta F, Winum J-Y, Zalubovskis R, Carradori S, Capasso C, Donald WA, Supuran CT. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem. 2021;36(1):561–580.
- Abdoli M, Giovannuzzi S, Supuran CT, Žalubovskis R. 4-(3-Alkyl/benzyl-uanidino)benzenesulfonamides as selective carbonic anhydrase VII inhibitors. J Enzyme Inhib Med Chem. 2022;37(1):1568–1576.
- Fares M, Eldehna WM, Bua S, Lanzi C, Lucarini L, Masini E, Peat TS, Abdel-Aziz HA, Nocentini A, Keller PA, et al. Discovery of potent dual-tailed benzenesulfonamide inhibitors of human carbonic anhydrases implicated in glaucoma and in vivo profiling of their intraocular pressure-lowering action. J Med Chem. 2020;63(6):3317–3326.
- Supuran CT, Alterio V, Di Fiore A, D' Ambrosio K, Carta F, Monti SM, De Simone G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev. 2018;38(6):1799–1836.
- Krasavin M, Sharonova T, Sharoyko V, Zhukovsky D, Kalinin S, Žalubovskis R, Tennikova T, Supuran CT. Combining carbonic anhydrase and thioredoxin reductase inhibitory motifs within a single molecule dramatically increases its cytotoxicity. J Enzyme Inhib Med Chem. 2020;35(1):665–671.
- Tawfik HO, Shaldam MA, Nocentini A, Salem R, Almahli H, Al-Rashood ST, Supuran CT, Eldehna WM. Novel 3-(6-methylpyridin-2-yl)coumarin-based chalcones as selective inhibitors of cancer-related carbonic anhydrases IX and XII endowed with antiproliferative activity. J Enzyme Inhib Med Chem. 2022;37(1):1043–1052.
- Alterio V, Tanc M, Ivanova J, Zalubovskis R, Vozny I, Monti SM, Di Fiore A, De Simone G, Supuran CT. X-ray crystallographic and kinetic investigations of 6-sulfamoyl-saccharin as a carbonic anhydrase inhibitor. Org Biomol Chem. 2015;13(13):4064–4069.
- Ivanova J, Carta F, Vullo D, Leitans J, Kazaks A, Tars K, Žalubovskis R, Supuran CT. N-Substituted and ring opened saccharin derivatives selectively inhibit transmembrane, tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem. 2017;25(13):3583–3589.
- Leitans J, Kazaks A, Balode A, Ivanova J, Zalubovskis R, Supuran CT, Tars K. Efficient expression and crystallization system of cancer-associated carbonic anhydrase isoform IX. J Med Chem. 2015;58(22):9004–9009.
- Ivanova J, Balode A, Žalubovskis R, Leitans J, Kazaks A, Vullo D, Tars K, Supuran CT. 5-Substituted-benzylsulfanyl-thiophene-2-sulfonamides with effective carbonic anhydrase inhibitory activity: solution and crystallographic investigations. Bioorg Med Chem. 2017;25(3):857–863.
- Pustenko A, Nocentini A, Gratteri P, Bonardi A, Vozny I, Žalubovskis R, Supuran CT. The antibiotic furagin and its derivatives are isoform-selective human carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2020;35(1):1011–1020.
- Wu Y, Xu J, Liu Y, Zeng Y, Wu G. A review on anti-tumor mechanisms of coumarins. Front Oncol. 2020;10:592853.
- Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: an up-to-date review. Bioorg Chem. 2020;103:104163.
- Maresca A, Temperini C, Vu H, Pham NB, Poulsen S-A, Scozzafava A, Quinn RJ, Supuran CT. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc. 2009;131(8):3057–3062.
- Žalubovskis R. In a search for selective inhibitors of carbonic anhydrases: coumarin and its bioisosteres – synthesis and derivatization. Chem Heterocycl Comp. 2015;51(7):607–612.
- Tars K, Vullo D, Kazaks A, Leitans J, Lends A, Grandane A, Zalubovskis R, Scozzafava A, Supuran CT. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem. 2013;56(1):293–300.
- Grandane A, Tanc M, Zalubovskis R, Supuran CT. Synthesis of 6-tetrazolyl-substituted sulfocoumarins acting as highly potent and selective inhibitors of the tumor-associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem. 2014;22(5):1522–1528.
- Grandane A, Tanc M, Zalubovskis R, Supuran CT. 6-Triazolyl-substituted sulfocoumarins are potent, selective inhibitors of the tumor-associated carbonic anhydrases IX and XII. Bioorg Med Chem Lett. 2014;24(5):1256–1260.
- Grandane A, Tanc M, Žalubovskis R, Supuran CT. Synthesis of 6-aryl-substituted sulfocoumarins and investigation of their carbonic anhydrase inhibitory action. Bioorg Med Chem. 2015;23(7):1430–1436.
- Grandane A, Tanc M, Di Cesare Mannelli L, et al. 6‑Substituted sulfocoumarins are selective carbonic anhdydrase IX and XII inhibitors with significant cytotoxicity against colorectal cancer cells. J Med Chem. 2015; 58:2975–2983.
- Grandāne A, Nocentini A, Domračeva I, Žalubovskis R, Supuran CT. Development of oxathiino[6,5-b]pyridine 2,2-dioxide derivatives as selective inhibitors of tumor-related carbonic anhydrases IX and XII. Eur J Med Chem. 2020; 200:112300.
- Krasavin M, Žalubovskis R, Grandāne A, Domračeva I, Zhmurov P, Supuran CT. Sulfocoumarins as dual inhibitors of human carbonic anhydrase isoforms IX/XII and of human thioredoxin reductase. J Enzyme Inhib Med Chem. 2020;35(1):506–510.
- Pustenko A, Stepanovs D, Žalubovskis R, Vullo D, Kazaks A, Leitans J, Tars K, Supuran CT. 3H-1,2-benzoxathiepine 2,2-dioxides: a new class of isoform-selective carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2017;32(1):767–775.
- Pustenko A, Nocentini A, Balašova A, Alafeefy A, Krasavin M, Žalubovskis R, Supuran CT. Aryl derivatives of 3H-1,2-benzoxathiepine 2,2-dioxide as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2020;35(1):245–254.
- Pustenko A, Nocentini A, Balašova A, Krasavin M, Žalubovskis R, Supuran CT. 7-Acylamino-3H-1,2-benzoxathiepine 2,2-dioxides as new isoform-selective carbonic anhydrase IX and XII inhibitors. J Enzyme Inhib Med Chem. 2020;35(1):650–656.
- Podolski-Renić A, Dinić J, Stanković T, Jovanović M, Ramović A, Pustenko A, Žalubovskis R, Pešić M. Sulfocoumarins, specific carbonic anhydrase IX and XII inhibitors, interact with cancer multidrug resistant phenotype through pH regulation and reverse P-glycoprotein mediated resistance. Eur J Pharm Sci. 2019; 138:105012.
- Grandane A, Nocentini A, Werner T, Zalubovskis R, Supuran CT. Benzoxepinones: a new isoform-selective class of tumor associated carbonic anhydrase inhibitors. Bioorg Med Chem. 2020;28(11):115496.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246(8):2561–2573.
- Nocentini A, Carta F, Tanc M, Selleri S, Supuran CT, Bazzicalupi C, Gratteri P. Deciphering the mechanism of human carbonic anhydrases inhibition with sulfocoumarins: computational and experimental studies. Chemistry. 2018;24(31):7840–7844.
- Angeli A, Carta F, Nocentini A, Winum JY, Zalubovskis R, Onnis V, Eldehna WM, Capasso C, Carradori S, Donald WA, et al. Response to perspectives on the classical enzyme carbonic anhydrase and the search for inhibitors. Biophys J. 2021;120(1):178–181.
- Bua S, Bozdag M, Del Prete S, Carta F, Donald WA, Capasso C, Supuran CT. Mono- and di-thiocarbamate inhibition studies of the δ-carbonic anhydrase TweCAδ from the marine diatom Thalassiosira weissflogii. J Enzyme Inhib Med Chem. 2018;33(1):707–713.
- Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1,3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem. 2018;33(1):1575–1580.
- Nocentini A, Bonardi A, Gratteri P, Cerra B, Gioiello A, Supuran CT. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J Enzyme Inhib Med Chem. 2018;33(1):1453–1459.
- Supuran CT. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem. 2018;33(1):485–495.
- Briganti F, Pierattelli R, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Part 37. Novel classes of carbonic anhydrase inhibitors and their interaction with the native and cobalt-substituted enzyme: kinetic and spectroscopic investigations. Eur J Med Chem. 1996;31(12):1001–1010.
- Pastorekova S, Casini A, Scozzafava A, Vullo D, Pastorek J, Supuran CT. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg Med Chem Lett. 2004;14(4):869–873.
- Carta F, Supuran CT, Scozzafava A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med Chem. 2014;6(10):1149–1165.
- Yıldırım A, Atmaca U, Keskin A, Topal M, Çelik M, Gülçin İ, Supuran CT. N-Acylsulfonamides strongly inhibit human carbonic anhydrase isoenzymes I and II. Bioorg Med Chem. 2015;23(10):2598–2605.
- Davis RA, Vullo D, Maresca A, Supuran CT, Poulsen S-A. Natural product coumarins that inhibit human carbonic anhydrases. Bioorg Med Chem. 2013;21(6):1539–1543.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181.
- Maresca A, Scozzafava A, Supuran CT. 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett. 2010;20(24):7255–7258.
- Yan Z, Wu B, Gao X, Chen M-W, Zhou Y-G. Enantioselective synthesis of α-amino phosphonates via Pd-catalyzed asymmetric hydrogenation. Org Lett. 2016;18(4):692–695.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777.
- Supuran CT. Carbonic anhydrase inhibitors in the treatment and prophylaxis of obesity. Expert Opin Ther Pat. 2003;13(10):1545–1550.
- Supuran CT. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs. 2012;17(1):11–15.
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2013;8(7):793–810.
- Nishimori I, Minakuchi T, Kohsaki T, Onishi S, Takeuchi H, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: The β-carbonic anhydrase from Helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. Bioorg Med Chem Lett. 2007;17(13):3585–3594.
- Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat. 2013;23(6):725–735.