Abstract
New thymol − 1,5-disubstitutedpyrazole hybrids were synthesised as dual COX-2/5-LOX inhibitors. Compounds 8b, 8g, 8c, and 4a displayed in vitro inhibitory activity against COX-2 (IC50 = 0.043, 0.045, 0.063, and 0.068 µM) nearly equal to celecoxib (IC50 = 0.045 µM) with high SI (316, 268, 204, and 151, respectively) comparable to celecoxib (327). All target compounds, 4a–c and 8a–i, showed in vitro 5-LOX inhibitory activity higher than reference quercetin. Besides, they possessed in vivo inhibition of formalin-induced paw oedema higher than celecoxib. In addition, compounds 4a, 4b, 8b, and 8g showed superior gastrointestinal safety profile (no ulceration) as celecoxib and diclofenac sodium in the population of fasted rats. In conclusion, compounds 4a, 8b, and 8g achieved the target goal. They elicited in vitro dual inhibition of COX-2/5-LOX higher than celecoxib and quercetin, in vivo potent anti-inflammatory activity higher than celecoxib and in vivo superior gastrointestinal safety profile (no ulceration) as celecoxib.
Introduction
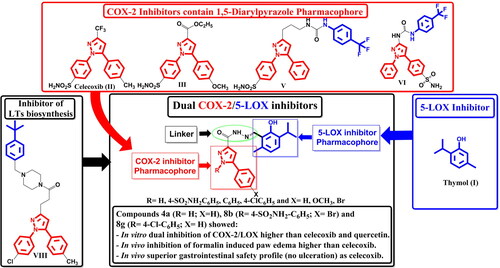
Inflammation includes variety of mechanisms and release of mediators which lead to drawbacks in the cardiovascular system and the renal apparatusCitation1. Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for the treatment of inflammation, pain, fever, and arthritis. NSAIDs decrease prostaglandins production via inhibition of cyclooxygenase (COX) enzymes. Classical NSAIDs inhibit both COX-1 and COX-2 enzymes which lead to anti-inflammatory activity as well as their side effectsCitation2–6. Accordingly, several selective COX-2 inhibitors such as celecoxib, valdecoxib, and rofecoxib have been developed and were approved for clinical use due to their low gastrointestinal side effects. However, their long-term use has been reported to cause cardiovascular side effects and was withdrawn from the marketCitation7–10. The inhibition of COX-1/COX-2 result in an increased formation of leukotrienes (LTs) through the lipoxygenase (LOX) pathwayCitation11. Overproduction of LTs induces asthmatic problems, gastric damage, and ulcerationCitation12–17. Moreover, 5-LOX has been related to several undesirable physiological effects which are involved in the progression of inflammation, osteoarthritis, and asthmaCitation18–20. Accordingly, dual inhibition of COX-2/5-LOX could provide anti-inflammatory effects with reduced side effects Citation21. Furthermore, the natural phenol derivative, thymol I, was proved to have COX-2 inhibition and anti-inflammatory activitiesCitation22–24. Moreover, thymol was found to abolish the activity of 5-LOX in human monocytic (THP-1) cell lineCitation25. On the other hand, several 1,5-diaryl pyrazoles were reported to have remarkable anti-inflammatory activity comparable to the lead celecoxib II. The anti-inflammatory activity of celecoxib remained in the 3-carboxylate derivative IIICitation26. Moreover, linking phenyl urea to pyrazole in compounds IV and V resulted in dual inhibition of COX-2 and Soluble Epoxide Hydrolase with favourable cardiovascular profile than celecoxibCitation27,Citation28. Furthermore, hybridisation of 1,5-diarylpyrazole with morpholine produced dual COX-2/5-LOX inhibitor with low toxicity, compound VICitation29. In addition, replacement of morpholine with its bioisostere piperazine and linking it to 4-tert-butylbenzyl moiety in compound VII showed potent inhibition of LT biosynthesis in activated human neutrophilsCitation30 (). Moreover, various N-acylhydrazone derivatives were reported to have analgesic, anti-inflammatory, and COX-2 inhibitory activitiesCitation31–34.
In the present investigation, the COX-2 inhibitor pharmacophore, 1,5-diarylpyrazole, was hybridised with thymol, a natural 5-LOX inhibitor, through N-acylhydrazone linker in order to simultaneously inhibit of the key inflammatory enzymes COX-2 and 5-LOX to get compounds having in vivo anti-inflammatory activity with low side effects and better safety profile ().
Experimental
Chemistry
Melting points were determined in open glass capillaries using a Griffin melting point apparatus or an electrothermal capillary tube melting point apparatus and are all uncorrected. Infra-red spectra (IR) were recorded, using KBr discs, by a Perkin-Elmer 1430 Infra-red spectrophotometer in the Central Laboratory, Faculty of Pharmacy, Alexandria University, and Schimadzu FT-IR Affinity-1 Spectrometer in Faculty of Pharmacy, Cairo University. Nuclear magnetic resonance (1H-NMR and 13C-NMR) were determined using Bruker High-Performance Digital FT-NMR Spectrometer Avance III 400 MHz, Faculty of Pharmacy, Cairo University in using deuterated dimethylsulphoxide as a solvent. The data were reported as chemical shifts or δ values (ppm) relative to tetramethylsilane (TMS) as internal standard. Signals are indicated by the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartette, and m = multiplet. Electron impact mass spectra (EIMS) were run on a gas chromatograph/mass spectrophotometer at Al-Azhar University (The Regional Centre of Microbiology and Biotechnology). Relative intensity % corresponding to the most characteristic fragments was recorded. Elemental microanalyses were performed at the microanalytical unit, Al-Azhar University (The Regional Centre of Microbiology and Biotechnology). Reaction progress was monitored by thin-layer chromatography (TLC) on silica gel sheets (60 GF254, Merck, Kenilworth, NJ 07033, USA). The spots were visualised by exposure to iodine vapour or UV-lamp at λ 254 nm for few seconds.
Compounds 1a–cCitation35,Citation36, 2a–cCitation37, 3Citation38–Citation41 5a–cCitation42–44, 6a–g,i,Citation26 and 7a–g,iCitation45 were prepared according to reported procedures.
General procedure for synthesis of N'-(2-Hydroxy-3-isopropyl-6-methylbenzylidene)-3-(substituted phenyl)-1H-pyrazole-5-carbohydrazides (4a–c)
A mixture of 2-formylthymol 3, appropriate hydrazides 2a–c, and few drops of glacial acetic acid in absolute ethanol was refluxed for 4–6 h, cooled to RT, the precipitate was filtered, washed with diethyl ether and recrystallised from ethanol.
N'-(2-Hydroxy-3-isopropyl-6-methylbenzylidene)-3-phenyl-1H-pyrazole-5-carbohydrazide; 4a
White crystals, yield 68%, m.p0.164–166 °C. IR (KBr, v cm−1): 3567.49 (OH); 3266.54 (NH); 1691.30(C=O); 1605.85, 1566.92 (C=N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.35(s, 3H, CH3 of 6-methylthymol); 3.25–3.28 (m, 1H, CH of isopropyl); 6.57(s, 1H, CH of C4 of pyrazole); 6.76(d, 1H, J= 7.72 Hz, C5 of thymol); 6.95–7.00(m, 3H, C3, C4, C5 of phenyl); 7.24(d, 1H, J= 7.72 Hz, C4 of thymol); 7.40 (d, 2H, J= 7.6 Hz, C2, C6 of phenyl); 9.27(br.s, 1H, CH of CH=N); 12.47(s, 1H, OH, D2O exchangeable); 12.60(s, 1H, NH of NH-N=, D2O exchangeable); 13.87(br.s, 1H of NH of pyrazole, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.26 (CH3 of 6-methylthymol); 22.86 (2CH3 of isopropyl); 26.61 (CH of isopropyl); 115.33 (C4 of pyrazole); 116.83 (C1 of thymol); 120.85 (C5 of thymol); 121.65 (C3 of thymol); 125.60 (C4 of thymol); 130.39 (C4 of phenyl); 132.94 (C3 and C5 of phenyl); 133.31 (C2 and C6 of phenyl); 133.79 (C1 of phenyl); 138.35 (C6 of thymol); 141.75 (C3 of pyrazole); 155.25 (C5 of pyrazole); 157.91 (CH of CH = N); 163.67 (C2 of thymol); 164.91 (C = O); EIMS, m/z (relative abundance %): 363.01(M+.+1) (8.3), 362.17 (M+.) (3.04), 187.06 (100.00); 171.28 (74.13); Analysis Calcd for C21H22N4O2 (362.17): C, 69.59; H, 6.12; N, 15.46. Found: C, 69.26; H, 5.88; N, 15.09.
3-(4-Bromophenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1H-pyrazole-5-carbohydrazide; 4b
Camel crystals, yield 75%, m.p0.260–262 °C. IR (KBr, v cm−1): 3444.15 (OH); 3228.94 (NH); 1666.98(C = O); 1614.04, 1599.59 (C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.84 Hz, 2CH3 of isopropyl); 2.38(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.71(d, 1H, J= 7.60 Hz, C5 of thymol); 7.13(d, 1H, J= 7.60 Hz, C4 of thymol); 7.32(s, 1H, CH of C4 of pyrazole); 7.70(d, 2H, J= 7.68 Hz, C3, C5 of bromophenyl); 7.82(d, 2H, J= 7.68 Hz, C2, C6 of bromophenyl); 9.06(br.s, 1H, CH of CH=N); 12.17(s, 1H, OH, D2O exchangeable); 12.61(s, 1H, NH of NH-N=, D2O exchangeable); 13.93(br.s, 1H of NH of pyrazole, D2O exchangeable); Analysis Calcd for C21H21BrN4O2 (441.33): C, 57.15; H, 4.80; N, 12.70. Found: C, 56.71; H, 4.64; N, 12.85.
N'-(2-Hydroxy-3-isopropyl-6-methylbenzylidene)-3–(4-methoxy phenyl)-1H-pyrazole-5-carbohydrazide; 4c
Linen crystals, yield 65%, m.p. 246–248 °C. IR (KBr, v cm−1): 3421.72(OH); 3245.12(NH); 1668.28(C = O); 1609.13, 1600.14(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J = 6.88 Hz, 2CH3 of isopropyl); 2.37(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 3.82(s, 3H, CH3 of OCH3); 6.70(d, 1H, J= 7.60 Hz, C5 of thymol); 7.06(d, 2H, J= 8.40 Hz, C3, C5 of p-methoxyphenyl); 7.11–7.15(m, 2H, C4 of thymol, CH of C4 of pyrazole); 7.80(d, 2H, J= 8.40 Hz, C2, C6 of p-methoxyphenyl); 9.07(br.s, 1H, CH of CH=N); 12.11(s, 1H, OH, D2O exchangeable); 12.64(s, 1H, NH of NH-N=, D2O exchangeable); 13.70(s, 1H of NH of pyrazole, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.12(CH3 of 6-methylthymol); 22.78(2CH3 of isopropyl); 26.49(CH of isopropyl); 55.74(CH3 of OCH3); 103.14(C4 of pyrazole); 114.94(2 C, C3, C5 of p-methoxyphenyl); 115.80(C1 of thymol); 121.23(C5 of thymol); 121.61(C1 of p-methoxyphenyl); 127.43(2 C, C2, C6 of p-methoxyphenyl); 128.23(C4 of thymol); 133.69(C3 of thymol); 136.01(C6 of thymol); 144.30(C3 of pyrazole); 146.53(C5 of pyrazole); 149.01(CH of CH = N); 156.42(C2 of thymol); 158.28(C = O); 160.01(C4 of p-methoxyphenyl); EIMS, m/z (relative abundance %): 393 (M+.+1) (13.58); 392 (M+.) (43.87); 217 (100.00); 216 (51.88); 201 (73.85); 200 (66.89); Analysis Calcd for C22H24N4O3 (392.46): C, 67.33; H, 6.16; N, 14.28. Found: C, 66.98; H, 6.45; N, 14.57.
General procedure for synthesis of Methyl 5-(4-substitutedphenyl)-1-(4-substitutedphenyl)-1H-pyrazole-3-carboxylates (6a–i)
A mixture of sodium salt of diketone 1a–c (1 mol) and substituted phenylhydrazine hydrochloride 5a–c (1 mol) in glacial acetic acid (30 ml) was refluxed for 24 h, then cooled to RT and poured on crushed ice, filtered, washed with water, air-dried and then washed with diethyl ether and recrystallised from methanol.
Methyl 5-(4-bromophenyl)-1-(4-chlorophenyl)-1H-pyrazole-3-carboxylate; 6h
Pale pink crystals, yield 80%, m.p0.96–98 °C. IR (KBr, v cm−1): 1749.15(C = O); 1587.70(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 3.87(s, 3H, CH3 of OCH3); 7.20(s, 1H, CH of C4 of pyrazole); 7.33(d, 2H, J= 8.84 Hz, C3, C5 of chlorophenyl); 7.53–7.56(m, 4H, C2, C6 of chlorophenyl, C3, C5 of bromophenyl); 7.74(d, 2H, J= 8.36 Hz, C2, C6 of bromophenyl); EIMS, m/z (relative abundance %): 394 (M+.+2) (28.07); 393 (M+.+1) (11.87); 392(M+.) (70.52); 391 (9.85); 129(21.55); 127 (91.87); 126 (17.15); 75(37.57); 74(34.46); 73(26.25); 55(52.76); 57(59.94); 43(100); 42(32.85); 41(30.96); Analysis Calcd for C17H12BrClN2O2 (391.65): C, 52.14; H, 3.09; N, 7.15. Found: C, 51.98; H, 3.34; N, 7.28.
General procedure for synthesis of 5-(4-Substitutedphenyl)-1-(4-substitutedphenyl)-1H-pyrazole-3-carbohydrazides (7a–i)
To methyl pyrazole-3-carboxylate 6a–i (1 mol) in ethanol (20–25 ml), hydrazine hydrate (5 mol) was added and the reaction mixture was refluxed for 7–9 h, then the solvent was evaporated under reduced pressure, then the precipitate was washed with cold water, then with ether, air-dried and recrystallised from ethanol.
5-(4-Bromophenyl)-1–(4-chlorophenyl)-1H-pyrazole-3-carbohydrazide; 7h
White crystals, yield 74%, m.p0.86–88 °C. IR (KBr, v cm−1): 3375.87, 3250.12(NH, NH2); 1755.55(C = O); 1571.75(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 4.49(s, 2H, NH2, D2O exchangeable); 7.01 (s, 1H, CH of C4 of pyrazole); 7.26–7.28(m, 2H, C3, C5 of chlorophenyl); 7.35(d, 2H, J= 8.76 Hz, C3, C5 of bromophenyl); 7.38–7.40(m, 2H, C2, C6 of chlorophenyl); 7.52(d, 2H, J= 8.76 Hz, C2, C6 of bromophenyl); 9.61(s, 1H, NH, D2O exchange- able); EIMS, m/z (relative abundance %): 394 (M+.+2) (19.90); 393 (M+.+1) (78.76); 392(M+.) (16.23); 391 (60.63); 363(27.69); 362(13.58); 361(100); 360(14.11); 359(41.32); 75(66.24); 111(50.79); Analysis Calcd for C16H12BrClN4O (391.65): C, 49.07; H, 3.09; N, 14.31. Found: C, 49.38; H, 3.23; N, 14.50.
General procedure for synthesis of 5–(4-Substitutedphenyl)-1-(4-substitutedphenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1H-pyrazole-3-carbohydrazides (8a–i)
Equimolar amount of 2-formylthymol 3 and appropriate hydrazide 7a–i in absolute ethanol and few drops of glacial acetic acid were refluxed for 4–6 h, cooled to RT, the precipitate was filtered, washed with diethyl ether, and recrystallised from ethanol.
4-(3-(2–(2-Hydroxy-3-isopropyl-6-methylbenzylidene)hydrazine-1-carbonyl)-5-phenyl-1H-pyrazol-1-yl)benzenesulfonamide; 8a
White crystals, yield 79%, m.p0.220–222 °C. IR (KBr, v cm−1): 3568.31 (OH); 3272.15 (NH); 1662.64(C = O); 1597.06(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.80 Hz, 2CH3 of isopropyl); 2.37(s, 3H, CH3 of 6-methylthymol); 3.1–3.2(m, 1H, CH of isopropyl); 6.71(d, 1H, J= 7.68 Hz, C5 of thymol); 7.14(d, 1H, J= 7.68 Hz, C4 of thymol); 7.24(s, 1H, CH of C4 of pyrazole); 7.35–7.36(m, 2H, C2, C6 of phenyl); 7.42–7.43(m, 3H, C3, C4, C5 of phenyl); 7.52(s, 2H of NH2 of sulfamoyl, D2O exchangeable); 7.61(d, 2H, J= 8.36 Hz, C3, C5 of sulfamoylphenyl); 7.61(d, 2H, J= 8.36 Hz, C2, C6 of sulfamoylphenyl); 9.07(s, 1H, CH of CH=N); 12.25(s, 1H, OH, D2O exchangeable); 12.58(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.13(CH3 of 6-methylthymol); 22.77(2CH3 of isopropyl); 26.50(CH of isopropyl); 109.42(C4 of pyrazole); 115.69(C1 of thymol); 121.30(C5 of thymol); 125.45(2C, C2, C6 of sulfamoylphenyl); 127.21(2C, C3, C5 of sulfamoylphenyl); 128.46(C4 of thymol); 129.22(2C, C3, C5 of phenyl); 129.26(C4 of phenyl); 129.32(2C, C2, C6 of phenyl);129.62(C1 of phenyl); 133.75(C3 of thymol); 136.16(C6 of thymol); 141.97(C4 of sulfamoylphenyl); 144.35(C3 of pyrazole); 145.40(C1 of sulfamoylphenyl); 146.54(C5 of pyrazole); 149.68(CH of CH = N); 156.49(C2 of thymol); 157.43(C = O); EIMS, m/z (relative abundance %): 518 (M+.+1) (14.15); 517 (M+.) (36.90); 326 (88.11); 160 (100.00); 77 (65.57); Analysis Calcd for C27H27N5O4S (517.60): C, 62.65; H, 5.26; N, 13.53; S, 6.19. Found: C, 62.84; H, 5.38; N, 13.79; S, 6.27.
4-(5-(4-Bromophenyl)-3-(2-(2-hydroxy-3-isopropyl-6-methylbenzylidene)hydrazine-1-carbonyl)-1H-pyrazol-1-yl)benzene sulphonamide; 8b
White crystals, yield 80%, m.p0.253–255 °C. IR (KBr, v cm−1): 3545.16(OH); 3263.56(NH); 1689.64(C = O); 1593.20(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.84 Hz, 2CH3 of isopropyl); 2.37(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.71(d, 1H, J= 7.68 Hz, C5 of thymol); 7.14(d, 1H, J= 7.68 Hz, C4 of thymol); 7.28–7.31(m, 3H, CH of C4 of pyrazole, C3, C5 of bromophenyl); 7.52(s, 2H of NH2 of sulfamoyl, D2O exchangeable); 7.62–7.65(m, 4H, C2, C6 of bromophenyl C3, C5 of sulfamoylphenyl); 7.93(d, 2H, J= 8.48 Hz, C2, C6 of sulfamoylphenyl); 9.06(s, 1H, CH of CH=N); 12.26(s, 1H, OH, D2O exchangeable); 12.57(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.13(CH3 of 6-methylthymol); 22.78(2CH3 of isopropyl); 26.50(CH of isopropyl); 109.71(C4 of pyrazole); 115.67(C1 of thymol); 121.31(C5 of thymol); 123.21(C4 of bromophenyl); 126.63(2C, C2, C6 of sulfamoylphenyl); 127.31(2C, C3, C5 of sulfamoylphenyl); 128.43(C1 of bromophenyl); 128.49(C4 of thymol); 131.31(2C, C2, C6 of bromophenyl); 132.30(2C, C3, C5 of bromophenyl); 133.75(C3 of thymol); 136.17(C6 of thymol); 141.73(C4 of sulfamoylphenyl); 144.25(C3 of pyrazole); 144.44(C1 of sulfamoylphenyl); 146.59(C5 of pyrazole); 149.74(CH of CH=N); 156.48(C2 of thymol); 157.33(C = O); EIMS, m/z (relative abundance %): 599 (M+.+2)(6.71); 598 (M+.+1) (4.23); 597 (M+.) (16.77); 423 (68.60); 421 (64.12); 420 (100.00); 406 (77.05); 404 (72.95); Analysis Calcd for C27H26 BrN5O4S (596.50): C, 54.37; H, 4.39; N, 11.74; S, 5.37. Found: C, 54.66; H, 4.60; N, 11.96; S, 5.51.
4-(3-(2-(2-Hydroxy-3-isopropyl-6-methylbenzylidene)hydrazine-1-carbonyl)-5-(4-methoxyphenyl)-1H-pyrazol-1-yl)benzenesulfonamide; 8c
Pale white crystals, yield 77%, m.p. 245–247 °C. IR (KBr, v cm−1): 3567.41 (OH); 3266.82 (NH); 1689.64 (C = O); 1597.06(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.12(d, 6H, J= 6.76 Hz, 2CH3 of isopropyl); 2.37(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 3.78(s, 3H, CH3 of OCH3); 6.71(d, 1H, J= 7.72 Hz, C5 of thymol); 6.98(d, 2H, J= 8.72 Hz, C3, C5 of p-methoxyphenyl); 7.14(d, 1H, J= 7.72 Hz, C4 of thymol); 7.15(s, 1H, CH of C4 of pyrazole); 7.28(d, 2H, J= 8.72 Hz, C2, C6 of p-methoxyphenyl); 7.52(s, 2H of NH2 of sulfamoyl, D2O exchangeable); 7.61(d, 2H, J= 8.52 Hz, C3, C5 of sulfamoylphenyl); 7.91(d, 2H, J= 8.52 Hz, C2, C6 of sulfamoylphenyl); 9.06(s, 1H, CH of CH=N); 12.22(s, 1H, OH, D2O exchangeable); 12.58(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.13(CH3 of 5-methyl thymol); 22.78(2CH3 of isopropyl); 26.50(CH of isopropyl); 55.73(CH3 of OCH3); 108.88(C4 of pyrazole); 114.78(2C, C3, C5 of p-methoxyphenyl); 115.70(C1 of thymol); 121.30(C5 of thymol); 121.39 (C1 of p-methoxyphenyl); 126.55(2C, C2, C6 of sulfamoyl phenyl); 127.20(2C, C3, C5 of sulfamoylphenyl); 128.45(C4 of thymol); 130.67(2C, C2, C6 of p-methoxyphenyl); 133.75(C3 of thymol); 136.15(C6 of thymol); 142.11 (C4 of sulfamoylphenyl); 144.23(C3 of pyrazole); 145.30(C1 of sulfamoyl phenyl); 146.43 (C5 of pyrazole); 149.62(CH of CH = N); 156.47(C2 of thymol); 157.49(C = O); 160.25(C4 of p-methoxyphenyl); EIMS, m/z (relative abundance %): 549 (M+.+1) (38.26); 548 (M+.) (63.25); 521 (72.07); 404 (78.65); 241 (70.30); 163 (100.00); 135 (63.36); Analysis Calcd for C28H29N5O5S (547.63): C, 61.41; H, 5.34; N, 12.79; S, 5.85. Found: C, 61.30; H, 5.56; N, 13.06; S, 5.97.
N'-(2-Hydroxy-3-isopropyl-6-methylbenzylidene)-1,5-diphenyl-1H-pyrazole-3-carbo-hydrazide; 8d
White crystals, yield 75%, m.p. 232–235 °C. IR (KBr, v cm−1): 3421.72 (OH); 3270.97 (NH); 1651.07(C = O); 1597.06(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.36(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.70(d, 1H, J= 7.72 Hz, C5 of thymol); 7.13(d, 1H, J= 7.72 Hz, C4 of thymol); 7.21(s, 1H, CH of C4 of pyrazole); 7.31–7.32(m, 2H, C2, C6 of phenyl); 7.37–7.38(m, 3H, C3, C4, C5 of phenyl); 7.41–7.43(m, 2H, C2, C6 of N-phenyl); 7.48–7.50(m, 3H, C3, C4, C5 of N-phenyl); 9.08(s, 1H, CH of CH=N); 12.22(s, 1H, OH, D2O exchangeable); 12.60(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.11(CH3 of 6-methylthymol); 22.78(2CH3 of isopropyl); 26.50(CH of isopropyl); 108.73(C4 of pyrazole); 115.73(C1 of thymol); 121.27(C5 of thymol); 126.48(2C, C2, C6 of N-phenyl); 128.39(C4 of thymol); 129.09(2C, C2, C6 of phenyl); 129.13(3C, C3, C5 of phenyl, C4 of N-phenyl); 129.29(C4 of phenyl); 129.46(C1 phenyl); 129.72(2C, C3, C5 of N-phenyl); 133.72(C3 of thymol); 136.12(C6 of thymol); 139.71(C1 of N-phenyl); 145.16(C3 of pyrazole); 145.91(C5 of pyrazole); 149.56(CH of CH = N); 156.46(C2 of thymol); 157.62(C = O); EIMS, m/z (relative abundance %): 439 (M+.+1) (20.10); 438 (M+.) (45.95); 247 (100.00); 245 (33.97); Analysis Calcd for C27H26N4O2 (438.53): C, 73.95; H, 5.98; N, 12.78. Found: C, 74.23; H, 5.87; N, 13.14.
5-(4-Bromophenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1-phenyl-1H-pyrazole-3-carbohydrazide; 8e
White crystals, yield 82%, m.p. 0.209–211 °C. IR (KBr, v cm−1): 3545.16(OH); 3278.69(NH); 1651.07(C = O); 1597.06(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.20(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.36(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.71(d, 1H, J= 7.72 Hz, C5 of thymol); 7.14(d, 1H, J= 7.72 Hz, C4 of thymol) ; 7.23(s, 1H, CH of C4 of pyrazole); 7.27(d, 2H, J= 8.00 Hz, C3, C5 of p-bromophenyl); 7.43–7.45(m, 2H, C2, C6 of N-phenyl); 7.51–7.53(m, 3H, C3, C4, C5 of N-phenyl); 7.59(d, 2H, J= 8.00 Hz, C2, C6 of p-bromophenyl); 9.07(s, 1H, CH of CH=N); 12.24(s, 1H, OH, D2O exchangeable); 12.59(s, 1H, NH of NH-N=, D2O exchangeable); EIMS, m/z (relative abundance %): 519 (M+.+2)(41.16); 518 (M+.+1) (62.23); 517 (M+.) (19.71); 516 (67.32); 341 (82.53); 327 (100.00); 325 (70.56); 44 (88.69); Analysis Calcd for C27H25BrN4O2 (517.43): C, 62.67; H, 4.87; N, 10.83. Found: C, 62.50; H, 4.98; N, 11.15.
5-(4-Methoxyphenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1-phenyl-1H-pyrazole-3-carbohydrazide; 8f
White crystals, yield 75%, m.p. 0.227–229 °C. IR (KBr, v cm−1): 3448.72 (OH); 3279.99(NH); 1685.79(C = O); 1597.06(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.35(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 3.76(s, 3H, CH3 of OCH3); 6.70(d, 1H, J= 7.72 Hz, C5 of thymol); 6.91(d, 2H, J= 8.60 Hz, C3, C5 of p-methoxyphenyl); 7.12–7.14(m, 2H, C4 of thymol, CH of C4 of pyrazole) ; 7.23(d, 2H, J= 8.60 Hz, C2, C6 of p-methoxyphenyl); 7.41–7.43(m, 2H, C2, C6 of N-phenyl); 7.48–7.50(m, 3H, C3, C4, C5 of N-phenyl); 9.07(s, 1H, CH of CH=N); 12.19(s, 1H, OH, D2O exchangeable); 12.61(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.10(CH3 of 6-methylthymol); 22.78(2CH3 of isopropyl); 26.50(CH of isopropyl); 55.66(CH3 of OCH3); 108.14(C4 of pyrazole); 114.58(2C, C3, C5 of p-methoxyphenyl); 115.72(C1 of thymol); 121.26(C5 of thymol); 121.68(C1 of p-methoxyphenyl); 126.47(2C, C2, C6 of N-phenyl); 128.36(C4 of thymol); 129.20(C4 of N-phenyl); 129.71(2C, C3, C5 of N-phenyl); 130.47(2C, C2, C6 of p-methoxyphenyl); 133.72(C3 of thymol); 136.10(C6 of thymol); 139.84(C1 of N-phenyl); 145.06(C3 of pyrazole); 145.81(C5 of pyrazole); 149.49(CH of CH = N); 156.46(C2 of thymol); 157.69(C = O); 160.01(C4 of p-methoxyphenyl); EIMS, m/z (relative abundance %): 469 (M+.+1) (39.25); 468 (M+.) (75.20); 467(19.50); 293(100.00); 277 (95.75); 276 (37.40).
Analysis Calcd for C28H28N4O3 (468.56): C, 71.78; H, 6.02; N, 11.96. Found: C, 72.11; H, 6.24; N, 11.87.
1-(4-Chlorophenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide; 8g
White crystals, yield 86%, m.p. 0.245–247 °C. IR (KBr, v cm−1): 3545.15 (OH); 3266.25(NH); 1738.54 (C = O); 1586.19 1570.32(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.82 Hz, 2CH3 of isopropyl); 2.35(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.72(d, 1H, J= 7.72 Hz, C5 of thymol); 7.14 (d, 1H, J= 7.72 Hz, C4 of thymol); 7.21(s, 1H, CH of C4 of pyrazole); 7.32–7.34(m, 2H, C3, C5 of phenyl); 7.40–7.42(m, 3H, C2, C4, C6 of phenyl); 7.46 (d, 2H, J= 8.62 Hz, C3, C5 of chlorophenyl); 7.57 (d, 2H, J= 8.62 Hz, C2, C6 of chlorophenyl); 9.08(s, 1H, CH of CH=N); 12.24(s, 1H, OH, D2O exchangeable); 12.58(s, 1H, NH of NH-N=, D2O exchangeable); Analysis Calcd for C26H23ClN4O2 (458.95): C, 68.04; H, 5.05; N, 12.21. Found: C, 68.19; H, 5.27; N, 12.09.
5-(4-Bromophenyl)-1-(4-chlorophenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1H-pyrazole-3-carbohydrazide; 8h
White crystals, yield 70%, m.p. 0.255–257 °C. IR (KBr, v cm−1): 3448.72 (OH); 3277.99(NH); 1744.95 (C = O); 1599.59, 1572.07(C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.36(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 6.71(d, 1H, J= 7.72 Hz, C5 of thymol); 7.13(d, 1H, J= 7.72 Hz, C4 of thymol); 7.25(s, 1H, CH of C4 of pyrazole); 7.28 (d, 2H, J= 8.3 Hz, C3, C5 of chlorophenyl); 7.47(d, 2H, J= 8.5 Hz, C3, C5 of bromophenyl); 7.58–7.64(m, 4H, C2, C6 of chlorophenyl, C2, C6 of bromophenyl); 9.07(s, 1H, CH of CH=N); 12.25(s, 1H, OH, D2O exchangeable); 12.58(s, 1H, NH of NH-N=, D2O exchangeable); EIMS, m/z (relative abundance %): 555 (M+.+4) (11.5); 553 (M+.+2)(30.84); 552 (M+.+1)(36.91); 551 (M+.) (100.00); 548 (58.43); 375 (62.53); Analysis Calcd for C27H24BrClN4O2 (551.87): C, 58.76; H, 4.38; N, 10.15. Found: C, 59.02; H, 4.51; N, 10.38.
5-(4-Methoxyphenyl)-1-(4-chlorophenyl)-N'-(2-hydroxy-3-isopropyl-6-methylbenzylidene)-1H-pyrazole-3-carbohydrazide; 8i
White crystals, yield 75%, m.p. 218–220 °C. IR (KBr, v cm−1): 3417.86 (OH); 3259.69 (NH); 1717.40(C = O); 1604.84, 1594.60 (C = N); 1H-NMR (400 MHz, DMSO-d6δ ppm): 1.19(d, 6H, J= 6.88 Hz, 2CH3 of isopropyl); 2.36(s, 3H, CH3 of 6-methylthymol); 3.27–3.31(m, 1H, CH of isopropyl); 3.77(s, 3H, CH3 of OCH3); 6.70(d, 1H, J= 7.72 Hz, C5 of thymol); 6.96(d, 2H, J= 8.60 Hz, C3, C5 of p-methoxyphenyl); 7.12–7.14(m, 2H, C4 of thymol, CH of C4 of pyrazole); 7.24(d, 2H, J= 8.60 Hz, C2, C6 of p-methoxyphenyl); 7.45(d, 2H, J= 8.60 Hz, C3, C5 of chlorophenyl); 7.57(d, 2H, J= 8.60 Hz, C2, C6 of chlorophenyl); 9.07(s, 1H, CH of CH=N); 12.21(s, 1H, OH, D2O exchangeable); 12.60(s, 1H, NH of NH-N=, D2O exchangeable); 13C-NMR (100 MHz, DMSO-d6δ ppm): 19.12(CH3 of 6-methylthymol); 22.77(2CH3 of isopropyl); 26.50(CH of isopropyl); 55.68(CH3 of OCH3); 108.45(C4 of pyrazole); 114.69(2C, C3, C5 of p-methoxyphenyl); 115.72(C1 of thymol); 121.27(C5 of thymol); 121.44(C1 of p-methoxyphenyl); 128.07(2C, C2, C6 of chlorophenyl); 128.39(C4 of thymol); 129.73(2C, C2, C6 of p-methoxyphenyl); 130.58(2C, C3, C5 of chlorophenyl); 133.55(C4 of chlorophenyl); 133.73(C3 of thymol); 136.12(C6 of thymol); 138.62(C1 of chlorophenyl); 145.19(C3 of pyrazole); 146.11(C5 of pyrazole); 149.56(CH of CH = N); 156.47(C2 of thymol); 157.57(C = O); 160.13(C4 of p-methoxyphenyl); EIMS, m/z (relative abundance %): 503 (M+.+1) (40.05); 502 (M+.) (100.00); 327 (77.20); 311 (37.85); Analysis Calcd for C28H27 ClN4O3 (503.00): C, 66.86; H, 5.41; N, 11.14. Found: C, 67.14; H, 5.56; N, 11.41.
Biological screening
In vitro COX-1 and COX-2 inhibitory assay
Compounds 4a–c and 8a–i were screened for their ability to inhibit COX-1 and COX-2 enzymes in vitro. This was carried out using Cayman colorimetric COX (ovine) inhibitor screening assay kit (Catalog No. 560131) supplied by Cayman chemicals, Ann Arbour, MI according to reported methodCitation46 (Page: S20, Supplementary file).
In vitro 5-LOX inhibitory assay
The newly synthesised compounds were screened for their ability to inhibit 5-LOX enzymes. This was carried out using Abnova 5-LOX inhibitor screening assay kit (Catalog No. 760700) according to reported methodCitation47 (Page: S21, Supplementary file).
In vivo anti-inflammatory activity
Formalin-induced paw oedema
Compounds that showed in vitro selectivity indices higher or nearly equivalent to reference drugs towards COX 2 enzyme, were further evaluated for their in vivo anti-inflammatory activity applying the formalin-induced paw oedema screening protocol as an acute inflammation model using celecoxib and Diclofenac sodium as reference drugs according to reported proceduresCitation48,Citation49 (Approved by AlexU-IACUC)Citation50 (Page: S22–23, Supplementary file).
Gastric ulcerogenic activity
Compounds were evaluated for acute gastric ulcerogenic effect in adult female Wistar rats. Gross examination was performed for any evidence of hyperaemia, haemorrhage, definite haemorrhagic erosion, or ulcer according to reported proceduresCitation49,Citation51 (Approved by AlexU-IACUC)Citation50 (Page: S24, Supplementary file).
Molecular modelling
The molecular modelling studies were performed using the Molecular Operating Environment (MOE 2016.08) software (Chemical Computing Group, Montreal, Canada).Citation52 and the crystal structures of the proteins were downloaded from the Protein Data Bank (PDB) website (Page: S24, Supplementary file).
Results and discussion
Chemistry
Scheme 1 illustrated the synthesis of the target thymol–1,5-disubstitutedpyrazole hybrids. The dioxobutanoate derivativesCitation53 1a–c were cyclised using reaction conditions used in Knorr pyrazole synthesisCitation54 and celecoxib synthesisCitation55 by reaction with either hydrazine hydrate or substituted phenylhydrazine hydrochloride in glacial acetic acid. Hydrazine hydrate resulted in both cyclisation and formation of the hydrazide derivatives 2a–c. On the other hand, substituted phenylhydrazine hydrochlorides produced only the cyclised methyl esters 6a–i which were further reacted with hydrazine hydrate to produce the corresponding hydrazides 7a–i. The hydrazides 2a–c and 7a–i were condensed with 2-formylthymolCitation38–41 3 to yield the target thymol-1,5-disubstitutedpyrazole hybrids 4a–c and 8a–i, respectively. IR spectra of compounds 4a–c showed absence of bands assigned to CHO and NH2 and presence of absorption bands assigned to C = N, as well as to OH. 1H-NMR spectra of compounds 4a–c were characterised by the absence of signals assigned to NH2 protons at their previously recorded positions, and presence of 2D2O exchangeable singlets of (NH) functional groups one for (CO-NH-N=) and the other for pyrazole at (12.61–12.64) ppm, (13.70–13.93) ppm, respectively. 13C-NMR spectrum of compounds 4a–c showed the presence of signals of (C = O) and (C3, C4, C5 of pyrazole) at expected chemical shifts. In addition, MS for 4c showed the molecular ion peak (M+.) at m/z 392. On the other hand, IR spectra of compounds 8a–i were characterised by the absence of absorption bands assigned to (NH2 of pyrazole) and (CHO) group and presence of absorption bands assigned to (NH), (C = N) and (C = O) groups as well as (OH) of thymol at their expected absorption regions. 1H-NMR spectra of compounds 8a–i were characterised by the absence of signals assigned to NH2 protons at their previously recorded positions, and presence of (CH) proton of C4 of pyrazole at (7.15–7.24) ppm as singlet signal, as well as D2O exchangeable singlet of NH proton at 12.57–12.60 ppm. 13C-NMR spectra of compounds 8a–i showed the presence of signals of (C3, C4, C5 of pyrazoles) and (C = N of thiazolidinone) at expected chemical shifts. Besides, MS of compounds 8a-i showed the molecular ion peak (M+.), for 8a at m/z 517, at 597 for 8b, at 548 for 8c, at 438 for 8d, at 517 for 8e, at 468 for 8f, at 551 for 8h, and at 502 for 8i (Scheme 1).
Biological screening
In vitro COX-1 and COX-2 inhibitory assay
Compounds 4a–c and 8a–i were screened for their in vitro inhibitory activity against COX-1 and COX-2 enzymes using Cayman colorimetric COX (ovine) inhibitor screening assay kit (Catalog No. 560131) supplied by Cayman chemicals, Ann Arbour, MICitation46. The half-maximal inhibitor concentrations (IC50µM) were determined and the selectivity index (SI) values were calculated as IC50 (COX-1)/IC50 (COX-2) and recorded in . All compounds showed high activity, in nanomolar range, against COX-2 and high SI to COX-2. Compounds 8b, 8g, 8c, and 4a displayed nearly equal activity to celecoxib with high SI (316, 268, 204, and 151, respectively) ().
Table 1. In vitro COX-1, COX-2, and 5-LOX enzyme inhibitory activities, aIC50 values, and bselectivity indices (SI) of the tested compounds.
In vitro 5-LOX inhibitory assay
Compounds 4a–c and 8a–i were screened for their in vitro ability to inhibit 5-LOX enzymes. This was carried out using Abnova 5-LOX inhibitor screening assay kit (Catalog No. 760700)Citation47. All compounds showed potent 5-LOX inhibitory activities higher than the reference quercetin. Consequently, compounds 8b, 8c, 8g, and 4a showed dual inhibitory activities against COX-2 and 5-LOX higher than the references celecoxib and quercetin ().
In vivo anti-inflammatory activity
Formalin-induced paw oedema test (acute inflammation model)
Compounds 4a–c and 8a–i were tested for their in vivo anti-inflammatory activity using formalin-induced paw oedema test (Acute inflammation model) (, ). All compounds showed higher % inhibition than celecoxib except 8b which revealed nearly equal activity to celecoxib. Compounds 4a and 8i exhibited double % inhibition exhibited by Celecoxib. All compounds showed higher % inhibition than that of Diclofenac sodium except 8a, 8b, 8f, and 8h which elicited slightly less % inhibition than that of Diclofenac sodium. Compound 4a was the most potent with % inhibition 81.93% comparing with 36.37% and 52.37% of celecoxib and diclofenac sodium, respectively.
Table 2. In vivo anti-inflammatory activities of selected compounds in formalin-induced rat paw oedema bioassay (acute inflammation model).
In vivo gastric ulcerogenic activity
Compounds 4a–c and 8a–i were evaluated for their ulcerogenic potential in rats. Gross examination revealed that compounds 4a, 4b, 8b, and 8g showed superior gastrointestinal safety profile (no ulceration) as the references celecoxib and diclofenac sodium in the population of fasted rats. On the other hand, compounds 4c, 8a, 8c–f, 8h, and 8i showed variable degrees of hyperaemia ().
Structure–activity relationship
The in vitro inhibition of COX-1/2 and 5-LOX assays and the in vivo anti-inflammatory testing showed that, substitution at para position of phenyl at position 5 of pyrazole by either electron donating group (OCH3) or electron withdrawing group (Br) or unsubstituted did not affect the activity. This could be due to the presence of the phenyl ring out of coplanarity with the pyrazole moiety so could not affect the electronic configuration of the whole molecule hence binding affinity and activity. On the other hand, substitution at N1 of pyrazole affected the activity. The unsubstituted derivative 4a showed in vitro COX-2/5-LOX inhibitory activity and in vivo potent anti-inflammatory activity. Furthermore, the activities of N1 aryl substituted derivatives were affected with the type of substitution at para position of N1 phenyl moiety. The substitution with either sulphonamide group or chloro showed higher in vitro COX-2/5-LOX inhibitory activity and in vivo anti-inflammatory activity than unsubstituted derivatives. This could be due to the importance of sulphonamide and chloro groups in polar and hydrophobic interactions with the active sites of the target COX-2/5-LOX enzymes.
Molecular modelling
MOE 2016.08 softwareCitation52 was used for performing molecular modelling studies for the most active compounds 4a, 8b, and 8g. These compounds were docked into the active site of COX-2 (PDB entry 3LN1) and 5-LOX (PDB entry 3V99). The results were illustrated in and and .
Figure 7. Mode of binding (2D) of celecoxib (A), 4a (B), 8b (C), and 8g (D) inside the active site of COX-2.

Figure 9. Overlay of compounds 4a (green), 8b (yellow), 8g (pink), and celecoxib (cyan) inside the active site of COX-2.

Figure 10. Mode of binding (3D) of celecoxib (A), 4a (B), 8b (C), and 8g (D) into COX-2 active site.

Figure 11. Mode of binding (2D) of Arachidonic acid (A), 4a (B), 8b (C), and 8g (D) inside the active site of 5-LOX.

Figure 12. Overlay of compounds 4a (green), 8b (yellow), 8g (pink), and arachidonic acid (cyan) inside the active site of 5-LOX.

Figure 13. Mode of binding (3D) of arachidonic acid (A), 4a (B), 8b (C), and 8g (D) inside the active site of 5-LOX.

Table 3. Docking results of the active compounds in COX-2 active site.
Table 4. Docking results of the active compounds in 5-LOX active site.
Docking to COX-2 active site
Compounds 4a, 8b, and 8g which showed dual in vitro COX-2/5-LOX inhibiting activity and the reference celecoxib were docked into COX-2 active site (pdb entry 3LN1)Citation56 using MOE version 2016.0802 softwareCitation52. The docking solutions of the compounds 4a, 8b, 8g, and celecoxib ( and ) confirmed the potential activities against COX-2 as the mode of interactions of the best poses were comparable to the reference celecoxib. All the target compounds interacted with hydrogen bond with the key amino acid Leu338. In addition, they showed potential polar interaction with the key amino acids Arg499 and Ser339Citation57 in the polar side pocket of COX-2 active site. Interaction with this small pocket’s amino acids is essential for the selective inhibition of the enzymeCitation57. Moreover, overlay of compounds 4a, 8b, 8g, and celecoxib inside the active site of COX-2 () revealed that, the pyrazole moiety of 4a and 1-arylpyrazole moiety of 8b and 8g were superposed on 1-arylpyrazole moiety of celecoxib with p-substituents interacted in the same position of trifluromethyl of celecoxib. Besides, the carbonyl group interacted in the same position of sulphonamide group of celecoxib.
Docking to 5-LOX active site
Compounds 4a, 8b, and 8g which showed dual in vitro COX-2/5-LOX inhibiting activity and the co-crystallised ligand arachidonic acid were docked into 5-LOX active site (pdb entry 3V99)Citation58 using MOE version 2016.0802 softwareCitation52. The docking solutions of the compounds 4a, 8b, 8g, and arachidonic acid ( and ) supported the potential activities against 5-LOX . It was reported that, 5-LOX active site comprises several anchors. Polar positively-charged anchor consists of His550, His367 and His372 which interact with Fe2+. Polar anchor form both electrostatic and hydrogen-bonding interactions comprises Asp176, Asn180 and Gln363. Hydrophobic anchor contains Phe177, Leu607, Ile673, Leu414, Phe421, Gly174, Val175, Leu368, and Ile406Citation59. Compounds 4a, 8b, and 8g interacted with various anchors in 5-LOX active site. All of them showed polar interaction with His367 beside additional interaction with His372 for 4a and His550 for 8b and 8g. Besides, all compounds elicited polar interaction with Gln363 in addition to hydrogen bond formed between N1-pyrazole of 4a and Asp176 as part of the second polar anchor. Furthermore, 4a, 8b, and 8g interacted by hydrophobic anchor with Phe177 and Leu607 in addition to hydrophobic interaction with Leu368 for 8b and 8g and Ile406 for 4a. It is worth mentioning that, arachidonic acid did not form hydrogen bond with 5-LOX active site, while the target compounds formed hydrogen bond and coordinate bond interactions with 5-LOX active site so the target compounds could have favourable binding affinity towards 5-LOX more than arachidonic acid.
Conclusion
Selective COX-2 inhibitors have many benefits in treatment of inflammation, but this selective inhibition resulted in accumulation of arachidonic acid at 5-LOX site which leads to overproduction of LTs which in turn induced asthmatic problems, gastric damage, and ulceration. New thymol − 1,5-disubstitutedpyrazole hybrids were synthesised as dual COX-2/5-LOX inhibitors to get safer anti-inflammatory therapy. Compounds 8b, 8g, 8c, and 4a displayed in vitro inhibitory activity against COX-2 nearly equal to celecoxib with high SI comparable to celecoxib. All compounds, 4a–c and 8a–i, showed 5-LOX inhibitory activity higher than reference quercetin. Furthermore, all compounds, 4a–c and 8a–i, showed in vivo inhibition of formalin induced paw oedema higher than celecoxib. Compound 4a was the most potent with inhibition percentage higher than celecoxib and diclofenac sodium. In addition, compounds 4a, 4b, 8b, and 8g showed superior gastrointestinal safety profile (no ulceration) as the references celecoxib and diclofenac sodium in the population of fasted rats. In silico docking studies in the COX-2 and 5-LOX active sites predicted that, the target compounds could have strong binding affinity to the target enzymes active sites in comparison with the reference celecoxib and arachidonic acid, respectively. In conclusion, compounds 4a, 8b, and 8g achieved the target goal. They elicited in vitro dual inhibition of COX-2/5-LOX higher than celecoxib and quercetin, in vivo potent anti-inflammatory activity higher than celecoxib and in vivo superior gastrointestinal safety profile (no ulceration) as celecoxib.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supplemental Material
Download PDF (3.1 MB)Acknowledgement
The author thank Alnukhba University College for their support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-lox and cox, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62(6):501–509.
- Yoshimura H, Sekine S, Adachi H, Uematsu Y, Mitani A, Futaki N, Shimizu N. High levels of human recombinant cyclooxygenase-1 expression in mammalian cells using a novel gene amplification method. Protein Expr Purif. 2011;80(1):41–46.
- Yao B, Xu J, Harris RC, Zhang MZ. Renal localization and regulation of 15-hydroxyprostaglandin dehydrogenase. Am J Physiol Ren Physiol. 2008;294(2):433–439.
- Haruna H, Shimizu T, Ohtsuka Y, Yarita Y, Fujii T, Kudo T, Yamashiro Y. Expression of cox-1, cox-2, and ppar-γ in the gastric mucosa of children with helicobacter pylori infection. Pediatr Int. 2008;50(1):1–6.
- Yoshida S, Ujiki M, Ding X-Z, Pelham C, Talamonti MS, Bell RH, Denham W, Adrian TE. Pancreatic stellate cells (pscs) express cyclooxygenase-2 (cox-2) and pancreatic cancer stimulates cox-2 in pscs. Mol Cancer. 2005;4:27–27.
- Abdelall EKA, Kamel GM. Synthesis of new thiazolo-celecoxib analogues as dual cyclooxygenase-2/15-lipoxygenase inhibitors: determination of regio-specific different pyrazole cyclization by 2d nmr. Eur J Med Chem. 2016;118(:250–258.
- Garcia-Rodriguez LA, Hernández-Diaz S. Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology. 2003;14:240–246.
- Ruan CH, So SP, Ruan KH. Inducible cox-2 dominates over cox-1 in prostacyclin biosynthesis: mechanisms of cox-2 inhibitor risk to heart disease. Life Sci. 2011;88(1–2):24–30.
- Khan M, Fraser A. Cox-2 inhibitors and the risk of cardiovascular thrombotic events. Ir Med J. 2012;105(4):119–121.
- Singh P, Prasher P, Dhillon P, Bhatti R. Indole based peptidomimetics as anti-inflammatory and anti-hyperalgesic agents: dual inhibition of 5-lox and cox-2 enzymes. Eur J Med Chem. 2015;97:104–123.
- Tries S, Neupert W, Laufer S. The mechanism of action of the new antiinflammatory compound ml3000: Inhibition of 5-lox and cox-1/2. Inflamm Res. 2002;51(3):135–143.
- Rainsford K. The effects of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by nonsteroidal antiinflammatory drugs in mice. Agents Actions. 1987;21(3–4):316–319.
- Rainsford K. Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions. 1993;39(S1):C24–C26.
- Laufer S. Discovery and development of ml3000. Inflammopharmacology. 2001;9(1–2):101–112.
- Hudson N, Balsitis M, Everitt S, Hawkey C. Enhanced gastric mucosal leukotriene b4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993;34(6):742–747.
- Burnett BP, Levy RM. 5-lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv Ther. 2012;29(2):79–98.
- Sala A, Zarini S, Bolla M. Leukotrienes: lipid bioeffectors of inflammatory reactions. Biochem NY Eng Trans Biokhimiya. 1998;63(1):84–92.
- Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (cox-2) and 5-lipoxygenase (5-lox) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38(7–8):645–659.
- Young RN. Inhibitors of 5-lipoxygenase: a therapeutic potential yet to be fully realized? Eur J Med Chem. 1999;34(9):671–685.
- Lamie PF, Ali WAM, Bazgier V, Rárová L. Novel n-substituted indole schiff bases as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase enzymes: synthesis, biological activities in vitro and docking study. Eur J Med Chem. 2016;123:803–813.
- Alvaro-Gracia JM. Licofelone—clinical update on a novel lox/cox inhibitor for the treatment of osteoarthritis. Rheumatology. 2004;43(90001):21i–25.
- Botelho MA, Barros G, Queiroz DB, Carvalho CF, Gouvea J, Patrus L, Bannet M, Patrus D, Rego A, Silva I, et al. Nanotechnology in phytotherapy: antiinflammatory effect of a nanostructured thymol gel from lippia sidoides in acute periodontitis in rats. Phytother Res. 2016;30(1):152–159.
- Marsik P, Kokoska L, Landa P, Nepovim A, Soudek P, Vanek T. In vitro inhibitory effects of thymol and quinones of nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin e2 biosyntheses. Planta Med. 2005;71(8):739–742.
- Meeran N, Fizur M, Javed H, Al Taee H, Azimullah S, Ojha SK. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol. 2017;8:380.
- Tsai ML, Lin CC, Lin WC, Yang CH. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci Biotechnol Biochem. 2011;75(10):1977–1983.
- Menozzi G, Merello L, Fossa P, Mosti L, Piana A, Mattioli F. 4-substituted 1, 5-diarylpyrazole, analogues of celecoxib: synthesis and preliminary evaluation of biological properties. Farmaco. 2003;58(9):795–808.
- Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD. Synthesis and structure − activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011;54(8):3037–3050.
- Abdelazeem AH, Safi El-Din AG, Abdel-Fattah MM, Amin NH, El-Moghazy SM, El-Saadi MT. Discovery of novel urea-diarylpyrazole hybrids as dual cox-2/seh inhibitors with improved anti-inflammatory activity and highly reduced cardiovascular risks. Eur J Med Chem. 2020;205:112662.
- Li Z, Wang ZC, Li X, Abbas M, Wu SY, Ren SZ, Liu QX, Liu Y, Chen PW, Duan YT, et al. Design, synthesis and evaluation of novel diaryl-1,5-diazoles derivatives bearing morpholine as potent dual cox-2/5-lox inhibitors and antitumor agents. Eur J Med Chem. 2019;169:168–184.
- Çalışkan B, Luderer S, Özkan Y, Werz O, Banoglu E. Pyrazol-3-propanoic acid derivatives as novel inhibitors of leukotriene biosynthesis in human neutrophils. Eur J Med Chem. 2011;46(10):5021–5033.
- Gorantla V, Gundla R, Jadav SS, Anugu SR, Chimakurthy J, Nidasanametla SK, Korupolu R. Molecular hybrid design, synthesis and biological evaluation of n-phenyl sulfonamide linked n-acyl hydrazone derivatives functioning as cox-2 inhibitors: new anti-inflammatory, anti-oxidant and anti-bacterial agents. New J Chem. 2017;41(22):13516–13532.
- Cordeiro NDM, Freitas RHCN, Fraga CAM, Fernandes PD. New 2-amino-pyridinyl-n-acylhydrazones: synthesis and identification of their mechanism of anti-inflammatory action. Biomed Pharmacother. 2020;123:109739.
- Ju Z, Su M, Hong J, La Kim E, Moon HR, Chung HY, Kim S, Jung JH. Design of balanced cox inhibitors based on anti-inflammatory and/or cox-2 inhibitory ascidian metabolites. Eur J Med Chem. 2019;180:86–98.
- Hernández P, Cabrera M, Lavaggi ML, Celano L, Tiscornia I, Rodrigues da Costa T, Thomson L, Bollati-Fogolín M, Miranda ALP, Lima LM, et al. Discovery of new orally effective analgesic and anti-inflammatory hybrid furoxanyl n-acylhydrazone derivatives. Bioorg Med Chem. 2012;20(6):2158–2171.
- Drysdale MJ, Hind SL, Jansen M, Reinhard JF. Synthesis and sar of 4-aryl-2-hydroxy-4-oxobut-2-enoic acids and esters and 2-amino-4-aryl-4-oxobut-2-enoic acids and esters: potent inhibitors of kynurenine-3-hydroxylase as potential neuroprotective agents. J Med Chem. 2000;43(1):123–127.
- Sheverdov V, Nasakin O, Andreev AY, Gein V, Tafeenko V. Synthesis of methyl 3-acyl-6-amino-5-cyano-4-phenyl-4h-pyran-2-carboxylates and their rearrangement into 2-hydroxy-4-[hydroxy (r) methylidene]-3-oxo-5-phenylcyclopent-1-ene-1-carbonitriles. Russ J Org Chem. 2011;47(7):1117–1118.
- Dias LRS, Salvador RRS. Pyrazole carbohydrazide derivatives of pharmaceutical interest. Pharmaceuticals (Basel). 2012;5(3):317–324. (
- Rajput J, Bagul S, Tadavi S, Karandikar P, Bendre R. Design, synthesis and biological evaluation of novel class diindolyl methanes (dims) derived from naturally occurring phenolic monoterpenoids. Med Chem. 2016;6(2):123–128.
- Duff JC. A new general method for the preparation of o-hydroxyaldehydes from phenols and hexamethylenetetramine. J Chem Soc. 1941;547–550.
- Casiraghi G, Casnati G, Cornia M, Pochini A, Puglia G, Sartori G, Ungaro R. Selective reactions using metal phenoxides. Part 1. Reactions with formaldehyde. J Chem Soc Perkin Trans 1. 1978;9(4):318–321.
- Casnati G, Casiraghi G, Puglia G, Sartori G, Terenghi G. Process for preparing 2-hydroxybenzoic aldehydes. Process for preparing 2-hydroxybenzoic aldehydes. Google Patents. 1979.
- Soliman R. Preparation and antidiabetic activity of some sulfonylurea derivatives of 3, 5-disubstituted pyrazoles. J Med Chem. 1979;22(3):321–325.
- Coleman GH. Phenylhydrazine. Org Synth Coll. 1941;1:432–435.
- Karczmarzyk Z, Mojzych M, Rykowski A. Synthesis and structure of p-chlorophenylhydrazone of 3-(methylthio)-5-propanoyl-1, 2, 4-triazine. J Chem Crystallogr. 2000;30(6):423–427.
- Kumar V, Kaur K, Gupta GK, Sharma AK. Pyrazole containing natural products: synthetic preview and biological significance. Eur J Med Chem. 2013;69(:735–753.
- Cayman colorimetric COX (ovine) inhibitor screening assay kit (Catalog No. 560131) supplied by Cayman chemicals, 1180 E. Ann Arbor, MI, USA.
- Lipoxygenase inhibitor screening assay kit (Catalog No. 760700) supplied by Cayman chemicals, 1180 E. Ann Arbor, MI, USA.
- Razmi A, Zarghi A, Arfaee S, Naderi N, Faizi M. Evaluation of anti-nociceptive and anti-inflammatory activities of novel chalcone derivatives. Iran J Pharm Res. 2013;12:153–159.
- Lakshmi V, Mishra V, Palit G. A new gastroprotective effect of limonoid compounds xyloccensins x and y from xylocarpus molluccensis in rats. Nat Prod Bioprospect. 2014;4(5):277–283.
- Hazzaa AA-E. Synthesis and biological evaluation of novel terpene derivatives. AlexU-IACUC (Member of ICLAS) 2019:AU-06-2019-9-30-2-58.
- Srivastava S, Nath C, Gupta M, Vrat S, Sinha J, Dhawan K, Gupta G. Protection against gastric ulcer by verapamil. Pharmacol Res. 1991;23(1):81–86.
- Molecular operating environment (moe) 2016.08, chemical computing group inc. 1010 Sherbrooke St. West suite #910, Montreal, QC, Canada, h3a2r7. www.Chemcomp.Com.
- Rafinejad A, Fallah Tafti A, Tiwari R, Shirazi AN, Mandal D, Parang K, Foroumadi A, Akbarzadeh T. Synthesis and evaluation of ethyl 2,4-dioxo-4-arylbutanoate derivatives as src kinase inhibitors. J Sci Islamic Republic Iran. 2015;26:321–325.
- Yoon JY, Lee SG, Shin H. Recent advances in the regioselective synthesis of pyrazoles. Curr Org Chem. 2011;15(5):657–674.
- Soliman WM, Abdellatif KRA, Knaus EE. Design, synthesis, biological evaluation, and nitric-oxide release studies of a novel series of celecoxib prodrugs possessing a nitric-oxide donor moiety. Braz J Pharm Sci. 2018;54(4):1–10.
- Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JRB, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett. 2010; 20(23):7159–7163.
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648.
- Gilbert NC, Rui Z, Neau DB, Waight MT, Bartlett SG, Boeglin WE, Brash AR, Newcomer ME. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at serine-663. FASEB J. 2012;26(8):3222–3229.
- Hsu KC, HuangFu WC, Lin TE, Chao MW, Sung TY, Chen YY, Pan SL, Lee JC, Tzou SC, Sun CM, et al. A site-moiety map and virtual screening approach for discovery of novel 5-lox inhibitors. Sci Rep. 2020;10(1):10510.