Abstract
Monoterpenoid indole alkaloids (MIAs) represent a major class of active ingredients from the plants of the genus Gelsemium. Gelsemium MIAs with diverse chemical structures can be divided into six categories: gelsedine-, gelsemine-, humantenine-, koumine-, sarpagine- and yohimbane-type. Additionally, gelsemium MIAs exert a wide range of bioactivities, including anti-tumour, immunosuppression, anti-anxiety, analgesia, and so on. Owing to their fascinating structures and potent pharmaceutical properties, these gelsemium MIAs arouse significant organic chemists’ interest to design state-of-the-art synthetic strategies for their total synthesis. In this review, we comprehensively summarised recently reported novel gelsemium MIAs, potential pharmacological activities of some active molecules, and total synthetic strategies covering the period from 2013 to 2022. It is expected that this study may open the window to timely illuminate and guide further study and development of gelsemium MIAs and their derivatives in clinical practice.
Introduction
Derivatives of monoterpenoid indole alkaloids (MIAs) represent a class of active secondary metabolites mostly isolated from the plants of the Gelsemium genus (Loganiaceae), mainly in three known Gelsemium species, including G. sempervirens, G. elegans and G. rankiniiCitation1. These plants are mainly distributed in southern China, Asia, and North America, and have been broadly used as a folk herbal medicine for clinical treatment for hundreds of yearsCitation2. MIAs are particularly concentrated in the roots of Gelsemium plants, accounting for the content of approximately 0.5%, whereas the stems, fruits, branches, and leaves also contain smaller amountsCitation3. Since the discovery of the first MIA in 1959, more than 100 kinds of representative MIAs with complex structures have been widely extracted from these Gelsemium plantsCitation4. The majority of gelsemium MIAs have been found to exhibit a plethora of notable pharmacological properties, especially anti-tumour, immunosuppressive, anxiolytic, and analgesic characteristics, reflecting their great potential as lead compounds in new drug developmentCitation5–8.
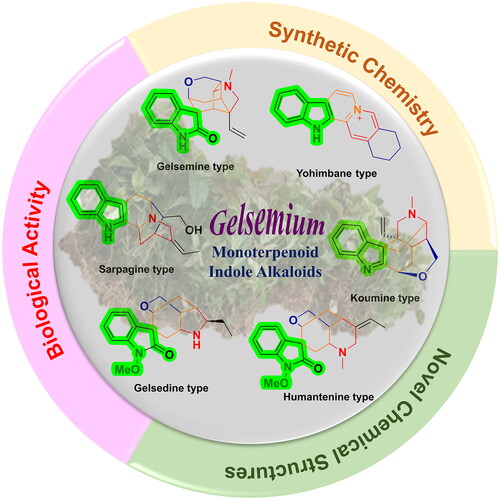
In terms of structure type, gelsemium MIAs possess characteristic chemical structures containing polycyclic monoterpene portions and indole, oxindole, or bisindole nucleiCitation9. Gelsemium MIAs can be classified into six categories: gelsedine-, gelsemine-, humantenine-, koumine-, sarpagine- and yohimbane-type, on the basis of their structural featuresCitation10 (. Among them, gelsemine-type, humantenine-type and gelsedine-type alkaloids bear peculiar spiro-indolinone nuclei, while koumine-, sarpagine- and yohimbane-type alkaloids have normal indole groups. The structural skeletons of their monoterpene parts incorporate sterically compact and dense polycyclic architectures and multiple stereocenters, forming privileged chemical diversity and structural complexity of gelsemium MIAs. These exceptional structural properties of gelsemium MIAs render them sophisticated challenges for total synthesis and structure modification and attracted considerable attention from synthetic scientists. Historically, a vast number of total synthetic works on gelsemium MIAs have been reportedCitation11–13.
Figure 1. Six representative members of gelsemium MIAs. Gelsemium MIAs can be classified into six categories: gelsedine-, koumine-, gelsemine-, humantenine-, sarpagine-, and yohimbane-type. Gelsemine-type, humantenine-type and gelsedine-type alkaloids bear peculiar spiro-indolinone nuclei, while koumine-, sarpagine- and yohimbane-type alkaloids have normal indole groups.

Figure 2. The chemical structures of novel gelsedine-type alkaloids.
Jin’s group (2014) previously reviewed the phytochemistry, pharmacology, and toxicology together with their traditional use of the genus Gelsemium, whereas Carter’s group (2019) described the synthetic strategies towards the gelsemine- and gelsedine-type MIAs between 2005 and 2016Citation14,Citation15. However, these reviews did not provide a comprehensive review of all types of gelsemium MIAs, especially in aspects of their frontier pharmacological effects as well as chemical structures and syntheses. A large number of breakthroughs in their novel compounds, biological activities, and more elegant total syntheses have been reported over the past decade. As such, this review is intended to comprehensively summarise the representative examples covering from 2013 to 2022 with the following novel objectives: (1) a comprehensive presentation of novel gelsemium MIAs and more elegant total synthetic methodologies; (2) a focus on recent their bioactivities of gelsemium MIAs, mainly involving specific biotarget and mechanism of actions; (3) an overview of advice how gelsemium MIAs can be utilised as promising candidates in further studies. Finally, we hope that this review will provide an insight into rational study and development of gelsemium MIAs and their derivatives in further clinical practice.
Novel chemical structures of gelsemium MIAs
Since 2013, a total of 70 novel MIAs have been isolated from the Gelsemium genus, mainly G. elegans. The structural types of gelsemium MIAs are mainly focussed on gelsedine-type, humantenine-type, and koumine-type. These gelsemium MIAs groups will be discussed in the following paragraphs ().
Table 1. Summary of novel MIAs from the genus of Gelsemium.
Gelsedine-type alkaloids
Gelsedine-type alkaloids occupy the largest portion of the gelsemium MIAs, whose intricate frameworks share a characteristic spiro-N-methoxyindoleone chromophore, an oxabicyclo[3.2.2]nonane ring system, and a versatile functionalised pyrrolidine ring inserted in their complex cage-like skeletons. The main differences among individual gelsedine-type members are different substituents at C11, 14, 15, and 20. Gelseleganin D (7), 19-hydroxygelselegine (8), and 14-hydroxygelselegine (9), belonging to classical gelsedine-type skeleton containing a methylol group at C20, were isolated from the different parts of G. elegansCitation16–18. 14β-Hydroxygelselenidine (10), gelseleganins A (11), and B (12) were obtained from the aerial parts of G. elegans. These compounds represented a rare class of MIAs carrying a 2,3-epoxybutane moiety at C20Citation17,Citation19. Gelsedethenine (13) and 11-methoxygelseziridine (14) were isolated from the roots and aerial parts of G. elegans, respectively, which structurally featured a particular trans-butenyl group at C20Citation19,Citation20. Gelsekoumidines A (15) and B (16) were two pairs of atropoisomeric bisindole alkaloids from the roots of G. elegans. Gelsekoumidines A and B represented an unprecedented class of seco-koumine-gelsedine-type alkaloids containing a unique 20,21-seco-koumine skeleton fused with a gelsedine scaffold via a double bond bridge. The only difference between these two compounds was that gelsekoumidine A had a hydroxyl group at C14Citation21. 14β-Hydroxygelsedethenine (17) and 14-hydroxygelseziridine (18) were obtained from the aerial parts of G. elegans. Structural comparison with gelsedine (1) displayed that the two compounds had an α-configuration of the 1,2-oxaziridine group located between C20 and N4Citation19,Citation22. Gelseleganin E (19) and 11-methoxy-14-hydroxygelsedilam (20) were isolated from the leaves and branches of G. elegans, which possessed a particular lactam ringCitation17,Citation23. Gelseleganin C (21) and gelsepyrrodines A (22), B (23), and C (24) were isolated from the leaves, branches, and roots of G. elegans, in which a pyrrole ring was incorporated into their gelsedine-type skeleton. The main difference was the replacement of an additional aldehyde in the pyrrole ring in the former two by an acetyl group in the latter twoCitation17,Citation24. Gelsecorydines A (25), B (26), C (27), D (28), and E (29), five bisindole alkaloids with novel chemical skeleton, were obtained from the fruits of G. elegans. Their heterodimeric framework incorporated a gelsedine-type alkaloid and a modified corynanthe-type monomer. Especially, gelsecorydine B possessed an unprecedented caged structure with a 6/5/7/6/5/6 heterohexacyclic ring system via a direct pyridine ring linkageCitation25. Gelserancines A (30), B (31), C (32), D (33), and E (34), five unusual gelsedine-type derivatives, were separated from the roots of G. elegans. The structure of gelserancine A incorporated a rare trimethyl-dihydrofuranone building block at C20. In gelserancines B and C, their gelsedine-type frameworks are bound with an additional pyridine ring with a 5-hydroxy-2-(hydroxymethyl)-3-methylcyclopentyl moiety at N4 and C19. Gelserancines D and E were a pair of E/Z tautomer, in which the 14-hydroxygelsenicine unit was connected to a 2-hydroxymethyl furan ring via a C19-C1' conjugated bridgeCitation26. Geleganimines A (35) and B (36), two trace nonsymmetric bisindole alkaloids, were isolated from the aerial parts of G. elegans. Geleganimines A and B belonged to two epimers consisting of gelsenicine and gelseziridine moieties via a 3-carbon alkanoic chainCitation27. 11-Methoxy-14,15-dihydroxygelsedine (37) and 11-methoxy-14,15-dihydroxy-19-oxogelsenicine (38) were got from the ethanol extracts of the leaves and branches of G. elegans. The difference between them was that the ethyl moiety in compound 37 was replaced by an acetyl group in compound 38Citation23. Gelselegandines A (39), B (40), and C (41), isolated from the roots of G. elegans, possess an unprecedented gelsedine-type core structure incorporating an additional C9 aromatic unit as a side chain. Gelselegandines A and C belonged to a pair of cis- and trans-isomers, whereas gelselegandine B existed in the replacement of the ethyl group by a vinyl moiety that was not in accordance with the two formersCitation28 (.
Humantenine-type alkaloids
The chemical structures of humantenine-type alkaloids are quite similar to those of gelsemine-type alkaloids, having an oxindole group, but adding a C21 carbon and a C19-C20 double bond. 19,20-Epoxyhumantenine (42), gelselegandines D (43), and E (44), with a rare epoxypropyl ring at C19 and 20, were isolated from the roots and stems of G. elegans. The difference was that the configuration of C19 near the epoxypropyl ring was S and R in gelselegandines D and E, respectivelyCitation20,Citation29. Geleganidines A (45) and C (46), two unusual humantenine-type alkaloids, were isolated from the roots of G. elegans. Particularly, geleganidine A carried a formamide moiety at N4 in its molecular structure. Geleganidine C was a novel dimer of geleganidine A connected by a carbonyl group to form a rare urea-containing substructureCitation30. 11-Hydroxyhumantenine N4-oxide (47) and N-desmethoxyhumantenine N4-oxide (48) were isolated from the stems of G. elegans. Both of them owned a N-O coordinate linkage at N4, however, the distinct difference was the methoxy group and hydroxy substitution at C11 and N1 in compound 47Citation18. N4-methyl-19,20-dihydrorankinidine (49) and gelstriamine A (50) were isolated from the roots and stems of G. elegans, respectively. In compound 49, the C20 olefin moiety was changed to an ethyl group, compared with humantenine. As mimics of compound 49, gelstriamine A featured a unique hexahydrooxazolo[4,5-b]pyridin-2(3H)-one moiety at C20 and C21, forming an abnormal 6/5/7/6/6/5 heterohexacyclic coreCitation16,Citation18. 14β,20α-Dihydroxydihydrorankinidine (51), 11-methoxy-19,20α-dihydroxydihydrorankinidin (52), and norhumantenine A (53) were purified from the leaves and vine stems of G. elegans. The structure of compounds 51 and 52 was similar to that of rankinidine, except for the reduction of the C19-C20 double bond with a location of a hydroxy group at C20. A comparison of structural differences showed the replacement of the C18/C21 subunits by those from an α,β-unsaturated formyl functionality in norhumantenine ACitation31 (.
Koumine-type alkaloids
Koumine-type alkaloids own an indole-fused cage-shaped scaffold having a terminal vinyl group that are biogenetically derived from sarpagine-type alkaloids. 19-Dehydrokouminol (54), koureamine (55), (4 R)-dihydrokoumine N4-oxide (56), (4S)-dihydrokoumine N4-oxide (57) were extracted from the roots of G. elegans. The terminal vinyl group at C20 in koumine (2) was substituted by an acetyl group in 19-dehydrokouminol. Koureamine represented the first koumine-type alkaloid with a urea group. (4 R)-Dihydrokoumine N4-oxide and (4S)-dihydrokoumine N4-oxide belonged to a pair of enantiomers of N4-oxide derivativesCitation32. N4-demethyl-21-dehydrokoumine (58), 21α-hydroxylkoumine (59), 21β-hydroxylkoumine (60), and (19S)-hydroxydihydrokoumine N4-oxide (61) were isolated from the leaves and vine stems of G. elegans. N4-Demethyl-21-dehydrokoumine represented the first koumine-type alkaloid without a N4-methyl group. Compound 61 comprised an α-hydroxylethyl group in place of the terminal vinyl group in compound 57Citation31. 21-Oxokoumine (62) and furanokoumine (63), two new gelsemium MIAs, were isolated from the roots of G. elegans. As compared with koumine, additional carbonyl oxygen was attached to C21 in 21-oxokoumine and a tetrahydrofuran ring is located at C20 and C21 in furanokoumineCitation33. 18, 19-Dihydro-21-oxokoumine (64), isodihydrokoumine-N1-oxide (65), and (4 R)-isodihydrokoumine-N4-oxide (66) were isolated from the roots of G. elegans. Structurally, these compounds differed from the prototype of koumine-type alkaloids existing in an ethyl group instead of the terminal vinyl group. Compound 64 embodied a carbonyl substitution at C21 that was similar to 21-oxokoumine. Compounds 65 and 66 belonged to N-oxide derivatives. More in detail, the N1-O dative covalent bond in the former was switched to N4-O one in the latterCitation16,Citation32 (.
Yohimbane-type alkaloids
In the core structure of yohimbane-type alkaloids, the indole unit is adjacent to a 7,8,9,10-tetrahydropyrido[1,2-b]isoquinolin-5-ium. Sempervirinoxide (67) and seco-semperviroic acid (68), two novel MIAs, were isolated from the leaves and vine stems of G. elegans. Sempervirinoxide possessed an additional O atom located at N1 to form a N-O dative covalent bond. In particular, the E ring in seco-semperviroic acid was opened in 9/9a. Sempervirinoxide and seco-semperviroic acid represented the first N1-oxide and the first seco-E-ring yohimbane-type alkaloids, respectivelyCitation31. Gelsechizines A (69) and B (70) with the usual three nitrogen atoms were isolated from the fruits of G. elegans. Differently, gelsechizine A was characterised with a methyl 1,2,6,7,12,12 b-hexahydroindolo[2,3-a]quinolizine-3-carboxylate core and an additional 4-methylpyridine unit located at C15. While, gelsechizine B featured a (4S,4aS,13bS,14aS)-methyl 3,4,4a,5,7,8,13,13b,14,14a-decahydro-4-methylindolo[2′,3′:3,4]pyrido [1,2-b][Citation2,Citation7]naphthyridine-1-carboxylate skeletonCitation34 ().
Sarpagine-type alkaloids
Sarpagine-type alkaloids feature an exocyclic (E)-ethylidene side chain and a cage-shaped scaffold that is made up of two bridged substructures, namely indole-fused azabicyclo[3.3.1]nonane and azabicyclo[2.2.2]octane. Of note, this type of alkaloids is especially high not only in Gelsemium genus, but also in Gardneria, Rauwolfia, and Alstonia genera (Apocynaceae)Citation35,Citation36. epi-Koumidine N4-oxide (71), isolated from the stems of G. elegans, had the presence of one more oxygen atom than that of epi-koumidine, indicating that the formation of a coordination bond was formed among oxygen and nitrogen atoms. It represented the first example of N4-oxide sarpagine-type alkaloid from the Gelsemium genusCitation18 ().
Gelsemine-type alkaloids
Gelsemine-type alkaloids with seven contiguous stereocenters bear a spiro-indoleone unit, a rare oxidised[3.2.1]bicyclic architecture, and a 1-methyl-3-vinylpyrrolidine ring compacted into a caged framework. (4 R)-19-Oxo-gelsevirine N4-oxide (72), a N4-oxide derivative was isolated from the roots of G. elegans. Compared to typical gelsevirine, the vinyl group in gelsemine was replaced by an acetyl group, and the N1 atom was substituted by a methoxyl group in (4 R)-19-oxo-gelsevirine N4-oxideCitation20 ().
Other-type alkaloids
10,11-Dimethoxy-N1-demethoxy-gelsemamide (73), 11-demethoxy-gelsemazonamide (74), geleganamide (75), and geleganidine B (76), four unusual MIAs, were isolated from the different parts of G. elegans. 10,11-Dimethoxy-N1-demethoxy-gelsemamide possessed a characteristic symmetrical dimeric N1-C2 seco-indole unit, whose skeleton might be derived from humantenine-type alkaloids. 11-Demethoxy-gelsemazonamide, geleganamide, and geleganidine B belonged to three rare dimeric derivatives of open-loop indole alkaloids. The difference between the three compounds was that the two formers were bridged by an azo group to form an aromatic azo-containing substructure, while the latter was connected by a biphenyl structure. The methoxy group at C11 in geleganidine B was missing in 11-demethoxy-gelsemazonamide that represented the first nonsymmetric aromatic azo-linked bisindole alkaloidCitation20,Citation27,Citation30 (.
Pharmacological activity
In recent years, a large number of studies have proven that gelsemium MIAs exhibit extensive beneficial pharmacological activities, which primarily focus on analgesic, anti-tumour, anxiolytic, immunosuppressive, and anti-inflammatory aspects. Among general gelsemium MIAs, the activity evaluations of gelsemine and koumine are the most intensively studied. These pharmacological activities could be briefly summarised as follows ().
Table 2. The pharmacological effects of gelsemium MIAs in vivo and in vitro experiments.
Analgesic activity
Several research has indicated that gelsemium MIAs exert analgesic properties in vivo and in vitro. Koumine (2) is the most abundant MIA of G. elegans. Its treatment displayed efficient analgesic activity against inflammatory and neuropathic pain in a variety of rodent modelsCitation37. Mechanistic studies revealed that koumine could function as a high-affinity ligand that interacted with translocator protein 18kda positive (TSPO) protein in microglia, thereby inducing TSPO allosteryCitation37,Citation38. TSPO allostery triggered the biosynthesis of neurosteroids, such as allopregnanolone in the spinal cords, which mediated the reduction of neuropathic painCitation39. Moreover, in vivo and in vitro studies also found that koumine enabled to inhibit the production of proinflammatory cytokines and glial activationCitation40,Citation41. Because TSPO is the typical marker of activated microglia, we conjectured that this anti-inflammatory action of koumine might also be related to TSPO allostery. Besides, koumine also increased astrocyte autophagy occurrence and decreased astrocyte-related inflammation, which was the mechanistic basis for its analgesic activityCitation42. In terms of improvement in diabetic neuropathic pain (DNP), the neuropathic pain behaviour and the injury of axon and myelin sheath of the sciatic nerve were greatly ameliorated in streptozocin (STZ)-induced diabetes rats after subcutaneous treatment with koumine (0.28, 1.4, and 7.0 mg/kg, for 7 days)Citation43. Its analgesic effects could be attributed to the modulation of spinal microglial M1 polarisation and proinflammatory mediators via inhibiting the Notch-RBP-Jκ signalling pathwayCitation44. Moreover, the study on pharmacokinetics indicated that koumine elimination was decreased in STZ-induced rats, suggesting koumine was retained for the treatment of DNP in vivoCitation45. Gelsemine (3), is the principal active alkaloid from G. sempervirens. Like koumine, gelsemine also was able to inhibit nociceptive pain and tonic pain in different pain models. Its mechanism for analgesic activity was that gelsemine might serve as a potential α3 glycine receptor (α3-GlyR) agonist to modulate the function of spinal α3-GlyRs and stimulate the biosynthesis of allopregnanolone through upregulation of the mRNA expression of 3α-hydroxysteroid oxidoreductaseCitation46–48. Where after, gelsemine’s analgesic effect was reported in partial sciatic nerve-ligated mice as its administrations (2.0 and 4.0 mg/kg, i.p.) alleviated both neuropathic pain and sleep disturbance, and upregulated c-Fos expression in the neurons of the anterior cingulate cortexCitation49. Additionally, intraperitoneal N-desmethoxyhumantenine N4-oxide (48) treatment at lower doses of 0.04 and 0.2 mg/kg alleviated acetic acid intraperitoneal injection-induced writhing of mice with inhibition rates of 67.6 and 76.1%, respectively, which were even stronger than those of the positive control, morphine. Additionally, gelstriamine A (50) treatment (1.0 mg/kg, i.p.) showed potent analgesic activity with a reduced rate of 64.7% in the same modelCitation18.
Antitumor activity
Sempervirine (6) displayed significant anti-cancer effects in several in vitro and in vivo cancer models. In glioma U251 cells, sempervirine (1.0, 4.0, and 8.0 μM) could inhibit tumour cell proliferation, suppress colony formation, and cause cellular G2/M phase arrest. Sempervirine also could promote the occurrence of cellular autophagy via the blockade of the Akt/mTOR signalling pathway. An in vivo experimental result showed that sempervirine (4.0 and 8.0 mg/kg, i.p., for 28 days) significantly decreased the growth of glioma cancer by 44.76% and 61.26%, respectivelyCitation50. In human hepatocellular carcinoma, sempervirine (0.1, 0.5, and 1.0 μM) induced HepG2 cells apoptosis and blocked the cell cycle in the G1 phase, accompanied by the upregulation of p53 and the downregulation of cyclin D1, cyclin B1, and CDK2. In the xenograft nude mice model, sempervirine treatment (1 mg/kg, i.p., for 18 days) substantially inhibited tumour growth and enhanced the anti-tumour effect of sorafenib. The underlying mechanism was involved in the inactivation of the Wnt/β-catenin pathwayCitation51. Sempervirine treatment (0.8–5.0 μM) triggered cell death in both p53-wildtype and p53-null testicular germ cell tumours (TGCT) cells. Mechanistically, sempervirine could translocate into the nucleus, where it bound rRNA to induce RNA polymerase I (RNA Pol I) degradation and disrupt ribosomal content. This cascade further mediated the MDM2 block to kill cancer cells via concomitant inhibition of the E2F1/pRB pathwayCitation52. 14β-Hydroxygelsedethenine (17) exhibited cytotoxicity against NCI-H1975, PC9, NCI-H460, NCI-H661, and H292 cell lines, with IC50 values ranging 8.3–90.3 μM, respectivelyCitation19. Gelseleganin C (21) displayed significant cytotoxic activities against A549, SPC-A, 1D356, OC3 Tca8113, SACC83, and MEC1 cell lines, with IC50 values less than 10 μMCitation17. 11-Methoxy-14,15-dihydroxygelsedine (37) displayed moderate cytotoxic activity with IC50 values of 12.1, 11.7, 10.9, and 11.4 μM towards Hep-2, LSC-1, TR-LCC-1, and FD-LSC-1 cell lines, respectivelyCitation23. Geleganidine C (46) displayed a growth inhibitory effect against PC-12 cells with an IC50 value of 16.1 μM, while geleganidine B (76) showed weak activity against MCF-7 cells, with an IC50 value of 38.4 μMCitation30. Norhumantenine A (53) and N4-demethyl-21-dehydrokoumine (58) exhibited moderate cytotoxicity against the six human tumours HL-60, SMMC-7721, A-549, MCF-7, SW480, and BEAS-2B cell lines, with IC50 values in the range 4.6–9.3 μMCitation31. Our in vivo and in vitro studies of colorectal cancer have reported that koumine (2) displayed an antitumor effect. Koumine could induce apoptosis and suppress glycolysis via inhibiting Akt/mTOR/HK2 pathway and promoting the disassociation of HK2 to VDAC-1 via interaction with PDK1Citation53.
Anxiolytic activity
Several pieces of studies have shown that gelsemium MIAs play an effective role in anxiolytic activity. Koumine (2) administration by gavage (0.25, 1, and 4 mg/kg) exhibited anxiolytic-like properties in the open-field tests of ICR mice. Additionally, subcutaneous koumine treatment at the same concentration released anti-punishment action like diazepam in the Vogel conflict test of ratsCitation54. Koumine treatment (0.167, 0.5, and 1.5 mg/kg, s.c.) mitigated anxiety-like behaviour of acute predatory sound stress-induced rats in the open field test and elevated plus maze test. Its treatment also led to an increase in progesterone and allopregnanolone levels in the prefrontal cortex and hippocampus and a decrease in ACTH and CORT levels in plasma, suggesting that its anxiolytic mechanism was related to mediating neurosteroids-HPA axisCitation55. In 2013, an in vitro study performed in rats reported that gelsemine (3) treatment at low doses (0.0001 and 1 μM, i.p., for 7 days) significantly improved anxiety-specific parameters, some of which even approached the activity of the positive control, benzodiazepine diazepamCitation56. Also, gelsemine when administered to chronic unpredictable mild stress-induced ICR mice by gavage (0.4, 2.0, and 10.0 mg/kg, for 9 days) substantially altered anxiety-like behavioural performance via inhibiting NLRP3-inflammasome and upregulating CREB and BDNF expression in the hypothalamusCitation57.
Anti-inflammatory activity
Koumine (2) treatment (0.8, 2.4, and 7.2 mg/kg) dramatically decreased the production of IL-1β in the MSU-induced peritonitis mice model, consistent with an inhibitory effect on NLRP3 inflammasome activation and NF-κB pathway. Additionally, an in vivo study confirmed that koumine treatment (50, 100, and 200 μM) antagonised inflammation in LPS-primed macrophages treated with ATP or MSU via blockage of the NF-κB/NLRP3 signalling pathwayCitation58. Gelsekoumidine B (16) exhibited concentration-dependent inhibition of LPS-induced NO production in RAW 264.7 macrophage cells, with an IC50 value of 33.2 μM (indomethacin was used as a positive control, IC50=23.1 μM)Citation21. Gelsecorydines A (25), C (27), D (28), and E (29) exhibited a dose-dependent inhibition on LPS-caused NO production in macrophage RAW 264.7 cells, with IC50 values of 14.7, 16.2, 13.7 and 4.2 μM, respectively, some of which were approximately 1.5- to 15.8-fold stronger than the positive control, indomethacin, with an IC50 value of 21.0 μMCitation25. Gelserancines B (31), C (32), and D (33) were evaluated for their anti-inflammatory effects in vivo, using dexamethasone as the positive control. Gelserancines B-D (50 μM) significantly reduced the neutrophil number in inflammatory sites in zebrafish acute inflammatory models which were induced by tail fin injury or CuSO4Citation26. Geleganimine B (36) decreased LPS-induced NO production in BV2 cells, with an IC50 value of 10.2 μM, suggesting the reduction of the pro-inflammatory stateCitation27. Gelsechizines A (69) and B (70) (12.5 and 25.0 μM) exerted potent anti-inflammatory effects on LPS-induced zebrafish by inhibiting the recruitment of neutrophils and macrophages. Furthermore, the two compounds were shown to significantly inhibit the secretion levels of TNF-α and IL-6 in LPS-stimulated RAW 264.7 macrophage cells. SAR study showed that the existence of β-N-acrylate moiety might be an important factor in their anti-inflammatory effect.Citation34. (4 R)-19-Oxo-gelsevirine N4-oxide (72) and 10,11-dimethoxy-N1-demethoxy-gelsemamide (73) exhibited a dose-dependent inhibitory effect on LPS-induced NO production in RAW 264.7 macrophage cells, with IC50 values of 6.2 and 12.2 μM, respectively (indomethacin was used as the positive control, IC50=21.7 μM)Citation20.
Anti-rheumatoid arthritis and immunosuppressive activities
Gelsevirine (77) has been well described to have an excellent anti-osteoarthritis effect. In IL-1β-stimulated mouse primary chondrocytes, its treatment (6.25 and 50.0 μM) dose-dependently enhanced cell viability and mitigated cell apoptosis. Moreover, it could downregulate the mRNA expression of MMPs and inflammatory factors and upregulate the mRNA expression of Col2A and IL-10 via suppression of STING activation. In an in vivo experiment, chronic exposure to gelsevirine (5.0 mg/kg, i.p., every 3 days for 10 weeks) could markedly reduce OARSI scores and MMP13 expression levels and increased cartilage area and Col2A expression levels in STING-deficient mice and the destabilisation of the medial meniscus-operated mice. Its latent mechanism was in conformity with the promotion of the K48-ubiquitination of STINGCitation59. Recent studies on collagen-induced rats of arthritis disclosed that treatment with koumine (2) alone (0.6, 3.0, or 15.0 mg/kg, i.g., for 10 days) exerted an inhibitory effect on joint pain, that concomitantly occurred with an improvement in the arthritis index scores, mechanical allodynia and volume of injected hind paw as well as the destruction of bone and cartilage. Moreover, koumine effectively ameliorated the production of proinflammatory cytokines in joint tissues and astrocyte activation in the spinal cords. Studies on its antirheumatic mechanism revealed that koumine suppressed the secretion of anti-CII antibody, which was produced by B lymphocytes and could damage joints via the occurrence of the inflammatory responseCitation60,Citation61. In 2022, koumine treatment decreased T cell-dependent and T cell-independent B cell immune response in vivo and in vitro, which might be an alternative mechanism for its anti-rheumatoid arthritis bioactivityCitation62. Such evidence was also demonstrated by an in vivo study in which koumine pre-treatment (4.0 and 8.0 mg/kg, p.o., for 3 weeks) exhibited a therapeutic effect on CIA in mice through regulation of RORγt/Foxp3 signal pathway and modulation of Th17/Treg immune imbalanceCitation63.
Total synthetic chemistry
Due to profuse and diverse effects along with their distinctive chemical structures, tremendous efforts have been devoted to synthetic approaches towards the total synthesis of gelsemium MIAs. Gelsemine and koumine-type alkaloids, the flagship members of gelsemium, have been widely studied by several synthetic chemists. Conversely, yohimbane-type alkaloids with relatively simple structures have received relatively less attention. According to the different molecular skeletons of gelsemium MIAs, these synthetic approaches were classified as follows. ( and )
Total syntheses of gelsedine-type alkaloids
Carreira’s total synthesis of (±)-gelsemoxonine (2013, 2015)
Carreira and co-workers achieved the total synthesis of (±)-gelsemoxonine using a ring contraction approach of a spirocyclopropane isoxazolidine to introduce the β-lactam intermediate, providing access to the unusual azetidineCitation64,Citation65. They began with aldehyde 78 (5 steps from cyclopropanone hemiacetal), which was converted into nitro-alcohol 79 in high yield through Henry reaction. Then, it was prepared to isoxazoline 82 via elimination followed by intramolecular Huisgen dipolar cycloaddition induced by Boc2O/DMAP between nitrone and alkene. It was then treated with DMDO to get an epoxy intermediate, which was attacked by ketene silyl acetal 83 via nucleophilic addition to generate alcohol 84. Its reaction with 1-bromo-1-propene 85 installed a 1-propynyl moiety furnished diastereomeric oxazolidine 86 using anhydrous CeCl3 and BF3·OEt2. 86 underwent the key ring contraction rearrangement to build the isoxazolidine ring in 87 employing TFA in 40–45% yield. After Boc group protection, treatment of the carbonyl group in 87 with Petasis’ olefination generated olefin 88 in situ, followed by a concomitant hydroboration reaction to yield the desired primary alcohol 89 with high diastereoselectivity. Subsequently, 89 was transformed to dialdehyde 90 via reduction with DIBAL-H along with oxidation of the diol under Swern conditions. Furthermore, the 7-membered ring was set up through an intramolecular aldol reaction catalysed by DL-proline, thus yielding aldol 91 as a single diastereomer. 91 was further advanced to unsaturated ester 92 via a multistep reaction sequence, including Pinnick oxidation, esterification, and hydroxyl elimination. Through hydrolysis using Me3SnOH, the condensation of 92 with N-(2-bromophenyl)hydroxylamine 93 proceeded to forge aryl bromide 94, whose exposure to reductive Heck conditions afforded oxindole 95 as a single diastereoisomer in 72% yield. After esterification and selective removal of the Boc group using K2CO3, the corresponding alcohol was immediately subjected to hydrosilylation employing [RuCl2(C6H6)]2 as catalysis to deliver vinylsilane 96 in 46% yield over 3 steps. Finally, Tamao-Fleming oxidation followed by removal of the N-Boc group using HCl provided the natural product (±)-gelsemoxonine (97). (Scheme 1)
Fukuyama’s total syntheses of (-)-gelsenicine, (-)-gelsedine, (-)-gelsedilam, (-)-14-hydroxygelsenicine, and (-)-14,15-dihydroxygelsenicine (2016)
In Fukuyama’s study, a flexible and unified synthetic route was developed to construct a library of gelsedine-type alkaloids through an enal intermediate bearing a versatile core structureCitation66. The key intermediate aldehyde 99 was synthesised from furfuryl alcohol 98 in 21 steps as reported in earlier Fukuyama’s studyCitation67. TMSCN/DBU-mediated redox isomerisation reaction led to the formation of acyl cyanide derivative 100. It was followed by a nucleophilic reaction with MeOH to afford methyl ester 101 (15 R/15S = 2.6:1), and the desired 15 R isomer was isolated in 57% yield. Deprotection of the Cbz group by TMSI and subsequent N-acylation reaction in the presence of DBU in DCE completed the intermolecular cyclisation, thereby furnishing (-)-gelsedilam (102). In parallel, the instalment of the ethyl group onto 99 using EtMgBr followed by IBX-induced hydroxyl oxidation afforded another key intermediate carbonyl 103. Then, Pd(OAc)2-catalysed conjugate reduction of the unsaturated ketone with in situ trapping as its silyl enol ether at base condition yielded the resulting 104. It was exposed to TBAF, and then the liberated amine and ketone were facilely cyclized to achieve the total synthesis of (-)-gelsenicine (105). It was further subjected to hydrogenation reaction by Adams’ catalyst to afford (-)-gelsedine (1). In addition, the exposed double bond of 103 was oxidised by catalytic OsO4/NMO in acetone/H2O to yield diol 106, which underwent similar two-step Pd(OAc)2/Et3SiH treatment and concomitant dehydrative cyclisation yielded (-)-14,15-dihydroxygelsenicine (107) in 43% yield over 2 steps. Meanwhile, the epoxy moiety was diastereoselectively introduced in treating TBHP/Triton B on C14 and C15 in 103 to afford epoxy 108. After the loss of the Cbz group by TMSI in DCM, the treatment of this substrate with reductive SmI2 in THF at −78 °C allowed the reduction of α,β-epoxy ketone to β-hydroxy ketone, further forming the samarium enolate 109. When it was protonated in MeOH, the Schiff’s base was simultaneously synthesised, eventually affording (-)-14-hydroxygelsenicine (110) in 37% yield over 3 steps. (Scheme 2)
Zhao’s total synthesis of gelsedilam (2016)
In 2016, Zhao and co-workers described the total synthesis of gelsedilam, by utilising a highly thiol-mediated diastereoselective conjugate addition-aldol reaction to construct the oxabicyclo[3.2.2]nonane ring systemCitation68. In the beginning, C3-substituted oxindole 113 was synthesised from N-OMe oxindole 111 and 2-(benzyloxy)acetaldehyde 112 via a sequence of aldol condensation, acylation, and reduction reaction in 70% yield over 3 steps. The following aldol reaction between 113 and aldehyde 114 occurred to construct compound 115 (dr = 1:1) using a mild base of K2CO3. Oxidation using Dess-Martin periodinane (DMP) and re-reduction subsequent were performed to switch the β-hydroxyl group to the α-hydroxyl group, and the diastereoselective ratio was noticeably improved from 1:1 to 5:1. Then, an intramolecular condensation employing TFA occurred to smoothly deliver lactone 116, which was further selectively reduced by DIBAL-H at −78 °C and removed the ketal group by p-TSA in acetone to yield pyrone 117 in 60% yield over 3 steps. Next, aldehyde 118 was synthesised over 3 steps. Cs2CO3-promoted Michael addition of thiol and conjugate addition-aldol reaction as key steps produced thiolated 119, which was removed from the thiolate group in the presence of AIBN/n-Bu3SnH in benzene yielded the corresponding 120 as a single diastereoisomer in moderate yield. Upon acetylation protection, the carbonyl group in 120 was transformed into triflyl enol in 121 by treatment with KHMDS. Subsequent Pd(OAc)2-catalysed carbonylation reaction in the presence of CO obtained unsaturated ester 122. Deprotection of the acetate group along with DMP oxidation furnished the ketone product, which was treated by hydroxylamine hydrochloride to obtain oxime 123. It was further elaborated to complete the reduction with NaBH4 with the use of NiCl2 and in situ lactam cyclisation in one pot process to yield the final gelsedilam (102). (Scheme 3)
Ferreira’s total synthesis of (-)-gelsenicine (2016, 2022)
Ferreira and co-workers reported the shortest approach towards the total synthesis of gelsenicine in 13 stepsCitation69,Citation70. Their synthesis commenced with the alkylation of (Z)-but-2-ene-1,4-diol 124 with 3-bromoprop-1-yne 125, along with Cu-catalysed oxidation and olefin isomerisation to forge aldehyde 127 (E/Z > 20:1) in satisfactory yield over 3 steps. Then, it underwent a Horner-Wadsworth-Emmons olefination with phosphonate 128 and phosphine-mediated alkene E/Z isomerisation to synthesise (E,E)-dienyne 129 in a high (E,E)/(E,Z) ratio of 8.2:1. Subsequent Cadiot-Chodkiewicz coupling of 129 with 1-bromo-1-propyne 130 allowed access to (E,E)-diyne 131. Under the optimised condition, Au-catalysed cycloisomerization provided the resulting product 132 with an outstanding yield in a 3.2:1 dr ratio, whereupon a strain-release Cope rearrangement was performed to obtain bicycle 135 in MeOH at 60 °C in 75% yield. Regioselective Kucherov alkyne hydration using HgSO4/H2SO4 catalysis then transformed 135 into enone 136. It was directly subjected to conjugate reduction to afford ketone 137 (dr = 4:1) using Stryker’s reagent, followed by a series of hydrolysis, acyl chloride, and amidation, thereby generating amide 138 with an overall yield of 73%. Oxime formation with hydroxylamine and benzoylation proceeded to install the oxindole unit, giving benzoyl-oxime 139. The oxindole moiety was then assembled through a ring closure of amide using PhI(OTFA)2 in TCM at 0 °C, thus giving rise to oxindole 140. Finally, the radical ring closure between benzoyl-oxime and olefin using Bu3SnH/AIBN at 120 °C successfully favoured (-)-gelsenicine (105). (Scheme 4)
Ma’s total syntheses of (-)-gelsedilam, (-)-gelsenicine, (-)-gelsedine, and (-)-gelsemoxonine (2018)
In 2018, Ma and co-workers developed and implemented a short total synthesis approach for four gelsedine-type alkaloidsCitation71. The synthesis started with an asymmetric Michael addition reaction of dihyropyranone 141 (4 steps from L-arabinose) and indole 142 (accessible from gramine in 3 steps), affording a 1:1 diastereoisomeric mixture of indolone 143. After the formation of indolone upon treatment with NCS/H2O, subsequent aldol cyclisation smoothly established the oxabicyclo[3.2.2]nonane skeleton in 146 as a single isomer in 90% yield. Bicyclic 146 underwent an oxonium ion-induced pinacol rearrangement in toluene/Et2O and heating under AlCl3 catalysis to afford the key intermediate 147. Then, the reaction of 147 with Mander’s reagent allowed the introduction of methyl formate at the α-carbon of the carbonyl group. The concomitant isomerisation of the α-position of the nitro group led to the formation of enol 148 as a 1:1 diastereomeric mixture. Subsequently, it was transformed into the corresponding enol triflate 149 under the conditions of Tf2O/DIPEA. Reductive removal of the triflate group using Pd(PPh3)4/Et3SiH afforded the corresponding α,β-unsaturated methyl ester 150. Next, (-)-gelsedilam (102) was synthesised following Zhao’s approach as illustrated in Scheme 3. In parallel, treatment 147 with KHMDS and freshly distilled NCCOOEt provided diketone 151 as a single diastereomer in 4 3 ∼ 55% yield after quenching with HCl. 151 underwent the same synthetic procedure as 150 to afford α,β-unsaturated methyl ester 153. Similarly, NiCl2/NaBH4-mediated nitro reduction accompanied by an intramolecular Schiff base formation provided (-)-gelsenicine (105). Furthermore, (-)-gelsedine (1) was synthesised via catalytic hydrogenation of 105 with PtO2 in the hydrogen atmosphere. In addition, the stereoselective epoxidation of 153 with mCPBA was followed by reduction of the nitro group with Zn in AcOH and furnished amino 155. Epoxide opening reaction in boiling ethanol and intramolecular cyclisation yielded the desired (-)-gelsemoxonine (97). (Scheme 5)
Takayama’s unified total syntheses of (-)-14-hydroxygelsenicine, (-)-14-hydroxygelsedilam, (-)-14-acetoxygelsedilam, (-)-gelsemolenine A, (-)-gelsefuranidine, (-)-gelselegandine and (-)-gelselegandine C (2019)
In 2019, Takayama and co-workers achieved the first concise and collective asymmetric total synthesis of (-)-gelsemolenine A and five gelsedine-type alkaloids with a hydroxy group at C14Citation72. The synthesis began with the stereoselective alkylation reaction of γ-lactone (S)-156 with alkyl iodide 157 using LiHMDS to obtain compound 158. It was subjected to ring-closing metathesis using Hoveyda-Grubbs II catalyst to forge cycloheptene 160. Aminolysis of the γ-lactone in 159 under the influence of PMBNH2/DIBAL led to amide 160 in 99% yield. It was transformed into the corresponding enone 161 over 3 steps, which underwent an intramolecular aza-Michael addition reaction to give bicyclic 162 under LiHMDS condition. Selective 1,4-reduction of the enone in 162 employing L-Selectride and successive exposure to McMurry reagent generated the key enoltriflate 163 in quantitative yield. Pd(OAc)2-catalysed carbonylation cross-coupling between 163 and N-(2-bromophenyl)-O-methylhydroxylamine 164 at CO atmosphere constructed amide 165. After cleavage of the TBDPS group, a Heck coupling reaction was performed to form spiro-N-methoxyoxindole 166 with high stereoselectivity, which upon an intramolecular alkoxymercuration-demercuration reaction gave alcohol 167. Removal of the p-methoxybenzyl group by TFA in anisole achieved the total synthesis of (-)-14-hydroxygelsedilam (168) in 77% yield. It was acetylated to yield (-)-14-acetoxygelsedilam (169), whose lactam was protected by the Boc group and then treated with ethyl magnesium bromide to install an ethyl moiety, thus yielding the resulting 170. It was deprotected using TFA to prepare (-)-14-hydroxygelsenicine (171) in 56% yield over 3 steps. (-)-Gelsemolenine A (172) was accessed by acetylation of 171 followed by treatment with aqueous HCl in methanol. Concurrently, 171 was condensed with 2-furaldehyde 173 and 3-vinylbenzaldehyde 176 under acidic conditions in 1,2-dichloroethane to obtain (-)-gelsefuranidine (174) and (-)-gelselegandine (176), respectively. Following a similar procedure, the condensation of 171 with 4-ethylbenzaldehyde 177 easily gave the product 178 having an E-configuration C19-C1′ double bond. Finally, photocatalytic riboflavin altered the olefin of E/Z configuration, and then led to the first total synthesis of (-)-gelselegandine C (179), albeit in 23% yield. (Scheme 6)
Scheme 6 Takayama’s unified total syntheses of six gelsedine-type alkaloids.
Total syntheses of gelsemine-type alkaloids
Qiu’s total synthesis of (+)-gelsemine (2015)
In 2015, Qiu and co-workers completed the asymmetric total synthesis of (+)-gelsemine using an organocatalytic Diels-Alder starting strategyCitation73. The synthesis was initiated by the linkage of methyl (Z)-4-oxobut-2-enoate 180 with dihydropyridine 181 through an organocatalytic Diels-Alder reaction. This process provided the intermediate 183 and by-product 182. Fortunately, 182 could be converted to lactone 183 through an intramolecular cyclisation in the presence of DBU in 97% yield. 183 was then transformed into hemiacetal 184 through selective reduction using DIBAL-H at −78 °C. It was then subjected to a Wittig reaction to yield a racemic mixture that occurred an electrophilic addition reaction by catalytic p-TSA in DCM to afford (S)-acetal 185 (dr = 13:1) in 93% overall yield. Subsequent ozonolysis of 185 employing ozone in DCM, accompanied by trans-annular aldol condensation of resulting dicarbonyl groups using sodium methanol afforded ketone 186. On subjecting reduction of 186 using NaBH4 led to hydroxyl 187, whose hydroxyl group was further methanesulfonylated with MsCl to afford disulfonate 188. Upon treatment of 188 with DBU in heating toluene, reduction of the Cbz protecting group to methyl group with LiAlH4 in THF resulted in the formation of olefin 189. Hemiacetal 190 was prepared via acid hydrolysis with HCl in THF in a 2:1 dr ratio. Then, 1-MOM-oxindole 191 was installed onto 190 via condensation reaction by catalytic piperidine to generate the resulting product 192. Using their optimised conditions, its treatment with LDA and subsequent SN2 substitution reaction using Et2AlCl constructed the configuration of the C7 quaternary carbon stereochemical centre and afforded the desired 193 as a single diastereoisomer in only 32% yield. Finally, acid hydrolysis of the methyl group from the MOM group and removal of the resulting hydroxymethyl group using Et3N furnished (+)-gelsemine (3) in 70% yield over 2 steps. (Scheme 7)
Vanderwal’s synthetic route to the polycyclic core of gelsemine (2015)
In the same year, Vanderwal’s and co-workers relied on a Zincke-aldehyde-based approach to prepare the polycyclic core intermediate to gelsemineCitation74. Sulfolene 195 was prepared by the bromination of 3-methylsulfolene 194 (from the adduct of isoprene and SO2) through 4 steps. Then, dienyl amine 196 could be accessed through the chelotropic extrusion of SO2 under microwave conditions in toluene at 150 °C. Subsequent reaction of 196 with pyridinium salt 197 triggered the pyridine ring-opening, affording Zincke aldehyde 198 in 85% yield. Next, 198 underwent a pericyclic cascade rearrangement including [Citation1,Citation5]-H sigmatropic shift and 6π electrocyclic ring opening to smoothly generate ketone 202 as a single diastereomer under microwave irradiation in 82% yield. With 202 in hand, a concomitant intramolecular Diels-Alder cyclisation was carried out to yield bicyclic lactam 203, which was prepared to a pair of the separable diastereomeric mixture of epoxide 204 after epoxidation with mCPBA. Ring-opening reaction using Nagata’s reagent followed by hydrogenation advanced 204 to the only one secyanoalcohol 205 with excellent diastereoselectivity. It was converted to the critical polycyclic skeleton 209 of gelsemine via a series of reactions involving the switch from TIPS to Ms protecting group, retro-aldol-type cleavage, and pyran ring closure. As the authors’ report, several attempts to partially or fully hydrolyse the nitrile prior to decomposition were not successful. (Scheme 8)
Total syntheses of koumine and sarpagine-type alkaloids
Takayama’s asymmetric total synthesis of koumine (2016)
In 2016, Takayama and co-workers published the asymmetric total synthesis of koumineCitation75. Their synthetic strategy relied on a stereoselective gold(I)-catalysed 6-exo-dig cyclisation reaction providing a key piperidine intermediator with an exocyclic (E)-ethylidene side chain. The approach utilised azabicyclononane 210 (4 steps from 1,5-cyclooctadiene) as the starting material, followed by Swern oxidation and secondary selective carbonyl reduction with the use of BH3 to generate alcohol 211. Then, it was transformed into amine 212 through 2 steps. The alkylation reaction of 212 with alkyne 213 resulted in the formation of ether 214, which was then converted into silyl enol ether ester 215. Gold(I)-catalysed 6-exo-dig cyclisation of 215 using Au catalysis/AgBF4 in MeCN/H2O at 80 °C furnished the common intermediate 216 in 85% yield. Deprotection of the acetyl group allowed the free hydroxyl group along with the conversion of a ketone into olefin on treating Tebbe reagent to form olefinic 217, which proceeded to yield ketone 218 after 3 steps. The reaction of 218 with phenylhydrazine 219 in the presence of pyridine·HCl initiated the construction of an indole unit to form N-benzyl indole 220 in 98% yield. Successively, a 9-BBN-induced borohydride reaction followed by oxidation with H2O2 was achieved to regio- and diastereoselectively introduce a hydroxyl group at C17 in 221. 221 was subjected to N-benzyl deprotection using Na/liquid NH3 in THF rendering the formation of indole 222. After the successful preparation of indole 222, Takayama and co-workers turned our attention to the synthesis of koumine. The C/D ring opening was achieved upon treatment with methyl chloroformate in THF/H2O to afford the resulting intermediate 223, which was transformed to 224 over 2 steps. Finally, NaH-treated indole ionisation followed by Pb(OAc)2-catalysed intermolecular indolyl addition to the allene chain built the C20-C7 bond, thus furnishing the desired koumine (2). (Scheme 9)
Kerr’s total synthesis of isodihydrokoumine and (4 R)-isodihydroukoumine N4-oxide (2018)
In 2018, Kerr’s total syntheses of isodihydrokoumine and (4 R)-isodihydroukoumine N4-oxide utilised an intramolecular [3 + 2] nitrone olefin cycloaddition and a Lewis acid-mediated cyclisation as the key steps to prepare their core structureCitation76. They commenced this synthetic study with the preparation of dihydropyranone 227. Hydrostannylation of alkyne 225 with n-Bu3SnH followed by Stille coupling with methyl (Z)-3-iodoacrylate 226 provided dihydropyranone 227 in 60% yield. Then, the copper-catalysed conjugate addition of 228 with vinyl magnesium bromide 228 led to the formation of lactone 229. After the smooth reduction of lactone, the allylic alcohol of the resulting product was substituted through Mitsunobu reaction to yield hydroxylamine 232 in 83% yield with high regioselectivity. Upon removal of the Boc groups, the relevant product was condensed with N-tosyl indole-3-acetaldehyde 233 in situ to provide nitrone 234, which underwent an intramolecular N-alkenyl nitrone dipolar cycloaddition upon heating in toluene to produce isoxazolidine 235 in a 23% yield of a 2:1 mixture of cis-diastereomer. Removal of the N-tosyl protecting group with Mg, followed by Swern oxidation, acetal protection as well as SmI2-mediated isoxazolidine reduction, and ring-opening smoothly delivered acetal 236 in 48% yield over 4 steps. Treatment of 236 with TMSCI in MeCN induced the cascade Friedel-Crafts and Conia-Ene cyclizations that forged the polycyclic cage skeleton in 240. Eschweiler-Clarke reaction installed a methyl group on an N4 atom and then furnished natural isodihydrokoumine (241) in a 57% yield. Oxidisation of the N4 atom in 241 with mCPBA generated the separable diastereomeric products in a dr ratio of 1.8:1, which was separated by chiral chromatography to afford the natural product (4 R)-isodihydrokoumine-N4-oxide (66) in a 35% yield. (Scheme 10)
De Paolis’ asymmetric synthetic route to the bicyclic core of koumine (2019)
In 2019, De Paolis and co-workers utilised an intramolecular vinylation reaction of an enolate to form a [3.3.1] bicyclononane frameworkCitation77. In their synthetic pathway, the aldehyde 243 was yielded from 2-nitrophenyl-1,3-cyclohexanedione 242 (1 step from 1,3-cyclohexanedione) through 2 steps. Then, 243 was condensed with ylide 244 through Wittig reaction to afford ester 245 in 83% yield with a high level of Z/E selectivity (4:1). Subsequent treatment of DIBAL-H/n-BuLi followed by reduction with NaBH4 in THF caused the construction of vinyl bromide 247. Intramolecular vinylation cyclisation using catalytic Pd(PPh3)4 initiated the formation of the crucial [3.3.1] bicyclononane core, thus giving the expected intermediator 248, albeit in 18% yield. With 248 in hand, there was an opportunity to employ this key core as the progenitor needed for the synthesis of koumine (2). It was envisioned that koumine eventually could be completed by subsequent closure of the piperidine and tetrahydropyrane ring as well as the assembly of the indole portion. (Scheme 11)
Tanja’s total synthesis of koumidine (2019)
In Tanja’s synthesis of koumidine, the late-stage enol-oxonium cyclisation sequence was used to construct the hexacyclic cage framework on a gram scaleCitation78. In their work, a highly diastereoselective 1,3-dipolar cycloaddition reaction of trans-2-methylene-1,3-dithiolane 1,3-dioxide 249 (from available 1,1,2-trimethoxyethane in 4 steps) with 3-oxidopyridinium 250 generated an inseparable mixture of tropane 251 and 252 in 2.5:1 dr ratio. Next, the bissulfoxides in the mixture of 251 and 252 were simultaneously reduced using TFAA/NaI to afford two separable regioisomers dithiolanes 253 and 254, with the latter capable of being transformed to ketone 255 via 1,4-reduction using L-selectride. Then, the Palladium-catalysed intramolecular coupling of vinyliodide and ketone fused the piperidine ring using potassium phenoxide in THF, thus affording tetracycle 256. Wittig reaction of 256 with triphenylmethylmethoxy-chloride in the presence of KHMDS happened to smoothly deliver the corresponding enol ether 257 with high efficiency. After removal of the ethylenethioacetal group using Meerwein’s reagent, the resulting bissulfonium intermediate was hydrolysed with CuSO4, followed by basification with NH4OH to yield the crude mixture, which was readily converted to keto-aldehyde 258 via acid hydrolysis in 82% yield over 3 steps. Subsequently, the chemoselective reduction of the aldehyde proceeded to yield alcohol 259 with excellent chemoselectivity that participated in a TMSCH2N2-involved expansion reaction followed by subsequent resulting TMS enol ether hydrolysis to furnish 6-membered ketone 260. With sufficient 260 in hand, the total synthesis of koumidine (5) was eventually completed by Fischer indole synthesis reaction with phenylhydrazine 261. (Scheme 12)
Zhang’s asymmetric total syntheses of (-)-koumimine, (-)-N-demethylkoumine, and (-)-koumine (2021)
In 2021, Zhang and co-workers disclosed a tandem sequential oxidative cyclopropanol ring-opening cyclisation and a cooperative organo/metal-assisted ketone α-allenylation for constructing the core skeleton of koumineCitation79. Methyl ester 264 was first prepared from L-tryptophan 262 in 3 steps involving by Pictet-Spengler reaction, N-arylation, and carboxylic acid esterification. 264 underwent Kulinkovich cyclopropanation of the carboxyl ester to furnish cyclopropanol 265, which was directly subjected to DEAD-promoted amine oxidation and subsequent CuCl2-catalysed cyclopropanol ring-opening cyclisation to set up the bicyclo[3.3.1]nonane in 267. Dearylation of 267 and secondary alkylation of the free amine with 268 afforded 269 in 60% yield for 2 steps. Under identical reaction conditions, treatment of 269 with pyrrolidine and AgNTf2 facilitated the formation of the bicyclo[2.2.2]octane to afford ketone 271 in 75% yield. Then, it could be converted to aldehyde 272 in 2 steps. Its treatment with TrocCl and an excess Na2CO3 led to alcohol 273 as a single diastereomer in 62% yield. The synthesis was further manipulated by a Gold-catalysed intramolecular coupling reaction between C7 and C20 positions, DBU-induced isomerisation of the aldehyde group, and sequential aldol condensation reaction with trapping of the hydroxyl group newly generated in situ to obtain the required hemiacetal 275 as a single diastereomer. Next, the hemiacetal and imine moieties were reduced by TFA/Et3SiH to produce the resulting 276. Removal of the Troc group and successive PhIO-induced oxidation gave (-)-koumimine (277) in 45% yield over 2 steps. Meanwhile, PhIO-exposed oxidation and subsequent deprotection of the Troc group took place to complete the synthesis of (-)-N-demethylkoumine (278) in 77% yield. Finally, a HCHO/NaBH3CN-assisted reductive methylation happened to afford (-)-koumine (2) in good yield. (Scheme 13)
Zhang’s total syntheses of akuammidine, 19-(Z)-akuammidine, komidine, dihydrokoumine and koumine (2022)
In 2022, Zhang and co-workers exploited a unified approach towards the asymmetric synthesis of sarpagine- and koumine-type alkaloids. Among them, akuammidine, 19-Z-akuammidine, and dihydrokoumine are synthesised for the first timeCitation80. The synthesis started with the preparation of sulfinamide 283 through Schiff base formation and a vinylogous Mannich reaction from (3S)-aldehyde 279 (from tryptophol for 2 steps) in 72% yield. Then, 283 was converted to unstable aldehyde 284 over 4 steps. The azabicyclo[3.3.1]nonane in 285 could be built through an intermolecular cyclisation in the presence of Lewis acid, which was further transformed to 286 over 3 steps. Alkylation of olefin with 3–(2-bromoacetyl)oxazolidin-2-one 287 was carried out to afford N-acyl-oxazolidinone 288. Next, SmI2-mediated asymmetric radical cyclisation smoothly proceeded to fuse the bridged piperidine-3-one ring, thus affording ketone 291 with excellent diastereoselectivity. Its olefination reaction with Julia reagent 292 exhibited preference for (Z)-olefin 293 as the common sarpagine-type skeleton in 80% yield with a dr ratio of 4.2:1. Treatment of 291 with Wittig reagent in THF in the presence of NaHMDS provided (E)-olefin 294 in 83% yield with a dr ratio of 5.5:1. Then, the first total synthesis of akuammidine (295) and 19-(Z)-akuammidine (296) were completed from 293 and 294 via Knoevenagel condensation reaction with formaldehyde followed by deprotection of the PMB group, respectively.
On the other hand, treatment of intermediate 294 with I2 in the presence of LDA yielded iodide 297. The required stereochemistry at C16 in 298 was fixed by light-induced radical reduction of 297 with catalytic [Ir(ppy)2(dtbbpy)]PF6 with the use of DIPEA and (TMS)2NH under blue LED at −60 °C in 47% yield. Koumidine (5) could be obtained from the removal of the PMB group of 298 with TFA along with reduction with LiAlH4 in 80% yield over 2 steps. Treatment of 5 with methyl chloroformate triggered the formation of amide 299 in a 69% isolated yield. NIS-induced cyclisation simultaneously set up two vicinal all-carbon quaternary stereocenters, thereby providing iodide 300 in 88% yield. Next, olefin 301 was obtained by eliminating the iodide in 300 with AgOAc in acetic acid. Upon reduction with LiAlH4, the first synthesis of dihydrokoumine (302) was eventually completed in 79% yield. Oxidation of 302 with PhIO in DCM gave rise to koumine (2) in nearly quantitative yield. (Scheme 14)
Scheme 14 Zhang’s total syntheses of sarpagine- and koumine-type alkaloids.
Total syntheses of sempervirine-type alkaloids
Malhotra’s total synthesis of sempervirine (2013)
In 2013, Malhotra and co-workers accomplished the concise total synthesis of sempervirine under microwave irradiation in one pot processCitation81. Quaternization of 1-methyl-9H-pyrido[3,4-b]indole 303 with ethyl bromoacetate 304 occurred to give the corresponding quaternary 305 in excellent yield. In a one-pot reaction, a Westphal condensation reaction with 1,2-cyclohexanedione 306 under microwave heating in MeOH in the presence of sodium methoxide, followed by ester hydrolysis and decarboxylation yielded a separable mixture of sempervirine precursor 309 in 53% yield. After acidification with dilute hydrochloric acid, the mixture was separated through silica gel chromatography to afford the expected sempervirine (6) in a 78% isolated yield. (Scheme 15)
Bannister’s total synthesis of sempervirine triflate (2016)
In 2016, Bannister and co-workers used Pd-catalysed Sonagashira coupling and Larock indole annulation reaction to efficiently synthesise sempervirine and its analogsCitation82. Regioselective semi-reduction of 3-isoquinolone 310 using PtO2 catalysis in TfOH/TFA generated the corresponding hydrogenated product, whose amide group was further alkylated to provide triflate 311 in 94% yield over 2 steps. Under optimised Sonagashira conditions, the addition of 311 to butyne-1-ol 312 smoothly gave alkyne 311 using Pd(PPh3)2Cl2 as a catalytic agent in 92% yield. Upon Larock indole synthesis of 313 with o-bromoaniline 314 catalysed by Pd(OAc)2, the 2-heteroaryl indole product 315 was prepared with a good yield. Subsequently, a triflate-mediated cyclisation cleanly furnished the intermediate pyridinium salt 316. Ultimately, it was converted to sempervirine triflate (317) through DDQ-promoted oxidation in 96% yield. (Scheme 16)
Conclusions and perspectives
At present, this review has summarised a total of 70 novel gelsemium MIAs, which greatly complemented the library of compounds from the Gelsemium genus. Although remarkable accomplishments to synthesise several gelsemium MIAs are successful, the separation from plants are a more economical route for access to these gelsemium MIAs and their analog, due to their abundance in plant sources. An increasing number of gelsemium MIAs are essential for mapping out their possible biosynthesis mechanisms and molecule transformations. Once a reasonable biosynthesis is proposed, it is conducive to satisfying the need of synthetic chemists for addressing the synthetic challenges of complex gelsemium MIAs depending on biomimetic synthesis inspired by nature. Therefore, it is necessary to deeply dig and identify unknown trace gelsemium MIAs from natural sources on a large scale. It is noteworthy that the structure-activity relationship of many gelsemium MIAs remains unclear, partly because of limited material availability. The structural modification of gelsemium MIAs using semi-synthesis directly starting from several key intermediates could effectively solve this issue as possible. Besides, these synthetic tactics also enable to provide novel gelsemium MIAs derivatives with better bioactivities. Further innovative total synthetic strategies with more concise steps and higher yields should be designed to construct the complicated skeletons of gelsemium MIAs.
Despite gelsemium MIAs’ promising bioactive potentials both in vitro and in vivo, their exact molecular mechanisms and specific targets in many types of diseases have long been limited; thus, such further studies are required. Bioinformatics and the integration analyses of transcriptome, genome, intestinal flora, and proteome are ripe for wide prediction and exploration of singling pathways and precise target proteinsCitation83–85. Molecular docking tools, which create ligand-target interaction, aid the prediction of binding sites of compounds and the delineation of SARsCitation86. These approaches greatly support the in-depth understanding of gelsemium MIAs-regulated molecular mechanisms. In addition, the extensive clinical applications of gelsemium MIAs and plants are still largely challenging due to their toxicity. Our study has reported that the combination treatment of koumine and Glycyrrhiza uralensis showed a significant low-toxic effect by upregulating cytochrome enzymes and mediating pharmacokineticsCitation87. Thus, the synergistic application with another detoxifying agent would be crucial to partly enhancing the curative effect and reducing the toxicity of individual gelsemium MIA or gelsemium extract. Regarding the tissue distribution, the koumine and gelsemine peak concentrations in the intestines and livers are higher than that in other tissues, thus indicating that gelsemium MIAs may bring greater advantages into full play in the treatment of digestive system diseasesCitation88,Citation89.
In conclusion, a historical account of relevant studies research advances on the structural diversity, potential bioactivity, and total syntheses of gelsemium MIAs covering the period from 2013 to 2022 has been described and discussed. We hope this review will help drive the future drug development of gelsemium MIAs as promising lead compounds with better safety and potency forward.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dutt V, Thakur S, Dhar VJ, Sharma A. The genus Gelsemium: an update. Pharmacogn Rev. 2010;4(8):185–194.
- Yamada Y, Kitajima M, Kogure N, Wongseripipatana S, Takayama H. Seven new monoterpenoid indole alkaloids from Gelsemium elegans. Chem Asian J. 2011;6(1):166–173.
- Zhang Z, Zhang Y, Wang Y, Zhang Q, Yan X, Di Y, He H, Hao X. Three novel β-carboline alkaloids from Gelsemium elegans. Fitoterapia. 2012;83(4):704–708.
- Lovell FM, Pepinsky R, Wilson AJC. X-ray analysis of the structure of gelsemine hydrohalides. Tetrahedron Lett. 1959;1(4):1–5.
- Liu M, Huang HH, Yang J, Su YP, Lin HW, Lin LQ, Liao WJ, Yu CX. The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology. 2013;225(4):839–851.
- Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang J, Su YP, Yu CX. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol Biochem Behav. 2012;101(3):504–514.
- Xu YK, Liao SG, Na Z, Hu HB, Li Y, Luo HR. Gelsemium alkaloids, immunosuppressive agents from Gelsemium elegans. Fitoterapia. 2012;83(6):1120–1124.
- Liu M, Shen J, Liu H, Xu Y, Su YP, Yang J, Yu CX. Gelsenicine from Gelsemium elegans attenuates neuropathic and inflammatory pain in mice. Biol Pharm Bull. 2011;34(12):1877–1880.
- Ishikura M, Abe T, Choshi T, Hibino S. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat Prod Rep. 2013;30(5):694–752.
- Lin H, Qiu H, Cheng Y, Liu M, Chen M, Que Y, Que W. Gelsemium elegans Benth: chemical components, pharmacological effects, and toxicity mechanisms. Molecules. 2021;26(23):7145.
- Zhou X, Xiao T, Iwama Y, Qin Y. Biomimetic total synthesis of (+)-gelsemine. Angew Chem Int Ed Engl. 2012;51(20):4909–4912.
- Zhou S, Xiao T, Song H, Zhou X. Studies toward the total synthesis of (+)-gelsemine and synthesis of spirocyclopentaneoxindole through intramolecular Michael cyclization. Tetrahedron Lett. 2012;53(42):5684–5687.
- Shimokawa J, Harada T, Yokoshima S, Fukuyama T. Total synthesis of gelsemoxonine. Pure Appl Chem. 2012;84(7):1643–1650.
- Jin G, Su Y, Liu M, Xu Y, Yang J, Liao K, Yu C. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)-A review of their phytochemistry, pharmacology, toxicology and traditional use. J Ethnopharmacol. 2014;152(1):33–52.
- Ghosh A, Carter RG. Recent syntheses and strategies toward polycyclic gelsemium alkaloids. Angew Chem Int Ed Engl. 2019;58(3):681–694.
- Zhang W, Zhang S, Yin Z, Wang L, Ye W. Monoterpenoid indole alkaloids from Gelsemium Elegans. Heterocycles. 2014;89(5):1245–1253.
- Wang L, Wang JF, Mao X, Jiao L, Wang XJ. Gelsedine-type oxindole alkaloids from Gelsemium elegans and the evaluation of their cytotoxic activity. Fitoterapia. 2017; 120:131–135.
- Jin P, Zhan G, Zheng G, Liu J, Peng X, Huang L, Gao B, Yuan X, Yao G. Gelstriamine A, a triamino monoterpene indole alkaloid with a caged 6/5/7/6/6/5 scaffold and analgesic alkaloids from Gelsemium elegans stems. J Nat Prod. 2021;84(4):1326–1334.
- Xue Q, Hu J, Liu X, Gu J. Cytotoxic gelsedine-type indole alkaloids from Gelsemium elegans. J Asian Nat Prod Res. 2020;22(12):1138–1144.
- Sun MX, Cui Y, Li Y, Meng WQ, Xu QQ, Zhao J, Lu JC, Xiao K. Indole alkaloids from Gelsemium elegans. Phytochemistry. 2019; 162:232–240.
- Zhang W, Xu W, Wang G, Gong X, Li N, Wang L, Ye W. Gelsekoumidines A and B: two pairs of atropisomeric bisindole alkaloids from the roots of Gelsemium elegans. Org Lett. 2017;19(19):5194–5197.
- Wei X, Huang XT, Zhang LY, Hu XY, Zhang W, Zhou YQ, Yu HF, Ding CF, Zhang LC, Liu X, et al. New oxindole alkaloids with selective osteoclast inhibitory activity from Gelsemium elegans. Nat Prod Res. 2021;36(10):2630–2636.
- Wang HT, Yang YC, Mao X, Wang Y, Huang R. Cytotoxic gelsedine-type indole alkaloids from Gelsemium elegans. J Asian Nat Prod Res. 2018;20(4):321–327.
- Sun M, Gao H, Zhao J, Zhang L, Xiao K. New oxindole alkaloids from Gelsemium elegans. Tetrahedron Lett. 2015;56(45):6194–6197.
- Li NP, Liu M, Huang XJ, Gong XY, Zhang W, Cheng MJ, Ye WC, Wang L. Gelsecorydines A-E, five gelsedine-corynanthe-type bisindole alkaloids from the fruits of Gelsemium elegans. J Org Chem. 2018;83(10):5707–5714.
- Gu J, Zhang W, Cai W, Fu X, Zhou H, Li N, Tian H, Liu J, Ye W, Wang L. Gelserancines A-E, monoterpenoid indole alkaloids with unusual skeletons from Gelsemium elegans. Org Chem Front. 2021;8(9):1918–1925.
- Qu J, Fang L, Ren XD, Liu Y, Yu SS, Li L, Bao XQ, Zhang D, Li Y, Ma SG. Bisindole alkaloids with neural anti-inflammatory activity from Gelsemium elegans. J Nat Prod. 2013;76(12):2203–2209.
- Wei X, Yang J, Ma H, Ding C, Yu H, Zhao Y, Liu Y, Khan A, Wang Y, Yang Z, et al. Antimicrobial indole alkaloids with adductive C-9 aromatic unit from Gelsemium elegans. Tetrahedron Lett. 2018;59(21):2066–2070.
- Wei X, Guo R, Wang X, Liang JJ, Yu HF, Ding CF, Feng TT, Zhang LY, Liu X, Hu XY, et al. New monoterpenoid indoles with osteoclast activities from Gelsemium elegans. Molecules. 2021;26(24):7457.
- Zhang W, Huang XJ, Zhang SY, Zhang DM, Jiang RW, Hu JY, Zhang XQ, Wang L, Ye WC. Geleganidines A-C, unusual monoterpenoid indole alkaloids from Gelsemium elegans. J Nat Prod. 2015;78(8):2036–2044.
- Xu YK, Yang L, Liao SG, Cao P, Wu B, Hu HB, Guo J, Zhang P. Koumine, humantenine, and yohimbane alkaloids from Gelsemium elegans. J Nat Prod. 2015;78(7):1511–1517.
- Zhang W, Zhang S, Wang G, Li N, Chen M, Gu J, Zhang D, Wang L, Ye W. Five new koumine-type alkaloids from the roots of Gelsemium elegans. Fitoterapia. 2017; 118:112–117.
- Sun M, Hou X, Gao H, Guo J, Xiao K. Two new koumine-type indole alkaloids from Gelsemium elegans Benth. Molecules. 2013;18(2):1819–1825.
- Li NP, Liu JS, Liu JW, Tian HY, Zhou HL, Zheng YR, Huang XJ, Cao JQ, Ye WC, Wang L. Monoterpenoid indole alkaloids from the fruits of Gelsemium elegans and their anti-inflammatory activities. Bioorg Chem. 2021; 107:104624.
- Liu L, Cao JX, Yao YC, Xu SP. Progress of pharmacological studies on alkaloids from Apocynaceae. J Asian Nat Prod Res. 2013;15(2):166–184.
- Pan L, Terrazas C, Muñoz Acuña U, Ninh TN, Chai H, Carcache de Blanco EJ, Soejarto DD, Satoskar AR, Kinghorn AD. Bioactive indole alkaloids isolated from Alstonia angustifolia. Phytochem Lett. 2014; 10:54–59.
- Xiong B, Jin G, Xu Y, You W, Luo Y, Fang M, Chen B, Huang H, Yang J, Lin X, et al. Identification of koumine as a translocator protein 18 kda positive allosteric modulator for the treatment of inflammatory and neuropathic pain. Front Pharmacol. 2021; 12:692917.
- Xiong B, You W, Luo Y, Wu JG, Xu M, Yang Y, Huang J, Yu H. C. Investigation of the possible allostery of koumine extracted from Gelsemium elegans benth. and analgesic mechanism associated with neurosteroids. Front Pharmacol. 2021;12:739618.
- Qiu HQ, Xu Y, Jin GL, Yang J, Liu M, Li SP, Yu CX. Koumine enhances spinal cord 3α-hydroxysteroid oxidoreductase expression and activity in a rat model of neuropathic pain. Mol Pain. 2015;11:46.
- Xiong BJ, Xu Y, Jin GL, Liu M, Yang J, Yu CX. Analgesic effects and pharmacologic mechanisms of the Gelsemium alkaloid koumine on a rat model of postoperative pain. Sci Rep. 2017;7(1):14269.
- Jin GL, He SD, Lin SM, Hong LM, Chen WQ, Xu Y, Yang J, Li SP, Yu CX. Koumine attenuates neuroglia activation and inflammatory response to neuropathic pain. Neural Plast. 2018;2018:9347696.
- Jin G, Yue R, He S, Hong L, Xu Y, Yu C. Koumine decreases astrocyte-mediated neuroinflammation and enhances autophagy, contributing to neuropathic pain from chronic constriction injury in rats. Front Pharmacol. 2018;9:989.
- Ling Q, Liu M, Wu MX, Xu Y, Yang J, Huang HH, Yu CX. Anti-allodynic and neuroprotective effects of koumine, a Benth alkaloid, in a rat model of diabetic neuropathy. Biol Pharm Bull. 2014;37(5):858–864.
- Jin G, Hong L, Liu H, Yue R, Shen Z, Yang J, Xu Y, Huang H, Li Y, Xiong B, et al. Koumine modulates spinal microglial M1 polarization and the inflammatory response through the Notch-RBP-J kappa signaling pathway, ameliorating diabetic neuropathic pain in rats. Phytomedicine. 2021;90:153640.
- Ye LX, Huang HH, Zhang SH, Lu JS, Cao DX, Wu DD, Chi PW, Hong LH, Wu MX, Xu Y, et al. Streptozotocin-induced hyperglycemia affects the pharmacokinetics of koumine and its anti-allodynic action in a rat model of diabetic neuropathic pain. Front Pharmacol. 2021;12:640318.
- Zhang J, Gong N, Huang J, Guo L, Wang Y. Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal alpha 3 glycine receptors. Pain. 2013;154(11):2452–2462.
- Lara CO, Murath P, Muñoz B, Marileo AM, Martín LS, San Martín VP, Burgos CF, Mariqueo TA, Aguayo LG, Fuentealba J, et al. Functional modulation of glycine receptors by the alkaloid gelsemine. Br J Pharmacol. 2016;173(14):2263–2277.
- Shoaib RM, Zhang J, Mao X, Wang Y. Gelsemine and koumine, principal active ingredients of Gelsemium, exhibit mechanical antiallodynia via spinal glycine receptor activation-induced allopregnanolone biosynthesis. Biochem Pharmacol. 2019;161:136–148.
- Wu YE, Li YD, Luo YJ, Wang TX, Wang HJ, Chen SN, Qu WM, Huang ZL. Gelsemine alleviates both neuropathic pain and sleep disturbance in partial sciatic nerve ligation mice. Acta Pharmacol Sin. 2015;36(11):1308–1317.
- Li G, Zhong Y, Wang W, Jia X, Zhu H, Jiang W, Song Y, Xu W, Wu S. Sempervirine mediates autophagy and apoptosis via the Akt/mTOR signaling pathways in glioma cells. Front Pharmacol. 2021;12:770667.
- Yue R, Liu H, Huang Y, Wang J, Shi D, Su Y, Luo Y, Cai P, Jin G, Yu C. Sempervirine inhibits proliferation and promotes apoptosis by regulating Wnt/β-Catenin pathway in human hepatocellular carcinoma. Front Pharmacol. 2021;12:806091.
- Caggiano C, Guida E, Todaro F, Bielli P, Mori M, Ghirga F, Quaglio D, Botta B, Moretti F, Grimaldi P, et al. Sempervirine inhibits RNA polymerase I transcription independently from p53 in tumor cells. Cell Death Discov. 2020;6(1):111.
- Wang L, Xu H, Liang J, Ding Y, Meng F. An integrated network, RNA sequencing, and experiment pharmacology approach reveals the active component, potential target, and mechanism of Gelsemium elegans in the treatment of colorectal cancer. Front Oncol. 2020; 10:616628.
- Chen C, Zhong Z, Xin Z, Hong L, Su Y, Yu C. Koumine exhibits anxiolytic properties without inducing adverse neurological effects on functional observation battery, open-field and Vogel conflict tests in rodents. J Nat Med. 2017;71(2):397–408.
- Xiong B, Zhong Z, Chen C, Huang H, Lin J, Xu Y, Yang J, Yu C. The anxiolytic effect of koumine on a predatory sound stress-induced anxiety model and its associated molecular mechanisms. Phytomedicine. 2022;103:154225.
- Meyer L, Boujedaini N, Patte-Mensah C, Mensah-Nyagan AG. Pharmacological effect of gelsemine on anxiety-like behavior in rat. Behav Brain Res. 2013;253:90–94.
- Yu H, Tang M, Zeng Z, Huang S, Zheng X, Liu Z. Suppressive effects of gelsemine on anxiety-like behaviors induced by chronic unpredictable mild stress in mice. Brain Sci. 2022;12(2):191.
- Luo Y, Xiong B, Liu H, Chen Z, Huang H, Yu C, Yang J. Koumine suppresses IL-1 beta secretion and attenuates inflammation associated with blocking ROS/NF-kappa B/NLRP3 axis in macrophages. Front Pharmacol. 2021;11:622074.
- Feng M, Kong D, Guo H, Xing C, Lv J, Bian H, Lv N, Zhang C, Chen D, Liu M, et al. Gelsevirine improves age-related and surgically induced osteoarthritis in mice by reducing STING availability and local inflammation. Biochem Pharmacol. 2022;198:114975.
- Yang J, Cai HD, Zeng YL, Chen ZH, Fang MH, Su YP, Huang HH, Xu Y, Yu CX. Effects of koumine on adjuvant- and collagen-induced arthritis in rats. J Nat Prod. 2016;79(10):2635–2643.
- Jin GL, Yang J, Chen WQ, Wang J, Qiu HQ, Xu Y, Yu CX. The analgesic effect and possible mechanisms by which koumine alters type II collagen-induced arthritis in rats. J Nat Med. 2019;73(1):217–225.
- Lin Y, Liu Q, Chen Z, Zheng F, Huang H, Yu C, Yang J. The immunomodulatory effect of koumine on B cells under dependent and independent responses by T cells. Eur J Pharmacol. 2022; 914:174690.
- Li Z, Zhang J, Zhang R, Kuang Y. Extraction of koumine from Gelsemium Elegans Benth. and its therapeutic effect on collagen-induced arthritis in mice. Food Sci Tech. 2022; 42:e10421–e10421.
- Diethelm S, Carreira EM. Total synthesis of (±)-Gelsemoxonine. J Am Chem Soc. 2013;135(23):8500–8503.
- Diethelm S, Carreira EM. Total synthesis of gelsemoxonine through a spirocyclopropane isoxazolidine ring contraction. J Am Chem Soc. 2015;137(18):6084–6096.
- Harada T, Shimokawa J, Fukuyama T. Unified total synthesis of five gelsedine-type alkaloids: (-)-gelsenicine, (-)-gelsedine, (-)-gelsedilam, (-)-14-hydroxygelsenicine, and (-)-14,15-dihydroxygelsenicine. Org Lett. 2016;18(18):4622–4625.
- Shimokawa J, Harada T, Yokoshima S, Fukuyama T. Total synthesis of gelsemoxonine. J Am Chem Soc. 2011;133(44):17634–17637.
- Huang YM, Liu Y, Zheng CW, Jin QW, Pan L, Pan RM, Liu J, Zhao G. Total synthesis of gelsedilam by means of a thiol-mediated diastereoselective conjugate addition-aldol reaction. Chemistry. 2016;22(51):18339–18342.
- Newcomb ET, Knutson PC, Pedersen BA, Ferreira EM. Total synthesis of gelsenicine via a catalyzed cycloisomerization strategy. J Am Chem Soc. 2016;138(1):108–111.
- Knutson PC, Ji H, Harrington CM, Ke Y, Ferreira EM. Chirality transfer and asymmetric catalysis: two strategies toward the enantioselective formal total synthesis of (+)-gelsenicine. Org Lett. 2022;24(27):4971–4976.
- Wang P, Gao Y, Ma D. Divergent entry to gelsedine-type alkaloids: total syntheses of (-)-gelsedilam, (-)-gelsenicine, (-)-gelsedine, and (-)-gelsemoxonine. J Am Chem Soc. 2018;140(37):11608–11612.
- Saito A, Kogure N, Kitajima M, Takayama H. Total synthesis of (-)-14-hydroxygelsenicine and six biogenetically related gelsemium alkaloids. Org Lett. 2019;21(17):7134–7137.
- Chen X, Duan S, Tao C, Zhai H, Qiu FG. Total synthesis of (+)-gelsemine via an organocatalytic Diels-Alder approach. Nat Commun. 2015; 6:7204.
- Lam JK, Joseph SB, Vanderwal CD. A Zincke aldehyde approach to gelsemine. Tetrahedron Lett. 2015;56(23):3165–3168.
- Kitajima M, Watanabe K, Maeda H, Kogure N, Takayama H. Asymmetric total synthesis of sarpagine-related indole alkaloids hydroxygardnerine, hydroxygardnutine, gardnerine, (E)-16-epi-normacusine b, and koumine. Org Lett. 2016;18(8):1912–1915.
- Kerkovius JK, Kerr MA. Total synthesis of isodihydrokoumine, (19Z)-taberpsychine, and (4R)-isodihydroukoumine N-4-oxide. J Am Chem Soc. 2018;140(27):8415–8419.
- Loya DR, Maddaluno J, De Paolis M. Study toward an asymmetric and catalytic synthesis of koumine. Heterocycles. 2019;99(2):1388–1397.
- Rebmann H, Gerlinger CKG, Gaich T. Gram-scale total synthesis of sarpagine alkaloids and non-natural derivatives. Chemistry. 2019;25(11):2704–2707.
- Yang Z, Tan Q, Jiang Y, Yang J, Su X, Qiao Z, Zhou W, He L, Qiu H, Zhang M. Asymmetric total synthesis of sarpagine and koumine alkaloids. Angew Chem Int Ed Engl. 2021;60(23):13105–13111.
- Chen W, Ma Y, He W, Wu Y, Huang Y, Zhang Y, Tian H, Wei K, Yang X, Zhang H. Structure units oriented approach towards collective synthesis of sarpagine-ajmaline-koumine type alkaloids. Nat Commun. 2022;13(1):908.
- Rao TSC, Saha S, Raolji GB, Patro B, Risbood P, Difilippantonio MJ, Tomaszewski JE, Malhotra SV. Microwave assisted Westphal condensation and its application to synthesis of sempervirine and related compounds. Tetrahedron Lett. 2013;54(6):487–490.
- Pan X, Yang C, Cleveland JL, Bannister TD. Synthesis and cytoxicity of sempervirine and analogues. J Org Chem. 2016;81(5):2194–2200.
- Jiang T, Wang H, Liu L, Song H, Zhang Y, Wang J, Liu L, Xu T, Fan R, Xu Y, et al. CircIL4R activates the PI3K/AKT signaling pathway via the miR-761/TRIM29/PHLPP1 axis and promotes proliferation and metastasis in colorectal cancer. Mol Cancer. 2021;20(1):167.
- Chu G, Shan W, Ji X, Wang Y, Niu H. Multi-omics analysis of novel signature for immunotherapy response and tumor microenvironment regulation patterns in urothelial cancer. Front Cell Dev Biol. 2021;9:764125.
- Zhou X, Zhang B, Zhao X, Lin Y, Zhuang Y, Guo J, Wang S. Chlorogenic acid prevents hyperuricemia nephropathy via regulating TMAO-related gut microbes and inhibiting the PI3K/AKT/mTOR pathway. J Agric Food Chem. 2022;70(33):10182–10193.
- Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18):4331.
- Wang L, Sun Q, Zhao N, Wen YQ, Song Y, Meng FH. Ultra-liquid chromatography tandem mass spectrometry (UPLC-MS/MS)-based pharmacokinetics and tissue distribution study of koumine and the detoxification mechanism of Glycyrrhiza uralensis Fisch on Gelsemium elegans Benth. Molecules. 2018;23(7):1693.
- Qi X, Zuo M, Huang S, Ma X, Wang Z, Liu Z. Metabolic profile and tissue distribution of humantenirine, an oxindole alkaloid from Gelsemium, after oral administration in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2021; 1181:122901.
- Zhang S, Hu S, Yang X, Shen J, Zheng X, Huang K, Xiang Z. Development of a liquid chromatography with mass spectrometry method for the determination of gelsemine in rat plasma and tissue: Application to a pharmacokinetic and tissue distribution study. J Sep Sci. 2015;38(6):936–942.