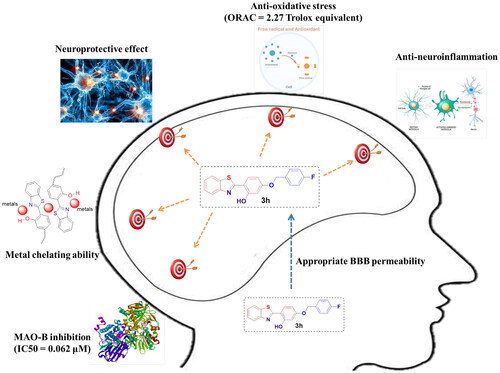
Abstract
To discover novel multifunctional agents for the treatment of Parkinson’s disease, a series of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives was designed, synthesized and evaluated. The results revealed that representative compound 3h possessed potent and selective MAO-B inhibitory activity (IC50 = 0.062 µM), and its inhibitory mode was competitive and reversible. Additionally, 3h also displayed excellent anti-oxidative effect (ORAC = 2.27 Trolox equivalent), significant metal chelating ability and appropriate BBB permeability. Moreover, 3h exhibited good neuroprotective effect and anti-neuroinflammtory ability. These results indicated that compound 3h was a promising candidate for further development against PD.
Introduction
Parkinson’s disease (PD), the second common neurodegenerative disease, is characterized by the loss of dopamine neurons and formation of Lewy bodies (LB)Citation1. The symptoms of PD include motor symptoms (tremor, rigidity, bradykinesia, and postural instability) and non-motor symptoms (anosmia, constipation, sleep disorders, dementia, and depression), causing immense agony to the patients and caregiversCitation2. Nowadays, more than 6.1 million people are suffering from PD, and this figure is increasing rapidly which makes PD become the fastest-growing neurological disorder in prevalence, disability, and deathsCitation3. However, there is no drug or therapy that can arrest or reverse the progression of PDCitation4. Therefore, it is urgent to discovery novel anti-PD agents.
The pathogenesis of PD is still not clear, but studies have revealed that several factors played important role in this process, such as abnormal aggregation of α-synuclein, increase in oxidative stress, neuroinflammation, bio-metals dyshomeostasis, decline in dopamine (DA) level and so onCitation5. The dopamine neurons in the brain of PD patients are pathologically degenerated, while the level of monoamine oxidase B (MAO-B) is conversely increasedCitation6. Because DA was predominantly produced by dopamine neurons and catalytically decomposed by MAO-B, these pathological changes could significantly reduce the DA level in PD patients and lead corresponding motor and non-motor syptomsCitation7. Besides, the deamination of DA catalysed by MAO-B could produce a large amount of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which would induce the increase in oxidative stressCitation8. On the one hand, the overproduced ROS and RNS can damage the lipid, protein and DNA, leading the death of cells; on the other hand, these reactive species can also promote the aggregation of α-synuclein and induce neuroinflammation, which have the ability to aggravate the oxidative stress vice versaCitation9. Additionally, bio-metals dyshomeostasis also contributes to the increase in oxidative stress levelCitation10. In detail, iron ions are abnormally elevated in the striatum of PD patients, and these iron ions can accelerate the production of ROS through Fenton reactionCitation11. Besides, iron ions can also induce the death of cells through ferroptosis.Citation12 Copper ion also has the ability to induce Fenton reaction, while its concentration is comparatively low in the brain of PD patientsCitation13. Therefore, drugs which possess MAO-B inhibitory activities, antioxidant abilities and metal chelating effects are necessary in PD patients.
Neuroinflammation is another hallmarker of PDCitation14. Under normal conditions, neuroinflammation helps us to defense against harmful invasion and eliminate waste, but, when neuroinflammation is chronic, it triggers neuronal damageCitation15. Unfortunately, chronic neuroinflammation is clearly existed in the brain of PD patientsCitation16. The microglias in the brain of PD patients are pathologically activated, and then excess inflammatory cytokines would be released, like tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), nitric oxide (NO), which have the ability to induce the dopaminergic neuronal death, increase in oxidative stress and α-synuclein aggregationCitation17. So, inhibiting neuroinflammation is an efficient way to cure PD.
PD is a complex disease, so drugs which can only intervene one single target are not efficient to cure PDCitation18. Current studies revealed that using “multi-target-directed ligands” (MTDLs) strategy to design small molecules which could interact with multiple targets of complex disease possessed natural advantagesCitation19. Especially, the agents with MAO-B inhibitory activity and other activities may have the ability to reverse the progression of PD while relieving the symptomsCitation20.
Benzothiazole is a versatile bicyclic heterocycle and benzothiazole derivatives exhibited various biologic activities, such as anti-inflammatory activity, antimicrobial activity, anticonvulsant activity, antioxidant activity and so onCitation21–24. Recent studies also revealed that some benzothiazole derivatives possessed good anti-PD effects. In particular, the indole-substituted benzothiazoles designed by Nam et al. generally exhibited excellent MAO-B inhibitory activities, and the representative compound even displayed a good therapeutic effect on Parkinsonian motor symptom in MPTP-induced PD modelCitation25. Therefore, benzothiazole is an ideal scaffold for the discovery of novel anti-PD agents. Furthermore, studies revealed that aryl benzyl ether is a multifunctional pharmacophore, and aryl benzyl ether derivatives showed many anti-PD activities, such as MAO-B inhibition, antioxidant activity, and neuroprotective effectCitation26–29. Therefore, benzothiazole skeleton was hybrided with aryl benzyl ether pharmacophore to afford a series of 2-(4-(benzyloxy) phenyl) benzothiazole derivatives firstly. Interestingly, the obtained derivatives possess much similar scaffold with imine resveratrols, while some imine resveratrol derivatives also displayed good metal-chelating ability when the ortho-position on the benzene ring was substituted by hydroxyl group, since this structure was similar with clioquinol (a metal chelator)Citation30. Therefore, hydroxyl group was further introduced to the ortho-position on the B benzene ring of 2-(4-(benzyloxy) phenyl) benzothiazole derivatives. Taken together, a series of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives were designed, and these derivatives were expected to act as MAO-B inhibitors with antioxidant activities, metal chelating abilities, neuroprotective effects, anti-neuroinflammatory activities and BBB permeation abilities. The design strategy is depicted in .
Results and discussion
Chemistry
2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives were synthesised by using 2-aminothiophenol (1) as starting material (Scheme 1). The condensation of compound 1 with 2,4-dihydroxybenzaldehyde in the presence of Na2S2O5 afforded 2-(2,4-(dihydroxyl) phenyl) benzothiazole (2)Citation31. Then, the subsequent reaction of key intermediate (2) with corresponding benzyl chlorides or benzyl bromides under the condition of NaHCO3/KI in CH3CN produced 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivativesCitation32. All the target compounds were characterised by the 1H NMR, 13C NMR, and ESI-MS in this study. In addition, the purity of target compounds was determined by high-performance liquid chromatography (HPLC) analysis to be over 97%.
Pharmacology
Evaluation of MAOs inhibitory activities
The MAOs inhibitory activities of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives were measured by kynuramine methodCitation33, with safinamide, rasagiline, iproniazid, and clorgyline as positive compounds. The result was shown in . Noticeably, all the derivatives exhibited excellent MAO-B inhibitory activities, while their MAO-A inhibitory effects were comparatively weak, so these derivatives are selective MAO-B inhibitors. Among the target derivatives, representative compound 3h displayed the most potent MAO-B inhibitory activity with an IC50 value of 0.062 µM, which is stronger than the IC50 value of rasagiline (IC50 = 0.0953 µM) but slightly weaker than the IC50 value of safinamide (IC50 = 0.0572 µM). Besides, compound 3n showed the greatest MAO-A inhibition with an IC50 value of 9.80 µM.
Table 1. Inhibitory effects on MAOs and antioxidative activities of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives and reference compounds in vitro.
The structure-activity relationship (SAR) analysis revealed that the substituent groups and their number as well as position played important role in the MAO-A/B inhibitory activities. Obviously, compared with compound 3a, derivatives 3b–3o showed stronger MAO-B inhibition, indicating the introduction of substituent groups on the benzyloxy ring is favourable for MAO-B inhibition. Besides, derivatives with fluoro substitution showed better MAO-B inhibition than compounds which bear other substituent groups on the same position, while derivatives with chlorine substitution exhibited more potent MAO-A inhibitory activities. This result reflected that the fluoro substitution on the benzyloxy ring was good for the interaction of target compounds with MAO-B, and chlorine substitution was beneficial for the derivatives to occupy the catalytic centre of MAO-A. Additionally, it is noticeable that derivatives (3h and 3i) with fluoro substitution or chlorine substitution on the para-position of benzyloxy ring exhibited stronger MAO-A/B inhibitory activity than compounds with fluoro substitution or chlorine substitution on the ortho-position and meta-position. This phenomenon revealed that it is more favourable for MAO-A/B inhibition when the halogenation occurs on the para-position of benzyloxy ring. Furthermore, 3m and 3n displayed superior MAO-A inhibitory activities than 3d, 3e, 3h, and 3i, so the dihalogenation on the 3,4-position of benzyloxy ring is favourable for the improvement of MAO-A inhibitory activities. Interestingly, the MAO-A/B inhibitory activities and SAR analysis results of these compounds are much similar with those of coumarin derivatives reported by Secci et al.Citation34, which may provide clue to find new MAOs inhibitors.
Kinetic study
Kinetic studyCitation35,Citation36 was conducted to figure out the MAO-B inhibitory mechanism of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives, with 3h as the test compound. The catalytic rates of MAO-B were measured at four concentrations of kynuramine (15, 30, 60, and 90 µM), and the Lineweaver-Burk plots were thus constructed in the absence or presence of three concentrations of 3h (2 × IC50, IC50, and 1/2 IC50). The graphical of the reciprocal Lineweaver-Burk plots was depicted in . It is noticeable that both the slopes (decreased Vmax) and intercepts (higher Km) were increased when the concentration of 3h was elevated, and these straight lines intersected at Y axis. This result revealed that the inhibition pattern of 3h was competitive, which provides the support for that 3h is a reversible MAO inhibitor. Besides, the graph obtained from the slopes of the Lineweaver-Burk plots vs. inhibitor concentration () revealed the competitive inhibition constant (Ki) of 3h was 0.102 µM, as the x-axis intercept is −Ki.
Reversibility study
To figure out the MAO-B inhibition mode of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives, dialysis methodCitation37 was carried out with 3h as the test compound. Safinamide (reversible MAO-B inhibitor) and rasagiline (irreversible MAO-B inhibitor) were used as control compounds. According to the literatureCitation38, the MAO-B inhibitory mode can be divided into three types according to the MAO-B catalytic activities in dialysis groups (reversible inhibition: MAO-B catalytic activity ≥ 80%; irreversible inhibition: MAO-B catalytic activity ≤ 20%; quasi-reversible inhibition: 20% < MAO-B catalytic activity < 80%).
The experimental result was shown in . Not surprisingly, the MAO-B catalytic activities in the undialysis groups were 27.6% (3h), 39.3% (safinamide) and 2.7% (rasagiline). Besides, the MAO-B activities in dialysis group of safinamide recovered to 93.0%, while the MAO-B activities in rasagiline dialysis group only recovered to 4.2%, reflecting their MAO-B inhibitory behaviour were reversible and irreversible, respectively. Compound 3h exhibited similar MAO-B inhibitory mode with safinamide, and the MAO-B catalytic activities in its dialysis group recovered to 80.9%. It revealed that 3h is a reversible MAO-B inhibitor.
Docking study
Molecular docking studyCitation39 was carried out to clarify the interaction modes of 3h with MAO-A/B by using the software packages of Autodock 4.2. The crystal structures of hMAO-A (PDB ID: 2Z5X) and hMAO-B (PDB ID: 2V5Z) were used for this study, and the result was depicted in . Obviously, representative compound 3h showed strong interactions with MAO-B. In detail, 3h induced one hydrogen bond interaction with Tyr435 (3h-OH•••O-Tyr435) and formed two parallel π–π interactions with Tyr435 and Tyr398. Besides, 3h also adopted T-shaped π–π interaction with flavin adenine dinucleotide (FAD), and exhibited hydrophobic interactions with the amino acid residues Leu164, Tyr326, Ile199, Ile198, Tyr188, Tyr60, and Gly434. Therefore, compound 3h had strong affinity to MAO-B and showed potent MAO-B inhibition.
Figure 4. (A) The docking mode of compound 3h with hMAO-A (PDB ID: 2Z5X), (B) The docking mode of compound 3h with hMAO-B (PDB ID: 2V5Z), 3h and amino acid residues that participate in the interactions were displayed as sticks and coloured by the element, hydrogen bonds are showed as green dashed lines.

As for the binding mode of 3h with MAO-A (), 3h formed parallel π–π interaction with Tyr444 and adopted T-shaped π–π interaction with FAD. Additionally, 3h also displayed hydrophobic interactions with the amino acid residues Cys323, Gly110, Ala111, Ile180, Asn181 and Tyr407, while no hydrogen bond interaction was observed in the docking complex of 3h-MAO-A. Therefore, 3h exhibited relatively weak inhibition on MAO-A, and displayed selective inhibition on MAO-B.
In vitro antioxidant activity study
Oxygen radical absorbance capacity assay which used fluorescein (ORAC-FL) was performed to measure the antioxidant activities of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivativesCitation40. Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), was used as a standard, and the result was shown in . Obviously, all the derivatives showed excellent antioxidant activities in this assay, with ORAC-FL values between 2.13 and 2.48 Trolox equivalents. In particular, 3k exhibited the most potent activity (ORAC-FL value = 2.48-fold of Trolox), and the representative compound 3h also displayed good antioxidant activity with an ORAC-FL value of 2.14 Trolox equivalents. Compared with compound 3a, all the other derivatives showed much higher ORAC-FL value, indicating the introduction of substituent groups on benzyloxy ring was favourable for the improvement of radical scavenge activities. Besides, compound 3k and 3l showed superior antioxidant activity than other derivatives. It reflected that introduction of alkyl substituents on benzyloxy ring was more beneficial for the improvement of in vitro antioxidant activity. However, the position and number of substituents seem to have little influence on the antioxidative activities.
Metal-chelating study
UV spectra methodCitation41 was performed to measure the metal chelating ability of 3h, and the result was shown in . Obviously, the UV spectra were significantly changed after the addition of Fe3+, Al3+, Zn2+, Cu2+, Fe2+, reflecting that 3h could interact with these biometals. Particularly, after the addition of FeSO4, maximum increase in absorption was detected. Besides, after the addition of CuCl2, maximum absorption peak shifted from 335.5 to 382.0 nm. Therefore, 3h showed strong interaction with Fe2+ and Cu2+ and can play the role in removing the excess metals in PD brain.
Figure 5. (A) UV spectra of compound 3h (75.0 μM in methanol) with or without FeSO4, ZnCl2, CuCl2, FeCl3, or AlCl3 (75.0 μM in methanol); (B) UV spectra of compound 3h (75.0 μM in methanol) with FeSO4 (0, 7.5, 15.0, 30.0, 45.0, 60.0, 75.0, 112.5, 150.0, and 187.5 μM); (C) Determination of the stoichiometry of 3h-Fe2+ complex by using the molar ratio method of titrating the methanol solution of compounds 3h with ascending amounts of FeSO4.

Because Fe2+ played a crucial role in the pathogenesis of PD, the stoichiometry of 3h-Fe2+ complex was further measured by using molar ratio method. 3h was incubated with various concentrations of FeSO4 (0–187.5 µM), and the electronic spectra were detected. As shown in , the absorbance at 385 nm initially increased linearly and then became plateaued with the increase of Fe2+ concentration. The point for the straight lines to intersect was determined to be at a mole fraction of 1.65, revealing an almost 2:3 stoichiometry for 3h-Fe2+ complex.
In vitro blood-brain barrier permeation study
A parallel artificial membrane permeation assay was performed to predict the blood-brain barrier (BBB) permeation ability of 3hCitation42. To gain the standard, the permeabilities of 11 drugs were measured firstly, and then the obtained results (Table S1) were compared with the values reported by literature. Not surprisingly, good linear correlation was observed: Pe (exp.) = 0.8792 × Pe (bibl.) − 0.0616 (R2 = 0.9555) (Figure S1). Further considering the limit established by Di et al.Citation43, we used 3.46 × 10−6 cm/s as the standard, and compounds which showed better permeabilities than the standard could cross BBB (Table S2). Then the predicted BBB permeability of 3h was measured, the result shown in revealed that the Pe value of 3h (4.03 × 10−6 cm/s) was higher than the standard, meaning that 3h could cross the BBB and reach its therapeutic targets.
Table 2. Permeability results Pe (×10−6 cm/s) for 3h and its predicted penetration into CNS.
In vitro neuroprotective effect study
MTT method was used to detect the in vitro neuroprotective effect of 3h on H2O2 induced PC-12 cells injury, with resveratrol as positive controlCitation44. Firstly, the cytotoxicity of 3h to PC-12 cells was measured. As shown in , the positive control resveratrol did not significantly alter the PC-12 cell viability at the concentration of 10.0 µM, and 3h also did not exhibit cytotoxicity up to the concentration of 50.0 µM. Then the neuroprotective effect of 3h was further tested, and the result was shown in . Noticeably, the cell viability was significantly reduced to 50.1% of control value when the PC-12 cells were exposed to H2O2 (100 µM), while treatment with resveratrol (10.0 µM) could reverse the cell viability to 64.3% of control value, indicating resveratrol possesses significant neuroprotective effect. Besides, treatment with 3h could also increase the cell viability to 57.2, 65.6, and 75.9% of control value at the concentrations of 2.5, 10.0, and 50.0 µM, respectively. Therefore, representative compound 3h exhibited similar neuroprotective effect with resveratrol. Moreover, the protective potency of 3h displayed a dose dependent manner, which also verified that 3h is not toxic towards the PC-12 cells up to the concentration of 50 µM. Overall, these results revealed that 3h possessed excellent neuroprotective effect because it could reduce the oxidative damage by acting as a scavenger of H2O2.
In vitro anti-neuroinflammation study
The in vitro anti-neuroinflammatory activity of 3h was detected by using LPS-induced inflammation mode in BV-2 cells, with resveratrol as the positive control compoundCitation45. The cytotoxicities of resveratrol and 3h to BV-2 cells were firstly detected by MTT method. As shown in , resveratrol and 3h did not significantly alter the BV-2 cell viability up to the concentration of 10.0 µM in the presence or absence LPS (1.0 µg/mL). It reflected that resveratrol and representative compound 3h possessed low cytotoxicity to BV-2 cells, and LPS (1.0 µg/mL) also did not influence the cell viability of BV-2 cells.
Figure 7. The effects of resveratrol and 3h on the cell viability of BV-2 cells (A) without LPS and (B) in the presence of LPS (1.0 μg/mL). Each assay was carried out at least three times and data are expressed as mean ± SD.

Then, Griess method was performed to measure the inhibitory effect of 3h on LPS-induced NO release in BV-2 cellsCitation46. The result was shown in . Noticeably, the concentration of NO in control group is comparative low, while it was remarkably increased when BV-2 cells were exposed to LPS (1.0 µg/mL). It reflected that LPS could induce the activation of BV-2 cells and induce neuroinflammation. Besides, treatment of resveratrol (10.0 µM) significantly reduced the LPS-induced NO production, indicating resveratrol have remarkable anti-inflammatory activities (51.2% inhibition at 10.0 µM). Additionally, treatment of 3h also decreased the NO production, and 3h exhibited more potent inhibitory effect than resveratrol, and its inhibitory rates were 15.8, 35.8, and 63.7% at the concentration of 0.5, 2.5, and 10.0 µM. Moreover, the effect of 3h showed a dose dependent manner, which verified that the inhibitory effect of 3h is not due to the cytotoxicity.
Figure 8. The inhibitory effects of resveratrol and 3h on NO release (A) and TNF-α production (B) in LPS-activated BV-2 microglia cells. The data are expressed as the mean ± SD from three independent experiments. #p < 0.05 vs. control group; *p < 0.05 vs. model group.

ELISA method was carried out to detect the inhibitory effects of 3h on LPS-induced TNF-α productionCitation47. As shown in , the level of TNF-α expression was dramatically raised when BV-2 cells were exposed to LPS (1.0 µg/mL). Besides, the treatment of resveratrol also significantly decreased the production of TNF-α, and its inhibitory rate was 50.3% at the concentration of 10.0 µM. Similarly, the inhibitory effect of 3h was more potent than resveratrol, with inhibition rates of 7.8, 25.7, and 52.5% at the concentration of 0.5, 2.5, and 10.0 µM, and the effect of 3h also showed a dose dependent manner. These results revealed that representative compound 3h possessed low cytotoxicity on BV-2 cells and good anti-neuroinflammatory activity in vitro.
Study of inhibitory effect on LPS-induced ROS release in vitro
The inhibitory effect of 3h on LPS-induced ROS release in BV-2 cells in vitro was detected by using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) as probe to quantify the ROS level in LPS-treated BV-2 cellsCitation48. The result was shown in . Noticeably, the fluorescence intensity of control group was relatively low, indicating low level of ROS. However, when the BV-2 cells were treated with LPS (1.0 µg/mL), the fluorescence intensity dramatically increased. It revealed that LPS (1.0 µg/mL) could trigger the release of ROS in BV-2 cells. Besides, the treatment of resveratrol and 3h significantly reduced the fluorescence intensity, so both resveratrol and 3h have the ability to relieve the rise in oxidative stress level induced by LPS. The fluorescence values were further detected by using microplate reader (), and then the inhibition rates were calculated. The inhibition rate of resveratrol was 59.8%, while the inhibition rates of 3h were 19.8 and 78.0% at the concentration of 2.5 and 10.0 µM. Therefore, 3h showed slightly more potent inhibitory effect than resveratrol on LPS-induced ROS release in vitro.
Figure 9. The inhibitory effects of resveratrol and 3h on LPS-induced ROS release in BV-2 microglia cells. (A) representative images, (B) relative fluorescence values obtained by microplate reader. The data are expressed as the mean ± SD from three independent experiments. ##p < 0.01 vs. control; *p < 0.05 vs. model group; **p < 0.01 vs. model group.

Conclusion
A series of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives was designed, synthesised and evaluated as multi-functional anti-PD agents. The biological screening indicated that most derivatives possessed potent MAO-B inhibitory activities and anti-oxidative abilities. Among them, compound 3h raised particular interest, because 3h showed the most potent MAO-B inhibitory activity (IC50 = 0.062 µM) and high antioxidant activity (ORAC = 2.27 Trolox equivalent). Further kinetic study and reversibility study revealed that its MAO-B inhibitory mode was competitive and reversible. Additionally, the molecular study disclosed the interaction modes of 3h with MAO-A and MAO-B. Besides, 3h also exhibited good metal chelating ability, and can form 3h-Fe2+ complex with an almost 2:3 stoichiometry. Furthermore, 3h displayed appropriate BBB permeability and significant neuroprotective effect. Moreover, in vitro anti-neuroinflammation study showed that 3h had the ability to inhibit the release of NO, TNF-α and ROS in LPS-treated BV-2 cells. Overall, these results revealed that 3h was a promising anti-PD agent, which is worthy of being further studied.
Experimental section
Chemistry
All reagents were purchased from commercial suppliers and were used without further purification. The reactions were monitored by thin-layer chromatography (TLC), and the spots in TCL were detected under UV light (254 or 365 nm). Column chromatography was carried out using silica gel (230–400 mesh) purchased from General-Reagent (China). The 1H NMR and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 on a Varian INOVA spectrometer at 25 °C with TMS as the internal standard. The mass spectra of target compounds were detected by the Shimadzu LC-MS 2020 Spectrometer. The purity of target compound was measured on a Shimadzu LC-10Avp plus system by using a Diamonsil C18 column (4.6 × 150 mm, 5 µM).
The synthesis of intermediate 2Citation31
To a solution of 2-aminothiophenol (0.43 ml, 3.98 mmol) and 2,4-dihydroxybenzaldehyde (605 mg, 4.38 mmol) in DMF (15 ml), Na2S2O5 (1.13 g, 5.94 mmol) was added. After addition, the mixture was refluxed under nitrogen atmosphere for 2 h. After the reaction was completed, the reaction mixture was allowed to cool and H2O (30 ml) was poured into it. The mixture was filtered, and the filter cake was collected and dried in vacuo to obtain crude intermediate 2, which was further purified by recrystallization from ethanol to afford the pure product as brown solid, yield 65.7%, mp > 220 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.68 (brs, 1H), 10.20 (brs, 1H), 8.09 (d, J = 8.0 Hz, 1H), 7.98–7.92 (m, 2H), 7.50 (t, J = 7.2 Hz, 1H), 7.39 (t, J = 7.2 Hz, 1H), 6.46 (brs, 2H).
General procedure for the synthesis of 3a ∼ oCitation32
Intermediate 2 (36.5 mg, 0.15 mmol), substituted benzyl chloride or benzyl bromide (0.16 mmol), NaHCO3 (18.9 mg, 0.23 mmol), acetonitrile (5.0 ml) and KI (catalytic amount) were added to a round bottom flask (10.0 ml) successively. The suspension was heated to 60 °C under a nitrogen atmosphere for 30 h. Then, the suspension was filtrated, and the filtrate was evaporated under reduced pressure. Subsequently, the residue was purified by column chromatography to afford corresponding target compounds.
2-(Benzo[d]thiazol-2-yl)-5-(benzyloxy)phenol (3a)
3a was obtained as brown solid, mp 131.5–132.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.10 (d, J = 7.6 Hz, 1H), 8.03 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H), 7.53–7.35 (m, 7H), 6.70 (d, J = 8.4 Hz, 1H), 6.69 (s, 1H), 5.18 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.3, 162.3, 158.6, 151.1, 137.0, 133.9, 130.4, 129.0 (2 C), 128.5, 128.2 (2 C), 126.9, 125.2, 122.4, 122.1, 112.1, 108.3, 102.6, 69.9. ESI-MS m/z: 332.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((2-fluorobenzyl)oxy)phenol (3b)
3b was obtained as brown solid, mp 107.4–108.6 °C. 1H NMR (400 MHz, CDCl3) δ 12.75 (brs, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.52–7.46 (m, 2H), 7.38–7.32 (m, 2H), 7.18 (t, J = 7.2 Hz, 1H), 7.11 (t, J = 8.8 Hz, 1H), 6.69 (s, 1H), 6.60 (d, J = 8.0 Hz, 1H), 5.18 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 169.2, 162.3, 161.7–159.3 (d, JC-F = 245.8 Hz), 159.9, 151.8, 132.2, 130.0–129.9 (d, JC-F = 8.2 Hz), 129.8, 129.7, 126.6, 125.1, 124.4–124.3 (d, JC-F = 3.5 Hz), 123.6–123.5 (d, JC-F = 14.1 Hz), 121.7, 121.4, 115.6–115.4 (d, JC-F = 20.8 Hz), 110.8, 108.1, 102.4, 63.9–63.8 (d, JC-F = 4.4 Hz). ESI-MS m/z: 350.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((2-chlorobenzyl)oxy)phenol (3c)
3c was obtained as brown solid, mp 113.9–135.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.87 (brs, 1H), 8.11 (d, J = 8.0 Hz, 1H), 8.08 (d, J = 8.4 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.63–7.61 (m, 1H), 7.56–7.50 (m, 2H), 7.45–7.40 (m, 3H), 6.75 (d, J = 2.4 Hz, 1H), 6.73–6.71 (m, 1H), 5.24 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.1, 162.1, 158.5, 151.9, 134.3, 134.0, 133.1, 130.6, 130.5, 130.4, 129.9, 127.9, 126.9, 125.2, 122.4, 122.1, 112.4, 108.2, 102.5, 87.5. ESI-MS m/z: 366.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3-fluorobenzyl)oxy)phenol (3d)
3d was obtained as brown solid, mp 101.7–102.3 °C. 1H NMR (400 MHz, CDCl3) δ 12.77 (brs, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 8.8 Hz, 1H), 7.48 (td, J1 = 8.0 Hz, J2 = 1.2 Hz, 1H), 7.38 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.22–7.16 (m, 2H), 7.04 (td, J1 = 8.4 Hz, J2 = 1.6 Hz, 1H), 6.65 (d, J = 2.4 Hz, 1H), 6.60 (dd, J1 = 8.4 Hz, J2 = 2.4 Hz, 1H), 5.11 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 169.2, 164.2–161.8 (d, JC-F = 244.9 Hz), 162.2, 159.9, 151.8, 138.9 (d, JC-F = 7.3 Hz), 132.2, 130.3–130.2 (d, JC-F = 8.2 Hz), 129.7, 126.6, 125.1, 122.8 (d, JC-F = 3.0 Hz), 121.7, 121.4, 115.2–115.0 (d, JC-F = 21.0 Hz), 114.4–114.2 (d, JC-F = 22.0 Hz), 110.8, 108.2, 102.4, 69.3 (d, JC-F = 1.9 Hz). ESI-MS m/z: 350.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3-chlorobenzyl)oxy)phenol (3e)
3e was obtained as brown solid, mp 123.0–123.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.89 (brs, 1H), 8.10 (d, J = 7.6 Hz, 1H), 8.03 (d, J = 8.8 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.54–7.49 (m, 2H), 7.45–7.39 (m, 4H), 6.73–6.68 (m, 2H), 5.20 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.3, 162.1, 158.6, 151.9, 139.7, 133.9, 133.6, 130.9, 130.4, 128.4, 127.8, 126.9, 126.7, 125.3, 122.4, 122.1, 112.2, 108.3, 102.7, 69.0. ESI-MS m/z: 366.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3-(trifluoromethyl)benzyl)oxy)phenol (3f)
3f was obtained as brown solid, mp 118.4–119.7 °C. 1H NMR (400 MHz, CDCl3) δ 12.78 (brs, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.72 (s, 1H), 7.65–7.59 (m, 3H), 7.53 (t, J = 8.0 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H), 7.38 (t, J = 8.0 Hz, 1H), 6.67 (d, J = 2.4 Hz, 1H), 6.61 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 5.16 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 169.1, 162.1, 159.9, 151.8, 137.4, 132.2, 131.3–130.9 (d, JC-F = 32.2 Hz), 130.6, 129.8, 129.2, 126.6, 125.4–122.7 (d, JC-F = 270.7 Hz), 125.1, 125.1–124.9 (q, JC-F = 3.7 Hz), 124.2–124.1 (q, JC-F = 3.7 Hz), 121.8, 121.4, 110.9, 108.1, 102.4, 69.3. ESI-MS m/z: 400.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3-methoxybenzyl)oxy)phenol (3g)
3g was obtained as brown solid, mp 90.5–92.1 °C. 1H NMR (400 MHz, CDCl3) δ 12.77 (brs, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.48 (td, J1 = 8.0 Hz, J2 = 1.2 Hz, 1H), 7.37 (td, J1 = 8.0 Hz, J2 = 0.8 Hz, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 6.99 (brs, 1H), 6.88 (dd, J1 = 8.0 Hz, J2 = 2.4 Hz, 1H), 6.67 (d, J = 2.4 Hz, 1H), 6.60 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 5.09 (s, 2H), 3.83 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.2, 162.5, 159.9, 151.9, 137.9, 132.2, 129.9, 129.8, 129.7, 126.6, 125.1, 121.7, 121.4, 119.7, 113.7, 112.9, 110.6, 108.2, 102.4, 70.0, 55.3. ESI-MS m/z: 362.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((4-fluorobenzyl)oxy)phenol (3h)
3h was obtained as brown solid, mp 135.1–136.5 °C. 1H NMR (400 MHz, CDCl3) δ 12.79 (brs, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.48 (dd, J1 = 8.0 Hz, J2 = 1.2 Hz, 1H), 7.44–7.40 (m, 2H), 7.37 (td, J1 = 8.0 Hz, J2 = 0.8 Hz, 1H), 7.12–7.06 (m, 2H), 6.66 (d, J2 = 2.8 Hz, 1H), 6.58 (dd, J1 = 8.8 Hz, J2 = 2.8 Hz, 1H), 5.06 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 169.2, 163.9–161.4 (d, JC-F = 245.0 Hz), 162.3, 159.9, 151.8, 132.2, 132.1–132.0 (d, JC-F = 3.2 Hz), 129.7, 129.5–129.4 (d, JC-F = 8.3 Hz, 2 C), 126.6, 125.1, 121.7, 121.4, 115.7–115.5 (d, JC-F = 21.4 Hz, 2 C), 110.7, 108.2, 102.3, 69.5. ESI-MS m/z: 350.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((4-chlorobenzyl)oxy)phenol (3i)
3i was obtained as brown solid, mp 132.8–133.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.90 (brs, 1H), 8.11 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.54–7.47 (m, 5H), 7.42 (td, J1 = 8.0 Hz, J2 = 0.8 Hz, 1H), 6.72 (d, J2 = 2.4 Hz, 1H), 6.69 (dd, J1 = 8.0 Hz, J2 = 2.4 Hz, 1H), 5.18 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.2, 162.1, 158.6, 151.9, 136.1, 133.9, 133.0, 130.4, 130.1 (2 C), 129.0 (2 C), 126.9, 125.2, 122.4, 122.1, 112.2, 108.3, 102.6, 69.1. ESI-MS m/z: 366.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((4-methylbenzyl)oxy)phenol (3j)
3j was obtained as brown solid, mp 145.5–146.2 °C. 1H NMR (400 MHz, CDCl3) δ 12.74 (brs, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.48 (t, J = 7.2 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.34 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 6.67 (t, J = 2.4 Hz, 1H), 6.69 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 5.07 (s, 2H), 2.37 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 169.3, 162.6, 159.9, 151.9, 138.1, 133.2, 132.2, 129.7, 129.4 (2 C), 127.7 (2 C), 126.6, 125.1, 121.7, 121.4, 110.5, 108.3, 102.3, 70.1, 21.3. ESI-MS m/z: 346.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((4-(tert-butyl)benzyl)oxy)phenol (3k)
3k was obtained as brown solid, mp 151.4–152.0 °C. 1H NMR (400 MHz, CDCl3) δ 12.73 (brs, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.50–7.35 (m, 6H), 6.68 (d, J = 2.4 Hz, 1H), 6.60 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 5.08 (s, 2H), 1.33 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 169.3, 162.7, 159.9, 151.9, 151.3, 133.3, 132.2, 129.6, 127.5 (2 C), 126.6, 125.6 (2 C), 125.0, 121.7, 121.4, 110.6, 108.3, 102.3, 70.1, 34.6, 31.3 (3 C). ESI-MS m/z: 388.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((4-nitrobenzyl)oxy)phenol (3l)
3l was obtained as brown solid, mp 128.5–129.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.25 (d, J = 8.4 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 8.03 (d, J = 8.4 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.50 (t, J = 7.6 Hz, 1H), 7.39 (t, J = 7.6 Hz, 1H), 6.70 (m, 2H), 5.34 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.2, 161.8, 151.9, 147.5, 145.1, 134.1, 130.3, 128.7 (2 C), 126.8, 125.1, 124.1 (2 C), 122.3, 122.0, 112.6, 107.7, 102.8, 68.7, 29.5. ESI-MS m/z: 377.1 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3,4-difluorobenzyl)oxy)phenol (3m)
3m was obtained as brown solid, mp 136.6–137.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.90 (brs, 1H), 8.11 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 8.8 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.60–7.40 (m, 4H), 7.35 (brs, 1H), 6.72–6.69 (m, 2H), 6.17 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.3, 162.0, 158.6, 151.9, 151.1–148.6 (dd, JC-F = 244.0 Hz, JC-F = 12.5 Hz), 150.8–148.3 (dd, JC-F = 244.2 Hz, JC-F = 12.1 Hz), 134.9–134.8 (q, JC-F = 3.0 Hz), 133.9, 130.4, 126.9, 125.3, 125.2–125.1 (q, JC-F = 3.0 Hz), 122.4, 122.1, 118.2–118.0 (d, JC-F = 17.0 Hz), 117.5–117.3 (d, JC-F = 17.0 Hz), 112.3, 108.3, 102.7, 68.6. ESI-MS m/z: 368.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3,4-dichlorobenzyl)oxy)phenol (3n)
3n was obtained as brown solid, mp 125.3–126.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.09 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 9.2 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.74 (s, 1H), 7.67 (d, J = 8.4 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.40 (t, J = 7.6 Hz, 1H), 6.68 (brs, 2H), 5.18 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.1, 161.9, 160.3, 152.0, 138.5, 134.2, 131.6, 131.2, 130.9, 130.1, 130.0, 128.3, 126.7, 124.8, 122.3, 121.9, 112.8, 107.1, 102.8, 68.2. ESI-MS m/z: 340.0 [M − H]−.
2-(Benzo[d]thiazol-2-yl)-5-((3-chloro-4-fluorobenzyl)oxy)phenol (3o)
3o was obtained as brown solid, mp 130.9–131.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.89 (brs, 1H), 8.10 (d, J = 7.6 Hz, 1H), 8.04 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.71 (d, J = 6.8 Hz,1H), 7.53–7.46 (m, 3H), 7.41 (t, J = 7.6 Hz, 1H), 7.72–6.69 (m, 2H), 5.16 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.3, 162.0, 158.6, 158.5–156.1 (d, JC-F = 245.4 Hz), 151.9, 135.1–135.0 (d, JC-F = 4.0 Hz), 133.9, 130.4 (2 C), 129.1–129.0 (d, JC-F = 7.7 Hz), 126.9, 125.3, 122.4, 122.1, 120.1–119.9 (d, JC-F = 17.7 Hz), 117.6–117.4 (d, JC-F = 20.8 Hz), 112.3, 108.3, 102.7, 68.5. ESI-MS m/z: 384.0 [M − H]−.
Pharmacology
Inhibition experiments of MAOsCitation33
Test compound solution (10 µL), MAO-A or MAO-B solution (30 µL, 12.5 µg/mL in PBS) were added to a black 96-wells plate successively. The mixture was incubated at 37 °C for 30 min. Then, kynuramine solution (10 µL, 224 µM for MAO-A or 150 µM for MAO-B in PBS) was added, the mixture was incubated at 37 °C for another 30 min. After incubation, NaOH solution (40 µL, 2 mol/l in water) and H2O (100 µL) were added to end the reaction, the fluorescence was measured with excitation and emission wavelengths at 310 and 400 nm on the multifunctional enzyme marker (Thermo Scientific), and the percentage inhibition was calculated by the formula: 100 − (IFi − IF0)/(IFc − IF0)*100. The IC50 was further detected if their percentage inhibition at the concentration of 10 µM was higher than 50%.
Kinetic studyCitation35,Citation36
The kinetic study of 3h was conducted by the method which is similar with the inhibition experiment of MAOs. Different concentrations of 3h (0.124, 0.062, and 0.031 µM) and kynuramine (15, 30, 60, and 90 µM) solutions were selected. The control experiment was performed without 3h. The method of data analysis has been described previously.
Reversibility studyCitation37
MAO-B solution (0.3 ml, 0.06 mg/mL in PBS containing 5% sucrose) and test compound solution (0.3 ml, 4 × IC50) were added to a vial. The mixture was incubated at 37 °C for 30 min and transferred to dialysis bag (MWCO: 10 000, flattening width: 24 mm, length: 8–10 cm). Then, this dialysis bag was placed in 200 ml PBS for 24 h, and the outer PBS was replaced with fresh at 3 and 7 h, respectively. As for the undialysis group, the mixture was placed at 4 °C for 24 h. After dialysis, the mixtures (40 µL) in the dialysis bag were taken out to measure the catalytic activity, and the test method was similar with the inhibition experiment of MAOs. Safinamide was used as positive control and rasagiline was used as negative control. The test compound solution was replaced with PBS buffer at the same volume in the blank group.
Molecular docking with MAOs
The molecular docking study was performed by using autodock 4.2 software packageCitation39. Firstly, the crystal structures of MAO-A (PDB ID: 2z5x) and MAO-B (PDB ID: 2v5z) were obtained from PDB website (https://www.rcsb.org/). Then, original ligands (harmine or safinamide) and water molecules were removed from the crystals, and the polar hydrogens were added to the obtained crystals. Subsequently, the chemical structure of 3h was plotted using chemdraw software, and then the lowest energy conformation of 3h was calculated in Chem3D and its torsion tree was defined in autodock 4.2 software. After preparation of macromolecules and ligand, the ligand was embed into the macromolecular receptor using autogrid. After the embedment, the autodock program was run, and the flexible docking program generally runs 100 times. The conformation with the highest populated cluster and lowest energy was chosen to the result discussion.
In vitro antioxidant activity studyCitation40
Test compounds solution (20 µL, 10 µM) and fluorescein solution (120 µL, 250 nM) were added to a black 96-wells plate in order. The mixture was incubated for 15 min at 37 °C. Then, AAPH solution (60 µL, 40 mM) was added through an automatic sampler. The fluorescence was recorded with excitation and emission wavelengths at 485 and 535 nm on the multifunctional enzyme marker (Thermo Scientific), and the area under the curve (AUC) was calculated automatically. Trolox was used as a reference, and the antioxidant activities of the compounds were expressed as Trolox equivalent. The test compound solution was replaced with PBS (20 µL) in blank well. The antioxidant activities of the test compounds were calculated by the formula: [(AUCsample − AUCblank)/(AUCtrolox − AUCblank)] × [(Trolox concentration/test sample concentration)].
Metal-chelating studiesCitation41
UV spectrum method was conducted to clarify the metal chelating ability of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives. Test compound solution (1.0 ml, 75 µM in methanol) and metal ion solution (1.0 ml, 75 µM in methanol) were added to a vial. The vial was placed at room temperature for 2 h. Then, the mixture was removed from the vial to a quartz cell. After transfer, the absorbance of the mixture at 200–800 nm was measured by using UV spectrophotometer (Shimadzu UV-1900i).
As the Fe2+ played important role in the pathogenetic process of PD, the stoichiometry of 3h-Fe2+ complex was detected by using molar ratio method. In detail, test compound solution (1.0 ml, 75 µM in methanol) was incubated with Fe2+ solution (1.0 ml, 7.5–187.5 µM in methanol) at 37 °C for 30 min. Then, the mixture was removed from the vial to a quartz cell, and the absorbance of mixture at 200–800 nm was measured by using UV spectrophotometer (Shimadzu UV-1900i).
In vitro blood-brain barrier permeation assay
The method described by Di et al.Citation43 was carried out to detect the blood-brain barrier permeation of 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives. Porcine brain lipid solution (4 µL, 20 mg/mL in n-dodecane) was added dropwise to a filter membrane. Then the membrane was placed still at room temperature for 5 min to form an artificial biomembrane. When this bionics membrane was formed, test compound solution (350 µL, 100 µg/mL) was added to the donor well which was over the artificial biomembrane, and the mixture of PBS and Ethanol (200 µL, 70/30, v/v) was added to receptor well which is under the bionics membrane. Then, the “sandwich” was incubated at 25 °C for 18 h. After incubation, 150 µL solution in both donor well and receptor well were taken out and added to a 96-well quartz cuvette, and the absorbance of the mixture at 200–800 nm was measured. The Pe value was calculated according to the formula depicted by the literature.
Study of neuroprotective effect on H2O2-mediated PC12 cells injury
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method was conducted to measure the in vitro neuroprotective effect of 3hCitation44. Firstly the cytotoxicity of 3h towards PC-12 cells was detected. In detail, PC-12 cells were seeded into 96-well cell culture plate in DMEM (100 µL, 1 × 105 cells per mL) firstly. Then, these cells were incubated at 37 °C for 24 h. After incubation, test compound solutions (10 µL) were added to the plate, and the mixture was incubated for another 2 h at 37 °C. Then, MTT solution (100 µL, 5 mg/ml in PBS) was added to each well, which would be incubated at 37 °C for final 4 h. After the final incubation, the supernatants were removed, and the formazan was dissolved in DMSO (150 µL). Then, the absorbance at 490 nm was determined by using a Varioskan Flash Multimode Reader (Thermo Scientific). As for the assay to evaluate the neuroprotective effect of 3h, the procedures were almost similar, but after the incubation of cells with 3h, H2O2 (100 µM) was added and the mixture was further incubated for 24 h.
Study of anti-neuroinflammatory effect in vitro
Firstly, the MTT method was carried out to determine the cytotoxicity of 3h towards BV-2 cell lineCitation45. BV-2 cells in the logarithmic phase were seeded into 96-well cell culture plate, followed by the incubation at 37 °C for 24 h at the atmosphere of 5% CO2. After incubation, the culture was replaced by fresh one (90 µL without serum), and test compound solution (10 µL) was added. Then, the mixture was incubated for another 30 min, and MTT (100 µL, 0.5 mg/ml in PBS) was added to each well. After the final incubation at 37 °C for 4 h, the supernatants were removed, and the formazan was dissolved in 200 µL of DMSO. Then, the absorbance at 490 nm was determined by using a Varioskan Flash Multimode Reader (Thermo Scientific). As for the method to evaluate the cytotoxicity of 3h towards LPS-stimulated BV-2 cells, the procedures were almost similar, but after the incubation of cells with 3h, LPS (10 µL, 1.0 µg/mL) was added, and the mixture was further incubated for 24 h.
ELISA and Griess reaction methods were performed to detect the inhibitory effect of 3h on the release of TNF-α and NO in LPS-stimulated BV-2 cellsCitation46,Citation47. BV-2 cells in the logarithmic phase were seeded into 96-well cell culture plate, and these cells were incubated at 37 °C for 24 h at the atmosphere of 5% CO2. After incubation, the culture solution was replaced by fresh one (90 µL without serum), and test compound solution (10 µL) was added. After addition, the plate was incubated for 30 min, then LPS (10 µL, 1.0 µg/ml in PBS) was added to each well, and the mixture would be incubated for another 24 h at 37 °C. After the final incubation, the supernatants were taken out to measure the level of TNF-α and NO according to the procedures depicted on the kits (kits were purchased from Nanjing Jiancheng Bioengineering Institute).
Study of inhibitory effect on LPS-induced ROS release in vitroCitation48
BV-2 cells were incubated with test compound solution (10 µL) for 30 min, then LPS (10 µL, 1.0 µg/ml in PBS) was added to each well, and each well would be incubated for another 30 min at 37 °C. Then, DCFH-DA (100 µL, 10 µM) was added, and the mixture was incubated for another 40 min at 37 °C. The fluorescence was measured with excitation and emission wavelengths at 488 and 525 nm on an inverted fluorescence microscope.
Supplemental Material
Download PDF (2.2 MB)Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021; 397(10291):2284–2303.
- Hayes MT. Parkinson’s disease and Parkinsonism. Am J Med. 2019;132(7):802–807.
- Dorsey ER, Elbaz A, Nichols E, Abbasi N, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi J-YJ, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):939–953.
- Azam F, El-Gnidi BA, Alkskas IA, Ahmed MA. Design, synthesis and anti-Parkinsonian evaluation of 3-alkyl/aryl-8-(furan-2-yl)thiazolo[5,4-e][1,2,4]triazolo[1,5-c]pyrimidine-2(3H)-thiones against neuroleptic-induced catalepsy and oxidative stress in mice. J Enzyme Inhib Med Chem. 2010;25(6):818–826.
- Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson’s disease: implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700.
- Tan YY, Jenner P, Chen SD. Monoamine oxidase-B inhibitors for the treatment of Parkinson’s disease: past, present, and future. J Parkinsons Dis. 2022;12(2):477–493.
- Group PMC. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196–1205.
- Santin Y, Resta J, Parini A, Mialet-Perez J. Monoamine oxidases in age-associated diseases: new perspectives for old enzymes. Ageing Res Rev. 2021;66:101256.
- Aborode AT, Pustake M, Awuah WA, Alwerdani M, Shah P, Yarlagadda R, Ahmad S, Silva Correia IF, Chandra A, Nansubuga EP, et al. Targeting oxidative stress mechanisms to treat Alzheimer’s and Parkinson’s disease: a critical review. Oxid Med Cell Longev. 2022;2022:7934442.
- Raj K, Kaur P, Gupta GD, Singh S. Metals associated neurodegeneration in Parkinson’s disease: insight to physiological, pathological mechanisms and management. Neurosci Lett. 2021;753:135873.
- Knorle R. Neuromelanin in Parkinson’s disease: from Fenton reaction to calcium signaling. Neurotox Res. 2018;33(2):515–522.
- Thapa K, Khan H, Kanojia N, Singh TG, Kaur A, Kaur G. Therapeutic insights on ferroptosis in Parkinson’s disease. Eur J Pharmacol. 2022;930:175133.
- Bisaglia M, Bubacco L. Copper ions and Parkinson’s disease: why is homeostasis so relevant? Biomolecules. 2020;10(2):195.
- Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022;22(11):657–673.
- Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157–172.
- Sarkar S, Nguyen HM, Malovic E, Luo J, Langley M, Palanisamy BN, Singh N, Manne S, Neal M, Gabrielle M, et al. Kv1.3 modulates neuroinflammation and neurodegeneration in Parkinson’s disease. J Clin Invest. 2020;130(8):4195–4212.
- Liu TW, Chen CM, Chang KH. Biomarker of neuroinflammation in Parkinson’s disease. Int J Mol Sci. 2022;23(8):4148.
- Sasaki NA, Sonnet P. A novel multi-target strategy to attenuate the progression of Parkinson’s disease by diamine hybrid AGE/ALE inhibitor. Future Med Chem. 2021;13(24):2185–2200.
- Carradori S, Ortuso F, Petzer A, Bagetta D, De Monte C, Secci D, De Vita D, Guglielmi P, Zengin G, Aktumsek A, et al. Design, synthesis and biochemical evaluation of novel multi-target inhibitors as potential anti-Parkinson agents. Eur J Med Chem. 2018;143:1543–1552.
- Anastassova N, Aluani D, Hristova-Avakumova N, Tzankova V, Kondeva-Burdina M, Rangelov M, Todorova N, Yancheva D. Study on the neuroprotective, radical-scavenging and MAO-B inhibiting properties of new benzimidazole arylhydrazones as potential multi-target drugs for the treatment of Parkinson’s disease. Antioxidants. 2022;11(5):884–1552.
- Ugwu DI, Okoro UC, Ukoha PO, Gupta A, Okafor SN. Novel anti-inflammatory and analgesic agents: synthesis, molecular docking and in vivo studies. J Enzyme Inhib Med Chem. 2018;33(1):405–415.
- Lad NP, Manohar Y, Mascarenhas M, Pandit YB, Kulkarni MR, Sharma R, Salkar K, Suthar A, Pandit SS. Methylsulfonyl benzothiazoles (MSBT) derivatives: search for new potential antimicrobial and anticancer agents. Bioorg Med Chem Lett. 2017;27(5):1319–1324.
- Siddiqui N, Alam MS, Sahu M, Naim MJ, Yar MS, Alam O. Design, synthesis, anticonvulsant evaluation and docking study of 2-[(6-substituted benzo[d]thiazol-2-ylcarbamoyl)methyl]-1-(4-substituted phenyl)isothioureas. Bioorg Chem. 2017;71:230–243.
- Payaz DU, Kucukbay FZ, Kucukbay H, Angeli A, Supuran CT. Synthesis carbonic anhydrase enzyme inhibition and antioxidant activity of novel benzothiazole derivatives incorporating glycine, methionine, alanine, and phenylalanine moieties. J Enzyme Inhib Med Chem. 2019;34(1):343–349.
- Nam M-H, Park M, Park H, Kim Y, Yoon S, Sawant VS, Choi JW, Park J-H, Park KD, Min S-J, et al. Indole-substituted benzothiazoles and benzoxazoles as selective and reversible MAO-B inhibitors for treatment of Parkinson’s disease. ACS Chem Neurosci. 2017;8(7):1519–1529.
- Sudevan ST, Rangarajan TM, Al-Sehemi AG, Nair AS, Koyiparambath VP, Mathew B. Revealing the role of the benzyloxy pharmacophore in the design of a new class of monoamine oxidase-B inhibitors. Arch Pharm. 2022;355(8):e2200084.
- Wang Z, Wu J, Yang X, Cai P, Liu Q, Wang KDG, Kong L, Wang X. Neuroprotective effects of benzyloxy substituted small molecule monoamine oxidase B inhibitors in Parkinson’s disease. Bioorg Med Chem. 2016;24(22):5929–5940.
- Hassan RM, Aboutabl ME, Bozzi M, El-Behairy MF, El Kerdawy AM, Sampaolese B, Desiderio C, Vincenzoni F, Sciandra F, Ghannam IAY, et al. Discovery of 4-benzyloxy and 4-(2-phenylethoxy) chalcone fibrate hybrids as novel PPARα agonists with anti-hyperlipidemic and antioxidant activities: design, synthesis and in vitro/in vivo biological evaluation. Bioorg Chem. 2021;115:105170.
- Yeon SK, Choi JW, Park J-H, Lee YR, Kim HJ, Shin SJ, Jang BK, Kim S, Bahn Y-S, Han G, et al. Synthesis and evaluation of biaryl derivatives for structural characterization of selective monoamine oxidase B inhibitors toward Parkinson’s disease therapy. Bioorg Med Chem. 2018;26(1):232–244.
- Li SY, Wang XB, Kong LY. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur J Med Chem. 2014;71:36–45.
- Yu Y, Xu H, Zhang W, Wang B, Jiang Y. A novel benzothiazole-based fluorescent probe for cysteine detection and its application on test paper and in living cells. Talanta. 2018;176:151–155.
- Ma J, Chen D, Lu K, Wang L, Han X, Zhao Y, Gong P. Design, synthesis, and structure-activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur J Med Chem. 2014;86:257–269.
- Weissbach H, Smith TE, Daly JW, Witkop B, Udenfriend S. A rapid spectrophotometric assay of monoamine oxidase based on the rate of disappearance of kynuramine. J Biol Chem. 1960;235(4):1160–1163.
- Chimenti F, Secci D, Bolasco A, Chimenti P, Bizzarri B, Granese A, Carradori S, Yáñez M, Orallo F, Ortuso F, et al. Synthesis, molecular modeling, and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J Med Chem. 2009;52(7):1935–1942.
- Legoabe LJ, Petzer A, Petzer JP. Selected C7-substituted chromone derivatives as monoamine oxidase inhibitors. Bioorg Chem. 2012;45:1–11.
- Chavarria D, Fernandes C, Silva V, Silva C, Gil-Martins E, Soares P, Silva T, Silva R, Remião F, Oliveira PJ, et al. Design of novel monoamine oxidase-B inhibitors based on piperine scaffold: structure-activity-toxicity, drug-likeness and efflux transport studies. Eur J Med Chem. 2020;185:111770.
- Legoabe LJ, Petzer A, Petzer JP. α-Tetralone derivatives as inhibitors of monoamine oxidase. Bioorg Med Chem Lett. 2014;24(12):2758–2763.
- Harfenist M, Heuser DJ, Joyner CT, Batchelor JF, White HL. Selective inhibitors of monoamine oxidase. 3. structure-activity relationship of tricyclics bearing imidazoline, oxadiazole, or tetrazole groups. J Med Chem. 1996;39(9):1857–1863.
- Li W, Yang X, Song Q, Cao Z, Shi Y, Deng Y, Zhang L. Pyridoxine-resveratrol hybrids as novel inhibitors of MAO-B with antioxidant and neuroprotective activities for the treatment of Parkinson’s disease. Bioorg Chem. 2020;97:103707.
- Chavarria D, Da Silva O, Benfeito S, Barreiro S, Garrido J, Cagide F, Soares P, Remião F, Brazzolotto X, Nachon F, et al. Fine-tuning the biological profile of multitarget mitochondriotropic antioxidants for neurodegenerative diseases. Antioxidants. 2021;10(2):329.
- Zhang L, Liu Y, Hu X, Wang Y, Xu M. Metal ion interactions with methyl gallate characterized by UV spectroscopic and computational methods. Food Chem. 2019;293:66–73.
- Tang Y-W, Shi C-J, Yang H-L, Cai P, Liu Q-H, Yang X-L, Kong L-Y, Wang X-B. Synthesis and evaluation of isoprenylation-resveratrol dimer derivatives against Alzheimer’s disease. Eur J Med Chem. 2019;163:307–319.
- Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood-brain barrier. Eur J Med Chem. 2003;38(3):223–232.
- Cao Z, Song Q, Yu G, Liu Z, Cong S, Tan Z, Deng Y. Novel 3-benzylidene/benzylphthalide Mannich base derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2021;35:116074.
- Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37.
- Tang JJ, Wang MR, Dong S, Huang LF, He QR, Gao JM. 1,10-Seco-eudesmane sesquiterpenoids as a new type of anti-neuroinflammatory agents by suppressing TLR4/NF-κB/MAPK pathways. Eur J Med Chem. 2021;224:113713.
- Fang Y, Xia W, Cheng B, Hua P, Zhou H, Gu Q, Xu J. Design, synthesis, and biological evaluation of compounds with a new scaffold as anti-neuroinflammatory agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;149:129–138.
- Ding T, Wang S, Zhang X, Zai W, Fan J, Chen W, Bian Q, Luan J, Shen Y, Zhang Y, et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53.