Abstract
Multi-target inhibitors represent useful anticancer agents with superior therapeutic attributes. Here in, two novel series of benzimidazole-triazole hybrids were designed, synthesised as multi-target EGFR, VEGFR-2 and Topo II inhibitors, and evaluated for anticancer activity. Compounds 5a and 6g were the most potent analogues against four cancer cell lines, HepG-2, HCT-116, MCF-7 and HeLa, and were further evaluated for EGFR, VEGFR-2, and Topo II inhibition. Compound 5a was especially good inhibitor for EGFR (IC50 = 0.086 µM) compared to Gefitinib (IC50 = 0.052 µM), moderate VEGFR-2 inhibitor (IC50 = 0.107 µM) compared to Sorafenib (IC50 = 0.0482 µM), and stronger Topo II inhibitor (IC50 = 2.52 µM) than Doxorubicin (IC50 = 3.62 µM). Compound 6g exhibited moderate EGFR and VEGFR-2 inhibition and weaker Topo II inhibition. DNA binding assay, cell cycle analysis, apoptotic induction, molecular docking, and physicochemical studies were additionally implemented to explore the plausible mechanism of the active compounds.
Introduction
Cancer is regarded as one of the most dreadful diseases worldwide. Development of more efficient drugs with multiple mechanisms became indispensable to control various cancer types, especially with the current innate ability of most cancer cells to evade the majority of the present chemotherapeuticsCitation1–3. Multi-targeting approaches give an ideal therapeutic paradigm to simultaneously interrupt more than one target to avoid prevailing drug resistance, giving insights for medicinal chemists to devote much effort for the design of new multi-targeted anticancer agentsCitation4.
Benzimidazole nucleus appeared as a crucial pharmacophore in cancer research; owing to its diverse anticancer potential with versatile mechanisms of tumour inhibition, beside its facile synthetic strategies to get assorted derivativesCitation2–9. Many reported anticancer drugs as well as different bioactive molecules contain the benzimidazole motifCitation10–15. It was manifested that the anticancer potential and selectivity of benzimidazole derivatives depended radically on different substitutions comprised by the benzimidazole scaffoldCitation2. This was the promising master key that unlocked all the doors for the development of novel target-specific and highly effective benzimidazole-based anticancer agents. In particular, 2-substituted benzimidazoles have been widely explored as anticancer agents with unique mechanisms, targeting not only different tyrosine kinases, but also other enzymesCitation5,Citation6.
As shown in , Bendamustine (I) is a nitrogen mustard benzimidazole based alkylating agent used in the treatment of chronic lymphomaCitation6. Dovitinib (II) is an orally active benzimidazole-quinolinone hybrid with potential antineoplastic activity as multiple receptor tyrosine kinases’ (RTKs) inhibitor. It strongly targets fibroblast growth factor receptor-1 (FGFR-1) (IC50 = 8 nM), vascular endothelial growth factor receptor-2 (VEGFR-2) (IC50 = 13 nM), and other RTKs involved in tumour growth and angiogenesis, like FGFR-3, VEGFR-1, VEGFR-3, and PDGFR. It also targets Topoisomerase II (Topo II) enzyme with IC50 of 13 μMCitation8. Dovitinib has been exclusively in-licensed worldwide by Novartis, who has completed phase-III study against renal cell carcinoma (RCC), in addition to several promising phase-II studies against liver, breast, endometrial cancer, and gastrointestinal stromal tumourCitation10–12.
Further, 2-substituted benzimidazole analogues (III) were assessed for their antitumor and antiangiogenic activities. They effectively antagonised VEGF-A165/NRP-1 binding, with IC50 values range of 0.05–0.40 μMCitation13. In addition, a new series of 2-aryl benzimidazoles was designed as multi-target RTKs inhibitors, and biologically evaluated against HepG-2 cells and different kinases; where compound (IV) gave 88% inhibition of epidermal growth factor receptor (EGFR), 33.1% inhibition of VEGFR-2, and a range of 42.7–52.6% inhibition concerning platelet-derived growth factor receptors (PDGFR-α,β)Citation6. Likewise, 2,5-disubstituted benzimidazole-indazole hybrids were designed and synthesised as multi-inhibitors of VEGFR-1, VEGFR-2, PDGFR, and FGFR-1, where compound (V) afforded potent effect against all the tested RTKs, with favourable pharmacokinetics and improved in vivo tumour growth inhibition properties that reached to about 88%Citation13.
Moreover, other 2-substituted benzimidazole derivatives were identified as potent Topo II inhibitorsCitation15,Citation16, like the 2-phenylthiomethylbenzimidazole (VI) that displayed enhanced activity against Topo II with IC50 of 17 μM, which is more potent than Etoposide reference drug (IC50 = 21.8 μM)Citation15.
On the other hand, 1,2,3‐triazole motifs have recently received considerable attention in drug discovery for the development of novel anticancer agents, since they represented potent pharmacophores that are implicated in numerous anticancer compoundsCitation17,Citation18. They have been reported to exert outstanding anticancer effect through different mechanisms via inhibition of various enzymes ()Citation17,Citation18. Triazole scaffold (VII) showed significant in vitro cytotoxicity against different human cancer cell lines via remarkable kinase inhibitory activity against EGFR (IC50 = 0.582 μM)Citation19. Similarly, the triazole derivative (VIII) offered potent inhibitory activity against EGFR (IC50 = 0.103 μM), compared to Erlotinib. The docking study revealed similar interactions with Erlotinib in the specified binding pocket, confirming its role as EGFR inhibitor. It formed a hydrogen bond with the Met769 amino acid residue via the triazole N3, in addition to double aromatic stabilisation with both Gly772 and Leu694 residues in the hinge region. Furthermore, the benzothiazole ring; that is considered to be a structural isostere of benzimidazole; presented good interaction with Leu820 and Asp831 key residuesCitation18.
An indole‐2‐one‐based 1,2,3‐triazole scaffold (IX) has displayed significant VEGFR inhibition on cancer cells (IC50 = 26.38 nM, better than Sunitinib, IC50 = 83.20 nM) whereas it was less toxic to human cellsCitation20. Furthermore, the docking studies of this scaffold confirmed that it is spatially embedded in a perfect way within the protein binding pocket, leading to potential VEGFR-2 inhibitionCitation20. Besides, the triazole derivative (X) displayed greater inhibitory activity against Topo IIB (IC50 = 0.52 μM) compared with Doxorubicin (Dox) (IC50 = 0.83 μM)Citation21.
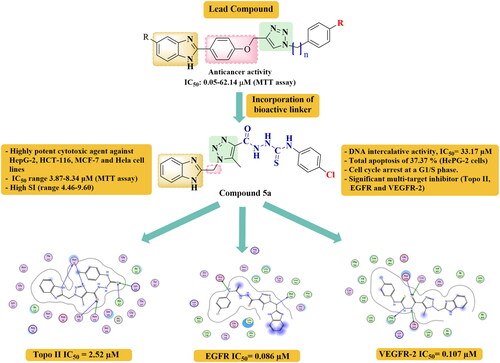
Accordingly, the attachment of the well reported anticancer pharmacophores; 2-substituted benzimidazole along with a 1,2,3-triazole backbone, based on a hybrid pharmacophore design, became an encouraging strategy to develop new highly effective anticancer candidates against both drug-resistant and drug-sensitive cancers due to their expected combined mechanisms. Over the past few years, benzimidazole-1,2,3-triazole hybrids (XI) were verified to exhibit considerable activity against A549, HeLa, CFPAC-1 (ductal pancreatic adenocarcinoma), and SW620 (metastatic colorectal adenocarcinoma) cells, with IC50 range of 0.05–62.14 μM, using MTT assay, where some compounds showed activity comparable to or even better than 5-Fluorouracil (IC50 range of 0.08–8.81), depending on certain variablesCitation22,Citation23. Structure-activity relationship (SAR) studies suggested that the shorter carbon spacer between 1,2,3-triazole and benzimidazole moiety was favourable to the activity, whereas the longer side chain between 1,2,3-triazole and other motifs was preferredCitation22–24.
Thus, taking together all these findings, we envisioned that a hybrid approach that combines benzimidazole at position 2 with 1,2,3-triazole via short (-CH2) group spacer unit, and using different elongated linkers between the triazole nucleus and other motifs in one structure might provide a new hybrid scaffold (XII) with potentiated multi-targeted; EGFR, VEGFR-2, and Topo II inhibitory activities (). Moreover, it was reported in different studies that the embodiment of (thio)ureido-moiety, like that present in Tivozanib, Sorafenib, and compound XIII, or azomethine connecting group, as in compound XIV, within the structure of a given compound, could enhance the antitumor activityCitation3,Citation25,Citation26. Therefore, our new scaffold (XII) was diversified by incorporating these active linkers as long spacers between 1,2,3-triazole and the aryl motif, hoping to get more potent anticancer candidates with multi-targeted molecular mechanisms (). On the other hand, connecting various substituents of different electronic properties to the phenyl ring was also accomplished, to explore their impact on activity. In brief; the rational design of our new compounds was based on the following considerations: (i) the benzimidazole scaffold itself, (ii) the presence of the effective 2- substituted position, (iii) linking of 1,2,3-triazole nucleus to the benzimidazole entity through short CH2- connecting group, which seems to play a crucial role in the cytotoxic activity, (iv) employing varied long spacers between the 1,2,3-triazole and different substituted phenyl rings to examine their cytotoxic behaviour, as shown in , and (v) investigate the chemical nature of different moieties and their hydrogen bond acceptor or donor properties, enclosed in the structures of the designed target compounds, which may contribute to the tolerability of these compounds within the binding pockets of targeted enzymes. Hopefully, our target compounds were designed to embrace the common structural requirements that can adequately fit with the three intended target enzymes; EGFR, VEGFR 2, and Topo II. The involvement of triazole ring was guaranteed to form the reported hydrogen bond with the Met793 amino acid residue, which is an essential feature for EGFR inhibitionCitation19. Further, all compounds contain the hydrogen bond domain and the hydrophobic tail, which are essential requirements for VEGFR 2 binding, like (thio)urea, or azomethine linkersCitation3,Citation25,Citation26. Moreover, the presence of benzimidazole nucleus in all derivatives was adopted as an essential scaffold for Topo II inhibition, where the benzimidazole nitrogen is reported to bind with the essential amino acid residues needed for activity, like Asn120, Asn95, Asn91, As150, Arg98, Ser148, or Lys157Citation15,Citation16.
Herein, we report our fruitful findings on the synthesis, characterisation and in vitro pharmacological evaluation of new series of benzimidazole-triazole hybrids, with different linkers, in order to produce potential multi-targeting anti-proliferative candidates. In an attempt to reveal the anticipated antitumor mechanism, cell cycle analysis beside EGFR, VEGFR-2, and Topo II inhibitory activities have been evaluated. Furthermore, molecular docking and physicochemical studies were assessed.
Results and discussion
Chemistry
The synthetic route used to access our target compounds 5a–h and 6a–g is shown in Scheme 1. 2-Chloro methyl benzimidazole 1 was transformed into the corresponding azide 2, using sodium azide in dry DMSOCitation23. Then, the formed azide 2 was condensed with ethyl acetoacetate (EAA) to afford the intermediate ester 3, which was further reacted with hydrazine hydrate to give the corresponding hydrazide 4Citation27,Citation28. The final target compounds 5a–h were obtained by reacting the hydrazide 4 with the appropriate substituted iso(thio)cyanates in THF at room temperature overnight. While compounds 6a–g were attained by reacting the hydrazide 4 with various benzaldehyde derivatives in refluxing ethanol, in the presence of catalytic amount of glacial acetic acid. The structures of all the title compounds were confirmed by IR, 1H-NMR, 13C-APT NMR, and elemental analysis. The 1H-NMR spectra of all synthesised target compounds were characterised by the disappearance of distinctive NH2 signal, formerly appearing at 4.45 ppm in the spectrum of the starting hydrazide 4. In addition, two characteristic singlet signals appeared in all compounds, at ∼ 2.64 and 5.94 ppm, corresponding to methyl protons (-CH3) and methylene protons (-CCH2N-), respectively. The formation of carbothio(oxa)amide derivatives 5a-h was also confirmed by the presence of remarkable singlet peaks, that appeared downfield in the range of 8.20–10.50 ppm, referring to the exchangeable NH protons (-NHNHCXNH-); while the formation of arylidene derivatives 6a-g was confirmed by the presence of characteristic singlet peak at ∼ 8.60 ppm, that corresponds to the azo methine proton (-N=CH-). Furthermore, 13C-APT NMR spectra revealed the appearance of two peaks at ∼157.0–163.0 ppm in compounds 5a-h, related to carbonyl/thione carbons or one peak at ∼157.5 ppm in derivatives 6a-g, related to carbonyl carbon. In addition, all final targets showed two characteristic peaks in the aliphatic region at approximately 8.9–45.9 ppm that were corresponded to the methyl and methylene carbons. All other spectral and analytical data were consistent with the assumed structures. The mass spectra of the final targets showed the correct molecular ion peaks (M+), as suggested by their molecular formulas. All compounds gave good CHNS quantitative elemental analysis results, in agreement with the calculated values.
Biological activity
In vitro studies
In vitro cytotoxic study against HepG-2, HCT-116, MCF-7, and HeLa cell lines
The newly synthesised compounds were screened for their cytotoxic activity against hepatocellular carcinoma (HepG-2), human colon carcinoma (HCT-116), breast adenocarcinoma (MCF-7), and cervical cell carcinoma (HeLa) at various concentrations via the standard MTT assay method, using Dox as a reference drug (). Inspection of results denoted that compounds 5a and 6g showed very strong cytotoxic activity against all the tested cancer cell lines. Within the first series (5a-h), compound 5g displayed very strong activity against HeLa and MCF-7 cells with IC50 values of 8.70 and 9.39 μM, respectively, and strong effect against HepG-2 and HCT-116 with IC50 values of 13.59 and 18.67 μM, respectively. Besides, compound 5e revealed strong activity against MCF-7 and HeLa cells with IC50 values of 16.57 and 19.14 µM, in succession, but gave moderate activity against HepG-2 and HCT-116. Compound 5d exhibited strong HeLa cell cytotoxic activity with IC50 = 12.39 µM, whereas it showed moderate activity against HCT-116 and MCF-7 and weak inhibitory effect against HepG-2. Concerning the second series (6a-g), compound 6f exhibited strong activity against HCT-116, MCF-7, and HepG-2 with IC50 values of 11.72, 14.69, and 18.31 μM, respectively, whereas it presented moderate activity against HeLa cells with IC50 = 22.75 μM. Also, compound 6e exerted strong cytotoxic activity against HCT-116 with IC50 = 19.69 µM, but gave only moderate activity against MCF-7, HeLa, and HepG-2 cells with IC50 values of, 23.73, 29.07, and 33.62 µM, respectively. Derivative 6a offered moderate activity towards the four tested cell lines, with IC50 range of 28.18–47.04 µM. Depending on the in vitro cytotoxic study, the tested compounds could be classified into four categories, ranging from weak to very strong active compounds, as shown in .
Table 1. IC50 values (µM) of target compounds 5a-h and 6a-g against four cancer cell lines in comparison with Dox.
SAR analysis
SAR analysis of the antitumor activity of the newly synthesised compounds against HepG-2, HCT-116, MCF-7, and HeLa cells revealed that the length of the designed spacer has an impact upon activity, where the first series 5a-h, having carbothio(oxa)amide moiety as a longer linker, providing 5-atom spacer, showed slightly better activity to those of the benzylidene derivatives 6a-g, that comprised 4-atom spacer.
Concerning the first carbothio(oxa)amide series 5a-h, results demonstrated that most of compounds containing thiourea moiety exhibited more powerful antitumor activity than those incorporating urea moiety.
Within the thiourea derivatives, it was deduced that the 4-chloro substitution in the aryl part gave the most potent compound 5a against all the tested cell lines. Besides, the 4-methoxyphenyl substituted compound 5g gave enhanced activity against both MCF-7 and HeLa cell lines. On the other hand, it was found that unsubstituted phenyl ring in compound 5d affected the activity that was reported to be exclusively strong against HeLa. The methyl-substituted compound 5f reported the least activity.
Regarding the urea-containing derivatives, the effect of various substituents was also investigated, where either aryl unsubstitution in 5h, 4-chlorosubstitution in 5b, or even replacement of the phenyl ring with a naphthyl one in 5c led to weak to moderate cytotoxic activity, while the 4-nitro-substituted compound 5e has revealed strong activity against MCF-7 and HeLa cells.
Concerning the second series, representing the arylidene derivatives 6a-g, it was detected that the presence of electron donating groups; like 3,4-dimethoxy, 4-dimethyl amino or 4-hydroxy, 3-methoxy groups; has notably enhanced the antitumor activity in compounds 6e-g, compared to either unsubstituted aryl derivative 6b, or those substituted with electron withdrawing groups; namely 4-chloro, 3-bromo, or 3-nitro groups like compounds 6a, 6c, and 6d. Compounds 6e-g displayed activity ranging from moderate to very strong activity. The dimethoxy derivative 6e exhibited moderate effect against HepG-2, MCF-7, and HeLa cell, whereas it showed strong effect against HCT-116. Compound 6f with the dimethyl amino-substitution also gave a moderate activity against HeLa cells, but was strongly active against the other three cell lines. The best activity in this series was obtained from the 4-hydroxy-3-methoxybenzylidene derivative 6g, which showed very strong cytotoxic activity against all the four cell lines.
In vitro cytotoxic activity of the most active compounds 5a and 6g against WI-38 cell line
Normal Caucasian fibroblast-like foetal lung cells (WI-38) were used for further investigation of the cytotoxic effect and the therapeutic safety of the two new hybrids 5a and 6g, having the highest potency against the formerly tested cancer cell lines. Dox was used as a standard anticancer drug for comparison. Both compounds 5a and 6g exhibited lower cytotoxicity against WI-38 cells with IC50 values of 37.16 and 43.28 μM, respectively, in addition to more improved selectivity indexes (SI), proving to be much safer on normal cells compared to Dox (IC50 = 6.72 μM) ().
Table 2. In vitro cytotoxic study of the most active compounds 5a and 6g against WI-38 cell line and their selectivity indexes.
In vitro enzyme inhibition assays
The effect of compounds 5a and 6g was screened against several molecular targets, namely: EGFR, VEGFR-2, and Topo II. Both compounds expressed reasonable inhibitory activity against the three enzymes, compared to the specified reference drugs ().
Table 3. In vitro EGFR-2, VEGFR-2, and Topo II inhibitory effects of the synthesised compounds 5a and 6g compared to reference drugs.
The new hybrid 5a showed remarkable inhibitory activity against EGFR with IC50 = 0.086 µM, that represented about 60% of activity of the reference drug Gefitinib (IC50 = 0.052 µM). Concerning VEGFR-2, compound 5a gave about 45% of the inhibitory activity of Sorafenib. It is of much interest that it further displayed strong Topo II inhibitory activity with IC50 of 2.52 µM, which is superior to that of the reference drug Dox (IC50 = 3.62 µM) by about 1.4 folds.
Referring to compound 6g, it conferred about 43% of activity of Dox towards Topo II, 40% of activity of Gefitinib against EGFR, while it was only 21% as potent as Sorafenib against VEGFR-2.
DNA binding activity assay
Several anticancer agents exert their effect throughout binding with DNA, and consequently inhibiting its synthesis. To assess the impact of the synthesised compounds on DNA binding properties, the most potent derivatives 5a and 6g were further evaluated for their DNA intercalating affinities, to be investigated as a potential mechanism for their anti-proliferative activities, using methyl green dye according to the reported techniqueCitation29. Displacement of methyl green, ionically bound to DNA by the drug has been suggested as a potential assay for drug-DNA interactionCitation29. As depicted in , the DNA binding results displayed that compound 5a exhibited nearly similar intercalative activity (IC50 = 33.17 µM) as Dox (IC50 = 31.54 µM). In addition, compound 6g showed DNA-binding activity with IC50 value of 42.03 µM. It is worthy noted that binding of these compounds to DNA can cause distortion of DNA helical structure, that leads to inhibition of its replication, transcription, and recombination, indicating that these compounds may act by such mechanism to attain their anticancer potential.
Table 4. DNA binding assay results (IC50, µM) of compounds 5a and 6g.
Cell cycle analysis
In order to gain more information regarding the mechanism of compounds 5a and 6g in growth inhibition of cancer cells, cell cycle distribution and induction of apoptosis on HepG-2 cells were evaluated using propidium iodide (PI) staining assayCitation30,Citation31. The obtained results ( and ) indicated that compounds 5a and 6g induced apoptosis in the G0-G1 phase by 36.93% and 38.91%, respectively, while untreated cells revealed 44.82% apoptosis. In the S phase, 5a reported high effect (49.06%) compared to the untreated cells (36.59%), while 6g exhibited 27.28% apoptotic effect. In G2/M phase, compound 5a showed 14.01% apoptotic effect whereas 6g revealed 33.81% inhibition and control cells exhibited 18.59%. Thus, it was well established that compound 5a arrested growth at the S phase, whereas compound 6g produces its apoptotic activity via arresting cell growth at G2/M.
Figure 4. Flow cytometry analysis of DNA ploidy in HepG-2 cells after treatment with compounds 5a and 6g.

Table 5. Effect of compounds 5a and 6g on the cell cycle distribution in HepG-2 cells.
Detection of apoptosis
The percentage of apoptosis induced by compounds 5a and 6g using HepG-2 cells was further determined using Annexin V-FITC/propidium iodide double staining flow cytometry assayCitation32. As shown in and and , compounds 5a and 6g induced the early apoptosis in HepG-2 cells after 24 h incubation by 12.24% and 11.02%, respectively, compared to the untreated cells (0.37%). In addition, the target compounds enhanced the late apoptotic induction by 25.13% and 19.85% compared to untreated control 0.12%. Moreover, they promoted necrosis of the cells by 7.12% and 5.41%, compared to untreated cells which showed 1.68%. Cumulatively, compounds 5a and 6g enhanced the total apoptosis by 37.37% and 30.87%, respectively in comparison to control cells (0.39).
Figure 5. Effect of compounds 5a and 6g on the percentage of Annexin V-FITC-positive staining in HepG-2 cells. The cells were treated with DMSO as a control, 5a and 6g for 24 h. Q1: Necrotic cells, Q2: Late apoptosis, Q3: Live cells, Q4: early apoptosis.

Figure 6. Early, late, total apoptosis, and necrosis induced by compounds 5a and 6g in HepG-2 cells compared to control.

Table 6. Apoptosis and necrosis induction analysis in HepG-2 cells after treatment with compounds 5a and 6g.
Molecular Docking study
Molecular docking has been shown to be useful tool in the field of drug discovery for explaining the interaction of small molecules with various biological targets, giving us the chance to optimise and develop better therapeutic agentsCitation33–38. Biological evaluation of the newly synthesised compounds revealed the outstanding potency of compound 5a against Topo II, VEGFR2, and EGFR; that was even higher or comparable to the standard inhibitors, while compound 6g showed moderate activity.
To inspect how far our main hypothesis in designing the target compounds was achieved in relation to the enzyme inhibitory activity, molecular docking study was utilised to investigate the potential binding mode of compounds under investigation and their interaction with the specified enzymes’ active sites. Both of compounds 5a and 6g showed good binding energy in comparison to the co-crystallised ligand, still compound 5a showed better higher affinity which agrees with the results of enzyme inhibition assay data as shown in .
Table 7. The binding energy of compound 5a and 6g in comparison to the co-crystallised ligand for each prospective target.
Analysis of binding mode of docked compounds in Topo II enzyme
ATP active site in Topo II enzyme was selected as the binding site for the molecular docking, both of compounds 5a and 6g showed good fitting to catalytic site of Topo II suggesting competitive inhibition mode like that reported for this type of inhibitorsCitation39,Citation40. However compound 5a showed different binding pattern than 6g where in case of the first, the benzimidazole ring was able to interact with the same amino acid residues interacting with adenine moiety of ATP, allowing hydrophobic interactions with Asn91, Ile125, and Phe142, also the triazole ring was in position to make extensive interaction with Ser148 and Asn150 through hydrogen bonding, mimicking ribose ring of ATP. Finally, the carbonyl and NH of carbothio(oxa)amide were able to interact through hydrogen bonding with Asn162, Asn163, Gly164, and Glu87, respectively, confirming the importance of such linker in achieving good inhibitory activity. In contrast, compound 6g showed inverted binding mode which could explain its lower inhibitory activity in comparison to 5a as shown in the enzyme inhibition assay. Nevertheless, the benzimidazole ring occupied the same site of triphosphate moiety; allowing the interaction with Arg162, Asn163, Gly164 through hydrogen bonding, and Gly166, Lys378 through hydrophobic interaction, still the triazole ring maintained its ability to form extensive hydrogen bonding with Ser148, Ser149, and Asn150 while the benzylidene formed hydrogen bond with Asn120 and Thr215. The interaction of compound 5a and 6g with ATP active site is depicted in and .
Analysis of binding mode of docked compounds in VEGFR-2 enzyme
Regarding the binding mode of compounds 5a and 6g in VEGFR-2, both of them showed the typical binding mode of VEGFR-2 inhibitors; where the heterocyclic ring formed hydrophobic interactions with ATP active site, also triazole rings forming hydrophobic interaction with Phe1045, Cys1043, and Val846 acting as bridge, allowing the carbothio(oxa)amide of 5a and hydrazide of 6g to form hydrogen bond with Asp1044 and Glu883 which is necessary to exert good inhibitory activity against this type of enzymesCitation41. Interestingly, it seems that carbothio(oxa)amide linker is responsible for the better enzyme inhibition activity observed experimentally; as it formed bidentate hydrogen bond with Glu883 and give the aromatic ring of 5a better chance to fully occupy the hydrophobic pocket of the allosteric site of VEGFR-2 interacting with Leu887, Glu883, and Gly1046 as demonstrated in . While in case of 6g the benzylidene moiety was able to exert hydrophobic interaction with the hydrophobic pocket but with less extent than 5a. It is worthy to note that unlike Sorafenib, a known inhibitor of VEFFR-2, both of 5a and 6g were not able to interact with Cys917, an interaction that is reported to promote the inhibitory activity to nanomolar range, which might explain the promoted potency of Sorafenib over both compounds in the experimental enzyme inhibition assay. The interactions of compounds 5a and 6g are demonstrated in and .
Analysis of binding mode of docked compounds in EGFR enzyme
Finally, the assessment of binding mode of compounds 5a and 6g revealed that compound 5a is more aligned to the co-crystallised ligand than 6g which protrude from its binding site, explaining the better enzyme inhibitory activity of 5a over 6g. Yet, both of them was not able to interact with Met793 residue through hydrogen bonding but 5a interacted with it through hydrophobic interaction which was reported to be important for good docking in the enzymeCitation42. This is in agree with experimental enzyme inhibition assay, where Gefitinib, an inhibitor for EGFR, showed better inhibition than compounds 5a and 6g. Even so, this was compensated by forming hydrogen bond with Cys797 and Phe795 for 5a and with Asp800 and Pro794 with 6g, allowing them to achieve comparable enzyme inhibition activity, which is shown in and .
Physicochemical properties and Lipinski’s rule
Compounds 5a and 6g that showed the highest cytotoxicity against the tested cancer cell lines; concomitant with good safety profiles on normal cells; were further evaluated for their compliance to Lipinski’s rule of five to inspect their physicochemical properties which are crucial for drug’s pharmacokinetics in the human body, and; hence, predicting their putative drug-likeness (). The specified parameters were calculated with the aid of the integrated online platform pkCSM (http://structure.bio.cam.ac.uk/pkcsm)Citation43. It was worthy noted that both compounds 5a and 6g had no violations for Lipinski’s rule, whereas Dox displayed three violations, since its molecular weight exceeded 500 Daltons, it had more than five hydrogen bond donors, in addition to possessing more than ten hydrogen bond acceptors. Furthermore, Sorafenib violated the rule concerning lipophilicity. Moreover, the topological surface area values for both compounds 5a and 6g are smaller than those of either Dox, Sorafenib, or Gefitinib, thus they may serve better passive oral absorption in comparison to Dox.
Table 8. Calculated Lipinski’s rule of five for compound 5a, 6g, Dox, Sorafenib, and Gefitinib.
Radar Plot
The bioavailability radar plot was employed to assess the dug-likeness via the SwissADME softwareCitation44. Compounds 5a and 6g exhibited enhanced parameters concerning size and polarity over Dox (). It was noted that degree of insaturation was slightly deviated than Dox, but better than that of Sorafenib, giving an overall good impact about their drug-likeness.
Conclusion
The main purpose of this study was to develop multi target-based benzimidazole-triazole hybrids 5a-h and 6a-g, aiming to promote future achievements in discovering target-specific anticancer drug candidates. The new series have been designed, prepared, and investigated as potential multi-targeting cytotoxic agents. The in vitro antitumor activity, EGFR, VEGFR-2 and Topo II inhibition, DNA binding assay, cell cycle analysis, and apoptotic induction have been evaluated. Among the tested hybrids, compounds 5a (IC50 ≃ 3.87–8.34 μM) and 6g (IC50 ≃ 3.34–10.92 μM) were the most potent antitumor agents against HepG-2, HCT-116, MCF-7, and HeLa cancer cells lines, with activity comparable to that of Dox (IC50 ≃ 4.17–5.57 μM). In addition, they showed good safety profiles on normal cells. Also, both compounds displayed good inhibitory activity against EGFR, VEGFR-2, and Topo II. The benzimidazole derivative 5a specially exhibited good inhibitory activity against EGFR (IC50 = 0.086 µM) in comparison with Gefitinib (IC50 = 0.052 µM). Moreover, it exerted strong inhibitory activity on Topo II (IC50 = 2.52 µM) which is better than Dox (IC50 = 3.62 µM). Whereas compound 6g exhibited moderate inhibitory activity (IC50 ≃ 0.131 − 8.37 μM) on EGFR, VEGFR-2, and Topo II. The most active compound, 5a, showed apoptosis-inducing activity of 44.49% using HepG-2 cancer cells and the cell cycle was arrested at a G1/S phase. Besides, compound 5a exhibited nearly similar DNA intercalative activity (IC50 = 33.17 µM) as Dox (IC50 = 31.54 µM), where compound 6g gave better binding than Dox with IC50 of 42.03 µM. The SAR indicated that carbothioamide linker in series 5a-h and the presence of electron donating groups in arylidene derivatives 6a-g gave much contribution to the cytotoxic activity. The antitumor activity, as well as EGFR, VEGFR-2, and Topo II inhibitory activities were further explained using molecular modelling studies, that apparently displayed good binding with the key active sites.
Experimental part
Chemistry
All melting points (°C) were measured on Stuart melting point apparatus (SMP 30) and are uncorrected. IR spectra (KBr) were recorded on FT-IR 200 spectrophotometer (ύ cm−1), Faculty of Pharmacy, Mansoura University. 1H-NMR and 13C-APT NMR spectra were recorded in (DMSO-d6) at 1HNMR (400 MHz), 13CNMR (100 MHz) on an NMR spectrometer (δ ppm) using TMS as an internal standard, NMR Unit, Faculty of Pharmacy, Mansoura University. Abbreviations are as follows: s, singlet; d, doublet; t, triplet; m, multiplet; br, broad. Mass spectra were carried out on direct inlet part to mass analyser in Thermo Scientific GCMS model ISQ at the Regional Centre for Mycology and Biotechnology (RCMB), Al-Azhar University, Egypt. Microanalyses were performed at Cairo University using Perkin-Elmer 240 elemental analyser for C, H, N, and S elements, and the results were within the acceptable range of the theoretical values. Compounds were detected with 254 nm UV lamp. All the chemicals and reagents used were purchased from Aldrich Chemicals Co, USA and commercial sources. Reaction times were determined using TLC on silica gel plates 60F245 E. Merk, using (EtOAc/Pet. ether; 1:1) as eluting system and the spots were visualised by UV (366–245 nm). The key precursor compound 1 and intermediate compound 2 were prepared according to the reported procedures in literatureCitation23.
General procedure for synthesis of ethyl 1-[(1H-benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carboxylate (3)
To a stirred solution of the azide 2 (1.2 g, 7 mmol) and ethyl acetoacetate (1.0 g, 7.7 mmol) in anhydrous DMSO (15 ml), K2CO3 (0.38 g, 2.8 mmol) was slowly added, where the resulting suspension was stirred at rt for 48 h. The reaction mixture was then diluted with H2O (60 ml), and the precipitated ester 3 was filtered and crystallised from pet. ether/EA mixture as pale-yellow crystals; (1.1 g, 65%). M.p. 212–214 °C. IR (ν max/cm−1): 3407 (NH), 1719 (C = O). 1HNMR (400 MHz, CDCl3) δ 7.81–7.70 (m, 2H), 7.56–7.44 (m, 2H), 6.45 (s, 2H), 4.30 (q, J = 7.1 Hz, 2H), 2.70 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H). Anal. Calcd. for C14H15N5O2 (285.30): C, 58.94; H, 5.30; N, 24.55. Found: C, 58.69; H, 5.42; N, 24.50%.
General procedure for synthesis of 1-[(1H-benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (4)
To a solution of the ester 3 (1.00 g, 3.5 mmol) in ethanol, hydrazine hydrate (0.5 g, 10.0 mmol) was added, then the mixture was stirred at rt overnight. The precipitated solid was filtered, crystallised from ethanol to afford the target hydrazide 4 as a white solid; (0.55 g, 58%). M.p. 245–247 °C. 1HNMR (400 MHz, DMSO) δ 12.67 (s, 1H), 9.67 (s, 1H), 7.55–7.65 (m, 2H), 7.19–7.20 (m, 2H), 5.88 (s, 2H), 4.45 (s, 2H), 2.57 (s, 3H). 13C-APT NMR (100 MHz, DMSO) δ 160.8, 148.6, 143.2, 137.9, 137.0, 134.7, 123.4, 122.1, 119.3, 112.0, 45.9, 8.9. Anal. Calcd. for C12H13N7O (271.28): C, 53.13; H, 4.83; N, 36.14. Found: C, 53.10; H, 4.75; N, 36.20%.
General procedure for synthesis of carbothio(oxa)amide derivatives 5a-h
To a solution of the hydrazide 4 (0.09 g, 0.35 mmol) in THF, the appropriate iso(thio)cyanate derivative (0.3 mmol) was added, where the reaction mixture was stirred overnight at room temperature. The precipitated solid was filtered, crystallised from ethanol to afford the target derivatives 5a-h.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(4-chlorophenyl) hydrazinecarbothioamide (5a)
White solid; (0.083 g, 59%). M.p. 153–155 °C. IR (ν max/cm−1): 3451, 3213 (NHs), 1684 (C = O). 1H NMR (400 MHz, DMSO) δ 12.73 (s, 1H), 10.52 (s, 1H), 9.83 (s, 1H), 9.81 (s, 1H), 7.61–7.47 (m, 4H), 7.37 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 5.92 (s, 2H), 2.62 (s, 3H). 13CNMR (100 MHz, DMSO) δ 161.6, 159.1, 148.9, 142.3, 139.3, 138.8, 136.7, 134.8, 128.9, 125.8, 123.1, 122.1, 120.5, 119.4, 112.4, 45.9, 8.9. MS m/z (%): 440.71 (M+, 27.65), 442.30 (M+ +2, 10.08). Anal. Calcd. for C19H17ClN8OS (440.91): C, 51.76; H, 3.89; N, 25.41; S, 7.27. Found: C, 51.88; H, 3.89; N, 25.45; S, 7.29%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(4-chlorophenyl) hydrazinecarboxamide (5b)
White solid; (0.102 g, 71%). M.p. 184–186 °C. IR (ν max/cm−1): 3537, 3323, 3252 (NHs), 1694 (C = O). 1HNMR (400 MHz, DMSO) δ 12.70 (s, 1H), 10.22 (s, 1H), 8.96 (s, 1H), 8.25 (s, 1H), 7.67–7.44 (m, 4H), 7.31 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H), 5.92 (s, 2H), 2.60 (s, 3H). 13CNMR (100 MHz, DMSO) δ 161.6, 156.1, 148.6, 143.3, 139.3, 138.2, 137.4, 134.8, 128.9, 125.8, 123.1, 122.1, 120.5, 119.4, 112.0, 45.9, 8.9. MS m/z (%): 424.99 (M+, 39.26), 426.30 (M++2, 11.08). Anal. Calcd. for C19H17ClN8O2 (424.84): C, 53.71; H, 4.03; N, 26.38. Found: C, 53.66; H, 4.23; N, 26.44%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(naphthalen-1-yl)hydrazinecarboxamide (5c)
White solid; (0.105 g, 79%). M.p. 233–235 °C. 1HNMR (400 MHz, DMSO) δ 12.70 (s, 1H), 10.33 (s, 1H), 8.86 (s, 1H), 8.46 (s, 1H), 8.12 (d, J = 7.7 Hz, 1H), 7.94 (d, J = 7.7 Hz, 1H), 7.86–7.70 (m, 1H), 7.67 (d, J = 8.1 Hz, 1H), 7.61–7.50 (m, 4H), 7.47 (d, J = 8.1 Hz, 1H), 7.20–7.19 (m, 2H), 5.93 (s, 2H), 2.62 (s, 3H). 13CNMR (100 MHz, DMSO) δ 161.5, 156.9, 148.6, 143.4, 138.3, 137.4, 134.8, 134.2, 128.8, 126.4, 126.3, 126.11, 124.00, 123.9, 123.2, 122.4, 122.3, 122.1, 121.7, 119.3, 112.1, 45.9, 8.9. MS m/z (%): 440.33 (M+, 31.51). Anal. Calcd. for C23H20N8O2 (440.46): C, 62.72; H, 4.58; N, 25.44. Found: C, 62.66; H, 4.68; N, 25.45%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-phenylhydrazinecarbothioamide (5d)
White solid; (0.088 g, 67%). M.p. 157–159 °C. 1HNMR (400 MHz, DMSO) δ 10.79 (s, 1H), 7.63 (d, J = 7.9 Hz, 2H), 7.61–7.56 (m, 2H), 7.41–7.37 (m, 2H), 7.23 (dd, J = 7.9, 3.1 Hz, 2H), 7.05–7.01 (m, 1H), 5.99 (s, 2H), 2.68 (s, 3H). 13CNMR (100 MHz, DMSO) δ 159.9, 152.7, 148.4, 139.0, 136.0, 131.5, 130.6, 129.6, 128.8, 126.4, 122.8, 122.4, 119.6, 117.5, 97.6, 46.2, 9.2. MS m/z (%): 406.42 (M+, 25.20). Anal. Calcd. for C19H18N8OS (406.46): C, 56.14; H, 4.46; N, 27.57; S, 7.89. Found: C, 56.16; H, 4.33; N, 27.59; S, 7.95%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(4-nitrophenyl) hydrazinecarboxamide (5e)
Yellow solid; (0.102 g, 71%). M.p. 247–249 °C. 1HNMR (400 MHz, DMSO) δ 12.71 (s, 1H), 10.33 (s, 1H), 9.62 (s, 1H), 8.57 (s, 1H), 8.19 (d, J = 9.1 Hz, 2H), 7.75 (d, J = 9.1 Hz, 2H), 7.63–7.48 (m, 2H), 7.27–7.09 (m, 2H), 5.92 (s, 2H), 2.60 (s, 3H). 13CNMR (100 MHz, DMSO) δ 161.8, 157.5, 153.8, 148.6, 147.0, 143.5, 141.5, 138.4, 137.3, 134.8, 125.5, 123.1, 122.1, 119.4, 112.1, 45.9, 8.9. MS m/z (%): 435.54 (M+, 22.26). Anal. Calcd. for C19H17N9O4 (435.40): C, 52.41; H, 3.94; N, 28.95. Found: C, 52.36; H, 3.98; N, 28.88%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(p-tolyl)hydrazinecarbothioamide (5f)
White solid; (0.090 g, 64%). M.p. 257–259 °C. 1H NMR (400 MHz, DMSO) δ 12.72 (s, 1H), 10.66 (s, 1H), 7.72–7.52 (m, 2H), 7.51 (d, J = 7.3 Hz, 2H), 7.31–7.23 (m, 2H), 7.18 (d, J = 7.3 Hz, 2H), 5.97 (s, 2H), 2.67 (s, 3H), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO) δ 160.0, 152.6, 148.4, 136.6, 136.0, 131.5, 131.3, 129.9, 123.2, 123.1 122.1 119.4, 119.3, 117.5, 112.1, 46.3, 20.8, 9.1. MS m/z (%): 420.31 (M+, 33.56). Anal. Calcd. for C20H20N8OS (420.49): C, 57.13; H, 4.79; N, 26.65; S, 7.63. Found: C, 57.26; H, 4.79; N, 26.69; S, 7.60%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-(4-methoxyphenyl)hydrazinecarbothioamide (5g)
White solid; (0.079 g, 56%). M.p. 235–237 °C. 1HNMR (400 MHz, DMSO) δ 12.71 (s, 1H), 10.55 (s, 1H), 7.64–7.54 (m, 2H), 7.52 (d, J = 7.9 Hz, 2H), 7.26–7.17 (m, 2H), 6.97 (d, J = 7.9 Hz, 2H), 5.97 (s, 2H), 3.75 (s, 3H), 2.66 (s, 3H). 13CNMR (101 MHz, DMSO) δ 162.9, 160.1, 154.9, 152.5, 148.4, 135.9, 132.3, 131.5, 123.2, 122.2, 119.9, 119.3, 119.0, 114.8, 112.1, 55.7, 46.2, 9.1. MS m/z (%): 436.02 (M+, 20.15). Anal. Calcd. for C20H20N8O2S (436.49): C, 55.03; H, 4.62; N, 25.67; S, 7.35. Found: C, 55.03; H, 4.60; N, 25.69; S, 7.25%.
2-{1-[(1h-Benzo[d]imidazol-2-yl)methyl]-5-methyl-1H-1,2,3-triazole-4-carbonyl}-N-phenylhydrazinecarboxamide (5h)
White solid; (0.085 g, 66%). M.p. 182–184 °C. 1HNMR (400 MHz, DMSO) δ 12.69 (s, 1H), 10.19 (s, 1H), 8.79 (s, 1H), 8.15 (s, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.52 (d, J = 7.0 Hz, 1H), 7.50–7.46 (m, 2H), 7.33–7.14 (m, 4H), 7.01–6.92 (m, 1H), 5.92 (s, 2H), 2.60 (s, 3H). 13CNMR (100 MHz, DMSO) δ 161.5, 155.8, 148.6, 143.3, 140.2, 138.2, 137.4, 134.8, 129.1, 123.1, 122.3, 122.1, 119.4, 118.9, 112.0, 45.9, 8.9. MS m/z (%): 390.35 (M+, 29.66). Anal. Calcd. for C19H18N8O2 (390.40): C, 58.45; H, 4.65; N, 28.70. Found: C, 58.26; H, 4.80; N, 28.65%.
General procedure for synthesis of benzylidene derivatives 6a-g
To a solution of hydrazide 4 (0.09 g, 0.35 mmol) in ethanol, containing catalytic drops of glacial acetic acid, the appropriate aldehyde (0.3 mmol) was added. The mixture was refluxed overnight. Then, the reaction mixture was cooled to rt. The precipitated solid was filtered, and crystallised from ethanol to afford pure derivatives 6a-g.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-(4-chlorobenzylidene)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6a)
White solid; (0.085 g, 61%). M.p. 280–282 °C. 1HNMR (400 MHz, DMSO) δ 12.70 (s, 1H), 12.18 (s, 1H), 8.57 (s, 1H), 7.73 (d, J = 8.1 Hz, 2H), 7.67–7.48 (m, 4H), 7.31 (d, J = 8.1 Hz, 2H), 5.94 (s, 2H), 2.64 (s, 3H). 13CNMR (100 MHz, DMSO) δ 157.9, 148.5, 147.1, 138.9, 137.6, 134.9, 133.9, 129.4, 129.3, 129.2, 129.1, 123.1, 122.1, 119.4, 112.1, 46.0, 9.0. MS m/z (%): 393.41 (M+, 30.25), 395.5 (M++2, 11.08). Anal. Calcd. for C19H16ClN7O (393.83): C, 57.94; H, 4.09; N, 24.90. Found: C, 57.84; H, 4.12; N, 24.88%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-benzylidene-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6b)
White solid; (0.072 g, 65%). M.p. 185–187 °C. IR (ν max/cm−1): 3300, 3221 (NHs), 1669 (C = O). 1H NMR (400 MHz, DMSO) δ 12.69 (s, 1H), 12.08 (s, 1H), 8.58 (s, 1H), 7.71 (d, J = 6.3 Hz, 2H), 7.65–7.38 (m, 5H), 7.29–7.07 (m, 2H), 5.94 (s, 2H), 2.65 (s, 3H). 13CNMR (100 MHz, DMSO) δ 157.8, 148.5, 148.4, 138.8, 137.7, 135.1, 134.9, 130.5, 130.4, 129.3, 129.3, 129.2, 127.6, 127.5, 127.5, 46.0, 9.0. MS m/z (%): 359.01 (M+, 25.33). Anal. Calcd. for C19H17N7O (359.38): C, 63.50; H, 4.77; N, 27.28. Found: C, 63.56; H, 4.78; N, 27.08%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-(3-bromobenzylidene)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6c)
White solid; (0.089 g, 64%). M.p. 152–154 °C. 1HNMR (400 MHz, DMSO) δ 12.79 (s, 1H), 12.24 (s, 1H), 8.55 (s, 1H), 7.91 (s, 1H), 7.67 (d, J = 6.7 Hz, 2H), 7.61–7.51 (m, 2H), 7.49–7.39 (m, 1H), 7.33–7.12 (m, 2H), 5.95 (s, 2H), 2.65 (s, 3H). 13CNMR (100 MHz, DMSO) δ 157.9, 148.5, 146.6, 138.9, 138.9, 137.6, 137.5, 137.4, 133.0, 133.0, 131.6, 131.5, 129.5, 129.4, 126.8, 126.7, 122.6, 46.0, 9.0. MS m/z (%): 438.34 (M+, 36.21), 440.14 (M++2, 30.91). Anal. Calcd. for C19H16BrN7O (438.28): C, 52.07; H, 3.68; N, 22.37. Found: C, 52.12; H, 3.55; N, 22.27%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-5-methyl-N′-(3-nitrobenzylidene)-1H-1,2,3-triazole-4-carbohydrazide (6d)
Yellow solid; (0.076 g, 58%). M.p. 163–165 °C. 1HNMR (400 MHz, DMSO) δ 12.70 (s, 1H), 12.37 (s, 1H), 8.69 (s, 1H), 8.53 (s, 1H), 8.28 (d, J = 7.6 Hz, 1H), 8.12 (d, J = 7.6 Hz, 1H), 7.87–7.72 (m, 1H), 7.72–7.44 (m, 2H), 7.33–7.12 (m, 2H), 5.95 (s, 2H), 2.66 (s, 3H). 13CNMR (100 MHz, DMSO) δ 158.0, 148.7, 146.0, 139.1, 139.0, 137.6, 137.5, 136.9, 136.8, 133.9, 131.0, 131.0, 124.7, 124.7, 122.7, 121.3, 121.2, 46.0, 9.1. MS m/z (%): 404.29 (M+, 39.55). Anal. Calcd. for C19H16N8O3 (404.38): C, 56.43; H, 3.99; N, 27.71. Found: C, 56.38; H, 3.92; N, 27.75%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-(3,4-dimethoxybenzylidene)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6e)
White solid; (0.093 g, 71%). M.p. 270–272 °C. 1HNMR (400 MHz, DMSO) δ 12.69 (s, 1H), 11.96 (s, 1H), 8.49 (s, 1H), 7.63–7.47 (m, 2H), 7.39 (s,1H), 7.34–7.22 (m, 2H), 7.17 (d, J = 8.2 Hz, 1H), 7.04 (d, J = 8.2 Hz, 1H), 5.94 (s, 2H), 3.84 (s, 3H), 3.82 (s, 3H), 2.64 (s, 3H). 13CNMR (100 MHz, DMSO) δ 157.7, 151.2, 149.5, 148.6, 148.5, 139.1, 138.6, 137.8, 137.7, 127.6, 127.5, 122.4, 122.3, 112.0, 111.9, 108.7, 108.6, 56.1, 55.9, 46.0, 9.1. MS m/z (%): 419.02 (M+, 21.56). MS m/z (%):419.64 (M+, 74.96). Anal. Calcd. for C21H21N7O3 (419.44): C, 60.13; H, 5.05; N, 23.38. Found: C, 60.11; H, 5.15; N, 23.32%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-[4-(dimethylamino)benzylidene]-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6f)
White solid; (0.082 g, 60%). M.p. 268–270 °C. 1HNMR (400 MHz, DMSO) δ 12.68 (s, 1H), 11.74 (s, 1H), 8.41 (s, 1H), 7.69–7.60 (m, 2H), 7.52 (d, J = 8.7 Hz, 2H), 7.35–7.12 (m, 2H), 6.77 (d, J = 8.7 Hz, 2H), 5.93 (s, 2H), 2.99 (s, 6H), 2.63 (s, 3H). 13CNMR (101 MHz, DMSO) δ 157.4, 151.9, 149.2, 148.6, 138.3, 138.0, 128.9, 128.8, 123.1, 122.2, 122.1, 119.4, 112.6, 112.3, 112.1, 46.1, 40.4, 9.0. MS m/z (%): 402.41 (M+, 28.36). Anal. Calcd. for C21H22N8O (402.45): C, 62.67; H, 5.51; N, 27.84. Found: C, 62.54; H, 5.50; N, 27.89%.
(E)-1-[(1H-Benzo[d]imidazol-2-yl)methyl]-N′-(4-hydroxy-3-methoxybenzylidene)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (6g)
White solid; (0.091 g, 75%). M.p. 228–230 °C. IR (ν max/cm−1): 3427 (OH), 3325 (NH), 1659 (C = O). 1HNMR (400 MHz, DMSO) δ 12.70 (s, 1H), 11.88 (s, 1H), 9.54 (s, 1H), 8.44 (s, 1H), 7.63–7.49 (m, 2H), 7.30 (s, 1H), 7.26–7.14 (m, 2H), 7.06 (d, J = 8.3 Hz, 1H), 6.85 (d, J = 8.3 Hz, 1H), 5.93 (s, 2H), 3.85 (s, 3H), 2.64 (s, 3H). 13CNMR (100 MHz, DMSO) δ 158.7, 151.2, 149.5, 148.6, 147.5, 139.1, 138.6, 137.8, 137.7, 127.6, 126.5, 122.4, 122.3, 112.0, 111.9, 108.7, 108.6, 55.7, 46.0, 9.1. MS m/z (%): 405.48 (M+, 50.06). Anal. Calcd. for C20H19N7O3 (405.41): C, 59.25; H, 4.72; N, 24.18. Found: C, 59.30; H, 4.62; N, 24.09%.
Biological evaluation
In vitro cytotoxic study against HepG-2, HCT-116, MCF-7, and HeLa cell lines
The MTT assay was performed to evaluate the in vitro antitumor activity of the newly synthesised compounds according to the reported methodCitation3,Citation45,Citation46.
In vitro cytotoxic activity of the most active compounds (5a, 6g) against WI-38 cell line
The cytotoxicity of compounds 5a and 6g was estimated according to the reported procedureCitation47.
In vitro enzyme inhibitory assays (against EGFR, VEGFR-2, and Topo II)
Enzyme inhibitory assays for the most active compounds 5a and 6g were carried out as described in the previous reports in the literatureCitation48.
DNA/methyl green colorimetric method for DNA binding assay
DNA binding assay was carried out according to the reported methodCitation29.
Flow cytometry analysis of the cell cycle distribution
Cell cycle analysis was performed using the HepG-2 cell lines stained with propidium iodide (PI) and FACSCalibur flow cytometer as mentioned in previous reportsCitation30,Citation31.
Analysis of cellular apoptosis
Apoptosis induction was performed using the HepG-2 cell lines and well-established Annexin 5-FITC/PI detection kit similar to the reported proceduresCitation30,Citation31.
Molecular docking study
Molecular docking of compounds 5a and 6g was done by preparing 3D structure of these compounds and the PDB of the selected targets, Top II (1ZXM) and VEGFR-2 (2OH4) and EGFR (2J6M) as previously reportedCitation49. Molecular docking was done using Leadit softwareCitation50,Citation51 and the active site was set as sphere with radius 6.5 Å around the co-crystallised ligand. The software was considered valid by redocking the co-crystallised ligand and the RMSD were shown to be no more than 1.5 Å ( and ). The 3D structure of the compounds was selected as a library and was docked to the active site. Finally, the docked poses were inspected using Discovery studio visualiser to study their interaction with the binding siteCitation52.
Physicochemical properties and Lipinski’s rule
The physicochemical parameters were calculated for compounds 5a, 6g, Dox, Sorafenib, and Gefitinib using the free online website of pkCSM (http://structure.bio.cam.ac.uk/pkcsm)Citation43.
Radar plot
SwissADME software was employed to investigate the dug-likeness of compounds 5a and 6g in comparison with the reference drugs Dox, Sorafenib, and Gefitinib via the bioavailability radar plotCitation53.
Appendix A. Supplementary data
Supplementary data related to this manuscript are found in a separate file.
Supplemental Material
Download PDF (6 MB)Acknowledgements
This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Goud NS, Kumar P, Bharath RD. Recent developments of target-based benzimidazole derivatives as potential anticancer agents. Mini Rev Med Chem. 2020;20(17):1754–1766.
- Mostafa AS, Gomaa RM, Elmorsy MA. Design and synthesis of 2‐phenyl benzimidazole derivatives as VEGFR‐2 inhibitors with anti‐breast cancer activity. Chem Biol Drug Des. 2019;93(4):454–463.
- Hamdi A, El-Shafey HW, Othman DI, El-Azab AS, AlSaif NA, Alaa A-M. Design, synthesis, antitumor, and VEGFR-2 inhibition activities of novel 4-anilino-2-vinyl-quinazolines: molecular modeling studies. Bioorg Chem. 2022;122:105710.
- Singh H, Kinarivala N, Sharma S. Multi-targeting anticancer agents: rational approaches, synthetic routes and structure activity relationship. Anticancer Agents Med Chem. 2019;19(7):842–874.
- Ibrahim HA, Refaat HM. Versatile mechanisms of 2-substituted benzimidazoles in targeted cancer therapy. Future J Pharm Sci. 2020;6(1):1–20.
- Li Y, Tan C, Gao C, Zhang C, Luan X, Chen X, Liu H, Chen Y, Jiang Y. Discovery of benzimidazole derivatives as novel multi-target EGFR, VEGFR-2 and PDGFR kinase inhibitors. Bioorg Med Chem. 2011;19(15):4529–4535.
- Cheson BD, Leoni L. Bendamustine: mechanism of action and clinical data. Clin Adv Hematol Oncol. 2011;9(8 Suppl 19):1–11.
- Hasinoff BB, Wu X, Nitiss JL, Kanagasabai R, Yalowich JC. The anticancer multi-kinase inhibitor dovitinib also targets topoisomerase I and topoisomerase II. Biochem Pharmacol. 2012;84(12):1617–1626.
- Ali AM, Tawfik SS, Mostafa AS, Massoud MAM. Benzimidazole-based protein kinase inhibitors: current perspectives in targeted cancer therapy. Chem Biol Drug Des. 2022;100(5):656–673.
- Musolino A, Campone M, Neven P, Denduluri N, Barrios CH, Cortes J, Blackwell K, Soliman H, Kahan Z, Bonnefoi H, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2− breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017;19(1):1–14.
- Nadhan R, Srinivas P, Pillai MR. RTKs in pathobiology of head and neck cancers. Adv Cancer Res. 2020;147:319–373.
- Woei-A-Jin FJSH, Weijl NI, Burgmans MC, Fariña Sarasqueta A, van Wezel JT, Wasser MNJM, Coenraad MJ, Burggraaf J, Osanto S. Neoadjuvant treatment with angiogenesis-inhibitor dovitinib prior to local therapy in hepatocellular carcinoma: a phase II study. Oncologist. 2021;26(10):854–864.
- Liu W-Q, Megale V, Borriello L, Leforban B, Montès M, Goldwaser E, Gresh N, Piquemal J-P, Hadj-Slimane R, Hermine O, et al. Synthesis and structure–activity relationship of non-peptidic antagonists of neuropilin-1 receptor. Bioorg Med Chem Lett. 2014;24(17):4254–4259.
- McBride CM, Renhowe PA, Heise C, Jansen JM, Lapointe G, Ma S, Piñeda R, Vora J, Wiesmann M, Shafer CM, et al. Design and structure–activity relationship of 3-benzimidazol-2-yl-1H-indazoles as inhibitors of receptor tyrosine kinases. Bioorg Med Chem Lett. 2006;16(13):3595–3599.
- Pinar A, Yurdakul P, Yildiz I, Temiz-Arpaci O, Acan NL, Aki-Sener E, Yalcin I. Some fused heterocyclic compounds as eukaryotic topoisomerase II inhibitors. Biochem Biophys Res Commun. 2004;317(2):670–674.
- Nawareg NA, Mostafa AS, El-Messery SM, Nasr MN. New benzimidazole based hybrids: synthesis, molecular modeling study and anticancer evaluation as TopoII inhibitors. Bioorg Chem. 2022; 127:106038.
- Xu Z, Zhao S-J, Liu Y. 1, 2, 3-Triazole-containing hybrids as potential anticancer agents: current developments, action mechanisms and structure-activity relationships. Eur J Med Chem. 2019;183:111700.
- Alam MM. 1, 2, 3‐Triazole hybrids as anticancer agents: a review. Arch Pharm. 2022;355(1):2100158.
- El-Sayed WA, Alminderej FM, Mounier MM, Nossier ES, Saleh SM, Kassem AF. Novel 1, 2, 3-triazole-coumarin hybrid glycosides and their tetrazolyl analogues: design, anticancer evaluation and molecular docking targeting EGFR, VEGFR-2 and CDK-2. Molecules. 2022;27(7):2047.
- Wang D-P, Liu K-L, Li X-Y, Lu G-Q, Xue W-H, Qian X-H, Mohamed O K, Meng F-H. Design, synthesis, and in vitro and in vivo anti-angiogenesis study of a novel vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitor based on 1, 2, 3-triazole scaffold. Eur J Med Chem. 2021;211:113083.
- Abdel-Hafez GA, Mohamed A-MI, Youssef AF, Simons C, Aboraia AS. Synthesis, computational study and biological evaluation of 9-acridinyl and 1-coumarinyl-1, 2, 3-triazole-4-yl derivatives as topoisomerase II inhibitors. J Enzyme Inhib Med Chem. 2022;37(1):502–513.
- Bistrović A, Krstulović L, Harej A, Grbčić P, Sedić M, Koštrun S, Pavelić SK, Bajić M, Raić-Malić S. Design, synthesis and biological evaluation of novel benzimidazole amidines as potent multi-target inhibitors for the treatment of non-small cell lung cancer. Eur J Med Chem. 2018;143:1616–1634.
- Sahay II, Ghalsasi PS. Synthesis of new 1, 2, 3-triazole linked benzimidazole molecules as anti-proliferative agents. Synth Commun. 2017;47(8):825–834.
- Cai M, Hu J, Tian J-L, Yan H, Zheng C-G, Hu W-L. Novel hybrids from N-hydroxyarylamide and indole ring through click chemistry as histone deacetylase inhibitors with potent antitumor activities. Chin Chem Lett. 2015;26(6):675–680.
- Nafie MS, Boraei AT. Exploration of novel VEGFR2 tyrosine kinase inhibitors via design and synthesis of new alkylated indolyl-triazole Schiff bases for targeting breast cancer. Bioorg Chem. 2022;122:105708.
- Al-Hussain SA, Farghaly TA, Zaki ME, Abdulwahab HG, Al-Qurashi NT, Muhammad ZA. Discovery of novel indolyl-1, 2, 4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity. Bioorg Chem. 2020;105:104330.
- Wang Z-J, Yang H-H, Tian L, Zhao W-G. Design, synthesis, and fungicidal activities of novel 5-methyl-1H-1, 2, 3-trizole-4-carboxyl amide analogues. Med Chem. 2016;12(3):290–295.
- Theocharis A, Alexandrou N, Terzis A. Generation and dienophilic properties of 1‐benzyl‐1H‐1, 2, 3‐triazolo [4, 5‐d] pyridazine‐4, 7‐dione. J Heterocycl Chem. 1990;27(6):1741–1744.
- Burres NS, Frigo A, Rasmussen RR, McAlpine JB. A colorimetric microassay for the detection of agents that interact with DNA. J Nat Prod. 1992;55(11):1582–1587.
- Tolba MF, Esmat A, Al-Abd AM, Azab SS, Khalifa AE, Mosli HA, Abdel-Rahman SZ, Abdel-Naim AB. Caffeic acid phenethyl ester synergistically enhances docetaxel and paclitaxel cytotoxicity in prostate cancer cells. IUBMB Life. 2013;65(8):716–729.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
- Kumar R, Saneja A, Panda AK. An annexin V-FITC—propidium iodide-based method for detecting apoptosis in a non-small cell lung cancer cell line. Lung Cancer. 2021;2279:213–223.
- Selim NM, Elgazar AA, Abdel-Hamid NM, El-Magd MRA, Yasri A, Hefnawy HME, Sobeh M. Chrysophanol, physcion, hesperidin and curcumin modulate the gene expression of pro-inflammatory mediators induced by LPS in HepG2: in silico and molecular studies. Antioxidants. 2019;8(9):371.
- Elsbaey M, Ibrahim MAA, Bar FA, Elgazar AA. Chemical constituents from coconut waste and their in silico evaluation as potential antiviral agents against SARS-CoV-2. S Afr J Bot. 2021;141:278–289.
- Elgazar AA, Selim NM, Abdel-Hamid NM, El-Magd MA, El Hefnawy HM. Isolates from Alpinia officinarum Hance attenuate LPS-induced inflammation in HepG2: evidence from in silico and in vitro studies. Phytother Res. 2018;32(7):1273–1288.
- Elgazar AA, Knany HR, Ali MS. Insights on the molecular mechanism of anti-inflammatory effect of formula from Islamic traditional medicine: an in-silico study. J Tradit Complement Med. 2019;9(4):353–363.
- Badria FA, Elgazar AA. Chapter 37 – revealing the molecular mechanism of Olea europaea L. in treatment of cataract. In: Preedy VR, Watson RR, editors. Olives and olive oil in health and disease prevention. 2nd ed. San Diego: Academic Press; 2021. p. 445–456.
- Othman DI, Hamdi A, Abdel-Aziz MM, Elfeky SM. Novel 2-arylthiazolidin-4-one-thiazole hybrids with potent activity against Mycobacterium tuberculosis. Bioorg Chem. 2022;124:105809.
- Yao B-L, Mai Y-W, Chen S-B, Xie H-T, Yao P-F, Ou T-M, Tan J-H, Wang H-G, Li D, Huang S-L, et al. Design, synthesis and biological evaluation of novel 7-alkylamino substituted benzo[a]phenazin derivatives as dual topoisomerase I/II inhibitors. Eur J Med Chem. 2015;92:540–553.
- Hu C-X, Zuo Z-L, Xiong B, Ma J-G, Geng M-Y, Lin L-P, Jiang H-L, Ding J. Salvicine functions as novel Topoisomerase II poison by binding to ATP pocket. Mol Pharmacol. 2006;70(5):1593–1601.
- Hasegawa M, Nishigaki N, Washio Y, Kano K, Harris PA, Sato H, Mori I, West RI, Shibahara M, Toyoda H, et al. Discovery of novel benzimidazoles as potent inhibitors of TIE-2 and VEGFR-2 tyrosine kinase receptors. J Med Chem. 2007;50(18):4453–4470.
- Verma N, Rai AK, Kaushik V, Brünnert D, Chahar KR, Pandey J, Goyal P. Identification of gefitinib off-targets using a structure-based systems biology approach; their validation with reverse docking and retrospective data mining. Sci Rep. 2016;6(1):1–12.
- Pires DE, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015;58(9):4066–4072.
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717.
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89(2):271–277.
- Hamdi A, Elhusseiny WM, Othman DIA, Haikal A, Bakheit AH, El-Azab AS, Al-Agamy MHM, Abdel-Aziz AA-M. Synthesis, antitumor, and apoptosis-inducing activities of novel 5-arylidenethiazolidine-2,4-dione derivatives: histone deacetylases inhibitory activity and molecular docking study. Eur J Med Chem. 2022;244:114827.
- Sofan MA, El‐Mekabaty A, Hasel AM, Said SB. Synthesis, cytotoxicity assessment and antioxidant activity of some new thiazol‐2‐yl carboxamides. J Heterocycl Chem. 2021;58(8):1645–1655.
- El-Mawgoud HK, Fouda AM, El-Nassag MA, Elhenawy AA, Alshahrani MY, El-Agrody AM. Discovery of novel rigid analogs of 2-naphthol with potent anticancer activity through multi-target topoisomerase I & II and tyrosine kinase receptor EGFR & VEGFR-2 inhibition mechanism. Chem Biol Interact. 2022;355:109838.
- Elimam DM, Elgazar AA, El-Senduny FF, El-Domany RA, Badria FA, Eldehna WM. Natural inspired piperine-based ureas and amides as novel antitumor agents towards breast cancer. J Enzyme Inhib Med Chem. 2022;37(1):39–50.
- Böhm H-J. Prediction of binding constants of protein ligands: a fast method for the prioritization of hits obtained from de novo design or 3D database search programs. J Comput Aided Mol Des. 1998;12(4):309–323.
- Kramer B, Rarey M, Lengauer T. Evaluation of the FLEXX incremental construction algorithm for protein–ligand docking. Proteins. 1999;37(2):228–241.
- Studio DJAISD, CA, USA. version 2.5; 2009.
- DeLano WL. Pymol: an open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002;40(1):82–92.