Abstract
A β-class carbonic anhydrase (CA, EC 4.2.1.1) present in the genome of the Monogenean platyhelminth Gyrodactylus salaris, a fish parasite, GsaCAβ, has been investigated for its inhibitory effects with a panel of sulphonamides and sulfamates, some of which in clinical use. Several effective GsaCAβ inhibitors were identified, belonging to simple heterocyclic sulphonamides, the deacetylated precursors of acetazolamide and methazolamide (KIsof 81.9–139.7 nM). Many other simple benezene sulphonamides and clinically used agents, such as acetazolamide, methazolamide, ethoxzolamide, dorzolamide, benzolamide, sulthiame and hydrochlorothiazide showed inhibition constants <1 µM. The least effective GsaCAβ inhibitors were 4,6-disubstituted-1,3-benzene disulfonamides, with KIs in the range of 16.9–24.8 µM. Although no potent GsaCAβ-selective inhibitors were detected so far, this preliminary investigation may be helpful for better understanding the inhibition profile of this parasite enzyme and for the potential development of more effective and eventually parasite-selective inhibitors.
Introduction
We have recently reported the cloning and characterisation of a β-class carbonic anhydrase (CA, EC 4.2.1.1) encoded in the genome of Gyrodactylus salaris, GsaCAβCitation1, a platyhelminth (flatworm) parasite attacking various fish speciesCitation2,Citation3. The Atlantic salmon (Salmo salar) is particularly sensitive to this parasite, which produced catastrophic losses in fish farms in Scandinavian countries and elsewhere, starting with the 1970sCitation3–5. By releasing proteolytic enzymes, the parasite attaches on the fish gills, fins or skin inducing the formation of wounds, which favour the emergence of infections, with debilitation and eventual death of the infected animalsCitation5,Citation6. There are no effective drugs for the treatment of this parasitic disease, although a variety of inorganic salts, synthetic compounds/drugs (e.g., praziquantel, levamisole, mebendazole and toltrazuril) and other approaches (manual removal of the worms) have been investigated, with rather unsuccessful resultsCitation7. Furthermore, many of these compounds/drugs induce serious host toxicity, raising thus significant human health concerns if such fish is to be consumedCitation7. Thus, as for other platyhelminth parasites producing infection in vertebrates including humans, such as Schistosoma haematobiumCitation8 or Schistosoma mansoniCitation9–11 there is a stringent need of alternative drug targets and efficient compounds to treat these infections.
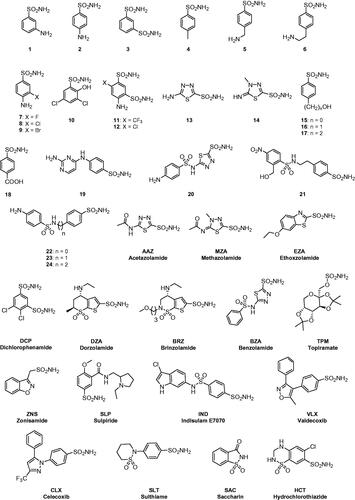
CAs are well known drug targets for the management of human diseasesCitation12–15, with their inhibitors acting as diureticsCitation16, antiepilepticsCitation17, antiglaucomaCitation18, antiobesityCitation19 and antitumor agentsCitation20. In the last decade, CAs from pathogens started to be considered as possible targets for the development of antiinfectives, for the management of diseases provoked by bacteriaCitation21, fungiCitation22, protozoaCitation23 and wormsCitation10,Citation11,Citation24. In the previous workCitation1 we have shown that GsaCAβ has a significant catalytic activity for the physiologic, CO2 hydration reaction, with a kcat of 1.1 × 105 s−1 and a kcat/Km of 7.58 × 106 M−1 × s−1. Furthermore, inorganic anions, a well-known class of CA inhibitors (CAIs)Citation14,Citation15 inhibit the enzyme in the millimolar range, as for other α- and β-CAs investigated for their interaction with such modulators of activityCitation14. Among the investigated such inhibitors, sulfamide (KI of 81 µM) and sulphamic acid (KI of 6.2 µM) showed the most efficient inhibitory actionCitation1. Both of them incorporate the SO2NH2 moiety found in the most investigated class of CAIs, the aromatic/heterocyclic sulphonamides and their isosteres (sulfamates, sulfamides)Citation14,Citation15. Thus in this work we report GsaCAβ inhibition studies with a panel of such compounds, many of which are clinically used drugs (.
Materials and methods
Chemistry
Compounds 1–24 and AAZ-HCT were commercially available, highest purity reagents from Sigma-Aldrich (Milan, Italy) or were synthesised as previously reportedCitation25.
Production of β-CA recombinant protein
Protein production was carried out according to the previously reported protocolCitation1.
Ca activity and inhibition measurements
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activityCitation26. Phenol red at a concentration of 0.2 mM was used as pH indicator, working at the absorbance maximum of 557 nm, with 10 mM TRIS (pH 8.3) as buffer, and in the presence of 10 mM NaClO4 for maintaining constant the ionic strength, following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors (10–20 mM) were prepared in distilled-deionized water and dilutions up to 0.01 µM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the enzyme-inhibitor complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng-Prusoff equation, whereas the kinetic parameters for the uninhibited enzymes from Lineweaver-Burk plots, as reported earlierCitation27,Citation28, and represent the mean from at least three different determinations. GsaCAβ concentration in the assay system was of 11.9 nM.
Results and discussion
GsaCAβ shows catalytic properties for the physiologic reaction similar to those of the slow human isoform hCA I, being however slightly less effective as a catalyst compared to hCA I (). On the other hand, it should be stressed that many CAs are among the most effective catalysts known in natureCitation14,Citation15, and even this level of activity is in fact quite significant.
Table 1. Kinetic parameters for the CO2 hydration reaction catalysed by α- and β-class CA enzymes: the human cytosolic isozymes hCA I and II (α-class) at 20 °C and pH 7.5 in 10 mM HEPES buffer, and GsaCAβ (measured at 20 °C, pH 8.3 in 20 mM TRIS buffer and 10 mM NaClO4) are shown. Inhibition data with the clinically used sulphonamide acetazolamide are also presented.
We have investigated the inhibition profile of GsaCAβ with a panel of sulphonamides and sulfamates () known to effectively inhibit many classes of CAs, with some of these derivatives being clinically used drugs for decades, in the treatment of a multitude of diseases, as shown in the introduction. The names of the relevant drugs are reported in , and as mentioned above, they are used as diuretics, antiglaucoma drugs, antiepileptics or for the management of other disorders connected with CA activity disbalancesCitation14,Citation15. The GsaCAβ inhibition data with these compounds, as well as those for hCA I and II (for comparison reasons), are shown in .
Table 2. Inhibition of β-CA from G. salaris and human isoforms hCA I and hCA II with sulphonamides 1–24 and the clinically used drugs AAZ-HCT, by a stopped-flow assayCitation26.
As seen from , where the inhibition data of the human α-class isoforms hCA I and II were also included for comparison, all investigated sulphonamides/sulfamates inhibited GsaCAβ, with inhibition constants raging between 81.9 nM and 24.8 µM. The following structure-activity relationship (SAR) should be noted regarding the inhibition data of :
The most effective GsaCAβ inhibitors were compounds 13 and 14, the deacetylated precursors of acetazolamide and methazolamide, which showed KI values of 81.9–139.7 nM, which is 5.1–5.6 times a better inhibitory activity compared to the clinically used derivatives AAZ and MZA (). As seen in , these precursors are less effective as hCA I and II inhibitors compared to the acetylated derivatives used as drugs.
A rather large number of derivatives, such as 1–3, 7, 15–20, 2–24, AAZ, MZA, EZA, DZA, BZA, SLT and HCT, showed less effective inhibition, but anyhow with KIs <1000 nM. The SAR is rather difficult to rationalise in this case as these compounds belong to very heterogeneous classes of sulphonamides, both aromatic (benzene sulphonamides) and heterocyclic derivatives. However, it seems that rather simple and elongated scaffolds lead to effective inhibition whereas the inclusion of bulkier substituents (e.g. in 21 compared to 22–24, or BRZ compared to DZA) is detrimental for the inhibitory activity.
Compounds showing low micromolar inhibition of GsaCAβ were 4–6, 8–10, 21, DCP, BRZ, TPM, ZNS, SLP; IND, VLX, CLX and SAC. These compounds had KIs in the range of 1.63–9.1 µM. As above, they belong to a large number of diverse chemotypes in order to draw a rationalisation of their SAR. Saccharin, also being a medium potency inhibitor, is among the most selective ones for inhibiting GsaCAβ over the human isoforms ().
4,6-disubstituted-1,3-benzene disulfonamides 11 and 12 were the least effective GsaCAβ inhibitors, with KIs in the range of 16.9–24.8 µM ().
The inhibition profile of GsaCAβ and hCA I/II are very different, obviously due to the fact that they belong to diverse genetic CA families. Unfortunately, no GsaCAβ-selective inhibitors (over the hCAs investigated here) were detected so far.
Conclusions
The Monogenean platyhelminth Gyrodactylus salaris, a fish parasite of salmon and other economically relevant aquaculture fish species, encodes for a β-class CA, GsaCAβ, which has been investigated here for its inhibition profile with sulphonamides/sulfamates, as a possible antiparasitic drug target. We identified several effective GsaCAβ inhibitors, belonging to simple heterocyclic sulphonamide derivatives, the deacetylated precursors of acetazolamide and methazolamide, which showed KI values of 81.9 − 139.7 nM. Many other simple benezenesulfonamides and clinically used agents, such as acetazolamide, methazolamide, ethoxzolamide, dorzolamide, benzolamide, sulthiame and hydrochlorothiazide showed inhibition constants <1 µM. The least effective GsaCAβ inhibitors were 4,6-disubstituted-1,3-benzene disulfonamides, with KIs in the range of 16.9 − 24.8 µM. Although no GsaCAβ-selective inhibitors were detected so far, this preliminary investigation may be helpful for better understanding the SAR for inhibition of this parasite enzyme and for the potential development of more effective and eventually parasite-selective inhibitors.
Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Aspatwar A, Barker H, Aisala H, et al. Cloning, purification, kinetic and anion inhibition studies of a recombinant β-carbonic anhydrase from the Atlantic salmon parasite platyhelminth Gyrodactylus salaris. J Enzyme Inhib Med Chem 2022;37:1577–86.
- Paladini G, Shinn AP, Taylor NGH, et al. Geographical distribution of Gyrodactylus salaris Malmberg, 1957 (Monogenea, Gyrodactylidae). Parasit Vectors 2021;14:34.
- Zueva KJ, Lumme J, Veselov AE, et al. Genomic signatures of parasite-driven natural selection in north European Atlantic salmon (Salmo salar). Mar Genomics 2018;39:26–38.
- Hansen H, Cojocaru CD, Mo TA. Infections with Gyrodactylus spp. (Monogenea) in Romanian fish farms: Gyrodactylus salaris Malmberg, 1957 extends its range. Parasit Vectors 2016;9:444.
- Ramírez R, Bakke TA, Harris PD. Same barcode, different biology: differential patterns of infectivity, specificity and pathogenicity in two almost identical parasite strains. Int J Parasitol 2014;44:543–9.
- Hopkins C. Introduced marine organisms in Norwegian waters, including Svalbard. Parasites and diseases. In: Leppakoski E, Gollasch S, Olenin S, eds. Invasive aquatic species of Europe. Distribution, impacts and management. Dordrecht: Springer Netherlands; 2002:13–25.
- (a) Schelkle B, Shinn AP, Peeler E, et al. Treatment of gyrodactylid infections in fish. Dis Aquat Organ 2009;86:65–75. (b) Soleng A, Poléo AB, Alstad NE, et al. Aqueous aluminium eliminates Gyrodactylus salaris (Platyhelminthes, Monogenea) infections in Atlantic salmon. Parasitology 1999;119 (Pt 1):19–25. (c) Schmahl G. The chemotherapy of monogeneans which parasitize fish: a review. Folia Parasitol (Praha) 1991;38:97–106.
- (a) Feldmeier H, Chitsulo L. Therapeutic and operational profiles of metrifonate and praziquantel in Schistosoma haematobium infection. Arzneimittelforschung 1999;49:557–65. (b) Kramer CV, Zhang F, Sinclair D, et al. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev 2014;2014:CD000053.
- (a) Skelly PJ, Nation CS, Da’Dara AA. Schistosoma mansoni and the purinergic halo. Trends Parasitol 2022;38:1080–8. (b) Skelly PJ, Da’dara AA. Schistosome secretomes. Acta Trop 2022;236:106676. (c) Nation CS, Da’Dara AA, Skelly PJ. NAD-catabolizing ectoenzymes of Schistosoma mansoni. Biochem J 2022;479:1165–80. (d) Acharya S, Da’dara AA, Skelly PJ. Schistosome immunomodulators. PLoS Pathog 2021;17:e1010064.
- (a) Da’dara AA, Angeli A, Ferraroni M, et al. Crystal structure and chemical inhibition of essential schistosome host-interactive virulence factor carbonic anhydrase SmCA. Commun Biol 2019;2:333. (b) Angeli A, Pinteala M, Maier SS, et al. Sulfonamide inhibition studies of an α-carbonic anhydrase from Schistosoma mansoni, a platyhelminth parasite responsible for schistosomiasis. Int J Mol Sci 2020;21:1842. (c) Angeli A, Ferraroni M, Da’dara AA, et al. Structural insights into Schistosoma mansoni Carbonic Anhydrase (SmCA) inhibition by selenoureido-substituted benzenesulfonamides. J Med Chem 2021;64:10418–28.
- (a) Ferraroni M, Angeli A, Carradori S, et al. Inhibition of Schistosoma mansoni carbonic anhydrase by the antiparasitic drug clorsulon: X-ray crystallographic and in vitro studies. Acta Crystallogr D Struct Biol 2022;78(Pt 3):321–7. (b) Angeli A, Ferraroni M, Carta F, et al. Development of praziquantel sulphonamide derivatives as antischistosomal drugs. J Enzyme Inhib Med Chem 2022;37:1479–94.
- Aspatwar A, Tolvanen MEE, Barker H, et al. Carbonic anhydrases in metazoan model organisms: molecules, mechanisms, and physiology. Physiol Rev 2022;102:1327–83.
- Hooper PL, Swenson ER, Johnson RJ. Carbonic anhydrase inhibitors for the treatment of high-altitude hypoxemia. Am J Med 2019;132:e799–800.
- Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- (a) Supuran CT. Emerging role of carbonic anhydrase inhibitors. Clin Sci (Lond)) 2021;135:1233–49. (b) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12. (c) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21. (d) Ferraroni M, Angeli A, Pinteala M, et al. Sulfonamide diuretic azosemide as an efficient carbonic anhydrase inhibitor. J Mol Struct 2022;1268:133672.
- (a) Mishra CB, Kumari S, Angeli A, et al. Discovery of potent carbonic anhydrase inhibitors as effective anticonvulsant agents: drug design, synthesis, and in vitro and in vivo investigations. J Med Chem 2021;64:3100–14. (b) Mishra CB, Kumari S, Angeli A, et al. Discovery of potent anti-convulsant carbonic anhydrase inhibitors: design, synthesis, in vitro and in vivo appraisal. Eur J Med Chem 2018;156:430–43. (c) Shukralla AA, Dolan E, Delanty N. Acetazolamide: old drug, new evidence? Epilepsia Open 2022;7:378–92. (d) Ciccone L, Cerri C, Nencetti S, et al. Carbonic anhydrase inhibitors and epilepsy: state of the art and future perspectives. Molecules 2021;26:6380.
- (a) Supuran CT. Multitargeting approaches involving carbonic anhydrase inhibitors: hybrid drugs against a variety of disorders. J Enzyme Inhib Med Chem 2021;36:1702–14. (b) Mincione F, Nocentini A, Supuran CT. Advances in the discovery of novel agents for the treatment of glaucoma. Expert Opin Drug Discov 2021;16:1209–25. (c) Bonardi A, Nocentini A, Bua S, et al. Sulfonamide inhibitors of human carbonic anhydrases designed through a three-tails approach: improving ligand/isoform matching and selectivity of action. J Med Chem 2020;63:7422–44.
- (a) Supuran CT. Anti-obesity carbonic anhydrase inhibitors: challenges and opportunities. J Enzyme Inhib Med Chem 2022;37:2478–88. (b) Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med Res Rev 2020;40:2485–565. (c) Muñoz W, Lamm A, Poppers D, et al. Acetazolamide promotes decreased consumption of carbonated drinks and weight loss. Oxf Med Case Reports 2018;2018:omy081. (d) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25.
- (a) Angeli A, Carta F, Nocentini A, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites 2020;10:412. (b) McDonald PC, Chafe SC, Supuran CT, et al. Cancer therapeutic targeting of hypoxia induced carbonic anhydrase IX: from bench to bedside. Cancers (Basel) 2022;14:3297. (c) Chafe SC, Vizeacoumar FS, Venkateswaran G, et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv 2021;7:eabj0364. (d) Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70. (e) Supuran CT. Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2021;30:1197–208.
- (a) Angeli A, Urbański LJ, Capasso C, et al. Activation studies with amino acids and amines of a β-carbonic anhydrase from Mammaliicoccus (Staphylococcus) sciuri previously annotated as Staphylococcus aureus (SauBCA) carbonic anhydrase. J Enzyme Inhib Med Chem 2022;37:2786–92. (b) An W, Holly KJ, Nocentini A, et al. Structure-activity relationship studies for inhibitors for vancomycin-resistant Enterococcus and human carbonic anhydrases. J Enzyme Inhib Med Chem 2022;37:1838–44. (c) Giovannuzzi S, Hewitt CS, Nocentini A, et al. Coumarins effectively inhibit bacterial α-carbonic anhydrases. J Enzyme Inhib Med Chem 2022;37:333–8. (d) Abutaleb NS, Elhassanny AEM, Nocentini A, et al. Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. J Enzyme Inhib Med Chem 2022;37:51–61. (e) Flaherty DP, Seleem MN, Supuran CT. Bacterial carbonic anhydrases: underexploited antibacterial therapeutic targets. Future Med Chem 2021;13:1619–22. (f) Hewitt CS, Abutaleb NS, Elhassanny AEM, et al. Structure-activity relationship studies of acetazolamide-based carbonic anhydrase inhibitors with activity against Neisseria gonorrhoeae. ACS Infect Dis 2021;7:1969–84. (g) De Luca V, Giovannuzzi S, Supuran CT, et al. May sulfonamide inhibitors of carbonic anhydrases from Mammaliicoccus sciuri Prevent antimicrobial resistance due to gene transfer to other harmful Staphylococci? Int J Mol Sci 2022;23:13827. (h) Supuran CT, Capasso C. Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin Ther Pat 2020;30:963–82.
- (a) Angeli A, Velluzzi A, Selleri S, et al. Seleno containing compounds as potent and selective antifungal agents. ACS Infect Dis 2022;8:1905–19. (b) De Luca V, Angeli A, Mazzone V, et al. Heterologous expression and biochemical characterisation of the recombinant β-carbonic anhydrase (MpaCA) from the warm-blooded vertebrate pathogen malassezia pachydermatis. J Enzyme Inhib Med Chem 2022;37:62–8. (c) Supuran CT, Capasso C. A highlight on the inhibition of fungal carbonic anhydrases as drug targets for the antifungal armamentarium. Int J Mol Sci 2021;22:4324.
- (a) Bonardi A, Parkkila S, Supuran CT. Inhibition studies of the protozoan α-carbonic anhydrase from Trypanosoma cruzi with phenols. J Enzyme Inhib Med Chem 2022; 37:2417–22. (b) Urbański LJ, Angeli A, Mykuliak VV, et al. Biochemical and structural characterization of beta-carbonic anhydrase from the parasite Trichomonas vaginalis. J Mol Med (Berl) 2022;100:115–24. (c) Syrjänen L, Vermelho AB, Rodrigues IDA, et al. Cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Leishmania donovani chagasi, the protozoan parasite responsible for leishmaniasis. J Med Chem 2013;56:7372–81. (d) Pal DS, Mondal DK, Datta R. Identification of metal dithiocarbamates as a novel class of antileishmanial agents. Antimicrob Agents Chemother 2015;59:2144–52. (e) Pal DS, Abbasi M, Mondal DK, et al. Interplay between a cytosolic and a cell surface carbonic anhydrase in pH homeostasis and acid tolerance of Leishmania. J Cell Sci 2017;130:754–66. (f) Reungprapavut S, Krungkrai SR, Krungkrai J. Plasmodium falciparum carbonic anhydrase is a possible target for malaria chemotherapy. J Enzyme Inhib Med Chem 2004;19:249–56. (g) Krungkrai J, Krungkrai SR, Supuran CT. Carbonic anhydrase inhibitors: inhibition of Plasmodium falciparum carbonic anhydrase with aromatic/heterocyclic sulfonamides-in vitro and in vivo studies. Bioorg Med Chem Lett 2008;18:5466–71.
- (a) Zolfaghari Emameh R, Kuuslahti M, Vullo D, et al. Ascaris lumbricoides β carbonic anhydrase: a potential target enzyme for treatment of ascariasis. Parasit Vectors 2015;8:479. (b) Zolfaghari Emameh R, Barker H, Hytönen VP, et al. Beta carbonic anhydrases: novel targets for pesticides and anti-parasitic agents in agriculture and livestock husbandry. Parasit Vectors 2014;7:403. (c) Zolfaghari Emameh R, Syrjänen L, Barker H, et al. Drosophila melanogaster: a model organism for controlling Dipteran vectors and pests. J Enzyme Inhib Med Chem 2015;30:505–13.
- (a) Abbate F, Winum JY, Potter BV, et al. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatase. Bioorg Med Chem Lett 2004;14:231–4. (b) Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999; 34:41–50. (c) Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem 2012;55:5591–600.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Nishimori I, Minakuchi T, Morimoto K, et al. Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. J Med Chem 2006;49:2117–26. (b) Zimmerman SA, Ferry JG, Supuran CT. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr Top Med Chem 2007;7:901–8.
- (a) Sarikaya SB, Gülçin I, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–20. (b) Supuran CT. Carbonic anhydrase inhibitors from marine natural products. Mar Drugs. 2022;20:721.