Abstract
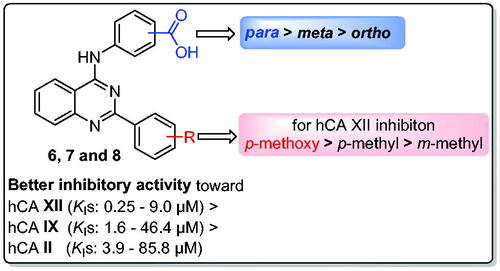
As part of our ongoing endeavour to identify novel inhibitors of cancer-associated CA isoforms IX and XII as possible anticancer candidates, here we describe the design and synthesis of small library of 2-aryl-quinazolin-4-yl aminobenzoic acid derivatives (6a–c, 7a–c, and 8a–c) as new non-classical CA inhibitors. On account of its significance in the anticancer drug discovery and in the development of effective CAIs, the 4-anilinoquinazoline privileged scaffold was exploited in this study. Thereafter, the free carboxylic acid functionality was appended in the ortho (6a–c), meta (7a–c), or para-positon (8a–c) of the anilino motif to furnish the target inhibitors. All compounds were assessed for their inhibitory activities against the hCA I, II (cytosolic), IX, and XII (trans-membrane, tumour-associated) isoforms. Moreover, six quinazolines (6a–c, 7b, and 8a–b) were chosen by the NCI-USA for in vitro anti-proliferative activity evaluation against 59 human cancer cell lines representing nine tumour subpanels.
Introduction
Carbonic anhydrases (CA, EC 4.2.1.1) are ubiquitous metalloenzymes that play a crucial role in catalysing the reversible hydration reaction of carbon dioxide to bicarbonate and protons.Citation1 This reaction, catalysed by Zn+2 ion, has a critical role in many physiological and pathological processes such as gluconeogenesis and tumorigenicity.Citation2,Citation3 So far, fifteen human CA (hCA) isoforms have been identified, with varying distributions across tissues and cells.Citation4 As a result of the dysfunction of different hCA isoforms activities, a number of pathological repercussions might occur, featuring these hCA isoforms as interesting pharmacological targets for a variety of therapeutic approaches using small molecule CA inhibitors (CAIs).Citation4 Thus, the pharmacological applications of CAIs are identified for the management of diverse disorders such as ophthalmologic problems,Citation5 epilepsy,Citation6 obesityCitation7 and human malignancies.Citation8
Sulphonamides and their sulfamides and sulfamate bioisosteres are considered as classical hCA inhibitors with a high affinity to the zinc ion in the active site.Citation3 It is worth to mention that although identification of several chemotypes of CAIs, like coumarins, phenols, thiocarbamates, and carboxylates,Citation9–11 only primary sulfonamide-tethered CAIs have been clinically used for glaucoma (such as acetazolamide and dorzolamide), and investigated in the clinical trials for the treatment of human malignancies (SLC-0111), .Citation12,Citation13 These sulfonamide-tethered CAIs produce strong CA inhibition, however, a number of them lack the necessary isoform selectivity. So, the design and synthesis of new nonclassical CAIs stands out as a promising strategy to discover effective and isoform-selective CAIs for the management of different diseases. The carboxylic acid-based derivatives represent an important non-classical CAIs chemotype that can exert the CA inhibitory effect through different modes of action, such as anchoring to the zinc-bound water-hydroxide ion through H-bonding, or direct binding to the catalytic zinc displacing bound water-hydroxide anion.Citation14–16
Figure 1. Structure of acetazolamide, dorzolamide, SLC-0111, non-classical CAIs (I–III), and the target inhibitors (6, 7, and 8).
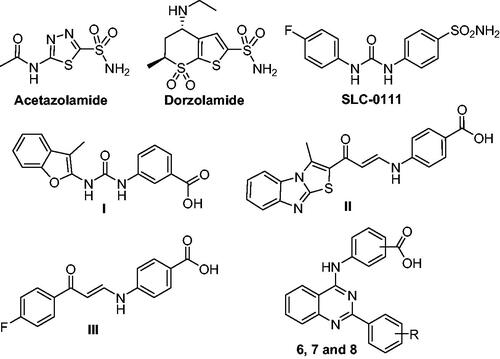
In the few last years, we have reported several carboxylic acid-tethered small molecules as new CAIs.Citation17–19 A novel series of benzofuran-based carboxylic acids was described as promising CA inhibitors in 2020.Citation20 Among these benzofuran derivatives, compound I () with a meta-benzoic acid moiety inhibited hCA IX at a submicromolar concentration (KI = 0.79 μM), as well as exerted good hCA XII inhibitory activity (KI = 2.3 μM). Also in the same year, we have developed a small library of methylthiazolo[3,2-a]benzimidazole-based carboxylic acid derivatives as novel CA inhibitors.Citation21 In particular, compound II () effectively suppressed CA isoforms IX and XII with inhibition constants equal 0.83 μM and 2.4 μM, respectively. Furthermore, we identified a new series of non-classical CA inhibitors that incorporates enaminone-based carboxylic acids.Citation22 Compound III () endowed with a para-benzoic acid motif showed submicromolar hCA IX inhibitory activity (KI = 0.92 µM) and good hCA XII inhibitory activity (KI = 1.1 µM).
Based on the findings described above, and as part of our ongoing endeavour to identify novel inhibitors of cancer-associated CA isoforms IX and XII as possible anticancer candidates,Citation23–29 here we describe the design and synthesis of a small library of 2-aryl-quinazolin-4-yl aminobenzoic acid derivatives (6a–c, 7a–c, and 8a–c) as new non-classical CA inhibitors (). On account of its significance in the anticancer drug discovery and development,Citation30–33 and in the development of effective CAIs,Citation34,Citation35 the 4-anilinoquinazoline privileged scaffold was exploited in this study. Thereafter, the free carboxylic acid functionality was appended in the ortho (6a–c), meta (7a–c), or para-positon (8a–c) of the anilino motif to furnish the target inhibitors.
All the newly synthesised quinazoline-based carboxylic acid derivatives (6a–c, 7a–c, and 8a–c) were assessed for their inhibitory activities against the hCA I, II (cytosolic), IX and XII (trans membrane, tumour associated) isoforms by the stopped-flow CO2 hydrase assay. Moreover, six quinazolines (6a–c, 7b, and 8a–b) were chosen by the NCI-USA for in vitro anti-proliferative activity evaluation against 59 human cancer cell lines representing nine tumour subpanels.
Experimental
Chemistry
Melting points (°C, uncorrected) were determined using a Stuart melting point apparatus. The IR spectra (KBr) were recorded on a SHIMADZU FT/IR spectrometer. The NMR spectra recorded by BRUKER 400 MHz NMR spectrometers using DMSO-d6 as the solvent. Chemical shifts were reported in parts per million (δ), and coupling constants (J) expressed in Hertz. 1H and 13C spectra were run at 400 and 101 MHz, respectively. Microanalytical data (C, H, and N) were obtained by FLASH 2000 CHNS/O analyser.
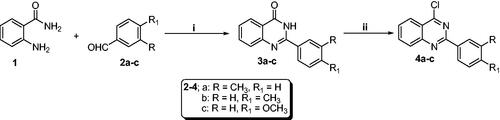
General procedures for the synthesis of 2-arylquinazolin-4(3H)-one derivatives (3a–c)
An aqueous solution of ferric chloride (5.4 g, 20 mmol) was added to a mixture of anthranilamide 1 (2.72 g, 20 mmol) and the appropriate aldehyde derivative 2a–c (20 mmol).Citation36 The mixture was heated at 80 °C for 3 h. After completion of the reaction, as indicated by TLC (n-hexane: ethyl acetate 1:1), the formed solid was filtrated, washed with water (4 × 5 ml), dried, and finally recrystallized from dioxane to produce 2-arylquinazolin-4(3H)-ones 3a–c.
General procedures for the synthesis of 2-aryl-4-chloroquinazolines (4a–c)
To a suspension of 2-arylquinazolinones 3a–c (1eq) in phosphorus oxychloride (10 eq), a catalytic amount of DMF was added.Citation36 The reaction mixture was then heated at 90 °C for 4h. After cooling, the mixture was added drop-wise to ice-water with stirring, neutralised by ammonium hydroxide, and extracted by methylene chloride. The organic layer was washed with cold water, dried over anhydrous Na2SO4, and evaporated in vacuo. The obtained solid was crystallised from isopropanol to afford the key 2-aryl-4-chloroquinazolines intermediates 4a–c.
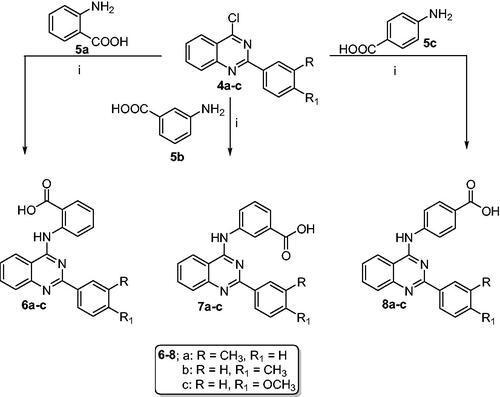
Synthesis of 2/3/4-((2-arylquinazolin-4-yl)amino)benzoic acid derivatives (6a–c, 7a–c and 8a–c)
To a stirred solution of 4-chloroquinazoline derivatives 4a–c (1 mmol) in refluxing isopropanol (5 ml) containing a few drops of HCl, the appropriate aminobenzoic acid derivative 5a–c (1 mmol) was added. The reaction mixture was heated under reflux for 2 h. The solid formed upon cooling was collected by filtration, dried, and recrystallized from ethanol to afford the target quinazolines (6a–c, 7a–c, and 8a–c).
2-((2-(m-Tolyl)quinazolin-4-yl)amino)benzoic acid (6a)
White crystals, (67%) yield; m.p. 198–200 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6): 2.42 (s, 3H, CH3), 7.47–7.51 (m, 3H, Ar-H), 7.80 (t, 1H, H-6 quinazoline, J = 7.2 Hz), 7.86 (t, 1H, H-7 quinazoline, J = 7.6 Hz), 8.09–8.16 (m, 3H, Ar-H), 8.24–8.26 (m, 2H, Ar-H), 8.38 (d, 1H, H-5, quinazoline, J = 8.4 Hz), 8.61 (d, 1H, H-8 quinazoline, J = 8.0 Hz), 12.34 (s, 1H, NH); 13C NMR (DMSO-d6): 21.47 (CH3), 113.31, 122.0, 124.02, 125.95, 126.68, 126.85, 128.93, 129.36, 130.10, 131.54, 132.34, 133.78, 134.24, 136.35, 138.06, 138.74, 157.99, 159.29, 168.84 (C=O); Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: C, 74.15; H, 4.79; N, 11.85.
2-((2-(p-Tolyl)quinazolin-4-yl)amino)benzoic acid (6b)
White crystals, (65%) yield; m.p. 200–202 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6): 2.41 (s, 3H, CH3), 7.41 (d, 2H, Ar-H, J = 8.0 Hz) , 7.47 (t, 1H, Ar-H, J = 7.2 Hz), 7.79 (t, 1H, H-6 quinazoline, J = 7.6 Hz), 7.85 (t, 1H, Ar-H, J = 7.6 Hz), 8.08–8.13 (m, 2H, Ar-H), 8.23–8.25 (m, 1H, Ar-H) , 8.28 (d, 2H, Ar-H, J = 8.4 Hz), 8.41 (d, 1H, H-5 quinazoline, J = 7.6 Hz), 8.61 (d, 1H,H-8 quinazoline, J = 8.4 Hz), 12.34 (s, 1H, NH); 13C NMR (DMSO-d6): 21.67 (CH3), 113.21, 124.08, 126.03, 126.77, 128.85, 129.71, 130.04, 131.53, 133.79, 136.39, 144.36, 157.78, 159.32, 168.80 (C=O); Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: 74.25; H, 4.80; N, 11.84.
2-((2–(4-Methoxyphenyl)quinazolin-4-yl)amino)benzoic acid (6c)
White crystals, (72%) yield; m.p. 204–206 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6): 3.87 (s, 3H, OCH3), 7.15 (d, 2H, Ar-H, J = 8.8 Hz), 7.49 (t, 1H, Ar-H, J = 7.2 Hz), 7.79 (t, 1H, H-6 quinazoline, J = 8.8 Hz), 7.83 (t, 1H, Ar-H, J = 8.8 Hz), 8.08–8.20 (m, 3H, Ar-H), 8.39 (d, 2H, Ar-H, J = 9.2 Hz), 8.45 (d, 1H, H-5 quinazoline, J = 8.4 Hz), 8.62 (d, 1H, H-8 quinazoline, J = 8.0 Hz), 12.28 (s, 1H, NH); 13C NMR (DMSO-d6): 56.02 (OCH3), 56.25, 114.56, 115.03, 120.92, 126.45, 126.99, 130.34, 131.34, 131.84, 133.75, 135.30, 136.57, 159.33, 162.52, 162.70; Anal. Calcd. For: C22H17N3O3 (371.40): C, 71.15; H, 4.61; N, 11.31; Found: 71.37; H, 4.60; N, 11.25.
3-((2-(m-Tolyl)quinazolin-4-yl)amino)benzoic acid (7a)
Yellow crystals, (72%) yield; m.p. 248–250 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6):2.42 (s, 3H, CH3), 7.47–7.53 (m, 2H, Ar-H), 7.63 (t, 1H, H-6 quinazoline, J = 8.0 Hz), 7.80 (t, 1H, H7 quinazoline, J = 7.6 Hz), 7.89 (d, 1H, Ar-H, J = 7.6 Hz), 8.07–8.11 (m, 2H, Ar-H), 8.30–8.31 (m, 2H, Ar-H) 8.40 (d, 1H, H-5 quinazoline, J = 8.4 Hz), 8.75 (s, 1H, Ar-H), 9.03 (d, 1H, H-8 quinazoline, J = 8.0 Hz),11.82 (s, 1H, NH); 13C NMR (DMSO-d6): 21.41 (CH3), 25.96, 62.49, 113.26, 125.15, 125.52, 126.96, 127.27, 128.57, 129.31, 130.34, 131.64, 134.48, 136.38, 137.81, 138.96, 157.61, 159.31, 167.49 (C=O); Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: 74.38; H, 4.85; N, 11.78.
3-((2-(p-Tolyl)quinazolin-4-yl)amino)benzoic acid (7b)
Yellow crystals, (67%) yield; m.p. 220–223 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6):2.42 (s, 3H, CH3), 7.40 (d, 2H, Ar-H, J = 8.0 Hz), 7.64 (t, 1H, Ar-H, J = 7.6 Hz), 7.80 (t, 1H, H-6 quinazoline, J = 7.6 Hz), 7.89 (d, 1H, Ar-H, J = 7.6 Hz), 8.07 (t, 1H, H-7 quinazoline, J = 8.0 Hz), 8.12 (d, 1H, Ar-H, J = 8.0 Hz), 8.37 (d, 2H, Ar-H, J = 8.0 Hz), 8.40 (d, 1H, H-5 quinazoline, J = 8.8 Hz), 8.69 (s, 1H, H-2, Ar-H), 9.00 (d, 1H, H-8 quinazoline, J = 8.4 Hz), 11.79 (s, 1H, NH); 13C NMR (DMSO-d6): 21.69, 25.97, 62.48, 113.20, 120.96, 125.15, 125.51, 127.28, 128.49, 128.73, 129.44, 129.87, 130.05, 131.70, 136.38, 137.82, 144.55, 157.46, 159.35, 167.41; Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: 74.51; H, 4.79; N, 11.86.
3-((2–(4-Methoxyphenyl)quinazolin-4-yl)amino)benzoic acid (7c)
Off white crystals, (75%) yield; m.p. 244–246 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6): 3.90 (s, 3H, OCH3), 7.18 (d, 2H, Ar-H, J = 8.8 Hz), 7.66 (t, 1H, H-6 quinazoline, J = 8.0 Hz), 7.77 (d, 1H, Ar-H, J = 7.2 Hz), 7.81 (t, 1H, H-7 quinazoline, J = 8.0 Hz), 7.91 (d, 1H, Ar-H, J = 7.6 Hz), 8.10 (d, 1H, Ar-H, J = 8.0 Hz), 8.37 (d, 1H, H-5 quinazoline, J = 8.4 Hz), 8.48 (d, 2H, Ar-H, J = 8.8 Hz), 8.69 (s, 1H, H-2, Ar-H), 8.93(d, 1H, H-8 quinazoline, J = 8.4 Hz), 11.70 (s, 1H, NH); 13C NMR (DMSO-d6): 56.25 (OCH3), 113.02, 115.00, 123.62, 125.06, 125.53, 125.99, 127.35, 128.36, 128.79, 129.54, 130.40, 131.76, 132.06, 132.49, 136.47, 137.82, 157.04, 159.28, 164.16, 167.07, 167.42; Anal. Calcd. For: C22H17N3O3 (371.40): C, 71.15; H, 4.61; N, 11.31; Found: C, 71.27; H, 4.64; N, 11.26.
4-((2-(m-Tolyl)quinazolin-4-yl)amino)benzoic acid (8a)
White crystals, (78%) yield; m.p. 201–204 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6):2.43 (s, 3H, CH3), 7.52–7.53 (m, 2H, Ar-H), 7.81 (t, 1H, H-6 quinazoline, J = 7.2 Hz), 8.05–8.12 (m, 5H, Ar-H), 8.20–8.22 (m, 1H, Ar-H) , 8.28 (s, 1H, Ar-H), 8.39 (d, 1H, H-5 quinazoline, J = 8.4 Hz), 9.01 (d, 1H, H-8 quinazoline, J = 8.0 Hz), 11.79 (s, 1H, NH); 13C NMR (DMSO-d6): 21.50 (CH3), 113.39, 124.27, 125.17, 127.03, 128.33, 128.59, 129.48, 130.11, 130.37, 132.13, 134.38, 136.43, 138.84, 141.66, 157.78, 159.42, 167.30 (C=O); Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: C, 74.32; H, 4.81; N, 11.86.
4-((2-(p-Tolyl)quinazolin-4-yl)amino)benzoic acid (8b)
Yellow crystals, (70%) yield; m.p. 306–308 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6):2.43 (s, 3H, CH3), 7.46 (d, 2H, Ar-H, J = 8.0 Hz), 7.82 (t, 1H, H-6 quinazoline, J = 8.0 Hz), 8.04 (d, 2H, Ar-H, J = 8.8 Hz), 8.09 (d, 2H, Ar-H, J = 4 Hz) , 8.11 (t, 1H, H-7 quinazoline, J = 2.8 Hz), 8.32 (d, 2H, Ar-H, J = 8.4 Hz), 8.36 (d, 1H, H-5 quinazoline, J = 8.4 Hz), 8.96 (d, 1H, H-8 quinazoline, J = 8.0 Hz) 11.72 (s, 1H, NH); 13C NMR (DMSO-d6): 21.70, 113.35, 124.26, 125.10, 128.32, 128.51, 129.75, 130.18, 130.44, 136.45, 141.67, 144.41, 157.70, 159.44, 167.30; Anal. Calcd. For: C22H17N3O2 (355.40): C, 74.35; H, 4.82; N, 11.82; Found: C, 74.21; H, 4.80; N, 11.84.
4-((2–(4-Methoxyphenyl)quinazolin-4-yl)amino)benzoic acid (8c)
Off white crystals, (78%) yield; m.p. >300 °C; IR (KBr) νmax/cm−1; 1H NMR (DMSO-d6): 3.90 (s, 3H, OCH3), 7.22 (d, 2H, Ar-H, J = 8.8 Hz,), 7.71 (d, 1H, Ar-H, J = 8.8 Hz), 7.81 (t, 1H, H-6 quinazoline, J = 7.6 Hz), 8.01 (d, 2H, Ar-H, J = 8.4 Hz), 8.09–8.13 (m, 3H, Ar-H and H-7 quinazoline), 8.42–8.47 (m, 3H, Ar-H and H-5 quinazoline), 8.97 (d, 1H, H-8 quinazoline, J = 8.4 Hz), 11.81 (s, 1H, NH); 13C NMR (DMSO-d6): 56.26, 113.06, 115.15, 120.53, 123.43, 124.56, 125.20, 128.44, 128.54, 130.45, 131.60, 132.08, 136.61, 141.48, 157.04, 159.39, 164.22, 167.29, 167.72; Anal. Calcd. For: C22H17N3O3 (371.40): C, 71.15; H, 4.61; N, 11.31; Found: C, 71.02; H, 4.59; N, 11.26.
Biological evaluation
CA inhibitory assay
All the newly synthesised quinazoline-based carboxylic acid derivatives (6a–c, 7a–c and 8a–c) were assessed for their CA catalysed CO2 hydration activities against hCA isoforms I, II, IX and XII by the stopped flow CO2 hydrase assay as reported previouslyCitation37–40 (Supporting Materials).
In vitro NCI-59 cancer cell lines assays
The NCI-USA anticancer assays was performed utilising the NCI, Bethesda, Drug Evaluation Branch protocol,Citation41–43 using the SRB cytotoxicity assay,Citation44 as desribed earlier.Citation45,Citation46
Results and discussion
Chemistry
The synthetic strategy to develop the target 2-aryl-quinazolin-4-yl aminobenzoic acid derivatives (6a–c, 7a–c, and 8a–c) were represented in Schemes 1 and 2. Synthesis of intermediates (3a–c) was carried out by reacting different aldehydes (2a–c) with anthranilamide (1) in an aqueous solution of FeCl3. The key intermediates (4a–c) were then synthesised via a chlorination reaction of quinazolinone derivatives (3a–c) with phosphrous oxychloride in the presence of the catalytic amount of N,N-dimethylformamide (Scheme 1).
Scheme 1. Synthesis of chloroquinazolines (4a–c): Reaction conditions (i) FeCl3/H2O/heating 80 °C/3h, (ii) POCl3/N,N-dimethylformamide (cat.)/heating 90 °C/4h.
The target 2-aryl-quinazolin-4-yl aminobenzoic acids (6a–c, 7a–c, and 8a–c) were obtained, with a yield of 65–86%, by reacting 2-aryl-4-chloroquinazoline derivatives (4a–c) with aminobenzoic acid derivatives (5a–c) in refluxing isopropanol containing few drops of HCl (Scheme 2).
Scheme 2. Synthesis of 2-aryl-quinazolin-4-yl aminobenzoic acids (6a–c, 7a–c and 8a–c): Reaction conditions (i) Isopropanol/HCl (cat.)/reflux/2h.
The target quinazoline derivatives (6a–c, 7a–c and 8a–c) were structurally confirmed by spectral and elemental analyses. The 1H NMR spectra of all compounds revealed a singlet signal around δ 11.70–12.34 ppm due to the proton of the NH group. Moreover, all compounds showed two doublet signals in the aromatic region around δ 7.49–8.40 and 8.61–9.03 ppm that are attributable to H5 and H8 of quinazoline moiety, respectively. In addition, 1H NMR spectra for derivatives (6a–b, 7a–b and 8a–b) showed another singlet signal for the CH3 group at the range of δ 2.41–2.43 ppm, whereas, the 1H NMR spectra for (6c, 7c and 8c) disclosed the singlet signal of the OCH3 group around δ 3.87–3.90 ppm. One the other hand, 13C NMR spectra for the target quinazoline derivatives confirmed the presence of the carboxylic C=O functionality at δ 162–170 ppm. Furthermore, 13C NMR spectra for compounds (6a–b, 7a–b, and 8a–b) showed a signal at δ 21.41–21.70 ppm for the CH3 carbon, whereas spectra of compounds (6c, 7c, and 8c) displayed a signal at δ 56.02–56.25 ppm for the OCH3 carbon.
Biological evaluation
Carbonic anhydrase inhibition
All the newly synthesised quinazoline-based carboxylic acid derivatives (6a–c, 7a–c, and 8a–c) were assessed for their inhibitory activities against the hCA I, II (cytosolic), IX and XII (trans membrane, tumour associated) isoforms by the stopped-flow CO2 hydrase assay.Citation37 Acetazolamide (AAZ) was used as a standard CA inhibitor. The data is summarised in .
Table 1. Inhibition data of hCA isoforms I, II, IX, XII, for carboxylic acids (6a–c, 7a–c, and 8a–c) by a stopped flow CO2 hydrase assay.
Only three of the tested quinazoline-based carboxylic acids (8a, 8b, and 8c) weakly inhibited the cytosolic hCA I isoform, with inhibition constants (KIs) equal 87.7, 73.2, and 66.3 µM, respectively, whereas quinazoline derivatives 6a–c and 7a–c could not inhibit hCA I up to 100 µM. These results revealed that grafting the carboxylic acids functionality at the para position (8a–c) could result in modest hCA I inhibitory activity, while shifting to ortho- (6a–c) or meta- (7a–c) positions resulted in the elimination of hCA I inhibitory activity (KIs > 100 M), .
The cytosolic hCA II was effectively inhibited by para-aminobenzoic acid-bearing quinazolines (8a–c) with KIs of 9.3, 3.9 and 4.6 µM, respectively, whereas, their ortho (6a–c) and meta (7a–c) regioisomers elicited modest inhibitory effects with inhibition constants spanning in the range of 26.0 − 85.8 µM. It is worth to mention that substitution of the 2-phenyl motif with a 4-methyl group, in series 8, led to compound 8b with the best hCA II inhibitory activity (KI = 3.9 µM).
Similar to the hCA I and hCA II inhibition profiles, the obtained KI values disclosed that the cancer-related hCA IX isoform was inhibited most effectively by para-aminobenzoic acid-bearing quinazolines (8a–c) with KIs equal 4.3, 1.6 and 4.5 µM, respectively. In addition, hCA IX was moderately affected by quinazolines decorated with ortho and meta aminobenzoic acid motifs with KIs ranging between 24.2 and 46.4 µM. The order of activities of target quinazoline-based carboxylic acids towards hCA IX was increased in the order of para isomers 8 (KIs: 1.6 − 4.5 µM) > meta isomers 7 (KIs: 24.2 − 31.6 µM) > ortho isomers 6 (KIs: 34.4 − 46.4 µM), . Regarding the impact of substitution on the 2-phenyl moiety, within series 6, 7, and 8, it was found that the order of hCA IX inhibitory activities was 4-methyl derivatives (6b, 7b, and 8b; KIs = 34.4, 24.2 and 1.6 µM) > 3-methyl derivatives (6a, 7a, and 8a; KIs = 42.5, 29.3 and 4.3 µM) > 4-methoxy derivatives (6c, 7c and 8c; KIs = 46.4, 31.6 and 4.5 µM), .
The second cancer-related isoform studied in this study is hCA XII, which is also the most vulnerable to the prepared molecules. All quinazoline-based carboxylic acids (6a–c, 7a–c, and 8a–c) exhibited good inhibition of hCA XII (KIs: 0.25 − 9.0 µM), as seen by the data in . In particular, the best hCA XII inhibitory effect was exerted by quinazoline 8c with a KI value equals 0.25 µM. Besides, quinazolines 7b, 7c, and 8b displayed also sub-micromolar inhibitory activity towards hCA XII with KI values 0.91, 0.48, and 0.42 µM, respectively. Similarly to the abovementioned deduced Structure-Activity Relationship (SAR) for hCA I, II, and IX isoforms, the order of hCA XII inhibitory activities was increased in the order of para isomers 8 (KIs: 0.25 − 3.8 µM) > meta isomers 7 (KIs: 0.48 − 4.8 µM) > ortho isomers 6 (KIs: 7.1–9 µM), . Also, it’s noteworthy that appending p-methoxyphenyl moiety at C-2 of quianzoline within series 7 and 8 (7c and 8c; KIs = 0.48 and 0.25 µM) resulted in a better hCA XII inhibitory activity than p-methylphenyl (7b and 8b; KIs = 0.91 and 0.42 µM) and m-methylphenyl (7a and 8a; KIs = 4.8 and 3.8 µM) moieties. The SAR for the inhibitory activity of the new quinazolines towards different hCA isoforms is summarised in .
As a result of the inhibitory profile for the reported quinazoline-based carboxylic acid derivatives (6a–c, 7a–c, and 8a–c) (), the selectivity index (SI) for each derivative was calculated and presented in . Regarding the selectivity towards CA IX and XII isoforms, most the examined quinazoline-based carboxylic acids (6a–c, 7a–c, and 8a–c) exhibited low to moderate selectivity, except compounds 7b and 7c that disclosed excellent selectivity towards hCA XII over hCA I (SI = 109.9 and 208.3, respectively), and hCA II (SI = 45.82 and 178.75, respectively), in addition, compounds 8b and 8c displayed outstanding selectivity towards hCA XII over hCA I (SI = 174.3 and 265.2, respectively), .
Table 2. Selectivity ratios for the inhibition of CA IX and XII isoforms over CA I and II isoforms for carboxylic acids (6a–c, 7a–c, and 8a–c) and acetazolamide.
In vitro anti-proliferative activity
The structures of all novel quinazoline based-carboxylic acids prepared in this study were submitted to the National Cancer Institute (NCI-USA), and six compounds (6a–c, 7b, and 8a–b) were chosen for in vitro anti-proliferative activity evaluation against fifty-nine human cancer cell lines representing nine tumour subpanels, according to Bethesda, Drug Evaluation Branch Protocol.Citation41–43
Preliminary single dose screening at 10 µM concentration
The anti-proliferative activities of the selected quinazoline derivatives (6a–c, 7b, and 8a–b) have been evaluated at single (10 μM) dose assay using the SRB protocol.Citation44 The obtained data was presented as a mean-graph of the percentage growth of the various treated cancer cells and was displayed in as the percentage growth inhibition (GI%) induced by the investigated compounds.
Examining the data in revealed that the tested quinazoline-based-carboxylic acids (6a–c, 7b, and 8a–b) demonstrated diverse patterns of sensitivity and selectivity against the various NCI cancer cell panels. Quinazoline derivatives featuring ortho aminobenzoic acid (6a–c) showed excellent broad cell growth inhibitory activity (GI % mean = 63, 84, and 52, respectively) against most of all cancer cell lines, whereas compounds (7b and 8a–b) with meta and para-aminobenzoic acid moiety showed fair selective activity (GI % mean = 14, 16 and 20, respectively) towards certain cancer cell lines as shown in .
Table 3. Percentage growth inhibition (GI%) of subpanel tumour cell lines at 10 μM concentration of the quinazoline based-carboxylic acids (6a–c, 7b, and 8a–b).
In particular, quinazoline derivative 6b stood out as the most effective anti-proliferative compound (GI % mean = 84). Compound 6b exhibited excellent activity with GI% more than 75% against the examined cancer cell lines from all subpanels, except non-small cell lung (A549, HOP-62, NCI-H322M and NCI-H522), colon (COLO 205), melanoma (SK-MEL-28 and UACC-257), ovarian (IGROV1, OVCAR-5, OVCAR-8 and SK-OV-3), renal (TK-10) and breast (MDA-MB-231) cancer cell lines. In addition, compound 6b showed good activity towards non-small cell lung (A549, HOP-62, NCI-H322M and NCI-H522), melanoma (SK-MEL-28 and UACC-257), ovarian (IGROV1, OVCAR-8, and SK-OV-3), breast (MDA-MB-231) cancer cell lines with GI% of 70, 72, 66, 67, 71, 71, 69, 69, 69 and 65% respectively. It is noteworthy to mention that quinazoline derivative 6b had a lethal cytotoxic effect against non-small cell lung (HOP-92, NCI-H23, and NCI-H460), melanoma (SK-MEL-5), and breast (HS 578 T, BT-549, and MDA-MB-468) cancer cell lines with GI% equal 120, 104, 109,124, 109, 123 and 126% respectively.
Moreover, quinazoline 6a disclosed a broad-spectrum anticancer effect against 56 cell lines representing all subpanels and emerged as the second most active compound in this assay (mean % GI = 63). Superiorly, quinazoline 6a exerted effective cell growth inhibitory activity with GI% more than 75% against leukaemia (CCRF-CEM and SR), non-small cell lung (EKVX, NCI-H23 and NCI-H460), colon (HCT-116 and HCT-15), CNS (SNB-19), melanoma (MDA-MB-435 and SK-MEL-5), ovarian (NCI/ADR-RES), renal (786–0) and breast (MCF7, HS 578 T and BT-549) cancer cell lines. In addition, compound 6a possessed a lethal impact towards non-small cell lung (HOP-92) and breast (MDA-MB-468) cancer cell lines with GI% of 105 and 115% respectively.
In vitro NCI 5-dose assay
The preliminary screening data showed that quinazoline-carboxylic acid 6b (NSC: 835857) was the most active anticancer molecule in this study, with promising activity against numerous tumour cell lines. Thus, 6b was selected by NCI for further evaluations at a 5-doses (0.01–100 µM) level. Three dose-response parameters (GI50, TGI, and LC50) were calculated and displayed in .
Table 4. GI50, TGI, and LC50 values of NCI five doses anticancer assay for 6b (NSC: 835857).
Results displayed in , disclosed that compound 6b exhibited good anti-proliferative activities towards all the examined human cancer cell subpanels with GI50 values range 1.4 − 19.9 μM, except for renal TK-10 cell line (GI50 = 29.3 μM). In particular, the best anti-proliferative activity was noticed for non-small cell (HOP-92), CNS (SNB-75), and melanoma (SK-MEL-2) cancer cell lines with GI50 values equal 2.9, 1.4, and 3.7 μM, respectively ().
Concerning the cytostatic impact of quinazoline 6b, it showed moderate to good effect towards melanoma (SK-MEL-2), non-small cell (HOP-92, NCI-H522, NCI-H460, and HOP-62), colon (HCT-15, COLO 205, HCC-2998, and HCT-116), CNS (U251, SF-539, and SF-295), melanoma (UACC-62, SK-MEL-5, and LOX IMVI), and breast (BT-549, and MDA-MB-468) with TGI range = 15.6– 42 μM. It is worthy to mention that 6b exhibited LC50 values more than 100 μM and considered as non-lethal towards all the examined cell lines except for non-small cell (NCI-H460), colon (COLO 205), CNS (SF-539 and SF-295), melanoma (UACC-62, SK-MEL-5, LOX IMVI, and SK-MEL-2), and breast (BT-549) that possessed weak lethal effect with LC50 = 84.0, 94.1, 67.2, 86.0, 71.6, 57.5, 97.7, 83.5, and 78.1 μM, respectively ().
With regard to the sensitivity of the examined cell lines, quinazoline 6b elicited comparatively homogenous growth inhibitory activity throughout all NCI panels, with good growth inhibition full panel GI50 (MG-MID) equals 11.99 μM, as well as subpanel GI50 (MG-MID) values spanning from 8.04 to 15.66 μM. In particular, the most susceptible subpanels were CNS and Leukaemia with MG-MID of 8.04 and 8.68 μM, respectively (). In order to assess the selectivity of 6b, its full panel MG-MID is divided by its individual subpanel MG-MID (). The selectivity index for compound 6b ranged from 0.76 to 1.49 which points out that 6b has non-selective broad-spectrum anti-proliferative activity towards all NCI cancer subpanels. It is worth to mention that the best anti-proliferative counterpart 6b is not the most active inhibitor against CA IX or XII, thus the target of this compound could be other than CAs.
Table 5. Median GI50 values (µM) for compound 6b on subpanel tumour cell lines.
Conclusions
Three sets of 2-aryl-quinazolin-4-yl aminobenzoic acid regioisomers (6a–c, 7a–c, and 8a–c) were designed and synthesised as new non-classical CA inhibitors. Their CA inhibitory activities towards isoforms I, II, IX, and XII were evaluated. Only three of the tested quinazoline-based carboxylic acids (8a, 8b, and 8c) weakly inhibited the cytosolic hCA I isoform, with inhibition constants (KIs) equal 87.7, 73.2, and 66.3 µM. The cytosolic hCA II was effectively inhibited by para-aminobenzoic acid-bearing quinazolines (8a–c) with KIs of 9.3, 3.9, and 4.6 µM, respectively, whereas, their ortho (6a–c) and meta (7a–c) regioisomers elicited modest inhibitory effects. Moreover, the cancer-related hCA IX isoform was inhibited most effectively by quinazolines (8a–c) with KIs equal 4.3, 1.6, and 4.5 µM, respectively. Also, the results revealed that the cancer-related hCA XII isoform is the most vulnerable to the prepared molecules. In particular, the best hCA XII inhibitory effect was exerted by quinazoline 8c (KI = 0.25 µM), also, quinazolines 7b, 7c, and and 8b displayed sub-micromolar hCA XII inhibitory activity (KI = 0.91, 0.48, and 0.42 µM, respectively). The SAR analysis highlighted that the order of hCA inhibitory activities was increased in the order of para isomers 8 > meta isomers 7 > ortho isomers 6. On the other hand, the anti-proliferative activities of the quinazoline derivatives (6a–c, 7b, and 8a–b) have been evaluated at single (10 μM) dose assay against 59 cancer cell lines in the NCI-USA. Quinazoline derivatives featuring ortho aminobenzoic acid (6a–c) showed excellent broad cell growth inhibitory activity (GI % mean = 63, 84 and 52, respectively) against most of all cancer cell lines, whereas compounds (7b and 8a–b) with meta and para aminobenzoic acid moiety showed fair selective activity (GI % mean = 14, 16, and 20, respectively) towards certain cancer cell lines. Thereafter, 6b was selected by NCI for further evaluations at 5-doses (0.01–100 µM) level. Quinazoline 6b elicited comparatively homogenous growth inhibitory activity throughout all NCI panels, with good growth inhibition full panel GI50 (MG-MID) equals 11.99 μM, as well as subpanel GI50 (MG-MID) values spanning from 8.04 to 15.66 μM. In particular, the most susceptible subpanels were CNS and Leukaemia with MG-MID of 8.04 and 8.68 μM, respectively.
Supplemental Material
Download PDF (1.1 MB)Disclosure statement
CT Supuran is Editor-in-Chief of the Journal of Enzyme Inhibition and Medicinal Chemistry. He was not involved in the assessment, peer review, or decision-making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J. 2016;473(14):2023–2032.
- Capasso C, Supuran CT. isoforms/isoforms. In: Supuran CT, Capasso C, editors. Targeting carbonic anhydrases. London: Future Science Ltd.; 2014. p. 7–16.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017;12(1):61–88.
- Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: Where are we today? Med Res Rev. 2020;40(6):2485–2565.
- Mincione F, Nocentini A, Supuran CT. Advances in the discovery of novel agents for the treatment of glaucoma. Expert Opin Drug Discov. 2021;16(10):1209–1225.
- Ciccone L, Cerri C, Nencetti S, Orlandini E. Carbonic anhydrase inhibitors and epilepsy: State of the art and future perspectives. Molecules. 2021;26(21):6380.
- Costa G, Carta F, Ambrosio FA, Artese A, Ortuso F, Moraca F, Rocca R, Romeo I, Lupia A, Maruca A, et al. A computer-assisted discovery of novel potential anti-obesity compounds as selective carbonic anhydrase VA inhibitors. Eur J Med Chem. 2019;181:111565.
- Angeli A, Carta F, Nocentini A, Winum J-Y, Zalubovskis R, Akdemir A, Onnis V, Eldehna WM, Capasso C, Simone GD, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites. 2020;10(10):412.
- Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg Chem. 2020;103:104163.
- Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Supuran CT. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun. 2012;48(13):1868–1870.
- Karioti A, Carta F, Supuran CT. Phenols and polyphenols as carbonic anhydrase inhibitors. Molecules. 2016;21(12):1649.
- Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat. 2013;23(6):705–716.
- A study of SLC-0111 and gemcitabine for metastatic pancreatic ductal cancer in subjects positive for CAIX (SLC-0111-17-01). 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03450018
- D'Ambrosio K, Carradori S, Monti SM, Buonanno M, Secci D, Vullo D, Supuran CT, De Simone G. Out of the active site binding pocket for carbonic anhydrase inhibitors. Chem Commun. 2015;51(2):302–305.
- Supuran CT. Novel carbonic anhydrase inhibitors. Future Med Chem. 2021;13:1935–1937.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31(3):345–360.
- Ibrahim HS, Abdelrahman MA, Nocentini A, Bua S, Abdel-Aziz HA, Supuran CT, Abou-Seri SM, Eldehna WM. Insights into the effect of elaborating coumarin-based aryl enaminones with sulfonamide or carboxylic acid functionality on carbonic anhydrase inhibitory potency and selectivity. Bioorg Chem. 2022;126:105888.
- Elimam DM, Elgazar AA, Bonardi A, Abdelfadil M, Nocentini A, El-Domany RA, Abdel-Aziz HA, Badria FA, Supuran CT, Eldehna WM. Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: design, synthesis and biological evaluation. Eur J Med Chem. 2021;225:113800.
- Cau Y, Vullo D, Mori M, Dreassi E, Supuran CT, Botta M. Potent and selective carboxylic acid inhibitors of tumor-associated carbonic anhydrases IX and XII. Molecules. 2017;23(1):17.
- Eldehna WM, Nocentini A, Elsayed ZM, Al-Warhi T, Aljaeed N, Alotaibi OJ, Al-Sanea MM, Abdel-Aziz HA, Supuran CT. Benzofuran-based carboxylic acids as carbonic anhydrase inhibitors and antiproliferative agents against breast cancer. ACS Med Chem Lett. 2020;11(5):1022–1027.
- Alkhaldi AAM, Al-Sanea MM, Nocentini A, Eldehna WM, Elsayed ZM, Bonardi A, Abo-Ashour MF, El-Damasy AK, Abdel-Maksoud MS, Al-Warhi T, et al. 3-Methylthiazolo [3,2-a]benzimidazole-benzenesulfonamide conjugates as novel carbonic anhydrase inhibitors endowed with anticancer activity: design, synthesis, biological and molecular modeling studies. Eur J Med Chem. 2020;207:112745.
- Abo-Ashour MF, Almahli H, Bonardi A, Khalil A, Al-Warhi T, Al-Rashood ST, Abdel-Aziz HA, Nocentini A, Supuran CT, Eldehna WM. Enaminone-based carboxylic acids as novel non-classical carbonic anhydrases inhibitors: design, synthesis and in vitro biological assessment. J Enzyme Inhib Med Chem. 2022;37(1):2256–2264.
- Shaldam M, Eldehna WM, Nocentini A, Elsayed ZM, Ibrahim TM, Salem R, El-Domany RA, Capasso C, Abdel-Aziz HA, Supuran CT. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur J Med Chem. 2021;216:113283.
- Bua S, Lomelino C, Murray AB, Osman SM, ALOthman ZA, Bozdag M, Abdel-Aziz HA, Eldehna WM, McKenna R, Nocentini A, et al. “A sweet combination”: developing saccharin and acesulfame K structures for selectively targeting the tumor-associated carbonic anhydrases IX and XII. J Med Chem. 2020;63(1):321–333.
- Shaldam M, Nocentini A, Elsayed ZM, Ibrahim TM, Salem R, El-Domany RA, Capasso C, Supuran CT, Eldehna WM. Development of novel quinoline-based sulfonamides as selective cancer-associated carbonic anhydrase isoform IX inhibitors. IJMS. 2021;22(20):11119.
- Al-Warhi T, Elbadawi MM, Bonardi A, Nocentini A, Al-Karmalawy AA, Aljaeed N, Alotaibi OJ, Abdel-Aziz HA, Supuran CT, Eldehna WM. Design and synthesis of benzothiazole-based SLC-0111 analogues as new inhibitors for the cancer-associated carbonic anhydrase isoforms IX and XII. J Enzyme Inhib Med Chem. 2022;37(1):2635–2643.
- Eldehna WM, Abdelrahman MA, Nocentini A, Bua S, Al-Rashood ST, Hassan GS, Bonardi A, Almehizia AA, Alkahtani HM, Alharbi A, et al. Synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5 (4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg Chem. 2019;90:103102.
- Eldehna WM, Taghour MS, Al-Warhi T, Nocentini A, Elbadawi MM, Mahdy HA, Abdelrahman MA, Alotaibi OJ, Aljaeed N, Elimam DM, et al. Discovery of 2, 4-thiazolidinedione-tethered coumarins as novel selective inhibitors for carbonic anhydrase IX and XII isoforms. J Enzyme Inhib Med Chem. 2022;37(1):531–541.
- Tawfik HO, Shaldam MA, Nocentini A, Salem R, Almahli H, Al-Rashood ST, Supuran CT, Eldehna WM. Novel 3-(6-methylpyridin-2-yl) coumarin-based chalcones as selective inhibitors of cancer-related carbonic anhydrases IX and XII endowed with anti-proliferative activity. J Enzyme Inhib Med Chem. 2022;37(1):1043–1052.
- Liu F, Tang B, Liu H, Li L, Liu G, Cheng Y, Xu Y, Chen W, Huang Y. 4-Anilinoquinazoline derivatives with epidermal growth factor receptor inhibitor activity. Anti-Cancer Agents Med Chem. 2016;16:1652–1664.
- Ahmad I., Shagufta An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm. 2017;8(5):871–885.
- Ismail RS, Abou-Seri SM, Eldehna WM, Ismail NS, Elgazwi SM, Ghabbour HA, Ahmed MS, Halaweish FT, Abou El Ella DA. Novel series of 6-(2-substitutedacetamido)-4-anilinoquinazolines as EGFR-ERK signal transduction inhibitors in MCF-7 breast cancer cells. Eur J Med Chem. 2018;155:782–796.
- Ismail RS, Ismail NS, Abuserii S, Abou El Ella DA. Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future J Pharm Sci. 2016;2(1):9–19.
- Nada H, Elkamhawy A, Abdellattif MH, Angeli A, Lee CH, Supuran CT, Lee K. 4-Anilinoquinazoline-based benzenesulfonamides as nanomolar inhibitors of carbonic anhydrase isoforms I, II, IX, and XII: design, synthesis, in-vitro, and in-silico biological studies. J Enzyme Inhib Med Chem. 2022;37(1):994–1004.
- Alafeefy AM, Ahmad R, Abdulla M, Eldehna WM, Al-Tamimi A-MS, Abdel-Aziz HA, Al-Obaid O, Carta F, Al-Kahtani AA, Supuran CT. Development of certain new 2-substituted-quinazolin-4-yl-aminobenzenesulfonamide as potential antitumor agents. Eur J Med Chem. 2016;109:247–253.
- Eldehna WM, Almahli H, Al-Ansary GH, Ghabbour HA, Aly MH, Ismael OE, Al-Dhfyan A, Abdel-Aziz HA. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J Enzyme Inhib Med Chem. 2017;32(1):600–613.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246(8):2561–2573.
- Abdelrahman MA, Ibrahim HS, Nocentini A, Eldehna WM, Bonardi A, Abdel-Aziz HA, Gratteri P, Abou-Seri SM, Supuran CT. Novel 3-substituted coumarins as selective human carbonic anhydrase IX and XII inhibitors: synthesis, biological and molecular dynamics analysis. Eur J Med Chem. 2021;209:112897.
- Fares M, Eldehna WM, Bua S, Lanzi C, Lucarini L, Masini E, Peat TS, Abdel-Aziz HA, Nocentini A, Keller PA, et al. Discovery of potent dual-tailed benzenesulfonamide inhibitors of human carbonic anhydrases implicated in glaucoma and in vivo profiling of their intraocular pressure-lowering action. J Med Chem. 2020;63(6):3317–3326.
- Said MA, Eldehna WM, Nocentini A, Bonardi A, Fahim SH, Bua S, Soliman DH, Abdel-Aziz HA, Gratteri P, Abou-Seri SM, et al. Synthesis, biological and molecular dynamics investigations with a series of triazolopyrimidine/triazole-based benzenesulfonamides as novel carbonic anhydrase inhibitors. Eur J Med Chem. 2020;185:111843.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, et al. Feasibility of a highflux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83(11):757–766.
- Boyd MR. Cancer. In: Teicher BA, editors. Drug discovery and development: anticancer drug development guide: preclinical screening, clinical trials and approval. 2nd ed. Totowa (NJ): Humana Press; 2014. p. 41–62.
- Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res. 1995;34(2):91–109.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112.
- Eldehna WM, Salem R, Elsayed ZM, Al-Warhi T, Knany HR, Ayyad RR, Traiki TB, Abdulla M-H, Ahmad R, Abdel-Aziz HA, et al. Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with apoptosis inducing mechanism in colon cancer. J Enzyme Inhib Med Chem. 2021;36(1):1423–1434.
- Elbadawi MM, Eldehna WM, Wang W, Agama KK, Pommier Y, Abe M. Discovery of 4-alkoxy-2-aryl-6, 7-dimethoxyquinolines as a new class of topoisomerase I inhibitors endowed with potent in vitro anticancer activity. Eur J Med Chem. 2021;215:113261.