Abstract
Inositol polyphosphates (IPs) are a group of inositol metabolites that act as secondary messengers for external signalling cues. They play various physiological roles such as insulin release, telomere length maintenance, cell metabolism, and aging. Inositol hexakisphosphate kinase 2 (IP6K2) is a key enzyme that produces 5-diphosphoinositol 1,2,3,4,6-pentakisphosphate (5-IP7), which influences the early stages of glucose-induced exocytosis. Therefore, regulation of IP6Ks may serve as a promising strategy for treating diseases such as diabetes and obesity. In this study, we designed, synthesised, and evaluated flavonoid-based compounds as new inhibitors of IP6K2. Structure-activity relationship studies identified compound 20s as the most potent IP6K2 inhibitor with an IC50 value of 0.55 μM, making it 5-fold more potent than quercetin, the reported flavonoid-based IP6K2 inhibitor. Compound 20s showed higher inhibitory potency against IP6K2 than IP6K1 and IP6K3. Compound 20s can be utilised as a hit compound for further structural modifications of IP6K2 inhibitors.
Introduction
Myo-inositol (inositol), a carbocyclic sugar with one axial and five equatorial hydroxyl groups, is an essential nutrient for human health.Citation1 The discovery of phospholipase C (PLC) has highlighted the biological significance of inositol polyphosphates (IPs), which are bioactive signalling secondary messengers.Citation2,Citation3 For example, inositol 1,4,5-trisphosphate (IP3) mediates cytosolic calcium release by opening an IP3-gated calcium channel.Citation2 IP3 is further metabolised into higher phosphorylated IPs, such as IP4, IP5, and IP6 by a group of IP kinases, such as IP3-kinases, IP5-2 kinase, and inositol polyphosphate multikinase (IPMK).Citation3–7
Among the many IP species commonly found in eukaryotic cells from yeast to humans, pyrophosphorylated-IPs (PP-IPs) have drawn attention because they have highly energetic phosphoanhydride bonds (pyrophosphates) at specific positions.Citation3,Citation7 PP-IP biosynthesis is catalysed by two groups of IP kinases, IP6 kinases (IP6Ks) and PPIP5 kinases (PPIP5Ks), which phosphorylate IP6 and IP7.Citation4,Citation5 In mammals, IP6Ks (IP6K1/2/3) phosphorylate IP6 at the 5-position to form 5-PP-IP5 (5-IP7),Citation8,Citation9 whereas PPIP5Ks (PPIP5K1/2) phosphorylate IP6 at the 1-position to produce 1-PP-IP5 (designated as 1-IP7).Citation10,Citation11 Compared with 5-IP7, 1-IP7 appears to be a better substrate for PP-IP phosphatases such as DIPP, which establishes higher levels of 5-IP7 than 1-IP7 in mammalian cells.Citation12,Citation13
As a signalling molecule, 5-IP7 modulates specific target proteins to control signalling events via different molecular interactions.Citation3,Citation7 For example, 5-IP7 allosterically interacts with the Akt PH domain, thereby inhibiting its recruitment to phosphatidylinositol 3,4,5-trisphosphates (PIP3) in the plasma membrane and its subsequent activation.Citation14 Other 5-IP7 binding proteins include synaptotagmin, phosphatidylinositol 3-kinase (PI3K) p85α, and casein kinase.Citation15–17 5-IP7 is also known to non-enzymatically transfer its β-phosphates to serine residues that have been primed by CK2-mediated phosphorylation.Citation18,Citation19 Accumulating evidence has demonstrated that 5-IP7 and IP6 kinases regulate various biological events including growth, apoptosis, male fertility, metabolic homeostasis, blood clotting, immunity, vesicle trafficking, and longevity.Citation3,Citation7,Citation20 Thus, increasing efforts are being undertaken for developing therapeutic options for managing pathological conditions such as obesity, type II diabetes, and cancer by pharmacologically targeting IP6Ks.Citation21–26
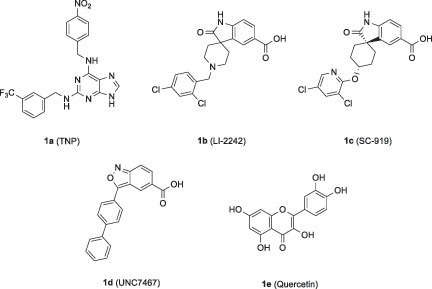
Since IP6K is considered as a potential target for obesity and metabolic diseases, several synthetic and natural IP6K inhibitors have been described to date.Citation22–25,Citation27,Citation28 One of the synthesised IP6K inhibitors is [N2−(m−(trifluoromethyl)benzyl) −N6−(p−nitrobenzyl)purine] (TNP) (1a), which acts as a competitive inhibitor by interacting with the ATP-binding site of IP6K ().Citation22 However, TNP has limitations for clinical use, including its inhibition of cytochrome P450 (CYP450), cellular Ca2+ fluxes, and several off-target kinases, such as CaMK1 and ERK.Citation24,Citation27,Citation29–31 The second compound is an oxindole analog, LI-2242 (1b), which was reported as a potent IP6K inhibitor (IP6K1 IC50: 31 nM, IP6K2 IC50: 42 nM, IP6K3 IC50: 8.7 nM).Citation25 Another oxindole analog, SC-919 (1c), was disclosed by Takeda Pharmaceuticals.Citation32 The latest IP6K inhibitor is a benzisoxazole analog, UNC7467 (1d), which was recently reported as a potent IP6K inhibitor (IP6K1 IC50: 8.9 nM, IP6K2 IC50: 4.9 nM, IP6K3 IC50: 1323 nM) with selectivity of IP6K1 and IP6K2 over IP6K3.Citation23
One of the natural IP6K2 inhibitors is quercetin (1e), a natural flavonoid.Citation24 Gu et. al., reported 17 natural dietary flavonoids with 5-OH or 5,7-di-OH substitutions as IP6K2 inhibitors.Citation24 Their inhibitory activity against IP6K2 varied according to the substitution pattern and number of hydroxyl groups in the A ring of the flavonoid scaffold. Generally, flavonoids with dihydroxyl groups at the A ring, such as quercetin and myricetin, exhibited stronger IP6K2 inhibition than monohydroxyl-substituted flavonoids. In addition, compounds with 3′,4′-di-OH substitution at the B ring inhibited IP6K2 more strongly than the corresponding compounds with 4′-OH substitution. Although the previous study identified natural flavonoids as hIP6K2 inhibitors with submicromolar IC50 values, a limited number of structure-activity relationship studies have been conducted on the 5- and 7-positions of the A ring. In this study, we designed, synthesised, and evaluated flavonoid-based analogs with a variation in the A ring to identify IP6K2 inhibitors that are more potent than natural flavonoids. Since synthetic IP6K inhibitors 1b-1d contained -COOH functional group, we also included it as a substituent to modify the flavonoid-based IP6K inhibitors.
Results and discussion
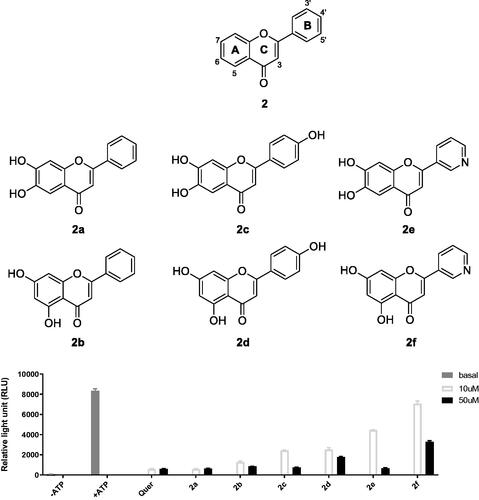
Previously, we reported flavonoid analogs as potential inhibitors of thymic stromal lymphopoietin (TSLP), an alarmin cytokine involved in allergic immune responses.Citation33 First, we screened the IP6K2-inhibitory activities of representative compounds (2a-2f, ) from an in-house compound library at concentrations of 10 and 50 µM using an in vitro ADP-Glo assay. We focussed on determining the effect of 5,7-di-OH substitution on IP6K2 inhibition compared to that of 6,7-di-OH substitution at the A ring, which is not common in natural flavonoids. Compounds (2a, 2c, and 2e) substituted with 6,7-di-OH were found to inhibit IP6K2 more strongly than the corresponding compounds (2b, 2d, and 2f) with 5,7-di-OH.
On the basis of preliminary studies, we designed and synthesised flavonoid analogs by replacing the -OH group of the A ring with the -F group to investigate the necessity of the hydroxyl group for IP6K2 inhibition. Scheme 1 describes the synthesis of fluoro-substituted flavonoid derivatives with various functional groups on the B ring. Commercial acetophenones 3–5 were used as starting materials for the synthesis of monofluoro-substituted compounds. In the case of difluoro-substituted compounds, starting materials 6 and 7 were prepared from 3,4-difluorophenol and 3,5-difluorophenol, respectively, by applying a reported synthetic procedure.Citation34,Citation35
Scheme 1. Synthesis of fluoro-substituted flavonoid analogs with a variation of the B ring. Reagents and conditions: (i) (a) Acetyl chloride, pyridine, CH2Cl2, rt, 30 min, (b) AlCl3, 150 °C, 10 min; (ii) appropriate aldehydes, Ba(OH)2, MeOH (or EtOH), 50 °C, 1–17 h; (iii) I2, DMSO, 110 °C, 6–24 h; (iv) BBr3, CH2Cl2, 50 °C, 14–18 h.
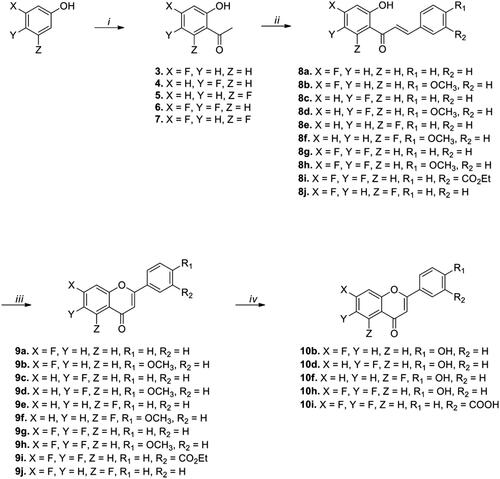
The reactions of compounds 3–7 and appropriate aldehydes with barium hydroxide in methanol (or ethanol) at 50 °C for 1–17 h afforded chalcone compounds 8a-8k (44-95% yield) via the Claisen-Schmidt condensation reaction. The intramolecular cyclisation of compounds 8a-8k with iodine (I2) at 110 °C for 6–24 h generated compounds 9a-9k (47-97% yield). Compounds substituted with a methoxy group or an ethyl ester were reacted with BBr3 to yield OH-substituted compounds (10b, 10d, 10f, and 10h) and COOH-substituted compound (10i), respectively. According to the results of the ADP-Glo assay, compounds (9a-10h) substituted with the -F group in the A ring showed considerably weaker IP6K2-inhibitory activity than the corresponding compounds with the -OH group at 10 and 50 μM concentrations (See Supporting Information Figures 1 & 2). Therefore, we maintained the -OH substituent in the A ring for further structural modification of the flavonoid-based IP6K2 inhibitors.
Next, we focussed on the structural modification of the B ring while maintaining the dihydroxyl substituents of the A ring at either the 5,7- or 6,7-positions. We introduced a polar carboxylic acid substituent at the meta- and para-positions of the B ring to generate potential ionic or hydrogen-bonding interactions. Scheme 2 describes the synthesis of flavonoid derivatives (16a-16d) with a carboxylic acid functional group in the B ring. The synthetic strategy for 16a-16d, which was similar to that for the F-substituted compounds (Scheme 1), included Claisen-Schmidt condensation and intramolecular cyclisation starting from methoxy-substituted acetophenones. The global dealkylation step was achieved using boron tribromide in dichloromethane at 50 °C, affording the final compounds 16a-16d (50-87% yield).
Scheme 2. Synthesis of dihydroxy-substituted flavonoid analogs with a variation of the B ring. Reagents and conditions: (i) Appropriate aldehydes, Ba(OH)2, MeOH (or EtOH), 50 °C, 11–20 h; (ii) I2, DMSO, 110 °C, 11–17 h; (iii) BBr3, CH2Cl2, 50 °C, 5–18 h.
The ADP-Glo kinase assay was conducted using the synthesised compounds 16a-16d (). Carboxylic-acid substitution at the meta position of the B-ring (16b and 16d) led to superior IP6K2 inhibition compared to para substitution (16a and 16c). Consistent with the results of the primary screening with 2a-2f, compounds 16a-16b with 6,7-dihydroxyl groups exhibited a stronger inhibitory effect on IP6K2 than the corresponding compounds 16c-16d with 5,7-dihydroxyl groups. Compounds 16a and 16b were as potent as quercetin, which possesses an -OH group at the 3-position of the C-ring.
Table 1. IC50 values of the synthesised compounds 16a-16d against IP6K2.
To increase IP6K2-inhibitory activity, we introduced a hydroxyl group at the 3-position of the C-ring. Scheme 3 describes the synthesis of compounds with an -OH group at the 3-position of the C ring. The reaction of 11 with the appropriate benzaldehydes under basic conditions (NaOMe in MeOH/THF) provided high yields of chalcone compounds (18a-18s and 21a-21c). The cyclized compounds (19a-19q and 22a-22c) were obtained via the Algar-Flynn-Oyamada (AFO) reaction of chalcone compounds with hydrogen peroxide (35% aq.) and sodium hydroxide (3 M aq.) in ethanol (or methanol) at 40 °C for 16-20 h. In the case of methylester compounds 19r and 19s, sodium methoxide was applied in the AFO reaction instead of sodium hydroxide. Reaction of the cyclized compounds with boron tribromide in dichloromethane at 50 °C for 6–16 h afforded compounds (20a-20s and 23a-23c) with substitution at the 3-OH of the C ring. The chemical structures and purities of the final compounds were confirmed using 1H NMR, LC/MS, and HPLC.
Scheme 3. Synthesis of flavonoid analogs with a 3-OH group on the C ring. Reagents and conditions: (i) Appropriate aldehydes, NaOMe, THF, rt, 16–20 h; (ii) a. H2O2, NaOH (or NaOMe), EtOH (or MeOH), 40 °C, b. HCl, 16–20 h; (iii) BBr3, CH2Cl2, 50 °C, 6-16 h.
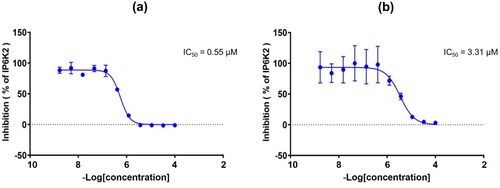
For accurate quantification of the IP6K2-inhibitory activity, the IC50 values of the synthesised compounds 20a-20s and 23a-23c were determined by performing an ADP-Glo kinase assay in a dose-dependent manner (). We confirmed the positional effect of OH groups in the A ring on IP6K2 inhibition by comparing compound 20a with quercetin. The IC50 value of compound 20a was 1.77 µM, while that of quercetin was 3.31 µM under the same experimental condition, consistently indicating that 6,7-substitution is preferred to 5,7-substitution in this series. The IC50 value of quercetin was reported as 0.70 µM.Citation24 However, under our experimental conditions, it was 3.31 µM. The difference could be due to the different experimental conditions including the IP6K2 enzyme concentration and reaction temperature.
Table 2. IC50 values of the synthesised compounds against IP6K2.
We examined the effects of substituents at the meta- or para-position of the B ring on IP6K2 inhibition. Compound 20b, with no substituents on the B-ring, was synthesised as a control compound. Compounds 20c-20e with a -CH3 group and compounds 20k-20l with a -Cl group were less potent than compound 20b. This result suggests that hydrophobic substituents on the B ring decrease IP6K2 inhibition. In addition, compounds 20f-20h with the -F group and the CF3-substituted compounds 20i-20j were less potent than 20b. In particular, the disubstituted compounds (e.g. 20e and 20h) completely lost their inhibitory activity against IP6K2. This may be due to the increased volume of the hydrophobic substituents. This negative effect of increased volume due to lipophilic substituents was confirmed for compounds (20m-20o) with bulky and hydrophobic substituents, such as the -Ph and -OPh groups.
In contrast, compound 20q, with a hydrophilic -OH group at the meta position in the B ring (20q), strongly inhibited IP6K2 with an IC50 value of 2.96 μM and showed a 10-fold greater inhibition than compound 20p, with the -OH group at the para position. Consistent with the result for analogs with a carboxylic acid substituent 16a-16d, this result implies that the introduction of a hydrophilic substituent, particularly at the m-position of the B ring, contributes to increased IP6K2 inhibition. As expected, the substitution of the carboxylic acid group in the B ring resulted in an increase in IP6K2 inhibition. Compounds 20r and 20s showed strong inhibitory activity against IP6K2. In particular, the carboxylic compound 20s was the most potent with an IC50 value of 0.55 µM, showing 5-fold more potent IP6K2 inhibition than quercetin ( and ). Compound 20s has a carboxylic acid moiety at the meta position in the B ring, which is approximately 7-fold more potent than compound 20r with the -COOH group at the para position. We additionally introduced heteroaromatic rings such as pyrazole (23b) and thiophene (23c). The pyrazole compound 23b exhibited an IC50 value of 1.79 µM, rendering it 2-fold more potent than quercetin.
Figure 3. (a) Dose-response curve of compound 20s against IP6K2. (b) Dose-response curve of quercetin against IP6K2.
To figure out the selectivity for IP6K2 over IP6K1 and IP6K3, we decided to evaluate the inhibition rate of quercetin and the most potent compound 20s. The IC50 value and the ratio of IP6K2 and other IP6Ks were shown in (See Supporting Information Figure 3). Compound 20s showed IC50 value of 2.87 µM against IP6K1 resulting 5.22-fold IC50 ratio of IP6K1/IP6K2 and IC50 value of 3.56 µM against IP6K3, resulting 6.47-fold IC50 ratio of IP6K3/IP6K2. When the final concentration of ATP and IP6 was adjusted to 10 μM, compound 20s showed 4.65-fold and 2.32-fold IC50 ratio of IP6K1/IP6K2 and IP6K3/IP6K2, respectively (See Supporting Information Figure 4). This result represented that compound 20s had selectivity against IP6K2 rather than IP6K1 or IP6K3 compared with quercetin. Overall, quercetin is a pan-IP6K inhibitor while compound 20s is a more selective IP6K2 inhibitor. Next, we determined the membrane permeability of 20s by an in vitro PAMPA permeability assay.Citation36 Compound 20s exhibited very low permeability with an apparent permeability coefficient of <1.8 nm/s while quercetin did with 5.4 nm/s (See Supporting Information Table 1). We further examined whether 20s can inhibit IP7 synthesis in human colorectal cell line (HCT116) after treatment of the compound (10 μM) for 6 h since IP6K2 has been reported as a major enzyme for cellular IP7 synthesis among three isoforms of IP6Ks in HCT116.Citation37 However, 20s failed to reduce cellular IP7 levels (See Supporting Information Figure 5), suggesting its low cell permeability.
Table 3. Inhibition data of quercetin and compound 20s against IP6K1/2/3.
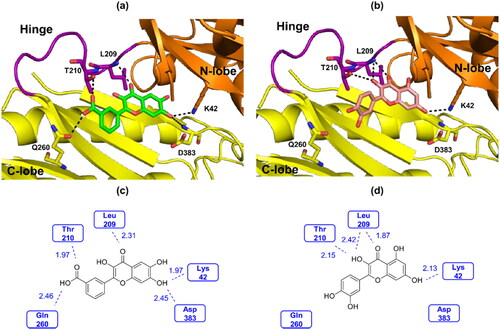
In silico molecular docking studies of compound 20s and quercetin were performed using the IP6K2 homology model. The homology model of human IP6K2 (UniProt: Q9UHH9) was downloaded from AlphaFold2 structure datable (https://alphafold.ebi.ac.uk). Quercetin has been reported as a ligand in the 3D crystal structure of IPMK (PDB ID: 6m89) and with that of DAPK1 (PDB ID: 5auw).Citation24,Citation38 According to the reported quercetin-bound protein crystal structures, quercetin can exist in two conformational isomers, designated 3,3′,4′,5,7-pentahydroxylflavone and 3,4′,5′,5,7-pentahydroxyflavone. Therefore, we performed the docking studies by using 3,3′,4′,5,7-pentahydroxylflavone and 3,4′,5′,5,7-pentahydroxyflavone for quercetin and 3′-(3,6,7-trihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid and 5′-(3,6,7-trihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid for compound 20s as initial ligand structures. Although two conformers of 20s were used as input files in the docking studies, only the docked pose exhibited with a conformation of 3′-(3,6,7-trihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (). On the contrary, two different conformers of quercetin were observed. The total score of the best-docked pose 20s was 4.3908 while that of quercetin was 3.7869. Both compound 20s and quercetin formed hydrogen bonds with Lys42 and with Leu209 (). However, compound 20s formed an additional hydrogen bond with Asp383 via the 7-OH group of the A ring compared to quercetin. In addition, the -COOH group in the B ring of compound 20s formed a hydrogen bond with Thr210 and additional hydrogen bond with Gln260, whereas the 3-OH group of quercetin formed one hydrogen bond with Thr210. This result supports a stronger IP6K2 inhibition of compound 20s than quercetin.
Conclusion
In the present study, new flavonoid-based IP6K2 inhibitors were designed, synthesised, and evaluated in vitro. Systemic structure-activity relationship studies for IP6K2 inhibition revealed the substituent preference for each ring in the flavonoid backbone as follows: -OH groups at the 6- and 7-positions in the A ring, -COOH group at the meta position of the B ring, and -OH group at the 3-position of the C ring. Furthermore, hydrophilic substituents such as -OH and -COOH in the B ring imparted stronger IP6K2-inhibitory activity than hydrophobic ones, including -CH3, -Cl, CF3, -Ph, and -OPh. Additionally, compounds with a substituent at the meta position of the B ring were more effective than the corresponding compounds with a substituent at the para position. Among the compounds synthesised, compound 20s was the most potent inhibitor with an IC50 value of 0.55 µM against IP6K2, which renders it 5-fold more potent than quercetin. In addition, compound 20s showed higher inhibitory potency against IP6K2 than IP6K1 and IP6K3. The molecular docking study showed that compound 20s formed additional hydrogen bonds with Gln260 and Asp383 compared to quercetin. Although compound 20s showed low membrane permeability, its physicochemical properties can be improved by a prodrug approach. Overall, compound 20s has the potential to be used as a hit compound for the structural optimisation of flavonoid-based IP6K2 inhibitors and as a tool compound for studying IP6K2 signalling pathways.
Experimental section
General
All chemicals and solvents used in the reaction were purchased from Sigma-Aldrich, TCI, and Acros and were used without further purification. Reaction progress was monitored by TLC on pre-coated silica gel plates with silica gel 60 F254 (Merck; Darmstadt, Germany) and visualised by UV254 light and/or KMnO4 staining for detection purposes. Column chromatography was performed on silica gel (Silica gel 60; 230–400 mesh ASTM, Merck, Darmstadt, Germany). A quantity of ∼1 mg was used for HSM experiment. Heating rate was 2 °C/minute and images were captured automatically every 1 min throughout the melting periods. Nuclear magnetic resonance (NMR) spectra were recorded at room temperature on a Bruker Ultrashield 600 MHz Plus (1H, 600 MHz; 13C, 150 MHz) spectrometer. All chemical shifts are reported in parts per million (ppm) from tetramethylsilane (δ = 0) and were measured relative to the solvent in which the sample was analysed (CDCl3: δ 7.26 for 1H NMR, δ 77.0 for 13C NMR; MeOH-d4: δ 3.31 for 1H NMR, δ 49.0 for 13C NMR; DMSO-d6: δ 2.50 for 1H NMR, δ 39.5 for 13C NMR). The 1H NMR shift values are reported as chemical shift (δ), the corresponding integral, multiplicity (s = singlet, br = broad, d = doublet, t = triplet, q = quartette, m = multiplet, dd = doublet of doublets, td = triplet of doublets, qd = quartette of doublets), coupling constant (J in Hz) and assignments. High-resolution mass spectra (HRMS) were recorded on an Agilent 6530 Accurate Mass Q-TOF LC/MS spectrometer. The purity of all final compounds was measured by analytical reverse-phase HPLC on an Agilent 1260 Infinity (Agilent) with a C18 column (Phenomenex, 150 mm × 4.6 mm, 3 μm, 110 Å). RP-HPLC was performed using various isocratic conditions: for method A, mobile phase was acetonitrile and water (55:45, v/v, 0.1% trifluoroacetic acid); for method B, mobile phase was acetonitrile and water (50:50, v/v, 0.1% trifluoroacetic acid); for method C, mobile phase was acetonitrile and water (45:55, v/v, 0.1% trifluoroacetic acid); for method D, mobile phase was acetonitrile and water (40:60, v/v, 0.1% trifluoroacetic acid); for method E, mobile phase was acetonitrile and water (35:65, v/v, 0.1% trifluoroacetic acid); for method F, mobile phase was acetonitrile and water (30:70, v/v, 0.1% trifluoroacetic acid); for method G, mobile phase was acetonitrile and water (25:75, v/v, 0.1% trifluoroacetic acid); for method H, mobile phase was acetonitrile and water (20:80, v/v, 0.1% trifluoroacetic acid); for method I, mobile phase was acetonitrile and water (15:85, v/v, 0.1% trifluoroacetic acid); for method J, mobile phase was acetonitrile and water (12:88, v/v, 0.1% trifluoroacetic acid). All compounds were eluted with a flow rate of 1 mL/min and monitored at a UV detector (220 nm or 254 nm). The purity of all tested compounds was >95%.
Chemical synthesis
General Procedure A for the synthesis of compounds 8a–8n and 14a–14d
To a stirred solution of substituted acetophenone (3–7 and 11–13) in methanol (MeOH) or ethanol (EtOH) was added barium hydroxide (2.0 eq) and appropriate aldehyde (1.2 eq) at room temperature. The reaction mixture was stirred under argon at 40–50 °C until complete conversion monitored by TLC analysis (typically 1–20 h), quenched with acetic acid, and extracted with EtOAc and H2O. The organic layer was washed with saturated aqueous NaHCO3, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography on a silica gel to furnish compounds 8a–8n and 14a–14d.
(E)-1–(4-Fluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8a)
Compound 8a was prepared in 72% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4-Fluoro-2-hydroxyphenyl)ethan-1-one (3) (200 mg, 1.3 mmol), benzaldehyde (159 μL, 1.2 eq), barium hydroxide (446 mg, 2.0 eq) in methanol (10 mL), stirring for 1 h at 50 °C. The crude residue was purified by column chromatography on silica gel (petroleum ether/ether = 50:1 to 20:1). Rf = 0.34 (petroleum ether/ether = 20:1). 1H NMR (600 MHz, CDCl3) δ 13.2 (s, 1H), 7.97 − 7.90 (m, 2H), 7.70 − 7.64 (m, 2H), 7.58 (d, J = 15.6 Hz, 1H), 7.48 − 7.43 (m, 3H), 6.71 (dd, J = 2.6 and 10.3 Hz, 1H), 6.69 − 6.65 (m, 1H).
(E)-1–(4-Fluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8b)
Compound 8b was prepared in 44% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4-Fluoro-2-hydroxyphenyl)ethan-1-one (3) (200 mg, 1.30 mmol), p-anisaldehyde (190 μL, 1.2 eq), barium hydroxide (445 mg, 2.0 eq) in methanol (10 mL), stirring for 17 h at 50 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 1:1). Rf = 0.50 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.33 (s, 1H), 7.96 − 7.89 (m, 2H), 7.63 (d, J = 8.7 Hz, 2H), 7.45 (d, J = 15.3 Hz, 1H), 6.96 (d, J = 8.6 Hz, 2H), 6.70 (dd, J = 2.5 and 10.3 Hz, 1H), 6.68 − 6.63 (m, 1H), 3.87 (s, 3H).
(E)-1–(5-Fluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8c)
Compound 8c was prepared in quantitative yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(5-Fluoro-2-hydroxyphenyl)ethan-1-one (4) (200 mg, 1.3 mmol), benzaldehyde (159 μL, 1.2 eq), barium hydroxide (446 mg, 2.0 eq) in methanol (8 mL), stirring for 1 h at 50 °C. The crude residue was purified by column chromatography on silica gel (petroleum ether/ether = 50:1 to 20:1). Rf = 0.45 (petroleum ether/ether = 20:1). 1H NMR (600 MHz, CDCl3) δ 12.53 (s, 1H), 7.95 (d, J = 15.4 Hz, 1H), 7.72 − 7.66 (m, 2H), 7.59 (dd, J = 3.0 and 9.0 Hz, 1H), 7.55 (d, J = 15.4 Hz, 1H), 7.47 − 7.44 (m, 3H), 7.29 − 7.22 (m, 2H), 7.0 (dd, J = 4.6 and 9.1 Hz, 1H).
(E)-1–(5-Fluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8d)
Compound 8d was prepared in 76% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4-Fluoro-2-hydroxyphenyl)ethan-1-one (4) (200 mg, 1.30 mmol), p-anisaldehyde (190 μL, 2 eq), barium hydroxide (445 mg, 2.0 eq) in methanol (10 mL), stirring for 4 h at 40 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 8:1 to 4:1). Rf = 0.56 (hexane/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3) δ 12.65 (s, 1H), 7.93 (d, J = 15.3 Hz, 1H), 7.65 (d, J = 8.6 Hz, 2H), 7.58 (dd, J = 2.9 and 9.1 Hz, 1H), 7.43 (d, J = 15.3 Hz, 1H), 7.25 − 7.21 (m, 1H), 7.02 − 6.94 (m, 3H), 3.88 (s, 3H).
(E)-1–(2-Fluoro-6-hydroxyphenyl)-3-phenylprop-2-en-1-one (8e)
Compound 8e was prepared in quantitative yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Fluoro-6-hydroxyphenyl)ethan-1-one (5) (166 μL, 1.3 mmol), benzaldehyde (159 μL, 1.2 eq), barium hydroxide (446 mg, 2.0 eq) in methanol (8 mL), stirring for 3 h at 50 °C. The crude residue was purified by column chromatography on silica gel (petroleum ether/ether = 50:1 to 20:1). Rf = 0.67 (hexane/EtOAc = 5:1). 1H NMR (600 MHz, CDCl3) δ 7.94 (dd, J = 3.5 and 16.0 Hz, 1H), 7.72 − 7.64 (m, 3H), 7.49 − 7.38 (m, 4H), 6.58 (d, J = 8.5 Hz, 1H), 6.71 − 6.61 (m, 1H); 13C NMR (150 MHz, CDCl3) δ 192.49, 164.68, 163.74, 162.05, 145.72, 136.07, 135.98, 134.66, 131.00, 129.04, 128.86, 125.39, 125.28, 114.47, 106.40, 106.23. HRMS m/z calculated for C15H11FO2 [M – H]–: 241.0670; found: 241.0681.
(E)-1–(2-Fluoro-6-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8f)
Compound 8f was prepared in quantitative yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Fluoro-6-hydroxyphenyl)ethan-1-one (5) (200 mg, 1.30 mmol), p-anisaldehyde (190 μL, 1.2 eq), barium hydroxide (445 mg, 2.0 eq) in methanol (10 mL), stirring for 6 h at 50 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 4:1). Rf = 0.33 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.17 (s, 1H), 7.93 (dd, J = 3.4 and 15.2 Hz, 1H), 7.62 (d, J = 8.7 Hz, 2H), 7.56 (dd, J = 1.8 and 15.4 Hz, 1H), 7.42 − 7.36 (m, 1H), 6.95 (d, J = 8.7 Hz, 2H), 6.81 (d, J = 8.4 Hz, 1H), 6.63 (dd, J = 8.3 and 12.1 Hz, 1H), 3.87 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 192.35, 164.68, 163.72, 162.13, 162.02, 145.77, 135.75, 135.67, 130.80, 130.78, 127.45, 122.99, 122.88, 114.55, 114.53, 114.40, 110.73, 110.63, 106.34, 106.18, 55.47. HRMS m/z calculated for C16H16F1O3 [M – H]–: 271.0776; found: 271.0780.
(E)-1–(4,5-Difluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8g)
Compound 8g was prepared in 84% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4,5-Difluoro-2-hydroxyphenyl)ethan-1-one (6) (200 mg, 1.16 mmol), benzaldehyde (142 μL, 1.2 eq), barium hydroxide (398 mg, 2.0 eq) in methanol (8 mL), stirring for 4 h at 50 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 12:1 to 6:1). Rf = 0.64 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.96 (d, J = 15.4 Hz, 1H), 7.76 − 7.70 (m, 1H), 7.69 − 7.65 (m, 2H), 7.51 − 7.42 (m, 4H), 6.83 (dd, J = 6.5 and 11.4 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ 192.02, 161.41, 156.36, 156.27, 146.61, 134.23, 131.35, 129.16, 128.83, 119.36, 116.97, 116.95, 116.85, 107.11, 106.99. HRMS m/z calculated for C15H10F2O2 [M – H]–: 259.0576; found: 259.0587.
(E)-1–(4,5-Difluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8h)
Compound 8h was prepared in 83% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4,5-Difluoro-2-hydroxyphenyl)ethan-1-one (6) (200 mg, 1.16 mmol), p-anisaldehyde (169 μL, 1.2 eq), barium hydroxide (398 mg, 2.0 eq) in methanol (8 mL), stirring for 4 h at 50 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 4:1). Rf = 0.43 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.09 (s, 1H), 7.93 (d, J = 15.1 Hz, 1H), 7.71 (dd, J = 8.6 and 10.8 Hz, 1H), 7.64 (d, J = 8.6 Hz, 2H), 7.34 (d, J = 15.4 Hz, 1H), 6.97 (d, J = 8.6 Hz, 2H), 6.81 (dd, J = 7.1 and 11.7 Hz, 1H), 3.88 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 192.02, 161.41, 156.36, 156.27, 146.61, 134.23, 131.35, 129.16, 12.83, 119.36, 116.97, 116.95, 116.5, 107.11, 106.9. HRMS m/z calculated for C16H12F2O3 [M – H]–: 289.0682; found: 289.0695.
(E)-Ethyl 3–(3-(4,5-difluoro-2-hydroxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (8i)
Compound 8i was prepared in 64% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(4,5-Difluoro-2-hydroxyphenyl)ethan-1-one (6) (150 mg, 0.87 mmol), methyl 3-formylbenzoate (172 mg, 1.2 eq), barium hydroxide (298 mg, 2.0 eq) in ethanol (10 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 500:1 to 5:1). Rf = 0.56 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.08 (s, 1H), 7.94 (d, J = 15.1 Hz, 2H), 7.71 (dd, J = 2.2 and 8.6 Hz, 1H), 7.64 (d, J = 8.64 Hz, 2H), 7.34 (d, J = 15.4 Hz, 1H), 6.98 (d, J = 8.64 Hz, 2H), 6.81 (dd, J = 4.6 and 7.1 Hz, 1H), 3.88 (s, 3H). HRMS m/z calculated for C18H14F2O4 [M – H]–: 331.0787; found: 331.0801.
(E)-1–(2,4-Difluoro-6-hydroxyphenyl)-3-phenylprop-2-en-1-one (8j)
Compound 8j was prepared in 95% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2,4-Difluoro-6-hydroxyphenyl)ethan-1-one (7) (200 mg, 1.16 mmol), benzaldehyde (142 μL, 1.2 eq), barium hydroxide (398 mg, 2.0 eq) in methanol (8 mL), stirring for 2 h at 50 °C. The crude residue was used in the next step without further purification. Rf = 0.74 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.98 (dd, J = 3.3 and 15.4 Hz, 1H), 7.71 − 7.66 (m, 2H), 7.64 (dd, J = 2.2 and 15.4 Hz, 1H), 7.50 − 7.44 (m, 3H), 6.59 − 6.53 (m, 1H), 6.47 − 6.41 (m, 1H); 13C NMR (150 MHz, CDCl3) δ 191.64, 191.60, 167.64, 166.78, 166.71, 165.09, 164.98, 163.39, 163.28, 146.20, 134.53, 131.13, 129.07, 128.88, 124.78, 124.67, 101.59, 101.43, 101.41, 96.27, 96.08, 95.90. HRMS m/z calculated for C15H10F2O2 [M – H]–: 259.0576; found: 259.0587.
(E)-1–(2,4-Difluoro-6-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8k)
Compound 8k was prepared in 85% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2,4-Difluoro-6-hydroxyphenyl)ethan-1-one (7) (200 mg, 1.16 mmol), p-anisaldehyde (169 μL, 1.2 eq), barium hydroxide (398 mg, 2.0 eq) in methanol (8 mL), stirring for 3 h at 50 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 6:1). Rf = 0.60 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.94 (dd, J = 3.5 and 15.4 Hz, 1H), 7.62 (d, J = 8.7 Hz, 2H), 7.50 (dd, J = 1.9 and 15.3 Hz, 1H), 6.95 (d, J = 8.8 Hz, 2H), 6.55 − 6.50 (m, 1H), 6.43 − 6.36 (m, 1H), 3.87 (s, 3H); 13C NMR (150 MHz, DMSO) δ 191.39, 162.23, 146.22, 130.82, 127.30, 122.29, 122.17, 114.55, 101.47, 101.34, 96.13, 95.94, 55.46. HRMS m/z calculated for C16H12F2O5 [M – H]–: 289.0682; found: 289.0696.
(E)-Ethyl 4–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14a)
Compound 14a was prepared in 70% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Hydroxy-4,5-dimethoxyphenyl)ethan-1-one (11) (200 mg, 1.02 mmol), methyl terephthalaldehydate (251 mg, 1.5 eq), barium hydroxide (350 mg, 2.0 eq) in ethanol (10 mL), stirring for 17 h at 70 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 8:1 to 4:1). Rf = 0.21 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.30 (s, 1H), 8.09 (d, J = 8.2 Hz, 2H), 7.88 (d, J = 15.4 Hz, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.57 (d, J = 15.5 Hz, 1H), 7.24 (s, 1H), 6.50 (s, 1H), 4.40 (q, J = 7.1 Hz, 2H), 3.93 (s, 3H), 3.92 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 191.01, 165.91, 162.01, 157.38, 142.93, 142.07, 138.86, 131.98, 130.12, 128.30, 122.46, 111.93, 110.88, 100.84, 61.26, 56.94, 56.24, 14.31. HRMS m/z calculated for C20H20O6 [M – H]–: 355.1187; found: 355.1203.
(E)-Ethyl 3–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14b)
Compound 14b was prepared in 26% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Hydroxy-4,5-dimethoxyphenyl)ethan-1-one (11) (200 mg, 1.02 mmol), methyl 3-formylbenzoate (200 mg, 1.2 eq), barium hydroxide (350 mg, 2.0 eq) in ethanol (10 mL), stirring for 20 h at 40 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 8:1 to 3:1). Rf = 0.28 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.33 (s, 1H), 8.35 (s, 1H), 8.09 (d, J = 7.7 Hz, 1H), 7.93 (d, J = 15.5 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.58 (d, J = 15.5 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 7.27 (d, J = 4.5 Hz, 2H), 6.53 (s, 1H), 4.43 (q, J = 7.1 Hz, 2H), 3.95 (s, 3H), 3.94 (s, 3H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 191.21, 166.08, 161.96, 157.32, 143.31, 142.07, 135.13, 132.88, 131.36, 131.32, 129.17, 129.11, 121.53, 111.95, 111.01, 100.86, 61.39, 57.04, 56.25, 14.34. HRMS m/z calculated for C20H20O6 [M + H]+: 357.1333; found: 357.1333.
(E)-Ethyl 4–(3-(2-hydroxy-4,6-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14c)
Compound 14c was prepared in 16% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Hydroxy-4,6-dimethoxyphenyl)ethan-1-one (12) (200 mg, 1.02 mmol), methyl terephthalaldehydate (200 mg, 1.2 eq), barium hydroxide (350 mg, 2.0 eq) in ethanol (10 mL), stirring for 18 h at 40 °C. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 8:1 to 3:1). Rf = 0.24 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 14.18 (s, 1H), 8.08 (d, J = 8.3 Hz, 2H), 7.95 (d, J = 15.6 Hz, 1H), 7.76 (d, J = 15.6 Hz, 1H), 7.65 (d, J = 8.2 Hz, 2H), 6.12 (d, J = 1.9 Hz, 1H), 5.98 (d, J = 2.3 Hz, 1H), 4.40 (q, J = 7.9 and 15.1 Hz, 2H), 3.93 (s, 3H), 3.85 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, MeOD) δ 192.29, 168.48, 166.52, 166.12, 162.52, 140.61, 139.80, 131.40, 130.06, 129.76, 128.07, 106.33, 93.83, 91.37, 61.18, 55.93, 55.65, 14.32. HRMS m/z calculated for C20H20O6 [M + H]+: 357.1333; found: 357.1343.
(E)-Ethyl 3–(3-(2-hydroxy-4,6-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14d)
Compound 14d was prepared in 83% yield as a yellow powder, following the same procedure as described in the general procedure A with 1–(2-Hydroxy-4,6-dimethoxyphenyl)ethan-1-one (12) (200 mg, 1.02 mmol), methyl 3-formylbenzoate (200 mg, 1.2 eq), barium hydroxide (350 mg, 2.0 eq) in ethanol (10 mL), stirring for 17 h at 40 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 200:1 to 100:1). Rf = 0.35 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 14.22 (s, 1H), 8.30 (s, 1H), 8.05 (d, J = 7.2 Hz, 1H), 7.97 (d, J = 15.6 Hz, 1H), 7.82 − 7.74 (m, 2H), 7.49 (t, J = 7.9 Hz, 1H), 6.12 (d, J = 2.0 Hz, 1H), 5.98 (d, J = 2.1 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 3.94 (s, 3H), 3.85 (s, 3H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 192.40, 1686.47, 166.43, 166.15, 162.53, 140.89, 135.93, 132.48, 131.19, 130.73, 129.12, 128.95, 128.85, 128.76, 93.82, 91.32, 61.23, 55.88, 55.63, 14.33. HRMS m/z calculated for C20H20O6 [M + H]+: 357.1333; found: 357.1328.
General Procedure B for compounds 9a–9k and 15a–15d
To a stirred solution of chalcone compound (8a–8k and 14a–14d.) in dimethylsulphoxide (DMSO) was added iodine (0.1 eq) at 25 °C. The reaction mixture was stirred under argon at 110 °C until complete conversion monitored by TLC analysis (typically 6–24h), quenched with 1M sodium thiosulphate solution, and extracted with EtOAc, and H2O. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography on a silica gel (hexane/EtOAc or CH2Cl2/MeOH)to furnish compounds 9a–9k and 15a–15d.
7-Fluoro-2-phenyl-4H-chromen-4-one (9a)
Compound 9a was prepared in 90% yield as a white powder, following the same procedure as described in the general procedure B (E)-1–(4-Fluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8a) (194 mg, 0.80 mmol), Iodine (20.3 mg, 0.1 eq) in DMSO (6 mL), stirring for 24 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 3:1). Rf = 0.27 (hexane/EtOAc = 4:1). m.p: 96–98 °C. 1H NMR (600 MHz, CDCl3) δ 8.26 (dd, J = 6.4 and 8.8 Hz, 1H), 7.91 (dd, J = 1.3 and 7.8 Hz, 2H), 7.60 − 7.51 (m, 3H), 7.28 (d, J = 2.2 Hz, 1H), 7.20 − 7.14 (m, 1H), 6.82 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 177.48, 166.60, 164.91, 163.75, 157.31, 157.23, 131.80, 131.46, 129.14, 128.30, 126.28, 120.87, 114.09, 113.94, 107.69, 104.92, 104.75. HRMS m/z calculated for C15H9FO2 [M + H]+: 241.0660; found: 241.0654. >95% purity (as determined by RP-HPLC, method C, tR = 11.30 min).
7-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9b)
Compound 9b was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(4-Fluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8b) (137 mg, 0.50 mmol), iodine (12.7 mg, 0.1 eq) in DMSO (7 mL), stirring for 16 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 5:1 to 1:1 and then CH2Cl2/MeOH = 10:1). Rf = 0.40 (hexane/EtOAc = 1:1). m.p: 228–230 °C. 1H NMR (600 MHz, CDCl3) δ 8.24 (dd, J = 6.4 and 8.8 Hz, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.24 (dd, J = 2.3 and 9.1 Hz, 1H), 7.17 − 7.12 (m, 1H), 7.04 (d, J = 8.9 Hz, 2H), 6.73 (s, 1H), 3.90 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 177.45, 166.49, 164.80, 163.77, 162.57, 157.22, 128.21, 128.14, 128.01, 123.67, 120.82, 114.56, 113.88, 113.74, 106.21, 104.80, 104.64, 55.55. HRMS m/z calculated for C16H11FO3 [M + H]+: 271.0765; found: 271.0753. >95% purity (as determined by RP-HPLC, method B, tR = 7.28 min).
6-Fluoro-2-phenyl-4H-chromen-4-one (9c)
Compound 9c was prepared in 91% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(5-Fluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8c) (304 mg, 1.25 mmol), iodine (31.7 mg, 0.1 eq) in DMSO (6 mL), stirring for 15 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 1:1). Rf = 0.46 (hexane/EtOAc = 4:1). m.p: 131–133 °C. 1H NMR (600 MHz, CDCl3) δ 7.93 (d, J = 7.1 Hz, 2H), 7.88 (dd, J = 3.2 and 8.1 Hz, 1H), 7.61 − 7.58 (m, 1H), 7.57 − 7.52 (m, 3H), 7.46 − 7.41 (m, 1H), 6.83 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 177.63, 163.71, 160.44, 158.81, 152.47, 131.82, 131.54, 129.12, 126.34, 125.21, 125.16, 122.01, 121.85, 120.22, 120.16, 110.74, 110.59, 106.92. HRMS m/z calculated for C15H9FO2 [M + H]+: 241.0660; found: 241.0648. >95% purity (as determined by RP-HPLC, method C, tR = 11.20 min).
6-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9d)
Compound 9d was prepared in 97% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(5-Fluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8d) (240 mg, 0.88 mmol), iodine (22.3 mg, 0.1 eq) in DMSO (10 mL), stirring for 15 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 6:1 to 1:1). Rf = 0.23 (hexane/EtOAc = 4:1). m.p: 160–162 °C. 1H NMR (600 MHz, CDCl3) δ 7.90 − 7.85 (m, 3H), 7.56 (dd, J = 4.1 and 9.1 Hz, 1H), 7.44 − 7.39 (m, 1H), 7.04 (d, J = 8.9 Hz, 2H), 6.74 (s, 1H), 3.90 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 177.55, 163.72, 162.58, 160.38, 158.74, 152.39, 128.07, 125.18, 123.75, 121.74, 121.57, 120.04, 119.99, 114.54, 110.72, 110.57, 105.52, 55.54. HRMS m/z calculated for C16H11FO3 [M + H]+: 271.0765; found: 271.0751. >95% purity (as determined by RP-HPLC, method B, tR = 7.12 min).
5-Fluoro-2-phenyl-4H-chromen-4-one (9e)
Compound 9e was prepared in 86% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(2-Fluoro-6-hydroxyphenyl)-3-phenylprop-2-en-1-one (8e) (200 mg, 0.83 mmol), iodine (21.1 mg, 0.1 eq) in DMSO (5 mL), stirring for 20 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 1:1). Rf = 0.27 (hexane/EtOAc = 4:1). m.p: 144–146 °C. 1H NMR (600 MHz, CDCl3) δ 7.91 (d, J = 7.8 Hz, 2H), 7.66 − 7.61 (m, 1H), 7.58 − 7.51 (m, 3H), 7.39 (d, J = 8.5 Hz, 1H), 7.12 − 7.05 (m, 1H), 6.78 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 176.79, 162.46, 133.74, 133.67, 131.81, 131.23, 129.13, 126.27, 114.06, 114.03, 112.23, 112.09, 108.69. HRMS m/z calculated for C15H9FO2 [M + H]+: 241.0660; found: 241.0647. >95% purity (as determined by RP-HPLC, method C, tR = 7.86 min).
5-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9f)
Compound 9f was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(2-Fluoro-6-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8f) (380 mg, 1.40 mmol), iodine (35.5 mg, 0.1 eq) in DMSO (10 mL), stirring for 16 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 5:1 to 1:1 and then CH2Cl2/MeOH = 10:1). Rf = 0.40 (hexane/EtOAc = 1:1). m.p: 178–180 °C. 1H NMR (600 MHz, CDCl3) δ 7.87 − 7.84 (m, 2H), 7.63 − 7.58 (m, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.08 − 7.04 (m, 1H), 7.04 − 7.01 (m, 2H), 6.69 (s, 1H), 3.90 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 176.63, 162.52, 162.35, 161.47, 159.72, 157.14, 157.12, 133.50, 133.43, 127.88, 123.20, 114.48, 114.28, 114.21, 113.94, 113.91, 112.02, 111.88, 107.04, 55.50. HRMS m/z calculated for C16H11FO3 [M + H]+: 271.0765; found: 271.0773. >95% purity (as determined by RP-HPLC, method C, tR = 7.46 min).
6,7-Difluoro-2-phenyl-4H-chromen-4-one (9g)
Compound 9 g was prepared in 88% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(4,5-Difluoro-2-hydroxyphenyl)-3-phenylprop-2-en-1-one (8 g) (145 mg, 0.56 mmol), iodine (14.1 mg, 0.1 eq) in DMSO (8 mL), stirring for 14 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 4:1). Rf = 0.38 (hexane/EtOAc = 4:1). m.p: 182–184 °C. 1H NMR (600 MHz, CDCl3) δ 8.04 − 7.98 (m, 1H), 7.93 − 7.87 (m, 2H), 7.59 − 7.52 (m, 3H), 7.42 (dd, J = 6.1 and 9.8 Hz, 1H), 6.81 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 176.68, 164.06, 154.93, 154.82, 153.21, 153.11, 152.49, 152.42, 149.49, 149.40, 147.83, 147.74, 131.98, 131.21, 129.18, 126.27, 120.97, 120.95, 113.14, 113.13, 113.02, 113.01, 107.30, 107.15, 107.09. HRMS m/z calculated for C15H8F2O2 [M – H]–: 259.0565; found: 259.0564. >95% purity (as determined by RP-HPLC, method C, tR = 14.19 min).
6,7-Difluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9h)
Compound 9h was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(4,5-Difluoro-2-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8h) (220 mg, 0.76 mmol), iodine (19.2 mg, 0.1 eq) in DMSO (7 mL), stirring for 15 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 5:1 to 1:1 and then CH2Cl2/MeOH = 10:1). Rf = 0.20 (hexane/EtOAc = 4:1). m.p: 200–202 °C. 1H NMR (600 MHz, DMSO) δ 8.10 − 8.02 (m, 3H), 7.93 (t, J = 9.2 Hz, 1H), 7.12 (d, J = 8.9 Hz, 2H), 6.99 (s, 1H), 3.85 (s, 3H); 13C NMR (150 MHz, DMSO) δ 176.05, 163.81, 162.85, 152.69, 128.80, 123.28, 121.12, 115.13, 112.65, 112.52, 108.87, 108.73, 105.39, 56.07. HRMS m/z calculated for C16H10F2O3 [M + H]+: 289.0671; found: 289.0656. >95% purity (as determined by RP-HPLC, method B, tR = 8.87 min).
Ethyl 3–(6,7-difluoro-4-oxo-4H-chromen-2-yl)benzoate (9i)
Compound 9i was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure B with (E)-Ethyl 3–(3-(4,5-difluoro-2-hydroxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (8i) (155 mg, 0.47 mmol), iodine (22.8 mg, 0.2 eq) in DMSO (8 mL), stirring for 6 h. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 20:1). Rf = 0.40 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 8.59 − 8.55 (m, 1H), 8.26 − 8.21 (m, 1H), 8.09 − 8.05 (m, 1H), 8.04 − 7.99 (m, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.47 (dd, J = 6.2 and 9.8 Hz, 1H), 6.87 (s, 1H)., 4.46 (q, J = 7.1 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 176.60, 165.59, 163.02, 155.05, 154.95, 153.33, 153.23, 152.48, 152.41, 149.61, 149.52, 147.94, 147.86, 132.72, 131.67, 131.62, 130.20, 129.37, 127.39, 120.93, 113.20, 113.19, 113.07, 107.61, 107.44, 107.29, 61.63, 14.34. HRMS m/z calculated for C18H12F2O4 [M + H]+: 331.0777; found: 331.0791.
6,7-Difluoro-2-phenyl-4H-chromen-4-one (9j)
Compound 9j was prepared in 88% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(2,4-Difluoro-6-hydroxyphenyl)-3-phenylprop-2-en-1-one (8j) (280 mg, 0.96 mmol), iodine (24.4 mg, 0.1 eq) in DMSO (10 mL), stirring for 14 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 1:1). Rf = 0.25 (hexane/EtOAc = 4:1). m.p: 158–160 °C. 1H NMR (600 MHz, CDCl3) δ 7.88 (d, J = 7.0 Hz, 2H), 7.59 − 7.51 (m, 3H), 7.10 (d, J = 8.8 Hz, 1H), 6.88 − 6.83 (m, 1H), 6.75 (s, 1H); 13C NMR (150 MHz, CDCl3) δ 175.75, 165.74, 165.64, 164.05, 163.95, 162.78, 162.68, 162.56, 161.01, 160.91, 158.07, 158.03, 157.96, 157.93, 131.97, 130.78, 129.17, 126.18, 111.58, 111.53, 111.51, 108.62, 102.19, 102.03, 101.86, 101.41, 101.38, 101.25, 101.22. HRMS m/z calculated for C15H8F2O2 [M – H]–: 259.0565; found: 259.0562. >95% purity (as determined by RP-HPLC, method C, tR = 9.89 min).
5,7-Difluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9k)
Compound 9k was prepared in 47% yield as a white powder, following the same procedure as described in the general procedure B with (E)-1–(2,4-Difluoro-6-hydroxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (8k) (270 mg, 0.93 mmol), iodine (23.6 mg, 0.1 eq) in DMSO (6 mL), stirring for 10 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 5:1 to 1:1). Rf = 0.20 (hexane/EtOAc = 4:1). m.p: 256–258 °C. 1H NMR (600 MHz, DMSO) δ 8.03 (d, J = 8.9 Hz, 2H), 7.62 (d, J = 9.4 Hz, 1H), 7.37 − 7.31 (m, 1H), 7.11 (d, J = 8.9 Hz, 2H), 6.88 (s, 1H), 3.85 (s, 3H); 13C NMR (150 MHz, DMSO) δ 175.01, 165.48, 162.88, 162.34, 128.69, 123.01, 115.14, 106.97, 102.55, 102.46, 102.43, 102.38, 102.29, 102.26, 102.21, 56.08. HRMS m/z calculated for C16H10F2O3 [M + H]+: 289.0671; found: 289.0657. >95% purity (as determined by RP-HPLC, method B, tR = 6.73 min).
Ethyl 4–(6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15a)
Compound 15a was prepared in 43% yield as a white powder, following the same procedure as described in the general procedure B with (E)-Ethyl 4–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14a) (241 mg, 0.70 mmol), iodine (18 mg, 0.1 eq) in DMSO (5 mL), stirring for 11 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 5:1 to 1:1 and then CH2Cl2/MeOH = 50:1 to 30:1). Rf = 0.34 (hexane/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3) δ 8.15 (d, J = 8.7 Hz, 2H), 7.94 (d, J = 8.6 Hz, 2H), 7.52 (s, 1H), 6.99 (s, 1H), 6.82 (s, 1H), 4.42 (q, J = 7.1 Hz, 2H), 4.03 (s, 3H), 3.97 (s, 3H), 1.44 (t, J = 6.7 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 177.44, 165.74, 161.44, 154.72, 152.33, 147.88, 135.85, 132.78, 130.12, 125.96, 117.40, 108.21, 104.36, 99.80, 61.45, 56.55, 56.39, 14.31. HRMS m/z calculated for C20H18O6 [M + H]+: 355.1176; found: 355.1184.
Ethyl 3–(6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15b)
Compound 15b was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure B with (E)-Ethyl 3–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14b) (70 mg, 0.19 mmol), iodine (5 mg, 0.1 eq) in DMSO (8 mL), stirring for 12 h. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 2:1 to 1:1 and then CH2Cl2/MeOH = 10:1). Rf = 0.19 (hexnae/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3) δ 8.61 (s, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.61 (t, J = 7.9 Hz, 1H), 7.58 (s, 1H), 7.06 (s, 1H), 6.86 (s, 1H), 4.46 (q, J = 7.1 Hz, 2H), 4.04 (s, 3H), 4.01 (s, 3H), 1.45 (t, J = 7.1 Hz, 3H). HRMS m/z calculated for C20H18O6 [M + H]+: 355.1176; found: 355.1184.
Ethyl 4–(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15c)
Compound 15c was prepared in 87% yield as a white powder, following the same procedure as described in the general procedure B with (E)-Ethyl 4–(3-(2-hydroxy-4,6-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14c) (116 mg, 0.34 mmol), iodine (8.7 mg, 0.1 eq) in DMSO (8 mL), stirring for 11 h. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 10:1). Rf = 0.28 (hexane/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3) δ 8.2 (d, J = 8.3 Hz, 2H), 7.94 (d, J = 8.3 Hz, 2H), 6.74 (s, 1H), 6.60 (d, J = 2.1 Hz, 1H), 6.40 (d, J = 2.1 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 3.97 (s, 3H), 3.93 (s, 3H), 1.43 (t, J = 7.1 Hz, 3H). HRMS m/z calculated for C20H18O6 [M + H]+: 355.1176; found: 355.1169.
Ethyl 3–(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15d)
Compound 15d was prepared in 81% yield as a white powder, following the same procedure as described in the general procedure B with (E)-Ethyl 3–(3-(2-hydroxy-4,6-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (14d) (271 mg, 0.76 mmol), iodine (19.3 mg, 0.1 eq) in DMSO (10 mL), stirring for 16 h. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 200:1 to 60:1). Rf = 0.37 (CH2Cl2/MeOH = 20:1). 1H NMR (600 MHz, CDCl3) δ 8.56 (s, 1H), 8.19 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 6.75 (s, 1H), 6.63 (d, J = 2.2 Hz, 1H), 6.40 (d, J = 2.1 Hz, 1H), 4.45 (q, J = 7.1 Hz, 2H), 3.99 (s, 3H), 3.93 (s, 3H), 1.45 (t, J = 7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ 177.36, 165.75, 164.19, 160.87, 159.83, 159.49, 131.94, 131.86, 131.35, 129.87, 129.08, 126.98, 109.49, 109.23, 96.34, 92.88, 61.47, 56.42, 55.84, 14.35. HRMS m/z calculated for C20H18O6 [M + H]+: 355.1176; found: 355.1174.
General Procedure C for compounds 10b–10k, 16a–16d, 20a–20s
To a stirred solution of cyclized compound (9b–9k, 15a–15d and 19a–19t) in dichloromethane (CH2Cl2) was added boron tribromide (5–12 eq) at 0 °C. The reaction mixture was stirred under argon at 50 °C until complete conversion monitored by TLC analysis (typically 14–20 h), quenched with ice water, and concentrated CH2Cl2. The crude residue was extracted with EtOAc. The organic layer was washed with saturated NaHCO3 solution, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude was purified by column chromatography on a silica gel (CH2Cl2/MeOH or hexane/EtOAc) or washed with organic solvents such as ether or hexane to furnish compounds 10b–10k, 16a–16d and 20a–20s.
7-Fluoro-2–(4-hydroxyphenyl)-4H-chromen-4-one (10b)
Compound 10b was prepared in quantitative yield as a brown powder, following the same procedure as described in the general procedure C with 7-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9b) (76 mg, 0.28 mmol) and boron tribromide (3.36 mL, 12 eq) in dichloromethane (30 mL), stirring for 18 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.38 (CH2Cl2/MeOH = 10:1). m.p: 310–312 °C. 1H NMR (600 MHz, DMSO) δ 10.32 (s, 1H), 8.07 (dd, J = 6.5 and 8.8 Hz, 1H), 7.97 − 7.92 (m, 2H), 7.69 (dd, J = 2.4 and 9.6 Hz, 1H), 7.37 − 7.32 (m, 1H), 6.95 − 6.90 (m, 2H), 6.86 (s, 1H); 13C NMR (150 MHz, DMSO) δ 176.54, 166.18, 164.51, 163.87, 161.56, 157.15, 157.06, 128.86, 128.03, 127.96, 121.75, 120.94, 116.43, 114.36, 114.20, 105.79, 105.62, 105.24. HRMS m/z calculated for C15H9FO3 [M + H]+: 257.0609; found: 257.0616. >95% purity (as determined by RP-HPLC, method D, tR = 7.67 min).
6-Fluoro-2–(4-hydroxyphenyl)-4H-chromen-4-one (10d)
Compound 10d was prepared in 97% yield as a yellow powder, following the same procedure as described in the general procedure C with 6-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9d) (100 mg, 0.37 mmol) and boron tribromide (4.44 mL, 12 eq) in dichloromethane (15 mL), stirring for 11 h. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 10:1). Rf = 0.21 (hexane/EtOAc = 4:1). m.p: 292–294 °C. 1H NMR (600 MHz, MeOD) δ 7.96 − 7.89 (m, 2H), 7.81 − 7.74 (m, 2H), 7.63 − 7.55 (m, 1H), 6.98 − 6.93 (m, 2H), 6.81 (s, 1H); 13C NMR (150 MHz, DMSO) δ 176.79, 164.00, 160.19, 158.62, 152.49, 128.98, 124.97, 122.56, 122.39, 121.65, 121.59, 116.47, 109.94, 109.79, 104.59. HRMS m/z calculated for C15H9FO3 [M − H]−: 257.0609; found: 257.0599. >95% purity (as determined by RP-HPLC, method D, tR = 6.26 min).
5-Fluoro-2–(4-hydroxyphenyl)-4H-chromen-4-one (10f)
Compound 10f was prepared in 96% yield as a white powder, following the same procedure as described in the general procedure C with 5-Fluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9f) (100 mg, 0.37 mmol) and boron tribromide (4.44 mL, 12 eq) in dichloromethane (30 mL), stirring for 18 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.38 (CH2Cl2/MeOH = 10:1). m.p: 272–274 °C. 1H NMR (600 MHz, DMSO) δ 10.31 (brs, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.81 − 7.73 (m, 1H), 7.57 (d, J = 8.5 Hz, 1H), 7.21 (dd, J = 8.3 and 10.7 Hz, 1H), 6.92 (d, J = 8.8 Hz, 2H), 6.79 (s, 1H); 13C NMR (150 MHz, DMSO) δ 175.81, 162.53, 161.56, 160.89, 159.16, 157.13, 134.81, 134.74, 128.84, 121.47, 116.44, 115.03, 115.01, 113.99, 113.92, 112.54, 112.41, 106.28. HRMS m/z calculated for C15H9FO3 [M + H]+: 257.0609; found: 257.0618. >95% purity (as determined by RP-HPLC, method D, tR = 6.12 min).
6,7-Difluoro-2–(4-hydroxyphenyl)-4H-chromen-4-one (10h)
Compound 10h was prepared in quantitative yield as a white powder, following the same procedure as described in the general procedure C with 6,7-Difluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9h) (123 mg, 0.43 mmol) and boron tribromide (5.12 mL, 12 eq) in dichloromethane (30 mL), stirring for 17 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 3:1). Rf = 0.60 (CH2Cl2/MeOH = 10:1). m.p: 302–304 °C. 1H NMR (600 MHz, DMSO) δ 10.34 (brs, 1H), 8.03 (dd, J = 6.5 and 10.8 Hz, 1H), 7.96 − 7.90 (m, 2H), 6.94 − 6.91 (m, 2H), 6.89 (s, 1H); 13C NMR (150 MHz, DMSO) δ 175.93, 164.19, 161.69, 152.52, 128.92, 121.59, 121.09, 116.45, 112.60, 112.47, 108.76, 108.61, 104.72. HRMS m/z calculated for C15H8F2O3 [M + H]+: 275.0515; found: 275.0527. >95% purity (as determined by RP-HPLC, method D, tR = 9.11 min).
3–(6,7-Difluoro-4-oxo-4H-chromen-2-yl)benzoic acid (10i)
Compound 10i was prepared in 31% yield as a white powder, following the same procedure as described in the general procedure C with ethyl 3–(6,7-Difluoro-4-oxo-4H-chromen-2-yl)benzoate (9i) (74 mg, 0.23 mmol) and boron tribromide (2.70 mL, 12 eq) in dichloromethane (15 mL), stirring for 18 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 3:1). Rf = 0.51 (CH2Cl2/MeOH = 10:1). m.p: 272–274 °C. 1H NMR (600 MHz, MeOD) δ 8.66 (s, 1H), 8.29 − 8.23 (m, 2H), 7.99 (t, J = 8.8 Hz, 1H), 7.85 (dd, J = 6.4 and 10.4 Hz, 1H), 7.71 (t, J = 7.9 Hz, 1H), 7.0 (s, 1H). HRMS m/z calculated for C16H8F2O4 [M − H]−: 301.0318; found: 301.0298. >95% purity (as determined by RP-HPLC, method D, tR = 8.00 min).
5,7-Difluoro-2–(4-hydroxyphenyl)-4H-chromen-4-one (10k)
Compound 10k was prepared in 94% yield as a white powder, following the same procedure as described in the general procedure C with 5,7-Difluoro-2–(4-methoxyphenyl)-4H-chromen-4-one (9k) (100 mg, 0.35 mmol) and boron tribromide (4.16 mL, 12 eq) in dichloromethane (30 mL), stirring for 14 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.60 (CH2Cl2/MeOH = 10:1). m.p: 228–230 °C. 1H NMR (600 MHz, DMSO) δ 10.34 (brs, 1H), 7.92 (d, J = 8.5 Hz, 2H), 7.58 (d, J = 9.0 Hz, 1H), 7.35 − 7.28 (m, 1H), 6.91 (d, J = 8.8 Hz, 2H), 6.78 (s, 1H); 13C NMR (150 MHz, DMSO) δ 175.07, 162.66, 161.77, 157.95, 128.84, 121.08, 116.47, 111.49, 106.15, 102.38, 49.07F10. HRMS m/z calculated for C15H8F2O3 [M + H]+: 275.0515; found: 275.0524. >95% purity (as determined by RP-HPLC, method E, tR = 8.99 min).
4–(6,7-Dihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (16a)
Compound 16a was prepared in 87% yield as a yellow powder, following the same procedure as described in the general procedure C with Ethyl 4–(6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15a) (82 mg, 0.23 mmol) and boron tribromide (2.78 mL, 12 eq) in dichloromethane (30 mL), stirring for 18 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was washed with ether, hexane and methanol. Rf = 0.19 (CH2Cl2/MeOH = 10:1). m.p: 500–502 °C. 1H NMR (600 MHz, MeOD) δ 8.18 (d, J = 8.5 Hz, 2H), 8.11 (d, J = 8.5 Hz, 2H), 7.42 (s, 1H), 7.08 (s, 1H), 6.90 (s, 1H). HRMS m/z calculated for C16H10O6 [M − H]−: 297.0404; found: 297.0418. >95% purity (as determined by RP-HPLC, method H, tR = 14.84 min).
3–(6,7-Dihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (16b)
Compound 16b was prepared in 87% yield as a yellow powder, following the same procedure as described in the general procedure C with Ethyl 3–(6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15b) (50.4 mg, 0.14 mmol) and boron tribromide (1.71 mL, 12 eq) in dichloromethane (15 mL), stirring for 17 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was washed with ether, hexane, and methanol. Rf = 0.24 (CH2Cl2/MeOH = 10:1). m.p: 416–418 °C. 1H NMR (600 MHz, MeOD) δ 8.64 (t, J = 1.6 Hz, 1H), 8.27 − 8.21 (m, 2H), 7.71 (t, J = 7.9 Hz, 1H), 7.45 (s, 1H), 7.12 (s, 1H), 6.92 (s, 1H); 13C NMR (150 MHz, MeOD) δ 178.28, 167.30, 162.78, 153.77, 152.44, 145.33, 132.24, 132.03, 131.75, 130.16, 129.19, 126.93, 115.79, 106.95, 105.41, 102.53. HRMS m/z calculated for C16H10O6 [M − H]−: 297.0404; found: 297.0399. >95% purity (as determined by RP-HPLC, method G, tR = 6.70 min).
4–(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (16c)
Compound 16c was prepared in 50% yield as a yellow powder, following the same procedure as described in the general procedure C with Ethyl 4–(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15c) (30 mg, 0.08 mmol) and boron tribromide (1.02 mL, 12 eq) in dichloromethane (15 mL), stirring for 18 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was washed with ether, hexane and methanol. Rf = 0.26 (CH2Cl2/MeOH = 10:1). m.p: 337–339 °C. 1H NMR (600 MHz, MeOD) δ 8.08 (d, J = 7.9 Hz, 2H), 8.00 (d, J = 7.5 Hz, 2H), 6.75 (s, 1H), 6.42 (s, 1H), 6.15 (s, 1H). HRMS m/z calculated for C16H10O6 [M − H]−: 297.0404; found: 297.0419. >95% purity (as determined by RP-HPLC, method E, tR = 7.99 min).
3–(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (16d)
Compound 16d was prepared in 70% yield as a yellow powder, following the same procedure as described in the general procedure C with Ethyl 3–(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (15d) (104 mg, 0.29 mmol) and boron tribromide (3.54 mL, 12 eq) in dichloromethane (15 mL), stirring for 18 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 10:1 to 3:1). Rf = 0.40 (CH2Cl2/MeOH = 10:1). m.p: 450–452 °C. 1H NMR (600 MHz, DMSO) δ 12.79 (s, 1H), 8.50 (s, 1H), 8.31 (d, J = 5.4 Hz, 1H), 8.15 (d, J = 6.0 Hz, 1H), 7.71 (s, 1H), 7.03 (s, 1H), 6.54 (s, 1H), 6.24 (s, 1H); 13C NMR (150 MHz, DMSO) δ 182.28, 167.22, 165.07, 162.78, 161.96, 157.95, 132.92, 132.57, 131.70, 131.06, 130.06, 127.26, 106.37, 104.48, 99.58, 94.62. HRMS m/z calculated for C16H10O6 [M − H]−: 297.0404; found: 297.0398. >95% purity (as determined by RP-HPLC, method E, tR = 7.18 min).
2–(3,4-Dihydroxyphenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20a)
Compound 20a was prepared in 89% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(3,4-dimethoxyphenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19a) (120 mg, 0.34 mmol) and boron tribromide (5.04 mL, 15 eq) in dichloromethane (20 mL), stirring for 15 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was washed with ether, hexane and methanol. Rf = 0.13 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.41 (s, 1H), 9.71 (s, 1H), 9.46 (s, 1H), 9.24 (s, 1H), 8.85 (s, 1H), 7.66 (d, J = 1.3 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.30 (s, 1H), 6.92 (s, 1H), 6.88 (d, J = 8.4 Hz, 1H). HRMS m/z calculated for C15H10O7 [M + H]+: 301.0354; found: 301.0351.
3,6,7-Trihydroxy-2-phenyl-4H-chromen-4-one (20b)
Compound 20b was prepared in 41% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2-phenyl-4H-chromen-4-one (19b) (40 mg, 0.11 mmol) and boron tribromide (1.13 mL, 10 eq) in dichloromethane (15 mL), stirring for 6 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.39 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.15 (d, J = 7.5 Hz, 2H), 7.54 (t, J = 7.7 Hz, 2H), 7.46 (t, J = 7.3 Hz, 1H), 7.32 (s, 1H), 6.97 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.83, 152.64, 150.27, 144.42, 143.70, 138.05, 131.71, 129.34, 128.46, 127.26, 114.04, 106.91, 102.65. HRMS m/z calculated for C15H10O5 [M + H]+: 269.0455; found: 269.0464. >95% purity (as determined by RP-HPLC, method F, tR = 13.617 min).
3,6,7-Trihydroxy-2-(p-tolyl)-4H-chromen-4-one (20c)
Compound 20c was prepared in 29% yield as a yellow powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2-(p-tolyl)-4H-chromen-4-one (19c) (90 mg, 0.29 mmol) and boron tribromide (3.46 mL, 12 eq) in dichloromethane (20 mL), stirring for 6 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 10:1 to 4:1). Rf = 0.23 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, MeOD) δ 8.12 (d, J = 8.3 Hz, 2H), 7.40 (s, 1H), 7.33 (d, J = 8.1 Hz, 2H), 6.98 (s, 1H), 2.41 (s, 3H). 13C NMR (150 MHz, DMSO) δ 171.75, 152.60, 150.20, 144.39, 143.94, 139.11, 137.73, 129.07, 128.94, 127.18, 114.00, 106.90, 102.64, 21.01. HRMS m/z calculated for C16H12O5 [M + H]+: 285.0758; found: 285.0766. >95% purity (as determined by RP-HPLC, method E, tR = 12.49 min).
3,6,7-Trihydroxy-2-(m-tolyl)-4H-chromen-4-one (20d)
Compound 20d was prepared in 20% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2-(m-tolyl)-4H-chromen-4-one (19d) (70 mg, 0.25 mmol) and boron tribromide (3.69 mL, 15 eq) in dichloromethane (20 mL), stirring for 13 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.50 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, MeOD) δ 8.06 − 7.97 (m, 2H), 7.38 (dd, J = 15.1, 7.4 Hz, 2H), 7.26 (d, J = 7.3 Hz, 1H), 6.98 (s, 3H), 2.42 (s, 3H); 13C NMR (150 MHz, MeOD) δ 174.31, 154.43, 152.87, 146.49, 145.91, 139.21, 133.01, 131.37, 129.36, 129.34, 129.03, 125.93, 115.45, 107.76, 103.46, 21.63. HRMS m/z calculated for C16H12O5 [M – H]–: 283.0612; found: 283.0606. >95% purity (as determined by RP-HPLC, method D, tR = 11.64 min).
2–(3,4-Dimethylphenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20e)
Compound 20e was prepared in 85% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(3,4-dimethylphenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19e) (40 mg, 0.12 mmol) and boron tribromide (1.22 mL, 10 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 30:1 to 4:1). Rf = 0.45 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 7.94 (s, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.32 (s, 1H), 7.30 (d, J = 8.0 Hz, 1H), 6.98 (s, 1H), 2.31 (d, J = 7.0 Hz, 3H), 2.29 (s, 3H); 13C NMR (150 MHz, DMSO) δ 171.72, 152.51, 150.20, 144.35, 144.12, 137.99, 137.70, 136.27, 129.58, 129.27, 128.03, 124.92, 114.03, 106.93, 102.67, 40.06, 39.94, 39.80, 39.66, 39.52, 39.38, 39.24, 39.10, 19.65, 19.39. HRMS m/z calculated for C17H14O5 [M + H]+: 299.0914; found: 299.0922. >95% purity (as determined by RP-HPLC, method D, tR = 10.22 min).
2–(4-Fluorophenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20f)
Compound 20f was prepared in 66% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(4-fluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19f) (40 mg, 0.13 mmol) and boron tribromide (1.26 mL, 10 eq) in dichloromethane (15 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.37 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.20 − 8.15 (m, 2H), 7.40 − 7.32 (m, 2H), 7.31 (s, 1H), 6.99 (s, 1H); 13C NMR (150 MHz, DMSO) δ 172.19, 168.24, 163.61, 161.96, 152.79, 150.65, 148.27, 144.63, 143.59, 137.96, 130.16, 130.11, 128.43, 116.04, 115.89, 114.43, 107.24, 103.01, 79.36. HRMS m/z calculated for C15H9FO5 [M – H]–: 287.0361; found: 287.0375. >95% purity (as determined by RP-HPLC, method E, tR = 9.18 min).
2–(3-Fluorophenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20g)
Compound 20 g was prepared in 92% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(3-fluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19 g) (110 mg, 0.35 mmol) and boron tribromide (5.2 mL, 15 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.35 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.53 (s, 1H), 9.81 (s, 1H), 9.47 (s, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 10.8 Hz, 1H), 7.58 (dd, J = 14.5, 7.9 Hz, 1H), 7.36 − 7.25 (m, 2H), 7.02 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.87, 162.87, 161.26, 152.85, 150.28, 144.55, 142.14, 138.64, 133.99, 130.61, 130.56, 123.25, 116.16, 116.02, 114.07, 113.89, 113.73, 106.90, 102.77. HRMS m/z calculated for C15H9FO5 [M – H]–: 287.0361; found: 287.0367. >95% purity (as determined by RP-HPLC, method E, tR = 10.17 min).
2–(3,4-Difluorophenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20h)
Compound 20h was prepared in 40% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(3,4-difluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19h) (40 mg, 0.12 mmol) and boron tribromide (1.20 mL, 10 eq) in dichloromethane (15 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.42 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.18 (s, 1H), 8.04 (s, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.31 (s, 1H), 7.07 − 6.96 (m, 1H); 13C NMR (150 MHz, DMSO) 171.81, 152.81, 150.2, 144.54, 141.47, 138.40, 129.29, 124.53, 117.87, 117.76, 116.32, 116.19, 114.05, 106.89, 102.78. HRMS m/z calculated for C15H8F2O5 [M – H]–: 305.0267; found: 305.0281. >95% purity (as determined by RP-HPLC, method E, tR = 12.85 min,).
3,6,7-Trihydroxy-2–(4-(trifluoromethyl)phenyl)-4H-chromen-4-one (20i)
Compound 20i was prepared in 35% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(4-(trifluoromethyl)phenyl)-4H-chromen-4-one (19i) (27 mg, 0.07 mmol) and boron tribromide (1.10 mL, 15 eq) in dichloromethane (20 mL), stirring for 13 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 4:1). Rf = 0.26 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.57 (s, 1H), 9.83 (s, 1H), 9.62 (s, 1H), 8.37 (d, J = 8.3 Hz, 2H), 7.90 (d, J = 8.4 Hz, 2H), 7.33 (s, 1H), 7.00 (s, 1H). 13C NMR (150 MHz, DMSO) δ 171.91, 152.97, 150.37, 144.62, 144.60, 141.84, 139.18, 135.71, 129.01, 128.81, 127.79, 125.39, 125.37, 114.10, 106.91, 102.67. HRMS m/z calculated for C16H9F3O5 [M – H]–: 337.0329; found: 337.0343. >95% purity (as determined by RP-HPLC, method D, tR = 13.83 min).
3,6,7-Trihydroxy-2–(3-(trifluoromethyl)phenyl)-4H-chromen-4-one (20j)
Compound 20j was prepared in 57% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(3-(trifluoromethyl)phenyl)-4H-chromen-4-one (19j) (40 mg, 0.11 mmol) and boron tribromide (1.64 mL, 15 eq) in dichloromethane (20 mL), stirring for 15 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 4:1). Rf = 0.21 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, MeOD) δ 8.57 (s, 1H), 8.47 (s, 1H), 7.76 − 7.69 (m, 2H), 7.42 (s, 1H), 7.01 (s, 1H). 13C NMR (150 MHz, DMSO) δ 171.85, 152.89, 150.31, 144.57, 141.82, 138.81, 132.78, 130.71, 129.78, 125.65, 125.02, 123.66, 123.22, 114.11, 106,90, 102.77. HRMS m/z calculated for C16H9F3O5 [M – H]–: 337.0329; found: 337.0332. >95% purity (as determined by RP-HPLC, method A, tR = 6.91 min).
2–(4-Chlorophenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20k)
Compound 20k was prepared in 55% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(4-chlorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19k) (40 mg, 0.12 mmol) and boron tribromide (1.20 mL, 10 eq) in dichloromethane (15 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.48 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.18 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.31 (s, 1H), 6.97 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.79, 152.85, 150.24, 144.53, 142.50, 138.35, 133.82, 130.62, 128.93, 128.59, 114.01, 106.87, 102.63. HRMS m/z calculated for C15H9ClO5 [M – H]–: 303.0066; found: 303.0078. >95% purity (as determined by RP-HPLC, method D, tR = 9.11 min).
2–(3-Chlorophenyl)-3,6,7-trihydroxy-4H-chromen-4-one (20l)
Compound 20 l was prepared in 66% yield as a brown powder, following the same procedure as described in the general procedure C with 2–(3-chlorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19 l) (65 mg, 0.20 mmol) and boron tribromide (2.92 mL, 15 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.39 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.21 (s, 1H), 8.11 (d, J = 7.5 Hz, 1H), 7.57 (t, J = 7.7 Hz, 1H), 7.53 (d, J = 7.3 Hz, 1H), 7.31 (s, 1H), 7.01 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.83, 152.88, 150.31, 144.56, 141.93, 138.67, 133.77, 133.28, 130.44, 129.03, 126.75, 125.62, 114.06, 106.86, 102.74. HRMS m/z calculated for C15H9ClO5 [M – H]–: 303.0066; found: 303.0077. >95% purity (as determined by RP-HPLC, method D, tR = 8.87).
2-([1,1'-Biphenyl]-4-yl)-3,6,7-trihydroxy-4H-chromen-4-one (20m)
Compound 20 m was prepared in 87% yield as a brown powder, following the same procedure as described in the general procedure C with 2-([1,1′-biphenyl]-4-yl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19 m) (300 mg, 0.80 mmol) and boron tribromide (4.0 mL, 5 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 30:1 to 10:1). Rf = 0.33 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.49 (s, 1H), 9.78 (s, 1H), 9.28 (s, 1H), 8.27 (d, J = 8.6 Hz, 2H), 7.86 (d, J = 8.6 Hz, 2H), 7.77 (d, J = 7.3 Hz, 2H), 7.52 (t, J = 7.7 Hz, 2H), 7.42 (t, J = 7.4 Hz, 1H), 7.33 (s, 1H), 7.01 (s, 1H); 13C NMR (150 MHz, DMSO) δ 172.24, 153.11, 150.72, 144.89, 143.91, 141.16, 139.73, 138.72, 131.22, 129.53, 128.42, 128.25, 127.20, 127.11, 114.57, 107.41, 103.16, 31.16. HRMS m/z calculated for C21H14O5 [M – H]–: 345.0708; found: 345.0739. >95% purity (as determined by RP-HPLC, method C, tR = 13.94 min).
3,6,7-Trihydroxy-2–(4-phenoxyphenyl)-4H-chromen-4-one (20n)
Compound 20n was prepared in 41% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(4-phenoxyphenyl)-4H-chromen-4-one (19n) (70 mg, 0.19 mmol) and boron tribromide (2.29 mL, 12 eq) in dichloromethane (20 mL), stirring for 6 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 4:1). Rf = 0.28 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.49 (s, 1H), 9.77 (s, 1H), 9.14 (s, 1H), 8.17 (d, J = 8.9 Hz, 2H), 7.44 (t, J = 7.9 Hz, 2H), 7.32 (s, 1H), 7.20 (t, J = 7.4 Hz, 1H), 7.12 (dd, J = 20.6, 8.4 Hz, 4H), 6.97 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.74, 157.63, 155.91, 152.54, 150.17, 144.41, 143.60, 137.61, 130.27, 130.25, 129.34, 126.66, 124.13, 119.25, 118.07, 114.10, 106.99, 102.66. HRMS m/z calculated for C21H14O6 [M – H]–: 361.0717; found: 361.0716. >95% purity (as determined by RP-HPLC, method C, tR = 11.28 min).
3,6,7-Trihydroxy-2–(3-phenoxyphenyl)-4H-chromen-4-one (20o)
Compound 20o was prepared in 22% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(3-phenoxyphenyl)-4H-chromen-4-one (19o) (180 mg, 0.46 mmol) and boron tribromide (2.29 mL, 12 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:1 to 10:1). Rf = 0.48 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 7.94 (d, J = 8.0 Hz, 1H), 7.88 − 7.81 (m, 1H), 7.54 (t, J = 7.9 Hz, 1H), 7.42 (t, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 1H), 7.17 (t, 1H), 7.08 (dd, J = 14.0, 4.9 Hz, 3H), 6.94 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.80, 156.57, 156.55, 152.83, 150.24, 144.51, 142.68, 138.40, 133.61, 130.26, 130.19, 130.14, 124.12, 123.61, 122.31, 119.48, 118.57, 117.55, 113.97, 106.86, 102.62. HRMS m/z calculated for C21H14O6 [M – H]–: 361.0717; found: 361.0727. >95% purity (as determined by RP-HPLC, method C, tR = 11.54 min).
3,6,7-Trihydroxy-2–(4-hydroxyphenyl)-4H-chromen-4-one (20p)
Compound 20p was prepared in 89% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(4-methoxyphenyl)-4H-chromen-4-one (19p) (180 mg, 0.633 mmol) and boron tribromide (6.33 mL, 10 eq) in dichloromethane (20 mL), stirring for 15 h at 50 °C. The reaction mixture was filtered through a filter paper and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 20:1 to 4:1). Rf = 0.30 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.41 (s, 1H), 9.98 (s, 1H), 9.71 (s, 1H), 8.87 (s, 1H), 8.01 (d, J = 7.9 Hz, 2H), 7.30 (s, 1H), 6.99 − 6.87 (m, 3H). HRMS m/z calculated for C15H10O6 [M – H]–: 285.0404; found: 285.0399.
3,6,7-Trihydroxy-2–(3-hydroxyphenyl)-4H-chromen-4-one (20q)
Compound 20q was prepared in 87% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(3-methoxyphenyl)-4H-chromen-4-one (19q) (96 mg, 0.292 mmol) and boron tribromide (4.38 mL, 15 eq) in dichloromethane (20 mL), stirring for 15 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 20:1 to 4:1). Rf = 0.32 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 7.61 − 7.58 (m, 2H), 7.32 (d, J = 14.4 Hz, 2H), 6.91 (s, 1H), 6.85 (d, J = 6.0 Hz 1H). HRMS m/z calculated for C15H10O6 [M – H]–: 285.0404; found: 285.0410.
4–(3,6,7-Trihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (20r)
Compound 20r was prepared in 9% yield as a brown powder, following the same procedure as described in the general procedure C with methyl 4–(3-hydroxy-6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (19r) (80 mg, 0.22 mmol) and boron tribromide (3.36 mL, 15 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was recrystallized from methanol. Rf = 0.05 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, MeOD) δ 8.33 (d, J = 8.4 Hz, 2H), 8.13 (d, J = 8.6 Hz, 2H), 7.41 (s, 1H), 7.01 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.83, 165.84, 153.50, 150.53, 144.77, 142.02, 139.24, 136.22, 129.53, 129.25, 127.26, 113.81, 106.64, 102.49. HRMS m/z calculated for C15H10Cl2O5 [M – H]–: 313.0354; found: 313.0342. >95% purity (as determined by RP-HPLC, method G, tR = 8.65 min).
3–(3,6,7-Trihydroxy-4-oxo-4H-chromen-2-yl)benzoic acid (20s)
Compound 20s was prepared in 26% yield as a brown powder, following the same procedure as described in the general procedure C with methyl methyl 3–(3-hydroxy-6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (19s) (76 mg, 0.21 mmol) and boron tribromide (3.20 mL, 15 eq) in dichloromethane (20 mL), stirring for 16 h at 50 °C. The crude residue was recrystallized from methanol. Rf = 0.08 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, MeOD) δ 8.87 (t, J = 1.6 Hz, 1H), 8.51 − 8.42 (m, 1H), 8.13 − 8.05 (m, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.41 (d, J = 4.3 Hz, 1H), 7.02 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.86, 167.06, 152.79, 150.29, 144.52, 142.70, 138.50, 132.13, 131.09, 131.05, 129.93, 128.95, 128.26, 114.11, 106.96, 102.65. HRMS m/z calculated for C16H10O7 [M – H]–: 313.0354; found: 313.0365. >95% purity (as determined by RP-HPLC, method G, tR = 10.22 min).
3,6,7-Trihydroxy-2-(naphthalen-2-yl)-4H-chromen-4-one (23a)
Compound 23a was prepared in 57% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2-(naphthalen-2-yl)-4H-chromen-4-one (22a) (35 mg, 0.11 mmol) and boron tribromide (1.07 mL, 10 eq) in dichloromethane (10 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 30:1 to 4:1). Rf = 0.45 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 8.75 (s, 1H), 8.29 (dd, J = 8.7, 1.6 Hz, 1H), 8.06 (d, J = 8.8 Hz, 2H), 8.02 − 7.96 (m, 1H), 7.66 − 7.52 (m, 2H), 7.36 (s, 1H), 7.06 (s, 1H); 13C NMR (150 MHz, DMSO) δ 172.29, 153.14, 150.84, 144.93, 144.17, 138.91, 133.38, 133.00, 129.72, 129.23, 128.36, 128.03, 127.75, 127.70, 127.17, 124.74, 114.60, 107.43, 103.20. HRMS m/z calculated for C19H12O5 [M – H]–: 319.0612; found: 319.0609. >95% purity (as determined by RP-HPLC, method C, tR = 12.43 min).
3,6,7-Trihydroxy-2–(1-methyl-1H-pyrazol-4-yl)-4H-chromen-4-one (23b)
Compound 23b was prepared in 27% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2–(1-methyl-1H-pyrazol-4-yl)-4H-chromen-4-one (22b) (177 mg, 0.59 mmol) and boron tribromide (5.85 mL, 10 eq) in dichloromethane (10 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 30:1 to 4:1). Rf = 0.34 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.35 (s, 1H), 9.65 (s, 1H), 9.10 (s, 1H), 8.30 (s, 1H), 8.01 (s, 1H), 7.30 (s, 1H), 6.91 (s, 1H), 3.94 (s, 3H); 13C NMR (150 MHz, DMSO) δ 171.29, 152.32, 150.05, 144.53, 142.53, 137.77, 135.76, 130.99, 114.99, 114.20, 107.65, 103.06, 49.07. HRMS m/z calculated for C13H10N2O5 [M – H]–: 273.0524; found: 273.0523. >95% purity (as determined by RP-HPLC, method H, tR = 5.97 min).
3,6,7-Trihydroxy-2-(thiophen-2-yl)-4H-chromen-4-one (23c)
Compound 23c was prepared in 39% yield as a brown powder, following the same procedure as described in the general procedure C with 3-hydroxy-6,7-dimethoxy-2-(thiophen-2-yl)-4H-chromen-4-one (22c) (75 mg, 0.25 mmol) and boron tribromide (2.46 mL, 10 eq) in dichloromethane (10 mL), stirring for 16 h at 50 °C. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 30:1 to 4:1). Rf = 0.45 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, DMSO) δ 10.47 (s, 1H), 9.79 (s, 1H), 9.75 (s, 1H), 7.91 − 7.85 (m, 1H), 7.83 (dd, J = 5.0, 0.8 Hz, 1H), 7.32 (s, 1H), 7.27 (dd, J = 4.9, 3.9 Hz, 1H), 6.95 (s, 1H); 13C NMR (150 MHz, DMSO) δ 171.55, 152.85, 150.13, 144.75, 142.36, 136.32, 133.29, 130.33, 128.13, 127.88, 114.93, 107.65, 103.04. HRMS m/z calculated for [M – H]–: 275.0019; found: 275.0026. >95% purity (as determined by RP-HPLC, method F, tR = 10.00 min).
General Procedure D for compounds 18a–18s
To a stirred solution of substituted acetophenone compound in tetrahydrofuran (THF) was added appropriate aldehyde (1.2 eq or 1.5 eq) at room temperature. The reaction mixture was added sodium methoxide (5.4 M, 1.2 eq) in methanol solution at 0 °C and stirred for 5 min. The reaction mixture was warmed to room temperature and stirred at room temperature until complete conversion monitored by TLC analysis (typically 12–16 h), quenched with acetic acid, and extracted with EtOAc and H2O. The organic layer was washed with saturated aqueous NaHCO3, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography on a silica gel to furnish compounds 18a–18t.
(E)-3–(3,4-Dimethoxyphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18a)
Compound 18a was prepared in 53% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (200 mg, 1.02 mmol), 3,4-dimethoxybenzaldehyde (202 mg, 1.2 eq), sodium methoxide solution (0.23 mL, 1.2 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was purified by column chromatography on a silica gel (hexane/EtOAc = 4:1 to 1:1). Rf = 0.54 (hexane/EtOAc = 2:1). 1H NMR (600 MHz, CDCl3) δ 7.87 (d, J = 15.3 Hz, 1H), 7.37 (d, J = 15.3 Hz, 1H), 7.28 (t, J = 4.1 Hz, 2H), 7.16 (s, 1H), 6.93 (d, J = 8.3 Hz, 1H), 6.52 (s, 1H), 3.97 (s, 3H), 3.94 (d, J = 3.1 Hz, 6H), 3.92 (s, 3H). HRMS m/z calculated for C19H20O6 [M + H]+: 345.1333; found: 345.1336.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3-phenylprop-2-en-1-one (18b)
Compound 18b was prepared in 97% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (300 mg, 1.53 mmol), benzaldehyde (187 μL, 1.2 eq), sodium methoxide solution (0.33 mL, 1.2 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 10:1 to 6:1). Rf = 0.63 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.91 (d, J = 15.4 Hz, 1H), 7.66 (dd, J = 6.4, 2.7 Hz, 2H), 7.51 (d, J = 11.7 Hz, 1H), 7.46 − 7.40 (m, 3H), 6.52 (s, 1H), 3.94 (s, 9H), 3.92 (s, 3H). HRMS m/z calculated for C17H16O4 [M + H]+: 285.1122; found: 285.1112.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3-(p-tolyl)prop-2-en-1-one (18c)
Compound 18c was prepared in 85% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (200 mg, 1.02 mmol), benzaldehyde (252 μL, 1.05 eq), sodium methoxide solution (0.56 mL, 1.2 eq) in THF (10 mL), stirring for 15 h at room temperature. The crude residue was purified by column chromatography on silica gel (hexane/EtOAc = 20:1 to 3:1). Rf = 0.30 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 13.41 (s, 1H), 7.89 (d, J = 15.4 Hz, 1H), 7.57 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 15.4 Hz, 1H), 7.26 (d, J = 1.5 Hz, 2H), 7.24 (d, J = 2.7 Hz, 1H), 6.52 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 2.41 (s, 3H). HRMS m/z calculated for C18H18O4 [M + H]+: 299.1278; found: 299.1283.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3-(m-tolyl)prop-2-en-1-one (18d)
Compound 18d was prepared in 65% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), m-tolubenzaldehyde (445 μL, 1.5 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 15 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.35 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.88 (d, J = 15.4 Hz, 1H), 7.49 (dd, J = 16.3, 11.6 Hz, 3H), 7.33 (t, J = 7.6 Hz, 1H), 7.27 (s, 1H), 7.24 (s, 1H), 6.52 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 2.42 (s, 3H). HRMS m/z calculated for C18H18O4 [M + H]+: 299.1278; found: 299.1270.
(E)-3–(3,4-Dimethylphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18e)
Compound 18e was prepared in 74% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), 3,4-dimethylbenzaldehyde (405 μL, 1.2 eq), sodium methoxide solution (0.56 mL, 1.2 eq) in THF (20 mL), stirring for 12 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.45 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.87 (d, J = 15.4 Hz, 1H), 7.47 (d, J = 15.4 Hz, 1H), 7.44 − 7.39 (m, 2H), 7.28 (d, J = 4.5 Hz, 1H), 7.20 (d, J = 7.7 Hz, 1H), 6.51 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 2.33 (s, 3H), 2.32 (s, 3H). HRMS m/z calculated for C19H20O4 [M + H]+: 313.1435; found: 313.1426.
(E)-3–(4-Fluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18f)
Compound 18f was prepared in 81% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (300 mg, 1.53 mmol), 4-fluorobenzaldehyde (196 μL, 1.2 eq), sodium methoxide solution (0.33 mL, 1.2 eq) in THF (20 mL), stirring for 12 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.28 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.91 (d, J = 15.4 Hz, 1H), 7.66 (dd, J = 6.4, 2.7 Hz, 2H), 7.52 (d, J = 15.4 Hz, 1H), 7.47 − 7.40 (m, 3H), 6.52 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C17H15FO4 [M + H]+: 303.1027; found: 303.1032.
(E)-3–(3-Fluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18g)
Compound 18 g was prepared in 91% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (400 mg, 2.04 mmol), 3-fluorobenzaldehyde (324 μL, 1.5 eq), sodium methoxide solution (0.56 mL, 1.5 eq) in THF (20 mL), stirring for 12 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.46 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.85 (d, J = 15.4 Hz, 1H), 7.50 (d, J = 15.4 Hz, 1H), 7.44 − 7.38 (m, 2H), 7.36 (d, J = 9.5 Hz, 1H), 7.24 (d, J = 3.9 Hz, 1H), 7.18 − 7.10 (m, 1H), 6.52 (s, 1H), 3.95 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C17H15FO4 [M + H]+: 303.1027; found: 303.1025.
(E)-3–(3,4-Difluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18h)
Compound 18h was prepared in 56% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (400 mg, 2.04 mmol), 3,4-difluorobenzaldehyde (337 μL, 1.5 eq), sodium methoxide solution (0.56 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.52 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.81 (d, J = 14.2 Hz, 1H), 7.54 − 7.46 (m, J = 15.5, 8.7, 5.0 Hz, 1H), 7.44 − 7.34 (m, 2H), 7.25 − 7.19 (m, 2H), 6.52 (s, 1H), 3.95 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C17H14F2O4 [M + H]+: 321.0933; found: 321.0918.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3–(4-(trifluoromethyl)phenyl)prop-2-en-1-one (18i)
Compound 18i was prepared in 89% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (200 mg, 1.02 mmol), 4-(Trifluoromethyl)benzaldehyde (508 μL, 1.5 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.49 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.90 (d, J = 15.5 Hz, 1H), 7.76 (d, J = 8.3 Hz, 2H), 7.70 (d, J = 8.3 Hz, 2H), 7.57 (d, 1H), 7.24 (s, 1H), 6.53 (s, 1H), 3.95 (s, 3H), 3.93 (s, 3H). HRMS m/z calculated for C18H15F3O4 [M + H]+: 353.0995; found: 353.0988.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3–(3-(trifluoromethyl)phenyl)prop-2-en-1-one (18j)
Compound 18j was prepared in 67% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (200 mg, 1.02 mmol), 3-(Trifluoromethyl)benzaldehyde (508 μL, 1.5 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.33 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 7.91 (d, J = 15.4 Hz, 2H), 7.82 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.57 (dd, J = 18.3, 11.6 Hz, 2H), 7.25 (s, 1H), 6.53 (s, 1H), 3.95 (s, 3H), 3.93 (s, 3H). HRMS m/z calculated for C18H15F3O4 [M + H]+: 353.0995; found: 353.0982.
(E)-3–(4-Chlorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18k)
Compound 18k was prepared in 86% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (300 mg, 1.53 mmol), 4-chlorobenzaldehyde (257 mg, 1.2 eq), sodium methoxide solution (0.33 mL, 1.2 eq) in THF (20 mL), stirring for 12 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.24 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.85 (d, J = 15.4 Hz, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 15.4 Hz, 1H), 7.41 (d, J = 8.4 Hz, 2H), 7.24 (s, 1H), 6.52 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C17H15ClO4 [M + H]+: 319.0732; found: 319.0736.
(E)-3–(3-Chlorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18l)
Compound 18 l was prepared in 68% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), 3-chlorobenzaldehyde (346 μL, 1.2 eq), sodium methoxide solution (0.56 mL, 1.2 eq) in THF (20 mL), stirring for 12 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.50 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.82 (d, J = 15.4 Hz, 1H), 7.65 (s, 1H), 7.51 (t, J = 10.4 Hz, 2H), 7.42 − 7.36 (m, 2H), 7.23 (s, 1H), 6.52 (s, 1H), 3.95 (s, 3H), 3.93 (s, 3H). HRMS m/z calculated for C17H15ClO4 [M + H]+: 319.0732; found: 319.0746.
(E)-3-([1,1'-Biphenyl]-4-yl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18m)
Compound 18 m was prepared in 52% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), Biphenyl-4-carboxaldehyde (557 mg, 1.2 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.44 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.95 (d, J = 15.4 Hz, 1H), 7.75 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 8.3 Hz, 2H), 7.66 − 7.62 (m, 2H), 7.56 (d, J = 15.4 Hz, 1H), 7.50 − 7.45 (m, 2H), 7.41 − 7.37 (m, 1H), 7.29 (s, 1H), 6.53 (s, 1H), 3.95 (s, 3H), 3.94 (s, 3H). HRMS m/z calculated for C23H20O4 [M + H]+: 361.1417; found: 361.1447.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3–(4-phenoxyphenyl)prop-2-en-1-one (18n)
Compound 18n was prepared in 89% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), 4-phenoxybenzaldehyde (658 μL, 1.5 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.44 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.89 (d, J = 15.4 Hz, 1H), 7.64 (d, J = 8.6 Hz, 2H), 7.48 − 7.35 (m, 3H), 7.25 (s, 1H), 7.18 (t, J = 7.4 Hz, 1H), 7.08 (d, J = 7.8 Hz, 2H), 7.03 (d, J = 8.6 Hz, 2H), 6.52 (s, 1H), 3.94 (s, 3H), 3.91 (s, 3H). HRMS m/z calculated for C23H20O5 [M + H]+: 377.1384; found: 377.1384.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3–(3-phenoxyphenyl)prop-2-en-1-one (18o)
Compound 18o was prepared in 35% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), 3-phenoxybenzaldehyde (658 μL, 1.5 eq), sodium methoxide solution (0.7 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.44 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.82 (d, J = 11.7 Hz, 1H), 7.46 (d, J = 11.7 Hz, 1H), 7.39 − 7.35 (m, 3H), 7.31 (s, 1H), 7.21 (s, 1H), 7.15 (t, J = 7.4 Hz, 1H), 7.10 − 7.02 (m, 2H), 6.51 (s, 2H), 3.94 (s, 3H), 3.90 (s, 3H). HRMS m/z calculated for C23H20O5 [M + H]+: 377.1384; found: 377.1393.
(E)-3–(4-Methoxyphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18p)
Compound 18p was prepared in 84% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (2350 mg, 12 mmol), p-anisaldehyde (1.747 mL, 1.2 eq), sodium methoxide solution (2.66 mL, 1.2 eq) in THF (36 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.33 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) 7.88 (d, J = 15.3 Hz, 1H), 7.63 (d, J = 8.7 Hz, 2H), 7.41 (d, J = 13.8 Hz, 1H), 7.26 (s, 1H), 6.96 (d, J = 8.7 Hz, 2H), 6.51 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 3.87 (s, 3H). HRMS (ESI) m/z calculated for C18H18O5 [M + H]+: 315.1227; found: 315.1141.
(E)-3–(3-Methoxyphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18q)
Compound 18q was prepared in 35% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 12 mmol), m-anisaldehyde (465 μL, 1.5 eq), sodium methoxide solution (0.84 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was purified by column chromatography on a silica gel (hexane/EtOAc = 10:1 to 4:1). Rf= 0.41 (hexane-E.A = 2:1, v/v). 1 H NMR (300 MHz, CDCl3) δ 13.48 (s, 1H), 7.88 (d, J = 15.3 Hz, 1H), 7.64 (d, J = 8.7 Hz, 1H), 7.63 (dd, J = 4.5 and 9.6 Hz, 1H), 7.41 (d, J = 15.3 Hz, 1H), 6.97 (d, J = 8.7 Hz, 1H), 6.96 (dd, J = 4.5 and 9.6 Hz, 1H), 6.51 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 3.87 (s, 3H). HRMS (ESI) m/z calculated for C18H18O5 [M + H]+: 315.1227; found: 315.1242.
(E)-Methyl 4–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (18r)
Compound 18r was prepared in 37% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (2350 mg, 12 mmol), Methyl terepthalaldehydate (2360 mg, 1.2 eq), sodium methoxide solution (3.28 mL, 1.2 eq) in THF (36 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.61 (hexane/EtOAc = 1:1). 1H NMR (600 MHz, CDCl3) δ 8.10 (d, J = 8.3 Hz, 2H), 7.90 (d, J = 15.5 Hz, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.58 (d, J = 15.5 Hz, 1H), 7.25 (s, 1H), 6.52 (s, 1H), 3.95 (s, 6H), 3.92 (s, 3H). HRMS m/z calculated for C19H18O6 [M + H]+: 343.1176; found: 343.1179.
(E)-Methyl 3–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (18s)
Compound 18s was prepared in 19% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (1000 mg, 5.10 mmol), Methyl 3-formylbenzoate (1000 mg, 1.2 eq), sodium methoxide solution (1.13 mL, 1.2 eq) in THF (20 mL), stirring for 16 h at room temperature The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.49 (hexane/EtOAc = 2:1). 1H NMR (600 MHz, CDCl3) δ 8.35 (s, 1H), 8.12 − 8.05 (m, 1H), 7.92 (d, J = 15.5 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.58 (d, 1H), 7.53 (t, J = 7.7 Hz, 1H), 7.27 (s, 1H), 6.52 (s, 1H), 3.97 (s, 3H), 3.95 (s, 3H), 3.94 (s, 3H). HRMS m/z calculated for C19H18O6 [M + H]+: 343.1176; found: 343.1181.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3-(naphthalen-2-yl)prop-2-en-1-one (21a)
Compound 21a was prepared in 54% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 2.55 mmol), 2-naphthaldehyde (477 mg, 1.2 eq), sodium methoxide solution (0.56 mL, 1.2 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.32 (hexane/EtOAc = 4:1). 1H NMR (600 MHz, CDCl3) δ 8.07 (d, J = 15.1 Hz, 2H), 7.98 − 7.84 (m, 3H), 7.81 (dd, J = 8.5, 1.2 Hz, 1H), 7.63 (d, J = 15.4 Hz, 1H), 7.59 − 7.50 (m, 2H), 7.32 (s, 1H), 6.53 (s, 1H), 3.95 (s, 6H). HRMS m/z calculated for C21H18O4 [M + H]+: 335.1278; found: 335.1259.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3–(1-methyl-1H-pyrazol-4-yl)prop-2-en-1-one (21b)
Compound 21b was prepared in 54% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (500 mg, 1.53 mmol), 1-methypyrazole-4-carboxaldehyde (0.52 mL, 1.5 eq), sodium methoxide solution (0.56 mL, 1.2 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.12 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 7.85 (s, 1H), 7.81 (d, J = 15.0 Hz, 1H), 7.67 (s, 1H), 7.26 (s, 1H), 7.24 (d, J = 15.6 Hz, 1H), 3.96 (s, 3H), 3.94 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C15H16N2O4 [M + H]+: 289.1183; found: 289.1195.
(E)-1–(2-Hydroxy-4,5-dimethoxyphenyl)-3-(thiophen-2-yl)prop-2-en-1-one (21c)
Compound 21c was prepared in 99% yield as a yellow powder, following the same procedure as described in the general procedure D with 1–(2-hydroxy-4,5-dimethoxyphenyl)ethanone (11) (300 mg, 1.53 mmol), 2-thiophenecarboxaldehyde (209 μL, 1.5 eq), sodium methoxide solution (0.43 mL, 1.5 eq) in THF (20 mL), stirring for 16 h at room temperature. The crude residue was recrystallized from ethyl acetate and hexane. Rf = 0.34 (hexane/EtOAc = 3:1). 1H NMR (600 MHz, CDCl3) δ 8.03 (d, J = 15.0 Hz, 1H), 7.44 (d, J = 3.6 Hz, 1H), 7.39 (d, J = 3.6 Hz, 1H), 7.30 (d, J = 3.6 Hz, 1H), 7.22 (s, 1H), 7.12 − 7.08 (m, 1H), 6.51 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H). HRMS m/z calculated for C15H14O4S [M + H]+: 291.0686; found: 291.0678.
General Procedure E for compounds 19a–19s and 22a–22c
To a stirred solution of chalcone compound (19a–19s and 22a–22c) in MeOH or EtOH were added sodium hydroxide (NaOH, 3 M aq.) or sodium methoxide (NaOCH3, 5.4 M in methanol solution, 4 eq) and hydrogen peroxide (H2O2, 35% aq., 4 eq) at room temperature. The reaction mixture was stirred under argon at 40 °C until complete conversion monitored by TLC analysis (typically 5–16 h). The reaction mixture was then poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography on a silica gel furnish compounds 19a–19s and 22a–22c.
2–(3,4-Dimethoxyphenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19a)
Compound 19a was prepared in 68% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(3,4-dimethoxyphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18a) (185 mg, 0.54 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 209 μL, 4 eq) in ethanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 8:1). Rf = 0.35 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 7.91 − 7.78 (m, 2H), 7.53 (s, 1H), 7.05 − 6.95 (m, 3H), 4.03 (s, 3H), 4.00 (s, 6H), 3.98 (d, J = 6.4 Hz, 3H). HRMS m/z calculated for C19H18O7 [M + H]+: 359.1126; found: 359.1132.
3-Hydroxy-6,7-dimethoxy-2-phenyl-4H-chromen-4-one (19b)
Compound 19b was prepared in 24% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3-phenylprop-2-en-1-one (18b) (200 mg, 0.70 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 273 μL, 4 eq) in ethanol (10 mL), stirring for 5 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.39 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.23 (d, J = 7.5 Hz, 2H), 7.57 − 7.51 (m, 3H), 7.46 (t, J = 7.4 Hz, 1H), 7.03 (s, 1H), 7.00 (s, 1H), 4.03 (s, 3H), 4.01 (s, 3H). HRMS m/z calculated for C17H14O5 [M + H]+: 299.0554; found: 299.0914.
3-Hydroxy-6,7-dimethoxy-2-(p-tolyl)-4H-chromen-4-one (19c)
Compound 19c was prepared in 58% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3-(p-tolyl)prop-2-en-1-one (18c) (180 mg, 0.60 mmol), NaOH (3 M aq.) 1.5 mL and hydrogen peroxide (35% aq., 274 μL, 4 eq) in ethanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). 1H NMR (600 MHz, CDCl3) δ 8.13 (d, J = 8.2 Hz, 2H), 7.53 (s, 1H), 7.34 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 4.4 Hz, 2H), 4.03 (s, 3H), 4.00 (s, 3H), 2.44 (s, 3H). HRMS m/z calculated for C18H16O5 [M + H]+: 313.1070; found: 313.1069.
3-Hydroxy-6,7-dimethoxy-2-(m-tolyl)-4H-chromen-4-one (19d)
Compound 19d was prepared in 16% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3-(m-tolyl)prop-2-en-1-one (18d) (480 mg, 1.60 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 625 μL, 4 eq) in methanol (10 mL), stirring for 20 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.21 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.04 (d, J = 8.9 Hz, 2H), 7.53 (s, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.28 (d, J = 7.7 Hz, 1H), 7.01 (s, 2H), 4.03 (s, 3H), 4.01 (s, 3H), 2.47 (s, 3H).
2–(3,4-Dimethylphenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19e)
Compound 19e was prepared in 32% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(3,4-dimethylphenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18e) (120 mg, 0.38 mmol), NaOH (3 M aq.) 1 mL and hydrogen peroxide (35% aq., 149 μL, 4 eq) in ethanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 20:1). Rf = 0.55 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.05 − 7.91 (m, 2H), 7.53 (s, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.00 (s, 1H), 6.97 (s, 1H), 4.03 (s, 3H), 4.00 (s, 3H), 2.38 (s, 3H), 2.35 (s, 3H). (s, 3H), 4.01 (s, 3H), 2.47 (s, 3H). HRMS m/z calculated for C19H18O5 [M + H]+: 327.1227; found: 327.1207.
2–(4-Fluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19f)
Compound 19f was prepared in 33% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(4-fluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18f) (300 mg, 0.99 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 386 μL, 4 eq) in ethanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.29 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.25 (dd, J = 8.8, 5.4 Hz, 2H), 7.53 (s, 1H), 7.22 (t, J = 8.7 Hz, 2H), 7.04 (s, 1H), 6.99 (s, 1H), 4.03 (s, 3H), 4.01 (s, 3H).
2–(3-Fluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19g)
Compound 19 g was prepared in 80% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(3-fluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18 g) (300 mg, 0.99 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 386 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.52 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.06 (d, J = 7.9 Hz, 1H), 7.96 (dd, J = 10.5, 1.9 Hz, 1H), 7.54 − 7.46 (m, 2H), 7.19 − 7.07 (m, J = 35.4, 20.7, 11.4 Hz, 2H), 7.00 (s, 1H), 4.04 (s, 3H), 4.01 (s, 3H).
2–(3,4-Difluorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19h)
Compound 19h was prepared in 29% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(3,4-difluorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18h) (200 mg, 0.99 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 242 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.27 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.14 − 8.04 (m, J = 11.9, 7.7, 2.2 Hz, 1H), 8.07 − 7.99 (m, 1H), 7.52 (s, 1H), 7.37 − 7.27 (m, 1H), 7.09 (d, J = 2.4 Hz, 1H), 6.99 (s, 1H), 4.04 (s, 3H), 4.01 (s, 3H).
3-Hydroxy-6,7-dimethoxy-2–(4-(trifluoromethyl)phenyl)-4H-chromen-4-one (19i)
Compound 19i was prepared in 16% yield as yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(4-(trifluoromethyl)phenyl)prop-2-en-1-one (18i) (220 mg, 0.62 mmol), NaOH (3 M aq.) 1.5 mL and hydrogen peroxide (35% aq., 242 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 30:1). Rf = 0.63 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.36 (d, J = 8.3 Hz, 6H), 7.78 (d, J = 8.4 Hz, 6H), 7.53 (s, 3H), 7.16 (s, 2H), 7.01 (s, 3H), 4.04 (s, 9H), 4.01 (s, 9H).
3-Hydroxy-6,7-dimethoxy-2–(3-(trifluoromethyl)phenyl)-4H-chromen-4-one (19j)
Compound 19j was prepared in 8% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(3-(trifluoromethyl)phenyl)prop-2-en-1-one (18j) (589 mg, 1.67 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 651 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 30:1). Rf = 0.32 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.48 (d, J = 6.5 Hz, 2H), 7.71 (d, J = 7.7 Hz, 1H), 7.66 (t, J = 8.1 Hz, 1H), 7.53 (s, 1H), 7.14 (s, 1H), 7.03 (s, 1H), 4.06 (s, 3H), 4.01 (s, 3H).
2–(4-Chlorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19k)
Compound 19k was prepared in 40% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(4-chlorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18k) (300 mg, 0.94 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 366 μL, 4 eq) in ethanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.3 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.19 (d, J = 8.6 Hz, 2H), 7.57 − 7.46 (m, 3H), 7.08 (s, 1H), 6.99 (s, 1H), 4.03 (s, 3H), 4.01 (s, 3H).
2–(3-Chlorophenyl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19l)
Compound 19 l was prepared in 29% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3–(3-chlorophenyl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18 l) (300 mg, 0.94 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 288 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.35 (CH2Cl2/MeOH = 100:1). 1H NMR (600 MHz, CDCl3) δ 8.21 (t, J = 1.7 Hz, 1H), 8.17 (d, J = 7.8 Hz, 1H), 7.52 (s, 1H), 7.50 − 7.39 (m, 2H), 7.09 (d, J = 11.1 Hz, 1H), 7.01 (s, 1H), 4.04 (s, 3H), 4.01 (s, 3H). HRMS m/z calculated for C17H13ClO5 [M + H]+: 333.0525; found: 333.0525.
2-([1,1'-Biphenyl]-4-yl)-3-hydroxy-6,7-dimethoxy-4H-chromen-4-one (19m)
Compound 19 m was prepared in 72% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-3-([1,1′-biphenyl]-4-yl)-1–(2-hydroxy-4,5-dimethoxyphenyl)prop-2-en-1-one (18 m) (400 mg, 1.11 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 431 μL, 4 eq) in methanol (20 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.29 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.32 (d, J = 8.2 Hz, 2H), 7.77 (d, J = 8.2 Hz, 2H), 7.68 (d, J = 7.7 Hz, 2H), 7.64 (d, J = 3.8 Hz, 1H), 7.55 (s, 1H), 7.48 (d, J = 7.4 Hz, 2H), 7.40 (t, J = 6.3 Hz, 1H), 7.11 (s, 1H), 7.03 (s, 1H), 4.04 (s, 3H), 4.02 (s, 3H). HRMS m/z calculated for C23H18O5 [M + H]+: 375.1227; found: 375.1240.
3-Hydroxy-6,7-dimethoxy-2–(4-phenoxyphenyl)-4H-chromen-4-one (19n)
Compound 19n was prepared in 16% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(4-phenoxyphenyl)prop-2-en-1-one (18n) (500 mg, 0.69 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 516 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.29 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.21 (d, J = 8.8 Hz, 2H), 7.53 (s, 1H), 7.39 (t, J = 7.9 Hz, 2H), 7.18 (t, J = 7.4 Hz, 1H), 7.13 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.2 Hz, 2H), 6.98 (s, 2H), 4.02 (s, 3H), 4.01 (s, 3H). HRMS m/z calculated for C23H18O6 [M + H]+: 391.1176; found: 391.1181.
3-Hydroxy-6,7-dimethoxy-2–(3-phenoxyphenyl)-4H-chromen-4-one (19o)
Compound 19o was prepared in 32% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(3-phenoxyphenyl)prop-2-en-1-one (18o) (800 mg, 0.69 mmol), NaOH (3 M aq.) 4 mL and hydrogen peroxide (35% aq., 990 μL, 4 eq) in methanol (20 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.33 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.02 (d, J = 7.9 Hz, 1H), 7.93 (s, 1H), 7.51 (s, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.37 (t, J = 7.9 Hz, 2H), 7.14 (t, J = 7.3 Hz, 1H), 7.10 − 7.01 (m, 4H), 6.97 (s, 1H), 4.01 (s, 3H), 4.00 (s, 3H). HRMS m/z calculated for C23H18O6 [M + H]+: 391.1176; found: 391.1191.
3-Hydroxy-6,7-dimethoxy-2–(4-methoxyphenyl)-4H-chromen-4-one (19p)
Compound 19p was prepared in 58% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(4-methoxyphenyl)prop-2-en-1-one (18p) (300 mg, 0.95 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 371 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 8:1). Rf = 0.21 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.20 (d, J = 9.0 Hz, 2H), 7.52 (d, J = 5.5 Hz, 1H), 7.05 (d, J = 9.0 Hz, 2H), 6.99 (s, 2H), 4.02 (s, 3H), 4.00 (s, 3H), 3.90 (s, 3H).
3-Hydroxy-6,7-dimethoxy-2–(3-methoxyphenyl)-4H-chromen-4-one (19q)
Compound 19q was prepared in 43% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(3-methoxyphenyl)prop-2-en-1-one (18q) (300 mg, 0.95 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 371 μL, 4 eq) in methanol (10 mL), stirring for 16 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 10:1). Rf = 0.16 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 7.88 − 7.77 (m, 2H), 7.53 (s, 1H), 7.45 (t, J = 8.1 Hz, 1H), 7.10 − 6.95 (m, 3H), 4.03 (s, 3H), 4.01 (s, 3H), 3.91 (s, 3H).
Methyl 4–(3-hydroxy-6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (19r)
Compound 19r was prepared in 17% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-methyl 4–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (18r) (800 mg, 0.68 mmol), NaOCH3 (5.4 M in methanol solution, 1.7 mL, 4 eq) and hydrogen peroxide (35% aq., 908 μL, 4 eq) in methanol (30 mL), stirring for 12 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 50:0 to 10:1). Rf = 0.44 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, CDCl3) δ 8.33 (d, J = 8.6 Hz, 2H), 8.18 (d, J = 8.6 Hz, 2H), 7.53 (s, 1H), 7.18 (s, 1H), 7.02 (s, 1H), 4.04 (s, 3H), 4.01 (s, 3H), 3.97 (s, 3H). HRMS m/z calculated for C19H16O7 [M + H]+: 357.0969; found: 357.0976.
Methyl 3–(3-hydroxy-6,7-dimethoxy-4-oxo-4H-chromen-2-yl)benzoate (19s)
Compound 19s was prepared in 18% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-methyl 3–(3-(2-hydroxy-4,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate (18s) (330 mg, 0.96 mmol), NaOCH3 (5.4 M in methanol solution, 0.7 mL, 4 eq) and hydrogen peroxide (35% aq., 374 μL, 4 eq) in methanol (10 mL), stirring for 12 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:1 to 8:1). Rf = 0.43 (CH2Cl2/MeOH = 10:1). 1H NMR (600 MHz, CDCl3) δ 8.84 (s, 1H), 8.48 (d, J = 6.8 Hz, 1H), 8.10 (d, J = 7.4 Hz, 1H), 7.60 (t, J = 7.5 Hz, 1H), 7.51 (s, 1H), 7.04 (s, 1H), 4.04 (s, 3H), 4.00 (s, 3H), 3.97 (d, J = 10.6 Hz, 3H). HRMS m/z calculated for C19H16O7 [M + H]+: 357.0969; found: 357.0969.
3-Hydroxy-6,7-dimethoxy-2-(naphthalen-2-yl)-4H-chromen-4-one (22a)
Compound 22a was prepared in 15% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3-(naphthalen-2-yl)prop-2-en-1-one (21a) (230 mg, 0.69 mmol), NaOH (3 M aq.) 1.5 mL and hydrogen peroxide (35% aq., 267 μL, 4 eq) in ethanol (10 mL), stirring for 15 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.58 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.78 (s, 1H), 8.32 (dd, J = 8.7, 1.7 Hz, 1H), 7.99 (dd, J = 11.3, 7.9 Hz, 2H), 7.93 − 7.86 (m, 1H), 7.62 − 7.52 (m, 3H), 7.14 (s, 1H), 7.08 (d, J = 6.2 Hz, 1H), 4.06 (s, 3H), 4.02 (s, 3H). HRMS m/z calculated for C21H16O5 [M + H]+: 349.1071; found: 349.1077.
3-Hydroxy-6,7-dimethoxy-2–(1-methyl-1H-pyrazol-4-yl)-4H-chromen-4-one (22b)
Compound 22b was prepared in 46% yield as a yellow powder, following the same procedure as described in the general procedure E with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3–(1-methyl-1H-pyrazol-4-yl)prop-2-en-1-one (21b) (338 mg, 1.35 mmol), NaOH (3 M aq.) 2 mL and hydrogen peroxide (35% aq., 523 μL, 4 eq) in ethanol (10 mL), stirring for 15 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.39 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 8.15 (s, 1H), 8.11 (s, 1H), 7.52 (s, 1H), 6.69 (s, 1H), 4.03 (s, 3H), 4.02 (s, 3H), 3.99 (s, 3H). HRMS m/z calculated for C15H14N2O5 [M + H]+: 303.0976; found: 303.0971.
3-Hydroxy-6,7-dimethoxy-2-(thiophen-2-yl)-4H-chromen-4-one (22c)
Compound 22c was prepared in 22% yield as a yellow powder, following the same procedure as described in the general procedure F with (E)-1–(2-hydroxy-4,5-dimethoxyphenyl)-3-(thiophen-2-yl)prop-2-en-1-one (21c) (273 mg, 0.94 mmol), NaOH (3 M aq.) 1.5 mL and hydrogen peroxide (35% aq., 365 μL, 4 eq) in ethanol (10 mL), stirring for 15 h. The reaction mixture was poured into H2O and acidified with 3N HCl (pH = 2) and extracted with CH2Cl2. The crude residue was purified by column chromatography on silica gel (CH2Cl2/MeOH = 100:0 to 30:1). Rf = 0.58 (CH2Cl2/MeOH = 30:1). 1H NMR (600 MHz, CDCl3) δ 7.98 − 7.90 (m, 1H), 7.59 (dd, J = 4.8 Hz and 1.2 Hz, 1H), 7.52 (s, 1H), 7.24 − 7.22 (m, 1H), 6.98 (s, 1H), 4.03 (s, 3H), 3.99 (s, 3H). HRMS m/z calculated for C15H12O5S [M + H]+: 305.0477; found: 305.0469.
In silico docking studies
Ligand preparation and optimisation: All ligands were generated as 2D and 3D structure by ChemDraw Ultra (ver. 12.0.2) and Chem3D Pro (ver. 11.0.1), respectively. Ligand preparation and optimisation was followed by “Sanitize” preparation protocol in SYBYL-X 2.1.1 (Tripos Inc., St Louis) to clean up of the structures involving filling valences, standardising, removing duplicates and producing only one molecule per input structure. The group of ligands was saved as .sdf file.
Protein preparation: For IP6K2, the protein model of human IP6K2 (UniProt: Q9UHH9) was downloaded from AlphaFold2 structure database (https://alphafold.ebi.ac.uk) as PDB and FASTA format. SYBYL-X 2.1.1 program was employed for protein preparation including conflicted side chains of amino acid residues fixation. Hydrogen atoms were added under the application of AMBER7 FF99 Force Field setting for IP6K2, respectively. Minimisation process was performed by POWELL method, and initial optimisation option was set to None for IP6K2. Termination gradient and max iteration were set 0.05 kcal/(mol*Å) and 100 times.
Docking and scoring function studies: The docking studies of all prepared ligands were performed by Surflex-Dock GeomX module in SYBYL-X 2.1.1. For CYP3A4, docking was guided by the Surflex-Dock protomol and docking site was defined by the “Ligand” method with the reported ligand quercetin. Surflex-Dock protomol set to “Residues” method with selected amino acids (Lys42, Leu206, Glu207, Asn208, Leu209, Thr210, Val 218, Leu219, Asp220, Leu221, Lys222, Asp383; radius setting: 2.2; Those amino acids were selected based on the active site of EhIP6KA.) was used to guide docking site for IP6K2 homology model. Two factors related with a generation of Protomol are Bloat(Å) and Threshold were set to 0.5 and 0, respectively. Other parameters were applied with its default settings in all runs.
ADP-Glo kinase assay
Inhibition activity of the synthesised compounds against IP6K2 was assessed using ADP-Glo™ Kinase Assay (Promega). Kinase reaction mixtures were first shaking-incubated with individual drugs (diluted in DMSO) for pre-incubation at RT for 15 min, and then ATP was added (final 10 µM) to initiate the reaction. 20 ng of recombinant human IP6K2 protein was used per 15 µL of IP6K2 reaction mixture (50 mM Tris-HCl, pH 6.8, 10 mM MgCl2, 2.5 mM DTT, 0.02% Triton X-100, 10 µM IP6). Kinase reaction was proceeded at 37 °C for 30 min with 100 rpm shaking, and the amount of ADP produced was measured on white plates using Mithras LB940 plate reader (Berthold) according to the manufacturer’s protocol. Each detection set was prepared on 96-well (25 µL kinase reaction) as duplicates. Resulting enzyme activity was calculated as following; Activity (%) = ([ADPexperimental]/[ADPDMSO])×100. For IC50 estimation of compounds, kinase assay was prepared on 384-well plates (20 ng of recombinant human IP6K2 protein was used per 5 µL kinase reaction mixture) as duplicates. The final concentrations for IP6K2, IP6, and ATP were 80 nM, 10 μM, and 10 μM, respectively. Kinase reaction was proceeded at 37 °C for 40 min with 300 rpm shaking, and the amount of ADP produced was measured using Synergy Neo microplate reader (Biotek) according to the manufacturer’s protocol. IP6K1 and IP6K3 assays were performed by Liao et al.’s conditions.Citation25 The final concentrations for IP6K1, IP6, and ATP were 60 nM, 100 μM, and 1 mM, respectively. The final concentrations for IP6K3, IP6, and ATP were 120 nM, 100 μM, and 1 mM, respectively. The mixed reaction plate was placed into a 37 °C incubator and rotated on an orbital shaker at 300 rpm for 30 min. All statistical analyses including IC50 estimation was performed using Prism 7 or Prism 9 (Graphpad Software).
Recombinant IP6K enzyme purification
Full-length human IP6K1, IP6K2, or IP6K3 cDNA was subcloned into pFASTBAC1 plasmid (Gibco) with an N-terminal FLAG epitope sequence, and baculovirus was generated according to the manufacturer’s instructions. Sf9 insect cells were infected with baculovirus and incubated for 72 h. The cells were resuspended in lysis buffer [20 mM Tris–HCl (pH 7.9), 500 mM NaCl, 4 mM MgCl2, 0.4 mM EDTA, 2 mM DTT, 20% glycerol, 1 mM PMSF, and protease inhibitor cocktail (Roche)] and then disrupted with a Dounce homogeniser (pestle A, 3 series of 10 strokes with 10 min interval). Clarified extracts were adjusted to 300 mM NaCl by adding dilution buffer [20 mM Tris–HCl (pH 7.9) and 10% glycerol], supplemented with final 0.1% NP-40, and then subjected to affinity purification on M2 agarose (Sigma). After extensive washing with wash buffer [20 mM Tris–HCl (pH 7.9), 150 mM NaCl, 2 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 15% glycerol, 1 mM PMSF, and 0.1% NP-40], FLAG-IP6K proteins were eluted with elution buffer (wash buffer containing 0.25 mg/mL FLAG-peptide and protease inhibitor cocktail) and stored at −80 °C (See Supporting Information Figure 5).
Extractions and quantification of intracellular inositol phosphates by HPLC
For HPLC analysis, 2 × 105 cells/60 mm dish of HCT116 were treated with 60 μCi [3H] myo-inositol (NET1177001MC, PerkinElmer). After 3 days, soluble inositol polyphosphates from HCT116 (human colorectal cancer cell line) were extracted and analysed as previously described.Citation39 Intracellular inositol phosphates were extracted with acid extraction buffer (1M HClO4, 3 mM EDTA, and 0.1 mg/mL IP6), and neutralised with neutralisation buffer (1M K2CO3 and 3 mM EDTA). The lysates were centrifuged for 10 min, and the soluble fraction was resolved by HPLC as described earlier.Citation39 The lipid pellet was lysed with 0.1% Triton X-100 in NaOH overnight. Each fraction was mixed with Ultima-Flo AP liquid scintillation cocktail (6013599, PerkinElmer), and radioactivity was counted in a scintillation counter. Quantity of inositol phosphates was presented as total counts/min (CPM) normalised by total lipid contents.
Supplemental Material
Download PDF (3.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6(1):563–597.
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21.
- Chakraborty A, Kim S, Snyder SH. Inositol pyrophosphates as mammalian cell signals. Sci Signal. 2011;4(188):re1.
- Hatch AJ, York JD. Snapshot: inositol phosphates. Cell. 2010;143(6):1030.e1.
- Shears SB, Wang H. Inositol phosphate kinases: Expanding the biological significance of the universal core of the protein kinase fold. Adv Biol Regul. 2019;71:118–127.
- Lee B, Park SJ, Hong S, Kim K, Kim S. Inositol polyphosphate multikinase signaling: multifaceted functions in health and disease. Mol Cells. 2021;44(4):187–194.
- Lee S, Kim M-G, Ahn H, Kim S. Inositol pyrophosphates: signaling molecules with pleiotropic actions in mammals. Molecules. 2020;25(9):2208.
- Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 1999;9(22):1323–1326.
- Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. Identification and characterization of a novel inositol hexakisphosphate kinase. J Biol Chem. 2001;276(42):39179–39185.
- Choi JH, Williams J, Cho J, Falck JR, Shears SB. Purification, sequencing, and molecular identification of a mammalian PP-insP5 kinase that is activated when cells are exposed to hyperosmotic stress. J Biol Chem. 2007;282(42):30763–30775.
- Fridy PC, Otto JC, Dollins DE, York JD. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J Bio Chem. 2007;282(42):30754–30762.
- Kilari RS, Weaver JD, Shears SB, Safrany ST. Understanding inositol pyrophosphate metabolism and function: kinetic characterization of the dipps. FEBS Lett. 2013;587(21):3464–3470.
- Gu C, Wilson MSC, Jessen HJ, Saiardi A, Shears SB. Inositol pyrophosphate profiling of two HCT116 cell lines uncovers variation in insp(8) levels. PLoS One. 2016;11(10):e0165286.
- Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, et al. Inositol pyrophosphates inhibit akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143(6):897–910.
- Lee T-S, Lee J-Y, Kyung JW, Yang Y, Park SJ, Lee S, Pavlovic I, Kong B, Jho YS, Jessen HJ, et al. Inositol pyrophosphates inhibit synaptotagmin-dependent exocytosis. Proc Natl Acad Sci USA. 2016;113(29):8314–8319.
- Chin AC, Gao Z, Riley AM, et al. The inositol pyrophosphate 5-insp(7) drives sodium-potassium pump degradation by relieving an autoinhibitory domain of PI3K p85 alpha. Sci Adv. 2020;6:eabb8542.
- Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, Tokhunts R, Koldobskiy MA, Fu C, Barrow R, et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol Cells. 2014;54(1):119–132.
- Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306(5704):2101–2105.
- Saiardi A. Protein pyrophosphorylation: Moving forward. Biochem J. 2016;473(21):3765–3768.
- Mukherjee S, Haubner J, Chakraborty A. Targeting the inositol pyrophosphate biosynthetic enzymes in metabolic diseases. Molecules. 2020;25(6):1403.
- Wormald MM, Ernst G, Wei H, Barrow JC. Synthesis and characterization of novel isoform-selective IP6K1 inhibitors. Bioorg Med Chem. 2019;29(19):126628.
- Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases use in defining biological roles and metabolic relationships of inositol pyrophosphates. J Biol Chem. 2009;284(16):10571–10582.
- Zhou Y, Mukherjee S, Huang D, Chakraborty M, Gu C, Zong G, Stashko MA, Pearce KH, Shears SB, Chakraborty A, et al. Development of novel IP6K inhibitors for the treatment of obesity and obesity-induced metabolic dysfunctions. J Med Chem. 2022;65(9):6869–6887.
- Gu C, Stashko MA, Puhl-Rubio AC, Chakraborty M, Chakraborty A, Frye SV, Pearce KH, Wang X, Shears SB, Wang H, et al. Inhibition of inositol polyphosphate kinases by quercetin and related flavonoids: a structure-activity analysis. J Med Chem. 2019;62(3):1443–1454.
- Liao G, Ye W, Heitmann T, Ernst G, DePasquale M, Xu L, Wormald M, Hu X, Ferrer M, Harmel RK, et al. Identification of small-molecule inhibitors of human inositol hexakisphosphate kinases by high-throughput screening. ACS Pharmacol Transl Sci. 2021;4(2):780–789.
- Lee S, Park BB, Kwon H, Kim V, Jeon JS, Lee R, Subedi M, Lim T, Ha H, An D, et al. TNP and its analogs: modulation of IP6K and CYP3A4 inhibition. J Enzyme Inhib Med Chem. 2022;37(1):269–279.
- Puhl-Rubio AC, Stashko MA, Wang H, Hardy PB, Tyagi V, Li B, Wang X, Kireev D, Jessen HJ, Frye SV, et al. Use of protein kinase-focused compound libraries for the discovery of new inositol phosphate kinase inhibitors. Slas Disc. 2018;23(9):982–988.
- Moritoh Y, Abe S-i, Akiyama H, Kobayashi A, Koyama R, Hara R, Kasai S, Watanabe M. The enzymatic activity of inositol hexakisphosphate kinase controls circulating phosphate in mammals. Nat Commun. 2021;12(1):4847.
- Ghoshal S, Zhu Q, Asteian A, Lin H, Xu H, Ernst G, Barrow JC, Xu B, Cameron MD, Kamenecka TM, et al. TNP [N2-(m-trifluorobenzyl), N6-(p-nitrobenzyl) purine] ameliorates diet induced obesity and insulin resistance via inhibition of the IP6K1 pathway. Mol Metab. 2016;5(10):903–917.
- Chang Y-T, Choi G, Bae Y-S, Burdett M, Moon H-S, Lee JW, Gray NS, Schultz PG, Meijer L, Chung S-K, et al. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. ChemBioChem. 2002;3(9):897–901.
- Sekar MC, Shahiwala K, Leloup L, Wells A. Modulation of epidermal growth factor stimulated erk phosphorylation and cell motility by inositol trisphosphate kinase. J Pharmaceut Sci Pharmacol. 2014;1(2):160–164.
- Terao Y, Takahashi M, Hara R, et al. Preparation of 4-aryloxy-2′-oxo-1′,2′- dihydrospiro[cyclohexane-1,3′-indole]-5′-carboxylic acid and 4-aryloxy-2′-oxo-1′,2′-dihydrospiro[cyclohexane-1,3′-pyrrolo[3,2-b]- pyridine]-5′-carboxylic acid derivatives as IP6K inhibitors. WO2018182051 A1, 2018.
- Park BB, Choi JW, Park D, Choi D, Paek J, Kim HJ, Son S-Y, Mushtaq AU, Shin H, Kim SH, et al. Structure-activity relationships of baicalein and its analogs as novel tslp inhibitors. Sci Rep. 2019;9(1):8762.
- Britton RG, Horner-Glister E, Pomenya OA, Smith EE, Denton R, Jenkins PR, Steward WP, Brown K, Gescher A, Sale S, et al. Synthesis and biological evaluation of novel flavonols as potential anti-prostate cancer agents. Eur J Med Chem. 2012;54:952–958.
- Vasselin DA, Westwell AD, Matthews CS, Bradshaw TD, Stevens MFG. Synthesis and biological properties of fluoro-, methoxyl-, and amino-substituted 3-phenyl-4h-1-benzopyran-4-ones and a comparison of their antitumor activities with the activities of related 2-phenylbenzothiazoles. J Med Chem. 2006;49(13):3973–3981.
- Oh MH, Lee HJ, Jo SH, Park BB, Park S-B, Kim E-y, Zhou Y, Jeon YH, Lee K. Development of cassette pampa for permeability screening. Biol Pharm Bull. 2017;40(4):419–424.
- Koldobskiy MA, Chakraborty A, Werner JK, Snowman AM, Juluri KR, Vandiver MS, Kim S, Heletz S, Snyder SH. Jr,. P53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 2010;107(49):20947–20951.
- Yokoyama T, Kosaka Y, Mizuguchi M. Structural insight into the interactions between death-associated protein kinase 1 and natural flavonoids. J Med Chem. 2015;58(18):7400–7408.
- Azevedo C, Saiardi A. Extraction and analysis of soluble inositol polyphosphates from yeast. Nat Protoc. 2006;1(5):2416–2422.