Abstract
In this article, emulsomes (EMLs) were fabricated to encapsulate the N-(5-nitrothiazol-2-yl)-carboxamido derivatives (3a–3g) in an attempt to improve their biological availability and antiviral activity. Next, both cytotoxicity and anti-SARS-CoV-2 activities of the examined compounds loaded EMLs (F3a–g) were assessed in Vero E6 cells via MTT assay to calculate the CC50 and inhibitory concentration 50 (IC50) values. The most potent 3e-loaded EMLs (F3e) elicited a selectivity index of 18 with an IC50 value of 0.73 μg/mL. Moreover, F3e was selected for further elucidation of a possible mode of action where the results showed that it exhibited a combination of virucidal (>90%), viral adsorption (>80%), and viral replication (>60%) inhibition. Besides, molecular docking and MD simulations towards the SARS-CoV-2 Mpro were performed. Finally, a structure–activity relationship (SAR) study focussed on studying the influence of altering the size, type, and flexibility of the α-substituent to the carboxamide in addition to compound contraction on SARS-CoV-2 activity.
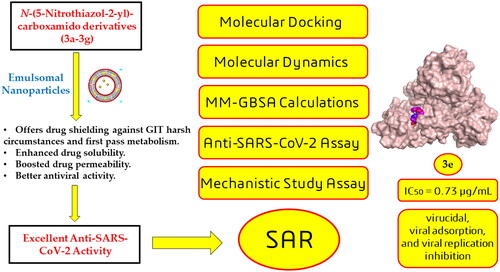
Emulsomes (EMLs) were fabricated to encapsulate the N-(5-nitrothiazol-2-yl)-carboxamido derivatives (3a–3g).
The most potent 3e-loaded EMLs (F3e) showed an IC50 value of 0.73 μg/mL against SARS-CoV-2.
F3e exhibited a combination of virucidal (>90%), viral adsorption (>80%), and viral replication (>60%) inhibition.
Molecular docking, molecular dynamics (MD) simulations, and MM-GBSA calculations were performed.
Structure–activity relationship (SAR) study was discussed to study the influence of altering the size, type, and flexibility of the α-substituent to the carboxamide on the anti-SARS-CoV-2 activity.
Highlights
Introduction
Since its emergence in late 2019, the COVID-19 pandemic (caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) has gained global attention due to its significant mortality and morbidity with over 661 million confirmed cases and almost 6.7 million deaths (https://www.worldometers.info/coronavirus/)Citation1. In the initial stages of the viral infection, symptoms are commonly mild and include fever, myalgia, and dry cough. At more advanced stages of the disease, pulmonary symptoms such as dyspnoea and hypoxia developCitation2. Although the viral load usually subsides by that time, the condition of some patients worsens due to an uncontrolled systemic inflammatory response (or a cytokine storm), resulting in long-term or life-threatening implications on lung tissues and other organsCitation3.
Like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 belongs to the genus β-coronavirus and is an enveloped positive-stranded RNA virus. Its coronavirus particle consists of four structural proteins, namely the nucleocapsid, envelope, membrane, and spikeCitation4. In addition, its viral genome comprises non-structural open reading frames that encode several non-structural proteins, such as enzymes needed for viral intracellular replicationCitation5,Citation6.
The entry of the SARS-CoV-2 into the host cell is mediated via the fusion of the viral spike (S) glycoprotein to cell surface angiotensinogen-converting enzyme 2 (ACE2) receptors. Subsequent conformational changes in the S protein promote the viral envelope fusion with the cell membrane through an endosomal pathwayCitation7. On entering the host cells, the viral genome is released as a positive single-stranded RNA that can be directly converted into viral polyproteins employing host cell protein translation machinery. These polyproteins are further hydrolysed by two viral proteases, namely the Mpro (also known as the 3-chymotrypsin-like protease (3CLpro)) and the papain-like protease (PLpro) to yield the viral structural and functional proteins, including the RNA-dependent RNA polymerase (RdRp)Citation5. Afterwards, RdRp synthesises a full-length negative-strand RNA template, utilised to produce more viral genomic RNA (by replicating the new positive-strand RNA) in addition to several subgenomic RNAs, which are later translated into relevant viral proteins. Subsequently, viral proteins and RNA genomes are assembled into virions that are released from the cell via exocytosisCitation7,Citation8.
Despite the huge leap in the development of vaccines worldwide, the demand for pharmacological treatments of COVID-19 disease is still urging as vaccines were found not 100% effective, especially with emerging variants, and could not fully prevent the transmission of the disease besides their slow reach to underdeveloped countriesCitation7. On the contrary, pharmacological treatments can be designed to address multiple targets that are highly conserved among variants of the virus such as Mpro, PLpro, non-structural protein 12 (nsp12), and RdRp in addition to host-related targetsCitation9. This would likely maintain broad-spectrum therapeutic effectiveness despite the continuously emerging viral mutations.
Within the last three years, several antiviral therapeutic approaches have been tailored to various druggable targets along the stages of viral entry and replication. Recently, the orally active nirmatrelvir (PF-07321332) in combination with ritonavir, was granted emergency use by the FDA in December 2021 for COVID-19 treatment under the trade name Paxlovid®Citation10. The combination elicited an efficacy of about 88% against hospitalisation or mortality in adult outpatients after its administration within five days of the onset of symptomsCitation11. Nirmatrelvir is a peptidomimetic inhibitor of the main protease Mpro that acts via a reversible covalent interaction with its key Cys-145 residueCitation12. Ritonavir lacks activity against SARS-CoV-2 Mpro but acts as a pharmacokinetic boosting agent by inhibiting the CYP3A-mediated metabolism of nirmatrelvirCitation13. The most prominent antivirals against viral genome replication include the FDA-approved Remdesivir (Veklury®) and the EUA-authorised Molnupiravir (Lagevrio®)Citation14.
Among all reported SARS-CoV-2 druggable targets, Mpro is not only the most cysteine protease conserved in structure but also its function in all known CoVsCitation15. It serves as the main protease for proteolytic cleavage of the two overlapping large polyprotein precursors (pp1a and pp1ab) into the 16 non-structural proteins required for viral replication and maturationCitation5. Furthermore, it is a key player in virus entry to host cells where its inhibition was reported to impede viral entry and the subsequent infectionCitation16. The substantial dependence of the virus on the proper functioning of this protease, along with the absence of a homologous human protease, makes Mpro one of the most pursued therapeutic targets for curbing coronavirus-associated diseasesCitation17–19.
Several studies, comprehensively reviewed in refs.Citation20–22, have successfully disclosed various covalent (e.g. N3Citation23 and 1Citation17), non-covalent (e.g. baicaleinCitation20, 2Citation24, and 3Citation25) and high-throughput screening (HTS) (e.g. GC-376Citation26 and cryptotanshinoneCitation27) potential SARS-CoV-2 Mpro inhibitors (). Among these, α-ketoamide inhibitors, peptide-based inhibitors, anilide-based inhibitors, indole carboxamide-based inhibitors, drugs from Chinese traditional medicine, phytochemicals, and microorganism-based inhibitors were the most extensively explored drug classesCitation20,Citation28.
Diverse inhibitors exhibited potent inhibitory activities against SARS-CoV-2; however, the majority of drugs are suspended in preclinical experiments and terminated within in vivo models due to pharmacodynamics or pharmacokinetics concernsCitation21. The main challenge in designing protease inhibitors is a dilemma between using highly potent and selective peptidomimetic/high molecular weight compounds but of poor drug-likeness versus using non-peptidomimetic/low molecular weight compounds of lower inhibitory potentialCitation29,Citation30. Accordingly, continuous endeavours on SARS-CoV-2 Mpro inhibitors aim at achieving the required pharmacodynamic/kinetic balance that ensures clinical effectiveness and tolerability.
Among the most frequent reasons for poor absorption and restricted oral bioavailability of candidate drugs are their diminished intestinal permeability besides their liability for extensive metabolism in GIT and liverCitation31. Accordingly, a lipidic vesicular system, i.e. liposome or noisome is usually adopted to overcome those pitfallsCitation32. Emulsomes (EMLs) are one of the vesicular systems that possess the characteristics of lipidic bilayered vesicles and nanoemulsions and even exhibit the advantages of bothCitation33. Emulsomes primarily comprise two basic components: solid lipid as core enveloped with phospholipid as an outer layer which augments the vesicle stability. Emulsomes can successfully accommodate both hydrophilic and lipophilic drugs, in addition to overcoming the defects of conventional vesicular systems such as the high liability of aggregation and elevated drug leakage rateCitation34. Moreover, surface PEGylation of EMLs could promote vesicle stability and prolong its duration in the systemic circulationCitation35. Accordingly, EMLs are deemed as a prosperous carrier for candidate drugs, boosting their antiviral activity and bioavailability.
The rationale for work design
Previously, a series of N-(5-nitrothiazol-2-yl)-carboxamido derivatives (3a–g) was designed based on the basic pharmacophoric features of the co-crystallised inhibitor of the SARS-CoV. This was done according to the fact that there is a close structural similarity between the two strains of SARS-CoV (1 and 2)Citation31. However, only compound 3b (Scheme 1) showed superior anti-SARS-CoV-2 (174.7 µg/mL) and almost the SARS-CoV-2 Mpro inhibition (5.12 µg/mL) activities as well. This was attributed to its proposed better penetration throughout SARS-CoV-2 infected cells. Interestingly, one of the previous recommendations was to apply a suitable formulation for compound 3d to explain its superior in silico stability in contrast to its lower anti-SARS-CoV-2 activity, compared to other membersCitation31. Herein, the proceeded study EMLs were fabricated to encapsulate the examined compounds (3a–g) in an attempt to improve their biological availability and antiviral activity as well.
Scheme 1. Chemical synthesis of the previously designed 3a–g candidates to combat COVID-19Citation31.

EMLs aid in promoting intestinal permeability and prolonging the mean residence time of the drug in the systemic circulation, accordingly promoting its targeted therapeutic effectCitation33. Besides, the authors continued their study to investigate the proposed anti-SARS-CoV-2 activities of the aforementioned candidates by applying deep in silico studies towards the SARS-CoV-2 Mpro target receptor (6Y2GCitation24) as a recommended mechanism of action. Collectively, the main pharmacophoric features of the co-crystallised inhibitor of SARS-CoV-2 Mpro could be suggested as follows ():
Figure 2. Rationale design for the synthesised N-(5-nitrothiazol-2-yl)-carboxamido derivatives as potential inhibitors of SARS-CoV-2 Mpro and the promising effect of the emulsomal nanoparticles.

One functional group forms a hydrogen bond with GLU_166.
A second functional group forms a hydrogen bond with CYS_145.
An additional moiety to interact with HIS_41, HIS_163, HIS_164, or GLY_143.
The N-(5-nitrothiazol-2-yl) carboxamide scaffold was kept unchanged in all examined compounds. The authors mainly focussed on studying the influence of altering the size, type, and flexibility of the α-substituent to the carboxamide in addition to compound contraction on SARS-CoV-2 activity. In other words, the α-substituent group to the carboxamide (R) was designed in variable sizes hoping to fit properly inside the target receptor pocket which could improve the binding pattern accordingly.
Materials and methods
Materials
Egg yolk l-α-phosphatidylcholine (PC) (lecithin) and tristearin were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). The Brij52 (polyoxyethylene (2) cetyl ether) was obtained from BASF Co. (Florham Park, NJ). The sodium chloride, potassium dihydrogen orthophosphate, methanol, sodium hydroxide, magnesium chloride, chloroform, and absolute ethanol were purchased from El-Gomhouria Chemical Co. (Cairo, Egypt). The dialysis membranes (Spectra/Pore®, cut-off 12 000–14 000) were purchased from Spectrum Laboratories Inc., Rancho Dominguez, CA). All chemicals and solvents were of analytical grade and were used as received.
Methods
Fabrication of 3b-loaded emulsomes
3b-loaded EMLs were fabricated adopting the thin-film hydration technique with minor modificationCitation36. Briefly, in a round-bottom flask, 20 mg drug, 50 mg or 100 mg tristearin as lipid core, 25 mg or 50 mg PC, 20.0 or 40.0 mg Brij52, and 10.0 mg cholesterol were dissolved in 10 mL solvent mixture chloroform:methanol (2:1) (). Then, in a rotatory evaporator, the organic solvent was completely dispelled under reduced pressure at 60 °C for 30 min. The acquired dry film was then hydrated using 10 mL phosphate-buffered saline (PBS) at 60 °C (that exceeded the transition temperature of the lipid phase (Tc)) for 45 min. Finally, an ultrasonicator (Elmasonic, model LC 60/H, Wetzlar, Germany) was employed for further reduction in the particle size (PS) of the resulting EMLs dispersions kept at room temperature for 10 min.
Table 1. 23 full factorial experimental designs; experimental runs, independent variables, and estimated responses of three loaded EMLs.
In vitro analysis and optimisation of 3b-loaded EMLs
Estimation of the entrapment efficiency percentage (EE%)
The percentage of compound 3b enclosed within the fabricated EMLs was assessed in triplicates, where 1 mL aliquots of the EMLs dispersion were first diluted with 5 mL distilled water then agitated for 2 min, and finally, 1 mL of the final dispersion was then subjected to 1 h cooling centrifugation (Beckman Instruments, Fullerton, CA) at 15 000 rpm and kept at 4 °CCitation37. The sedimented pellets were washed off twice with distilled water and recentrifuged for 30 min. The vesicles were disrupted using methanol and then sonicated for 2 h at room temperature. The analysis of the total concentration of embedded 3b within the vesicles was conducted using UV spectrophotometry at λmax 254 nm versus methanol as the blanks. The EE% of the formulae was computed using the formula: EE% of 3b entrapped = (amount of 3b enclosed/overall amount of 3b) × 100.
Estimation of zeta potential (ZP), PS, and polydispersity index (PDI)
The prepared 3b-loaded EMLs were analysed in triplicates for determination of particle (or vesicle) size (PS), ZP, and PDI using a Zetasizer ZS (Malvern Instruments, Malvern, UK) (). 0.1 mL of the formulae dispersions were diluted to 10 mL with distilled water and vortexed for 5 min. The analysis was conducted at 25 °C using dynamic laser scattering with a 45 mm focus lens and a beam length of 2.4 mmCitation38.
Experimental design and choice of the optimal 3b-loaded EMLs
Design Expert® Version 13 (Stat Ease, Inc., Minneapolis, MN) was involved in the investigation of the influence of altering various formulation aspects of EMLs on their responses. Eight experimental runs were attained from the manipulated design adopting 23 experimental designs. Three factors were considered as the independent variables: lipid core amount (A), PC amount (B), and Brij52 amount (C), where EE% (Y1), PS (Y2), and ZP (Y3) were set as the dependent variables. The selection of the optimal 3b-loaded EML formulation was conducted based on the highest EE%, ZP, and lowest PS values. The consideration of the main effects and their relative significance were explored according to ANOVA statistical analyses. Furthermore, the optimal formulation with the highest desirability value was picked and involved in further assessments. Finally, the composition of the optimised formula was used in the preparation of the other compounds loaded emulsomal nanoparticles (EMLs) in order to investigate the effect of formulation of all compounds (3a–g) on their anti-SARS-CoV-2 activity which represent one of the prime targets of the study.
In vitro investigation of the optimum 3b-loaded EMLs
Lyophilisation of the optimised EML formula
Freeze-dryer (Alpha 2-4, CHRIST, Osterode am Harz, Germany) was employed in the solidification of the optimised 3b-loaded EML formula. To impede the lysis and fusion of the vesicles, mannitol (5% w/v) was added as the cryoprotectant. The EML dispersion was kept overnight at −80 °C, and it was dried for 24 h under a vacuum. The attained emulsomal powder was preserved in a desiccator in tightly closed amber-coloured glass tubes for further characterisation.
Differential scanning calorimetry (DSC)
DSC (DSC-50, Shimadzu, Kyoto, Japan) was involved in the assessment of the thermal behaviour of each pure compound 3b, plain optimum formula, and 3b-loaded EMLs. The calibration of the instrument was conducted using purified indium (99.9%). The thermal behaviour of the samples was investigated in a temperature range of 20–400 °C at a scan rate of 10 °C/min under nitrogenCitation39.
In vitro compound 3b release experiment
The release pattern of compound 3b from the optimum formula compared to the drug dispersion was investigated as follows: 1 mL of the 3b-loaded EML dispersions were diluted with 1 mL of Sorensen phosphate buffer (pH 7.4), then 1 mL of the produced dispersion (comprising 1 mg of 3b) was added to a 2.5 cm diameter 10 cm glass cylinder closed from one side with a pre-soaked cellulose membrane. Then the glass cylinder was attached to the shaft of the dissolution tester (Copley, DIS 8000, Nottingham, UK) and left to rotate at a speed of 50 rpm in 900 mL of the same Sorensen phosphate buffer kept at 37 ± 0.5 °C. At different scheduled time intervals, a 5 mL sample was withdrawn and replaced with an equal volume of freshly prepared dissolution medium for keeping a constant volume and maintaining constant sink conditions. The percentages of 3b released were measured spectrophotometrically at 254 nm and were computed in triplicate.
In vitro studies
MTT assay
To determine the cytotoxic concentration CC50 of each examined compound in VERO-E6 cells, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was conducted as previously describedCitation40. The MTT assay method was performed with minor changes to calculate the newly synthesised candidates’ minimum concentrations that cause 50% toxicity to the cells (CC50). The full methodology was elucidated in the supplementary material (S1).
Inhibitory concentration 50 (IC50)
The IC50 for each examined compound formula (F3a–g), which is equivalent to the minimum concentration to inhibit the virus infectivity by 50% compared to the virus control, was calculatedCitation41. The complete methodology was depicted in the supplementary material (S2).
Mode of action against SARS-CoV-2
The possible mode of action for the most potent EMLs formula (F3e) towards SARS-CoV-2 inhibition was examined at three different stages of the virus propagation cycle and based on three main possible modes of action:
The direct effect of each extract is to inactivate the virus viability (virucidal activity).
The ability of each extract to inhibit the attachment of the virus to infected cells – membrane fusion is known to block the viral entry (viral adsorption).
Inhibition of budding and viral replication.
Additionally, the above-mentioned modes of action could account for the recorded antiviral activities either independently or in combination. In this regard, the interaction between F3e and SARS-CoV-2 could be explained through the aforementioned three different modes of action. Therefore, the plaque infectivity reduction assay was performed according to the reported procedure (S3).
In silico studies
Docking studies
Molecular docking for the examined candidates (3a–3g) towards the binding site of SARS-CoV-2 Mpro was carried out using the MOE 2019.0102Citation42. This was done to investigate the proposed SARS-CoV-2 Mpro inhibitory activity of the studied compounds compared to the co-crystallised (Co) inhibitor of the target receptor.
The aforementioned compounds were sketched individually in the working window of MOE, hydrogenated in 3D orientation, and energy minimised according to the described methodologyCitation43. All the prepared derivatives together with the Co inhibitor were inserted in one database to be ready for the docking process. Then, the X-ray structure of SARS-CoV-2 Mpro (PDB ID: 6Y2GCitation24) was downloaded from the Protein Data Bank (www.rcsb.org). It was opened within the working window of MOE, corrected for the missed parts, and finally, energy was minimised as discussed earlierCitation44. A general docking process was performed by inserting the previously mentioned database in place of the ligand icon and adjusting the program specifications as mentioned beforeCitation45.
Molecular dynamics simulations and MM-GBSA calculations
The MD simulationsCitation46 were carried out through the Desmond package (Schrödinger LLC, New York, NY)Citation47. Also, the MM-GBSA calculations of SchrodingerCitation48 were applied to calculate the energies for all the studied complexesCitation49. The full methodology is described in detail in the supplementary material (S4 and S5).
Results and discussion
Chemistry
Previously, 3a–g were designed and synthesised by reacting N-acyl benzotriazoles (1a–g) and 5-nitrothiazol-2-amine (2) in the presence of TEA in acetonitrile at R.T. for 1 h as depicted in Scheme 1Citation31.
Experimental design and statistical evaluation
The investigation of the impact of the fabrication variables on the supposed responses was conducted using a 23-full-factorial design. The composition of the eight attained experimental runs and their related EE, PS, and ZP responses are demonstrated in . The suitability of the model to manoeuvre the design space was deduced using model precision valueCitation36. As shown in , a precision value exceeding four was perceived for all the dependent variables. Furthermore, the gap between predicted and adjusted R2 should not exceed a value of 0.20 to confirm a reasonable agreement. From , it can be depicted that the predicted R2 values were consistent with adjusted R2 values for all the dependent variables. Based on the superiority of previously reported IC50 against SARS-CoV-2, compound 3b was selected as a drug model to be endorsed in the formulation and optimisation of fabrication variablesCitation31.
Table 2. 23 Output factorial analysis data of 3b-loaded EMLs and the predicted, observed responses, and deviation percent of the Optimum formula (F6).
Influence of the compounding variables on EE%
The assessment of the EE% is beneficial in the estimation of the loading capability of compound 3b within the formulated vesicles. The encapsulation percentages of 3b within the vesicles ranged from 63.1 ± 2.4 to 93.3 ± 3.1%. In addition, the model with all variables for lipid core amount (factor A), PC amount (factor B), and Brij52 amount (factor C) significantly impacted (p = 0.0153) the EE%, as illustrated in the 3D plots ().
Figure 3. 3D surface plot of the impact of (A) lipid core amount, (B) PC amount, and (C) Brij 52 amount on EE% of 3b-loaded EMLs.

The increase in the amount of the lipid core (factor A) from 50 mg to 100 mg resulted in a significant (p = 0.0062) improvement in the entrapment of compound 3b. It was previously stated that the increase in the lipid core amount leads to an increase in the lipophilic space within the vesicles which will accommodate the drugCitation50. Furthermore, the viscosity of the fabricated vesicular dispersion was elevated with the increase in the amount of the lipid core; thus, the boosted viscosity can hinder the drug diffusion to the external hydrophilic phase, leading to a higher EE%Citation51. Moreover, increasing the amount of PC (factor C) from 25 to 50 mg predisposed to a significant increase (p = 0.0137) in the EE%. The increase in PC content resulted in the assembly of PC multilayers around the lipid core, thus allowing the drug to be efficiently enclosed within these bilayers. In addition, a higher amount of PCs led to the subsequent enhancement in the vesicle rigidity and compactness, thus impeding drug leakageCitation52. ANOVA statistical analysis declared a significant negative impact on EE% (p = 0.0142) upon increasing the amount of Brij from 20 mg to 40 mg. This could be credited to the development of more pores (voids) in the bilayers; moreover, the elevation in the amount of Brij predisposed to an increase in the vesicular fluidity, thus resulting in increased drug leakage and subsequent diminished EE% values.
The polydispersity index and the influence of the compounding variables on particle size
The extent of sample homogeneity along with the level of monodispersity can be deduced via PDI values, whereas the PDI values getting close to zero indicate monodispersity, while those getting close to 1 indicate PDI. The PDI values of the 3b-loaded EMLs shown in ranged from 0.24 ± 0.06 to 0.61 ± 0.1. Thus, the values of the PDI for the prepared formulae shifted towards PDI, but within an appropriate rangeCitation53. Generally, the drug’s permeation across intestinal membranes along with its fate and duration in blood circulation is strongly related to PS. Thus, promoted drug permeation, prolonged retention time, and boosted therapeutic activity are the significant results of decreasing the PS. As illustrated in , Y2, the PS of the fabricated 3b-loaded EMLs ranged from 212.9 ± 17 to 428.6 ± 24 nm. The significance of the whole model with all three variables (factors A–C) was confirmed based on ANOVA analysis; this is graphically illustrated in the 3D plots (). The rationale behind the effect of each variable on the PS can be discussed as follows.
Figure 4. 3D surface plot of the impact of (A) lipid core amount, (B) PC amount, and (C) Brij 52 amount on PS of 3b-loaded EMLs.

Regarding the lipid core amount (factor A), the increase in the lipid core amount from 50 mg to 100 mg predisposed to a prominent increase in PS (p = 0.0049). This may be attributed to the supposed increase in the viscosity of the fabricated emulsion resulting from the elevation in the amount of lipid involved in the formulationCitation36. Also increasing the amount of PC (factor B) resulted in a subsequent significant elevation in PS (p = 0.0075). This finding may be due to the increase in the thickness of the attained PC multiple bilayers which surround the lipidic core and this came in harmony with the results of Aldawsari et al., who studied the impact of the increase in the amount of the phospholipid on the PS of the fabricated raloxifene EMLsCitation33. Moreover, the alignment of the PC at the interface between aqueous and oily phases provides the vesicles with the extra capability to endorse higher amounts of the target drug, leading to greater sizes of the formulated EMLsCitation52.
On the other hand, the increase in the amount of Brij (factor C) led to a significant suppression in vesicle PS (p = 0.0115). Owing to the surface active properties of Brij, the higher amount of Brij will consequently diminish the interfacial tension of the system and alter the alignment t of the attained vesiclesCitation54. Additionally, the assembly of the Brij molecules at the surface of the vesicle sterically stabilises the vesicles by prohibiting their aggregation and improving their stabilityCitation36.
The influence of the compounding variables on zeta potentials
Based on the ZPs values, the stability of the vesicular system can be deduced, where ZP values fall in the range of ±30 mV and are assumed to attain adequate electrostatic stabilisationCitation55. This guarantees a sufficient electric repulsion to be produced between the vesicles, thus impeding their agglomeration and promoting their stabilityCitation56. In the present study, ZP values of the prepared EMLs ranged from −12.2 ± 1.4 to −49.7 ± 5.8 mV (). It was found that ZP was significantly influenced by all the variables (factors A–C) as depicted from the results of ANOVA and displayed as 3D plots (). The influence of each variable can be justified as follows.
Figure 5. 3D surface plot of the impact of (A) lipid core amount, (B) PC amount, and (C) Brij 52 amount on ZP of 3b-loaded EMLs.

According to ANOVA results, the increase in lipid core amount (factor A) led to a significant increase in ZP as an absolute value (p = 0.041). Increasing the amount of lipid permitted the diffusion of a larger amount of negatively charged drug into the lipid core, thus imparting higher negative charges imparted on the surface of the EMLs. Furthermore, increasing the amount of PC (factor B) from 25 to 50 mg predisposed to a significant increase (p = 0.038) in ZP values as an absolute value. The same findings were previously reported by El Zaafarany et al., who correlated this increase to the arrangement of the negatively charged PC on the outer surfaceCitation52. Consequently, the elevation in the amounts of PC increased the overall values of the ZPsCitation33. Additionally, ANOVA results revealed that the elevation in the amount of Brij52 (factor C) prominently increased (p = 0.018) the overall ZP values. This may be attributed to the alignment of a higher number of negatively charged PEG moieties as an electronegative coat that wraps the vesicles on increasing the amount of Brij 52; hence, elevation of the overall ZP values was noticedCitation57.
Selection and validation of the optimal 3b-loaded EMLs formulation
The selection of the optimal formula along with the investigation of the impact of the formulation variables on the characteristics of the fabricated vesicles was carried out based on the statistical analysis utilising Design Expert®. The formula (F6) composed of 100 mg of the lipid core, 25 mg of PC, and 40 mg of Brij52 was found to be the optimal formulation as it has the highest desirability value (0.628). The composition of the optimal formula was then used in the formulation of the rest compounds’ EMLs. Additionally, assessing the percentage differences between the observed and predicted values of the selected responses; EE%, PS, and ZP were involved in assuring the validities of the models tested. As noticed in , the low deviation percentage as an absolute value (less than 10%) between the predicted and observed values for all responses emphasises the suitability of the statistical design in the analysis of the findingsCitation36.
In vitro investigations of the optimum 3b-loaded EML formula
Differential scanning calorimetry
DSC study was conducted in order to estimate the change in the physical state and the degree of crystallinity of pure compound 3b compared to 3b-loaded EMLs. displays the thermal behaviour of the pure 3b compared to that of the blank lyophilised formula and optimum lyophilised 3b-loaded optimum EMLs (F6). Compound 3b discloses a sharp characteristic endothermic peak at 265.2 °C which is related to compound 3b’s melting point. Meanwhile, the thermograms of both lyophilised blank formula and F6 disclosed the disappearance of any characteristic peak of either compound 3b or the components incorporated in the vesicular formulation. Accordingly, this affirmed the total transformation of the drug along with the other components of the formula to an amorphous state instead of the crystalline one, implying the capability of the formulated vesicles to enclose the drug.
Comparative 3b in vitro release experiment
reveals a comparative release profile of the optimised 3b-loaded EML formula (F6) relative to 3b dispersion to denote the release pattern of the drug and the degree of stability of the prepared vesicles. The release pattern of the drug from F6 exhibited more controlled and prolonged release relative to the drug dispersion over 24 h, where the cumulative amount released from the optimised formula F6 after 24 h was 96.2% ± 5.1%. This can be rationalised by the drug pool effect of the vesicles enclosing the drug from which the drug was released in two successive phases: the first rapid burst release followed by the delayed and more controlled phaseCitation58. Additionally, the PEGylated coat resulted from Brij 52 inclusion in the formulation, which wrapped the bilayer and imparted more drug solubilisation and a higher release rate owing to the solubilisation effect and elevated hydrophilicity of PEG moietiesCitation59. Meanwhile, in the case of 3b dispersion, the drug was mostly released after 5 h.
In vitro studies
SARS-CoV-2 inhibitory assay
Both cytotoxicity and anti-SARS-CoV-2 activity of all examined compounds loaded EMLs (F3a–g) were assessed in Vero E6 cells via MTT assay. Half maximal cytotoxic concentrations (CC50) were 14.29 (F3a), 57.33 (F3b), 19.71 (F3c), 8.48 (F3d), 13.4 (F3e), 37.84 (F3f), and 58.98 μg/mL (F3g). In addition, half maximal inhibitory concentrations (IC50) were calculated using dose–response curves as follows; 2.87 (F3a), 1.51 (F3d), 0.73 (F3e), 6.22 (F3f), and 1.56 μg/mL (F3g). On the other hand, both F3b and F3c showed higher IC50 values compared with their respective CC50 values, and Table S1 (supplementary material). The most potent F3e elicited a selectivity index (SI = CC50/IC50) of 18 while F3g demonstrated the highest SI reaching 37.
Figure 8. In vitro assessment of cytotoxicity and anti-SARS-CoV-2 activity of F3a–g in Vero E6 cells via MTT assay (hCoV-19/Egypt/NRC-03/2020 (accession number on GSAID: EPI_ISL_430820)).

On the other hand, both F3b and F3c showed higher IC50 values compared with their respective CC50 values undermining their potential as anti-SARS-CoV-2 therapeutic agents.
The enhanced antiviral activity of the compounds after formulation may be attributed to the capability of attachment of the tailored vesicles to viral cells either by endocytosis or by fusion. This conjugation aided in the accumulation and creation of concentration and thermodynamic gradient at the viral membrane, accordingly, predisposed to boosted penetration of the compounds across virus cellular membranes. Moreover, the lipids and surfactant incorporated in the formulation of EMLs conveyed a vital in enhancing the compound’s antiviral activity, as they augment the diffusion of the drugs through the viral cellsCitation36.
Mode of action against SARS-CoV-2
The most potent hit against SARS-CoV-2 (F3e) was selected for further elucidation of a possible mode of action. Using an array of four different safe concentrations, three potential anti-viral mechanisms were investigated; direct virucidal activity, blockade of viral adsorption to host cell receptors, and inhibition of intracellular viral replication. Results showed that F3e exhibited a combination of virucidal (>90%), viral adsorption (>80%), and viral replication (>60%) inhibition as the principal mechanisms against SARS-CoV-2 ().
In silico studies
Molecular docking studies
A molecular docking study for the studied compounds (3a–3g) towards the SARS-CoV-2 Mpro target (6Y2GCitation24) was performed to investigate their expected inhibitory effects. Besides, the co-crystallised inhibitor (Co) of the target receptor was inserted as a reference standard. Briefly, the most active candidates (3d, 3e, and 3g) and Co were selected for further investigations.
The docked Co of SARS-CoV-2 Mpro formed two hydrogen bonds with the crucial amino acids (GLU_166 and GLN_192) important for producing the antagonistic effect within the binding pocket of SARS-CoV-2 Mpro. Its binding score was recorded to be −8.19 kcal/mol (RMSD = 1.66 Å). On the other hand, compound 3d was able to form two hydrogen bonds with the same crucial amino acid (GLU_166), and its binding score was found to be −7.32 kcal/mol (RMSD = 1.75 Å). Moreover, compound 3e was observed to form one hydrogen bond with the first crucial amino acid (GLU_166). Also, it formed two pi-hydrogen interactions; one of them with the second crucial amino acid (GLN_192) and the other one with ALA_191 amino acid. 3e achieved a binding score of −7.50 kcal/mol (RMSD = 1.24 Å). Furthermore, compound 3g formed two hydrogen bonds with the same crucial amino acid (GLU_166), and also it bound another crucial amino acid (HIS_41) with a pi–pi bond. The binding score of 3g was observed to be −7.79 kcal/mol (RMSD = 1.77 Å) ().
Table 3. 3D interactions and positioning of the Co inhibitor of SARS-CoV-2 Mpro (PDB ID: 6Y2G) binding pocket and the most active candidates (3d, 3e, and 3g).
Molecular dynamics simulations
To investigate the exact behaviour of the examined candidates towards the binding pocket of SARS-CoV-2 Mpro, MD simulations were performed. MD studies were applied for 200 ns and under the same conditions of the physiological environment. All the tested docked complexes were subjected to MD simulations for 200 ns and compared to the co-crystallised ligand (Co).
RMSD analysis
The RMSD was described to compare the deviation degree of the studied protein complexes compared to its initial one in a quantitative manner. This is important to judge each system’s stability during the simulation time.
All the studied eight protein complexes showed promising RMSD values <2.7 Å, except for 3e and 3d protein complexes which showed RMSD values <3.2 Å. This indicates good stability behaviours for all the examined complexes during the 200 ns of the simulation time ().
Figure 10. The RMSD of complexes (3a, 3b, 3c, 3d, 3e, 3f, 3g, and Co) for SARS-CoV-2 Mpro as a function of simulation time (200 ns).

On the other hand, to compare the exact behaviour of each ligand within the binding pocket of SARS-CoV-2 Mpro, the ligand RMSD values within protein complexes were recorded as well (). It was clear that ligands 3a, 3b, 3c, 3d, 3e, 3f, 3g, and Co moved inside the binding pocket of Mpro within the ranges of 9, 12.5, 11, 6.4, 9, 4.8, 10.5, and 7.2, respectively.
Figure 11. The RMSD of ligands (3a, 3b, 3c, 3d, 3e, 3f, 3g, and Co) for SARS-CoV-2 Mpro, respectively, as a function of simulation time (200 ns).

The RMSD behaviours of 3d, 3e, and 3g were selected due to their superior biological activities – to be compared to that of Co and discussed in more detail.
Ligand 3d showed very stable behaviour within the binding pocket of SARS-CoV-2 Mpro from the start till the end of the simulation time. It fluctuated within the range of 3.2 Å, indicating superior stability compared to that of the docked Co. Where the RMSD of Co showed lower stability and fluctuated within the range of 4 Å during the 200 ns of the simulation time. However, ligand 3e showed good stability and fluctuated within the range of 6 Å. It showed the highest fluctuations from 10 to 35 ns of the simulation time, where it returned to stable behaviour till the end of the simulation time. Besides, ligand 3g showed moderate stability where it fluctuated within the range of 7 Å, where it showed higher values from 100 to 200 ns of the simulation time (). Based on the above discussion, we can conclude the very promising behaviour of the aforementioned ligands (3d, 3e, and 3g) with respect to that of Co.
Figure S1 (supplementary material) reports the RMSF (root mean square fluctuation), where most proteins fluctuated within the range of 4–5 Å, with C- and N-terminals fluctuating at around 7 Å. Such change is acceptable within such a large system.
Binding interactions analysis (histogram and heat map)
First, the histograms of the three selected complexes (3d, 3e, and 3g) besides that of Co were analysed to describe the SARS-CoV-2 Mpro-ligand interactions during the simulation time of 200 ns ().
Figure 12. Histogram describing the binding interactions between the SARS-CoV-2 Mpro and its ligand during the simulation time of 200 ns for (A) 3d, (B) 3e, (C) 3g, and (D) Co.

3d-complex histogram showed that GLU_166, HIS_41, and GLY_143 contributed with 110, 100, and 90% of the interactions. GLU_166, HIS_41, and GLY_143 contributed mainly through ionic bonds (>70%), hydrophobic interactions (60%), and hydrogen bonds (>80%), respectively (). Besides, the 3e-complex histogram represented that GLU_166 was the main amino acid that contributed to the interactions (180%) through hydrogen bonds (80%), ionic bonds (45%), and water bridges (55%) followed by HIS_41, which contributed by 75% through hydrophobic interactions mainly (). Moreover, the histogram of 3g-complex showed that GLU_166 was the principal amino acid that contributed to the interactions (100%) through hydrogen bonds (<5%), ionic bonds (>90%), and water bridges (5%). Also, GLY_143 contributed with >90% of the interactions mainly through hydrogen bonds (). On the other hand, the Co-complex histogram showed that GLN_189 was the main amino acid in the interactions (120%) through hydrogen bonds (80%) and water bridges (40%). However, GLU_166 was the second amino acid that contributed to the interactions (110%) through hydrogen bonds (20%) and water bridges (90%), as depicted in . According to the previous discussion, we can conclude the great significance of GLU_166, HIS_41, and GLY_143 (especially GLU_166) in the binding pocket of SARS-CoV-2 Mpro. Therefore, the superior anti-SARS-CoV-2 biological effects of the studied compounds towards the binding site of SARS-CoV-2 Mpro can be justified.
On the other hand, the heat maps which describe the total contacts with respect to the simulation time (200 ns) for the four studied complexes (3d, 3e, 3g, and Co) are represented in .
Figure 13. Heat map showing the total number of SARS-CoV-2 Mpro-ligand interactions all over the simulation time of 200 ns for (A) 3d, (B) 3e, (C) 3g, and (D) Co.

For the 3d-complex, it was clear that GLU_166 and HIS_41 interactions were all over the simulation time. However, GLY_143 interactions were nearly absent before 10 ns and from 75 to 85 ns (). Moreover, the heat map of the 3e-complex showed that the binding interactions of GLU_166 extended throughout the whole time of the simulation. But, HIS_41 interactions started to be intense from 35 ns till the end of the simulation time (). Furthermore, the 3g-complex heat map showed that GLU_166 interactions were intense from the beginning till the end of the simulation time. Besides, the binding interactions of GLY_143 started to be more intense from 20 ns till the end of the simulation time (). Finally, for the Co-complex histogram, it was obvious that both GLN_189 and GLU_166 contributed to the interactions from the beginning till the end of the simulation time ().
MM-GBSA calculations
The average MM-GBSA binding energiesCitation60 for all the studied complexes were calculated using the thermal_mmgbsa.py python script of SchrodingerCitation48. represents the ΔG binding, hydrogen-bonding, lipophilic, covalent binding, Coulomb, van der Waals, and the generalised Born electrostatic solvation energies for all complexes.
Table 4. Prime MM-GBSA energies for complexes (3a, 3b, 3c, 3d, 3e, 3f, 3g, and Co) of SARS-CoV-2 Mpro protein.
Based on the introduced results in , we can observe that both compounds 3d and 3e achieved superior ΔG binding energies (–56.97 and −58.73 kcal/mol, respectively) compared to that of Co inhibitor (–55.25 kcal/mol). This indicates better stability for 3d and 3e within the binding pocket of SARS-CoV-2 Mpro compared to its Co inhibitor. Moreover, compounds 3b, 3d, and 3g showed superior Coulomb energies (–16.59, −13.63, and −15.55 kcal/mol, respectively) compared to that of the Co inhibitor (–11.77 kcal/mol). The best covalent energies were recorded for 3d, 3f, and 3g (4.44, 3.36, and 4.60 kcal/mol) compared to that of the Co inhibitor (1.59 kcal/mol). Notably, all compounds (3b–3g) showed better hydrogen-bonding energies with respect to that of the Co inhibitor. Besides, the bind packaging energies for compounds 3b, 3d, 3f, and 3g (–1.50, −1.54, −2.26, and −2.45 kcal/mol, respectively) exceeded that of the Co inhibitor (–1.48 kcal/mol) as well. However, only compound 3g achieved the superior generalised Born electrostatic solvation energy (23.92 kcal/mol) compared to the Co inhibitor (23.25 kcal/mol). Finally, the best van der Waals energy was recorded for compound 3e (–50.94 kcal/mol) compared to the Co inhibitor (–50.81 kcal/mol). Therefore, these findings confirm the superior affinities and subsequent intrinsic activities of the examined compounds (especially 3d, 3e, and 3g) towards the binding pocket of SARS-CoV-2 Mpro.
Structure–activity relationship (SAR) study of the examined compounds loaded EMLs (F3a–g)
The N-(5-nitrothiazol-2-yl) carboxamide scaffold was kept unchanged in all examined compounds. We mainly focussed on studying the influence of altering the size, type, and flexibility of the α-substituent to the carboxamide in addition to compound contraction on SARS-CoV-2 activity. Based on both the in vitro () and the in silico results ( and and ), the following SAR conclusions can be made (summarised in ).
Figure 14. SAR summary for the synthesised N-(5-nitrothiazol-2-yl)-carboxamido derivatives as potential inhibitors of SARS-CoV-2 Mpro.

Effect of α-substituent size
An increase in the size of substituent probed at the α-position to the N-(5-nitrothiazol-2-yl)-carboxamide scaffold was generally accompanied by a boost in the anti-SARS-CoV-2 activity. When compared to the unsubstituted compound 3b (IC50 = 116.8 μg/mL), substitution with an aliphatic α-methyl group (compound 3c) led to an almost twofold increase in SARS-CoV-2 inhibitory activity (IC50 = 63.13 μg/mL). Interestingly, a 77-fold drop in IC50 was observed upon substitution with the aliphatic but bulkier α-isopropyl group (compound 3d) with an IC50 reaching 1.51 μg/mL. The use of bulkier aromatic α-substituents maintained such a boost in SARS-CoV-2 inhibitory activity where compound 3g with methylene-(1H-indole-3-yl) group exhibited an IC50 of 1.56 μg/mL while employing a benzyl substituent in compound 3e granted the best anti-SARS-CoV-2 activity among all examined candidates (IC50 = 0.73 μg/mL). Furthermore, enlarging the α-substituent size was found to induce better selectivity profiles for compounds 3d, 3e, and 3g (selectivity indices; 5.61, 18, and 37, respectively). On the contrary, compounds 3b and 3c possessed higher IC50 values than their corresponding CC50 values, further thwarting their applicability as anti-SARS-CoV-2 agents.
Effect of α-chain cyclisation
Chain cyclisation is one of the most commonly adopted drug design strategies as it may enhance binding affinity and/or stabilise target binding patternsCitation61. Herein, we investigated the effect of cyclising the benzyl carbamate NH with the α-carbon via a propyl spacer forming the conformationally restricted pyrrolidine moiety (compound 3f). Unfortunately, such a rigidification did not improve the anti-SARS-CoV-2 activity where 3f demonstrated an IC50 of 6.22 μg/mL which is almost fourfold higher than compound 3d having analogous side chain carbons. This could be attributed to a cyclisation-induced twist in the main scaffold that led to the deviation of compound 3f away from key residues in the CYS-HIS dyad of the SARS-CoV-2 Mpro active site. Moreover, the capability to form an H-bond with the crucial amino acid GLU_166 was lost by cyclisation (supplementary material, Figure S2), further contributing to the observed diminished activity of compound 3f.
Effect of compound contraction
The effect of removing the benzyl carbamate moiety and directly acylating the aminothiazole with a tolyl ring was explored (compound 3a) as well. Intriguingly, a good SARS-CoV-2 inhibitory potency was achieved with an IC50 of 2.87 μg/mL. One plausible justification for this is that the essential H-bond with CYS_145 was maintained (supplementary material, Figure S3). In addition, compound extension to reach border residues such as GLN_192, ALA_191, and HIS_41 is seemingly not crucial for anti-SARS-CoV-2 activity as the contraction in compound 3a was quite tolerated.
Conclusions
The implemented experimental design declared that formula (F6) was an optimum formula, thus its composition (100 mg of the lipid core, 25 mg of PC, and 40 mg of Brij52) was involved in the fabrication of all compounds loaded EMLs. EMLs of N-(5-nitrothiazol-2-yl)-carboxamido derivatives (F3a–g) showed superior anti-SARS-CoV-2 activities compared to the previous unformulated ones. The IC50 values were recorded to be 2.87 (F3a), 1.51 (F3d), 0.73 (F3e), 6.22 (F3f), and 1.56 μg/mL (F3g). Obviously, the formulae of compounds 3d, 3e, and 3g showed superior IC50 values indicating their potential as promising anti-SARS-CoV-2 drug delivery panels. Besides, the mode of action for the most potent formula (F3e) showed that it exhibited a combination of virucidal (>90%), viral adsorption (>80%), and viral replication (>60%) inhibition. Furthermore, molecular docking studies clarified that the most active candidates (3d, 3e, and 3g) kept the interaction with the crucial amino acid of the SARS-CoV-2 Mpro target receptor (GLU_166). Also, the histogram analysis clarified the great importance of GLU_166 in the binding pocket of SARS-CoV-2 Mpro. Therefore, we can justify the recommended antagonistic effect of the studied compounds, which explains their superior anti-SARS-CoV-2 biological effects. Furthermore, the ligand RMSD described that both 3d and 3e candidates exhibited stable behaviours within the binding pocket of SARS-CoV-2 Mpro during the 200 ns of the simulation time. Moreover, the MM-GBSA calculations showed that both 3d and 3e achieved superior ΔG binding energies (–56.97 and −58.73 kcal/mol, respectively) compared to that of the Co inhibitor (–55.25 kcal/mol). Finally, the SAR analysis clarified that increasing the size of α-substituent to the carboxamide boosts both anti-SARS-CoV-2 activity and selectivity with benzyl substitution being optimum (compound 3e). Finally, compound 3e-loaded EMLs (F3e) could be proposed as a reliable system with boosted anti-SARS-CoV-2 activity.
Author contributions
Conceptualisation: A.A.A-K; formal analysis: A.A.A-K., D.S.E-G., R.E-S., M.S., R.A-n., O.K., Y.M., M.E-a., S.T.A-R., F.A.B., W.M.E-d., A.M.N., and M.Y.Z.; funding acquisition: S.T.A-R., F.A.B., and W.M.E-d.; methodology: A.A.A-K., D.S.E-G., R.E-S., M.S., R.A-N., M.E-A., O.K., Y.M., A.M.N., and M.Y.Z.; project administration: A.A.A-K. and M.Y.Z.; software: A.A.A-K., R.E-S., M.S., R.A-N., and M.Y.Z.; supervision: A.A.A-K.; validation: A.A.A-K., M.S., R.A-N., and M.Y.Z.; writing – original draft: A.A.A-K., D.S.E-G., R.E-S., M.S., R.A-n., W.M.E-d., A.M.N., and M.Y.Z.; writing – review and editing: all authors revised and approved the final version of the manuscript.
Supplemental Material
Download PDF (500.4 KB)Acknowledgements
The authors acknowledge financial support from the Researchers Supporting Project number (RSP-2023/103), King Saud University, Riyadh, Saudi Arabia. Also, the authors acknowledge the HPC, University of Cape Town, for using supercomputing facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Patel KP, Vunnam SR, Patel PA, Krill KL, Korbitz PM, Gallagher JP, Suh JE, Vunnam RR. Transmission of SARS-CoV-2: an update of current literature. Eur J Clin Microbiol Infect Dis. 2020;39(11):2005–2011.
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389.
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407.
- Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19(11):685–700.
- Fehr AR, Perlman SJC. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1–23.
- Abd-Alla HI, Kutkat O, Sweelam H-tM, Eldehna WM, Mostafa MA, Ibrahim MT, Moatasim Y, GabAllah M, Al-Karmalawy AA. Investigating the potential anti-SARS-CoV-2 and anti-MERS-CoV activities of yellow necklacepod among three selected medicinal plants: extraction, isolation, identification, in vitro, modes of action, and molecular docking studies. Metabolites. 2022;12(11):1109.
- Menendez JC. Approaches to the potential therapy of COVID-19: a general overview from the medicinal chemistry perspective. Molecules. 2022;27(3):658.
- Elmaaty AA, Eldehna WM, Khattab M, Kutkat O, Alnajjar R, El-Taweel AN, Al-Rashood ST, Abourehab MAS, Binjubair FA, Saleh MA, et al. Anticoagulants as potential SARS-CoV-2 Mpro inhibitors for COVID-19 patients: in vitro, molecular docking, molecular dynamics, DFT, and SAR studies. Int J Mol Sci. 2022;23(20):12235.
- Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347.
- Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593.
- Akinosoglou K, Schinas G, Gogos C. Oral antiviral treatment for COVID-19: a comprehensive review on nirmatrelvir/ritonavir. Viruses. 2022;14(11):2540.
- Hoffman RL, Kania RS, Brothers MA, Davies JF, Ferre RA, Gajiwala KS, He M, Hogan RJ, Kozminski K, Li LY, et al. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J Med Chem. 2020;63(21):12725–12747.
- Cooper CL, van Heeswijk RP, Gallicano K, Cameron DW. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin Infect Dis. 2003;36(12):1585–1592.
- Ashour NA, Elmaaty AA, Sarhan AA, Elkaeed EB, Moussa AM, Erfan IA, Al-Karmalawy AA. A systematic review of the global intervention for SARS-CoV-2 combating: from drugs repurposing to molnupiravir approval. Drug Des Devel Ther. 2022;16:685–715.
- Yan F, Gao F. An overview of potential inhibitors targeting non-structural proteins 3 (PL(pro) and Mac1) and 5 (3CL(pro)/M(pro)) of SARS-CoV-2. Comput Struct Biotechnol J. 2021;19:4868–4883.
- Jain R, Mujwar S. Repurposing metocurine as main protease inhibitor to develop novel antiviral therapy for COVID-19. Struct Chem. 2020;31(6):2487–2499.
- Dai W, Zhang B, Jiang XM, Su H, Li J, Zhao Y, Xie X, Jin Z, Peng J, Liu F, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335.
- Aljuhani A, Ahmed HEA, Ihmaid SK, Omar AM, Althagfan SS, Alahmadi YM, Ahmad I, Patel H, Ahmed S, Almikhlafi MA, et al. In vitro and computational investigations of novel synthetic carboxamide-linked pyridopyrrolopyrimidines with potent activity as SARS-CoV-2-MPro inhibitors. RSC Adv. 2022;12(41):26895–26907.
- El-Masry RM, Al-Karmalawy AA, Alnajjar R, Mahmoud SH, Mostafa A, Kadry HH, Abou-Seri SM, Taher AT. Newly synthesized series of oxoindole–oxadiazole conjugates as potential anti-SARS-CoV-2 agents: in silico and in vitro studies. New J Chem. 2022;46(11):5078–5090.
- Mengist HM, Dilnessa T, Jin T. Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. Front Chem. 2021;9:622898.
- Sabbah DA, Hajjo R, Bardaweel SK, Zhong HA. An updated review on SARS-CoV-2 main proteinase (M(Pro)): protein structure and small-molecule inhibitors. Curr Top Med Chem. 2021;21(6):442–460.
- Zhu G, Zhu C, Zhu Y, Sun F. Minireview of progress in the structural study of SARS-CoV-2 proteins. Curr Res Microb Sci. 2020;1:53–61.
- Zheng L, Zhang L, Huang J, Nandakumar KS, Liu S, Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur J Med Chem. 2020;205:112687.
- Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368(6489):409–412.
- Shie JJ, Fang JM, Kuo CJ, Kuo TH, Liang PH, Huang HJ, Yang WB, Lin CH, Chen JL, Wu YT, et al. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J Med Chem. 2005;48(13):4469–4473.
- Gilbert NC, Gerstmeier J, Schexnaydre EE, Börner F, Garscha U, Neau DB, Werz O, Newcomer ME. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat Chem Biol. 2020;16(7):783–790.
- Orhan IE, Senol Deniz FS. Natural products as potential leads against coronaviruses: could they be encouraging structural models against SARS-CoV-2? Nat Prod Bioprospect. 2020;10(4):171–186.
- Razali R, Asis H, Budiman C. Structure–function characteristics of SARS-CoV-2 proteases and their potential inhibitors from microbial sources. Microorganisms. 2021;9(12):2481.
- Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5(9):785–799.
- Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9(9):690–701.
- Elagawany M, Elmaaty AA, Mostafa A, Abo Shama NM, Santali EY, Elgendy B, Al-Karmalawy AA. Ligand-based design, synthesis, computational insights, and in vitro studies of novel N-(5-nitrothiazol-2-yl)-carboxamido derivatives as potent inhibitors of SARS-CoV-2 main protease. J Enzyme Inhib Med Chem. 2022;37(1):2112–2132.
- Gregoriadis G. Liposomes in drug delivery: how it all happened. Pharmaceutics. 2016;8(2):19.
- Aldawsari HM, Ahmed OA, Alhakamy NA, Neamatallah T, Fahmy UA, Badr-Eldin SM. Lipidic nano-sized emulsomes potentiates the cytotoxic and apoptotic effects of raloxifene hydrochloride in MCF-7 human breast cancer cells: factorial analysis and in vitro anti-tumor activity assessment. Pharmaceutics. 2021;13(6):783.
- Hegazy H, Amin MM, Fayad W, Zakaria MY. TPGS surface modified bilosomes as boosting cytotoxic oral delivery systems of curcumin against doxorubicin resistant MCF-7 breast cancer cells. Int J Pharm. 2022;619:121717.
- Dubey S, Vyas S. Emulsomes for lipophilic anticancer drug delivery: development, optimization and in vitro drug release kinetic study. Int J App Pharm. 2021;13:114–121.
- Zakaria MY, Georghiou PE, Banoub JH, Beshay BY. Inclusion of a phytomedicinal flavonoid in biocompatible surface-modified chylomicron mimic nanovesicles with improved oral bioavailability and virucidal activity: molecular modeling and pharmacodynamic studies. Pharmaceutics. 2022;14(5):905.
- Abdelbary GA, Amin MM, Zakaria MY. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017;24(1):309–319.
- Abd El-Halim SM, Abdelbary GA, Amin MM, Zakaria MY, Shamsel-Din HA, Ibrahim AB. Stabilized oral nanostructured lipid carriers of adefovir dipivoxil as a potential liver targeting: estimation of liver function panel and uptake following intravenous injection of radioiodinated indicator. Daru. 2020;28(2):517–532.
- Anwer KE, El-Sattar NEA, Shamaa MM, Zakaria MY, Beshay BY. Design, green synthesis and tailoring of vitamin E TPGS augmented niosomal nano-carrier of pyrazolopyrimidines as potential anti-liver and breast cancer agents with accentuated oral bioavailability. Pharmaceuticals. 2022;15(3):330.
- Mahmoud DB, Bakr MM, Al-Karmalawy AA, Moatasim Y, El Taweel A, Mostafa A. Scrutinizing the feasibility of nonionic surfactants to form isotropic bicelles of curcumin: a potential antiviral candidate against COVID-19. AAPS PharmSciTech. 2022;23(1):1–12.
- Marques NP, Lopes CS, Marques NCT, Cosme-Silva L, Oliveira TM, Duque C, Sakai VT, Hanemann JAC. A preliminary comparison between the effects of red and infrared laser irradiation on viability and proliferation of SHED. Lasers Med Sci. 2019;34(3):465–471.
- Chemical Computing Group Inc. Molecular operating environment (MOE). Montreal (Canada): Chemical Computing Group Inc.; 2016. p. 1010.
- Al-Karmalawy AA, Farid MM, Mostafa A, Ragheb AY, Mahmoud SH, Shehata M, Shama NMA, GabAllah M, Mostafa-Hedeab G, Marzouk MM. Naturally available flavonoid aglycones as potential antiviral drug candidates against SARS-CoV-2. Molecules. 2021;26(21):6559.
- Khattab M, Al-Karmalawy AA. Computational repurposing of benzimidazole anthelmintic drugs as potential colchicine binding site inhibitors. Future Med Chem. 2021;13(19):1623–1638.
- Elebeedy D, Badawy I, Elmaaty AA, Saleh MM, Kandeil A, Ghanem A, Kutkat O, Alnajjar R, Abd El Maksoud AI, Al-Karmalawy AA. In vitro and computational insights revealing the potential inhibitory effect of tanshinone IIA against influenza A virus. Comput Biol Med. 2022;141:105149.
- Elmaaty AA, Darwish KM, Chrouda A, Boseila AA, Tantawy MA, Elhady SS, Shaik AB, Mustafa M, Al-Karmalawy AA. In silico and in vitro studies for benzimidazole anthelmintics repurposing as VEGFR-2 antagonists: novel mebendazole-loaded mixed micelles with enhanced dissolution and anticancer activity. ACS Omega. 2022;7(1):875–899.
- Schrödinger Release. 3: Desmond molecular dynamics system, DE Shaw research, New York, NY, 2017. New York (NY): Maestro-Desmond Interoperability Tools, Schrödinger; 2017.
- Maestro-Desmond Interoperability Tools. New York (NY): Schrödinger; 2017.
- Farouk F, Elmaaty AA, Elkamhawy A, Tawfik HO, Alnajjar R, Abourehab MAS, Saleh MA, Eldehna WM, Al‐Karmalawy AA. Investigating the potential anticancer activities of antibiotics as topoisomerase II inhibitors and DNA intercalators: in vitro, molecular docking, molecular dynamics, and SAR studies. J Enzyme Inhib Med Chem. 2023;38(1):2171029.
- Zakaria MY, Zaki I, Alhomrani M, Alamri AS, Abdulaziz O, Abourehab MA. Boosting the anti MERS-CoV activity and oral bioavailability of resveratrol via PEG-stabilized emulsomal nano-carrier: factorial design, in-vitro and in-vivo assessments. Drug Deliv. 2022;29(1):3155–3167.
- Albash R, Yousry C, Al-Mahallawi AM, Alaa-Eldin AA. Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: in-vitro characterization, statistical optimization, and in-vivo assessment. Drug Deliv. 2021;28(1):1–9.
- El Zaafarany GM, Awad GA, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397(1–2):164–172.
- Stetefeld J, McKenna SA, Patel TR. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev. 2016;8(4):409–427.
- Zeb A, Qureshi OS, Kim H-S, Cha J-H, Kim H-S, Kim J-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int J Nanomedicine. 2016;11:3813–3824.
- Deng P, Felemban A, Masoud RE, Alamoudi WM, Zakaria MY. Employment of PEGylated ultra-deformable transferosomes for transdermal delivery of tapentadol with boosted bioavailability and analgesic activity in post-surgical pain. Int J Pharm. 2022;628:122274.
- Zakaria MY, Abd El-Halim SM, Beshay BY, Zaki I, Abourehab MA. ‘Poly phenolic phytoceutical loaded nano-bilosomes for enhanced caco-2 cell permeability and SARS-CoV 2 antiviral activity’: in-vitro and in-silico studies. Drug Deliv. 2023;30(1):2162157.
- Muthu MS, Kulkarni SA, Xiong J, Feng S-S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int J Pharm. 2011;421(2):332–340.
- Zakaria MY, Fayad E, Althobaiti F, Zaki I, Abu Almaaty AH. Statistical optimization of bile salt deployed nanovesicles as a potential platform for oral delivery of piperine: accentuated antiviral and anti-inflammatory activity in MERS-CoV challenged mice. Drug Deliv. 2021;28(1):1150–1165.
- Alemi A, Zavar Reza J, Haghiralsadat F, Zarei Jaliani H, Haghi Karamallah M, Hosseini SA, Haghi Karamallah S. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J Nanobiotechnol. 2018;16(1):1–20.
- Elkamhawy A, Son S, Lee HY, El-Maghrabey MH, Hamd MAE, Alshammari SO, Abdelhameed AA, Alshammari QA, Abdeen A, Ibrahim SF, et al. Design, synthesis, biological evaluation, and molecular dynamics studies of novel lapatinib derivatives. Pharmaceuticals. 2022;16(1):43.
- Tang K, Wang S, Gao W, Song Y, Yu B. Harnessing the cyclization strategy for new drug discovery. Acta Pharm Sin B. 2022;12(12):4309–4326.