Abstract
In this study, based on the effect of compounds on the activation of NF-κB and NO release, compound 51 was discovered as the best one with NO release inhibition IC50 value was 3.1 ± 1.1 μM and NF-κB activity inhibition IC50 value was 172.2 ± 11.4 nM. Compound 51 could inhibit the activation of NF-κB through suppressing phosphorylation and nuclear translocation of NF-κB, and suppress LPS-induced inflammatory response in RAW264.7 cells, such as the over-expression of TNF-α and IL-6, which were target genes of NF-κB. This compound also showed preferable anti-inflammatory activity in vivo, including alleviating significantly gastric distention and splenomegaly caused by LPS stimulation, reducing the level of oxidative stress induced by LPS, and inhibiting the expression of IL-6 and TNF-α in serum. Thus, it’s reasonable to consider that this compound is a promising small molecule with anti-inflammatory effect for inhibiting the NF-κB signalling pathway.
Introduction
Inflammation is a series of complex defense-related response caused by various injury factors, with red, swelling, heat, pain as the main characteristicsCitation1. The body’s inflammatory response causes cellular changes and immune responses that result in repair of the damaged tissue and cellular proliferation at the injured sitesCitation2. When inflammation becomes chronic or lasts too long, it may be harmful and lead to disease, even creates an environment that is suitable for the development of various of cancersCitation3. Various signalling pathways are key contributors in causing epigenetic changes, and switching on these internal mutationsCitation4. Therefore, treating the inflammatory causes is always important.
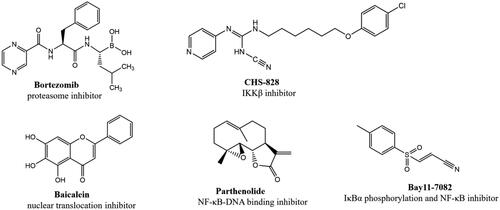
Nuclear factor-κB (NF-κB) is a nuclear transcription factor that plays an important role in cell growth, proliferation, differentiation, apoptosis and carcinogenesisCitation5–8. The NF-κB pathway has long been considered as a typical inflammatory signalling pathway, it regulates proinflammatory cytokine production, leukocyte recruitment, or cell survival, which are important contributors to the inflammatory responseCitation9,Citation10. It’s reported that the activation of NF-κB will induce expression of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), and interleukin-6 (IL-6), which lead to inflammatory diseases, including arthritisCitation11, psoriasisCitation12, chronic obstructive pulmonary disease (COPD)Citation13 and inflammatory bowel disease (IBD)Citation14. Thus, inhibiting the activation of NF-κB is a promising approach to alleviate inflammation. Currently, several compounds have been identified as NF-κB inhibitorsCitation15–18 ().
It has been reported that LPS induced the activation of NF-κB, promoting the release of proinflammatory cytokines, such as NO, IL-6 and TNF-αCitation19. Excessive NO can induce the development of inflammatory diseasesCitation20,Citation21. Therefore, inhibiting the excessive secretion of NO effectively is one of the important measures to control the inflammatory response. In this study, we used LPS-stimulated RAW264.7 cell model to measure the effect of compounds on NO release, and a dual-luciferase reporter assay to detect the effect of compounds on NF-κB activity in HEK293T cells, which is a cell line with high efficiency of transfection. Through preliminary evaluation of anti-inflammatory activity, we found that compound 6 with the structural characteristics of polysubstituted pyridine in a library of more than 3000 compounds with structural diversity showed good activity and has the potential for modification. Through a series of structural optimisation and biological evaluation, compound 51 (NO release inhibition activity IC50=3.1 ± 1.1 μM, NF-κB transcriptional inhibition activity IC50=172.2 ± 11.4 nM) was selected as our title compound. Further studies showed that compound 51 could inhibit LPS-induced phosphorylation of NF-κB p65 and IκB in RAW264.7 cells. In HEK293T cells, compound 51 could block the nuclear translocation of p65 and p50, which induced by TNF-α. Above results indicated that compound 51 could inhibit the activation of NF-κB signal. We also verified the effect of compound 51 on target genes of NF-κB, such as TNF-α and IL-6, the results showed that compound 51 inhibited the expression of TNF-α and IL-6.The results of the in vitro and in vivo experiments showed that the compound is a promising anti-inflammatory compound.
Result and discussion
Synthesis of aryl amides (6–52)
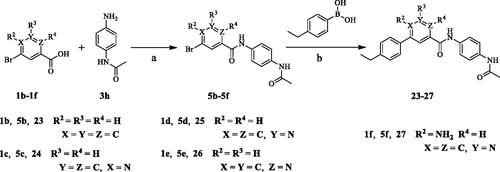
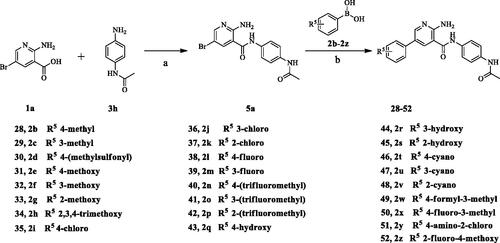
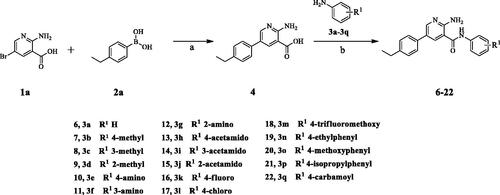
The target compounds (6–52) are synthesised according to the route shown in Schemes 1–3. The intermediate 4 can be obtained by Suzuki reactionCitation22,Citation23 between the key raw materials 2-amino-5-bromonetinic acid (1a) and (4-ethylphenyl)boronic acid(2a). The title compound 6–22 are prepared by amide condensation reaction between different substituted aniline (3a–3q) and carboxylic acid intermediate 4. The preparation of intermediates (5a–5f) and target compounds (23–27, 28–52) followed the same reaction conditions.
Scheme 1. Synthesis of aryl amide (6–22). Reagents and conditions: (a) 1a (1.0 eq.), 2a (1.0 eq.), K3PO4 (1.5 eq.), Pd(PPh3)4 (0.05 eq.), 80 °C, 1,4-dioxane/H2O (3:1), Ar2 atmosphere, 12 h; (b) 5 (1.0 eq.), aniline (1.1 eq.), DIPEA (4.0 eq.), HATU (1.2 eq.), room temperature, DCM, 18 h.
Anti-inflammatory activity and SAR study
Through screening the in-house compound library, compound 6 was found to have effective anti-inflammatory activity, moderate inhibition on LPS-induced NO release, but with undesirable inhibition effect on NF-κB transcriptional activity(NO release inhibition IC50=19.7 ± 2.6 μM, NF-κB activity inhibition IC50=1619.7 ± 13.2 nM). Therefore, we identified compound 6 as the HIT compound in this study. In order to obtain more excellent lead compound with anti-inflammatory activity, three rounds of structural modification of compound 6 were successfully implemented.
As listed in , we first considered to replace the group on the benzene ring at C3 position of pyridine core to observe the change of anti-inflammatory activity. The introduction of methyl (7–9) did not change the activity (NO IC50 = 15.2–30.2 μM), but the introduction of amino (10–12) significantly improved the inhibitions of NO release (NO IC50 = 0.9–2.3 μM) and NF-κB reporter activity (NF-κB reporter IC50 = 95.8–570.9 nM), in which the inhibition of NO release was increased by 10–20 folds, and the inhibition of NF-κB reporter was increased by 3–16 folds. Unfortunately, these three compounds showed strong cytotoxicity, which may not be suitable as lead compounds for the development of anti-inflammatory drugs. Further, the amino groups were replaced with acetylamino groups to obtain compound 13–15. Similar to compounds 10–12, compounds 14 (NO IC50 = 3.2 μM, NF-κB reporter IC50 = 167.4 nM) and 15 (NO IC50 = 1.1 μM, NF-κB reporter IC50 = 532.5 nM) also showed strong anti-inflammatory activity and strong cytotoxicity. It is gratifying to note that the anti-inflammatory activity of compound 13 (NO IC50 = 10.2 μM, NF-κB IC50 = 713.9 nM) was not significantly improved as that of other compounds, but the cytotoxicity is reduced. Next, halogens, trifluoromethoxy, ethyl, methoxy, isopropyl and carbamoyl were introduced to the para position of benzene ring to obtain compounds 16–22. The introduction of halogens (16–17), trifluoromethoxy (18) and carbamoyl (22) led to the loss of anti-inflammatory activity of the compounds. The introduction of ethyl (19), methoxy (20) and isopropyl (21) did not cause the fluctuation of anti-inflammatory activity, which was consistent with the introduction of methyl (7). Considering the changes in activity and cytotoxicity, compound 13 was identified as the node compound for the next round of transformation, although its activity was not the best.
Table 1. Structure-activity relationship study at position 3 of the pyridine core.
Next, the 2-aminopyridine core was replaced to give compounds 23–27 to explore the importance of pyridine core. As listed in , any changes about the 2-aminopyridine led to decreased of inhibitions of NO release and NF-κB reporter activity (). Therefore, in the third round of transformation, we continued to keep the core of compound 13 unchanged.
Table 2. Structure-activity relationship study of the core.
Finally, we began to discuss the effect of substituents on the benzene ring at C5 position of pyridine core on the anti-inflammatory activity of compounds. As listed in , after replacing ethyl with methyl (28, 29) and methoxy (31–34), the anti-inflammatory activity decreased significantly. The introduction of chlorine atom on the para position of benzene ring significantly improved the anti-inflammatory activity of the compound (35 NO IC50 = 4.2 μM, NF-κB reporter IC50 = 206.5 nM), but the introduction of chlorine atoms on the meta and ortho positions led to the opposite result. Compounds 38 and 39 showed a similar trend. In addition, compounds 40–48 were obtained by introducing trifluoromethyl, hydroxyl and cyano groups at different positions of the benzene ring. Unfortunately, with the exception of compound 41 (NO IC50 = 8.4 μM, NF-κB reporter IC50 = 306.6 nM), none of these compounds exhibited effective anti-inflammatory activity. Finally, in order to explore the possibility of activity change, we obtained compounds 49–52 by introducing poly-substituted benzene rings. Surprisingly, compounds 50 (NO IC50 = 8.6 μM, NF-κB reporter IC50 = 577.1 nM) and 51 (NO IC50 = 3.1 μM, NF-κB reporter IC50 = 172.7 nM) showed superior anti-inflammatory activity, of which compound 51 was the best. Compared with HIT compound 6, its inhibition of NO release was increased by 6-folds, and the inhibition of NF-κB transcriptional activity was increased by nine-folds. In addition, the compound did not show obvious cytotoxicity. With comprehensive consideration, 51 was identified as the optimal one for further study.
Table 3. Structure-activity relationship study at position 5 of the pyridine core.
Compound 51 suppressed NF-κB signalling activation
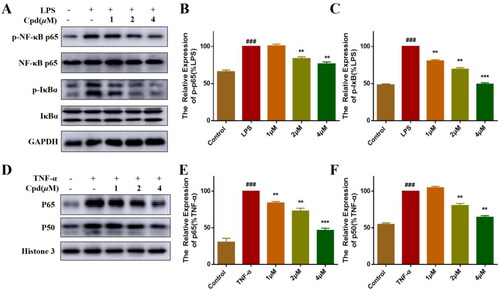
Under normal conditions, inactivated NF-κB is located in the cytoplasm and binds to IκB inhibitorsCitation24. Activation by inflammatory stimuli leads to the release and nuclear translocation of NF-κB, which shows as the breakdown and phosphorylation of the p65-IκBα complexCitation25. As shown in , LPS induced phosphorylation of p65 and IκBα protein, and after pre-treatment with different concentrations of compound 51, the LPS-induced phosphorylation of p65 and IκBα was suppressed significantly in a dose dependent manner. Studies reported that TNFα-activated NF-κB transcriptional activity is accompanied by the nuclear translocation of p65 and p50. Therefore, we tested the effects of compound 51 on TNFα-induced nuclear translocation of p65 and p50 in HEK293T cells by isolating the nuclear fraction. As the result shown, TNF-α induced the appearance of RELA (p65) and NFKB1 (p50) subunits of NF-κB in the nucleus, and this effect of TNF-α was inhibited by compound in a dose dependent manner ().
Figure 2. Compound 51 suppressed the activation of NF-κB. (A–C) Compound 51 inhibited the phosphorylation of P65 and IκBα. RAW264.7 cells were treated with compound 51 for different concentrations and then stimulated by LPS. The cell lysates were analysed using WB. ###p < 0.001 compared with control group. **p < 0.01, ***p < 0.001 compared to LPS group. (D–F) Compound 51 inhibited TNFα-induced nuclear translocation of p65 and p50. HEK293T were treated with compound 51 for different concentrations and then stimulated by TNF-α. The nuclear protein was analysed using WB. ###p < 0.001 compared with control group. **p < 0.01, ***p < 0.001 compared to TNF-α group.
Compound 51 suppressed activation of MAPK signalling pathway
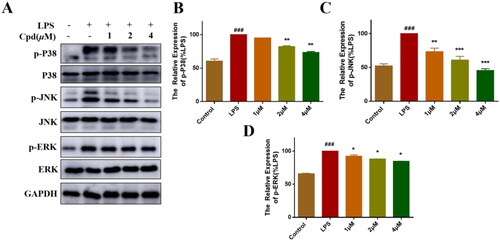
MAPKs signalling pathway also plays a key role in the regulation of inflammationCitation26. LPS-stimulation activate NF-κB/MAPKs signalling pathway, allowing the transcription factors AP-1 translocate into the nucleus and promoting the production of inflammatory mediatorsCitation27. Therefore, we measured the effects of compound 51 on LPS-regulated MAPK signalling activation by WB. As shown in , LPS increased the expression of phosphorylation of P38, JNK and ERK, after treated with compound 51, the phosphorylation level of MAPKs was significantly decreased.
Figure 3. Compound 51 inhibited the activation of MAPKs signalling. (A–D) Compound 51 inhibited the phosphorylation of MAPKs. RAW264.7 cells were treated with compound 51 for different concentrations and then stimulated by LPS. The cell lysates were analysed using WB. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group.
Compound 51 suppressed inflammatory response
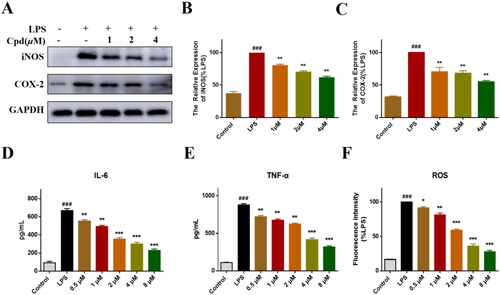
iNOS is activated in the inflammatory response, which catalyses NO productionCitation28. COX-2 is also an inflammatory mediator, regulates the activity of NF-κB and other transcription factors, then participates in a series of physiological and pathological processesCitation29. We found that the expression of iNOS and COX-2 was decreased significantly after treated with compound 51 (). Activation of NF-κB triggers transcription of various inflammatory factor genes, leading to the release of many inflammation factors such as TNF-α and IL-6, enhancing and amplifying the inflammatory responseCitation30,Citation31. We detected effect of compound 51 on TNF-α and IL-6. As expected, compound 51 decreased the levels of TNF-α and IL-6 significantly in a dose dependent manner (). In addition, LPS stimulation will enhance the production of ROS, accumulation of ROS also triggered a series of inflammatory responseCitation32. We found that compound could decrease the level of ROS obviously (), which indicated that compound can alleviate the oxidative stress response induced by LPS. Above results suggested compound 51 could suppress the inflammatory response which induced by NF-κB activation.
Figure 4. Compound 51 inhibited LPS-induced inflammatory response. (A–C) The expression of iNOS and COX-2. RAW264.7 cells were treated with compound 51 for 1 h, then stimulated by LPS for further 24 h. The samples were analysed by WB. (D). The level of IL-6. (E) The level of TNF-α. (F) The level of ROS. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group.
Compound 51 alleviated LPS-induced inflammation in vivo
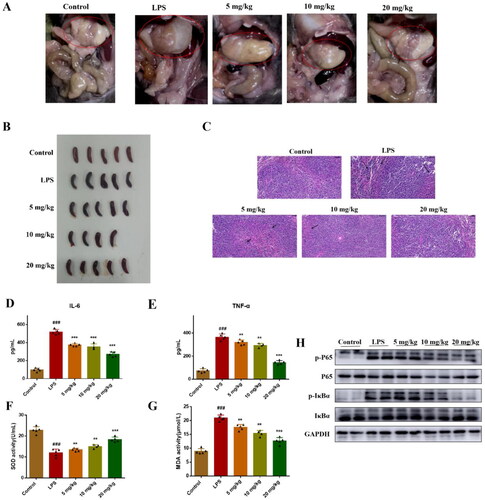
In vitro activity showed that compound 51 significantly inhibited TNF-α induced NF-κB transcriptional activity and LPS-induced inflammatory response. To further evaluate in vivo anti-inflammatory activity of compound 51, we established LPS-induced inflammation model of mice. We found that there were significant changes in organ tissues in mice treated with LPS, including gastric distention and splenomegaly (), and these symptoms were relieved after mice were treated with compound. The result of HE staining showed that LPS induced obvious injury of spleen, including broken of nuclear and numerous extramedullary haematopoietic cells. Compound 51 alleviated these injury in a dose-dependent manner (). In addition, after treated with 10 mg/kg LPS, the levels of IL-6 and TNF-α in blood of mice were increased, which suggested there is inflammatory response in mice. While IL-6 and TNF-α levels in mice treated with compound 51 were decreased obviously (). SOD and MDA were used as important indicators to evaluate the level of oxidative stress in antioxidant and oxidative capacity, respectively. We found that when compared with control group, SOD activity was decreased and MDA activity was increased in LPS group, compound 51 reversed their activity (). To confirm compound 51 exert anti-inflammatory effect in vivo through inhibiting NF-κB signalling pathway, we detected the expression of NF-κB in spleen. As shown in , compound 51 suppressed the activation of NF-κB in splenic organ. The results indicated that compound 51 could alleviate LPS-induced inflammation through inhibiting NF-κB signalling pathway in vivo.
Figure 5. Compound 51 suppressed LPS-induced inflammatory response in vivo. (A-B) The picture of mice organs. (C) HE staining of spleen tissues. Scan bar:50 μM. (D-E) The levels of IL-6 and TNF-α in blood. The samples were analysed by ELISA kits. (F–G) The SOD and MDA activity in blood. ###p < 0.001 compared with control group. *p < 0.1, **p < 0.01, ***p < 0.001 compared to LPS group. (H) The expression of NF-κB in splenic organ. The samples were analysed by WB.
Conclusion
The NF-κB pathway is implicated as a typical inflammatory signalling pathway, its activation leads to a series of inflammatory response. Thus, inhibiting NF-κB activation has long been considered as a promising approach to alleviate many inflammatory diseases. In this study, we synthesised a series of compounds, among them, compound 51 showed favourable NO release inhibition activity (IC50=3.1 μM) and inhibited NF-κB transcriptional activity strongly. Further studies showed that this compound suppressed LPS and TNF-α induced NF-κB activation, including the effect on phosphorylation of and nuclear translocation of NF-κB. In addition, title compound also exert inhibition effect on MAPKs signal, the expression of phosphorylation of P38, JNK and ERK was significantly decreased. NF-κB activation triggered the downstream inflammatory response, we found that compound 51 decrease the levels of IL-6, TNF-α and ROS, which indicated this compound could alleviate LPS and TNF-α induced inflammatory response through inhibiting NF-κB activation. In vivo study, we evaluated the anti-inflammatory activity of compound 51 using animal model of acute inflammation induced by LPS. LPS stimulation caused changes in organ tissues in vivo, compound reduced inflammatory symptoms, including gastric distention and splenomegaly. The result also showed that title compound decreased the levels of IL-6 and TNF-α in blood. In addition, it was confirmed that this compound could decrease ROS level in vitro, which indicated it could alleviate oxidative stress. So we also measured the activity of SOD and MDA in vivo, two key indicators of evaluate the level of oxidative stress. We found that treatment of compound increased SOD activity and decreased MDA activity. What’s more, title compound suppressed the activation of NF-κB signalling in spleen, which indicated that this compound inhibited LPS-induced inflammation through inhibiting NF-κB activity. Overall, we obtained a novel compound with favourable anti-inflammatory activity in vitro and in vivo, which is meaningful for the development of NF-κB inhibitors and anti-inflammatory drugs.
Experimental section (see supplementary data)
Statistical analysis
The results were expressed as the means ± SDs (SPSS software), and the differences between groups were evaluated using Turkey’s method (GraphPad Software). Differences between data were considered significant when the p values was <0.05.
Supplemental Material
Download PDF (13.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852.
- Kaplan AP, Joseph K, Shibayama Y, Nakazawa Y, Ghebrehiwet B, Reddigari S, Silverberg M. Bradykinin formation—plasma and tissue pathways and cellular interactions. Clin Rev Allergy Immunol. 1998;16(4):403–429.
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14(6):433–439.
- Kaplan AP, Joseph K, Silverberg M. Pathways for bradykinin formation and inflammatory disease. J Inflamm Res. 2022;15:3083–3094.
- Li QT, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734.
- Zhang YD, Ma CY, Liu CS, Wu W. NF-κB promotes osteoclast differentiation by overexpressing MITF via down regulating microRNA-1276 expression. Life Sci. 2020;258:118093.
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Transl Res. 2018;197:43–56.
- Chen TL, Zhang XD, Zhu GL, Liu HF, Chen JR, Wang Y, He XL. Quercetin inhibits TNF-a induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway in vitro. Medicine (Baltimore). 2020;99(38):e22241.
- Mitchell S, Vargas J, Hoffmann A. Signaling via the NF-κB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8(3):227–241.
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
- Ilchovska D, Barrow M. An overview of the NF-κB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-κB ligand RANKL and related nutritional interventions. Autoimmun Rev. 2021;20(2):102741.
- Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-κB: An essential transcription factor in psoriasis. J Dermatol Sci. 2013;69(2):89–94.
- Alharbi KS, Fuloria NK, Fuloria S, Rahman SB, Al-Malki WH, Javed Shaikh MA, Thangavelu L, Singh SK, Rama Raju Allam VS, Jha NK, et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem Biol Interact. 2021;345:109568.
- Dejban P, Nikravangolsefid N, Chamanara M, Dehpour A, Rashidian A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother Res. 2021;35(2):835–845.
- Fernández Y, Miller TP, Denoyelle C, Esteban JA, Tang W-H, Bengston AL, Soengas MS. Chemical Blockage of the Proteasome Inhibitory Function of Bortezomib. J Biol Chem. 2006;281(2):1107–1118.
- Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R, Zhu T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14:19.
- Cerna D, Li H, Flaherty S, Takebe N, Coleman CN, Yoo SS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) activity by small molecule GMX1778 regulates reactive oxygen species (ROS)-mediated cytotoxicity in a p53- and nicotinic acid phosphoribosyltransferase1 (NAPRT1)-dependent manner. J Biol Chem. 2012;287(26):22408–22417.
- Nakshatri H, Appaiah HN, Anjanapp M, Gilley D, Tanaka H, Badve S, Crooks PA, Mathews W, Sweeney C, Bhat-Nakshatri P. NF-κB-dependent and -independent epigenetic modulation using the novel anti-cancer agent DMAPT. Cell Death Dis. 2015;6(1):e1608.
- Welters ID, Fimiani C, Bilfinger TV, Stefano GB. NF-κB, nitric oxide and opiate signaling. Med Hypotheses. 2000;54(2):263–268.
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–487.
- Ghasemi M. Nitric oxide: antidepressant mechanisms and inflammation. Adv Pharmacol. 2019;86:121–152.
- Kanchana US, Diana EJ, Mathew TV, Anilkumar G. Cyclodextrin based palladium catalysts for Suzuki reaction. Carbohydr Res. 2020;489:107954.
- Kwiatkowski J, Liu B, Tee DHY, Chen G, Ahmad NHB, Wong YX, Poh ZY, Ang SH, Tan ESW, Ong EH, et al. Fragment-based drug discovery of potent protein kinase C iota inhibitors. J Med Chem. 2018;61(10):4386–4396.
- Karin M, Ben-Neria Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663.
- Valovka T, Hottiger MO. p65 controls NF-κB activity by regulating cellular localization of IκBβ. Biochem J. 2011;434(2):253–263.
- Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692.
- Hsia C-H, Jayakumar T, Lu W-J, Sheu J-R, Hsia C-W, Saravana Bhavan P, Manubolu M, Huang W-C, Chang Y. Auraptene, a monoterpene coumarin, inhibits LTA-induced inflammatory mediators via modulating NF-κB/MAPKs signaling pathways. Evid Based Complement Alternat Med. 2021;2021:5319584.
- Rapôso C, Luna R. L D A, Nunes AKS, Thomé R, Peixoto CA. Role of iNOS-NO-cGMP signaling in modulation of inflammatory and myelination processes. Brain Res Bull. 2014;104:60–73.
- Mingghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63(9):901–910.
- Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014;26(3):253–266.
- KobayashiNagino SM, Komatsu S, Naruse AK, Nimura Y, Nakanishi M, Sokabe M. Stretch-induced IL-6 secretion from endothelial cells requires NF-κB activation. Biochem Biophys Res Commun. 2003;308(2):306–312.
- Ma YF, Tang T, Sheng LL, Wang ZQ, Tao H, Zhang Q, Zhang Y, Qi ZL. Aloin suppresses lipopolysaccharide-induced inflammation by inhibiting JAK1-STAT1/3 activation and ROS production in RAW264.7 cells. Int J Mol Med. 2018;42(4):1925–1934.