Abstract
In this study, 21 new honokiol derivatives were synthesised, and their anti-cancer properties were investigated. Among these, compound 1g exhibited the most potent cytotoxic activity against human nasopharyngeal carcinoma CNE-2Z cells, human gastric cancer SGC7901 cells, human breast cancer MCF-7 cells, and mouse leydig testicular cancer I-10 lines with IC50 values of 6.04, 7.17, 6.83, and 5.30 μM, respectively. Compared to the parental compound, 1g displayed up to 5.18-fold enhancement of the cytotoxic effect on CNE-2Z cells. We further demonstrated that 1g inhibited cell growth, suppressed migration and invasion, and induced apoptosis of CNE-2Z cells by down-regulating HIF-1α, MMP-2, MMP-9, Bcl-2, Akt and up-regulating Bax protein levels. Transfection of CNE-2Z cells with HIF-1α siRNA reduced cell migration and invasion. In addition, in vivo experiments confirmed that 1g inhibited tumour growth in CNE-2Z cell-xenografted nude mice with low toxicity. Thus, our data suggested that 1g was a potent and safe lead compound for nasopharyngeal carcinoma therapy.
Introduction
Cancer is one of the leading causes of death, and it is a critical issue in the worldwide. The International Agency for Research on Cancer (IARC) estimated that there were 19.3 million new cancer cases and approximately 10 million cancer-related deaths in 2020.Citation1 In Southeast Asia, North Africa, and Southern China, nasopharyngeal carcinoma (NPC) is one of the common malignant tumours of the head and neck, which endangered human health.Citation2,Citation3 Although most patients with NPC respond well to radiotherapy/chemotherapy, with an improved 5-year survival rate of approximately 80%, local recurrence and distant metastasis are still primarily the causes of treatment failure and death associated with NPC.Citation4,Citation5 Therefore, it is necessary to discover new therapeutic targets and effective agents to prevent the invasion and metastasis of NPC.
Hypoxia is a common characteristic of most solid tumours, including NPC.Citation6 Hypoxia-inducible factor-1α (HIF-1α) is a potential therapeutic target for many types of cancer.Citation7,Citation8 Recently, several studies have provided convincing evidence for a strong correlation between increased HIF-1α levels and tumour metastasis, angiogenesis, poor patient prognosis, and cancer drug resistance.Citation9–12 In addition, meta-analysis demonstrated that HIF-1α could be an appropriate prognostic biomarker for NPC patients.Citation13
Natural products play an important role in drug discovery and research.Citation14,Citation15 Currently, approximately 80% of small molecule anti-cancer agents on the market are originate natural products or their modified derivatives.Citation16 Honokiol (HNK), a biphenyl neolignan extracted from Magnolia species, exhibited several pharmacological effects, including anti-cancer, anti-inflammatory, anti-oxidant, anti- infectious, and neuroprotective activities.Citation17,Citation18 These findings promote interest in the application of HNK and its analogs in the treatment of cancer, including anti-proliferation, induction of programmed cell death, suppression of invasion and metastasis, and inhibition of angiogenesis.Citation19–22 Many studies have demonstrated that the combination of HNK with other anti-cancer agents or ionising radiation could generate better therapeutic effects.Citation23–25 This evidence suggests that HNK could serve as a potential lead compound candidate for the further development and application of anti-cancer drugs.
Previous studies have reported that a series of HNK derivatives exhibited higher cytotoxic activity than HNK.Citation26–30 These studies indicated that free phenolic hydroxyl groups and hydrophobic side chains were necessary active groups for HNK to exert anti-cancer properties. Aminoguanidine is a functional moiety with high polarity and the capacity for hydrogen bonding with critical amino acid residues and metal ions.Citation31 Aminoguanidine derivatives (e.g. metformin) have been the focus of many studies because of their diverse pharmacolgical properties, including anti-cancer, anti-microbial, and anti-inflammatory effects.Citation32–35 In the present study, we modified the phenolic hydroxyl group of HNK and preserved the aminoguanidine moiety to obtain a series of novel HNK derivatives, and investigated the anti-NPC effects of these compounds in vitro and in vivo.
Results
Chemistry
As shown in Scheme 1, a series of HNK derivatives were designed and synthesised using honokiol as the starting compound. Compounds 1a–1c, 2a–2c, and 3a–3c were synthesised from HNK through Williamson alkylation of alkyl halides. Next, compounds 1d–3d and 1e–3e were synthesised via C-formylation using NaOH/tetrabutylammonium bromide (TBAB) in CHCl3 as the catalyst. The latter compounds 1d–3d and 1e–3e were further reacted with aminoguanidine bicarbonate in the presence of catalytic amounts of hydrochloric acid to provide 1f–3f and 1g–3g, respectively. The chemical structures of all compounds were characterised by 1H-NMR, 13C-NMR, and HR-ESI-MS. The detailed synthesis processes and an overview of the physical and analytical data were described in the experimental section (see Supplementary Materials).
Biological evaluation
Cytotoxic activity of HNK derivatives
All synthesised HNK derivatives were evaluated for their cytotoxic activities against the four cancer cell lines using the methyl thiazolyl tetrazolium (MTT) assay (). We observed that the O-alkylation of the substituted F atoms on the benzyl group of 2-OH or 4′-OH slightly improved the cytotoxic effect against cancer cells, whereas both 2-OH and 4′-OH alkylated derivatives were inactive at 50 μM. We further demonstrated that the HNK derivatives conjugated with aminoguanidine units exhibited better cytotoxicity than the parent compound. Among these, compounds 1e, 1f, 1g, 2f, 2g, 3e, 3f, and 3g showed excellent cytotoxicity against CNE-2Z, SG7901, MCF-7 and I-10 cells, with IC50 values of 5.30 to 20.14 μM. Compared to HNK, 1g displayed 5.18-, 4.02-, 6.12-, and 5.87-fold enhancement of the cytotoxic effects on CNE-2Z, SG7901, MCF-7, and I-10 cells, respectively. Taken together, 1g showed relatively low cytotoxic effect against normal mouse testicular leydig cells (TM3 cells), with IC50 values of 42.29 μM, indicating its selective inhibition (SI) to cancer cells (). The results indicated that 1g had the most potent in vitro cytotoxic activity against the four cancer cell lines with low toxicity.
Table 1. Anti-proliferative activities of compounds (72 h, IC50, μM).
Table 2. Cytotoxic activity of 1g on TM cells for 72 h (IC50, μM).
Anti-proliferative activity of 1g in CNE-2Z cells
To understand the in vitro anti-cancer effect of 1g in NPC, we analysed the anti-proliferative activity of 1g on CNE-2Z cells after treated with different concentrations (0, 1, 3, 6, 9, and 12 μM) for three time points (24, 48, and 72 h). As shown in , 1g significantly reduced the viability of CNE-2Z cells in a concentration- and time-dependent manner. Similarly, the colony-formation assay also revealed that 1g inhibited cell growth at low concentrations (0, 0.5, 1, and 2 μM) (P < 0.05) (). These results indicated that compound 1g exhibited potent anti-proliferative activity in CNE-2Z cells.
Figure 1. Inhibitory effect of 1g on cell viability in CNE-2Z cells. (a) Cytotoxic activity of 1g was measured using MTT assay. (b) Effects of 1g on the ability of cells to form colonies in CNE-2Z cells using colony-formation assay. (c) Quantification of the colony-formation assay. *P < 0.05 compared with the control.

1g suppressed migration and invasion of CNE-2Z cells
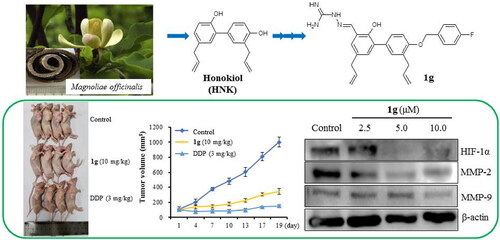
Transwell assays were performed to investigate the effects of 1g on the migration and invasion abilities of CNE-2Z cells (). As shown in , 1g treatments significantly reduced the number of cancer cells that crossed the membrane of the transwell chamber compared to the control (P < 0.05, P < 0.01). These results suggested that 1g inhibited the migration and invasion of CNE-2Z cells in a concentration-dependent manner. Western blotting data showed that treated with 1g significantly down-regulated the expression of HIF-1α, MMP-2, and MMP-9 ().
Figure 2. Effects of compound 1g on the migration and invasion of CNE-2Z cells by a transwell assays. (a) CNE-2Z cells were seeded into a transwell chamber and exposed to 1g (1.0, 3.0, and 6.0 μM) for 24 h to evaluate the migration and invasion activities. (b) Quantification analysis presented as the mean ± standard deviation. (c) Western blotting analyses of HIF-1α, MMP-2, and MMP-9 protein levels in CNE-2Z cells treated with various concentrations (2.5, 5.0, and 10.0 μM) of 1g. β-Actin was used as an internal control. *P < 0.05, **P < 0.01compared with the control.

Effect of HIF-1α knockdown on migration and invasion of CNE-2Z cells by siRNA
To characterise the mechanisms, we investigated the effect after HIF-1α knockdown in CNE-2Z cells with invasive and metastatic potential. As shown in , siRNA2107 can gradually down-regulated expression of HIF-1α, while the migration and invasion of CNE-2Z cells were significantly decreased after knockdown of HIF-1α. These results suggested that 1g suppressed migration and invasion of CNE-2Z cells, which may be mimicked by down-regulation of HIF-1α.
Figure 3. Knock-down of HIF-1α with siRNA protected against 1g inhibited migration and invasion in CNE-2Z cells. (a) The cells were transfected with HIF-1α siRNA, and whole cell lysates were subjected to western blotting analysis. (b) Transwell assay was used to detect cell migration and invasion after 24 h transfection with HIF-1α siRNA (original magnification 200×).

1g induced apoptosis in CNE-2Z cells
To clarify whether 1g had the ability to induce apoptosis in CNE-2Z cells, we stained the cells with Annexin V-FITC and PI and performed flow cytometry. After treatment with increasing concentration of 1g (2.5, 5, and 10 μM), the percentages of apoptotic cells increase to 12.12%, 12.07%, and 47.57%, respectively (). In addition, with an increase in 1g concentration, the expression of the pro-apoptotic protein Bax gradually increased, while that of the anti-apoptotic proteins Bcl-2 and Akt decreased (). These results indicated that 1g induced apoptosis in CNE-2Z cells.
Figure 4. Compound 1g induced cell death in human nasopharyngeal carcinoma CNE-2Z cells. (a) Flow cytometric analysis of cell death after treatment with various concentrations (2.5, 5.0, and 10.0 μM) of 1g using annexin V-FITC/PI dual staining. (b) Western blotting analysis of Bax, Bcl-2, and Akt protein levels.

In vivo anti-tumour efficacy of 1g
The in vivo anti-tumour efficacy of 1g was evaluated in nude mice bearing CNE-2Z xenograft tumours, and the mice were treated with 1g (10 mg/kg/3 days) for 19 days by intraperitoneal injection. DDP was used as a positive control. We observed that 1g significantly prevented tumour growth compared with the control (). As shown in showed potent inhibition of tumour growth and tumour volume to 343.59 mm3 compared with 1002.06 mm3 in the control group, accounting for a 65.71% decrease in tumour volume (P < 0.01). Compound 1g was well tolerated as it did not affect body weight loss (). As shown in , the enzymatic activity assay revealed that 1g had nearly no effect on the GPT and GOT levels in serum. In addition, H&E staining of the liver, kidneys, and lungs revealed no serious damage after treatment with 1g (). These results indicated that 1g may be a potent anti-NPC agent with low totoxicity.
Figure 5. Anti-tumour efficacy of 1g in CNE-2Z cell xenograft in nude mice (n = 4). (a) Representative tumours from each treatment group. (b) Tumour volume of each treatment group. (c) Body weight changes of nude mice. (d) The GPT and GOT of blood serum samples were determined by assay kit and the GPT and GOT activities are expressed as U/L. (e) H&E stained sections of the liver, kidney, lung, and tumour from the mice after treated with saline, DDP (3 mg/kg) and 1g (10 mg/kg).

Discussion
Several studies have reported that the four main treatments for NPC, including radiation, chemotherapy, molecule targeted therapy, and immune checkpoint therapy.Citation3,Citation36 However, the main challenges in NPC treatment include distant metastasis, chemo-resistance, and local recurrence.Citation5 Recently, HIF-1α has been recognised as a promising biomarker for NPC treatment.Citation13 Thus, small molecules HIF-1α inhibitors could show significant anti-tumour effects in NPC.
In our previous studies, we had found that HNK and its derivative exhibited potential inhibitory effect of HIF-1α and anti-tumour properties.Citation30,Citation37 Current literature on the anti-tumour properties and mechanisms of action of HNK derivatives has uncovered their potential in prevention and chemotherapy. In an attempt to improve its therapeutic profile, there has been an interest in obtaining novel HNK derivatives with enhanced anti-cancer activity, increased cell selectivity, and identification of the precise mechanisms of action in cell death.Citation28,Citation29 Based on the previous studies, we aimed to enhance the cytotoxic activity of the parent compound focusing on developing HNK derivatives containing aminoguanidine units and preliminarily assessing their mechanisms of action in CNE-2Z cells.
Highly electronegative and small fluorine atoms can play a significant role in medicinal chemistry and structural modification of natural products.Citation38 Fluorinated drug candidates can improve several physicochemical and pharmacokinetic properties, such as membrane permeation, metabolic stability, and binding affinity to target proteins.Citation39 In this study, we designed and synthesised a series of novel HNK derivatives based on fluorobenzyl-modified HNK conjugated with an aminoguanidine unit. As shown , the O-fluorobenzylation of 2-OH or 4′-OH might slightly improve cytotoxic effect against four cancer cell lines. Consistent with previous report, it was observed that O-fluorobenzylation of 4′-hydroxy group of HNK more than O-fluorobenzylation of 2-hydroxy group slightly improved the cytotoxic activity against four cancer cells.Citation40 Our results suggest that the 2-OH and 4′-OH of HNK were part of the pharmacophoric moiety in the inhibiting of tumour growth. However, the position of the fluorine atom had little effect on cytotoxicity in cancer cells. Among these, compound 1g, a novel HNK derivative conjugated with an aminoguanidine moiety, exhibited the highest cytotoxic activity against CNE-2Z, SGC7901, MCF-7, and I-10 cells with IC50 values of 6.04, 7.17, 6.83, and 5.30 μM, respectively.
The increased cytotoxic activity and cell sensitivity of compound 1g illustrated that HNK associated with the aminoguanidine moiety is an effective strategy for structural modification. During structural modifications, reducing the toxicity and side effects of compounds is as important as increasing their biological activity and sensitivity.Citation41 Relative to the strong cytotoxicity of compound 1g against CNE-2Z cells, it showed low toxicity in normal TM-3 cells, with an SI value of 7.0. Together, 1g also induced apoptosis in CNE-2Z cells by up-regulating of Bax and down-regulating of Bcl-2 and Akt protein expression. In addition, the in vivo experiment showed that 1g caused less damage to the liver, kidney, and lung tissues at therapeutic doses.
The underlying detailed mechanisms of the anti-cancer properties of 1g were explored. The invasion and metastasis of cell are the main reasons for the failure of NPC treatment.Citation5,Citation42 We observed that 1g significantly inhibited migration and invasion in CNE-2Z cells involving decreasing MMP-2, MMP-9, as well as HIF-1α protein levels. The key hypoxic regulator HIF-1α, has been shown to be highly expressed in NPC and was a promising involve in cancer cell survival, proliferation, apoptosis, invasion, metastasis etc.Citation13,Citation43,Citation44 To date, although many small molecule HIF-1α inhibitors have emerged, there are no clinically available HIF-1α inhibitors.Citation45–47 Transfection of CNE-2Z cells with HIF-1α siRNA resulted in reduced cell invasion and migration. In the present study, we synthesised a novel HIF-1α inhibitor from a natural source, compound 1g, which exhibited potent anti-NPC properties in vitro and in vivo.
Conclusions
Twenty-one novel HNK derivatives were synthesised and investigated for their cytotoxic activity. Among these HNK derivavites, 1g showed significant broad-spectrum and potent cytotoxic activity. Transwell assay revealed that 1g could prevent migration and invasion of CNE-2Z cells by down-regulating of HIF-1ɑ, MMP-2 and MMP-9. In addition, 1g also significantly induced apoptosis of CNE-2Z cells. Furthermore, a CNE-2Z xenograft model experiment demonstrated that 1g significantly inhibited tumour growth with low toxicity. Collectively, our findings suggest that 1g is a potential anti-NPC agent that warrants further investigation.
Experimental section
Reagents and instruments
HNK (>98% by HPLC) was purchased from Xinmingtai Chemical Co., Ltd (Hubei, China). All reagents and solvents were of analytical grade and were purchased from commercial sources. All NMR spectra of the compounds were measured using Bruker equipment (Fallanden, Switzerland). Mass spectra of the compounds were analysed using an Agilent 1290/6538 accurate Q-TOF mass spectrometer (Agilent Technologies, USA). The purity of all derivatives was determined by HPLC (Shimadzu, Tokyo, Japan) and found to be in the 96–99% range.
Synthesis of HNK derivatives 1a–3a, 1b–3c, and 1c–3c
HNK (2.1 mmol) was dissolved in dimethyl formamide (DMF solution, 5 ml), and then sodium carbonate solution (25%) was added at room temperature and stirred for another 0.5 h. The 4-fluorobenzyl-chloride (2.1 mmol) or 3-fluorobenzylchloride (2.1 mmol) or 5-fluorobenzylchloride (2.1 mmol) were slowly added to react and kept stirred for 3–6 h at 70–75 °C, and then detected by TLC analysis, respectively. The reaction was quenched with water, the pH was adjusted to weakly acidic with 1 M HCl and the aqueous layers were extracted twice with ethyl acetate. At the same time, the ethyl acetate layer was washed three times with saturated NaCl solution to wash away the residual DMF solvent. The combined extract was dried over Na2SO4 and concentrated under reduced pressure. Finally, the residue was purified by silica gel chromatography using petroleum ether: ethyl acetate = 100: 1 to 20: 1 to afford 1a–3a, 1b–3c, and 1c–3c, respectively.
2-((4-Fluorobenzyl)oxy)-honokiol (1a)
Yellow solid, yield: 18%; 1H-NMR (400 MHz, CDCl3): δ 7.45–7.38 (m, 2H), 7.28 − 7.25 (m, 2H), 7.11 − 7.01 (m, 4H), 6.97 (d, J = 9.0 Hz, 1H), 6.89 (d, J = 7.9 Hz, 1H), 6.06–5.91 (m, 2H), 5.20–5.09 (m, 2H), 5.07 (s, 2H), 5.06–5.02 (m, 2H), 3.47 (d, J = 6.2 Hz, 2H), 3.34 (d, J = 6.7 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 163.70, 155.98, 150.85, 137.83, 136.46, 132.87, 132.23, 130.76, 130.26, 130.10, 129.61, 129.05, 128.97, 128.85, 127.96, 127.78, 116.06, 115.65, 115.65, 115.61, 115.43, 112.26, 69.47, 39.46, 34.58. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + Na]+ 397.15744.
4'-((4-fluorobenzyl)oxy)-honokiol (2a)
Yellow solid, yield: 15%; 1H-NMR (400 MHz, CDCl3): δ 7.54 (t, J = 7.1 Hz, 1H), 7.36–7.25 (m, 3H), 7.18 (t, J = 7.5 Hz, 1H), 7.14–7.07 (m, 1H), 7.07–7.01 (m, 3H), 6.89 (d, J = 8.0 Hz, 1H), 6.07–5.92 (m, 2H), 5.19 (s, 2H), 5.13–5.01 (m, 4H), 3.48 (d, J = 6.7 Hz, 2H), 3.34 (d, J = 6.7 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 161.59, 155.90, 150.85, 137.81, 136.45, 132.19, 130.70, 130.23, 130.19, 129.66, 129.58, 129.38, 128.83, 127.98, 127.77, 124.28, 116.02, 115.61, 115.57, 115.45, 115.24, 112.27, 63.92, 39.44, 34.55. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + H]+ 375.17551.
4',2-Di-((4-fluorobenzyl)oxy)-honokiol (3a)
Yellow solid, yield: 13%; 1H-NMR (400 MHz, CDCl3): δ 7.38–7.32 (m, 1H), 7.28–7.15 (m, 4H), 7.06–6.98 (m, 3H), 6.95 (d, J = 8.1 Hz, 1H), 6.89 (d, J = 8.0 Hz, 1H), 6.08–5.91 (m, 2H), 5.14–5.03 (m, 4H), 5.11 (s, 2H), 3.50 (d, J = 6.8 Hz, 2H), 3.34 (d, J = 6.8 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 164.27, 155.84, 150.85, 139.78, 137.82, 136.42, 132.24, 130.82, 130.26, 130.21, 130.13, 129.72, 128.86, 127.97, 127.75, 122.42, 116.11, 115.65, 115.59, 114.90, 114.08, 112.22, 69.29, 39.45, 34.57. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + Na]+ 397.15738.
2-((2-Fluorobenzyl)oxy)-honokiol (1b)
Yellow solid, yield: 21%; 1H-NMR (400 MHz, CDCl3): δ 7.36–7.26 (m, 4H), 7.15 (d, J = 2.2 Hz, 1H), 7.07 (dd, J = 8.3, 2.3 Hz, 1H), 7.03–6.96 (m, 2H), 6.93 (d, J = 8.3 Hz, 1H), 6.84 (d, J = 8.1 Hz, 1H), 6.09–5.91 (m, 2H), 5.22–5.03 (m, 4H), 4.97 (s, 2H), 3.42 (d, J = 6.1 Hz, 2H), 3.37 (d, J = 6.8 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 163.48, 153.83, 153.25, 137.68, 136.37, 133.10, 133.08, 133.05, 131.74, 131.16, 131.04, 129.02, 128.83, 128.75, 128.00, 124.59, 116.56, 115.67, 115.45, 115.35, 115.14, 113.69, 70.17, 39.47, 35.27. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + Na]+ 397.15747.
4'-((2-Fluorobenzyl)oxy)-honokiol (2b)
Yellow solid, yield: 32%; 1H-NMR (600 MHz, CD3OD): δ 7.55 (td, J = 7.5, 1.8 Hz, 1H), 7.36–7.34 (m, 1H), 7.32 (d, J = 2.3 Hz, 1H), 7.19 (td, J = 7.5, 1.1 Hz, 1H), 7.15–7.11 (m, 1H), 7.03 (d, J = 8.4 Hz, 1H), 7.01 (d, J = 2.3 Hz, 1H), 6.92 (dd, J = 8.2, 2.3 Hz, 1H), 6.79 (d, J = 8.2 Hz, 1H), 6.03 − 5.92 (m, 2H), 5.17 (s, 2H), 5.08 − 4.95 (m, 4H), 3.42 (d, J = 1.6 Hz, 2H), 3.30 (d, J = 1.5 Hz, 2H). 13C-NMR (150 MHz, CD3OD): δ 161.44, 155.08, 152.03, 138.14, 136.97, 131.71, 131.14, 130.67, 130.20, 129.62, 129.52, 128.24, 127.93, 127.62, 124.56, 123.97, 115.54, 114.85, 114.71, 114.18, 114.01, 111.28, 63.72, 39.03, 34.18. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + H]+ 375.17554
4',2-Di-((4-fluorobenzyl)oxy)-honokiol (3b)
Yellow solid, yield: 27%; 1H-NMR (600 MHz, CD3OD): δ 7.38 (td, J = 8.0, 5.8 Hz, 1H), 7.35–7.33 (m, 2H), 7.27 (d, J = 7.6 Hz, 1H), 7.21 (ddd, J = 9.9, 2.6, 1.5 Hz, 1H), 7.05–7.01 (m, 1H), 7.01 (d, J = 2.4 Hz, 1H), 6.99–6.96 (m, 1H), 6.94–6.90 (m, 1H), 6.79 (d, J = 8.2 Hz, 1H), 6.06–5.91 (m, 2H), 5.13 (s, 2H), 5.11–4.95 (m, 4H), 3.46 (dt, J = 6.5, 1.6 Hz, 2H), 3.29 (dt, J = 6.5, 1.5 Hz, 2H). 13C- NMR (150 MHz, CD3OD): δ 163.80, 155.02, 152.03, 138.13, 136.99, 131.63, 131.14, 130.73, 130.20, 129.88, 129.82, 128.16, 128.04, 127.93, 127.62, 122.42, 115.54, 114.22, 114.01, 113.84, 113.48, 111.23, 68.81, 39.02, 34.23. HR-ESI-MS: molecular formula C25H23FO2 as revealed at m/z [M + Na]+ 397.15741.
2-((5-Fluorobenzyl)oxy)-honokiol (1c)
Yellow solid, yield: 24%; 1H-NMR (600 MHz, CD3OD): δ 7.49–7.45 (m, 2H), 7.44–7.40 (m, 1H), 7.32–7.26 (m, 4H), 7.12–7.06 (m, 4H), 7.03–6.97 (m, 3H), 6.00–5.90 (m, 2H), 5.08 (s, 2H), 5.08–4.95 (m, 4H), 4.95 (s, 2H), 3.39 (dt, J = 6.6, 1.5 Hz, 2H), 3.35 (dd, J = 6.7, 1.5 Hz, 2H). 13C-NMR (150 MHz, CD3OD): δ 161.72, 160.09, 153.78, 152.35, 136.33, 135.39, 132.11, 131.47, 129.70, 129.52, 128.91, 128.84, 127.53, 127.51, 127.47, 127.45, 126.53, 126.25, 113.56, 113.41, 113.31, 113.16, 113.13, 112.99, 112.80, 112.75, 112.19, 109.73, 68.36, 67.51, 37.50, 32.64. HR-ESI-MS: molecular formula C32H28F2O2 as revealed at m/z [M + Na]+ 505.19501.
4'-((5-Fluorobenzyl)oxy)-honokiol (2c)
Yellow solid, yield: 25%; 1H-NMR (600 MHz, CD3OD): δ 7.55 (td, J = 7.6, 1.8 Hz, 1H), 7.38–7.28 (m, 5H), 7.19 (td, J = 7.5, 1.1 Hz, 1H), 7.14 (ddd, J = 10.3, 8.3, 1.1 Hz, 1H), 7.11–7.08 (m, 3H), 7.08–7.04 (m, 2H), 7.02 (d, J = 8.4 Hz, 1H), 6.02–5.88 (m, 2H), 5.18 (s, 2H), 5.10–4.91 (m, 6H), 3.38–3.35 (m, 4H). 13C-NMR (150 MHz, CD3OD): δ 161.46, 159.64, 155.22, 153.78, 137.81, 136.82, 133.10, 131.33, 131.11, 131.03, 130.44, 129.63, 129.54, 129.48, 129.33, 127.99, 127.79, 124.42, 123.96, 123.85, 114.86, 114.71, 114.68, 114.54, 114.32, 114.23, 113.53, 111.25, 64.34, 63.72, 39.00, 34.09. HR-ESI-MS: molecular formula C32H28F2O2 as revealed at m/z [M + Na]+ 505.19516.
4',2-Di-((5-fluorobenzyl)oxy)-honokiol (3c)
Yellow solid, yield: 20%; 1H-NMR (600 MHz, CD3OD): δ 7.39 (td, J = 7.9, 5.9 Hz, 1H), 7.34 (d, J = 2.3 Hz, 1H), 7.32–7.26 (m, 3H), 7.24–7.19 (m, 1H), 7.12–7.06 (m, 3H), 7.05–6.95 (m, 5H), 6.01–5.93 (m, 2H), 5.15 (s, 2H), 5.09–4.95 (m, 6H), 3.46– 3.41 (dt, J = 6.7, 1.5 Hz, 2H), 3.35 (dt, J = 6.7, 1.5 Hz, 2H). 13C-NMR (150 MHz, CD3OD): δ 163.81, 162.06, 155.22, 153.75, 137.81, 136.81, 133.05, 131.28, 131.09, 130.43, 129.89, 129.70, 128.04, 127.88, 127.79, 122.46, 122.40, 114.36, 114.31, 114.01, 113.87, 113.84, 113.69, 113.55, 113.51, 113.39, 113.36, 111.22, 69.67, 68.86, 38.99, 34.10. HR-ESI-MS: molecular formula C32H28F2O2 as revealed at m/z [M + Na]+ 505.19507.
Synthesis of honokiol derivatives 1d–3d
Compounds 1a, 2a, and 3a were used as intermediates for the synthesis of 5′-formylation derivatives. In brief, the compounds and 25% NaOH/TBAB in CHCl3 were stirred for 2–3 h at 65 °C, and then detected by TLC analysis. When the reaction was complete, the mixture was neutralised with hydrochloric acid and extracted twice with ethyl acetate. The combined extract was dried over Na2SO4 and concentrated under reduced pressure. Finally, the residue was purified by silica gel chromatography using an eluent (petroleum ether: ethyl acetate = 120: 1 to 20: 1) to obtain 1d, 2d, and 3d, respectively.
2-((4-Fluorobenzyl)oxy)-5'-formy-honokiol (1d)
Yellow solid, yield: 33.5%; 1H-NMR (400 MHz, CDCl3): δ 11.30 (s, 1H), 9.84 (s, 1H), 7.37–7.29 (m, 5H), 7.25 (d, J = 2.2 Hz, 1H), 7.01 (t, J = 8.6 Hz, 2H), 6.89 (d, J = 8.4 Hz, 1H), 6.05–5.79 (m, 2H), 5.10–4.95 (m, 6H), 3.41 (d, J = 6.6 Hz, 2H), 3.34 (d, J = 6.7 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 196.81, 157.48, 157.33, 155.89, 138.18, 136.81, 136.75, 133.02, 132.09, 131.39, 130.90, 130.21, 128.99, 128.94, 128.91, 128.89, 128.23, 120.67, 117.84, 116.51, 115.72, 115.55, 115.34, 111.45, 69.43, 39.02, 34.56. HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M + H]+ 403.17087.
2-((2-Fluorobenzyl)oxy)-5'-formy-honokiol (2d)
Yellow solid, yield: 21.3%; 1H-NMR (400 MHz, CDCl3): δ 11.37 (s, 1H), 9.91 (s, 1H), 7.55 (t, J = 7.6 Hz, 1H), 7.45–7.40 (m, 3H), 7.35–7.29 (m, 2H), 7.18 (t, J = 7.4 Hz, 1H), 7.12–7.08 (m, 1H), 7.01 (d, J = 8.4 Hz, 1H), 6.10–5.93 (m, 2H), 5.20 (s, 2H), 5.15–5.04 (m, 4H), 3.50 (d, J = 7.0 Hz, 2H), 3.42 (d, J = 6.6 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 196.80, 161.54, 157.34, 155.80, 138.20, 136.82, 136.77, 132.08, 131.38, 130.87, 130.24, 129.52, 129.35, 129.01, 128.95, 128.26, 124.27, 120.67, 116.51, 115.71, 115.37, 115.16, 111.43, 63.88, 39.02, 34.57. HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M + H]+ 403.1718.
2-((5-Fluorobenzyl)oxy)-5'-formy-honokiol (3d)
Yellow solid, yield: 17.6%; 1H-NMR (400 MHz, CDCl3): δ 11.30 (s, 1H), 9.84 (s, 1H), 7.37–7.30 (m, 3H), 7.30–7.24 (m, 2H), 7.17–7.09 (m, 2H), 6.94 (td, J = 8.5, 2.6 Hz, 1H), 6.87 (d, J = 8.3 Hz, 1H), 6.05–5.83 (m, 2H), 5.10–4.96 (m, 6H), 3.44 (d, J = 6.6 Hz, 2H), 3.34 (d, J = 6.7 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 195.77, 160.85, 156.29, 154.71, 137.15, 135.77, 135.67, 131.07, 130.36, 129.92, 129.15, 129.09, 129.00, 128.00, 127.85, 127.21, 121.35, 119.63, 115.48, 114.75, 113.74, 113.53, 110.35, 68.20, 37.98, 33.53. HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M-H]- 401.1558.
Synthesis of honokiol derivatives 1e–3e
Compounds 1b, 2b, and 3b were used as intermediates for the synthesis of 3-formylation derivatives. In brief, the compounds and 25% NaOH/TBAB in CHCl3 were stirred for 3 h at 65 °C, and then detected by TLC analysis. When the reaction was complete, the mixture was neutralised with hydrochloric acid and extracted twice with ethyl acetate for twice. The combined extract was dried over Na2SO4 and concentrated under reduced pressure. Finally, the residue was purified by silica gel chromatography using an eluent (petroleum ether: ethyl acetate = 120: 1 to 20: 1) to obtain 1e, 2e, and 3e, respectively.
4'-((4-Fluorobenzyl)oxy)-3-formy-honokiol (1e)
Yellow solid, yield: 27%; 1H-NMR (600 MHz, CD3OD): δ 9.94–9.90 (m, 1H), 7.48 (q, J = 6.2, 4.5 Hz, 2H), 7.46–7.43 (m, 1H), 7.42 (q, J = 2.3 Hz, 1H), 7.38 (dt, J = 8.3, 2.2 Hz, 1H), 7.36 (t, J = 1.7 Hz, 1H), 7.12–7.09 (m, 2H), 7.05–7.00 (m, 1H), 6.06– 5.94 (m, 2H), 5.15–4.97 (m, 6H), 3.47–3.39 (m, 4H). 13C-NMR (150 MHz, CD3OD): δ 197.43, 163.23, 156.71, 155.75, 137.56, 137.10, 136.80, 133.51, 132.02, 131.64, 130.60, 129.81, 129.04, 129.02, 128.96, 128.32, 128.02, 120.89, 115.07, 114.83, 114.69, 114.35, 111.35, 69.02, 38.47, 34.14. HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M + H]+ 403.1711.
4'-((2-Fluorobenzyl)oxy)-3-formy-honokiol (2e)
Yellow solid, yield: 22.5%; 1H-NMR (400 MHz, CDCl3): δ 11.20 (s, 1H), 9.78 (s, 1H), 7.58–7.50 (m, 2H), 7.29–7.18 (m, 2H), 7.11–7.05 (m, 2H), 7.04–6.93 (m, 3H), 6.00–5.81 (m, 2H), 5.07–4.93 (m, 6H), 3.38–3.34 (m, 2H), 3.33–3.28 (m, 2H). 13C-NMR (100 MHz, CDCl3): δ 195.80, 160.57, 157.50, 152.72, 137.64, 136.48, 134.72, 132.20, 131.66, 129.61, 128.92, 128.67, 128.54, 128.37, 127.69, 127.11, 123.13, 119.00, 115.21, 114.79, 114.32, 114.11, 112.17, 63.64, 38.36, 32.12. HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M-H]- 401.1543
4'-((5-Fluorobenzyl)oxy)-3-formy-honokiol (3e)
Yellow solid, yield: 20.4%; 1H-NMR (400 MHz, CDCl3): δ 11.22 (s, 1H), 9.80 (s, 1H), 7.58 (d, J = 2.2 Hz, 1H), 7.52 (d, J = 2.2 Hz, 1H), 7.20 (td, J = 7.9, 5.8 Hz, 1H), 7.10 − 7.03 (m, 2H), 6.99 (d, J = 7.7 Hz, 1H), 6.96–6.85 (m, 3H), 5.96–5.83 (m, 2H), 5.07– 4.95 (m, 6H), 3.38 (d, J = 6.6 Hz, 2H), 3.31 (d, J = 6.7 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 195.72, 163.08, 157.59, 152.66, 137.62, 136.44, 134.66, 132.26, 131.52, 129.71, 129.02, 128.94, 128.89, 128.44, 127.68, 127.22, 121.36, 119.01, 115.35, 114.82, 113.75, 113.04, 112.21, 68.90, 38.35, 32.04. HR-ESI-MS: 401.1526 [M + H]+, (calcd for C26H23FO3, 403.1631). HR-ESI-MS: molecular formula C26H23FO3 as revealed at m/z [M-H]- 401.1562.
Synthesis of honokiol derivatives 1f–3f and 1g–3g
Compounds 1d–3d and 1e–3e were used as intermediates for the synthesis of hydrozinecarboximidamide-HNK derivatives. In brief, the compounds 1d–3d (0.05–0.28 mmol) and acetic acid/aminoguanidine carbonate (0.18–0.39 mmol) in ethanol were stirred for 3–4 h at 65 °C, and then detected by TLC analysis, respectively. The reaction was quenched with water and extracted twice with ethyl acetate. The combined extract was dried over Na2SO4 and concentrated under reduced pressure. Finally, the residue was purified by a 2535Q semi-prep HPLC system (Waters, USA) using a SunFireTM C18 column (250 × 10 mm) with 35–55%% acetonitrile to afford 1f–3f and 1g–3g.
2-((4-Fluorobenzyl)oxy)-5'-methylene-hydrozinecarboximidamide-honokiol (1f)
Yellow solid, yield: 24.7%; 1H-NMR (400 MHz, CDCl3): δ 8.20 (s, 1H), 7.37–7.27 (m, 5H), 7.25–7.14 (s, 4H), 7.06–7.00 (m, 3H), 6.91 (s, 1H), 6.86 (d, J = 8.3 Hz, 1H), 6.04–5.76 (m, 2H), 5.06–4.95 (m, 6H), 3.41 (d, J = 6.6 Hz, 2H), 3.21 (d, J = 6.6 Hz, 2H). 13C-NMR (100 MHz, CDCl3): δ 163.58, 155.64, 155.58, 152.79, 151.90, 137.12, 136.79, 133.51, 132.98, 131.48, 130.91, 129.95, 129.89, 129.34, 128.97, 128.89, 128.78, 128.24, 117.58, 116.10, 115.68, 115.49, 115.28, 111.38, 69.33, 39.06, 34.60. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.21939.
2-((2-Fluorobenzyl)oxy)-5'-methylene-hydrozinecarboximidamide-honokiol (2f)
Brown solid, yield: 27.3%; 1H-NMR (600 MHz, CD3OD): δ 8.31 (s, 1H), 7.46 (d, J = 2.2 Hz, 1H), 7.43–7.40 (m, 1H), 7.38–7.29 (m, 2H), 7.16–7.05 (m, 5H), 6.03–5.92 (m, 2H), 5.13–4.96 (m, 6H), 3.45–3.41 (m, 2H), 3.40–3.36 (m, 2H). 13C-NMR (150 MHz, CD3OD): δ 161.08, 154.89, 153.49, 153.47, 150.91, 137.44, 135.83, 133.92, 132.91, 130.04, 129.93, 129.85, 129.41, 129.21, 127.92, 126.99, 123.98, 123.52, 116.78, 114.47, 114.43, 114.33, 114.09, 113.19, 64.15, 38.65, 33.09. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.2207.
2-((5-Fluorobenzyl)oxy)-5'-methylene-hydrozinecarboximidamide-honokiol (3f)
Yellow solid, yield: 27.3%; 1H-NMR (400 MHz, CDCl3): δ 8.22 (s, 1H), 7.33 (s, 4H), 7.36–7.21 (m, 4H), 7.18–7.06 (m, 3H), 7.03–6.81 (m, 3H), 6.09–5.77 (m, 2H), 5.13–4.86 (m, 6H), 3.45 (d, J = 6.5 Hz, 2H), 3.29–3.14 (m, 2H). 13C-NMR (100 MHz, CDCl3): δ 164.18, 155.52, 155.52, 152.78, 151.89, 139.93, 137.09, 136.74, 133.66, 131.56, 130.99, 130.08, 129.99, 129.95, 129.38, 128.80, 128.25, 122.40, 117.47, 116.14, 115.75, 114.72, 114.01, 111.34, 69.14, 39.06, 34.60. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.2191.
4'-((4-Fluorobenzyl)oxy)-3-methylene-hydrozinecarboximidamide-honokiol(1g)
Yellow solid, yield: 23.2%; 1H-NMR (400 MHz, DMSO-d6): δ 11.21 (s, 1H), 8.27 (s, 1H), 7.61–7.48 (m, 2H), 7.38 (dd, J = 8.4, 2.3 Hz, 1H), 7.34 (d, J = 2.3 Hz, 1H), 7.28–7.22 (m, 2H), 7.18 (d, J = 2.2 Hz, 1H), 7.09 (d, J = 8.5 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 6.64–6.16 (m, 4H), 6.04–5.93 (m, 2H), 5.16 (s, 2H), 5.14–4.97 (m, 4H), 3.41 (d, J = 6.6 Hz, 2H), 3.33 (d, J = 6.8 Hz, 2H). 13C-NMR (100 MHz, DMSO-d6): δ 163.35, 158.34, 155.27, 152.77, 148.51, 138.45, 137.35, 134.08, 131.14, 131.02, 130.83, 130.81, 129.94, 129.85, 128.85, 128.81, 128.66, 128.05, 120.39, 116.12, 116.07, 115.84, 115.62, 112.24, 69.04, 39.07, 34.62. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.2192.
4'-((2-Fluorobenzyl)oxy)-3-methylene-hydrozinecarboximidamide-honokiol(2g)
Yellow solid, yield: 20.3%; 1H-NMR (600 MHz, CD3OD): δ 8.37 (s, 1H), 7.58–7.55 (m, 1H), 7.38 (d, J = 2.3 Hz, 1H), 7.34 (t, J = 2.5 Hz, 2H), 7.22–7.13 (m, 4H), 7.09 (d, J = 8.3 Hz, 1H), 6.05 − 5.97 (m, 2H), 5.21 (s, 2H), 5.11–4.98 (m, 4H), 3.44 (d, J = 6.7 Hz, 2H), 3.38 (d, J = 6.7 Hz, 2H). 13C-NMR (150 MHz, CD3OD): δ 161.48, 155.58, 155.26, 152.26, 150.31, 137.46, 136.78, 133.60, 131.78, 130.75, 130.28, 129.92, 129.64, 129.58, 128.74, 128.58, 128.07, 123.99, 118.45, 114.90, 114.76, 114.69, 114.32, 111.42, 63.72, 38.69, 34.17. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.2205.
4'-((5-Fluorobenzyl)oxy)-3-methylene-hydrozinecarboximidamide-honokiol(3g)
Yellow solid, yield: 16.5%; 1H-NMR (400 MHz, DMSO-d6): δ 11.22 (s, 1H), 8.26 (s, 1H), 7.46 (td, J = 7.9, 6.0 Hz, 1H), 7.38 (dd, J = 8.4, 2.3 Hz, 1H), 7.35–7.28 (m, 3H), 7.19–7.14 (m, 2H), 7.06 (d, J = 8.5 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 6.27 (s, 4H), 6.06–5.93 (m, 2H), 5.21 (s, 2H), 5.13–5.01 (m, 4H), 3.44 (d, J = 6.6 Hz, 2H), 3.33 (d, J = 6.9 Hz, 3H). 13C-NMR (100 MHz, DMSO-d6): δ 163.90, 158.25, 155.13, 152.76, 148.50, 138.45, 137.37, 131.19, 131.10, 131.01, 130.93, 130.89, 130.85, 128.83, 128.69, 128.02, 123.54, 120.40, 116.12, 116.09, 115.01, 114.34, 114.12, 112.16, 68.84, 45.94, 34.63. HR-ESI-MS: molecular formula C27H27FN4O2 as revealed at m/z [M + H]+ 459.2210.
Cell culture
The human nasopharyngeal carcinoma cell line (CNE-2Z), gastric cancer cell line (SGC7901), breast cancer cell line (MCF-7), mouse leydig testicular cancer cell line (I-10), and mouse leydig cell line (TM3) were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, USA) supplemented with 10% foetal bovine serum (FBS, Hangzhou Sijiqing Co., Ltd., China) and 1% penicillin/streptomycin (Gibco, USA). Cell culture was conducted in an incubator at 37 °C and 5% CO2.
MTT assay
All the cell lines were incubated in 96-well plates at a density of 5 × 103 cells per well and cultured overnight at 37 °C. After adherence, the cells were treated with various concentrations of the test compounds for 24, 48, and 72 h. After the mentioned time points, cell viability was examined using a standard MTT assay. The cytotoxic activity of compounds was expressed as IC50 value that inhibited cell growth to 50% of control. Cis-platinum (DDP) was used as the positive control.
Colony-formation assay
CNE-2Z cells were seeded in 6-well plates at a density of 6 × 103 cells/well and cultured overnight. When colonies formed, the medium was replaced with fresh medium containing 1g at various concentrations (0, 0.5, 1, and 2 μM), and the cells were cultured for 7 days. After 7 days, the cells were washed with phosphate buffer saline (PBS), fixed with 4% paraformaldehyde for 10 min, followed by stained with crystal violet for 10 min and photographed.
Cell migration assay
The migration assay was assessed using a 24-well plate with 8.0 μm pore membrane inserts (Corning, NY) without matrigel. CNE-2Z cells were added to the upper chambers at a concentration of 5 × 104/well, and incubated for 24 h, followed by treated with various concentrations (0, 1, 3, and 6 μM) of 1g. The lower chambers were filled with the conditioned media. After 24 h, the cells that had migrated were stained with 0.1% crystal violet and photographed under a light microscope at 200× magnification. The number of migratory cells was counted and analysed to determine statistically significant differences. The migration assay was performed independently three times. DDP was used as a positive control.
Cell invasion assay
The invasion assay was assessed using a 24-well plate with 8.0 μm pore membrane inserts that were coated with 50 μl of matrigel (BD, USA) and incubated at 37 °C for 1 h. CNE-2Z cells (5 × 104/well) were added to the upper chambers and incubated with various concentrations (0, 1, 3, and 6 μM) of 1g for 36 h. The remainder of the procedure was same as that described for the cell migration experiment.
Western blotting
CNE-2Z cells were cultured in 6-well plates at a density of 6 × 105 cells/well. After adherence, the cells were treatment with various concentrations of 1g (0, 2.5, 5, and 10 μM) and incubated for 24 h. The cells were harvested, washed with PBS, and proteins were extracted and quantified. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. The PVDF membrane was blocked with 5% skimmed milk and incubated with primary antibodies at 4 °C for overnight. After washing with TBST buffer, the membranes were incubated with the corresponding secondary antibodies at room temperature for 2.5 h. Finally, the protein bands were visualized using a chemiluminescence kit and detected using a gel imaging system (Bio-Rad, USA). Anti-β-actin was used as an internal control.
Small interfering RNA (siRNA) transfection
HIF-1a siRNAs were purchased from Gene-Pharma (China) and were transiently transfected into CNE-2Z cells in 6-well plates using Lipofectamine 2000 reagent (Invitrogen, USA). After 48 h of transfection, the cells were collected for western blotting as described previously. The siRNA sequences used for experiments with human HIF-1a were as follows: Negative control sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′; Positive control sense, 5′-UGA CCU CAA CUA CAU GGU UTT-3′ and antisense, 5′-AAC CAU GUA GUU GAG GUC ATT-3′; HIF-1a homo 2107 sense, 5′-CCA GCA GAC UCA AAU ACA ATT-3′ and antisense, 5′-UUG UAU UUG AGU CUG CUG GTT-3′.
Flow cytometry with Annexin V/PI staining
CNE-2Z cells were cultured in a 12-well plate at a density of 2.5 × 105 cells per well and incubated overnight. After adherence, the cells were treated with various concentrations of 1g (0, 2.5, 5, and 10 μM), and cultured for 24 h. The cells were harvested from each well, washed with PBS, and stained with Annexin V-FITC solution, followed by the addition of PI staining solution and subsequent incubation at room temperature in the dark for 12 min. The percentage of apoptotic cells was analyzed using flow cytometry (BD Biosciences, USA).
In vivo anti-tumour experiments
To evaluate whether anti-tumour efficacy was delivered by 1g, we used nude mice (female BALB/c nude mice, 4–5 weeks of age, obtained from Canes Laboratory, Changzhou, China) to perform experiment. CNE-2Z cells (5 × 106 cells/animal) were injected subcutaneously to induce tumour formation. All experimental procedures and protocols were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. When approximately 100 mm3 of tumour volume was measured, the mice were randomly divided into three groups (four mice per group), Vehicle control, 1g (10 mg/kg), and DDP (3 mg/kg) injected intraperitoneally every 3 days for 19 days. DDP was used as a positive control drug. Tumour volume (calculated as tumour length × width2/2) and body weight were monitored every 3 days. After treatment for 19 days, the mice were sacrificed, and serum glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) levers were measured. In addition, the liver, kidneys, lungs, and solid tumours were removed, preserved in 4% formalin solution, that were stained with haematoxylin and eosin (H&E).
Statistical analysis
Data are presented as the means ± SD from three independent experiments. SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA) was used for the data analysis. Statistical Comparisons between groups were performed using one-way ANOVA followed by the Student’s t-test. *P < 0.05, **P < 0.01 were considered statistically significant.
Supplemental Material
Download PDF (4.6 MB)Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Cao SM, Xu YJ, Lin GZ, Huang QH, Wei KR, Xie SH, Liu Q. Estimation of cancer burden in Guangdong Province, China in 2009. Chin J Cancer. 2015; 34 (12):594–601.
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
- Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364.
- Peng H, Chen L, Zhang Y, Li WF, Mao YP, Liu X, Zhang F, Guo R, Liu LZ, Tian L, et al. The tumor response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. Sci Rep. 2016;6:24835.
- Hong B, Lui VW, Hashiguchi M, Hui EP, Chan AT. Targeting tumor hypoxia in nasopharyngeal carcinoma. Head Neck. 2013;35(1):133–145.
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732.
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389.
- Xu L, Huan L, Guo T, Wu Y, Liu Y, Wang Q, Huang S, Xu Y, Liang L, He X. LncRNA SNHG11 facilitates tumor metastasis by interacting with and stabilizing HIF-1α. Oncogene. 2020;39(46):7005–7018.
- Al-Ostoot FH, Sherapura A, Vigneshwaran V, Basappa G, Vivek HK, Prabhakar BT, Khanum SA. Targeting HIF-1α by newly synthesized indolephenoxyacet- amide (IPA) analogs to induce anti-angiogenesis-mediated solid tumor suppression. Pharmacol Rep. 2021;73(5):1328–1343.
- Huang ZY, Zhang LH, Zhao C, Liu R, Tong H, Gan C, Lan T, Tang CW, Gao JH. High HIF-1α expression predicts poor prognosis of patients with colon adenocarcinoma. Int J Clin Exp Pathol. 2018;11(12):5635–5646.
- Wei T-T, Lin Y-T, Tang S-P, Luo C-K, Tsai C-T, Shun C-T, Chen C-C. Metabolic targeting of HIF-1α potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene. 2020;39(2):414–427.
- Xie W, Liu L, He H, Yang K. Prognostic value of hypoxia-inducible factor-1 alpha in nasopharyngeal carcinoma: a meta-analysis. Int J Biol Markers. 2018; 33(4):1724600818778756.
- Zhang L, Song J, Kong L, Yuan T, Li W, Zhang W, Hou B, Lu Y, Du G. The strategies and techniques of drug discovery from natural products. Pharmacol Ther. 2020;216:107686.
- Min HY, Jang HJ, Park KH, Hyun SY, Park SJ, Kim JH, Son J, Kang SS, Lee HY. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019;10(11):810.
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803.
- Fujita M, Itokawa H, Sashida Y. Honokiol, a new phenolic compound isolated from the bark of Magnolia obovata Thunb. Chem Pharm Bull. 1972;20(1):212–213.
- Rauf A, Olatunde A, Imran M, Alhumaydhi FA, Aljohani ASM, Khan SA, Uddin MS, Mitra S, Emran TB, Khayrullin M, et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine. 2021;90:153647.
- Luo LX, Li Y, Liu ZQ, Fan XX, Duan FG, Li RZ, Yao XJ, Leung EL, Liu L. Honokiol induces apoptosis, G1 arrest, and autophagy in KRAS mutant lung cancer cells. Front Pharmacol. 2017;8:199.
- Tian W, Xu D, Deng YC. Honokiol, a multifunctional tumor cell death inducer. Pharmazie. 2012;67(10):811–816.
- Wang WD, Shang Y, Li Y, Chen SZ. Honokiol inhibits breast cancer cell metastasis by blocking EMT through modulation of Snail/Slug protein translation. Acta Pharmacol Sin. 2019; 40(9):1219–1227.
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278(37):35501–35507.
- Chio CC, Tai YT, Mohanraj M, Liu SH, Yang ST, Chen RM. Honokiol enhances temozolomide-induced apoptotic insults to malignant glioma cells via an intrinsic mitochondrion-dependent pathway. Phytomedicine. 2018;49:41–51.
- Sabarwal A, Wedel J, Liu K, Zurakowski D, Chakraborty S, Flynn E, Briscoe DM, Balan M, Pal S. A combination therapy using an mTOR inhibitor and honokiol effectively induces autophagy through the modulation of AXL and rubicon in renal cancer cells and restricts renal tumor growth following organ transplantation. Carcinogenesis. 2022;43(4):360–370.
- Ponnurangam S, Mammen JM, Ramalingam S, He Z, Zhang Y, Umar S, Subramaniam D, Anant S. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther. 2012;11(4):963–972.
- Lin D, Yan Z, Chen A, Ye J, Hu A, Liu J, Peng J, Wu X. Anti-proliferative activity and structure-activity relationship of honokiol derivatives. Bioorg Med Chem. 2019;27(16):3729–3734.
- Ma L, Chen J, Wang X, Liang X, Luo Y, Zhu W, Wang T, Peng M, Li S, Jie S, et al. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: the evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship. J Med Chem. 2011;54(19):6469–6481.
- Xu T, Tian W, Zhang Q, Liu J, Liu Z, Jin J, Guo Y, Bai LP. Novel 1,3,4-thiadia- zole/oxadiazole-linked honokiol derivatives suppress cancer via inducing PI3K/Akt/mTOR-dependent autophagy. Bioorg Chem. 2021;115:105257.
- Shi X, Zhang T, Lou H, Song H, Li C, Fan P. Anticancer effects of honokiol via mitochondrial dysfunction are strongly enhanced by the mitochondria-targeting carrier berberine. J Med Chem. 2020;63(20):11786–11800.
- Zhu M, Li B, Ma H, Huang X, Wang H, Dai Y, Li Y, Li HM, Wu CZ. Synthesis and in vitro antitumor evaluation of honokiol derivatives. Bioorg Med Chem Lett. 2020;30(2):126849.
- Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999;48 (10):509–515.
- Deng X, Song M. Synthesis, antibacterial and anticancer activity, and docking study of aminoguanidines containing an alkynyl moiety. J Enzyme Inhib Med Chem. 2020;35(1):354–364.
- Liu DC, Gao MJ, Huo Q, Ma T, Wang Y, Wu CZ. Design, synthesis, and apoptosis-promoting effect evaluation of novel pyrazole with benzo[d]thiazole derivatives containing aminoguanidine units. J Enzyme Inhib Med Chem. 2019; 34(1):829–837.
- Song M, Wang S, Wang Z, Fu Z, Zhou S, Cheng H, Liang Z, Deng X. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N- arysulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur J Med Chem. 2019;166:108–118.
- Wei ZY, Chi KQ, Yu ZK, Liu HY, Sun LP, Zheng CJ, Piao HR. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg Med Chem Lett. 2016;26(24):5920–5925.
- Guan S, Wei J, Huang L, Wu L. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur J Med Chem. 2020; 207:112758.
- Li HM, Miao J, Zhu M, Gao M, Dai Y, Huo Q, Ma T, Wu CZ. Bishonokiol A inhibits breast cancer cell invasion and migration by suppressing hypoxia inducible factor-1α. J Bioenerg Biomembr. 2019;51(3):239–248.
- Richardson P. Fluorination methods for drug discovery and development. Expert Opin Drug Discov. 2016;11(10):983–999.
- Shah P, Westwell AD. The role of fluorine in medicinal chemistry. J Enzyme Inhib Med Chem. 2007;22(5):527–540.
- Lin JM, Prakasha GA, Sharma AK, Amin S. In vitro growth inhibition of human cancer cells by novel honokiol analgos. Bioorg Med Chem. 2012;20(10):3202–3211.
- Liu HL, Wang J, Lin DZ, Liu H. Lead compound optimization strategy (2)– structure optimization strategy for reducing toxicity risks in drug design. Yao Xue Xue Bao. 2014;49(1):1–15.
- Huang CC, Su CW, Wang PH, Lu YT, Ho YT, Yang SF, Hsin CH, Lin CW. Dihydromyricetin inhibits cancer cell migration and matrix metalloproteinases-2 expression in human nasopharyngeal carcinoma through extracellular signal-regulated kinase signaling pathway. Environ Toxicol. 2022;37(5):1244–1253.
- Rashid M, Zadeh LR, Baradaran B, Molavi O, Ghesmati Z, Sabzichi M, Ramezani F. Up-down regulation of HIF-1α in cancer progression. Gene. 2021; 798:145796.
- Sung WW, Chu YC, Chen PR, Liao MH, Lee JW. Positive regulation of HIF-1α expression by EBC oncoprotein LMP1 in nasopharyngeal carcinoma cells. Cancer Lett. 2016;382(1):21–31.
- Tang W, Zhao G. Small molecules targeting HIF-1α pathway for cancer therapy in recent years. Bioorg Med Chem. 2020;28(2):115235.
- Xu R, Wang F, Yang H, Wang Z. Action sites and clinical application of HIF-1α inhibitors. Molecules. 2022;27(11):3426.
- McAleese CE, Choudhury C, Butcher NJ, Minchin RF. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021;502:189–199.