Abstract
Conjugation of drugs with biotin is a widely studied strategy for targeted drug delivery. The structure–activity relationship (SAR) studies through H3-biotin competition experiments conclude with the presence of a free carboxylic acid being essential for its uptake via the sodium-dependent multivitamin transporter (SMVT, the major biotin transporter). However, biotin conjugation with a payload requires modification of the carboxylic acid to an amide or ester group. Then, there is the question as to how/whether the uptake of biotin conjugates goes through the SMVT. If not, then what is the mechanism? Herein, we present known uptake mechanisms of biotin and its applications reported in the literature. We also critically analyse possible uptake mechanism(s) of biotin conjugates to address the disconnect between the results from SMVT-based SAR and “biotin-facilitated” targeted drug delivery. We believe understanding the uptake mechanism of biotin conjugates is critical for their future applications and further development.
Introduction
Even with the tremendous development in the area of drug discovery and development during the last 50 yearsCitation1, there is still a large number of diseases that do not have effective treatmentCitation2. One can safely say that the levels of challenges are becoming progressively higher in dealing with the remaining problems, even after taking into consideration of new technologies and new scientific discoveries/insights. Future work is almost entirely focused on targeted therapies of various types, including modulation of activities of the molecular target(s) implicated in the relevant pathologies and/or targeted delivery based on molecular biomarkers. The latter often involves conjugation of a therapeutic molecule with a targeting ligand such as peptidesCitation3–5, nucleic acidsCitation6,Citation7, micronutrientsCitation8–11, and antibodiesCitation12,Citation13 as well as the use of nanomaterialsCitation14,Citation15 to achieve the goals of targeted delivery. Such targeting ligands act as vectors and offer distinct physicochemicalCitation16–19 and pharmacologicalCitation8,Citation20–22 features resulting in improved pharmacodynamic and pharmacokinetic profiles of the appended drugsCitation2. We have a long-standing interest in developing novel drug delivery approaches including targeted drug deliveryCitation17,Citation23–30. Along this line, we have an interest in biotin-mediated targeted delivery of drugs to organs such as the kidney, colon, and the lung as well as cancerCitation31–33.
BiotinCitation34 is a cofactor for enzymes collectively referred to as "biotin-dependent carboxylases," which are involved in the metabolism of amino acids, fatty acids, carbohydrates, and ureaCitation35–37. In addition, the roles of biotin also extend to the regulation of cellular processes, including gene expression and cell signallingCitation38. Physiological concentrations of biotin have been reported to be in the low nanomolar rangeCitation39–44. It has been reported that cancer cells overexpress biotin uptake system(s), which often leads to enhanced intracellular uptake of biotinCitation8,Citation31,Citation41,Citation45. A few examples are ovarian, lung, renal, colon, and breast cancers as well as leukaemiaCitation8,Citation38,Citation46. Conceivably, the over-expression of biotin uptake system(s) in a given type of cells provides a valuable strategy to selectively target the relevant cell types with a biotin analogue that are recognised by these “uptake system(s)”. The very first example of “biotin-mediated” drug delivery with the aim of targeting the biotin uptake system was published in 1990 describing work in plant cells (soybean)Citation47. Along this line, it should be noted that earlier work using biotin–avidin/streptavidin interactions for drug delivery was not intended to target the biotin uptake systemCitation48,Citation49. Interestingly, the very first paper on using biotin conjugates for drug delivery in mammalian cells was not for its ability to bind to the biotin uptake proteinCitation50–58. The very first mention of synthesising a biotin conjugate for taking advantage of the biotin uptake system in animals was reported in 2001 on the oral absorption properties of biotinylated retro-inverso Tat nonapeptidesCitation59. This study demonstrated an enhancement of up to 500-fold in transporting the biotinylated version of the peptide. Such transport was shown to be concentration dependent, saturable, and inhibitable by biotin. In 2004, a comparative study by Russell-Jones et al.Citation8 examined the uptake of fluorescently labelled polymers conjugated with biotin, folic acid, and vitamin B12 in 18 different tumour cell lines in vitro and in vivo. Compared to the non-targeted polymer, an enhanced uptake of all the ligand-conjugated polymers was found in various cell lines. For instance, Colo-26 cells (murine colon tumour) treated with vitamin B12-conjugated and biotin-conjugated polymers showed >2-fold more fluorescence intensity than that of non-targeted polymers. Of note, compared to the folic acid-conjugated and vitamin B12-conjugated polymers, the uptake of biotin-conjugated polymers was found to be >3-fold higher in M109 cells (murine lung carcinoma). Since then, biotin has been extensively studied as a vector for targeted delivery of anticancer agentsCitation31,Citation46,Citation60–64. For information on biotin metabolism, biological functions, and its medical applications, readers are referred to several comprehensive reviewsCitation31,Citation32,Citation34–36,Citation45,Citation65–78.
While there has been very impressive progress in using biotin conjugates for targeted delivery applications in cell culture and animal models, much less is known about the mechanistic aspects of such success. Foremost, it is not clear whether such targeted delivery relies on a biotin transporter or receptor. Though literature discussions seem to use the terms biotin “receptor” and “transporter” without a clear distinction, these are indeed two distinctly different conceptsCitation46,Citation79–86. It is commonly accepted that “transporters mediate the passage of molecules across cell membranes by alternating between inward- and outward-facing states, while receptors undergo intracellular structural rearrangements that initiate signaling cascadesCitation87.” Known biotin transporters almost universally require the presence of a free carboxylic acid group for the recognition of biotin ()Citation33,Citation72,Citation82,Citation88–92, and yet the biotin conjugates used in targeted delivery transforms the carboxyl group to an amide or esterCitation32,Citation45, which is supposed to abolish the ability for a biotin transporter to recognise such a conjugateCitation72. At the same time, no known “biotin receptor” has been identified, only transportersCitation72,Citation73,Citation88,Citation91,Citation93. Further, there is a report of the “anti-inhibition” effects of biotin in the binding of a biotin conjugate with cells that express the biotin transporterCitation94. In addition, a study by Wei and coworkers reported the lack of competition between 2000-fold excess of biotin and biotin conjugate during the uptake experiment in E. coliCitation95. All these suggest the need for much more mechanistic studies on how biotin conjugation allows for “biotin-receptor/transporter”-mediated delivery. In this review, we provide a brief overview of the known biotin transporters, discuss known structure–activity relationships (SARs) in binding between a substrate and a biotin transporter, explore the disconnect between the SAR results and “biotin receptor/transporter-mediated" delivery of biotin conjugate, and suggest various possibilities. All these are aimed to help future design of biotin conjugates for efficient delivery with a firm understanding of the biology behind it. Below, we provide our critical analysis by starting with a description of the various known biotin transport systems.
Known biotin uptake systems
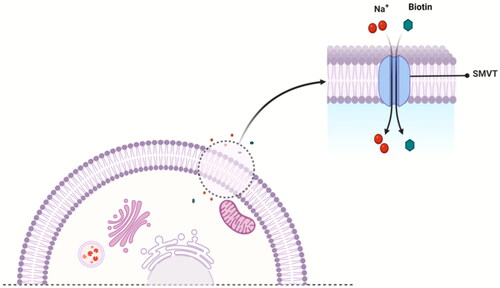
The uptake of biotin into cells occurs mainly via the sodium-dependent multivitamin transporter (SMVT)Citation96, which employs a transmembrane Na+ gradient for its translocation ()Citation32. In addition, a few studies have revealed a carrier system other than SMVT that might mediate biotin uptake in human peripheral blood mononuclear cells (PBMC)Citation97 and keratinocytesCitation98. In the following subsections, we briefly discuss all the known biotin uptake systems in the mammalian cell.
Sodium-dependent multivitamin transporter
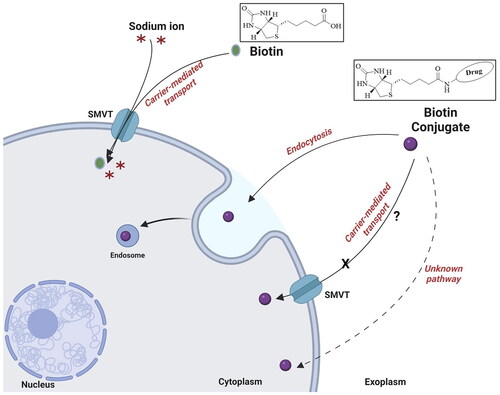
SMVT, a product of the solute carrier family-5 member-6 (SLC5A6) gene, is responsible for the transportation of biotin, lipoic acid, and pantothenic acid () across the cell membraneCitation70,Citation72,Citation99. This transport is coupled with Na+ fluxCitation99. As proposed based on topographical analysisCitation100,Citation101, the SMVT consists of 635 amino acids and has 12 putative transmembrane domains with N- and C-termini towards the cytosol. The nucleotide sequence of the cDNA predicts a molecular weight of 68.6 kDaCitation70,Citation102. It also has four N-linked glycosylation sites in extracellular domains and two protein kinase C-dependent phosphorylation sites in cytoplasmic domainsCitation100,Citation103. SMVT distributes ubiquitously in the human body and is most abundantly expressed in the absorptive tissues of the liver, intestine, placenta, pancreas, and kidneyCitation100,Citation104. Functionally, SMVT transports solutes such as biotin across the cell membrane against an electrochemical gradient of Na+. One biotin molecule is co-transported with two sodium ions in a single transport cycle ()Citation100. Again, SMVT has been widely studied for targeted delivery of biotin conjugates into cells overexpressing SMVTCitation105; although, known SAR binding for SMVT does not support such delivery.
Monocarboxylate transporter-1 (MCT-1)
The MCT family of proteins belongs to the proton-dependent transport protein family (SLC16A), with 14 members based on sequence homologyCitation106,Citation107. These transporters mediate the uptake of short-chain monocarboxylates such as lactate, α-hydroxybutyrate, pyruvate, and biotin in mammalian cellsCitation96. Among these proton-dependent transporters, MCT-1 to MCT-4 mediate the uptake of endogenous monocarboxylates into the brain, kidney, and intestinal cells through the electroneutral co-transport of one monocarboxylate molecule with one proton (1:1)Citation108. MCT-1 has been reported as an alternative biotin transporter in mammalian lymphoid cells; although, these cells also express SMVTCitation97. MCT-1 utilises a sodium-dependent co-transport mechanism for uptake into the PBMCsCitation109. The Michaelis–Menten constant (Km) was reported to be 2.6 nM, ∼1000-times lower for MCT-1-mediated biotin transport in PMBCs than SMVT-mediated biotin transportCitation97,Citation109. Of note, carrier-mediated transport exhibits Michaelis–Menten kinetics; hence, Km is a widely used parameter to indicate the binding affinity (alternatively, specificity) of the substrates with the carrier proteinsCitation110–113.
Miscellaneous: uptake of biotin into keratinocytes
The liver, intestinal epithelia, kidney, and placenta express SMVT for biotin uptakeCitation97. In addition, Grafe et al.Citation98 studied the uptake of biotin in the human HaCaT cell line and native non-transformed keratinocytes and revealed that lipoic acid and pantothenic acid inhibit the Na+-dependent biotin uptake in keratinocytes. SMVT showed an apparent Michaelis–Menten constant of 23 µM for biotin, 1 µM for pantothenic acid, and 4.6 µM for lipoic acid (oxidised form). In addition, kinetic studies showed the presence of a second uptake system for biotin in the human keratinocytes with an apparent Michaelis–Menten constant of 2.6 nM. Interestingly, this value is same as that for the MCT-1-mediated biotin transport in PMBCs. This uptake system seems specific for biotin because the uptake was not inhibited by lipoic acid and pantothenic acid. In comparison, SMVT-mediated biotin uptake was significantly affected by both lipoic acid and pantothenic acid. Hence, the study concluded that human keratinocytes express both the SMVT and another biotin-specific carrier-mediated transport system.
The structure–activity relationship: what might the SAR imply in terms of uptake mechanism(s)?
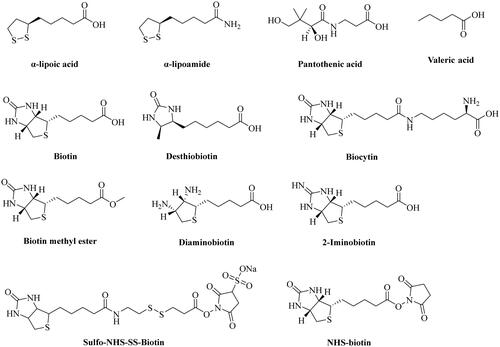
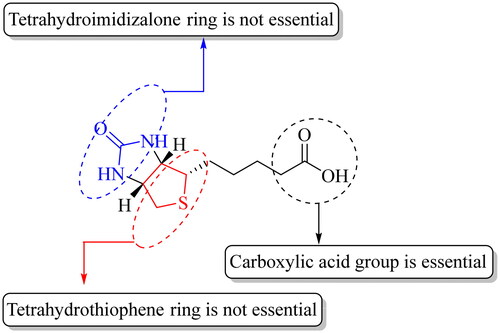
SMVT exhibits broad ligand specificity as it recognises a range of molecules such as biotin, pantothenic acid (vitamin B5), α-lipoic acid, and iodide for their cellular uptakeCitation31,Citation70,Citation114. Another transporter MCT-1 recognises short-chain aliphatic acids such as lactate, pyruvate, butyrate, acetoacetate, β-hydroxybutyrate, and γ-hydroxybutyrate and seems to be an even more versatile transporter than SMVTCitation96,Citation106. Because MCT-1 is largely involved in the transport of endogenous aliphatic carboxylates and has less prominent roles in biotin transport, it has not been as widely studied as the SMVT in terms of understanding the SAR for their binding characteristics. Therefore, we analyse the SAR of SMVT substrate/ligand characteristics as summarised in . The substrate specificity of SMVT has been studied by determining the uptake kinetics of radiolabeled biotin in the presence of unlabelled biotin and its structural analogues such as α-lipoic acid, α-lipoic amide (α-lipoamide), biocytin, desthiobiotin, biotin methyl ester, and pantothenic acid ()Citation89,Citation91,Citation92,Citation116. For instance, Nylander and coworkers examined the uptake of biotin (5 µM) in the presence of its structural analogues in human intestinal brush border membrane (entry 1, )Citation91. At 20 and 50 µM, desthiobiotin inhibited the uptake of biotin to 5.25 ± 0.18 pmol.mg protein−1.20 s−1 and 4.88 ± 0.14 pmol.mg protein−1.20 s−1, respectively, when compared to the control group (9.80 ± 0.60 pmol.mg protein−1.20 s−1). Similarly, α-lipoic acid also diminished the uptake to 5.60 ± 0.22 pmol.mg protein−1.20 s−1 and 4.70 ± 0.27 pmol.mg protein−1.20 s−1 at 20 and 50 µM concentrations, respectively in comparison to the control group. However, uptake studies using biotin analogues having modified carboxylic acid such as biotin methyl ester and α-lipoic amide did not result in a marked decrease in biotin uptake by intestinal cells. Another study done by Mitra and coworkers investigated the uptake of 10 nM [3H]biotin in a human-derived retinoblastoma cell line (Y-79) (entry 4, )Citation93. In the presence of 1 mM of unlabelled biotin, valeric acid, pantothenic acid, α-lipoic acid, and desthiobiotin, the uptake of [3H]biotin decreased to 9.03 ± 1.51%, 60.39 ± 4.02%, 9.12 ± 1.39%, 6.97 ± 0.82%, and 15.61 ± 2.41% of the control group, respectively. Treatment with 1 mM of biocytin and biotin methyl ester did not produce any inhibitory effect on [3H]biotin uptake. Therefore, it is clear that SMVT does not interact with biotin analogues having modified carboxyl group. Further, Said and coworkers conducted a similar study in the human intestinal cell line Caco-2 (entry 2, )Citation88. Specifically, incubation of cells with [3H]biotin (4 nM) with 25 µM of biotin and desthiobiotin resulted in the decrease of [3H]biotin uptake from 1.69 ± 0.04 pmol.mg protein−1.3 min−1 to 0.664 ± 0.03 and 0.95 ± 0.02 pmol.mg protein−1.3 min−1, respectively. However, no significant inhibitory effects on [3H]biotin uptake were observed upon incubation with 25 µM of diaminobiotin, biotin methyl ester, and biocytin. A few conclusions can be drawn. First, desthiobiotin (devoid of the tetrahydrothiophene ring present in biotin) shows strong inhibition of biotin uptake by SMVT (entries 1–5)Citation33,Citation88,Citation89,Citation91. Similarly, α-lipoic acid (devoid of the tetrahydroimidazolone ring present in biotin) competes strongly with biotin for SMVT-mediated transport (entries 1, 3, and 4)Citation33,Citation91,Citation93. Such results indicate that the presence of two fused five-membered rings is not essential. Second, diaminobiotin with two ionisable (protonation) amino groups on a single five-membered ring does not compete strongly with biotin (entry 2, ). In addition, the 2-iminobiotin compound () that contains fused rings do not compete with biotin for SMVT-mediated transport. Obviously, 2-iminobiotin has a protonatable guanidine moiety instead of a urea moiety in the second five-membered ring. Though the SAR for biotin interaction with the SMVT seems to be complexCitation59,Citation88,Citation100, the presence of a protonatable functional group clearly is not favourable for such interactions. Third, valeric acid was also found to be a strong inhibitor of biotin transport by the SMVT although it does not contain fused tetrahydroimidazolone and tetrahydrothiophene rings in its structure (entries 3 and 4, )Citation33,Citation93. The presence of a free aliphatic carboxylic acid group is the only common feature that exists among these structurally diverse substrates of SMVTCitation117. As expected, modification of the carboxylic acid group to an ester (e.g. biotin methyl ester) or amide (e.g. biocytin and α-lipoic amide) led to the abolishment of the ability to compete with biotin for transport by SMVT (entries 1, 2, and 5, )Citation88,Citation89,Citation91,Citation118.
Table 1. Uptake studies of biotin in various cell lines.
An extensive study by Finn and coworkersCitation119 demonstrates the high specificity of SMVT towards modification in the structure of pantothenic acid (). In the study, pantothenic acid derivatives were tested for competitive reduction of [3H]biotin uptake by SMVT in human embryonic kidney (HEK) cells transfected with human SMVT using LC–MS/MS. The SAR analysis (totally 18 derivatives) showed that modification of the carboxylic acid to an amide, ester, alcohol, or triazole of the β-alanine fragment of the pantothenic acid resulted in a significant loss of biotin uptake inhibitory activity. Further, it was found that only pantothenic acid derivatives with a free carboxyl group were tolerated by the transporter as shown by inhibition of biotin uptake and sodium-dependent transport of these derivatives. Overall, this study shows the high specificity of SMVT towards the modifications in the pantothenic acid structure and delineates the importance of the free carboxylic acid group of pantothenic acid for SMVT-mediated transport. Such results further corroborate the data from biotin uptake studies discussed in the previous SAR section, which emphasises the need for a free carboxylic acid group for SMVT-mediated transport.
All the studies point to one thing: the presence of a carboxylic acid group is essentialCitation72. If one examines the mechanism through which SMVT transports a substrate, the requirement for a carboxylic acid is consistent with the driving force being electrochemical via a sodium ion gradient (). In this regard, and provide a summary of results from various competition experiments to show the structural properties of biotin crucial for its recognition by SMVT.
One of the central questions for this review is the vast body of literature examples of various drug–biotin conjugates synthesised through amidation or esterification of the biotin carboxylic acid groupCitation8,Citation31,Citation45,Citation120,Citation121. Despite the requirement of a free carboxyl group for SMVT-mediated transport and the lack of a carboxyl group in commonly used biotin–drug conjugates, most studies attribute the targeted delivery of such conjugates due to binding to a “biotin receptor,” which is sometimes inaccurately used to mean biotin transporter. As discussed earlier, the concept of a “receptor” is different from that of a “transporter”. In the subsequent sections, we provide a brief description of such examples and analyse the apparent inconsistency between the structural need for SMVT transport and the key features of such biotin conjugates.
Biotin conjugates in delivery applications
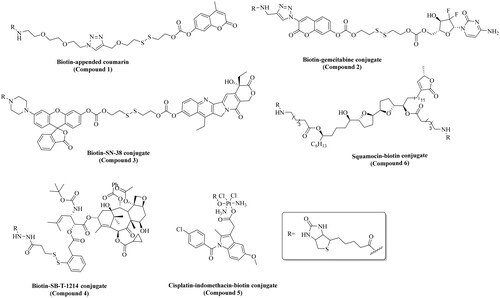
Biotin has been extensively investigated for various applications including delivery of both therapeutic and imaging agentsCitation31,Citation45,Citation69,Citation78,Citation86,Citation122. Without being comprehensive, we herein attempt to succinctly show the extent of applications of biotin conjugates. displays examples of various types of applications of biotin conjugates. Mostly, biotin has been used as a vector to deliver a payload to cancer cellsCitation31,Citation86. For instance, Kim and coworkers published their work on the development of a biotin-conjugated theranostic agent (compound 2) containing gemcitabine (an anticancer drug) and coumarin (a fluorescent reporter) (entry 2, )Citation123. As proposed by the authors, this multifunctional compound undergoes thiol-triggered release of gemcitabine and biotin-conjugated coumarin under physiological conditions. Briefly, compound 2 (10 µM) was incubated for 15 min with A549 cells (human lung carcinoma epithelial cells) and WI38 cells (Caucasian fibroblast-like foetal lung cells). Markedly, higher fluorescence was observed in A549 cells compared to the WI38 cells. Further, compound 2 was tested for anticancer activity using a cell viability assay. In comparison to the control group (no treatment), compound 2 reduced the cell viability by >80% at 1 µM, while non-biotinylated gemcitabine-coumarin conjugate reduced the cell viability by ∼50%. In addition, gemcitabine alone (1 µM) treatment resulted in ∼30% reduction of the cell viability compared to the control group (untreated). Subsequently, Kim and coworkers also reported biotin–coumarin conjugate (compound 1) for selective labelling of cancer cells (entry 1, )Citation121. In this study, biotin-conjugated coumarin (5 µM) showed a high level (quantitative data not provided) of fluorescence in A549 cells compared to WI38 cells after 20 min of incubation. Additional cell imaging studies showed a relatively high level of fluorescence (quantitative data not provided) in A549 cells for the biotin-conjugated compound when compared to its non-biotinylated derivative after 20 min of incubation. Overall, results from both articles show the ability of biotin to selectively deliver compounds to cancer cells. Though the enhancement effects seem small, biotinylation of anticancer drugs indeed provides beneficial effects. Additionally, biotin conjugation has been also extensively exploited for enhancing cancer cell accumulation of anticancer compounds such as SN-38 (entry 3, ), SB-T-1214 (entry 4, ), cisplatin (entry 5, ), curcumin (entry 8, ), squamocin (entry 9, ), and paclitaxel (entry 10, )Citation46,Citation80,Citation124,Citation126–128. Generally, such biotin conjugates are synthesised through amidation or esterification of the biotin carboxylic acid (). As discussed in the previous section, such transformations are contraindicated for interaction with the SMVT based on SAR studies and beg the question as to whether transport of such biotin conjugates is through the SMVT. In the subsequent sections, we probe the consequences of modification of the carboxylic acid on the transport of biotin conjugates reported in the literature for various applications.
Table 2. Applications of biotin in the development of drugs and diagnostics.
Activated biotin for cell-surface protein labelling work
Relevant to the discussion of biotin-mediated drug delivery issues, it is important to address another seemingly contradictory application of biotin: selective covalent labelling of (only) cell-surface proteins using biotinylated reactive agentsCitation129–136. Such experiments utilise the high acylating reactivity of N-hydroxysuccinimide–biotin conjugates (NHS–biotin) and sulfo-NHS-SS-biotin () to specifically conjugate and subsequently extract cell-surface proteins using streptavidin beadsCitation130,Citation131,Citation134,Citation137. Such a design is predicated on two characteristics of biotin: low passive permeability across the cell membrane (and thus only labels cell surface proteins) and high affinity for streptavidin (and avidin)Citation122, which are bacterial proteins with high affinity for biotin and are often used in isolation of biotinylated conjugates. Furthermore, such protein labelling work is not limited to cells without biotin transporter(s). A natural question is whether such protein labelling experiments would contradict the idea of transporter-mediated uptake of biotin conjugates. The answer seems to be with the temperature-dependent nature of the biotin transportCitation33,Citation46,Citation79,Citation89,Citation93. Generally, cell-surface labelling experimental protocols apply low temperature (generally performed at 4 °C) during the incubation of biotinylating reagents with cells to minimise uptake and afford selectivity for surface proteinsCitation129,Citation133,Citation138. However, there are also examples of labelling experiments conducted at room temperature or 37 °CCitation139–142. It is not clear whether the success of such experiments is correlated with low or lack of expression of the biotin uptake system. At this point, this issue needs to be examined to understand the mechanistic implications. It is possible that the acylating reaction is kinetically faster than transport, leading to the selective labelling of cell surface proteins. Another possibility is for NHS–biotin to acylate any biotin transporter present, leading to covalent inhibition. All these also suggest the possibility of using NHS–biotin or similarly activated biotin-based acylating agent for identifying any unknown “biotin receptor”.
Intriguing possibilities
With all the reports of targeted drug delivery by using biotin conjugates, there are very important unresolved issues. First, most of the SAR studies in biotin transporter binding uniformly specify the need for a carboxyl group. This is described in detail in section “The structure–activity relationship: what might the SAR imply in terms of uptake mechanism(s)?” ( and ). However, with all the biotin conjugates, the carboxyl group is used for conjugation through the formation of an amide (or occasionally an ester) bond. This means the elimination of the free carboxyl group. Then, there is the question as to why a conjugate with a modified carboxyl group binds to a transporter that has the structural requirement of a free carboxyl group in its substrate. Do these biotin conjugates really rely on the biotin transporter(s) for targeting and delivery of a payload? If not, what is/are the molecular target(s) for these biotin conjugates? Second, in the literature, the term “biotin receptor” is often usedCitation79–81,Citation83,Citation85,Citation86. However, there has not been a “biotin receptor” reportedCitation72,Citation73,Citation75,Citation77,Citation91,Citation92,Citation109,Citation118. Here, there is the need to emphasise that “transporter” and “receptor” are two different concepts in biology. Again, structurally “transporters mediate the passage of molecules across cell membranes by alternating between inward- and outward-facing states, while receptors undergo intracellular structural rearrangements that initiate signaling cascadesCitation87”. There will be a need to conduct extensive studies of this issue. If biotin-based drug delivery does not rely on a biotin transporter (or specifically SMVT), then why is the reported correlation of biotin transporter expression level with the ability for biotin conjugation-based targeting? Third, the biotin transporters tend to have Km in the low to mid-micromolar rangeCitation88,Citation90,Citation92,Citation102,Citation143. Then why some biotin conjugates in the nanomolar range are effective in targeted delivery? Lastly, SMVT has a molecular weight of 68.6 kDa and transports small molecules such as biotin, α-lipoic acid, pantothenic acid, and valproic acid effectivelyCitation70,Citation102. However, there have been reports of biotin-mediated transport of payloads of high molecular weight and/or size such as biotin-conjugated polypeptides, synthetic polymers, and nanoparticlesCitation31,Citation84,Citation103,Citation144. Molecular size plays a vital role in determining its cellular uptake mechanism. For example, nanoparticles tend to undergo an endocytic uptake mechanism due to their large size while small molecules may enter cells passively or via active transport depending on their physiochemical propertiesCitation145–147. In the following subsections, we pose these questions and present our analysis.
Transporter vs. receptor
Again, earlier SAR studies have uniformly shown the critical role of the carboxylic acid group for SMVT-mediated uptake of biotin. However, biotin conjugates in targeted delivery studies uniformly lack this functional group. For instance, Chen et al.Citation103 developed biotinylated chitosan surface-modified poly(d,l-lactide-co-glycolide) nanoparticles (Bio-PLGA NPs) to deliver epirubicin (anticancer agent) to MCF-7 cells in vitro. Compared to epirubicin-loaded PLGA (54.5%), cell viability was somewhat lower (36.1%) when treated with Bio-PLGA NPs for 120 h. The cytotoxic effect exerted by Bio-PLGA NPs was attributed to the enhanced accumulation of the drug inside cancer cells. Cellular internalisation of NPs was investigated by measuring epirubicin fluorescence signals using flow cytometer. In MCF-7 cells, a ∼41% increase in fluorescence was observed after treatment with Bio-PLGA NPs for 2 h in comparison to epirubicin-loaded PLGA. Furthermore, experiments were carried out to understand the mechanism of the cellular accumulation of Bio-PLGA NPs. In this regard, cells were pre-treated with 10 µg/mL (40.93 µM) of biotin in serum-free media at 37 °C. After 24 h, cells were washed and treated with Bio-PLGA NPs at epirubicin concentration of 10 µg/mL (18.4 µM) for 2 h and further analysed by flow cytometry. Pre-treatment with biotin reduced the uptake of Bio-PLGA NPs to ∼60% of the control group. On the surface, the results seem to be consistent with biotin transporter-mediated accumulation. Further, endocytic inhibitors chlorpromazine, filipin, and amiloride were used to probe the mechanism of receptor-mediated endocytosis. In all the endocytosis experiments, endocytic inhibitors were incubated with MCF-7 cells for 24 h at 37 °C prior to the incubation with NPs for 2 h before flow cytometry analysis. Chlorpromazine is commonly used to inhibit clathrin-mediated endocytosis, which is widely regarded as a major pathway to transport cargo through endocytosisCitation148–151. Amiloride acts as an inhibitor of micropinocytosis by inhibiting Na+/H+ exchange proteins on the cell membraneCitation152. In addition, caveolae-mediated endocytosis can be inhibited by filipinCitation153. These pathways have been classified as fundamental routes for intracellular trafficking of various extracellular molecules including nutrients, hormones, and nanoparticleCitation149,Citation154,Citation155. By measuring epirubicin fluorescence using flow cytometry, a >50% reduction of cellular internalisation of Bio-PLGA NPs was noted after pre-treatment with chlorpromazine (10 µg/mL) compared to the control group. About 15% and 30% reduction of Bio-PLGA NPs cellular uptake were noted after pre-treatment with filipin (5 µg/mL) and amiloride (10 µg/mL), respectively, when compared to the respective control groups. The inhibitory effect of amiloride on biotin transport is well-known as the blockade of the Na+ channel, suggesting a possibility of impairment of sodium-dependent transport of biotin by SMVTCitation117. Interestingly, results also suggest a receptor-mediated process, since SMVT transporter does not require endocytosis for the translocation of biotin into the cells. Endocytosis is part of the receptor-mediated trafficking of cargoCitation156,Citation157. However, the identity of this “receptor” is not known and is probably not the biotin transporter. In contrast, a study by Mitra and coworkers reported that the intracellular uptake of [3H]biotin remains unaltered in human-derived prostate cancer cells (PC-3) after pre-treatment with colchicine (100 µM) compared to the control groupCitation117. Noteworthy, colchicine inhibits endocytosis by disrupting microtubule assemblyCitation109. Sinko and coworkersCitation158 also presented evidence against endocytosisCitation103 being the uptake mechanism. Briefly, enhanced accumulation of biotinylated polyethylene glycol (PEG)-based Tat9 peptide (PEG:(R.I-Cys-K(biotin)-Tat9)8) (29 kDa) was observed in Chinese hamster ovary (CHO) cells transfected with human SMVT (CHO/hSMVT). In the competition experiments, biotin-conjugated peptide (0.1 µM) was co-incubated with 50 µM of various compounds. After 10 min coincubation, free biotin diminished the uptake of biotin-conjugated peptide to ∼35%; desthiobiotin and biocytin inhibited the uptake to ∼45% as compared to the control group. Further, pantothenate, PEG–biotin (PEG MW = 3400), and biotin–PEG–biotin (PEG polymer containing two biotin molecules) reduced the uptake of the biotin-conjugated peptide to ∼55–60% of the control group. Such results show competitive inhibition of biotin-conjugated peptides by molecules with a free carboxyl group (biotin, desthiobiotin, and pantothenate) as well as molecules with an amide group (biocytin, PEG–biotin, and PEG–biotin–biotin). Overall, results from these studies further emphasise the need to carefully look into the molecular mechanism(s) of biotin transport. Along this line, Russell-Jones et al. published a comprehensive study investigating the participation of an SMVT-independent uptake system in the absorption of biotin-conjugated rhodamine-labelled hydroxypropyl-methacrylamide (Bio-Rh-HPMA; 22 kDa) and biotin-conjugated quantum dots (B-Qdots; 10–12 nm)Citation116. Bio-Rh-HPMA was synthesised through conjugation using biotin’s carboxylic group. Further, different tumour cells such as ID8, RENCA, MMT, and Ov2008 were chosen to study the in vitro uptake mechanism of Bio-Rh-HPMA (25 µL of 20 mg/mL) using fluorescent microscopy. After 4 h incubation, histological examination showed punctate staining of the tumour cells due to the accumulation of the fluorescent Bio-Rh-HPMA in vesicle-like structures, implying endosomal uptake of the Bio-Rh-HPMA. Further, experiments were also carried out in vitro with B-Qdots. RD995 cells were incubated with 2.5 µL B-Qdots in 500 µL culture medium and monitored at 1 h and 5 h time points. The concentration B-Qdots was not provided in the article. However according to the catalogue (catalogue number: Q10321MP) from the manufacturer (Invitrogen, Carlsbad, CA), it should be 2 µM (stock solution). Hence, cells were incubated with 10 nM B-Qdots. A time course experiment using a fluorescent microscope showed clustering of the fluorescent B-Qdots on the cell surface at 1 h, with minimal internalisation. B-Qdots in subcellular vesicles were observed after 5 h. The phenomenon of surface-clustering and endosomal uptake are the characteristics of receptor-mediated uptake of cargoCitation156,Citation157,Citation159–161. Such results are not strongly consistent with polymer uptake by SMVT. Furthermore, the uptake of Bio-Rh-HPMA (100 µL of 20 mg/mL for 6 h) in M109 metastatic tumour-bearing mice was also studied. Imaging studies showed that Bio-Rh-HPMA was predominantly absorbed by the tumour cells growing immediately adjacent to normal intestinal tissue. As normal intestinal cells also express SMVTCitation32, the selective uptake of fluorescent molecules by tumour cells suggests the possibility of an SMVT-independent uptake system responsible for the absorption of Bio-Rh-HPMA in tumour cells. Clearly, analysis of these studies raises several questions that warrant further investigations to delineate the cellular uptake mechanism of biotin and its conjugates.
Adding to the perplexity, a study by Smirnov and coworkersCitation94 revealed unique results in a competition assay between biotin and biotin-conjugate with PEG through an amide bond. In this study, attachment of HeLa cells onto the flat glass surface was examined. The glass surface was decorated with 1% biotin through a PEG linker. For the competition assay, the cell suspension was mixed with 0.8 mM biotin. Within 1 min, the cells were further incubated for 15 min with modified glass slides to probe the attachment of the cells on the surface. Unlike the reduction of biotin-conjugate uptake in the presence of biotin as reported by Chen et al.Citation103 and other researchersCitation46,Citation63,Citation162–164, a 3–4-fold enhancement in attachment of cells onto the modified glass surface was recorded when compared to experiments without added biotin. Similarly, an augmentation of attachment of cells onto the modified glass surface was also observed with MCF-7 cells in the competition experiment. Incidentally, a study by Wei and coworkers reported that excess biotin (4 mM) did not impair the uptake of biotinylated Atto565 (2 µM) in a competition assay in E. coliCitation95. In this study, the authors investigated the impact of biotin conjugation on the accumulation of Atto565 (a commercially available fluorescent dye) in E. coli. Interestingly, fluorescence measurement of the biotin-conjugated Atto565 showed ∼2-fold higher accumulation of Atto565 upon biotinylation. Once again, such unexpected results highlight the differential mechanism involved in the interaction of biotin and biotin-derivatised molecules with their specific uptake system(s). Given the wide-spread interest in using biotin conjugates in targeted drug delivery, these results suggest the need for further mechanistic studies to probe the cellular uptake of biotin conjugates.
The Michaelis–Menten constant
Similar to enzymes, SMVT also displays Michaelis–Menten saturation kinetics to transfer substrates across the cell membraneCitation33,Citation113,Citation158. Using this Michaelis–Menten equation, several researchers have extensively studied SMVT-mediated uptake of biotin. Previously, the Km value of biotin was calculated to be 9.5 µM in Caco-2 cellsCitation88, 32.52 µM in rabbit corneal epithelial cells, 63.8 µM in Statens SerumInstitut Rabbit Cornea (SIRC) cellsCitation90, and 19.7 µM in human colonic epithelial NCM460 cellsCitation92. Along this direction, Sinko and coworkers have investigated the uptake of a biotin conjugate, i.e. [3H]PEG-biotin (PEG MW = 3400) in Caco-2 cellsCitation158. The kinetic studies showed significant inhibition of [3H]PEG-biotin uptake by free biotin with an inhibition constant (Ki) of 6.78 µM. The Ki for desthiobiotin, biocytin, and biotin–PEG–biotin was calculated to be 11.47, 14.01, and 19.08 µM, respectively. The Km value of biotin–PEG was calculated to be 6.61 µM in Caco-2 cells. Once again, these results clearly show competitive inhibition of biotin–PEG by biotin and its derivatives without a free carboxylic acid. Noteworthy, biocytin has been reported to be a weak competitor of biotin transport by SMVT in T47D cells (entry 5, )Citation89, Caco-2Citation88, and other cell linesCitation89,Citation118. The Km value of biotin–PEG was also found to be similar as shown by biotin towards SMVT in Caco-2 cellsCitation88. Based on the Km values, authors stated that both biotin and its conjugates devoid of a carboxylic acid group share the same uptake mechanism. Simultaneously, such results also contradict earlier findings, which indicate the essential nature of a free carboxylic acid group for SMVT-mediated transport. Hence, this example further emphasises the need to carefully examine the molecular mechanism(s) of biotin transport.
Small vs. large molecule
In the past two decades, biotin has been widely used as a targeting ligand for delivering small molecules such as doxorubicin, gemcitabine, and large molecules such as polypeptides, polysaccharides, nanoparticles, and micellesCitation31,Citation45,Citation116,Citation162,Citation165. If we take a look at the molecular weights of the currently known SMVT substrates, all of these molecules are small molecules (). Of note, the molecular weight of SMVT is calculated to be 68.6 kDaCitation70,Citation102. Generally, molecules <1 kDa molecular weight are categorised as small moleculesCitation145,Citation147. However, it is imperative to ask the question of whether biotin-conjugated small molecules share the same uptake mechanism as conjugated macromolecules or particles. For instance, Chen et al. applied Bio-PLGA NPs (10–30 kDa) to selectively deliver epirubicin to cancer cellsCitation103. These nanoparticles have 50–150 times higher molecular weights than biotin. Generally speaking, there is the question of whether a transporter meant for a small molecule is capable of transporting a nanoparticle. As described in the previous section, Bio-PLGA NPs undergo the endocytosis pathway to enter cells as demonstrated by the experiments applying endocytosis inhibitors, viz., chlorpromazine, filipin, and amiloride. Similarly, experiments conducted by Russell-Jones et al. employing Bio-Rh-HPMA (22 kDa) also indicate the participation of endocytic pathways to deliver Bio-Rh-HPMA to tumour cells as demonstrated by imaging studiesCitation116. Therefore, these findings point to the possibility of the presence of multiple mechanisms for the uptake of such biotin-conjugates that are devoid of a carboxylate group and comprise high molecular weight. Endocytosis for the trafficking of biotinylated cargos seems to be a distinct possibility as shown by the experiments applying endocytosis inhibitors.
Conclusions
Biotin has been widely studied as a vector for targeted drug delivery. SMVT has been recognised as the major transporter of biotin. Based on SAR studies, it has been found that the free aliphatic carboxylic acid of biotin plays a vital role in its recognition by SMVT. Importantly, the modification of the carboxylic acid of biotin to an amide or ester functional group seems to abolish its SMVT-mediated uptake. However, biotin-mediated drug delivery relies on the payload moiety being conjugated through amidation or esterification of the free carboxylic acid of biotin. Despite being devoid of free carboxylic acid, these biotin conjugates are reported to target biotin uptake system(s) for enhanced cell permeation. The results from the competition assay of biotin conjugates with free biotin and endocytosis experiments imply the involvement of multiple mechanisms for the uptake of biotin conjugates. In this regard, further studies are warranted to delineate the cellular uptake mechanism of biotin conjugates. A clear understanding of such mechanism(s) will be critical for the future design and development of biotin-based therapeutics.
Author contributions
The manuscript was prepared with contributions from all authors. RT and AG led the manuscript preparation work and contributed equally; RG assisted in the process. BW conceived the idea of the manuscript, guided the preparation process, revised the manuscript, and gave final approval of the manuscript. All authors have given approval to the submitted draft of the manuscript.
Acknowledgements
We gratefully acknowledge the National Institutes of Health (R01DK119202 and R01DK128823) for partial financial support of our drug delivery work in general. We would also like to thank the Molecular Basis of Disease program for a graduate fellowship to R. Tripathi, internal resources at Georgia State University, the Georgia Research Alliance for an Eminent Scholar endowment, and the Dr. Frank Hannah endowment fund for providing financial support to our drug delivery and drug discovery programs in general. The graphical abstract and Figure 2 were created using BioRender.com.
Disclosure statement
Binghe Wang is an associated editor of the Journal of Enzyme Inhibition and Medicinal Chemistry. There is no other potential conflict of interest to be declared by the authors.
Additional information
Funding
References
- Bebenek I, Bannister R, Dubinion J, Fortin M, Liu M, Motter AL, Rohde CM, Wrzesinski C. COVID-19 therapeutics and vaccines: a race to save lives. Toxicol Sci. 2022;185(2):119–127.
- Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–967.
- Liu M, Fang X, Yang Y, Wang C. Peptide-enabled targeted delivery systems for therapeutic applications. Front Bioeng Biotechnol. 2021;9:701504.
- Majumdar S, Siahaan TJ. Peptide-mediated targeted drug delivery. Med Res Rev. 2012;32(3):637–658.
- Firer MA, Gellerman G. Targeted drug delivery for cancer therapy: the other side of antibodies. J Hematol Oncol. 2012;5(1):70.
- Tan X, Jia F, Wang P, Zhang K. Nucleic acid-based drug delivery strategies. J Control Release. 2020;323:240–252.
- Chen K, Zhang Y, Zhu L, Chu H, Shao X, Asakiya C, Huang K, Xu W. Insights into nucleic acid-based self-assembling nanocarriers for targeted drug delivery and controlled drug release. J Control Release. 2022;341:869–891.
- Russell-Jones G, McTavish K, McEwan J, Rice J, Nowotnik D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J Inorg Biochem. 2004;98(10):1625–1633.
- Narmani A, Rezvani M, Farhood B, Darkhor P, Mohammadnejad J, Amini B, Refahi S, Abdi Goushbolagh N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424.
- Xiao W, Fu Q, Zhao Y, Zhang L, Yue Q, Hai L, Guo L, Wu Y. Ascorbic acid-modified brain-specific liposomes drug delivery system with "lock-in" function. Chem Phys Lipids. 2019;224:104727.
- Clardy SM, Allis DG, Fairchild TJ, Doyle RP. Vitamin B12 in drug delivery: breaking through the barriers to a B12 bioconjugate pharmaceutical. Expert Opin Drug Deliv. 2011;8(1):127–140.
- Chahibi Y, Akyildiz IF, Song OS. Antibody-based molecular communication for targeted drug delivery systems. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5707–5710.
- Awwad S, Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018;10(3):83.
- Gu L, Duan Z, Li X, Li X, Li Y, Li X, Xu G, Gao P, Zhang H, Gu Z, et al. Enzyme-triggered deep tumor penetration of a dual-drug nanomedicine enables an enhanced cancer combination therapy. Bioact Mater. 2023;26:102–115.
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124.
- Battogtokh G, Choi YS, Kang DS, Park SJ, Shim MS, Huh KM, Cho YY, Lee JY, Lee HS, Kang HC. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm Sin B. 2018;8(6):862–880.
- Zheng Y, Ji X, Yu B, Ji K, Gallo D, Csizmadia E, Zhu M, Choudhury MR, De La Cruz LKC, Chittavong V, et al. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat Chem. 2018;10(7):787–794.
- Yue Y, Huo F, Lee S, Yin C, Yoon J. A review: the trend of progress about pH probes in cell application in recent years. Analyst. 2016;142(1):30–41.
- Ambiliraj DB, Francis B, Reddy MLP. Lysosome-targeting luminescent lanthanide complexes: from molecular design to bioimaging. Dalton Trans. 2022;51(20):7748–7762.
- Gregor A, Lind M, Newman H, Grant R, Hadley DM, Barton T, Osborn C. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J Neurooncol. 1999;44(2):137–145.
- Liu LB, Xue YX, Liu YH. Bradykinin increases the permeability of the blood–tumor barrier by the caveolae-mediated transcellular pathway. J Neurooncol. 2010;99(2):187–194.
- Hampl R, Bičíková M, Sosvorová L. Hormones and the blood–brain barrier. Horm Mol Biol Clin Investig. 2015;21(3):159–164.
- Yang W, Gao S, Gao X, Karnati VV, Ni W, Wang B, Hooks WB, Carson J, Weston B. Diboronic acids as fluorescent probes for cells expressing sialyl Lewis X. Bioorg Med Chem Lett. 2002;12(16):2175–2177.
- Gao X, Zhu M, Fan H, Yang W, Ni W, Karnati VV, Gao S, Carson J, Weston B, Wang B. A fluorescent bisboronic acid compound that selectively labels cells expressing oligosaccharide Lewis X. Bioorg Med Chem Lett. 2015;25(12):2501–2504.
- Chu Y, Wang D, Wang K, Liu ZL, Weston B, Wang B. Fluorescent conjugate of sLe(x)-selective bisboronic acid for imaging application. Bioorg Med Chem Lett. 2013;23(23):6307–6309.
- Yang W, Fan H, Gao X, Gao S, Karnati VV, Ni W, Hooks WB, Carson J, Weston B, Wang B. The first fluorescent diboronic acid sensor specific for hepatocellular carcinoma cells expressing sialyl Lewis X. Chem Biol. 2004;11(4):439–448.
- Li S, Yu B, Wang J, Zheng Y, Zhang H, Walker MJ, Yuan Z, Zhu H, Zhang J, Wang PG, et al. Biomarker-based metabolic labeling for redirected and enhanced immune response. ACS Chem Biol. 2018;13(6):1686–1694.
- Jiang J, Wang W, Sane DC, Wang B. Synthesis of RGD analogs as potential vectors for targeted drug delivery. Bioorg Chem. 2001;29(6):357–379.
- Berreau LM. Targeted delivery of carbon monoxide. In: Wang B, Otterbein LE, editors. Carbon monoxide in drug discovery: basics, pharmacology, and therapeutic potential. Hoboken (NJ): John Wiley and Sons; 2022. p. 259–285. ISBN: 9781119783404.
- Das SK, Ghilzai NMK, Scolaro KL. Drug delivery: principles and applications. Am J Pharm Educ. 2006;70(4):94.
- Ren WX, Han J, Uhm S, Jang YJ, Kang C, Kim JH, Kim JS. Recent development of biotin conjugation in biological imaging, sensing, and target delivery. Chem Commun. 2015;51(52):10403–10418.
- Vadlapudi AD, Vadlapatla RK, Mitra AK. Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr Drug Targets. 2012;13(7):994–1003.
- Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multivitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm. 2006;3(3):329–339.
- McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22(1):221–239.
- León-Del-Río A. Biotin in metabolism, gene expression, and human disease. J Inherit Metab Dis. 2019;42(4):647–654.
- Zempleni J, Wijeratne SS, Hassan YI. Biotin. Biofactors. 2009;35(1):36–46.
- Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci. 2013;70(5):863–891.
- Dakshinamurti K, Chalifour LE. The biotin requirement of HeLa cells. J Cell Physiol. 1981;107(3):427–438.
- Zempleni J, Helm RM, Mock DM. In vivo biotin supplementation at a pharmacologic dose decreases proliferation rates of human peripheral blood mononuclear cells and cytokine release. J Nutr. 2001;131(5):1479–1484.
- Luong JHT, Vashist SK. Chemistry of biotin–streptavidin and the growing concern of an emerging biotin interference in clinical immunoassays. ACS Omega. 2020;5(1):10–18.
- Crisp SE, Griffin JB, White BR, Toombs CF, Camporeale G, Said HM, Zempleni J. Biotin supply affects rates of cell proliferation, biotinylation of carboxylases and histones, and expression of the gene encoding the sodium-dependent multivitamin transporter in JAr choriocarcinoma cells. Eur J Nutr. 2004;43(1):23–31.
- Schenker S, Hu ZQ, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM. Human placental biotin transport: normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993;17(3):566–575.
- Weiner D, Wolf B. Biotin uptake, utilization, and efflux in normal and biotin-deficient rat hepatocytes. Biochem Med Metab Biol. 1991;46(3):344–363.
- Mock DM, Malik MI. Distribution of biotin in human plasma: most of the biotin is not bound to protein. Am J Clin Nutr. 1992;56(2):427–432.
- Maiti S, Paira P. Biotin conjugated organic molecules and proteins for cancer therapy: a review. Eur J Med Chem. 2018;145:206–223.
- Chen S, Zhao X, Chen J, Chen J, Kuznetsova L, Wong SS, Ojima I. Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release. Bioconjug Chem. 2010;21(5):979–987.
- Horn MA, Heinstein PF, Low PS. Biotin-mediated delivery of exogenous macromolecules into soybean cells. Plant Physiol. 1990;93(4):1492–1496.
- Strydom S, Van Jaarsveld P, Van Helden E, Ariatti M, Hawtrey A. Studies on the transfer of DNA into cells through use of avidin–polylysine conjugates complexed to biotinylated transferrin and DNA. J Drug Target. 1993;1(2):165–174.
- Sakahara H, Saga T. Avidin–biotin system for delivery of diagnostic agents. Adv Drug Deliv Rev. 1999;37(1–3):89–101.
- Lindgren M, Gallet X, Soomets U, Hällbrink M, Bråkenhielm E, Pooga M, Brasseur R, Langel U. Translocation properties of novel cell penetrating transportan and penetratin analogues. Bioconjug Chem. 2000;11(5):619–626.
- Lee HJ, Pardridge WM. Pharmacokinetics and delivery of tat and tat–protein conjugates to tissues in vivo. Bioconjug Chem. 2001;12(6):995–999.
- Chen LL, Frankel AD, Harder JL, Fawell S, Barsoum J, Pepinsky B. Increased cellular uptake of the human immunodeficiency virus-1 Tat protein after modification with biotin. Anal Biochem. 1995;227(1):168–175.
- Ferraretto A, Sonnino S, Soria MR, Masserini M. Characterization of biotinylated liposomes sensitive to temperature and pH: new tools for anti-cancer drug delivery. Chem Phys Lipids. 1996;82(2):133–139.
- Loughrey HC, Ferraretto A, Cannon AM, Acerbis G, Sudati F, Bottiroli G, Masserini M, Soria MR. Characterisation of biotinylated liposomes for in vivo targeting applications. FEBS Lett. 1993;332(1–2):183–188.
- Soria MR, Loughrey H, Ferraretto A, Cannon A-M, Acerbis G, Sudati F, Bottiroli G, Masserini M. Targeting applications of biotinylated liposomes. J Liposome Res. 1993;3(3):543–549.
- Urdal DL, Hakomori S. Tumor-associated ganglio-N-triosylceramide. Target for antibody-dependent, avidin-mediated drug killing of tumor cells. J Biol Chem. 1980;255(21):10509–10516.
- Bayer EA, Rivnay B, Skutelsky E. On the mode of liposome-cell interactions. Biotin-conjugated lipids as ultrastructural probes. Biochim Biophys Acta. 1979;550(3):464–473.
- Choudhury I, Wang J, Rabson AB, Stein S, Pooyan S, Stein S, Leibowitz MJ. Inhibition of HIV-1 replication by a Tat RNA-binding domain peptide analog. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(2):104–111.
- Ramanathan S, Pooyan S, Stein S, Prasad PD, Wang J, Leibowitz MJ, Ganapathy V, Sinko PJ. Targeting the sodium-dependent multivitamin transporter (SMVT) for improving the oral absorption properties of a retro-inverso Tat nonapeptide. Pharm Res. 2001;18(7):950–956.
- Yang W, Cheng Y, Xu T, Wang X, Wen LP. Targeting cancer cells with biotin–dendrimer conjugates. Eur J Med Chem. 2009;44(2):862–868.
- Lu R, Zhou L, Yue Q, Liu Q, Cai X, Xiao W, Hai L, Guo L, Wu Y. Liposomes modified with double-branched biotin: a novel and effective way to promote breast cancer targeting. Bioorg Med Chem. 2019;27(14):3115–3127.
- Nosrati H, Barzegari P, Danafar H, Kheiri Manjili H. Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artif Cells Nanomed Biotechnol. 2019;47(1):104–114.
- Lee Y, Lee S, Jon S. Biotinylated bilirubin nanoparticles as a tumor microenvironment-responsive drug delivery system for targeted cancer therapy. Adv Sci. 2018;5(6):1800017.
- Kim SY, Cho SH, Lee YM, Chu L-Y. Biotin-conjugated block copolymeric nanoparticles as tumor-targeted drug delivery systems. Macromol Res. 2007;15(7):646–655.
- Beckett D. Biotin sensing: universal influence of biotin status on transcription. Annu Rev Genet. 2007;41(1):443–464.
- Gravel RA, Narang MA. Molecular genetics of biotin metabolism: old vitamin, new science. J Nutr Biochem. 2005;16(7):428–431.
- Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol. 2015;93(12):1091–1096.
- León-Del-Río A. Biotin-dependent regulation of gene expression in human cells. J Nutr Biochem. 2005;16(7):432–434.
- Lesch HP, Kaikkonen MU, Pikkarainen JT, Ylä-Herttuala S. Avidin–biotin technology in targeted therapy. Expert Opin Drug Deliv. 2010;7(5):551–564.
- Quick M, Shi L. The sodium/multivitamin transporter: a multipotent system with therapeutic implications. Vitam Horm. 2015;98:63–100.
- Riveron-Negrete L, Fernandez-Mejia C. Pharmacological effects of biotin in animals. Mini Rev Med Chem. 2017;17(6):529–540.
- Said HM. Cell and molecular aspects of human intestinal biotin absorption. J Nutr. 2009;139(1):158–162.
- Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem. 2012;56:1–19.
- Sirithanakorn C, Cronan JE. Biotin, a universal and essential cofactor: synthesis, ligation and regulation. FEMS Microbiol Rev. 2021;45(4):fuab003.
- Zempleni J, Teixeira DC, Kuroishi T, Cordonier EL, Baier S. Biotin requirements for DNA damage prevention. Mutat Res. 2012;733(1–2):58–60.
- Zempleni J. Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr. 2005;25(1):175–196.
- Zempleni J, Kuroishi T. Biotin. Adv Nutr. 2012;3(2):213–214.
- Mock DM. Biotin: from nutrition to therapeutics. J Nutr. 2017;147(8):1487–1492.
- Fam KT, Collot M, Klymchenko AS. Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chem Sci. 2020;11(31):8240–8248.
- Bhuniya S, Maiti S, Kim EJ, Lee H, Sessler JL, Hong KS, Kim JS. An activatable theranostic for targeted cancer therapy and imaging. Angew Chem Int Ed Engl. 2014;53(17):4469–4474.
- Li K, Dong W, Liu Q, Lv G, Xie M, Sun X, Qiu L, Lin J. A biotin receptor-targeted silicon(IV) phthalocyanine for in vivo tumor imaging and photodynamic therapy. J Photochem Photobiol B. 2019;190:1–7.
- Bongarzone S, Sementa T, Dunn J, Bordoloi J, Sunassee K, Blower PJ, Gee A. Imaging biotin trafficking in vivo with positron emission tomography. J Med Chem. 2020;63(15):8265–8275.
- Cetin M, Youn YS, Capan Y, Lee KC. Preparation and characterization of salmon calcitonin–biotin conjugates. AAPS PharmSciTech. 2008;9(4):1191–1197.
- Doerflinger A, Quang NN, Gravel E, Pinna G, Vandamme M, Ducongé F, Doris E. Biotin-functionalized targeted polydiacetylene micelles. Chem Commun. 2018;54(29):3613–3616.
- Vinothini K, Rajendran NK, Munusamy MA, Alarfaj AA, Rajan M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater Sci Eng C Mater Biol Appl. 2019;100:676–687.
- Kunjiappan S, Pavadai P, Vellaichamy S, Ram Kumar Pandian S, Ravishankar V, Palanisamy P, Govindaraj S, Srinivasan G, Premanand A, Sankaranarayanan M, et al. Surface receptor-mediated targeted drug delivery systems for enhanced cancer treatment: a state-of-the-art review. Drug Dev Res. 2021;82(3):309–340.
- Del Alamo D, Sala D, McHaourab HS, Meiler J. Sampling alternative conformational states of transporters and receptors with AlphaFold2. Elife. 2022;11:e75751.
- Ma TY, Dyer DL, Said HM. Human intestinal cell line Caco-2: a useful model for studying cellular and molecular regulation of biotin uptake. Biochim Biophys Acta. 1994;1189(1):81–88.
- Vadlapudi AD, Vadlapatla RK, Pal D, Mitra AK. Biotin uptake by T47D breast cancer cells: functional and molecular evidence of sodium-dependent multivitamin transporter (SMVT). Int J Pharm. 2013;441(1–2):535–543.
- Janoria KG, Hariharan S, Paturi D, Pal D, Mitra AK. Biotin uptake by rabbit corneal epithelial cells: role of sodium-dependent multivitamin transporter (SMVT). Curr Eye Res. 2006;31(10):797–809.
- Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport for biotin in human intestinal brush-border membrane vesicles. Am J Physiol. 1987;253(5 Pt 1):G631–G636.
- Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275(5):C1365–C1371.
- Kansara V, Luo S, Balasubrahmanyam B, Pal D, Mitra AK. Biotin uptake and cellular translocation in human derived retinoblastoma cell line (Y-79): a role of hSMVT system. Int J Pharm. 2006;312(1–2):43–52.
- Subedi D, Ashley AK, Chavez MV, Smirnov SN. Mixed silane monolayers reveal the disparity of biotin and folate in targeting cancer cells. ACS Appl Nano Mater. 2020;3(6):5372–5380.
- Pandeya A, Yang L, Alegun O, Karunasena C, Risko C, Li Z, Wei Y. Biotinylation as a tool to enhance the uptake of small molecules in Gram-negative bacteria. PLOS One. 2021;16(11):e0260023.
- Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20(10):1487–1498.
- Daberkow RL, White BR, Cederberg RA, Griffin JB, Zempleni J. Monocarboxylate transporter 1 mediates biotin uptake in human peripheral blood mononuclear cells. J Nutr. 2003;133(9):2703–2706.
- Grafe F, Wohlrab W, Neubert RH, Brandsch M. Transport of biotin in human keratinocytes. J Invest Dermatol. 2003;120(3):428–433.
- Holling T, Nampoothiri S, Tarhan B, Schneeberger PE, Vinayan KP, Yesodharan D, Roy AG, Radhakrishnan P, Alawi M, Rhodes L, et al. Novel biallelic variants expand the SLC5A6-related phenotypic spectrum. Eur J Hum Genet. 2022;30(4):439–449.
- Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem. 1999;274(21):14875–14883.
- Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am J Physiol. 1999;277(4):C605–C613.
- Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1998;273(13):7501–7506.
- Chen H, Xie LQ, Qin J, Jia Y, Cai X, Nan W, Yang W, Lv F, Zhang QQ. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf B Biointerfaces. 2016;138:1–9.
- Uchida Y, Ito K, Ohtsuki S, Kubo Y, Suzuki T, Terasaki T. Major involvement of Na+-dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J Neurochem. 2015;134(1):97–112.
- Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev. 2011;63(1–2):3–23.
- Halestrap AP. The monocarboxylate transporter family – structure and functional characterization. IUBMB Life. 2012;64(1):1–9.
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–299.
- Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271(2):861–868.
- Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am J Physiol. 1998;275(2):C382–C388.
- Sudo K. Enzyme kinetics for enzyme immunoassay. Nihon Rinsho. 1995;53(9):2134–2139.
- Su D, Kosciuk T, Yang M, Price IR, Lin H. Binding affinity determines substrate specificity and enables discovery of substrates for N-myristoyltransferases. ACS Catal. 2021;11(24):14877–14883.
- Schultz SG. Membrane transport, general concepts. In: Lennarz WJ, Lane MD, editors. Encyclopedia of biological chemistry. 2nd ed. Academic Press; 2013. p. 49–51.
- Stillwell W. Membrane transport. In: An introduction to biological membranes; 2016. p. 423–451.
- de Carvalho FD, Quick M. Surprising substrate versatility in SLC5A6: Na+-coupled I– transport by the human Na+/multivitamin transporter (hSMVT). J Biol Chem. 2011;286(1):131–137.
- Wang W, Ackermann D, Mehlich AM, König S. False labelling due to quenching failure of N-hydroxy-succinimide-ester-coupled dyes. Proteomics. 2010;10(7):1525–1529.
- Russell-Jones G, McTavish K, McEwan J. Preliminary studies on the selective accumulation of vitamin-targeted polymers within tumors. J Drug Target. 2011;19(2):133–139.
- Patel M, Vadlapatla RK, Shah S, Mitra AK. Molecular expression and functional activity of sodium dependent multivitamin transporter in human prostate cancer cells. Int J Pharm. 2012;436(1–2):324–331.
- Baur B, Baumgartner ER. Biotin and biocytin uptake into cultured primary calf brain microvessel endothelial cells of the blood–brain barrier. Brain Res. 2000;858(2):348–355.
- Chirapu SR, Rotter CJ, Miller EL, Varma MV, Dow RL, Finn MG. High specificity in response of the sodium-dependent multivitamin transporter to derivatives of pantothenic acid. Curr Top Med Chem. 2013;13(7):837–842.
- Saha S, Majumdar R, Hussain A, Dighe RR, Chakravarty AR. Biotin-conjugated tumour-targeting photocytotoxic iron(III) complexes. Philos Trans A Math Phys Eng Sci. 2013;371(1995):20120190.
- Jung D, Maiti S, Lee JH, Lee JH, Kim JS. Rational design of biotin–disulfide–coumarin conjugates: a cancer targeted thiol probe and bioimaging. Chem Commun. 2014;50(23):3044–3047.
- McConnell DB. Biotin’s lessons in drug design. J Med Chem. 2021;64(22):16319–16327.
- Maiti S, Park N, Han JH, Jeon HM, Lee JH, Bhuniya S, Kang C, Kim JS. Gemcitabine–coumarin–biotin conjugates: a target specific theranostic anticancer prodrug. J Am Chem Soc. 2013;135(11):4567–4572.
- Hu W, Fang L, Hua W, Gou S. Biotin–Pt (IV)–indomethacin hybrid: a targeting anticancer prodrug providing enhanced cancer cellular uptake and reversing cisplatin resistance. J Inorg Biochem. 2017;175:47–57.
- Zhang X, Qi J, Lu Y, He W, Li X, Wu W. Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine. 2014;10(1):167–176.
- Zhu W, Song Z, Wei P, Meng N, Teng F, Yang F, Liu N, Feng R. Y-shaped biotin-conjugated poly (ethylene glycol)–poly (epsilon-caprolactone) copolymer for the targeted delivery of curcumin. J Colloid Interface Sci. 2015;443:1–7.
- Shi JF, Wu P, Jiang ZH, Wei XY. Synthesis and tumor cell growth inhibitory activity of biotinylated annonaceous acetogenins. Eur J Med Chem. 2014;71:219–228.
- Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC, Lee WJ, Sun IC, Kwon IK. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials. 2012;33(3):856–866.
- Huang GN. Biotinylation of cell surface proteins. Bio Protoc. 2012;2(9):e170.
- Li Y, Wang Y, Mao J, Yao Y, Wang K, Qiao Q, Fang Z, Ye M. Sensitive profiling of cell surface proteome by using an optimized biotinylation method. J Proteomics. 2019;196:33–41.
- Li M, Peng F, Wang G, Liang X, Shao M, Chen Z, Chen Y. Coupling of cell surface biotinylation and SILAC-based quantitative proteomics identified myoferlin as a potential therapeutic target for nasopharyngeal carcinoma metastasis. Front Cell Dev Biol. 2021;9:621810.
- Hörmann K, Stukalov A, Müller AC, Heinz LX, Superti-Furga G, Colinge J, Bennett KL. A surface biotinylation strategy for reproducible plasma membrane protein purification and tracking of genetic and drug-induced alterations. J Proteome Res. 2016;15(2):647–658.
- Kirkemo LL, Elledge SK, Yang J, Byrnes JR, Glasgow JE, Blelloch R, Wells JA. Cell-surface tethered promiscuous biotinylators enable comparative small-scale surface proteomic analysis of human extracellular vesicles and cells. Elife. 2022;11:e73982.
- Hong M, Xu W, Yoshida T, Tanaka K, Wolff DJ, Zhou F, Inouye M, You G. Human organic anion transporter hOAT1 forms homooligomers. J Biol Chem. 2005;280(37):32285–32290.
- Niinae T, Ishihama Y, Imami K. Biotinylation-based proximity labelling proteomics: basics, applications and technical considerations. J Biochem. 2021;170(5):569–576.
- Liu G, Choi MH, Ma H, Guo X, Lo PC, Kim J, Zhang L. Bioorthogonal conjugation-assisted purification method for profiling cell surface proteome. Anal Chem. 2022;94(3):1901–1909.
- Kähne T, Ansorge S. Non-radioactive labelling and immunoprecipitation analysis of leukocyte surface proteins using different methods of protein biotinylation. J Immunol Methods. 1994;168(2):209–218.
- Karhemo PR, Ravela S, Laakso M, Ritamo I, Tatti O, Mäkinen S, Goodison S, Stenman UH, Hölttä E, Hautaniemi S, et al. An optimized isolation of biotinylated cell surface proteins reveals novel players in cancer metastasis. J Proteomics. 2012;77:87–100.
- Langó T, Kuffa K, Tóth G, Turiák L, Drahos L, Tusnády GE. Comprehensive discovery of the accessible primary amino group-containing segments from cell surface proteins by fine-tuning a high-throughput biotinylation method. Int J Mol Sci. 2022;24(1):273.
- Alandejani SA, Malaczynska J, Bluth MJ, Das B, Norin AJ. Moesin: a novel receptor on NK lymphocytes binds to TOMM40 on K562 leukemia cells initiating cytolysis. Hum Immunol. 2022;83(5):418–427.
- Kumar V, Nguyen TB, Tóth B, Juhasz V, Unadkat JD. Optimization and application of a biotinylation method for quantification of plasma membrane expression of transporters in cells. AAPS J. 2017;19(5):1377–1386.
- Belleannee C, Belghazi M, Labas V, Teixeira-Gomes AP, Gatti JL, Dacheux JL, Dacheux F. Purification and identification of sperm surface proteins and changes during epididymal maturation. Proteomics. 2011;11(10):1952–1964.
- Prasad PD, Ganapathy V. Keratinocytes join forces with immune cells in the prosecution of SMVT as a "false" biotin transporter. J Invest Dermatol. 2003;120(3):xi–xii.
- Li H, Bruce G, Childerhouse N, Keegan G, Mantovani G, Stolnik S. Biotin receptor-mediated intracellular delivery of synthetic polypeptide–protein complexes. J Control Release. 2023;357:333–341.
- Mosquera J, García I, Liz-Marzán LM. Cellular uptake of nanoparticles versus small molecules: a matter of size. Acc Chem Res. 2018;51(9):2305–2313.
- Sun XY, Gan QZ, Ouyang JM. Size-dependent cellular uptake mechanism and cytotoxicity toward calcium oxalate on Vero cells. Sci Rep. 2017;7(1):41949.
- Matsson P, Kihlberg J. How big is too big for cell permeability? J Med Chem. 2017;60(5):1662–1664.
- Montizaan D, Yang K, Reker-Smit C, Salvati A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells. Nanomedicine. 2020;30:102300.
- Rennick JJ, Johnston APR, Parton RG. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16(3):266–276.
- Bitsikas V, Corrêa IR Jr., Nichols BJ. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife. 2014;3:e03970.
- Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326.
- Bobulescu IA, Di Sole F, Moe OW. Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr Opin Nephrol Hypertens. 2005;14(5):485–494.
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127(5):1217–1232.
- Schwartz AL. Receptor cell biology: receptor-mediated endocytosis. Pediatr Res. 1995;38(6):835–843.
- Shen Z, Ye H, Kröger M, Li Y. Aggregation of polyethylene glycol polymers suppresses receptor-mediated endocytosis of PEGylated liposomes. Nanoscale. 2018;10(9):4545–4560.
- Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 2005;102(27):9469–9474.
- Liu AP, Aguet F, Danuser G, Schmid SL. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol. 2010;191(7):1381–1393.
- Ramanathan S, Qiu B, Pooyan S, Zhang G, Stein S, Leibowitz MJ, Sinko PJ. Targeted PEG-based bioconjugates enhance the cellular uptake and transport of a HIV-1 TAT nonapeptide. J Control Release. 2001;77(3):199–212.
- Li M, Yu Y. Innate immune receptor clustering and its role in immune regulation. J Cell Sci. 2021;134(4):134.
- Duke T, Graham I. Equilibrium mechanisms of receptor clustering. Prog Biophys Mol Biol. 2009;100(1–3):18–24.
- Caré BR, Soula HA. Impact of receptor clustering on ligand binding. BMC Syst Biol. 2011;5(1):48.
- Rompicharla SVK, Kumari P, Bhatt H, Ghosh B, Biswas S. Biotin functionalized PEGylated poly(amidoamine) dendrimer conjugate for active targeting of paclitaxel in cancer. Int J Pharm. 2019;557:329–341.
- Dutta D, Alex SM, Bobba KN, Maiti KK, Bhuniya S. New insight into a cancer theranostic probe: efficient cell-specific delivery of SN-38 guided by biotinylated poly(vinyl alcohol). ACS Appl Mater Interfaces. 2016;8(49):33430–33438.
- Yellepeddi VK, Kumar A, Palakurthi S. Biotinylated poly(amido)amine (PAMAM) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer Res. 2009;29(8):2933–2943.
- Tian X, Yin H, Zhang S, Luo Y, Xu K, Ma P, Sui C, Meng F, Liu Y, Jiang Y, et al. Bufalin loaded biotinylated chitosan nanoparticles: an efficient drug delivery system for targeted chemotherapy against breast carcinoma. Eur J Pharm Biopharm. 2014;87(3):445–453.