Abstract
Two series of N-alkyl isatins and N-alkyl indoles varying in size of the alkyl group were synthesised and evaluated for inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Among the N-alkyl isatins 4a-j, the addition of the N-alkyl group improved inhibition potency towards AChE and BChE compared to isatin. Selectivity towards inhibition of BChE was observed, and the increase in size of the N-alkyl group positively correlated to improved inhibition potency. The most potent inhibitor for BChE was 4i (IC50 = 3.77 µM, 22-fold selectivity for BChE over AChE). N-alkyl indoles 5a-j showed similar inhibition of AChE, the most potent being 5g (IC50 = 35.0 µM), but 5a-j lost activity towards BChE. This suggests an important role of the 3-oxo group on isatin for BChE inhibition, and molecular docking of 4i with human BChE indicates a key hydrogen bond between this group and Ser198 and His438 of the BChE catalytic triad.
Introduction
Acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC 3.1.1.8) are well-studied enzymes with structural, mechanistic, and physiological similaritiesCitation1. Both enzymes contain three important structural features: a catalytic active site (CAS), gorge region, and peripheral anionic site (PAS). The CAS of each enzyme contains the Ser-His-Glu catalytic triad common to serine esterases, as well as anionic, oxyanion hole, and acyl pocket subsites. The gorge is about 20 Å long and connects the CAS at the enzyme interior to the PAS at the enzyme surface. Aromatic amino acids are prominent in the CAS, gorge, and PAS of both AChE and BChE. However, key amino acid substitutions in BChE relative to AChE are worth mentioning. First, the replacement of two Phe residues with Leu and Val in the acyl pocket allows BChE to bind larger substrates. Additionally, several aromatic residues are substituted with hydrophobic residues in the lining of the BChE gorgeCitation2–5.
AChE and BChE are both well-known enzymatic targets for treatment of Alzheimer’s disease (AD), and AChE and BChE inhibitors (AChEI and BChEI, respectively) have long been of interest. Under normal conditions, AChE hydrolyses acetylcholine (ACh) to regulate neurotransmissionCitation6. Inhibition of this enzyme boosts ACh levels to treat symptoms of AD, and it may also affect other AD etiologiesCitation7–9. Inhibition of BChE, which shows increased levels in advanced stages of AD, can have a similar beneficial effectCitation10,Citation11. Our lab has an ongoing interest in exploring new or understudied chemical scaffolds as dual inhibitors of AChE and BChE or selective inhibitors of one or the other.
Nitrogen-containing heterocycles show diverse biological activities in the literature, and they are well-known scaffolds for the design of AChEI and BChEI, which has recently been reviewedCitation12,Citation13. Isatin (indoline-2,3-dione) and indole (), and derivatives thereof, are no exception with various reports of inhibition of AChE and BChECitation14–17. We recently reported isatin dimer 1 and 3-indolyl-3-hydroxy-2-oxindole dimer 2 as selective BChEI (IC50 = 7.56 and 4.49 µM, respectively) (.Citation18 Prior, we reported on the AChE inhibition of isatin-linked thiazoline-2-thiol 3 (IC50 = 18.2 µM) (.Citation19 In the course of this previous work, we noted that 1-butylindoline-2,3-dione (4d, see Scheme 1), in which the nitrogen of isatin has been substituted with a butyl group, showed weak inhibition of AChE (IC50 = 540 µM). Compound 4d was prepared in the context of using it as a control for inhibition by comparison with 3 and was expected to be inactive. However, the weak inhibition caught our attention, and further investigation of 4d showed selectivity for BChE inhibition (IC50 = 73.7 µM) (previously unpublished). Naturally, this led us to wonder about the structure-activity relationship (SAR) between size of the alkyl group and inhibition of AChE and/or BChE. To further complement this N-alkyl SAR investigation, we also chose to investigate the role of the 2-oxo and 3-oxo groups of isatin by making a comparable indole series. Despite other biological activities being known for N-alkyl isatins and indoles, to our knowledge, there is no comprehensive report in the literature addressing the effect of N-alkylation of isatin or indole on the ability of these compounds to act as AChEI and BChEI. Herein, we report investigations into this matter.
Materials and methods
General
Consumable chemicals for synthesis and biochemical testing were purchased from Sigma-Aldrich (St. Louis, MO), VWR International (Radnor, PA), Beantown Chemical (Hudson, NH), AK Scientific (Union City, CA), and Tokyo Chemical Industry (Tokyo, Japan). A Bruker Avance 400 spectrometer was used to collect all 1H and 13C NMR spectra, and a Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap Mass Spectrometer was used to collect HRMS. UV-Vis absorbance in enzymatic assay was monitored on a Dynex Technologies Opsys MR™ Microplate Reader using flat-bottomed 96-well plates (VWR International).
General procedure for synthesis of N-alkyl isatins (4a-j)
Isatin (200 mg, 1.36 mmol), iodomethane (1.5 eq) or 1-bromoalkane (1.5 eq), K2CO3 (2 eq), and DMF (3 ml) were combined and stirred at rt for 24 h. The reaction mixture was then diluted with water (100 ml) and extracted with DCM (3 × 15 ml). The combined organic layers were washed with brine (50 ml), dried (MgSO4), and concentrated under reduced pressure to afford the crude product, which was then purified by flash column chromatography (SiO2; 3:1/hexanes:EtOAc) to yield the desired product.
1-Methylindoline-2,3-dione (4a)
Yield = 63% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.67 (td, 1H, J1 = 7.9 Hz, J2 = 1.1 Hz Ar-H), 7.54 (d, 1H, J = 7.3 Hz, Ar-H), 7.13 (m, 2H, Ar-H), 3.14 (s, 3H, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.4 (C = O), 158.2 (C = O), 151.4 (Ar-C), 138.2 (Ar-C), 124.2 (Ar-C), 123.2 (Ar-C), 117.4 (Ar-C), 110.6 (Ar-C), 26.0 (-CH3); HRMS (ESI) m/z calcd for C9H7NO2: 161.0471, found 162.0550 [M + H]+.
1-Ethylindoline-2,3-dione (4b)
Yield = 64% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.8 Hz, J2 = 0.9 Hz, Ar-H), 7.55 (d, 1H, J = 7.4 Hz, Ar-H), 7.18 (d, 1H, J = 8.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.5 Hz, Ar-H), 3.69 (q, 2H, J = 7.2 Hz, -CH2-), 1.17 (t, 3H, J = 7.2 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.7 (C = O), 157.8 (C = O), 150.4 (Ar-C), 138.3 (Ar-C), 124.6 (Ar-C), 123.2 (Ar-C), 115.5 (Ar-C), 110.7 (Ar-C), 34.3 (-CH2-), 12.3 (-CH3); HRMS (ESI) m/z calcd for C10H9NO2: 175.0628, found 176.0706 [M + H]+.
1-Propylindoline-2,3-dione (4c)
Yield = 53% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.9 Hz, J2 = 1.0 Hz, Ar-H), 7.53 (d, 1H, J = 7.4 Hz, Ar-H), 7.19 (d, 1H, J = 8.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.5 Hz, Ar-H), 3.62 (t, 2H, J = 7.1 Hz, -CH2-), 1.62 (sextet, 2H, J = 7.4 Hz, -CH2-), 0.90 (t, 3H, J = 7.4 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.2 (Ar-C), 124.5 (Ar-C), 123.1 (Ar-C), 117.4 (Ar-C), 110.8 (Ar-C), 41.1 (-CH2-), 20.2 (-CH2-), 11.2 (-CH3); HRMS (ESI) m/z calcd for C11H11NO2: 189.0784, found 190.0862 [M + H]+.
1-Butylindoline-2,3-dione (4d)
Yield = 15% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.8 Hz, J2 = 1.0 Hz, Ar-H), 7.54 (d, 1H, J = 7.4 Hz, Ar-H), 7.18 (d, 1H, J = 8.0 Hz, Ar-H), 7.12 (t, 1H, J = 7.5 Hz, Ar-H), 3.65 (t, 2H, J = 7.1 Hz, -CH2-), 1.58 (br pent, 2H, J = 7.1 Hz, -CH2-), 1.33 (sextet, 2H, J = 7.4 Hz, -CH2-), 0.90 (t, 3H, J = 7.3 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.5 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.7 (-CH2-, confirmed by DEPT135), 28.9 (-CH2-), 19.5 (-CH2-), 13.6 (-CH3); HRMS (ESI) m/z calcd for C12H13NO2: 203.0941, found 204.1019 [M + H]+.
1-Pentylindoline-2,3-dione (4e)
Yield = 25% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.6 Hz, J2 = 0.8 Hz, Ar-H), 7.54 (d, 1H, J = 7.3 Hz, Ar-H), 7.17 (d, 1H, J = 8.0 Hz, Ar-H), 7.12 (t, 1H, J = 7.5 Hz, Ar-H), 3.64 (t, 2H, J = 7.1 Hz, -CH2-), 1.59 (br pent, 2H, J = 7.0 Hz, -CH2-), 1.29 (m, 4H, -(CH2)2-), 0.85 (t, 3H, J = 6.6 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.5 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 28.4 (-CH2-), 26.5 (-CH2-), 21.9 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C13H15NO2: 217.1097, found 218.1175 [M + H]+.
1-Hexylindoline-2,3-dione (4f)
Yield = 9% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.8 Hz, J2 = 1.0 Hz, Ar-H), 7.54 (d, 1H, J = 7.4 Hz, Ar-H), 7.17 (d, 1H, J = 8.0 Hz, Ar-H), 7.12 (t, 1H, J = 7.5 Hz, Ar-H), 3.64 (t, 2H, J = 7.1 Hz, -CH2-), 1.58 (br pent, 2H, J = 7.0 Hz, -CH2-), 1.29 (m, 6H, -(CH2)3-), 0.84 (t, 3H, J = 6.8 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.5 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 30.9 (-CH2-), 26.7 (-CH2-), 25.9 (-CH2-), 22.1 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C14H17NO2: 231.1254, found 232.1331 [M + H]+.
1-Heptylindoline-2,3-dione (4 g)
Yield = 23% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (td, 1H, J1 = 7.8 Hz, J2 = 0.9 Hz, Ar-H), 7.54 (d, 1H, J = 7.4 Hz, Ar-H), 7.17 (d, 1H, J = 8.0 Hz, Ar-H), 7.12 (t, 1H, J = 7.5 Hz, Ar-H), 3.64 (t, 2H, J = 7.1 Hz, -CH2-), 1.59 (br pent, 2H, J = 6.8 Hz, -CH2-), 1.29 (m, 4H, -(CH2)2-), 1.25 (m, 4H, -(CH2)2-), 0.86 (t, 3H, J = 7.7 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.5 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 31.2 (-CH2-), 28.4 (-CH2-), 26.8 (-CH2-), 26.2 (-CH2-), 22.1 (-CH2-), 14.0 (-CH3); HRMS (ESI) m/z calcd for C15H19NO2: 245.1410, found 246.1488 [M + H]+.
1-Octylindoline-2,3-dione (4h)
Yield = 10% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.65 (t, 1H, J = 7.8 Hz, Ar-H), 7.53 (d, 1H, J = 7.4 Hz, Ar-H), 7.17 (d, 1H, J = 7.9 Hz, Ar-H), 7.12 (t, 1H, J = 7.5 Hz, Ar-H), 3.64 (t, 2H, J = 7.0 Hz, -CH2-), 1.58 (br pent, 2H, J = 6.9 Hz, -CH2-), 1.28 (m, 4H, -(CH2)2-), 1.25 (m, 6H, -(CH2)3-), 0.83 (t, 3H, J = 6.9 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.7 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.6 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 31.3 (-CH2-), 28.71 (-CH2-), 28.67 (-CH2-), 26.8 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 14.0 (-CH3); HRMS (ESI) m/z calcd for C16H21NO2: 259.1567, found 260.1646 [M + H]+.
1-Nonylindoline-2,3-dione (4i)
Yield = 40% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.64 (td, 1H, J1 = 7.5 Hz, J2 = 0.7 Hz Ar-H), 7.53 (d, 1H, J = 7.1 Hz, Ar-H), 7.17 (d, 1H, J = 7.9 Hz, Ar-H), 7.11 (t, 1H, J = 7.5 Hz, Ar-H), 3.64 (t, 2H, J = 7.1 Hz, -CH2-), 1.57 (br pent, 2H, J = 6.9 Hz, -CH2-), 1.27 (m, 4H, -(CH2)2-), 1.21 (m, 8H, -(CH2)4-), 0.83 (t, 3H, J = 6.4 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.6 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 31.3 (-CH2-), 28.9 (-CH2-), 28.74 (-CH2-), 28.69 (-CH2-), 26.8 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 14.0 (-CH3); HRMS (ESI) m/z calcd for C17H23NO2: 273.1723, found 274.1802 [M + H]+.
1-Decylindoline-2,3-dione (4j)
Yield = 52% as an orange solid; 1H NMR (DMSO-d6, 400 MHz) δ 7.64 (t, 1H, J = 7.8 Hz, Ar-H), 7.53 (d, 1H, J = 7.4 Hz, Ar-H), 7.17 (d, 1H, J = 8.0 Hz, Ar-H), 7.11 (t, 1H, J = 6.4 Hz, Ar-H), 3.63 (t, 2H, J = 7.0 Hz, -CH2-), 1.57 (br pent, 2H, J = 6.7 Hz, -CH2-), 1.27 (m, 4H, -(CH2)2-), 1.20 (m, 10H, -(CH2)5-), 0.82 (t, 3H, J = 6.4 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 183.6 (C = O), 158.1 (C = O), 150.8 (Ar-C), 138.3 (Ar-C), 124.5 (Ar-C), 123.2 (Ar-C), 117.5 (Ar-C), 110.8 (Ar-C), 39.9 (-CH2-, confirmed by DEPT135), 31.3 (-CH2-), 29.0 (-(CH2)2-), 28.7 (-(CH2)2-), 26.8 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 14.0 (-CH3); HRMS (ESI) m/z calcd for C18H25NO2: 287.1880, found 288.1958 [M + H]+.
General procedure for synthesis of N-alkyl indoles (5a-j)
Indole (200 mg, 1.71 mmol), iodomethane (1 eq) or 1-bromoalkane (1 eq), NaOH (2 eq), and DMSO (3 ml) were combined and stirred at rt for 24 h. The reaction mixture was then diluted with water (100 ml) and extracted with EtOAc (3 × 15 ml). The combined organic layers were washed with brine (50 ml), dried (MgSO4), and concentrated under reduced pressure to afford the crude product, which was then purified by flash column chromatography (SiO2; hexanes to 10:1/hexanes:EtOAc) to yield the desired product.
1-Methyl-1H-indole (5a)
Yield = 50% as pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.54 (d, 1H, J = 7.9 Hz, Ar-H), 7.42 (d, 1H, J = 8.2 Hz, Ar-H), 7.31 (d, 1H, J = 3.0 Hz, Ar-H), 7.14 (td, 1H, J1 = 7.3 Hz, J2 = 0.6, Ar-H), 7.02 (td, 1H, J1 = 7.7 Hz, J2 = 0.6, Ar-H), 6.41 (d, 1H, J = 2.4 Hz, Ar-H), 3.78 (s, 3H, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 136.3 (Ar-C), 129.5 (Ar-C), 128.0 (Ar-C), 121.0 (Ar-C), 120.3 (Ar-C), 118.9 (Ar-C), 109.6 (Ar-C), 100.2 (Ar-C), 32.4 (-CH3); HRMS (ESI) m/z calcd for C9H9N: 131.0730, found 132.0808 [M + H]+.
1-Ethyl-1H-indole (5b)
Yield = 13% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.46 (d, 1H, J = 8.2 Hz, Ar-H), 7.37 (d, 1H, J = 3.1 Hz, Ar-H), 7.12 (t, 1H, J = 7.9 Hz, Ar-H), 7.00 (t, 1H, J = 7.8 Hz, Ar-H), 6.41 (d, 1H, J = 2.6 Hz, Ar-H), 4.20 (q, 2H, J = 7.0 Hz, -CH2-), 1.35 (t, 3H, J = 7.2 Hz, -CH3-); 13C NMR (DMSO-d6, 100 MHz) δ 135.3 (Ar-C), 128.1 (Ar-C), 127.9 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.6 (Ar-C), 100.4 (Ar-C), 40.2 (-CH2-), 15.5 (-CH3); HRMS (ESI) m/z calcd for C10H11N: 145.0886, found 146.0963 [M + H]+.
1-Propyl-1H-indole (5c)
Yield = 20% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.45 (d, 1H, J = 8.2 Hz, Ar-H), 7.34 (d, 1H, J = 3.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.9 Hz, Ar-H), 7.00 (t, 1H, J = 7.1 Hz, Ar-H), 6.41 (d, 1H, J = 2.6 Hz, Ar-H), 4.12 (t, 2H, J = 7.0 Hz, -CH2-), 1.75 (sextet, 2H, J = 7.3 Hz, -CH2-), 0.82 (t, 3H, J = 7.4 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.7 (Ar-C), 128.7 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.8 (Ar-C), 100.3 (Ar-C), 47.1 (-CH2-), 23.2 (-CH2-), 11.2 (-CH3); HRMS (ESI) m/z calcd for C11H13N: 159.1043, found 160.1120 [M + H]+.
1-Butyl-1H-indole (5d)
Yield = 58% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.54 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.35 (d, 1H, J = 3.1 Hz, Ar-H), 7.12 (td, 1H, J1 = 7.3 Hz, J2 = 0.5, Ar-H), 7.01 (td, 1H, J1 = 7.1 Hz, J2 = 0.6 Ar-H), 6.41 (d, 1H, J = 2.6 Hz, Ar-H), 4.14 (t, 2H, J = 7.0 Hz, -CH2-), 1.71 (pent, 2H, J = 7.1 Hz, -CH2-), 1.22 (sextet, 2H, J = 7.5 Hz, -CH2-) 0.87 (t, 3H, J = 7.4 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.7 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.2 (-CH2-), 32.0 (-CH2-), 19.5 (-CH2-), 13.6 (-CH3); HRMS (ESI) m/z calcd for C12H15N: 173.1199, found 174.1277 [M + H]+.
1-Pentyl-1H-indole (5e)
Yield = 17% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.34 (d, 1H, J = 3.1 Hz, Ar-H), 7.11 (t, 1H, J = 7.8 Hz, Ar-H), 7.00 (t, 1H, J = 7.7 Hz, Ar-H), 6.41 (d, 1H, J = 2.6 Hz, Ar-H), 4.15 (t, 2H, J = 7.0 Hz, -CH2-), 1.74 (pent, 2H, J = 7.2 Hz, -CH2-), 1.30 (sextet, 2H, J = 6.1 Hz, -CH2-) 1.20 (m, 2H, J = 7.4 Hz, -CH2-) 0.83 (t, 3H, J = 7.0 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 29.6 (-CH2-), 28.5 (-CH2-), 21.8 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C13H17N: 187.1356, found 188.1433 [M + H]+.
1-Hexyl-1H-indole (5f)
Yield = 26% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.35 (d, 1H, J = 3.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.7 Hz, Ar-H), 7.00 (t, 1H, J = 7.6 Hz, Ar-H), 6.40 (d, 1H, J = 2.7 Hz, Ar-H), 4.15 (t, 2H, J = 7.0 Hz, -CH2-), 1.74 (m, 2H, -CH2-), 1.24 (br m, 6H, -(CH2)3-), 0.82 (m, 3H, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.7 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 30.8 (-CH2-), 29.8 (-CH2-), 25.9 (-CH2-), 22.0 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C14H19N: 201.1512, found 202.1589 [M + H]+.
1-Heptyl-1H-indole (5 g)
Yield = 69% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.34 (d, 1H, J = 3.1 Hz, Ar-H), 7.11 (t, 1H, J = 7.8 Hz, Ar-H), 6.99 (t, 1H, J = 7.6 Hz, Ar-H), 6.40 (d, 1H, J = 2.6 Hz, Ar-H), 4.15 (t, 2H, J = 7.0 Hz, -CH2-), 1.73 (m, 2H, -CH2-), 1.21 (br m, 8H, -(CH2)4-), 0.83 (m, 3H, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 31.2 (-CH2-), 29.9 (-CH2-), 28.3 (-CH2-), 26.2 (-CH2-), 22.0 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C15H21N: 215.1669, found 216.1747 [M + H]+.
1-Octyl-1H-indole (5h)
Yield = 26% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.35 (d, 1H, J = 3.1 Hz, Ar-H), 7.11 (t, 1H, J = 7.3 Hz, Ar-H), 7.00 (t, 1H, J = 7.3 Hz, Ar-H), 6.40 (d, 1H, J = 2.8 Hz, Ar-H), 4.15 (t, 2H, J = 7.0 Hz, -CH2-), 1.74 (br pent, 2H, J = 7.2 Hz, -CH2-), 1.22 (br m, 10H, -(CH2)5-), 0.83 (t, 3H, J = 6.5 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.9 (Ar-C), 120.4 (Ar-C), 118.8 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 31.2 (-CH2-), 29.8 (-CH2-), 28.64 (-CH2-), 28.59 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C16H23N: 229.1825, found 230.1904 [M + H]+.
1-Nonyl-1H-indole (5i)
Yield = 70% as a pale yellow oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.34 (d, 1H, J = 3.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.7 Hz, Ar-H), 6.99 (t, 1H, J = 7.6 Hz, Ar-H), 6.40 (d, 1H, J = 2.6 Hz, Ar-H), 4.14 (t, 2H, J = 7.0 Hz, -CH2-), 1.72 (br pent, 2H, J = 7.2 Hz, -CH2-), 1.20 (br m, 12H, -(CH2)6-), 0.83 (t, 3H, J = 6.5 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.6 (Ar-C), 128.1 (Ar-C), 120.8 (Ar-C), 120.4 (Ar-C), 118.7 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 31.2 (-CH2-), 29.8 (-CH2-), 28.9 (-CH2-), 28.63 (-CH2-), 28.60 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 13.9 (-CH3); HRMS (ESI) m/z calcd for C17H25N: 243.1982, found 244.2061 [M + H]+.
1-Decyl-1H-indole (5j)
Yield = 76% as a green oil; 1H NMR (DMSO-d6, 400 MHz) δ 7.53 (d, 1H, J = 7.8 Hz, Ar-H), 7.44 (d, 1H, J = 8.2 Hz, Ar-H), 7.34 (d, 1H, J = 3.0 Hz, Ar-H), 7.11 (t, 1H, J = 7.4 Hz, Ar-H), 6.99 (t, 1H, J = 7.6 Hz, Ar-H), 6.40 (d, 1H, J = 2.7 Hz, Ar-H), 4.14 (t, 2H, J = 7.0 Hz, -CH2-), 1.73 (br pent, 2H, J = 7.1 Hz, -CH2-), 1.20 (br m, 14H, -(CH2)7-), 0.84 (t, 3H, J = 6.5 Hz, -CH3); 13C NMR (DMSO-d6, 100 MHz) δ 135.6 (Ar-C), 128.5 (Ar-C), 128.1 (Ar-C), 120.8 (Ar-C), 120.4 (Ar-C), 118.7 (Ar-C), 109.7 (Ar-C), 100.3 (Ar-C), 45.4 (-CH2-), 31.2 (-CH2-), 29.8 (-CH2-), 29.0 (-CH2-), 28.9 (-CH2-), 28.7 (-CH2-), 28.6 (-CH2-), 26.3 (-CH2-), 22.1 (-CH2-), 14.0 (-CH3); HRMS (ESI) m/z calcd for C18H27N: 257.2138, found 258.2220 [M + H]+.
AChE and BChE inhibition and kinetics assays
Inhibition of AChE (Sigma-Aldrich, Product No. C2888, from Electrophorus electricus) and BChE (Lee Biosolutions, Product No. 130–10-1, from equine serum) was carried out as previously described by our labCitation18,Citation19. Briefly, compounds to be tested were prepared in sodium phosphate (dibasic) buffer (0.1 M) containing 10% DMSO and then diluted with phosphate buffer across the wells of a 96-well plate to reach desired concentrations (50 µL total well volume). AChE (50 µL, 400 U/L, in phosphate buffer) or BChE (50 µL, 400 U/L, in phosphate buffer) was added to the appropriate wells. After incubation, the enzymatic reaction was initiated with 100 µL of a 1:1 (v/v) solution of acetylthiocholine (ATC, 2 mM in phosphate buffer) or butyrylthiocholine (BTC, 2 mM in phosphate buffer) and 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 1 mM in phosphate buffer). The change in absorbance at 405 nm was monitored over 10 min and used to graph percent inhibition vs concentration of inhibitor (log transformed). IC50 values were calculated using GraphPad Prism 9.1.1.
Kinetics assays of 4i inhibition with AChE and BChE were also carried out as previously describedCitation18,Citation19. Briefly, varying concentrations of 4i were incubated with AChE and BChE before initiating the enzymatic reactions by addition of 100 µL of a 1:1 (v/v) solution of ATC or BTC (0.4 mM, 0.5 mM, 1 mM, or 2 mM in phosphate buffer) and DTNB (1 mM in phosphate buffer) to all wells. The change in absorbance at 405 nm over 10 min was used to calculate the rate of reaction (mean ΔAbs/min ± SEM for three replicates). Rate vs concentration was fitted to a Lineweaver-Burk plot using GraphPad Prism 9.1.1.
Molecular modelling
Molecular modelling was run using the modelling program AutoDock Vina (http://vina.scripps.edu/) as previously described by our labCitation18,Citation19. Briefly, the crystal structures of TcAChE (PDB ID: 1ACJ, 2.8 Å resolution) and human BChE (PDB ID: 4BDS, 2.1 Å resolution) were edited in PyMOL and AutoDock Tools. Ligands 4i and 5i were prepared using ChemDraw and Chem3D, as well as AutoDock Tools. The scoring grid (36 × 36 × 36 Å) for AutoDock Vina was centred as follows: AChE – x-center: 4.766, y-center: 65.514, z-center: 56.822; BChE – x-center: 138.790, y-center: 123.636, z-center: 38.646. PyMOL was used for results visualisation.
Results and discussion
The synthesis of the target molecules 4a-j and 5a-j was carried out in straightforward fashion by substitution of isatin or indole with iodomethane or 1-bromoalkanes in the presence of K2CO3 and DMF or NaOH and DMSO (Scheme 1). Yields ranged from 9–76%, and structures were confirmed by 1H NMR,13C NMR, and HRMS. In 13C NMR (DMSO-d6, 400 MHz) spectra for 4d-j, the residual DMSO signal overlapped with the signal from the methylene carbon adjacent to the nitrogen. A DEPT135 experiment was run to differentiate this signal (negative) from the solvent (positive) (see supplementary data), as we have employed previosulyCitation18. The synthesised compounds were then evaluated as AChEI and BChEI using the established Ellman methodCitation20. Results are reported as IC50 values in .
Table 1. Inhibition of AChE and BChE by N-alkyl isatins 4a-j and indoles 5a-j.
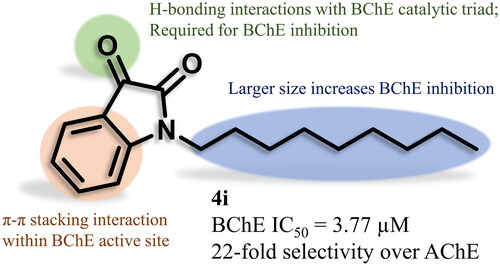
The best AChEI from each series was 4j (n = 9, IC50 = 62.2 µM) and 5 g (n = 6, IC50 = 35.0 µM). Regarding AChE IC50, there is no well-defined SAR for alkyl chain length in either series. N-Alkyl isatins 4a-j show a loose trend in that longer alkyl chains (4 g, 4i, 4j) show better inhibition of AChE than shorter chains (4a-e), but the N-alkyl indoles 5a-j show little difference between the shortest alkyl chain (5a) and the longest (5j). The best BChEI was 4i (n = 8, IC50 = 3.77 µM). N-Alkyl isatins 4a-j showed a SAR that as the alkyl chain gets longer, the BChE IC50 decreases. Interestingly, none of the N-alkyl indoles 5a-j showed significant inhibition of BChE at the concentrations tested. Thus, the indole series selectively inhibited AChE while the isatin series selectively inhibited BChE.
To put these IC50 values in context, they were compared to isatin and two well-known inhibitors, rivastigmine and tacrine, which we have previously used for comparison ()Citation18,Citation19. In most cases, the addition of an alkyl group to isatin significantly improves activity over the parent compound for both enzymes. The best AChEI (5 g) and BChEI (4i) from the compounds under investigation were comparable in activity to rivastigmine, a clinically used inhibitor for AD, against both AChE and BChE. However, neither 5g or 4i was as active against AChE or BChE as tacrine, an inhibitor extensively studied in the literature as an AChEI and BChEI and formerly used clinically.
As mentioned earlier, there was, to the best of our knowledge, no comprehensive report of N-alkyl isatins or indoles addressing the effect of alkyl chain length on inhibition of these enzymes. However, 4a was previously reported by Hyatt et al. to have a Ki > 100 µM for both AChE and BChECitation21. This same group also reported that the N-dodecyl and N-hexadecyl isatins have Ki values > 100 µM for both AChE and BChE. However, these were the only three N-alkyl isatins tested. Interestingly, and more relevant to the discussion of the current study, Hyatt et al. reached the conclusion that the inhibitory potency of isatin compounds against carboxylesterases was related to their hydrophobicity, with analogs showing a clogP > 5 commonly having nM range Ki values. This trend and conclusion certainly apply to the current study, particularly among 4a-j regarding BChE inhibitory potency. Our observations are also like a previous report of N-alkyl tacrines, in which longer alky chains improved inhibition of AChE, and, in all cases except one, the N-alkyl tacrines showed better AChE inhibition than the parent tacrine moleculeCitation22.
Compound 4i was chosen as a representative compound to investigate mode of inhibition through Lineweaver-Burk analysis. As shown in and Citation3, 4i displayed a non-competitive inhibition pattern with AChE and a mixed inhibition pattern with BChE. Previously we reported similar observations for isatin- and indole-based inhibitors. Namely, 3 showed non-competitive inhibition with AChE, while 1 and 2 showed mixed inhibition with BChECitation18,Citation19. Thus, the current results are in agreement with these previous findings.
Figure 2. Lineweaver-Burk plot showing non-competitive inhibition of AChE with respect to acetylthiocholine (ATC) for compound 4i.

Figure 3. Lineweaver-Burk plot showing mixed inhibition of BChE with respect to butyrylthiocholine (BTC) for compound 4i.

To better understand the results of the enzyme inhibition studies, molecular modelling of 4i and 5i with TcAChE (PDB: 1ACJ) and hBChE (PDB: 4BDS) was performed using AutoDock Vina by a method previously reportedCitation18,Citation19,Citation23–25. Results are shown in . Active site residues of TcAChE include the catalytic triad (Ser200, His440, Glu327; gold), anionic site (Trp84, Tyr130, Phe330, and Phe331; green), oxyanion hole (Gly118, Gly119, Ala201; purple), acyl pocket (Phe288, Phe290; blue), and PAS (Tyr70, Asp72, Tyr121, Ser122, Trp279, Tyr334; magenta). Active site residues of hBChE include the catalytic triad (Ser198, His438, Glu325; gold), anionic site (Trp82, Tyr128, Phe329; green), oxyanion hole (Gly116, Gly117, Ala199; purple), acyl pocket (Trp231, Leu286, Val288; blue), and PAS (Asp70, Tyr332; magenta).
Figure 4. Docking of TcAChE (PDB: 1ACJ; A and C) and hBChE (PDB: 4BDS; B and D) with 1-nonylindoline-2,3-dione (4i, cyan) and 1-nonyl-1H-indole (5i, yellow). Active site residues of TcAChE include the catalytic triad (Ser200, His440, Glu327; gold), anionic site (Trp84, Tyr130, Phe330, and Phe331; green), oxyanion hole (Gly118, Gly119, Ala201; purple), acyl pocket (Phe288, Phe290; blue), and PAS (Tyr70, Asp72, Tyr121, Ser122, Trp279, Tyr334; magenta). Active site residues of hBChE include the catalytic triad (Ser198, His438, Glu325; gold), anionic site (Trp82, Tyr128, Phe329; green), oxyanion hole (Gly116, Gly117, Ala199; purple), acyl pocket (Trp231, Leu286, Val288; blue), and PAS (Asp70, Tyr332; magenta). Orange dashes indicate π-π stacking interactions, and yellow dashes indicate H-bonding interactions. All distances shown are in Å. O, N, and H atoms are shown in red, blue, and white, respectively.

4i was predicted to bind to the active site of TcAChE with a parallel π-π stacking between the isatin moiety and Trp84, as well as a H-bonding interaction between the 2-oxo group and side chain OH of Ser122 (). In comparison, 4i was predicted to bind to the active site of hBChE with a perpendicular π-π stacking between the isatin moiety and Phe329, and a H-bonding interaction was also indicated between the 3-oxo group and side chain OH and NH of Ser198 and His438, respectively (). This direct H-bonding interaction between 4i and Ser198 and His438, which are part of the catalytic triad, may account for the enhanced potency seen against BChE (22-fold selectivity). Parallel π-π stacking with Trp84 and Phe330 and perpendicular π-π stacking with Tyr334 were predicted for the indole moiety of 5i when binding to the active site of TcAChE (). When binding to hBChE, the indole moiety of 5i was predicted to form perpendicular π-π stacking with Trp231 and Phe329 (). In all cases, the alkyl chain of the inhibitors has no binding interactions with the enzymes (expected due to lack of functionality). The alkyl chain may enhance binding to the enzyme active site by a hydrophobic effect or a steric directing effect or a combination of the two. This likely accounts for the observations that longer alkyl chains increase inhibition.
The shift from the isatin moiety to the indole moiety (4i vs. 5i) results in the loss of the 2-oxo and 3-oxo groups that participate in H-bonding interactions between 4i and the active sites of both enzymes. In the case of TcAChE, it appears that the lost H-bond with Ser122 is compensated for by the gain of π-π interactions with Phe330 and Tyr334 ( vs. Citation4(C)), which may explain the similar potency of 4i and 5i towards AChE in the inhibition assay. However, in the case of hBChE, it appears that the lost H-bonds with Ser198 and His438 cannot be compensated for by the gain of a π-π interaction with Trp231 ( vs. Citation4(D)). This likely accounts for the significant loss of potency (>17-fold) towards BChE for 5i compared to 4i in the inhibition assay.
Lastly, recognising that targeting AChE and BChE in the context of AD means that compounds in question must be able to cross the blood-brain barrier (BBB), a BBB permeability was predicted using the PreADMET application (https://preadmet.qsarhub.com/). Results are shown in where compounds were assigned a low, moderate, or high BBB permeability. Of note, all compounds in this study (4a-j and 5a-j) were predicted to have moderate or high BBB permeability.
Conclusion
Overall, the current study demonstrates that the addition of an N-alkyl chain to isatin increases inhibition of both AChE and BChE. For BChE, there is a clear trend indicating that the inhibitory potency increases as the length of the alkyl chain increases. Shifting to an indole scaffold resulted in a loss of BChE potency, but AChE potency was maintained. This observation, supported by molecular modelling, indicates a key role for the 3-oxo group of isatin in BChE inhibition. Results do not discount the strategy of linking isatin to another moiety by an alkyl linker, as in 1–3, as this additional moiety may offer other properties to make these compounds relevant to AD (e.g. metal binding, reactive oxygen species inhibition). However, results do show that adding a simple alkyl chain can lead to significantly improved inhibition of AChE and BChE.
Author contributions
TJE is responsible for conception and design of this study. All synthesised compounds were prepared by KNA. All enzyme inhibition assays were conducted by IAO. TJE performed molecular modelling and BBB preditions. MDP and TJE performed NMR analysis and wrote the initial draft of the manuscript. All authors assisted in the revision and final approval of the manuscript, and all authors agree to be accountable for all aspects of the work.
Supplemental Material
Download PDF (2.2 MB)Acknowledgements
The authors thank Dr. Tatiana Laremore and Zilia Koshkina for help with HRMS sample preparation, data collection, and analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, TJE, upon reasonable request.
Additional information
Funding
References
- Taylor P, Radic Z. The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol. 1994;34(1):281–320.
- Vellom DC, Radic Z, Li Y, Pickering NA, Camp S, Taylor P. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry. 1993;32:12–17. https://pubs.acs.org/sharingguidelines.
- Li S, Li AJ, Travers J, Xu T, Sakamuru S, Klumpp-Thomas C, Huang R, Xia M. Identification of Compounds for Butyrylcholinesterase Inhibition. SLAS DISCOVERY Adv Sci Drug Disc. 2021;26(10):1355–1364.
- Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem. 2003;278(42):41141–41147.
- De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC. A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry. 2001;40(35):10447–10457.
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s Disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–115.
- Hampel H, Mesulam M-M, Cuello AC, Khachaturian AS, Vergallo A, Farlow MR, Snyder PJ, Giacobini E, Khachaturian ZS. Revisiting the cholinergic hypothesis in alzheimer’s disease: emerging evidence from translational and clinical research. J Prev Alzheimers Dis. 2018;6(1):1–14.
- Bartolini M, Bertucci C, Cavrini V, Andrisano V. β-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem Pharmacol. 2003;65(3):407–416.
- Kochi A, Eckroat TJ, Green KD, Mayhoub AS, Lim MH, Garneau-Tsodikova S. A novel hybrid of 6-chlorotacrine and metal–amyloid-β modulator for inhibition of acetylcholinesterase and metal-induced amyloid-β aggregation. Chem Sci. 2013;4(11):4137–4145.
- Li Q, Yang H, Chen Y, Sun H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur J Med Chem. 2017;132:294–309.
- Macdonald IR, Maxwell SP, Reid GA, Cash MK, DeBay DR, Darvesh S. Quantification of butyrylcholinesterase activity as a sensitive and specific biomarker of Alzheimer’s disease. J Alzheimer’s Dis. 2017;58:491–505.
- Obaid RJ, Mughal EU, Naeem N, Al-Rooqi MM, Sadiq A, Jassas RS, Moussa Z, Ahmed SA. Pharmacological significance of nitrogen-containing five and six-membered heterocyclic scaffolds as potent cholinesterase inhibitors for drug discovery. Process Biochemistry. 2022;120:250–259.
- Obaid RJ, Naeem N, Mughal EU, Al-Rooqi MM, Sadiq A, Jassas RS, Moussa Z, Ahmed SA. Inhibitory potential of nitrogen, oxygen and sulfur containing heterocyclic scaffolds against acetylcholinesterase and butyrylcholinesterase. RSC Adv. 2022;12(31):19764–19855.
- Taha M, Rahim F, Uddin N, Khan IU, Iqbal N, Anouar EH, Salahuddin M, Farooq RK, Gollapalli M, Khan KM, et al. Exploring indole-based-thiadiazole derivatives as potent acetylcholinesterase and butyrylcholinesterase enzyme inhibitors. Int J Biol Macromol. 2021;188:1025–1036.
- Khorana N, Changwichit K, Ingkaninan K, Utsintong M. Prospective acetylcholinesterase inhibitory activity of indole and its analogs. Bioorg Med Chem Lett. 2012;22(8):2885–2888.
- Kaur K, Utreja D, Dhillon NK, Pathak RK, Singh K. N-alkyl isatin derivatives: Synthesis, nematicidal evaluation and protein target identifications for their mode of action. Pestic Biochem Physiol. 2021;171:104736.
- Chen G, Wang Y, Hao X, Mu S, Sun Q. Simple isatin derivatives as free radical scavengers: Synthesis, biological evaluation and structure-activity relationship. Chem Cent J. 2011;5(1):37.
- Reiland KM, Eckroat TJ. Selective butyrylcholinesterase inhibition by isatin dimers and 3-indolyl-3-hydroxy-2-oxindole dimers. Bioorg Med Chem Lett. 2022;77:129037.
- Davis SM, Eckroat TJ. Isatin-linked 4,4-dimethyl-5-methylene-4,5-dihydrothiazole-2-thiols for inhibition of acetylcholinesterase. Med Chem Res. 2021;30(12):2289–2300.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95.
- Hyatt JL, Moak T, Hatfield MJ, Tsurkan L, Edwards CC, Wierdl M, Danks MK, Wadkins RM, Potter PM. Selective inhibition of carboxylesterases by isatins, indole-2,3-diones. J Med Chem. 2007;50(8):1876–1885.
- Eckroat TJ, Green KD, Reed RA, Bornstein JJ, Garneau-Tsodikova S. Investigation of the role of linker moieties in bifunctional tacrine hybrids. Bioorg Med Chem. 2013;21(12):3614–3623.
- Trott O, Olson AJ. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem. 2010;31:455–461.
- Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, Renard P-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem J. 2013;453(3):393–399.
- Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA. 1993;90(19):9031–9035.