Abstract
Colon cancer remains a clinical challenge in industrialised countries. Its treatment with 5-Flurouracil (5-FU) develops many side effects and resistance. Thus, several strategies have been undertaken so far, including the use of drug cocktails and polypharmacology. Heme oxygenase-1 (HO-1) is an emerging molecular target in the treatment of various cancers. We recently demonstrated that a combination of HO-1 inhibitors with 5-FU and the corresponding hybrids SI1/17, SI1/20, and SI1/22, possessed anticancer activity against prostate and lung cancer cells. In this work, we evaluated these hybrids in a model of colon cancer and found that SI1/22 and the respective combo have greater potency than 5-FU. Particularly, compounds inhibit HO-1 activity in cell lysates, increase ROS and the expression of HO-1, SOD, and Nrf2. Moreover, we observed a decrease of pro-caspase and an increase in cleaved PARP-1 and p62, suggesting apoptotic and autophagic cell death and potential application of these drugs as anticancer agents.
Introduction
A novel target for anticancer therapy is the HO family of enzymes (HO-1, HO-2, and HO-3) devoted to the catabolism of hemeCitation1, and the production of iron, carbon monoxide, biliverdin, and bilirubinCitation2,Citation3. Although HO has mainly a cytoprotective role, overexpression of the inducible isoform HO-1 has been observed in many types of cancers, contributing to cancer spread and invasivenessCitation4; therefore, HO-1 inhibitors have been developed in the last years as new anticancer agents both alone and combined with chemotherapeutic drugsCitation5–9.
Colorectal cancer (CRC) is the third most common cancer diagnosed, and the second cause of cancer deathCitation10. In CRC, HO-1 has been demonstrated to be involved in cancer induction and spreading. Different studies pointed out a remarkable high HO-1 expression and activity in tumour tissue from CRC patientsCitation11 and, more specifically, the highest HO-1 expression has been detected in well-differentiated tumour areas of CRC. High nuclear localisation of HO-1 was also shown in human HCT116 cells where it activates transcription factors responsible for protection from oxidative stress and involved in cell proliferation, such as AP-1, AP-2, and Brn-3Citation12. CRC is treated with different chemotherapeutic drugs, including 5-fluorouracil (5-FU), capecitabine, irinotecan, oxaliplatin, trifluridine and tipiracilCitation13. Despite the substantial advances achieved in the last years with chemotherapeutic agents against CRC, severe side effects, toxicity, and drug resistance are the major clinical problemsCitation14,Citation15.
5-FU is an essential medicine on the WHO listCitation16, belonging to the chemical class of pyrimidine antimetabolitesCitation17; it is prescribed as first-line therapy for CRCCitation18,Citation19. 5-FU exerts its cytotoxic activity through the inhibition of thymidylate synthase and the incorporation of its metabolites into nucleic acids, causing DNA damage; however, this action is not selective against cancer cellCitation20–22. In addition, unfavourable pharmacokinetic (PK) properties negatively contribute to the 5-FU drug profileCitation23. To overcome these limits, 5-FU is often used in combined therapy for the treatment of CRC (e.g. FOLFOX and FOLFIRI)Citation13, but still exhibits severe cytotoxic effects and some drawbacksCitation24.
In recent years, combination therapy often used in cancer treatment is being replaced by the use of hybrids or multitarget compounds. These last are novel molecules that, combining in a single chemical entity two or more chemical features needed to act on different molecular targets, work as combined therapy reducing limitations related to combination therapyCitation25. A particular type of multitarget drug is the mutual prodrug, which is designed to release the original parent drugs after in vivo administrationCitation26. This approach was successfully applied in the case of 5-FU-based polypharmacologyCitation27.
We recently synthesised three innovative 5-FU/HO-1 hybrids named SI1/17, SI1/20, and SI1/22 (). The rationale for their design was to link, in the same chemical entity, the antimetabolite 5-FU and our most potent arylethanolimidazoles (i.e. SI1/09, LS4/28, and LS6/42, respectively, ) selected from an in-house library of non-competitive HO-1 inhibitors since the alcohol functional group can be easily esterified with a cleavable linker of succinic acid which in turn is linked to 5-FU. Accordingly, hybrids were developed to release the parent components after hydrolytic or enzymatic cleavage, thus acting as mutual prodrugs. These compounds, previously tested against prostate (DU145) and lung (A549) cancer cells, gave interesting results and were shown to release, in vitro, 5-FU, and HO-1 inhibitorsCitation28,Citation29.
Figure 1. Chemical structures and HO inhibitory activity in spleen and brain microsomal fractions of tested compounds.
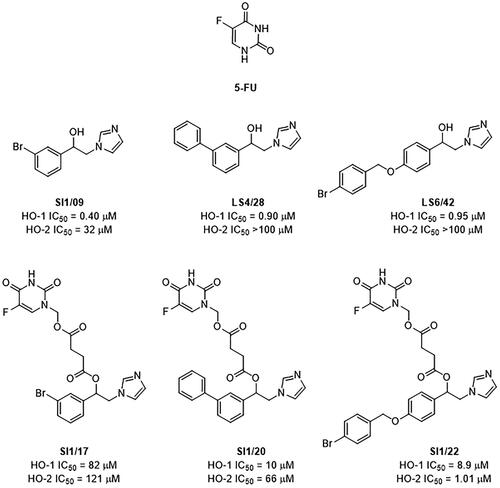
Being 5-FU the first-choice drug for CRC and being HO-1 involved in the proliferation of CRC cellsCitation30–32, in this work we aimed to evaluate the antiproliferative effects of the hybrids, as well as the physical combinations of 5-FU and corresponding HO-1 inhibitors (combo treatment), in a colon cancer model. Previously, we demonstrated that in colon cancer cells the targeting of intracellular redox balance commits to cell damage and deathCitation33,Citation34. Thus, some important antioxidant and apoptotic pathways were investigated to preliminarily analyse the mechanism of action of both the hybrids and combo treatments.
Materials and methods
Drugs preparation
5-FU was purchased from Fluorochem (product code F003241). Imidazole-based HO-1 inhibitors (SI1/09, LS4/28, and LS6/42)Citation35,Citation36 and key intermediates 4-[(5-fluoro-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)methoxy]-4-oxobutanoic acid (5-FU-succ) were sourced from our compounds library. 5-FU/HO-1 hybrids (SI1/17, SI1/20, and SI1/22) were resynthesized as previously described with minor modificationsCitation28,Citation29. Drugs were dissolved in dimethyl sulfoxide (DMSO) to give a 20 mM stock solution, and stored at −20 °C. Before use, all different stock solutions were diluted in RPMI culture medium to obtain the proper final concentrations.
Chemistry
Solvents and reagents were purchased from commercial vendors. Flash column chromatography was performed on Merck silica gel 60, 0.040–0.063 mm. Melting point was determined in an IA9200 Electrothermal apparatus equipped with a digital thermometer in glass capillary tubes and is uncorrected. The infra-red (IR) spectrum was recorded in KBr disc on a Perkin Elmer 1600 Series FT-IR spectrometer.1H NMR spectra was recorded on a Varian Inova Unity 200 spectrometer, using dimethyl sulfoxide-d6 (DMSO-d6) solution with tetramethylsilane as an internal standard. Chemical shifts are given in δ values (ppm), while coupling constants (J) are given in hertz (Hz). Elemental analysis for C, H, and N was within ± 0.4% of theoretical value and was performed on a Carlo Erba Elemental Analyser Mod. 1108 apparatus.
Details regarding the synthesis of the most representative compound SI1/22 is reported herein. Briefly, a mixture of 5-FU-succ (101 mg, 0.39 mmol), 3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (74 mg, 0.39 mmol) and 4-(dimethylamino)pyridine (5 mg, 0.04 mmol) were added to a stirred suspension of LS6/42 (111 mg, 0.32 mmol) in 10 ml of acetonitrile anhydrous. The reaction was left to stir at room temperature overnight, and the solvent removed under vacuum. The crude was purified by silica gel column chromatography using a mixture of acetone-cyclohexane (9:1, v/v) as eluent to give 1‐(1‐{4‐[(4‐bromophenyl)methoxy]phenyl}‐2‐(1H‐imidazol‐1‐yl)ethyl) 4‐(5‐fluoro‐2,4‐dioxo‐1,2,3,4‐tetrahydropyrimidin‐1‐yl)methyl butanedioate (33 mg), as a white solid: mp 143.5–144.0 °C; IR (KBr, selected lines): cm-1 3448, 3081, 2960, 1756, 1611, 1513, 1458, 1408, 1357, 1245, 1133, 1001, 832, 754; 1H NMR (200 MHz, DMSO-d6) δ 8.11 (d, JH-F = 6.6 Hz, 1H), 7.60 (d, J = 8.2 Hz, 2H), 7.51 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.26 (d, J = 8.3 Hz, 2H), 7.11 (s, 1H), 6.99 (d, J = 8.3 Hz, 2H), 6.84 (s, 1H), 5.90 (t, J = 6.1 Hz, 1H), 5.55 (s, 2H), 5.09 (s, 2H), 4.34 (d, J = 7.0 Hz, 2H), 2.58 (s, 4H). Anal. Calcd. for C27H24BrFN4O7: C, 52.70; H, 3.93; N, 9.10. Found: C, 52.53; H, 3.92; N, 9.08.
Measurement of HO-1 enzymatic activity
HO-1’s enzymatic activity was measured in cell lysates as the difference in absorbance between 464 and 530 nm of the bilirubin produced. Cells were cultured in RPMI supplemented with 10% FBS and 1% pen-strep solution, then cells were treated for 48/72 h with compounds (LS6/42 - SI1/22) at a concentration of 5 µM. After treatment cells were harvested and HO-1 enzymatic activity was assessed. The reaction mixtures consisted of 20 mM Tris-HCl at pH 7.4 (2 mg/mL), the cell lysate, 0.5–2 mg/mL biliverdin reductase, 1 mM NADPH, 2 mM glucose 6-phosphate (G6P), 1 U G6P dehydrogenase and 25 µM hemin. Incubation was carried out in a circulating water bath in the dark for 1 h at 37 °C. The reaction was stopped by adding chloroform. After recovering the chloroform phase, the amount of bilirubin that was formed was measured with a double-beam spectrophotometer at OD 464–530 nm (extinction coefficient: 40 mM/cm−1 for bilirubin). One unit of the enzyme was defined as the amount of enzyme catalysing the formation of 1 nmol of bilirubin/mg protein/h.
Cells and culture conditions
HCT116 colon cancer cells were obtained from Interlab Cell Line Collection (ICLC, Genoa, Italy) (accession number ICLC HTL95025). Cells were maintained in RPMI1640 medium enriched with 2 mM glutamine and 10% of heat-inactivated foetal bovine serum (FBS). A solution of 100 U/mL penicillin and 100 µg/mL streptomycin was added to the medium. Cells were incubated at 37 °C, in the presence of 5% CO2 and humidified atmosphere. Once cell confluence was reached, cells were seeded in opportune culture plates at the density of 2 × 104 cells/cm2 and exposed to the different drugs or vehicle (DMSO) alone (indicated in the text as control) for the established time. DMSO concentration never exceeded 0.3% (v/v), a concentration that did not affect HCT116 cells viability.
Cell viability assay
The colorimetric assay using MTT (3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Merck-Sigma Aldrich, MI, Italy), was employed to measure cellular metabolic activity as an indicator of cell viability and proliferation, as previously describedCitation37. For the experiments, cells were seeded in 96-well plates, treated with the compounds at different concentrations (5, 10, or 15 µΜ) or vehicle alone (control) for the indicated time, and incubated with 5 mg/ml of MTT for 2 h. The formazan crystals were dissolved in a lysis solution and the staining intensity was quantified by measuring the absorbance at 570 nm at a microplate reader (OPSYS MR, Dynex Technologies, Chantilly, VA, USA).
Cell cycle analysis
To analyse cell cycle distribution, after treatment with the compounds, cells were harvested by trypsinization (0.025% trypsin-EDTA; Life Technologies Ltd, Monza, Italy) and resuspended in a hypotonic solution containing 25 μg/ml propidium iodide, 0.1% sodium citrate, 0.01% Nonidet P-40 and 10 μg/ml RNase A for 2 h at 4° C. Cell cycle distribution was evaluated by using a FACScanto cytometer and data elaborated by Flowing software.
Western blotting analysis
After treatment, cells were lysed in RIPA buffer and protein extracted were quantified using Bradford method. Equal amount of proteins (30 μg) was separated by SDS-PAGE, electrotransferred to a nitrocellulose membrane and exposed to specific primary antibodies. HO-1 (GTX101147) antibody was purchased from Gene Tex (GeneTex, Prodotti Gianni, MI, Italy); anti-SOD (sc-133); anti-p62 (P0068) and anti-Actin (A4700) from Merck Millipore (Merck-Sigma Aldrich, MI, Italy); anti Caspase-3 (96625) and anti Nrf-2 (sc-518033) from Cell Signalling Technology (Danvers, MA, USA); anti-PARP1 from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Molecular weight markers were from Biogenerica, Catania, Italy (catalogue number RPN 800E rainbow molecular weight markers, full-range Mr 12000–225000). After incubation with specific HRP-conjugated secondary antibodies (Merck-Sigma Aldrich, MI, Italy), the blots were developed using the ECL chemiluminescent labelling systems. Optical densities of the bands were analysed by using ImageJ Software. In some cases, proteins were analysed on the same filter after stripping and re-hybridization with the specific antibodies.
ROS evaluation
The reactive oxygen species (ROS) produced after treatment were detected through the oxidation of the cell-permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probe, Life Technologies, Eugene, OR, USA) dye, as reported beforeCitation38. Cells were seeded in 24-well plates at the density of 2 × 104/cm2, treated and stained with 2 μM H2DCFDA dye for 15 min in the dark and in the presence of 5% CO2 at 37° C. After washing, the fluorescent 2′,7′-dichlorofluorescein (DCF), produced by intracellular oxidation, were analysed under a fluorescence microscope using FITC filter. Images were acquired by using an Optika IM3F fluorescence microscope equipped with camera.
Statistical analysis
Data, expressed as mean ± SD, were evaluated with Student’s t-test using Microsoft Excel software. The analysis of multiple groups of samples was performed with ANOVA test. P values <0.05 was considered statistically significant.
Results and discussion
Cytotoxicity and morphology of HCT116 CRC cells after treatment with 5-FU, HO-1 inhibitors, 5-FU/HO-1 hybrids and respective combo
Initially, we evaluated the effect of HO-1 inhibitors and the respective 5-FU/HO-1 hybrids in HCT116 cancer cells, which represent a human colon cancer model widely employed in therapeutic research and drug screening. Compounds were tested by performing an MTT assay to evaluate their effect on HCT116 cell viability. shows a screening over time of the effects exerted by the compounds employed at 5 µM concentration. The tested dose was chosen according to previous findings on the significant antiproliferative activity against different cancer cells exerted by HO-1 inhibitors at a similar concentrationCitation8,Citation29. The HO-1 inhibitor LS6/42 and the corresponding SI1/22 hybrid resulted in the most efficacious molecules in inhibiting HCT116 cell viability, after 72 h of treatment.
Table 1. Time course of HCT116 cell viability after treatment with 5 µM of the different drugs at different times.
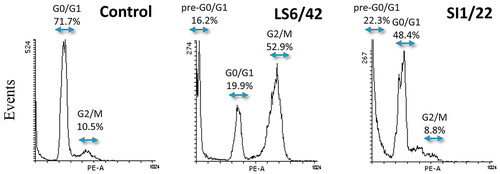
The analysis of cell cycle distribution profiles of more efficacious compounds showed that SI1/22 induced an increase in the pre-G0/G1 population seen to the left of the G0/G1 peak, indicating DNA fragmentation that can be associated with apoptotic cell death. Differently, LS6/42 treatment induced an increase of G2/M peak which was accompanied by a moderate increase of pre-G0/G1 peak ().
Figure 2. Flow cytometry analysis of the cell cycle phase distribution of HCT116 cells after 72 h incubation with 5 μM SI1/22 or LS6/42. Hypotonic propidium iodide staining was used to evaluate DNA content as described in Methods employing Flowing software.
These promising data prompted us to investigate the action of novel compounds in more detail and the effects of the treatment using HO-1 inhibitors alone or in combination with 5-FU on HCT116 cell viability (). As a general trend, HO-1 inhibitors showed a low to moderate effect on cell viability, while the hybrids produced a significant dose-dependent effect comparable to that of 5-FU. Among the tested hybrids SI1/22, bearing a bulkier and more hydrophobic 4-bromobenzyloxy group at the para-position of the phenylethyl moiety, exerted better antiproliferative activity than the 3-bromo (SI1/17) and the 3-phenyl (SI1/20) analogs, respectively; also compared to the reference drug 5-FU. As a result, a correlation between increased lipophilicity and higher antiproliferative potency was observed, likely due to the promotion of cellular uptake and considering the higher predicted permeability value of SI1/22 (). Particularly, SI1/22 was more potent than 5-FU at all tested concentrations resulting in the best compound among the series, as well as the combo administration of LS6/42 and 5-FU resulted in even more effective in terms of antiproliferative action (). Also, agreeing with extensive structure-activity relationship studies (SARs) performed on different azole-based analogs, and agreeing with the solved crystal structure of the protein in complex with non-competitive HO-1 inhibitors (e.g. QC-80)Citation39, the simultaneous accommodation of bulky groups (i.e. 5-FU-succinyl and 4-bromobenzyloxy moiety as for SI1/22) into the two main hydrophobic pockets of HO-1 is generally well-tolerated and seems to be an optimal combination for stabilising the inactive conformation of the enzyme.
Figure 3. Effect of 5-FU, HO-1 inhibitors, hybrids, and combo treatment on HCT116 cell viability and morphology. (A) Evaluation of cell viability by MTT assay after 72 h of treatment using different concentrations of the drugs. The results represent the mean ± SD of almost three separate experiments done in triplicate. Significant vs untreated control cells: *p < 0.05; **p < 0.01; ***p < 0.001. (B) Representative images of HCT116 cells after 72 h of treatment with 15 µM of the compounds. Cells were visualised under a light microscope (200× magnification) and the pictures were acquired by IM50 Leica Software (Leika Microsystems, Wetzlar, Germany).
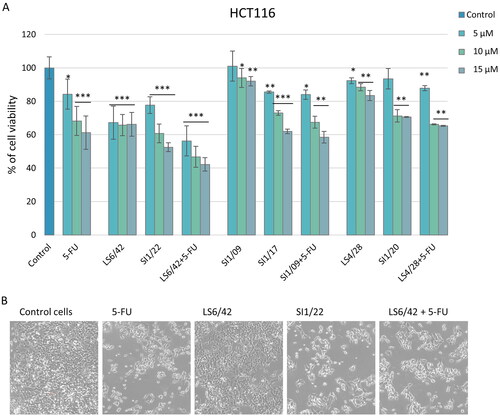
Table 2. Predicted lipophilicity/permeability and experimental antiproliferative effects in HCT116 cells of hybrids.
Based on these results, the HO-1 inhibitor LS6/42 alone or in combination with 5-FU and the corresponding hybrid SI1/22 were selected for subsequent experiments. The morphological observation of the treated cells with 15 µM of each compound, and the corresponding drug combination, after 72 h exposure, showed that LS6/42 did not induce a significant morphological change in cancer cells, while SI1/22 and the LS6/42 + 5-FU combo produced a significant reduction of cell density and a slight morphological variation, as the cells appeared more rounded and less close to one another, thus supporting an anti-proliferative effect ().
Effect on HO-1 enzymatic activity, ROS production and Nrf2-dependent antioxidant pathways
Then, we wanted to confirm that hybrid SI1/22 was able to inhibit HO-1 activity not only in microsomal preparation but also in cell lysate, an experimental condition that better reproduces a cell environment. For this purpose, bilirubin formation was measured in HCT116 cell lysates after 72 h of treatment. SI1/22 was able to reduce HO-1 activity similarly to the positive control LS6/42 (i.e., 78.65% vs. 66.9%) (). This result suggests that hybrid SI1/22 can cross cellular membranes and then it is cleaved to regenerate parental drugs and inhibit HO activity.
Table 3. Inhibition of HO-1 enzymatic activity in HCT116 treated cells with LS6/42 and SI1/22.
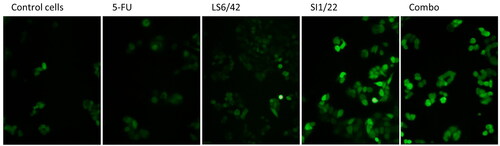
Additionally, to correlate HO-1 inhibition to oxidative stress conditions, ROS levels and antioxidant proteins expression were evaluated. Results depicted in clearly show that, after 3 h exposure, ROS levels highly increased after both treatments with hybrid SI1/22 and the LS6/42 + 5-FU combo, while no remarkable differences were observed for LS6/42 and 5-FU as a stand-alone treatment. Although 5-FU is known to increase ROS cellular levelsCitation40, there was no increase in our experimental conditions. On the other hand, hybrid SI1/22 and combo treatment were able to significantly induce ROS production even at low concentrations and after just 3 h of exposure.
Figure 4. Effect of 5-FU, HO-1 inhibitors, hybrids, and combo treatment on oxidative stress. Assessment of ROS levels in HCT116 cells treated with 15 µM of tested drugs for 3 h; the images were acquired under a fluorescence microscope at 200 x magnification.
Consequently, due to the high and early increase in ROS levels, the activation of an antioxidant response was analysed by measuring the expression levels of two antioxidant enzymes involved in oxidative stress, such as HO-1 and SOD. Western blotting and densitometric analysis highlighted an increase in both enzyme expressions compared to the control group (). These data were consistent with the previously observed ROS production with a major effect detected in the case of LS6/42 + 5-FU combo treatment.
Figure 5. Analysis of apoptosis and autophagy-related proteins. Representative images and relative densitometric analysis of western blotting against different proteins. HO-1, SOD, pro-caspase-3, p62 (A) and PARP (C) protein expression levels were evaluated after 72 h of treatment, Nrf2 (B) protein after 48 h. The histograms are related to relative intensities over actin. *p < 0.05; **p < 0.01; ***p < 0.001 vs untreated cells (control).
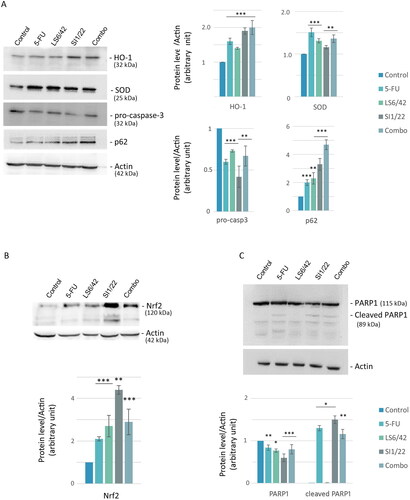
Therefore, the modulation of antioxidant pathways was analysed by measuring the expression level of Nrf2, which is one of the major transcription factors that promotes cellular defense against oxidative stress through the expression of many antioxidant genes including HMOX1 encoding for HO-1Citation41. Collected data depicted in showed an increased Nrf2 level when cells were treated with both hybrid SI1/22 and 5-FU + LS6/42 combo compared to the control, in accordance with the increase of HO-1 levels ().
Involvement of apoptotic or autophagic mechanism in drugs-induced cell death
To investigate what type of cell death pathway was induced by the compounds, we first analysed the levels of pro-caspase 3 as indicative of its activation to caspase-3. Caspase-3 is a well-known protease that catalyses the specific cleavage of many key proteins responsible for apoptosis. Western blotting and densitometric analysis suggested that all treatments decreased the pro-caspase-3 expression level in HCT116 cells, suggesting a possible promotion of apoptosis through the caspase-3-dependent pathway (). Active caspase-3 is known to cleave PARP1, a nuclear protein involved in DNA damage repair systemsCitation42. PARP1 and cleaved-PARP1 levels were also evaluated, showing a significant increase in cleaved isoform especially after SI1/22 and combo treatments ().
Additionally, to further investigate the mechanism of action of the hybrid compound we analysed p62 expression, a protein involved in ROS-dependent cell stress and autophagic processCitation41. Interestingly, treatment with LS6/42, SI1/22, and LS6/42 + 5-FU combo highly induced p62 accumulation in HCT116 cells (), suggesting an involvement of autophagy in the mechanism of action of the compounds. Since the autophagic pathway can play an opposite role (pro- or anti-survival) in cellsCitation43, it will be of interest to investigate deeper how the hybrids or the combination influence autophagy in colon cancer cell models.
Conclusions
In this work we aimed to evaluate whether the 5-FU/HO-1 hybrids SI1/17, SI1/20, and SI1/22 previously developed as antiproliferative agents against prostate and lung cancer cells, might work also against CRC, being this last treated with 5-FU as a first-choice drug. Here we have demonstrated that the simultaneous use of an inhibitor of HO-1 and 5-FU, both as a combo treatment or as resulting from hybrid cleavage, in the case of SI1/22, exerted antiproliferative activity greater than that 5-FU itself, thus allowing the use of lower and therefore potentially less toxic doses. Despite cells increased expression of HO-1, SOD, and Nrf-2 in response to the oxidative stress, the inhibition of HO-1 activity is maintained in the cellular environment after treatment with our compounds. Our preliminary study on the mechanism of cell death has highlighted that apoptosis and autophagy are involved, paving the way for new applications in the development of innovative CRC treatments.
Author contributions
Conceptualisation, M.G. and S.I.; methodology, L.S., A.N., V.S., M.G., S.I.; validation M.G. and S.I.; synthesis, purification, and compounds characterisation, L.S., V.P., S.I.; contributed reagents, materials, and analysis tools, V.S., L.V., M.G., S.I.; formal analysis, L.S., V.S. M.G., S.I.; investigation, A.N., V.C., F.A., V.P., L.V.; data curation, L.S., A.N., F.A., V.C., M.G., S.I.; writing original draft preparation, L.S., M.G., S.I.; writing, review and editing, L.S., M.G., S.I.; visualisation, A.N., L.V., V.C.; supervision, L.S., M.G., S.I.; funding acquisition, M.G. and S.I. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The data that support this study are available from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Surh YJ, Chung HT, Na HK, Dulak J, Stec DE. Progress in heme oxygenase research. Arch Biochem Biophys. 2020;685:1.
- Intagliata S, Salerno L, Ciaffaglione V, Leonardi C, Fallica AN, Carota G, Amata E, Marrazzo A, Pittalà V, Romeo G. Heme oxygenase-2 (ho-2) as a therapeutic target: activators and inhibitors. Eur J Med Chem. 2019;183:111703.
- Salerno L, Floresta G, Ciaffaglione V, Gentile D, Margani F, Turnaturi R, Rescifina A, Pittalà V. Progress in the development of selective heme oxygenase-1 inhibitors and their potential therapeutic application. Eur J Med Chem. 2019;167:439–10.
- Luu Hoang KN, Anstee JE, Arnold JN. The diverse roles of heme oxygenase-1 in tumor progression. Front Immunol. 2021;12:658315.
- Ciaffaglione V, Intagliata S, Pittalà V, Marrazzo A, Sorrenti V, Vanella L, Rescifina A, Floresta G, Sultan A, Greish K, et al. New arylethanolimidazole derivatives as ho-1 inhibitors with cytotoxicity against mcf-7 breast cancer cells. Int J Mol Sci. 2020;21(6):21.
- Mucha O, Podkalicka P, Mikulski M, Barwacz S, Andrysiak K, Biela A, Mieczkowski M, Kachamakova-Trojanowska N, Ryszawy D, Białas A, et al. Development and characterization of a new inhibitor of heme oxygenase activity for cancer treatment. Arch Biochem Biophys. 2019;671:130–142.
- Fallica AN, Sorrenti V, D’Amico AG, Salerno L, Romeo G, Intagliata S, Consoli V, Floresta G, Rescifina A, D’Agata V, et al. Discovery of novel acetamide-based heme oxygenase-1 inhibitors with potent in vitro antiproliferative activity. J Med Chem. 2021;64(18):13373–13393.
- Romeo G, Ciaffaglione V, Amata E, Dichiara M, Calabrese L, Vanella L, Sorrenti V, Grosso S, D’Amico AG, D’Agata V, et al. Combination of heme oxygenase-1 inhibition and sigma receptor modulation for anticancer activity. Molecules. 2021; 26(13):3860.
- Sorrenti V, Pittalà V, Romeo G, Amata E, Dichiara M, Marrazzo A, Turnaturi R, Prezzavento O, Barbagallo I, Vanella L, et al. Targeting heme oxygenase-1 with hybrid compounds to overcome imatinib resistance in chronic myeloid leukemia cell lines. Eur J Med Chem. 2018;158(:937–950.
- Torp SH, Solheim O, Skjulsvik AJ. The who 2021 classification of central nervous system tumours: A practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien)). 2022;164(9):2453–2464.
- Yin H, Fang J, Liao L, Maeda H, Su Q. Upregulation of heme oxygenase-1 in colorectal cancer patients with increased circulation carbon monoxide levels, potentially affects chemotherapeutic sensitivity. BMC Cancer. 2014;14(1):436. (
- Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282(28):20621–20633.
- NIH. Drugs approved for colon and rectal cancer National Cancer institute:Updated: January 23, 2023 (Cited: October 14,23.
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117.
- Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: A review. Ther Adv Med Oncol. 2016;8(1):57–84.
- World health organization. Model list of essential medicines - 23rd list. 2023. WHO: Geneva.:Retrieved 14 October 2023 from https://iris.who.int/bitstream/handle/10665/371090/WHO-MHP-HPS-EML-2023.02-eng.pdf?sequence=1(.
- Parker WB. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem Rev. 2009;109(7):2880–2893.
- Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16(4):215–237.
- Tanaka F, Fukuse T, Wada H, Fukushima M. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr Pharm Biotechnol. 2000;1(2):137–164.
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338.
- Macdonald JS. Toxicity of 5-fluorouracil. Oncology (Williston Park). 1999;13(7 Suppl 3):33–34.
- Shiga T, Hiraide M. Cardiotoxicities of 5-fluorouracil and other fluoropyrimidines. Curr Treat Options Oncol. 2020;21(4):27.
- Fraile RJ, Baker LH, Buroker TR, Horwitz J, Vaitkevicius VK. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980;40(7):2223–2228.
- Mohelnikova-Duchonova B, Melichar B, Soucek P. Folfox/folfiri pharmacogenetics: The call for a personalized approach in colorectal cancer therapy. World J Gastroenterol. 2014;20(30):10316–10330.
- de Oliveira Pedrosa M, Duarte da Cruz RM, de Oliveira Viana J, de Moura RO, Ishiki HM, Barbosa Filho JM, Diniz MF, Scotti MT, Scotti L, Bezerra Mendonca FJ. Hybrid compounds as direct multitarget ligands: a review. Curr Top Med Chem. 2017;17(9):1044–1079.
- Das N, Dhanawat M, Dash B, Nagarwal RC, Shrivastava SK. Codrug: An efficient approach for drug optimization. Eur J Pharm Sci. 2010;41(5):571–588.
- Ciaffaglione V, Modica MN, Pittalà V, Romeo G, Salerno L, Intagliata S. Mutual prodrugs of 5-fluorouracil: From a classic chemotherapeutic agent to novel potential anticancer drugs. ChemMedChem. 2021;16(23):3496–3512.
- Salerno L, Vanella L, Sorrenti V, Consoli V, Ciaffaglione V, Fallica AN, Canale V, Zajdel P, Pignatello R, Intagliata S. Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-fu/ho-1 hybrid): Design and preliminary in vitro evaluation. J Enzyme Inhib Med Chem. 2021;36(1):1378–1386.
- Salerno L, Sorrenti V, Pittalà V, Consoli V, Modica MN, Romeo G, Marrazzo A, Giuliano M, Zajdel P, Vanella L, et al. Discovery of si 1/20 and si 1/22 as mutual prodrugs of 5-fluorouracil and imidazole-based heme oxygenase 1 inhibitor with improved cytotoxicity in du145 prostate cancer cells. ChemMedChem. 2023;18(8):e202300047.
- Akhdar H, Loyer P, Rauch C, Corlu A, Guillouzo A, Morel F. Involvement of nrf2 activation in resistance to 5-fluorouracil in human colon cancer ht-29 cells. Eur J Cancer. 2009;45(12):2219–2227.
- Cernigliaro C, D’Anneo A, Carlisi D, Giuliano M, Marino Gammazza A, Barone R, Longhitano L, Cappello F, Emanuele S, Distefano A, et al. Ethanol-mediated stress promotes autophagic survival and aggressiveness of colon cancer cells via activation of nrf2/ho-1 pathway. Cancers (Basel). 2019;11(4):11.
- Seo GS, Jiang WY, Chi JH, Jin H, Park WC, Sohn DH, Park PH, Lee SH. Heme oxygenase-1 promotes tumor progression and metastasis of colorectal carcinoma cells by inhibiting antitumor immunity. Oncotarget. 2015;6(23):19792–19806.
- Lo Galbo V, Lauricella M, Giuliano M, Emanuele S, Carlisi D, Calvaruso G, De Blasio A, Di Liberto D, D’Anneo A. Redox imbalance and mitochondrial release of apoptogenic factors at the forefront of the antitumor action of mango peel extract. Molecules. 2021; 26(14):4328.
- Notaro A, Lauricella M, Di Liberto D, Emanuele S, Giuliano M, Attanzio A, Tesoriere L, Carlisi D, Allegra M, De Blasio A, et al. A deadly liaison between oxidative injury and p53 drives methyl-gallate-induced autophagy and apoptosis in hct116 colon cancer cells. Antioxidants (Basel). 2023;12(6):12.
- Salerno L, Amata E, Romeo G, Marrazzo A, Prezzavento O, Floresta G, Sorrenti V, Barbagallo I, Rescifina A, Pittalà V. Potholing of the hydrophobic heme oxygenase-1 western region for the search of potent and selective imidazole-based inhibitors. Eur J Med Chem. 2018;148(:54–62.
- Greish KF, Salerno L, Al Zahrani R, Amata E, Modica MN, Romeo G, Marrazzo A, Prezzavento O, Sorrenti V, Rescifina A, et al. Novel structural insight into inhibitors of heme oxygenase-1 (ho-1) by new imidazole-based compounds: Biochemical and in vitro anticancer activity evaluation. Molecules. 2018;23(5):1209.
- Lauricella M, D’Anneo A, Giuliano M, Calvaruso G, Emanuele S, Vento R, Tesoriere G. Induction of apoptosis in human osteosarcoma saos-2 cells by the proteasome inhibitor mg132 and the protective effect of prb. Cell Death Differ. 2003;10(8):930–932.
- Celesia A, Morana O, Fiore T, Pellerito C, D’Anneo A, Lauricella M, Carlisi D, De Blasio A, Calvaruso G, Giuliano M, et al. Ros-dependent er stress and autophagy mediate the anti-tumor effects of tributyltin (iv) ferulate in colon cancer cells. Int J Mol Sci. 2020;21(21):21.
- Rahman MN, Vlahakis JZ, Vukomanovic D, Szarek WA, Nakatsu K, Jia Z. X-ray crystal structure of human heme oxygenase-1 with (2r,4s)-2-[2-(4-chlorophenyl)ethyl]-2-[(1h-imidazol-1-yl)methyl]-4[((5-trifluoromethylpyridin-2-yl)thio)methyl]-1,3-dioxolane: A novel, inducible binding mode. J Med Chem. 2009;52(15):4946–4950.
- Fu Y, Yang G, Zhu F, Peng C, Li W, Li H, Kim HG, Bode AM, Dong Z, Dong Z. Antioxidants decrease the apoptotic effect of 5-fu in colon cancer by regulating src-dependent caspase-7 phosphorylation. Cell Death Dis. 2014;5(1):e983–e983.
- Emanuele S, Celesia A, D’Anneo A, Lauricella M, Carlisi D, De Blasio A, Giuliano M. The good and bad of nrf2: An update in cancer and new perspectives in covid-19. Int J Mol Sci. 2021;(15):22.
- Pandey A, Trigun SK. Fisetin induces apoptosis in colorectal cancer cells by suppressing autophagy and down-regulating nuclear factor erythroid 2-related factor 2 (nrf2). J Cell Biochem. 2023;124(9):1289–1308.
- Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134(:116–137.

