Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death. FGFR4 has been implicated in HCC progression, making it a promising therapeutic target. We introduce an approach for identifying novel FGFR4 inhibitors by sequentially adding fragments to a common warhead unit. This strategy resulted in the discovery of a potent inhibitor, 4c, with an IC50 of 33 nM and high selectivity among members of the FGFR family. Although further optimisation is required, our approach demonstrated the potential for discovering potent FGFR4 inhibitors for HCC treatment, and provides a useful method for obtaining hit compounds from small fragments.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, which is the sixth most common cause of cancer-related death in 2022Citation1. HCC is reported to be typically developed due to chronic liver inflammation caused by hepatitis B or C infection or non-alcoholic steatohepatitis (NASH) or cirrhosisCitation2–3. Regarding therapies for advanced HCC, multi-kinase inhibitors (TKIs), sorafenibCitation4 and regorafenibCitation5 have been approved for clinical use. Although they significantly improve the overall survival and quality of life of patients over conventional chemotherapies, the median survival time is less than one yearCitation6.
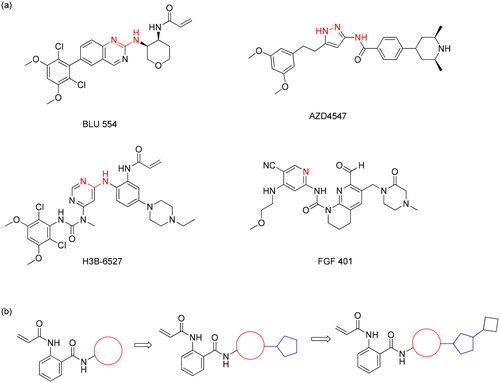
The Fibroblast Growth Factor Receptor 4 (FGFR4), a member of the FGFR family with tyrosine kinase domains, is activated by its specific ligand, FGF19Citation7. FGFR4 plays an important role in the regulation of cell growth, differentiation, and survival. Aberrant expression or dysregulation of FGFR4 has been observed in several solid tumours and has been implicated to the development and progression of various types of cancer, including liver, lung, and breast cancerCitation8. Abnormal activation of FGF19-FGFR4 signalling is closely associated with HCC progressionCitation9. FGFR4 inhibitors have demonstrated promise as a potential treatment for HCCCitation10. In a phase I trial, fisogatinib (BLU-554), a highly potent and selective FGFR4 inhibitor, showed anti-tumor activity in patients with overexpression of FGF19 in advanced HCCCitation11. FGF401, a reversible-covalent inhibitor for FGFR4, was investigated in clinical trial, and its treatment was safe and showed preliminary clinical efficacyCitation12. H3B-6527 is a potent and selective FGFR4 inhibitor that has shown promising results in preclinical studiesCitation13 and is being evaluated in clinical trials for the treatment of advanced HCCCitation14. Therefore, inhibition of FGFR4 can be an effective strategy for treating HCC. In addition, Studies on the binding mode of the known inhibitors have suggested that the 1,3-diamino group for BLU554Citation15 and H3B-6527Citation13 or the pyridine group for FGF401Citation16 would be placed towards the hinge region. We tried to discover novel FGFR4 inhibitors through an extension strategy as a hit identification strategy, starting from a small and commonly used warhead moiety that was expected to be favourable for target binding. Herein, we described the discovery of novel FGFR4 inhibitors through building up from a small fragment. This strategy commenced with the addition of heterocycle, which may be involved in hydrogen bonding interactions, as shown in .
Materials and methods
Chemistry
General information
Unless otherwise noted, all reagents and solvents were purchased from Alfa Aesar, Combi Blocks, Fisher Scientific, Samchun Pure Chemical, Sigma Aldrich, or TCI and used without additional processing. Reactions were monitored by thin-layer chromatography (TLC) using Merck TLC silica gel 60 F254 250 µm plates. Flash column chromatography was performed using ZEOprep 60 silica gel (Zeochem, 40–63 µm) and a CombiFlash system (Teledyne ISCO) loaded with pre-packed silica gel flash column cartridges (Welux™).1H and 13C NMR spectra were obtained using a 600 MHz NMR spectrometer (JEOL) using tetramethylsilane (TMS) as an internal standard. Chemical shifts are reported in parts per million (ppm, δ) downfield of TMS, and the coupling constant (J) is reported in hertz (Hz). Splitting patterns are reported with the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartette; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; m, multiplet; b, broad signal.
Methyl 2-acrylamidobenzoate (1a)
To a solution of methyl anthranilate (130 μL, 1 mmol) in anhydrous dichloromethane (2 ml) at 0 °C, was added acryloyl chloride (100 μL, 1.2 mmol) and triethylamine (280 μL, 2 mmol). Then the reaction mixture was purged with N2 gas and stirred at 25 °C for 3 h. After confirming the reaction was complete by TLC, the mixture was concentrated in vacuo and solid loaded without work up. It purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 10: 90 to 20: 80) to obtain 1a as a white solid (90 mg, 44%).
1H-NMR (600 MHz, CDCl3) δ 11.34 (s, 1H), 8.81 (d, J = 7.2 Hz, 1H), 8.03 (dd, J = 8.4, 1.2 Hz, 1H), 7.56 (ddd, J = 8.4, 7.2, 1.2 Hz, 1H), 7.09 (t, J = 7.2 Hz, 1H), 6.43 (dd, J = 16.8, 1.2 Hz, 1H), 6.32 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (dd, J = 10.2, 1.8 Hz, 1H), 3.92 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.8, 164.1, 141.5, 134.7, 132.5, 130.9, 127.2, 122.6, 120.4, 115.0, 52.3.
2-(Propanoyl amino)-N-pyridazin-3-benzamide (1b)
To a solution of the compound 2a (106.2 mg, 0.5 mmol) in methanol (5 ml) at 25 °C, was added palladium on carbon (30.8 mg, 0.3 wt.%). Then the reaction mixture was purged with H2 gas and stirred at 25 °C for 3 h. After confirming the reaction was complete by TLC, the mixture was filtered through CeliteTM and concentrated in vacuo to obtain 1b as a white solid (100.3 mg, 97%).
1H-NMR (600 MHz, CDCl3) δ 8.74 (d, J = 8.3 Hz, 1H), 8.03 (dd, J = 8.3, 1.4 Hz, 1H), 7.53–7.56 (m, 1H), 7.06–7.09 (m, 1H), 3.93 (s, 3H), 2.49 (q, J = 7.6 Hz, 2H), 1.28 (t, J = 7.6 Hz, 3H)
2-Acrylamido-N-(pyridazin-3-yl)benzamide (2a)
To a solution of the compound 1a (40 mg, 0.2 mmol), 3-aminopyridazine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2a as a pale yellow solid (6.3 mg, 12%).
1H-NMR (600 MHz, CDCl3) δ 11.05 (s, 1H), 9.25 (s, 1H), 9.01 (d, J = 4.2 Hz, 1H), 8.80 (d, J = 7.8 Hz, 1H), 8.54 (d, J = 9 Hz, 1H), 7.81 (d, J = 7.2 Hz, 1H), 7.61 (td, J = 7.8, 1.2 Hz, 1H), 7.58 (dd, J = 9.6, 4.8 Hz, 1H), 7.21 (td, J = 7.8, 1.2 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 164.1, 155.1, 149.1, 140.6, 134.4, 132.3, 128.5, 127.7, 127.3, 127.0, 123.5, 122.2, 119.3, 119.1; HRMS (ESI): m/z calcd for C14H12N4O2 [M-H]- 267.0887, found 267.0899.
2-Acrylamido-N-(pyridin-2-yl)benzamide (2b)
To a solution of the compound 1a (40 mg, 0.2 mmol), 2-aminopyridine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2b as a white solid (5.8 mg, 11%).
1H-NMR (600 MHz, CDCl3) δ 11.13 (s, 1H), 8.76–8.78 (m, 2H), 8.29–8.30 (m, 2H), 7.79 (td, J = 7.8, 1.8 Hz, 1H), 7.71 (dd, J = 7.2, 1.2 Hz, 1H), 7.57 (td, J = 7.8, 1.2 Hz, 1H), 7.17 (td, J = 7.8, 1.8 Hz, 1H), 7.11–7.13 (m, 1H), 6.44 (dd, J = 16.8, 1.2 Hz, 1H), 6.32 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (dd, J = 10.2, 1.2 Hz, 1H)); 13C-NMR (150 MHz, CDCl3) δ 167.7, 164.2, 151.1, 148.3, 140.2, 138.7, 133.7, 132.4, 127.6, 127.0, 123.3, 122.1, 120.6, 120.2, 114.5; HRMS (ESI): m/z calcd for C15H13N3O2 [M-H]- 266.0935, found 266.0939.
2-Acrylamido-N-(pyridin-3-yl)benzamide (2c)
To a solution of the compound 1a (40 mg, 0.2 mmol), 3-aminopyridine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2c as a white solid (5.7 mg, 11%).
1H-NMR (600 MHz, CDCl3) δ 10.7 (s, 1H), 9.06 (s, 1H), 8.93 (d, J = 3 Hz, 1H), 8.46 (dd, J = 4.8, 1.8 Hz, 1H), 8.39 (d, J = 7.8 Hz, 1H), 8.24 (dt, J = 14.4, 2.4 Hz, 1H), 7.52 (dd, J = 8.4, 1.2 Hz, 1H), 7.38 (dd, J = 8.4, 4.2 Hz, 1H), 7.32 (td, J = 7.8, 1.2 Hz, 1H), 7.02 (t, J = 7.8 Hz, 1H), 6.47 (d, J = 16.8 Hz, 1H), 6.26 (dd, J = 16.8, 10.2 Hz, 1H), 5.84 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 167.8, 164.6, 146.0, 142.2, 138.7, 134.8, 132.7, 132.0, 128.2, 127.7, 127.6, 123.9, 123.5, 122.3, 121.4;
HRMS (ESI): m/z calcd for C15H13N3O2 [M-H]- 266.0935, found 266.0936.
2-Acrylamido-N-(pyrimidin-2-yl)benzamide (2d)
To a solution of the compound 1a (40 mg, 0.2 mmol), 2-aminopyrimidine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2d as a white solid (7.4 mg, 14%).
1H-NMR (600 MHz, CDCl3) δ 11.12 (s, 1H), 8.92 (s, 1H), 8.77 (d, J = 8.4 Hz, 1H), 8.71 (d, J = 4.8 Hz, 2H), 7.73 (dd, J = 7.2, 1.2 Hz, 1H), 7.57 (td, J = 8.4, 1.2 Hz, 1H), 7.16 (t, J = 7.2, Hz, 1H), 7.13 (t, J = 4.8 Hz, 1H), 6.45 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.78 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 167.1, 164.4, 158.7, 157.6, 140.7, 134.1, 132.2, 128.0, 127.4, 123.3, 122.3, 119.7, 117.5; HRMS (ESI): m/z calcd for C14H12N4O2 [M-H]- 267.0887, found 267.0899.
2-Acrylamido-N-(pyrimidin-4-yl)benzamide (2e)
To a solution of the compound 1a (40 mg, 0.2 mmol), 4-aminopyrimidine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2e as a pale yellow solid (18.8 mg, 35%).
1H-NMR (600 MHz, CDCl3) δ 10.88 (s, 1H), 9.06 (s, 1H), 8.92 (d, J = 1.8 Hz, 1H), 8.73 (s, 1H), 8.72 (s, 1H), 8.25 (dd, J = 3.6, 1.2 Hz, 1H), 7.68 (dd, J = 7.8, 1.2 Hz, 1H), 7.55 (td, J = 7.8, 1.8 Hz, 1H), 7.16 (td, J = 7.8, 1.2 Hz, 1H), 6.45 (d, J = 16.8 Hz, 1H), 6.25 (dd, J = 16.8, 10.2 Hz, 1H), 5.82 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.5, 164.2, 158.8, 158.7, 157.1, 140.2, 134.3, 132.2, 127.9, 127.3, 123.4, 122.3, 119.4, 110.7; HRMS (ESI): m/z calcd for C14H12N4O2 [M-H]- 267.0887, found 267.0899.
2-Acrylamido-N-(pyrazin-2-yl)benzamide (2f)
To a solution of the compound 1a (40 mg, 0.2 mmol), 2-aminopyrazine (19 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2f as a white solid (8.1 mg, 15%).
1H-NMR (600 MHz, CDCl3) δ 10.98 (s, 1H), 9.62 (d, J = 1.2 Hz, 1H), 8.86 (s, 1H), 8.72 (d, J = 9 Hz, 1H), 8.43 (d, J = 3 Hz, 1H), 8.32 (dd, J = 3, 1.2 Hz, 1H), 7.69 (dd, J = 7.2, 1.2 Hz, 1H), 7.53 (td, J = 7.8, 1.2 Hz, 1H), 7.15 (t, J = 7.8 Hz, 1H), 6.45 (d, J = 16.8 Hz, 1H), 6.32 (dd, J = 16.8, 10.2 Hz, 1H), 5.82 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 167.6, 164.3, 148.1, 142.5, 141.0, 140.2, 137.5, 134.0, 132.3, 127.8, 127.3, 123.4, 122.3, 119.5; HRMS (ESI): m/z calcd for C14H12N4O2 [M-H]- 267.0887, found 267.09.
2-Acrylamido-N-(6-chloropyridazin-3-yl)benzamide (2 g)
To a solution of the compound 1a (40 mg, 0.2 mmol), 3-amino-6-chloropyridazine (26 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2 g as a white solid (7.1 mg, 11%).
1H-NMR (600 MHz, CDCl3) δ 10.90 (s, 1H), 9.40 (s, 1H), 8.77 (d, J = 7.8 Hz, 1H), 8.55 (d, J = 10.8 Hz, 1H), 7.81 (dd, J = 8.4, 1.2 Hz, 1H), 7.59–7.62 (m, 2H), 7.21 (td, J = 7.2, 1.8 Hz, 1H), 6.43 (d, J = 16.8 Hz, 1H), 6.29 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.3, 164.1, 154.3, 153.0, 140.5, 134.5, 132.3, 130.1, 127.8, 127.4, 123.5, 122.2, 121.7, 119.0; HRMS (ESI): m/z calcd for C14H11ClN4O2 [M-H]- 301.0497, found 301.0504
2-Acrylamido-N-(6-ethynylpyridin-3-yl)benzamide (2h)
To a solution of the compound 1a (40 mg, 0.2 mmol), 6-ethynylpyridin-3-amine (24 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2h as an ivory solid (19.7 mg, 34%).
1H-NMR (600 MHz, CDCl3) δ 10.64 (s, 1H), 9.12 (s, 1H), 8.88 (d, J = 3 Hz, 1H), 8.34 (d, J = 8.4 Hz, 1H), 8.27 (dd, J = 9, 2.4 Hz, 1H), 7.55 (d, J = 9 Hz, 1H), 7.50 (dd, J = 7.8, 1.2 Hz, 1H), 7.32 (td, J = 7.8, 1.2 Hz, 1H), 7.02 (t, J = 7.2 Hz, 1H), 6.47 (d, J = 16.8 Hz, 1H), 6.26 (dd, J = 16.8, 10.2 Hz, 1H), 5.85 (d, J = 10.2 Hz, 1H), 3.18 (s, 1H); 13C-NMR (150 MHz, CDCl3) δ 167.7, 164.7, 142.1, 138.7, 138.1, 134.7, 132.8, 132.0, 128.3, 127.9, 127.6, 127.3, 123.6, 122.5, 121.4, 82.7, 76.8; HRMS (ESI): m/z calcd for C17H13N3O2 [M-H]- 290.0934, found 290.0934.
2-Acrylamido-N-(6-ethynylpyridazin-3-yl)benzamide (2i)
To a solution of the compound 1a (40 mg, 0.2 mmol), 6-ethynylpyridazin-3-amine (24 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2i as a pale yellow solid (8.1 mg, 14%).
1H-NMR (600 MHz, CDCl3) δ 11.00 (s, 1H), 9.19 (s, 1H), 8.80 (d, J = 9 Hz, 1H), 8.54 (d, J = 9 Hz, 1H), 7.78 (d, J = 6.6 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.62 (td, J = 7.8, 1.2 Hz, 1H), 7.22 (td, J = 7.8, 1.2 Hz, 1H), 6.43 (d, J = 16.8 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 3.42 (s, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.1, 164.1, 153.5, 144.4, 140.7, 134.6, 132.3, 132.0, 127.8, 127.2, 123.5, 122.3, 118.9, 118.1, 81.6, 79.7; HRMS (ESI): m/z calcd for C16H12N4O2 [M-H]- 291.0887, found 291.0894.
2-Acrylamido-N-(5-methylisoxazol-3-yl)benzamide (2j)
To a solution of the compound 1a (40 mg, 0.2 mmol), 3-amino-5-methylisoxazole (25 mg, 0.24 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2j as a white solid (12.1 mg, 22%).
1H-NMR (600 MHz, CDCl3) δ 11.11 (s, 1H), 9.81 (s, 1H), 8.75 (d, J = 9.6 Hz, 1H), 7.80 (dd, J = 7.8, 1.2 Hz, 1H), 7.57 (td, J = 7.8, 1.2 Hz, 1H), 7.17 (td, J = 7.8, 1.8 Hz, 1H), 6.82 (s, 1H), 6.43 (dd, J = 16.8, 1.2 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (d, J = 10.2 Hz, 1H), 2.46 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 170.4, 167.4, 164.2, 158.2, 140.4, 134.0, 132.4, 127.8, 127.6, 123.3, 122.0, 119.1, 97.0, 12.9; HRMS (ESI): m/z calcd for C14H13N3O3 [M-H]- 270.0883, found 270.0889.
2-Acrylamido-N-(1H-indazol-3-yl)benzamide (2k)
To a solution of the compound 1a (40 mg, 0.2 mmol), 1H-indazol-3-amine (32 mg, 0.24 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2k as a white solid (20.0 mg, 33%).
1H-NMR (600 MHz, CDCl3) δ 11.10 (s, 1H), 9.60 (s, 1H), 8.67 (d, J = 1.8 Hz, 1H), 7.90 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.45 (t, J = 7.8 Hz, 1H), 7.37 (s, 1H), 7.37 (s, 1H), 7.15–7.17 (m, 1H), 7.04 (t, J = 7.8 Hz, 1H), 6.39 (d, J = 16.8 Hz, 1H), 6.21 (dd, J = 16.8, 10.2 Hz, 1H), 5.70 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 167.8, 164.5, 141.7, 140.6, 139.9, 133.4, 132.2, 128.0, 127.8, 127.8, 123.2, 122.4, 122.1, 121.2, 120.1, 116.4, 110.3; HRMS (ESI): m/z calcd for C17H14N4O2 [M-H]- 305.1043, found 305.1056.
2-Acrylamido-N-(quinazolin-4-yl)benzamide (2 l)
To a solution of the compound 1a (40 mg, 0.2 mmol), quinazolin-4-ylamine (35 mg, 0.24 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 2 l as a white solid (19.1 mg, 30%).
1H-NMR (600 MHz, CDCl3) δ 12.26 (s, 1H), 8.83 (d, J = 8.4 Hz, 1H), 8.72 (d, J = 6 Hz, 1H), 8.67 (d, J = 4.2 Hz, 1H), 8.32 (s, 1H), 7.94 (td, J = 7.8, 1.8 Hz, 1H), 7.89 (d, J = 8.4 Hz, 1H), 7.69 (t, J = 7.8 Hz, 1H), 7.56 (td, J = 7.8, 1.8 Hz, 1H), 7.17 (td, J = 7.8, 1.2 Hz, 1H), 6.45 (dd, J = 16.8, 1.2 Hz, 1H), 6.36 (dd, J = 16.8, 10.2 Hz, 1H), 5.78 (dd, J = 10.2, 1.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 164.2, 157.4, 148.8, 141.6, 141.2, 135.9, 134.3, 132.9, 132.6, 132.6, 128.6, 128.4, 127.2, 126.4, 122.9, 122.8, 121.5, 120.7; HRMS (ESI): m/z calcd for C18H14N4O2 [M-H]- 317.1043, found 317.1055.
6–(3-(Dimethylamino)prop-1-yn-1-yl)pyridazin-3-amine (3a)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), N, N-dimethylpropargylamine (107 μL, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3a as a brown solid (93 mg, 53%).
1H-NMR (600 MHz, CDCl3) δ 7.25 (d, J = 9 Hz, 1H), 6.82 (d, J = 9 Hz, 1H), 5.80 (b, 2H), 3.53 (s, 2H), 2.40 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 158.6, 139.3, 131.4, 114.0, 86.4, 82.6, 48.5, 44.3.
6–(3-Methoxyprop-1-yn-1-yl)pyridazin-3-amine (3b)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), methyl propargyl ether (84 μL, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3b as a yellow solid (132 mg, 80%).
1H-NMR (600 MHz, CDCl3) δ 7.29 (d, J = 9.6 Hz, 1H), 6.91 (d, J = 8.4 Hz, 1H), 5.71 (b, 2H), 4.34 (s, 2H), 3.45 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.5, 139.1, 131.5, 114.3, 87.1, 83.2, 60.3, 58.0.
6-((4-Methoxyphenyl)ethynyl)pyridazin-3-amine (3c)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 4-ethynylanisole (132 mg, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3c as a yellow solid (166 mg, 74%).
1H-NMR (600 MHz, CDCl3) δ 7.47 (d, J = 9 Hz, 2H), 7.33 (d, J = 9 Hz, 1H), 7.04 (d, J = 9 Hz, 1H), 6.87 (d, J = 9 Hz, 2H), 5.96 (b, 2H), 3.82 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.2, 158.3, 137.8, 133.3, 131.5, 114.8, 114.2, 114.0, 92.5, 84.9, 55.4.
6-((3-Methoxyphenyl)ethynyl)pyridazin-3-amine (3d)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 3-ethynylanisole (127 μL, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3d as a brown gel (180 mg, 80%).
1H-NMR (600 MHz, CDCl3) δ 7.35 (d, J = 9 Hz, 1H), 7.26 (t, J = 7.8 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.12 (d, J = 7.2 Hz, 1H), 7.05 (s,1H), 6.92 (dd, J = 8.4, 2.4 Hz, 1H), 6.25 (b, 2H), 3.79 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 159.1, 158.5, 138.8, 131.2, 129.4, 123.9, 122.7, 116.3, 115.3, 114.5, 90.7, 85.7, 55.1.
6-((2-Methoxyphenyl)ethynyl)pyridazin-3-amine (3e)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 1-ethynyl-2-methoxybenzene (130 μL, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3e as a yellow solid (160 mg, 71%).
1H-NMR (600 MHz, CDCl3) δ 7.52 (dd, J = 7.2, 1.2 Hz, 1H), 7.41 (d, J = 9 Hz, 1H), 7.36 (td, J = 8.4, 1.2 Hz, 1H), 7.09 (d, J = 9.6 Hz, 1H), 6.95 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 9 Hz, 1H), 5.80 (b, 2H), 3.91 (s, 2H); 13C-NMR (150 MHz, CDCl3) δ 160.3, 158.0, 139.8, 133.8, 131.9, 130.8, 120.7, 115.0, 111.2, 110.9, 89.7, 88.3, 55.9.
6-((3,5-Dimethoxyphenyl)ethynyl)pyridazin-3-amine (3f)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 1-ethynyl-3,5-dimethyoxybenzene (162 mg, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3f as a yellow gel (183.7 mg, 82%).
1H-NMR (600 MHz, CDCl3) δ 7.35 (d, J = 9 Hz, 1H), 7.10 (d, J = 9 Hz, 1H), 6.67 (d, J = 2.4 Hz, 2H), 6.47 (t, J = 1.8 Hz, 1H), 6.29 (b, 2H), 3.77 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 160.3, 158.6, 137.5, 131.3, 123.1, 114.5, 109.3, 102.1, 90.9, 85.5, 55.3.
6-((3-(Dimethylamino)phenyl)ethynyl)pyridazin-3-amine (3 g)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 3-ethynyl-N, N-dimethylaniline (145 mg, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3 g as a yellow gel (207 mg, 87%).
1H-NMR (600 MHz, CDCl3) δ 7.35 (d, J = 8.4 Hz, 1H), 7.20 (t, J = 8.4 Hz, 1H), 7.06 (d, J = 9 Hz, 1H), 6.86–6.88 (m, 2H), 6.73 (dd, J = 8.4, 3 Hz, 1H), 6.05 (b, 2H), 2.94 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 159.4, 158.4, 150.2, 137.7, 131.6, 129.1, 122.2, 119.7, 115.2, 113.4, 95.5, 84.8, 40.4.
6-((4-(Dimethylamino)phenyl)ethynyl)pyridazin-3-amine (3h)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 4′-dimethylaminophenylacetylene (145 mg, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 8: 92 to 10: 90) to obtain 3h as a brown solid (166 mg, 70%).
1H-NMR (600 MHz, CDCl3) δ 7.40 (d, J = 9 Hz, 2H), 7.37 (d, J = 9.6 Hz, 1H), 7.13 (d, J = 9 Hz, 1H), 6.65 (d, J = 9 Hz, 2H), 3.01 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 157.8, 150.6, 137.8, 133.0, 131.7, 129.5, 115.6, 111.5, 93.6, 83.9, 40.1.
6-(Phenylethynyl)pyridazin-3-amine (3i)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), ethynylbenzene (131 μL, 1.2 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3i as a yellow solid (185 mg, 95%).
1H-NMR (600 MHz, CDCl3) δ 7.56–7.57 (m, 2H), 7.35–7.37 (m, 4H), 6.79 (d, J = 9 Hz, 1H), 5.52 (b, 2H); 13C-NMR (150 MHz, CDCl3) δ 158.2, 140.1, 132.0, 131.5, 129.1, 128.5, 122.3, 113.9, 91.3, 86.3.
6-(Pyridin-3-ylethynyl)pyridazin-3-amine (3j)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 3-ethynylpyridine (103 mg, 1 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3j as a yellow solid (122 mg, 62%).
1H-NMR (600 MHz, DMSO-d6) δ 8.80 (s, 1H), 8.63 (d, J = 4.2 Hz, 1H), 8.02 (dt, J = 8.4, 1.8 Hz, 1H), 7.49–7.52 (m, 2H), 6.94 (s, 2H), 6.84 (d, J = 8.4 Hz, 1H); 13C-NMR (150 MHz, DMSO-d6) δ 159.4, 151.6, 149.2, 138.6, 137.0, 130.9, 123.7, 119.0, 112.8, 90.4, 86.3.
6-(Cyclohexylethynyl)pyridazin-3-amine (3k)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), ethynylcyclohexane (131 μL, 1.2 mmol), and TEA (420 μL, 3 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (70 mg, 0.1 mmol) and CuI (38 mg, 0.2 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3k as a yellow gel (178 mg, 89%).
1H-NMR (600 MHz, CDCl3) δ 7.24 (d, J = 9 Hz, 1H), 7.05 (d, J = 9.6 Hz, 1H), 5.96 (b, 2H), 2.61 (tt, J = 9.6, 3.6 Hz, 1H), 1.88–1.90 (m, 2H), 1.74–1.76 (m, 2H), 1.51–1.56 (m, 3H), 1.33–1.37 (m, 3H); 13C-NMR (150 MHz, CDCl3) δ 158.2, 137.7, 131.6, 117.5, 115.0, 96.9, 32.2, 29.5, 25.6, 24.8.
6-(Naphthalen-1-ylethynyl)pyridazin-3-amine (3 l)
6-ethynylpyridazin-3-amine (36 mg, 0.3 mmol), 1-iodonaphthalene (44 μL, 0.3 mmol), and TEA (120 μL, 0.9 mmol) were dissolved in dry THF (2 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (21 mg, 0.03 mmol) and CuI (12 mg, 0.014 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 2: 98 to 5: 95) to obtain 3 l as a yellow solid (60 mg, 81%).
1H-NMR (600 MHz, CDCl3) δ 8.46 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 3.6 Hz, 1H), 7.87 (d, J = 3.6 Hz, 1H), 7.82 (d, J = 7.2 Hz, 1H), 7.60 (t, J = 7.8 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.46–7.49 (m, 2H), 6.85 (d, J = 9 Hz, 1H), 5.28 (b, 2H); 13C-NMR (150 MHz, CDCl3) δ 158.1, 140.4, 133.3, 133.2, 131.5, 131.1, 129.6, 128.4, 127.1, 126.7, 126.3, 125.4, 120.0, 113.6, 91.2, 89.4.
6–(3-(3-Methoxyphenoxy)prop-1-yn-1-yl)pyridazin-3-amine (3 m)
The solution of 3-methyoxyphenol (120 μL, 1 mmol), propargyl bromide (80 μL, 1 mmol), potassium carbonate (276 mg, 2 equiv) and acetone was stirred at 25 °C for 6 h. Then the reaction mixture was filtered, and the filtrate was evaporated in vacuo. To reaction vial, 6-iodopyridazin-3-amine (154 mg, 0.7 mmol), and TEA (280 μL, 2.0 mmol) were dissolved in dry THF (3 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (49 mg, 0.07 mmol) and CuI (27 mg, 0.14 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3 m as a brown solid (111 mg, 44%).
1H-NMR (600 MHz, CDCl3) δ 7.17–7.21 (m, 2H), 6.74 (d, J = 9 Hz, 1H), 6.60 (dd, J = 8.4, 2.4 Hz, 1H), 6.56 (t, J = 1.8 Hz, 1H), 6.54 (dd, J = 7.8, 1.8 Hz, 1H), 5.78 (b, 2H), 4.88 (s, 2H), 3.77 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.9, 158.9, 158.7, 138.7, 131.5, 130.1, 114.0, 107.3, 107.0, 101.6, 85.9, 84.0, 56.5, 55.4.
6–(3-Methoxyphenoxy)pyridazin-3-amine (3n)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 3-methoxyphenol (118 μL, 1 mmol), picolinic acid (25 mg, 0.2 mmol), potassium phosphate (424 mg, 2 mmol) and CuI (20 mg, 0.1 mmol) were dissolved in DMSO (3 ml), purged by N2 gas, stirred at 100 °C for overnight. Then a reaction mixture was added distilled water and the aqueous layer was washed with dichloromethane. The organic layer was evaporated and purified by MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 80: 20) to obtain 3n as a brown solid (113 mg, 52%).
1H-NMR (600 MHz, CDCl3) δ 6.75 (t, J = 8.4 Hz, 1H), 6.51 (d, J = 9 Hz, 1H), 6.46 (d, J = 9 Hz, 1H), 6.20 (dd, J = 8.4, 1.2 Hz, 1H), 6.15–6.16 (m, 2H), 5.31 (b, 2H), 3.29 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 159.7, 158.6, 157.8, 155.5, 129.0, 119.6, 118.8, 110.7, 108.6, 104.7, 54.3.
6–(3-Methoxyphenyl)pyridazin-3-amine (3o)
6-Iodopyridazin-3-amine (221 mg, 1 mmol), 3-methoxyphenyl boronic acid (180 mg, 1.2 mmol) and potassium carbonate (276 mg, 2 mmol) were dissolved in the solution that mixed THF and distilled water by 2 ml and 1 ml, then degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)4 (110 mg, 0.1 mmol) and purged by N2 gas, heated with stirring in microwave reactor at 130 °C for 1 h. The mixture was filtered over Celite and the filtrate was evaporated in vacuo and purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3o as a white solid (137 mg, 68%).
1H-NMR (600 MHz, CDCl3) δ 7.59–7.62 (m, 2H), 7.44 (d, J = 7.8 Hz, 1H), 7.37 (t, J = 7.8 Hz, 1H), 6.96 (dd, J = 8.4, 3 Hz, 1H), 6.85 (d, J = 9 Hz, 1H), 5.09 (b, 2H), 3.88 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 160.2, 158.8, 152.3, 137.9, 129.9, 126.3, 118.5, 115.1, 115.0, 111.3, 55.5.
6-[2–(3-Fluoro-4-methoxyphenyl)ethynyl]pyridazin-3-amine (3r)
2-fluoro-4-iodo-1-methoxybenzene(63.5 mg, 0.25 mmol), 6-ethynylpyridazin-3-amine(30 mg, 0.25 mmol)), and diisopropylamine(71 μL, 0.504 mmol) were dissolved in dry THF (2 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (17.7 mg, 0.025 mmol) and CuI (9.6 mg, 0.05 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 3: 97 to 6: 94) to obtain 3r as a yellow solid (25 mg, 41%).
1H-NMR (600 MHz, MeOD-d4) δ 7.43 (d, J = 9.6 Hz, 1H), 7.27–7.34 (m, 2H), 7.11 (t, J = 8.6 Hz, 1H), 6.88–6.92 (m, 1H), 3.91 (s, 3H)
6-[2–(1,3-Benzodioxol-5-yl)ethynyl]pyridazin-3-amine (3s)
5-Ethynyl-1,3-benzodioxole (128.1 mg, 0.88 mmol), 6-Iodopyridazin-3-amine (194 mg, 0.88 mmol)), and TEA(368 μL, 2.64 mmol) were dissolved in dry THF (2 ml) and degassed with N2 balloon for 5 min. To a mixture were added Pd(PPh3)2Cl2 (63.17 mg, 0.09 mmol) and CuI (34.3 mg, 0.18 mmol), purged by N2 gas, stirred at 25 °C for 24 h. The mixture was filtered over Celite and the filtrate was purified using MPLC with gradient concentration of ethyl acetate/hexane (v: v = 60: 40 to 80: 20) to obtain 3s as a yellow solid (48 mg, 23%).
1H-NMR (600 MHz, DMSO-d6) δ 7.40 (d, J = 9.0 Hz, 1H), 7.09–7.11 (m, 2H), 6.98 (d, J = 8.3 Hz, 1H), 6.75 (d, J = 9.0 Hz, 3H), 6.09 (s, 2H)
2-Acrylamido-N-(6–(3-(dimethylamino)prop-1-yn-1-yl)pyridazin-3-yl)benzamide (4a)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3a (35 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 5: 95 to 8: 92) to obtain 4a as a brown solid (7.0 mg, 10%).
1H-NMR (600 MHz, CDCl3) δ 11.02 (s, 1H), 9.32 (b, 1H), 8.79 (d, J = 8.4 Hz, 1H), 8.50 (d, J = 9.6 Hz, 1H), 7.80 (d, J = 7.2 Hz, 1H), 7.62 (d, J = 9 Hz, 1H), 7,61 (td, J = 7.8, 1.2 Hz, 1H), 7.21 (t, J = 8.4 Hz, 1H), 6.43 (d, J = 16.8 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 3.58 (s, 2H), 2.42 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 153.2, 145.2, 140.6, 134.5, 132.3, 131.7, 127.7, 127.3, 123.5, 122.2, 119.1, 118.3, 89.9, 81.8, 48.7, 44.5; HRMS (ESI): m/z calcd for C19H19N5O2 [M-H]- 348.1465, found 348.1482.
2-Acrylamido-N-(6–(3-methoxyprop-1-yn-1-yl)pyridazin-3-yl)benzamide (4b)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3b (33 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 5: 95 to 8: 92) to obtain 4b as a yellow solid (7.4 mg, 11%).
1H-NMR (600 MHz, CDCl3) δ 11.00 (s, 1H), 9.31 (s, 1H), 8.79 (d, J = 8.4 Hz, 1H), 8.52 (d, J = 9 Hz, 1H), 7.80 (d, J = 7.2 Hz, 1H), 7.65 (d, J = 9.6 Hz, 1H), 7.61 (td, J = 7.8, 1.2 Hz, 1H), 7.21 (td, J = 7.8, 1.2 Hz, 1H), 6.43 (d, J = 16.8 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 4.41 (s, 2H), 3.50 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 153.4, 144.7, 140.6, 134.5, 132.3, 131.7, 127.8, 127.3, 123.5, 122.2, 119.0, 118.3, 89.7, 82.6, 60.3, 58.3; HRMS (ESI): m/z calcd for C18H16N4O3 [M-H]- 335.1149, found 335.1163.
2-Acrylamido-N-(6-((4-methoxyphenyl)ethynyl)pyridazin-3-yl)benzamide (4c)
To a solution of compound 1a (40 mg, 0.2 mmol), 3c (45 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4c as a pale yellow solid (2.7 mg, 3.3%).
1H-NMR (600 MHz, CDCl3) δ 11.05 (s, 1H), 9.22 (s, 1H), 8.81 (d, J = 8.4 Hz, 1H), 8.54 (d, J = 9 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.70 (d, J = 9 Hz, 1H), 7.62 (td, J = 8.4, 1.2 Hz, 1H), 7.59 (d, J = 9 Hz, 2H), 7.23 (t, J = 7.2 Hz, 1H), 6.93 (d, J = 8.4 Hz, 2H), 6.44 (d, J = 17.4 Hz, 1H), 6.32 (dd, J = 17.4, 10.2 Hz, 1H), 5.81 (d, J = 10.2 Hz, 1H), 3.86 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.0, 164.1, 160.7, 152.8, 145.7, 140.6, 134.4, 133.8, 132.3, 131.4, 127.7, 127.1, 123.4, 122.2, 119.0, 118.1, 114.3, 113.6, 94.1, 84.5, 55.5; HRMS (ESI): m/z calcd for C23H18N4O3 [M-H]- 397.1305, found 397.1320.
2-Acrylamido-N-(6-((3-methoxyphenyl)ethynyl)pyridazin-3-yl)benzamide (4d)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3d (45 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4d as a yellow solid (5.1 mg, 6%).
1H-NMR (600 MHz, CDCl3) δ 11.04 (s, 1H), 9.25 (s, 1H), 8.81 (d, J = 8.4 Hz, 1H), 8.55 (d, J = 9 Hz, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 9 Hz, 1H), 7.62 (td, J = 7.8, 1.2 Hz, 1H), 7.31 (t, J = 7.8 Hz, 1H), 7.21–7.25 (m, 2H), 7.17 (dd, J = 3, 1.2 Hz, 1H), 6.98 (ddd, J = 8.4, 3, 1.8 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.81 (d, J = 10.2 Hz, 1H), 3.85 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.1, 164.2, 159.6, 153.1, 145.5, 140.7, 134.5, 132.3, 131.7, 129.8, 127.8, 127.2, 124.8, 123.5, 122.6, 122.2, 119.1, 118.2, 116.9, 116.5, 93.6, 85.3, 55.5; HRMS (ESI): m/z calcd for C23H18N4O3 [M-H]- 397.1305, found 397.1324.
2-Acrylamido-N-(6-((2-methoxyphenyl)ethynyl)pyridazin-3-yl)benzamide (4e)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3e (45 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4e as a white solid (16.4 mg, 21%).
1H-NMR (600 MHz, CDCl3) δ 11.05 (s, 1H), 9.49 (s, 1H), 8.79 (d, J = 7.2 Hz, 1H), 8.53 (d, J = 8.4 Hz, 1H), 7.87 (dd, J = 8.4, 1.2 Hz, 1H), 7.75 (d, J = 9 Hz, 1H), 7.59–7.62 (m, 2H), 7.40 (td, J = 8.4, 1.8 Hz, 1H), 7.23 (t, J = 8.4 Hz, 1H), 6.99 (t, J = 7.8 Hz, 1H), 6.95 (d, J = 7.8 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.32 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 3.95 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.2, 160.7, 153.1, 145.7, 140.5, 134.4, 134.2, 132.3, 131.8, 131.3, 127.7, 127.5, 123.5, 122.1, 120.7, 119.2, 118.4, 118.4, 110.9, 90.5, 89.4, 56.0; HRMS (ESI): m/z calcd for C23H18N4O3 [M-H]- 397.1305, found 397.1315.
2-Acrylamido-N-(6-((3,5-dimethoxyphenyl)ethynyl)pyridazin-3-yl)benzamide (4f)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3f (51 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4f as a yellow solid (15.1 mg, 18%).
1H-NMR (600 MHz, CDCl3) δ 11.03 (s, 1H), 9.42 (s, 1H), 8.79 (d, J = 9 Hz, 1H), 8.54 (d, J = 9 Hz, 1H), 7.84 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 9 Hz, 1H), 7.61 (td, J = 7.8, 1.2 Hz, 1H), 7.22 (t, J = 7.2 Hz, 1H), 6.79 (d, J = 1.8 Hz, 2H), 6.54 (t, J = 2.4 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.81 (d, J = 10.2 Hz, 1H), 3.82 (s, J = 6H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 160.8, 153.2, 145.3, 140.6, 134.5, 132.3, 131.7, 127.8, 127.4, 123.5, 122.9, 122.2, 119.1, 118.4, 110.0, 103.3, 93.6, 85.0, 55.7; HRMS (ESI): m/z calcd for C24H20N4O4 [M-H]- 427.1411, found 427.1429.
2-Acrylamido-N-(6-((3-(dimethylamino)phenyl)ethynyl)pyridazin-3-yl)benzamide (4 g)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3 g (47 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4 g as a yellow solid (10.0 mg, 12%).
1H-NMR (600 MHz, CDCl3) δ 11.04 (s, 1H), 9.34 (s, 1H), 8.80 (d, J = 7.8 Hz, 1H), 8.53 (d, J = 9 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 9.6 Hz, 1H), 7.61 (t, J = 7.8 Hz, 1H), 7.21–7.25 (m, 2H), 6.98–7.00 (m, 2H), 6.79 (dd, J = 7.8, 1.8 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 2.98 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 153.0, 150.4, 145.7, 140.6, 134.4, 132.3, 131.7, 129.3, 127.7, 127.4, 123.5, 122.2, 122.0, 120.3, 119.1, 118.3, 115.8, 114.1, 94.9, 84.5, 40.6; HRMS (ESI): m/z calcd for C24H21N5O2 [M-H]- 410.1622, found 410.1631.
2-Acrylamido-N-(6-((4-(dimethylamino)phenyl)ethynyl)pyridazin-3-yl)benzamide (4h)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 4h (47 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4h as a yellow solid (13.8 mg, 17%).
1H-NMR (600 MHz, CDCl3) δ 11.07 (s, 1H), 9.40 (s, 1H), 8.79 (d, J = 8.4 Hz, 1H), 8.48 (d, J = 9 Hz, 1H), 7.84 (d, J = 7.2 Hz, 1H), 7.66 (d, J = 9.6 Hz, 1H), 7.59 (t, J = 8.4 Hz, 1H), 7.51 (d, J = 9 Hz, 1H), 7.21 (t, J = 7.2 Hz, 1H), 6.67 (d, J = 9 Hz, 2H), 6.43 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 3.02 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 168.1, 164.1, 152.6, 151.0, 146.2, 140.5, 134.3, 133.6, 132.4, 131.3, 127.7, 127.5, 123.5, 122.1, 119.2, 118.5, 111.8, 107.9, 96.0, 84.3, 40.2; HRMS (ESI): m/z calcd for C24H21N5O2 [M-H]- 410.1622, found 410.1637.
2-Acrylamido-N-(6-(phenylethynyl)pyridazin-3-yl)benzamide (4i)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 4i (39 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4i as a yellow solid (22.5 mg, 31%).
1H-NMR (600 MHz, CDCl3) δ 11.03 (s, 1H), 9.60 (s, 1H), 8.78 (d, J = 8.4 Hz, 1H), 8.53 (d, J = 9.6 Hz, 1H), 7.87 (dd, J = 8.4, 1.2 Hz, 1H), 7.72 (d, J = 9 Hz, 1H), 7.64 (dd, J = 7.8, 2.4 Hz, 1H), 7.59 (t, 7.8 Hz, 1H), 7.39–7.43 (m, 3H), 7.21 (t, J = 7.8 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.3, 164.1, 153.2, 145.4, 140.5, 134.4, 132.3, 132.3, 131.7, 129.7, 128.7, 127.7, 127.6, 123.5, 122.1, 121.7, 119.2, 118.6, 93.6, 85.5; HRMS (ESI): m/z calcd for C22H16N4O2 [M-H]- 367.1200, found 367.1209.
2-Acrylamido-N-(6-(pyridin-3-ylethynyl)pyridazin-3-yl)benzamide (4j)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3j (40 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 8: 92 to 10: 90) to obtain 4j as a yellow solid (16.3 mg, 22%).
1H-NMR (600 MHz, CDCl3) δ 11.00 (s, 1H), 9.55 (s, 1H), 8.88 (s, 1H), 8.79 (d, J = 9 Hz, 1H), 8.65 (s, 1H), 8.58 (d, J = 9 Hz, 1H), 7.93 (dt, J = 8.4, 1.8 Hz, 1H), 7.85 (dd, J = 7.2, 1.2 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.61 (td, J = 7.8, 1.2 Hz, 1H), 7.36 (dd, J = 7.8, 6.4 Hz, 1H), 7.22 (t, J = 7.2 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.81 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.3, 164.1, 153.5, 152.7, 149.9, 144.8, 140.6, 139.1, 134.5, 132.3, 131.7, 127.8, 127.5, 123.5, 123.4, 122.2, 119.1, 119.0, 118.4, 89.9, 88.6; HRMS (ESI): m/z calcd for C21H15N5O2 [M-H]- 368.1152, found 368.1166.
2-Acrylamido-N-(6-(cyclohexylethynyl)pyridazin-3-yl)benzamide (4k)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3k (40 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with with gradient concentration of ethyl acetate/hexanes (v: v = 20: 80 to 50: 50) to obtain 4k as a red gel (12.7 mg, 17%).
1H-NMR (600 MHz, CDCl3) δ 11.04 (s, 1H), 9.50 (s, 1H), 8.77 (d, J = 7.8 Hz, 1H), 8.45 (d, J = 9.6 Hz, 1H), 7.84 (dd, J = 7.8, 1.2 Hz, 1H), 7.56–7.60 (m, 2H), 7.20 (t, J = 8.4 Hz, 1H), 6.43 (dd, J = 16.8, 1.2 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (d, J = 10.2 Hz, 1H), 2.68 (tt, J = 9.6, 4.2 Hz, 1H), 1.93–1.95 (m, 2H), 1.77–1.81 (m, 2H), 1.58–1.63 (m, 3H), 1.37–1.41 (m, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 152.9, 145.8, 140.4, 134.2, 132.3, 131.6, 127.6, 127.5, 123.4, 122.0, 119.1, 118.5, 99.6, 60.5, 32.3, 29.9, 25.9, 25.0; HRMS (ESI): m/z calcd for C22H22N4O2 [M-H]- 373.1669, found 373.1679.
2-Acrylamido-N-(6-(naphthalen-1-ylethynyl)pyridazin-3-yl)benzamide (4 l)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3 l (49 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4 l as a white solid (19.2 mg, 23%).
1H-NMR (600 MHz, CDCl3) δ 11.04 (s, 1H), 9.38 (s, 1H), 8.80 (d, J = 8.4 Hz, 1H), 8.58 (d, J = 9.6 Hz, 1H), 8.48 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.90 (d, J = 8.4 Hz, 1H), 7.89 (d, J = 6 Hz, 1H), 7.85 (dd, J = 8.4, 1.8 Hz, 1H), 7.82 (d, J = 9 Hz, 1H), 7.56–7.66 (m, 3H), 7.50 (t, J = 7.2 Hz, 1H), 7.23 (td, J = 7.8, 1.2 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.32 (dd, J = 16.8, 10.2 Hz, 1H), 5.81 (d, J = 10.2 Hz, 1H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.2, 153.2, 145.6, 140.6, 134.5, 133.4, 133.3, 132.3, 131.8, 131.7, 130.4, 128.6, 127.8, 127.5, 127.4, 126.9, 126.2, 125.4, 123.5, 122.2, 119.3, 119.1, 118.4, 92.0, 90.3; HRMS (ESI): m/z calcd for C26H18N4O2 [M-H]- 417.1356, found 417.1370.
2-Acrylamido-N-(6–(3-(3-methoxyphenoxy)prop-1-yn-1-yl)pyridazin-3-yl)benzamide (4 m)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3 m (51 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4 m as a yellow solid (9.4 mg, 11%).
1H-NMR (600 MHz, CDCl3) δ 11.00 (s, 1H), 9.16 (s, 1H), 8.80 (d, J = 8.4 Hz, 1H), 8.51 (d, J = 9 Hz, 1H), 7.77 (d, J = 7.8 Hz, 1H), 7.63 (d, J = 9 Hz, 1H), 7.61 (td, J = 7.8, 1.2 Hz, 1H), 7.23 (t, J = 8.4 Hz, 1H), 7.21 (t, J = 7.8 Hz, 1H), 6.64 (dd, J = 7.8, 1.8 Hz, 1H), 6.57–6.60 (m, 2H), 6.43 (dd, J = 16.8, 1.2 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 4.98 (s, 2H), 3.81 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.1, 164.1, 161.1, 158.9, 153.4, 144.5, 140.7, 134.6, 132.3, 131.9, 130.2, 127.8, 127.2, 123.5, 122.3, 119.0, 118.1, 107.5, 107.0, 101.7, 88.5, 83.2, 56.5, 55.5; HRMS (ESI): m/z calcd for C24H20N4O4 [M-H]- 427.1411, found 427.1431.
2-Acrylamido-N-(6–(3-methoxyphenoxy)pyridazin-3-yl)benzamide (4n)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3n (49 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 5: 95 to 8: 92) to obtain 4n as a red gel (20.9 mg, 27%).
1H-NMR (600 MHz, CDCl3) δ 11.02 (s, 1H), 9.70 (s, 1H), 8.72 (d, J = 9 Hz, 1H), 8.49 (d, J = 9.6 Hz, 1H), 7.81 (d, J = 7.8 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.32 (t, J = 8.4 Hz, 1H), 7.27 (d, J = 9.6 Hz, 1H), 7.08 (t, J = 7.2 Hz, 1H), 6.75–6.80 (m, 3H), 6.41 (d, J = 16.8 Hz, 1H), 6.28 (dd, J = 16.8, 10.2 Hz, 1H), 5.77 (d, J = 10.2 Hz, 1H), 3.81 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.2, 164.1, 163.8, 161.1, 154.9, 152.3, 140.3, 134.0, 132.4, 130.4, 127.7, 127.6, 124.2, 123.4, 122.0, 119.7, 119.4, 113.0, 111.2, 107.1, 55.6; HRMS (ESI): m/z calcd for C21H18N4O4 [M-H]- 389.1255, found 389.1269.
2-Acrylamido-N-(6–(3-methoxyphenyl)pyridazin-3-yl)benzamide (4o)
To a solution of the compound 1a (40 mg, 0.2 mmol), compound 3o (40 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4o as a white solid (20.5 mg, 27%).
1H-NMR (600 MHz, CDCl3) δ 11.11 (s, 1H), 9.87 (s, 1H), 8.79 (d, J = 8.4 Hz, 1H), 8.57 (d, J = 8.4 Hz, 1H), 7.95–7.96 (m, 2H), 7.67 (s, 1H), 7.55–7.59 (m, 2H), 7.44 (t, J = 7.8 Hz, 1H), 7.17 (t, J = 7.8 Hz, 1H), 7.05 (dd, J = 7.8, 2.4 Hz, 1H), 6.44 (d, J = 16.8 Hz, 1H), 6.31 (dd, J = 16.8, 10.2 Hz, 1H), 5.80 (d, J = 10.2 Hz, 1H), 3.90 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 168.6, 164.1, 160.4, 156.9, 154.2, 140.5, 137.3, 134.2, 132.4, 130.3, 127.8, 127.7, 126.2, 123.4, 122.0, 120.0, 119.4, 119.2, 116.2, 112.0, 55.6; HRMS (ESI): m/z calcd for C21H18N4O3 [M-H]- 373.1305, found 373.1317.
2-Acrylamido-N-(6–(3-methoxyphenethyl)pyridazin-3-yl)benzamide (4p)
Compound 3d (45 mg, 0.2 mmol) was dissolved in MeOH as a solvent, add Pd/C to a mixture, purged by H2 gas and stirred for 3 h at 25 °C. The solution was filtered, and the filtrate was evaporated in vacuo. To the vial, a solution of the compound 1a (40 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 40: 60 to 70: 30) to obtain 4p as a white solid (4.4 mg, 5%).
1H-NMR (600 MHz, CDCl3) δ 11.07 (b, 1H), 9.17 (s, 1H), 8.79 (d, J = 8.4 Hz, 1H), 8.38 (d, J = 9 Hz, 1H), 7.81 (d, J = 7.2 Hz, 1H), 7.60 (td, J = 7.8, 1.2 Hz, 1H), 7.28 (d, J = 9 Hz, 1H), 7.19–7.23 (m, 2H), 6.79 (d, J = 7.8 Hz, 1H), 6.76–6.77 (m, 2H), 6.43 (d, J = 16.8 Hz, 1H), 6.30 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (d, J = 10.2 Hz, 1H), 3.79 (s, 3H), 3.28 (dd, J = 9.6, 7.2 Hz, 2H), 3.10 (dd, J = 9, 6.6 Hz, 2H); 13C-NMR (150 MHz, CDCl3) δ 164.1, 159.9, 153.4, 151.1, 142.3, 140.5, 134.2, 132.4, 129.7, 128.7, 127.6, 127.3, 123.5, 122.1, 121.0, 119.2, 114.5, 113.9, 111.8, 107.5, 55.3, 37.5, 35.8; HRMS (ESI): m/z calcd for C23H22N4O3 [M-H]- 401.1618, found 401.1632.
2-Acrylamido-N-(6–(3-(dimethylamino)phenethyl)pyridazin-3-yl)benzamide (4q)
Compound 3 g (47 mg, 0.2 mmol) was dissolved in MeOH as a solvent, add Pd/C to a mixture, purged by H2 gas and stirred for 3 h at 25 °C. The solution was filtered, and the filtrate was evaporated in vacuo. To the vial, a solution of the compound 1a (40 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (150 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of methanol/dichloromethane (v: v = 8: 92 to 10: 90) to obtain 4q as a white solid (7.0 mg, 8%).
1H-NMR (600 MHz, CDCl3) δ 11.09 (s, 1H), 9.24 (s, 1H), 8.79 (d, J = 7.8 Hz, 1H), 8.37 (b, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.59 (td, J = 7.8, 1.2 Hz, 1H), 7.31 (d, J = 9.6 Hz, 1H), 7.16–7.21 (m, 2H), 6.61 (dd, J = 7.2, 2.4 Hz, 1H), 6.58–6.59 (m, 2H), 6.43 (d, J = 16.8 Hz, 1H), 6.26 (dd, J = 16.8, 10.2 Hz, 1H), 5.79 (d, J = 10.2 Hz, 1H), 3.29 (t, J = 7.8 Hz, 2H), 3.06 (t, J = 7.8 Hz, 2H), 2.93 (s, 6H); 13C-NMR (150 MHz, CDCl3) δ 164.1, 153.5, 151.0, 143.0, 141.6, 140.5, 134.2, 132.4, 129.4, 128.8, 127.6, 127.4, 127.4, 123.4, 122.1, 119.5, 119.3, 117.0, 112.9, 110.8, 40.8, 37.7, 36.4; HRMS (ESI): m/z calcd for C24H25N5O2 [M-H]- 414.1935, found 414.1939.
2-Acrylamido-N-(6–(2-(3-fuloro-4-methoxyphenylethnyl)pyridazin-3-yl)benzamide (4r)
To a solution of the compound 1a (21 mg, 0.1 mmol), compound 3r (25 mg, 0.1 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (80 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 80 °C for 20 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with with gradient concentration of ethyl acetate/hexanes (v: v = 50: 50 to 70: 30) to obtain 4r as a white solid (6.3 mg, 15%).
1H-NMR (600 MHz, CDCl3) δ 11.03 (s, 1H), 9.27 (s, 1H), 8.80 (d, J = 7.6 Hz, 1H), 8.54 (d, J = 9.0 Hz, 1H), 7.80–7.82 (m, 1H), 7.69–7.70 (m, 1H), 7.60–7.63 (m, 1H), 7.39–7.40 (m, 1H), 7.35 (dd, J = 11.7, 2.1 Hz, 1H), 7.21–7.23 (m, 1H), 6.97 (t, J = 8.6 Hz, 1H), 6.44 (d, J = 17.9 Hz, 1H), 6.31 (dd, J = 17.2, 10.3 Hz, 1H), 5.80 (d, J = 10.3 Hz, 1H), 3.94 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 164.15, 153.07, 151.11, 145.39, 140.63, 134.49, 132.33, 131.56, 129.21, 129.19, 127.76, 127.28, 123.51, 122.24, 119.80, 119.66, 119.07, 118.26, 114.05, 113.39, 92.60, 85.03, 56.41; HRMS (ESI): m/z calcd for C23H17FN4O [M-H]- 415.1212, found 415.1227.
2-Acrylamido-N-(6–(2-(1,3-benzodioxol-5-yl)ethynyl)pyridazin-3-yl)benzamide (4s)
To a solution of the compound 1a (41 mg, 0.2 mmol), compound 3s (48 mg, 0.2 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (203 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 80 °C for 10 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with with gradient concentration of ethyl acetate/hexanes (v: v = 20: 80 to 50: 50) to obtain 4s as a white solid (5.8 mg, 7%).
1H-NMR (600 MHz, DMSO-d6) δ 8.39 (d, J = 9.6 Hz, 1H), 7.97 (d, J = 9.0 Hz, 1H), 7.92 (d, J = 9.0 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H), 7.56 (t, J = 7.2 Hz, 1H), 7.21–7.27 (m, 4H), 7.03 (d, J = 8.3 Hz, 1H), 6.42 (dd, J = 17.2, 10.3 Hz, 1H), 6.17–6.21 (m, 1H), 6.12 (s, 2H); 13C-NMR (150 MHz, DMSO-d6) δ 167.84, 163.30, 154.24, 148.80, 147.54, 143.79, 136.93, 131.97, 131.32, 129.34, 127.15, 127.06, 125.48, 123.83, 122.62, 118.90, 118.05, 113.99, 111.27, 108.99, 101.78, 92.22, 84.67, 54.90 HRMS (ESI): m/z calcd for C23H16N4O4 [M-H]- 411.1098, found 411.1117.
N-[6-[2–(4-methoxyphenyl)ethynyl]pyridazin-3-yl]-2-(propanoylamino)benzamide (4t)
To a solution of compound 1b (100 mg, 0.5 mmol), 3c (109 mg, 0.5 mmol) in dry toluene purged with N2 gas, was added trimethylaluminum (360 μL, 2.0 M in toluene) dropwise. The reaction mixture was heated at 110 °C for 3 h in the sand bath. After confirming the reaction was complete by TLC, the mixture was cooled at 25 °C, concentrated in vacuo, and solid loaded without work up. It was purified using MPLC with gradient concentration of ethyl acetate/hexanes (v: v = 0: 100 to 60: 40) to obtain 4t as a pale white solid (44 mg, 22.9%).
1H-NMR (600 MHz, CDCl3) δ 10.75 (s, 1H), 9.13 (s, 1H), 8.72 (d, J = 8.3 Hz, 1H), 8.53 (d, J = 9.6 Hz, 1H), 7.76 (d, J = 6.9 Hz, 1H), 7.69 (d, J = 9.6 Hz, 1H), 7.57–7.63 (m, 3H), 7.18–7.21 (m, 1H), 6.92 (d, J = 9.3 Hz, 2H), 3.86 (d, J = 6.9 Hz, 3H), 2.48 (q, J = 7.6 Hz, 2H), 1.28 (t, J = 7.6 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 172.87, 168.24, 160.77, 153.07, 145.62, 140.54, 134.21, 133.93, 131.49, 127.49, 122.01, 119.11, 118.51, 114.66, 114.14, 113.63, 94.02, 84.59, 77.16, 55.51, 31.65, 9.71; HRMS (ESI): m/z calcd for C23H20N4O3 [M-H]- 399.14624, found 399.1461.
In vitro kinase assay
In vitro kinase assay of compound 1, 24–56, 58 were performed through Kinase HotSpot Profiling service of Reaction Biology Corp. (USA). % inhibition of compounds was measured in the presence of a Km concentration of ATP at 0.1 and 1 μM.
Metabolic stability assay
Two types of liver microsomes (Human, Mouse, 0.5 mg/ml; Corning, cat No. #452117, #452701) were pre-incubated for 5 min at 37 °C after adding compounds at a concentration of 1 μM in 0.1 M phosphate buffer (pH 7). Subsequently, the reaction was initiated by adding the NADPH Regeneration System solution (Promega, #V9510) and allowed to incubate for 30 min at 37 °C. To terminate the reaction, an acetonitrile solution containing an internal standard (chlorpropamide) was added, followed by centrifugation for 15 min at 15,000 rpm and 4 °C. The supernatant was then injected into the LC-MS/MS system (Nexera XR system, Shimadzu) for the analysis of the parent compound. The experiments were conducted in duplicate, and the variation was within 15%. The results were quantified by comparing the values obtained after a 30-min reaction with those at 0 min, expressed as % Remaining (note that when results are unstable, the deviation may exceed 15%). To confirm the accuracy of the experiments, a positive control group, verapamil (1 μM), was conducted only in human microsomes, and the internal reference value was within 15% (±1), confirming the suitability of the experimental results.
Results and discussion
As shown in Scheme 1, key intermediate 1a was prepared by coupling with acryloyl chloride. Then, first blocks were introduced by adding a variety of aryl amines in the presence of trimethylaluminum to provide 2a-2l. The second blocks were linked to pyridazine group via metal catalysed coupling reactions, as described in Scheme 2. A variety of acetylenes 3a-m, 3r, 3s, arylether 3n and aryl derivative 3o were synthesised by Sonogashira reaction, Ullmann type reaction, and Suzuki-Miyaura coupling reaction, respectively. Amide formation in the presence of Lewis acid provided the target derivatives 4a-s. Saturated alkyl derivatives 4p and 4q were prepared by hydrogenation of 3d and 3 g. Finally, compound 4t, a propionamide derivative with a reduced vinyl group in the acrylamide moiety, was synthesised through reduction of compound la.
Scheme 1. Reagents and conditions: (a) acryloyl chloride, Et3N, CH2Cl2, rt, 3 h, 44%; (b) arylamine, trimethylaluminum 2 M in toluene, dry toluene, 110 °C, 3 h, 11–35%.
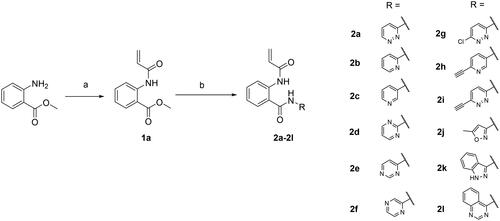
Scheme 2. Reagents and conditions: (a) arylacetylene, (Ph3P)2PdCl2, CuI, dry 1,4-dioxane, rt, overnight, 44–95%; (b) 3-methoxyphenol, picolinic acid, K3PO4, CuI, DMSO, 100 °C, overnight, 52%; (c) 3-methoxyphenylboronic acid, Pd(PPh3)4, K2CO3, THF, water, 130 °C, 1 h, microwave irradiation, 68%; (d) 1a, Al(CH3)3 2 M in toluene, dry toluene, 110 °C, 3 h, 11–31%. (e) i) Pd/C, H2, CH3OH, rt, 3 h; ii) 1a, Al(CH3)3 2M in toluene, dry toluene, 110 °C, 3 h, 5–8%. (f) Pd/C, H2, CH3OH, rt, 3 h, 97%; (g) 3c, Al(CH3)3 2M in toluene, dry toluene, 110 °C, 3 h, 23%.
First, small aromatic fragments were added to the acrylamidophenyl moiety as a common irreversible warhead essential for activity. As shown in , the introduction of pyridazine displayed slightly better activity compared to pyridine (2b, 2c), and pyrimidines (2d, 2e) and pyrazine (2f). The addition of an acetylene group (2h and 2i) or chlorination (2 g) led to increased activity, but methylisoxazole (2j) and bicyclic compounds (2k and 2 l) did not exhibit any activity. Therefore, further addition of fragments was carried out at terminal end of the acetylene moiety of 2i. As shown in , the introduction of non-aromatic groups (4a and 4b) exhibited weak activity, but p-methoxyphenyl derivative 4c displayed significant activity, surpassing all other alkoxy phenyl derivatives (4d, 4e and 4f). Replacement of methoxy group with dimethylamino group did not improve activity, although p-amino derivative 4h retained its activity. Unsubstituted phenyl derivative 4i showed similar activity to 4h, indicating that meta substitution at the terminal phenyl group would be unfavourable. cyclohexyl (4k) and pyridinyl (4j) and naphthyl (4 l) derivatives showed poor activity. One-carbon-extension (4 m) led to a decrease activity. Removal of acetylene moiety (4n and 4o) did not improve activity either. Hydrogenation of acetylene (4p and 4q) resulted in a significant decrease in activity. The introduction of fluoride (4r) decreased activity by half, while the alkoxy group (4s) caused a slight decrease in activity.
Table 1. In vitro inhibitory activity of compound 24–32, 54–56 against FGFR4 kinase at 1 μM.
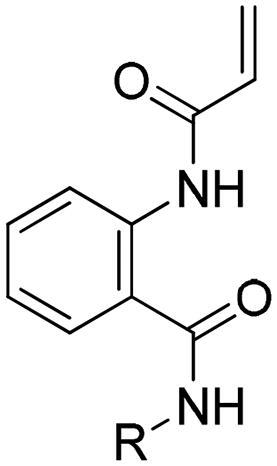
Table 2. In vitro inhibitory activity of compounds 4a-4s against FGFR4 kinase at 1 μM.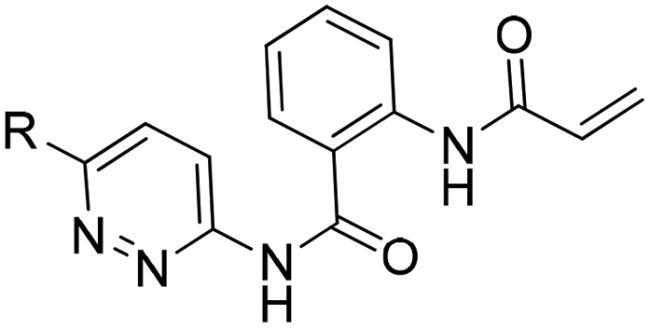
IC50 values for some selected compounds were described in . IC50 value of 4c exhibited about a 10-fold improvement compared to that of other derivatives and was 5-fold more potent than staurosporin (). As shown in , at 1 µM 4c demonstrated excellent selectivity of for the FGFR family. However, its metabolic stability was not great (). In contrast, 4s showed improved stability, suggesting that methoxyphenyl group would be susceptible to metabolic processes. Further investigation would be necessary to optimise its pharmacokinetic properties.
Table 3. IC50 values of selected compounds (4a, 4d, 4h and 4s).
Table 4. Kinase selectivity of 4c against FGFR subtypes.
Table 5. Metabolic stability of 4c in human and mouse liver microsomes.
Conclusion
We have successfully identified potent and selective FGFR4 inhibitors using a strategy that involves the sequential addition and selection of fragments to a covalent warhead unit. Among the approaches employed to discover active compounds, our method has demonstrated its effectiveness for obtaining hit compounds from small fragments. This approach would be useful to researchers who do not have access to a substantial chemical library.
Authors contributions
J.K. mainly conducted the experimental work with support from C.G.I., K.O., J.M.L., and F.A.R. in the preparation of some derivatives, as well as in the data collection and interpretation. K.H.M. conceptualised and designed the experiment, and prepared the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
- Ito T, Nguyen MH. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J Hepatocell Carcinoma. 2023;10:413–428.
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
- Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–240.
- Heo YA, Syed YY. Regorafenib: A review in hepatocellular carcinoma. Drugs. 2018;78(9):951–958.
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–149.
- Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25(3):552–563.
- Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D, Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12(1):56.
- Lu X, Chen H, Patterson AV, Smaill JB, Ding K. Fibroblast Growth Factor Receptor 4 (FGFR4) Selective Inhibitors as Hepatocellular Carcinoma Therapy: Advances and Prospects. J Med Chem. 2019;62(6):2905–2915.
- Kim RD, Sarker D, Meyer T, Yau T, Macarulla T, Park JW, Choo SP, Hollebecque A, Sung MW, Lim HY, et al. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discov. 2019;9(12):1696–1707.
- Chan SL, Schuler M, Kang YK, Yen CJ, Edeline J, Choo SP, Lin CC, Okusaka T, Weiss KH, Macarulla T, et al. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. J Exp Clin Cancer Res. 2022;41(1):189.
- Joshi JJ, Coffey H, Corcoran E, Tsai J, Huang CL, Ichikawa K, Prajapati S, Hao MH, Bailey S, Wu J, et al. H3B-6527 is a potent and selective inhibitor of FGFR4 in FGF19-driven hepatocellular carcinoma. Cancer Res. 2017;77(24):6999–7013.
- Rioux N, Kim A, Nix D, Bowser T, Warmuth M, Smith PG, Schindler J. Effect of a high-fat meal on the relative bioavailability of H3B-6527, a novel FGFR4 inhibitor, in healthy volunteers. Cancer Chemother Pharmacol. 2019;83(1):91–96.
- Zhang X, Wang Y, Ji J, Si D, Bao X, Yu Z, Zhu Y, Zhao L, Li W, Liu J. Discovery of 1,6-Naphthyridin-2(1H)-one derivatives as novel, potent, and selective FGFR4 inhibitors for the treatment of hepatocellular carcinoma. J Med Chem. 2022;65(11):7595–7618.
- Zhou Z, Chen X, Fu Y, Zhang Y, Dai S, Li J, Chen L, Xu G, Chen Z, Chen Y. Characterization of FGF401 as a reversible covalent inhibitor of fibroblast growth factor receptor 4. Chem Commun. 2019;55(42):5890–5893.