ABSTRACT
Introduction: Managing the polio endgame requires access to sufficient quantities of poliovirus vaccines. After oral poliovirus vaccine (OPV) cessation, outbreaks may occur that require outbreak response using monovalent OPV (mOPV) and/or inactivated poliovirus vaccine.
Areas covered: We review the experience and challenges with managing vaccine supplies in the context of the polio endgame. Building on models that explored polio endgame risks and the potential mOPV needs to stop outbreaks from live poliovirus reintroductions, we conceptually explore the potential demands for finished and bulk mOPV doses from a stockpile in the context of limited shelf-life of finished vaccine and time delays to convert bulk to finished vaccine. Our analysis suggests that the required size of the mOPV stockpile varies by serotype, with the highest expected needs for serotype 1 mOPV. Based on realizations of poliovirus risks after OPV cessation, the stockpile required to eliminate the chance of a stock-out appears considerably larger than the currently planned mOPV stockpiles.
Expert commentary: The total required stockpile size depends on the acceptable probability of a stock-out, and increases with longer times to finish bulk doses and shorter shelf-lives of finished doses. Successful polio endgame management will require careful attention to poliovirus vaccine supplies.
1. Introduction
In 1988, the World Health Assembly resolved to eradicate wild polioviruses (WPVs) and end all paralytic poliomyelitis (polio) cases caused by live polioviruses by the year 2000 [Citation1]. The Global Polio Eradication Initiative (GPEI) continues to work toward achieving these objectives. Eradicating WPVs requires that all countries vaccinate their populations with high enough coverage to raise population immunity to transmission to stop and prevent the circulation of all three WPV serotypes [Citation2]. Ending all polio cases caused by live polioviruses requires ending all use of the existing live, attenuated oral poliovirus vaccine (OPV) in a globally coordinated way [Citation3]. Success of the complex polio endgame depends on managing multiple risks [Citation4–Citation6] and preparedness to respond to any reintroduced polioviruses [Citation7]. While underappreciated by many, success will also depend on managing significant changes in the global demand and expected supply of different poliovirus vaccines while recognizing the long time delays that occur in vaccine production.
This review provides an overview of poliovirus epidemiology as of the end of 2016, the current use of poliovirus vaccines, and recent challenges associated with the poliovirus vaccine supply. We explore the important role of poliovirus vaccine stockpiles and illustrate key factors that influence the optimal vaccine stockpile composition.
2. Poliovirus epidemiology as of the end of 2016
The GPEI reported the lowest ever annual number of polio cases in 2016, with 37 reported cases caused by serotype 1 WPV (WPV1) in three countries (i.e. Afghanistan, Nigeria, and Pakistan) and five cases caused by circulating vaccine-derived polioviruses (cVDPVs) in three countries (i.e. Laos, Nigeria, and Pakistan) [Citation8]. The last reported case caused by serotype 3 WPV occurred in northern Nigeria with onset in November 2012 [Citation9], although the GPEI has not yet certified WPV3 eradication. Following the certification of serotype 2 WPV eradication in 2015 [Citation10], in April to May 2016, the GPEI globally coordinated the cessation of all use of OPV containing serotype 2 (i.e. OPV2 cessation) [Citation11]. During this short period, all OPV-using countries stopped using trivalent OPV (tOPV, containing serotypes 1, 2, and 3) and switched to bivalent OPV (bOPV, containing serotypes 1 and 3). The GPEI currently hopes to stop WPV1 circulation by the end of 2017 or 2018 and to coordinate cessation of all bOPV use in 2021 or 2022 [Citation12], although the option to certify WPV3 eradication and switch from bOPV to monovalent OPV (mOPV) of serotype 1 (mOPV1) still theoretically exists [Citation13,Citation14].
3. Poliovirus vaccination as of 2017
3.1. OPV
The workhorse of the GPEI, OPV continues to serve as the primary poliovirus vaccine used by most countries [Citation15]. A live attenuated virus vaccine, OPV causes infection in vaccine recipients, which can spread to contacts to induce immunity. Easy-to-deliver and relatively inexpensive, OPV offers the advantage of inducing mucosal intestinal immunity. OPV infection provides life-long protection from paralysis if reinfected and reduces the probability, duration, and infectiousness of reinfection. However, OPV comes with a low, but non-zero, risk of causing vaccine-associated paralytic polio (VAPP) in a small fraction of individuals first exposed to poliovirus by OPV [Citation4,Citation16]. In addition, in populations with low immunity, OPV can continue to infect individuals and over time lose its attenuating mutations and evolve toward cVDPVs that behave like WPVs and can cause outbreaks [Citation4,Citation5,Citation17]. Some individuals with B-cell primary immunodeficiencies may also experience long-term OPV infections that evolve to immunodeficiency-associated vaccine-derived polioviruses (iVDPVs), which could potentially trigger outbreaks after OPV cessation [Citation4,Citation6,Citation18]. In the context of ongoing WPV circulation, the risks of OPV appear relatively small. However, following WPV eradication, the risks of OPV become much more visible as its use causes continued cases of polio. Prior to 2005, OPV-using countries relied on tOPV for all routine immunization and supplemental immunization activities (SIAs), and serotype 1 OPV caused the largest cVDPV outbreaks [Citation5]. The GPEI subsequently prioritized first mOPV1 and serotype 3 mOPV (mOPV3) and later bOPV for SIAs, which resulted in gaps in population immunity to serotype 2 transmission and led to a significant increase in the size and relative proportion of serotype 2 cVDPV (cVDPV2) outbreaks [Citation5]. Since globally coordinated OPV2 cessation in mid-2016, only bOPV remains in use for routine immunization and SIAs, and serotype 2 mOPV (mOPV2) became available for outbreak response. All OPV-using countries should use bOPV to maintain high population immunity to transmission for both serotypes 1 and 3 [Citation19]. Use of mOPV2 in the event of a serotype 2 outbreak requires explicit approval from the Director-General of the World Health Organization to release the vaccine from an existing emergency outbreak response stockpile, and after coordinated bOPV cessation, mOPV1 and mOPV3 would similarly become available for outbreak response [Citation20].
Many OPV-using countries conducted SIAs using tOPV during 2015 and 2016 to increase their population immunity to serotype 2 transmission prior to OPV2 cessation and reduce cVDPV2 risks after OPV2 cessation [Citation21]. While it appears that most countries successfully prevented cVDPV2 emergences after the switch, several recent events demonstrate the failure to stop and prevent cVDPV2s in some places. Since March 2016, the GPEI detected the circulation of cVDPV2s in Borno and Sokoto, Nigeria and in Balochistan, Pakistan [Citation8,Citation22]. Thus, following global efforts to stop all OPV2 use and destroy tOPV at all levels in the vaccine supply chain [Citation11], these areas must use an OPV2-containing vaccine (i.e. mOPV2) to stop the cVDPV2 outbreaks, while both countries simultaneously need to address ongoing WPV1 transmission.
3.2. Inactivated poliovirus vaccine
Like OPV, inactivated poliovirus vaccine (IPV) offers life-long protection to vaccine recipients from paralysis in the event of a reinfection [Citation23]. However, as an injectable, killed vaccine, IPV only protects the vaccine recipient, costs much more than OPV to produce and administer, and provides mainly humoral immunity, which does not significantly reduce the probability, duration, or infectiousness of intestinal infections [Citation24]. All commercially available IPV comes in a trivalent form. To eliminate VAPP, most high-income countries transitioned their routine immunization schedules from OPV-only to IPV-only or to a sequential schedule of IPV followed by OPV [Citation25]. The sequential schedule protects vaccine recipients from VAPP while still inducing intestinal mucosal immunity. As a prerequisite to OPV2 cessation, the GPEI recommended that all countries using OPV-only introduce at least one IPV dose into their routine immunization schedules to provide new birth cohorts with some serotype 2 immunity following OPV2 cessation [Citation20]. Some countries, including India and Sri Lanka, introduced fractional (one-fifth) IPV dose schedules [Citation26], which may attain similar immunogenicity to a single full IPV dose [Citation27–Citation31].
4. Prior studies of poliovirus vaccine stockpiles and needs
Several prior studies addressed poliovirus vaccine needs and stockpile issues. An initial discussion of posteradication outbreak response considerations proposed a first estimate for a stockpile of 500 million total doses of each serotype, which could cover three global annual birth cohorts, including 100 million finished (i.e. filled, tested, labeled, and packaged) doses ready for immediate use [Citation32]. Later plans aimed for 500 million mOPV2 doses and 300 million mOPV1 and mOPV3 doses [Citation20]. Recognizing that OPV cessation would end OPV production but not eliminate all risks, another study made the case for globally coordinating OPV cessation and global cooperation to create stockpiles of mOPV for outbreak response [Citation33]. Rapid response to outbreaks offers the best strategy to contain them [Citation34], but it requires rapid access to an adequate supply of vaccine [Citation35]. Consideration of stockpile dynamics demonstrated that the stockpile size interacts with vaccine demand, because if the stockpile does not hold sufficient quantities of vaccine to contain outbreaks, then this leads to increased demand due to uncontrolled outbreaks, which further exacerbates the vaccine shortage [Citation35]. A recent global model for long-term poliovirus risk management explored health and economic outcomes of different endgame vaccination policies for 2013–2052 [Citation36], and related analyses using the same model characterized the associated expected OPV needs until OPV cessation of each serotype [Citation37]. Recognizing the risks of reintroducing large amounts of OPV viruses to respond to a potential outbreak long after OPV cessation, another analysis based on the same model argued for the creation of an IPV stockpile for future outbreak response [Citation38], which could operate as a rotating stock as long as IPV production for routine immunization continues [Citation39]. The analysis further recognized that a failure to use enough tOPV prior to OPV2 cessation to prevent cVDPV2s would imply the need for increased use of mOPV2 from the stockpile [Citation38]. For base case outbreak risk and response assumptions, the analysis found a probability of over 25% of a stock-out of at least one mOPV serotype for a stockpile of 100 million finished and 400 million bulk doses, assuming that it takes 1 year to convert bulk into finished product. Based on these findings, the analysis urged the creation of a larger stockpile and efforts to reduce the finishing time [Citation38]. Building on the lessons learned from OPV2 cessation, another study explored the importance of maintaining high population immunity to transmission for serotypes 1 and 3 prior to bOPV cessation [Citation19].
5. Recent poliovirus vaccine supply challenges
The GPEI faced significant challenges with respect to forecasting poliovirus vaccine demand and ensuring sufficient supplies of different formulations. Notably, the GPEI strategic plan for 2013–2018, which covered the OPV2 cessation time period, did not include tOPV intensification as a requirement [Citation20]. Accordingly, OPV supply planning from 2013 through late 2014 reflected a decreasing trend in expected tOPV demand to minimize overstock risks [Citation40,Citation41]. However, motivated by modeling results [Citation21,Citation42], the GPEI subsequently recognized the need to conduct more tOPV SIAs in 2015 and 2016 leading up to OPV2 cessation to minimize the probability of cVDPV2 outbreaks after OPV2 cessation [Citation37]. This led to tOPV production at maximum capacity to ensure short-term tOPV needs to support the tOPV intensification efforts and develop the mOPV2 stockpile needed at the time of tOPV cessation, which strained the system [Citation43]. This situation highlighted the supply challenges associated with a short OPV intensification strategy prior to OPV cessation, as opposed to a policy of maintaining constant high coverage and OPV supplies [Citation19]. The experience with tOPV intensification motivates a policy of bOPV maintenance prior to bOPV cessation, which also results in lower outbreak risks until bOPV cessation [Citation19]. The decision by Pakistan not to revise its SIA schedule to conduct sufficient tOPV SIAs prior to OPV2 cessation as recommended [Citation44] resulted not only in the cVDPV2 that recently emerged in Balochistan but also in excess tOPV available after OPV2 cessation. Unfortunately, the GPEI asked manufacturers to destroy these tOPV doses shortly after OPV2 cessation to comply with the tOPV withdrawal policy, instead of asking manufacturers to retain them until expiry. Consequently, tOPV became unavailable for use to respond to subsequent simultaneous WPV1 and cVDPV2 outbreaks in Nigeria and Pakistan, although tOPV appeared the best option to respond to the circulation of both serotypes in these areas [Citation38]. Instead, health authorities must now find a way to administer both bOPV and mOPV2 in the context of limited access to key populations due to security challenges.
The relatively higher-income countries that began using IPV prior to 2013 generally continue to shift toward IPV-containing combination vaccines, and the demand and supply of these vaccines will likely stabilize as countries using sequential IPV/OPV shift to IPV-only routine immunization schedules during the polio endgame. Consistent with the recommendation that OPV-only-using countries should introduce at least one IPV dose into their routine immunization schedules at least 6 months prior to OPV2 cessation (i.e. in 2015), the GPEI supported OPV-only-using countries by creating demand and supporting studies for low-cost IPV, raising resources to fund IPV introduction, and seeking to increase IPV supply [Citation20]. While the efforts to increase IPV demand and offer it at a relatively low price largely succeeded, scaling up IPV production by existing manufacturers proved challenging and led to lower availability of IPV than OPV-only-using countries demanded between 2015 and 2017 [Citation45]. The shortage in supply led the GPEI to prioritize IPV for countries considered at highest risk of cVDPV2s [Citation45], while other countries did not receive any IPV or faced delayed IPV introduction and/or stock-outs [Citation46]. As of the beginning of 2017, IPV shortages continue to limit the introduction and use of IPV by all countries, with insufficient supply expected to continue until at least 2018 [Citation26,Citation46].
Creation of an mOPV2 stockpile of at least 500 million doses for outbreak response represented one of the prerequisites for OPV2 cessation [Citation13,Citation20]. The GPEI successfully established both a bulk and a finished mOPV2 stockpile, with at least 100 million doses available in different finishing stages by the end of 2016 [Citation43]. However, the 2016 cVDPV2 outbreaks in the very populous countries of Nigeria and Pakistan already put pressure on the available mOPV2 supply from the stockpile.
6. Managing future poliovirus vaccine supplies
Past and ongoing vaccine supply issues illustrate the importance and complexity of managing vaccine supplies. With respect to IPV supply for previously OPV-only-using countries, expanding the use of fractional IPV doses where feasible could alleviate the shortage [Citation26]. Current use of fractional IPV focuses on administering two fractional instead of one full IPV dose, which results in a reduction by up to 60% in the IPV antigen needed to vaccinate a child with IPV. The programmatic reduction in IPV antigen needs may remain less because the higher effective vial size (i.e. 25 or 50 doses per vial, instead of 5 or 10 for full IPV) may translate into higher wastage rates [Citation24]. Resolving the supply shortages further depends on overcoming the real production capacity constraints faced by manufacturers, which takes time. Offering manufacturers a fair market price for IPV going forward will help to create incentives to ramp up production and add capacity, although uncertainty about how long donors and countries will remain willing to pay for routine IPV for previously OPV-using countries complicates the decisions of manufacturers to invest in new IPV production capacity. With IPV supply currently still limited, the GPEI should prioritize all IPV to ensure that the maximum number of countries can introduce IPV in routine immunization to protect children born since OPV2 cessation from paralysis. Prioritizing IPV use for SIAs, in contrast, would administer most IPV doses to older children with preexisting immunity for serotype 2 and would offer very little additional benefit compared to OPV-alone, even in the context of ongoing transmission [Citation47]. As the demand for combination vaccines containing IPV from relatively higher-income countries continues to increase, the manufacturers currently supplying stand-alone IPV to relatively lower-income countries will likely shift more of their IPV supply toward combination vaccines. This may further limit access of relatively low-income countries to low-cost IPV.
With respect to bOPV supply, the experience with tOPV in the context of OPV2 cessation provides important lessons. First, the GPEI must develop a plan that includes sufficient bOPV to cover all endgame activities leading up to bOPV cessation. With insecurity and programmatic quality and performance challenges still impeding the interruption of WPV1 in three endemic countries, bOPV use in routine immunization and SIAs remains important in low- and middle-income countries to prevent new WPV1 important outbreaks and to reduce the risk of serotype 1 and 3 cVDPV outbreaks before or after bOPV cessation [Citation19]. Moreover, unlike a policy of OPV intensification shortly before its cessation, maintaining a regular demand of bOPV for SIAs up until bOPV cessation would greatly facilitate bOPV supply management [Citation19]. A premature emphasis on transitioning polio eradication resources (or on inappropriately relying on IPV for population immunity to poliovirus transmission that it cannot provide) will encourage manufacturers to ramp down bOPV production, which will increase the risk of bOPV supply shortages and complicate the establishment of sufficiently large mOPV1 and mOPV3 emergency outbreak response stockpiles. With more countries relying on IPV-only and WPV1 still circulating, we could see more events like the episode of widespread asymptomatic WPV1 circulation in Israel in 2013 despite high IPV coverage, which motivated the country to respond with bOPV SIAs and reintroduce bOPV into its routine immunization schedule [Citation48,Citation49]. The experience with OPV2 cessation also provides a reminder that insecurity and programmatic quality issues affect vaccination against all serotypes. Notably, the geographic areas that continue to sustain endemic WPV1 circulation also experienced cVDPV2 transmission after OPV2 cessation. Following the destruction of all tOPV stocks remaining with manufacturers at OPV2 cessation, these areas must now use both bOPV and mOPV2, both of which remain in relatively short supply. Given this experience, the GPEI should prepare for the possibility of simultaneous serotype 1 and 3 outbreaks after bOPV cessation by avoiding the destruction of bOPV stocks if feasible and possibly plan to establish a stockpile of finished bOPV product using any supplies held by manufacturers after bOPV cessation. Recognizing the low-probability, but high-consequence event of needing to restart OPV, the GPEI should develop an appropriate strategy for such an occurrence [Citation7].
After OPV cessation, OPV supply management changes from the management of rotating stocks in the context of ongoing production to the management of a stockpile of previously produced OPV. The stockpile may address the dual purpose of responding to outbreaks after OPV cessation and providing a long-term reserve of OPV to cover vaccine demand over the period of time it would take to restart OPV production in the event of uncontrolled outbreaks [Citation36]. In theory, IPV manufacturers that use OPV as seed strains (i.e. Sabin IPV producers) [Citation50–Citation52] could provide a ‘warm base’ for restarting OPV, but facilitating the use of their OPV requires licensing and potentially prequalifying their OPV to make this a viable option.
The size of the emergency outbreak response stockpile primarily depends on the expected risk of outbreaks after OPV cessation and the expected amount of mOPV required to control these outbreaks, which in turn depends on how aggressively we respond to post-OPV-cessation outbreaks [Citation34,Citation35,Citation38]. Aggressive outbreak response activities require the deployment of finished mOPV product and trigger new orders to convert bulk to finished product. Due to the limited shelf-life of finished mOPV doses, stockpile management plans must further anticipate the replacement of any remaining finished mOPV product due to expire, taking into account the time delay to convert bulk into finished product. Prior work illustrated these realities based on possible realizations of post-OPV cessation outbreaks and assuming a 1-year finishing time and a 2-year shelf-life based on typical properties of finished OPV [Citation38]. One of the two manufacturers that currently maintain part of the mOPV2 stockpile recently developed the option of stockpiling semifinished (i.e. filled but not labeled or packaged) mOPV2 doses. The shelf-life for semifinished doses of 5 years and the time to convert semifinished into fully finished product of 5 months make this an attractive option. With this new option and the reality of mOPV2 supply constraints affecting the ability to conduct current mOPV2 outbreak response SIAs, questions remain related to the optimal stockpile size and composition going forward.
7. Illustration of factors influencing stockpile size and composition
We use assumptions about outbreak risks and responses based on 1000 stochastic iterations (i.e. possible realizations of random poliovirus introduction and long-range exportation events) [Citation53] from a global model for long-term poliovirus risk management [Citation36] to illustrate different factors that influence stockpile size and composition. For outbreak response, the model assumed the use of 4–6 SIAs with homotypic mOPV targeting children under 5 years of age among populations of 10 or 100 million people, depending on poliovirus transmissibility in the outbreak population [Citation36]. The model reflected assumptions as of 2015 that all countries would introduce at least one IPV dose in their routine immunization schedule and that all countries with low routine immunization coverage would conduct sufficient tOPV SIAs prior to OPV2 cessation to prevent subsequent cVDPV2 outbreaks. The GPEI did not meet these requirements. In addition, ongoing efforts continue to develop tools that may reduce other risks, including polio antiviral drugs to treat iVDPVs [Citation53] and new poliovirus vaccines [Citation54]. The model base case assumed no use of polio antiviral drugs and found outbreaks related to iVDPVs and other potential poliovirus releases of at least one serotype in >90% of iterations at any time from OPV cessation through 2052 [Citation53]. Most iVDPV outbreaks in the model occurred in years 2 to 4 after homotypic OPV cessation after sufficient decrease in population immunity to transmission in countries with a high risk of iVDPV excretors. Unlike the actual experience of insufficient tOPV intensification resulting in persistent cVDPV2 transmission and new cVDPV2 emergences requiring outbreak response, none of the base case iterations involved mOPV2 use due to a serotype 2 outbreak during the year of OPV2 cessation (i.e. 2016). Thus, we emphasize the illustrative and conceptual nature of our analysis as providing insights related to key concepts.
shows the cumulative distribution functions of the number of mOPV doses of each serotype based on 1000 global model iterations. The mOPV needs reflect outbreak response activities during the first 5 years after OPV cessation of each serotype because we assumed that outbreak response would no longer use mOPV beyond that point [Citation36]. illustrates the differences between the three serotypes, with the largest needs for mOPV1 due primarily to the assumption that serotype 1 VDPVs and WPVs exhibit the highest transmissibility among the three serotypes [Citation55]. This finding remains consistent with the reality that serotype 1 cVDPV caused the largest outbreaks before SIA strategies began prioritizing OPV containing only serotype 1 and/or 3 [Citation5] and with the ability of WPV1 to continue to circulate despite intense vaccination efforts. The vertical lines reflect the initially proposed stockpile sizes of 100 million finished and 500 million total mOPV doses of each serotype. Between approximately 75% (mOPV1) and 90% (mOPV3) of iterations do not require more than 100 million doses if we respond aggressively to all outbreaks, suggesting that the planned stockpile remains large enough for most possible futures, regardless of when the outbreaks occur and as long as the ordering strategy ensures maintenance of a stock of 100 million finished doses. For iterations requiring more than 100 million doses of finished OPV, the ability to meet the outbreak response needs with the planned stockpile depends on when the outbreak response demand occurs (i.e. spread evenly over the 5-year period vs. concentrated during a single year) and how this interacts with expiry and orders of new finished doses. For the 2.5% of iterations that require more than 500 million mOPV1 doses, the originally planned stockpile remains insufficient to meet the outbreak response demand regardless of the ordering strategy or timing of outbreaks.
Figure 1. Cumulative distribution functions of the number of monovalent oral poliovirus vaccine (mOPV) doses of each serotype used in 1,000 iterations of a global model for long-term poliovirus risk management with base case assumptions for outbreak probabilities and outbreak response [Citation36,Citation53]
![Figure 1. Cumulative distribution functions of the number of monovalent oral poliovirus vaccine (mOPV) doses of each serotype used in 1,000 iterations of a global model for long-term poliovirus risk management with base case assumptions for outbreak probabilities and outbreak response [Citation36,Citation53]](/cms/asset/8fcb02fe-29be-44d5-88f3-78dab49b02aa/ierv_a_1322514_f0001_oc.jpg)
To determine the implications of the mOPV needs in for stockpile management, we explicitly consider an ordering strategy. Specifically, we define two types of orders: (i) replacement, to address decreases in fully finished mOPV stocks due to expiry of doses, and (ii) replenishment, to address decreases in fully finished mOPV stocks due to the use of doses for outbreak response. We assume that replacement orders occur at the time that precedes the expiry date by the finishing time so that new finished doses arrive exactly in time to replace expiring finished doses. We subtract from the replacement orders any pending replenishment orders. We define the number of desired finished doses (DF) as the amount of finished vaccine that the GPEI aims to keep available for outbreak response, and we assume that replenishment orders to maintain the stockpile at the DF occur on the first day of each outbreak response SIA. We subtract from the replenishment orders any pending replacement orders. For simplicity, we assume a constant DF consistent with the notion of an insurance policy that guarantees the same coverage against a risk over time. We define a stock-out as the occurrence of demand for mOPV for an outbreak response SIA at any point in time during the first 5 years after OPV cessation for which insufficient fully finished mOPV doses exist in the stockpile.
Figure 2. (a) Relationship between the number of desired finished doses, the total stockpile size, and the probability of a stock-out for serotype 1 monovalent oral poliovirus vaccine, based on the 1,000 iterations depicted in , a finishing time of 5 months, and an effective shelf-life of 18 months. (b) Lowest attainable stock-out probability by the number of desired finished doses and corresponding minimum total stockpile size
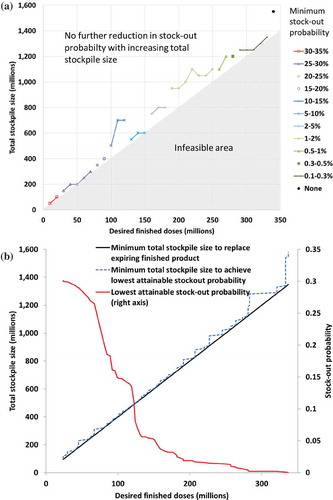
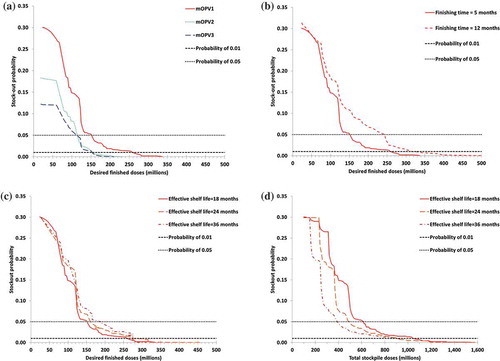
Figure 3. Relationship between the stockpile size and lowest attainable probability of a stock-out based on the 1,000 iterations depicted in , for different assumptions. (a) Results by monovalent oral poliovirus vaccine (mOPV) serotype, with a finishing time of 5 months and a shelf life of 18 months (b) Results for serotype 1 mOPV by finishing time, with a shelf life of 18 months (c) Results for serotype 1 mOPV by shelf-life and as a function of the number of desired finished doses, with a finishing time of 5 months (d) Results for serotype 1 mOPV by shelf-life and as a function of total stockpile size, with a finishing time of 5 months
Given that the 5-year shelf-life for semifinished product covers the entire assumed time horizon for mOPV use in outbreak response, we assume that the emergency outbreak response stockpile starts with all semifinished or fully finished product (excluding any additional bulk exclusively reserved to cover demand until OPV production resumes in the event of a need to restart OPV production). We further assume an effective shelf-life of 18 months for fully finished product, which reflects the current reality that the total 2-year shelf-life includes some time needed to process finished product and make it available for deployment from the stockpile. To illustrate the influence of these factors on stockpile management, we independently vary these assumptions. maps the relationship between DF, the total stockpile size, and the probability of a stock-out for mOPV1, based on the same 1000 iterations as depicted in .
To generate ), we varied the total stockpile size from 50 million to 2 billion doses at 50 million dose increments and the DF from 10 million doses at 10 million increments until the total stockpile size can no longer meet all replacement orders that would occur over 5 years in the absence of any replenishment orders. For example, with an effective shelf-life of 18 months, we need to reorder the DF four times (i.e. at 0, 18, 36, and 54 months), so the DF cannot exceed the total stockpile size divided by 4. ) shows the different possible minimum probabilities of stock-outs as a function of the DF and total stockpile size. At low DF, a total stockpile of over 500 million doses can always meet the replacement orders. However, a large probability exists that the outbreak response needs exceed the relatively small available finished vaccine stock at some point during the 5 years after homotypic OPV cessation, regardless of the total stockpile size, and this leads to relatively high stock-out probabilities. Increasing the DF reduces the stock-out probability, but requires increasing the total stockpile size to ensure the ability to fill replacement orders, as shown by the shaded area in ) that reflects insufficient total vaccine stockpile to replace the DF in the absence of any demand for outbreak response. The minimum total stockpile size may or may not accommodate the additional demand for outbreak response and therefore further increasing the total stockpile size sometimes results in a further decrease of the stock-out probability. Consequently, the minimum stock-out probability sometimes does not occur at the edge of the infeasible area. To generate ), we used finer increments in DF of 1 million doses to find the best combinations of DF and total stockpile size. To limit the probability of stock-outs to less than 5%, we need a DF of at least 154 million doses and a total stockpile size of at least 620 million doses. To avoid all mOPV1 stock-outs in all 1000 iterations, we need a DF of at least 337 million doses and a total stockpile size of at least 1550 million doses.
) shows the minimum stock-out probability by DF for each mOPV serotype. Consistent with , we find that attaining any given stock-out probability requires a larger stockpile of mOPV1 than mOPV2 and mOPV3. However, we recognize that the probabilities depend on OPV cessation risk management activities, and as discussed, the global model does not include the response to cVDPV2 outbreaks that already occurred because the model assumed that pre-cessation activities would have prevented these outbreaks. (b) shows the influence of the finishing time on the mOPV1 stock-out probability, with the solid curve (finishing time = 5 months) representing an assumption that we can finish all vaccine from semifinished product and the dashed curve (finishing time = 12 months) representing an assumption that we can finish vaccine only directly from bulk product without access to a semifinished option. Clearly, the shorter finishing time from semifinished product translates into substantially lower stock-out probabilities for any given total stockpile size. To achieve a lower than 5% stock-out probability, the semifinished option requires a DF of 154 million instead of 244 million mOPV1 doses for a finishing time of 12 months and a total stockpile size of 620 million instead of 980 million doses. Similarly, to achieve 0 stock-out probability, the semifinished option requires a DF of 337 million instead of 542 million mOPV1 doses for a finishing time of 12 months and a total stockpile size of 1550 million instead 2200 million doses. This analysis does not consider the implicit cost of the limitation that the semifinished product will last for only 5 years (with any unused doses destroyed after expiry) and cannot return to bulk to contribute to the reserve of vaccine available in case of the need to restart OPV globally.
(c–d) shows the potential impact of efforts to extend the effective shelf-life of finished product to eliminate the delays between finishing and making the vaccine available for immediate use so that the effective shelf-life becomes equal to the labeled 2-year shelf-life or to further extend it to 3 years, reflecting the possibility that stability data may suggest even longer potency of finished vials. Remarkably, extending the shelf-life but not changing the DF may result in an increase in the probability of a stock-out because the same DF would need to cover a longer time period and therefore more potential mOPV demand for outbreak response ((c)). However, extending the shelf-life implies less finished stock turnover and expiry, and therefore, the total stockpile size required to remain below any desired stock-out probability decreases with increasing shelf-life ((d)). Put differently, for a given total stockpile size, a longer shelf-life can accommodate a higher DF to effectively lower the stock-out probability. Due to the counteracting effect of longer shelf-life on meeting outbreak response demands for any given DF, the benefit of extending the shelf-life remains lower than that of shortening the finishing time.
lists the optimal stockpile composition for different desired stock-out probabilities based on a finishing time of 5 months and an effective shelf-life of fully finished mOPV of 18 months for the 1000 model runs. shows that even with a desired maximum stock-out probability of 5%, the required DF exceeds the originally planned DF of 100 million finished doses [Citation32]. The required total stockpile size for a maximum stock-out probability of 5% also exceeds the originally planned stockpile size of 500 million mOPV1 doses [Citation32] or the currently held 300 million mOPV1 and mOPV3 doses [Citation20]. The required total stockpile size for a maximum stock-out probability of 1% or less exceeds the planned total stockpile size for each mOPV serotype.
Table 1. Optimal stockpile composition for each monovalent oral poliovirus vaccine (mOPV) serotype for different stock-out probabilities, assuming a finishing time of 5 months and an effective shelf life of 18 months, based on 1000 iterations of a global model [Citation36,Citation53]
8. Discussion
Vaccine supply management involves uncertain future demand, long time delays, and a dangerous reinforcing feedback between insufficient vaccine supplies and future demand [Citation35]. Recent polio eradication experience and integrated modeling highlight the critical importance of long-term planning to manage vaccine supplies and maximize the probability of a successful polio endgame. Although inherent poliovirus risks, vaccine choices, and programmatic inabilities to reach children in access-compromised areas represent well-recognized challenges for the GPEI, a real possibility exists that a failure to manage vaccine supplies could ultimately cause an OPV restart and undermine a successful polio endgame. With insufficient IPV available to include it in routine immunization schedules in all countries, increasing numbers of children did not receive any individual protection for serotype 2. This represents a particular concern in the context of ongoing cVDPV2 outbreaks and the likely existence of serotype 2 iVDPV excretors in middle-income countries that currently do not receive IPV. More importantly, the finite mOPV2 supply further compounds the risks. While it may seem counterintuitive, conducting a small-scale response due to a fear of insufficient mOPV2 supply may ultimately require more mOPV2 as uncontrolled cVDPV2 outbreaks spread geographically. Based on 1000 realizations of a global model for long-term risk management [Citation36,Citation53], we find the need for larger stockpile requirements than currently planned to limit to possibility of stock-outs, particularly for mOPV1. Shorter finishing delays and longer shelf-lives reduce the stockpile needs, but planning a stockpile based on improved properties requires action to make these possible in practice.
We highlight a few important limitations of this review. First, we implicitly assumed that the main goal of the emergency outbreak response stockpile focuses on preventing stock-outs, but we did not address the consequences of stock-outs. Stock-outs may or may not lead to uncontrolled outbreaks [Citation38], and therefore, true optimization of the stockpile depends on balancing the health and financial consequences of stock-outs against the stockpile costs [Citation35]. While stock-outs may not lead to an eventual OPV restart if a delayed outbreak response still succeeds in stopping the outbreak, we emphasize that in practice even low stock levels may negatively affect outbreak response efforts by generating a counterproductive hesitancy to use mOPV. Second, the model underlying the stockpile examples made several more optimistic assumptions about compliance to the OPV2 cessation prerequisites than justified by the current experience while also excluding new risk management opportunities that may soon become available. Thus, while the insights remain robust at a conceptual level, future studies will need to update the global model to provide better quantitative estimates of expected doses required. Third, we assume that the DF would not change over time, although we recognize that outbreak risks and response needs may change over time and that experience may lead to updating and changing the DF. Fourth, we ignored the presumed need to destroy expiring semifinished doses 5 years after homotypic OPV cessation, while an emergency outbreak response stockpile relying on bulk could keep those doses as an extra reserve to cover a potential OPV restart.
Despite the limitations, this work highlights the risks associated with insufficient poliovirus vaccine supply. For the polio endgame, we should plan for more supply than we may think we need to account for uncertainties. Destroying unneeded vaccine should represent a preferred option to failing to develop enough vaccine supply to respond to the significant risks that may materialize and could lead to an OPV restart.
9. Expert commentary
We recognize the critical importance of acknowledging vaccine manufacturers as partners in the polio endgame and of good forecasting of vaccine demand by the GPEI and national immunization programs because success will require sufficient quantities of poliovirus vaccines. The complex reality of supply chain dynamics means that countries, manufacturers, and UNICEF should remain in close communication about vaccine inventories and expected needs. With the vaccine available now reflecting production that started 2 years ago, the significant time delays necessitate good planning.
Although OPV2 cessation aimed to eventually eliminate serotype 2 risks, national health leaders need to appreciate that delaying the mOPV2 response to a serotype 2 outbreak increases the risk of not controlling the outbreak [Citation56], forcing future needs to use mOPV2 at a time of lower global population immunity to serotype 2 transmission [Citation57,Citation58], and creating more future mOPV2 demand than planned for by the stockpile.
10. Five-year view
As the world enters an unprecedented era without any ongoing exposure to serotype 2 live poliovirus, we will observe the extent to which post-OPV cessation risks materialize. Global population immunity to serotype 2 transmission will continue to decrease, and in some countries, limited IPV supply means that cohorts who did not receive any serotype 2 vaccine will begin to accumulate. Limited IPV supply will continue to restrict IPV use in some routine immunization programs until at least 2018. Unfortunately, this means that we may observe some risks sooner, and their realization may influence planning for bOPV cessation, with important lessons already learned [Citation19,Citation56,Citation59]. At the same time, the GPEI must continue efforts to eradicate WPV1, with ongoing challenges possibly leading to further delays and debate about continued investments. In spite of, or because of the uncertainty about the future OPV needs, manufacturers will plan to sunset OPV production facilities, making stockpile planning even more urgent. Remarkably, OPV manufacturers extended the production plant lives to continue to support the GPEI and country demands for OPV long after the original timing of polio eradication (i.e. 2000). Five years from now, we expect the poliovirus vaccine market to look very different than it looks today.
The decrease in global population immunity to serotype 2 transmission implies that any new outbreaks will spread quickly, making outbreak response more critical. The experience with current outbreaks will inform the highly uncertain risks of using mOPV2 after OPV2 cessation [Citation38,Citation53]. Further research will need to address the appropriate vaccine for outbreak response long after OPV cessation, with the possibility of new OPV vaccines with lower risks offering an important potential option [Citation54,Citation60,Citation61]. Although time-limited IPV use appears economically justified, continued IPV use and production in middle-income countries come with risks and economic trade-offs [Citation36]. Significant differences exist between relatively lower- and relatively higher-income countries in the long-term risks of reintroductions of live polioviruses (e.g. iVDPVs, containment failures) and the long-term benefits of IPV. These differences provide long-term justification for relatively higher-income countries to continue to invest in IPV immunization, while such expenditures in relatively lower-income countries appear relatively less favorable, particularly in the context of competing opportunities to use scarce resources. Experience with OPV2 cessation and ongoing research will further inform the optimal schedule, formulation, and duration of IPV use after cessation of the last OPV serotype for countries of different income levels and risks. Upper middle- and high-income countries may increasingly shift to combination vaccines, which will likely reduce the supply of the single-antigen IPV that the GPEI currently supports for use in lower middle and low income countries. In the absence of long-term support and justification for continued use of IPV in relatively lower-income countries, that market may largely disappear. The long-term polio endgame should include the development and maintenance of appropriate poliovirus vaccine stockpiles (i.e. OPV, IPV) and infrastructure that can support emergency response activities anywhere in the world if needed. However, as the GPEI dissolves, the institutional support for these remains uncertain. Given the numerous uncertainties and realities of vaccine production, supply issues for both IPV and OPV will continue to represent critical determinants of the ultimate success of the polio endgame.
Key issues
Insufficient poliovirus vaccine supplies could potentially threaten the ultimate success of polio eradication and the GPEI must actively work with vaccine manufacturers
Management of vaccine supplies for bOPV cessation should consider vaccine supply lessons from recent globally coordinated OPV2 cessation
Currently planned mOPV stockpiles may prove insufficient to prevent stock-outs
The time to convert bulk to finished product represents a key determinant of stockpile size requirements
Among the three mOPV serotypes, modeling suggest the highest stockpile requirements for mOPV1
Maximizing the success of the polio endgame requires planning for more vaccine supply than may ultimately be needed, but this should represent an accepted cost of risk management
Global supplies of IPV will continue to experience shortages for the next several years, and in the absence of long-term programmatic and financial commitments to using IPV, this situation will likely persist
The GPEI should recognize time-limited opportunities, time delays, and other challenges in the poliovirus vaccine supply chain and commit to building sufficient poliovirus vaccine stockpiles
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- World Health Assembly. Global eradication of poliomyelitis by the year 2000 (resolution 41.28). Geneva: World Health Organization; 1988.
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, et al. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Anal. 2013;33:647–663.
- World Health Assembly. Poliomyelitis: mechanism for management of potential risks to eradication (resolution 61.1). Geneva: World Health Organization; 2008.
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006;26:1471–1505.
- Duintjer Tebbens RJ, Pallansch MA, Kim J-H, et al. Review: oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal. 2013;23:680–702.
- Duintjer Tebbens RJ, Pallansch MA, Thompson KM. Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infect Dis. 2015;15:379.
- Thompson KM, Duintjer Tebbens RJ. How should we prepare for an outbreak of reintroduced live polioviruses? Future Virol. 2017;12:41–44.
- World Health Organization. Polio this week as of 8 February 2017. [cited 2017 Feb 11]. Available from: http://polioeradication.org/polio-today/polio-now/this-week/.
- Kew OM, Cochi SL, Jafari HS, et al. Possible eradication of wild poliovirus type 3 – worldwide, 2012. MMWR Morb Mortal Wkly Rep. 2014;63:1031–1033.
- Global Polio Eradication Initiative. Global eradication of wild poliovirus type 2 declared 2015 [updated September 20, 2015; cited 2015 November 30]. Available from: http://www.polioeradication.org/mediaroom/newsstories/Global-eradication-of-wild-poliovirus-type-2-declared/tabid/526/news/1289/Default.aspx
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine – worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:934–938.
- UNICEF. RFP-DAN-2016-502330 Bivalent oral polio vaccines 2017 [cited 2017 January 13]. Available from: https://www.unicef.org/supply/index_94239.html.
- Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for OPV cessation. Expert Rev Vaccines. 2012;11:449–459.
- Thompson KM, Duintjer Tebbens RJ. Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes. BMC Infect Dis. 2015;15:376.
- Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine – live. Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. Sixth ed ed. Philadelphia:Saunders Elsevier; 2013. p.598–645.
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. 2014;210:S380–9.
- Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296:356–359.
- Kew O, Sutter R, Nottay B, et al. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J Clin Microbiol. 1998;36:2893.
- Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, et al. Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. J Vaccines Vaccin. 2016;7:340.
- World Health Organization. Global polio eradication initiative: polio eradication and endgame strategic plan (2013–2018). Geneva: World Health Organization; 2013. (no. WHO/POLIO/13.02).
- Thompson KM, Duintjer Tebbens RJ. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis. 2014;210:S475–S84.
- Etsano A, Damisa E, Shuaib F, et al. Environmental isolation of circulating vaccine-derived poliovirus after interruption of wild poliovirus transmission – Nigeria, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:770–773.
- Vidor E. Plotkin SA. Poliovirus vaccine – inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Sixth ed ed. Philadelphia: Saunders Elsevier; 2013. p. 573–597.
- Thompson KM, Duintjer Tebbens RJ. National choices related to inactivated poliovirus vaccine, innovation, and the end game of global polio eradication. Exp Rev Vaccines. 2014;13:221–234.
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, et al. Pre-eradication vaccine policy options for poliovirus infection and disease control. Risk Anal. 2013;33:516–543.
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, October 2016 – conclusions and recommendations. Wkly Epidemiol Rec. 2016;91:561–584.
- Cadorna-Carlos J, Vidor E, Bonnet MC. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. Int J Infect Dis. 2012;16:e110–e6. Epub 2011/12/14.
- Mohammed AJ, Al Awaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med. 2010;362:2351–2359. Epub 2010/06/25
- Resik S, Tejeda A, Lago PM, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis. 2011;201:1344–1352. Epub 2010/03/31
- Resik S, Tejeda A, Mach O, et al. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba. Vaccine. 2015;33:307–313.
- Anand A, Zaman K, Estivariz CF, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine. 2015;33:6816–6822.
- Fine PEM, Sutter RW, Orenstein WA. Stopping a polio outbreak in the post-eradication era. Dev Biol. 2001;105:129–147.
- Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Medscape J Med. 2008;10:190.
- Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal. 2006;26:1541–1556.
- Duintjer Tebbens RJ, Pallansch MA, Alexander JP Jr., et al. Optimal vaccine stockpile design for an eradicated disease: application to polio. Vaccine. 2010;28:4312–4327. Epub April 27.
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, et al. An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect Dis. 2015;15:389.
- Duintjer Tebbens RJ, Thompson KM. Managing the risk of circulating vaccine-derived poliovirus during the endgame: oral poliovirus vaccine needs. BMC Infect Dis. 2015;15:390.
- Duintjer Tebbens RJ, Pallansch MA, Wassilak SGF, et al. Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame. BMC Infect Dis. 2016;16:137.
- Thompson KM, Duintjer Tebbens RJ. Framework for optimal global vaccine stockpile design for vaccine-preventable diseases: application to measles and cholera vaccines as contrasting examples. Risk Anal. 2016;36:1487–1509.
- Current UNICEF OPV supply & outlook – July 2013.[cited 2017 Feb 14]. Available from: https://www.unicef.org/supply/files/Oral_polio_vaccine_update.pdf.
- UNICEF. Oral polio vaccine supply outlook. September 2014.[cited 2017 Feb 14]. Available from: https://www.unicef.org/supply/files/Oral_Polio_Vaccine_Outlook__September_2014.pdf.
- Duintjer Tebbens RJ, Thompson KM. Modeling the potential role of inactivated poliovirus vaccine to manage the risks of oral poliovirus vaccine cessation. J Infect Dis. 2014;210:S485–S97.
- UNICEF. Oral polio vaccine supply outlook – December 2015 [cited 2017 Feb 14]. Available from: https://www.unicef.org/supply/files/Oral_Polio_Vaccine_Outlook__December_2015.pdf.
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2015 - conclusions and recommendations. Wkly Epidemiol Rec. 2015;90:681–700.
- UNICEF. Inactivated poliovirus vaccine supply alert – May 2016. [cited 2017 Feb 14]. Available from: https://www.unicef.org/supply/files/Inactivated_Polio_Vaccine_(IPV)_-_may_2015_banner.pdf.
- UNICEF. Inactivated poliovirus vaccine: Supply update – September 2016. [cited 2017 Feb 14]. Available from: https://www.unicef.org/supply/files/Inactivated_Polio_Vaccine_(IPV)_-_september_2016.pdf.
- Duintjer Tebbens RJ, Thompson KM. Costs and benefits of including inactivated in addition to oral poliovirus vaccine in outbreak response after cessation of oral poliovirus vaccine use. Medical Decision Making Policy & Practice. 2017;2:1–13.
- Anis E, Kopel E, Singer S, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill. 2013;18:pii=20586.
- Kalkowska DA, Duintjer Tebbens RJ, Grotto I, et al. Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. J Infect Dis. 2015;211:1800–1812.
- Liao G, Li R, Li C, et al. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J Infect Dis. 2012;205:237–243. Epub 2011/12/14
- Resik S, Tejeda A, Fonseca M, et al. Decay of Sabin inactivated poliovirus vaccine (IPV)-boosted poliovirus antibodies. Trials Vaccinol. 2015;4:71–74.
- Simizu B, Abe S, Yamamoto H, et al. Development of inactivated poliovirus vaccine derived from Sabin strains. Biologicals. 2006;34:151–154.
- Duintjer Tebbens RJ, Thompson KM. Comprehensive screening for immunodeficiency-associated vaccine-derived poliovirus: an essential OPV cessation risk management strategy. Epidemiol Infect. 2017;145:217–226.
- Duintjer Tebbens RJ, Thompson KM. The potential benefits of a new poliovirus vaccine for long-term poliovirus risk management. Future Microbiol. 2016;11:1549–1561.
- Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, et al. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal. 2013;23:703–749.
- Thompson KM, Duintjer Tebbens RJ. Lessons from globally-coordinated cessation of serotype 2 oral poliovirus vaccine for the remaining serotypes. J Infect Dis. DOI:10.1093/infdis/jix128
- Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation. BMC Infect Dis. 2016;16:237.
- Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infect Dis. 2016;16:231.
- Thompson KM, Duintjer Tebbens RJ. Lessons from the polio endgame: overcoming the failure to vaccinate and the role of subpopulations in maintaining transmission. J Infect Dis. 2017. DOI:10.1093/infdis/jix108
- Adeyemi OO, Nicol C, Stonehouse NJ, et al. Increasing type 1 poliovirus capsid stability by thermal selection. J Virol. 2017;91(4):e01586-16. DOI:10.1128/JVI.01586-16
- Macadam AJ, Ferguson G, Stone DM, et al. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J Virol. 2006;80:8653–8663. Epub 2006/08/17

