ABSTRACT
Introduction
Rotavirus (RV) disease remains a prominent cause of disease burden in children <5 years of age worldwide. However, implementation of RV vaccination has led to significant reductions in RV mortality, compared to the pre-vaccination era. This review presents 15 years of real-world experience with the oral live-attenuated human RV vaccine (HRV; Rotarix). HRV is currently introduced in ≥80 national immunization programs (NIPs), as 2 doses starting from 6 weeks of age.
Areas covered
The clinical development of HRV and post-marketing experience indicating the impact of HRV vaccination on RV disease was reviewed.
Expert opinion
In clinical trials, HRV displayed an acceptable safety profile and efficacy against RV-gastroenteritis, providing broad protection against heterotypic RV strains by reducing the consequences of severe RV disease in infants. Real-world evidence shows substantial, rapid reduction in the number of RV infections and associated hospitalizations following introduction of HRV in NIPs, regardless of economic setting. Indirect effects against RV disease are also observed, such as herd protection, decrease in nosocomial infections incidence, and a reduction of disease-related societal/healthcare costs. However, not all countries have implemented RV vaccination. Coverage remains suboptimal and should be improved to maximize the benefits of RV vaccination.
PLAIN LANGUAGE SUMMARY
What is the context?
Rotaviruses are a leading cause of diarrheal diseases worldwide. They account for substantial morbidity and mortality among young children under 5 years, with developing countries showing a higher disease burden.
Although rotavirus vaccines have contributed to reductions in disease burden, they have not substantially averted deaths from rotavirus infection in the regions with the highest burden due to low coverage
What is new?
We reviewed almost 15 years of data from national immunization programs and private use settings to summarize the global experience with the rotavirus vaccine Rotarix (GSK).
We showed that vaccination with Rotarix:
Has a long term and substantial impact on disease burden in diverse geographic and socio-economic settings
Significantly reduces diarrhea-related hospitalizations and death rates
Provides indirect benefits such as herd protection to children who are too old or too young to be vaccinated; the reduction of hospital-acquired infections; and significant cost savings for healthcare systems, households and society.
Why is this important?
Despite promising real-world data and a favorable benefit-risk profile, the overall uptake of Rotarix is still suboptimal
Increasing awareness of the high disease burden and significant vaccine impact is critical for motivating healthcare professionals to advocate for rotavirus vaccination and convincing policymakers to implement national rotavirus immunization
1. Background
1.1. Disease burden
Rotavirus (RV) is the leading etiology for diarrhea mortality among children younger than 5 years [Citation1,Citation2]. Although the incidence of RV infection in developed and developing countries is similar [Citation3], a higher mortality burden is observed in developing countries (approx. 80% of deaths attributed to RV), because of difficult access to health care, poor sanitation and potential concomitant childhood infections [Citation1,Citation2,Citation4,Citation5]. While the introduction of RV vaccines more than one decade ago has led to substantial progress in reducing the global disease burden, the reduction has not been equal across settings. The regions with the highest needs have not substantially averted deaths from RV infection, as shown the 185,300 child deaths from RV estimated globally in 2017, nearly all in low- and middle-income countries [Citation1,Citation2,Citation6].
1.2. How to tackle the burden?
For most causes of diarrhea, improvements in living conditions, including in water supply, sanitation, and hygiene have led to significant decline in related infections. However, RV is a highly contagious virus with marked resistance in the environment, and sanitation measures are not sufficient to decrease risk of exposure. Vaccination has been identified as the best method to prevent RV infections, reducing the associated mortality and morbidity burden of the disease.
After the first recommendation in 2006, the World Health Organization (WHO) issued a reinforcement in 2009 that RV vaccination should be offered to infants in all regions worldwide. The first dose should be administered as soon as possible starting from 6 weeks of age, to ensure induction of protection prior to natural RV infection [Citation7]. The use of 2 oral RV vaccines is globally established for more than a decade: the live-attenuated human RV vaccine (HRV; Rotarix GSK, Belgium) and the live-attenuated human-bovine reassortant vaccine (HBRV; RotaTeq Merck, United States [US]). Two other RV vaccines, Rotasiil (Serum Institute of India Pvt. Ltd.) and Rotavac (Bharat Biotech International Limited India), used in universal mass vaccination in India have been recently prequalified by the WHO [Citation8]. New RV vaccines to be developed employ a similar approach, or are exploring new concepts such as inactivation or virus-like particles. Other vaccines are licensed and used locally (for instance the Lanzhou lamb RV vaccine in China [Citation9], and Rotavin M1 [Polyvac] in Vietnam [Citation10]), while others (of which an oral human rotavirus vaccine RV3-BB that can be administered at birth [Citation11]) are in different phases of their clinical development [Citation12,Citation13].
1.3. What is the causative agent?
RVs belong to the Reoviridae family, and are classified into 10 genetically distinct groups/species (A–J); however, group A RV is the most common cause of human disease [Citation14]. The RV RNA encodes 6 structural viral (VP1-VP4, VP6, VP7) and 6 non-structural proteins (NSP1-NSP6). RV strains are usually genotyped based on differences in the RNA sequences encoding 2 of the VPs (VP7 and VP4). There are six predominant circulating group A RV strains (G1P [Citation8], G2P [Citation4], G3P [Citation8], G4P [Citation8], G9P [Citation8] and G12P [Citation8] genotypes), that are causing >90% of the severe RV disease cases [Citation14,Citation15]. RV epidemiology is sero-diverse across settings and constantly fluctuating both in the pre- and post-vaccination eras. Surveillance is ongoing to understand these changes.
1.4. What is the aim of this review?
This review focuses on the 15 years of experience with HRV, covering the clinical development of the vaccine and post-marketing evidence of the impact of vaccination on RV disease following the introduction of HRV in national immunization programs (NIPs). Of note, in cases where the contribution of HRV could not be clearly distinguished from that of HBRV (for instances in countries/regions where both vaccines have been used for long periods), data were presented as such and reflect the impact of vaccination with both RV vaccines. A plain language summary contextualizing the findings and potential clinical research relevance and impact is presented in .
2. HRV: A rotavirus vaccine with global impact
2.1. Rationale for the development of HRV
Exposure to natural RV infection has previously been noted to reduce the severity of subsequent RV disease. Two infections confer virtually complete protection against moderate-to-severe RV disease [Citation16]. The protection afforded by natural infection appears to be broad, extending to other RV genotypes causing subsequent infections. These observations were the basis for the development of a 2-dose single-strain G1P [Citation8], live-attenuated human rotavirus vaccine, mimicking natural infection and thus providing protection against both homotypic and heterotypic RV strains. Another characteristic of RV infection is that almost all children will experience RVGE before the age of 5 years. While the majority of RVGE episodes are mild or moderate, severe RVGE requiring hospitalization is mainly observed in young children (0–36 months), due to their vulnerability to dehydration. In fact, very low to virtually no disease is observed in children aged >5 years, which are protected through prior exposure to RV () [Citation17]. Therefore, vaccination should be administered as early as possible (6 weeks of age) to replace natural exposure and ensure protection against severe consequences of the disease.
Figure 2. Distribution of cases of rotavirus infection incidence per age group (data collected from 16 European Union countries between 2006 and 2016 [Citation17])
![Figure 2. Distribution of cases of rotavirus infection incidence per age group (data collected from 16 European Union countries between 2006 and 2016 [Citation17])](/cms/asset/062ce652-9f95-4ba0-852d-05a1189f28fd/ierv_a_1800459_f0002_oc.jpg)
HRV was developed from a human G1P [Citation8] RV strain, named 89–12, initially obtained from an infant naturally infected with RV in the 1988–1989 RV season in the US. The strain was observed to provide protection against severe infections in subsequent RV seasons. The virus was attenuated by multiple passages in cell culture and the final vaccine, obtained at GSK, was lyophilized [Citation18–20]. Subsequently, a liquid preparation of HRV was also made available and is currently prequalified by the WHO [Citation21].
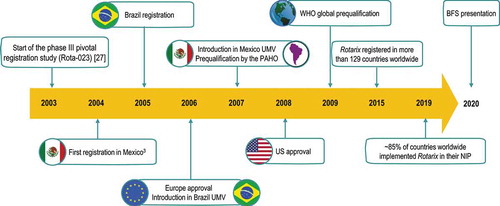
The milestones of HRV’ development and worldwide approval are summarized in .
2.2. HRV design – differences from other RV vaccines
Different concepts have been used for the design and development of a RV vaccine, such as using human RV attenuated strains versus an animal strain incorporating human VPs. These differences inevitably lead to distinct ways in which the immune response is activated/elicited and evolves over time.
The first RV vaccine (RotaShield, Wyeth), a human-rhesus tetravalent reassortant vaccine, was licensed in 1998 and withdrawn from the market less than one year after [Citation22], due to its association with an increased risk of intussusception (IS) – intestinal invagination that can result in bowel obstruction – following vaccination [Citation23]. HBRV, containing 5 human-bovine rotavirus reassortants (type G1, G2, G3, G4, and P1A [Citation8]) produced in Vero cells, was developed to induce immunity against 5 prevalent RV genotypes causing disease in humans, while avoiding virus circulation and clinical symptoms through the use of the bovine backbone. The vaccine is administered as a 3-dose schedule from 6 to 32 weeks of age [Citation24].
To date, HRV and HBRV are the most widely used RV vaccines with proven records of safety and efficacy [Citation25,Citation26]. Moreover, efficacy against various RV strains including G1P [Citation8], G2P [Citation4], G3P [Citation8], G4P [Citation8] and G9P[Citation8]) has been demonstrated for both vaccines, despite them being conceptually different [Citation24,Citation27].
Two doses of HRV are indicated, while the other WHO-prequalified RV vaccines are administered as a 3-dose schedule. In fact, the majority of vaccinated children achieve anti-RV IgA seroconversion (IgA concentration ≥20 U/mL) following the first dose of HRV, with the second dose demonstrating a catch-up effect [Citation28]. However, the full 3-dose vaccination course seems to be needed for HBRV to reach maximum seroresponse [Citation29], indicating a different dynamics in the immune responses elicited by human and reassortant RV vaccines. Despite these differences, comparable efficacy against RV and severe diarrhea cases is observed for 2-dose HRV and 3-dose HBRV vaccination [Citation13,Citation29]. A similar vaccine effectiveness was also reported in Australia, where the 2 vaccines were implemented in different states/territories. Data from Western Australia, where HRV was used during 2007–2009 and HBRV during 2009–2011, showed comparable vaccine effectiveness estimates for the complete vaccination series: 73% (95% confidence interval [CI] 55–83) and 82% (95% CI: 59–92), respectively [Citation30]. In New South Wales, 2-dose HRV vaccine effectiveness estimates were reported to decline from 89.5% (95% CI: 84.3–93.0) within the first year post-introduction to 77% (95% CI: 67.5–83.7) at 5–<10 years following implementation, although the decrease was not consistent over the years. Notably, the highest vaccine effectiveness (83%) was observed in 2017, when an RV outbreak was reported in New South Wales [Citation31]. Since July 2017, HRV is the only RV vaccine used in the Australian immunization program.
To date, HRV remains the only globally-available oral RV vaccine approved and WHO-prequalified as a 2-dose vaccination series against RVGE. Since its first licensure in Mexico in 2004, the vaccine is registered for use in 129 countries worldwide and is preferred in ≥80% of countries implementing RV vaccination in their NIPs [Citation32].
2.3. Robust clinical research and development
HRV provided the first example of a new strategy in global vaccine research and development, focusing on the introduction of the vaccine with precedence in countries with the most pronounced medical needs. The clinical development program for HRV included phase I–III multicenter trials in over 100,000 infants worldwide. The vaccine was shown to be well tolerated, immunogenic, and efficacious [Citation27].
Latin American countries were the first target for the introduction of the vaccine, based on the high burden of RV-caused disease, but also due to the well-documented epidemiology and the existence of reliable infrastructure for conducting well-designed clinical trials [Citation33]. Several phase II and III clinical studies evaluated efficacy, immunogenicity, and safety of HRV vaccination in Latin American children [Citation25,Citation34–36]. In a pivotal phase III study including 15,183 healthy infants from 10 Latin American countries, the combined 2-year period efficacy against severe RVGE was 80.5–82.1% against wild-type G1, 77.5% against pooled non-G1, and 80.5% against pooled non-G1P [Citation8] strains. A 2-dose vaccine schedule beginning at 6–13 weeks of age was also shown to have an acceptable safety profile [Citation34]. The high efficacy against severe RVGE and acceptable safety profile were maintained when HRV was co-administered with routine vaccines included in the expanded program on immunization [Citation37].
In clinical trials in Latin American, Europe and Asia, during 1–3 years of follow-up, 85–96% protection against severe RVGE caused by G1 and non-G1 genotypes was observed, together with a decrease of RVGE hospitalizations by 40–75% [Citation38]. An overall lower -but still significant- protective efficacy was achieved in clinical trials conducted in Africa [Citation19,Citation33,Citation38]. However, due to the high RV-disease burden and associated mortality, a higher number of prevented cases/deaths are expected; consequently, the public health impact is likely to be greater in this region.
Long-term follow-up studies confirmed a sustained efficacy against severe RVGE up to 2 years post-vaccination. Up to 92% efficacy observed in Japan in the first 2 years of life [Citation39]; in South African children, vaccine efficacy was estimated at 59% over 2 consecutive rotavirus seasons since vaccination [Citation40]. In a large study in European children, vaccine efficacy against severe RVGE was 90.4% over a mean duration of 17 months post-vaccination with 2 doses of HRV [Citation41]. Representative worldwide efficacy data for HRV from placebo-controlled studies are presented in .
Table 1. Vaccine efficacy data for HRV from phase III clinical trials
There is a considerable amount of research ongoing to understand the reduced efficacy and impact of RV vaccination in developing countries when compared with developed regions. Several hypotheses are currently advanced, such as differences in gut microbiota, malnutrition, micronutrient deficiencies, avitaminoses, co-infections, and maternal antibodies [Citation45], but still need to be carefully assessed. The timing and interval between doses may also impact vaccine efficacy, with recent data suggesting that a slight increase in interval between HRV doses, when the series begins at approximately 10 weeks of age, may improve its performance in low- and middle-income countries [Citation46]. However, an analysis of data accumulated so far on HRV vaccination (timing and interval between doses, dose concentration, or administration of a booster dose) does not indicate the need to change the vaccine’s current administration profile [Citation47–51].
Given the successful pre-licensure development, HRV was first registered for use in Mexico in 2004, followed by Brazil in 2005 and introduced in universal mass vaccination programs in Brazil and Mexico in 2006–2007. The data on the impact of RV vaccination generated in these countries further supported the subsequent registration of HRV in Europe (2006) and the US (2008). After more than a decade of use, the vast ever-growing real-world evidence across continents continues to support the global use of HRV.
2.4. Real-world evidence supporting the global impact of HRV vaccination
Mortality and morbidity associated with RVGE vary greatly with socio-economic setting [Citation1,Citation6]. In developing countries, a higher exposure to infection and transmission due to high RV prevalence, coupled with poor access to healthcare facilities, higher co-morbidity rates, and sometimes malnutrition status is likely to make the high mortality the main driver for vaccination.
In industrialized countries, RV prevalence follows a seasonal pattern parallel with that of other pathogens which cause infectious diseases in children (such as influenza, pneumococcal disease or respiratory syncytial virus infections), leading to compounded access to healthcare resources during winter months. While mortality due to RVGE remains low, the burden of disease is still important and implementation of vaccination against RV infections mainly contributes to the reduction in associated medical and non-medical cost [Citation52].
During its clinical development, as well as in real-life settings, HRV was already demonstrated to afford high and broad protection against RV genotypes sustained in children aged <2 years, when protection is needed the most [Citation53–56]. This justifies its current worldwide use and efforts to increase vaccine coverage in regions where RV vaccination is not yet implemented in NIPs.
2.4.1. General considerations across the world
The use of routine RV vaccination had an enormous impact on RV-related disease and its associated burden. Real-world data gathered so far in various geographical regions confirm the benefit of RV vaccination and further strengthen its introduction in all childhood immunization programs.
As of May 2019, more than 15 Latin American countries introduced HRV in their expanded programs on immunization. Following the introduction of HRV in Brazil, El Salvador, Mexico, Nicaragua, a significant reduction in all-cause diarrhea death rates in children was observed [Citation57]. In Mexico, where HRV was the only vaccine used in the NIP from 2006 and 2011, a 40% reduction of death rates was demonstrated between the pre- (2003–2006) and post-vaccination (2008–2010) periods, in children <5 years, for an estimated vaccine coverage of ~90% among <2-year-olds [Citation58]. The reduction has been sustained for 4 years following implementation of HRV vaccination [Citation59]. In El Salvador, where HRV coverage was 61%, all-cause diarrhea death rates decreased by 49.5% from 2002–2005 to 2006–2009 [Citation57]. In Panama, gastroenteritis-related mortality and hospitalization were shown to decrease by 50% and 30%, respectively, in 2007–2008 compared with the pre-vaccination data (2000–2005) [Citation60]. More post-licensure data continue to emerge from the Latin American experience, supporting the use of HRV in universal mass vaccination conditions [Citation33]. After a decade from their introduction in the US immunization program, a pooled analysis of HRV and HBRV showed substantial vaccine impact. A substantial decline in the incidence of RV disease and related hospitalization (55% reduction of diarrheal-related hospitalizations in children aged <5 years from 2000–2006 to 2008–2012 [Citation61]) and indirect protection in non-vaccinated groups were observed, leading to a noticeable decrease in health care costs [Citation55]. This was achieved even in the context of a vaccination uptake of approximately 75%, which is relatively low compared to that of other childhood vaccines in the US. Similar impact was observed for Europe, confirming results anticipated from the clinical trials [Citation53]. A rapid and substantial reduction in RV hospitalizations and infections was noted following introduction of HRV vaccination in the NIP of countries worldwide, both when HRV was used as the only vaccine in the NIP or in parallel with HBRV ().
Figure 4. Reduction in rotavirus gastroenteritis cases and hospitalizations following introduction of HRV vaccination worldwide HRV, human rotavirus vaccine; HBRV, human-bovine reassortant vaccine; UK, United Kingdom; RV, rotavirus; VE, vaccine effectiveness [Citation61–74]
![Figure 4. Reduction in rotavirus gastroenteritis cases and hospitalizations following introduction of HRV vaccination worldwide HRV, human rotavirus vaccine; HBRV, human-bovine reassortant vaccine; UK, United Kingdom; RV, rotavirus; VE, vaccine effectiveness [Citation61–74]](/cms/asset/f3adab59-7fc5-4232-b129-250f016a0067/ierv_a_1800459_f0004_oc.jpg)
Overall higher values for HRV effectiveness against RVGE-related hospitalization among children aged <5 years were estimated for countries with low (84%) and median (75%) mortality than those with high child mortality (57%) [Citation75]. While the exact causes for this difference are still unclear [Citation45], the impact of RV vaccination, including with HRV, remains substantial in developing countries because of the high disease burden in these areas. There is evidence of onset of protection with 1 dose of HRV [Citation56,Citation75]; however, the complete 2-dose vaccination series provides optimal protection.
Sub-Saharan countries also experienced declines of 35–80% for RVGE-related diarrhea hospitalization in children aged <5 years, 27–46% for in-hospital mortality due to diarrhea in infants and proved cost-effective [Citation76]. For instance, in Malawi, at 3 years post-vaccine introduction, a 70.6% and 31.7% vaccine effectiveness was estimated in the first and second year of life, and RV-related hospitalization decreased by 54.2% since vaccine introduction in infants, but not in older children [Citation71]. HRV effectiveness against diarrhea-associated -mortality was 34% [Citation77]. Reductions of 20–56% in RVGE-related hospitalizations were also reported in Ghana [Citation78] and Zambia [Citation79]. While data are indicating a clear reduction of RV-related hospitalization and deaths in African countries which introduced vaccination, implementation of RV vaccination in remaining African countries would still prevent a substantial amount of cases and fatalities [Citation80].
2.4.2. Specific lessons from Belgium
In Belgium, HRV is available from 2006 and HBRV since 2007. Since its introduction in the NIP, coverage of RV vaccines has fluctuated slightly, but remained high, at 86% [Citation64]. In this context, data for up to 7 years post-RV vaccine introduction among young children in 11 Belgian hospitals showed a significant impact. A decrease of 70–80% in RV-positive tests compared with a 2-year period pre-introduction was observed in the first 2 years after vaccine introduction and with an additional decline between 10 and 15% sustained in the 5 subsequent years [Citation81,Citation82]. In children aged <1 year, a decrease of 72% and 83% in the number of RVGE hospitalizations was observed by 2 and 4 years post-RV vaccine introduction [Citation64].
2.4.3. Specific lessons from the United Kingdom
A high RV vaccination coverage with HRV was also maintained in the United Kingdom (UK) since its introduction in July 2013, with estimated data for 2016 of 94% for one-dose coverage and 90% for 2-dose coverage among 6-month-olds [Citation83].
Initial data in vaccinated infants showed a 77% decline in laboratory-confirmed RV infections and a 26% reduction of all-cause acute gastroenteritis (AGE)-associated hospitalization for the 2013–2014 RV season [Citation67], with an overall 15% decrease in AGE incidence achieved within the first 2 years of the vaccination program (July 2013–April 2015) compared to the pre-vaccination era [Citation84]. A large analysis of the impact of HRV vaccination covering a 3-years post-introduction period compared to 3–11 years pre-vaccine, showed a decrease of gastroenteritis hospitalizations following introduction of the vaccine, with the highest reduction observed in vaccine-eligible age groups. In children <5 years, RVGE hospitalization was reduced by 80% and AGE-hospitalization by 44%, and a decrease in emergency department (23%), walk-in center (32%) and general practitioner (13%) visits was also observed [Citation85]. For the 2-year period post vaccination, a vaccine effectiveness of 77% and 69% was estimated against confirmed RV infection in young children for the 2 and 1-dose series, respectively [Citation86]. The impact of HRV vaccination on health-care utilization for gastroenteritis was greater than anticipated from modeling studies [Citation87].
2.4.4. Safety
During its clinical development and after years of post-marketing surveillance, HRV has displayed acceptable tolerability and safety profile [Citation27]. The WHO Global Advisory Committee on Vaccine Safety (GACVS) has been monitoring the safety of RV vaccination since 2011, to evaluate a potential association with occurrence of IS. In 2013, based on data from the US and Australia, the GACVS concluded that a risk of IS following administration of RV vaccines might exist, especially during the first 7 days following the first dose [Citation88]. In a meta-analysis of post-licensure surveillance studies, the relative risk of IS during the 7-day post-vaccination period was estimated at 5.4 and 1.8, after the first and second dose of HRV, respectively [Citation89]. Despite the risk of IS, the GAVCS considered the safety profile to be acceptable, with the benefits of vaccination exceeding its risks. Of note, in an analysis of data from 7 sub-Saharan African countries, no increase in short-term (up to 7 days post-vaccination) risk for IS was established after HRV vaccination, when compared to the background risk [Citation90]. Moreover, any short-term increased risk of IS following vaccination does not seem to translate into an overall long-term increased risk of IS in countries with routine RV vaccination [Citation91,Citation92]. Given these considerations, the GACVS reemphasized that the benefit/risk profiles of both HRV and HBRV remain favorable, with the benefits outweighing the risk of IS and pointed out that the latter was not always observed in large cohort studies [Citation93].
2.4.5. Benefits of a 2-dose schedule
Children generally experience more than one RV infection in the early months of life, with the first one being the most serious. Due to the vulnerability of young children to fluid loss, RV-related mortality peaks in the first months of life [Citation94,Citation95]. Moreover, in infants from developing countries, RV infections seem to occur at an even earlier age than in infants in industrialized countries [Citation94,Citation96] which emphasizes the need for vaccination starting within the first 2 months of life.
HRV is administered as a 2-dose series, with vaccination beginning as early as 6 weeks of age and a minimum of 4 weeks interval between doses [Citation27]. While the recommended maximum age for HRV vaccination is 24 weeks, the 2-dose vaccination schedule can be completed within 10 weeks of age, affording protection in a period of time when the infant is most susceptible to RV (re)infection. This timeframe could also lower the risk of IS, with several modeling studies estimating an association between an increased risk and administration of RV vaccines at older ages [Citation97,Citation98].
The dosing schedule of HRV may provide additional advantages over other RV vaccine schedules with >2 doses. First, it allows the completion of the vaccination series at an earlier age, therefore providing more timely protection against RV disease [Citation24,Citation27]. Second, evidence for a higher compliance and completion of the vaccination schedule emerged from several database studies. For instance, an estimated 65.3% versus 46.4% of vaccinated infants with 2- and 3-dose schedules, respectively, were reported to receive the full vaccination series in studies conducted in the US [Citation99,Citation100]. The 2-dose vaccination was also estimated to be more cost-effective in a modeling study in Argentina, in a birth cohort of 746,460 children >5 years [Citation101]. A nation-wide study comparing 2-dose vaccination in 2010 with 3-dose vaccination in 2012 in Mexico reported a higher coverage and compliance for the former, with 93.7% versus 71.1% of infants completing the full series and 75.5% versus 70.9% adhering to the schedule [Citation102]. Third, the 2-dose schedule may be more easily accommodated in the already-crowded NIPs than a 3-dose one. Fourth, vaccination-associated costs are lower for the 2-dose compared to the 3-dose series. Nevertheless, all these considerations must be weighed appropriately against country-specific circumstances.
2.4.6. Impact of HRV vaccination on global RV epidemiology
Both globally-used RV vaccines have proved to be effective in reducing the burden of disease due to the most common circulating RV strains [Citation103]. RV strain fluctuations over time occur naturally and so far, there is no conclusive evidence that they are driven by RV vaccination. Moreover, based on the latest surveillance reports from Europe [Citation17,Citation104] and Australia [Citation105], no new strain causing large outbreaks were detected, nor a vaccine-induced selective pressure occurrence could be unequivocally demonstrated. For instance, in Belgium, the G2P [Citation4] genotype rapidly became predominant after the introduction of RV vaccination, but its prevalence decreased to ~11% 8 years later, only to increase again to ~34% in the epidemiological year 2017/2018 [Citation17,Citation104]. In Australia, no single predominant RV genotype was observed over the first 5 years from RV vaccine introduction [Citation105]. However, between 2012 and 2015, G2P [Citation4] was more prominent in states implementing HRV vaccination compared to those using HBRV (26.7% versus 16.6% of tested strains), while G12P [Citation8] was dominant in HBRV states but not in those using HRV (30.5% versus 1.1% of tested strains) [Citation106]. From 2015, no consistent trend in genotype distribution was observed [Citation106]. In Brazil, a cyclic circulation pattern was noted in the post-vaccination era, with 10-year and 2–3–year reoccurrence intervals for DS-1-like genotypes and Wa-like types, respectively [Citation107]. Isolated reports on an increase in G2P [Citation4] prevalence following HRV introduction are still emerging, however data from long-term, ongoing systematic surveillance seem to disprove a potential association.
So far, surveillance data from all continents indicate that the strain distribution is constantly evolving both pre- and post-vaccination among moderate-to-severe RVGE cases in the pediatric population, highlighting the importance of continuous monitoring. The differences in the relative distribution of genotypes in the post-vaccine era should be interpreted within the context of the natural changes in diversity observed pre-rotavirus vaccination. Also, it is important that strain evolution is considered in the context of a significantly reduced burden of disease. Strain diversity persists and an increase in the relative prevalence of non-vaccine strains has been identified almost globally, including in regions where RV vaccines have not been introduced.
Besides, the lack of a correlate of protection and a limited understanding of the role of neutralizing antibodies is still a ground for various hypotheses on strain-specific RV vaccine efficacy, a discussion which might prove irrelevant. Knowledge on the severity and length of disease is also still lacking to assess if strains truly play a role in this outcome. Surveillance, genetic characterization and quantifiable new-protection tests are needed to conclude further and to allow timely detection of any changes in the distribution of disease-causing strains, together with a better assessment of the impact of RV vaccination.
3. Indirect effects following introduction of HRV
Through the 15 years of post-marketing experience, HRV vaccination was documented to provide benefits beyond those already estimated during the clinical development.
3.1. Benefits from a medical perspective
3.1.1. Herd effect
Available data from real-life setting indicate a herd effect following introduction of HRV in the NIP. In Belgium, the reduction in RV-positive tests in the post-vaccine introduction period compared with the pre-vaccination era was also observed in infants aged <2 months, not eligible for vaccination; the herd effect was maintained for more than 5 years following vaccine introduction. However, herd protection in older age groups is expected to wane over time and eventually disappear, as more and more children become directly protected by vaccination [Citation82]. In Austria, where both HRV and HBRV are used, a decrease in RV-related hospitalization was reported in 2008–2009 compared to 2001–2005 even in age groups too old or too young to be vaccinated, indicating a herd effect [Citation108]. In the US, over a 6-year period post-RV vaccine introduction, RV-hospitalization rates significantly declined even for the 5–19-year and 20–59-year age groups, in the context of a vaccine coverage of 73% among children 19–35-months of age [Citation109].
3.1.2. Reduced incidence of nosocomial infections
Nosocomial RV infections represent an emerging health concern, being responsible for a relatively large part of hospital-acquired AGE in children. While the interpretation of data is limited by discrepancies in the case definition used, reported incidences in Europe varied from 198 to 333 nosocomial RV infections/100,000 children <5 years of age in 2006 [Citation110], and represented 0.3–27.7% of all hospital admissions before RV vaccination was introduced [Citation111].
In Belgium, in the first 2 years after vaccine introduction, nosocomial RVGE was shown to decrease by 76% in children aged ≤5 years [Citation81]. A similar impact on nosocomial infections was maintained up to 7 years post-RV vaccination introduction, with the number of cases decreasing from 221 in 2005 to 33 in 2012 [Citation112]. In Austria, a reduction of 71.9% in the number of nosocomial RVGE yearly cases was observed post-RV vaccination introduction compared to the pre-vaccination period [Citation113].
3.1.3. Impact on incidence of childhood seizures
RV and other diarrheal illnesses often manifest beyond extra-intestinal symptoms and are associated with central nervous system complications, including seizures, although the latter are usually benign when following RVGE. While data are still scarce, there is growing evidence that RV vaccination might reduce the incidence of childhood seizure.
In a study in Spain, significant decreases in annual hospitalization rates for any kind of seizure were reported in children <5 years of age, ranging from 16.2% in 2007 to 34.0% in 2010 as compared with the median rates during the pre-vaccination period (2003–2006) [Citation114]. However, a recent observational study in England did not detect any statistically significant association between the introduction of RV vaccination and childhood seizures [Citation115]. In contrast, a different study conducted in English children <5 years reported reductions in the incidence of any seizures (23%) and febrile seizures with AGE (31%) post-HRV introduction, compared with the pre-vaccine era [Citation116].
These findings need to be interpreted with caution. Emerging evidence seems to point out to a reduction of seizures in the context of RV vaccination; however, a potential association needs to be systematically investigated, using a common methodological approach across studies.
3.1.4. Long-term sequelae of diarrhea
In developing countries, repeated episodes of severe diarrhea can lead to malnutrition and long-term sequalae such as growth stunting [Citation117] and poor physiological development [Citation118]. Likewise, diarrheal diseases have important consequences on early childhood cognition. A study conducted in a Brazilian shantytown showed that episodes of persistent diarrhea in children aged 2 years were associated with a delay in school readiness [Citation119]. The impact of RV vaccination on the prevention of one of the important causes of acute diarrhea is hence important.
Any potential effect of RV vaccination on long-term sequalae of diarrhea is still to be assessed, but is likely to be substantial, based on the reduction in severe RVGE hospitalization cases observed so far in post-RV vaccine introduction.
3.2. Benefits from an economic/health care perspective
3.2.1. Impact on loss of work days/absenteeism
Another aspect of the disease burden of RV is productivity loss, with parents needing to be absent from work while caring for children with RVGE, thus increasing the costs associated with RV disease for both employers and employees. Data obtained in 7 European countries prior to the introduction of RV vaccines showed that RVGE hospitalizations, emergency department cases and primary-care cases were associated with a mean number of absent days from work of 2.3–6.4, 2.5–4.4 and 3.4–7.5, respectively [Citation120].
The impact of RV vaccination on work absenteeism has been so far evaluated in female administrative employees in Belgium, over a 3-year period since RV vaccine introduction [Citation121]. Vaccination resulted in a reduction of absenteeism among mothers with a first child during the first, second, and third RV epidemic periods after birth, with a cumulative gain of 2.25 days per woman, translated into a net cost gain of €187 for the employer [Citation121].
Of note, the benefit of paid RV vaccination was investigated among working mothers in Hungary and Poland, through discrete choice experiment survey. Working mothers were found to be more likely than non-working mothers to vaccinate their children against RV. In addition, a remarkable finding was the importance placed on better time management afforded by vaccination, even if not free-of charge, by avoiding medical services when exposed to severe diarrhea events [Citation122].
3.2.2. Impact on hospital quality of care
Overcrowding and decrease in the quality of care in hospitals is a serious concern especially during seasonal peaks in the incidence of pediatric infections, including RVGE. Introduction of RV vaccination was shown to improve the quality of care in Belgian hospitals during winter months in terms of bed-day occupancy, bed-day turnover and unplanned readmissions for AGE in the post-vaccination (2007–2009) compared with the pre-vaccination era (2004–2006) () [Citation123]. The resulting reduced pressure on healthcare resources may favor the management of other typical winter diseases. However, a similar study in the UK did not show a significant reduction in overall bed occupancy, but demonstrated a decrease in nosocomial infections in the post-vaccination era [Citation124]. Of note, in a different study in the UK, the impact of HRV vaccination at 1 year after introduction was higher in most resource-deprived communities than in least deprived, despite a lower vaccine uptake in the former [Citation85].
Figure 5. Impact of RV vaccination on hospital bed occupancy in Belgium [Citation123]
![Figure 5. Impact of RV vaccination on hospital bed occupancy in Belgium [Citation123]](/cms/asset/e2acbae9-25d9-4a94-aeed-133b6b609468/ierv_a_1800459_f0005_oc.jpg)
3.2.3. Cost-effectiveness of rotavirus vaccination
The global cost of RV infections, including both outpatient visits and hospitalizations, was estimated to be higher than 200 USD million each year, from both an individual and a societal perspective [Citation125]. Families and households typically incur costs related to practitioner visits, treatment, transportation, and missed wages, due to work absenteeism. While even in developed countries these costs are known to be important [Citation126], studies in developing countries have shown a significant economic burden of RV infections. For instance, in Uganda, household costs are estimated at 10% of the average monthly income for one episode of severe RVGE [Citation127,Citation128] while in Malaysia the costs can amount to 26% [Citation129].
From a healthcare and societal perspective, the costs associated with RV infections can be both direct (consisting of expenditure related to hospitalization, out-patient care, and practitioner visits) and indirect (mainly related to productivity loss, transportation, and accommodation during treatment). Several studies assessing RV-associated expenses in developing countries estimated the health care costs at more than 150 USD million each year in India, 34 USD million in Malaysia and 19 USD million in Senegal, while societal costs amounted to 32 USD million in Iran over a 5-year period and 50 USD million in Malaysia [Citation125].
Numerous studies have evaluated the cost-effectiveness of RV vaccination and most of them showed a positive economic impact [Citation128]. Most studies assessing HRV found vaccination to be cost-effective in both developed [Citation52] and developing countries [Citation130], although comparisons are hindered by the different methodologies used.
4. Status, challenges and new chapters to explore
RV mass vaccination has a rapid and substantial impact, detectable within a year from implementation. While the benefits of RV vaccination are clear, there are still gaps in the coverage of RV vaccination worldwide, which can be addressed by looking at the main determinants of vaccination coverage, the 5As: access, affordability, awareness, acceptance, and activation [Citation131].
As of 2019, RV vaccination was introduced in more than 100 countries, with most countries reporting high (80–89%) and very high (90–100%) vaccine coverage shortly after introduction [Citation132]. However, of the 10 countries with the highest RV-associated mortality, only 6 have implemented RV vaccination, and it is estimated that over 70 million infants still do not have access to a RV vaccine [Citation2].
Surprisingly, vaccination uptake remains moderate in Europe and the US. Moreover, RV vaccination is still not introduced in the NIP and is available only in the private sector in large regions worldwide, including EU countries, the Russian Federation, and most Asian countries. The reasons behind this maybe multifaced. A potential barrier to the introduction of RV vaccination, especially in Europe, is the very low mortality rate due to RV infection which probably generated a misperception of the true burden of disease, enhanced by the lack of comparative analyses with other diseases to demonstrate the relative value of RV vaccination. In addition, the incomplete awareness of cost-effectiveness and the mediatization of adverse events (and particularly, IS) risk following vaccination lead to a perceivably-low benefit of RV vaccination, which needs to be corrected. Healthcare providers should be thoroughly educated on the acceptable safety profile of RV vaccines and the rare incidence of IS following vaccination, which should always be accompanied by parental counseling, leading to timely diagnosis and treatment [Citation17]. Moreover, early RV vaccination, outside the peak period of onset for natural IS, may help reduce the risk of IS [Citation90]. Parents and healthcare providers should also be made aware of the benefits of RV mass vaccination, including cost-effectiveness. Current modeling studies may underestimate the economic value of RV vaccination in developed countries, since they do not account for herd effects, long-lasting protection, societal costs due to loss of productivity and costs incurred by the families of children with RVGE during treatment [Citation133]. A recent study using a Social Accounting Matrix model showed that the cost of government-supported vaccination can be counterbalanced by an increase in tax revenue and lower expenses related to treatment, with an economic surplus observed when RV vaccination is implemented [Citation134]. Affordability can be further increased by alternative filling and packaging technology like the use of blow-fill-seal technology in the manufacturing process of RV vaccines [Citation135], which in addition would facilitate sustainable delivery of the vaccine.
Future vaccination strategies should also consider high-risk populations, such as preterm infants, who are at increased risk of severe RV disease, due to reduced maternal antibody levels, as placental transmission starts at 28 gestational weeks and peaks after 36 weeks [Citation136]. Low birth-weight and severe congenital abnormalities, like cardiovascular disorders or metabolic diseases, have been identified as additional risk factors for RVGE in this high-risk category [Citation136,Citation137]. Vaccination practices of preterm infants vary considerably. In the US, RV vaccination is recommended only at discharge, which has led to missing the vaccination window, especially for low birth-weight infants [Citation138]. In other countries, such as the UK, Australia, or New Zealand, vaccination is not deferred, even if the infant is still hospitalized. Data so far indicate that HRV administration in premature infants is immunogenic and well tolerated [Citation139], in line with observations from clinical trials in more than 1000 infants born at 27–36 weeks gestation [Citation140]. Evidence also continues to accumulate to demonstrate the need and cost-effectiveness of vaccination in preterm infants and other high-risk categories [Citation137]. Taking this into account, including at-risk populations in the programmatic RV vaccination will allow RV vaccines to achieve their maximum impact.
5. Conclusion
With a post-licensure proven beneficial impact and effectiveness against RVGE in both developed and developing countries, data obtained post-HRV vaccination introduction confirmed the global reduction in the burden of disease anticipated based on the results obtained during the clinical development. As of April 2020, more than 600 million vaccine doses have been distributed since first licensure, potentially protecting 300 million children against RV disease.
6. Expert opinion
At 15 years since the introduction of HRV, the global experience has provided us with important lessons on the prevention of RV diseases: it showed how a vaccine design mimicking natural infection and subsequent immunity can provide direct and indirect benefits toward reduction of the RVGE burden across the world.
The rapid impact afforded on the burden of RV disease by introduction of RV vaccines suggests that the dosing schedule, mode of administration, vaccination adherence, and compliance are important considerations to the success of vaccination programs. Herd effects following mass RV vaccination, although temporary, have also contributed to the success of RV NIPs, and need to be factored-in when evaluating the health economic benefits. Overall clinical and real-world evidence should give decision makers the much-needed confidence in RV prevention, as there are still barriers in uptake and global vaccination coverage remains low.
With efforts deployed toward RV vaccination, evidence will continue to accumulate and contribute to our overall knowledge on the prevention of RV disease: the increasing effectiveness and impact of vaccination in developing countries, the potential evolution of RV strains post-vaccination and its impact on disease control by current vaccines, and specific concerns about vaccination safety – including one of the most important barriers in RV vaccination uptake, ignorance on the true burden disease and increased risk of IS. Other added benefits of RV vaccination, such as the impact on the incidence of seizures, and potentially, type 1 diabetes or celiac disease [Citation141,Citation142] are being investigated. However, further supportive evidence is needed to clearly establish an association between RV vaccination and these outcomes.
The first 2 years of life are critical for the child’s physiological, neurological, and emotional growth and development. Therefore, prevention of infections like RV can have an important role in a healthy childhood and adulthood. The short window of opportunity for early vaccination and the fact that licensed vaccines are available only for infants <32 weeks of age require a strong commitment from healthcare providers to prioritize RV vaccination for the infant and make parents aware of the importance of RVGE prevention through early vaccination. Health authorities, healthcare providers and vaccine manufacturers must equally commit to the continued improvement of RV vaccination uptake, which will significantly contribute to the control of diarrheal diseases and the associated mortality among children <5 years of age worldwide.
While more than 90 countries have currently included RV vaccination in their NIPs, there are still many for which this is not a priority. The impact on mortality is highest in resources-deprived areas and morbidity is more relevant in high-income countries. The 5 ”A’s” – the main determinants of vaccination coverage: access, affordability, awareness, acceptance, and activation [Citation131] – need to be addressed while considering the specificities of each setting, since barriers to uptake may differ considerably from one country to another.
The next 5 to 10 years will be key in establishing clear directions to improve the impact of RV vaccination in developing countries, as well as to overcome barriers to global vaccine uptake. In developed countries, RV prevention is not prioritized as a need, as well as the current available evidence of the success of mass RV vaccination, are most likely underestimated. Overall, continued efforts to increase awareness on the substantial impact of RV vaccination are critical in motivating healthcare professionals and parents to vaccinate and in convincing policy-makers to implement national RV immunization. In that respect, the lessons learnt from these first decades of HRV mass vaccination on early, effective and broad protection advocate the substantial impact of RV vaccination.
Of note, the vaccine efficacy outcome currently used to measure vaccine impact might be a limited way of describing mass RV vaccination benefits, which should also reflect external factors that are influenced through vaccination. The adaptation of measured outcomes to specific countries or resource limitations or socioeconomic status needs further research. At present, increasing the reach and coverage of mass vaccination with available vaccines may already provide the optimum impact on RV disease, and circumvent the need to develop new vaccines.
Another point to consider relates to the immunological mechanisms underlying the protection afforded by natural and vaccine-induced immunity. Broad protection has been demonstrated for both multi- and single-strain RV but needs further investigation. A deeper understanding of the nature of the immune response, the reservoirs of rotavirus infection, the dynamics of transmission of disease across different age groups (vaccinated and unvaccinated individuals, including adults) would play a major role in deciphering observed patterns and predicting the long-term impact of vaccination and its effect on RV disease control. Continued RV strain surveillance – already implemented in several regions worldwide – is paramount to identify a clear, quantifiable measure to assess and define potential differences in strain-specific significant clinical outcomes. This in turn will allow to understand how disease caused by new virus strains can be controlled by vaccination.
Finally, the development of new RV vaccines, based on inactivated or neonatal human RV strains, or employing innovative approaches (such as, subunit vaccines based on recombinant proteins or virus-like particles) is ongoing and is likely to evolve further. These vaccines can present several advantages, among which the possibility of parenteral administration (thus reducing the risk of IS by bypassing the contact of the vaccine with the gut), reducing the risk of emergence of reassortant strains, a better immunogenicity [Citation143], or administration in special at-risk categories for which live-attenuated vaccines are not recommended (e.g., children with severe combined immunodeficiency [Citation144]). However, it is worth mentioning that given the availability of the well-established RV vaccines, already proven to be effective, increasing coverage with those should be prioritized. In addition, new production technologies [Citation135], such as the blow-fill-seal technique, arising from the need for improved vaccine delivery leading to higher vaccination coverage and affordability, are also emerging and are supported by the WHO.
Article highlights
A 2-dose series of the single-strain G1P[8], live-attenuated human rotavirus vaccine Rotarix provides broad protection against both homotypic and heterotypic rotavirus strains.
Real world evidence supports the impact of HRV mass vaccination, with rapid and substantial reductions in rotavirus infections and related hospitalizations being observed on a global scale.
Additional benefits of HRV vaccination include herd effect, reduction in nosocomial infections and economic/healthcare benefits.
The impact of rotavirus vaccination can be further improved by increasing vaccination coverage worldwide.
Author contributions
All authors participated in thedevelopment of this manuscript and gave final approval before submission.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
All authors are employees of GlaxoSmithKline and hold shares in the GSK group of companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Trademark statement
Rotarix is a trademark owned by the GSK group of companies. RotaTeq is a trademark of Merck Inc.
Additional information
Funding
References
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1211–1228.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. Rota Council. Rotavirus disease and immunization: series of briefs. Cover of epidemiology and burden brief; 2019 cited 2019 Dec 30. Available from: http://rotacouncil.org/wp-content/uploads/2019/05/ROTA-Brief3-Burden-SP-1.pdf
- Pan American Health Organization (PAHO). About rotavirus; [ cited 2019 Dec 30]. Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=1861:2009-about-rotavirus&Itemid=1621&lang=en
- Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105.
- Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect. 2009;137(5):607–616.
- Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–965.
- Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64.
- GAVI. The vaccine alliance. Detailed product profiles. Rotavirus vaccines; 2020 cited 2020 May 5. Available from: http://www.gavi.org/library/gavi-documents/supply-procurement/detailed-product-profiles/
- Zhen SS, Li Y, Wang SM, et al. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg Microbes Infect. 2015;4(10):e64.
- Dang DA, Nguyen VT, Vu DT, et al. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine. 2012;30(Suppl 1):A114–121.
- Bines JE, At Thobari J, Satria CD, et al. Human neonatal rotavirus vaccine (RV3-BB) to target rotavirus from birth. N Engl J Med. 2018;378(8):719–730.
- O’Ryan M. Rotavirus vaccines: a story of success with challenges ahead. F1000Res. 2017;6:1517.
- Burke RM, Tate JE, Kirkwood CD, et al. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32(5):435–444.
- Crawford SE, Ramani S, Tate JE, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083.
- Gentsch JR, Laird AR, Bielfelt B, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192(Suppl 1):S146–159.
- Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–1028.
- European Centre for Disease Prevention and Control. Expert opinion on rotavirus vaccination in infancy; 2017 cited 2019 Oct 14. Available from: https://ecdc.europa.eu/sites/portal/files/documents/rotavirus-vaccination-expert%20opinion-september-2017.pdf
- Bernstein DI. Live attenuated human rotavirus vaccine, Rotarix. Semin Pediatr Infect Dis. 2006;17(4):188–194.
- O’Ryan M. Rotarix (RIX4414): an oral human rotavirus vaccine. Expert Rev Vaccines. 2007;6(1):11–19.
- Ward RL, Bernstein DI. Rotarix: a rotavirus vaccine for the world. Clin Infect Dis. 2009;48(2):222–228.
- World Health Organization. WHO prequalified vaccines listing; [ updated 2019 May 30; cited 2019 Oct 14]. Available from: http://extranet.who.int/gavi/PQ_Web/
- Centers for Disease Control and Prevention. Suspension of rotavirus vaccine after reports of intussusception–United States, 1999. MMWR Morb Mortal Wkly Rep. 2004;53(34):786–789.
- Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–572.
- European Medicines Agency. RotaTeq SmPC 01/ 03/2016EMA EPAR update; 2016 cited 2019 Oct 14. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000669/WC500054185.pdf
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22.
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33.
- European Medicines Agency. Rotarix SmPC 31/ 03/2016EMA EPAR update; 2016 cited 2019 Oct 14. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000639/WC500054789.pdf
- Vesikari T, Karvonen A, Korhonen T, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine. 2004;22(21–22):2836–2842.
- Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521.
- Fathima P, Snelling TL, Gibbs RA. Effectiveness of rotavirus vaccines in an Australian population: A case-control study. Vaccine. 2019;37(41):6048–6053.
- Maguire JE, Glasgow K, Glass K, et al. Rotavirus epidemiology and monovalent rotavirus vaccine effectiveness in Australia: 2010–2017. Pediatrics. 2019;144(4):e20191024.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub RV- Vaccine introduction. Current dosing schedule; 2019. cited 2019 Oct 14. Available from: http://view-hub.org/viz/?YXBwaWQ9MSZpbmRpY2F0b3JpZD01NSZvdmVybGF5aWQ9NA==
- Perez Schael I, O’Ryan M, Sáez-Llorens X, et al. Clinical development, registration, and introduction of human rotavirus vaccine: the Latin American experience. Trials Vaccinol. 2012;1:10–20.
- Linhares AC, Velazquez FR, Perez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–1189.
- Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis. 2007;196(4):537–540.
- Salinas B, Perez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24(9):807–816.
- Tregnaghi MW, Abate HJ, Valencia A, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30(6):e103–108.
- O’Ryan M, Linhares AC. Update on Rotarix: an oral human rotavirus vaccine. Expert Rev Vaccines. 2009;8(12):1627–1641.
- Kawamura N, Tokoeda Y, Oshima M, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29(37):6335–6341.
- Madhi SA, Kirsten M, Louw C, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl 1):A44–51.
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–1763.
- Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298.
- Phua KB, Lim FS, Lau YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30(30):4552–4557.
- Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11–18.
- Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens. 2017;6(4):65.
- Gruber JF, Becker-Dreps S, Hudgens MG, et al. Timing of rotavirus vaccine doses and severe rotavirus gastroenteritis among vaccinated infants in low- and middle-income countries. Epidemiology. 2018;29(6):867–875.
- Burnett E, Lopman BA, Parashar UD. Potential for a booster dose of rotavirus vaccine to further reduce diarrhea mortality. Vaccine. 2017;35(51):7198–7203.
- Dai X, Bai R, Jian M, et al. Immunogenicity of different dosing schedules of the human live attenuate rotavirus vaccine (RV1) in infants and children: a meta-analysis. Hum Vaccin Immunother. 2019;15(6):1228–1236.
- Dennehy PH, Brady RC, Halperin SA, et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J. 2005;24(6):481–488.
- Kompithra RZ, Paul A, Manoharan D, et al. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine. 2014;32(Suppl 1):A129–133.
- Lee B, Dickson DM, Alam M, et al. The effect of increased inoculum on oral rotavirus vaccine take among infants in Dhaka, Bangladesh: A double-blind, parallel group, randomized, controlled trial. Vaccine. 2020;38(1):90–99.
- Plosker GL. Rotavirus vaccine RIX4414 (Rotarix): a pharmacoeconomic review of its use in the prevention of rotavirus gastroenteritis in developed countries. Pharmacoeconomics. 2011;29(5):439–454.
- Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine. 2015;33(18):2097–2107.
- Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis. 2015;61(12):1792–1799.
- Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States - vaccine uptake, effectiveness, and impact. Expert Rev Vaccines. 2018;17(7):593–606.
- Willame C, Vonk Noordegraaf-Schouten M, Gvozdenovic E, et al. Effectiveness of the oral human attenuated rotavirus vaccine: A systematic review and meta-analysis-2006–2016. Open Forum Infect Dis. 2018;5(11):ofy292.
- Paternina-Caicedo A, Parashar UD, Alvis-Guzman N, et al. Effect of rotavirus vaccine on childhood diarrhea mortality in five Latin American countries. Vaccine. 2015;33(32):3923–3928.
- Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med. 2011;365(8):772–773.
- Gastanaduy PA, Sanchez-Uribe E, Esparza-Aguilar M, et al. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics. 2013;131(4):e1115–1120.
- Bayard V, DeAntonio R, Contreras R, et al. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis. 2012;16(2):e94–98.
- Leshem E, Tate JE, Steiner CA, et al. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA. 2015;313(22):2282–2284.
- Uhlig U, Kostev K, Schuster V, et al. Impact of rotavirus vaccination in Germany: rotavirus surveillance, hospitalization, side effects and comparison of vaccines. Pediatr Infect Dis J. 2014;33(11):e299–304.
- Doll MK, Buckeridge DL, Morrison KT, et al. Effectiveness of monovalent rotavirus vaccine in a high-income, predominant-use setting. Vaccine. 2015;33(51):7307–7314.
- Standaert B, Gomez JA, Raes M, et al. Impact of rotavirus vaccination on hospitalisations in Belgium: comparing model predictions with observed data. PLoS One. 2013;8(1):e53864.
- Araki K, Hara M, Tsugawa T, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Japanese children. Vaccine. 2018;36(34):5187–5193.
- Sahakyan G, Grigoryan S, Wasley A, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis. 2016;62(Suppl 2):S147–154.
- Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis. 2016;213(2):243–249.
- Inns T, Trindall A, Dunling-Hall S, et al. Introduction of a new rotavirus vaccine: initial results of uptake and impact on laboratory confirmed cases in Anglia and Essex, United Kingdom, July 2015. Hum Vaccin Immunother. 2016;12(4):1040–1044.
- Gheorghita S, Birca L, Donos A, et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis. 2016;62(Suppl 2):S140–146.
- Yen C, Armero Guardado JA, Alberto P, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30(1 Suppl):S6–S10.
- Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis. 2016;62(Suppl 2):S213–219.
- Pendleton A, Galic M, Clarke C, et al. Impact of rotavirus vaccination in Australian children below 5 years of age: a database study. Hum Vaccin Immunother. 2013;9(8):1617–1625.
- Msimang VM, Page N, Groome MJ, et al. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr Infect Dis J. 2013;32(12):1359–1364.
- Safadi MA, Berezin EN, Munford V, et al. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr Infect Dis J. 2010;29(11):1019–1022.
- Jonesteller CL, Burnett E, Yen C, et al. Effectiveness of rotavirus vaccination: A systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis. 2017;65(5):840–850.
- Mwenda JM, Parashar UD, Cohen AL, et al. Impact of rotavirus vaccines in Sub-Saharan African countries. Vaccine. 2018;36(47):7119–7123.
- Bar-Zeev N, King C, Phiri T, et al. Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study. Lancet Glob Health. 2018;6(9):e1036–e1044.
- Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis. 2016;62(Suppl 2):S200–207.
- Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis. 2016;62(Suppl 2):S175–182.
- Shah MP, Tate JE, Mwenda JM, et al. Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev Vaccines. 2017;16(10):987–995.
- Raes M, Strens D, Vergison A, et al. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J. 2011;30(7):e120–125.
- Standaert B, Strens D, Alwan A, et al. Medium- to long-term impact of rotavirus vaccination on hospital care in Belgium: A 7-year follow-up of the Rotavirus Belgium Impact Study (RotaBIS). Infect Dis Ther. 2016;5(1):31–44.
- Public Health England. Health protection report; 2016. cited 2019 Dec 30. Available from: http://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/555048/hpr3216_rtvrs_VC.pdf
- Thomas SL, Walker JL, Fenty J, et al. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35(4):680–686.
- Hungerford D, Vivancos R, Read JM, et al. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med. 2018;16(1):10.
- Walker JL, Andrews NJ, Atchison CJ, et al. Effectiveness of oral rotavirus vaccination in England against rotavirus-confirmed and all-cause acute gastroenteritis. Vaccine X. 2019;1:100005.
- Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine. 2007;25(20):3971–3979.
- WHO. Global advisory committee on vaccine safety, 11–12 December 2013. Wkly Epidemiol Rec. 2014;89(7):53–60.
- Rosillon D, Buyse H, Friedland LR, et al. Risk of intussusception after rotavirus vaccination: meta-analysis of postlicensure studies. Pediatr Infect Dis J. 2015;34(7):763–768.
- Tate JE, Mwenda JM, Armah G, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med. 2018;378(16):1521–1528.
- Burke RM, Tate JE, Dahl RM, et al. Does rotavirus vaccination affect longer-term intussusception risk in US infants? J Pediatric Infect Dis Soc. 2020;9(2):257–260.
- Hawken S, Ducharme R, Rosella LC, et al. Assessing the risk of intussusception and rotavirus vaccine safety in Canada. Hum Vaccin Immunother. 2017;13(3):703–710.
- WHO. Global advisory committee on vaccine safety, 6–7 December 2017. Wkly Epidemiol Rec. 2018;93(2):17–30.
- Cunliffe N, Zaman K, Rodrigo C, et al. Early exposure of infants to natural rotavirus infection: a review of studies with human rotavirus vaccine RIX4414. BMC Pediatr. 2014;14:295.
- Patel MM, Clark AD, Sanderson CFB, et al. Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis. PLoS Med. 2012;9(10):e1001330.
- Steele AD, Madhi SA, Cunliffe NA, et al. Incidence of rotavirus gastroenteritis by age in African, Asian and European children: relevance for timing of rotavirus vaccination. Hum Vaccin Immunother. 2016;12(9):2406–2412.
- Yung CF, Chong CY, Thoon KC. Age at first rotavirus vaccination and risk of intussusception in infants: A public health modeling analysis. Drug Saf. 2016;39(8):745–748.
- Koch J, Harder T, von Kries R, et al. Risk of intussusception after rotavirus vaccination. Dtsch Arztebl Int. 2017;114(15):255–262.
- Krishnarajah G, Davis EJ, Fan Y, et al. Rotavirus vaccine series completion and adherence to vaccination schedules among infants in managed care in the United States. Vaccine. 2012;30(24):3717–3722.
- Krishnarajah G, Landsman-Blumberg P, Eynullayeva E. Rotavirus vaccination compliance and completion in a Medicaid infant population. Vaccine. 2015;33(3):479–486.
- Marti SG, Alcaraz A, Valanzasca P, et al. Cost effectiveness evaluation of a rotavirus vaccination program in Argentina. Vaccine. 2015;33(42):5684–5690.
- Luna-Casas G, Juliao P, Carreno-Manjarrez R, et al. Vaccine coverage and compliance in Mexico with the two-dose and three-dose rotavirus vaccines. Hum Vaccin Immunother. 2019;15(6):1251–1259.
- Banyai K, Estes MK, Martella V, et al. Viral gastroenteritis. Lancet. 2018;392(10142):175–186.
- EuroRotaNet. Annual report 2018; 2019 [cited 2020 May Oct 6]. Available from: http://www.eurorotanet.com/wp-content/uploads/2019/09/EuroRotaNet_report-Sept_2019_v1.pdf
- Roczo-Farkas S, Kirkwood CD, Bines JE. Australian rotavirus surveillance program annual report, 2015. Commun Dis Intell Q Rep. 2016;40(4):E527–E538.
- Roczo-Farkas S, Kirkwood CD, Cowley D, et al. The impact of rotavirus vaccines on genotype diversity: A comprehensive analysis of 2 decades of Australian surveillance data. J Infect Dis. 2018;218(4):546–554.
- Carvalho-Costa FA, de Assis RMS, Fialho AM, et al. The evolving epidemiology of rotavirus A infection in Brazil a decade after the introduction of universal vaccination with Rotarix. BMC Pediatr. 2019;19(1):42.
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 2011;29(15):2791–2796.
- Baker JM, Tate JE, Steiner CA, et al. Longer-term direct and indirect effects of infant rotavirus vaccination across all ages in the United States in 2000–2013: analysis of a large hospital discharge dataset. Clin Infect Dis. 2018;68(6):976–983.
- Gervasi G, Capanna A, Mita V, et al. Nosocomial rotavirus infection: an up to date evaluation of European studies. Hum Vaccin Immunother. 2016;12(9):2413–2418.
- Gleizes O, Desselberger U, Tatochenko V, et al. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006;25(1 Suppl):S12–21.
- Standaert B, Strens D, Li X, et al. The sustained rotavirus vaccination impact on nosocomial infection, duration of hospital stay, and age: the RotaBIS study (2005–2012). Infect Dis Ther. 2016;5(4):509–524.
- Zlamy M, Kofler S, Orth D, et al. The impact of rotavirus mass vaccination on hospitalization rates, nosocomial rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis. 2013;13:112.
- Pardo-Seco J, Cebey-Lopez M, Martinon-Torres N, et al. Impact of rotavirus vaccination on childhood hospitalization for seizures. Pediatr Infect Dis J. 2015;34(7):769–773.
- Biggart R, Finn A, Marlow R. Lack of impact of rotavirus vaccination on childhood seizure hospitalizations in England - An interrupted time series analysis. Vaccine. 2018;36(31):4589–4592.
- Hungerford DJ, French N, Iturriza-Gomara M, et al. Reduction in hospitalisations for acute gastroenteritis-associated childhood seizures since introduction of rotavirus vaccination: a time-series and change-point analysis of hospital admissions in England. J Epidemiol Community Health. 2019;73(11):1020–1025.
- Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37(4):816–830.
- Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–1338.
- Lorntz B, Soares AM, Moore SR, et al. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25(6):513–520.
- Van der Wielen M, Giaquinto C, Gothefors L, et al. Impact of community-acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL study. BMC Fam Pract. 2010;11:22.
- Standaert B, Van de Mieroop E, Nelen V. Exploring the potential impact of rotavirus vaccination on work absenteeism among female administrative personnel of the City of Antwerp through a retrospective database analysis. BMJ Open. 2015;5(6):e007453.
- Poulos C, Standaert B, Sloesen B, et al. Preferences for vaccines against children’s diarrheal illness among mothers in Poland and Hungary. Vaccine. 2018;36(40):6022–6029.
- Standaert B, Alwan A, Strens D, et al. Improvement in hospital quality of care (QoC) after the introduction of rotavirus vaccination: an evaluation study in Belgium. Hum Vaccin Immunother. 2015;11(9):2266–2273.
- Heinsbroek E, Hungerford D, Cooke RPD, et al. Do hospital pressures change following rotavirus vaccine introduction? A retrospective database analysis in a large paediatric hospital in the UK. BMJ Open. 2019;9(5):e027739.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health, Rota Council. The economic burden; 2017 [cited 2019 Oct 14]. Available from: http://rotacouncil.org/rotavirus-disease/global-burden/
- Standaert B, Harlin O, Desselberger U. The financial burden of rotavirus disease in four countries of the European Union. Pediatr Infect Dis J. 2008;27(1):S20–S27.
- Sigei C, Odaga J, Mvundura M, et al. Cost-effectiveness of rotavirus vaccination in Kenya and Uganda. Vaccine. 2015;33(Suppl 1):A109–118.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. Technical information for national-level rotavirus vaccine policy recommendations. Summary of available evidence to guide decision-making; 2017 [cited 2019 Oct 14]. Available from: http://www.jhsph.edu/ivac/wp-content/uploads/2018/05/RVVTechnicalBriefings-Jan2017.pdf
- Chai PF, Lee WS. Out-of-pocket costs associated with rotavirus gastroenteritis requiring hospitalization in Malaysia. Vaccine. 2009;27(Suppl 5):F112–115.
- Plosker GL. Pharmacoeconomic spotlight on rotavirus vaccine RIX4414 (Rotarix) in the prevention of rotavirus gastroenteritis in developing countries. Paediatr Drugs. 2012;14(6):429–433.
- Thomson A, Robinson K, Vallee-Tourangeau G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine. 2016;34(8):1018–1024.
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub; [ cited 2020 May 27]. Available from: www.view-hub.org
- Poelaert D, Pereira P, Gardner R, et al. A review of recommendations for rotavirus vaccination in Europe: arguments for change. Vaccine. 2018;36(17):2243–2253.
- Kotsopoulos N, Haitsma G, Connolly MP, et al. Estimating the money flow in the economy attributed to rotavirus disease and vaccination in the Netherlands using a Social Accounting Matrix (SAM) framework. Expert Rev Pharmacoecon Outcomes Res. 2019;1–10.
- Sedita J, Perrella S, Morio M, et al. Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats. Vaccine. 2018;36(12):1700–1709.
- Kilich E, Sadarangani M. Use of rotavirus vaccines in preterm babies on the neonatal unit. Expert Rev Vaccines. 2016;15(12):1463–1465.
- Bruijning-Verhagen P, Mangen MJJ, Felderhof M, et al. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11:112.
- Stumpf KA, Thompson T, Sanchez PJ. Rotavirus vaccination of very low birth weight infants at discharge from the NICU. Pediatrics. 2013;132(3):e662–665.
- Jaques SC, Ogley L, Duffy D, et al. Rotavirus immunisation in NICU: a 1-year experience in a UK tertiary neonatal surgical unit postvaccine introduction. Arch Dis Child Fetal Neonatal Ed. 2015;100(2):F186–187.
- Omenaca F, Sarlangue J, Szenborn L, et al. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European Infants: a randomized phase IIIb study. Pediatr Infect Dis J. 2012;31(5):487–493.
- Hemming-Harlo M, Lahdeaho ML, Maki M, et al. Rotavirus vaccination does not Increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J. 2019;38(5):539–541.
- Perrett KP, Jachno K, Nolan TM, et al. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatr. 2019;173(3):280–282; Erratum in JAMA Pediatr. 2019;2173(2019):2895.
- Changotra H, Vij A. Rotavirus virus-like particles (RV-VLPs) vaccines: an update. Rev Med Virol. 2017;27(6):e1954.
- Centers for Disease Control and Prevention. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep. 2010;59(22):687–688.