ABSTRACT
Objectives
The emergence of human pathogens with pandemic potential motivates rapid vaccine development. We explore the role of vaccines in control and eradication of a novel emerging pathogen.
Methods
We hypothetically simulate emergence of a novel wild poliovirus (nWPV) in 2020 assuming an immunologically naïve population. Assuming different nonpharmaceutical interventions (NPIs), we explore the impacts of vaccines resembling serotype-specific oral poliovirus vaccine (OPV), novel OPV (nOPV), or inactivated poliovirus vaccine (IPV).
Results
Vaccines most effectively change the trajectory of an emerging disease when disseminated early, rapidly, and widely in the background of ongoing strict NPIs, unless the NPIs successfully eradicate the emerging pathogen before it establishes endemic transmission. Without strict NPIs, vaccines primarily reduce the burden of disease in the remaining susceptible individuals and in new birth cohorts. Live virus vaccines that effectively compete with the nWPVs can reduce disease burdens more than other vaccines. When relaxation of existing NPIs occurs at the time of vaccine introduction, nWPV transmission can counterintuitively increase in the short term.
Conclusions
Vaccines can increase the probability of disease eradication in the context of strict NPIs. However, successful eradication will depend on specific immunization strategies used and a global commitment to eradication.
1. Introduction
As we experience the evolution of Coronavirus Disease 2019 (COVID-19) from an emerging viral infection to an endemic infectious disease [Citation1], efforts to develop, produce, and administer a vaccine represent an international global health priority. The opportunity to learn from existing models of familiar vaccine-preventable diseases offers the possibility to appreciate the potential value and limitations of vaccines [Citation2]. Building on extensive experience modeling poliovirus transmission, as well as the health and economic impacts of poliovirus vaccines [Citation3], we consider polio and poliovirus vaccines useful surrogates for studying and managing the expectations of COVID-19 vaccines and vaccination strategies, as well as potential future pathogens.
While phylogenetically and structurally different viruses, polioviruses and severe acute respiratory syndrome coronaviruses, including SARS-CoV-1 (which caused the SARS multinational outbreak) and SARS-CoV-2 (which caused the COVID-19 pandemic), share some clinical and epidemiological similarities. For instance, both groups of viruses result in respiratory and gastrointestinal infections, exhibit substantial asymptomatic or subclinical infections with a small subset of significant morbidity and mortality requiring intensive care, transmit through multiple routes, including droplets and aerosols, and include the potential for reinfection. Specifically, while we acknowledge significant differences between these viruses, we postulate that policy insights gained from control and eradication efforts are relevant to both, as well as future emerging pathogens of comparable (albeit not identical) characteristics.
Since the global experience with polio includes three poliovirus serotypes with distinct biological and clinical phenotypes, as well as two classes of vaccines, also with distinct characteristics, the global polio model provides an opportunity to explore multiple diseases and vaccine combinations and to demonstrate the potential use of modeling in decision support. The insights from this modeling may prove useful for many clinically significant emerging viruses, including SARS-CoV-2, since some of the key characteristics of the pathogens and vaccines will likely overlap.
1.1. Prior modeling of the role of vaccines in responding to a pandemic threat
As a foundation for exploring the role of vaccines in managing an emerging viral pandemic, we separately applied an existing global poliovirus transmission model to explore the dynamic health and policy consequences of the introduction of a hypothetical novel wild poliovirus (nWPV) with disease emergence in early 2020 and biological properties like each of the existing three known wild poliovirus serotypes (i.e., nWPV1, nWPV2, and nWPV3) [Citation4]. The study reviewed prior modeling literature related to using nonpharmaceutical interventions (NPIs), which can reduce reproduction numbers and exportations until die out, and it demonstrated the establishment of endemic transmission for each of the three nWPVs in the absence of a global commitment to contain and eradicate the nWPV [Citation4].
Numerous other studies explored the role of vaccines as one of many potential interventions (part of a combination of strategies) used to respond to a pandemic caused by an emerging virus after a delay showing mixed results of the impacts of vaccine [Citation5–10]. For pandemic influenza, seminal reports and policies emphasized the importance of rapid vaccine development [Citation11–13]. Recognition of the need for incentives for manufacturers to develop vaccines following the experiences with SARS, Ebola, and Zika led to the creation of the Coalition for Epidemic Preparedness and Innovations (CEPI) [Citation14]. Recently, global experience with SARS-CoV-2 led to numerous modeling studies that explore the potential role for SARS-CoV-2 vaccines [Citation15–18]. The value of vaccines in responding to an emerging pathogen depends on the time required to develop, produce, distribute, and administer the vaccine, and its properties (i.e., effectiveness, the nature and durability of immunity induced, and coverage in susceptible individuals and/or individuals who contribute the most substantially to transmission). The process of vaccine development changed significantly over time.
1.2. Historical development of poliovirus vaccines
The actual historical development of poliovirus vaccines occurred over decades as shown in and led to two different classes of vaccines with different biological and clinical properties: (1) oral poliovirus vaccine (OPV) and (2) inactivated poliovirus vaccine (IPV). Although the process of polio vaccine development involved many complexities [Citation19] and some false starts [Citation20], we highlight the key milestones related to three major challenges related to the development of a safe and effective polio vaccine.
Figure 1. Historical timelines of polio (blue font), SARS (green font) and COVID-19 (red font) highlight significantly different pathways to disease discovery and vaccine development. Critical polio milestones spanned nearly a century, and vaccine development preceded molecular characterization of the virus by decades. SARS was contained with no contribution from vaccines, and COVID-19 continues on a path distinct from polio and SARS. For context, global population growth is represented by gray circles drawn to scale from 1.57B in 1890 to 7.79B in 2020 [Citation21]. Superimposed on gray circles are blue circles drawn to the same scale showing growth in global airline passenger volume from 0.31B in 1970 to 4.54B in 2019 [Citation22,Citation23]. The relationship between disease, world population and travel changed substantially between polio, SARS, and COVID-19, with progressively larger global population with significant increase in mixing as seen in air passenger volume
![Figure 1. Historical timelines of polio (blue font), SARS (green font) and COVID-19 (red font) highlight significantly different pathways to disease discovery and vaccine development. Critical polio milestones spanned nearly a century, and vaccine development preceded molecular characterization of the virus by decades. SARS was contained with no contribution from vaccines, and COVID-19 continues on a path distinct from polio and SARS. For context, global population growth is represented by gray circles drawn to scale from 1.57B in 1890 to 7.79B in 2020 [Citation21]. Superimposed on gray circles are blue circles drawn to the same scale showing growth in global airline passenger volume from 0.31B in 1970 to 4.54B in 2019 [Citation22,Citation23]. The relationship between disease, world population and travel changed substantially between polio, SARS, and COVID-19, with progressively larger global population with significant increase in mixing as seen in air passenger volume](/cms/asset/677f2ae1-7e9d-46b7-96d9-8283feab3b90/ierv_a_1891889_f0001_oc.jpg)
Critical to polio vaccine development, multiple landmark studies suggested the existence of three distinct poliovirus serotypes [Citation24,Citation25]. Recognition of different serotypes led to a frenzy of confirmatory work over the subsequent few years. These studies confirmed in 1951 that nearly 200 different strains of poliovirus tested in thousands of monkeys fell into three distinct serotypes conferring protective immunity. Serotype 1 accounted for 82% of the strains, serotype 2 for 10%, and serotype 3 for 8%, which implied that a vaccine containing these 3 serotypes of polioviruses would cover the entirety of the disease spectrum [Citation19]. Thus, a vaccination strategy involving these three serotypes of polioviruses would cover the entirety of the disease spectrum. Around the same time, tissue culture methods for in vitro cultivation of polioviruses overcame substantial safety and manufacturing hurdles to vaccine development [Citation26].
Building on these key basic science advances, the race for the mass development and deployment of polio vaccines followed two distinct strategies (IPV and OPV). IPV development built on tissue culture techniques [Citation27] that enabled large-scale production of polioviruses followed by formaldehyde inactivation. The first human trials of IPV involved small cohorts of institutionalized children in 1953, followed by large-scale field trials in the US in 1954 involving more than 600,000 school children [Citation28]. In spite of limited effectiveness against serotype 1 poliovirus infection (68%), this study received acclaim as a triumph over poliomyelitis and led to IPV licensure in 1955 [Citation29] and contracts for widescale production [Citation30]
During the same time, work began on OPV by Koprowski followed by work by Sabin (reviewed in [Citation31]). In spite of methodological differences in clinical studies, the principle of live virus attenuation remains the same; namely, polioviruses tend to lose neurovirulence after multiple passages in cell culture. As a live attenuated virus vaccine, OPV induces a subclinical infection and provides robust immunity against its wild-type neurovirulent counterparts. Although the US did not conduct large-scale trials using OPV, millions of children received OPV in the Soviet Union (Sabin OPV), as well as Poland, Croatia, and the Belgian Congo (Koprowski OPV) by the early 1960s. The Sabin OPV strains considered as the least neurovirulent based on studies in monkeys received approval for a US vaccine trial in 1960, and OPV became the preferred poliovirus vaccine of choice in the US [Citation32] and for most countries by 1963 [Citation33]. Over the subsequent decades, refinements of IPV and OPV vaccination formulations helped to increase vaccine safety and efficacy, but the fundamental principles of immunization against polioviruses remain the same [Citation34]. Global production, distribution, and use of OPV and IPV followed extensive efforts by manufacturers to meet demand largely driven by the 1988 global commitment to polio eradication [Citation35] and efforts of the Global Polio Eradication Initiative (GPEI) [Citation36–39].
Once individual countries, particularly relatively higher-income countries, achieved high immunization coverage with OPV, they successfully stopped indigenous transmission of WPVs [Citation32]. These countries began to only observe paralytic polio cases associated with OPV use (i.e., vaccine-associated paralytic polio or VAPP cases [Citation40]), which motivated them to switch from using OPV to using IPV for their national immunization programs [Citation32,Citation41,Citation42]. In some countries that eliminated the indigenous transmission of WPVs, the continued use of OPV with low coverage led to the observation of paralytic cases caused by vaccine-derived polioviruses (VDPVs) [Citation40,Citation43,Citation44]. The potential for OPV to spread secondarily, evolve into circulating VDPVs (cVDPVs) in populations with low coverage, and cause outbreaks like WPVs motivated a 2008 global commitment to coordinate cessation of all OPV use following successful eradication of all WPVs [Citation45]. Global health leaders certified the eradication of WPV2 in 2015 [Citation46] and WPV3 in 2019 [Citation47]. In mid-2016, the GPEI globally coordinated the cessation of all serotype 2 OPV (OPV2) use and recommended the introduction of a minimum of one dose of IPV into national immunization programs in all countries [Citation48]. Prior to OPV2 cessation, the GPEI developed a stockpile of monovalent OPV2 (mOPV2) for use in rapid outbreak response in the event of detection of continued serotype 2 live poliovirus transmission after OPV2 cessation [Citation49]. Unfortunately, OPV2 cessation did not lead to complete die out of serotype 2 live poliovirus transmission and the GPEI and countries continue to respond to cVDPV2 outbreaks and to develop contingency plans [Citation50–52].
The GPEI current strategic plan to manage cVDPV2 risks [Citation53] focuses on the development and deployment of a novel OPV (nOPV) for serotype 2 (i.e., nOPV2). Developers engineered the nOPVs to make the attenuated Sabin OPV backbone less likely to lose its attenuating mutations and thus less likely to revert to cVDPVs [Citation54]. Ideally, the nOPVs would retain the highly desirable ability of OPV to induce both mucosal and humoral immunity and to spread secondarily, but reduce the probability of genetic reversion to wild-type virus phenotype and the associated risks of VAPP and VDPVs [Citation55]. Although the public health benefits of such engineered vaccines compared to currently licensed OPV would appear minimal during a pandemic, nOPVs could represent a highly desirable option for management of nWPV as an endemic disease or during outbreak response. The relative benefits of nOPVs compared to OPV will depend on their actual properties when used in real populations, which remain uncertain [Citation54].
1.3. Likely development pathway of a vaccine if a wild poliovirus newly emerged in 2020
In contrast to the polio vaccine development timeline and milestones, shows how the approach and timeline for a COVID-19 vaccine fundamentally differed. This figure reflects the vastly different biomedical technologies available in 2020 compared to those available in the early to mid-20th century. Most importantly, approximately 1 month after the first report of an epidemic emerging in China, the identification of an RNA virus as the causative agent of COVID-19 received classification as a new member of the severe acute respiratory distress-related coronavirus (SARS-related CoV) species of the genus betacoronaviruses (β-CoV) [Citation56]. Shortly thereafter, molecular studies generated significant evidence about the molecular structure of the virus, and nearly two dozen vaccine candidates entered pre-clinical studies [Citation57]. In less than 12 months after identification of the virus, multiple vaccine candidates entered phase 3 clinical studies, and several vaccines started mass production following successful clinical trials [Citation58–61]. As of early 2021, multiple COVID-19 vaccines have been licensed for emergency use and countries continue to administer the vaccine doses as quickly as possible. We postulate that if polio emerged as a novel disease in 2020, researchers would pursue the same approach as was used for COVID-19 for a rational vaccine design [Citation62]. Notably, none of the first COVID-19 vaccines in use are live virus vaccines because modern vaccine development strategies favor new technologies. However, live virus vaccines with potential for secondary spread could prove highly desirable when rapid and large-scale vaccine deployment of vaccines would help to shut down transmission more quickly, as in the case of global response to an emerging pandemic. Of note, SARS vaccine development efforts were essentially aborted since the disease was eliminated through strict NPIs within a few months of emergence ().
Since the global experience with the COVID-19 pandemic demonstrates substantial vaccine research and development efforts undertaken for an emerging pathogen of global concern, we postulate that if a nWPV had emerged in 2020, the global commitment to develop a vaccine against nWPV would occur with similar magnitude and scale. Thus, we assume for this analysis that investments in vaccine development would lead to the licensed use of a poliovirus vaccine at some point in early 2021. For this analysis, we model the introduction of serotype-specific OPV, nOPV, or IPV assuming their prompt licensure for emergency use and sufficient production to allow for their broad distribution at different points in time after the isolation of the nWPV. The analysis provides some context for the potential benefits of introducing a vaccine to respond to a novel emerging pathogen with pandemic potential as a function of the NPIs used prior to vaccine introduction. Although we use a polio-specific model, we emphasize that this hypothetical analysis is not relevant to the current situation with polio or the efforts of the GPEI.
2. Methods
2.1. Global structure
We apply our existing global poliovirus transmission model [Citation63,Citation64], assuming nWPV emergence as a novel pathogen in 2020 in China, with no prior population immunity to polioviruses [Citation4]. The technical appendix provides details about the model (see Supplementary data and Tables A1a-A1h). For this analysis, we use the full global poliovirus transmission model structure [Citation63,Citation65], which includes a set of constant model inputs (Table A1a) and serotype-specific inputs for the 8 model immunity states (Table A1b). The model requires the use of different immunity states to capture the very different nature of the immunological protection induced by OPV versus IPV. Building on the prior analysis [Citation4], we demonstrate the expected health benefits of the new vaccines for the same four policy scenarios: (i) no response (NR), (ii) less strict flatten-the-curve (FTC1), (iii) more strict flatten-the-curve (FTC2), and (iv) contain and eradicate (C&E). Table A1e summarizes the model inputs used to characterize the effects of each of these restrictions or NPIs. Briefly, NR assumes no specific policy response to affect mixing of individuals, while C&E represents very strict and global action to limit mixing of individuals and exportation of the disease. FTC1 and FTC2 are intermediate scenarios that result in different levels of decrease in mixing and exportation (see additional detail elsewhere [Citation4]). We emphasize that for this analysis and the prior one [Citation4], the actual NPIs do not matter for this hypothetical analysis, because the scenarios modeled focus on bounding the overall space between doing nothing and acting as aggressively as possible. Since this analysis does not seek to characterize costs or effectiveness of NPIs or prioritize among them, we simply highlight that the use of different NPIs with different levels of effectiveness can affect the expected health benefits of vaccines. Consistent with the prior results, following the establishment of endemic transmission in a population, most subsequent cases will occur predominantly in relatively younger children as new birth cohorts increasingly account for the bulk of the remaining susceptible individuals.
The model structure stratifies the world into 72 blocks of 10 subpopulations each [Citation63]. Within each subpopulation, we assume people mix heterogeneously by age, but otherwise homogeneously within the population of approximately 10.7 million [Citation63]. The subpopulations correspond abstractly to countries or parts of countries, and we classify each block by a World Bank income level (i.e. low-income, LI; lower middle-income, LMI; upper middle-income, UMI; high-income, HI [Citation66]). We assume block- or subpopulation-specific dependent transmission model inputs (Table A1c) and some income level-dependent model inputs, including vaccine take rates (Table A1d) [Citation63].
2.2. Vaccine assumptions and vaccination scenarios
We assume the introduction of the poliovirus vaccine occurs 1 year after the isolation of the nWPV in HI and UMI blocks and we assume a 6-month delay to begin vaccination in LMI and LI blocks. We run scenarios that show the impacts of introducing a vaccine with the characteristics of OPV, ‘ideal’ nOPV as described elsewhere [Citation54], or IPV for each serotype. For this analysis, we assume the same initial secondary spread characteristics for both OPV and nOPV. However, ideal nOPV does not cause VAPP or lose its attenuating mutations, so it never increases in fitness or neurovirulence like OPV [Citation54]. We demonstrate the impacts of introducing these vaccines assuming coverage at the current DTP3 coverage levels [Citation67], initially assuming 1 dose of vaccine given in routine immunization (RI) at the age of 3 months and a one-time mass campaign (a catch-up supplementary immunization activity (SIA)) dose targeting all individuals older than 3 months that occurs over a period of 6 months from the start of the first RI. Table A1f summarizes the immunization inputs for each block and Tables A1g and A1h provide the associated SIA coverage by SIA impact level and assumed OPV take rates. The model varies the IPV take rates by income level and OPV take rates by population based on reviews of the available evidence [Citation65,Citation68].
For the OPV, nOPV, and IPV scenarios, we demonstrate the impacts of vaccinating all individuals within assumed age groups, although we recognize the possibility that serological testing would offer an option to vaccinate only individuals who remain susceptible at the time of vaccine introduction and that some individuals will refuse immunization. For IPV, we also explore the implications of longer delay in vaccine introduction (i.e., IPV late), which introduces IPV 2 years after the isolation of the nWPV in HI and UMI, followed by a 6-month delay to reach LMI and LI blocks.
We coded the model in the general-purpose programming language JAVATM in the integrated development environment EclipseTM. We perform 100 stochastic iterations for each scenario using the Amazon Elastic Compute Cloud (Amazon EC2). For each scenario, we report the probability of eradication, the expected value of the speed of global spread, and the expected cumulative incidence of paralytic cases over time.
3. Results
present the impacts of various vaccination strategies on the average speed of virus spread through the 720 model subpopulations and the growth of the expected cumulative global incidence of paralytic polio case for nWPV1, nWPV2, and nWPV3, respectively. In each figure, panels on the left represent the speed of virus spread, and the panels on the right present the cumulative incidence of paralytic polio. Each figure is further subdivided into 4 groups, representing 4 NPIs including (a) NR, (b) FTC1, (c) FTC2, and (d) C&E. reports the probability of eradication for each of the scenarios shown in . The results highlight several key differences between different poliovirus serotypes, due to differences in virus phenotype. For additional details about the expected cumulative incidence of paralytic cases and the number of affected subpopulations under each vaccination strategy and NPI by serotype see Table A2.
Figure 2. The average speed of nWPV1 spread through 720 subpopulations (left) and growth of expected cumulative global incidence of paralytic polio (right) over time as a function of interventions and method of vaccination
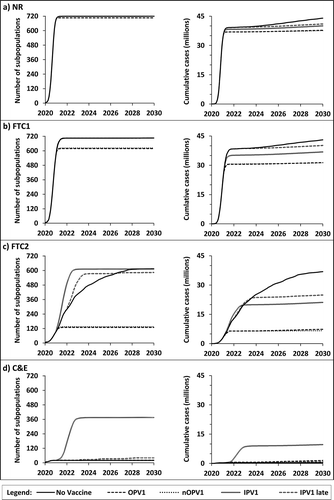
Figure 3. The average speed of nWPV2 spread through 720 subpopulations (left) and growth of expected cumulative global incidence of paralytic polio (right) over time as a function of interventions and method of vaccination
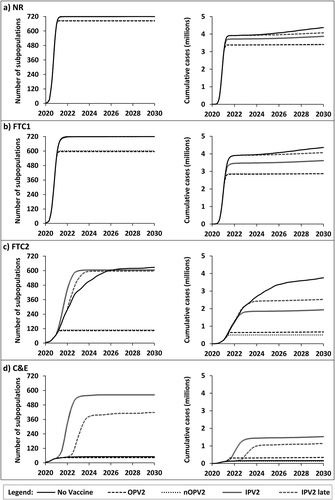
Figure 4. The average speed of nWPV3 spread through 720 subpopulations (left) and growth of expected cumulative global incidence of paralytic polio (right) over time as a function of interventions and method of vaccination
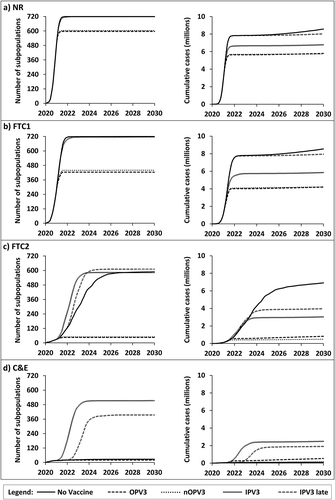
Table 1. Probability of eradication (%) for each nWPV serotype as a function of modeled scenarios for the period January 1, 2020 to January 1, 2030. Green boxes highlight probabilities of eradication higher than 95%. Yellow boxes highlight probabilities of eradication between 75% and 95%. Red boxes show probabilities of eradication below 20%
3.1. Effects of vaccine introduction on expected spread and cumulative incidence
Comparison of the results for highlight several common patterns in response to various vaccination strategies with significant health and policy implications for the model time horizon of 2020–2030. First, all serotypes and all scenarios studied show that the extent of NPIs and timing of vaccine introduction substantially affect the global, long-term outcomes of the emerging pandemic. Notably, in the absence of effective NPIs, as occurs with NR and FTC1, vaccines developed and deployed on a timeline similar to the current COVID-19 vaccine timelines do not increase the probability of eradication (), and only result in a modest decrease in global spread and the expected cumulative burden of the disease (, NR and FTC1 panels). This effect becomes more pronounced for the less infectious and less virulent nWPV3 () than the more infectious and more virulent nWPV1 () due to the relatively smaller proportion of individuals who remain susceptible at the time of vaccine introduction in . In addition, the live oral vaccines that come with the benefits of secondary spread (i.e. OPV and nOPV) will result in a modest reduction in the global spread of the disease only when vaccine introduction occurs before the virus reaches all 720 global subpopulations (e.g. compare with ). Again, this effect depends on the virus phenotype, with the largest impact seen with nWPV3 (e.g. compare with ). Perhaps the most important health impact of vaccines in the absence of effective NPIs would come from protection of vulnerable populations (primarily represented by the immunologically naïve newborns) after the disease establishes endemic transmission. Comparing the solid lines (no vaccine) and dashed lines (OPV) for the expected cumulative cases between 2024 and 2030 in panels a and b of shows the impact of the vaccines as and after the nWPV establishes endemic transmission.
Second, live attenuated viral vaccines that can spread secondarily as represented by OPV (, dashed lines) and nOPV (, dotted lines) consistently outperform the IPV scenarios. These results highlight the importance of vaccine coverage in managing an emerging infection, and contrast with current non-transmissible COVID-19 vaccine leading candidates. The results show the impact of delayed introduction of IPV introduction (i.e., double lines compared to dashed double lines) (), such that later vaccine introduction implies fewer susceptible individuals that may benefit from IPV. Both IPV vaccination strategies (double lines and dashed double lines) result in counterintuitive increases in global spread of the pandemic (panel d, ) and significantly reduced probability of eradication (). This model behavior occurs due to the model assumptions that NPIs stop at the time of vaccine introduction (Table A1e). The same behavior occurs with different dynamics for FTC2 scenarios in which the use of IPV results in acceleration of the spread of the virus during 2021 to 2024–2025 (panel c, left side, ), even though expected cumulative paralytic cases increase less because of the use of the vaccine (panel c, right side, ). These findings highlight an important public policy and behavioral issue. Specifically, if the availability and distribution of vaccines result in premature relaxation of the NPIs, the pandemic may paradoxically become worse. This may unintentionally result in loss of confidence in the vaccine and vaccination strategy, and lead to lowered acceptance of vaccines, because the increase in cases could look like ineffectiveness of the vaccine instead of increased risk of exposure due to the relaxed NPIs. Perhaps surprisingly, in the absence of effective NPIs (i.e. NR and FTC1 in ), vaccines play a less substantial expected role in protecting most of the population, although they will still serve to protect new birth cohorts and prevent substantial burdens of disease caused by endemic transmission in later years.
The third general insight relates to OPV and nOPV and builds on the history oral polio vaccine development and incidence of VAPP and cVDPV [Citation54]. For this analysis, which assumes that OPV and nOPV perform equally well in preventing the spread of polioviruses for all three serotypes, the results overlap in the left panels of (see dashed (OPV) and dotted (nOPV) lines). In contrast, FTC2 shown in panel c of shows much higher expected impact of oral vaccines in preventing the spread of the virus, because the introduction of OPV or nOPV in 2021 reduces the exportation of the nWPV to naïve populations, and substantially decreases the cumulative incidence of paralytic polio cases (right panels). However, the sustained use of OPV, but not nOPV, shows a subtle increase in the incidence of paralytic cases over time due to VAPP cases and in some cases reversion of OPV strains to cVDPVs, depending on the population transmission dynamics and coverage. Panel d of shows these effects.
3.2. Effects of vaccine introduction on the probability of eradication
Finally, the results show a substantial and arguably non-intuitive interplay between virus phenotype, NPIs, and vaccines in the overall probability of success in achieving eradication of the emerging nWPV during the model time horizon (). Importantly, most global policy actions, including significant flatten-the-curve strategies, as well as development and deployment of vaccines, result in less than a 1 in 5 chance of success in disease eradication (, light red boxes). Conversely, a high probability of success (≥95%) in disease eradication depends on global coordinated efforts to contain and eradicate an emerging pathogen through NPIs early on that limit population mixing and exportation for prolonged periods of time (, green boxes). While these may seem unrealistic, we note that countries such as China, Singapore, and New Zealand deployed these strategies in response to COVID-19 with reasonable success. However, in the absence of coordinated global action, such local or national efforts must continue in perpetuity to battle disease importation from other population centers. Eradication is an all or nothing game. Again, as noted earlier, vaccines may counterintuitively result in a reduction in the probability of success in disease eradication if their deployment coincides with relaxation of NPIs (, yellow boxes). Specifically, for the C&E NPIs, shows the decrease in the probability of eradication when introducing vaccines, which reflects the model assumptions of relaxation of the strict NPIs that would otherwise continue. The introduction of vaccines while maintaining the strict NPIs in these scenarios would result in unchanged or increased probabilities of eradication, although long-term use of OPV with low coverage would lead to ongoing observation of VAPP and potential VDPV cases.
Consideration of the impacts of vaccine introduction does not affect one of the key findings from modeling of NPIs [Citation4]. Specifically, rapid, decisive, and coordinated global action to limit mixing of individuals and to limit disease exportations represent the most effective strategies to contain and eradicate an emerging disease (, green boxes). Even with modern technology, vaccines require time to develop, produce, and administer, and thus would likely play a minor role in eradication of an emerging disease in the absence of strict NPIs. A false sense of reassurance in the form of relaxing NPIs with the availability of vaccines such as IPV may in fact result in a paradoxical increase in transmission and disease incidence (panel d, left side, ) and lower the probability of eradication (, yellow boxes). On the other hand, highly effective vaccines such as OPV and nOPV that result in rapid population immunity can substantially reduce disease transmission, morbidity, and mortality and increase the probability of eradication when strict NPIs fall short (e.g. FTC2 or C&E) (). For example, the probability of eradication for serotype 2 for FTC2 increases from a low of 13% without vaccination to a high of 84% after the introduction of OPV or nOPV. The introduction of vaccines will yield the greatest health benefits if introduced as early as possible such that they can potentially protect more susceptible individuals, and if NPIs remain in place during the administration of the initial vaccine doses.
For a virus with characteristics similar to WPV1 (Table A1), which would arguably present a more serious threat than COVID-19, virtually no probability exists of disease eradication within 10 years of emergence as a nWPV in 2020 without effective NPIs, and the probability of eradication remains very low with some NPIs even after vaccination (, NR and FTC1 columns). The chance of eradication increases slightly with a significantly less aggressive viral pathogen such as WPV3, but remains less than 10% even with a highly effective vaccine such as OPV as used in the model.
4. Discussion
The global experience with COVID-19, which stands in stark contrast with successful eradication of a similar emerging pathogen less than two decades ago (SARS), raises questions about the collective global ability to combat emerging pathogens. A companion study [Citation4] shows that in the absence of decisive and coordinated global action, an emerging nWPV would likely spread rapidly through the global population and become endemic, which we anticipate would lead to substantial costs to human health and economies. We see these findings again in the ‘No Vaccine’ results shown in and of this study. Notably, the introduction of vaccines as a tool in global efforts to combat an emerging hypothetical nWPV in an immunologically naïve population does not change the importance of using early and effective NPIs to stop and slow transmission. In contrast, highly effective and strict NPIs can lead to eradication of the emerging disease before it becomes established, and this eliminates demand and the market for any vaccines (as occurred with SARS). As a consequence of highly effective and coordinated multinational NPIs in 2003, development of a SARS vaccine did not progress into clinical trials despite favorable candidates due to no market demand.
Although our studies investigate the hypothetical emergence of polio as a novel disease in 2020, the global model and all input variables related to the viruses, vaccines, and vaccination strategies derive from decades of real-life experience supporting the GPEI and extensive use to support actual global policy decision making [Citation2,Citation3,Citation69]. Nonetheless, the model and insights remain limited by the assumptions, model structure, and finite number of stochastic iterations performed [Citation63]. In spite of these limitations, no other existing model for a vaccine-preventable disease exists with this level of maturity as a living policy model. As such, our insights could potentially inform the global policy decisions regarding COVID-19 vaccines and vaccination strategies and future discussions about the role of vaccination for emerging human diseases. No model can perfectly predict the actual course of events that would occur, which will depend on the choices, actions, and stochastic events that impact local transmission and the aggregation of these to the overall trajectory of the pandemic.
As in the reality with polio, the available polio vaccines could achieve eradication, but this depends on an effective vaccination strategy. Specifically, the GPEI certified eradication of endemic transmission of WPV2 and WPV3 by using a 3 or 4 dose childhood routine immunization schedule with OPV in most countries and by performing supplementary immunization activities (SIAs) with OPV that aimed to increase coverage in young children to increase the population immunity to transmission. Prior modeling demonstrates the dynamics of population immunity to transmission and die out [Citation70,Citation71]. The GPEI immunization strategy increased population immunity to levels high enough that the WPVs died out, such that as of 2020, all countries successfully interrupted indigenous transmission of all WPVs, except for Pakistan and Afghanistan where WPV1 transmission continues to date [Citation63]. Thus, the development of vaccine tools can support the eradication of some emerging and established diseases, but achieving eradication depends on the vaccine properties and coverage achieved with the specific vaccine strategies. Eradication also depends on overcoming the ‘weak links’ [Citation72], which continues to become increasingly difficult as the population and international travel increases substantially over time (). Arguably, the success of SARS control and eradication in 2003 and failure of COVID-19 control and eradication in 2020 are in part due to substantial growth in global population in the context of an even larger increase in travel, rendering SARS-like NPIs less effective in the battle against COVID-19.
With respect to insights relevant to COVID-19, these results suggest that the introduction of vaccines may help to control transmission, but not lead to eradication depending on the properties of the vaccines developed and the coverage achievable and achieved. Thus, the eradication of SARS-CoV-2, if even possible using the vaccine tools ultimately developed and distributed, may require immunization activities that go beyond the initial pandemic vaccine outbreak response plans. The motivation to even consider a commitment to eradicate SARS-CoV-2 may also not exist given its expected trajectory [Citation1]. The ability of some large countries to protect their populations from transmission (e.g., China) and prevent national establishment of the disease would substantially reduce the vaccine doses needed to achieve eradication. However, those countries that successfully prevent establishment or eliminate transmission will face continued pressures of importations until eradication, as currently occurs with polio and measles.
5. Conclusion
The role of vaccines introduced in response to a pandemic will depend not only on the properties of the vaccines and the coverage achieved, but also on the NPIs taken prior to vaccine introduction and during the vaccination campaign. Relaxation of NPIs prior to widespread administration of vaccines may lead to increases in transmission and cases shortly after vaccine introduction, which could negatively affect perceptions about the effectiveness of the vaccines.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
KMT, DAK, and KB conceived the study and contributed to the writing of the first draft and all subsequent revisions. KMT and DAK developed the model used. DAK performed all of the modeling. KB designed the figures. KMT acquired the funding for the study. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (583.8 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021 Jan 12;371(6530):741–745.
- Thompson KM, Kalkowska DA. Review of poliovirus modeling performed from 2000 to 2019 to support global polio eradication. Expert Rev Vaccines. 2020;19(7):661–686.
- Thompson KM, Kalkowska DA. Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Anal. 2020;41(2):229–247.
- Thompson KM, Kalkowsa DA, Badizadegan K. Hypothetical emergence of poliovirus in 2020: 1. consequences of policy decisions to respond using nonpharmaceutical interventions. Expert Rev Vaccines. 2021; doi:10.1080/14760584.2021.1891888 .
- Pourbohloul B, Meyers LA, Skowronski DM, et al. Modeling control strategies of respiratory pathogens. Emerg Infect Dis. 2005 Aug;11(8):1249–1256.
- Ferguson NM, Cummings DA, Fraser C, et al. Strategies for mitigating an influenza pandemic. Nature. 2006 Jul 27;442(7101):448–452.
- Germann TC, Kadau K, Longini IM Jr., et al. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006 Apr 11;103(15):5935–5940.
- Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci USA. 2008 Mar 25;105(12):4639–4644.
- Gumel AB, McCluskey CC, An WJ. SVEIR model for assessing potential impact of an imperfect anti-SARS vaccine. Math Biosci Eng. 2006 Jul;3(3):485–512.
- Podder CN, Gumel AB, Bowman CS, et al. Mathematical study of the impact of quarantine, isolation and vaccination in curtailing an epidemic. J Biol Syst. 2007;15(2):185–202.
- Institute of Medicine. Modeling community containment for pandemic influenza: A letter report Washington, DC: National Academies Press; 2006 [cited 2020 Nov 1]. Available from: https://www.nap.edu/catalog/11800/modeling-community-containment-for-pandemic-influenza-a-letter-report
- Centers for Disease Control and Prevention. Interim pre-pandemic planning guidance: community strategy for pandemic influenza mitigation in the United States: early, targeted, layered use of nonpharmaceutical interventions 2007 [cited 2020 Nov 1]. Available from: https://stacks.cdc.gov/view/cdc/11425
- World Health Organization. WHO global influenza preparedness plan: the role of WHO and recommendations for national measures before and during pandemics 2005 [cited 2020 Nov 12]. Available from: https://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5.pdf
- Plotkin SA. Vaccines for epidemic infections and the role of CEPI. Hum Vaccin Immunother. 2017 Dec 2;13(12):2755–2762.
- Moore S, Hill EM, Dyson L, et al. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. medRxiv. 2020.09.22.20194183; doi:10.1101/2020.09.22.20194183.
- Gallagher ME, Sieben AJ, Nelson KN, et al. Considering indirect benefits is critical when evaluating SARS-CoV-2 vaccine candidates. medRxiv. 2020:2020.08.07.20170456 2020; 10.1101/2020.08.07.20170456
- Makhoul M, Ayoub HH, Chemaitelly H, et al. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines (Basel). 2020 Nov 9;8(4):4.
- Bubar KM, Reinholt K, Kissler SM, et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021 Feb 26;371(6532):916–921. 10.1126/science.abe6959
- Oshinsky DM. Polio: an American Story. New York: Oxford University Press; 2005.
- Offit PA. The cutter incident: how America’s first polio vaccine led to the growing vaccine crisis. New Haven: Yale University Press; 2005.
- Roser M, Ritchie H, Ortiz-Ospina E. World population growth 2013 [ updated May 2019; cited 2020 Oct 16]. Available from: https://ourworldindata.org/world-population-growth
- Mazareanu J. Global air traffic - scheduled passengers 2004-2021 [updated Nov 2020; cited 2020 Nov 8]. Available from: https://www.statista.com/statistics/564717/airline-industry-passenger-traffic-globally/#statisticContainer
- Anon. World development indicators: air transport, passengers carried: the World Bank; 2020 [cited 2020 Nov 8]. Available from: https://data.worldbank.org/indicator/IS.AIR.PSGR?end=2018&name_desc=false&start=1970
- Bodian D. Neutralization of three immunological types of poliomyelitis virus by human gamma Globulin. Proc Soc Exp Biol Med. 1949 Oct;72(1):259–261.
- Bodian D, Morgan IM, Howe HA. Differentiation of types of poliomyelitis viruses; the grouping of 14 strains into three basic immunological types. Am J Hyg. 1949 Mar;49(2):234–245.
- Enders JF, Weller TH, Robbins FC. Cultivation of the lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949 Jan 28;109(2822):85–87.
- Salk JE, Youngner JS, Ward EN. Use of color change of phenol red as the indicator in titrating poliomyelitis virus or its antibody in a tissue-culture system. Am J Hyg. 1954 Sep;60(2):214–230.
- Meldrum M. ‘A calculated risk’: the salk polio vaccine field trials of 1954. BMJ. 1998 Oct 31;317(7167):1233–1236.
- Marks HM. The 1954 salk poliomyelitis vaccine field trial. Clin Trials. 2011 Apr;8(2):224–234.
- Anon. Polio and Eli Lilly and Company. Indiana historical society; 2012. [cited 2020 Nov 15]. Available from: https://www.indianahistory.org/wp-content/uploads/361af7a2729f0dceacf4e2d77469e737.pdf
- Koprowski H. First decade (1950–1960) of studies and trials with the polio vaccine. Biologicals. 2006;34(2):81–86. 2006/06/01/
- Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006;26(6):1423–1440.
- Sutter RW, Kew OM, Cochi SL, et al. Poliovirus vaccine – live. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Plotkin’s vaccines. Sixth ed, Philadelphia: Saunders Elsevier; 2017. p. 866–917.
- Bandyopadhyay AS, Garon J, Seib K. et al. Polio vaccination: past, present and future. Future Microbiol. 2015;10(5):791–808.
- World Health Assembly. Global eradication of poliomyelitis by the year 2000 (resolution 41.28) Geneva: world health organization; 1988 [cited 2019 Jun 4]. Available from: http://www.who.int/csr/ihr/polioresolution4128en.pdf
- World Health Organization. Global Polio Eradication Initiative: Strategic plan 2004-2008 Geneva 2004 [cited 2021 Feb 10]. Available from: https://apps.who.int/iris/handle/10665/42850
- World Health Organization. Global Polio Eradication Initiative - Strategic Plan 2010-2012 Geneva2010 [cited 2021 Feb 10]. Available from: https://apps.who.int/iris/handle/10665/70373
- World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame Strategic Plan (2013-2018) 2013 [cited 2019 Jun 4]. Available from: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf
- World Health Organization Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2019-2023) 2019 [cited 2019 Jun 4]. Available from: http://polioeradication.org/wp-content/uploads/2019/05/polio-endgame-strategy-2019-2023.pdf
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 2006;26(6):1471–1505.
- Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines. 2012 Apr;11(4):449–459.
- Thompson KM, Duintjer Tebbens RJ. National choices related to inactivated poliovirus vaccine, innovation, and the end game of global polio eradication. Expert Rev Vaccines. 2014;13(2):221–234.
- Kew OM, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359.
- Duintjer Tebbens RJ, Pallansch MA, Kim J-H, et al. Review: oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal. 2013;23(4):680–702.
- World Health Assembly. Poliomyelitis: mechanism for management of potential risks to eradication (resolution 61.1) Geneva: world Health Organization; 2008 [cited 2019 Jun 4]. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf
- World Health Organization. 14th meeting of the global commission for certification of poliomyelitis eradication (GCC), Bali, Indonesia, 20-21 September, 2015 Summary of findings, decisions and recommendations 2015 [cited 2020 Jun 1]. Available from: http://polioeradication.org/wp-content/uploads/2016/07/1Report.pdf
- World Health Organization. Report from the twentieth meeting of the global commission for certification of poliomyelitis eradication, Geneva, Switzerland, 17-18 October 2019 2019 [cited 2019 Dec 31]. Available from: http://polioeradication.org/wp-content/uploads/2016/07/20th-meeting-of-the-Global-Commission-for-the-Certification-of-Eradication-of-Poliomyelitis-17-18-October-2019.pdf
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - Worldwide, 2016. MMWR. 2016 September 9;65(35):934–938.
- Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Med J Med. 2008;10(8):190.
- Duintjer Tebbens RJ, Thompson KM. Poliovirus vaccination during the endgame: insights from integrated modeling. Expert Rev Vaccines. 2017;16(6):577–586.
- Duintjer Tebbens RJ, Thompson KM. Polio endgame risks and the possibility of restarting the use of oral poliovirus vaccine. Expert Rev Vaccines. 2018;17(8): 739–751. 2018/08/03
- Thompson KM, Kalkowska DA. Logistical challenges and assumptions for modeling the failure of global cessation of oral poliovirus vaccine (OPV). Expert Rev Vaccines. 2019;18(7): 725–736. 2019/07/03
- World Health Organization Global Polio Eradication Initiative. Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: Addendum to the Polio eradication and endgame strategic plan (2019-2023) 2020 [ cited 2020 Mar 10]. Available from: http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf
- Kalkowska DA, Pallansch MA, Wilkinson A, et al. Updated characterization of poliovirus outbreak response strategies for 2019-2029: impacts of the use of novel OPV2 strains. Risk Anal. 2021;41(2):329–348.
- Duintjer Tebbens RJ, Thompson KM. The potential benefits of a new poliovirus vaccine for long-term poliovirus risk management. Future Microbiol 2016;11(12):1549–1561.
- Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol 2020;5:536–544.
- Le T T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020 May;19(5):305–306.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416.
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111.
- Zimmer C, Corum J, Wee S-L. Coronavirus vaccine tracker: New York Times; 2020 [cited 2020 Nov 9]. Available from: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- Burton DR, Walker LM. Rational vaccine design in the time of COVID-19. Cell Host Microbe. 2020 May 13;27(5):695–698.
- Kalkowska DA, Wassilak SGF, Cochi SL, et al. Global transmission of live polioviruses: updated integrated dynamic modeling of the polio endgame. Risk Anal. 2021;41(2):248–265.
- Kalkowska DA, Pallansch MA, Cochi SL, et al. Updated characterization of post-OPV cessation risks: lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Anal. 2021;41(2):320–328.
- Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, et al. Characterizing poliovirus transmission and evolution: insights from modeling experiences with wild and vaccine-related polioviruses. Risk Anal. 2013;23(4):703–749.
- World Bank. World Bank list of economies (June 2019) 2019 [ cited 2019 Jul 17]. Available from: http://databank.worldbank.org/data/download/site-content/CLASS.xls
- World Health Organization. WHO/UNICEF estimated coverage time series: WHO and UNICEF; 2020 [cited 2020 Jul 24]. Available from: http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, et al. Preeradication vaccine policy options for poliovirus infection and disease control. Risk Anal. 2013;33(4):516–543.
- Thompson KM, Duintjer Tebbens RJ, Pallansch MA, et al. Polio eradicators use integrated analytical models to make better decisions. Interfaces. 2015 January-February;45(1):5–25.
- Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet. 2007 Apr 21;369(9570):1363–1371.
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, et al. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Anal. 2013;33(4):647–663.
- Barrett S. Polio eradication: strengthening the weakest links. Health Aff. 2009;28(4):1079–1090.

