ABSTRACT
Introduction
COVID-19 vaccines have been highly effective in reducing morbidity and mortality during the pandemic. However, the emergence of the Omicron variant and subvariants as the globally dominant strains have raised doubts about the effectiveness of currently available vaccines and prompted debate about potential future vaccination strategies.
Areas covered
Using the publicly available IVAC VIEW-hub platform, we reviewed 52 studies on vaccine effectiveness (VE) after booster vaccinations. VE were reported for SARS-CoV-2 symptomatic infection, severe disease and death and stratified by vaccine schedule and age. In addition, a non-systematic literature review of safety was performed to identify single or multi-country studies investigating adverse event rates for at least two of the currently available COVID-19 vaccines.
Expert opinion
Booster shots of the current COVID-19 vaccines provide consistently high protection against Omicron-related severe disease and death. Additionally, this protection appears to be conserved for at least 3 months, with a small but significant waning after that. The positive risk-benefit ratio of these vaccines is well established, giving us confidence to administer additional doses as required. Future vaccination strategies will likely include a combination of schedules based on risk profile, as overly frequent boosting may be neither beneficial nor sustainable for the general population.
1. Introduction
As of 29 August 2022, over 12.5 billion doses of COVID-19 vaccines have been administered worldwide [Citation1,Citation2] to more than 67% of the global population [Citation3]. Yet, over 585 million cases of SARS-CoV-2 infections have been reported since the start of the pandemic. Over 6.4 million COVID-related deaths have been recorded, but the World Health Organization (WHO) estimates that the real death toll associated directly or indirectly with the COVID-19 pandemic (‘excess deaths’) could be approximately 14.9 million [Citation4]. Between July 12 and August 9, over 28.9 million new cases and over 63,000 deaths were recorded globally [Citation1,Citation5], so much still needs to be done to contain the COVID-19 pandemic, particularly as only 20% of people in low-income countries have received at least one dose compared to more than 80% in upper-middle- and high-income countries [Citation3]. These inequities between lower and higher income countries leave many of the most vulnerable populations in low-income countries unprotected, and thus may contribute to prolonging the pandemic.
We previously analyzed epidemiological data from the International Vaccine Access Center [(IVAC; Johns Hopkins Bloomberg School of Public Health, United States (U.S.)] [Citation6] on the most used mRNA vaccines [BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and combinations of both), as well as vector vaccines [AZD1222 (ChAdOx1 nCoV-19; AstraZeneca)]. Our results showed a consistently high (>90%) and comparable level of protection against serious clinical outcomes for all the vaccines, including hospitalizations and deaths after primary schedule (two-dose) vaccination. This high vaccine effectiveness (VE) was maintained against the Delta (B.1.617.2) SARS-CoV-2 variant of concern (VoC) and remained high and comparable between vaccine types [Citation7]. However, the Omicron (B.1.1.529) VoC has now overtaken Delta and has quickly advanced from an emerging to a globally dominant strain [Citation8,Citation9], replete with sublineages (BA.1, BA.2, BA.2.12.1, BA.4, and BA.5). Cumulative data suggest that in vaccinated individuals, Omicron causes milder symptoms [Citation10–15] like headaches, runny nose, and fatigue [Citation16,Citation17]. One observational study reported 66% lower infection fatality rates with Omicron than with Delta [Citation18] (other studies reported similar findings – 0.27% vs. 1.07% [Citation19]), along with a 78.7% reduction in case fatality rates compared to previous variants [Citation20]. However, at many points during Omicron surges around the world, daily average deaths matched or surpassed the peaks reached by Delta [Citation21–26], and its infectivity [Citation21] and immune evasiveness [Citation27–30] continue to hamper efforts to halt the pandemic. While Omicron is assumed to have decreased clinical severity compared to earlier variants, higher vaccination coverage and increased exposure to natural infection will increase individual and community-level protection from disease. Thus, observations of reduced severity of Omicron-induced clinical illness may not be an accurate reflection of this virus. Recent accounts of significant mortality due to Omicron in elderly, under-vaccinated populations suggest that Omicron may not be significantly milder than previous variants [Citation31,Citation32], per se. Notably, as primary vaccination programs were implemented, waning VE [Citation8,Citation9,Citation33–39], particularly against mild or symptomatic infections, was reported alongside the emergence of Omicron [Citation10,Citation11,Citation40,Citation41] and suggested that vaccines were less impactful against virus transmission. This general and widespread perception of Omicron’s reduced disease severity and mortality may be contributing to a slowing pace of vaccination in many regions [Citation42–44]. Additionally, there are now sufficient data demonstrating that antibody neutralization activity is more reduced against Omicron than against previous strains [Citation45–48], and that Omicron sub-lineages have developed a significant capacity to escape immune protection conferred by vaccination [Citation49] and previous infection [Citation50,Citation51]. The speed at which these variants and sublineages have emerged should cause concern given that, even within its first week of emergence, 24 countries had detected Omicron [Citation52], including in previously infected or two-dose vaccinated individuals [Citation53]. Furthermore, low and unequal vaccine access produces an undesirable selective pressure that may drive the emergence of new variants. These factors alone can further strain healthcare systems and resources. For example, the initial surge of Omicron in December and January in the United Kingdom (UK) and U.S. overburdened healthcare systems and increased the risk of hospitalization and death for elderly, unvaccinated or comorbid individuals [Citation54–57]. The general public are now being warned that complacency in getting boosters risks prolonging or worsening the pandemic [Citation58–62].
Moreover, some confidence in COVID-19 vaccines has been eroded by misinterpretations of their safety and performance. Vaccine safety is a critical aspect of public acceptance, and ultimately the success of mass vaccination programs. While adverse reactions (ARs) and adverse events (AEs) can occur with all medical interventions, most are mild and self-limiting or resolve quickly. Like all vaccinations, COVID-19 vaccines are commonly associated with transient side effects like injection site pain and swelling, fevers, chills, fatigue, and headaches [Citation63–65]. They are also occasionally associated with rare or very rare serious side effects [Citation7,Citation66,Citation67] such as thrombosis, myositis, or allergic reactions related to vaccine components. Given the unprecedented scale of COVID-19 vaccination programs worldwide, rare ARs that would not have been detected in smaller-cohort randomized clinical trials have inevitably emerged. Decision-makers confronted with these challenges must consider the data on COVID-19 vaccine safety in the context of their associated benefits, and balance those against the risk of SARS-CoV-2 infections.
Given these issues, organizations like the U.S. Centers for Disease Control and the WHO started to provide guidance on booster vaccinations from as early as August 2021 [Citation68,Citation69]. Although less than 50% of Latin America and under 40% of the Asia Pacific region had received an additional dose (beyond that prescribed by vaccine manufacturer’s protocol) [Citation70] by 7 August 2022 [Citation71], over 2 billion additional doses have now been administered globally. However, booster uptake remains low and uneven in Asia and Latin America [Citation72,Citation73], as well as in the U.S. and Europe [Citation74–76].
When formulating future booster vaccination strategies, many factors need to be evaluated, including how quickly VE is waning, whether VE varies in subpopulations and risk groups, and how to balance the risks of COVID-19 complications, SARS-CoV-2 exposure, and SARS-CoV-2 variants. There is also a need to understand the safety of booster doses, and their effectiveness in reducing COVID-19 incidence, hospitalizations and/or deaths. In areas of low vaccine uptake or distribution, it is also critical to increase vaccine coverage, since the combination of high community transmission and low vaccination increases the risk of new variants emerging.
The updated WHO Global COVID-19 Vaccination Strategic Vision brief [Citation77] now recommends collectively pursuing an ‘all adults global vaccination goal with risk mitigation’ for 2022; specifically, the goals of reducing disease burden, limiting health system impacts and putting countries on a trajectory toward resuming socioeconomic activity. Establishing this as a collective, global goal, levels the playing field for all countries to advance together, leaving no country behind. This brief also proposes establishing risk mitigation strategies that secure the systems and investments necessary to reach these goals (e.g. should boosters be needed) or advance further (e.g. in younger age groups) if deemed necessary and based on robust, long-ranging scientific evidence. To this end, we sought to analyze the real-world effectiveness and safety data of the world’s most frequently used vaccines. By reviewing the real-world VE of COVID-19 vaccines against variants of concern to date, we hope to provide evidence to support timely, global, public health responses, policies and vaccination programs, particularly in low- and middle-income countries.
2. Methods
2.1. VE study selection
VE data were extracted from the IVAC VIEW-hub database (https://view-hub.org/covid-19/effectiveness-studies) [Citation6] up to the data-lock point of 30 June 2022.
As per the strict IVAC inclusion/exclusion criteria, only observational study effectiveness results were included if they appeared in at least a detailed report or preprint, and if the comparison group included concurrent individuals (no modeled or historic controls), laboratory-confirmed outcomes, a study design that accounted for confounding, self-reported vaccination status comprising no more than 10% of participants, reporting of confidence intervals, no significant bias, and unvaccinated controls (e.g. excluded if ‘unvaccinated’ included days 0–12 post-vaccination). Only studies comparing persons with and without the clinical outcome under investigation, and with and without vaccination, were included.
Only VE data of booster dose schedules against specific disease endpoints [symptomatic disease, severe disease, hospitalization, emergency department/intensive care unit (ICU)/hospital admission and death] were extracted. Effectiveness against the Omicron variant was confirmed if a variant in all cases contributed to an estimate, or the variant caused the majority of cases in a study cohort or population. (Supplementary Figure 1)
As few studies measured mortality as an endpoint, severe disease, hospitalization, ICU admission and death were combined into a composite ‘severe disease’ endpoint for the analysis.
One datapoint was omitted from the analysis due to suspicion of reporting error. One study showed a high level of VE (83.8%) during 0–1 week of booster dose, followed by a significant drop (32.7%) and increase (86.9%) during 2–4 weeks and 5–8 week respectively. This datapoint was excluded from the analysis due to contradiction with the natural process of immunity waning over time, suggesting a high probability of error in the datapoint [Citation78].
In general, a ‘booster dose’ refers to a third dose following a two-dose primary series. However, we do report VE estimate data including the Ad26.COV2.S vaccine (Janssen), either as a primary or booster vaccine are after 2 doses only. Our analysis also includes fourth-dose data extracted from the IVAC database. Note that all fourth-dose VE was estimated compared to a fully vaccinated and boosted population more than 3 months prior to receiving a fourth dose.
2.2. Data visualization and statistical analysis
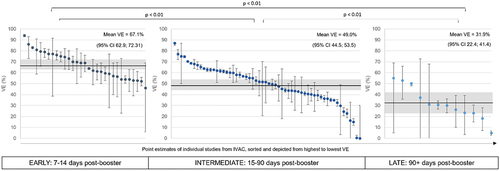
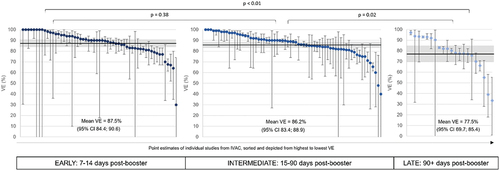
VE estimates were combined and stratified across time since booster dose, booster vaccine type, age, and prior SARS-CoV-2 infection. VE point-estimates are presented graphically from highest to lowest with respective confidence intervals. If a meaningful estimate of the time since vaccination was extracted, the potential waning of VE over time was assessed. Studies were stratified according to VE estimates recorded as ‘early’ (7–14 days post-booster dose), ‘intermediate’ (15–90 days post-booster dose) and ‘late’ (90+ days post-booster dose).
To allow discussions on vaccine and vaccine platform comparisons, means and confidence intervals were calculated for all figures. Statistical comparisons were conducted using independent t-tests or one-way ANOVA tests (for >2 group comparisons) with a significance level of 5%. Non-parametric tests (Mann-Whitney U and Kruskal-Wallis rank sum test) or Welch t-test were conducted when violations of normality or homogeneity of variance assumptions were detected. Weighted means were not calculated due to missing or incomplete sample size data for many studies. Analyses were done using R statistical software (Version 3.5.2).
2.3. Other relevant VE data
The IVAC dataset has several gaps in its VE data, including limited data on fourth-dose booster and in patients with comorbidities or who are immunocompromised. To enable discussion and recommendations around the highly relevant fourth dose, we addressed these gaps by additionally reviewing one of the only publicly available datasets at the time of publication (a preprint from Northern Thailand, led by one of this paper’s authors [Citation79]).
2.4. Safety data
The IVAC database does not currently include safety studies. To facilitate discussion of vaccine safety and vaccination strategies for immunocompromised patients, we also performed non-systematic literature reviews to collate relevant data published during 2021 and 2022. For safety analyses, any single or multi-country studies were considered only if they investigated AE rates for at least two of the currently available COVID-19 vaccines or vaccine platforms. Seven studies were selected for analysis.
3. Results
IVAC included 325 point-estimates of VE from 52 studies with data on post-booster dose VE against SARS-CoV-2 infection, COVID-19-related symptomatic infection, severe disease, hospitalization or death (Omicron variant only). Most of the studies were from North America, Europe and the United Kingdom where the mRNA vaccines were by far the most studied as a booster. Three studies were included from South America (all from Brazil) with no data from the Southeast Asia region available for inclusion ().
Table 1. Characteristics of studies included in our review.
3.1. VE against symptomatic infection
Our analysis confirms emerging real-world data suggesting that VE against symptomatic infection due to Omicron is lower for all COVID-19 vaccines than the Delta variant, even following a booster dose. VE estimates against Omicron-related symptomatic infection range between 0% and 94% with a mean VE of 52.2% (95%CI: 48.8; 56.3). Further stratification of VE point-estimates based on the time since booster dose indicates significant waning of VE within the first 3 months post-booster dose (). Stratification by booster vaccine type suggests that there is no significant difference in VE against symptomatic infection between mRNA vaccines (mRNA-1273, BNT162b2), the inactivated and viral vector vaccines [Ad26.COV2.S booster (p = 0.052), AZD1222 booster (p = 0.13) and CoronaVac booster (p = 0.13).] These findings were consistent with a Latin America-specific sub-analysis detailed in Supplementary Table 1.
3.2. VE against severe disease and death
VE against Omicron-related severe disease (hospitalizations and death) is high (mean VE of 85.4%, 95%CI: 83.3, 87.5) and is conserved for at least 3 months post-booster dose with mean VE of 87.5% (95%CI: 84.4, 90.6) for 7–14 days post-booster, and a mean VE of 86.2% (95%CI: 83.4, 88.9) for 15–90 days post-booster (). A small but significant waning of VE is seen more than 3 months post-booster compared to 7–14 (p < 0.01) and 15–90 days post-booster (p = 0.02). We found no significant difference in booster VE among people with and without prior SARS-CoV-2 infection (symptomatic disease: p = 0.16 and severe disease and death: p = 0.42). These findings were consistent with a Latin America-specific sub-analysis detailed in Supplementary Table 1.
3.3. VE against severe disease and death by vaccine schedule
VE against severe disease and death was comparable for both heterologous and homologous vaccine schedules amongst individuals who received a COVID-19 vaccine booster dose (p = 0.13). There were no statistically significant differences between a three-dose mRNA homologous schedule and a three-dose AZD1222 homologous schedule (p = 0.19) or a two-dose Ad26.COV2.S homologous schedule (p = 0.09) respectively. However, VE after a three-dose mRNA homologous schedule was significantly greater than a homologous three-dose CoronaVac schedule (p < 0.01). There were no statistically significant differences between any heterologous booster schedules (p = 0.19) ().
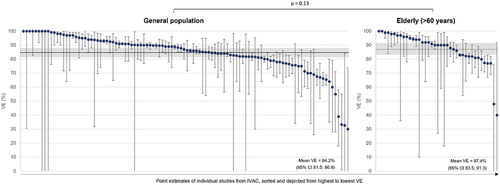
3.4. VE in elderly populations
In age-stratified analyses, there was no statistically significant difference in booster vaccine effectiveness against severe disease and death between the general population and the elderly (p = 0.13), indicating that VE is maintained against hospitalizations and death in the elderly, irrespective of booster vaccine (). In our stratifications, elderly is defined as those >60 years of age. The use of this cutoff is in part due to the availability and stratification of data within the IVAC dataset.
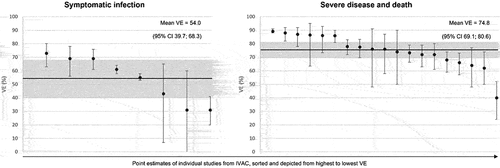
Mean incremental VE against symptomatic disease was 54% (95%:CI 39.7,68.3) after a fourth dose. Incremental VE against severe disease and death was 74.8% (95% CI: 69.1,80.6) (). All fourth-dose VE datapoints were evaluated in elderly populations relative to a fully vaccinated and boosted population more than 3 months prior to receiving the fourth dose, hence this is described as ‘incremental’ VE.
Mean VE and 95% Cis for all stratifications included is documented in Supplementary Table 1.
4. Expert opinion
4.1. Vaccine effectiveness against omicron VoC
4.1.1. VE against symptomatic infection
Our comprehensive analysis provides evidence that many of the currently used vaccines, even after 3 doses, provide limited and rapidly waning protection against SARS-CoV-2 infections caused by the Omicron variant and its sublineages, which is consistent with the published literature [Citation80–84]. Although no statistically significant difference in VE was seen between the different vaccine schedules, in absolute numerical terms, an mRNA booster dose did appear to confer a greater level of protection against infection than an inactivated or viral vector vaccine booster. However, the increasing and prevalent use of mRNA vaccines as boosters over inactivated and viral vector vaccines limits data on the latter and makes it difficult to make conclusions on their comparative efficacy.
4.1.2. VE against severe disease and death
Of greatest consequence to the public health and infectious disease community is our analysis showing that regardless of vaccine schedule, VE after a booster dose consistently prevented severe disease, hospital admission, and death. Crucially, VE was high and sustained against severe outcomes for all booster vaccines up to and beyond 90 days, with limited waning observed. With the exception of a three-dose CoronaVac schedule, that was associated with a lower VE compared to a three-dose mRNA homologous schedule, our analysis confirms that both homologous and heterologous schedules confer similar levels of protection, irrespective of vaccine platform.
Moreover, a previous SARS-CoV-2 infection was found to have no impact on VE. Based on the current immunological understanding of this virus, this Expert Working Group anticipated that the combination of a previous SARS-CoV-2 infection with a recommended vaccination course would confer some protective advantage from severe disease, but this was not observed in our analyses. We believe that there may be, in general, a bias or inflation of expected protection [Citation85–87] in both the study and control (unvaccinated) groups, rendering the vaccine advantage less perceptible.
4.1.3. VE against severe disease and death in the elderly
Encouragingly, our analyses show no decline in VE against severe disease and death in the elderly compared to the general population and indicate that vaccinations maintain a high level of protection in this risk group. In instances where vaccine performance in other immunocompromised populations is sought, data in the elderly may serve as an informative proxy. Further investigation of the studies included in our analyses is required to tease out reasons for the lack of decline in VE, given that immune responses in the elderly are expected to be less robust than in younger populations. However, this is not currently possible with the information available from IVAC.
4.1.4. Fourth-dose VE
Several studies have shown that the decline in two-dose mRNA vaccine VE [Citation40,Citation88] against Omicron could be increased or restored by a third vaccine dose. For example, the US VISION Network found that adults with a third dose had higher VE against hospitalizations during the Omicron surge than the Delta surge, even though VE waned with increasing post-vaccination duration [Citation88] and may have accelerated potentially because of immune escape [Citation89]. Importantly, the durability of VE was likely to be impacted by time from last inoculation [Citation90], given that a faster decline was observed with a third dose than a second dose of vaccine [Citation38,Citation91,Citation92], and that VE and duration of protection against Omicron significantly decreased at each month post-vaccination with a third dose of BNT162b2 [Citation90].
Currently, most studies on VE of booster doses are based on mRNA vaccines; data for inactivated or viral vector vaccines remain sparse [Citation93,Citation94]. Studies from Israel [Citation95–99] and Canada [Citation100] during an Omicron-dominant period found that the VE of a fourth-dose mRNA vaccine depended on the VE initially achieved by the three-dose schedule and the extent of waning that occurred thereafter. However, these studies were limited by a short follow-up of up to ten weeks after the fourth dose.
Recent studies from Chang Mai, Northern Thailand, not yet included in the IVAC database, present encouraging data on the VE of fourth-dose boosters [Citation79]. Importantly, protection against Omicron infection after a fourth dose improved substantially (adjusted VE 75%, 95%CI:71.00, 80.00), and the observed VE for infection during both Delta- and Omicron-dominant periods was consistent across age groups. After a third dose, the adjusted VE demonstrated an 89% reduction in risk of severe COVID-19 (HR:0.11; 95%CI: 0.07, 0.17) and mortality (HR:0.11, 95%CI: 0.06, 0.21), versus the unvaccinated group. Also, amongst patients given a fourth dose, no severe outcomes or deaths were observed after a median follow-up of 53 days (IQR 29–75) after the last vaccine dose. The protection offered against severe COVID-19 outcomes did not differ significantly across age groups and all three vaccine types used for boosting in Thailand (AZD1222, BNT162b2 and mRNA-1273). Comparing this to our VE estimates requires caution given the comparator group in the IVAC fourth-dose studies is a fully vaccinated group who received a third dose four months prior. Regardless, the data shows encouraging cumulative fourth-dose effectiveness, particularly in older individuals.
4.1.5. VE in special populations
To formulate the optimal vaccination strategy, decision-makers need sufficient data on VE in higher risk populations (e.g. the elderly, those with comorbidities, immunocompromised) that may need to be prioritized. One systematic review and meta-analysis [Citation101] of eleven vaccines (including mRNA-1273, BNT162b2, AZD1222, Ad26.COV2.S, CoronaVac, and BBIBP-CorV) found that VE was 59.7% (95%CI: 45.00, 70.40) in older adults. Another review [Citation102] of BNT162b2, mRNA-1273, Ad26.COV2.S and AZD1222 in immunocompromised groups found a wide range in VE – 64% to 90% against infection, 73% to 84% against symptomatic disease, 70% to 100% against severe disease, and 63% to 100% against hospitalizations. Of note, VE in immunocompromised populations was usually lower than in immunocompetent populations, and importantly, was not significantly influenced by age, vaccine type or time of evaluation, but varied considerably among immunocompromised patient subgroups [Citation103].
Many questions remain to be answered in these populations, such as whether poorer immune responses induce lower levels of antibodies, a less robust immune memory and a faster waning of protection. Moreover, if improvement in VE is only marginal, it is prudent to ascertain the number of booster shots necessary to maintain protection without being insufferable to these high-risk populations. The most effective alternative and adjuvant protection strategies for these populations also needs to be established. This Expert Working Group proposes that more studies in high-risk, comorbid, immunocompromised, and elderly populations, be performed as a priority, as these are the most vulnerable to severe outcomes, and as the pandemic evolves into an endemic situation.
Another group currently under-represented in global effectiveness and safety data are pediatric populations. The most interesting data missing for this group relates to waning of immunity and duration of protection, and addresses the issue of whether their levels of immunity persist for longer than that of the general population. To confidently propose regular vaccination strategies covering the whole population, more data will be needed in pediatric and adolescent populations.
4.1.6. Studies reporting markedly low VE against severe disease and death
This Expert review of IVAC data raised concerns over several studies reporting markedly lower VE against severe disease and death. An additional review of outlier datapoints with VE against severe outcomes less than 60% were conducted. Three of the six outlier datapoints had a sample size of less than 20, suggesting that results are likely distorted by random error [Citation104,Citation105]. In Denmark, a significantly lower third-dose VE was found in two studies of the general population compared to the elderly. While the discrepancy could be attributed to differences in behaviors, it is more plausible that low VE was caused by selection bias in adults who were first to receive the third-dose vaccine. Immunocompromised individuals were among the first to receive third-dose vaccines in August 2021, followed by the elderly and healthcare workers in October 2021. Therefore, immunocompromised individuals or healthcare workers may be potentially over-represented among those who received third-dose vaccines during the study period (December 2021 to March 2022), as both are at higher risk of severe COVID-19 outcomes. It is thus possible that the true VE among those aged 18–49 years is underestimated due to an existing selection bias [Citation106]. We also included one test-negative study that was conducted during an Omicron peak in the United States [Citation107], but a test-negative design can be prone to bias during an outbreak peak due to differential recruitment of self-reporting individuals and disease contact-tracing [Citation108]. In this setting, people who are more likely to get vaccinated and tested are also more likely to access healthcare systems. Thus, VE could be underestimated due to over-representation of vaccinated individuals with positive COVID-19 tests [Citation109].
4.1.7. Limitations of this study
Our study has some limitations. First, our analyses included a few datapoints from the same study. Although this scenario occurred in <5% of our datapoints, it is worth noting that the assumption of independent cannot be guaranteed for these datapoints. Second, our analyses included 5 types of COVID-19 vaccines. While there are currently 10 types of COVID-19 vaccines approved by the WHO, no evidence on other types of COVID-19 vaccines were available. Third, most studies did not specify whether omicron cases were confirmed genotypically. Hence, vaccine effectiveness may be under or overestimated following the assumption that all cases were Omicron cases during the Omicron waves. Fourth, age groups were not clearly defined in the IVAC dataset which added difficulties in age stratifications of our analyses.
4.2. Safety of available COVID-19 vaccines
Vaccine clinical trials enroll fewer individuals than are seen in mass vaccination programs, so rare side effects may only be observed in the latter scenario. For example, vaccination programs have reported anaphylaxis (at an unknown frequency, although documented across many other types of vaccines) [Citation110–112], capillary leak [Citation113] syndrome (extremely rare), myocarditis or pericarditis [Citation114] and thromboembolic events with concurrent thrombocytopenia (very rare) [Citation115]. Rather than attempt to reconcile the risks associated with one vaccine for a specific adverse event, and compare to another vaccine for different events, we reviewed studies evaluating multiple COVID-19 vaccines against comparable endpoints. Data for key AEs are available from seven studies. The UK COV-BOOST [Citation116] evaluated the safety and immunogenicity of seven COVID-19 vaccines as a booster dose after a primary series of AZD1222 or BNT162b2 in 2,878 adults in the UK. While this small study could not capture rare events, it provided data on the rate of common AEs according to priming and booster schedules compared to a meningococcal control. Overall, approximately 5% of participants reported AEs, with a slight increase in AEs for BNT162b2 prime-AZD1222 boost or BNT162b2 prime-Ad26.COV2.S boost recipients, as well as in recipients of AZD1222 prime-mRNA-1273 boost, AZD1222 prime-CoronaVac boost, BNT162b2 prime-mRNA-1273-boost or BNT162b2 prime-CoronaVac boost. This study suggests that AEs and ARs were slightly more frequent with some vaccines used in heterologous boosting than in homologous boosting. Notably, BNT162b2 prime-mRNA-1273 boost gave slightly higher (though mainly local and systemic) adverse responses. General AEs were slightly higher for heterologous schedules than homologous schedules [Citation116,Citation117].
Very recently, Patone et al [Citation118] assessed the risks of more severe events associated with COVID-19 vaccination or SARS-CoV-2 infection in over 37 million UK adults; specifically, myocarditis, pericarditis, and cardiac arrhythmias. Myocarditis risk was elevated by two events per million people within the first seven days of a first dose of AZD1222, or by one event per million people with a second dose of BNT162b2, or by six events per million people with both doses of mRNA-1273. In contrast to all of these vaccinations, there was a significantly increased risk of myocarditis for one month post-SARS-CoV-2 infection. The risk of pericarditis did not appear to be elevated in this analysis, although it was increased during the first two weeks post-SARS-CoV-2 infection. While the cardiac arrhythmia risk was increased in the first seven days of a second dose of mRNA-1273, this risk was elevated for over a month post-SARS-CoV-2 infection. In addition, in the first 28 days after a second vaccine dose, only mRNA-1273 produced a small excess risk of myocarditis (ten events per million vaccinated individuals); however, stratification for younger age also uncovered a small excess risk of myocarditis with BNT162b2. Interestingly, the second dose of mRNA-1273 was associated with a higher risk of myocarditis than SARS-CoV-2 infection. Excess event risks for pericarditis and myocarditis were not reported for other vaccines.
Whiteley et al [Citation119] also evaluated major venous, arterial or thrombocytopenic events associated with AZD1222 and BNT162b2 in over 46 million individuals in the UK. The hazard ratios for most venous, arterial and thrombocytopenia events were lower in most vaccinated individuals (than in baseline cohorts), but the risk of intracranial venous events was slightly elevated for AZD1222, and the risk of thrombocytopenia and related events was slightly elevated for AZD1222 in those younger than 70 years. The adjusted HRs (aHRs) for AZD1222-vaccinated or unvaccinated individuals younger than 70 years and individuals older than 70 years were 0.90 and 0.76 for arterial thromboses, and 0.97 and 0.58 for venous thromboses, respectively. Correspondingly, the aHRs for BNT162b2 were 0.94 and 0.72 for arterial thromboses, and 0.81 and 0.57 for venous thromboses, respectively. There was no added risk of stroke events in this age group. Notably, any increase in high-risk events never exceeded a two-fold level, representing a small, elevated risk in this large population study. In those older than 70 years, COVID-19 vaccinations generally reduced the risk of embolic events or stroke, compared to baseline.
Burn et al [Citation120,Citation121] evaluated the incidence of thrombosis and thrombocytopenia after SARS-CoV-2 infection or vaccination with AZD1222 and BNT162b2 in two European Medicines Agency-funded studies involving over 6.1 million individuals in the UK and over 6.9 million individuals in Spain. In the UK, the overall standardized incidence rates of arterial and venous thromboembolism (ATE and VTE) were similar between AZD1222 and BNT162b2 and comparable to the background rate: 1.0 to 8.5 events per 100,000 person-years for deep vein thrombosis (DVT) with thrombocytopenia, 0.5 to 20.8 for pulmonary embolism (PE) with thrombocytopenia, 0.1 to 2.5 for splanchnic vein thrombosis with thrombocytopenia, and 1.0 to 43.4 for myocardial infarction or ischemic stroke with thrombocytopenia. While a potential safety signal for PE was identified for both vaccines, this only represented a 1.2-fold higher incidence of post-vaccination PE than the background, while SARS-CoV-2 infections increased this incidence rate by 15-fold over the background. In younger males, AZD1222 slightly increased the risks of DVT and VTE. Both vaccines were associated with a small, elevated risk of immune thrombocytopenia (two-fold or less) but again, this risk was greater in individuals experiencing a SARS-CoV-2 infection. Very small increases in the number of cases versus expected were seen for cerebral venous sinus thrombosis (with an incidence of 0.1 per 100,000 person-years) and stroke with thrombocytopenia in 1.8 million people vaccinated with AZD1222 in a secondary analysis, but these were not significantly higher than background. The standardized incidence rates in Spain were similar to that in the UK – for example, 1.29 and 0.90 for VTE after first- and second-dose BNT162b2, and 1.15 after first-dose AZD1222; 1.35 and 1.19 for thrombocytopenia after first- and second-dose BNT162b2, and 1.03 after first-dose AZD1222. Similar safety profiles were also observed for both vaccines – for example, the rate of most events (DVT, PE, VTE, immune thrombocytopenia, VTE with thrombocytopenia) was far higher in individuals with SARS-CoV-2 infections. In the Spanish cohort, BNT162b2 was associated with slightly higher-than-expected rates of DVT, PE and VTE after a first dose and of thrombocytopenia after either dose. Neither vaccine increased the risk of thrombosis with thrombocytopenia syndrome.
Rahman et al [Citation122] reported AEs in case-based safety monitoring after AZD1222, CoronaVac, and BNT162b2, in over 20 million individuals in Malaysia. This report is relevant for many low- to middle-income countries due to its comprehensive study of CoronaVac in a large population, and its focus on severe AEs requiring hospitalization in individuals who received at least one dose of vaccine (although dose number stratification was not performed). Among all vaccines, a higher age-standardized absolute event rate was observed with BNT162b2. However, CoronaVac appeared to cause higher absolute rates for many events than AZD1222, although inactivated vaccines are widely perceived to have a better safety profile [Citation123]. Older BNT162b2 recipients had an unexpectedly higher rate of myocarditis than younger recipients (2.9 events with BNT162b2, 0.3 events with CoronaVac and 1.9 events with AZD1222 in those aged 40 to 59 years per million doses, versus 1.2, 1.2 and none, respectively, in those aged 18–29 years), and a thrombocytopenia rate twice that of UK populations in other studies (8 cases after BNT162b2 versus 4 cases with other vaccines). Incidence rate ratios (IRR) showed no significantly increased risk for myocarditis/pericarditis, Bell’s palsy, stroke, and myocardial infarction in the 21 days following either vaccine dose, in all vaccine platforms. A slight elevation in IRRs was observed for BNT162b2 recipients in the risk of venous thromboembolism, arrhythmia, and convulsion/seizure while CoronaVac vaccine recipients showed slightly increased IRR for arrhythmia overall, and slightly elevated risks for myocardial infarction, arrhythmia and hemorrhagic stroke after the first dose. Similarly, AZD1222 showed elevated IRRs for thrombocytopenia and venous thromboembolism, but the published confidence intervals were wide due to a small number of events in this group.
Finally, a recent European multi-country study evaluated 29 adverse events of special interest in a large cohort study of over 25 million subjects. After adjustment for factors associated with severe COVID-19, 10 statistically significant associations of pooled IRRs remained based on dose 1 and 2 combined [Citation124]. Anaphylaxis was observed after AZD1222, TTS after both AZD1222 and Ad26.COV2.S, erythema multiforme after mRNA-1273, Guillain-Barré syndrome and single organ cutaneous vasculitis after Ad26.COV2.S, thrombocytopenia after Ad26.COV2.S and mRNA-1273, and VTE after mRNA-1273 and BNT1262b2. Importantly, the pooled rate ratio remained less than three-fold increased for all events with all vaccines except for Ad26.COV2.S. A comparative table of AE incidence rates reported in these studies is included as Supplementary Table 2.
Our analyses were limited by a lack of data on booster dose safety. With Omicron surges resulting in calls for further booster doses, it remains to be seen if AEs will increase correspondingly. Regardless, these studies reinforce the overall, relative safety of the current vaccines in use globally. While the overall safety profiles are largely similar based on studies that evaluate multiple vaccines for similar events, certain safety signals should continue to be anticipated, like myocarditis in younger recipients. These remain relatively rare, not severe, can be managed clinically, and have a good prognosis. Similarly, signals like thrombocytopenia are rare or very rare, are more severe, and should also be anticipated. Appropriate training and management would thus be important to ensure better outcomes. In environments where vaccine choice exists, the effectiveness of mRNA and viral vector vaccines in both primary and booster schedules appears very similar, so safety profiles will continue to be an important factor in vaccine selection.
4.3. Additional population-wide strategies for future proofing against SARS-CoV-2 infection
As governments seek to transition to ‘living with COVID-19,’ the choice of vaccination strategy will depend on a country’s income level classification. Equitable access to vaccines across the four dimensions of production, affordability, allocation and deployment, is a crucial deciding factor for low-to-middle-income countries (LMIC). For example, cold-chain transport and storage of vaccines may be prioritized during vaccine choice decisions in many LMICs (as represented by this paper’s authorship) with sizable rural or geographically disparate communities [Citation125]. Cost-effectiveness of the most commonly used vaccines has been demonstrated via several national modeling studies [Citation126,Citation127]. However, cost-effectiveness comparisons between vaccines are complicated by the not-for-profit provision of many vaccines to LMICs. As we transition into an endemic status, costs will become a key consideration for stakeholders seeking to maintain vaccination programs and manage changes in funding models. Beyond these considerations, broad vaccine coverage is further hampered by vaccine hesitancy, ‘pandemic fatigue’ [Citation128,Citation129] and general complacency, possibly due to the misperception that Omicron is a less harmful variant [Citation18]. Vaccination challenges will be compounded if governments shift the cost of COVID-19 vaccines to individuals, thus underscoring a need to understand the obstacles to vaccine uptake, and devise solutions to overcome these.
Although the IVAC database contains few studies from LATAM and Africa, our findings and conclusions include studies from many LMIC, and accurately reflect our COVID-19 vaccination challenges. In the absence of this analysis, we have chosen to briefly detail our obstacles to achieving herd immunity to SARS-CoV-2 in our regions. We have consistently and substantially higher vaccine acceptance [Citation130–132] than in countries like Russia and the United States, and lower vaccine hesitancy than in higher income countries [Citation133]. Yet, vaccine hesitancy in LMIC is driven by worries about vaccine side effects and adverse events. It is thus still necessary to persist with efforts to increase confidence and trust in vaccines however appropriate or necessary, e.g. through healthcare providers or even celebrities [Citation130]. Other countries with existing infrastructure for childhood immunizations (e.g. Bangladesh and Ghana [Citation134]), require assistance or funding to improve or adapt these to facilitate large-scale, population-wide COVID-19 vaccination rollouts. The challenge of equitable vaccine distribution must be addressed to help LMIC push quickly toward a collective immunity. In LATAM, Argentina, Bolivia, Brazil, Chile, Peru and Uruguay have the requisite two-dose vaccine quantities needed to immunize their populations [Citation135], but Honduras, Nicaragua, and Venezuela remain unable to provide their entire population with a first vaccine dose. Many others struggle with vaccine hesitancy, insufficient distribution and storage, and issues with local vaccine manufacturing or distribution [Citation6,Citation136,Citation137]. Incorporating a heterologous dose into primary vaccination schedules to mitigate against delayed vaccine supplies may be a prudent measure in these countries.
Finally, while Novavax, Sputnik and CanSino were not included in our study, these vaccines are also in use in worldwide, including in LMIC. In the UK, Novavax’s recombinant spike glycoprotein nanoparticle vaccine (NVX‐CoV2373/Covovax) [Citation138] produced a VE of 95.6% or 85.6% against a non-VOC or VOC, respectively, while in South Africa, Novavax had a VE of 60%[Citation139]. The heterologous recombinant adenovirus (rAd)-based Gam-COVID-Vac vaccine (Sputnik V), also gave robust and reliable protection in all age groups in a phase III trial[Citation140], with an efficacy of 91.6%. CanSino Biologics’ single-shot, virus-vector Ad5-nCoV vaccine [Citation141] has also effectively prevented symptomatic disease with 65.7% VE in phase III trials in Pakistan. Where equitable and ready access to COVID-19 vaccines remains difficult, these vaccines will be an important contributor to achieving herd immunity against SARS-CoV-2.
5. Conclusion – future vaccine strategy
The results of our analysis suggest that the most effective way to achieve national vaccination coverage targets, particularly in resource-limited settings, would be to consider booster vaccines which have good safety and comparable effectiveness profiles against severe outcomes. The consistency of VE of current vaccines for severe disease against all variants, including Omicron, suggests a limited requirement for variant-specific vaccines at this time. Given the rate of mutation of the virus, pursuing variant or subvariant-specific vaccine development may distract from the immediate needs. Resources should instead be diverted toward improving coverage and access to currently available COVID-19 vaccines and challenging hesitancy and complacency.
This review reinforces the value of real-world evidence to support efforts advocating for the completion of primary series and booster vaccinations where appropriate, especially to restore waning VE against the more infectious Omicron variant and protect populations from severe outcomes, hospital admissions, and longer lasting post-COVID-19 complications, as well as mortality. Whilst acknowledging the limitations of our analysis, based on these findings and our own clinical experience, it is the opinion of this Expert Working Group that future vaccination strategies could incorporate annual booster shots for the general population alongside evaluation of the incremental benefits of the next generation of vaccines. Vaccination programs could include more regular, shorter interval, 4-6-monthly booster shots based on risk i.e. those who are elderly, and/or with comorbid conditions that increase the risk associated with a SARS-CoV-2 infection, as well as the use of a three-dose primary series to prolong the duration of protection, although data to support these recommendations is still lacking. The notion of more regular boosting than this would likely be neither beneficial nor sustainable for the general population. As we face up to an endemic COVID-19 future, important considerations must be given to optimizing protection in high-risk and immunocompromised populations that either respond poorly to vaccines or cannot be vaccinated.
Article highlights
All COVID-19 vaccines have a lower VE against symptomatic infection due to Omicron than to the Delta variant, even following a booster dose – VE significantly wanes within the first 3 months post-booster dose.
Regardless of vaccine schedule, VE after a booster dose consistently prevented severe disease, hospital admission and death. VE against Omicron-related severe disease and death is high (85.4%) and is conserved for at least 3 months post-booster dose with a mean VE of 87.5% for 7-14 days post-booster, and a mean VE of 86.2% for 15-90 days post-booster. A small but significant waning of VE is seen more than 3 months post-booster compared to more than 7-14 and 15-90-days post-booster.
We found no significant difference in booster VE among people with and without prior SARS-CoV-2 infection or between the general population and the elderly.
VE against severe disease and death was comparable for both heterologous and homologous vaccine schedules amongst individuals who received a COVID-19 vaccine booster dose.
There were no statistically significant differences between a three-dose mRNA homologous schedule and a three-dose AZD1222 homologous schedule or a two-dose Ad26.COV2.S homologous schedule.
More studies in high-risk, comorbid, immunocompromised, and elderly populations, should be performed as a priority, as these are the most vulnerable to severe outcomes, particularly as the pandemic evolves into an endemic situation.
Large population-level safety studies reinforce the overall, relative safety of the current vaccines in use globally, and emphasize the favorable risk-benefit profiles of all of these vaccines.
Future vaccination strategies could incorporate annual booster shots for the general population alongside evaluation of the incremental benefits of the next generation of vaccines.
Vaccination programs could include more regular, shorter interval, 4-6 monthly booster shots based on risk i.e. those who are elderly, and/or with comorbid conditions that increase the risk associated with a SARS-CoV-2 infection, as well as the use of a three-dose primary series to prolong the duration of protection, although data to support these recommendations is still lacking.
Declaration of Interest
Following International Committee of Medical Journal Editors’ (ICMJE) guidelines, A Ong-Lim reports honoraria for lectures from Moderna and is a member of the Technical Advisory Group, Department of Health in the Philippines. C Alvarez-Moreno has received a grant from World Health Organization (WHO) to conduct the Solidarity Vaccine Trial in Colombia, he participates on the Colombian Data Safety Monitoring Board, and he reports receiving honoraria for lectures from Pfizer and AstraZeneca. C Huu Nghia, KP Hwang, R Solante, AJ. Rodriguez-Morales and S Chariyalertsak report consulting fees from AstraZeneca. J Ortiz Ibarra reports receiving consulting fees, meeting attendance support and speaker honoraria from AstraZeneca and meeting attendance support from Pfizer. D Do-Van reports consulting fees and honoraria for advisory board attendance by AstraZeneca. NC Chiu reports consulting fees from AstraZeneca and honoraria for scientific meeting travel and lecture from multiple companies. He is also a member of the Taiwan Vaccine Injury Compensation Program and Pediatric Infectious Disease Society of Taiwan. PI Lee reports grants from the Taiwan Center for Disease Control for COVID-19 vaccine immunogenicity studies, consulting fees from AstraZeneca, Merck Sharp & Dohme (MSD), and GlaxoSmithKline and payment for lectures from MSD. He also serves as the Chair, Advisory Committee on Immunization Practice in Taiwan. Prasad S. Kulkarni is employed by Serum Institute of India Pvt Ltd which manufactures a COVID-19 vaccine (Covishield) that is sub-licensed from AstraZeneca. R Crisenio Lobo reports consulting fees from AstraZeneca and honoraria for lectures from Menarini Philippines, Nestle, Mead Johnson, and Novartis. He is also the vice-chair of the National Adverse Events Following Immunization committee in the Philippines. S Kiertiburanakul reports consulting fees from AstraZeneca and honoraria for lectures from AstraZeneca, Pfizer and Zuellig Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (101.4 KB)Acknowledgments
The authors wish to thank Shawna Tan of Medical Writers Asia for manuscript writing and Glen Halliwell and Sharon Pang of GCI Health for analysis of IVAC study and data visualizations.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2143347
Additional information
Funding
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 2020;20(5):533–534.
- WHO Coronavirus. (COVID-19) dashboard [cited 2022 Aug 7].https://covid19.who.int/?adgroupsurvey={adgroupsurvey}.
- Coronavirus (COVID-19) Vaccinations. ourworldindata.org. https://ourworldindata.org/covid-vaccinations. cited 2022 Aug 7.
- 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. World Health Organisation. https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021. cited 2022 Aug 29.
- COVID-19 Dashboard. Johns Hopkins University Coronavirus Resource Center. [cited 2022 Aug 29] https://coronavirus.jhu.edu/map.html
- VIEW-Hub. [cited 2022 Aug 29] https://view-hub.org/covid-19/effectiveness-studies
- Chuenkitmongkol S, Solante R, Burhan E, et al. Expert review on global real-world vaccine effectiveness against SARS-CoV-2 [published online ahead of print. Expert Rev Vaccines. 2022 Jun 30;1–14. 10.1080/14760584.2022.2092472.
- Horne EMF, Hulme WJ, Keogh RH, et al. Waning effectiveness of BNT162b2 and ChAdOx1 covid-19 vaccines over six months since second dose: openSAFELY cohort study using linked electronic health records. BMJ. 2022;378:e071249.
- Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021 cited 2021 Aug 21; Preprint. 10.1101/2021.08.06.21261707
- Hyams C, Challen R, Marlow R, et al. Severity of Omicron (B.1.1.529) and Delta (B.1.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study. 2022 Jul 07.
- Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761.
- Bager P, Wohlfahrt J, Fonager J, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. Lancet Infect Dis. 2021;21(11):1507–1517.
- Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;39911303–1312.
- Strasser Z, Hadavand A, Murphy S, et al. SARS-CoV-2 omicron variant is as deadly as previous waves after adjusting for vaccinations, demographics, and comorbidities. Res Square. 2022.
- Cuadros DF, Moreno CM, Musuka G, et al. Association between vaccination coverage disparity and the dynamics of the COVID-19 delta and omicron waves in the US. Front Med (Lausanne). 2022;9:898101. cited 2022 Jun 14.
- Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22(7):1002–1010.
- Spector T. What are the symptoms of omicron? 13 Jan 2022. [cited 2022 Aug 10] https://www1.racgp.org.au/newsgp/clinical/what-are-the-symptoms-of-omicron
- Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2. Retrospective Cohort Study BMJ. 2022;378:e070695
- Larrauri B, Malbrán A, Larrauri JA. Omicron and vaccines: an analysis on the decline in COVID-19 mortality. medRxiv. 2022. 10.1101/2022.05.20.222753966
- Liu Y, Yu Y, Zhao Y, et al. Reduction in the infection fatality rate of Omicron variant compared with previous variants in South Africa. Int J Infect Dis. 2022;120:146–149.
- Asia’s outbreaks show that Omicron is deadly in unvaccinated people. The Economist. [cited 2022 Aug 10] https://www.economist.com/graphic-detail/2022/04/09/asias-outbreaks-show-that-omicron-is-deadly-in-unvaccinated-people
- Kamp J, Onque R, Stancati M. Omicron deaths in U.S. exceed delta’s peak as covid-19 optimism rises in Europe [cited 22 Aug 10] https://www.wsj.com/articles/omicron-deaths-in-u-s-exceed-deltas-peak-as-covid-19-optimism-rises-in-europe-11643201653
- Fenit Nirappil F, Keating K. Covid deaths highest in a year as omicron targets the unvaccinated and elderly. [cited 22 Aug 10] https://www.washingtonpost.com/health/2022/02/08/omicron-deaths-covid/
- Shelton J. Omicron variant caused more excess deaths in massachusetts than delta. [cited 22 Aug 10] https://news.yale.edu/2022/05/20/omicron-variant-caused-more-excess-deaths-massachusetts-delta
- COVID-19 mortality in Australia, deaths registered to 2022 Jan 31. Australian Bureau of Statistics. https://www.abs.gov.au/articles/covid-19-mortality-australia-deaths-registered-31-january-2022#deaths-due-to-covid-19-in-australia.
- Weekly COVID-19 epidemiological update – region of the Americas. Issue 17, cited 2022 Jun 01. Pan American Health Organization. https://iris.paho.org/bitstream/handle/10665.2/56110/COVID-19Summary1June_eng.pdf?sequence=1&isAllowed=y.
- Mahase E. Omicron sub-lineage BA.2 may have “substantial growth advantage,” UKHSA reports. BMJ. 2022;376:o263.
- Callaway E. Why does the omicron sub-variant spread faster than the original? Nature. 2022;602(7898):556–557.
- UK Health Security Agency. COVID-19 vaccine surveillance report Week 4. 2022 Jan 27. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf.
- Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86–88.
- Taylor L. Covid-19: hong kong reports world’s highest death rate as zero covid strategy fails. BMJ. 2022;376(o420):35177535.
- Control Centres for Disease Control. COVID-19 (SARS-CoV-2 infection) dashboard Taiwan2019 cited 2022 08 Aug 2022]. Available from 2022 08 Aug 2022 Aug 08: https://www.cdc.gov.tw/En.
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416.
- Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. 2021;27(12):2108–2110.
- Cohn BA, Cirillo PM, Murphy CC, et al. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331–336.
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84.
- Pouwels KB, Pritchard E, Matthews PC, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127–2135.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83.
- Mahumud RA, Ali MA, Kundu S, et al. Effectiveness of COVID-19 vaccines against delta variant (B.1.617.2): a meta-analysis. Vaccines (Basel). 2022;10(2):277.
- Feikin DR, Abu-Raddad LJ, Andrews N, et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the world health organization. Vaccine. 2022;40(26):3516–3527.
- Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28(5):1063–1071.
- Reeves J. US vaccination drive is bottoming out as omicron subsides. [cited 22 Aug 10] https://apnews.com/article/coronavirus-pandemic-health-us-news-alabama-4c0026679a346ea83a6a04f475a518ef
- Bariyo N, Parkinson J. As omicron surges, Africa’s covid-19 vaccination drive sputters. [cited 2022 Aug 10] https://www.wsj.com/articles/as-omicron-surges-africas-covid-19-vaccination-drive-sputters-11640358002
- Pradhan R, Recht H. As omicron surges, effort to vaccinate young children stalls. [cited 2022 Aug 10] https://www.nbcnews.com/health/health-news/omicron-surges-effort-vaccinate-young-children-stalls-rcna12173
- Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS-CoV-2 omicron variant BA.1 following homologous and heterologous coronavac or BNT162b2 vaccination. Nat Med. 2022;28(3):486–489.
- Bonura F, Genovese D, Amodio E, et al. Neutralizing antibodies response against SARS-CoV-2 variants of concern elicited by prior infection or mRNA BNT162b2 Vaccination. Vaccines (Basel). 2022 May 30;10(6):874.
- Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 delta and omicron variants following heterologous coronavac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–485.
- van Gils MJ, Lavell A, van der Straten K, et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: a prospective cohort study. PLoS Med. 2022 May 17;19(5):e1003991.
- Dolgin E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature. 2022;601(7893):311.
- Prillaman M. One coronavirus infection wards off another - but only if it’s a similar variant [published online ahead of print, 2022 Jul 14]. Nature. 2022. 10.1038/d41586-022-01914-6
- Patel DR, Field CJ, Septer KM, et al. Transmission and protection against reinfection in the ferret model with the SARS-CoV-2 USA-WA1/2020 reference isolate. J Virol. 2021;95(13):e0223220.
- Dyer O. Covid-19: south Africa’s surge in cases deepens alarm over omicron variant. BMJ. 2021;375:n3013.
- Chen J, Wang R, Gilby NB, et al. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–422.
- Enhancing response to Omicron SARS-CoV-2. variant: technical brief and priority actions for member states World Health Organization HQ: headquarters, Geneva, Switzerland. World Health Organization [cited 2022 Aug 10]. https://www.who.int/docs/default-source/coronaviruse/2022-01-07-global-technical-brief-and-priority-action-on-omicron—corr2.pdf?sfvrsn=918b09d_20
- Potential rapid increase of omicron variant infections in the United States [cited 2022 Aug 10]. https://www.cdc.gov/coronavirus/2019-ncov/science/forecasting/mathematical-modeling-outbreak.html
- Covid News: U.S. Hospitalizations break record as omicron surges [cited 2022 Aug 10]. https://www.nytimes.com/live/2022/01/10/world/omicron-covid-testing-vaccines
- Kupferschmidt P, Vogel G. Omicron cases are exploding. Scientists still don’t know how bad the wave will be [cited 2022 Aug 10]. https://www.science.org/content/article/omicron-cases-are-exploding-scientists-still-don-t-know-how-bad-wave-will-be
- Butler M. No time for complacency in battle with COVID-19 [cited 2022 Aug 10]. https://www.health.gov.au/ministers/the-hon-mark-butler-mp/media/no-time-for-complacency-in-battle-with-covid-19
- The Editorial Board. Living with Covid-19 requires caution, not complacency [cited 2022 Aug 10]. https://www.ft.com/content/89451b07-9121-4a6d-b2f8-097c8cb02b67
- Lovett S. Covid vaccines: government warned of ‘dangerous complacency’ as millions skip boosters [cited 2022 Aug 10]. https://www.independent.co.uk/news/health/covid-booster-jab-vaccine-latest-uk-b2124200.html
- Wirawan GBS, Harjana NPA, Nugrahani NW, et al. Health beliefs and socioeconomic determinants of COVID-19 booster vaccine acceptance: an Indonesian cross-sectional study. Vaccines (Basel). 2022 May 5;10(5):724.
- Navin MC, Oberleitner LM, Lucia VC, et al. COVID-19 vaccine hesitancy among healthcare personnel who generally accept vaccines. J Community Health. 2022;47(3):519–529.
- Vanegas E, Robles-Velasco K, Osorio MF, et al. Adverse reactions following COVID−19 vaccination: an ecuadorian experience. Ann Med Surg (Lond). 2021 Dec; 72:103044. Epub 2021 NOV 18.
- Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV−19) covid−19 vaccine. N Engl J Med. 2021 Dec 16;385(25):2348–2360. Epub 2021 Sep 29.
- Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV−2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021 Jul;21(7):939–949. Epub 2021 Apr 27.
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID−19 vaccine type and adverse effects following vaccination. JAMA Network Open. 2021 Dec 1;4(12):e2140364.
- Fragkou PC, Dimopoulou D. Serious complications of COVID-19 vaccines: a mini-review. Metabol Open. 2021 Dec;12:100145Epub 2021 Oct 30. PMID: 34746732; PMCID: PMC8556676.
- Oliver S. Considerations for booster doses of COVID-19 vaccines. MSPH ACIP Meeting 2021 Aug 13 https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-13/05-COVID-Oliver-508.pdf
- Interim statement on the use of additional booster doses of emergency use listed mRNA vaccines against COVID-19 2022 May 17 https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19.
- How many vaccine booster doses have been administered? [cited 2022 Aug 10]. https://ourworldindata.org/covid-vaccinations#how-many-vaccine-doses-have-been-administered-in-total
- Holder J. Tracking coronavirus vaccinations around the world [cited 2022 Aug 10]. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
- Urrunaga-Pastor D, Fernandez-Guzman D, Caira-Chuquineyra B, et al. Prevalence and factors associated with not receiving the booster dose of the COVID-19 vaccine in adults in Latin America and the Caribbean [published online ahead of print. Travel Med Infect Dis. 2022 Aug 9;50:102409.
- Wee SL, Cave D, Dooley B. How asia, once a vaccination laggard, is revving up inoculations [cited 2022 Aug 10]. https://www.nytimes.com/2021/09/30/business/economy/asia-covid-vaccinations.html
- Gaffney A, Himmelstein DU, McCormick D, et al. Disparities in COVID-19 vaccine booster uptake in the USA: december 2021–February 2022. J Gen Intern Med. 2022;37(11):2918–2921.
- European centre for disease prevention and control COVID-19 vaccine tracker [cited 2022 Aug 10]. https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
- European centre for disease prevention and control country overview report: week 31 2022. [cited 2022 Aug 10] https://covid19-country-overviews.ecdc.europa.eu/
- Global COVID-19 vaccination strategy in a changing world: july 2022 update. https://www.who.int/publications/m/item/global-covid-19-vaccination-strategy-in-a-changing-world–july-2022-update cited 22 Aug 10 .
- Cerqueira-Silva T, Shah SA, Robertson C , et al. Waning of mRNA boosters after homologous primary series with BNT162b2 or ChadOx1 against symptomatic infection and severe COVID-19 in Brazil and Scotland: a test-negative design case-control study. cited 22 Aug 10. Available at SSRN: 10.2139/ssrn.4082927.
- Chariyalertsak S, Intawong K, Chalom K, et al. Effectiveness of heterologous 3rd and 4th dose COVID-19 vaccine schedules for SARS-CoV-2 infection during delta and omicron predominance in Thailand. Res Square. 2022.
- Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) Variant. N Engl J Med. 2022 Apr 21;38616:1532–1546. Epub 2022 Mar 2.
- Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022 Jun 14;327(22):2210–2219.
- UK Health Security Agency. COVID-19 vaccine surveillance report: week 6, 10 February 2022. cited 2022 Feb 13. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1054071/vaccine-surveillance-report-week-6.pdf
- Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651.
- Florentino PTV, Millington T, Cerqueira-Silva T, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study [published online ahead of print, 2022 Aug 8]. Lancet Infect Dis. 2022;22(11):1577–1586.
- De-Leon H, Aran D. Over- and under-estimation of vaccine effectiveness. medRxiv. 2022. DOI:10.1101/2022.01.24.22269737
- Williams LR, Ferguson NM, Donnelly CA, et al. Measuring vaccine efficacy against infection and disease in clinical trials: sources and magnitude of bias in COVID-19 vaccine efficacy estimates [published online ahead of print, 2021 Oct 26]. Clin Infect Dis. 2021. DOI:10.1093/cid/ciab914
- Tran TN, Wikle NB, Yang F, et al. SARS-CoV-2 attack rate and population immunity in Southern New England, march 2020 to may 2021. JAMA Network Open. 2022;5(5):e2214171.
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 states, august 2021-january 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263.
- Girard B, Tomassini JE, Deng W, et al. mRNA-1273 vaccine-elicited neutralization of SARS-CoV-2 omicron in adolescents and children. medRxiv [Preprint]. 2022 Jan; 25. 10.1101/2022.01.24.22269666.
- Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):3203.
- Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl J Med. 2021;385(24):e85.
- Veneti L, Berild JD, Watle SV, et al. Vaccine effectiveness with BNT162b2 (comirnaty, Pfizer-BioNTech) vaccine against reported SARS-CoV-2 delta and omicron infection among adolescents, Norway, August 2021 to January 2022. medRxiv. 2022. 10.1101/2022.03.24.22272854.
- Jara A, Undurraga EA, Zubizarreta JR, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10(6):e798–e806.
- Kanokudom S, Assawakosri S, Suntronwong N, et al. Safety and immunogenicity of the third booster dose with inactivated, viral vector, and mRNA COVID-19 vaccines in fully immunized healthy adults with inactivated vaccine. Vaccines (Basel). 2022;10(1):86.
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386(18):1712–1720.
- Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–1614.
- Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022;28(7):1486–1490.
- Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N Engl J Med. 2022;386(14):1377–1380.
- Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative. case-control study BMJ. 2022;377:e071113.
- Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of COVID-19 vaccine among long-term care residents in Ontario, Canada. medRxiv. 2022. 10.1101/2022.04.15.22273846.
- Zeng B, Gao L, Zhou Q, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis BMC Med 2022 cited 2022 May 23; 20(1) 200
- Di Fusco M, Lin J, Vaghela S, et al. COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies. Expert Rev Vaccines. 2022 Apr;21(4):435–451. Epub 2022 Feb 3
- Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COIVD-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults – nine states, january-september 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553–1559.
- Baum U, Poukka E, Leino T, et al. High vaccine effectiveness against severe Covid-19 in the elderly in Finland before and after the emergence of Omicron. MedRxiv. 2022.
- Adams K, Rhoads JP, Surie D, et al. Vaccine effectiveness of primary series and booster doses against omicron variant COVID-19-associated hospitalization in the United States. medRxiv. 2022.
- Mutambudzi M, Niedzwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78(5):307–314.
- Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022;10(7):689–699.
- Pearson CAB, Edmunds WJ, Hladish TJ, et al. Potential test-negative design study bias in outbreak settings: application to Ebola vaccination in Democratic Republic of Congo. Int J Epidemiol. 2022;51(1):265–278.
- Dean NE, Hogan JW, Schnitzer ME. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(15):1431–1433.
- European Medicines Agency. 2021 Apr 14 COVID-19 vaccine safety update VAXZEVRIA AstraZeneca AB.https://www.ema.europa.eu/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-14-april-2021_en.pdf
- UK Medicines and Healthcare Products Regulatory Agency. Decision ARCHIVE: information for healthcare professionals on COVID-19 vaccine Pfizer/BioNTech (regulation 174). cited 2022 Aug 16. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-healthcare-professionals-on-pfizerbiontech-covid-19-vaccine.
- UK Medicines and Healthcare Products Regulatory Agency. Decision. summary of product characteristics for spikevax. cited 2022 Jun 17 https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna/information-for-healthcare-professionals-on-covid-19-vaccine-moderna.
- MHRA. Coronavirus vaccine - weekly summary of Yellow Card reporting. cited Apr 29. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. cited 2021 May 20
- U.S. Centers for disease control and prevention. Myocarditis and Pericarditis. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. cited 22 Aug 10
- UK Medicines and Healthcare Products Regulatory Agency. Decision. summary of product characteristics for vaxzevria. cited 2022 Jun 27. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-covid-19-vaccine-astrazeneca.
- Munro APS, Janani L, Cornelius V, Munro APS, Janani L, Cornelius V, et al. COV-BOOST study group. Safety and immunogenicity of seven COVID-19. vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 Dec 18;398(10318):2258–2276.
- Heterologous primary and booster COVID-19 vaccination. Evidence based regulatory considerations. https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf. cited 22 Aug 10
- Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022 Feb;28(2):410–422.Epub 2021 Dec 14
- Whiteley WN, Ip S, Cooper JA, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PloS Med. 2022 Feb 22;19(2):e1003926.
- Burn E, Li X, Delmestri A, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2: a population-based cohort analysis. medRxiv. 2021; Preprint. 10.1101/2021.07.29.21261348.
- Burn E, Roel E, Pistillo A, et al. Thromboembolic events and thrombosis with thrombocytopenia after COVID-19 infection and vaccination in Catalonia. Spain, 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3886421
- Ab Rahman N, Lim MT, Lee FY, et al. Risk of serious adverse events after the BNT162b2, coronavac, and ChAdOx1 vaccines in Malaysia: a self-controlled case series study. Vaccine. 2022 Jul 30; 40(32):4394–4402.Epub 2022 Jun 3
- Sanders B, Koldijk M, Schuitemaker H. Inactivated viral vaccines. vaccine analysis: strategies. Principles Control. 2014Nov;28:45–80.
- Sturkenboom M, Messina D, Paoletti O, et al. Cohort monitoring of 29 adverse events of special interest prior to and after COVID-19 vaccination in four large European electronic healthcare data sources. medRxiv. 2022.
- Yamey G, Garcia P, Hassan F, et al. It is not too late to achieve global covid-19 vaccine equity. BMJ. 2022;376:e070650.
- Vaezi A, Meysamie A. COVID-19 vaccines (basel) cost-effectiveness analysis: a scenario for Iran. Vaccines (Basel). 2022;10:37.
- Wang WC, Fann JC, Chang RE, et al. Economic evaluation for mass vaccination against COVID-19. J. J Formos Med Assoc. 2021;120 Suppl 1:S95–S105.
- Reicher S, Drury J. Pandemic fatigue? How adherence to covid-19 regulations has been misrepresented and why it matters. BMJ. 2021;372:n137.
- Bodas M, Kaim A, Velan B, et al. Overcoming the effect of pandemic fatigue on vaccine hesitancy-Will belief in science triumph? [published online ahead of print, 2022 Apr 7]. J Nurs Scholarsh. 2022. https://sigmapubs.onlinelibrary.wiley.com/doi/10.1111/jnu.12778
- Sanders B, Koldijk M, Schuitemaker H. Inactivated Viral Vaccines. Vaccine Analysis. 2014Nov;28:45–80.
- Jaramillo-Monge J, Obimpeh M, Vega B, et al. COVID-19 vaccine acceptance in azuay province, ecuador: a cross-sectional online survey. Vaccines (Basel). 2021;9(6):678.
- Matos CCSA, Gonçalves BA, Couto MT. Vaccine hesitancy in the global south: towards a critical perspective on global health. Glob Public Health. 2022Jun;17(6):1087–1098. Epub 2021 Apr 11. PMID: 33843459.
- Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. Medline:33082575.
- Tagoe ET, Sheikh N, Morton A, et al. COVID-19 vaccination in lower-middle income countries: national stakeholder views on challenges, barriers, and potential solutions. Front Public Health. 2021;9:709127.
- Fernandez-Guzman D, Chavez-Cruzado E, Diaz-Velez C, et al. Advances and challenges in COVID-19 vaccination in Latin American: a public health perspective. Infectio. 2022;26(4):441–449.
- Slaoui M, Hepburn M. Developing safe and effective covid vaccines — operation warp speed’s strategy and approach. N Engl J Med. 2020;383(18):1701–1703.
- Rodríguez M. BBC News Mundo – los países en América Latina en los que desarrollan proyectos de vacunas contra la covid-19. 2021. https://www.bbc.com/mundo/noticias-america-latina-56545330 cited 22 Aug 10.
- Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020 Dec 10;383(24):2320–2332. Epub 2020 Sep 2. PMID: 32877576; PMCID: PMC7494251.
- Novavax. Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. 2021, reuters. Available at https://www.reuters.com/article/brief-novavax-covid-19-vaccine-demonstra-idUSB8N2ES01Q [cited 2022 Oct 24].
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al., Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 Feb 20;397(10275):671–681.
- Peshimam GN, Farooq U. CanSinoBIO’s COVID-19 vaccine 65.7% effective in global trials, Pakistan official says. 2021, Reuters. Available at https://www.reuters.com/article/us-health-coronavirus-vaccine-pakistan-idUSKBN2A81N0 cited 2021 Feb 26.