ABSTRACT
Introduction
Vaccines prevent disease and disability; save lives and represent a good assessment of health interventions. Several systematic reviews on the efficacy and effectiveness of COVID-19 vaccines have been published, but the immunogenicity and safety of these vaccines should also be addressed.
Areas covered
This systemic investigation sought to explain the efficacy, immunogenicity, and safety of new vaccination technologies against SARS-CoV-2 in people over 18 years old. Original research studying the effectiveness on mRNA, protein subunit vaccines, and viral vector vaccines against SARS-CoV-2 in people over 18 years old was analyzed. Several databases (Web of Science, Scopus, MEDLINE and EMBASE) were searched between 2012 and November 2022 for English-language papers using text and MeSH terms related to SARS-CoV-2, mechanism, protein subunit vaccine, viral vector, and mRNA. The protocol was registered on PROSPERO, CRD42022341952. Study quality was assessed using the NICE methodology. We looked at a total of six original articles. All studies gathered and presented quantitative data.
Expert opinion
Our results suggest that new vaccinations could have more than 90% efficacy against SARS-CoV-2, regardless of the technology used. Furthermore, adverse reactions go from mild to moderate, and good immunogenicity can be observed for all vaccine types.
1. Introduction
Vaccination has significantly reduced infectious diseases. In effect, the World Health Organization (WHO) has shown that vaccines are safer than therapeutic medicines [Citation1,Citation2].
The vaccination programs reach into nearly every region, neighborhood, and household to protect against several acute infectious diseases and their long-term complications, which range from congenital rubella syndrome to Hepatitis B Virus (HBV), Human Papilloma Virus related cancers [Citation3], and severe coronavirus disease 2019 (COVID-19) [Citation4].
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has brought a hard and dangerous pandemic. In addition, the world’s health and economy have been disrupted by this extremely infectious virus [Citation5]. The SARS-CoV-2 prevalence and mortality rates are still fluctuating daily [Citation6]. About 8,000,000,000 doses of the COVID-19 vaccine have been distributed globally to reduce the rate of SARS-CoV-2 illnesses and cases of COVID-19 [Citation7].
Vaccines prevent infection and disability, save lives, and they also represent a good assessment of health interventions. Vaccines using live, attenuated viruses; inactivated or killed viruses; toxoid, or another mechanism, have demonstrated their benefits, eradicating diseases such as polio, measles, and smallpox [Citation8].
Based on vaccine efficacy findings from randomized controlled trials, regulatory authorities and the WHO have granted emergency use listing or emergency use authorization for COVID-19 vaccines [Citation9]. New technologies in vaccines include messenger RNA vaccines (mRNA), protein subunit vaccine and adenovirus vector vaccines [Citation10–12].
mRNA platforms include mRNA-1273, BNT162b1, and BNT162b2. The mRNA-1273 vaccine is a lipid nanoparticle encapsulated mRNA vaccine that encodes the S-2P antigen glycoprotein, which is the S2 component, with two consecutive proline changes at amino acid positions 986 and 987 [Citation13–15]. By using several significantly altered mRNA sequences, BNT162b1 and BNT162b2 produce long-lasting and abundant target protein expression. Both approaches use different sequences around the start codon, such as GCCACCAUG rather than GCCRCCAUGG. R and G residues at the 4th and 10th positions, respectively, are removed to improve translational initiation at a downstream AUG start codon. Following the start codon, the mRNA in BNT162b1 and BNT162b2 have a tiny flanking area with secondary structure, but the mRNA-1273 has a significantly more obvious secondary structure [Citation16].
Full-length S proteins or their antigenic components, such as the S1 subunit and receptor-binding domain (RBD), are typically used as antigen targets in the development of SARS-CoV-2 protein subunit vaccines [Citation17]. ZF2001 is composed of an antigen-recombinant, full-length S protein produced in a baculovirus-Sf9 system and the adjuvant MatrixM [Citation17,Citation18]. A dimeric form of the SARS-CoV-2 RBD serves as the antigen in this protein subunit vaccine. The SARS-CoV-2 RBD antigen is encoded in two copies in tandem repeat dimeric form by the ZF2001 recombinant vaccine.
ChAdOx1 is a serotype Y25 chimpanzee adenovirus vector with additional modifications that substitute E4 regions with that of Ad5 to increase virus yields [Citation19]. The AZD1222 vaccine carries sequences encoding the spike protein of SARS-CoV-2 and a tissue plasminogen activator leader sequence [Citation13,Citation20]. Importantly, AZD1222-induced antibodies can facilitate antibody-dependent neutrophil/monocyte phagocytosis, complement deposition and NK cell activation [Citation21], which may effectively control SARS-CoV-2 infection.
Some reviews of COVID-19 effectiveness and efficacy studies have been published [Citation22–27], but the immunogenicity and safety of these vaccines should also be addressed. In addition, sex-related factors, influencing both the subjects’ immunity and the vaccine outcomes, are still neglected in clinical research [Citation28,Citation29]. Therefore, we aimed to evaluate the efficacy, immunogenicity and safety of mRNA, protein subunit vaccine or viral vector vaccines against SARS-CoV-2 in adults over 18 years old and to carefully evaluate the time of immunization provided by COVID-19 vaccinations.
2. Methods
A systematic review of quantitative studies, evaluating the efficacy of new vaccination technologies against SARS-CoV-2 according to the mechanism of modulation of immune responses in humans older than 18 years old. The protocol was listed in PROSPERO, CRD42022341952. The review is reported according to PRISMA [Citation30].
2.1. Search strategy and selection criteria
2.1.1. Search strategy
From January 2012 to November 2022, several databases (Web of Science, Scopus, MEDLINE as well as EMBASE) were searched for original studies in English, using MeSH terms (‘SARS-CoV-2’ AND ‘mechanism’ AND ‘protein subunit vaccine’ AND ‘viral vector’ AND ‘mRNA’) and text words relating to the effectiveness of vaccines against SARS-CoV-2 related to the mechanism of immune response modulation in accordance with the investigation question. The searches were part of a broader search for a series of articles on topics such as the number of doses, SARS-CoV-2 variants, vaccine immunogenicity and vaccine safety. The included studies and relevant reviews’ reference lists were also searched.
2.1.2. Identification of relevant studies
Titles, abstracts, and papers were screened for inclusion by two reviewers (Daniela Guerrero and Joham Muñoz). Differences in results between reviewers were resolved through discussion with another reviewer.
2.1.3. Types of study and design
The following inclusion criteria were used: 1. Original quantitative research (using inferential or descriptive statistics methods, either non-parametric or parametric): cross-sectional research, retrospective or randomized controlled trials; reporting the effectiveness of vaccines against SARS-CoV-2 according to the mechanism of modulation of immune responses or the number of doses, SARS-CoV-2 variants, and type of vaccine-induced antibodies; and 2. Studies in English. The following exclusion criteria were used: 1. Did not include or specify numerical data; 2. Were not original investigations published in full; 3. Were not published in a peer-reviewed journal; 4. Conference abstracts; 5. Systematic reviews; 6. Editor letters; and 7. Studies on populations younger than 18 years old; and 8. Studies not focused on the effectiveness of vaccines against SARS-CoV-2 according to the mechanism of modulation of immune responses, or that do not describe the dose number, SARS-CoV-2 variants, and type of vaccine-induced antibodies.
2.1.4. Population
Men and women in the community over the age of 18, including healthy individuals with or without a history of SARS-CoV-2 infection. Everyone in the intervention group should have had the COVID-19 vaccine. Pregnant women were excluded. In addition, mental or seizure illness history; allergy to the vaccine’s ingredients; gastrointestinal symptoms within the previous seven days or acute febrile illness within the previous 24 h before vaccination; acquired or congenital immune diseases; serious chronic disease; a positive test for HBV, Hepatitis C Virus, Human Immunodeficiency Virus (HIV), or syphilis; a history of a tumor or cancer; and receipt of any blood products in the previous three months were all disqualified.
2.1.5. Quality assessment/risk of bias
One reviewer (Joham Muñoz) assessed quality using the National Institute for Health and Care Excellence (NICE) methodology and analyzed it for accuracy another reviewer (Daniela Guerrero) [Citation31]. Disparities between the authors were solved by discussion. No studies were excluded based on assessment.
2.1.6. Data extraction and synthesis
Two reviewers extracted and checked data from the included studies’ populations and study characteristics ().
Table 1. Characteristics of included studies.
Two researchers went line by line through the results and discussion sections of each text to look for data involving the effectiveness of vaccines against SARS-CoV-2 according to the mechanism of modulation of immune responses such as predominant Th1 response, measured by the production of Th1 cytokines IFNγ, TNF, and IL-2 [Citation33]; a Th2-biased response, as indicated by the production of IL-4, IL-5 and IL-13; or antibodies against RBD [Citation34]. The text was reviewed in greater detail and rearranged into topics (). These were included if the study’s authors built their interpretation and concepts from the initial data.
Table 2. Variables involved in the efficacy of mRNA, protein subunit vaccine and viral vectors vaccines against SARS-CoV-2 in people over 18 years old.
3. Results
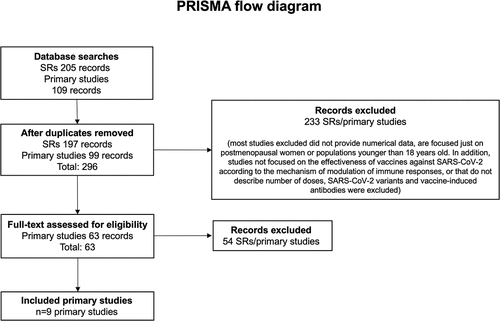
The flow chart for the selection process of the studies is shown in [Citation11,Citation15,Citation35–41]. lists the studies that were included, along with the demographics, environments, and contexts in which they were carried out.
3.1. Description of included studies
A total of nine original articles were analyzed. The papers collected and reported quantitative data through clinical trials (). All studies were conducted on both male and female participants [Citation11,Citation15,Citation35–41].
Details of the vaccine involved, population, intervention details, investigated outcomes, and study aims are shown in . One study used protein subunit vaccine [Citation38], another five used mRNA-based COVID-19 vaccines [Citation11,Citation15,Citation35,Citation37,Citation41], while three used viral vector vaccine [Citation36,Citation39,Citation40].
3.2. Quality assessment
Supplementary Table 1 displays the quality assessment outcomes and evaluation standards for studies. Studies’ overall quality for internal and external validity was often high or moderate. No studies were disqualified due to poor quality.
3.3. New technologies for vaccines
Platforms of mRNA, protein subunit vaccine and viral vector vaccines against SARS-CoV-2 were studied (). mRNA-based COVID-19 vaccines, such as the mRNA-1273, BNT162b1, and BNT162b2 vaccines [Citation11,Citation15,Citation35,Citation37,Citation41]; protein subunit vaccines – which contain S protein or their antigenic components [Citation38]; while viral vector based-vaccines with additional modifications that substitute E4 regions with that of Ad5 and allows to recognize and fight the virus that causes COVID-19 [Citation36,Citation39,Citation40].
3.4. Efficacy
In a controlled clinical study, the efficacy of a vaccination is determined by comparing the proportion of recipients of the vaccine who developed the illness to the proportion of those who received the placebo. In this sense, the goal of mRNA-1273 vaccination in participants who were seronegative at baseline is to avoid the occurrence of COVID-19 for at least 14 days after the second dose [Citation11,Citation35]. Thus, for the primary analysis, a total of 3.3 per 1000 person-years (95% CI 1.7 to 6.0) was found in the vaccine group; and 56.5 per 1000 person-years in the placebo group, demonstrating an efficacy higher than >94% in the prevention of SARS-CoV-2 infection after mRNA-1273 vaccination (p < 0.001) [Citation11]. However, it is important to note that a higher efficacy was found in men between 18 and 64 years old (95.6% CI 90.6–97.9) in comparison to females older than 65 years old (93.1% CI 85.2–96.8) [Citation11]. Likewise, in participants who received a LD/SD schedule the efficacy was higher at 90.0% [Citation36]. However, in the individuals following two standard-dose of ChAdOx1 vaccine, vaccine efficacy was 53.4% and 65.4% after less than 6 weeks’ interval between doses and after at least six weeks interval, respectively [Citation36]. Likewise, the efficacy of the vaccine was 78% in patients who got their second dose at a prolonged dosing interval [Citation39]. Although ZF2001 produced higher neutralizing GMTs than convalescent samples, the vaccine’s protective efficacy has yet to be shown [Citation38].
3.5. Safety
The vaccinations for COVID-19 that have been licensed are both safe and efficacious. COVID-19 vaccinations have exceedingly uncommon, significant, or long-term adverse effects.
Local incidents occurred more frequently following vaccine inoculation in the mRNA-1273, BNT162b1 and/or BNT162b2 groups [Citation11,Citation15,Citation37,Citation41]. However, the adverse effects were mostly graded 1 or 2 [Citation11,Citation15,Citation37,Citation41]. Toxicity grade for erythema is defined as: G1 = 25–50 mm; G2 = 51–100 mm; G3 = >100 mm. Toxicity grade for fever is defined as: G1 = 38–38.4°C; G2 = 38.5–38.9°C; G3 = 39–40°C; G4 = >40°C. Toxicity grading scales for solicited systemic and local adverse events are shown in Supplementary Table 2.
Most studies have not found serious adverse events or withdrawals due to related adverse events [Citation35]. However, although vaccines have a good safety profile, three studies have shown serious adverse events across the vaccine and placebo groups [Citation36,Citation39,Citation40].
The two-dose vaccination series was generally safe, with mild-to-moderate systemic adverse events following the first inoculation [Citation15,Citation37–41]. Frequency of adverse reaction is shown in .
Table 3. Adverse reactions after vaccination.
3.6. Immunogenicity
While vaccine immunogenicity varies a little depending on the age group, good immunogenicity can be seen for all vaccine types. In this sense, vaccines like mRNA-1273 have not shown short-term evidence of increased respiratory disease following infection [Citation11,Citation15]. However, although antibody titers decrease with age [Citation35], it is important to note that immunogenicity data shows similar immune responses in older adults following vaccination with two doses of mRNA-1273, ChAdOx1, or ZF2001 vaccine [Citation11,Citation15,Citation35–41]. In fact, although high neutralizing titers were attained 14 days following the booster immunization in participants between 18 or older [Citation39,Citation40]; a non-neutralizing level was found in the LD/LD group in participants aged 70 and older [Citation40].
The mRNA-1273 vaccine’s rapid and robust immunologic responses are most likely due to an innovative structure-based vaccine antigen design, as well as the prevention of early intracellular activation of interferon-associated genes [Citation11,Citation15]. The serologic responses elicited by BNT162b1 and BNT162b2 were similar [Citation37,Citation41]. Specifically, the highest neutralization titers were on day 7 or 14 after the second dose, with similar tendencies observed for the 50% and 90% neutralizing titers, respectively [Citation37,Citation41]. Meanwhile, overall vaccine efficacy across both groups was 70.4% (95.8% CI 54.8–80.6) after ChAdOx1 vaccination [Citation36,Citation39,Citation40].
After 30 days of the first dose, geometric mean titers (GMTs) of ZF2001 vaccine-induced RBD-binding IgG were higher than 23.1 IU/ml and 40.8 IU/ml in the 25 μg and 50 μg dose groups. Then, after 30 days of the second dose, the values increased to 1077.0 IU/ml and 825.5 IU/ml in the 25 μg dose and the 50 μg dose groups. Finally, after 30 days of the third dose, GMTs increased to 2719.5 IU/ml and 2776.8 IU/ml in the 25 μg and 50 μg groups [Citation38]. For AZD1222 vaccination, after 28 days following the booster vaccination, all two-dose groups had similar antibody titers, independent of age or vaccine dosage (p = 0.68) and were greater than those who did not get a booster immunization. Anti-RBD antibodies had similar findings [Citation40,Citation41].
4. Discussion
This review collates and synthetizes evidence from six quantitative studies relating to the efficacy, safety, and immunogenicity of mRNA, protein subunit vaccine, and viral vector vaccines against SARS-CoV-2 in people over 18 years old.
4.1. Summary of key findings and interpretation
It is essential to remember that our searches were aimed mainly at mRNA, protein subunit vaccine, and viral vector vaccines against SARS-CoV-2 because they are poorly investigated compared to the live/attenuated or inactivated/killed vaccines.
Between the advantages of new technologies for vaccines, we found they are easier to design, have a high degree of adaptableness, and produce robust cellular and humoral immune responses [Citation42]. In addition, they have high potency, rapid development capability, potential for low-cost manufacturing, and safe administration [Citation43,Citation44]. However, among the disadvantages, we could find that ChAdOx1 vaccines possibly present preexisting immunity [Citation42]. Thus, it is very important to consider the previous infection, adverse reactions, dose injected, and the type of vaccine applied to evaluate the efficacy, immune response, and immunogenicity.
In relation to the efficacy of mRNA, protein subunit vaccines, and viral vector vaccines against SARS-CoV-2, our findings have shown that the vaccines based on mRNA have an efficacy greater than 90% against the symptomatic disease of SARS-CoV-2. In fact, previous studies have found that a two-dose regimen of BNT162b2 and mRNA-1273 was found to be safe and >90% effective against COVID-19 between seven days and six months after the second dose [Citation10,Citation35,Citation37,Citation39,Citation41,Citation45,Citation46]. In addition, the vaccines met both main efficacy endpoints with a greater than 99.99% probability of efficacy [Citation10,Citation45]. Finally, the efficacy of the BNT162b2 vaccine against severe disease with an onset after receiving the first dose was approximately 97% [Citation47]. However, the higher efficacy has been found in men between 18 and 64 years of age after mRNA-1273 vaccination [Citation11]. The efficacy of ChAdOx1 vaccine after two standard-dose was 53.4% after less than 6 weeks’ interval between doses and 65.4% after at least six weeks interval [Citation36]. In addition, ChAdOx1 vaccine efficacy seems to be better when a standard dose is applied after a low dose [Citation36,Citation39]. In relation to ZF2001 vaccination, even if higher neutralizing GMTs have been shown after vaccination, the percentage efficacy has not yet been described [Citation38].
In relation to safety, our results have shown vaccines against COVID-19 can cause adverse reactions of different types, from mild to moderate [Citation11,Citation15,Citation30,Citation35,Citation36,Citation41]. In fact, previous studies have described local and systemic events that were generally mild to moderate in severity and typically resolved within 1 to 2 days [Citation40,Citation48,Citation49]. However, three studies found major adverse effects in both the vaccine and placebo groups, even though vaccines have a high safety record [Citation36,Citation39,Citation40].
The strong and rapid immunogenicity profile of mRNA, protein subunit vaccine and viral vector vaccines against SARS-CoV-2 is the most expected outcome of a novel structure-based vaccine antigen design, coupled with a powerful lipid-nanoparticle release system and the use of improved nucleotides that prevent early intracellular stimulation of interferon-associated genes [Citation11,Citation15]. In fact, GMT titers of IgG antibodies bound to S-2P, and the receptor-binding domain boosted quickly after the first vaccination [Citation49,Citation50] and increased substantially 7 days after the second dose administered on day 21 [Citation39,Citation41,Citation49]. In this sense, our searches have shown higher neutralizing titers antibody and/or GMTs of vaccine-induced RBD-binding IgG against SARS-CoV-2 [Citation15,Citation37,Citation40,Citation41]. However, these antibody titers as expected have lower levels in people over 60 years old [Citation35,Citation37,Citation40].
4.2. Scope and limitations
The goal of this study was to seek an explanation that would reconcile prior conflicting findings concerning the efficacy, safety, and immunogenicity of new technologies of vaccination against SARS-CoV-2. Most of the studies identified by this review were conducted on people receiving mRNA-1273, BNT162b1/BNT162b2, ZF2001 or ChAdOx1 nCoV-19 vaccine. Specifically, it was to test the hypothesis that mRNA platforms, protein subunit vaccines and viral vector vaccines against SARS-CoV-2 are effective, safe, and can increase antibody titers to protect against the illness in people over 18 years old. Fortunately, most of the results provide support for this notion. We found a trend toward enhanced geometric mean titers of RBD-binding IgG against SARS-CoV-2 and higher neutralizing titers of antibody. Considering these data and adverse reactions observed after vaccination, it appears that mRNA platforms, protein subunit vaccines and viral vector vaccines against SARS-CoV-2 could be used in people over 18 years old with or without previous infection.
Our review had some limitations, i.e. the studies did not use the same methodological model, included only people without previous infection, or used the same vaccination scheme. In addition, most studies have not considered sex- and age-related factors, which influence both the subjects’ immunity and the vaccine outcomes. Thus, the titers of RBD-binding IgG and neutralizing titers of antibodies could not be compared among different studies. In addition, it is important to note the virus can mutate and evolve over time; therefore, the vaccine can become seasonal protection. Finally, it is important to consider that the response against different vaccines that are based on the same platform can be very different because some vaccines use a combination of two different vectors.
5. Conclusions
The pandemic caused by SARS-CoV-2 is a public health problem. Therefore, the development and production of vaccines for its control are necessary. Safety and reactogenicity are essential aspects that must be considered for the development, production, and approval of different vaccines before their massive administration.
Our results suggest that mRNA, protein subunit vaccines, and viral vector vaccines had durable effectiveness in reducing the risks of hospitalization and death in people over 18 years of age after SARS-CoV-2 vaccination. In addition, they showed protection against symptomatic disease, and a good efficacy, safety, and immune response. However, both declining immunity as the gradual development of the SARS-CoV-2 variations contributed to the declining level of infection resistance. Therefore, despite all the advances that have been made, the efficacy of mRNA platforms and viral vectors should continue to be studied through clinical trials.
6. Expert opinion
The development of vaccines to prevent the symptomatic disease of COVID-19 has happened at an unprecedented rate [Citation51–54]. For this reason, it is almost impossible to identify the true impact of mRNA, protein subunit, and viral vector-based vaccines against SARS-CoV-2 variants, the use of a mixed or heterologous vaccine schedule, or even third or fourth immunization. Therefore, we assess the effectiveness of mRNA platforms, protein subunit vaccine, and viral vectors vaccines against SARS-CoV-2. The results showed that the new technologies of vaccination are a bit studied due to lack of clinical trials; however, among them the most studied vaccines, mRNA-1273, BNT162b1, BNT162b2, ZF2001, and ChAdOx1 nCoV-19, had comparable efficacy profiles, particularly against severe outcomes, underscoring the importance of real-world data in a growing clinical practice.
Preclinical research indicates that new vaccine technologies will most likely meet many of the criteria for the ideal clinical vaccine: they have a favorable safety profile in animals, are adaptable and quick to design for emerging infectious diseases and can be produced using scalable good manufacturing practices. Unlike inactivated or dead vaccinations, several mRNA, protein subunit, and viral vector-based vaccines generate robust immune responses. Most likely, due to the capacity of vaccinations to elicit significant CD4 + T cell responses, as well as the presentation of endogenously produced antigens on major histocompatibility complex class I molecules, Th1/Th2 cytokines or antibodies against RBD. These vaccines have been proven to induce significant neutralizing antibody responses in animals with only one or two low-dose doses of immunizations. Due to the protective immunity elicited by vaccines based on mRNA, protein subunits, and viral vectors against a variety of infectious diseases in animal models, there is a great deal of trust in their efficacy. However, the mRNA COVID-19 vaccines cause a greater increase in neutralizing antibodies after the second dose compared to the ChAdOx1 nCoV-19 COVID-19 vaccine, despite having less neutralizing antibody activity after the first dose.
One of the most notable advantages of mRNA technology is its biological usage as a template for protein translation. Unlike traditional vaccination techniques, which rely on the mass synthesis of the protein antigen in a bioreactor using, i.e. mammalian cells, mRNA vaccines are created just once in a patient’s cells. Essentially, mRNA uses the human body as its own vaccine production facility, with a variety of additional advantages.
The COVID-19 pandemic has put conventional vaccine manufacturing methods to the test, creating a new environment for research into mRNA, protein subunit, and viral vector vaccines. Because of the public health emergency, manufacturers sought ways to reduce time to the clinic (for example, by parallelizing different parts of the serial development process, minimizing pilot studies, and conducting minimal product-quality release testing); conversely, extensive validation of the new mRNA technology against established vaccines was prioritized.
Using based on mRNA technology; cancer vaccines have lately gained popularity. Cancer vaccines can be created to target tumor-associated antigens, such as growth factors that are selectively generated in cancer cells, or antigens that are particular to sick cells owing to somatic mutation. Human mRNA vaccination targets have been developed using these neoantigens or the neoepitopes inside them. Most cancer vaccines are therapeutic rather than preventive in nature, trying to stimulate cell-mediated responses, such as those produced by cytotoxic T lymphocytes that can eliminate or reduce tumor burden. More than two decades ago, the first proof-of-concept experiments that not only confirmed the notion of mRNA cancer vaccines but also offered evidence of their viability were published. Since then, several preclinical and clinical studies have confirmed the effectiveness of mRNA vaccines in cancer therapy.
Therefore, due to advances in manufacturing methods because of the pandemic produced by SARS-CoV-2; in the coming years, we should expect significant advances against diseases such as HIV/AIDS and cancer, thus reducing the human and monetary resources involved annually in World Public Health.
Article highlights
New technologies for vaccines have a high degree of adaptableness and produce robust cellular and humoral immune responses.
The mRNA-1273 and ChAdOx1 vaccines have an efficacy greater than 90% and >50% against SARS-CoV-2, respectively.
mRNA, protein subunit vaccine and viral vectors vaccines against SARS-CoV-2 can cause adverse reactions from mild to moderate.
The vaccines based on mRNA, protein subunit and viral vectors against SARS-CoV-2 exhibit a strong and rapid immunogenicity profile and prevent early intracellular stimulation of interferon-associated genes.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the conception and design of the study, analysis, and interpretation of the relevant literature, writing, as well as the creation of this manuscript and the associated enhancements. Before submission, all authors had full access to the data analysis and gave their final approval of the paper and the associated enhancements.
Supplemental Material
Download MS Word (29.3 KB)Supplemental Material
Download MS Word (34.3 KB)Supplemental Material
Download MS Word (36.2 KB)Acknowledgments
Authors thanks to SmartC-BIOREN (Service Management Analytical Research and Training Center), CCSS210005 Project, Agencia Nacional de Investigación y Desarrollo de Chile (ANID) for English Editing Services; and Programa de Formación de Investigadores Postdoctorales 2022, Universidad de La Frontera for design support.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2156861
Additional information
Funding
References
- World Health Organization. Global advisory committee on vaccine safety, 3-4 Dec 2003. Weekly Epidemiological Rec. 2004;79(3):16–20. https://apps.who.int/iris/handle/10665/232366
- Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunisation: vaccine adverse event reporting system (VAERS) – United States, 1991–2001. MMWR Surveill Summ. 2003;52:1–24.
- Schuchat A. Human vaccines and their importance to public health. Procedia Vaccinol. 2011;5:120–126.
- Tavako S, Alavijeh MS, Seifalian AM. COVID-19 vaccines on clinical trials and their mode of action for immunity against the virus. Curr Pharm Des. 2021;27(13):1553–1563.
- Meo SA, Alhowikan AM, Al-Khlaiwi T, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–2019.
- Meo SA, Al-Khlaiwi T, Usmani AM, et al. Biological and epidemiological trends in the prevalence and mortality due to outbreaks of novel coronavirus COVID-19. J King Saud Univ Sci. 2020;32:2495–2499.
- Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Human Behav. 2021;27:1–7.
- Tourni M, Ricciardi W. The economic value of vaccination: why prevention is wealth. J Mark Access Health Policy. 2015;3:29414.
- World Health Organization. 2021. Guidance document: status of COVID-19 vaccines within WHO EUL/PQ evaluation process. [cited 2022 Jun 05]: Available at: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_15July2021.pdf
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–2201.
- Le TT, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306.
- Van Riel D, Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19:810–812.
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931.
- Xia X. Detailed dissection and critical evaluation of the pfizer/biontech and moderna mRNA vaccines. Vaccines (Basel). 2021;9(7):734.
- Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82.
- Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12(1):372.
- Dicks MD, Spencer AJ, Edwards NJ, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7(7):e40385.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478.
- Barrett JR, Belij-Rammerstorfer S, Dold C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288.
- Harder T, Koch J, Vygen-Bonnet S, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26(28):2100563.
- Harder T, Külper-Schiek W, Reda S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26(41):2100920.
- Higdon MM, Wahl B, Jones CB, et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. Open Forum Infect Dis. 2021;9(6):ofac138.
- Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29:1075–1090.
- Meggiolaro A, Schepisi MS, Nikolaidis G, et al. Effectiveness of vaccination against symptomatic and asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Vaccines (Basel). 2022;10(2):157.
- Zeng B, Gao L, Zhou Q, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200.
- McCartney PR. Sex-Based vaccine response in the context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020;49:405–408.
- Vijayasingham L, Bischof E, Wolfe J. Sex-Disaggregated data in COVID-19 vaccine trials. Lancet. 2021;397:966–967.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097.
- National Institute for Health and Care Excellence (ed). 2012. Appendix F Quality appraisal checklist – quantitative intervention studies. London: Methods for the Development of NICE Public Health Guidance. https://www.nice.org.uk/process/pmg4/chapter/about-this-document.
- Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11: 2601-2601.
- Corbett KS, Edwards DK, Leist SR, et al. Sars-Cov-2 MRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571.
- Sun Z, Ren K, Zhang X, et al. Mass spectrometry analysis of newly emerging coronavirus HCoV-19 spike S protein and human ACE2 reveals camouflaging glycans and unique post-translational modifications. Engineering (Beijing). 2021;7(10):1441–1451.
- Kontopoulou K, Ainatzoglou A, Ifantidou A, et al. Immunogenicity after the first dose of the BNT162b2 mRNA Covid-19 vaccine: real-world evidence from Greek healthcare workers. J Med Microbiol. 2021;70(8):001387.
- Voysey M, Clemens S, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
- Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450.
- Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–1119.
- Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360.
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993.
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593.
- Shang W, Yang Y, Rao Y, et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020;5:18.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nature Rev Drug Discov. 2018;17(4):261–279.
- Doerfler W. Adenoviral Vector DNA- and SARS-CoV-2 mRNA-Based Covid-19 vaccines: possible integration into the human genome - are adenoviral genes expressed in vector-based vaccines? Virus Res. 2021;302:198466.
- Lundstrom K. Viral vectors for COVID-19 vaccine development. Viruses. 2021;13(2):317.
- Fiolet T, Kherabi Y, MacDonald CJ, et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221.
- Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773.
- Coggins SA, Laing ED, Olsen CH, et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. Open Forum Infect Dis. 2021;9(1):ofab575.
- Frenck RW, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250.
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438.
- Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973.
- Lurie N, Sharfstein JM, Goodman JL. The development of COVID-19 vaccines: safeguards needed. JAMA. 2020;324(5):439–440.
- Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395:1751–1752.
- Collins FS, Stoffels P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA. 2020;323:2455–2457.