 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Limited data are available describing the global impact of COVID-19 vaccines. This study estimated the global public health and economic impact of COVID-19 vaccines before the emergence of the Omicron variant.
Methods
A static model covering 215 countries/territories compared the direct effects of COVID-19 vaccination to no vaccination during 13 December 2020–30 September 2021. After adjusting for underreporting of cases and deaths, base case analyses estimated total cases and deaths averted, and direct outpatient and productivity costs saved through averted health outcomes. Sensitivity analyses applied alternative model assumptions.
Results
COVID-19 vaccines prevented an estimated median (IQR) of 151.7 (133.7–226.1) million cases and 620.5 (411.1–698.1) thousand deaths globally through September 2021. In sensitivity analysis applying an alternative underreporting assumption, median deaths averted were 2.1 million. Estimated direct outpatient cost savings were $21.2 ($18.9–30.9) billion and indirect savings of avoided productivity loss were $135.1 ($121.1–206.4) billion, yielding a total cost savings of $155 billion globally through averted infections.
Conclusions
Using a conservative modeling approach that considered direct effects only, we estimated that COVID-19 vaccines have averted millions of infections and deaths, generating billions of cost savings worldwide, which underscore the continued importance of vaccination in public health response to COVID-19.
1. Introduction
The Coronavirus disease 2019 (COVID-19) pandemic has resulted in over 610 million cases and 6.5 million deaths globally as of 22 September 2022 and has disrupted economies worldwide [Citation1–3]. Cutler and Summers estimated that the economic cost of the pandemic in the United States (US) would be approximately $16 trillion, assuming that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) activity would be substantially contained by Fall 2021 [Citation4]. In the early phase of the pandemic, in the US, a single symptomatic COVID-19 case was estimated to have a median direct medical cost of $3,045 during the course of the acute illness [Citation5]. Further, productivity losses may result from short-term and long-term absenteeism from work due to disability and premature deaths, lockdowns, and other pandemic-related restrictions. These losses carry significant costs not only to individuals and their families but also to the society while disproportionately affecting some groups more than the others [Citation6–9].
According to Our World in Data (OWID), 6.3 billion COVID-19 vaccine doses had been administered worldwide by the end of September 2021. Vaccine uptake has since doubled to 12.8 billion doses as of September 2022. Despite this, more than 30% of the world population remains unvaccinated, with 77% of individuals in low-income countries are still unvaccinated as of October 2022 [Citation10].
The clinical and socioeconomic impact of safe and effective COVID-19 vaccines during the on-going pandemic has been enormous [Citation11]. However, limited empirical data are available to quantify the clinical and economic impact of the rapid roll-out of COVID-19 vaccines globally. Several studies have estimated country-specific public health and economic impacts of COVID-19 vaccines [Citation12–15]. Few studies, however, have assessed public health impact on a global scale using empirical data, and in particular, limited data are available on the direct and indirect economic impacts of COVID-19 vaccination, which may inform future public health decision-making and healthcare budget allocation [Citation11,Citation13,Citation16,Citation17]. Hence, the aim of this study was to quantify the global health and economic impact of vaccination programs against COVID-19, prior to the emergence of the SARS-CoV-2 Omicron variant.
2. Methods
2.1. Study design
A static Susceptible-Infectious-Recovered (SIR) model was developed to estimate the global health and economic impact of the primary series of any type of authorized or approved COVID-19 vaccine as defined per the product label [e.g. two doses of messenger ribonucleic acid (mRNA) vaccines or one dose of JNJ-78436735 vaccine] compared to no vaccination in the general population. The analysis period started on 13 December 2020, the date of the first vaccination, and ended on 30 September 2021. Public health impact was estimated as the number of COVID-19 cases and deaths averted. Economic outcomes included total cost savings in 2021 US dollars (USD), based on estimated outpatient costs and productivity loss avoided corresponding to the averted health outcomes, from the healthcare and societal perspective, respectively. Models were developed in Microsoft Excel that estimated country-specific impacts, which were then aggregated to the World Health Organization (WHO)-defined regions, and finally to the global level over the defined study period.
2.2. Model structure
A bottom-up approach was used to develop the static SIR model (Supplementary Figure S1). This approach has been employed previously in analyses on pneumococcal conjugate vaccination [Citation18], and the impact of COVID-19 vaccines on mortality among individuals aged 60 years and older in the United Kingdom (UK) [Citation19].
Using reported numbers of daily COVID-19-related cases and deaths from the OWID database [Citation10], we estimated the number of cases that would have occurred without vaccination (i.e. counterfactual scenario), and derived country-specific numbers of cases and deaths averted, which were then aggregated to obtain a total global impact estimate.
Estimates were derived using the following equation:
where CasesNV,c,t were counterfactual cases in country c at time t, when all factors from observed cases were held constant. Casesv,c,t ,defined as observed cases in country c at time t, was a function of the disease environment, non-pharmaceutical interventions (NPIs), testing and policies as well as vaccine coverage and VE in country c at time t. 1 − Covc,t*VEt was defined as the proportion of the population susceptible in country c at time t, wherein Covc,t and VEt referred to vaccine coverage and VE in country c at time t, respectively. ∑t-1∆%CasesNV,c was the additional population that would have natural immunity through infection in the counterfactual scenario in county c through time t-1. Hence, the observed cases were a function of the number of unvaccinated cases, re-infections, and breakthrough cases. Finally, public health impact, defined as the averted outcomes, was estimated as the difference between observed cases and counterfactual cases.
No. of cases averted = Casesv,c,t –CasesNV,c,t
For economic impact, estimates of direct outpatient cost savings were derived using the Number of cases averted * Average cost per outpatient visit * Number of visits (2 visits [Citation20]), while hospitalization-related cost savings were estimated using the Number of hospitalizations averted * Length of stay (8 days [Citation21–24]) * Inpatient cost per day. Indirect cost savings from averting cases were estimated using the Labor participation rate * Cases averted * Weekly income per person* Two weeks (assumption [Citation25,Citation26]). All costs were expressed in 2021 USD.
2.3. Model inputs
The key clinical and economic inputs used in the base case analysis are shown in .
Table 1. Model inputs for the base case analysis.
2.3.1. Clinical inputs
Our World in Data (OWID)’s coronavirus pandemic data hub provides data for 226 individual countries and territories and also grouped by the six WHO-defined regions of North America, South America, Europe, Asia, Africa, and Oceania [Citation10]. The hub reports country-specific COVID-19-related cases, deaths, and vaccination status for all ages only, while hospitalization data are limited, and reported mostly for Europe and the US. Hence, the OWID hub was selected as the primary source for clinical inputs on daily total COVID-19 cases observed and daily total COVID-19-related-deaths reported for each country starting from the date the first individual was fully vaccinated in the country to the end of the study period on 30 September 2021 (database accessed on 20 April 2022). We assumed that NPIs and testing practices, which impact the incidence of COVID-19, would have remained unchanged during the study period in the absence of vaccination. The total number of individuals receiving COVID-19 primary series vaccination was also obtained from the OWID database [Citation10].
During the study period, 12 COVID-19 vaccines were authorized or approved for administration. However, data on the relative proportions of doses administered by vaccine brand in each country were not available. Hence, we first identified the authorized/approved COVID-19 vaccines for each country from the COVID-19 vaccine tracker (accessed on 22 April 2022) [Citation27] and assumed an equal distribution of use across vaccine brands. This assumption was considered necessary from a modeling perspective because data on the proportion of vaccines given by brand were available for only 42 countries. However, a sensitivity analysis of this assumption for those 42 countries was performed as described in Section 2.4.3. Brand-specific VE against infection and severe disease (i.e. hospitalization or death) used estimates from the Institute for Health Metrics and Evaluation (IHME) [Citation28]. Finally, for each country, above-brand VE was calculated as the median VE of all the COVID-19 vaccines available in that particular country. Waning VE was not incorporated into the model due to the relatively short duration of the study period.
2.3.2. Economic inputs
Inpatient and outpatient costs per infection were not available for most countries from a single standard source, and previous country-specific studies have reported widely varying costs [Citation20,Citation29–35]. To standardize the cost inputs, WHO CHOICE 2008 estimates of average cost per inpatient day and outpatient visit were adopted [Citation36]. The WHO CHOICE reported costs were in 2008 USD, which were adjusted to 2021 USD using the US consumer price index [Citation37]. Although it is acknowledged that inflation may differ across countries, it was adopted in similar exercises previously [Citation11]. The average length of stay (LOS) for COVID-related hospitalization was assumed to be 8 days based on LOS assumptions used in the prior COVID-19-specific inpatient studies [Citation12,Citation21–24], while two outpatient visits were assumed per infection based on a prior study by Di Fusco et al. [Citation20]. For productivity loss, it was assumed that 2 weeks would be lost due to infection. Workforce participation rates and gross national income (GNI) per capita for each country were sourced from the World Bank data to define region-specific rates [Citation38,Citation39]. To avoid double counting, productivity loss related to reported deaths was excluded from estimates for overall cost savings and for productivity loss. Region-specific average costs per outpatient visit and inpatient bed day are listed in supplementary Table ST1, and average GNI and labor force participation rates are provided in supplementary Table ST2.
2.4. Analysis
2.4.1. Base-case analysis
The base case analysis estimated the health outcomes averted due to COVID-19 vaccination in terms of the number of COVID-19 cases and deaths prevented over the study period, along with the corresponding direct outpatient and productivity costs saved, compared with no vaccination, from the healthcare and societal perspective, respectively.
2.4.2. Adjustment for underreporting of COVID-19 cases and deaths
Evidence from COVID-19 case studies has raised concerns regarding underreporting of COVID-19-related deaths and infections across countries. Underreporting may result from a lack of COVID-19 testing or reporting infrastructure, or cultural or financial barriers. Rahmandad et al. [Citation40] concluded that infections and deaths were widely underreported across 92 countries, with an estimated ratio of actual to reported cumulative cases of 7.03 (10th to 90th percentile: 3.2 to 18.0). Further, Lau et al. [Citation41] estimated that only 1–2% of total infections were detected in the United States, Italy, Spain, and France. Prior published dynamic models estimating COVID-19 burden have also adjusted for underreporting [Citation13,Citation17].
We, therefore, adjusted for underreporting of cases and deaths using the ratios reported by Rahmandad et al. [Citation40]. Point estimates were extracted to compute the medians and interquartile ranges (IQR) for underreporting of deaths and cases among the countries within each region (supplementary Table ST3 and ST4). Base case results were adjusted by the median underreporting ratios as well as the 25th (Q1) and 75th (Q3) percentiles (). Subsequently, cost savings due to avoided outpatient costs and productivity loss were calculated for all regions.
Table 2. Base case analysis: public health and economic impact of COVID-19 vaccines.
2.4.3. Scenario analysis
Since data on COVID-19-related incident hospitalizations were available only for 26 countries, cost savings from preventing hospitalizations were assessed in a scenario analysis to understand the scale of economic impact. Because underreporting ratios for hospitalization were not available, we used the median (Q1-Q3) underreporting ratios for deaths reported by Rahmandad et al. [Citation40] as a proxy to estimate the impact of vaccines on COVID-19-related hospitalization and associated costs .
2.4.4. Sensitivity analyses
2.4.4.1. Sensitivity analysis using an alternative VE assumption
The base case analysis used an above-brand VE that assumed equal use of all available COVID-19 vaccine brands in a particular country. Data were available from a total of 42 countries on the relative proportions of each vaccine brand administered in that country [Citation42]. A sensitivity analysis was performed applying an alternative above-brand VE assumption, wherein brand-specific VEs were weighted according to their relative proportion of administration.
2.4.4.2. Sensitivity analysis using an alternative underreporting assumption for estimating deaths
Estimated underreporting of COVID-19-related deaths by WHO region, based on the Rahmandad et al. [Citation40] reported ratios for deaths, was relatively low. With the exception of Europe, all regions had a median of 100% underreporting for deaths. In contrast, the median value for Europe indicated no underreporting of deaths. Hence, we further conducted a sensitivity analysis using the 25th percentile of each region’s case underreporting ratio as an aggressive scenario to estimate the COVID-19-related deaths.
3. Results
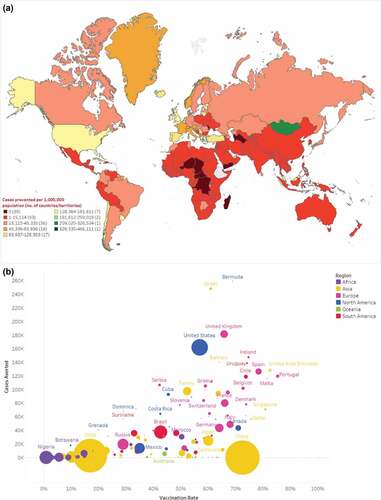
Between 13 December 2020 and 30 September 2021, there were an estimated 1.1 billion infections and 4.7 million deaths (medians) across 215 countries/territories after adjusting reported data for underreporting (). At the regional level, North America had the highest incidence of infections (99,900 per one million population), while Africa had the lowest (1,622 per one million population). By September 2021, an estimated 2.6 billion individuals had completed COVID-19 primary series vaccination (). Vaccination uptake was generally highest in Europe with Portugal having the highest uptake (85%) and lowest in Africa where several countries had less than 1% uptake (). These patterns aligned with the start dates of vaccine campaign rollout. For example, most African countries did not begin vaccination campaigns until late spring or summer 2021, and therefore had shorter follow-up time compared to other areas (median: 4.3 months; range: 0.3–7.9 months). In contrast, nearly all European countries had started vaccination campaigns by March 2021, with a median follow-up of 8.4 months (range: 3.4–9.3 months) ().
Figure 1. a estimated median number of Covid-19 cases prevented per 1,000,000 population as of September 2021; b scatterplot of estimated median number of COVID-19 cases prevented per 1,000,000 population and COVID-19 vaccine uptake as of September 2021.
3.1. Base-case analysis
3.1.1. Health outcomes averted
As of September 2021, COVID-19 vaccines prevented an estimated median (Q1-Q3) of 151.7 (133.7–226.1) million infections and 620.5 (411.1–698.1) thousand deaths globally, over a median follow-up of 5.8 months ().
Country-specific estimates of averted infections and deaths per one million population are shown in and supplementary Figure S2, respectively. Scatterplots showing the unadjusted relationship between vaccine uptake and clinical outcomes at the country-level are provided in and supplementary Figure S3, and by WHO region in supplementary Figures S4-10. Total estimated cases and deaths averted were highest for North America [median (Q1-Q3): 59.2 (53.6–106.3) million] and Europe [median (Q1-Q3): 45.7 (40.5–46.9) million], and lowest in Oceania [median (Q1-Q3): 170.0 (170.0–170.0) thousand] and Africa [median (Q1-Q3): 2.0 (1.8–2.8) million] (). Per one million population, estimated median COVID-19 infections prevented were highest for North America, followed by Europe (99,900 and 60,860, respectively) with both regions having similar vaccine uptake (48.3% and 52.2%, respectively) (, and S4). North America also had the highest number of averted median deaths per one million population, followed by South America (481 and 281, respectively), but vaccine uptake in South America was lower at 30.8% (Figure S2-4).
Estimated infections and deaths averted were substantially higher for high-income countries, especially the US and EU-5 countries (UK, France, Germany, Spain, and Italy), than for low- and middle-income countries (LMIC) (Figure S5-10). Estimates for health outcomes averted varied widely for high-income countries despite having similar vaccine uptake and follow-up. For instance, vaccine uptake in the US, UK, and France was similar (56.9%, 65.8%, and 64.1%, respectively) but the number of deaths averted per million population was much higher for the US (750, 153, and 120, respectively), while the number of infections averted per million population was much lower for France (161,598; 181,611; and 80,207, respectively). Estimated total health outcomes averted for China were substantially low (median infections: 9,818; deaths: 0) despite the high vaccine uptake of 72.6%, but limited median follow-up of only 1.3 months.
3.1.2. Economic outcomes averted
COVID-19 vaccines saved an estimated median (Q1-Q3) of $21.1 ($18.9–30.9) billion through averted direct costs related to outpatient care, and an additional $135.1 ($121.1–206.4) billion in indirect costs through averted COVID-19-related productivity loss ().
Country-specific estimated total cost savings related to the outpatient care and productivity loss are shown in , respectively. Regionally, median direct outpatient cost savings through prevented COVID-19 infections ranged from $29.8 million for Oceania to $11.0 billion for North America. By country, the US, UK, and France had the highest estimated median direct outpatient cost savings of $10.6 billion, $2.3 billion, and $1.1 billion, respectively. Thirty-seven countries/territories were estimated to have no direct cost savings (i.e. $0) since there were no observed clinical benefits (Table ST5). Of these 37 countries/territories, 24 (66.7%) had populations of less than 200,000, while four countries from Asia and Africa with populations of more than 5 million had very low vaccine uptake (≤4%). Additionally, nine countries/territories had missing data on clinical inputs, and hence corresponding economic outcomes could not be assessed for these countries.
Figure 2. Estimated median cost savings (million, 2021 USD) through averted direct outpatient costs as of September 2021.
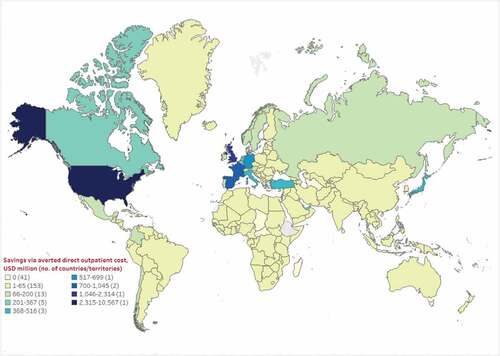
Figure 3. Estimated median cost savings (million, 2021 USD) through averted productivity loss as of September 2021.
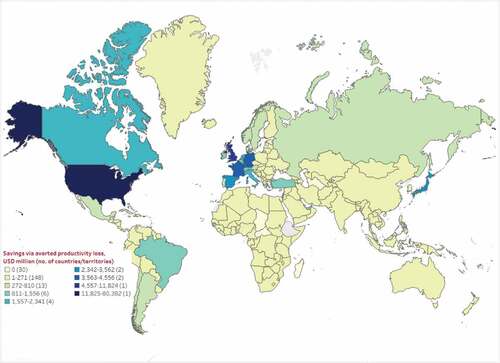
Median indirect cost savings from averted COVID-19-related productivity loss ranged from $147.6 million in Africa to $83.0 billion in North America. As expected, based on the results for direct cost savings, the US, UK, and France also had the highest median cost savings through avoided productivity loss at $80.4 billion, $11.8 billion, and $4.6 billion, respectively (Table ST5). Twenty-five countries/territories were estimated to have no indirect cost savings (i.e. $0) based on no observed health outcomes averted, while 22 countries/territories did not have clinical and/or labor participation/income data, and hence corresponding productivity loss-related cost savings could not be assessed for these countries.
3.2. Scenario analysis
A total of 26 countries (21 in Europe, 2 in Asia, South Africa, Chile, and the US) had data available on COVID-19-related hospitalization rates. During the study period, approximately 1% of infections were hospitalized in these countries. A total of 6.6 million COVID-19-related hospitalizations were reported, of which the US accounted for 4.4 million hospitalizations. Vaccine campaigns started between 13 December 2020 and 24 February 2021, average vaccine uptake was 60%, and follow-up time for these countries ranged from 7.2 to 9.6 months (). Assuming underreporting ratios for hospitalizations to be the same as for deaths, COVID-19 vaccines prevented an estimated median (Q1-Q3) of 2.4 (1.5–2.5) million hospitalizations, ranging from a median of only 6 in Luxembourg to 1.8 million in the US (). Consequently, median total hospitalization costs averted had wide variability, ranging from a low of $0.2 million (Luxembourg) to a high of $17.9 billion (United States). Median (Q1-Q3) total COVID-19-related outpatient or inpatient cost savings were approximately $39.2 ($28.1–48.3) billion, including $21.1 ($12.4–21.9) billion saved in hospitalization costs and $18.1 ($16.2–26.4) billion saved in outpatient costs for these 26 countries.
Table 3. Scenario analysis: global impact of COVID-19 vaccines on hospitalization and costs.
3.3. Sensitivity analyses
3.3.1. A) Sensitivity analysis using an alternative VE assumption
After applying an alternative VE assumption that accounted for the relative proportions of vaccine brands administered (available for 42 countries), the sensitivity analysis indicated substantial variability in estimated clinical and economic outcomes averted compared to the base case, ranging from −23% to +19% for the three outcomes of cases, outpatient costs, and productivity loss; and from −40% to +20% for deaths (supplementary Table ST6).
Chile, followed by Uruguay and Ecuador, had the highest (negative) discrepancies compared to the base case, suggesting that base case analyses for South America may have applied an overestimated above brand VE. South Africa had the highest positive discrepancy for all four outcomes, which suggests that the clinical and economic impacts for South Africa may be up to 20% higher than that estimated in the base case analysis.
3.3.2. B) Sensitivity analysis using an alternative underreporting assumption for estimating deaths
After adjusting for underreporting of COVID-19-related deaths by applying the region-specific 25th percentile for underreporting ratios for COVID-19 cases reported by Rahmandad et al. [Citation40] COVID-19 vaccines prevented an estimated 2.1 million COVID-19-related deaths, a difference of 1.5 million more deaths compared to 620.5 thousand averted deaths in the base case (supplementary table ST7) .
4. Discussion
Using a conservative approach that considered only the direct impacts of vaccination, our results show that COVID-19 vaccines have provided immense public health and economic benefits globally. We estimated that COVID-19 vaccines prevented a median of 151 million infections and 620 thousand deaths worldwide by September 2021. Our estimates for prevented deaths, however, were very sensitive to under-reporting assumptions, which increased by up to 2.1 million after applying a less conservative assumption. In addition, we estimated that $155 billion in costs related to outpatient care and COVID-19-related productivity loss were saved through averted infections. The global cost savings would be substantially higher, given that we estimated a median savings of $21 billion due to inpatient costs alone across the 26 countries with available data on hospital admissions. The highest estimated benefits in terms of both COVID-19-related deaths and infections averted and outpatient costs and productivity losses saved were observed in high-income countries, potentially due to earlier vaccine roll-out and higher vaccine uptake in these countries compared to LMICs.
Our findings are consistent with previous studies that have estimated the public health impact of COVID-19 vaccination at the country or regional level [Citation11–15,Citation19,Citation43,Citation44]. Notably, our results are very similar to estimates reported by Steele et al. [Citation13] on the impact of COVID-19 vaccination (defined as 2 doses of mRNA vaccines or 1 dose of JNJ-78436735 vaccine) among US adults aged 18 years or older during 1 December 2020 to 30 September 2021. Steele et al. [Citation13] estimated that approximately 1.6 million hospitalizations and 235,000 deaths were prevented, which is consistent with our estimates of 1.8 million hospitalizations and 249,546 deaths averted among the US population of all ages during a similar time period. However, our estimate of averted infections was substantially higher than that reported by Steele et al. [Citation13] (53.7 million averted among all ages vs 27 million among adults), largely owing to methodological differences. Our estimates for averted infections were based on observed numbers of cases, while Steele et al. [Citation13] estimated averted infections by applying an age-specific ratio of infection-to-hospitalization. After applying the same age-weighted infection-to-hospitalization ratio of 16.7 to our model, we estimated that 30.6 million infections were averted among the US population, in close alignment with the results of Steele et al. [Citation13].
The modeling study by Watson et al. [Citation17] estimated that COVID-19 vaccination averted 14.4 million COVID-19-related deaths globally between 8 December 2020 and 8 December 2021 [Citation17]. This estimate is substantially higher than our estimate for deaths averted and is likely due to several major methodological differences between the studies. First, Watson et al. [Citation17] used an age-structured susceptible-exposed-infectious-recovered-susceptible (SEIRS) model that captured the indirect impact of COVID-19 vaccination, including benefits from reduced SARS-CoV-2 transmission (i.e. assuming post-vaccination infections would be less infectious than infections among unvaccinated individuals) [Citation45], while we employed a static model that considered only the direct impacts of COVID-19 vaccination. Second, Watson et al. [Citation17] assumed a vaccination scenario that prioritized individuals most at risk (e.g. healthcare workers), whereas we relied solely on observed vaccine roll-out data and did not differentiate groups based on clinical risk factors, occupation or age. Third, our study had a time horizon that was shorter by 3 months. Lastly, we evaluated the impact of receiving a complete primary series, while Watson et al. [Citation17] assessed the impact of receiving any COVID-19 vaccination (regardless of the number of doses received). Data have shown that individuals who are partially vaccinated are less protected against asymptomatic and symptomatic infection and severe disease compared to the primary series completion; although in the early phase of the pandemic due to vaccine supply constraints, some countries used dose allocation strategies that prioritized vaccinating a larger proportion of the population.
This study is among the first to quantify cost savings in the outpatient setting and through averted productivity loss at a global level, as well as savings through avoided hospitalization costs for countries with available data. Previous economic evaluations have confirmed the cost-effectiveness of COVID-19 vaccines across countries [Citation46–50]. Few studies, however, have quantified the economic benefits due to COVID-19 vaccine programs, which may inform global health budget allocation. Quantifying the economic benefits is challenging, as COVID-related healthcare cost data are sparse and cost estimates can vary considerably according to patient characteristics and access to care, even within the same country/region, particularly in the in-hospital setting [Citation20,Citation21,Citation31,Citation33,Citation51,Citation52]. Given the limited COVID-specific cost data and the need for consistency in cost estimates across countries, we used standardized WHO CHOICE data [Citation36], which have been widely acknowledged as robust inputs for economic analysis [Citation53,Citation54]. Nonetheless, our results likely underestimate the true economic benefits of COVID-19 vaccination. Future studies quantifying the economic benefits of vaccination are warranted as emerging cost data continue to accumulate in the literature. Additionally, data on hospital admissions were available for only 26 countries, which limited our economic evaluation of severe cases that are known to consume the most healthcare resources. Bell et al. [Citation11] recently examined the clinical and economic impact of COVID-19 vaccination on hospitalization in 92 countries. Using a linear modeling of IHME-reported epidemiological data and projections, the authors estimated that COVID-19 vaccination had averted 6 million hospitalizations and saved hospital resources worth $59 billion from January 2021 through December 2021 across the 92 countries [Citation11].
To our knowledge, this study is the first to quantify the global impact of COVID-19 disease and of vaccination on work productivity. Wang et al. used data from the U.S. Research and Development Survey (RANDS), and found that weekly productivity or loss in earnings due to oneself or a family member being sick from COVID-19 was up to $588 for adults aged 45–64 years, and up to $611 for adults aged ≥65 years during May 2021–June 2021 [Citation55]. The COVID-19 pandemic has resulted in substantial temporary loss of human capital due to absences from work and reduced productivity of the working population [Citation56]. As such, it is critical to adopt a broad societal perspective when evaluating the value of COVID-19 vaccination programs. An economic evaluation of COVID-19 vaccination in Denmark has suggested that the cost-effectiveness of vaccinating the younger population is highly dependent on whether productivity losses are included in the analyses [Citation57]. In the present study, we estimated that globally, COVID-19 vaccination has averted $135.1 billion related to productivity loss, which underscores the economic benefits and importance of continued vaccination campaigns among working age adults, whose physical health is a critical determinant of productivity and earnings. Worth noting, however, is that when estimating cost savings through averted COVID-19-related productivity loss, we assumed an average of 2 weeks of lost working days due to infection for all COVID-19 cases based upon average duration of COVID-19 illness and quarantine recommendations, in the absence of age-specific and country-specific data [Citation25,Citation26]. In the real-world setting, however, work loss likely varies by age-group, occupation, and geography.
The results of this study should be considered in the context of several assumptions and the following limitations. First, while most model inputs were derived from surveillance data and published literature, one of the biggest challenges was limited data at the global level on COVID-19 burden, vaccination coverage, and COVID-specific economic inputs. COVID-19-related hospitalization data were available only for 26 countries. Thus, our estimates for COVID-19 inpatient-related cost savings were very conservative. Further, data on the relative proportion of administered doses by vaccine brand were not available for most countries, which potentially made determining precise estimates of vaccine effectiveness difficult in many countries. Although the NPIs might be different across countries (e.g. China’s zero COVID policy [Citation58]), we assumed that the synergetic effect of vaccination and NPIs would remain unchanged during the study period in the absence of vaccination. Further research is warranted to better understand their individual effects. In addition, we used a static SIR model based on the OWID data for reported COVID-19 burden, while dynamic models have been used in other studies [Citation13,Citation17]. Hence, the impact of vaccination on reducing transmission could not be incorporated in our static model, which might underestimate the vaccination benefits. Another limitation is that observed COVID-19 burden may be underreported, hence we adjusted for underreporting of COVID-19 infections and deaths by applying median under-reporting factors previously reported by Rahmandad et al. [Citation40]. Additionally, due to the lack of subgroup-specific data on vaccine coverage, and VE against COVID-19 infections and deaths across countries, vaccine uptake and VE against health outcomes were assumed to be the same for all individuals, regardless of age and other patient characteristics (e.g. immunocompromised status, occupation), which might underestimate the benefits of vaccination in high-risk groups. We also did not account for variant-specific VE or waning immunity in the model due to the limited study period. Further, we did not consider reinfections in our model since information was not available on whether cases reported in the OWID dataset included reinfections. The end of the model time horizon corresponded to approximately the same time as the emergence of the Delta variant, when re-infections were less common relative to the current Omicron era [Citation59]. Additionally, our estimates for COVID-19 infections and severe disease averted focused specifically on the direct clinical benefits of COVID-19 vaccination among vaccinated individuals, without accounting for the benefits of partial vaccination or broader societal value of vaccination [Citation60]. Hence, our estimates represent only a fraction of the overall burden of COVID-19 averted by primary series COVID-19 vaccination and should be interpreted in that context. Moreover, vaccine hesitancy or perception across COVID-19 vaccine types was not considered in the model explicitly, and was assumed to be implicitly reflected in vaccine uptake [Citation61]. We also did not account for COVID-19 vaccine-related adverse events or side effects in the model. Finally, as the analytic time horizon of this study preceded the rollout of booster vaccination, our model did not consider the potential benefits of booster vaccines nor vaccination on the long-term complications of COVID-19, both of which likely provide important additional value and should be quantified in future studies.
5. Conclusion
COVID-19 vaccines have provided immense and far-ranging global public health and economic benefits. Millions of infections and deaths and billions of dollars in economic costs have been averted through vaccination. Despite these benefits, more than 30% of the world’s population and more than 75% of the population in low-income countries remain unvaccinated as of October 2022. Continued improvement in uptake of COVID-19 vaccines remains our most important tool to ensure pandemic preparedness and to limit the impact of SARS-CoV-2 on health systems globally.
Author contributions
All authors contributed to the study conception, design, data acquisition, analysis, interpretation, and drafting and revising the manuscript.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Availability of data and materials
Data generated or analyzed during this study are available upon request.
Declaration of interests
J Yang, M Di Fusco, TL Wiemken, MH Kyaw, JM. McLaughlin, and JL Nguyen are employees of Pfizer Inc. and may hold stock or stock options. S Vaghela is an employee of HealthEcon Consulting Inc. and an external consultant for Pfizer who has received consulting fees from Pfizer in connection with the development of this study and manuscript. B Yarnoff and S De Boisvilliers are employees of Evidera, which was a paid consultant to Pfizer in connection with the development of this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work.
Ethical approval
These analyses used data from previously conducted studies and did not include data from any new studies with human participants or animals performed by any of the authors, hence ethical approval was not required.
Supplemental Material
Download MS Word (1.8 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2157817
Additional information
Funding
References
- Kaye AD, Okeagu CN, Pham AD, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: international perspectives. Best Pract Res Clin Anaesthesiol. 2021;35(3):293–306.
- The impact of COVID-19 on health and health systems [Internet]. OECD. 2022 cited 2022 Jul 24]. Available from 2022 Jul 24: https://www.oecd.org/health/covid-19.htm.
- Coronavirus (COVID-19) Dashboard [Internet]. World Health Organization. 2022 cited 2022 Jul 24]. Available from 2022 Jul 24: https://covid19.who.int/.
- Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–1496.
- Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Affairs. 2020;39(6):927–935.
- Gaunt ER, Harvala H, McIntyre C, et al. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52(3):215–221.
- Murray CJL, Lopez AD, World Health O, et al. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary/edited. 1996. Murray CJL, Lopez AD. Geneva: World Health Organization
- Kempler C How covid-19 mitigation measures disproportionately impacted women and girls 2022 [Internet]. Johns Hopkins Bloomberg School of Public Health. 2022 cited 2022 Jul 23]. Available from 2022 Jul 23: https://publichealth.jhu.edu/2022/covid-19-shutdowns-disproportionately-harmed-women.
- COVID-19 response health equity strategy: accelerating progress towards reducing covid-19 disparities and achieving health equity 2022 [Internet]. Centers for Disease Control and Prevention (CDC). 2022 cited 2022 Jun 20]. Available from 2022 Jun 20: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/cdc-strategy.html.
- Coronavirus Pandemic (COVID-19) [Internet]. Our World In Data. 2022 cited 2022 Apr 22]. Available from 2022 Apr 22: https://ourworldindata.org/coronavirus.
- Bell E, Brassel S, Oliver E, et al. Estimates of the global burden of COVID-19 and the value of broad and equitable access to COVID-19 vaccines. Vaccines (Basel). 2022;10(8):1320.
- Di Fusco M, Marczell K, Deger KA, et al., Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. J Med Econ. 2022. 25(1): 605–617.
- Steele MK, Couture A, Reed C, et al., Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. JAMA Network Open. 2022. 5(7): e2220385–e.
- Sacco C, Mateo-Urdiales A, Petrone D, et al. Estimating averted COVID-19 cases, hospitalisations, intensive care unit admissions and deaths by COVID-19 vaccination, Italy, January−September 2021. Euro surveillance. 2021. 26(47): 2101001.
- Arbel R, Moore CM, Sergienko R, et al. How many lives do COVID vaccines save? Evidence from Israel. Am J Infect Control. 2022;50(3):258–261.
- Li Z, Liu X, Liu M, et al. The effect of the COVID-19 vaccine on daily cases and deaths based on global vaccine data. Vaccines (Basel). 2021;9(11):1328.
- Watson OJ, Barnsley G, Toor J, et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022. 22(9): 1293–1302.
- Chapman R, Sutton K, Dillon-Murphy D, et al. Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: a modelling analysis. Vaccine. 2020;38(45):7138–7145.
- Impact of COVID-19 vaccines on mortality in England: December 2020 to March 2021 [Internet]. Public Health of England. 2021 cited 2022 Jun 2]. Available from 2022 Jun 2: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/977249/PHE_COVID-19_vaccine_impact_on_mortality_March.pdf
- Scott A, Chambers R, Reimbaeva M, et al. Real-world retrospective analysis of patient characteristics, healthcare resource utilization, costs, and treatment patterns among unvaccinated adults with COVID-19 diagnosed in outpatient settings in the United States. J Med Econ. 2022;25(1):287–298.
- Ohsfeldt RL, Choong CK, Mc Collam PL, et al. Inpatient hospital costs for COVID-19 patients in the United States. Adv Ther. 2021;38(11):5557–5595.
- Yeates EO, Nahmias J, Chinn J, et al. Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure. PLOS ONE. 2021;16(6):e0253767.
- Patone M, Thomas K, Hatch R, et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: an observational cohort study. Lancet Infect Dis. 2021;21(11):1518–1528.
- Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275–1287.
- Faramarzi A, Javan-Noughabi J, Tabatabaee SS, et al. The lost productivity cost of absenteeism due to COVID-19 in health care workers in Iran: a case study in the hospitals of mashhad university of medical sciences. BMC Health Serv Res. 2021;21(1):1169.
- Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). The World Health Organization. 2020. . cited 2022 Nov 28]. Available from 2022 Nov 28: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- COVID-19 vaccine tracker [internet]. VIPER group COVID19 vaccine tracker. 2022 [cited 2022, May 31]. Available from 2022 May 31: https://covid19.trackvaccines.org/.
- COVID-19 vaccine efficacy summary [internet]. The institute for health metrics and evaluation (IHME). 2022 [cited 2022 Jun 1]. Available from 2022 Jun 1: https://www.healthdata.org/covid/covid-19-vaccine-efficacy-summary.
- Barasa E, Kairu A, Ng’ang’a W, et al. Examining unit costs for COVID-19 case management in Kenya. BMJ Glob Health. 2021;6(4):e004159.
- Ismaila H, Asamani JA, Lokossou VK, et al. The cost of clinical management of SARS-COV-2 (COVID-19) infection by level of disease severity in Ghana: a protocol-based cost of illness analysis. BMC Health Serv Res. 2021;21(1):1115.
- Jin H, Wang H, Li X, et al. Economic burden of COVID-19, China, January-March, 2020: a cost-of-illness study. Bull World Health Organ. 2021;99(2):112–124.
- López-Valcárcel BG, Vallejo-Torres L. The costs of COVID-19 and the cost-effectiveness of testing. Appl Econ. 2021;29(85):77–89.
- Maltezou HC, Giannouchos TV, Pavli A, et al. Costs associated with COVID-19 in healthcare personnel in Greece: a cost-of-illness analysis. J Hosp Infect. 2021;114:126–133.
- Nakhaei K, Jalilian H, Arab-Zozani M, et al. Direct and indirect cost of COVID-19 patients in Iran. Health Policy Technol. 2021;10(4):100572.
- Tsai Y, Vogt TM, Zhou F. Patient characteristics and costs associated with COVID-19-related medical care among medicare fee-for-service beneficiaries. Ann Intern Med. 2021;174(8):1101–1109.
- WHO-CHOICE estimates of cost for inpatient and outpatient health service delivery 2021 [Internet]. World Health Organization (WHO). 2008 cited 2022 Jun 10]. Available from 2022 Jun 10: https://www.who.int/publications/m/item/who-choice-estimates-of-cost-for-inpatient-and-outpatient-health-service-delivery.
- Statistics USBoL - consumer price index [Internet]. US Bureau of Labor Statistics. 2022 cited 2022 Jul 1]. Available from 2022 Jul 1: https://www.bls.gov/cpi/.
- Labor Force Participation Rates [Internet]. World Bank. 2022 cited 2022 Jun 27]. Available from 2022 Jun 27: https://data.worldbank.org/indicator/SL.TLF.CACT.ZS.
- GNI per capita, atlas method (current US$) [Internet]. World Bank. 2022 cited 2022 Jun 27]. Available from 2022 Jun 27: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD.
- Rahmandad H, Lim TY, Sterman J. Behavioral dynamics of COVID-19: estimating underreporting, multiple waves, and adherence fatigue across 92 nations. Syst Dyn Rev. 2021;37(1):5–31.
- Lau H, Khosrawipour T, Kocbach P, et al. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology. 2021;27(2):110–115.
- COVID-19 vaccine doses administered by manufacturer [Internet]. Our World in Data. 2022 cited 2022 Jun 15. Available from: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer?country=~European+Union.
- Tan ST, Park HJ, Rodríguez-Barraquer I, et al. COVID-19 vaccination and estimated public health impact in California. JAMA Network Open. 2022;5(4):e228526–e.
- Vilches TN, Moghadas SM, Sah P, et al. Estimating COVID-19 infections, hospitalizations, and deaths following the US vaccination campaigns during the pandemic. JAMA Network Open. 2022;5(1):e2142725–e.
- Eyre DW, Taylor D, Purver M, et al. Effect of covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022;386(8):744–756.
- Kohli M, Maschio M, Becker D, et al. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–1164.
- Sandmann FG, Davies NG, Vassall A, et al. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis. 2021;21(7):962–974.
- Reddy KP, Fitzmaurice KP, Scott JA, et al. Clinical outcomes and cost-effectiveness of COVID-19 vaccination in South Africa. Nat Commun. 2021;12(1):6238.
- Wang WC, Fann JC, Chang RE, et al. Economic evaluation for mass vaccination against COVID-19. J Formos Med Assoc. 2021;120 Suppl 1:S95–s105.
- Vaezi A, Meysamie A. COVID-19 vaccines cost-effectiveness analysis: a scenario for Iran. Vaccines (Basel). 2021;10(1). 10.3390/vaccines10010037
- Calderón-Moreno J, Juárez-Vela R, Delgado-Rodríguez MJ, et al. Approximation to the consumption of healthcare resources and the economic cost of SARS-CoV-2 patient management: a retrospective study. Front Public Health. 2022;10:843751.
- Thant PW, Htet KT, Win WY, et al. Cost estimates of COVID-19 clinical management in Myanmar. BMC Health Serv Res. 2021;21(1):1365.
- Stenberg K, Lauer JA, Gkountouras G, et al. Econometric estimation of WHO-CHOICE country-specific costs for inpatient and outpatient health service delivery. Cost Eff Resour Alloc. 2018;16(1):11.
- Zhang S, Incardona B, Qazi SA, et al. Cost-effectiveness analysis of revised WHO guidelines for management of childhood pneumonia in 74 Countdown countries. J Glob Health. 2017;7(1):010409.
- Wang F, Wang JD. Estimating US earnings loss associated with COVID-19 based on human capital calculation. Int J Environ Res Public Health. 2022;19(2):1015.
- Nurchis MC, Pascucci D, Sapienza M, et al. Impact of the burden of COVID-19 in Italy: results of disability-adjusted life years (DALYs) and productivity loss. Int J Environ Res Public Health. 2020;17(12). 10.3390/ijerph17124233.
- Debrabant K, Grønbæk L, Kronborg C. The cost-effectiveness of a COVID-19 vaccine in a Danish context. Clin Drug Investig. 2021;41(11):975–988.
- Bai W, Sha S, Cheung T, et al. Optimizing the dynamic zero-COVID policy in China. Int J Biol Sci. 2022;18(14):5314–5316.
- Özüdoğru O, Bahçe YG, Acer Ö. 2022. SARS CoV-2 reinfection rate is higher in the Omicron variant than in the Alpha and Delta variants. Ir J Med Sci. 1971. 10.1007/s11845-022-03060-4.
- Kirson N, Swallow E, Lu J, et al. The societal economic value of COVID-19 vaccines in the United S tates. J Med Econ. 2022;25(1):119–128.
- Kutasi K, Koltai J, Szabó-Morvai Á, et al. Understanding hesitancy with revealed preferences across COVID-19 vaccine types. Sci Rep. 2022;12(1):13293.

