ABSTRACT
Introduction
Hexaxim is a hexavalent vaccine approved as primary and booster vaccination in infants 6 weeks and older, protecting against diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B and Haemophilus influenzae type b.
Areas Covered
To evaluate the immunogenicity and reactogenicity (safety) of Hexaxim (Hexyon, Hexacima) in primary and booster vaccine schedules; long-term antibody persistence; concomitant use with other childhood vaccines and use in immunocompromised infants. Hexaxim was found to be noninferior to other licensed hexavalent vaccines, being highly immunogenic for all toxoids/antigens and with an acceptable safety profile. It can be administered concomitantly with other childhood vaccines. Hexaxim can be given as a booster for infants primed with Infanrix Hexa and given in a pentavalent-hexavalent-pentavalent series. Hexaxim elicits a similar immune response and safety profile in human immunodeficiency virus (HIV) positive infants. It has the benefit of being a ready-to-use liquid formulation, minimizing dosage errors and preparation time.
Expert Opinion
Hexaxim has an acceptable safety profile and provides immunity against all six targeted diseases. It is an acceptable alternative to other hexavalent vaccines on the market. Further studies are required on the use of immunocompromised patients as well as the antibody persistence of each of the vaccine components.
1. Introduction
1.1. History of multivalent vaccines
Since the first vaccine against smallpox was developed in 1796, the number of both vaccine-preventable diseases has rapidly increased. Combination vaccinations containing more than one microbial antigen have been used since 1948 when diphtheria, tetanus, and pertussis antigens were grouped into a single product to vaccinate infants [Citation1,Citation2]. The need for multivalent vaccines was highlighted in 1995 when the term ‘pincushions’ was frequently used to describe childhood vaccination regimes where multiple injections of vaccines were required in single visits [Citation3]. Combination vaccines now include a diphtheria and tetanus toxoid base (DT) with either whole-cell pertussis (DTwP) or acellular pertussis (DTaP). Various antigens have been added, such as inactivated poliomyelitis (IPV) and Haemophilus influenzae type b (Hib) making pentavalent vaccines; Hepatitis B (HepB) is now often added to form a hexavalent vaccine covering six different diseases [Citation2].
1.2. Licensed hexavalent vaccines
Four hexavalent vaccines containing aP are currently licensed in Europe with three available on the European market. The first two aP-containing hexavalent vaccines licensed in 2000 were Hexavac (Sanofi Pasteur) and Infanrix Hexa (GSK) (DT3aP-IPV-Hib-HBV). Marketing authorization for Hexavac was suspended in 2005 and subsequently withdrawn in 2012 by the European Medicines Agency (EMA) because of concerns of insufficient protection against HepB [Citation4]. Hexaxim (Sanofi Pasteur) was licensed in 2012 outside of the European Union and in 2013 in Europe. Vaxelis (MCM) was approved in Europe in 2016 [Citation5]. These hexavalent vaccines protect against six of the nine recommended diseases in children less than 12 months of age [Citation6].
1.3. Hexaxm, hexyon, hexacima
Hexaxim (also referred to as Hexyon and Hexacima) (DT2aP-IPV-Hib-HBV) is a thiomersal free, hexavalent vaccination containing diphtheria toxoid (D), tetanus toxoid (T), and acellular pertussis (aP), with additional IPV, Hib antigens and Hep B virus. A vial (0.5 mL) contains: Diphtheria toxoid (not less than 20IU); Tetanus toxoid (not less than 40IU); Bordetella pertussis antigens including Pertussis Toxoid (PT) (25 µg) and Filamentous Hemagglutinin (FHA) (25 µg); Inactivated Poliovirus type 1 (40D antigen units), type 2 (8D antigen units) and type 3 (32D antigen units); HepB surface antigen (10 µg); and Hib polysaccharide polyribosylribitol (PRP) (12 µg) conjugated to Tetanus protein (22–33 µg) [Citation7]. The latest review of DT2aP-IPV-Hib-HBV was published in 2019 [Citation8] with additional data becoming available and included in this review.
1.4. Diphtheria
Before the 1940s, diphtheria was common in the UK. The introduction of diphtheria vaccination in 1942 led to a decrease in notified cases and deaths from 61,000 cases and 3,283 deaths in 1940, to 38 cases and 6 deaths in 1957 [Citation9]. Diphtheria is now a rare infection in the United Kingdom with only 33 cases reported between 2009 and 2017, 18 caused by C. diphtheriae and 15 by C. ulcerans. Twenty-three (67%) of the patients were not adequately vaccinated and the most common risk factor for infection was travel to endemic areas for C. diphtheriae and contact with an animal for C. ulcerans [Citation10].
Diphtheria vaccination is usually in the form of a DTaP containing vaccine with other antigens included. The primary series can be administered as early as six weeks with doses at least four weeks apart and the last dose given prior to six months. A booster dose is recommended at 12–23 months, 4–7 years, and 9–15 years of age [Citation11].
Immunological protection against diphtheria is antibody mediated. The disease is due to the diphtheria toxin, and immunity is dependent on antitoxin which is primarily IgG and measured in IU/mL by the in vitro seroneutralization assay. Other assays include the passive hemagglutination test and ELISA. The level of circulating diphtheria antitoxin to provide clinical immunity against disease, as determined by seroneutralization is 0.01IU/mL [Citation12].
1.5. Tetanus
In 2015, there were 56,743 deaths due to Clostridium tetani infection, of which 19,937 occurred in neonates and 36,806 in older children and adults. Deaths were in South Asia (45–47%) and sub-Saharan Africa (36–44%). From 1990 to 2015 the global death rate in neonates decreased by 90% [Citation13]. In the UK, there were 96 identified cases of tetanus between 2001 and 2014, of which the case fatality rate was 11%. The deaths were all in partly vaccinated adults over 45 years, showing the need for maintaining tetanus vaccination [Citation14]. In 2021, there were 11 cases of tetanus identified, with 7 cases in 2020 and 4 in 2019 [Citation15].
The minimum circulating antitoxin that ensures immunity against tetanus is assay-specific. There are several different methods available for measuring immunity against tetanus, including the in vivo neutralization and in vitro passive hemagglutination assays. All clinical trials discussed in this review measured tetanus immunity by ELISA, with a minimum protective level of antibody 0.01IU/mL, although, cases of tetanus have been reported in people with antitoxin concentrations above 0.01IU/mL [Citation16].
1.6. Pertussis immunity
Globally, there were more than 151,000 cases of pertussis in 2018. Pertussis is spread easily from person to person via droplets from coughing or sneezing. Patients are contagious up to 3 weeks from the beginning of the cough [Citation17]. In European Union/European Economic Area (EU/EEA) countries there were 35,627 cases in 2018 with 72% of the notified cases coming from 5 countries: Germany, The Netherlands, Norway, Spain, and the UK [Citation18]. In 2020, there were 994 cases reported, while in 2021 only 49 new cases. The COVID-19 pandemic and implementation of social distancing and lockdown in the UK from March 2020 significantly impacted both the spread and detection of pertussis infections. Since the introduction of the maternal vaccination program, there has been a decline in infantile Pertussis from 234/100,000 in 2012, to 0.7/100,000 in 2021 [Citation19].
Immunity to two virulence factors measured for DT2aP-IPV-Hib-HBV in clinical trials are the pertussis toxin (PT) and filamentous hemoglobin (FHA) [Citation5,Citation6]. Whole-cell pertussis (wP) vaccines can contain whole non-viable bacterial cells with all antigens and virulence factors being components of them. Conversely, acellular pertussis vaccines (such as DT2aP-IPV-Hib-HBV) use purified antigens, such as PT and FHA in the case of DT2aP-IPV-Hib-HBV. There are no generally accepted seroprotection thresholds for pertussis antigens, so a ≥ 4-fold increase from baseline antibody concentration was used as a surrogate of protection for PT and FHA [Citation20].
1.7. Polio immunity
In 1988, the World Health Assembly developed a Global Polio Eradication Initiative (GPEI) to eradicate polio worldwide [Citation21]. Since then, cases of polio have dropped 99% from an estimated 350,000 wild poliovirus cases in 1988 across 125 endemic countries, to only 6 cases reported in 2021. There are two main types of vaccines against polio, the live attenuated oral poliovirus vaccine (OPV) and the inactivated poliovirus vaccine (IPV). OPV is still in use in certain countries due to its lower cost, easier transportation, and the superior intestinal immunity induced when compared to IPV. Despite the advantages of OPV, developed countries have switched to IPV, due to OPV rarely causing paralytic disease [Citation22]. The virus has three serotypes, all of which are included in the DT2aP-IPV-Hib-HBV vaccine [Citation7,Citation23]. In June 2022, the UK Health Security Agency (UKHSA) announced that vaccine-derived poliovirus type 2 (VDPV2) had been repeatedly detected in sewage in London, UK [Citation24]. Several weeks later, the New York State Department of Health reported a case of acute flaccid paralysis caused by VDPV2 in an unvaccinated individual; the first case of poliomyelitis in almost a decade in the USA [Citation25]. A similar virus has also been detected in sewage from Jerusalem, Israel [Citation26]. Vaccines elicit serum antibodies that neutralize poliovirus infection with titers of 1:4, measured using sero-neutralization [Citation27].
1.8. Haemophilus influenzae type b
A total of 3,982 cases of invasive H. influenzae from 30 EU/EEA countries were reported in 2018 with France, Germany, and the UK accounting for 57%. Of these confirmed cases, 2,266 had a known serotyping result, 153 (3%) of which were type b. The notification rate has increased from 0.5/100,000 in 2010–2012, to 0.8 in 2018. Vaccination against Hib has led to a continued reduction in Hib infections with only 7% of the cases with a known type in 2018 being type b [Citation28].
Hib vaccines are based on the capsular ribosyl, and ribitol-phosphate polymers conjugated with either a protein carrier, diphtheria toxin, Neisseria meningitidis serogroup B membrane complex or a tetanus toxoid (PRP-T) as is the case with DT2aP-IPV-Hib-HBV. The occurrence of Hib is relatively low in the first few months due to the transfer of maternal IgG antibodies that are specific for PRP via the placenta. Once the maternal IgG antibodies wane, disease incidence increases and then decreases again after 5 years of age, peaking again in late adulthood. Due to the varying epidemiology of Hib disease globally, different vaccination schedules have been adopted by public health departments. The schedule used directly influences the immune response to vaccination with a 2-month interval between doses providing a better response than a 1-month interval. For vaccination to confer protection, the antibody threshold of ≥0.15 µg/mL must be met for short-term immunity whilst a level of ≥1.0 µg/mL is commonly used for long-term protection, these are measured using ELISA-based immunoassays [Citation29].
1.9. Hepatitis B
HepB is most frequently found in the Western Pacific with 116 and chronically infected, and African Regions with 81 million people infected. In endemic regions, HepB commonly spread perinatally from mother to child and from child to child via infected blood in the first few years of life. HepB can also be spread via needlestick injuries, piercings, tattoos, and bodily fluids. Infection acquired during childhood leads to chronic hepatitis in approximately 95% of cases, compared with infection acquired in adulthood which only leads to chronic infection in 5% of patients [Citation30]. WHO estimates that 296 million people were chronically infected with Hep B in 2019, with 1.5 million new cases/annum. A total of 820,000 deaths from HepB occurred in 2019, predominantly from cirrhosis and hepatocellular carcinoma [Citation30].
Vaccination against HepB is safe and effective with protective rates between 98% and 100%. Only the monovalent HepB vaccine is used in the neonatal period, as antigens in multivalent vaccines are not approved for use at this age. Vaccine efficacy studies show complete protection against both acute and chronic HepB with anti-HB levels >10IU/L (10mIU/mL) measured 1–3 months post-primary HepB vaccination schedule. This is commonly measured using commercial assays such as VITROS [Citation31].
1.10. Review aims
The aim of this systematic literature review was to evaluate the immunogenicity, long-term immunity and safety data currently available on DT2aP-IPV-Hib-HBV vaccination in infants. The immune response and level of protection was compared to alternative vaccines on the market, specifically Infanrix Hexa with its different pertussis and Hep B components.
2. Methods
This systematic literature review was performed using PubMed and ClinicalTrials.gov with the following keywords: ‘Hexaxim,’ ‘Hexacima,’ Hexacima,’ ‘DTaP-IPV-Hep B-PRP-T,’ ‘DTaP-IPV-Hep B-PRP~T,’ ‘DTaP-IPV-HB-PRP-T,’ and ‘DTaP-IPV-HB-PRP~T.’ The Sanofi Clinical Trials website [Citation32] was also reviewed for relevant studies. Articles were included if they were randomized trials, clinical trials, case–control studies, or cohort studies of patients given Hexaxim as a primary series, booster dosage, or both and were written in English. The immunogenicity and safety data of the included articles were evaluated.
3. Results
3.1. Overview
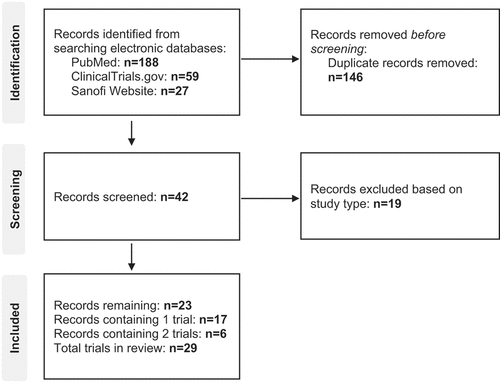
Twenty-three original articles, containing 29 individual trials were included in this systematic review (). There were 17 trials comparing the immunogenicity and safety of DT2aP-IPV-Hib-HBV with related vaccines () [Citation33–45], nine with DT3aP-IPV-Hib-HBV () [Citation36–38,Citation40–42,Citation45]. The remaining trials evaluated the immunogenicity and safety of DT2aP-IPV-Hib-HBV without comparing it to an equivalent hexavalent vaccine () [Citation46–52]. Several trials and observational studies evaluated the long-term persistence of antibodies post primary and diphtheria immunogenicity booster vaccine schedules [Citation53–55].
Table 1. Clinical trial characteristics comparing the immunogenicity and safety of primary and booster DT2aP-IPV-Hib-HBV with comparator vaccines.
Table 2. Immune response of DT2aP-IPV-Hib-HBV vs DT3aP-IPV-Hib-HBVfor each antigen post primary or booster vaccination.
Table 3. Clinical trial characteristics of studies without comparator vaccines.
3.2. Diphtheria immunity
The immunogenicity against diphtheria toxoid was evaluated in 26 trials [Citation33–43,Citation45–52,Citation54]. DT2aP-IPV-Hib-HBV was non-inferior when compared with other alternative hexavalent vaccines such as Infanrix Hexa [Citation36,Citation37,Citation40–42,Citation45] and the pentavalent vaccine Pentaxim in combination with a monovalent Hep B vaccine [Citation34,Citation35,Citation38,Citation39]. Anti-diphtheria antibody titers were similar in groups receiving DT2aP-IPV-Hib-HBV and alternative vaccines, including 13-valent pneumococcal conjugate vaccine (PCV13) [Citation34], PCV13 and Rotateq [Citation39] or pneumococcal 7-valent conjugate vaccine (PCV7) and Rotarix [Citation40].
The anti-diphtheria antibody seroconversion rates (≥0.01IU/mL) were 96–100% and 99–100% with Hexaxim and DT3aP-IPV-Hib-HBV, respectively. The percentage of participants with anti-diphtheria antibody levels ≥0.1IU/mL was comparable between each group at 62–100% and 56–100%, respectively [Citation36,Citation37,Citation40–42,Citation45]. The percentage of participants with anti-D ≥ 1.0IU/mL was higher for Hexaxim (26.5%) (95% CI 20.3; 33.3) than DT3aP-IPV-Hib-HBV (12.1%) (95% CI 7.8; 17.6) [Citation41]. Antipyretics given around the time of the Hexaxim or the alternative vaccine Infanrix Hexa, did not significantly affect immunogenicity of the vaccines [Citation40].
3.3. Tetanus immunogenicity
The immunogenicity against tetanus with DT2aP-IPV-Hib-HBV and alternative vaccines was also comparable, with 100% seroconversion rates (≥0.01IU/mL) for both DT2aP-IPV-Hib-HBV and DT3aP-IPV-Hib-HBV. The response rate of individuals with antibody concentrations ≥0.10 IU/mL was also high at 86–100% and 87–100% for DT2aP-IPV-Hib-HBV and DT3aP-IPV-Hib-HBV, respectively [Citation36,Citation37,Citation40–42].
A study by Kosalaraksa et al. (2011) [Citation41] showed that the seroconversion was higher (≥1.0IU/mL) in participants given DT3aP-IPV-Hib-HBV 88% (95% CI 82–92) than in those receiving DT2aP-IPV-Hib-HBV 71% (95% CI 64–77). When comparing DT2aP-IPV-Hib-HBV given alone or co-administration with the MenC-TT vaccine, there were notable differences in anti-T seroprotection and GMT at 12 months (≥0.10IU/mL). The group given only DT2aP-IPV-Hib-HBV saw a higher rate of protection at 100% (95% CI 98–100) than the group also given MenC-TT at 92% (95% CI 86–96), however there was no difference observed after the booster dose was given [Citation47]. There was also no difference in the protection observed when participants received antipyretics [Citation40].
3.4. Pertussis immunogenicity
DT2aP-IPV-Hib-HBV was found to be noninferior to other alternative vaccines with respect to the two pertussis antigens included within the vaccine and provided acceptable levels of immunity against pertussis [Citation33–43,Citation46–52,Citation54]. In the absence of a universally accepted correlation of protection, this was antibody levels ≥4 times the baseline (pre-vaccination) level. As shown in , the immune response in participants receiving DT2aP-IPV-Hib-HBV was not significantly different to the alternative vaccine Infanrix Hexa, with 79–94% and 80–96% achieving a 4-fold increase in anti-PT antibody titers, and 60–98% and 76–97% achieving a 4-fold increase in anti-FHA antibodies, respectively.
Three studies compared the immune response of DT2aP-IPV-Hib-HBV with an alternative vaccine containing whole cell pertussis rather than the acellular pertussis as with DT2aP-IPV-Hib-HBV [Citation33,Citation43,Citation54]. Madhi et al., 2013 [Citation43] demonstrated that vaccination with an acellular Pertussis-based vaccine (DT2aP-IPV-Hib-HBV) provided a similar rate of putatively protected participants than a whole-cell pertussis vaccination (91% vs 84%).
3.5. Polio immunogenicity
The rates of seroconversion for inactivated polioviruses 1, 2 and 3 were similar in all studies that evaluated poliomyelitis immunogenicity (33–43, 46–52, 54). Seroprotection (≥1:8) for IPV 1, 2 and 3 was achieved in 99–100% of study participants given both DT2aP-IPV-Hib-HBV and Infanrix Hexa [Citation36,Citation37,Citation40–42].
3.6. Haemophilus influenzae type b immunogenicity
Haemophilus influenzae type b immunogenicity was similar and adequate in groups receiving Hexaxim and the alternative vaccines [Citation33–43,Citation45–52,Citation54]. The immune response was shown to be noninferior, when comparing the proportion of people obtaining anti-Hib antibody levels higher than the correlation of protection (≥0.15 µg/mL), with 91–100% of the subjects receiving Hexaxim and 86–100% of the subjects receiving DT3aP-IPV-Hib-HBV, achieving ≥0.15 µg/mL anti-Hib antibody levels [Citation36,Citation37,Citation40–42].
Eighty-five percent (95% CI 79–89) of subjects in the DT2aP-IPV-Hib-HBV group achieved ≥1.0 µg/mL anti-Hib antibody titers compared to 71% (95% CI 64–77) receiving DT3aP-IPV-Hib-HBV [Citation41]. Similarly, anti-PRP-T antibodies ≥1.0 µg/mL were higher in participants post-primary vaccination schedule who received DT2aP-IPV-Hib-HBV at 59% (95% CI 52–65) compared with those given Infanrix Hexa at 37% (95% CI 31–44) [Citation42].
GMC levels were similar, but some studies observed differences between groups, for example, the GMC (µg/mL) of anti-PRP-T antibodies were higher with the alternative vaccine group than the group given Hexaxim, with GMT levels of 5.18 (95% CI 4.47–6.00) and 3.31 (95% CI 2.69–4.08) [Citation33]. Conversely, the GMC levels have been shown to be higher when given DT2aP-IPV-Hib-HBV than DT3aP-IPV-Hib-HBV [Citation41].
3.7. Hepatitis B immunogenicity
HepB immunogenicity was measured in all studies [Citation33–55] and similar protective responses were found in participants given DT2aP-IPV-Hib-HBV and alternative vaccines. Immunogenicity (≥10mIU/mL anti-HepB antibodies) achieved with DT2aP-IPV-Hib-HBV and DT3aP-IPV-Hib-HBV vaccine courses were not significantly different, with 96–100% and 99–100% reaching this level of protection, respectively [Citation36,Citation37,Citation40–42]. The proportion of participants with ≥100mIU/mL anti-Hep B antibodies was 87–100% in the DT3aP-IPV-Hib-HBV group, similar when compared with 72–99% of the participants who received DT2aP-IPV-Hib-HBV. Differences noted in levels of anti-Hep B antibody persistence were noted in long-term studies in section 3.12 [Citation35,Citation43,Citation53–55].
3.8. Studies in HIV infected subjects
A post-licensure commitment of DT2aP-IPV-Hib-HBV was to evaluate the primary series, antibody persistence, and booster response in those who are HIV positive. A phase III, open-label, randomized study was performed at a single center in the Republic of South Africa [Citation50]. DT2aP-IPV-Hib-HBV was administered to HIV-exposed infected (Group A: N = 14) and HIV-exposed uninfected (Group B: N = 50) infants as a 6, 10, 14 week primary series with a toddler booster at 15–18 months of age. Immunogenicity of each antigen was measured using validated assays. In each group, primary series and booster immune seroprotection rates were strong, and pre-booster antibody persistence was good, although anti-HepB ≥10 mIU/mL in Group A was 78.6% post-primary series, 58.3% pre-booster, and 75.0% post-booster.
3.9. Injection site reactions
The overall safety profiles of DT2aP-IPV-Hib-HBV and alternative vaccines were similar and acceptable, with the incidence of injection site reactions within seven days of any injection being similar in most trials performed [Citation33,Citation35–39,Citation42–45,Citation47,Citation49,Citation51–54]. In the study by López et al. (2017) [Citation40], erythema was reported in the DT2aP-IPV-Hib-HBV group 44% (95% CI 40–47) of the time and 34% (95% CI 29–40) in the alternative vaccine group following the primary series vaccination, although the rates were similar in the follow-up booster vaccine trial [Citation40]. Additionally, the incidence of solicited injection site swelling in the group who received DT2aP-IPV-Hib-HBV was similar at 41% compared with 32% in the group who received DT3aP-IPV-Hib-HBV [Citation41]. In a trial comparing the immunogenicity and reactogenicity of DT2aP-IPV-Hib-HBV in patients who are HIV positive and negative, solicited injection site reactions were reported in those who were HIV positive and compared to those free of infection. During the primary series, solicited injection site reactions were reported 29% of the time in HIV positive participants and 56% of the time in HIV negative participants; during the booster phase of the trial, the rates were 42% and 38%, respectively [Citation50].
3.10. Systemic adverse events
The overall systemic reactogenicity safety profile of DT2aP-IPV-Hib-HBV was similar to that of alternative vaccines in all the trials, which evaluated the safety of the vaccines. Although this was the case, there were some statistically significant differences observed in the frequency of specific systemic reactions across various trials detailed below. Systemic reactions included but were not limited to pyrexia, vomiting, crying, somnolence, anorexia, and irritability.
Post-dose 1, the incidence of crying was higher in infants given DT2aP-IPV-Hib-HBV (49%, 95% CI 43–55) vs Pentaxim with Engerix B Pediatrico (36%, 95% CI 31–42). The incidence of pyrexia was also higher in infants given DT2aP-IPV-Hib-HBV (37%, 95% CI 32–43) than those given Pentaxim with Engerix B Pediatrico (23%, 95% CI 18–28). These differences were not observed in subsequent doses [Citation34]. Aquino et al. (2012) [Citation36] also showed the frequency of abnormal crying in the group given DT2aP-IPV-Hib-HBV was 67% (95% CI 64–70) and 55% (95% CI 47–63) in the Infanrix Hexa group; all other reactions occurred at a similar frequency in this trial. The incidence of pyrexia (all grades) was higher in children given DT2aP-IPV-Hib-HBV was 20% (95% CI 14–28) and 8% (95% CI 4–13) in those given Pentaxim with a separate HepB vaccination (38). Fever occurred similarly in infants given DT2aP-IPV-Hib-HBV and Pentaxim combined with Engerix B with 41% and 28% of infants having a fever in each group, respectively. The incidence of grade 3 fever was also similar (2%) in both groups [Citation39].
3.11. Concomitant administration with other vaccines
Various trials have been conducted that have evaluated the immune response and safety profile of DT2aP-IPV-Hib-HBV given concomitantly with other childhood vaccines. Some include PCV13 [Citation37], PCV13 and Rotateq [Citation42], PCV7 & Rotarix [Citation40], Measles, Mumps, Rubella (MMR) & Varicella [Citation43], NeisVac-C [Citation46] and MenACWY [Citation47].
DT2aP-IPV-Hib-HBV or DT3aP-IPV-Hib-HBVwere given in a 3-, 5- and 11–12-months schedule alongside PCV13. The safety and immunogenicity profiles were acceptable, with DT2aP-IPV-Hib-HBV showing noninferiority to DT3aP-IPV-Hib-HBV [Citation37]. In another 2-part trial, DT2aP-IPV-Hib-HBVwas given to infants in a 2, 4, 6 months, and 12–24-month booster schedule alongside PCV7 with Rotarix given at 2 and 4 months and showed a similar and acceptable immunogenicity and safety profile [Citation40]. Additionally, a 2-part trial where DT2aP-IPV-Hib-HBV was given to children in a 2, 3, 4-month primary schedule with a 11 to 15-month booster in conjunction with PCV13 showed an overall acceptable safety profile and immune response [Citation42].
A trial was conducted to compare the immunogenicity and reactogenicity of DT2aP-IPV-Hib-HBVwith or without NeisVac-C concomitant vaccination in a 2, 3, 4-month primary schedule with NeisVac-C given at 2 and 4 months in the comparator group [Citation46]. One-month after the primary vaccination schedule, there were no observable differences between the two groups in the seroprotection rates for any of the antigens. The proportion of children displaying antibody responses above the established correlates of protection for HepB, Dip, Tet, Hib, and polio were comparable and high in both groups at 98–100% with NeisVac-C and 94–100% without NeisVac-C; the anti-PT and anti-FHA responses were also similar between the groups (>99%). Additionally, the GMTs and GMCs were comparable between the two groups, although higher in the group with NeisVac-C for anti-Hib and anti-tetanus antibodies. The higher levels are due to NeisVac-C using a tetanus toxoid carrier protein which has been demonstrated to induce in itself anti-tetanus antibodies as well as enhanced immune responses to Hib conjugate vaccines [Citation56]. The overall safety profiles were also comparable with the only notable difference between groups being that crying, 85% (95% CI 79–90) vs 71% (95% CI 64–78) was reported more often in the group with NeisVac-C than without [Citation46].
A booster trial was conducted that compared the concomitant administration of the MenACWY vaccination with DT2aP-IPV-Hib-HBV following the primary schedule detailed in the study by Vesikari et al. (2017) [Citation37]. Participants were randomized into three groups and offered either: DT2aP-IPV-Hib-HBV & MenACWY-TT at 12 months, PCV13 at 13 months; DT2aP-IPV-Hib-HBV at 12 months, MenACWY-TT & PCV13 at 13 months; or MenACWY-TT at 12 months, PCV13 and DT2aP-IPV-Hib-HBV at 13 months. All participants were offered an optional M-M-RvaxPro at 13 months of age [Citation47]. One-month post-booster dose, there were no observable differences between the group given DT2aP-IPV-Hib-HBV with MenACWY-TT at 12 months (A) with the group given MenACWY-TT alone at 12 months (C), in terms of rates of seroprotection (98–100% vs 99–100%) for any of the DT2aP-IPV-Hib-HBV vaccine antigens. Injection site reactions within seven days after DT2aP-IPV-Hib-HBV vaccination occurred in 66% of Group A and 60% in Group B (DT2aP-IPV-Hib-HBValone at 12 months). MenACWY-TT was associated with reactions in 48% in Group A and 32% in Group C. Systemic reactions were reported for group A and group C for crying (51% vs 30%), irritability (77% vs 49%), pyrexia (30% vs 11%), and somnolence (52% vs 32%) [Citation47].
3.12. Vaccine batch comparisons
Several studies compared different batches (lots) of DT2aP-IPV-Hib-HBV to ensure there was an equivalent immune and safety response [Citation36,Citation40,Citation44]. Infants were randomized to receive one of the three batches of DT2aP-IPV-Hib-HBV or DT3aP-IPV-Hib-HBVat 2, 4 and 6 months in addition to a booster vaccination at 15–18 months [Citation36]. After the primary schedule, ≥96% of the infants in both the DT2aP-IPV-Hib-HBV and DT3aP-IPV-Hib-HBVgroups were protected against all antigens in the vaccine. The seroprotection and seroconversion rates of the three DT2aP-IPV-Hib-HBV batches were equivalent as the 90% confidence intervals between batch differences for each antigen was entirely within the predefined clinically acceptable range (±5% for IPV, ±10% for all other antigens).
Another study where infants received HepB vaccine at birth were randomized to receive 1 of 3 batches of DT2aP-IPV-Hib-HBV or a licensed comparator (DT3aP-IPV-Hib-HBV) at 2, 4 and 6 months, concomitantly with PCV7 (2, 4 and 6 months) and Rotarix (2 and 4 months). Booster doses of either DT2aP-IPV-Hib-HBV or DT3aP-IPV-Hib-HBV were given at 12–24 months with PCV7 [Citation40]. The three batches of DT2aP-IPV-Hib-HBV were deemed to be equivalent and therefore pooled and shown to be non-inferior to DT3aP-IPV-Hib-HBVfor each antigen.
In a study evaluating the safety profile and immunogenicity for HepB only, 3 randomized batches of DT2aP-IPV-Hib-HBV were given to infants in Peru at 2, 4 and 6 months [Citation44]. The seroprotection rates for HepB above the 10mIU/mL threshold was 100% within each of the 3 batches of DT2aP-IPV-Hib-HBV, with 92–98% of infants receiving each batch having anti-HepB antibody levels ≥100mIU/mL. The three groups also displayed similar GMCs at 1108, 969, and 1169, although the GMC level was approximately threefold higher in the control group who received DTwP-Hep B//Hib (Tritanrix-Hep B/Hib) than in the pooled DT2aP-IPV-Hib-HBV group.
3.13. Long-term antibody persistence
Trials have evaluated the antibody persistence in infants previously vaccinated with DT2aP-IPV-Hib-HBV primary and booster schedules, but only four studies assessed antibody persistence at 3.5, 4.5, 6 and 9–10 years [Citation53–55]. A trial was conducted evaluating long-term anti-Hep-B antibody persistence and the response to an additional HepB vaccination at 9–10 years of age [Citation53]. Participants in this trial had either previously received a primary vaccination series of DT2aP-IPV-Hib-HBV or DT3aP-IPV-Hib-HBVin Thailand [Citation41]. The anti-HepB surface antigen antibody seroprotection rates were high and comparable in each group one-month post the primary schedule. The antibody GMCs declined 6–12 months after the primary schedule with seroprotection rates remaining similar between groups. The anti-HepB antibody levels decreased at the 9–10 years’ time point post-primary series; this drop rate was similar in each group. The seroprotection rate in terms of the threshold ≥10mIU/mL at 9–10 years post-primary schedule was 49% and 43%, respectively. The participants were boosted with a single HepB, with both groups showing a similar anamnestic response with 93% and 99% seroprotected, respectively.
Observational studies were performed on two cohorts that were participants in previous studies in South Africa [Citation33,Citation43] and Latin America [Citation40]. The South African study participants had not received HepB at birth and followed a 6, 10, 14-week primary series with a booster at 15–18 months; the Latin American study participants did receive HepB vaccination at birth and followed a 2, 4, 6-month primary series followed by a 12 to 24-month booster. The participants of each study were then followed up at 3.5 and 4.5 years to assess antibody persistence for each antigen. The participants who received HepB vaccination at birth showed an increased anti-HepB antibody persistence (≥10mIU/mL) following DT2aP-IPV-Hib-HBV primary and booster vaccination from 76% (95% CI 69–82) with no birth dosage to 96% (95% CI 90–99) with a birth HepB dosage at 3.5 years. In those who did not receive a HepB birth dosage, the persistence was high and comparable in each group. All other antigens showed no notable differences between groups or studies for both 3.5 and 4.5 years post-primary vaccination schedules [Citation54].
Anti-HepB antibody persistence was evaluated [Citation55] in a separate study at 6 years of age following a primary vaccination schedule of 3, 5, and 11–12 months with either DT2aP-IPV-Hib-HBV or DT3aP-IPV-Hib-HBV [Citation36]. Seroprotection (≥10mIU/mL) for Hep B was observed at 6 years being 54% and 74%, which increased to 97% and 96% post-HepB revaccination, respectively; this confirms that a strong anamnestic response occurs in infants previously primed with DT2aP-IPV-Hib-HBV.
4. Discussion
This review evaluated 23 original articles, containing 29 separate trials, focusing on the immunogenicity and/or safety of the hexavalent vaccine DT2aP-IPV-Hib-HBV, which provides protection against six childhood diseases. The reviewed data demonstrate that DT2aP-IPV-Hib-HBV has a good safety and immunogenicity profile when given during childhood and that this is comparable with the safety and immunogenicity profiles of other licensed vaccines such as DT3aP-IPV-Hib-HBV. The immune response was also comparable when given in different schedules such as 2, 4, 6 months; 6, 10, 14 weeks, and other various schedules. These trials were performed in various regions in Europe, Asia, and Africa, with the immune response and safety profile being acceptable regardless of the country the trials were performed in. Although the responses were similar and above the predefined correlate or surrogates of protection, some minor differences were observed in the post primary and post-booster antibody concentrations.
Long-term antibody persistence studies [Citation53–55] showed that antibodies persist into early childhood, but data were limited due to DT2aP-IPV-Hib-HBV only being licensed since 2013 in Europe. It has been demonstrated that the more HepB doses given (particularly when given at birth), the higher the HepB antibody concentration later in childhood was [Citation54]. It has also been shown that vaccination with HepB at 6 years old creates a strong anamnestic response and restores protection levels to above the correlation of protection [Citation55]. Despite these encouraging findings, there are few studies evaluating long-term antibody persistence post DT2aP-IPV-Hib-HBV vaccination, there are no studies that evaluate the effects of booster vaccination in late childhood or antibody persistence in adulthood due to the vaccine only being around for less than 10 years when compared to DT3aP-IPV-Hib-HBVwhich has been licensed for over 20 years.
DT2aP-IPV-Hib-HBV was licensed based on studies that demonstrated noninferiority in terms of immunogenicity in comparison to already licensed alternative vaccines such as DT3aP-IPV-Hib-HBV. The studies available do not demonstrate equivalence and have not assessed the superiority of immune responses of Hexaxim against alternative vaccines.
A review of three Hexavalent vaccines: DT2aP-IPV-Hib-HBV, Vaxelis and DT3aP-IPV-Hib-HBV, was performed in 2021 that highlighted similarities and differences in these vaccines by looking at head-to-head trials [576]. This review determined that the three hexavalent vaccines are similar in terms of immunogenicity, but some differences were determined that may be due to the differences in composition of the three vaccines; the clinical relevance of these findings was not established. DT2aP-IPV-Hib-HBV was determined to be more reactogenic than DT3aP-IPV-Hib-HBV, although both vaccines displayed acceptable safety profiles. This was also highlighted in the review of Mukherjee et al. [Citation57,Citation58]. Anti-FHA (pertussis) and anti-PRP (Hib) concentrations tended to be higher in DT2aP-IPV-Hib-HBV subjects when compared with DT3aP-IPV-Hib-HBV. Additionally, anti-HepB and anti-PT concentrations tended to be lower in DT2aP-IPV-Hib-HBV when compared directly with concentrations in DT3aP-IPV-Hib-HBVrecipients.
A recent review focussing on the immunogenicity and safety of DT2aP-IPV-Hib-HBV specifically, and not hexavalent vaccines in general, was performed in 2019 [Citation8]. This review had similar findings, with DT2aP-IPV-Hib-HBV demonstrating non-inferiority in all the trials evaluated. They also observed that DT2aP-IPV-Hib-HBVm was well tolerated, with a safety profile similar to already licensed alternative vaccines such as DT3aP-IPV-Hib-HBV. Since this review was performed in 2019, new data have become available surrounding long-term antibody persistence [Citation55] and the use of DT2aP-IPV-Hib-HBV in patients with HIV [Citation50].
This review also pointed out the similarities in immune response when compared to quadrivalent combination vaccines such as CombAct-Hib with Engerix B and OPV [Citation33]. CombAct-Hib consists of a DTwP liquid suspension, which is that required to reconstitute freeze-dried Hib vaccine. This study allowed for the comparison of aP vs wP, IPV vs OPV and Hexyon vaccination with or without a dose of HepB at birth. DT2aP-IPV-Hib-HBV was non-inferior to the CombAct-Hib/Engerix B/OPV combination regarding the post-primary schedule seroprotection rates for anti-D, anti-T, anti-polio (1–3), anti-HepB, and anti-Hib.
The pentavalent vaccine, Pentaxim, was also compared with DT2aP-IPV-Hib-HBV, across numerous studies [Citation34,Citation35,Citation38,Citation39]. Pentaxim is a DTaP-IPV liquid suspension that is used to reconstitute freeze-dried Hib vaccine and must be administered with a standalone, monovalent Hep B vaccine such as Engerix B [Citation34,Citation35,Citation39] or Euvax B [Citation38]. DT2aP-IPV-Hib-HBV has also been compared with the pentavalent vaccine, Tritanrix-Hep B/Hib, and OPV, but only safety and Hep B immunogenicity was evaluated in this trial [Citation44]. DT2aP-IPV-Hib-HBV was non-inferior to Pentaxim regardless of the choice of standalone HepB vaccination administered alongside it. DT2aP-IPV-Hib-HBV was more reactogenic and after the first dose, crying, and pyrexia were seen more frequently in children given DT2aP-IPV-Hib-HBV compared with Pentaxim and Engerix B [Citation34]. Pyrexia was also more common in infants receiving DT2aP-IPV-Hib-HBV than in those who received Pentaxim with Euvax B [Citation38]. Additionally, crying was also seen to be more common in infants who received DT2aP-IPV-Hib-HBV in comparison to DT3aP-IPV-Hib-HBV [Citation36].
The vaccines differ slightly in their composition, with DT3aP-IPV-Hib-HBVhaving a higher quantity of diphtheria toxoid (DT) (≥30IU) compared with both DT2aP-IPV-Hib-HBV and Vaxelis (≥20IU). DT2aP-IPV-Hib-HBV targets only two pertussis antigens (PT and FHA), whereas DT3aP-IPV-Hib-HBVtargets three and Vaxelis targets five. In some regions where Pertussis is still a large cause of morbidity and mortality, wP is recommended despite the risk of paralysis, due to its superior immunological protection. Children who received aP vaccines containing only three pertussis antigens such as DT3aP-IPV-Hib-HBV, were more likely to be diagnosed with pertussis than those who received wP containing vaccines. In addition, those who received aP vaccines with five pertussis antigens, such as Vaxelis, showed a similar risk of contracting pertussis compared with wP [Citation59]. It would be beneficial for more comparisons to be made with DT2aP-IPV-Hib-HBV and wP containing vaccines to determine if the risk of contracting pertussis is higher due to DT2aP-IPV-Hib-HBV only containing two pertussis antigens.
Previous Hep B vaccination has been based on recombinant HepB surface antigen (HBsAg) produced from the recombinant yeast Saccharomices cerevisiae or Pichia pastoris. HepB vaccines are also commonly produced from recombinant mammalian cells such as Chinese Hamster Ovary (CHO). The Hep B portion of the DT2aP-IPV-Hib-HBV vaccine differs from other alternative vaccines such as DT3aP-IPV-Hib-HBV, as it is derived from the yeast Hansenula polymorpha [Citation7]. These vaccines, including Hexaxim with the H. polymorpha derived Hep B have all been shown to be highly immunogenic [Citation60]. The HepB seroprotection rates in direct head-to-head studies with Hexaxim and DT3aP-IPV-Hib-HBVshow that although they are both high and DT2aP-IPV-Hib-HBV has been shown to be noninferior that the seroprotection rates are numerically higher in DT3aP-IPV-Hib-HBVrecipients (99–100%) than in DT2aP-IPV-Hib-HBV recipients (96–100%). This discrepancy is more defined when the rate of participants with anti-HepB antibodies ≥100mIU/mL is observed with those getting DT3aP-IPV-Hib-HBV being 87–100% vs only 72–99% of DT2aP-IPV-Hib-HBV [Citation36–38,Citation40–42,Citation45].
Another difference between DT2aP-IPV-Hib-HBV and DT3aP-IPV-Hib-HBV, is that DT2aP-IPV-Hib-HBV is a fully liquid ready to use preparations, whereas DT3aP-IPV-Hib-HBV requires reconstitution of the Hib component prior to administration. There is evidence to suggest that fully liquid vaccines such as DT2aP-IPV-Hib-HBV, have a lower error rate and reduced preparation time in comparison to reconstituted preparations [Citation61]. A study has shown that the average preparation time of fully liquid vaccines is 36 seconds and the average for the reconstituted vaccines is 70 seconds. Additionally, they found that out of the 192 preparations, 57 immunization errors occurred, of which 47 were in the reconstituted group and only 10 in the fully liquid group [Citation62].
Healthcare providers have also been surveyed regarding their opinion on fully liquid and reconstituted vaccines and it was found that reducing the probability of dosage errors was the most important factor when choosing a vaccine to both nurses and physicians. It was also noted that both nurses and physicians preferred fully liquid vaccines and a reduction in preparation time [Citation63]. A survey completed by 151 pediatricians and 201 general practitioners (GP) discovered that 70% of pediatricians and 57% of GPs think vaccine reconstitution is a complicating factor. It also found that overall, 28% had occasionally omitted the reconstitution step of a pentavalent or hexavalent vaccine completely, and 60% have occasionally not fully reconstituted a vaccine [Citation64].
A post-marketing study was performed in the Apulia region of Italy, into the administration of DT2aP-IPV-Hib-HBV in preterm infants (PTIs). The PTIs were born between January and June of 2017, with the timeliness and coverage of vaccination analyzed in addition to interviewing parents to investigate adverse events. They discovered that 92% of PTIs had received the first dose of a hexavalent vaccine, with 58% vaccinated by the third month of age, as is recommended. Of the PTIs who received a hexavalent vaccine, 81% received DT2aP-IPV-Hib-HBV. The safety side of the study revealed that local pain was the most common symptom (36%) with other common symptoms such as erythema and swelling present in 25% of the infants. They concluded that more than 40% of PTIs received a delayed hexavalent vaccination, but that the safety profile was reassuring [Citation65].
Although there are numerous trials that show DT2aP-IPV-Hib-HBV is noninferior to other hexavalent vaccines that have been on the market for over 20 years such as DT3aP-IPV-Hib-HBV, there are no studies that have determined the equivalence of DT2aP-IPV-Hib-HBV to other hexavalent vaccines such as Infanrix Hexa or Vaxelis.
There are only a few studies that have evaluated the long-term antibody persistence in infants who received primary and booster DT2aP-IPV-Hib-HBV vaccinations. Of these, the more recently completed study only evaluated anti-HepB antibodies, not providing a full picture of the antibody persistence for antibodies against the remaining five diseases [Citation55].
There are few data analyzing the use of DT2aP-IPV-Hib-HBV in immunocompromised patients, with only one trial currently available focussing on HIV infected individuals [Citation50]. The sample size of this trial was much smaller than typical clinical trials due to the nature of the exclusion criteria. This trial had a comparatively low number of individuals in the HIV positive group (n = 14) in comparison to the HIV uninfected group (n = 50), so any comparisons made in this trial need to be taken with care.
5. Summary
DT2aP-IPV-Hib-HBV (Hexyon, Hexacima) is immunogenic and safe when given to pediatric patients in both primary and booster vaccination schedules. It is non-inferior to other hexavalent vaccines on the market, including DT3aP-IPV-Hib-HBV. When DT2aP-IPV-Hib-HBV is given concomitantly with other childhood vaccinations, including: PCV13; PCV13 and Rotateq; PCV7 and Rotarix; MMR and Varicella; NeisVac-C; or MenACWY, it remains immunogenic and has no adverse effect on the immune response to the other vaccines given. Batch to batch comparisons show that DT2aP-IPV-Hib-HBV provides a consistent immune response across batches. Antibodies to each antigen persist to preschool (3.5 to 4.5) years, regardless of the primary and booster vaccination schedule. Long-term Hep B antibody persistence is similar to other hexavalent vaccines on the market, with antibody levels dropping at a similar rate. Anti-Hep B antibody levels can be brought back to above the correlation of protection with a single booster dosage in children aged 9–10 years, showing an anamnestic response. The immunogenicity and safety profile of DT2aP-IPV-Hib-HBV in HIV positive infants were like those who were uninfected. Antipyretics had no negative effect on the immune responses elicited from DT2aP-IPV-Hib-HBV vaccination.
6. Expert opinion
Hexavalent vaccines have demonstrated the ability to reduce the global disease burden of Diphtheria, Tetanus, Pertussis, Polio, HepB and disease caused by Hib. Infants have an increased risk of developing severe symptoms or having a fatal infection due to their immature immune system as they are developing. Prevention is more effective than treatment, and so preventing these infections is crucial to reduce the morbidity and mortality caused by these diseases. The WHO recommends that infants should be protected against the six diseases that DT2aP-IPV-Hib-HBV provides immunological protection from, so ensuring high rates of vaccination in infants is vital. Our review determined that DT2aP-IPV-Hib-HBV is a good choice of hexavalent vaccine to use for the protection against these childhood diseases as it provides a good, lasting immune response and has an acceptable safety profile. It also found that concomitant administration with other childhood vaccinations does not reduce its effectiveness. The vaccine can be given as either a primary or booster vaccination and has demonstrated effectiveness in a variety of different populations across Asia, Africa, and Europe. Long-term immunity is provided, similarly to other hexavalent vaccines on the market, but the anti-HepB antibody levels can be boosted with an additional HepB dose in late childhood. Finally, infants with HIV can also be given DT2aP-IPV-Hib-HBV as it has a similar immune response and safety profile when compared with HIV uninfected infants.
6.1. Five-year view
Future studies should look at evaluating not just the noninferiority of DT2aP-IPV-Hib-HBV against alternative vaccines, but whether it has a superior or equivalent immune response and safety profile. All studies so far have been in infants with a lack of trials looking into the effect of boosters in later childhood or adulthood. There are few studies available with data on long-term antibody persistence, so more controlled studies following infants into late childhood and adulthood should be considered to elucidate the longevity of immune memory in those given primary and booster courses of DT2aP-IPV-Hib-HBV when infants. These studies could be completed in tandem with those evaluating the effect of booster dosages later in life, to determine how many doses may be required to maintain immunity into adulthood. More trials are required to investigate the immunogenicity and safety of DT2aP-IPV-Hib-HBV in immunocompromised patients. There is currently only one study into those with HIV and this has a small sample size, making comparisons difficult. Larger, controlled trials comparing healthy and immunocompromised patients, including those with HIV, would help aid decision-making for public health departments for the vaccination of vulnerable infants. There is also a lack of published efficacy data for DT2aP-IPV-Hib-HBV, this is of particular importance due to this hexavalent vaccine only containing 2 pertussis components. A study from Sweden in the 1990s demonstrated that after three doses in infancy, the efficacy of the vaccines with respect to pertussis linked to a laboratory-confirmed case of pertussis or contact with an infected household member with paroxysmal cough for ≥ = 21 days was 58.9% for an experimental two-component aP vaccine (95% CI 50.9 to 65.9), 85.2% for an experimental five-component aP vaccine (95% CI, 80.6 to 88.8), and 48.3% for the licensed whole-cell vaccine (95% CI, 37.0 to 57.6) [Citation66].
Article highlights
Twenty-three original articles, containing 29 individual trials were included in this systematic review
Hexaxim (DT2aP-IPV-Hib-HBV) was licensed based on studies that demonstrated noninferiority in terms of immunogenicity in comparison to already licensed alternatives such as Infanrix Hexa
DT2aP-IPV-Hib-HBV is a fully liquid ready to use vaccine
DT2aP-IPV-Hib-HBV provides protection against six childhood diseases
DT2aP-IPV-Hib-HBV has an acceptable safety profile
DT2aP-IPV-Hib-HBV elicits a similar immune response and safety profile in Human immunodeficiency virus (HIV) positive infants
Declaration of interest
R Borrow performs contract research on behalf of UK Health Security Agency for GSK, Pfizer and Sanofi Pasteur. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Skibinski DA, Baudner BC, Singh M, et al. Combination vaccines. J Glob Infect Dis. 2011;3(1):63–72.
- Obando-Pacheco P, Rivero-Calle I, Gómez-Rial J, et al. New perspectives for hexavalent vaccines. Vaccine. 2018;36(36):5485–5494.
- Woodin KA, Rodewald LE, Humiston SG, et al. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med. 1995;149(8):845–849.
- European Medicines Agency. Public statement on hexavac: withdrawal of marketing authorisation in the European Union. 2012 [cited 2022 Dec 23]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/hexavac
- Dolhain J, Janssens W, Mesaros N, et al. Hexavalent vaccines: increasing options for policy-makers and providers. A review of the data supporting interchangeability (substitution with vaccines containing fewer antigens) and mixed schedules from the same manufacturer. Expert Rev Vaccines. 2018;17(6):513–524.
- World Health Organisation. Table 2: summary of WHO position papers - recommended routine immunizations for children. (2021). https://www.who.int/docs/default-source/immunization/immunization_schedules/immunization-routine-table2
- European Medicines Agency. Hexyon: EPAR - Product Information. 2022 [cited 2022 Dec 23]. https://www.ema.europa.eu/en/documents/product-information/hexyon-epar-product-information_en.pdf
- Syed YY. DTaP-IPV-HepB-Hib vaccine (hexyon®): an updated review of its use in primary and booster vaccination. Paediatr Drugs. 2019;21(5):397–408.
- World Health Organization. Immunological basis for immunization: module 2: diphtheria - update 2009 [cited 2022 Dec 23]. https://apps.who.int/iris/bitstream/handle/10665/44094/9789241597869_eng.pdf?sequence=1&isAllowed=y
- United Kingdom Health Security Agency. Public health control and management of diphtheria in England: 2022 Guidelines. 2022 [cited 2022 Dec 23]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1083946/Diphtheria-guidelines-2022-v16.1.pdf
- World Health Organization. Diphtheria vaccine: WHO position paper, August 2017 - recommendations. Vaccine. 2018;36(2):199–201.
- United Kingdom Health Security Agency. Diphtheria. In: The Green Book: Immunisation against infectious disease. 2013 [cited 2022 Dec 23]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/147952/Green-Book-Chapter-15.pdf
- Kyu HH, Mumford JE, Stanaway JD, et al. Mortality from tetanus between 1990 and 2015: findings from the global burden of disease study 2015. BMC Public Health. 2017;17(1):179.
- Collins S, Amirthalingam G, Beeching NJ, et al. Current epidemiology of tetanus in England, 2001-2014. Epidemiol Infect. 2016;144(16):3343–3353.
- United Kingdom Health Security Agency. Tetanus in England: 2021 - GOV.UK [Internet]. 2021 [cited 2022 Dec 23]. Available from: https://www.gov.uk/government/publications/tetanus-in-england-annual-reports/tetanus-in-england-2021.
- World Health Organization. The immunological basis for immunization series: module 3: tetanus. World Health Org. 2018. https://apps.who.int/iris/bitstream/handle/10665/275340/9789241513616-eng.pdf?sequence=1&isAllowed=y
- World Health Organisation. Pertussis [Internet]. 2022 [cited 2022 Dec 23]. Available from: https://www.who.int/health-topics/pertussis.
- European Centre for Disease Prevention and Control. Pertussis In: ECDC. Annual epidemiological report for 2018. 2020 [cited 2022 Dec 23]. https://www.ecdc.europa.eu/en/publications-data/pertussis-annual-epidemiological-report-2018#:~:text=Pertussis%20In%3A%20ECDC.-,Annual%20epidemiological%20report%20for,Stockholm%3A%20ECDC%3B%202020.&text=In%202018%2C%20there%20were%2035,72%25%20of%20all%20notified%20cases
- United Kingdom Health Security Agency. Laboratory confirmed cases of pertussis in England: annual report for 2021 - GOV.UK [Internet]. 2022 [cited 2022 Dec 23]. Available from: https://www.gov.uk/government/publications/pertussis-laboratory-confirmed-cases-reported-in-england-2021/laboratory-confirmed-cases-of-pertussis-in-england-annual-report-for-2021.
- World Health Organization. The immunological basis for immunization series: module 4: pertussis. World Health Org. 2010. https://apps.who.int/iris/bitstream/handle/10665/259388/9789241513173-eng.pdf?sequence=1&isAllowed=y
- World Health Organisation. Poliomyelitis [Internet]. 2022 [cited 2022 Dec 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis.
- Bandyopadhyay AS, Garon J, Seib K, et al. Polio vaccination: past, present and future. Future Microbiol. 2015;10(5):791–808.
- Mehndiratta MM, Mehndiratta P, Pande R. Poliomyelitis: historical facts, epidemiology, and current challenges in eradication. Neurohospitalist. 2014;4(4):223–229.
- Wise J. Poliovirus is detected in sewage from north and east London. BMJ. 2022;377:o1546.
- New York State Department. New York state department of health and rockland county department of health alert the public to a case of polio in the county. 2022 [cited 2022 Dec 23]. https://health.ny.gov/press/releases/2022/2022-07-21_polio_rockland_county.htm.
- Global Polio Laboratory Network. Short report on type 2 polioviruses detected in the USA, Israel and the UK. 2022 [cited 2022 Dec 23]. https://polioeradication.org/wp-content/uploads/2022/07/VP1-narrative-ISR-NY-UK-29072022.pdf
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065.
- European Centre for Disease Prevention and Control. Haemophilus influenzae. ECDC. In: Annual epidemiological report for 2018.2020. https://www.ecdc.europa.eu/en/publications-data/haemophilus-influenzae-annual-epidemiological-report-2018
- World Health Organization. Immunological basis for immunization: haemophilus influenzae type b vaccines. 2007 [cited 2022 Dec 23]. https://www.who.int/publications/i/item/who-immunological-basis-for-immunization-series-module-9-haemophilus-influenzae-type-b
- World Health Organization. Hepatitis B [Internet]. 2022 [cited 2022 Dec 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- World Health Organization. The immunological basis for immunization series: module 22: hepatitis B. 2011 [cited 2022 Dec 23]. https://www.who.int/publications/i/item/who-immunological-basis-for-immunization-series-module-22-hepatitis-b
- Sanofi. Clinical study results pasteur - sanofi [Internet]. 2022 [cited 2022 Dec 23]. Available from: https://www.sanofi.com/en/science-and-innovation/clinical-trials-and-results/our-disclosure-commitments/pasteur
- Madhi SA, Mitha I, Cutland C, et al. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatr Infect Dis J. 2011;30(4):68–74.
- Tregnaghi MW, Zambrano B, Santos-Lima E. Immunogenicity and safety of an investigational hexavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B-Haemophilus influenzae b conjugate combined vaccine in healthy 2-, 4-, and 6-month-old Argentinean infants. Pediatr Infect Dis J. 2011;30(6):88–96.
- Tregnaghi M, Zambrano B, Santos-Lima E. Antibody persistence after a primary series of a new DTaP-IPV-Hep B-PRP-T combined vaccine or separate DTaP-IPV//PRP-T and hepatitis B vaccines at 2, 4, and 6 months of age and the effect of a subsequent DTaP-IPV//PRP-T booster vaccination at 18 months of age in healthy Argentinean infants. Pediatr Infect Dis J. 2012;31(1):24–30.
- Aquino AGB, Brito MG, Doniz CEA, et al. A fully liquid DTaP-IPV-Hep B-PRP-T hexavalent vaccine for primary and booster vaccination of healthy mexican children. Vaccine. 2012;30(45):6492–6500.
- Vesikari T, Silfverdal S-A, Jordanov E, et al. A randomized, controlled study of DTaP-IPV-HB-PRP-T, a fully liquid hexavalent vaccine, administered in a 3-, 5- and 11- to 12-month schedule. Pediatr Infect Dis J. 2017;36(1):87–93.
- Kim Y-K, Vidor E, Kim HM, et al. Immunogenicity and safety of a fully liquid DTaP-IPV-HB-PRP∼T hexavalent vaccine compared with the standard of care in infants in the Republic Of Korea. Vaccine. 2017;35(32):4022–4028.
- Ceyhan M, Yıldırım İ, Tezer H, et al. A fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine for primary and booster vaccination of healthy Turkish infants and toddlers. Turk J Med Sci. 2017;47(4):1247–1256.
- López P, Arguedas Mohs A, Abdelnour Vásquez A, et al. A randomized controlled study of a fully liquid DTaP-IPV-HB-PRP-T Hexavalent vaccine for primary and booster vaccinations of healthy infants and toddlers in Latin America. Pediatr Infect Dis J. 2017;36(11):272–282.
- Kosalaraksa P, Thisyakorn U, Benjaponpitak S, et al. Immunogenicity and safety study of a new DTaP-IPV-Hep B-PRP-T combined vaccine compared to a licensed DTaP-IPV-Hep B//PRP-T comparator, both concomitantly administered with a 7-valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. Int J Infect Dis. 2011;15(4):249–256.
- Prymula R, Kieninger D, Feroldi E, et al. Immunogenicity and safety of primary and booster vaccinations of a fully liquid DTaP-IPV-HB-PRP-T Hexavalent vaccine in healthy infants and toddlers in Germany and the Czech Republic. Pediatr Infect Dis J. 2018;37(8):823–830.
- Madhi SA, Koen A, Cutland C, et al. Antibody persistence and booster vaccination of a fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15-18 months of age in healthy South African infants. Pediatr Infect Dis J. 2013;32(8):889–897.
- Macías M, Lanata CF, Zambrano B, et al. Safety and immunogenicity of an investigational fully liquid hexavalent DTaP-IPV-Hep B-PRP-T vaccine at two, four and six months of age compared with licensed vaccines in Latin America. Pediatr Infect Dis J. 2012;31(8):126–132.
- Lanata C, Zambrano B, Ecker L. Immunogenicity and safety of a fully liquid DTaP-IPV-Hep B-PRP-T vaccine at 2-4-6 months of age in peru. J Vaccines Vaccin. 2012;3(1). https://www.walshmedicalmedia.com/open-access/immunogenicity-and-safety-of-a-fully-liquid-dtap-ipv-hep-b-prp-t-vaccine-at-2-4-6-months-of-age-in-peru-2157-7560.1000128.pdf.
- Vesikari T, Borrow R, Da Costa X, et al. Concomitant administration of a fully liquid, ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal serogroup C conjugate vaccine in infants. Vaccine. 2017;35(3):452–458.
- Vesikari T, Borrow R, Da Costa X, et al. Concomitant administration of a fully liquid ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal ACWY conjugate vaccine in toddlers. Vaccine. 2018;36(52):8019–8027.
- Chhatwal J, Lalwani S, Vidor E. Immunogenicity and safety of a liquid hexavalent vaccine in Indian infants. Indian Pediatr. 2017;54(1):15–20.
- Martinón-Torres F, Diez-Domingo J, Feroldi E, et al. Evaluation of a hexavalent-pentavalent-hexavalent infant primary vaccination series followed by a pentavalent booster vaccine in healthy infants and toddlers. Pediatr Infect Dis J. 2019;38(3):317–322.
- Koen A, Madhi S, Lyabis O, et al. Immunogenicity and safety of a hexavalent pediatric vaccine in HIV-exposed infected and uninfected infants in Republic of South Africa. Hum Vaccin Immunother. 2012;17(6):1770–1778.
- Sanofi. Immunogenicity and safety of sanofi pasteur’s combined vaccine given as a three-dose primary series at 2, 3,4 months of age and followed by a booster dose given at 16 to 17 months of age in vietnamese infants who previously received a dose of Hepatitis B vaccine at birth or within 1 week after birth - full text view - clinicaltrials.gov [Internet]. 2022 [cited 2022 Dec 23]. https://www.clinicaltrials.gov/ct2/show/NCT02428491?term=A3L35&rank=1.
- Sanofi. EudraCT number 2013-003267-55 - clinical trial results - eu clinical trials register [internet]. 2017 [cited 2022 Dec 23]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-003267-55/results.
- Kosalaraksa P, Chokephaibulkit K, Benjaponpitak S, et al. Persistence of hepatitis B immune memory until 9-10 years of age following hepatitis B vaccination at birth and DTaP-IPV-HB-PRP∼T vaccination at 2, 4 and 6 months. Hum Vaccin Immunother. 2018;14(5):1257–1265.
- Madhi SA, López P, Zambrano B, et al. Antibody persistence in pre-school children after hexavalent vaccine infant primary and booster administration. Hum Vaccin Immunother. 2019;15(3):658–668.
- Virta M, Soininen A, Patel DM, et al. Persistence of hepatitis B immune memory until 6 years of age following hexavalent DTaP-IPV-HB-PRP~T vaccination in a 3-, 5- and 11- to 12-month schedule and response to a subsequent Hepatitis B challenge vaccination. Pediatr Infect Dis J. 2021;40(1):28–30.
- Borrow R, Dagan R, Zepp F, et al. Glycoconjugate vaccines and immune interactions, and implications for vaccination schedules. Expert Rev Vaccines. 2011;10(11):1621–1631.
- Knuf M, Haas H, Garcia-Corbeira P, et al. Hexavalent vaccines: what can we learn from head-to-head studies? Vaccine. 2021;39(41):6025–6036.
- Mukherjee P, Hervé Akpo E, Kuznetsova A, et al. Hexavalent vaccines in infants: a systematic literature review and meta-analysis of the solicited local and systemic adverse reactions of two hexavalent vaccines. Expert Rev Vaccines. 2021;20(3):319–330.
- Harriman K, Healy CM. How important is the type of acellular pertussis vaccine? Clin Infect Dis. 2020;70(2):208–209.
- Tregnaghi MW, Voelker R, Santos-Lima E, et al. Immunogenicity and safety of a novel yeast Hansenula polymorpha-derived recombinant Hepatitis B candidate vaccine in healthy adolescents and adults aged 10-45 years. Vaccine. 2010;28(20):3595–3601.
- Lee YH, Harris RC, Oh HW, et al. Vaccine-related errors in reconstitution in south korea: a national physicians’ and nurses’ survey. Vaccines (Basel). 2021;9(2):117.
- De Coster I, Fournie X, Faure C, et al. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33(32):3976–3982.
- Lloyd AJ, Nafees B, Ziani E, et al. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence. 2015;9:1517–1524.
- Bakhache P, Virey B, Bienenfeld C. Knowledge and practices regarding infant vaccination: results of a survey of French physicians. Eur J Pediatr. 2019;178(4):533–540.
- Martinelli D, Fortunato F, Del Matto G, et al. Post-marketing surveillance study of the DTaP2-IPV-HB-Hib (Hexyon) vaccine administered in preterm infants in the Apulia region, Italy, in 2017. Vaccine. 2020;38(33):5148–5153.
- Gustafsson L, Hallander HO, Olin P, et al. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334(6):349–355.